CIC
-
 Decoding Brain Signals to Control a Robotic Arm
Advanced brain-machine interface system successfully interprets arm movement directions from neural signals in the brain
Researchers have developed a mind-reading system for decoding neural signals from the brain during arm movement. The method, described in the journal Applied Soft Computing, can be used by a person to control a robotic arm through a brain-machine interface (BMI).
A BMI is a device that translates nerve signals into commands to control a machine, such as a computer or a robotic limb. There are two main techniques for monitoring neural signals in BMIs: electroencephalography (EEG) and electrocorticography (ECoG).
The EEG exhibits signals from electrodes on the surface of the scalp and is widely employed because it is non-invasive, relatively cheap, safe and easy to use. However, the EEG has low spatial resolution and detects irrelevant neural signals, which makes it difficult to interpret the intentions of individuals from the EEG.
On the other hand, the ECoG is an invasive method that involves placing electrodes directly on the surface of the cerebral cortex below the scalp. Compared with the EEG, the ECoG can monitor neural signals with much higher spatial resolution and less background noise. However, this technique has several drawbacks.
“The ECoG is primarily used to find potential sources of epileptic seizures, meaning the electrodes are placed in different locations for different patients and may not be in the optimal regions of the brain for detecting sensory and movement signals,” explained Professor Jaeseung Jeong, a brain scientist at KAIST. “This inconsistency makes it difficult to decode brain signals to predict movements.”
To overcome these problems, Professor Jeong’s team developed a new method for decoding ECoG neural signals during arm movement. The system is based on a machine-learning system for analysing and predicting neural signals called an ‘echo-state network’ and a mathematical probability model called the Gaussian distribution.
In the study, the researchers recorded ECoG signals from four individuals with epilepsy while they were performing a reach-and-grasp task. Because the ECoG electrodes were placed according to the potential sources of each patient’s epileptic seizures, only 22% to 44% of the electrodes were located in the regions of the brain responsible for controlling movement.
During the movement task, the participants were given visual cues, either by placing a real tennis ball in front of them, or via a virtual reality headset showing a clip of a human arm reaching forward in first-person view. They were asked to reach forward, grasp an object, then return their hand and release the object, while wearing motion sensors on their wrists and fingers. In a second task, they were instructed to imagine reaching forward without moving their arms.
The researchers monitored the signals from the ECoG electrodes during real and imaginary arm movements, and tested whether the new system could predict the direction of this movement from the neural signals. They found that the novel decoder successfully classified arm movements in 24 directions in three-dimensional space, both in the real and virtual tasks, and that the results were at least five times more accurate than chance. They also used a computer simulation to show that the novel ECoG decoder could control the movements of a robotic arm.
Overall, the results suggest that the new machine learning-based BCI system successfully used ECoG signals to interpret the direction of the intended movements. The next steps will be to improve the accuracy and efficiency of the decoder. In the future, it could be used in a real-time BMI device to help people with movement or sensory impairments.
This research was supported by the KAIST Global Singularity Research Program of 2021, Brain Research Program of the National Research Foundation of Korea funded by the Ministry of Science, ICT, and Future Planning, and the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education.
-PublicationHoon-Hee Kim, Jaeseung Jeong, “An electrocorticographic decoder for arm movement for brain-machine interface using an echo state network and Gaussian readout,” Applied SoftComputing online December 31, 2021 (doi.org/10.1016/j.asoc.2021.108393)
-ProfileProfessor Jaeseung JeongDepartment of Bio and Brain EngineeringCollege of EngineeringKAIST
2022.03.18 View 13559
Decoding Brain Signals to Control a Robotic Arm
Advanced brain-machine interface system successfully interprets arm movement directions from neural signals in the brain
Researchers have developed a mind-reading system for decoding neural signals from the brain during arm movement. The method, described in the journal Applied Soft Computing, can be used by a person to control a robotic arm through a brain-machine interface (BMI).
A BMI is a device that translates nerve signals into commands to control a machine, such as a computer or a robotic limb. There are two main techniques for monitoring neural signals in BMIs: electroencephalography (EEG) and electrocorticography (ECoG).
The EEG exhibits signals from electrodes on the surface of the scalp and is widely employed because it is non-invasive, relatively cheap, safe and easy to use. However, the EEG has low spatial resolution and detects irrelevant neural signals, which makes it difficult to interpret the intentions of individuals from the EEG.
On the other hand, the ECoG is an invasive method that involves placing electrodes directly on the surface of the cerebral cortex below the scalp. Compared with the EEG, the ECoG can monitor neural signals with much higher spatial resolution and less background noise. However, this technique has several drawbacks.
“The ECoG is primarily used to find potential sources of epileptic seizures, meaning the electrodes are placed in different locations for different patients and may not be in the optimal regions of the brain for detecting sensory and movement signals,” explained Professor Jaeseung Jeong, a brain scientist at KAIST. “This inconsistency makes it difficult to decode brain signals to predict movements.”
To overcome these problems, Professor Jeong’s team developed a new method for decoding ECoG neural signals during arm movement. The system is based on a machine-learning system for analysing and predicting neural signals called an ‘echo-state network’ and a mathematical probability model called the Gaussian distribution.
In the study, the researchers recorded ECoG signals from four individuals with epilepsy while they were performing a reach-and-grasp task. Because the ECoG electrodes were placed according to the potential sources of each patient’s epileptic seizures, only 22% to 44% of the electrodes were located in the regions of the brain responsible for controlling movement.
During the movement task, the participants were given visual cues, either by placing a real tennis ball in front of them, or via a virtual reality headset showing a clip of a human arm reaching forward in first-person view. They were asked to reach forward, grasp an object, then return their hand and release the object, while wearing motion sensors on their wrists and fingers. In a second task, they were instructed to imagine reaching forward without moving their arms.
The researchers monitored the signals from the ECoG electrodes during real and imaginary arm movements, and tested whether the new system could predict the direction of this movement from the neural signals. They found that the novel decoder successfully classified arm movements in 24 directions in three-dimensional space, both in the real and virtual tasks, and that the results were at least five times more accurate than chance. They also used a computer simulation to show that the novel ECoG decoder could control the movements of a robotic arm.
Overall, the results suggest that the new machine learning-based BCI system successfully used ECoG signals to interpret the direction of the intended movements. The next steps will be to improve the accuracy and efficiency of the decoder. In the future, it could be used in a real-time BMI device to help people with movement or sensory impairments.
This research was supported by the KAIST Global Singularity Research Program of 2021, Brain Research Program of the National Research Foundation of Korea funded by the Ministry of Science, ICT, and Future Planning, and the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education.
-PublicationHoon-Hee Kim, Jaeseung Jeong, “An electrocorticographic decoder for arm movement for brain-machine interface using an echo state network and Gaussian readout,” Applied SoftComputing online December 31, 2021 (doi.org/10.1016/j.asoc.2021.108393)
-ProfileProfessor Jaeseung JeongDepartment of Bio and Brain EngineeringCollege of EngineeringKAIST
2022.03.18 View 13559 -
 New Structural Insight into Neurodegenerative Disease
A research team from the Korea Advanced Institute of Science and Technology (KAIST) released their results on the structure and molecular details of the neurodegenerative disease-associated protein Ataxin-1. Mutations in Ataxin-1 cause the neurological disease, Spinocerebella Ataxia Type 1 (SCA1), which is characterized by a loss of muscular coordination and balance (ataxia), as is seen in Parkinson’s, Alzheimer’s, and Huntington’s diseases.
SCA1-causing mutations in the ATAXIN1 gene alter the length of a glutamine stretch in the Ataxin-1 protein. The research team provides the first structural insight into the complex formation of ATAXIN-1 with its binding partner, Capicua (CIC). The team, led by Professor Ji-Joon Song from the Department of Biological Sciences at KAIST, solved the structure of Ataxin-1 and CIC complex in atomic level revealing molecular details of the interaction between Ataxin-1 and CIC.
Professor Song explained his recent research work,
“We are able to see the intricate process of complex formation and reconfiguration of the two proteins when they interact with each other. Our work, we expect, will provide a new therapeutic target to modulate SCA1 neurodegenerative disease.”
Understanding structural and molecular details of proteins at the atomic level will help researchers to track the molecular pathogenesis of the disease and, ultimately, design targeted therapies or treatments for patients, rather than just relieving the symptoms of diseases.
Professor Song’s research paper, entitled “Structural Basis of Protein Complex Formation and Reconfiguration by Polyglutamine Disease Protein ATAXIN-1 and Capicua,” will be published in the March 15th issue of Genes & Development (www.genesdev.org).
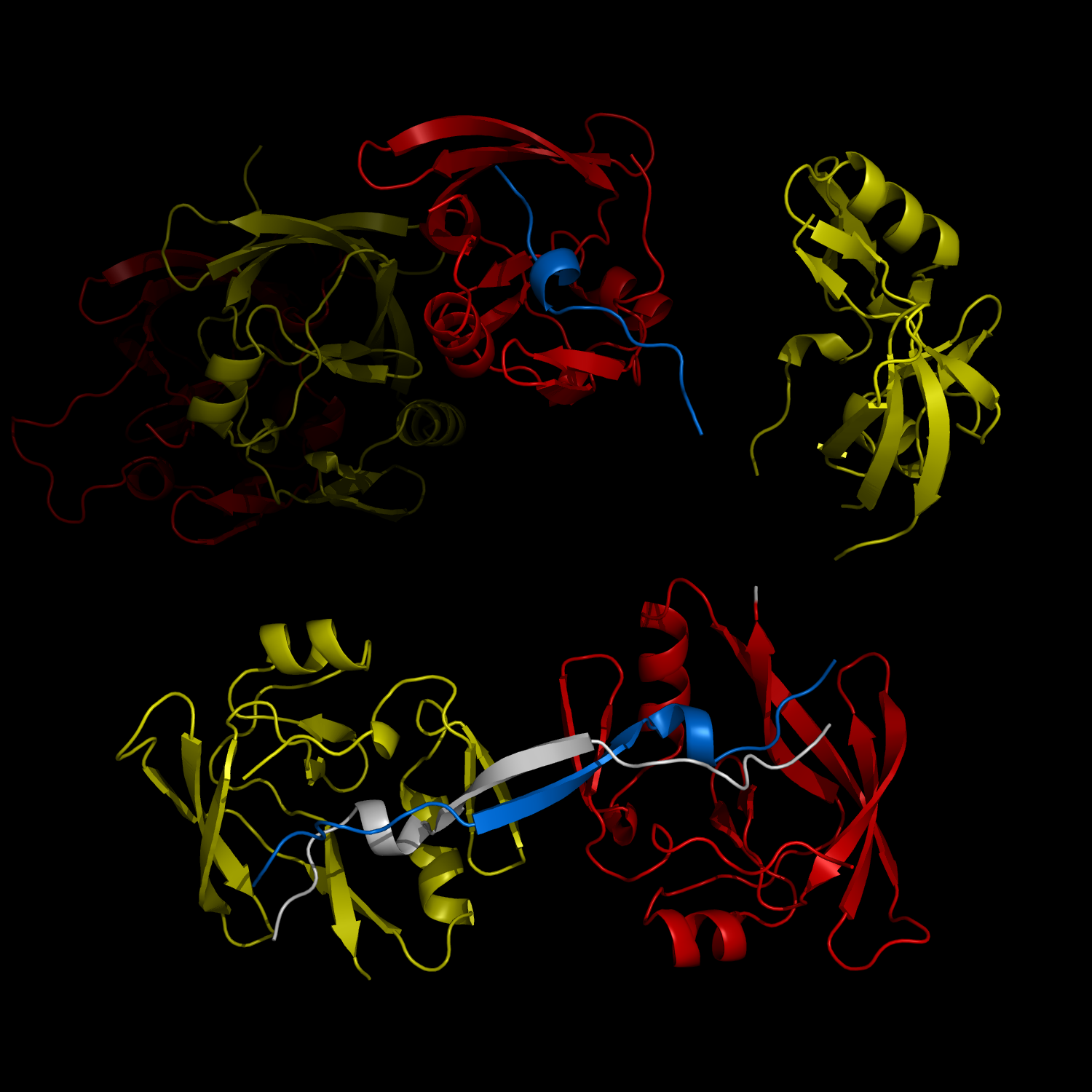
Complex Formation and Reconfiguration of ATAXIN-1 and Capicua
The complex formation between a polyglutamine disease protein, ATXIN-1 and the transcriptional repressor Capicua (CIC) plays a critical role in SCA 1 pathogenesis. The image shows that the homodimerization of ATXIN-1 (yellow and red) is disrupted upon binding of CIC (blue). Furthermore, the binding of CIC to the ATXIN-1 induces a new form of ATXIN-1 dimerization mediated by CICs (ATXIN-1 AXH domains are shown in yellow and red, and CIC peptides shown in blue and white).
2013.04.02 View 10272
New Structural Insight into Neurodegenerative Disease
A research team from the Korea Advanced Institute of Science and Technology (KAIST) released their results on the structure and molecular details of the neurodegenerative disease-associated protein Ataxin-1. Mutations in Ataxin-1 cause the neurological disease, Spinocerebella Ataxia Type 1 (SCA1), which is characterized by a loss of muscular coordination and balance (ataxia), as is seen in Parkinson’s, Alzheimer’s, and Huntington’s diseases.
SCA1-causing mutations in the ATAXIN1 gene alter the length of a glutamine stretch in the Ataxin-1 protein. The research team provides the first structural insight into the complex formation of ATAXIN-1 with its binding partner, Capicua (CIC). The team, led by Professor Ji-Joon Song from the Department of Biological Sciences at KAIST, solved the structure of Ataxin-1 and CIC complex in atomic level revealing molecular details of the interaction between Ataxin-1 and CIC.
Professor Song explained his recent research work,
“We are able to see the intricate process of complex formation and reconfiguration of the two proteins when they interact with each other. Our work, we expect, will provide a new therapeutic target to modulate SCA1 neurodegenerative disease.”
Understanding structural and molecular details of proteins at the atomic level will help researchers to track the molecular pathogenesis of the disease and, ultimately, design targeted therapies or treatments for patients, rather than just relieving the symptoms of diseases.
Professor Song’s research paper, entitled “Structural Basis of Protein Complex Formation and Reconfiguration by Polyglutamine Disease Protein ATAXIN-1 and Capicua,” will be published in the March 15th issue of Genes & Development (www.genesdev.org).
Complex Formation and Reconfiguration of ATAXIN-1 and Capicua
The complex formation between a polyglutamine disease protein, ATXIN-1 and the transcriptional repressor Capicua (CIC) plays a critical role in SCA 1 pathogenesis. The image shows that the homodimerization of ATXIN-1 (yellow and red) is disrupted upon binding of CIC (blue). Furthermore, the binding of CIC to the ATXIN-1 induces a new form of ATXIN-1 dimerization mediated by CICs (ATXIN-1 AXH domains are shown in yellow and red, and CIC peptides shown in blue and white).
2013.04.02 View 10272 -
 A Breakthrough for Cardiac Monitoring: Portable Smart Patch Makes It Possible for Real-time Observation of Heart Movement
Newly invented device makes the monitoring easier and convenient.
Professor Hoi-Jun Yoo of KAIST, Department of Electrical Engineering, said that his research team has invented a smart patch for cardiac monitoring, the first of its kind in the world. Adhesive and can be applied directly to chest in human body, the patch is embedded with a built-in high performance semiconductor integrated circuit (IC), called Healthcare IC, and with twenty five electrodes formed on the patch’s surface.
The 25-electrodes, with a capability of creating various configurations, can detect cardiac contractions and relaxations and collect electrocardiogram (ECG) signals. The Healthcare IC monitors ECG signals and sends the information to a portable data terminal like mobile phones, making it possible for a convenient, easy check up on cardiac observations.
The key technologies used for the patch are the Healthcare IC that measures cardiovascular impedance and ECG signals, and the electronic circuit board made of four layers of fabric, between which electrodes, wireless antenna, circuit board, and flexible battery are installed. With the P-FCB (Planar Fashionable Circuit Board) technology, the research team explained, electrodes and a circuit board are directly stacked into the fabric. Additionally, the Healthcare IC (size: 5mm x 5mm), which has components of electrode control unit, ECG and cardiovascular resistance detection unit, data compression unit, Static Random Access Memory (SRAM), and wireless transmitter receiver, is attached on the fabric. The Healthcare IC is operated by an ultra-low electrical power.
Like a medicated patch commonly used to relieve arthritis pains, the surface of smart patch is adhesive so that people can carry it around without much hassle. A finished product will be 15cm x 15 cm in size and 1mm high in thickness. The Healthcare IC can measure cardiovascular impedance variances with less than 0.81% distortion in 16 different configurations through differential current injectors and reconfigurable high sensitivity detection circuitry.
“The patch will be ideal for patients who suffer a chronic heart disease and need to receive a continuous care for their condition. Once commercialized, the patch will allow the patients to conduct a self-diagnosis at anytime and anywhere,” said Yan Long, a member of the research team.
There has been a continuously growing demand worldwide since 2000 for the development of technology that provides a suitable healthcare management to patients with a chronic heart disease (e.g., cardiovascular problems), but most of the technology developed today are only limited to monitoring electrical signals of heart activity. Cardiovascular monitors, commonly used at many of healthcare places nowadays, are too bulky to use and give uncomfortable feelings to patients when applied. Besides, the current monitors are connected to an electrical line for power supply, and they are unable to have a low power communication with an outdoor communication gadget, thus unavailable for wide use.
Professor Yoo gave his presentation on this new invention at an international conference, International Solid-State Circuits Conference, held on February 8-10 in San Francisco. The subject of his presentation was “A 3.9mW 25-electorde Reconfigurable Thoracic Impedance/ECG SoC with Body-Channel Transponder.”
(Picture 1) Structure of Smart Patch
(Picture 2) Smart patch when applied onto human body
(Picture 3) Data received from smart patch
(Picture 4) Healthcare IC
2010.02.17 View 17479
A Breakthrough for Cardiac Monitoring: Portable Smart Patch Makes It Possible for Real-time Observation of Heart Movement
Newly invented device makes the monitoring easier and convenient.
Professor Hoi-Jun Yoo of KAIST, Department of Electrical Engineering, said that his research team has invented a smart patch for cardiac monitoring, the first of its kind in the world. Adhesive and can be applied directly to chest in human body, the patch is embedded with a built-in high performance semiconductor integrated circuit (IC), called Healthcare IC, and with twenty five electrodes formed on the patch’s surface.
The 25-electrodes, with a capability of creating various configurations, can detect cardiac contractions and relaxations and collect electrocardiogram (ECG) signals. The Healthcare IC monitors ECG signals and sends the information to a portable data terminal like mobile phones, making it possible for a convenient, easy check up on cardiac observations.
The key technologies used for the patch are the Healthcare IC that measures cardiovascular impedance and ECG signals, and the electronic circuit board made of four layers of fabric, between which electrodes, wireless antenna, circuit board, and flexible battery are installed. With the P-FCB (Planar Fashionable Circuit Board) technology, the research team explained, electrodes and a circuit board are directly stacked into the fabric. Additionally, the Healthcare IC (size: 5mm x 5mm), which has components of electrode control unit, ECG and cardiovascular resistance detection unit, data compression unit, Static Random Access Memory (SRAM), and wireless transmitter receiver, is attached on the fabric. The Healthcare IC is operated by an ultra-low electrical power.
Like a medicated patch commonly used to relieve arthritis pains, the surface of smart patch is adhesive so that people can carry it around without much hassle. A finished product will be 15cm x 15 cm in size and 1mm high in thickness. The Healthcare IC can measure cardiovascular impedance variances with less than 0.81% distortion in 16 different configurations through differential current injectors and reconfigurable high sensitivity detection circuitry.
“The patch will be ideal for patients who suffer a chronic heart disease and need to receive a continuous care for their condition. Once commercialized, the patch will allow the patients to conduct a self-diagnosis at anytime and anywhere,” said Yan Long, a member of the research team.
There has been a continuously growing demand worldwide since 2000 for the development of technology that provides a suitable healthcare management to patients with a chronic heart disease (e.g., cardiovascular problems), but most of the technology developed today are only limited to monitoring electrical signals of heart activity. Cardiovascular monitors, commonly used at many of healthcare places nowadays, are too bulky to use and give uncomfortable feelings to patients when applied. Besides, the current monitors are connected to an electrical line for power supply, and they are unable to have a low power communication with an outdoor communication gadget, thus unavailable for wide use.
Professor Yoo gave his presentation on this new invention at an international conference, International Solid-State Circuits Conference, held on February 8-10 in San Francisco. The subject of his presentation was “A 3.9mW 25-electorde Reconfigurable Thoracic Impedance/ECG SoC with Body-Channel Transponder.”
(Picture 1) Structure of Smart Patch
(Picture 2) Smart patch when applied onto human body
(Picture 3) Data received from smart patch
(Picture 4) Healthcare IC
2010.02.17 View 17479