ICA
-
 Quantum Laser Turns Energy Loss into Gain
A new laser that generates quantum particles can recycle lost energy for highly efficient, low threshold laser applications
Scientists at KAIST have fabricated a laser system that generates highly interactive quantum particles at room temperature. Their findings, published in the journal Nature Photonics, could lead to a single microcavity laser system that requires lower threshold energy as its energy loss increases.
The system, developed by KAIST physicist Yong-Hoon Cho and colleagues, involves shining light through a single hexagonal-shaped microcavity treated with a loss-modulated silicon nitride substrate. The system design leads to the generation of a polariton laser at room temperature, which is exciting because this usually requires cryogenic temperatures.
The researchers found another unique and counter-intuitive feature of this design. Normally, energy is lost during laser operation. But in this system, as energy loss increased, the amount of energy needed to induce lasing decreased. Exploiting this phenomenon could lead to the development of high efficiency, low threshold lasers for future quantum optical devices.
“This system applies a concept of quantum physics known as parity-time reversal symmetry,” explains Professor Cho. “This is an important platform that allows energy loss to be used as gain. It can be used to reduce laser threshold energy for classical optical devices and sensors, as well as quantum devices and controlling the direction of light.”
The key is the design and materials. The hexagonal microcavity divides light particles into two different modes: one that passes through the upward-facing triangle of the hexagon and another that passes through its downward-facing triangle. Both modes of light particles have the same energy and path but don’t interact with each other.
However, the light particles do interact with other particles called excitons, provided by the hexagonal microcavity, which is made of semiconductors. This interaction leads to the generation of new quantum particles called polaritons that then interact with each other to generate the polariton laser. By controlling the degree of loss between the microcavity and the semiconductor substrate, an intriguing phenomenon arises, with the threshold energy becoming smaller as energy loss increases. This research was supported by the Samsung Science and Technology Foundation and Korea’s National Research Foundation.
-PublicationSong,H.G, Choi, M, Woo, K.Y. Yong-Hoon Cho Room-temperature polaritonic non-Hermitian system with single microcavityNature Photonics (https://doi.org/10.1038/s41566-021-00820-z)
-ProfileProfessor Yong-Hoon ChoQuantum & Nanobio Photonics Laboratoryhttp://qnp.kaist.ac.kr/
Department of PhysicsKAIST
2021.07.07 View 11532
Quantum Laser Turns Energy Loss into Gain
A new laser that generates quantum particles can recycle lost energy for highly efficient, low threshold laser applications
Scientists at KAIST have fabricated a laser system that generates highly interactive quantum particles at room temperature. Their findings, published in the journal Nature Photonics, could lead to a single microcavity laser system that requires lower threshold energy as its energy loss increases.
The system, developed by KAIST physicist Yong-Hoon Cho and colleagues, involves shining light through a single hexagonal-shaped microcavity treated with a loss-modulated silicon nitride substrate. The system design leads to the generation of a polariton laser at room temperature, which is exciting because this usually requires cryogenic temperatures.
The researchers found another unique and counter-intuitive feature of this design. Normally, energy is lost during laser operation. But in this system, as energy loss increased, the amount of energy needed to induce lasing decreased. Exploiting this phenomenon could lead to the development of high efficiency, low threshold lasers for future quantum optical devices.
“This system applies a concept of quantum physics known as parity-time reversal symmetry,” explains Professor Cho. “This is an important platform that allows energy loss to be used as gain. It can be used to reduce laser threshold energy for classical optical devices and sensors, as well as quantum devices and controlling the direction of light.”
The key is the design and materials. The hexagonal microcavity divides light particles into two different modes: one that passes through the upward-facing triangle of the hexagon and another that passes through its downward-facing triangle. Both modes of light particles have the same energy and path but don’t interact with each other.
However, the light particles do interact with other particles called excitons, provided by the hexagonal microcavity, which is made of semiconductors. This interaction leads to the generation of new quantum particles called polaritons that then interact with each other to generate the polariton laser. By controlling the degree of loss between the microcavity and the semiconductor substrate, an intriguing phenomenon arises, with the threshold energy becoming smaller as energy loss increases. This research was supported by the Samsung Science and Technology Foundation and Korea’s National Research Foundation.
-PublicationSong,H.G, Choi, M, Woo, K.Y. Yong-Hoon Cho Room-temperature polaritonic non-Hermitian system with single microcavityNature Photonics (https://doi.org/10.1038/s41566-021-00820-z)
-ProfileProfessor Yong-Hoon ChoQuantum & Nanobio Photonics Laboratoryhttp://qnp.kaist.ac.kr/
Department of PhysicsKAIST
2021.07.07 View 11532 -
 Wearable Device to Monitor Sweat in Real Time
An on-skin platform for the wireless monitoring of flow rate, cumulative loss, and temperature of sweat in real time
An electronic patch can monitor your sweating and check your health status. Even more, the soft microfluidic device that adheres to the surface of the skin, captures, stores, and performs biomarker analysis of sweat as it is released through the eccrine glands.
This wearable and wireless electronic device developed by Professor Kyeongha Kwon and her collaborators is a digital and wireless platform that could help track the so-called ‘filling process’ of sweat without having to visually examine the device. The platform was integrated with microfluidic systems to analyze the sweat’s components.
To monitor the sweat release rate in real time, the researchers created a ‘thermal flow sensing module.’ They designed a sophisticated microfluidic channel to allow the collected sweat to flow through a narrow passage and a heat source was placed on the outer surface of the channel to induce a heat exchange between the sweat and the heated channel.
As a result, the researchers could develop a wireless electronic patch that can measure the temperature difference in a specific location upstream and downstream of the heat source with an electronic circuit and convert it into a digital signal to measure the sweat release rate in real time. The patch accurately measured the perspiration rate in the range of 0-5 microliters/minute (μl/min), which was considered physiologically significant. The sensor can measure the flow of sweat directly and then use the information it collected to quantify total sweat loss. Moreover, the device features advanced microfluidic systems and colorimetric chemical reagents to gather pH measurements and determine the concentration of chloride, creatinine, and glucose in a user's sweat.
Professor Kwon said that these indicators could be used to diagnose various diseases related with sweating such as cystic fibrosis, diabetes, kidney dysfunction, and metabolic alkalosis. “As the sweat flowing in the microfluidic channel is completely separated from the electronic circuit, the new patch overcame the shortcomings of existing flow rate measuring devices, which were vulnerable to corrosion and aging,” she explained.
The patch can be easily attached to the skin with flexible circuit board printing technology and silicone sealing technology. It has an additional sensor that detects changes in skin temperature. Using a smartphone app, a user can check the data measured by the wearable patch in real time.
Professor Kwon added, “This patch can be widely used for personal hydration strategies, the detection of dehydration symptoms, and other health management purposes. It can also be used in a systematic drug delivery system, such as for measuring the blood flow rate in blood vessels near the skin’s surface or measuring a drug’s release rate in real time to calculate the exact dosage.”
-PublicationKyeongha Kwon, Jong Uk Kim, John A. Rogers, et al. “An on-skin platform for wireless monitoring of flow rate, cumulative loss and temperature of sweat in real time.” Nature Electronics (doi.org/10.1038/s41928-021-00556-2)
-ProfileProfessor Kyeongha KwonSchool of Electrical EngineeringKAIST
2021.06.25 View 11930
Wearable Device to Monitor Sweat in Real Time
An on-skin platform for the wireless monitoring of flow rate, cumulative loss, and temperature of sweat in real time
An electronic patch can monitor your sweating and check your health status. Even more, the soft microfluidic device that adheres to the surface of the skin, captures, stores, and performs biomarker analysis of sweat as it is released through the eccrine glands.
This wearable and wireless electronic device developed by Professor Kyeongha Kwon and her collaborators is a digital and wireless platform that could help track the so-called ‘filling process’ of sweat without having to visually examine the device. The platform was integrated with microfluidic systems to analyze the sweat’s components.
To monitor the sweat release rate in real time, the researchers created a ‘thermal flow sensing module.’ They designed a sophisticated microfluidic channel to allow the collected sweat to flow through a narrow passage and a heat source was placed on the outer surface of the channel to induce a heat exchange between the sweat and the heated channel.
As a result, the researchers could develop a wireless electronic patch that can measure the temperature difference in a specific location upstream and downstream of the heat source with an electronic circuit and convert it into a digital signal to measure the sweat release rate in real time. The patch accurately measured the perspiration rate in the range of 0-5 microliters/minute (μl/min), which was considered physiologically significant. The sensor can measure the flow of sweat directly and then use the information it collected to quantify total sweat loss. Moreover, the device features advanced microfluidic systems and colorimetric chemical reagents to gather pH measurements and determine the concentration of chloride, creatinine, and glucose in a user's sweat.
Professor Kwon said that these indicators could be used to diagnose various diseases related with sweating such as cystic fibrosis, diabetes, kidney dysfunction, and metabolic alkalosis. “As the sweat flowing in the microfluidic channel is completely separated from the electronic circuit, the new patch overcame the shortcomings of existing flow rate measuring devices, which were vulnerable to corrosion and aging,” she explained.
The patch can be easily attached to the skin with flexible circuit board printing technology and silicone sealing technology. It has an additional sensor that detects changes in skin temperature. Using a smartphone app, a user can check the data measured by the wearable patch in real time.
Professor Kwon added, “This patch can be widely used for personal hydration strategies, the detection of dehydration symptoms, and other health management purposes. It can also be used in a systematic drug delivery system, such as for measuring the blood flow rate in blood vessels near the skin’s surface or measuring a drug’s release rate in real time to calculate the exact dosage.”
-PublicationKyeongha Kwon, Jong Uk Kim, John A. Rogers, et al. “An on-skin platform for wireless monitoring of flow rate, cumulative loss and temperature of sweat in real time.” Nature Electronics (doi.org/10.1038/s41928-021-00556-2)
-ProfileProfessor Kyeongha KwonSchool of Electrical EngineeringKAIST
2021.06.25 View 11930 -
 Mobile Clinic Module Wins Red Dot and iF Design Awards
The Mobile Clinic Module (MCM), an inflatable negative pressure ward building system developed by the Korea Aid for Respiratory Epidemic (KARE) initiative at KAIST, gained international acclaim by winning the prestigious Red Dot Design Award and iF Design Award.
The MCM was recognized as one of the Red Dot Product Designs of the Year. It also won four iF Design Awards in communication design, interior architecture, user interface, and user experience. Winning the two most influential design awards demonstrates how product design can make a valuable contribution to help contain pandemics and reflects new consumer trends for dealing with pandemics.
Designed to be patient friendly, even in the extreme medical situations such as pandemics or triage, the MCM is the result of collaborations among researchers in a variety of fields including mechanical engineering, computing, industrial and systems engineering, medical hospitals, and engineering companies. The research team was led by Professor Tek-Jin Nam from the Department of Industrial Design.
The MCM is expandable, moveable, and easy to store through a combination of negative pressure frames, air tents, and multi-functional panels. Positive air pressure devices supply fresh air from outside the tent. An air pump and controller maintain air beam pressure, while filtering exhausted air from inside. An internal air information monitoring system efficiently controls inside air pressure and purifies the air. It requires only one-fourth of the volume of existing wards and takes up approximately 40% of their weight. The unit can be transported in a 40-foot container truck.
MCMs are now located at the Korea Institute of Radiological & Medical Sciences and Jeju Vaccine Center and expect to be used at many other facilities. KARE is developing antiviral solutions and devices such as protective gear, sterilizers, and test kits to promptly respond to the pandemic. More than 100 researchers at KAIST are collaborating with industry and clinical hospitals to develop antiviral technologies that will improve preventive measures, diagnoses, and treatments.
Professor Nam said, “Our designers will continue to identify the most challenging issues, and try to resolve them by realizing user-friendly functions. We believe this will significantly contribute to relieving the drastic need for negative pressure beds and provide a place for monitoring patients with moderate symptoms. We look forward to the MCM upgrading epidemic management resources around the globe.”
(END)
2021.04.21 View 11006
Mobile Clinic Module Wins Red Dot and iF Design Awards
The Mobile Clinic Module (MCM), an inflatable negative pressure ward building system developed by the Korea Aid for Respiratory Epidemic (KARE) initiative at KAIST, gained international acclaim by winning the prestigious Red Dot Design Award and iF Design Award.
The MCM was recognized as one of the Red Dot Product Designs of the Year. It also won four iF Design Awards in communication design, interior architecture, user interface, and user experience. Winning the two most influential design awards demonstrates how product design can make a valuable contribution to help contain pandemics and reflects new consumer trends for dealing with pandemics.
Designed to be patient friendly, even in the extreme medical situations such as pandemics or triage, the MCM is the result of collaborations among researchers in a variety of fields including mechanical engineering, computing, industrial and systems engineering, medical hospitals, and engineering companies. The research team was led by Professor Tek-Jin Nam from the Department of Industrial Design.
The MCM is expandable, moveable, and easy to store through a combination of negative pressure frames, air tents, and multi-functional panels. Positive air pressure devices supply fresh air from outside the tent. An air pump and controller maintain air beam pressure, while filtering exhausted air from inside. An internal air information monitoring system efficiently controls inside air pressure and purifies the air. It requires only one-fourth of the volume of existing wards and takes up approximately 40% of their weight. The unit can be transported in a 40-foot container truck.
MCMs are now located at the Korea Institute of Radiological & Medical Sciences and Jeju Vaccine Center and expect to be used at many other facilities. KARE is developing antiviral solutions and devices such as protective gear, sterilizers, and test kits to promptly respond to the pandemic. More than 100 researchers at KAIST are collaborating with industry and clinical hospitals to develop antiviral technologies that will improve preventive measures, diagnoses, and treatments.
Professor Nam said, “Our designers will continue to identify the most challenging issues, and try to resolve them by realizing user-friendly functions. We believe this will significantly contribute to relieving the drastic need for negative pressure beds and provide a place for monitoring patients with moderate symptoms. We look forward to the MCM upgrading epidemic management resources around the globe.”
(END)
2021.04.21 View 11006 -
 Professor Jihee Kim Wins the Lucas Prize for Her Income Inequality Theory
Professor Jihee Kim from the School of Business and Technology Management at KAIST was announced as one of two winners of the 2021 Robert E. Lucas Jr. Prize. Professor Kim was recognized for having provided an empirical analysis on engines of income growth, sources of income inequality, and their rich interplay in her paper published in the Journal of Political Economy (JPE) in October 2018. The co-author of this study, Professor Charles I. Jones at Stanford University, was honored to be another awardee of this year’s Lucas Prize.
The Robert E. Lucas Jr. Prize, simply known as the Lucas Prize, is awarded biannually for the most interesting paper in the area of Dynamic Economics published in the leading economics journal JPE in the preceding two years. The prize was established in 2016 in celebration of the 1995 Nobel Prize in Economics Laureate Dr. Lucas’s seminal contributions to economics. The two former prizes were presented in 2019 and 2017 respectively.
Professor Kim and Professor Jones, in their award-winning paper titled 'A Schumpeterian Model of Top Income Inequality', observed that top income inequality was relatively low and stable between 1960 and 1980, but then rose sharply in some countries, including the United States and the United Kingdom.
The authors focused on entrepreneurial activities and the resulting income as the driving force of income inequality. They assumed that the forces that increased the efforts of fast-growing entrepreneurs to improve their products or increased productivity of their efforts could increase income inequality. On the other hand, the forces that enhanced creative destruction or that raised the rate at which high-growth entrepreneurs lost that status could decrease income inequality, according to the authors’ theory.
Professor Kim explained, “Various economic forces due to globalization, the advancement in AI and IT technologies, taxes, and policies related to innovation blocking may explain the varied patterns in income inequality.”
“Through follow-up research, I will continue developing economic theory models that can analyze the impact of changes such as income tax rates and salary negotiations on income inequality,” she added.
Professor Kim received her bachelor’s degree from the KAIST School of Computing in 2005 and pursued her graduates studies at Stanford University, acquiring a master’s degree in economics in 2011 and a doctoral degree in management science and engineering in 2013.
(END)
2021.03.26 View 9070
Professor Jihee Kim Wins the Lucas Prize for Her Income Inequality Theory
Professor Jihee Kim from the School of Business and Technology Management at KAIST was announced as one of two winners of the 2021 Robert E. Lucas Jr. Prize. Professor Kim was recognized for having provided an empirical analysis on engines of income growth, sources of income inequality, and their rich interplay in her paper published in the Journal of Political Economy (JPE) in October 2018. The co-author of this study, Professor Charles I. Jones at Stanford University, was honored to be another awardee of this year’s Lucas Prize.
The Robert E. Lucas Jr. Prize, simply known as the Lucas Prize, is awarded biannually for the most interesting paper in the area of Dynamic Economics published in the leading economics journal JPE in the preceding two years. The prize was established in 2016 in celebration of the 1995 Nobel Prize in Economics Laureate Dr. Lucas’s seminal contributions to economics. The two former prizes were presented in 2019 and 2017 respectively.
Professor Kim and Professor Jones, in their award-winning paper titled 'A Schumpeterian Model of Top Income Inequality', observed that top income inequality was relatively low and stable between 1960 and 1980, but then rose sharply in some countries, including the United States and the United Kingdom.
The authors focused on entrepreneurial activities and the resulting income as the driving force of income inequality. They assumed that the forces that increased the efforts of fast-growing entrepreneurs to improve their products or increased productivity of their efforts could increase income inequality. On the other hand, the forces that enhanced creative destruction or that raised the rate at which high-growth entrepreneurs lost that status could decrease income inequality, according to the authors’ theory.
Professor Kim explained, “Various economic forces due to globalization, the advancement in AI and IT technologies, taxes, and policies related to innovation blocking may explain the varied patterns in income inequality.”
“Through follow-up research, I will continue developing economic theory models that can analyze the impact of changes such as income tax rates and salary negotiations on income inequality,” she added.
Professor Kim received her bachelor’s degree from the KAIST School of Computing in 2005 and pursued her graduates studies at Stanford University, acquiring a master’s degree in economics in 2011 and a doctoral degree in management science and engineering in 2013.
(END)
2021.03.26 View 9070 -
 Professor Jae Kyoung Kim to Lead a New Mathematical Biology Research Group at IBS
Professor Jae Kyoung Kim from the KAIST Department of Mathematical Sciences was appointed as the third Chief Investigator (CI) of the Pioneer Research Center (PRC) for Mathematical and Computational Sciences at the Institute for Basic Science (IBS). Professor Kim will launch and lead a new research group that will be devoted to resolving various biological conundrums from a mathematical perspective. His appointment began on March 1, 2021.
Professor Kim, a rising researcher in the field of mathematical biology, has received attention from both the mathematical and biological communities at the international level. Professor Kim puts novel and unremitting efforts into understanding biological systems such as cell-to-cell interactions mathematically and designing mathematical models for identifying causes of diseases and developing therapeutic medicines.
Through active joint research with biologists, mathematician Kim has addressed many challenges that have remained unsolved in biology and published papers in a number of leading international journals in related fields. His notable works based on mathematical modelling include having designed a biological circuit that can maintain a stable circadian rhythm (Science, 2015) and unveiling the principles of how the biological clock in the body maintains a steady speed for the first time in over 60 years (Molecular Cell, 2015). Recently, through a joint research project with Pfizer, Professor Kim identified what causes the differences between animal and clinical test results during drug development explaining why drugs have different efficacies in different people (Molecular Systems Biology, 2019).
The new IBS biomedical mathematics research group led by Professor Kim will further investigate the causes of unstable circadian rhythms and sleeping patterns. The team will aim to present a new paradigm in treatments for sleep disorders.
Professor Kim said, “We are all so familiar with sleep behaviors, but the exact mechanisms behind how such behaviors occur are still unknown. Through cooperation with biomedical scientists, our group will do its best to discover the complicated, fundamental mechanisms of sleep, and investigate the causes and cures of sleep disorders.”
Every year, the IBS selects young and promising researchers and appoints them as CIs. A maximum of five selected CIs can form each independent research group within the IBS PRC, and receive research funds of 1 billion to 1.5 billion KRW over five years.
(END)
2021.03.18 View 11065
Professor Jae Kyoung Kim to Lead a New Mathematical Biology Research Group at IBS
Professor Jae Kyoung Kim from the KAIST Department of Mathematical Sciences was appointed as the third Chief Investigator (CI) of the Pioneer Research Center (PRC) for Mathematical and Computational Sciences at the Institute for Basic Science (IBS). Professor Kim will launch and lead a new research group that will be devoted to resolving various biological conundrums from a mathematical perspective. His appointment began on March 1, 2021.
Professor Kim, a rising researcher in the field of mathematical biology, has received attention from both the mathematical and biological communities at the international level. Professor Kim puts novel and unremitting efforts into understanding biological systems such as cell-to-cell interactions mathematically and designing mathematical models for identifying causes of diseases and developing therapeutic medicines.
Through active joint research with biologists, mathematician Kim has addressed many challenges that have remained unsolved in biology and published papers in a number of leading international journals in related fields. His notable works based on mathematical modelling include having designed a biological circuit that can maintain a stable circadian rhythm (Science, 2015) and unveiling the principles of how the biological clock in the body maintains a steady speed for the first time in over 60 years (Molecular Cell, 2015). Recently, through a joint research project with Pfizer, Professor Kim identified what causes the differences between animal and clinical test results during drug development explaining why drugs have different efficacies in different people (Molecular Systems Biology, 2019).
The new IBS biomedical mathematics research group led by Professor Kim will further investigate the causes of unstable circadian rhythms and sleeping patterns. The team will aim to present a new paradigm in treatments for sleep disorders.
Professor Kim said, “We are all so familiar with sleep behaviors, but the exact mechanisms behind how such behaviors occur are still unknown. Through cooperation with biomedical scientists, our group will do its best to discover the complicated, fundamental mechanisms of sleep, and investigate the causes and cures of sleep disorders.”
Every year, the IBS selects young and promising researchers and appoints them as CIs. A maximum of five selected CIs can form each independent research group within the IBS PRC, and receive research funds of 1 billion to 1.5 billion KRW over five years.
(END)
2021.03.18 View 11065 -
 Acoustic Graphene Plasmons Study Paves Way for Optoelectronic Applications
- The first images of mid-infrared optical waves compressed 1,000 times captured using a highly sensitive scattering-type scanning near-field optical microscope. -
KAIST researchers and their collaborators at home and abroad have successfully demonstrated a new methodology for direct near-field optical imaging of acoustic graphene plasmon fields. This strategy will provide a breakthrough for the practical applications of acoustic graphene plasmon platforms in next-generation, high-performance, graphene-based optoelectronic devices with enhanced light-matter interactions and lower propagation loss.
It was recently demonstrated that ‘graphene plasmons’ – collective oscillations of free electrons in graphene coupled to electromagnetic waves of light – can be used to trap and compress optical waves inside a very thin dielectric layer separating graphene from a metallic sheet. In such a configuration, graphene’s conduction electrons are “reflected” in the metal, so when the light waves “push” the electrons in graphene, their image charges in metal also start to oscillate. This new type of collective electronic oscillation mode is called ‘acoustic graphene plasmon (AGP)’.
The existence of AGP could previously be observed only via indirect methods such as far-field infrared spectroscopy and photocurrent mapping. This indirect observation was the price that researchers had to pay for the strong compression of optical waves inside nanometer-thin structures. It was believed that the intensity of electromagnetic fields outside the device was insufficient for direct near-field optical imaging of AGP.
Challenged by these limitations, three research groups combined their efforts to bring together a unique experimental technique using advanced nanofabrication methods. Their findings were published in Nature Communications on February 19.
A KAIST research team led by Professor Min Seok Jang from the School of Electrical Engineering used a highly sensitive scattering-type scanning near-field optical microscope (s-SNOM) to directly measure the optical fields of the AGP waves propagating in a nanometer-thin waveguide, visualizing thousand-fold compression of mid-infrared light for the first time.
Professor Jang and a post-doc researcher in his group, Sergey G. Menabde, successfully obtained direct images of AGP waves by taking advantage of their rapidly decaying yet always present electric field above graphene. They showed that AGPs are detectable even when most of their energy is flowing inside the dielectric below the graphene.
This became possible due to the ultra-smooth surfaces inside the nano-waveguides where plasmonic waves can propagate at longer distances. The AGP mode probed by the researchers was up to 2.3 times more confined and exhibited a 1.4 times higher figure of merit in terms of the normalized propagation length compared to the graphene surface plasmon under similar conditions.
These ultra-smooth nanostructures of the waveguides used in the experiment were created using a template-stripping method by Professor Sang-Hyun Oh and a post-doc researcher, In-Ho Lee, from the Department of Electrical and Computer Engineering at the University of Minnesota.
Professor Young Hee Lee and his researchers at the Center for Integrated Nanostructure Physics (CINAP) of the Institute of Basic Science (IBS) at Sungkyunkwan University synthesized the graphene with a monocrystalline structure, and this high-quality, large-area graphene enabled low-loss plasmonic propagation.
The chemical and physical properties of many important organic molecules can be detected and evaluated by their absorption signatures in the mid-infrared spectrum. However, conventional detection methods require a large number of molecules for successful detection, whereas the ultra-compressed AGP fields can provide strong light-matter interactions at the microscopic level, thus significantly improving the detection sensitivity down to a single molecule.
Furthermore, the study conducted by Professor Jang and the team demonstrated that the mid-infrared AGPs are inherently less sensitive to losses in graphene due to their fields being mostly confined within the dielectric. The research team’s reported results suggest that AGPs could become a promising platform for electrically tunable graphene-based optoelectronic devices that typically suffer from higher absorption rates in graphene such as metasurfaces, optical switches, photovoltaics, and other optoelectronic applications operating at infrared frequencies.
Professor Jang said, “Our research revealed that the ultra-compressed electromagnetic fields of acoustic graphene plasmons can be directly accessed through near-field optical microscopy methods. I hope this realization will motivate other researchers to apply AGPs to various problems where strong light-matter interactions and lower propagation loss are needed.”
This research was primarily funded by the Samsung Research Funding & Incubation Center of Samsung Electronics. The National Research Foundation of Korea (NRF), the U.S. National Science Foundation (NSF), Samsung Global Research Outreach (GRO) Program, and Institute for Basic Science of Korea (IBS) also supported the work.
Publication:
Menabde, S. G., et al. (2021) Real-space imaging of acoustic plasmons in large-area graphene grown by chemical vapor deposition. Nature Communications 12, Article No. 938. Available online at https://doi.org/10.1038/s41467-021-21193-5
Profile:
Min Seok Jang, MS, PhD
Associate Professorjang.minseok@kaist.ac.krhttp://jlab.kaist.ac.kr/
Min Seok Jang Research GroupSchool of Electrical Engineering
http://kaist.ac.kr/en/Korea Advanced Institute of Science and Technology (KAIST)Daejeon, Republic of Korea
(END)
2021.03.16 View 15967
Acoustic Graphene Plasmons Study Paves Way for Optoelectronic Applications
- The first images of mid-infrared optical waves compressed 1,000 times captured using a highly sensitive scattering-type scanning near-field optical microscope. -
KAIST researchers and their collaborators at home and abroad have successfully demonstrated a new methodology for direct near-field optical imaging of acoustic graphene plasmon fields. This strategy will provide a breakthrough for the practical applications of acoustic graphene plasmon platforms in next-generation, high-performance, graphene-based optoelectronic devices with enhanced light-matter interactions and lower propagation loss.
It was recently demonstrated that ‘graphene plasmons’ – collective oscillations of free electrons in graphene coupled to electromagnetic waves of light – can be used to trap and compress optical waves inside a very thin dielectric layer separating graphene from a metallic sheet. In such a configuration, graphene’s conduction electrons are “reflected” in the metal, so when the light waves “push” the electrons in graphene, their image charges in metal also start to oscillate. This new type of collective electronic oscillation mode is called ‘acoustic graphene plasmon (AGP)’.
The existence of AGP could previously be observed only via indirect methods such as far-field infrared spectroscopy and photocurrent mapping. This indirect observation was the price that researchers had to pay for the strong compression of optical waves inside nanometer-thin structures. It was believed that the intensity of electromagnetic fields outside the device was insufficient for direct near-field optical imaging of AGP.
Challenged by these limitations, three research groups combined their efforts to bring together a unique experimental technique using advanced nanofabrication methods. Their findings were published in Nature Communications on February 19.
A KAIST research team led by Professor Min Seok Jang from the School of Electrical Engineering used a highly sensitive scattering-type scanning near-field optical microscope (s-SNOM) to directly measure the optical fields of the AGP waves propagating in a nanometer-thin waveguide, visualizing thousand-fold compression of mid-infrared light for the first time.
Professor Jang and a post-doc researcher in his group, Sergey G. Menabde, successfully obtained direct images of AGP waves by taking advantage of their rapidly decaying yet always present electric field above graphene. They showed that AGPs are detectable even when most of their energy is flowing inside the dielectric below the graphene.
This became possible due to the ultra-smooth surfaces inside the nano-waveguides where plasmonic waves can propagate at longer distances. The AGP mode probed by the researchers was up to 2.3 times more confined and exhibited a 1.4 times higher figure of merit in terms of the normalized propagation length compared to the graphene surface plasmon under similar conditions.
These ultra-smooth nanostructures of the waveguides used in the experiment were created using a template-stripping method by Professor Sang-Hyun Oh and a post-doc researcher, In-Ho Lee, from the Department of Electrical and Computer Engineering at the University of Minnesota.
Professor Young Hee Lee and his researchers at the Center for Integrated Nanostructure Physics (CINAP) of the Institute of Basic Science (IBS) at Sungkyunkwan University synthesized the graphene with a monocrystalline structure, and this high-quality, large-area graphene enabled low-loss plasmonic propagation.
The chemical and physical properties of many important organic molecules can be detected and evaluated by their absorption signatures in the mid-infrared spectrum. However, conventional detection methods require a large number of molecules for successful detection, whereas the ultra-compressed AGP fields can provide strong light-matter interactions at the microscopic level, thus significantly improving the detection sensitivity down to a single molecule.
Furthermore, the study conducted by Professor Jang and the team demonstrated that the mid-infrared AGPs are inherently less sensitive to losses in graphene due to their fields being mostly confined within the dielectric. The research team’s reported results suggest that AGPs could become a promising platform for electrically tunable graphene-based optoelectronic devices that typically suffer from higher absorption rates in graphene such as metasurfaces, optical switches, photovoltaics, and other optoelectronic applications operating at infrared frequencies.
Professor Jang said, “Our research revealed that the ultra-compressed electromagnetic fields of acoustic graphene plasmons can be directly accessed through near-field optical microscopy methods. I hope this realization will motivate other researchers to apply AGPs to various problems where strong light-matter interactions and lower propagation loss are needed.”
This research was primarily funded by the Samsung Research Funding & Incubation Center of Samsung Electronics. The National Research Foundation of Korea (NRF), the U.S. National Science Foundation (NSF), Samsung Global Research Outreach (GRO) Program, and Institute for Basic Science of Korea (IBS) also supported the work.
Publication:
Menabde, S. G., et al. (2021) Real-space imaging of acoustic plasmons in large-area graphene grown by chemical vapor deposition. Nature Communications 12, Article No. 938. Available online at https://doi.org/10.1038/s41467-021-21193-5
Profile:
Min Seok Jang, MS, PhD
Associate Professorjang.minseok@kaist.ac.krhttp://jlab.kaist.ac.kr/
Min Seok Jang Research GroupSchool of Electrical Engineering
http://kaist.ac.kr/en/Korea Advanced Institute of Science and Technology (KAIST)Daejeon, Republic of Korea
(END)
2021.03.16 View 15967 -
 Professor Mu-Hyun Baik Honored with the POSCO TJ Park Prize
Professor Mu-Hyun Baik at the Department of Chemistry was honored to be the recipient of the 2021 POSCO TJ Park Prize in Science. The POSCO TJ Park Foundation awards every year the individual or organization which made significant contribution in science, education, community development, philanthropy, and technology.
Professor Baik, a renowned computational chemist in analyzing complicated chemical reactions to understand how molecules behave and how they change. Professor Baik was awarded in recognition of his pioneering research in designing numerous organometallic catalysts with using computational molecular modelling. In 2016, he published in Science on the catalytic borylation of methane that showed how chemical reactions can be carried out using the natural gas methane as a substrate.
In 2020, he reported in Science that electrodes can be used as functional groups with adjustable inductive effects to change the chemical reactivity of molecules that are attached to them, closely mimicking the inductive effect of conventional functional groups. This constitutes a potentially powerful new way of controlling chemical reactions, offering an alternative to preparing derivatives to install electron-withdrawing functional groups.
Joined at KAIST in 2015, Professor Baik also serves as associate director at the Center for Catalytic Hydrocarbon Functionalization at the Institute for Basic Science (IBS) since 2015. Among the many recognitions and awards that he received include the Kavli Fellowship by the Kavli Foundation and the National Academy of Science in the US in 2019 and the 2018 Friedrich Wilhelm Bessel Award by the Alexander von Humboldt Foundation in Germany.
2021.03.11 View 10076
Professor Mu-Hyun Baik Honored with the POSCO TJ Park Prize
Professor Mu-Hyun Baik at the Department of Chemistry was honored to be the recipient of the 2021 POSCO TJ Park Prize in Science. The POSCO TJ Park Foundation awards every year the individual or organization which made significant contribution in science, education, community development, philanthropy, and technology.
Professor Baik, a renowned computational chemist in analyzing complicated chemical reactions to understand how molecules behave and how they change. Professor Baik was awarded in recognition of his pioneering research in designing numerous organometallic catalysts with using computational molecular modelling. In 2016, he published in Science on the catalytic borylation of methane that showed how chemical reactions can be carried out using the natural gas methane as a substrate.
In 2020, he reported in Science that electrodes can be used as functional groups with adjustable inductive effects to change the chemical reactivity of molecules that are attached to them, closely mimicking the inductive effect of conventional functional groups. This constitutes a potentially powerful new way of controlling chemical reactions, offering an alternative to preparing derivatives to install electron-withdrawing functional groups.
Joined at KAIST in 2015, Professor Baik also serves as associate director at the Center for Catalytic Hydrocarbon Functionalization at the Institute for Basic Science (IBS) since 2015. Among the many recognitions and awards that he received include the Kavli Fellowship by the Kavli Foundation and the National Academy of Science in the US in 2019 and the 2018 Friedrich Wilhelm Bessel Award by the Alexander von Humboldt Foundation in Germany.
2021.03.11 View 10076 -
 Deep-Learning and 3D Holographic Microscopy Beats Scientists at Analyzing Cancer Immunotherapy
Live tracking and analyzing of the dynamics of chimeric antigen receptor (CAR) T-cells targeting cancer cells can open new avenues for the development of cancer immunotherapy. However, imaging via conventional microscopy approaches can result in cellular damage, and assessments of cell-to-cell interactions are extremely difficult and labor-intensive. When researchers applied deep learning and 3D holographic microscopy to the task, however, they not only avoided these difficultues but found that AI was better at it than humans were.
Artificial intelligence (AI) is helping researchers decipher images from a new holographic microscopy technique needed to investigate a key process in cancer immunotherapy “live” as it takes place. The AI transformed work that, if performed manually by scientists, would otherwise be incredibly labor-intensive and time-consuming into one that is not only effortless but done better than they could have done it themselves. The research, conducted by the team of Professor YongKeun Park from the Department of Physics, appeared in the journal eLife last December.
A critical stage in the development of the human immune system’s ability to respond not just generally to any invader (such as pathogens or cancer cells) but specifically to that particular type of invader and remember it should it attempt to invade again is the formation of a junction between an immune cell called a T-cell and a cell that presents the antigen, or part of the invader that is causing the problem, to it. This process is like when a picture of a suspect is sent to a police car so that the officers can recognize the criminal they are trying to track down. The junction between the two cells, called the immunological synapse, or IS, is the key process in teaching the immune system how to recognize a specific type of invader.
Since the formation of the IS junction is such a critical step for the initiation of an antigen-specific immune response, various techniques allowing researchers to observe the process as it happens have been used to study its dynamics. Most of these live imaging techniques rely on fluorescence microscopy, where genetic tweaking causes part of a protein from a cell to fluoresce, in turn allowing the subject to be tracked via fluorescence rather than via the reflected light used in many conventional microscopy techniques.
However, fluorescence-based imaging can suffer from effects such as photo-bleaching and photo-toxicity, preventing the assessment of dynamic changes in the IS junction process over the long term. Fluorescence-based imaging still involves illumination, whereupon the fluorophores (chemical compounds that cause the fluorescence) emit light of a different color. Photo-bleaching or photo-toxicity occur when the subject is exposed to too much illumination, resulting in chemical alteration or cellular damage.
One recent option that does away with fluorescent labelling and thereby avoids such problems is 3D holographic microscopy or holotomography (HT). In this technique, the refractive index (the way that light changes direction when encountering a substance with a different density—why a straw looks like it bends in a glass of water) is recorded in 3D as a hologram.
Until now, HT has been used to study single cells, but never cell-cell interactions involved in immune responses. One of the main reasons is the difficulty of “segmentation,” or distinguishing the different parts of a cell and thus distinguishing between the interacting cells; in other words, deciphering which part belongs to which cell.
Manual segmentation, or marking out the different parts manually, is one option, but it is difficult and time-consuming, especially in three dimensions. To overcome this problem, automatic segmentation has been developed in which simple computer algorithms perform the identification.
“But these basic algorithms often make mistakes,” explained Professor YongKeun Park, “particularly with respect to adjoining segmentation, which of course is exactly what is occurring here in the immune response we’re most interested in.”
So, the researchers applied a deep learning framework to the HT segmentation problem. Deep learning is a type of machine learning in which artificial neural networks based on the human brain recognize patterns in a way that is similar to how humans do this. Regular machine learning requires data as an input that has already been labelled. The AI “learns” by understanding the labeled data and then recognizes the concept that has been labelled when it is fed novel data. For example, AI trained on a thousand images of cats labelled “cat” should be able to recognize a cat the next time it encounters an image with a cat in it. Deep learning involves multiple layers of artificial neural networks attacking much larger, but unlabeled datasets, in which the AI develops its own ‘labels’ for concepts it encounters.
In essence, the deep learning framework that KAIST researchers developed, called DeepIS, came up with its own concepts by which it distinguishes the different parts of the IS junction process. To validate this method, the research team applied it to the dynamics of a particular IS junction formed between chimeric antigen receptor (CAR) T-cells and target cancer cells. They then compared the results to what they would normally have done: the laborious process of performing the segmentation manually. They found not only that DeepIS was able to define areas within the IS with high accuracy, but that the technique was even able to capture information about the total distribution of proteins within the IS that may not have been easily measured using conventional techniques.
“In addition to allowing us to avoid the drudgery of manual segmentation and the problems of photo-bleaching and photo-toxicity, we found that the AI actually did a better job,” Professor Park added.
The next step will be to combine the technique with methods of measuring how much physical force is applied by different parts of the IS junction, such as holographic optical tweezers or traction force microscopy.
-Profile
Professor YongKeun Park
Department of Physics
Biomedical Optics Laboratory
http://bmol.kaist.ac.kr
KAIST
2021.02.24 View 13946
Deep-Learning and 3D Holographic Microscopy Beats Scientists at Analyzing Cancer Immunotherapy
Live tracking and analyzing of the dynamics of chimeric antigen receptor (CAR) T-cells targeting cancer cells can open new avenues for the development of cancer immunotherapy. However, imaging via conventional microscopy approaches can result in cellular damage, and assessments of cell-to-cell interactions are extremely difficult and labor-intensive. When researchers applied deep learning and 3D holographic microscopy to the task, however, they not only avoided these difficultues but found that AI was better at it than humans were.
Artificial intelligence (AI) is helping researchers decipher images from a new holographic microscopy technique needed to investigate a key process in cancer immunotherapy “live” as it takes place. The AI transformed work that, if performed manually by scientists, would otherwise be incredibly labor-intensive and time-consuming into one that is not only effortless but done better than they could have done it themselves. The research, conducted by the team of Professor YongKeun Park from the Department of Physics, appeared in the journal eLife last December.
A critical stage in the development of the human immune system’s ability to respond not just generally to any invader (such as pathogens or cancer cells) but specifically to that particular type of invader and remember it should it attempt to invade again is the formation of a junction between an immune cell called a T-cell and a cell that presents the antigen, or part of the invader that is causing the problem, to it. This process is like when a picture of a suspect is sent to a police car so that the officers can recognize the criminal they are trying to track down. The junction between the two cells, called the immunological synapse, or IS, is the key process in teaching the immune system how to recognize a specific type of invader.
Since the formation of the IS junction is such a critical step for the initiation of an antigen-specific immune response, various techniques allowing researchers to observe the process as it happens have been used to study its dynamics. Most of these live imaging techniques rely on fluorescence microscopy, where genetic tweaking causes part of a protein from a cell to fluoresce, in turn allowing the subject to be tracked via fluorescence rather than via the reflected light used in many conventional microscopy techniques.
However, fluorescence-based imaging can suffer from effects such as photo-bleaching and photo-toxicity, preventing the assessment of dynamic changes in the IS junction process over the long term. Fluorescence-based imaging still involves illumination, whereupon the fluorophores (chemical compounds that cause the fluorescence) emit light of a different color. Photo-bleaching or photo-toxicity occur when the subject is exposed to too much illumination, resulting in chemical alteration or cellular damage.
One recent option that does away with fluorescent labelling and thereby avoids such problems is 3D holographic microscopy or holotomography (HT). In this technique, the refractive index (the way that light changes direction when encountering a substance with a different density—why a straw looks like it bends in a glass of water) is recorded in 3D as a hologram.
Until now, HT has been used to study single cells, but never cell-cell interactions involved in immune responses. One of the main reasons is the difficulty of “segmentation,” or distinguishing the different parts of a cell and thus distinguishing between the interacting cells; in other words, deciphering which part belongs to which cell.
Manual segmentation, or marking out the different parts manually, is one option, but it is difficult and time-consuming, especially in three dimensions. To overcome this problem, automatic segmentation has been developed in which simple computer algorithms perform the identification.
“But these basic algorithms often make mistakes,” explained Professor YongKeun Park, “particularly with respect to adjoining segmentation, which of course is exactly what is occurring here in the immune response we’re most interested in.”
So, the researchers applied a deep learning framework to the HT segmentation problem. Deep learning is a type of machine learning in which artificial neural networks based on the human brain recognize patterns in a way that is similar to how humans do this. Regular machine learning requires data as an input that has already been labelled. The AI “learns” by understanding the labeled data and then recognizes the concept that has been labelled when it is fed novel data. For example, AI trained on a thousand images of cats labelled “cat” should be able to recognize a cat the next time it encounters an image with a cat in it. Deep learning involves multiple layers of artificial neural networks attacking much larger, but unlabeled datasets, in which the AI develops its own ‘labels’ for concepts it encounters.
In essence, the deep learning framework that KAIST researchers developed, called DeepIS, came up with its own concepts by which it distinguishes the different parts of the IS junction process. To validate this method, the research team applied it to the dynamics of a particular IS junction formed between chimeric antigen receptor (CAR) T-cells and target cancer cells. They then compared the results to what they would normally have done: the laborious process of performing the segmentation manually. They found not only that DeepIS was able to define areas within the IS with high accuracy, but that the technique was even able to capture information about the total distribution of proteins within the IS that may not have been easily measured using conventional techniques.
“In addition to allowing us to avoid the drudgery of manual segmentation and the problems of photo-bleaching and photo-toxicity, we found that the AI actually did a better job,” Professor Park added.
The next step will be to combine the technique with methods of measuring how much physical force is applied by different parts of the IS junction, such as holographic optical tweezers or traction force microscopy.
-Profile
Professor YongKeun Park
Department of Physics
Biomedical Optics Laboratory
http://bmol.kaist.ac.kr
KAIST
2021.02.24 View 13946 -
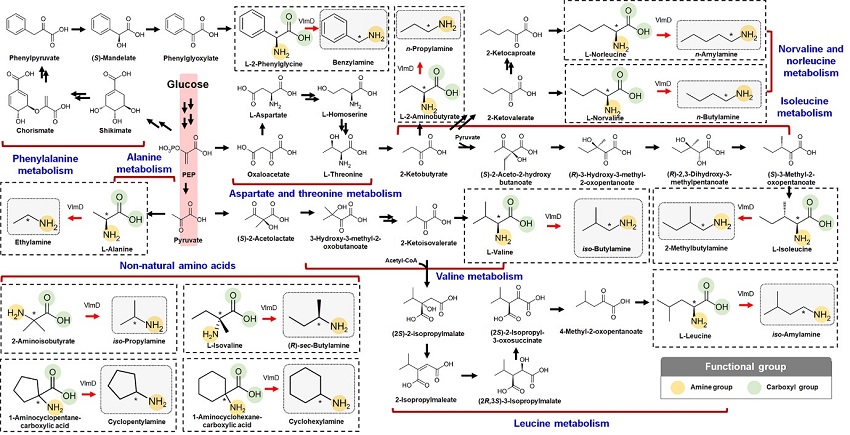 Expanding the Biosynthetic Pathway via Retrobiosynthesis
- Researchers reports a new strategy for the microbial production of multiple short-chain primary amines via retrobiosynthesis. -
KAIST metabolic engineers presented the bio-based production of multiple short-chain primary amines that have a wide range of applications in chemical industries for the first time. The research team led by Distinguished Professor Sang Yup Lee from the Department of Chemical and Biomolecular Engineering designed the novel biosynthetic pathways for short-chain primary amines by combining retrobiosynthesis and a precursor selection step.
The research team verified the newly designed pathways by confirming the in vivo production of 10 short-chain primary amines by supplying the precursors. Furthermore, the platform Escherichia coli strains were metabolically engineered to produce three proof-of-concept short-chain primary amines from glucose, demonstrating the possibility of the bio-based production of diverse short-chain primary amines from renewable resources. The research team said this study expands the strategy of systematically designing biosynthetic pathways for the production of a group of related chemicals as demonstrated by multiple short-chain primary amines as examples.
Currently, most of the industrial chemicals used in our daily lives are produced with petroleum-based products. However, there are several serious issues with the petroleum industry such as the depletion of fossil fuel reserves and environmental problems including global warming. To solve these problems, the sustainable production of industrial chemicals and materials is being explored with microorganisms as cell factories and renewable non-food biomass as raw materials for alternative to petroleum-based products. The engineering of these microorganisms has increasingly become more efficient and effective with the help of systems metabolic engineering – a practice of engineering the metabolism of a living organism toward the production of a desired metabolite. In this regard, the number of chemicals produced using biomass as a raw material has substantially increased.
Although the scope of chemicals that are producible using microorganisms continues to expand through advances in systems metabolic engineering, the biological production of short-chain primary amines has not yet been reported despite their industrial importance. Short-chain primary amines are the chemicals that have an alkyl or aryl group in the place of a hydrogen atom in ammonia with carbon chain lengths ranging from C1 to C7. Short-chain primary amines have a wide range of applications in chemical industries, for example, as a precursor for pharmaceuticals (e.g., antidiabetic and antihypertensive drugs), agrochemicals (e.g., herbicides, fungicides and insecticides), solvents, and vulcanization accelerators for rubber and plasticizers. The market size of short-chain primary amines was estimated to be more than 4 billion US dollars in 2014.
The main reason why the bio-based production of short-chain primary amines was not yet possible was due to their unknown biosynthetic pathways. Therefore, the team designed synthetic biosynthetic pathways for short-chain primary amines by combining retrobiosynthesis and a precursor selection step. The retrobiosynthesis allowed the systematic design of a biosynthetic pathway for short-chain primary amines by using a set of biochemical reaction rules that describe chemical transformation patterns between a substrate and product molecules at an atomic level.
These multiple precursors predicted for the possible biosynthesis of each short-chain primary amine were sequentially narrowed down by using the precursor selection step for efficient metabolic engineering experiments.
“Our research demonstrates the possibility of the renewable production of short-chain primary amines for the first time. We are planning to increase production efficiencies of short-chain primary amines. We believe that our study will play an important role in the development of sustainable and eco-friendly bio-based industries and the reorganization of the chemical industry, which is mandatory for solving the environmental problems threating the survival of mankind,” said Professor Lee.
This paper titled “Microbial production of multiple short-chain primary amines via retrobiosynthesis” was published in Nature Communications. This work was supported by the Technology Development Program to Solve Climate Changes on Systems Metabolic Engineering for Biorefineries from the Ministry of Science and ICT through the National Research Foundation (NRF) of Korea.
-Publication
Dong In Kim, Tong Un Chae, Hyun Uk Kim, Woo Dae Jang, and Sang Yup Lee. Microbial production of multiple short-chain primary amines via retrobiosynthesis. Nature Communications ( https://www.nature.com/articles/s41467-020-20423-6)
-Profile
Distinguished Professor Sang Yup Lee
leesy@kaist.ac.kr
Metabolic &Biomolecular Engineering National Research Laboratory
http://mbel.kaist.ac.kr
Department of Chemical and Biomolecular Engineering
KAIST
2021.01.14 View 13488
Expanding the Biosynthetic Pathway via Retrobiosynthesis
- Researchers reports a new strategy for the microbial production of multiple short-chain primary amines via retrobiosynthesis. -
KAIST metabolic engineers presented the bio-based production of multiple short-chain primary amines that have a wide range of applications in chemical industries for the first time. The research team led by Distinguished Professor Sang Yup Lee from the Department of Chemical and Biomolecular Engineering designed the novel biosynthetic pathways for short-chain primary amines by combining retrobiosynthesis and a precursor selection step.
The research team verified the newly designed pathways by confirming the in vivo production of 10 short-chain primary amines by supplying the precursors. Furthermore, the platform Escherichia coli strains were metabolically engineered to produce three proof-of-concept short-chain primary amines from glucose, demonstrating the possibility of the bio-based production of diverse short-chain primary amines from renewable resources. The research team said this study expands the strategy of systematically designing biosynthetic pathways for the production of a group of related chemicals as demonstrated by multiple short-chain primary amines as examples.
Currently, most of the industrial chemicals used in our daily lives are produced with petroleum-based products. However, there are several serious issues with the petroleum industry such as the depletion of fossil fuel reserves and environmental problems including global warming. To solve these problems, the sustainable production of industrial chemicals and materials is being explored with microorganisms as cell factories and renewable non-food biomass as raw materials for alternative to petroleum-based products. The engineering of these microorganisms has increasingly become more efficient and effective with the help of systems metabolic engineering – a practice of engineering the metabolism of a living organism toward the production of a desired metabolite. In this regard, the number of chemicals produced using biomass as a raw material has substantially increased.
Although the scope of chemicals that are producible using microorganisms continues to expand through advances in systems metabolic engineering, the biological production of short-chain primary amines has not yet been reported despite their industrial importance. Short-chain primary amines are the chemicals that have an alkyl or aryl group in the place of a hydrogen atom in ammonia with carbon chain lengths ranging from C1 to C7. Short-chain primary amines have a wide range of applications in chemical industries, for example, as a precursor for pharmaceuticals (e.g., antidiabetic and antihypertensive drugs), agrochemicals (e.g., herbicides, fungicides and insecticides), solvents, and vulcanization accelerators for rubber and plasticizers. The market size of short-chain primary amines was estimated to be more than 4 billion US dollars in 2014.
The main reason why the bio-based production of short-chain primary amines was not yet possible was due to their unknown biosynthetic pathways. Therefore, the team designed synthetic biosynthetic pathways for short-chain primary amines by combining retrobiosynthesis and a precursor selection step. The retrobiosynthesis allowed the systematic design of a biosynthetic pathway for short-chain primary amines by using a set of biochemical reaction rules that describe chemical transformation patterns between a substrate and product molecules at an atomic level.
These multiple precursors predicted for the possible biosynthesis of each short-chain primary amine were sequentially narrowed down by using the precursor selection step for efficient metabolic engineering experiments.
“Our research demonstrates the possibility of the renewable production of short-chain primary amines for the first time. We are planning to increase production efficiencies of short-chain primary amines. We believe that our study will play an important role in the development of sustainable and eco-friendly bio-based industries and the reorganization of the chemical industry, which is mandatory for solving the environmental problems threating the survival of mankind,” said Professor Lee.
This paper titled “Microbial production of multiple short-chain primary amines via retrobiosynthesis” was published in Nature Communications. This work was supported by the Technology Development Program to Solve Climate Changes on Systems Metabolic Engineering for Biorefineries from the Ministry of Science and ICT through the National Research Foundation (NRF) of Korea.
-Publication
Dong In Kim, Tong Un Chae, Hyun Uk Kim, Woo Dae Jang, and Sang Yup Lee. Microbial production of multiple short-chain primary amines via retrobiosynthesis. Nature Communications ( https://www.nature.com/articles/s41467-020-20423-6)
-Profile
Distinguished Professor Sang Yup Lee
leesy@kaist.ac.kr
Metabolic &Biomolecular Engineering National Research Laboratory
http://mbel.kaist.ac.kr
Department of Chemical and Biomolecular Engineering
KAIST
2021.01.14 View 13488 -
 Extremely Stable Perovskite Nanoparticles Films for Next-Generation Displays
Researchers have reported an extremely stable cross-linked perovskite nanoparticle that maintains a high photoluminescence quantum yield (PLQY) for 1.5 years in air and harsh liquid environments. This stable material’s design strategies, which addressed one of the most critical problems limiting their practical application, provide a breakthrough for the commercialization of perovskite nanoparticles in next-generation displays and bio-related applications.
According to the research team led by Professor Byeong-Soo Bae, their development can survive in severe environments such as water, various polar solvents, and high temperature with high humidity without additional encapsulation. This development is expected to enable perovskite nanoparticles to be applied to high color purity display applications as a practical color converting material. This result was published as the inside front cover article in Advanced Materials.
Perovskites, which consist of organics, metals, and halogen elements, have emerged as key elements in various optoelectronic applications. The power conversion efficiency of photovoltaic cells based on perovskites light absorbers has been rapidly increased. Perovskites are also great promise as a light emitter in display applications because of their low material cost, facile wavelength tunability, high (PLQY), very narrow emission band width, and wider color gamut than inorganic semiconducting nanocrystals and organic emitters. Thanks to these advantages, perovskites have been identified as a key color-converting material for next-generation high color-purity displays. In particular, perovskites are the only luminescence material that meets Rec. 2020 which is a new color standard in display industry.
However, perovskites are very unstable against heat, moisture, and light, which makes them almost impossible to use in practical applications. To solve these problems, many researchers have attempted to physically prevent perovskites from coming into contact with water molecules by passivating the perovskite grain and nanoparticle surfaces with organic ligands or inorganic shell materials, or by fabricating perovskite-polymer nanocomposites. These methods require complex processes and have limited stability in ambient air and water. Furthermore, stable perovskite nanoparticles in the various chemical environments and high temperatures with high humidity have not been reported yet.
The research team in collaboration with Seoul National University develops siloxane-encapsulated perovskite nanoparticle composite films. Here, perovskite nanoparticles are chemically crosslinked with thermally stable siloxane molecules, thereby significantly improving the stability of the perovskite nanoparticles without the need for any additional protecting layer.
Siloxane-encapsulated perovskite nanoparticle composite films exhibited a high PLQY (> 70%) value, which can be maintained over 600 days in water, various chemicals (alcohol, strong acidic and basic solutions), and high temperatures with high humidity (85℃/85%). The research team investigated the mechanisms impacting the chemical crosslinking and water molecule-induced stabilization of perovskite nanoparticles through various photo-physical analysis and density-functional theory calculation.
The research team confirmed that displays based on their siloxane-perovskite nanoparticle composite films exhibited higher PLQY and a wider color gamut than those of Cd-based quantum dots and demonstrated perfect color converting properties on commercial mobile phone screens. Unlike what was commonly believed in the halide perovskite field, the composite films showed excellent bio-compatibility because the siloxane matrix prevents the toxicity of Pb in perovskite nanoparticle.
By using this technology, the instability of perovskite materials, which is the biggest challenge for practical applications, is greatly improved through simple encapsulation method.
“Perovskite nanoparticle is the only photoluminescent material that can meet the next generation display color standard. Nevertheless, there has been reluctant to commercialize it due to its moisture vulnerability. The newly developed siloxane encapsulation technology will trigger more research on perovskite nanoparticles as color conversion materials and will accelerate early commercialization,” Professor Bae said.
This work was supported by the Wearable Platform Materials Technology Center (WMC) of the Engineering Research Center (ERC) Project, and the Leadership Research Program funded by the National Research Foundation of Korea.
-Publication:
Junho Jang, Young-Hoon Kim, Sunjoon Park, Dongsuk Yoo, Hyunjin Cho, Jinhyeong Jang, Han Beom Jeong, Hyunhwan Lee, Jong Min Yuk, Chan Beum Park, Duk Young Jeon, Yong-Hyun Kim, Byeong-Soo Bae, and Tae-Woo Lee. “Extremely Stable Luminescent Crosslinked Perovskite Nanoparticles under Harsh Environments over 1.5 Years” Advanced Materials, 2020, 2005255. https://doi.org/10.1002/adma.202005255.
Link to download the full-text paper:
https://onlinelibrary.wiley.com/doi/10.1002/adma.202005255
-Profile: Prof. Byeong-Soo Bae (Corresponding author)
bsbae@kaist.ac.kr
Lab. of Optical Materials & Coating
Department of Materials Science and Engineering
Korea Advanced Institute of Science and Technology (KAIST)
2020.12.29 View 15147
Extremely Stable Perovskite Nanoparticles Films for Next-Generation Displays
Researchers have reported an extremely stable cross-linked perovskite nanoparticle that maintains a high photoluminescence quantum yield (PLQY) for 1.5 years in air and harsh liquid environments. This stable material’s design strategies, which addressed one of the most critical problems limiting their practical application, provide a breakthrough for the commercialization of perovskite nanoparticles in next-generation displays and bio-related applications.
According to the research team led by Professor Byeong-Soo Bae, their development can survive in severe environments such as water, various polar solvents, and high temperature with high humidity without additional encapsulation. This development is expected to enable perovskite nanoparticles to be applied to high color purity display applications as a practical color converting material. This result was published as the inside front cover article in Advanced Materials.
Perovskites, which consist of organics, metals, and halogen elements, have emerged as key elements in various optoelectronic applications. The power conversion efficiency of photovoltaic cells based on perovskites light absorbers has been rapidly increased. Perovskites are also great promise as a light emitter in display applications because of their low material cost, facile wavelength tunability, high (PLQY), very narrow emission band width, and wider color gamut than inorganic semiconducting nanocrystals and organic emitters. Thanks to these advantages, perovskites have been identified as a key color-converting material for next-generation high color-purity displays. In particular, perovskites are the only luminescence material that meets Rec. 2020 which is a new color standard in display industry.
However, perovskites are very unstable against heat, moisture, and light, which makes them almost impossible to use in practical applications. To solve these problems, many researchers have attempted to physically prevent perovskites from coming into contact with water molecules by passivating the perovskite grain and nanoparticle surfaces with organic ligands or inorganic shell materials, or by fabricating perovskite-polymer nanocomposites. These methods require complex processes and have limited stability in ambient air and water. Furthermore, stable perovskite nanoparticles in the various chemical environments and high temperatures with high humidity have not been reported yet.
The research team in collaboration with Seoul National University develops siloxane-encapsulated perovskite nanoparticle composite films. Here, perovskite nanoparticles are chemically crosslinked with thermally stable siloxane molecules, thereby significantly improving the stability of the perovskite nanoparticles without the need for any additional protecting layer.
Siloxane-encapsulated perovskite nanoparticle composite films exhibited a high PLQY (> 70%) value, which can be maintained over 600 days in water, various chemicals (alcohol, strong acidic and basic solutions), and high temperatures with high humidity (85℃/85%). The research team investigated the mechanisms impacting the chemical crosslinking and water molecule-induced stabilization of perovskite nanoparticles through various photo-physical analysis and density-functional theory calculation.
The research team confirmed that displays based on their siloxane-perovskite nanoparticle composite films exhibited higher PLQY and a wider color gamut than those of Cd-based quantum dots and demonstrated perfect color converting properties on commercial mobile phone screens. Unlike what was commonly believed in the halide perovskite field, the composite films showed excellent bio-compatibility because the siloxane matrix prevents the toxicity of Pb in perovskite nanoparticle.
By using this technology, the instability of perovskite materials, which is the biggest challenge for practical applications, is greatly improved through simple encapsulation method.
“Perovskite nanoparticle is the only photoluminescent material that can meet the next generation display color standard. Nevertheless, there has been reluctant to commercialize it due to its moisture vulnerability. The newly developed siloxane encapsulation technology will trigger more research on perovskite nanoparticles as color conversion materials and will accelerate early commercialization,” Professor Bae said.
This work was supported by the Wearable Platform Materials Technology Center (WMC) of the Engineering Research Center (ERC) Project, and the Leadership Research Program funded by the National Research Foundation of Korea.
-Publication:
Junho Jang, Young-Hoon Kim, Sunjoon Park, Dongsuk Yoo, Hyunjin Cho, Jinhyeong Jang, Han Beom Jeong, Hyunhwan Lee, Jong Min Yuk, Chan Beum Park, Duk Young Jeon, Yong-Hyun Kim, Byeong-Soo Bae, and Tae-Woo Lee. “Extremely Stable Luminescent Crosslinked Perovskite Nanoparticles under Harsh Environments over 1.5 Years” Advanced Materials, 2020, 2005255. https://doi.org/10.1002/adma.202005255.
Link to download the full-text paper:
https://onlinelibrary.wiley.com/doi/10.1002/adma.202005255
-Profile: Prof. Byeong-Soo Bae (Corresponding author)
bsbae@kaist.ac.kr
Lab. of Optical Materials & Coating
Department of Materials Science and Engineering
Korea Advanced Institute of Science and Technology (KAIST)
2020.12.29 View 15147 -
 Electrosprayed Micro Droplets Help Kill Bacteria and Viruses
With COVID-19 raging around the globe, researchers are doubling down on methods for developing diverse antimicrobial technologies that could be effective in killing a virus, but harmless to humans and the environment.
A recent study by a KAIST research team will be one of the responses to such efforts. Professor Seung Seob Lee and Dr. Ji-hun Jeong from the Department of Mechanical Engineering developed a harmless air sterilization prototype featuring electrosprayed water from a polymer micro-nozzle array. This study is one of the projects being supported by the KAIST New Deal R&D Initiative in response to COVID-19. Their study was reported in Polymer.
The electrosprayed microdroplets encapsulate reactive oxygen species such as hydroxyl radicals, superoxides that are known to have an antimicrobial function. The encapsulation prolongs the life of reactive oxygen species, which enable the droplets to perform their antimicrobial function effectively. Prior research has already proven the antimicrobial and encapsulation effects of electrosprayed droplets.
Despite its potential for antimicrobial applications, electrosprayed water generally operates under an electrical discharge condition, which can generate ozone. The inhalation of ozone is known to cause damage to the respiratory system of humans. Another technical barrier for electrospraying is the low flow rate problem. Since electrospraying exhibits the dependence of droplet size on the flow rate, there is a limit for the amount of water microdroplets a single nozzle can produce.
With this in mind, the research team developed a dielectric polymer micro-nozzle array to perform the multiplexed electrospraying of water without electrical discharge. The polymer micro-nozzle array was fabricated using the MEMS (Micro Electro-Mechanical System) process. According to the research team, the nozzle can carry five to 19 micro-nozzles depending on the required application.
The high aspect ratio of the micro-nozzle and an in-plane extractor were proposed to concentrate the electric field at the tip of the micro-nozzle, which prevents the electrical discharge caused by the high surface tension of water. A micro-pillar array with a hydrophobic coating around the micro-nozzle was also proposed to prevent the wetting of the micro-nozzle array.
The polymer micro-nozzle array performed in steady cone jet mode without electrical discharge as confirmed by high-speed imaging and nanosecond pulsed imaging. The water microdroplets were measured to be in the range of six to 10 μm and displayed an antimicrobial effect on Escherichia coli and Staphylococcus aureus.
Professor Lee said, “We believe that this research can be applied to air conditioning products in areas that require antimicrobial and humidifying functions.”
Publication:
Jeong, J. H., et al. (2020) Polymer micro-atomizer for water electrospray in the cone jet mode. Polymer. Vol. No. 194, 122405. Available online at https://doi.org/10.1016/j.polymer.2020.122405
Profile: Seung Seob Lee, Ph.D.
sslee97@kaist.ac.kr
http://mmst.kaist.ac.kr/
Professor
Department of Mechanical Engineering (ME)
Korea Advanced Institute of Science and Technology (KAIST)
https://www.kaist.ac.kr
Daejeon 34141, Korea
Profile: Ji-hun Jeong, Ph.D.
jiuni6022@kaist.ac.kr
Postdoctoral researcher
Department of Mechanical Engineering (ME)
Korea Advanced Institute of Science and Technology (KAIST)
https://www.kaist.ac.kr
Daejeon 34141, Korea
(END)
2020.12.21 View 15422
Electrosprayed Micro Droplets Help Kill Bacteria and Viruses
With COVID-19 raging around the globe, researchers are doubling down on methods for developing diverse antimicrobial technologies that could be effective in killing a virus, but harmless to humans and the environment.
A recent study by a KAIST research team will be one of the responses to such efforts. Professor Seung Seob Lee and Dr. Ji-hun Jeong from the Department of Mechanical Engineering developed a harmless air sterilization prototype featuring electrosprayed water from a polymer micro-nozzle array. This study is one of the projects being supported by the KAIST New Deal R&D Initiative in response to COVID-19. Their study was reported in Polymer.
The electrosprayed microdroplets encapsulate reactive oxygen species such as hydroxyl radicals, superoxides that are known to have an antimicrobial function. The encapsulation prolongs the life of reactive oxygen species, which enable the droplets to perform their antimicrobial function effectively. Prior research has already proven the antimicrobial and encapsulation effects of electrosprayed droplets.
Despite its potential for antimicrobial applications, electrosprayed water generally operates under an electrical discharge condition, which can generate ozone. The inhalation of ozone is known to cause damage to the respiratory system of humans. Another technical barrier for electrospraying is the low flow rate problem. Since electrospraying exhibits the dependence of droplet size on the flow rate, there is a limit for the amount of water microdroplets a single nozzle can produce.
With this in mind, the research team developed a dielectric polymer micro-nozzle array to perform the multiplexed electrospraying of water without electrical discharge. The polymer micro-nozzle array was fabricated using the MEMS (Micro Electro-Mechanical System) process. According to the research team, the nozzle can carry five to 19 micro-nozzles depending on the required application.
The high aspect ratio of the micro-nozzle and an in-plane extractor were proposed to concentrate the electric field at the tip of the micro-nozzle, which prevents the electrical discharge caused by the high surface tension of water. A micro-pillar array with a hydrophobic coating around the micro-nozzle was also proposed to prevent the wetting of the micro-nozzle array.
The polymer micro-nozzle array performed in steady cone jet mode without electrical discharge as confirmed by high-speed imaging and nanosecond pulsed imaging. The water microdroplets were measured to be in the range of six to 10 μm and displayed an antimicrobial effect on Escherichia coli and Staphylococcus aureus.
Professor Lee said, “We believe that this research can be applied to air conditioning products in areas that require antimicrobial and humidifying functions.”
Publication:
Jeong, J. H., et al. (2020) Polymer micro-atomizer for water electrospray in the cone jet mode. Polymer. Vol. No. 194, 122405. Available online at https://doi.org/10.1016/j.polymer.2020.122405
Profile: Seung Seob Lee, Ph.D.
sslee97@kaist.ac.kr
http://mmst.kaist.ac.kr/
Professor
Department of Mechanical Engineering (ME)
Korea Advanced Institute of Science and Technology (KAIST)
https://www.kaist.ac.kr
Daejeon 34141, Korea
Profile: Ji-hun Jeong, Ph.D.
jiuni6022@kaist.ac.kr
Postdoctoral researcher
Department of Mechanical Engineering (ME)
Korea Advanced Institute of Science and Technology (KAIST)
https://www.kaist.ac.kr
Daejeon 34141, Korea
(END)
2020.12.21 View 15422 -
 Three Professors Named to Highly Cited Researchers 2020 List
Distinguished Professor Sukbok Chang from the Department of Chemistry, Distinguished Professor Sang-Yup Lee from the Department of Chemical & Biomolecular Engineering, and Professor Jiyong Eom from the College of Business were named to Clarivate’s Highly Cited Researchers 2020 list.
Clarivate announced the researchers who rank in the top 1% of citations by field and publication year in the Web of Science citation index. A total of 6,167 researchers from more than 60 countries were listed this year and 37 Korean scholars made the list.
The methodology that determines the “Who’s Who” of influential researchers draws on data and analyses performed by bibliometric experts and data scientists at the Institute for Scientific Information at Clarivate. It also uses the tallies to identify the countries and research institutions where these scientific elite are based. More than 6,000 researchers from 21 fields in the sciences, social sciences, and cross field categories were selected based on the number of highly cited papers they produced over an 11-year period from January 2009 to December 2019.
Professor Chang made the list six years in a row, while Professor Lee made it for four consecutive years, and Professor Eom for the last two years. Professor Chang’s group (http://sbchang.kaist.ac.kr) investigates catalytic hydrocarbon functionalization. Professor Lee (http://mbel.kaist.ac.kr) is a pioneering scholar in the field of metabolic engineering, systems, and synthetic biology. Professor Eom’s (https://kaistceps.quv.kr) research extends to energy and environmental economics and management, energy big data, and green information systems.
2020.11.30 View 10776
Three Professors Named to Highly Cited Researchers 2020 List
Distinguished Professor Sukbok Chang from the Department of Chemistry, Distinguished Professor Sang-Yup Lee from the Department of Chemical & Biomolecular Engineering, and Professor Jiyong Eom from the College of Business were named to Clarivate’s Highly Cited Researchers 2020 list.
Clarivate announced the researchers who rank in the top 1% of citations by field and publication year in the Web of Science citation index. A total of 6,167 researchers from more than 60 countries were listed this year and 37 Korean scholars made the list.
The methodology that determines the “Who’s Who” of influential researchers draws on data and analyses performed by bibliometric experts and data scientists at the Institute for Scientific Information at Clarivate. It also uses the tallies to identify the countries and research institutions where these scientific elite are based. More than 6,000 researchers from 21 fields in the sciences, social sciences, and cross field categories were selected based on the number of highly cited papers they produced over an 11-year period from January 2009 to December 2019.
Professor Chang made the list six years in a row, while Professor Lee made it for four consecutive years, and Professor Eom for the last two years. Professor Chang’s group (http://sbchang.kaist.ac.kr) investigates catalytic hydrocarbon functionalization. Professor Lee (http://mbel.kaist.ac.kr) is a pioneering scholar in the field of metabolic engineering, systems, and synthetic biology. Professor Eom’s (https://kaistceps.quv.kr) research extends to energy and environmental economics and management, energy big data, and green information systems.
2020.11.30 View 10776