Organoids
-
 KAIST Research Team Develops Stretchable Microelectrodes Array for Organoid Signal Monitoring
< Photo 1. (From top left) Professor Hyunjoo J. Lee, Dr. Mi-Young Son, Dr. Mi-Ok Lee(In the front row from left) Doctoral student Kiup Kim, Doctoral student Youngsun Lee >
On January 14th, the KAIST research team led by Professor Hyunjoo J. Lee from the School of Electrical Engineering in collaboration with Dr. Mi-Young Son and Dr. Mi-Ok Lee at Korea Research Institute of Bioscience and Biotechnology (KRIBB) announced the development of a highly stretchable microelectrode array (sMEA) designed for non-invasive electrophysiological signal measurement of organoids.
Organoids* are highly promising models for human biology and are expected to replace many animal experiments. Their potential applications include disease modeling, drug screening, and personalized medicine as they closely mimic the structure and function of humans.
*Organoids: three-dimensional in vitro tissue models derived from human stem cells
Despite these advantages, existing organoid research has primarily focused on genetic analysis, with limited studies on organoid functionality. For effective drug evaluation and precise biological research, technology that preserves the three-dimensional structure of organoids while enabling real-time monitoring of their functions is needed. However, it’s challenging to provide non-invasive ways to evaluate the functionalities without incurring damage to the tissues. This challenge is particularly significant for electrophysiological signal measurement in cardiac and brain organoids since the sensor needs to be in direct contact with organoids of varying size and irregular shape. Achieving tight contact between electrodes and the external surface of the organoids without damaging the organoids has been a persistent challenge.
< Figure 1. Schematic image of highly stretchable MEA (sMEA) with protruding microelectrodes. >
The KAIST research team developed a highly stretchable microelectrode array with a unique serpentine structure that contacts the surface of organoids in a highly conformal fashion. They successfully demonstrated real-time measurement and analysis of electrophysiological signals from two types of electrogenic organoids (heart and brain). By employing a micro-electromechanical system (MEMS)-based process, the team fabricated the serpentine-structured microelectrode array and used an electrochemical deposition process to develop PEDOT:PSS-based protruding microelectrodes. These innovations demonstrated exceptional stretchability and close surface adherence to various organoid sizes. The protruding microelectrodes improved contact between organoids and the electrodes, ensuring stable and reliable electrophysiological signal measurements with high signal-to-noise ratios (SNR).
< Figure 2. Conceptual illustration, optical image, and fluorescence images of an organoid captured by the sMEA with protruding microelectrodes.>
Using this technology, the team successfully monitored and analyzed electrophysiological signals from cardiac spheroids of various sizes, revealing three-dimensional signal propagation patterns and identifying changes in signal characteristics according to size. They also measured electrophysiological signals in midbrain organoids, demonstrating the versatility of the technology. Additionally, they monitored signal modulations induced by various drugs, showcasing the potential of this technology for drug screening applications.
< Figure 3. SNR improvement effect by protruding PEDOT:PSS microelectrodes. >
Prof. Hyunjoo Jenny Lee stated, “By integrating MEMS technology and electrochemical deposition techniques, we successfully developed a stretchable microelectrode array adaptable to organoids of diverse sizes and shapes. The high practicality is a major advantage of this system since the fabrication is based on semiconductor fabrication with high volume production, reliability, and accuracy. This technology that enables in situ, real-time analysis of states and functionalities of organoids will be a game changer in high-through drug screening.”
This study led by Ph.D. candidate Kiup Kim from KAIST and Ph.D. candidate Youngsun Lee from KRIBB, with significant contributions from Dr. Kwang Bo Jung, was published online on December 15, 2024 in Advanced Materials (IF: 27.4).
< Figure 4. Drug screening using cardiac spheroids and midbrain organoids.>
This research was supported by a grant from 3D-TissueChip Based Drug Discovery Platform Technology Development Program (No. 20009209) funded by the Ministry of Trade, Industry & Energy (MOTIE, Korea), by the Commercialization Promotion Agency for R&D Outcomes (COMPA) funded by the Ministry of Science and ICT (MSIT) (RS-2024-00415902), by the K-Brain Project of the National Research Foundation (NRF) funded by the Korean government (MSIT) (RS-2023-00262568), by BK21 FOUR (Connected AI Education & Research Program for Industry and Society Innovation, KAIST EE, No. 4120200113769), and by Korea Research Institute of Bioscience and Biotechnology (KRIBB) Research Initiative Program (KGM4722432).
2025.01.14 View 3792
KAIST Research Team Develops Stretchable Microelectrodes Array for Organoid Signal Monitoring
< Photo 1. (From top left) Professor Hyunjoo J. Lee, Dr. Mi-Young Son, Dr. Mi-Ok Lee(In the front row from left) Doctoral student Kiup Kim, Doctoral student Youngsun Lee >
On January 14th, the KAIST research team led by Professor Hyunjoo J. Lee from the School of Electrical Engineering in collaboration with Dr. Mi-Young Son and Dr. Mi-Ok Lee at Korea Research Institute of Bioscience and Biotechnology (KRIBB) announced the development of a highly stretchable microelectrode array (sMEA) designed for non-invasive electrophysiological signal measurement of organoids.
Organoids* are highly promising models for human biology and are expected to replace many animal experiments. Their potential applications include disease modeling, drug screening, and personalized medicine as they closely mimic the structure and function of humans.
*Organoids: three-dimensional in vitro tissue models derived from human stem cells
Despite these advantages, existing organoid research has primarily focused on genetic analysis, with limited studies on organoid functionality. For effective drug evaluation and precise biological research, technology that preserves the three-dimensional structure of organoids while enabling real-time monitoring of their functions is needed. However, it’s challenging to provide non-invasive ways to evaluate the functionalities without incurring damage to the tissues. This challenge is particularly significant for electrophysiological signal measurement in cardiac and brain organoids since the sensor needs to be in direct contact with organoids of varying size and irregular shape. Achieving tight contact between electrodes and the external surface of the organoids without damaging the organoids has been a persistent challenge.
< Figure 1. Schematic image of highly stretchable MEA (sMEA) with protruding microelectrodes. >
The KAIST research team developed a highly stretchable microelectrode array with a unique serpentine structure that contacts the surface of organoids in a highly conformal fashion. They successfully demonstrated real-time measurement and analysis of electrophysiological signals from two types of electrogenic organoids (heart and brain). By employing a micro-electromechanical system (MEMS)-based process, the team fabricated the serpentine-structured microelectrode array and used an electrochemical deposition process to develop PEDOT:PSS-based protruding microelectrodes. These innovations demonstrated exceptional stretchability and close surface adherence to various organoid sizes. The protruding microelectrodes improved contact between organoids and the electrodes, ensuring stable and reliable electrophysiological signal measurements with high signal-to-noise ratios (SNR).
< Figure 2. Conceptual illustration, optical image, and fluorescence images of an organoid captured by the sMEA with protruding microelectrodes.>
Using this technology, the team successfully monitored and analyzed electrophysiological signals from cardiac spheroids of various sizes, revealing three-dimensional signal propagation patterns and identifying changes in signal characteristics according to size. They also measured electrophysiological signals in midbrain organoids, demonstrating the versatility of the technology. Additionally, they monitored signal modulations induced by various drugs, showcasing the potential of this technology for drug screening applications.
< Figure 3. SNR improvement effect by protruding PEDOT:PSS microelectrodes. >
Prof. Hyunjoo Jenny Lee stated, “By integrating MEMS technology and electrochemical deposition techniques, we successfully developed a stretchable microelectrode array adaptable to organoids of diverse sizes and shapes. The high practicality is a major advantage of this system since the fabrication is based on semiconductor fabrication with high volume production, reliability, and accuracy. This technology that enables in situ, real-time analysis of states and functionalities of organoids will be a game changer in high-through drug screening.”
This study led by Ph.D. candidate Kiup Kim from KAIST and Ph.D. candidate Youngsun Lee from KRIBB, with significant contributions from Dr. Kwang Bo Jung, was published online on December 15, 2024 in Advanced Materials (IF: 27.4).
< Figure 4. Drug screening using cardiac spheroids and midbrain organoids.>
This research was supported by a grant from 3D-TissueChip Based Drug Discovery Platform Technology Development Program (No. 20009209) funded by the Ministry of Trade, Industry & Energy (MOTIE, Korea), by the Commercialization Promotion Agency for R&D Outcomes (COMPA) funded by the Ministry of Science and ICT (MSIT) (RS-2024-00415902), by the K-Brain Project of the National Research Foundation (NRF) funded by the Korean government (MSIT) (RS-2023-00262568), by BK21 FOUR (Connected AI Education & Research Program for Industry and Society Innovation, KAIST EE, No. 4120200113769), and by Korea Research Institute of Bioscience and Biotechnology (KRIBB) Research Initiative Program (KGM4722432).
2025.01.14 View 3792 -
 KAIST Succeeds in the Real-time Observation of Organoids using Holotomography
Organoids, which are 3D miniature organs that mimic the structure and function of human organs, play an essential role in disease research and drug development. A Korean research team has overcome the limitations of existing imaging technologies, succeeding in the real-time, high-resolution observation of living organoids.
KAIST (represented by President Kwang Hyung Lee) announced on the 14th of October that Professor YongKeun Park’s research team from the Department of Physics, in collaboration with the Genome Editing Research Center (Director Bon-Kyoung Koo) of the Institute for Basic Science (IBS President Do-Young Noh) and Tomocube Inc., has developed an imaging technology using holotomography to observe live, small intestinal organoids in real time at a high resolution.
Existing imaging techniques have struggled to observe living organoids in high resolution over extended periods and often required additional treatments like fluorescent staining.
< Figure 1. Overview of the low-coherence HT workflow. Using holotomography, 3D morphological restoration and quantitative analysis of organoids can be performed. In order to improve the limited field of view, which is a limitation of the microscope, our research team utilized a large-area field of view combination algorithm and made a 3D restoration by acquiring multi-focus holographic images for 3D measurements. After that, the organoids were compartmentalized to divide the parts necessary for analysis and quantitatively evaluated the protein concentration measurable from the refractive index and the survival rate of the organoids. >
The research team introduced holotomography technology to address these issues, which provides high-resolution images without the need for fluorescent staining and allows for the long-term observation of dynamic changes in real time without causing cell damage.
The team validated this technology using small intestinal organoids from experimental mice and were able to observe various cell structures inside the organoids in detail. They also captured dynamic changes such as growth processes, cell division, and cell death in real time using holotomography.
Additionally, the technology allowed for the precise analysis of the organoids' responses to drug treatments, verifying the survival of the cells.
The researchers believe that this breakthrough will open new horizons in organoid research, enabling the greater utilization of organoids in drug development, personalized medicine, and regenerative medicine.
Future research is expected to more accurately replicate the in vivo environment of organoids, contributing significantly to a more detailed understanding of various life phenomena at the cellular level through more precise 3D imaging.
< Figure 2. Real-time organoid morphology analysis. Using holotomography, it is possible to observe the lumen and villus development process of intestinal organoids in real time, which was difficult to observe with a conventional microscope. In addition, various information about intestinal organoids can be obtained by quantifying the size and protein amount of intestinal organoids through image analysis. >
Dr. Mahn Jae Lee, a graduate of KAIST's Graduate School of Medical Science and Engineering, currently at Chungnam National University Hospital and the first author of the paper, commented, "This research represents a new imaging technology that surpasses previous limitations and is expected to make a major contribution to disease modeling, personalized treatments, and drug development research using organoids."
The research results were published online in the international journal Experimental & Molecular Medicine on October 1, 2024, and the technology has been recognized for its applicability in various fields of life sciences. (Paper title: “Long-term three-dimensional high-resolution imaging of live unlabeled small intestinal organoids via low-coherence holotomography”)
This research was supported by the National Research Foundation of Korea, KAIST Institutes, and the Institute for Basic Science.
2024.10.14 View 4857
KAIST Succeeds in the Real-time Observation of Organoids using Holotomography
Organoids, which are 3D miniature organs that mimic the structure and function of human organs, play an essential role in disease research and drug development. A Korean research team has overcome the limitations of existing imaging technologies, succeeding in the real-time, high-resolution observation of living organoids.
KAIST (represented by President Kwang Hyung Lee) announced on the 14th of October that Professor YongKeun Park’s research team from the Department of Physics, in collaboration with the Genome Editing Research Center (Director Bon-Kyoung Koo) of the Institute for Basic Science (IBS President Do-Young Noh) and Tomocube Inc., has developed an imaging technology using holotomography to observe live, small intestinal organoids in real time at a high resolution.
Existing imaging techniques have struggled to observe living organoids in high resolution over extended periods and often required additional treatments like fluorescent staining.
< Figure 1. Overview of the low-coherence HT workflow. Using holotomography, 3D morphological restoration and quantitative analysis of organoids can be performed. In order to improve the limited field of view, which is a limitation of the microscope, our research team utilized a large-area field of view combination algorithm and made a 3D restoration by acquiring multi-focus holographic images for 3D measurements. After that, the organoids were compartmentalized to divide the parts necessary for analysis and quantitatively evaluated the protein concentration measurable from the refractive index and the survival rate of the organoids. >
The research team introduced holotomography technology to address these issues, which provides high-resolution images without the need for fluorescent staining and allows for the long-term observation of dynamic changes in real time without causing cell damage.
The team validated this technology using small intestinal organoids from experimental mice and were able to observe various cell structures inside the organoids in detail. They also captured dynamic changes such as growth processes, cell division, and cell death in real time using holotomography.
Additionally, the technology allowed for the precise analysis of the organoids' responses to drug treatments, verifying the survival of the cells.
The researchers believe that this breakthrough will open new horizons in organoid research, enabling the greater utilization of organoids in drug development, personalized medicine, and regenerative medicine.
Future research is expected to more accurately replicate the in vivo environment of organoids, contributing significantly to a more detailed understanding of various life phenomena at the cellular level through more precise 3D imaging.
< Figure 2. Real-time organoid morphology analysis. Using holotomography, it is possible to observe the lumen and villus development process of intestinal organoids in real time, which was difficult to observe with a conventional microscope. In addition, various information about intestinal organoids can be obtained by quantifying the size and protein amount of intestinal organoids through image analysis. >
Dr. Mahn Jae Lee, a graduate of KAIST's Graduate School of Medical Science and Engineering, currently at Chungnam National University Hospital and the first author of the paper, commented, "This research represents a new imaging technology that surpasses previous limitations and is expected to make a major contribution to disease modeling, personalized treatments, and drug development research using organoids."
The research results were published online in the international journal Experimental & Molecular Medicine on October 1, 2024, and the technology has been recognized for its applicability in various fields of life sciences. (Paper title: “Long-term three-dimensional high-resolution imaging of live unlabeled small intestinal organoids via low-coherence holotomography”)
This research was supported by the National Research Foundation of Korea, KAIST Institutes, and the Institute for Basic Science.
2024.10.14 View 4857 -
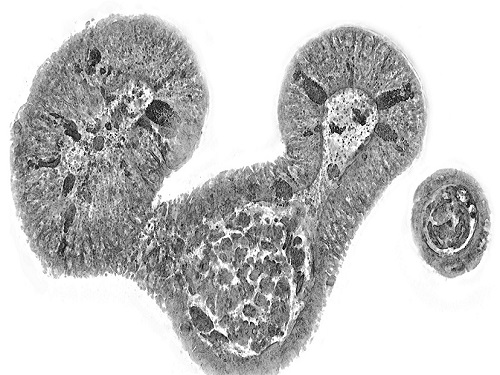 KAIST presents strategies for Holotomography in advanced bio research
Measuring and analyzing three-dimensional (3D) images of live cells and tissues is considered crucial in advanced fields of biology and medicine. Organoids, which are 3D structures that mimic organs, are particular examples that significantly benefits 3D live imaging. Organoids provide effective alternatives to animal testing in the drug development processes, and can rapidly determine personalized medicine. On the other hand, active researches are ongoing to utilize organoids for organ replacement.
< Figure 1. Schematic illustration of holotomography compared to X-ray CT. Similar to CT, they share the commonality of measuring the optical properties of an unlabeled specimen in three dimensions. Instead of X-rays, holotomography irradiates light in the visible range, and provides refractive index measurements of transparent specimens rather than absorptivity. While CT obtains three-dimensional information only through mechanical rotation of the irradiating light, holotomography can replace this by applying wavefront control technology in the visible range. >
Organelle-level observation of 3D biological specimens such as organoids and stem cell colonies without staining or preprocessing holds significant implications for both innovating basic research and bioindustrial applications related to regenerative medicine and bioindustrial applications.
Holotomography (HT) is a 3D optical microscopy that implements 3D reconstruction analogous to that of X-ray computed tomography (CT). Although HT and CT share a similar theoretical background, HT facilitates high-resolution examination inside cells and tissues, instead of the human body. HT obtains 3D images of cells and tissues at the organelle level without chemical or genetic labeling, thus overcomes various challenges of existing methods in bio research and industry. Its potential is highlighted in research fields where sample physiology must not be disrupted, such as regenerative medicine, personalized medicine, and infertility treatment.
< Figure 2. Label-free 3D imaging of diverse live cells. Time-lapse image of Hep3B cells illustrating subcellular morphology changes upon H2O2 treatment, followed by cellular recovery after returning to the regular cell culture medium. >
This paper introduces the advantages and broad applicability of HT to biomedical researchers, while presenting an overview of principles and future technical challenges to optical researchers. It showcases various cases of applying HT in studies such as 3D biology, regenerative medicine, and cancer research, as well as suggesting future optical development. Also, it categorizes HT based on the light source, to describe the principles, limitations, and improvements of each category in detail. Particularly, the paper addresses strategies for deepening cell and organoid studies by introducing artificial intelligence (AI) to HT.
Due to its potential to drive advanced bioindustry, HT is attracting interest and investment from universities and corporates worldwide. The KAIST research team has been leading this international field by developing core technologies and carrying out key application researches throughout the last decade.
< Figure 3. Various types of cells and organelles that make up the imaging barrier of a living intestinal organoid can be observed using holotomography. >
This paper, co-authored by Dr. Geon Kim from KAIST Research Center for Natural Sciences, Professor Ki-Jun Yoon's team from the Department of Biological Sciences, Director Bon-Kyoung Koo's team from the Institute for Basic Science (IBS) Center for Genome Engineering, and Dr. Seongsoo Lee's team from the Korea Basic Science Institute (KBSI), was published in 'Nature Reviews Methods Primers' on the 25th of July. This research was supported by the Leader Grant and Basic Science Research Program of the National Research Foundation, the Hologram Core Technology Development Grant of the Ministry of Science and ICT, the Nano and Material Technology Development Project, and the Health and Medical R&D Project of the Ministry of Health and Welfare.
2024.07.30 View 5433
KAIST presents strategies for Holotomography in advanced bio research
Measuring and analyzing three-dimensional (3D) images of live cells and tissues is considered crucial in advanced fields of biology and medicine. Organoids, which are 3D structures that mimic organs, are particular examples that significantly benefits 3D live imaging. Organoids provide effective alternatives to animal testing in the drug development processes, and can rapidly determine personalized medicine. On the other hand, active researches are ongoing to utilize organoids for organ replacement.
< Figure 1. Schematic illustration of holotomography compared to X-ray CT. Similar to CT, they share the commonality of measuring the optical properties of an unlabeled specimen in three dimensions. Instead of X-rays, holotomography irradiates light in the visible range, and provides refractive index measurements of transparent specimens rather than absorptivity. While CT obtains three-dimensional information only through mechanical rotation of the irradiating light, holotomography can replace this by applying wavefront control technology in the visible range. >
Organelle-level observation of 3D biological specimens such as organoids and stem cell colonies without staining or preprocessing holds significant implications for both innovating basic research and bioindustrial applications related to regenerative medicine and bioindustrial applications.
Holotomography (HT) is a 3D optical microscopy that implements 3D reconstruction analogous to that of X-ray computed tomography (CT). Although HT and CT share a similar theoretical background, HT facilitates high-resolution examination inside cells and tissues, instead of the human body. HT obtains 3D images of cells and tissues at the organelle level without chemical or genetic labeling, thus overcomes various challenges of existing methods in bio research and industry. Its potential is highlighted in research fields where sample physiology must not be disrupted, such as regenerative medicine, personalized medicine, and infertility treatment.
< Figure 2. Label-free 3D imaging of diverse live cells. Time-lapse image of Hep3B cells illustrating subcellular morphology changes upon H2O2 treatment, followed by cellular recovery after returning to the regular cell culture medium. >
This paper introduces the advantages and broad applicability of HT to biomedical researchers, while presenting an overview of principles and future technical challenges to optical researchers. It showcases various cases of applying HT in studies such as 3D biology, regenerative medicine, and cancer research, as well as suggesting future optical development. Also, it categorizes HT based on the light source, to describe the principles, limitations, and improvements of each category in detail. Particularly, the paper addresses strategies for deepening cell and organoid studies by introducing artificial intelligence (AI) to HT.
Due to its potential to drive advanced bioindustry, HT is attracting interest and investment from universities and corporates worldwide. The KAIST research team has been leading this international field by developing core technologies and carrying out key application researches throughout the last decade.
< Figure 3. Various types of cells and organelles that make up the imaging barrier of a living intestinal organoid can be observed using holotomography. >
This paper, co-authored by Dr. Geon Kim from KAIST Research Center for Natural Sciences, Professor Ki-Jun Yoon's team from the Department of Biological Sciences, Director Bon-Kyoung Koo's team from the Institute for Basic Science (IBS) Center for Genome Engineering, and Dr. Seongsoo Lee's team from the Korea Basic Science Institute (KBSI), was published in 'Nature Reviews Methods Primers' on the 25th of July. This research was supported by the Leader Grant and Basic Science Research Program of the National Research Foundation, the Hologram Core Technology Development Grant of the Ministry of Science and ICT, the Nano and Material Technology Development Project, and the Health and Medical R&D Project of the Ministry of Health and Welfare.
2024.07.30 View 5433 -
 'Mini-Lungs' Reveal Early Stages of SARS-CoV-2 Infection
Researchers in Korea and the UK have successfully grown miniature models of critical lung structures called alveoli, and used them to study how the coronavirus that causes COVID-19 infects the lungs.
To date, there have been more than 40 million cases of COVID-19 and almost 1.13 million deaths worldwide. The main target tissues of SARS-CoV-2, the virus that causes COVID-19, especially in patients that develop pneumonia, appear to be alveoli – tiny air sacs in the lungs that take up the oxygen we breathe and exchange it with carbon dioxide to exhale.
To better understand how SARS-CoV-2 infects the lungs and causes disease, a team of Professor Young Seok Ju from the Graduate School of Medical Science and Engineering at KAIST in collaboration with the Wellcome-MRC Cambridge Stem Cell Institute at the University of Cambridge turned to organoids – ‘mini-organs’ grown in three dimensions to mimic the behaviour of tissue and organs.
The team used tissue donated to tissue banks at the Royal Papworth Hospital NHS Foundation Trust and Addenbrooke’s Hospital, Cambridge University NHS Foundations Trust, UK, and Seoul National University Hospital to extract a type of lung cell known as human lung alveolar type 2 cells. By reprogramming these cells back to their earlier ‘stem cell’ stage, they were able to grow self-organizing alveolar-like 3D structures that mimic the behaviour of key lung tissue.
“The research community now has a powerful new platform to study precisely how the virus infects the lungs, as well as explore possible treatments,” said Professor Ju, co-senior author of the research.
Dr. Joo-Hyeon Lee, another co-senior author at the Wellcome-MRC Cambridge Stem Cell Institute, said: “We still know surprisingly little about how SARS-CoV-2 infects the lungs and causes disease. Our approach has allowed us to grow 3D models of key lung tissue – in a sense, ‘mini-lungs’ – in the lab and study what happens when they become infected.”
The team infected the organoids with a strain of SARS-CoV-2 taken from a patient in Korea who was diagnosed with COVID-19 on January 26 after traveling to Wuhan, China. Using a combination of fluorescence imaging and single cell genetic analysis, they were able to study how the cells responded to the virus.
When the 3D models were exposed to SARS-CoV-2, the virus began to replicate rapidly, reaching full cellular infection just six hours after infection. Replication enables the virus to spread throughout the body, infecting other cells and tissue.
Around the same time, the cells began to produce interferons – proteins that act as warning signals to neighbouring cells, telling them to activate their antiviral defences. After 48 hours, the interferons triggered the innate immune response – its first line of defence – and the cells started fighting back against infection.
Sixty hours after infection, a subset of alveolar cells began to disintegrate, leading to cell death and damage to the lung tissue.
Although the researchers observed changes to the lung cells within three days of infection, clinical symptoms of COVID-19 rarely occur so quickly and can sometimes take more than ten days after exposure to appear. The team say there are several possible reasons for this. It may take several days from the virus first infiltrating the upper respiratory tract to it reaching the alveoli. It may also require a substantial proportion of alveolar cells to be infected or for further interactions with immune cells resulting in inflammation before a patient displays symptoms.
“Based on our model we can tackle many unanswered key questions, such as understanding genetic susceptibility to SARS-CoV-2, assessing relative infectivity of viral mutants, and revealing the damage processes of the virus in human alveolar cells,” said Professor Ju. “Most importantly, it provides the opportunity to develop and screen potential therapeutic agents against SARS-CoV-2 infection.”
“We hope to use our technique to grow these 3D models from cells of patients who are particularly vulnerable to infection, such as the elderly or people with diseased lungs, and find out what happens to their tissue,” added Dr. Lee.
The research was a collaboration involving scientists from KAIST, the University of Cambridge, Korea National Institute of Health, Institute for Basic Science (IBS), Seoul National University Hospital and Genome Insight in Korea.
- ProfileProfessor Young Seok JuLaboratory of Cancer Genomics https://julab.kaist.ac.kr the Graduate School of Medical Science and EngineeringKAIST
2020.10.26 View 12761
'Mini-Lungs' Reveal Early Stages of SARS-CoV-2 Infection
Researchers in Korea and the UK have successfully grown miniature models of critical lung structures called alveoli, and used them to study how the coronavirus that causes COVID-19 infects the lungs.
To date, there have been more than 40 million cases of COVID-19 and almost 1.13 million deaths worldwide. The main target tissues of SARS-CoV-2, the virus that causes COVID-19, especially in patients that develop pneumonia, appear to be alveoli – tiny air sacs in the lungs that take up the oxygen we breathe and exchange it with carbon dioxide to exhale.
To better understand how SARS-CoV-2 infects the lungs and causes disease, a team of Professor Young Seok Ju from the Graduate School of Medical Science and Engineering at KAIST in collaboration with the Wellcome-MRC Cambridge Stem Cell Institute at the University of Cambridge turned to organoids – ‘mini-organs’ grown in three dimensions to mimic the behaviour of tissue and organs.
The team used tissue donated to tissue banks at the Royal Papworth Hospital NHS Foundation Trust and Addenbrooke’s Hospital, Cambridge University NHS Foundations Trust, UK, and Seoul National University Hospital to extract a type of lung cell known as human lung alveolar type 2 cells. By reprogramming these cells back to their earlier ‘stem cell’ stage, they were able to grow self-organizing alveolar-like 3D structures that mimic the behaviour of key lung tissue.
“The research community now has a powerful new platform to study precisely how the virus infects the lungs, as well as explore possible treatments,” said Professor Ju, co-senior author of the research.
Dr. Joo-Hyeon Lee, another co-senior author at the Wellcome-MRC Cambridge Stem Cell Institute, said: “We still know surprisingly little about how SARS-CoV-2 infects the lungs and causes disease. Our approach has allowed us to grow 3D models of key lung tissue – in a sense, ‘mini-lungs’ – in the lab and study what happens when they become infected.”
The team infected the organoids with a strain of SARS-CoV-2 taken from a patient in Korea who was diagnosed with COVID-19 on January 26 after traveling to Wuhan, China. Using a combination of fluorescence imaging and single cell genetic analysis, they were able to study how the cells responded to the virus.
When the 3D models were exposed to SARS-CoV-2, the virus began to replicate rapidly, reaching full cellular infection just six hours after infection. Replication enables the virus to spread throughout the body, infecting other cells and tissue.
Around the same time, the cells began to produce interferons – proteins that act as warning signals to neighbouring cells, telling them to activate their antiviral defences. After 48 hours, the interferons triggered the innate immune response – its first line of defence – and the cells started fighting back against infection.
Sixty hours after infection, a subset of alveolar cells began to disintegrate, leading to cell death and damage to the lung tissue.
Although the researchers observed changes to the lung cells within three days of infection, clinical symptoms of COVID-19 rarely occur so quickly and can sometimes take more than ten days after exposure to appear. The team say there are several possible reasons for this. It may take several days from the virus first infiltrating the upper respiratory tract to it reaching the alveoli. It may also require a substantial proportion of alveolar cells to be infected or for further interactions with immune cells resulting in inflammation before a patient displays symptoms.
“Based on our model we can tackle many unanswered key questions, such as understanding genetic susceptibility to SARS-CoV-2, assessing relative infectivity of viral mutants, and revealing the damage processes of the virus in human alveolar cells,” said Professor Ju. “Most importantly, it provides the opportunity to develop and screen potential therapeutic agents against SARS-CoV-2 infection.”
“We hope to use our technique to grow these 3D models from cells of patients who are particularly vulnerable to infection, such as the elderly or people with diseased lungs, and find out what happens to their tissue,” added Dr. Lee.
The research was a collaboration involving scientists from KAIST, the University of Cambridge, Korea National Institute of Health, Institute for Basic Science (IBS), Seoul National University Hospital and Genome Insight in Korea.
- ProfileProfessor Young Seok JuLaboratory of Cancer Genomics https://julab.kaist.ac.kr the Graduate School of Medical Science and EngineeringKAIST
2020.10.26 View 12761