bio
-
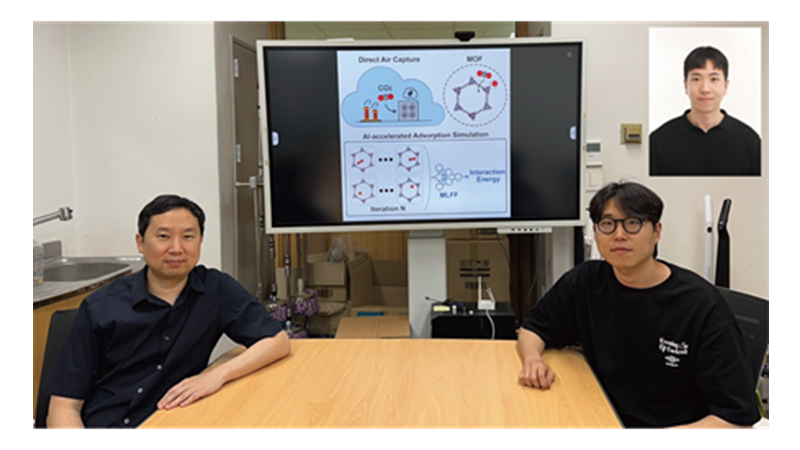 KAIST Develops AI to Easily Find Promising Materials That Capture Only CO₂
< Photo 1. (From left) Professor Jihan Kim, Ph.D. candidate Yunsung Lim and Dr. Hyunsoo Park of the Department of Chemical and Biomolecular Engineering >
In order to help prevent the climate crisis, actively reducing already-emitted CO₂ is essential. Accordingly, direct air capture (DAC) — a technology that directly extracts only CO₂ from the air — is gaining attention. However, effectively capturing pure CO₂ is not easy due to water vapor (H₂O) present in the air. KAIST researchers have successfully used AI-driven machine learning techniques to identify the most promising CO₂-capturing materials among metal-organic frameworks (MOFs), a key class of materials studied for this technology.
KAIST (President Kwang Hyung Lee) announced on the 29th of June that a research team led by Professor Jihan Kim from the Department of Chemical and Biomolecular Engineering, in collaboration with a team at Imperial College London, has developed a machine-learning-based simulation method that can quickly and accurately screen MOFs best suited for atmospheric CO₂ capture.
< Figure 1. Concept diagram of Direct Air Capture (DAC) technology and carbon capture using Metal-Organic Frameworks (MOFs). MOFs are promising porous materials capable of capturing carbon dioxide from the atmosphere, drawing attention as a core material for DAC technology. >
To overcome the difficulty of discovering high-performance materials due to the complexity of structures and the limitations of predicting intermolecular interactions, the research team developed a machine learning force field (MLFF) capable of precisely predicting the interactions between CO₂, water (H₂O), and MOFs. This new method enables calculations of MOF adsorption properties with quantum-mechanics-level accuracy at vastly faster speeds than before.
Using this system, the team screened over 8,000 experimentally synthesized MOF structures, identifying more than 100 promising candidates for CO₂ capture. Notably, this included new candidates that had not been uncovered by traditional force-field-based simulations. The team also analyzed the relationships between MOF chemical structure and adsorption performance, proposing seven key chemical features that will help in designing new materials for DAC.
< Figure 2. Concept diagram of adsorption simulation using Machine Learning Force Field (MLFF). The developed MLFF is applicable to various MOF structures and allows for precise calculation of adsorption properties by predicting interaction energies during repetitive Widom insertion simulations. It is characterized by simultaneously achieving high accuracy and low computational cost compared to conventional classical force fields. >
This research is recognized as a significant advance in the DAC field, greatly enhancing materials design and simulation by precisely predicting MOF-CO₂ and MOF-H₂O interactions.
The results of this research, with Ph.D. candidate Yunsung Lim and Dr. Hyunsoo Park of KAIST as co-first authors, were published in the international academic journal Matter on June 12.
※Paper Title: Accelerating CO₂ direct air capture screening for metal–organic frameworks with a transferable machine learning force field
※DOI: 10.1016/j.matt.2025.102203
This research was supported by the Saudi Aramco-KAIST CO₂ Management Center and the Ministry of Science and ICT's Global C.L.E.A.N. Project.
2025.06.29 View 222
KAIST Develops AI to Easily Find Promising Materials That Capture Only CO₂
< Photo 1. (From left) Professor Jihan Kim, Ph.D. candidate Yunsung Lim and Dr. Hyunsoo Park of the Department of Chemical and Biomolecular Engineering >
In order to help prevent the climate crisis, actively reducing already-emitted CO₂ is essential. Accordingly, direct air capture (DAC) — a technology that directly extracts only CO₂ from the air — is gaining attention. However, effectively capturing pure CO₂ is not easy due to water vapor (H₂O) present in the air. KAIST researchers have successfully used AI-driven machine learning techniques to identify the most promising CO₂-capturing materials among metal-organic frameworks (MOFs), a key class of materials studied for this technology.
KAIST (President Kwang Hyung Lee) announced on the 29th of June that a research team led by Professor Jihan Kim from the Department of Chemical and Biomolecular Engineering, in collaboration with a team at Imperial College London, has developed a machine-learning-based simulation method that can quickly and accurately screen MOFs best suited for atmospheric CO₂ capture.
< Figure 1. Concept diagram of Direct Air Capture (DAC) technology and carbon capture using Metal-Organic Frameworks (MOFs). MOFs are promising porous materials capable of capturing carbon dioxide from the atmosphere, drawing attention as a core material for DAC technology. >
To overcome the difficulty of discovering high-performance materials due to the complexity of structures and the limitations of predicting intermolecular interactions, the research team developed a machine learning force field (MLFF) capable of precisely predicting the interactions between CO₂, water (H₂O), and MOFs. This new method enables calculations of MOF adsorption properties with quantum-mechanics-level accuracy at vastly faster speeds than before.
Using this system, the team screened over 8,000 experimentally synthesized MOF structures, identifying more than 100 promising candidates for CO₂ capture. Notably, this included new candidates that had not been uncovered by traditional force-field-based simulations. The team also analyzed the relationships between MOF chemical structure and adsorption performance, proposing seven key chemical features that will help in designing new materials for DAC.
< Figure 2. Concept diagram of adsorption simulation using Machine Learning Force Field (MLFF). The developed MLFF is applicable to various MOF structures and allows for precise calculation of adsorption properties by predicting interaction energies during repetitive Widom insertion simulations. It is characterized by simultaneously achieving high accuracy and low computational cost compared to conventional classical force fields. >
This research is recognized as a significant advance in the DAC field, greatly enhancing materials design and simulation by precisely predicting MOF-CO₂ and MOF-H₂O interactions.
The results of this research, with Ph.D. candidate Yunsung Lim and Dr. Hyunsoo Park of KAIST as co-first authors, were published in the international academic journal Matter on June 12.
※Paper Title: Accelerating CO₂ direct air capture screening for metal–organic frameworks with a transferable machine learning force field
※DOI: 10.1016/j.matt.2025.102203
This research was supported by the Saudi Aramco-KAIST CO₂ Management Center and the Ministry of Science and ICT's Global C.L.E.A.N. Project.
2025.06.29 View 222 -
 Distinguished Professor Sang Yup Lee Wins 2025 Global Metabolic Engineering Award
< Distinguished Professor Sang Yup Lee (Senior Vice President for Research) from the Department of Chemical & Biomolecular Engineering >
KAIST announced on the 20th that Professor Sang Yup Lee, who serves as the Vice President for Research and a Distinguished Professor at our university, has been awarded the '2025 Gregory N. Stephanopoulos Award for Metabolic Engineering' by the International Metabolic Engineering Society (IMES). Professor Lee delivered his award lecture at the 16th Metabolic Engineering Conference (ME16), held in Copenhagen, Denmark, from June 15th to 19th.
This award was established through contributions from the American Institute of Chemical Engineers (AIChE) Foundation, as well as fellow colleagues and acquaintances, to honor the achievements of Dr. Gregory Stephanopoulos, widely recognized as one of the pioneers of metabolic engineering. Presented biennially, the award recognizes scientists who have successfully commercialized fundamental research in metabolic engineering or have made outstanding contributions to the quantitative analysis, design, and modeling of metabolic pathways.
Professor Sang Yup Lee boasts an impressive record of over 770 journal papers and more than 860 patents. His groundbreaking research in metabolic engineering and biochemical engineering is highly acclaimed globally.
Throughout his 31 years as a professor at KAIST, Professor Lee has developed various metabolic engineering-based technologies and strategies. These advancements have been transferred to industries, facilitating the production of bulk chemicals, polymers, natural products, pharmaceuticals, and health functional foods. He has also founded companies and actively engages in advisory roles with various enterprises.
The International Metabolic Engineering Society (IMES) defines metabolic engineering as the manipulation of metabolic pathways in microorganisms or cells to produce useful substances (such as pharmaceuticals, biofuels, and chemical products). It utilizes tools like systems biology, synthetic biology, and computational modeling with the aim of enhancing the economic viability and sustainability of bio-based processes.
Furthermore, Professor Lee previously received the Merck Metabolic Engineering Award, a prominent international award in the field, in 2008. In 2018, he was honored with the Eni Award, often referred to as the Nobel Prize in energy, presented by the President of Italy.
Professor Sang Yup Lee remarked, "Metabolic engineering is a discipline that leads the current and future of biotechnology. It is a tremendous honor to receive this meaningful award at a time when the transition to a bio-based economy is accelerating. Together with my students and fellow researchers, we have generated numerous patents and transferred technologies to industry, and also established startups in the fields of biofuels, wound healing, and cosmetics. I will continue to pursue research that encompasses both fundamental research and technological commercialization."
The 'International Metabolic Engineering Society (IMES)' is a specialized society under the American Institute of Chemical Engineers. Its mission is to enable the production of various bio-based products, including pharmaceuticals, food additives, chemicals, and fuels, through metabolic engineering. The society hosts the Metabolic Engineering Conference biennially, offering researchers opportunities for knowledge exchange and collaboration.
2025.06.20 View 657
Distinguished Professor Sang Yup Lee Wins 2025 Global Metabolic Engineering Award
< Distinguished Professor Sang Yup Lee (Senior Vice President for Research) from the Department of Chemical & Biomolecular Engineering >
KAIST announced on the 20th that Professor Sang Yup Lee, who serves as the Vice President for Research and a Distinguished Professor at our university, has been awarded the '2025 Gregory N. Stephanopoulos Award for Metabolic Engineering' by the International Metabolic Engineering Society (IMES). Professor Lee delivered his award lecture at the 16th Metabolic Engineering Conference (ME16), held in Copenhagen, Denmark, from June 15th to 19th.
This award was established through contributions from the American Institute of Chemical Engineers (AIChE) Foundation, as well as fellow colleagues and acquaintances, to honor the achievements of Dr. Gregory Stephanopoulos, widely recognized as one of the pioneers of metabolic engineering. Presented biennially, the award recognizes scientists who have successfully commercialized fundamental research in metabolic engineering or have made outstanding contributions to the quantitative analysis, design, and modeling of metabolic pathways.
Professor Sang Yup Lee boasts an impressive record of over 770 journal papers and more than 860 patents. His groundbreaking research in metabolic engineering and biochemical engineering is highly acclaimed globally.
Throughout his 31 years as a professor at KAIST, Professor Lee has developed various metabolic engineering-based technologies and strategies. These advancements have been transferred to industries, facilitating the production of bulk chemicals, polymers, natural products, pharmaceuticals, and health functional foods. He has also founded companies and actively engages in advisory roles with various enterprises.
The International Metabolic Engineering Society (IMES) defines metabolic engineering as the manipulation of metabolic pathways in microorganisms or cells to produce useful substances (such as pharmaceuticals, biofuels, and chemical products). It utilizes tools like systems biology, synthetic biology, and computational modeling with the aim of enhancing the economic viability and sustainability of bio-based processes.
Furthermore, Professor Lee previously received the Merck Metabolic Engineering Award, a prominent international award in the field, in 2008. In 2018, he was honored with the Eni Award, often referred to as the Nobel Prize in energy, presented by the President of Italy.
Professor Sang Yup Lee remarked, "Metabolic engineering is a discipline that leads the current and future of biotechnology. It is a tremendous honor to receive this meaningful award at a time when the transition to a bio-based economy is accelerating. Together with my students and fellow researchers, we have generated numerous patents and transferred technologies to industry, and also established startups in the fields of biofuels, wound healing, and cosmetics. I will continue to pursue research that encompasses both fundamental research and technological commercialization."
The 'International Metabolic Engineering Society (IMES)' is a specialized society under the American Institute of Chemical Engineers. Its mission is to enable the production of various bio-based products, including pharmaceuticals, food additives, chemicals, and fuels, through metabolic engineering. The society hosts the Metabolic Engineering Conference biennially, offering researchers opportunities for knowledge exchange and collaboration.
2025.06.20 View 657 -
 KAIST Develops Glare-Free, Heat-Blocking 'Smart Window'... Applicable to Buildings and Vehicles
• Professor Hong Chul Moon of the Department of Chemical and Biomolecular Engineering develops RECM, a next-generation smart window technology, expecting cooling energy savings and effective indoor thermal management.
• When using the developed RECM, a significantly superior temperature reduction effect is observed compared to conventional windows.
• With a 'pedestrian-friendly smart window' design that eliminates glare by suppressing external reflections, it is expected to be adapted in architectural structures, transportation, and more.
< (From left) First author Hoy Jung Jo, Professor Hong Chul Moon >
In the building sector, which accounts for approximately 40% of global energy consumption, heat ingress through windows has been identified as a primary cause of wasted heating and cooling energy. Our research team has successfully developed a 'pedestrian-friendly smart window' technology capable of not only reducing heating and cooling energy in urban buildings but also resolving the persistent issue of 'light pollution' in urban living.
On the 17th of June, Professor Hong Chul Moon's research team at KAIST's Department of Chemical and Biomolecular Engineering announced the development of a 'smart window technology' that allows users to control the light and heat entering through windows according to their intent, and effectively neutralize glare from external sources.
Recently, 'active smart window' technology, which enables free adjustment of light and heat based on user operation, has garnered significant attention. Unlike conventional windows that passively react to changes in temperature or light, this is a next-generation window system that can be controlled in real-time via electrical signals.
The next-generation smart window technology developed by the research team, RECM (Reversible Electrodeposition and Electrochromic Mirror), is a smart window system based on a single-structured *electrochromic device that can actively control the transmittance of visible light and near-infrared (heat).
*Electrochromic device: A device whose optical properties change in response to an electrical signal.
In particular, by effectively suppressing the glare phenomenon caused by external reflected light—a problem previously identified in traditional metal *deposition smart windows—through the combined application of electrochromic materials, a 'pedestrian-friendly smart window' suitable for building facades has been realized.
*Deposition: A process involving the electrochemical reaction to coat metal ions, such as Ag+, onto an electrode surface in solid form.
The RECM system developed in this study operates in three modes depending on voltage control.
Mode I (Transparent Mode) is advantageous for allowing sunlight to enter the indoor space during winter, as it transmits both light and heat like ordinary glass.
In Mode II (Colored Mode), *Prussian Blue (PB) and **DHV+• chemical species are formed through a redox (oxidation-reduction) reaction, causing the window to turn a deep blue color. In this state, light is absorbed, and only a portion of the heat is transmitted, allowing for privacy while enabling appropriate indoor temperature control.
*Prussian Blue: An electrochromic material that transitions between colorless and blue upon electrical stimulation.
**DHV+•: A radical state colored molecule generated upon electrical stimulation.
Mode III (Colored and Deposition Mode) involves the reduction and deposition of silver (Ag+) ions on the electrode surface, reflecting both light and heat. Concurrently, the colored material absorbs the reflected light, effectively blocking glare for external pedestrians.
The research team validated the practical indoor temperature reduction effect of the RECM technology through experiments utilizing a miniature model house. When a conventional glass window was installed, the indoor temperature rose to 58.7°C within 45 minutes. Conversely, when RECM was operated in Mode III, the temperature reached 31.5°C, demonstrating a temperature reduction effect of approximately 27.2°C.
Furthermore, since each state transition is achievable solely by electrical signals, it is regarded as an active smart technology capable of instantaneous response according to season, time, and intended use.
< Figure 1. Operation mechanism of the RECM smart window. The RECM system can switch among three states—transparent, colored, and colored & deposition—via electrical stimulation. At -1.6 V, DHV•+ and Prussian Blue (PB) are formed, blocking visible light to provide privacy protection and heat blocking. At -2.0 V, silver (Ag) is deposited on the electrode surface, reflecting light and heat, while DHV•+ and Prussian Blue absorb reflected light, effectively suppressing external glare. Through this mechanism, it functions as an active smart window that simultaneously controls light, heat, and glare. >
Professor Hong Chul Moon of KAIST, the corresponding author of this study, stated, "This research goes beyond existing smart window technologies limited to visible light control, presenting a truly smart window platform that comprehensively considers not only active indoor thermal control but also the visual safety of pedestrians." He added, "Various applications are anticipated, from urban buildings to vehicles and trains."
< Figure 2. Analysis of glare suppression effect of conventional reflective smart windows and RECM. This figure presents the results comparing the glare phenomenon occurring during silver (Ag) deposition between conventional reflective smart windows and RECM Mode III. Conventional reflective devices resulted in strong reflected light on the desk surface due to their high reflectivity. In contrast, RECM Mode III, where the colored material absorbed reflected light, showed a 33% reduction in reflected light intensity, and no reflected light was observed from outside. This highlights the RECM system's distinctiveness and practicality as a 'pedestrian-friendly smart window' optimized for dense urban environments, extending beyond just heat blocking. >
The findings of this research were published on June 13, 2025, in Volume 10, Issue 6 of 'ACS Energy Letters'. The listed authors for this publication are Hoy Jung Jo, Yeon Jae Jang, Hyeon-Don Kim, Kwang-Seop Kim, and Hong Chul Moon.
※ Paper Title: Glare-Free, Energy-Efficient Smart Windows: A Pedestrian-Friendly System with Dynamically Tunable Light and Heat Regulation
※ DOI: 10.1021/acsenergylett.5c00637
< Figure 3. Temperature reduction performance verification in a miniature model house. The actual heat blocking effect was evaluated by applying RECM devices to a model building. Under identical conditions, the indoor temperature with ordinary glass rose to 58.7°C, whereas with RECM in Mode III, it reached 31.5°C, demonstrating a maximum temperature reduction effect of 27.2°C. The indoor temperature difference was also visually confirmed through thermal images, which proves the potential for indoor temperature control in urban buildings. >
This research was supported by the Nano & Material Technology Development Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT and the internal research program of the Korea Institute of Machinery and Materials.
2025.06.20 View 1940
KAIST Develops Glare-Free, Heat-Blocking 'Smart Window'... Applicable to Buildings and Vehicles
• Professor Hong Chul Moon of the Department of Chemical and Biomolecular Engineering develops RECM, a next-generation smart window technology, expecting cooling energy savings and effective indoor thermal management.
• When using the developed RECM, a significantly superior temperature reduction effect is observed compared to conventional windows.
• With a 'pedestrian-friendly smart window' design that eliminates glare by suppressing external reflections, it is expected to be adapted in architectural structures, transportation, and more.
< (From left) First author Hoy Jung Jo, Professor Hong Chul Moon >
In the building sector, which accounts for approximately 40% of global energy consumption, heat ingress through windows has been identified as a primary cause of wasted heating and cooling energy. Our research team has successfully developed a 'pedestrian-friendly smart window' technology capable of not only reducing heating and cooling energy in urban buildings but also resolving the persistent issue of 'light pollution' in urban living.
On the 17th of June, Professor Hong Chul Moon's research team at KAIST's Department of Chemical and Biomolecular Engineering announced the development of a 'smart window technology' that allows users to control the light and heat entering through windows according to their intent, and effectively neutralize glare from external sources.
Recently, 'active smart window' technology, which enables free adjustment of light and heat based on user operation, has garnered significant attention. Unlike conventional windows that passively react to changes in temperature or light, this is a next-generation window system that can be controlled in real-time via electrical signals.
The next-generation smart window technology developed by the research team, RECM (Reversible Electrodeposition and Electrochromic Mirror), is a smart window system based on a single-structured *electrochromic device that can actively control the transmittance of visible light and near-infrared (heat).
*Electrochromic device: A device whose optical properties change in response to an electrical signal.
In particular, by effectively suppressing the glare phenomenon caused by external reflected light—a problem previously identified in traditional metal *deposition smart windows—through the combined application of electrochromic materials, a 'pedestrian-friendly smart window' suitable for building facades has been realized.
*Deposition: A process involving the electrochemical reaction to coat metal ions, such as Ag+, onto an electrode surface in solid form.
The RECM system developed in this study operates in three modes depending on voltage control.
Mode I (Transparent Mode) is advantageous for allowing sunlight to enter the indoor space during winter, as it transmits both light and heat like ordinary glass.
In Mode II (Colored Mode), *Prussian Blue (PB) and **DHV+• chemical species are formed through a redox (oxidation-reduction) reaction, causing the window to turn a deep blue color. In this state, light is absorbed, and only a portion of the heat is transmitted, allowing for privacy while enabling appropriate indoor temperature control.
*Prussian Blue: An electrochromic material that transitions between colorless and blue upon electrical stimulation.
**DHV+•: A radical state colored molecule generated upon electrical stimulation.
Mode III (Colored and Deposition Mode) involves the reduction and deposition of silver (Ag+) ions on the electrode surface, reflecting both light and heat. Concurrently, the colored material absorbs the reflected light, effectively blocking glare for external pedestrians.
The research team validated the practical indoor temperature reduction effect of the RECM technology through experiments utilizing a miniature model house. When a conventional glass window was installed, the indoor temperature rose to 58.7°C within 45 minutes. Conversely, when RECM was operated in Mode III, the temperature reached 31.5°C, demonstrating a temperature reduction effect of approximately 27.2°C.
Furthermore, since each state transition is achievable solely by electrical signals, it is regarded as an active smart technology capable of instantaneous response according to season, time, and intended use.
< Figure 1. Operation mechanism of the RECM smart window. The RECM system can switch among three states—transparent, colored, and colored & deposition—via electrical stimulation. At -1.6 V, DHV•+ and Prussian Blue (PB) are formed, blocking visible light to provide privacy protection and heat blocking. At -2.0 V, silver (Ag) is deposited on the electrode surface, reflecting light and heat, while DHV•+ and Prussian Blue absorb reflected light, effectively suppressing external glare. Through this mechanism, it functions as an active smart window that simultaneously controls light, heat, and glare. >
Professor Hong Chul Moon of KAIST, the corresponding author of this study, stated, "This research goes beyond existing smart window technologies limited to visible light control, presenting a truly smart window platform that comprehensively considers not only active indoor thermal control but also the visual safety of pedestrians." He added, "Various applications are anticipated, from urban buildings to vehicles and trains."
< Figure 2. Analysis of glare suppression effect of conventional reflective smart windows and RECM. This figure presents the results comparing the glare phenomenon occurring during silver (Ag) deposition between conventional reflective smart windows and RECM Mode III. Conventional reflective devices resulted in strong reflected light on the desk surface due to their high reflectivity. In contrast, RECM Mode III, where the colored material absorbed reflected light, showed a 33% reduction in reflected light intensity, and no reflected light was observed from outside. This highlights the RECM system's distinctiveness and practicality as a 'pedestrian-friendly smart window' optimized for dense urban environments, extending beyond just heat blocking. >
The findings of this research were published on June 13, 2025, in Volume 10, Issue 6 of 'ACS Energy Letters'. The listed authors for this publication are Hoy Jung Jo, Yeon Jae Jang, Hyeon-Don Kim, Kwang-Seop Kim, and Hong Chul Moon.
※ Paper Title: Glare-Free, Energy-Efficient Smart Windows: A Pedestrian-Friendly System with Dynamically Tunable Light and Heat Regulation
※ DOI: 10.1021/acsenergylett.5c00637
< Figure 3. Temperature reduction performance verification in a miniature model house. The actual heat blocking effect was evaluated by applying RECM devices to a model building. Under identical conditions, the indoor temperature with ordinary glass rose to 58.7°C, whereas with RECM in Mode III, it reached 31.5°C, demonstrating a maximum temperature reduction effect of 27.2°C. The indoor temperature difference was also visually confirmed through thermal images, which proves the potential for indoor temperature control in urban buildings. >
This research was supported by the Nano & Material Technology Development Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT and the internal research program of the Korea Institute of Machinery and Materials.
2025.06.20 View 1940 -
 High-Resolution Spectrometer that Fits into Smartphones Developed by KAIST Researchers
- Professor Mooseok Jang's research team at the Department of Bio and Brain Engineering develops an ultra-compact, high-resolution spectrometer using 'double-layer disordered metasurfaces' that generate unique random patterns depending on light's color.
- Unlike conventional dispersion-based spectrometers that were difficult to apply to portable devices, this new concept spectrometer technology achieves 1nm-level high resolution in a device smaller than 1cm, comparable in size to a fingernail.
- It can be utilized as a built-in spectrometer in smartphones and wearable devices in the future, and can be expanded to advanced optical technologies such as hyperspectral imaging and ultrafast imaging.
< Photo 1. (From left) Professor Mooseok Jang, Dong-gu Lee (Ph.D. candidate), Gookho Song (Ph.D. candidate) >
Color, as the way light's wavelength is perceived by the human eye, goes beyond a simple aesthetic element, containing important scientific information like a substance's composition or state. Spectrometers are optical devices that analyze material properties by decomposing light into its constituent wavelengths, and they are widely used in various scientific and industrial fields, including material analysis, chemical component detection, and life science research. Existing high-resolution spectrometers were large and complex, making them difficult for widespread daily use. However, thanks to the ultra-compact, high-resolution spectrometer developed by KAIST researchers, it is now expected that light's color information can be utilized even within smartphones or wearable devices.
KAIST (President Kwang Hyung Lee) announced on the 13th that Professor Mooseok Jang's research team at the Department of Bio and Brain Engineering has successfully developed a reconstruction-based spectrometer technology using double-layer disordered metasurfaces*.
*Double-layer disordered metasurface: An innovative optical device that complexly scatters light through two layers of disordered nanostructures, creating unique and predictable speckle patterns for each wavelength.
Existing high-resolution spectrometers have a large form factor, on the order of tens of centimeters, and require complex calibration processes to maintain accuracy. This fundamentally stems from the operating principle of traditional dispersive elements, such as gratings and prisms, which separate light wavelengths along the propagation direction, much like a rainbow separates colors. Consequently, despite the potential for light's color information to be widely useful in daily life, spectroscopic technology has been limited to laboratory or industrial manufacturing environments.
< Figure 1. Through a simple structure consisting of a double layer of disordered metasurfaces and an image sensor, it was shown that speckles of predictable spectral channels with high spectral resolution can be generated in a compact form factor. The high similarity between the measured and calculated speckles was used to solve the inverse problem and verify the ability to reconstruct the spectrum. >
The research team devised a method that departs from the conventional spectroscopic paradigm of using diffraction gratings or prisms, which establish a one-to-one correspondence between light's color information and its propagation direction, by utilizing designed disordered structures as optical components. In this process, they employed metasurfaces, which can freely control the light propagation process using structures tens to hundreds of nanometers in size, to accurately implement 'complex random patterns (speckle*)'.
*Speckle: An irregular pattern of light intensity created by the interference of multiple wavefronts of light.
Specifically, they developed a method that involves implementing a double-layer disordered metasurface to generate wavelength-specific speckle patterns and then reconstructing precise color information (wavelength) of the light from the random patterns measured by a camera.
As a result, they successfully developed a new concept spectrometer technology that can accurately measure light across a broad range of visible to infrared (440-1,300nm) with a high resolution of 1 nanometer (nm) in a device smaller than a fingernail (less than 1cm) using only a single image capture.
< Figure 2. A disordered metasurface is a metasurface with irregularly arranged structures ranging from tens to hundreds of nanometers in size. In a double-layer structure, a propagation space is placed between the two metasurfaces to control the output speckle with high degrees of freedom, thereby achieving a spectral resolution of 1 nm even in a form factor smaller than 1 cm. >
Dong-gu Lee, a lead author of this study, stated, "This technology is implemented in a way that is directly integrated with commercial image sensors, and we expect that it will enable easy acquisition and utilization of light's wavelength information in daily life when built into mobile devices in the future."
Professor Mooseok Jang said, "This technology overcomes the limitations of existing RGB three-color based machine vision fields, which only distinguish and recognize three color components (red, green, blue), and has diverse applications. We anticipate various applied research for this technology, which expands the horizon of laboratory-level technology to daily-level machine vision technology for applications such as food component analysis, crop health diagnosis, skin health measurement, environmental pollution detection, and bio/medical diagnostics." He added, "Furthermore, it can be extended to various advanced optical technologies such as hyperspectral imaging, which records wavelength and spatial information simultaneously with high resolution, 3D optical trapping technology, which precisely controls light of multiple wavelengths into desired forms, and ultrafast imaging technology, which captures phenomena occurring in very short periods."
This research was collaboratively led by Dong-gu Lee (Ph.D. candidate) and Gookho Song (Ph.D. candidate) from the KAIST Department of Bio and Brain Engineering as co-first authors, with Professor Mooseok Jang as the corresponding author. The findings were published online in the international journal Science Advances on May 28, 2025.* Paper Title: Reconstructive spectrometer using double-layer disordered metasurfaces* DOI: 10.1126/sciadv.adv2376
This research was supported by the Samsung Research Funding and Incubation Center of Samsung Electronics grant, the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT), and the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MSIT).
2025.06.13 View 1150
High-Resolution Spectrometer that Fits into Smartphones Developed by KAIST Researchers
- Professor Mooseok Jang's research team at the Department of Bio and Brain Engineering develops an ultra-compact, high-resolution spectrometer using 'double-layer disordered metasurfaces' that generate unique random patterns depending on light's color.
- Unlike conventional dispersion-based spectrometers that were difficult to apply to portable devices, this new concept spectrometer technology achieves 1nm-level high resolution in a device smaller than 1cm, comparable in size to a fingernail.
- It can be utilized as a built-in spectrometer in smartphones and wearable devices in the future, and can be expanded to advanced optical technologies such as hyperspectral imaging and ultrafast imaging.
< Photo 1. (From left) Professor Mooseok Jang, Dong-gu Lee (Ph.D. candidate), Gookho Song (Ph.D. candidate) >
Color, as the way light's wavelength is perceived by the human eye, goes beyond a simple aesthetic element, containing important scientific information like a substance's composition or state. Spectrometers are optical devices that analyze material properties by decomposing light into its constituent wavelengths, and they are widely used in various scientific and industrial fields, including material analysis, chemical component detection, and life science research. Existing high-resolution spectrometers were large and complex, making them difficult for widespread daily use. However, thanks to the ultra-compact, high-resolution spectrometer developed by KAIST researchers, it is now expected that light's color information can be utilized even within smartphones or wearable devices.
KAIST (President Kwang Hyung Lee) announced on the 13th that Professor Mooseok Jang's research team at the Department of Bio and Brain Engineering has successfully developed a reconstruction-based spectrometer technology using double-layer disordered metasurfaces*.
*Double-layer disordered metasurface: An innovative optical device that complexly scatters light through two layers of disordered nanostructures, creating unique and predictable speckle patterns for each wavelength.
Existing high-resolution spectrometers have a large form factor, on the order of tens of centimeters, and require complex calibration processes to maintain accuracy. This fundamentally stems from the operating principle of traditional dispersive elements, such as gratings and prisms, which separate light wavelengths along the propagation direction, much like a rainbow separates colors. Consequently, despite the potential for light's color information to be widely useful in daily life, spectroscopic technology has been limited to laboratory or industrial manufacturing environments.
< Figure 1. Through a simple structure consisting of a double layer of disordered metasurfaces and an image sensor, it was shown that speckles of predictable spectral channels with high spectral resolution can be generated in a compact form factor. The high similarity between the measured and calculated speckles was used to solve the inverse problem and verify the ability to reconstruct the spectrum. >
The research team devised a method that departs from the conventional spectroscopic paradigm of using diffraction gratings or prisms, which establish a one-to-one correspondence between light's color information and its propagation direction, by utilizing designed disordered structures as optical components. In this process, they employed metasurfaces, which can freely control the light propagation process using structures tens to hundreds of nanometers in size, to accurately implement 'complex random patterns (speckle*)'.
*Speckle: An irregular pattern of light intensity created by the interference of multiple wavefronts of light.
Specifically, they developed a method that involves implementing a double-layer disordered metasurface to generate wavelength-specific speckle patterns and then reconstructing precise color information (wavelength) of the light from the random patterns measured by a camera.
As a result, they successfully developed a new concept spectrometer technology that can accurately measure light across a broad range of visible to infrared (440-1,300nm) with a high resolution of 1 nanometer (nm) in a device smaller than a fingernail (less than 1cm) using only a single image capture.
< Figure 2. A disordered metasurface is a metasurface with irregularly arranged structures ranging from tens to hundreds of nanometers in size. In a double-layer structure, a propagation space is placed between the two metasurfaces to control the output speckle with high degrees of freedom, thereby achieving a spectral resolution of 1 nm even in a form factor smaller than 1 cm. >
Dong-gu Lee, a lead author of this study, stated, "This technology is implemented in a way that is directly integrated with commercial image sensors, and we expect that it will enable easy acquisition and utilization of light's wavelength information in daily life when built into mobile devices in the future."
Professor Mooseok Jang said, "This technology overcomes the limitations of existing RGB three-color based machine vision fields, which only distinguish and recognize three color components (red, green, blue), and has diverse applications. We anticipate various applied research for this technology, which expands the horizon of laboratory-level technology to daily-level machine vision technology for applications such as food component analysis, crop health diagnosis, skin health measurement, environmental pollution detection, and bio/medical diagnostics." He added, "Furthermore, it can be extended to various advanced optical technologies such as hyperspectral imaging, which records wavelength and spatial information simultaneously with high resolution, 3D optical trapping technology, which precisely controls light of multiple wavelengths into desired forms, and ultrafast imaging technology, which captures phenomena occurring in very short periods."
This research was collaboratively led by Dong-gu Lee (Ph.D. candidate) and Gookho Song (Ph.D. candidate) from the KAIST Department of Bio and Brain Engineering as co-first authors, with Professor Mooseok Jang as the corresponding author. The findings were published online in the international journal Science Advances on May 28, 2025.* Paper Title: Reconstructive spectrometer using double-layer disordered metasurfaces* DOI: 10.1126/sciadv.adv2376
This research was supported by the Samsung Research Funding and Incubation Center of Samsung Electronics grant, the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT), and the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MSIT).
2025.06.13 View 1150 -
 KAIST Successfully Develops High-Performance Water Electrolysis Without Platinum, Bringing Hydrogen Economy Closer
< Photo 1. (Front row, from left) Jeesoo Park (Ph.D. Candidate), Professor Hee-Tak Kim (Back row, from left) Kyunghwa Seok (Ph.D. Candidate), Dr. Gisu Doo, Euntaek Oh (Ph.D. Candidate) >
Hydrogen is gaining attention as a clean energy source that emits no carbon. Among various methods, water electrolysis, which splits water into hydrogen and oxygen using electricity, is recognized as an eco-friendly hydrogen production method. Specifically, proton exchange membrane water electrolysis (PEMWE) is considered a next-generation hydrogen production technology due to its ability to produce high-purity hydrogen at high pressure. However, existing PEMWE technology has faced limitations in commercialization due to its heavy reliance on expensive precious metal catalysts and coating materials. Korean researchers have now proposed a new solution to address these technical and economic bottlenecks.
KAIST (President Kwang Hyung Lee) announced on June 11th that a research team led by Professor Hee-Tak Kim of the Department of Chemical and Biomolecular Engineering, in a joint study with Dr. Gisu Doo of the Korea Institute of Energy Research (KIER, President Chang-keun Lee), has developed a next-generation water electrolysis technology that achieves high performance without the need for expensive platinum (Pt) coating.
The research team focused on the primary reason why 'iridium oxide (IrOx),' a highly active catalyst for water electrolysis electrodes, fails to perform optimally. They found that this is due to inefficient electron transfer and, for the first time in the world, demonstrated that performance can be maximized simply by controlling the catalyst particle size.
In this study, it was revealed that the reason iridium oxide catalysts do not exhibit excellent performance without platinum coating is due to 'electron transport resistance' that occurs at the interface between the catalyst, the ion conductor (hereinafter referred to as ionomer), and the Ti (titanium) substrate—core components inherently used together in water electrolysis electrodes.
Specifically, they identified that the 'pinch-off' phenomenon, where the electron pathway is blocked between the catalyst, ionomer, and titanium substrate, is the critical cause of reduced conductivity. The ionomer has properties close to an electron insulator, thereby hindering electron flow when it surrounds catalyst particles. Furthermore, when the ionomer comes into contact with the titanium substrate, an electron barrier forms on the surface oxide layer of the titanium substrate, significantly increasing resistance.
< Figure 1. Infographic related to electron transport resistance at the catalyst layer/diffusion layer interface >
To address this, the research team fabricated and compared catalysts of various particle sizes. Through single-cell evaluation and multiphysics simulations, they demonstrated, for the first time globally, that when iridium oxide catalyst particles with a size of 20 nanometers (nm) or larger are used, the ionomer mixed region decreases, ensuring an electron pathway and restoring conductivity.
Moreover, they successfully optimized the interfacial structure through precise design, simultaneously ensuring both reactivity and electron transport. This achievement demonstrated that the previously unavoidable trade-off between catalyst activity and conductivity can be overcome through meticulous interfacial design.
This breakthrough is expected to be a significant milestone not only for the development of high-performance catalyst materials but also for the future commercialization of proton exchange membrane water electrolysis systems that can achieve high efficiency while drastically reducing the amount of precious metals used.
Professor Hee-Tak Kim stated, "This research presents a new interface design strategy that can resolve the interfacial conductivity problem, which was a bottleneck in high-performance water electrolysis technology." He added, "By securing high performance even without expensive materials like platinum, it will be a stepping stone closer to realizing a hydrogen economy."
This research, with Jeesoo Park, a Ph.D. student from the Department of Chemical and Biomolecular Engineering at KAIST, as the first author, was published on June 7th in 'Energy & Environmental Science' (IF: 32.4, 2025), a leading international journal in the energy and environmental fields, and was recognized for its innovativeness and impact. (Paper title: On the interface electron transport problem of highly active IrOx catalysts, DOI: 10.1039/D4EE05816J).
This research was supported by the New and Renewable Energy Core Technology Development Project of the Ministry of Trade, Industry and Energy.
2025.06.11 View 1555
KAIST Successfully Develops High-Performance Water Electrolysis Without Platinum, Bringing Hydrogen Economy Closer
< Photo 1. (Front row, from left) Jeesoo Park (Ph.D. Candidate), Professor Hee-Tak Kim (Back row, from left) Kyunghwa Seok (Ph.D. Candidate), Dr. Gisu Doo, Euntaek Oh (Ph.D. Candidate) >
Hydrogen is gaining attention as a clean energy source that emits no carbon. Among various methods, water electrolysis, which splits water into hydrogen and oxygen using electricity, is recognized as an eco-friendly hydrogen production method. Specifically, proton exchange membrane water electrolysis (PEMWE) is considered a next-generation hydrogen production technology due to its ability to produce high-purity hydrogen at high pressure. However, existing PEMWE technology has faced limitations in commercialization due to its heavy reliance on expensive precious metal catalysts and coating materials. Korean researchers have now proposed a new solution to address these technical and economic bottlenecks.
KAIST (President Kwang Hyung Lee) announced on June 11th that a research team led by Professor Hee-Tak Kim of the Department of Chemical and Biomolecular Engineering, in a joint study with Dr. Gisu Doo of the Korea Institute of Energy Research (KIER, President Chang-keun Lee), has developed a next-generation water electrolysis technology that achieves high performance without the need for expensive platinum (Pt) coating.
The research team focused on the primary reason why 'iridium oxide (IrOx),' a highly active catalyst for water electrolysis electrodes, fails to perform optimally. They found that this is due to inefficient electron transfer and, for the first time in the world, demonstrated that performance can be maximized simply by controlling the catalyst particle size.
In this study, it was revealed that the reason iridium oxide catalysts do not exhibit excellent performance without platinum coating is due to 'electron transport resistance' that occurs at the interface between the catalyst, the ion conductor (hereinafter referred to as ionomer), and the Ti (titanium) substrate—core components inherently used together in water electrolysis electrodes.
Specifically, they identified that the 'pinch-off' phenomenon, where the electron pathway is blocked between the catalyst, ionomer, and titanium substrate, is the critical cause of reduced conductivity. The ionomer has properties close to an electron insulator, thereby hindering electron flow when it surrounds catalyst particles. Furthermore, when the ionomer comes into contact with the titanium substrate, an electron barrier forms on the surface oxide layer of the titanium substrate, significantly increasing resistance.
< Figure 1. Infographic related to electron transport resistance at the catalyst layer/diffusion layer interface >
To address this, the research team fabricated and compared catalysts of various particle sizes. Through single-cell evaluation and multiphysics simulations, they demonstrated, for the first time globally, that when iridium oxide catalyst particles with a size of 20 nanometers (nm) or larger are used, the ionomer mixed region decreases, ensuring an electron pathway and restoring conductivity.
Moreover, they successfully optimized the interfacial structure through precise design, simultaneously ensuring both reactivity and electron transport. This achievement demonstrated that the previously unavoidable trade-off between catalyst activity and conductivity can be overcome through meticulous interfacial design.
This breakthrough is expected to be a significant milestone not only for the development of high-performance catalyst materials but also for the future commercialization of proton exchange membrane water electrolysis systems that can achieve high efficiency while drastically reducing the amount of precious metals used.
Professor Hee-Tak Kim stated, "This research presents a new interface design strategy that can resolve the interfacial conductivity problem, which was a bottleneck in high-performance water electrolysis technology." He added, "By securing high performance even without expensive materials like platinum, it will be a stepping stone closer to realizing a hydrogen economy."
This research, with Jeesoo Park, a Ph.D. student from the Department of Chemical and Biomolecular Engineering at KAIST, as the first author, was published on June 7th in 'Energy & Environmental Science' (IF: 32.4, 2025), a leading international journal in the energy and environmental fields, and was recognized for its innovativeness and impact. (Paper title: On the interface electron transport problem of highly active IrOx catalysts, DOI: 10.1039/D4EE05816J).
This research was supported by the New and Renewable Energy Core Technology Development Project of the Ministry of Trade, Industry and Energy.
2025.06.11 View 1555 -
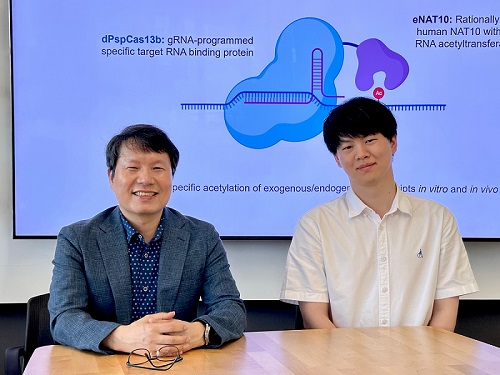 KAIST develops technology for selective RNA modification in living cells and animals
· A team led by Professor Won Do Heo from the Department of Biological Sciences, KAIST, has developed a pioneering technology that selectively acetylates specific RNA molecules in living cells and tissues.
· The platform uses RNA-targeting CRISPR tools in combination with RNA-modifying enzymes to chemically modify only the intended RNA.
· The method opens new possibilities for gene therapy by enabling precise control of disease-related RNA without affecting the rest of the transcriptome.
< Photo 1. (From left) Professor Won Do Heo and Jihwan Yu, a Ph.D. Candidate of the Department of Biological Sciences >
CRISPR-Cas13, a powerful RNA-targeting technology is gaining increasing attention as a next-generation gene therapy platform due to its precision and reduced side effects. Utilizing this system, researchers at KAIST have now developed the world’s first technology capable of selectively acetylating (chemically modifying) specific RNA molecules among countless transcripts within living cells. This breakthrough enables precise, programmable control of RNA function and is expected to open new avenues in RNA-based therapeutic development.
KAIST (President Kwang Hyung Lee) announced that a research team led by Professor Won Do Heo in the Department of Biological Sciences has recently developed a groundbreaking technology capable of selectively acetylating specific RNA molecules within the human body using the CRISPR-Cas13 system—an RNA-targeting platform gaining increasing attention in the fields of gene regulation and RNA-based therapeutics.
RNA molecules can undergo chemical modifications—the addition of specific chemical groups—which alter their function and behavior without changing the underlying nucleotide sequence. However, some of these modifications, a critical layer of post-transcriptional gene regulation, remain poorly understood. Among them, N4-acetylcytidine (ac4C) has been particularly enigmatic, with ongoing debate about its existence and function in human messenger RNA (mRNA), the RNA that encodes proteins.
To address this gap, the KAIST research team developed a targeted RNA acetylation system, named dCas13-eNAT10. This platform combines a catalytically inactive Cas13 enzyme (dCas13) that guides the system to specific RNA targets, with a hyperactive variant of the NAT10 enzyme (eNAT10), which performs RNA acetylation. This approach enables precise acetylation of only the desired RNA molecules among the vast pool of transcripts within the cell.
< Figure 1. Development of hyperactive variant eNAT10 through NAT10 protein engineering. By engineering the NAT10 protein, which performs RNA acetylation in human cells, based on its domain and structure, eNAT10 was developed, showing approximately a 3-fold increase in RNA acetylation activity compared to the wild-type enzyme. >
Using this system, the researchers demonstrated that guide RNAs could direct the dCas13-eNAT10 complex to acetylate specific RNA targets, and acetylation significantly increased protein expression from the modified mRNA. Moreover, the study revealed, for the first time, that RNA acetylation plays a role in intracellular RNA localization, facilitating the export of RNA from the nucleus to the cytoplasm—a critical step in gene expression regulation.
To validate its therapeutic potential, the team successfully delivered the targeted RNA acetylation system into the livers of live mice using adeno-associated virus (AAV), a commonly used gene therapy vector. This marks the first demonstration of in vivo RNA modification, extending the applicability of RNA chemical modification tools from cell culture models to living organisms.
< Figure 2. Acetylation of various RNA in cells using dCas13-eNAT10 fusion protein. Utilizing the CRISPR-Cas13 system, which can precisely target specific RNA through guide RNA, a dCas13-eNAT10 fusion protein was created, demonstrating its ability to specifically acetylate various endogenous RNA at different locations within cells. >
Professor Won Do Heo, who previously developed COVID-19 treatment technology using RNA gene scissors and technology to activate RNA gene scissors with light, stated, "Existing RNA chemical modification research faced difficulties in controlling specificity, temporality, and spatiality. However, this new technology allows selective acetylation of desired RNA, opening the door for accurate and detailed research into the functions of RNA acetylation." He added, "The RNA chemical modification technology developed in this study can be widely used as an RNA-based therapeutic agent and a tool for regulating RNA functions in living organisms in the future."
< Figure 3. In vivo delivery of targeted RNA acetylation system. The targeted RNA acetylation system was encoded in an AAV vector, commonly used in gene therapy, and delivered intravenously to adult mice, showing that target RNA in liver tissue was specifically acetylated according to the guide RNA. >
This research, with Ph.D. candidate Jihwan Yu from the Department of Biological Sciences at KAIST as the first author, was published in the journal Nature Chemical Biology on June 2, 2025. (Title: Programmable RNA acetylation with CRISPR-Cas13, Impact factor: 12.9, DOI: https://doi.org/10.1038/s41589-025-01922-3)
This research was supported by the Samsung Future Technology Foundation and the Bio & Medical Technology Development Program of the National Research Foundation of Korea.
2025.06.10 View 1072
KAIST develops technology for selective RNA modification in living cells and animals
· A team led by Professor Won Do Heo from the Department of Biological Sciences, KAIST, has developed a pioneering technology that selectively acetylates specific RNA molecules in living cells and tissues.
· The platform uses RNA-targeting CRISPR tools in combination with RNA-modifying enzymes to chemically modify only the intended RNA.
· The method opens new possibilities for gene therapy by enabling precise control of disease-related RNA without affecting the rest of the transcriptome.
< Photo 1. (From left) Professor Won Do Heo and Jihwan Yu, a Ph.D. Candidate of the Department of Biological Sciences >
CRISPR-Cas13, a powerful RNA-targeting technology is gaining increasing attention as a next-generation gene therapy platform due to its precision and reduced side effects. Utilizing this system, researchers at KAIST have now developed the world’s first technology capable of selectively acetylating (chemically modifying) specific RNA molecules among countless transcripts within living cells. This breakthrough enables precise, programmable control of RNA function and is expected to open new avenues in RNA-based therapeutic development.
KAIST (President Kwang Hyung Lee) announced that a research team led by Professor Won Do Heo in the Department of Biological Sciences has recently developed a groundbreaking technology capable of selectively acetylating specific RNA molecules within the human body using the CRISPR-Cas13 system—an RNA-targeting platform gaining increasing attention in the fields of gene regulation and RNA-based therapeutics.
RNA molecules can undergo chemical modifications—the addition of specific chemical groups—which alter their function and behavior without changing the underlying nucleotide sequence. However, some of these modifications, a critical layer of post-transcriptional gene regulation, remain poorly understood. Among them, N4-acetylcytidine (ac4C) has been particularly enigmatic, with ongoing debate about its existence and function in human messenger RNA (mRNA), the RNA that encodes proteins.
To address this gap, the KAIST research team developed a targeted RNA acetylation system, named dCas13-eNAT10. This platform combines a catalytically inactive Cas13 enzyme (dCas13) that guides the system to specific RNA targets, with a hyperactive variant of the NAT10 enzyme (eNAT10), which performs RNA acetylation. This approach enables precise acetylation of only the desired RNA molecules among the vast pool of transcripts within the cell.
< Figure 1. Development of hyperactive variant eNAT10 through NAT10 protein engineering. By engineering the NAT10 protein, which performs RNA acetylation in human cells, based on its domain and structure, eNAT10 was developed, showing approximately a 3-fold increase in RNA acetylation activity compared to the wild-type enzyme. >
Using this system, the researchers demonstrated that guide RNAs could direct the dCas13-eNAT10 complex to acetylate specific RNA targets, and acetylation significantly increased protein expression from the modified mRNA. Moreover, the study revealed, for the first time, that RNA acetylation plays a role in intracellular RNA localization, facilitating the export of RNA from the nucleus to the cytoplasm—a critical step in gene expression regulation.
To validate its therapeutic potential, the team successfully delivered the targeted RNA acetylation system into the livers of live mice using adeno-associated virus (AAV), a commonly used gene therapy vector. This marks the first demonstration of in vivo RNA modification, extending the applicability of RNA chemical modification tools from cell culture models to living organisms.
< Figure 2. Acetylation of various RNA in cells using dCas13-eNAT10 fusion protein. Utilizing the CRISPR-Cas13 system, which can precisely target specific RNA through guide RNA, a dCas13-eNAT10 fusion protein was created, demonstrating its ability to specifically acetylate various endogenous RNA at different locations within cells. >
Professor Won Do Heo, who previously developed COVID-19 treatment technology using RNA gene scissors and technology to activate RNA gene scissors with light, stated, "Existing RNA chemical modification research faced difficulties in controlling specificity, temporality, and spatiality. However, this new technology allows selective acetylation of desired RNA, opening the door for accurate and detailed research into the functions of RNA acetylation." He added, "The RNA chemical modification technology developed in this study can be widely used as an RNA-based therapeutic agent and a tool for regulating RNA functions in living organisms in the future."
< Figure 3. In vivo delivery of targeted RNA acetylation system. The targeted RNA acetylation system was encoded in an AAV vector, commonly used in gene therapy, and delivered intravenously to adult mice, showing that target RNA in liver tissue was specifically acetylated according to the guide RNA. >
This research, with Ph.D. candidate Jihwan Yu from the Department of Biological Sciences at KAIST as the first author, was published in the journal Nature Chemical Biology on June 2, 2025. (Title: Programmable RNA acetylation with CRISPR-Cas13, Impact factor: 12.9, DOI: https://doi.org/10.1038/s41589-025-01922-3)
This research was supported by the Samsung Future Technology Foundation and the Bio & Medical Technology Development Program of the National Research Foundation of Korea.
2025.06.10 View 1072 -
 A 10-Month Journey of Tiny Flaps Completed: A Special Family Returns to KAIST Duck Pond
On the morning of June 9, 2025, gentle activity stirred early around the KAIST campus duck pond. It was the day a special family of ducks—and two goslings—were to be released back into the pond after spending a month in a temporary shelter. One by one, the ducklings cautiously emerged from their box, waddling toward the water's edge and scanning their surroundings, followed closely by their mother.
< The landscape manager from the KAIST Facilities Team releases the ducks and goslings. >
The mother duck, once a rescued loner who couldn’t integrate with the flock, returned triumphantly as the head of a new family—caring for both ducklings and goslings. Students and faculty looked on quietly, welcoming them back and reflecting on their remarkable 10-month journey.
The story began in July 2024, as a student filed a report of spotting two ducklings wandering near the pond without a mother. Based on their soft down, flat beaks, and lack of fear around humans, it was presumed they had been abandoned. Professor Won Do Heo of the Department of Biological Sciences—affectionately known as the “Goose Dad”—and the KAIST Facilities Team quickly stepped in to rescue them. After about a month of care, the ducklings were released back into the pond.
< On June 9, the day of the release, KAIST President Kwang-Hyung Lee (left), the former “Goose Dad,” and Professor Won Do Heo (right), the current “Goose Dad,” watched the flock as they freely wobbled about. >
At first, the ducklings seemed to adapt, but they started distancing themselves from the established goose flock. One eventually disappeared, and the remaining duckling was found injured by the pond during winter. Although KAIST typically avoids making human interference in the natural ecosystem, an exception was made to save the young duck’s life. It was put under the care of Professor Heo and the Facilities Team to regain its health within a month.
In the spring, the healed duck began laying eggs. Professor Heo supported the process by adjusting its diet, avoiding further intervention. On Children’s Day, May 5, the duck’s eggs hatched. The once-isolated duck had become a mother. Ten days later, on May 15, four goslings also hatched from the resident goose flock. With new life flourishing, the pond was more vibrant than ever.
< Rescued baby goslings near the pond, alongside the duck family that took them in. The mother duck—once a vulnerable duckling herself—had grown strong enough to care for others in need. >
But just days later, the mother goose disappeared, and two goslings—still unable to swim—were found shivering by the pond. Dahyeon Byeon, a student from Seoul National University who came for a visit on that day, reported this upon sighting, prompting another rescue. The vulnerable goslings were brought to the shelter to stay with the duck family.
Initially, the interspecies cohabitation was uneasy. But the mother duck did not reject the goslings. Slowly, they began to eat and sleep together, forming a new kind of family. After a month, they were released together into the pond—and to everyone’s surprise, the existing goose flock accepted both the goslings and the duck family.
< A peaceful moment for the duck family. The baby goslings naturally followed the mother duck. >
It took ten months for this family to return. From abandonment and injury to healing, birth, and unexpected bonds, this was more than a story of survival. It was a journey of transformation. The duck family’s ten-month saga is a quiet miracle—written in small moments of crisis, care, and connection—and a lasting memory on the KAIST campus.
< The resident goose flock at KAIST’s pond naturally accepted the returning duck and goslings as part of their group. >
2025.06.10 View 1148
A 10-Month Journey of Tiny Flaps Completed: A Special Family Returns to KAIST Duck Pond
On the morning of June 9, 2025, gentle activity stirred early around the KAIST campus duck pond. It was the day a special family of ducks—and two goslings—were to be released back into the pond after spending a month in a temporary shelter. One by one, the ducklings cautiously emerged from their box, waddling toward the water's edge and scanning their surroundings, followed closely by their mother.
< The landscape manager from the KAIST Facilities Team releases the ducks and goslings. >
The mother duck, once a rescued loner who couldn’t integrate with the flock, returned triumphantly as the head of a new family—caring for both ducklings and goslings. Students and faculty looked on quietly, welcoming them back and reflecting on their remarkable 10-month journey.
The story began in July 2024, as a student filed a report of spotting two ducklings wandering near the pond without a mother. Based on their soft down, flat beaks, and lack of fear around humans, it was presumed they had been abandoned. Professor Won Do Heo of the Department of Biological Sciences—affectionately known as the “Goose Dad”—and the KAIST Facilities Team quickly stepped in to rescue them. After about a month of care, the ducklings were released back into the pond.
< On June 9, the day of the release, KAIST President Kwang-Hyung Lee (left), the former “Goose Dad,” and Professor Won Do Heo (right), the current “Goose Dad,” watched the flock as they freely wobbled about. >
At first, the ducklings seemed to adapt, but they started distancing themselves from the established goose flock. One eventually disappeared, and the remaining duckling was found injured by the pond during winter. Although KAIST typically avoids making human interference in the natural ecosystem, an exception was made to save the young duck’s life. It was put under the care of Professor Heo and the Facilities Team to regain its health within a month.
In the spring, the healed duck began laying eggs. Professor Heo supported the process by adjusting its diet, avoiding further intervention. On Children’s Day, May 5, the duck’s eggs hatched. The once-isolated duck had become a mother. Ten days later, on May 15, four goslings also hatched from the resident goose flock. With new life flourishing, the pond was more vibrant than ever.
< Rescued baby goslings near the pond, alongside the duck family that took them in. The mother duck—once a vulnerable duckling herself—had grown strong enough to care for others in need. >
But just days later, the mother goose disappeared, and two goslings—still unable to swim—were found shivering by the pond. Dahyeon Byeon, a student from Seoul National University who came for a visit on that day, reported this upon sighting, prompting another rescue. The vulnerable goslings were brought to the shelter to stay with the duck family.
Initially, the interspecies cohabitation was uneasy. But the mother duck did not reject the goslings. Slowly, they began to eat and sleep together, forming a new kind of family. After a month, they were released together into the pond—and to everyone’s surprise, the existing goose flock accepted both the goslings and the duck family.
< A peaceful moment for the duck family. The baby goslings naturally followed the mother duck. >
It took ten months for this family to return. From abandonment and injury to healing, birth, and unexpected bonds, this was more than a story of survival. It was a journey of transformation. The duck family’s ten-month saga is a quiet miracle—written in small moments of crisis, care, and connection—and a lasting memory on the KAIST campus.
< The resident goose flock at KAIST’s pond naturally accepted the returning duck and goslings as part of their group. >
2025.06.10 View 1148 -
 KAIST-UIUC researchers develop a treatment platform to disable the ‘biofilm’ shield of superbugs
< (From left) Ph.D. Candidate Joo Hun Lee (co-author), Professor Hyunjoon Kong (co-corresponding author) and Postdoctoral Researcher Yujin Ahn (co-first author) from the Department of Chemical and Biomolecular Engineering of the University of Illinois at Urbana-Champaign and Ju Yeon Chung (co-first author) from the Integrated Master's and Doctoral Program, and Professor Hyun Jung Chung (co-corresponding author) from the Department of Biological Sciences of KAIST >
A major cause of hospital-acquired infections, the super bacteria Methicillin-resistant Staphylococcus aureus (MRSA), not only exhibits strong resistance to existing antibiotics but also forms a dense biofilm that blocks the effects of external treatments. To meet this challenge, KAIST researchers, in collaboration with an international team, successfully developed a platform that utilizes microbubbles to deliver gene-targeted nanoparticles capable of break ing down the biofilms, offering an innovative solution for treating infections resistant to conventional antibiotics.
KAIST (represented by President Kwang Hyung Lee) announced on May 29 that a research team led by Professor Hyun Jung Chung from the Department of Biological Sciences, in collaboration with Professor Hyunjoon Kong's team at the University of Illinois, has developed a microbubble-based nano-gene delivery platform (BTN MB) that precisely delivers gene suppressors into bacteria to effectively remove biofilms formed by MRSA.
The research team first designed short DNA oligonucleotides that simultaneously suppress three major MRSA genes, related to—biofilm formation (icaA), cell division (ftsZ), and antibiotic resistance (mecA)—and engineered nanoparticles (BTN) to effectively deliver them into the bacteria.
< Figure 1. Effective biofilm treatment using biofilm-targeting nanoparticles controlled by microbubbler system. Schematic illustration of BTN delivery with microbubbles (MB), enabling effective permeation of ASOs targeting bacterial genes within biofilms infecting skin wounds. Gene silencing of targets involved in biofilm formation, bacterial proliferation, and antibiotic resistance leads to effective biofilm removal and antibacterial efficacy in vivo. >
In addition, microbubbles (MB) were used to increase the permeability of the microbial membrane, specifically the biofilm formed by MRSA. By combining these two technologies, the team implemented a dual-strike strategy that fundamentally blocks bacterial growth and prevents resistance acquisition.
This treatment system operates in two stages. First, the MBs induce pressure changes within the bacterial biofilm, allowing the BTNs to penetrate. Then, the BTNs slip through the gaps in the biofilm and enter the bacteria, delivering the gene suppressors precisely. This leads to gene regulation within MRSA, simultaneously blocking biofilm regeneration, cell proliferation, and antibiotic resistance expression.
In experiments conducted in a porcine skin model and a mouse wound model infected with MRSA biofilm, the BTN MB treatment group showed a significant reduction in biofilm thickness, as well as remarkable decreases in bacterial count and inflammatory responses.
< Figure 2. (a) Schematic illustration on the evaluation of treatment efficacy of BTN-MB gene therapy. (b) Reduction in MRSA biofilm mass via simultaneous inhibition of multiple genes. (c, d) Antibacterial efficacy of BTN-MB over time in a porcine skin infection biofilm model. (e) Schematic of the experimental setup to verify antibacterial efficacy in a mouse skin wound infection model. (f) Wound healing effects in mice. (g) Antibacterial effects at the wound site. (h) Histological analysis results. >
These results are difficult to achieve with conventional antibiotic monotherapy and demonstrate the potential for treating a wide range of resistant bacterial infections.
Professor Hyun Jung Chung of KAIST, who led the research, stated, “This study presents a new therapeutic solution that combines nanotechnology, gene suppression, and physical delivery strategies to address superbug infections that existing antibiotics cannot resolve. We will continue our research with the aim of expanding its application to systemic infections and various other infectious diseases.”
< (From left) Ju Yeon Chung from the Integrated Master's and Doctoral Program, and Professor Hyun Jung Chung from the Department of Biological Sciences >
The study was co-first authored by Ju Yeon Chung, a graduate student in the Department of Biological Sciences at KAIST, and Dr. Yujin Ahn from the University of Illinois. The study was published online on May 19 in the journal, Advanced Functional Materials.
※ Paper Title: Microbubble-Controlled Delivery of Biofilm-Targeting Nanoparticles to Treat MRSA Infection ※ DOI: https://doi.org/10.1002/adfm.202508291
This study was supported by the National Research Foundation and the Ministry of Health and Welfare, Republic of Korea; and the National Science Foundation and National Institutes of Health, USA.
2025.05.29 View 1649
KAIST-UIUC researchers develop a treatment platform to disable the ‘biofilm’ shield of superbugs
< (From left) Ph.D. Candidate Joo Hun Lee (co-author), Professor Hyunjoon Kong (co-corresponding author) and Postdoctoral Researcher Yujin Ahn (co-first author) from the Department of Chemical and Biomolecular Engineering of the University of Illinois at Urbana-Champaign and Ju Yeon Chung (co-first author) from the Integrated Master's and Doctoral Program, and Professor Hyun Jung Chung (co-corresponding author) from the Department of Biological Sciences of KAIST >
A major cause of hospital-acquired infections, the super bacteria Methicillin-resistant Staphylococcus aureus (MRSA), not only exhibits strong resistance to existing antibiotics but also forms a dense biofilm that blocks the effects of external treatments. To meet this challenge, KAIST researchers, in collaboration with an international team, successfully developed a platform that utilizes microbubbles to deliver gene-targeted nanoparticles capable of break ing down the biofilms, offering an innovative solution for treating infections resistant to conventional antibiotics.
KAIST (represented by President Kwang Hyung Lee) announced on May 29 that a research team led by Professor Hyun Jung Chung from the Department of Biological Sciences, in collaboration with Professor Hyunjoon Kong's team at the University of Illinois, has developed a microbubble-based nano-gene delivery platform (BTN MB) that precisely delivers gene suppressors into bacteria to effectively remove biofilms formed by MRSA.
The research team first designed short DNA oligonucleotides that simultaneously suppress three major MRSA genes, related to—biofilm formation (icaA), cell division (ftsZ), and antibiotic resistance (mecA)—and engineered nanoparticles (BTN) to effectively deliver them into the bacteria.
< Figure 1. Effective biofilm treatment using biofilm-targeting nanoparticles controlled by microbubbler system. Schematic illustration of BTN delivery with microbubbles (MB), enabling effective permeation of ASOs targeting bacterial genes within biofilms infecting skin wounds. Gene silencing of targets involved in biofilm formation, bacterial proliferation, and antibiotic resistance leads to effective biofilm removal and antibacterial efficacy in vivo. >
In addition, microbubbles (MB) were used to increase the permeability of the microbial membrane, specifically the biofilm formed by MRSA. By combining these two technologies, the team implemented a dual-strike strategy that fundamentally blocks bacterial growth and prevents resistance acquisition.
This treatment system operates in two stages. First, the MBs induce pressure changes within the bacterial biofilm, allowing the BTNs to penetrate. Then, the BTNs slip through the gaps in the biofilm and enter the bacteria, delivering the gene suppressors precisely. This leads to gene regulation within MRSA, simultaneously blocking biofilm regeneration, cell proliferation, and antibiotic resistance expression.
In experiments conducted in a porcine skin model and a mouse wound model infected with MRSA biofilm, the BTN MB treatment group showed a significant reduction in biofilm thickness, as well as remarkable decreases in bacterial count and inflammatory responses.
< Figure 2. (a) Schematic illustration on the evaluation of treatment efficacy of BTN-MB gene therapy. (b) Reduction in MRSA biofilm mass via simultaneous inhibition of multiple genes. (c, d) Antibacterial efficacy of BTN-MB over time in a porcine skin infection biofilm model. (e) Schematic of the experimental setup to verify antibacterial efficacy in a mouse skin wound infection model. (f) Wound healing effects in mice. (g) Antibacterial effects at the wound site. (h) Histological analysis results. >
These results are difficult to achieve with conventional antibiotic monotherapy and demonstrate the potential for treating a wide range of resistant bacterial infections.
Professor Hyun Jung Chung of KAIST, who led the research, stated, “This study presents a new therapeutic solution that combines nanotechnology, gene suppression, and physical delivery strategies to address superbug infections that existing antibiotics cannot resolve. We will continue our research with the aim of expanding its application to systemic infections and various other infectious diseases.”
< (From left) Ju Yeon Chung from the Integrated Master's and Doctoral Program, and Professor Hyun Jung Chung from the Department of Biological Sciences >
The study was co-first authored by Ju Yeon Chung, a graduate student in the Department of Biological Sciences at KAIST, and Dr. Yujin Ahn from the University of Illinois. The study was published online on May 19 in the journal, Advanced Functional Materials.
※ Paper Title: Microbubble-Controlled Delivery of Biofilm-Targeting Nanoparticles to Treat MRSA Infection ※ DOI: https://doi.org/10.1002/adfm.202508291
This study was supported by the National Research Foundation and the Ministry of Health and Welfare, Republic of Korea; and the National Science Foundation and National Institutes of Health, USA.
2025.05.29 View 1649 -
 Life Springs at KAIST: A Tale of Two Special Campus Families
A Gift of Life on Teachers' Day: Baby Geese Born at KAIST Pond
On Teachers' Day, a meaningful miracle of life arrived at the KAIST campus. A pair of geese gave birth to two goslings by the duck pond.
< On Teachers' Day, a pair of geese and their goslings leisurely swim in the pond. >
The baby goslings, covered in yellow down, began exploring the pond's edge, scurrying about, while their aunt geese steadfastly stood by. Their curious glances, watchful gazes, playful hops on waterside rocks, and the procession of babies swimming behind their parents in the water melted the hearts of onlookers.
< As night falls on the duck pond, the goose family gathers among the reeds. >
This special new life, born on Teachers' Day, seems to symbolize the day's meaning of "care" and "growth." This wondrous scene of life brought warm comfort and joy to KAIST members, adding the inspiration of nature to a campus that is a space for research and learning.
< Under the protection of the adult geese, the goslings take their first steps, exploring the pond's grassy areas and rocks. >
This adorable family is already roaming the area leisurely, like the pond's owners. With the joy of life added to the spring-filled pond, warm smiles are spreading across the KAIST campus.
< The geese look around, surveying their surroundings, while caring for their goslings. >
The pond has now become a small but special haven for students and staff. This goose family, arriving on Teachers' Day, quietly reminds us of the meaning of care and learning conveyed by nature.
< The goose family shows care and growth, and warm moments together are anticipated. >
---
On Children's Day 2025, a Duck Becomes a Mother
In July 2024, a special guest arrived at the KAIST campus. With soft yellow down, waddling gait, and a flat beak, it was undeniably a baby duck. However, for some reason, its mother was nowhere to be seen. Given that it wasn't afraid of people and followed them well, it was clear that someone had abandoned the duck.
Fortunately, the baby duck was safely rescued thanks to prompt reporting by students.
< Two ducks found on a corner of campus, immediately after their rescue in summer 2024. >
The ducks, newly integrated into KAIST, seemed to adapt relatively peacefully to campus life. As new additions, they couldn't blend in with the existing goose flock that had settled on campus, but the geese didn't ostracize them either. Perhaps because they were awkward neighbors, there was hope that the ducks would soon join the existing goose flock.
< Following their rescue based on a student's report in summer 2024, the ducks adapted to campus life under the protection of the campus facility team and Professor Won Do Heo. >
Professor Won Do Heo of the Department of Biological Sciences, widely known as "Goose Dad," stepped forward to protect them along with the KAIST facility team. Professor Heo is well-known for consistently observing and protecting the campus geese and ducks, which are practically symbols of KAIST. Thanks to the care of the staff and Professor Heo, the two ducks were safely released back onto campus approximately one month after their rescue.
< A moment on campus: Before winter, the ducks lived separately from the goose flock, maintaining a certain distance. While there were no conflicts, they rarely socialized. >
However, as winter passed, sad news arrived. One duck went missing, and the remaining one was found injured by the pond. While the policy of the facility team and Professor Heo was to minimize intervention to allow campus animals to maintain their natural state, saving the injured duck was the top priority. After being isolated again for a month of recovery, the duck fully recovered and was able to greet spring under the sun.
< The mother duck left alone in winter: One went missing, and the remaining one was found injured. After indoor isolation and recovery, she was released back onto campus in the spring. >
As spring, the ducks' breeding season, began, Professor Heo decided to offer a little more help. When signs of egg-laying appeared, he consistently provided "special meals for pregnant mothers" throughout March. On the morning of May 5th, Children's Day, 28 days after the mother duck began incubating her eggs with the care and attention of KAIST members, new life finally hatched. It was a precious outcome achieved solely by the duck that had survived abandonment and injury, with no special protection other than food.
The duck, having overcome hardship and injury to stand alone, has now formed a new family. Although there is still some distance from the existing goose flock, it is expected that they will naturally find their place in the campus ecosystem, as KAIST's geese are not aggressive or exclusive. The KAIST goose flock already has experience protecting and raising five ducklings.
< A new beginning by the pond on Children's Day: On the morning of May 5th, the 28th day of incubation, four ducklings hatched by the pond. This was a natural hatching, achieved without protective equipment. >
A single duck brought a special spring to the KAIST campus on Children's Day. The outcome achieved by that small life, leading to the birth of a new family, also symbolizes the harmonious coexistence of people and animals on the KAIST campus. The careful intervention of KAIST members, providing only the necessary assistance from rescue to hatching, makes us reconsider what "desirable coexistence between animals and people" truly means.
2025.05.21 View 2199
Life Springs at KAIST: A Tale of Two Special Campus Families
A Gift of Life on Teachers' Day: Baby Geese Born at KAIST Pond
On Teachers' Day, a meaningful miracle of life arrived at the KAIST campus. A pair of geese gave birth to two goslings by the duck pond.
< On Teachers' Day, a pair of geese and their goslings leisurely swim in the pond. >
The baby goslings, covered in yellow down, began exploring the pond's edge, scurrying about, while their aunt geese steadfastly stood by. Their curious glances, watchful gazes, playful hops on waterside rocks, and the procession of babies swimming behind their parents in the water melted the hearts of onlookers.
< As night falls on the duck pond, the goose family gathers among the reeds. >
This special new life, born on Teachers' Day, seems to symbolize the day's meaning of "care" and "growth." This wondrous scene of life brought warm comfort and joy to KAIST members, adding the inspiration of nature to a campus that is a space for research and learning.
< Under the protection of the adult geese, the goslings take their first steps, exploring the pond's grassy areas and rocks. >
This adorable family is already roaming the area leisurely, like the pond's owners. With the joy of life added to the spring-filled pond, warm smiles are spreading across the KAIST campus.
< The geese look around, surveying their surroundings, while caring for their goslings. >
The pond has now become a small but special haven for students and staff. This goose family, arriving on Teachers' Day, quietly reminds us of the meaning of care and learning conveyed by nature.
< The goose family shows care and growth, and warm moments together are anticipated. >
---
On Children's Day 2025, a Duck Becomes a Mother
In July 2024, a special guest arrived at the KAIST campus. With soft yellow down, waddling gait, and a flat beak, it was undeniably a baby duck. However, for some reason, its mother was nowhere to be seen. Given that it wasn't afraid of people and followed them well, it was clear that someone had abandoned the duck.
Fortunately, the baby duck was safely rescued thanks to prompt reporting by students.
< Two ducks found on a corner of campus, immediately after their rescue in summer 2024. >
The ducks, newly integrated into KAIST, seemed to adapt relatively peacefully to campus life. As new additions, they couldn't blend in with the existing goose flock that had settled on campus, but the geese didn't ostracize them either. Perhaps because they were awkward neighbors, there was hope that the ducks would soon join the existing goose flock.
< Following their rescue based on a student's report in summer 2024, the ducks adapted to campus life under the protection of the campus facility team and Professor Won Do Heo. >
Professor Won Do Heo of the Department of Biological Sciences, widely known as "Goose Dad," stepped forward to protect them along with the KAIST facility team. Professor Heo is well-known for consistently observing and protecting the campus geese and ducks, which are practically symbols of KAIST. Thanks to the care of the staff and Professor Heo, the two ducks were safely released back onto campus approximately one month after their rescue.
< A moment on campus: Before winter, the ducks lived separately from the goose flock, maintaining a certain distance. While there were no conflicts, they rarely socialized. >
However, as winter passed, sad news arrived. One duck went missing, and the remaining one was found injured by the pond. While the policy of the facility team and Professor Heo was to minimize intervention to allow campus animals to maintain their natural state, saving the injured duck was the top priority. After being isolated again for a month of recovery, the duck fully recovered and was able to greet spring under the sun.
< The mother duck left alone in winter: One went missing, and the remaining one was found injured. After indoor isolation and recovery, she was released back onto campus in the spring. >
As spring, the ducks' breeding season, began, Professor Heo decided to offer a little more help. When signs of egg-laying appeared, he consistently provided "special meals for pregnant mothers" throughout March. On the morning of May 5th, Children's Day, 28 days after the mother duck began incubating her eggs with the care and attention of KAIST members, new life finally hatched. It was a precious outcome achieved solely by the duck that had survived abandonment and injury, with no special protection other than food.
The duck, having overcome hardship and injury to stand alone, has now formed a new family. Although there is still some distance from the existing goose flock, it is expected that they will naturally find their place in the campus ecosystem, as KAIST's geese are not aggressive or exclusive. The KAIST goose flock already has experience protecting and raising five ducklings.
< A new beginning by the pond on Children's Day: On the morning of May 5th, the 28th day of incubation, four ducklings hatched by the pond. This was a natural hatching, achieved without protective equipment. >
A single duck brought a special spring to the KAIST campus on Children's Day. The outcome achieved by that small life, leading to the birth of a new family, also symbolizes the harmonious coexistence of people and animals on the KAIST campus. The careful intervention of KAIST members, providing only the necessary assistance from rescue to hatching, makes us reconsider what "desirable coexistence between animals and people" truly means.
2025.05.21 View 2199 -
 Decoding Fear: KAIST Identifies An Affective Brain Circuit Crucial for Fear Memory Formation by Non-nociceptive Threat Stimulus
Fear memories can form in the brain following exposure to threatening situations such as natural disasters, accidents, or violence. When these memories become excessive or distorted, they can lead to severe mental health disorders, including post-traumatic stress disorder (PTSD), anxiety disorders, and depression. However, the mechanisms underlying fear memory formation triggered by affective pain rather than direct physical pain have remained largely unexplored – until now.
A KAIST research team has identified, for the first time, a brain circuit specifically responsible for forming fear memories in the absence of physical pain, marking a significant advance in understanding how psychological distress is processed and drives fear memory formation in the brain. This discovery opens the door to the development of targeted treatments for trauma-related conditions by addressing the underlying neural pathways.
< Photo 1. (from left) Professor Jin-Hee Han, Dr. Junho Han and Ph.D. Candidate Boin Suh of the Department of Biological Sciences >
KAIST (President Kwang-Hyung Lee) announced on May 15th that the research team led by Professor Jin-Hee Han in the Department of Biological Sciences has identified the pIC-PBN circuit*, a key neural pathway involved in forming fear memories triggered by psychological threats in the absence of sensory pain. This groundbreaking work was conducted through experiments with mice.*pIC–PBN circuit: A newly identified descending neural pathway from the posterior insular cortex (pIC) to the parabrachial nucleus (PBN), specialized for transmitting psychological threat information.
Traditionally, the lateral parabrachial nucleus (PBN) has been recognized as a critical part of the ascending pain pathway, receiving pain signals from the spinal cord. However, this study reveals a previously unknown role for the PBN in processing fear induced by non-painful psychological stimuli, fundamentally changing our understanding of its function in the brain.
This work is considered the first experimental evidence that 'emotional distress' and 'physical pain' are processed through different neural circuits to form fear memories, making it a significant contribution to the field of neuroscience. It clearly demonstrates the existence of a dedicated pathway (pIC-PBN) for transmitting emotional distress.
The study's first author, Dr. Junho Han, shared the personal motivation behind this research: “Our dog, Lego, is afraid of motorcycles. He never actually crashed into one, but ever since having a traumatizing event of having a motorbike almost run into him, just hearing the sound now triggers a fearful response. Humans react similarly – even if you didn’t have a personal experience of being involved in an accident, a near-miss or exposure to alarming media can create lasting fear memories, which may eventually lead to PTSD.”
He continued, “Until now, fear memory research has mainly relied on experimental models involving physical pain. However, much of real-world human fears arise from psychological threats, rather than from direct physical harm. Despite this, little was known about the brain circuits responsible for processing these psychological threats that can drive fear memory formation.”
To investigate this, the research team developed a novel fear conditioning model that utilizes visual threat stimuli instead of electrical shocks. In this model, mice were exposed to a rapidly expanding visual disk on a ceiling screen, simulating the threat of an approaching predator. This approach allowed the team to demonstrate that fear memories can form in response to a non-nociceptive, psychological threat alone, without the need for physical pain.
< Figure 1. Artificial activation of the posterior insular cortex (pIC) to lateral parabrachial nucleus (PBN) neural circuit induces anxiety-like behaviors and fear memory formation in mice. >
Using advanced chemogenetic and optogenetic techniques, the team precisely controlled neuronal activity, revealing that the lateral parabrachial nucleus (PBN) is essential to form fear memories in response to visual threats. They further traced the origin of these signals to the posterior insular cortex (pIC), a region known to process negative emotions and pain, confirming a direct connection between the two areas.
The study also showed that inhibiting the pIC–PBN circuit significantly reduced fear memory formation in response to visual threats, without affecting innate fear responses or physical pain-based learning. Conversely, artificially activating this circuit alone was sufficient to drive fear memory formation, confirming its role as a key pathway for processing psychological threat information.
< Figure 2. Schematic diagram of brain neural circuits transmitting emotional & physical pain threat signals. Visual threat stimuli do not involve physical pain but can create an anxious state and form fear memory through the affective pain signaling pathway. >
Professor Jin-Hee Han commented, “This study lays an important foundation for understanding how emotional distress-based mental disorders, such as PTSD, panic disorder, and anxiety disorder, develop, and opens new possibilities for targeted treatment approaches.”
The findings, authored by Dr. Junho Han (first author), Ph.D. candidate Boin Suh (second author), and Dr. Jin-Hee Han (corresponding author) of the Department of Biological Sciences, were published online in the international journal Science Advances on May 9, 2025.※ Paper Title: A top-down insular cortex circuit crucial for non-nociceptive fear learning. Science Advances (https://doi.org/10.1101/2024.10.14.618356)※ Author Information: Junho Han (first author), Boin Suh (second author), and Jin-Hee Han (corresponding author)
This research was supported by grants from the National Research Foundation of Korea (NRF-2022M3E5E8081183 and NRF-2017M3C7A1031322).
2025.05.15 View 2716
Decoding Fear: KAIST Identifies An Affective Brain Circuit Crucial for Fear Memory Formation by Non-nociceptive Threat Stimulus
Fear memories can form in the brain following exposure to threatening situations such as natural disasters, accidents, or violence. When these memories become excessive or distorted, they can lead to severe mental health disorders, including post-traumatic stress disorder (PTSD), anxiety disorders, and depression. However, the mechanisms underlying fear memory formation triggered by affective pain rather than direct physical pain have remained largely unexplored – until now.
A KAIST research team has identified, for the first time, a brain circuit specifically responsible for forming fear memories in the absence of physical pain, marking a significant advance in understanding how psychological distress is processed and drives fear memory formation in the brain. This discovery opens the door to the development of targeted treatments for trauma-related conditions by addressing the underlying neural pathways.
< Photo 1. (from left) Professor Jin-Hee Han, Dr. Junho Han and Ph.D. Candidate Boin Suh of the Department of Biological Sciences >
KAIST (President Kwang-Hyung Lee) announced on May 15th that the research team led by Professor Jin-Hee Han in the Department of Biological Sciences has identified the pIC-PBN circuit*, a key neural pathway involved in forming fear memories triggered by psychological threats in the absence of sensory pain. This groundbreaking work was conducted through experiments with mice.*pIC–PBN circuit: A newly identified descending neural pathway from the posterior insular cortex (pIC) to the parabrachial nucleus (PBN), specialized for transmitting psychological threat information.
Traditionally, the lateral parabrachial nucleus (PBN) has been recognized as a critical part of the ascending pain pathway, receiving pain signals from the spinal cord. However, this study reveals a previously unknown role for the PBN in processing fear induced by non-painful psychological stimuli, fundamentally changing our understanding of its function in the brain.
This work is considered the first experimental evidence that 'emotional distress' and 'physical pain' are processed through different neural circuits to form fear memories, making it a significant contribution to the field of neuroscience. It clearly demonstrates the existence of a dedicated pathway (pIC-PBN) for transmitting emotional distress.
The study's first author, Dr. Junho Han, shared the personal motivation behind this research: “Our dog, Lego, is afraid of motorcycles. He never actually crashed into one, but ever since having a traumatizing event of having a motorbike almost run into him, just hearing the sound now triggers a fearful response. Humans react similarly – even if you didn’t have a personal experience of being involved in an accident, a near-miss or exposure to alarming media can create lasting fear memories, which may eventually lead to PTSD.”
He continued, “Until now, fear memory research has mainly relied on experimental models involving physical pain. However, much of real-world human fears arise from psychological threats, rather than from direct physical harm. Despite this, little was known about the brain circuits responsible for processing these psychological threats that can drive fear memory formation.”
To investigate this, the research team developed a novel fear conditioning model that utilizes visual threat stimuli instead of electrical shocks. In this model, mice were exposed to a rapidly expanding visual disk on a ceiling screen, simulating the threat of an approaching predator. This approach allowed the team to demonstrate that fear memories can form in response to a non-nociceptive, psychological threat alone, without the need for physical pain.
< Figure 1. Artificial activation of the posterior insular cortex (pIC) to lateral parabrachial nucleus (PBN) neural circuit induces anxiety-like behaviors and fear memory formation in mice. >
Using advanced chemogenetic and optogenetic techniques, the team precisely controlled neuronal activity, revealing that the lateral parabrachial nucleus (PBN) is essential to form fear memories in response to visual threats. They further traced the origin of these signals to the posterior insular cortex (pIC), a region known to process negative emotions and pain, confirming a direct connection between the two areas.
The study also showed that inhibiting the pIC–PBN circuit significantly reduced fear memory formation in response to visual threats, without affecting innate fear responses or physical pain-based learning. Conversely, artificially activating this circuit alone was sufficient to drive fear memory formation, confirming its role as a key pathway for processing psychological threat information.
< Figure 2. Schematic diagram of brain neural circuits transmitting emotional & physical pain threat signals. Visual threat stimuli do not involve physical pain but can create an anxious state and form fear memory through the affective pain signaling pathway. >
Professor Jin-Hee Han commented, “This study lays an important foundation for understanding how emotional distress-based mental disorders, such as PTSD, panic disorder, and anxiety disorder, develop, and opens new possibilities for targeted treatment approaches.”
The findings, authored by Dr. Junho Han (first author), Ph.D. candidate Boin Suh (second author), and Dr. Jin-Hee Han (corresponding author) of the Department of Biological Sciences, were published online in the international journal Science Advances on May 9, 2025.※ Paper Title: A top-down insular cortex circuit crucial for non-nociceptive fear learning. Science Advances (https://doi.org/10.1101/2024.10.14.618356)※ Author Information: Junho Han (first author), Boin Suh (second author), and Jin-Hee Han (corresponding author)
This research was supported by grants from the National Research Foundation of Korea (NRF-2022M3E5E8081183 and NRF-2017M3C7A1031322).
2025.05.15 View 2716 -
 KAIST Discovers Protein Switch that Turns Anti-Viral Immune Response On and Off
Even after the COVID-19 pandemic, various new infectious diseases continue to emerge, posing ongoing viral threats that demand robust and sustained immune defenses. However, excessive immune reactions can also harm body tissues, causing significant health issues. KAIST and an international research team have discovered a critical protein that acts as a 'switch' regulating immune responses to viruses. This breakthrough is expected to lay the groundwork for future infectious disease responses and autoimmune disease treatment strategies.
KAIST (President Kwang-Hyung Lee) announced on May 14 that a joint research team led by Professor Yoosik Kim from the Department of Chemical and Biomolecular Engineering at KAIST and Professor Seunghee Cha from University of Florida has discovered the mechanism by which double-stranded RNA derived from mitochondria amplifies immune responses. They identified the protein SLIRP as an 'immune switch' that regulates this process, playing a crucial role in both viral infections and autoimmune diseases.
< (From left) Master's candidate Yewon Yang, Professor Yoosik Kim and Ph.D. candidate Doyeong Ku of the Department of Chemical and Biomolecular Engineering >
Autoimmune diseases arise when the immune system fails to differentiate between external pathogens and the body's own molecules, leading to self-directed attacks. Despite extensive research, the precise causes of excessive inflammatory conditions like Sjögren’s syndrome and systemic lupus erythematosus remain unclear, and effective treatments are still limited.
To uncover the molecular mechanisms driving immune hyperactivation and to identify potential regulatory factors, the research team led by Professor Yoosik Kim focused on mitochondrial double-stranded RNA (mt-dsRNA), a genetic immunogenic material produced within cellular organelles. Since mt-dsRNA structurally resembles viral RNA, it can mistakenly trigger immune responses even in the absence of an actual viral infection.
The team discovered that SLIRP, a key regulator of mt-dsRNA, amplifies immune responses by stabilizing the RNA. They confirmed that SLIRP expression increases in experimental models simulating the tissues of autoimmune disease patients and viral infections. Conversely, suppressing SLIRP significantly reduced the immune response, underscoring its role as a critical factor in immune amplification.
This study also demonstrated the dual function of SLIRP in different contexts. In cells infected with human beta coronavirus OC43 and encephalomyocarditis virus (EMCV), SLIRP suppression led to reduced antiviral responses and increased viral replication. Meanwhile, in the blood and salivary gland cells of Sjögren’s syndrome patients, where both SLIRP and mt-dsRNA levels were elevated, suppressing SLIRP alleviated the abnormal immune response.
These findings highlight SLIRP as a key molecular switch that regulates immune responses in both infections and autoimmune diseases.
< Figure 1. Schematic diagram of antiviral signal amplification by SLIRP: SLIRP-based mt-dsRNA induction, cytoplasmic accumulation, and strong interferon response induction by positive feedback of immune response activation. Confirmation of the immune regulatory function of SLIRP in defense against autoimmune diseases Sjögren's syndrome, coronavirus, and encephalomyocarditis virus infection. >
Professor Yoosik Kim remarked, "Through this study, we have identified SLIRP as a crucial protein that drives immune amplification via mt-dsRNAs. Given its dual role in autoimmune diseases and viral infections, SLIRP presents a promising target for immune regulation therapies across various inflammatory disease contexts."
The study, with Ph.D. student Do-Young Ku (first author) and M.S. student Ye-Won Yang (second author) from the Department of Chemical and Biomolecular Engineering at KAIST as primary contributors, was published online in the journal Cell Reports on April 19, 2025.
※ Paper title: SLIRP amplifies antiviral signaling via positive feedback regulation and contributes to autoimmune diseases※ Main authors: Do-Young Ku (KAIST, first author), Ye-Won Yang (KAIST, second author), Seunghee Cha (University of Florida, corresponding author), Yoosik Kim (KAIST, corresponding author)
This study was supported by the Ministry of Health and Welfare's Public Health Technology Research Program and the National Institutes of Health (NIH) through Research Project (R01) funding.
2025.05.14 View 2403
KAIST Discovers Protein Switch that Turns Anti-Viral Immune Response On and Off
Even after the COVID-19 pandemic, various new infectious diseases continue to emerge, posing ongoing viral threats that demand robust and sustained immune defenses. However, excessive immune reactions can also harm body tissues, causing significant health issues. KAIST and an international research team have discovered a critical protein that acts as a 'switch' regulating immune responses to viruses. This breakthrough is expected to lay the groundwork for future infectious disease responses and autoimmune disease treatment strategies.
KAIST (President Kwang-Hyung Lee) announced on May 14 that a joint research team led by Professor Yoosik Kim from the Department of Chemical and Biomolecular Engineering at KAIST and Professor Seunghee Cha from University of Florida has discovered the mechanism by which double-stranded RNA derived from mitochondria amplifies immune responses. They identified the protein SLIRP as an 'immune switch' that regulates this process, playing a crucial role in both viral infections and autoimmune diseases.
< (From left) Master's candidate Yewon Yang, Professor Yoosik Kim and Ph.D. candidate Doyeong Ku of the Department of Chemical and Biomolecular Engineering >
Autoimmune diseases arise when the immune system fails to differentiate between external pathogens and the body's own molecules, leading to self-directed attacks. Despite extensive research, the precise causes of excessive inflammatory conditions like Sjögren’s syndrome and systemic lupus erythematosus remain unclear, and effective treatments are still limited.
To uncover the molecular mechanisms driving immune hyperactivation and to identify potential regulatory factors, the research team led by Professor Yoosik Kim focused on mitochondrial double-stranded RNA (mt-dsRNA), a genetic immunogenic material produced within cellular organelles. Since mt-dsRNA structurally resembles viral RNA, it can mistakenly trigger immune responses even in the absence of an actual viral infection.
The team discovered that SLIRP, a key regulator of mt-dsRNA, amplifies immune responses by stabilizing the RNA. They confirmed that SLIRP expression increases in experimental models simulating the tissues of autoimmune disease patients and viral infections. Conversely, suppressing SLIRP significantly reduced the immune response, underscoring its role as a critical factor in immune amplification.
This study also demonstrated the dual function of SLIRP in different contexts. In cells infected with human beta coronavirus OC43 and encephalomyocarditis virus (EMCV), SLIRP suppression led to reduced antiviral responses and increased viral replication. Meanwhile, in the blood and salivary gland cells of Sjögren’s syndrome patients, where both SLIRP and mt-dsRNA levels were elevated, suppressing SLIRP alleviated the abnormal immune response.
These findings highlight SLIRP as a key molecular switch that regulates immune responses in both infections and autoimmune diseases.
< Figure 1. Schematic diagram of antiviral signal amplification by SLIRP: SLIRP-based mt-dsRNA induction, cytoplasmic accumulation, and strong interferon response induction by positive feedback of immune response activation. Confirmation of the immune regulatory function of SLIRP in defense against autoimmune diseases Sjögren's syndrome, coronavirus, and encephalomyocarditis virus infection. >
Professor Yoosik Kim remarked, "Through this study, we have identified SLIRP as a crucial protein that drives immune amplification via mt-dsRNAs. Given its dual role in autoimmune diseases and viral infections, SLIRP presents a promising target for immune regulation therapies across various inflammatory disease contexts."
The study, with Ph.D. student Do-Young Ku (first author) and M.S. student Ye-Won Yang (second author) from the Department of Chemical and Biomolecular Engineering at KAIST as primary contributors, was published online in the journal Cell Reports on April 19, 2025.
※ Paper title: SLIRP amplifies antiviral signaling via positive feedback regulation and contributes to autoimmune diseases※ Main authors: Do-Young Ku (KAIST, first author), Ye-Won Yang (KAIST, second author), Seunghee Cha (University of Florida, corresponding author), Yoosik Kim (KAIST, corresponding author)
This study was supported by the Ministry of Health and Welfare's Public Health Technology Research Program and the National Institutes of Health (NIH) through Research Project (R01) funding.
2025.05.14 View 2403 -
 KAIST Develops Novel Catalyst With 100-Fold Platinum Efficiency
Propylene, a key building block used in producing plastics, textiles, automotive components, and electronics, is essential to the petrochemical industry. A KAIST research team has developed a novel catalyst that dramatically enhances the efficiency of propylene production while significantly reducing costs.
< Photo. Professor Minkee Choi (left), and Ph.D. Candidate Susung Lee (right) of the Department of Chemical and Biomolecular Engineering >
KAIST (represented by President Kwang-Hyung Lee) announced on the 12th of May that a research group led by Professor Minkee Choi from the Department of Chemical and Biomolecular Engineering has successfully developed a new catalyst using inexpensive metals—gallium (Ga) and alumina (Al₂O₃)—with only a trace amount of platinum (100 ppm, or 0.01%). Remarkably, this new catalyst outperforms conventional industrial catalysts that use high concentrations of platinum (10,000 ppm).
Propylene is commonly produced through the propane dehydrogenation (PDH) process, which removes hydrogen from propane. Platinum has long been used as a catalyst in PDH due to its high efficiency in breaking carbon-hydrogen bonds and facilitating hydrogen removal. However, platinum is costly and suffers from performance degradation over repeated use.
To address this, the KAIST team engineered a catalyst that incorporates only a minimal amount of platinum, relying on gallium and alumina as the primary components.
< Figure 1. Schematic diagram showing the catalytic cooperation between gallium (Ga) and platinum (Pt) >
The core mechanism of the catalyst involves a cooperative function between the metals: gallium activates the C–H bond in propane to produce propylene, while platinum bonds the residual hydrogen atoms on the surface to form hydrogen gas (H₂), which is then released. This division of roles allows for high catalytic efficiency despite the drastic reduction in platinum content.
The researchers identified an optimal platinum-to-gallium ratio that delivered peak performance and provided a scientific rationale and quantitative metrics to predict this ideal composition.
Additionally, the team addressed a major limitation of traditional platinum catalysts: sintering—the agglomeration of platinum particles during repeated use, which causes performance loss. By adding a small amount of cerium (Ce), the researchers successfully suppressed this aggregation. As a result, the new catalyst maintained stable performance even after more than 20 reaction-regeneration cycles.
< Figure 2. Performance comparison of KAIST's newly developed catalyst (100 ppm platinum) and existing commercial platinum catalyst (10,000 ppm platinum) >
Professor Choi stated, “This research demonstrates the possibility of reducing platinum usage to 1/100th of current levels without compromising, and even enhancing, performance. It presents significant economic and environmental advantages, including lower catalyst costs, extended replacement intervals, and reduced catalyst waste.”
He added, “We are planning to evaluate this technology for large-scale process demonstration and commercialization. If adopted in industry, it could greatly improve the economic viability and efficiency of propylene production.”
The study was led by Professor Minkee Choi as corresponding author, with Ph.D. candidate Susung Lee as the first author. The findings were published in the Journal of the American Chemical Society (JACS), a leading journal in chemistry and chemical engineering, on February 13.※ Paper title: Ideal Bifunctional Catalysis for Propane Dehydrogenation over Pt-Promoted Gallia-Alumina and Minimized Use of Precious Elements※ DOI: https://pubs.acs.org/doi/10.1021/jacs.4c13787
The research was supported by the National Research Foundation of Korea and Hanwha Solutions Corporation.
2025.05.12 View 1493
KAIST Develops Novel Catalyst With 100-Fold Platinum Efficiency
Propylene, a key building block used in producing plastics, textiles, automotive components, and electronics, is essential to the petrochemical industry. A KAIST research team has developed a novel catalyst that dramatically enhances the efficiency of propylene production while significantly reducing costs.
< Photo. Professor Minkee Choi (left), and Ph.D. Candidate Susung Lee (right) of the Department of Chemical and Biomolecular Engineering >
KAIST (represented by President Kwang-Hyung Lee) announced on the 12th of May that a research group led by Professor Minkee Choi from the Department of Chemical and Biomolecular Engineering has successfully developed a new catalyst using inexpensive metals—gallium (Ga) and alumina (Al₂O₃)—with only a trace amount of platinum (100 ppm, or 0.01%). Remarkably, this new catalyst outperforms conventional industrial catalysts that use high concentrations of platinum (10,000 ppm).
Propylene is commonly produced through the propane dehydrogenation (PDH) process, which removes hydrogen from propane. Platinum has long been used as a catalyst in PDH due to its high efficiency in breaking carbon-hydrogen bonds and facilitating hydrogen removal. However, platinum is costly and suffers from performance degradation over repeated use.
To address this, the KAIST team engineered a catalyst that incorporates only a minimal amount of platinum, relying on gallium and alumina as the primary components.
< Figure 1. Schematic diagram showing the catalytic cooperation between gallium (Ga) and platinum (Pt) >
The core mechanism of the catalyst involves a cooperative function between the metals: gallium activates the C–H bond in propane to produce propylene, while platinum bonds the residual hydrogen atoms on the surface to form hydrogen gas (H₂), which is then released. This division of roles allows for high catalytic efficiency despite the drastic reduction in platinum content.
The researchers identified an optimal platinum-to-gallium ratio that delivered peak performance and provided a scientific rationale and quantitative metrics to predict this ideal composition.
Additionally, the team addressed a major limitation of traditional platinum catalysts: sintering—the agglomeration of platinum particles during repeated use, which causes performance loss. By adding a small amount of cerium (Ce), the researchers successfully suppressed this aggregation. As a result, the new catalyst maintained stable performance even after more than 20 reaction-regeneration cycles.
< Figure 2. Performance comparison of KAIST's newly developed catalyst (100 ppm platinum) and existing commercial platinum catalyst (10,000 ppm platinum) >
Professor Choi stated, “This research demonstrates the possibility of reducing platinum usage to 1/100th of current levels without compromising, and even enhancing, performance. It presents significant economic and environmental advantages, including lower catalyst costs, extended replacement intervals, and reduced catalyst waste.”
He added, “We are planning to evaluate this technology for large-scale process demonstration and commercialization. If adopted in industry, it could greatly improve the economic viability and efficiency of propylene production.”
The study was led by Professor Minkee Choi as corresponding author, with Ph.D. candidate Susung Lee as the first author. The findings were published in the Journal of the American Chemical Society (JACS), a leading journal in chemistry and chemical engineering, on February 13.※ Paper title: Ideal Bifunctional Catalysis for Propane Dehydrogenation over Pt-Promoted Gallia-Alumina and Minimized Use of Precious Elements※ DOI: https://pubs.acs.org/doi/10.1021/jacs.4c13787
The research was supported by the National Research Foundation of Korea and Hanwha Solutions Corporation.
2025.05.12 View 1493