SNARE
-
 Dr. Ryu of KAIST Receives the S-Oil Outstanding Paper Award
Dr. Je-Kyung Ryu of KAIST’s Department of Physics has been awarded the S-Oil Outstanding Paper Award for his doctoral dissertation’s originality and applicability.
Professor Tae-Young Yoon of Physics is his doctoral advisor.
The award ceremony took place on November 25, 2015 at the Press Center in Seoul.
This S-Oil Outstanding Paper Award, jointly sponsored by the Korean Academy of Science and Technology (KAST) and the Scholastic University Presidential Association, was established to foster young talented scientists in basic science and to advance the field.
The award is given every other year for each of the fields of physics, chemistry, mathematics, biology, and earth sciences.
With the award, Dr. Ryu received a research grant of USD 8,600.
He discovered, for the first time in the world, how NSF (N-ethylmaleimide-sensitive factor), a protein involved in a vesicular transport in cellular activities, disassembles a SNARE (soluble NSF attachment protein receptor) complex, using a unimolecular biophysics method.
Unlike the existing studies, he proposed a model in which NSF disassembles SNARE complexes at one step, and as a result, provided evidence of how the SNARE complex influenced the fusion of biological membranes.
His research was published in the scientific journal Science issued on March 27, 2015. The title of the paper is “Spring-loaded Unraveling of a Single SNARE Complex by NSF in One Round of ATP Turnover.”
2015.11.27 View 11036
Dr. Ryu of KAIST Receives the S-Oil Outstanding Paper Award
Dr. Je-Kyung Ryu of KAIST’s Department of Physics has been awarded the S-Oil Outstanding Paper Award for his doctoral dissertation’s originality and applicability.
Professor Tae-Young Yoon of Physics is his doctoral advisor.
The award ceremony took place on November 25, 2015 at the Press Center in Seoul.
This S-Oil Outstanding Paper Award, jointly sponsored by the Korean Academy of Science and Technology (KAST) and the Scholastic University Presidential Association, was established to foster young talented scientists in basic science and to advance the field.
The award is given every other year for each of the fields of physics, chemistry, mathematics, biology, and earth sciences.
With the award, Dr. Ryu received a research grant of USD 8,600.
He discovered, for the first time in the world, how NSF (N-ethylmaleimide-sensitive factor), a protein involved in a vesicular transport in cellular activities, disassembles a SNARE (soluble NSF attachment protein receptor) complex, using a unimolecular biophysics method.
Unlike the existing studies, he proposed a model in which NSF disassembles SNARE complexes at one step, and as a result, provided evidence of how the SNARE complex influenced the fusion of biological membranes.
His research was published in the scientific journal Science issued on March 27, 2015. The title of the paper is “Spring-loaded Unraveling of a Single SNARE Complex by NSF in One Round of ATP Turnover.”
2015.11.27 View 11036 -
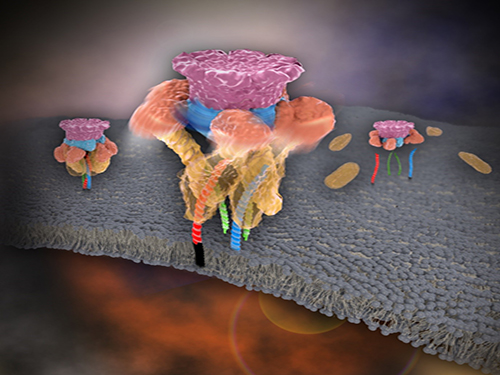 Mystery in Membrane Traffic How NSF Disassembles Single SNAR Complex Solved
KAIST researchers discovered that the protein N-ethylmaleimide-sensitive factor (NSF) unravels a single SNARE complex using one round ATP turnover by tearing the complex with a single burst, contradicting a previous theory that it unwinds in a processive manner.
In 2013, James E. Rothman, Randy W. Schekman, and Thomas C. Südhof won the Nobel Prize in Physiology or Medicine for their discoveries of molecular machineries for vesicle trafficking, a major transport system in cells for maintaining cellular processes. Vesicle traffic acts as a kind of “home-delivery service” in cells. Vesicles package and deliver materials such as proteins and hormones from one cell organelle to another. Then it releases its contents by fusing with the target organelle’s membrane. One example of vesicle traffic is in neuronal communications, where neurotransmitters are released from a neuron. Some of the key proteins for vesicle traffic discovered by the Nobel Prize winners were N-ethylmaleimide-sensitive factor (NSF), alpha-soluble NSF attachment protein (α-SNAP), and soluble SNAP receptors (SNAREs).
SNARE proteins are known as the minimal machinery for membrane fusion. To induce membrane fusion, the proteins combine to form a SNARE complex in a four helical bundle, and NSF and α-SNAP disassemble the SNARE complex for reuse. In particular, NSF can bind an energy source molecule, adenosine triphosphate (ATP), and the ATP-bound NSF develops internal tension via cleavage of ATP. This process is used to exert great force on SNARE complexes, eventually pulling them apart. However, although about 30 years have passed since the Nobel Prize winners’ discovery, how NSF/α-SNAP disassembled the SNARE complex remained a mystery to scientists due to a lack in methodology.
In a recent issue of Science, published on March 27, 2015, a research team, led by Tae-Young Yoon of the Department of Physics at the Korea Advanced Institute of Science and Technology (KAIST) and Reinhard Jahn of the Department of Neurobiology of the Max-Planck-Institute for Biophysical Chemistry, reports that NSF/α-SNAP disassemble a single SNARE complex using various single-molecule biophysical methods that allow them to monitor and manipulate individual protein complexes.
“We have learned that NSF releases energy in a burst within 20 milliseconds to “tear” the SNARE complex apart in a one-step global unfolding reaction, which is immediately followed by the release of SNARE proteins,” said Yoon.
Previously, it was believed that NSF disassembled a SNARE complex by unwinding it in a processive manner. Also, largely unexplained was how many cycles of ATP hydrolysis were required and how these cycles were connected to the disassembly of the SNARE complex.
Yoon added, “From our research, we found that NSF requires hydrolysis of ATPs that were already bound before it attached to the SNAREs—which means that only one round of an ATP turnover is sufficient for SNARE complex disassembly. Moreover, this is possible because NSF pulls a SNARE complex apart by building up the energy from individual ATPs and releasing it at once, yielding a “spring-loaded” mechanism.”
NSF is a member of the ATPases associated with various cellular activities family (AAA+ ATPase), which is essential for many cellular functions such as DNA replication and protein degradation, membrane fusion, microtubule severing, peroxisome biogenesis, signal transduction, and the regulation of gene expression. This research has added valuable new insights and hints for studying AAA+ ATPase proteins, which are crucial for various living beings.
The title of the research paper is “Spring-loaded unraveling of a single SNARE complex by NSF in one round of ATP turnover.” (DOI: 10.1126/science.aaa5267)
Youtube Link: https://www.youtube.com/watch?v=FqTSYHtyHWE&feature=youtu.be
Picture 1. Working model of how NSF/α-SNAP disassemble a single SNARE complex
Picture 2. After neurotransmitter release, NSF disassembles a single SNARE complex using a single round of ATP turnover in a single burst reaction.
2015.03.28 View 11730
Mystery in Membrane Traffic How NSF Disassembles Single SNAR Complex Solved
KAIST researchers discovered that the protein N-ethylmaleimide-sensitive factor (NSF) unravels a single SNARE complex using one round ATP turnover by tearing the complex with a single burst, contradicting a previous theory that it unwinds in a processive manner.
In 2013, James E. Rothman, Randy W. Schekman, and Thomas C. Südhof won the Nobel Prize in Physiology or Medicine for their discoveries of molecular machineries for vesicle trafficking, a major transport system in cells for maintaining cellular processes. Vesicle traffic acts as a kind of “home-delivery service” in cells. Vesicles package and deliver materials such as proteins and hormones from one cell organelle to another. Then it releases its contents by fusing with the target organelle’s membrane. One example of vesicle traffic is in neuronal communications, where neurotransmitters are released from a neuron. Some of the key proteins for vesicle traffic discovered by the Nobel Prize winners were N-ethylmaleimide-sensitive factor (NSF), alpha-soluble NSF attachment protein (α-SNAP), and soluble SNAP receptors (SNAREs).
SNARE proteins are known as the minimal machinery for membrane fusion. To induce membrane fusion, the proteins combine to form a SNARE complex in a four helical bundle, and NSF and α-SNAP disassemble the SNARE complex for reuse. In particular, NSF can bind an energy source molecule, adenosine triphosphate (ATP), and the ATP-bound NSF develops internal tension via cleavage of ATP. This process is used to exert great force on SNARE complexes, eventually pulling them apart. However, although about 30 years have passed since the Nobel Prize winners’ discovery, how NSF/α-SNAP disassembled the SNARE complex remained a mystery to scientists due to a lack in methodology.
In a recent issue of Science, published on March 27, 2015, a research team, led by Tae-Young Yoon of the Department of Physics at the Korea Advanced Institute of Science and Technology (KAIST) and Reinhard Jahn of the Department of Neurobiology of the Max-Planck-Institute for Biophysical Chemistry, reports that NSF/α-SNAP disassemble a single SNARE complex using various single-molecule biophysical methods that allow them to monitor and manipulate individual protein complexes.
“We have learned that NSF releases energy in a burst within 20 milliseconds to “tear” the SNARE complex apart in a one-step global unfolding reaction, which is immediately followed by the release of SNARE proteins,” said Yoon.
Previously, it was believed that NSF disassembled a SNARE complex by unwinding it in a processive manner. Also, largely unexplained was how many cycles of ATP hydrolysis were required and how these cycles were connected to the disassembly of the SNARE complex.
Yoon added, “From our research, we found that NSF requires hydrolysis of ATPs that were already bound before it attached to the SNAREs—which means that only one round of an ATP turnover is sufficient for SNARE complex disassembly. Moreover, this is possible because NSF pulls a SNARE complex apart by building up the energy from individual ATPs and releasing it at once, yielding a “spring-loaded” mechanism.”
NSF is a member of the ATPases associated with various cellular activities family (AAA+ ATPase), which is essential for many cellular functions such as DNA replication and protein degradation, membrane fusion, microtubule severing, peroxisome biogenesis, signal transduction, and the regulation of gene expression. This research has added valuable new insights and hints for studying AAA+ ATPase proteins, which are crucial for various living beings.
The title of the research paper is “Spring-loaded unraveling of a single SNARE complex by NSF in one round of ATP turnover.” (DOI: 10.1126/science.aaa5267)
Youtube Link: https://www.youtube.com/watch?v=FqTSYHtyHWE&feature=youtu.be
Picture 1. Working model of how NSF/α-SNAP disassemble a single SNARE complex
Picture 2. After neurotransmitter release, NSF disassembles a single SNARE complex using a single round of ATP turnover in a single burst reaction.
2015.03.28 View 11730