Vascular+Research
-
 Newly Identified Meningeal Lymphatic Vessels Answers the Key Questions on Brain Clearance
(Figure: Schematic images of location and features of meningeal lymphatic vessels and their changes associated with ageing.)
Just see what happens when your neighborhood’s waste disposal system is out of service. Not only do the piles of trash stink but they can indeed hinder the area’s normal functioning. That is also the case when the brain’s waste management is on the blink.
The buildup of toxic proteins in the brain causes a massive damage to the nerves, leading to cognitive dysfunction and increased probability of developing neurodegenerative disorders such as Alzheimer's disease. Though the brain drains its waste via the cerebrospinal fluid (CSF), little has been understood about an accurate route for the brain’s cleansing mechanism.
Medical scientists led by Professor Gou Young Koh at the Graduate School of Medical Science and Engineering have reported the basal side of the skull as the major route, so called “hotspot” for CSF drainage.
They found that basal meningeal lymphatic vessels (mLVs) function as the main plumbing pipes for CSF. They confirmed macromolecules in the CSF mainly runs through the basal mLVs. Notably, the team also revealed that the brain’s major drainage system, specifically basal mLVs are impaired with aging. Their findings have been reported in the journal Nature on July 24.
Throughout our body, excess fluids and waste products are removed from tissues via lymphatic vessels. It was only recently discovered that the brain also has a lymphatic drainage system. mLVs are supposed to carry waste from the brain tissue fluid and the CSF down the deep cervical lymph nodes for disposal. Still scientist are left with one perplexing question — where is the main exit for the CSF? Though mLVs in the upper part of the skull (dorsal meningeal lymphatic vessels) were reported as the brain’s clearance pathways in 2014, no substantial drainage mechanism was observed in that section.
“As a hidden exit for CSF, we looked into the mLVs trapped within complex structures at the base of the skull,” says Dr. Ji Hoon Ahn, the first author of this study. The researchers used several techniques to characterize the basal mLVs in detail. They used a genetically engineered lymphatic-reporter mouse model to visualize mLVs under a fluorescence microscope. By performing a careful examination of the mice skull, they found distinctive features of basal mLVs that make them suitable for CSF uptake and drainage. Just like typical functional lymphatic vessels, basal mLVs are found to have abundant lymphatic vessel branches with finger-like protrusions. Additionally, valves inside the basal mLVs allow the flow to go in one direction. In particular, they found that the basal mLVs are closely located to the CSF. Dr. Hyunsoo Cho, the first author of this study explains, “All up, it seemed a solid case that basal mLVs are the brain’s main clearance pathways.
The researchers verified such specialized morphologic characteristics of basal mLVs indeed facilitate the CSF uptake and drainage. Using CSF contrast-enhanced magnetic resonance imaging in a rat model, they found that CSF is drained preferentially through the basal mLVs. They also utilized a lymphatic-reporter mouse model and discovered that fluorescence-tagged tracer injected into the brain itself or the CSF is cleared mainly through the basal mLVs. Jun-Hee Kim, the first author of this study notes, “We literally saw that the brain clearance mechanism utilizing basal outflow route to exit the skull.
It has long been suggested that CSF turnover and drainage declines with ageing. However, alteration of mLVs associated with ageing is poorly understood. In this study, the researchers observed changes of mLVs in young (3-month-old) and aged (24~27-months-old) mice. They found that the structure of the basal mLVs and their lymphatic valves in aged mice become severely flawed, thus hampering CSF clearance. The corresponding author of this study, Dr. Koh says, “By characterizing the precise route for fluids leaving the brain, this study improves our understanding on how waste is cleared from the brain. Our findings also provide further insights into the role of impaired CSF clearance in the development of age-related neurodegenerative diseases.”
Many current therapies for Alzheimer’s disease target abnormally accumulated proteins, such as beta-amyloid. By mapping out a precise route for the brain’s waste clearance system, this study may be able to help find ways to improve the brain’s cleansing function. Such breakthrough might become quite a sensational strategy for eliminating the buildup of aging-related toxic proteins. “It definitely warrants more extensive investigation of mLVs in patients with age-related neurodegenerative disease such as Alzheimer’s disease prior to clinical investigation,” adds Professor Koh.
2019.07.25 View 33649
Newly Identified Meningeal Lymphatic Vessels Answers the Key Questions on Brain Clearance
(Figure: Schematic images of location and features of meningeal lymphatic vessels and their changes associated with ageing.)
Just see what happens when your neighborhood’s waste disposal system is out of service. Not only do the piles of trash stink but they can indeed hinder the area’s normal functioning. That is also the case when the brain’s waste management is on the blink.
The buildup of toxic proteins in the brain causes a massive damage to the nerves, leading to cognitive dysfunction and increased probability of developing neurodegenerative disorders such as Alzheimer's disease. Though the brain drains its waste via the cerebrospinal fluid (CSF), little has been understood about an accurate route for the brain’s cleansing mechanism.
Medical scientists led by Professor Gou Young Koh at the Graduate School of Medical Science and Engineering have reported the basal side of the skull as the major route, so called “hotspot” for CSF drainage.
They found that basal meningeal lymphatic vessels (mLVs) function as the main plumbing pipes for CSF. They confirmed macromolecules in the CSF mainly runs through the basal mLVs. Notably, the team also revealed that the brain’s major drainage system, specifically basal mLVs are impaired with aging. Their findings have been reported in the journal Nature on July 24.
Throughout our body, excess fluids and waste products are removed from tissues via lymphatic vessels. It was only recently discovered that the brain also has a lymphatic drainage system. mLVs are supposed to carry waste from the brain tissue fluid and the CSF down the deep cervical lymph nodes for disposal. Still scientist are left with one perplexing question — where is the main exit for the CSF? Though mLVs in the upper part of the skull (dorsal meningeal lymphatic vessels) were reported as the brain’s clearance pathways in 2014, no substantial drainage mechanism was observed in that section.
“As a hidden exit for CSF, we looked into the mLVs trapped within complex structures at the base of the skull,” says Dr. Ji Hoon Ahn, the first author of this study. The researchers used several techniques to characterize the basal mLVs in detail. They used a genetically engineered lymphatic-reporter mouse model to visualize mLVs under a fluorescence microscope. By performing a careful examination of the mice skull, they found distinctive features of basal mLVs that make them suitable for CSF uptake and drainage. Just like typical functional lymphatic vessels, basal mLVs are found to have abundant lymphatic vessel branches with finger-like protrusions. Additionally, valves inside the basal mLVs allow the flow to go in one direction. In particular, they found that the basal mLVs are closely located to the CSF. Dr. Hyunsoo Cho, the first author of this study explains, “All up, it seemed a solid case that basal mLVs are the brain’s main clearance pathways.
The researchers verified such specialized morphologic characteristics of basal mLVs indeed facilitate the CSF uptake and drainage. Using CSF contrast-enhanced magnetic resonance imaging in a rat model, they found that CSF is drained preferentially through the basal mLVs. They also utilized a lymphatic-reporter mouse model and discovered that fluorescence-tagged tracer injected into the brain itself or the CSF is cleared mainly through the basal mLVs. Jun-Hee Kim, the first author of this study notes, “We literally saw that the brain clearance mechanism utilizing basal outflow route to exit the skull.
It has long been suggested that CSF turnover and drainage declines with ageing. However, alteration of mLVs associated with ageing is poorly understood. In this study, the researchers observed changes of mLVs in young (3-month-old) and aged (24~27-months-old) mice. They found that the structure of the basal mLVs and their lymphatic valves in aged mice become severely flawed, thus hampering CSF clearance. The corresponding author of this study, Dr. Koh says, “By characterizing the precise route for fluids leaving the brain, this study improves our understanding on how waste is cleared from the brain. Our findings also provide further insights into the role of impaired CSF clearance in the development of age-related neurodegenerative diseases.”
Many current therapies for Alzheimer’s disease target abnormally accumulated proteins, such as beta-amyloid. By mapping out a precise route for the brain’s waste clearance system, this study may be able to help find ways to improve the brain’s cleansing function. Such breakthrough might become quite a sensational strategy for eliminating the buildup of aging-related toxic proteins. “It definitely warrants more extensive investigation of mLVs in patients with age-related neurodegenerative disease such as Alzheimer’s disease prior to clinical investigation,” adds Professor Koh.
2019.07.25 View 33649 -
 Distinguished Professor Koh Donates His Ho-Am Prize Money
(From left: Distinguished Professor Gou Young Koh and KAIST President Sung-Chul Shin)
Distinguished Professor Gou Young Koh from the Graduate School of Medical Science and Engineering donated one hundred million KRW to KAIST that he received for winning the Ho-Am Prize.
Professor Koh, who is widely renowned for angiogenesis, was appointed as the 2018 laureate of the 28th Ho-Am Prize for demonstrating the effective reduction of tumor progression and metastasis via tumor vessel normalization.
He made the donation to the Graduate School of Medical Science and Engineering, where he conducted his research. “As a basic medical scientist, it is my great honor to receive this prize for the recognition of my research outcome. I will give impetus to research for continuous development,” Professor Koh said.
Professor Koh also received the 5th Asan Award in Medicine in 2012 and the 7th Kyung-Ahm Award in 2011. He was also the awardee of the 17th Wunsch Medical Award. He has donated cash prizes to the school every time he is awarded.
KAIST President Sung-Chul Shin said, “I would like to express my gratitude to the professor for his generous donation to the school. It will be a great help fostering outstanding medical scientists.
Professor Koh received his MD-PhD from the Medical School of Chonbuk National University. After finishing his post-doctoral program at Cornell University and Indiana State University, he was appointed as a professor at Chonbuk National University and POSTECH. Currently, he holds the position of distinguished professor at KAIST and director of the IBS Center for Vascular Research.
2018.06.20 View 6380
Distinguished Professor Koh Donates His Ho-Am Prize Money
(From left: Distinguished Professor Gou Young Koh and KAIST President Sung-Chul Shin)
Distinguished Professor Gou Young Koh from the Graduate School of Medical Science and Engineering donated one hundred million KRW to KAIST that he received for winning the Ho-Am Prize.
Professor Koh, who is widely renowned for angiogenesis, was appointed as the 2018 laureate of the 28th Ho-Am Prize for demonstrating the effective reduction of tumor progression and metastasis via tumor vessel normalization.
He made the donation to the Graduate School of Medical Science and Engineering, where he conducted his research. “As a basic medical scientist, it is my great honor to receive this prize for the recognition of my research outcome. I will give impetus to research for continuous development,” Professor Koh said.
Professor Koh also received the 5th Asan Award in Medicine in 2012 and the 7th Kyung-Ahm Award in 2011. He was also the awardee of the 17th Wunsch Medical Award. He has donated cash prizes to the school every time he is awarded.
KAIST President Sung-Chul Shin said, “I would like to express my gratitude to the professor for his generous donation to the school. It will be a great help fostering outstanding medical scientists.
Professor Koh received his MD-PhD from the Medical School of Chonbuk National University. After finishing his post-doctoral program at Cornell University and Indiana State University, he was appointed as a professor at Chonbuk National University and POSTECH. Currently, he holds the position of distinguished professor at KAIST and director of the IBS Center for Vascular Research.
2018.06.20 View 6380 -
 Professor Gou Young Koh, 2018 Laureate of Ho-Am Prize
Distinguished Professor Gou Young Koh from the Graduate School of Medical Science and Engineering was appointed a 2018 laureate in medicine of the Ho-Am Prize by the Ho-Am Foundation. Professor Koh is a renowned expert in the field of tumor angiogenesis by exploring the hidden nature of capillary and lymphatic vessels in human organs.
He was recognized for demonstrating the effective reduction of tumor progression and metastasis via tumor vessel normalization. This counterintuitive study result is regarded as a stepping stone for a drug discovery to prevent microvascular diseases.
Besides Professor Koh, Professor Hee Oh from Yale University (Science), Professor Nam-Gyu Park from Sungkyunkwan University (Engineering), Opera Singer Kwangchul Youn (The Arts) and Sister Carla Kang (Community Service) received awards.
The Ho-Am Prize is presented to individuals who have contributed to academics, the arts, and social development, or furthered the welfare of humanity, and commemorates the noble spirit of public service espoused by the late Chairman Byung-chull Lee, who used the pen name Ho-Am.
It was established in 1990 by Kun-Hee Lee, the chairman of Samsung. Awards have been presented to 143 individuals worth a total of 24.4 billion KRW.
2018.04.11 View 9432
Professor Gou Young Koh, 2018 Laureate of Ho-Am Prize
Distinguished Professor Gou Young Koh from the Graduate School of Medical Science and Engineering was appointed a 2018 laureate in medicine of the Ho-Am Prize by the Ho-Am Foundation. Professor Koh is a renowned expert in the field of tumor angiogenesis by exploring the hidden nature of capillary and lymphatic vessels in human organs.
He was recognized for demonstrating the effective reduction of tumor progression and metastasis via tumor vessel normalization. This counterintuitive study result is regarded as a stepping stone for a drug discovery to prevent microvascular diseases.
Besides Professor Koh, Professor Hee Oh from Yale University (Science), Professor Nam-Gyu Park from Sungkyunkwan University (Engineering), Opera Singer Kwangchul Youn (The Arts) and Sister Carla Kang (Community Service) received awards.
The Ho-Am Prize is presented to individuals who have contributed to academics, the arts, and social development, or furthered the welfare of humanity, and commemorates the noble spirit of public service espoused by the late Chairman Byung-chull Lee, who used the pen name Ho-Am.
It was established in 1990 by Kun-Hee Lee, the chairman of Samsung. Awards have been presented to 143 individuals worth a total of 24.4 billion KRW.
2018.04.11 View 9432 -
 Professor Ko Kyu Young Appointed as a Distinguished Professor at KAIST
Professor Ko Kyu Young of the Graduate School of Medical Sciences was appointed as the Distinguished Professor at KAIST.
Professor Ko is famous internationally for his work on the catalyst for blood vessel growth COMP-ANG1, and also for his research on blood vessel growth and lymph duct growth control.
Professor Ko developed the Double Anti-Angiogenic Protein (DAAP) which effectively restricts the blood vessels from growing, opening a new approach to curing caner. The paper was published in ‘Cancer Cell’ as the cover paper (2010 August 17th edition) and is widely recognized as the marker that sums up the new paradigm of cure for cancer.
In addition, his work on explaining how the new antigen interacts with the T-lymphocyte during a vaccination lead to the possibility of the increase of the efficiency of vaccination. The result of the research was published as the cover paper in ‘Immunity’ magazine.
As is obvious to see his work with blood vessel growth and lymph duct growth and control is being published in major scientific journals. In addition he is continuously invited to international conferences as guest speakers and leader, effectively leading the field. As a result, he was appointed as the editor of ‘Blood’ magazine, the world’s best journal in the field of hematology and received ‘2010 KAISTian of the Year’ Award.
The title Distinguished Professor is appointed to those who have made world-class research results and educational results and actively lead their respective field. They are provided with extra incentives and can even continue on with the professorship after retirement.
It is only limited to 3% of the professors at KAIST and has to be someone recommended by the President, Vice-President, and the Deans of department and their worthiness is scrutinized by a foreign expert.
2011.03.25 View 12727
Professor Ko Kyu Young Appointed as a Distinguished Professor at KAIST
Professor Ko Kyu Young of the Graduate School of Medical Sciences was appointed as the Distinguished Professor at KAIST.
Professor Ko is famous internationally for his work on the catalyst for blood vessel growth COMP-ANG1, and also for his research on blood vessel growth and lymph duct growth control.
Professor Ko developed the Double Anti-Angiogenic Protein (DAAP) which effectively restricts the blood vessels from growing, opening a new approach to curing caner. The paper was published in ‘Cancer Cell’ as the cover paper (2010 August 17th edition) and is widely recognized as the marker that sums up the new paradigm of cure for cancer.
In addition, his work on explaining how the new antigen interacts with the T-lymphocyte during a vaccination lead to the possibility of the increase of the efficiency of vaccination. The result of the research was published as the cover paper in ‘Immunity’ magazine.
As is obvious to see his work with blood vessel growth and lymph duct growth and control is being published in major scientific journals. In addition he is continuously invited to international conferences as guest speakers and leader, effectively leading the field. As a result, he was appointed as the editor of ‘Blood’ magazine, the world’s best journal in the field of hematology and received ‘2010 KAISTian of the Year’ Award.
The title Distinguished Professor is appointed to those who have made world-class research results and educational results and actively lead their respective field. They are provided with extra incentives and can even continue on with the professorship after retirement.
It is only limited to 3% of the professors at KAIST and has to be someone recommended by the President, Vice-President, and the Deans of department and their worthiness is scrutinized by a foreign expert.
2011.03.25 View 12727 -
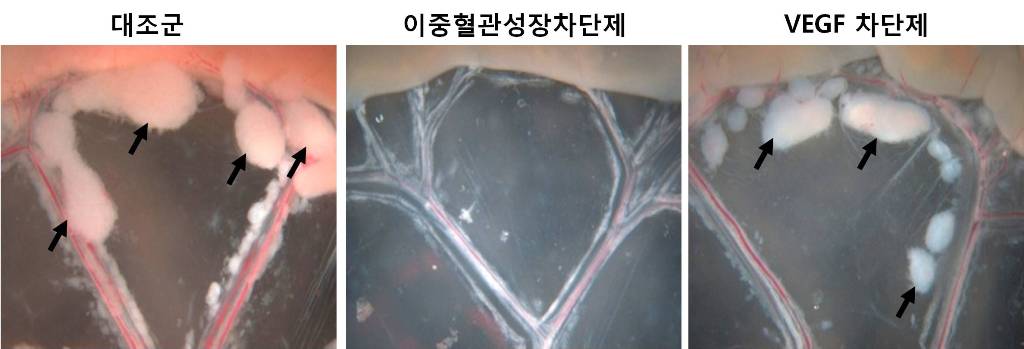 An internationally renowned academic journal published the research result produced by a KAST research team on its cover.
Fc DAAP VEGF-Trap
Photograph showing the gross features of tumor growth along the mesentery-intestinal border. T: tumor. Scale bars represent 5 mm.
Professor Gou-Young Koh of the Biological Sciences Department, KAIST, and his research team published their research result in Cancer Cell, a peer-review scientific journal, as a cover article dated August 17, 2010. It is the first time for the journal to pick up a paper written by a Korean research team and publish it as the cover.
It has been known that a vascular growth factor (VEGF) is closely related to the growth of a tumor. The research team recently discovered that in addition to VEGF, another growth factor, angiopoietin-2 (Ang2), is also engaged with the increase of tumors.
Professor Koh said, “VEGF and the angiopoietins play critical roles in tumor progression and metastasis, and a single inhibitor targeting both factors have not been available.”
The team led by Professor Koh has developed a double anti-angiogenic protein (DAAP) that can simultaneously bind VEGF-A and the angiopoietins and block their actions.
Professor Koh said in his paper, “DAAP is a highly effective molecule for regressing tumor angiogenesis and metastasis in implanted and spontaneous solid tumor; it can also effectively reduce ascites formation and vascular leakage in an ovarian carcinoma model. Thus, simultaneous blockade of VEGF-A and angiopoietins with DAAP is an effective therapeutic strategy for blocking tumor angiogenesis, metastasis, and vascular leakage.”
So far, cancer patients have received Avastin, anticancer drug, to inhibit VEGF, but the drug has not successfully restrained the growth of cancer tumors and brought to some of the patients with serious side effects instead.
Professor Koh said, “DAAP will be very effective to control the expansion of tumor growth factors, which will open up a new possibility for the development of more helpful cancer medicine with low side effects.”
2010.08.20 View 13953
An internationally renowned academic journal published the research result produced by a KAST research team on its cover.
Fc DAAP VEGF-Trap
Photograph showing the gross features of tumor growth along the mesentery-intestinal border. T: tumor. Scale bars represent 5 mm.
Professor Gou-Young Koh of the Biological Sciences Department, KAIST, and his research team published their research result in Cancer Cell, a peer-review scientific journal, as a cover article dated August 17, 2010. It is the first time for the journal to pick up a paper written by a Korean research team and publish it as the cover.
It has been known that a vascular growth factor (VEGF) is closely related to the growth of a tumor. The research team recently discovered that in addition to VEGF, another growth factor, angiopoietin-2 (Ang2), is also engaged with the increase of tumors.
Professor Koh said, “VEGF and the angiopoietins play critical roles in tumor progression and metastasis, and a single inhibitor targeting both factors have not been available.”
The team led by Professor Koh has developed a double anti-angiogenic protein (DAAP) that can simultaneously bind VEGF-A and the angiopoietins and block their actions.
Professor Koh said in his paper, “DAAP is a highly effective molecule for regressing tumor angiogenesis and metastasis in implanted and spontaneous solid tumor; it can also effectively reduce ascites formation and vascular leakage in an ovarian carcinoma model. Thus, simultaneous blockade of VEGF-A and angiopoietins with DAAP is an effective therapeutic strategy for blocking tumor angiogenesis, metastasis, and vascular leakage.”
So far, cancer patients have received Avastin, anticancer drug, to inhibit VEGF, but the drug has not successfully restrained the growth of cancer tumors and brought to some of the patients with serious side effects instead.
Professor Koh said, “DAAP will be very effective to control the expansion of tumor growth factors, which will open up a new possibility for the development of more helpful cancer medicine with low side effects.”
2010.08.20 View 13953 -
 KAIST Research Team Identified Promising New Source to Obtain Stem Cells
KAIST Research Team Identified Promising New Source to Obtain Stem Cells
A research team at KAIST led by Professor Gou-Young Koh, M.D. and Ph.D., of the Department of Biological Sciences, has found evidence that fat tissue, known as adipose tissue, may be a promising new source of valuable and easy-to-obtain regenerative cells called hematopoietic stem and progenitor cells (HSPCs).
HSPCs are adult stem cells that have the ability to generate and develop into many different kinds of cells. They are now used to repair damaged tissues and are being studied for their potential to treat a vast array of chronic and degenerative conditions such as leukemia. Mostly found in bone marrow but with a limited quantity, HSPCs are hard to cultivate in vitro, thus becoming an obstacle to use them for research and therapeutic purposes.
Within the adipose tissue is a special cell population known as the stromal vascular fraction (SVF), which share similar properties to those in the bone marrow. Cells in the bone marrow and SVF have the ability to differentiate into several cell types. In addition, both adipose and bone marrow offer similar environments for optimal stem cell growth and reproduction.
Given the fact that adipose and bone marrow tissues share similar properties, Dr. Koh and his team conducted a research, injecting granulocyte colony-stimulating factor (G-CSF), a growth hormone used to encourage the development of stem cells, into an adipose tissue of a mouse whose bone marrow is damaged. As a result, the team has found that the SVF derived from adipose tissue contains functional HSPCs capable of generating hematopoietic (blood-forming) cells to repair the damaged bone morrow.
The Ministry of Education, Science and Technology nominated the KAIST research as one of its sponsoring 21st Century Frontier R&D Programs. Director Dong-Wook Kim of Stem Cell Research Center (SCRS) that oversees the KAIST team expressed a possibility to use the adipose tissue as an alternative source to obtain stem cells for regeneration medicine.
Dr. Koh also said, “It’s been a well known method to extract HSPCs from the bone morrow or blood, but it’s the first time to identify adipose tissue, before considered useless, as a new possible supplier for functional and transplantable HSPCs.”
The study results have received an important recognition from the academia—the American Society of Hematology published the research as a main article in its official journal, Blood, for the February 4th, 2010 issue, which is the most citied peer-reviewed publication in the field.
2010.02.05 View 14038
KAIST Research Team Identified Promising New Source to Obtain Stem Cells
KAIST Research Team Identified Promising New Source to Obtain Stem Cells
A research team at KAIST led by Professor Gou-Young Koh, M.D. and Ph.D., of the Department of Biological Sciences, has found evidence that fat tissue, known as adipose tissue, may be a promising new source of valuable and easy-to-obtain regenerative cells called hematopoietic stem and progenitor cells (HSPCs).
HSPCs are adult stem cells that have the ability to generate and develop into many different kinds of cells. They are now used to repair damaged tissues and are being studied for their potential to treat a vast array of chronic and degenerative conditions such as leukemia. Mostly found in bone marrow but with a limited quantity, HSPCs are hard to cultivate in vitro, thus becoming an obstacle to use them for research and therapeutic purposes.
Within the adipose tissue is a special cell population known as the stromal vascular fraction (SVF), which share similar properties to those in the bone marrow. Cells in the bone marrow and SVF have the ability to differentiate into several cell types. In addition, both adipose and bone marrow offer similar environments for optimal stem cell growth and reproduction.
Given the fact that adipose and bone marrow tissues share similar properties, Dr. Koh and his team conducted a research, injecting granulocyte colony-stimulating factor (G-CSF), a growth hormone used to encourage the development of stem cells, into an adipose tissue of a mouse whose bone marrow is damaged. As a result, the team has found that the SVF derived from adipose tissue contains functional HSPCs capable of generating hematopoietic (blood-forming) cells to repair the damaged bone morrow.
The Ministry of Education, Science and Technology nominated the KAIST research as one of its sponsoring 21st Century Frontier R&D Programs. Director Dong-Wook Kim of Stem Cell Research Center (SCRS) that oversees the KAIST team expressed a possibility to use the adipose tissue as an alternative source to obtain stem cells for regeneration medicine.
Dr. Koh also said, “It’s been a well known method to extract HSPCs from the bone morrow or blood, but it’s the first time to identify adipose tissue, before considered useless, as a new possible supplier for functional and transplantable HSPCs.”
The study results have received an important recognition from the academia—the American Society of Hematology published the research as a main article in its official journal, Blood, for the February 4th, 2010 issue, which is the most citied peer-reviewed publication in the field.
2010.02.05 View 14038