Angewandte+Chemie+International+Edition
-
 Light Driven Drug-Enzyme Reaction Catalytic Platform Developed
Low Cost Dye Used, Hope for Future Development of High Value Medicinal Products to Treat Cardiovascular Disease and Gastric Ulcers
A KAIST research team from the Departments of Materials Science and Engineering and of Chemical and Biomolecular Engineering, led respectively by Professors Chan Beum Park and Ki Jun Jeong, has developed a new reaction platform to induce drug-enzyme reaction using light.
The research results were published in the journal Angewandte Chemie, International Edition, as the back cover on 12 January 2015.
Applications of this technology may enable production of high value products such as medicine for cardiovascular disease and gastric ulcers, for example Omeprazole, using an inexpensive dye.
Cytochrome P450 is an enzyme involved in oxidative response which has an important role in drug and hormone metabolism in organisms. It is known to be responsible for metabolism of 75% of drugs in humans and is considered a fundamental factor in new drug development.
To activate cytochrome P450, the enzyme must receive an electron by reducing the enzyme. In addition, NADPH (a coenzyme) needs to be present. However, since NADPH is expensive, the use of cytochrome P450 was limited to the laboratory and has not yet been commercialized.
The research team used photosensitizer eosin Y instead of NADPH to develop “Whole Cell Photo-Biocatalysis” in bacteria E. coli. By exposing inexpensive eosin Y to light, cytochrome P450 reaction was catalyzed to produce the expensive metabolic material.
Professor Park said, “This research enabled industrial application of cytochrome P450 enzyme, which was previous limited.” He continued, “This technology will help greatly in producing high value medical products using cytochrome P450 enzyme.”
The research was funded by the National Research Foundation of Korea and KAIST's High Risk High Return Project (HRHRP).
Figure 1: Mimetic Diagram of Electron Transfer from Light to Cytochrome P450 Enzyme via Eosin Y, EY
Figure 2: The back cover of Angewandte Chemie published on 12 January 2015, showing the research results
2015.01.26 View 11465
Light Driven Drug-Enzyme Reaction Catalytic Platform Developed
Low Cost Dye Used, Hope for Future Development of High Value Medicinal Products to Treat Cardiovascular Disease and Gastric Ulcers
A KAIST research team from the Departments of Materials Science and Engineering and of Chemical and Biomolecular Engineering, led respectively by Professors Chan Beum Park and Ki Jun Jeong, has developed a new reaction platform to induce drug-enzyme reaction using light.
The research results were published in the journal Angewandte Chemie, International Edition, as the back cover on 12 January 2015.
Applications of this technology may enable production of high value products such as medicine for cardiovascular disease and gastric ulcers, for example Omeprazole, using an inexpensive dye.
Cytochrome P450 is an enzyme involved in oxidative response which has an important role in drug and hormone metabolism in organisms. It is known to be responsible for metabolism of 75% of drugs in humans and is considered a fundamental factor in new drug development.
To activate cytochrome P450, the enzyme must receive an electron by reducing the enzyme. In addition, NADPH (a coenzyme) needs to be present. However, since NADPH is expensive, the use of cytochrome P450 was limited to the laboratory and has not yet been commercialized.
The research team used photosensitizer eosin Y instead of NADPH to develop “Whole Cell Photo-Biocatalysis” in bacteria E. coli. By exposing inexpensive eosin Y to light, cytochrome P450 reaction was catalyzed to produce the expensive metabolic material.
Professor Park said, “This research enabled industrial application of cytochrome P450 enzyme, which was previous limited.” He continued, “This technology will help greatly in producing high value medical products using cytochrome P450 enzyme.”
The research was funded by the National Research Foundation of Korea and KAIST's High Risk High Return Project (HRHRP).
Figure 1: Mimetic Diagram of Electron Transfer from Light to Cytochrome P450 Enzyme via Eosin Y, EY
Figure 2: The back cover of Angewandte Chemie published on 12 January 2015, showing the research results
2015.01.26 View 11465 -
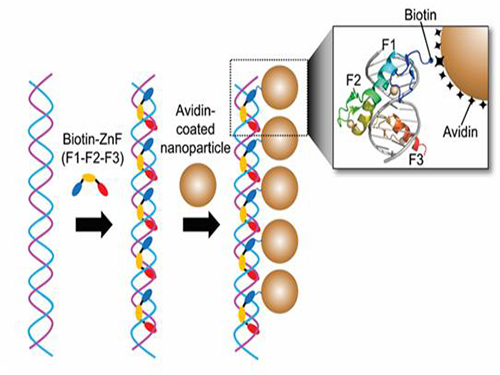 Nanoparticle Cluster Manufacturing Technique Using DNA Binding Protein Developed
Professor Hak-Sung Kim of the Department of Biological Sciences at KAIST and Yiseul Ryu, a doctoral candidate, used the Zinc Finger protein that specifically binds to target DNA sequence to develop a new manufacturing technique for size-controllable magnetic Nanoparticle Clusters (NPCs). Their research results were published in Angewandte Chemie International Edition online on 25 November 2014.
NPCs are structures consisting of magnetic nanoparticles, gold nanoparticles, and quantum dots, each of which are smaller than 100 nm (10-9m). NPCs have a distinctive property of collectivity not seen in single nanoparticles.
Specifically NPCS differ in physical and optical properties such as Plasmon coupling absorbance, energy transfers between particles, electron transfers, and conductivity. Therefore, NPCs can be employed in biological and medical research as well as the development of nanoelectric and nanoplasmon devices.
To make use of these novel properties, the size and the composition of the cluster must be exquisitely controlled. However, previous techniques relied on chemical binding which required complex steps, making it difficult to control the size and composition of NPCs.
Professor Kim’s team used Zinc Finger, a DNA binding protein, to develop a NPCs manufacturing technique to create clusters of the desired size easily. The Zinc Finger protein contains a zinc ion and specifically recognizes DNA sequence upon binding, which allows the exquisite control of the size and the cluster composition. The technique is also bio-friendly.
Professor Kim’s team created linear structure of different sizes of NPCs using Zinc Finger proteins and three DNA sequences of different lengths. The NPCs they produced confirmed their ability to control the size and structure of the cluster by using different DNA lengths.
The NPCs showed tripled T2 relaxation rates compared to the existing MRI contrast media (Feridex) and effectively transported to targeted cells. The research findings show the potential use of NPCs in biological and medical fields such as MRI contrast media, fluorescence imaging, and drug transport.
The research used the specific binding property of protein and DNA to develop a new method to create an inorganic nanoparticle’s supramolecular assembly. The technique can be used and applied extensively in other nanoparticles for future research in diagnosis, imaging, and drug and gene delivery.
Figure 1. A Mimetic Diagram of NPCs Manufacturing Technique Using DNA Binding Protein Zinc Finger
Figure 2. Transmission Electron Microscopy Images showing different sizes of NPCs depending on the length of the DNA
2014.12.04 View 14490
Nanoparticle Cluster Manufacturing Technique Using DNA Binding Protein Developed
Professor Hak-Sung Kim of the Department of Biological Sciences at KAIST and Yiseul Ryu, a doctoral candidate, used the Zinc Finger protein that specifically binds to target DNA sequence to develop a new manufacturing technique for size-controllable magnetic Nanoparticle Clusters (NPCs). Their research results were published in Angewandte Chemie International Edition online on 25 November 2014.
NPCs are structures consisting of magnetic nanoparticles, gold nanoparticles, and quantum dots, each of which are smaller than 100 nm (10-9m). NPCs have a distinctive property of collectivity not seen in single nanoparticles.
Specifically NPCS differ in physical and optical properties such as Plasmon coupling absorbance, energy transfers between particles, electron transfers, and conductivity. Therefore, NPCs can be employed in biological and medical research as well as the development of nanoelectric and nanoplasmon devices.
To make use of these novel properties, the size and the composition of the cluster must be exquisitely controlled. However, previous techniques relied on chemical binding which required complex steps, making it difficult to control the size and composition of NPCs.
Professor Kim’s team used Zinc Finger, a DNA binding protein, to develop a NPCs manufacturing technique to create clusters of the desired size easily. The Zinc Finger protein contains a zinc ion and specifically recognizes DNA sequence upon binding, which allows the exquisite control of the size and the cluster composition. The technique is also bio-friendly.
Professor Kim’s team created linear structure of different sizes of NPCs using Zinc Finger proteins and three DNA sequences of different lengths. The NPCs they produced confirmed their ability to control the size and structure of the cluster by using different DNA lengths.
The NPCs showed tripled T2 relaxation rates compared to the existing MRI contrast media (Feridex) and effectively transported to targeted cells. The research findings show the potential use of NPCs in biological and medical fields such as MRI contrast media, fluorescence imaging, and drug transport.
The research used the specific binding property of protein and DNA to develop a new method to create an inorganic nanoparticle’s supramolecular assembly. The technique can be used and applied extensively in other nanoparticles for future research in diagnosis, imaging, and drug and gene delivery.
Figure 1. A Mimetic Diagram of NPCs Manufacturing Technique Using DNA Binding Protein Zinc Finger
Figure 2. Transmission Electron Microscopy Images showing different sizes of NPCs depending on the length of the DNA
2014.12.04 View 14490 -
 Eggshell-like Cell Encapsulation and Degradation Technology Developed
Some bacteria form endospores on cell walls to protect their DNA in case of nutrient deficiency. When an endospore meets a suitable environment for survival, the cell can revert to the original state from which it can reproduce.
The technique that can artificially control such phenomenon was developed by an international team of researchers. At first, a cell is wrapped and preserved like an egg. When the cell is needed, the technique allows the endospore to decompose while it is alive. Future applications for this technique include cell-based biosensor, cell therapy, and biocatalyst processes.
Professors Insung Choi and Younghoon Lee from the Department of Chemistry at KAIST as well as and Professor Frank Caruso from the University of Melbourne developed this technique which permits a cell to stay alive by coating it with film on a nanometer scale and then to be decomposed while it is alive.
The research finding was published in the November 10th issue of Angewandte Chemie International Edition as the lead article.
Cell encapsulation allows researchers to capture a cell in a tight capsule while it is alive. It is highly recognized in cell-based applications where the control of cell stability and cell-division is the biggest issue.
Traditional cell encapsulation methods utilized organic film or inorganic capsules that are made of organic film moldings. Although these films tightly closed around the cell, because they were not easily decomposable, it was difficult to apply the method.
The research team succeeded in encapsulating each cell in a metal-polyphenol film by mixing tannic acid and iron ion solution with yeast cells.
Usually extracted from oak barks or grape peels, tannic acid is a natural substance. It forms a metal-polyphenol film within ten seconds when it meets iron ions due to its high affinity with cells. Cells encapsulated with this film presented high survival rates. Since the film forms quickly in a simple manner, it was possible to obtain large amount of encapsulated cells.
The research team also found that the metal-polyphenol film was stable in neutral pH, but is easily degradable under a weak acidic condition. Using this property, they were able to control cell division by restoring the cell to its pre-encapsulated state at a desired moment.
Protecting the cell from the external environment like an egg shell, the metal-polyphenol film protected the cell against foreign conditions such as lytic enzymes, extended exposure to UV radiation, and silver nanoparticles. The research indicated that the encapsulated cells had a high survival rate even under extreme environments.
Professor Lee said that “not only the cells remain alive during the encapsulation stage, but also they can be protected under extreme environment.” He added, “This is an advanced cell encapsulation technology that allows controlling cell-division of those cells through responsive shell degradation on-demand.”
Professor Choi commented, “Although the cell encapsulation technology is still in its infancy, as the technology matures the application of cell-manipulation technology will be actualized.” He highlighted that “it will serve as a breakthrough to problems faced by cell-based applications.”
Sponsored by the Ministry of Science, ICT and Future Planning and the National Research Foundation of Korea, the research was led by two Master’s candidates, Ji Hun Park and Kyung Hwan Kim, under the joint guidance of research professors from KAIST and the University of Melbourne.
Figure 1: Lead article of Angewandte Chemie
Background: Shows a live native yeast (in green) encapsulated in a metal-polyphenol film (in red) illustrating the vitality of the yeast
Front: A native yeast at each encapsulation stage
Pictured on the bottom left is a cell prior to encapsulation. Following the red arrow, the native yeast is in purple to show metal-polyphenol film formed around the cell. The cell after the green arrow is a visualization of the degradation of the film in weak acidic condition.
Figure 2: A mimetic diagram of cell encapsulation with a metal-polyphenol film
Top: A native yeast before encapsulation
Middle: A native yeast encapsulated with Tannic Acid-Fe (III) Nanoshell – cell-division of the encapsulated cell is controlled by pH and the shell is protected against silver nanoparticle, lytic enzyme, and UV-C
Bottom: Shell degradation on-demand depending on pH
2014.11.18 View 11276
Eggshell-like Cell Encapsulation and Degradation Technology Developed
Some bacteria form endospores on cell walls to protect their DNA in case of nutrient deficiency. When an endospore meets a suitable environment for survival, the cell can revert to the original state from which it can reproduce.
The technique that can artificially control such phenomenon was developed by an international team of researchers. At first, a cell is wrapped and preserved like an egg. When the cell is needed, the technique allows the endospore to decompose while it is alive. Future applications for this technique include cell-based biosensor, cell therapy, and biocatalyst processes.
Professors Insung Choi and Younghoon Lee from the Department of Chemistry at KAIST as well as and Professor Frank Caruso from the University of Melbourne developed this technique which permits a cell to stay alive by coating it with film on a nanometer scale and then to be decomposed while it is alive.
The research finding was published in the November 10th issue of Angewandte Chemie International Edition as the lead article.
Cell encapsulation allows researchers to capture a cell in a tight capsule while it is alive. It is highly recognized in cell-based applications where the control of cell stability and cell-division is the biggest issue.
Traditional cell encapsulation methods utilized organic film or inorganic capsules that are made of organic film moldings. Although these films tightly closed around the cell, because they were not easily decomposable, it was difficult to apply the method.
The research team succeeded in encapsulating each cell in a metal-polyphenol film by mixing tannic acid and iron ion solution with yeast cells.
Usually extracted from oak barks or grape peels, tannic acid is a natural substance. It forms a metal-polyphenol film within ten seconds when it meets iron ions due to its high affinity with cells. Cells encapsulated with this film presented high survival rates. Since the film forms quickly in a simple manner, it was possible to obtain large amount of encapsulated cells.
The research team also found that the metal-polyphenol film was stable in neutral pH, but is easily degradable under a weak acidic condition. Using this property, they were able to control cell division by restoring the cell to its pre-encapsulated state at a desired moment.
Protecting the cell from the external environment like an egg shell, the metal-polyphenol film protected the cell against foreign conditions such as lytic enzymes, extended exposure to UV radiation, and silver nanoparticles. The research indicated that the encapsulated cells had a high survival rate even under extreme environments.
Professor Lee said that “not only the cells remain alive during the encapsulation stage, but also they can be protected under extreme environment.” He added, “This is an advanced cell encapsulation technology that allows controlling cell-division of those cells through responsive shell degradation on-demand.”
Professor Choi commented, “Although the cell encapsulation technology is still in its infancy, as the technology matures the application of cell-manipulation technology will be actualized.” He highlighted that “it will serve as a breakthrough to problems faced by cell-based applications.”
Sponsored by the Ministry of Science, ICT and Future Planning and the National Research Foundation of Korea, the research was led by two Master’s candidates, Ji Hun Park and Kyung Hwan Kim, under the joint guidance of research professors from KAIST and the University of Melbourne.
Figure 1: Lead article of Angewandte Chemie
Background: Shows a live native yeast (in green) encapsulated in a metal-polyphenol film (in red) illustrating the vitality of the yeast
Front: A native yeast at each encapsulation stage
Pictured on the bottom left is a cell prior to encapsulation. Following the red arrow, the native yeast is in purple to show metal-polyphenol film formed around the cell. The cell after the green arrow is a visualization of the degradation of the film in weak acidic condition.
Figure 2: A mimetic diagram of cell encapsulation with a metal-polyphenol film
Top: A native yeast before encapsulation
Middle: A native yeast encapsulated with Tannic Acid-Fe (III) Nanoshell – cell-division of the encapsulated cell is controlled by pH and the shell is protected against silver nanoparticle, lytic enzyme, and UV-C
Bottom: Shell degradation on-demand depending on pH
2014.11.18 View 11276 -
 Explanation for the polymerized nucleic acid enzyme's abnormal activation found
KAIST’s Professor Park Hyun Kyu of the Department of Bio Chemical Engineering revealed on the 23rd of December 2010 that his team had successfully developed the technology that uses the metal ions to control the abnormal activation of nucleic acids’ enzymes and using this, created a logic gate, which is a core technology in the field of future bio electrons.
The polymerized nucleic acid enzyme works to increase the synthesis of DNA and kicks into action only when the target DNA and primers form complimentary pairs (A and T, C and G).
Professor Park broke the common conception and found that it is possible for none complimentary pairs like T-T and C-C to initiate the activation of the enzyme and thus increase the nucleic acid production, given that there are certain metal ions present.
What Professor Park realized is that the enzymes mistake the uncomplimentary T-T and C-C pairs (with stabilized structures due to the bonding with mercury and silver ions) as being complimentary base pairs. Professor Park described this phenomenon as the “illusionary polymerase activity.”
The research team developed a logic gate based on the “illusionary polymerase activity’ phenomenon.” The logic gate paves the way to the development of future bio electron needed for bio computers and high performance memories.
Professor Park commented, “The research is an advancement of the previous research carried on about metal ions and nucleic acid synthesis. Our research is the first attempt at merging the concepts of the two previously separately carried out researches and can be adapted for testing presence of metal ions and development of a new single nucleotide polymorphic gene analysis technology.”
Professor Park added that, “Our research is a great stride in the field of nano scale electron element research as the results made possible the formation of accurate logic gates through relatively cost efficient and simple system designs.”
On a side note, the research was funded by Korea Research Foundation (Chairman: Park Chan Mo) and was selected as the cover paper for the December issue of ‘Angewandte Chemie International Edition’.
2011.01.18 View 13496
Explanation for the polymerized nucleic acid enzyme's abnormal activation found
KAIST’s Professor Park Hyun Kyu of the Department of Bio Chemical Engineering revealed on the 23rd of December 2010 that his team had successfully developed the technology that uses the metal ions to control the abnormal activation of nucleic acids’ enzymes and using this, created a logic gate, which is a core technology in the field of future bio electrons.
The polymerized nucleic acid enzyme works to increase the synthesis of DNA and kicks into action only when the target DNA and primers form complimentary pairs (A and T, C and G).
Professor Park broke the common conception and found that it is possible for none complimentary pairs like T-T and C-C to initiate the activation of the enzyme and thus increase the nucleic acid production, given that there are certain metal ions present.
What Professor Park realized is that the enzymes mistake the uncomplimentary T-T and C-C pairs (with stabilized structures due to the bonding with mercury and silver ions) as being complimentary base pairs. Professor Park described this phenomenon as the “illusionary polymerase activity.”
The research team developed a logic gate based on the “illusionary polymerase activity’ phenomenon.” The logic gate paves the way to the development of future bio electron needed for bio computers and high performance memories.
Professor Park commented, “The research is an advancement of the previous research carried on about metal ions and nucleic acid synthesis. Our research is the first attempt at merging the concepts of the two previously separately carried out researches and can be adapted for testing presence of metal ions and development of a new single nucleotide polymorphic gene analysis technology.”
Professor Park added that, “Our research is a great stride in the field of nano scale electron element research as the results made possible the formation of accurate logic gates through relatively cost efficient and simple system designs.”
On a side note, the research was funded by Korea Research Foundation (Chairman: Park Chan Mo) and was selected as the cover paper for the December issue of ‘Angewandte Chemie International Edition’.
2011.01.18 View 13496