bio
-
 Astrocytes Eat Connections to Maintain Plasticity in Adult Brains
Developing brains constantly sprout new neuronal connections called synapses as they learn and remember. Important connections — the ones that are repeatedly introduced, such as how to avoid danger — are nurtured and reinforced, while connections deemed unnecessary are pruned away. Adult brains undergo similar pruning, but it was unclear how or why synapses in the adult brain get eliminated.
Now, a team of KAIST researchers has found the mechanism underlying plasticity and, potentially, neurological disorders in adult brains. They published their findings on December 23 in Nature.
“Our findings have profound implications for our understanding of how neural circuits change during learning and memory, as well as in diseases,” said paper author Won-Suk Chung, an assistant professor in the Department of Biological Sciences at KAIST. “Changes in synapse number have strong association with the prevalence of various neurological disorders, such as autism spectrum disorder, schizophrenia, frontotemporal dementia, and several forms of seizures.”
Gray matter in the brain contains microglia and astrocytes, two complementary cells that, among other things, support neurons and synapses. Microglial are a frontline immunity defense, responsible for eating pathogens and dead cells, and astrocytes are star-shaped cells that help structure the brain and maintain homeostasis by helping to control signaling between neurons. According to Professor Chung, it is generally thought that microglial eat synapses as part of its clean-up effort in a process known as phagocytosis.
“Using novel tools, we show that, for the first time, it is astrocytes and not microglia that constantly eliminate excessive and unnecessary adult excitatory synaptic connections in response to neuronal activity,” Professor Chung said. “Our paper challenges the general consensus in this field that microglia are the primary synapse phagocytes that control synapse numbers in the brain.”
Professor Chung and his team developed a molecular sensor to detect synapse elimination by glial cells and quantified how often and by which type of cell synapses were eliminated. They also deployed it in a mouse model without MEGF10, the gene that allows astrocytes to eliminate synapses. Adult animals with this defective astrocytic phagocytosis had unusually increased excitatory synapse numbers in the hippocampus. Through a collaboration with Dr. Hyungju Park at KBRI, they showed that these increased excitatory synapses are functionally impaired, which cause defective learning and memory formation in MEGF10 deleted animals.
“Through this process, we show that, at least in the adult hippocampal CA1 region, astrocytes are the major player in eliminating synapses, and this astrocytic function is essential for controlling synapse number and plasticity,” Chung said.
Professor Chung noted that researchers are only beginning to understand how synapse elimination affects maturation and homeostasis in the brain. In his group’s preliminary data in other brain regions, it appears that each region has different rates of synaptic elimination by astrocytes. They suspect a variety of internal and external factors are influencing how astrocytes modulate each regional circuit, and plan to elucidate these variables.
“Our long-term goal is understanding how astrocyte-mediated synapse turnover affects the initiation and progression of various neurological disorders,” Professor Chung said. “It is intriguing to postulate that modulating astrocytic phagocytosis to restore synaptic connectivity may be a novel strategy in treating various brain disorders.”
This work was supported by the Samsung Science & Technology Foundation, the National Research Foundation of Korea, and the Korea Brain Research Institute basic research program.
Other contributors include Joon-Hyuk Lee and Se Young Lee, Department of Biological Sciences, Korea Advanced Institute of Science and Technology (KAIST); Ji-young Kim, Hyoeun Lee and Hyungju Park; Research Group for Neurovascular Unit, Korea Brain Research Institute (KBRI); Seulgi Noh, and Ji Young Mun, Research Group for Neural Circuit, KBRI. Kim, Noh and Park are also affiliated with the Department of Brain and Cognitive Sciences, Daegu Gyeongbuk Institute of Science and Technology (DGIST).
-Profile
Professor Won-Suk Chung
Department of Biological Sciences
Gliabiology Lab (https://www.kaistglia.org/)
KAIST
-Publication
"Astrocytes phagocytose adult hippocampal synapses for circuit homeostasis"
https://doi.org/10.1038/s41586-020-03060-3
2020.12.24 View 12824
Astrocytes Eat Connections to Maintain Plasticity in Adult Brains
Developing brains constantly sprout new neuronal connections called synapses as they learn and remember. Important connections — the ones that are repeatedly introduced, such as how to avoid danger — are nurtured and reinforced, while connections deemed unnecessary are pruned away. Adult brains undergo similar pruning, but it was unclear how or why synapses in the adult brain get eliminated.
Now, a team of KAIST researchers has found the mechanism underlying plasticity and, potentially, neurological disorders in adult brains. They published their findings on December 23 in Nature.
“Our findings have profound implications for our understanding of how neural circuits change during learning and memory, as well as in diseases,” said paper author Won-Suk Chung, an assistant professor in the Department of Biological Sciences at KAIST. “Changes in synapse number have strong association with the prevalence of various neurological disorders, such as autism spectrum disorder, schizophrenia, frontotemporal dementia, and several forms of seizures.”
Gray matter in the brain contains microglia and astrocytes, two complementary cells that, among other things, support neurons and synapses. Microglial are a frontline immunity defense, responsible for eating pathogens and dead cells, and astrocytes are star-shaped cells that help structure the brain and maintain homeostasis by helping to control signaling between neurons. According to Professor Chung, it is generally thought that microglial eat synapses as part of its clean-up effort in a process known as phagocytosis.
“Using novel tools, we show that, for the first time, it is astrocytes and not microglia that constantly eliminate excessive and unnecessary adult excitatory synaptic connections in response to neuronal activity,” Professor Chung said. “Our paper challenges the general consensus in this field that microglia are the primary synapse phagocytes that control synapse numbers in the brain.”
Professor Chung and his team developed a molecular sensor to detect synapse elimination by glial cells and quantified how often and by which type of cell synapses were eliminated. They also deployed it in a mouse model without MEGF10, the gene that allows astrocytes to eliminate synapses. Adult animals with this defective astrocytic phagocytosis had unusually increased excitatory synapse numbers in the hippocampus. Through a collaboration with Dr. Hyungju Park at KBRI, they showed that these increased excitatory synapses are functionally impaired, which cause defective learning and memory formation in MEGF10 deleted animals.
“Through this process, we show that, at least in the adult hippocampal CA1 region, astrocytes are the major player in eliminating synapses, and this astrocytic function is essential for controlling synapse number and plasticity,” Chung said.
Professor Chung noted that researchers are only beginning to understand how synapse elimination affects maturation and homeostasis in the brain. In his group’s preliminary data in other brain regions, it appears that each region has different rates of synaptic elimination by astrocytes. They suspect a variety of internal and external factors are influencing how astrocytes modulate each regional circuit, and plan to elucidate these variables.
“Our long-term goal is understanding how astrocyte-mediated synapse turnover affects the initiation and progression of various neurological disorders,” Professor Chung said. “It is intriguing to postulate that modulating astrocytic phagocytosis to restore synaptic connectivity may be a novel strategy in treating various brain disorders.”
This work was supported by the Samsung Science & Technology Foundation, the National Research Foundation of Korea, and the Korea Brain Research Institute basic research program.
Other contributors include Joon-Hyuk Lee and Se Young Lee, Department of Biological Sciences, Korea Advanced Institute of Science and Technology (KAIST); Ji-young Kim, Hyoeun Lee and Hyungju Park; Research Group for Neurovascular Unit, Korea Brain Research Institute (KBRI); Seulgi Noh, and Ji Young Mun, Research Group for Neural Circuit, KBRI. Kim, Noh and Park are also affiliated with the Department of Brain and Cognitive Sciences, Daegu Gyeongbuk Institute of Science and Technology (DGIST).
-Profile
Professor Won-Suk Chung
Department of Biological Sciences
Gliabiology Lab (https://www.kaistglia.org/)
KAIST
-Publication
"Astrocytes phagocytose adult hippocampal synapses for circuit homeostasis"
https://doi.org/10.1038/s41586-020-03060-3
2020.12.24 View 12824 -
 Researchers Report Longest-lived Aqueous Flow Batteries
New technology to overcome the life limit of next-generation water-cell batteries
A research team led by Professor Hee-Tak Kim from the Department of Chemical and Biomolecular Engineering has developed water-based zinc/bromine redox flow batteries (ZBBs) with the best life expectancy among all the redox flow batteries reported by identifying and solving the deterioration issue with zinc electrodes.
Professor Kim, head of the Advanced Battery Center at KAIST's Nano-fusion Research Institute, said, "We presented a new technology to overcome the life limit of next-generation water-cell batteries. Not only is it cheaper than conventional lithium-ion batteries, but it can contribute to the expansion of renewable energy and the safe supply of energy storage systems that can run with more than 80 percent energy efficiency."
ZBBs were found to have stable life spans of more than 5,000 cycles, even at a high current density of 100 mA/cm2. It was also confirmed that it represented the highest output and life expectancy compared to Redox flow batteries (RFBs) reported worldwide, which use other redox couples such as zinc-bromine, zinc-iodine, zinc-iron, and vanadium.
Recently, more attention has been focused on energy storage system (ESS) that can improve energy utilization efficiency by storing new and late-night power in large quantities and supplying it to the grid if necessary to supplement the intermittent nature of renewable energy and meet peak power demand.
However, lithium-ion batteries (LIBs), which are currently the core technology of ESSs, have been criticized for not being suitable for ESSs, which store large amounts of electricity due to their inherent risk of ignition and fire. In fact, a total of 33 cases of ESSs using LIBs in Korea had fire accidents, and 35% of all ESS facilities were shut down. This is estimated to have resulted in more than 700 billion won in losses.
As a result, water-based RFBs have drawn great attention. In particular, ZBBs that use ultra-low-cost bromide (ZnBr2) as an active material have been developed for ESSs since the 1970s, with their advantages of high cell voltage, high energy density, and low price compared to other RFBs. Until now, however, the commercialization of ZBBs has been delayed due to the short life span caused by the zinc electrodes. In particular, the uneven "dendrite" growth behavior of zinc metals during the charging and discharging process leads to internal short circuits in the battery which shorten its life.
The research team noted that self-aggregation occurs through the surface diffusion of zinc nuclei on the carbon electrode surface with low surface energy, and determined that self-aggregation was the main cause of zinc dendrite formation through quantum mechanics-based computer simulations and transmission electron microscopy. Furthermore, it was found that the surface diffusion of the zinc nuclei was inhibited in certain carbon fault structures so that dendrites were not produced.
Single vacancy defect, where one carbon atom is removed, exchanges zinc nuclei and electrons, and is strongly coupled, thus inhibiting surface diffusion and enabling uniform nuclear production/growth. The research team applied carbon electrodes with high density fault structure to ZBBs, achieving life characteristics of more than 5,000 cycles at a high charge current density (100 mA/cm2), which is 30 times that of LIBs.
This ESS technology, which can supply eco-friendly electric energy such as renewable energy to the private sector through technology that can drive safe and cheap redox flow batteries for long life, is expected to draw attention once again.
Publication:
Ju-Hyuk Lee, Riyul Kim, Soohyun Kim, Jiyun Heo, Hyeokjin Kwon, Jung Hoon Yang, and Hee-Tak Kim. 2020. Dendrite-free Zn electrodeposition triggered by interatomic orbital hybridization of Zn and single vacancy carbon defects for aqueous Zn-based flow batteries. Energy and Environmental Science, 2020, 13, 2839-2848.
Link to download the full-text paper:http://xlink.rsc.org/?DOI=D0EE00723D
Profile: Prof. Hee-Tak Kimheetak.kim@kaist.ac.krhttp://eed.kaist.ac.krAssociate ProfessorDepartment of Chemical & Biomolecular EngineeringKAIST
2020.12.16 View 14790
Researchers Report Longest-lived Aqueous Flow Batteries
New technology to overcome the life limit of next-generation water-cell batteries
A research team led by Professor Hee-Tak Kim from the Department of Chemical and Biomolecular Engineering has developed water-based zinc/bromine redox flow batteries (ZBBs) with the best life expectancy among all the redox flow batteries reported by identifying and solving the deterioration issue with zinc electrodes.
Professor Kim, head of the Advanced Battery Center at KAIST's Nano-fusion Research Institute, said, "We presented a new technology to overcome the life limit of next-generation water-cell batteries. Not only is it cheaper than conventional lithium-ion batteries, but it can contribute to the expansion of renewable energy and the safe supply of energy storage systems that can run with more than 80 percent energy efficiency."
ZBBs were found to have stable life spans of more than 5,000 cycles, even at a high current density of 100 mA/cm2. It was also confirmed that it represented the highest output and life expectancy compared to Redox flow batteries (RFBs) reported worldwide, which use other redox couples such as zinc-bromine, zinc-iodine, zinc-iron, and vanadium.
Recently, more attention has been focused on energy storage system (ESS) that can improve energy utilization efficiency by storing new and late-night power in large quantities and supplying it to the grid if necessary to supplement the intermittent nature of renewable energy and meet peak power demand.
However, lithium-ion batteries (LIBs), which are currently the core technology of ESSs, have been criticized for not being suitable for ESSs, which store large amounts of electricity due to their inherent risk of ignition and fire. In fact, a total of 33 cases of ESSs using LIBs in Korea had fire accidents, and 35% of all ESS facilities were shut down. This is estimated to have resulted in more than 700 billion won in losses.
As a result, water-based RFBs have drawn great attention. In particular, ZBBs that use ultra-low-cost bromide (ZnBr2) as an active material have been developed for ESSs since the 1970s, with their advantages of high cell voltage, high energy density, and low price compared to other RFBs. Until now, however, the commercialization of ZBBs has been delayed due to the short life span caused by the zinc electrodes. In particular, the uneven "dendrite" growth behavior of zinc metals during the charging and discharging process leads to internal short circuits in the battery which shorten its life.
The research team noted that self-aggregation occurs through the surface diffusion of zinc nuclei on the carbon electrode surface with low surface energy, and determined that self-aggregation was the main cause of zinc dendrite formation through quantum mechanics-based computer simulations and transmission electron microscopy. Furthermore, it was found that the surface diffusion of the zinc nuclei was inhibited in certain carbon fault structures so that dendrites were not produced.
Single vacancy defect, where one carbon atom is removed, exchanges zinc nuclei and electrons, and is strongly coupled, thus inhibiting surface diffusion and enabling uniform nuclear production/growth. The research team applied carbon electrodes with high density fault structure to ZBBs, achieving life characteristics of more than 5,000 cycles at a high charge current density (100 mA/cm2), which is 30 times that of LIBs.
This ESS technology, which can supply eco-friendly electric energy such as renewable energy to the private sector through technology that can drive safe and cheap redox flow batteries for long life, is expected to draw attention once again.
Publication:
Ju-Hyuk Lee, Riyul Kim, Soohyun Kim, Jiyun Heo, Hyeokjin Kwon, Jung Hoon Yang, and Hee-Tak Kim. 2020. Dendrite-free Zn electrodeposition triggered by interatomic orbital hybridization of Zn and single vacancy carbon defects for aqueous Zn-based flow batteries. Energy and Environmental Science, 2020, 13, 2839-2848.
Link to download the full-text paper:http://xlink.rsc.org/?DOI=D0EE00723D
Profile: Prof. Hee-Tak Kimheetak.kim@kaist.ac.krhttp://eed.kaist.ac.krAssociate ProfessorDepartment of Chemical & Biomolecular EngineeringKAIST
2020.12.16 View 14790 -
 A Comprehensive Review of Biosynthesis of Inorganic Nanomaterials Using Microorganisms and Bacteriophages
There are diverse methods for producing numerous inorganic nanomaterials involving many experimental variables. Among the numerous possible matches, finding the best pair for synthesizing in an environmentally friendly way has been a longstanding challenge for researchers and industries.
A KAIST bioprocess engineering research team led by Distinguished Professor Sang Yup Lee conducted a summary of 146 biosynthesized single and multi-element inorganic nanomaterials covering 55 elements in the periodic table synthesized using wild-type and genetically engineered microorganisms. Their research highlights the diverse applications of biogenic nanomaterials and gives strategies for improving the biosynthesis of nanomaterials in terms of their producibility, crystallinity, size, and shape.
The research team described a 10-step flow chart for developing the biosynthesis of inorganic nanomaterials using microorganisms and bacteriophages. The research was published at Nature Review Chemistry as a cover and hero paper on December 3.
“We suggest general strategies for microbial nanomaterial biosynthesis via a step-by-step flow chart and give our perspectives on the future of nanomaterial biosynthesis and applications. This flow chart will serve as a general guide for those wishing to prepare biosynthetic inorganic nanomaterials using microbial cells,” explained Dr.Yoojin Choi, a co-author of this research.
Most inorganic nanomaterials are produced using physical and chemical methods and biological synthesis has been gaining more and more attention. However, conventional synthesis processes have drawbacks in terms of high energy consumption and non-environmentally friendly processes. Meanwhile, microorganisms such as microalgae, yeasts, fungi, bacteria, and even viruses can be utilized as biofactories to produce single and multi-element inorganic nanomaterials under mild conditions.
After conducting a massive survey, the research team summed up that the development of genetically engineered microorganisms with increased inorganic-ion-binding affinity, inorganic-ion-reduction ability, and nanomaterial biosynthetic efficiency has enabled the synthesis of many inorganic nanomaterials.
Among the strategies, the team introduced their analysis of a Pourbaix diagram for controlling the size and morphology of a product. The research team said this Pourbaix diagram analysis can be widely employed for biosynthesizing new nanomaterials with industrial applications.Professor Sang Yup Lee added, “This research provides extensive information and perspectives on the biosynthesis of diverse inorganic nanomaterials using microorganisms and bacteriophages and their applications. We expect that biosynthetic inorganic nanomaterials will find more diverse and innovative applications across diverse fields of science and technology.”
Dr. Choi started this research in 2018 and her interview about completing this extensive research was featured in an article at Nature Career article on December 4.
-ProfileDistinguished Professor Sang Yup Lee leesy@kaist.ac.krMetabolic &Biomolecular Engineering National Research Laboratoryhttp://mbel.kaist.ac.krDepartment of Chemical and Biomolecular EngineeringKAIST
2020.12.07 View 12263
A Comprehensive Review of Biosynthesis of Inorganic Nanomaterials Using Microorganisms and Bacteriophages
There are diverse methods for producing numerous inorganic nanomaterials involving many experimental variables. Among the numerous possible matches, finding the best pair for synthesizing in an environmentally friendly way has been a longstanding challenge for researchers and industries.
A KAIST bioprocess engineering research team led by Distinguished Professor Sang Yup Lee conducted a summary of 146 biosynthesized single and multi-element inorganic nanomaterials covering 55 elements in the periodic table synthesized using wild-type and genetically engineered microorganisms. Their research highlights the diverse applications of biogenic nanomaterials and gives strategies for improving the biosynthesis of nanomaterials in terms of their producibility, crystallinity, size, and shape.
The research team described a 10-step flow chart for developing the biosynthesis of inorganic nanomaterials using microorganisms and bacteriophages. The research was published at Nature Review Chemistry as a cover and hero paper on December 3.
“We suggest general strategies for microbial nanomaterial biosynthesis via a step-by-step flow chart and give our perspectives on the future of nanomaterial biosynthesis and applications. This flow chart will serve as a general guide for those wishing to prepare biosynthetic inorganic nanomaterials using microbial cells,” explained Dr.Yoojin Choi, a co-author of this research.
Most inorganic nanomaterials are produced using physical and chemical methods and biological synthesis has been gaining more and more attention. However, conventional synthesis processes have drawbacks in terms of high energy consumption and non-environmentally friendly processes. Meanwhile, microorganisms such as microalgae, yeasts, fungi, bacteria, and even viruses can be utilized as biofactories to produce single and multi-element inorganic nanomaterials under mild conditions.
After conducting a massive survey, the research team summed up that the development of genetically engineered microorganisms with increased inorganic-ion-binding affinity, inorganic-ion-reduction ability, and nanomaterial biosynthetic efficiency has enabled the synthesis of many inorganic nanomaterials.
Among the strategies, the team introduced their analysis of a Pourbaix diagram for controlling the size and morphology of a product. The research team said this Pourbaix diagram analysis can be widely employed for biosynthesizing new nanomaterials with industrial applications.Professor Sang Yup Lee added, “This research provides extensive information and perspectives on the biosynthesis of diverse inorganic nanomaterials using microorganisms and bacteriophages and their applications. We expect that biosynthetic inorganic nanomaterials will find more diverse and innovative applications across diverse fields of science and technology.”
Dr. Choi started this research in 2018 and her interview about completing this extensive research was featured in an article at Nature Career article on December 4.
-ProfileDistinguished Professor Sang Yup Lee leesy@kaist.ac.krMetabolic &Biomolecular Engineering National Research Laboratoryhttp://mbel.kaist.ac.krDepartment of Chemical and Biomolecular EngineeringKAIST
2020.12.07 View 12263 -
 Simulations Open a New Way to Reverse Cell Aging
Turning off a newly identified enzyme could reverse a natural aging process in cells.
Research findings by a KAIST team provide insight into the complex mechanism of cellular senescence and present a potential therapeutic strategy for reducing age-related diseases associated with the accumulation of senescent cells.
Simulations that model molecular interactions have identified an enzyme that could be targeted to reverse a natural aging process called cellular senescence. The findings were validated with laboratory experiments on skin cells and skin equivalent tissues, and published in the Proceedings of the National Academy of Sciences (PNAS).
“Our research opens the door for a new generation that perceives aging as a reversible biological phenomenon,” says Professor Kwang-Hyun Cho of the Department of Bio and Brain engineering at the Korea Advanced Institute of Science and Technology (KAIST), who led the research with colleagues from KAIST and Amorepacific Corporation in Korea.
Cells respond to a variety of factors, such as oxidative stress, DNA damage, and shortening of the telomeres capping the ends of chromosomes, by entering a stable and persistent exit from the cell cycle. This process, called cellular senescence, is important, as it prevents damaged cells from proliferating and turning into cancer cells. But it is also a natural process that contributes to aging and age-related diseases. Recent research has shown that cellular senescence can be reversed. But the laboratory approaches used thus far also impair tissue regeneration or have the potential to trigger malignant transformations.
Professor Cho and his colleagues used an innovative strategy to identify molecules that could be targeted for reversing cellular senescence. The team pooled together information from the literature and databases about the molecular processes involved in cellular senescence. To this, they added results from their own research on the molecular processes involved in the proliferation, quiescence (a non-dividing cell that can re-enter the cell cycle) and senescence of skin fibroblasts, a cell type well known for repairing wounds. Using algorithms, they developed a model that simulates the interactions between these molecules. Their analyses allowed them to predict which molecules could be targeted to reverse cell senescence.
They then investigated one of the molecules, an enzyme called PDK1, in incubated senescent skin fibroblasts and three-dimensional skin equivalent tissue models. They found that blocking PDK1 led to the inhibition of two downstream signalling molecules, which in turn restored the cells’ ability to enter back into the cell cycle. Notably, the cells retained their capacity to regenerate wounded skin without proliferating in a way that could lead to malignant transformation.
The scientists recommend investigations are next done in organs and organisms to determine the full effect of PDK1 inhibition. Since the gene that codes for PDK1 is overexpressed in some cancers, the scientists expect that inhibiting it will have both anti-aging and anti-cancer effects.
-Profile
Professor Kwang-Hyun Cho
Laboratory for Systems Biology and Bio-Inspired Engineering
http://sbie.kaist.ac.kr
Department of Bio and Brain Engineering
KAIST
2020.11.26 View 14131
Simulations Open a New Way to Reverse Cell Aging
Turning off a newly identified enzyme could reverse a natural aging process in cells.
Research findings by a KAIST team provide insight into the complex mechanism of cellular senescence and present a potential therapeutic strategy for reducing age-related diseases associated with the accumulation of senescent cells.
Simulations that model molecular interactions have identified an enzyme that could be targeted to reverse a natural aging process called cellular senescence. The findings were validated with laboratory experiments on skin cells and skin equivalent tissues, and published in the Proceedings of the National Academy of Sciences (PNAS).
“Our research opens the door for a new generation that perceives aging as a reversible biological phenomenon,” says Professor Kwang-Hyun Cho of the Department of Bio and Brain engineering at the Korea Advanced Institute of Science and Technology (KAIST), who led the research with colleagues from KAIST and Amorepacific Corporation in Korea.
Cells respond to a variety of factors, such as oxidative stress, DNA damage, and shortening of the telomeres capping the ends of chromosomes, by entering a stable and persistent exit from the cell cycle. This process, called cellular senescence, is important, as it prevents damaged cells from proliferating and turning into cancer cells. But it is also a natural process that contributes to aging and age-related diseases. Recent research has shown that cellular senescence can be reversed. But the laboratory approaches used thus far also impair tissue regeneration or have the potential to trigger malignant transformations.
Professor Cho and his colleagues used an innovative strategy to identify molecules that could be targeted for reversing cellular senescence. The team pooled together information from the literature and databases about the molecular processes involved in cellular senescence. To this, they added results from their own research on the molecular processes involved in the proliferation, quiescence (a non-dividing cell that can re-enter the cell cycle) and senescence of skin fibroblasts, a cell type well known for repairing wounds. Using algorithms, they developed a model that simulates the interactions between these molecules. Their analyses allowed them to predict which molecules could be targeted to reverse cell senescence.
They then investigated one of the molecules, an enzyme called PDK1, in incubated senescent skin fibroblasts and three-dimensional skin equivalent tissue models. They found that blocking PDK1 led to the inhibition of two downstream signalling molecules, which in turn restored the cells’ ability to enter back into the cell cycle. Notably, the cells retained their capacity to regenerate wounded skin without proliferating in a way that could lead to malignant transformation.
The scientists recommend investigations are next done in organs and organisms to determine the full effect of PDK1 inhibition. Since the gene that codes for PDK1 is overexpressed in some cancers, the scientists expect that inhibiting it will have both anti-aging and anti-cancer effects.
-Profile
Professor Kwang-Hyun Cho
Laboratory for Systems Biology and Bio-Inspired Engineering
http://sbie.kaist.ac.kr
Department of Bio and Brain Engineering
KAIST
2020.11.26 View 14131 -
 Researchers Control Multiple Wavelengths of Light from a Single Source
KAIST researchers have synthesized a collection of nanoparticles, known as carbon dots, capable of emitting multiple wavelengths of light from a single particle. Additionally, the team discovered that the dispersion of the carbon dots, or the interparticle distance between each dot, influences the properties of the light the carbon dots emit. The discovery will allow researchers to understand how to control these carbon dots and create new, environmentally responsible displays, lighting, and sensing technology.
Research into nanoparticles capable of emitting light, such as quantum dots, has been an active area of interest for the last decade and a half. These particles, or phosphors, are nanoparticles made out of various materials that are capable of emitting light at specific wavelengths by leveraging quantum mechanical properties of the materials. This provides new ways to develop lighting and display solutions as well as more precise detection and sensing in instruments.
As technology becomes smaller and more sophisticated, the usage of fluorescent nanoparticles has seen a dramatic increase in many applications due to the purity of the colors emitting from the dots as well as their tunability to meet desired optical properties.
Carbon dots, a type of fluorescent nanoparticles, have seen an increase in interest from researchers as a candidate to replace non-carbon dots, the construction of which requires heavy metals that are toxic to the environment. Since they are made up of mostly carbon, the low toxicity is an extremely attractive quality when coupled with the tunability of their inherent optical properties.
Another striking feature of carbon dots is their capability to emit multiple wavelengths of light from a single nanoparticle. This multi-wavelength emission can be stimulated under a single excitation source, enabling the simple and robust generation of white light from a single particle by emitting multiple wavelengths simultaneously.
Carbon dots also exhibit a concentration-dependent photoluminescence. In other words, the distance between individual carbon dots affects the light that the carbon dots subsequently emit under an excitation source. These combined properties make carbon dots a unique source that will result in extremely accurate detection and sensing.
This concentration-dependency, however, had not been fully understood. In order to fully utilize the capabilities of carbon dots, the mechanisms that govern the seemingly variable optical properties must first be uncovered. It was previously theorized that the concentration-dependency of carbon dots was due to a hydrogen bonding effect.
Now, a KAIST research team, led by Professor Do Hyun Kim of the Department of Chemical and Biomolecular Engineering has posited and demonstrated that the dual-color-emissiveness is instead due to the interparticle distances between each carbon dot. This study was made available online in June 2020 ahead of final publication in the 36th Issue of Physical Chemistry Chemical Physics on September 28, 2020.
First author of the paper, PhD candidate Hyo Jeong Yoo, along with Professor Kim and researcher Byeong Eun Kwak, examined how the relative light intensity of the red and blue colors changed when varying the interparticle distances, or concentration, of the carbon dots. They found that as the concentration was adjusted, the light emitted from the carbon dots would transform. By varying the concentration, the team was able to control the relative intensity of the colors, as well as emit them simultaneously to generate a white light from a single source (See Figure).
“The concentration-dependence of the photoluminescence of carbon dots on the change of the emissive origins for different interparticle distances has been overlooked in previous research. With the analysis of the dual-color-emission phenomenon of carbon dots, we believe that this result may provide a new perspective to investigate their photoluminescence mechanism,” Yoo explained.
The newly analyzed ability to control the photoluminescence of carbon dots will likely be heavily utilized in the continued development of solid-state lighting applications and sensing.
Publication:
Yoo, H. J., Kwak, B. E., and Kim. D. H. (2020) Interparticle distance as a key factor for controlling the dual-emission properties of carbon dots. Physical Chemistry Chemical Physics, Issue 36, Pages 20227-20237. Available online at https://doi.org/10.1039/d0cp02120b
Profile:
Do Hyun Kim, Sc.D.
Professor
dokim@kaist.ac.kr
http://procal.kaist.ac.kr/
Process Analysis Laboratory
Department of Chemical and Biomolecular Engineering
https://www.kaist.ac.kr
Korea Advanced Institute of Science and Technology (KAIST)Daejeon, Republic of Korea
(END)
2020.11.23 View 11844
Researchers Control Multiple Wavelengths of Light from a Single Source
KAIST researchers have synthesized a collection of nanoparticles, known as carbon dots, capable of emitting multiple wavelengths of light from a single particle. Additionally, the team discovered that the dispersion of the carbon dots, or the interparticle distance between each dot, influences the properties of the light the carbon dots emit. The discovery will allow researchers to understand how to control these carbon dots and create new, environmentally responsible displays, lighting, and sensing technology.
Research into nanoparticles capable of emitting light, such as quantum dots, has been an active area of interest for the last decade and a half. These particles, or phosphors, are nanoparticles made out of various materials that are capable of emitting light at specific wavelengths by leveraging quantum mechanical properties of the materials. This provides new ways to develop lighting and display solutions as well as more precise detection and sensing in instruments.
As technology becomes smaller and more sophisticated, the usage of fluorescent nanoparticles has seen a dramatic increase in many applications due to the purity of the colors emitting from the dots as well as their tunability to meet desired optical properties.
Carbon dots, a type of fluorescent nanoparticles, have seen an increase in interest from researchers as a candidate to replace non-carbon dots, the construction of which requires heavy metals that are toxic to the environment. Since they are made up of mostly carbon, the low toxicity is an extremely attractive quality when coupled with the tunability of their inherent optical properties.
Another striking feature of carbon dots is their capability to emit multiple wavelengths of light from a single nanoparticle. This multi-wavelength emission can be stimulated under a single excitation source, enabling the simple and robust generation of white light from a single particle by emitting multiple wavelengths simultaneously.
Carbon dots also exhibit a concentration-dependent photoluminescence. In other words, the distance between individual carbon dots affects the light that the carbon dots subsequently emit under an excitation source. These combined properties make carbon dots a unique source that will result in extremely accurate detection and sensing.
This concentration-dependency, however, had not been fully understood. In order to fully utilize the capabilities of carbon dots, the mechanisms that govern the seemingly variable optical properties must first be uncovered. It was previously theorized that the concentration-dependency of carbon dots was due to a hydrogen bonding effect.
Now, a KAIST research team, led by Professor Do Hyun Kim of the Department of Chemical and Biomolecular Engineering has posited and demonstrated that the dual-color-emissiveness is instead due to the interparticle distances between each carbon dot. This study was made available online in June 2020 ahead of final publication in the 36th Issue of Physical Chemistry Chemical Physics on September 28, 2020.
First author of the paper, PhD candidate Hyo Jeong Yoo, along with Professor Kim and researcher Byeong Eun Kwak, examined how the relative light intensity of the red and blue colors changed when varying the interparticle distances, or concentration, of the carbon dots. They found that as the concentration was adjusted, the light emitted from the carbon dots would transform. By varying the concentration, the team was able to control the relative intensity of the colors, as well as emit them simultaneously to generate a white light from a single source (See Figure).
“The concentration-dependence of the photoluminescence of carbon dots on the change of the emissive origins for different interparticle distances has been overlooked in previous research. With the analysis of the dual-color-emission phenomenon of carbon dots, we believe that this result may provide a new perspective to investigate their photoluminescence mechanism,” Yoo explained.
The newly analyzed ability to control the photoluminescence of carbon dots will likely be heavily utilized in the continued development of solid-state lighting applications and sensing.
Publication:
Yoo, H. J., Kwak, B. E., and Kim. D. H. (2020) Interparticle distance as a key factor for controlling the dual-emission properties of carbon dots. Physical Chemistry Chemical Physics, Issue 36, Pages 20227-20237. Available online at https://doi.org/10.1039/d0cp02120b
Profile:
Do Hyun Kim, Sc.D.
Professor
dokim@kaist.ac.kr
http://procal.kaist.ac.kr/
Process Analysis Laboratory
Department of Chemical and Biomolecular Engineering
https://www.kaist.ac.kr
Korea Advanced Institute of Science and Technology (KAIST)Daejeon, Republic of Korea
(END)
2020.11.23 View 11844 -
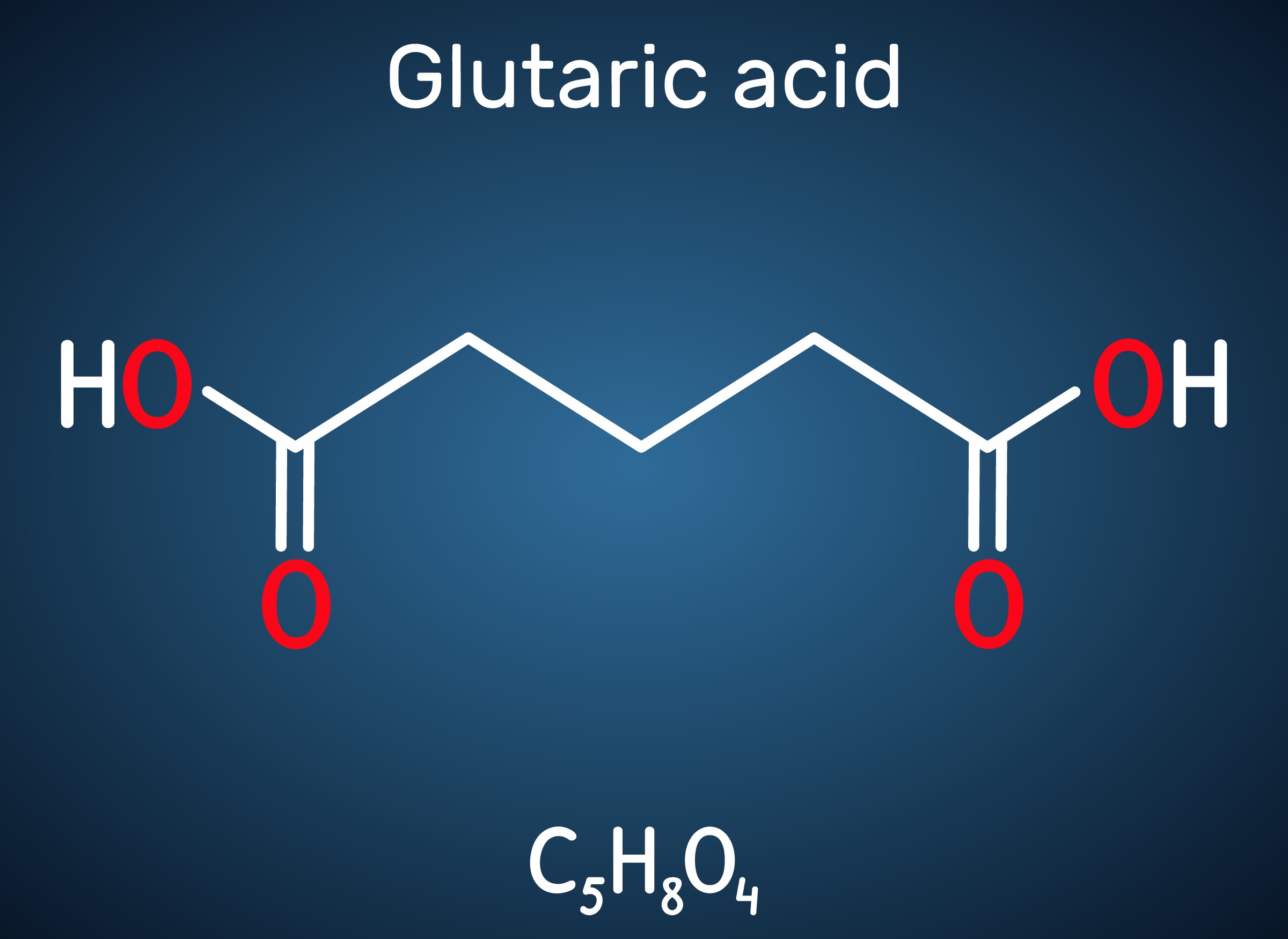 Engineered C. glutamicum Strain Capable of Producing High-Level Glutaric Acid from Glucose
An engineered C. glutamicum strain that can produce the world’s highest titer of glutaric acid was developed by employing systems metabolic engineering strategies
A metabolic engineering research group at KAIST has developed an engineered Corynebacterium glutamicum strain capable of producing high-level glutaric acid without byproducts from glucose. This new strategy will be useful for developing engineered micro-organisms for the bio-based production of value-added chemicals.
Glutaric acid, also known as pentanedioic acid, is a carboxylic acid that is widely used for various applications including the production of polyesters, polyamides, polyurethanes, glutaric anhydride, 1,5-pentanediol, and 5-hydroxyvaleric acid. Glutaric acid has been produced using various petroleum-based chemical methods, relying on non-renewable and toxic starting materials. Thus, various approaches have been taken to biologically produce glutaric acid from renewable resources. Previously, the development of the first glutaric acid producing Escherichia coli by introducing Pseudomonas putida genes was reported by a research group from KAIST, but the titer was low. Glutaric acid production by metabolically engineered Corynebacterium glutamicum has also been reported in several studies, but further improvements in glutaric acid production seemed possible since C. glutamicum has the capability of producing more than 130 g/L of L-lysine.
A research group comprised of Taehee Han, Gi Bae Kim, and Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering addressed this issue. Their research paper “Glutaric acid production by systems metabolic engineering of an L-lysine-overproducing Corynebacterium glutamicum” was published online in PNAS on November 16, 2020.
This research reports the development of a metabolically engineered C. glutamicum strain capable of efficiently producing glutaric acid, starting from an L-lysine overproducer. The following novel strategies and approaches to achieve high-level glutaric acid production were employed. First, metabolic pathways in C. glutamicum were reconstituted for glutaric acid production by introducing P. putida genes. Then, multi-omics analyses including genome, transcriptome, and fluxome were conducted to understand the phenotype of the L-lysine overproducer strain. In addition to systematic understanding of the host strain, gene manipulation targets were predicted by omics analyses and applied for engineering C. glutamicum, which resulted in the development of an engineered strain capable of efficiently producing glutaric acid. Furthermore, the new glutaric acid exporter was discovered for the first time, which was used to further increase glutaric acid production through enhancing product excretion. Last but not least, culture conditions were optimized for high-level glutaric acid production. As a result, the final engineered strain was able to produce 105.3 g/L glutaric acid, the highest titer ever reported, in 69 hours by fed-batch fermentation.
Professor Sang Yup Lee said, “It is meaningful that we were able to develop a highly efficient glutaric acid producer capable of producing glutaric acid at the world’s highest titer without any byproducts from renewable carbon sources. This will further accelerate the bio-based production of valuable chemicals in pharmaceutical/medical/chemical industries.” This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation and funded by the Ministry of Science and ICT.
-Profile
Distinguished Professor Sang Yup Lee
leesy@kaist.ac.kr
http://mbel.kaist.ac.kr
Department of Chemical and Biomolecular Engineering
KAIST
2020.11.17 View 8119
Engineered C. glutamicum Strain Capable of Producing High-Level Glutaric Acid from Glucose
An engineered C. glutamicum strain that can produce the world’s highest titer of glutaric acid was developed by employing systems metabolic engineering strategies
A metabolic engineering research group at KAIST has developed an engineered Corynebacterium glutamicum strain capable of producing high-level glutaric acid without byproducts from glucose. This new strategy will be useful for developing engineered micro-organisms for the bio-based production of value-added chemicals.
Glutaric acid, also known as pentanedioic acid, is a carboxylic acid that is widely used for various applications including the production of polyesters, polyamides, polyurethanes, glutaric anhydride, 1,5-pentanediol, and 5-hydroxyvaleric acid. Glutaric acid has been produced using various petroleum-based chemical methods, relying on non-renewable and toxic starting materials. Thus, various approaches have been taken to biologically produce glutaric acid from renewable resources. Previously, the development of the first glutaric acid producing Escherichia coli by introducing Pseudomonas putida genes was reported by a research group from KAIST, but the titer was low. Glutaric acid production by metabolically engineered Corynebacterium glutamicum has also been reported in several studies, but further improvements in glutaric acid production seemed possible since C. glutamicum has the capability of producing more than 130 g/L of L-lysine.
A research group comprised of Taehee Han, Gi Bae Kim, and Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering addressed this issue. Their research paper “Glutaric acid production by systems metabolic engineering of an L-lysine-overproducing Corynebacterium glutamicum” was published online in PNAS on November 16, 2020.
This research reports the development of a metabolically engineered C. glutamicum strain capable of efficiently producing glutaric acid, starting from an L-lysine overproducer. The following novel strategies and approaches to achieve high-level glutaric acid production were employed. First, metabolic pathways in C. glutamicum were reconstituted for glutaric acid production by introducing P. putida genes. Then, multi-omics analyses including genome, transcriptome, and fluxome were conducted to understand the phenotype of the L-lysine overproducer strain. In addition to systematic understanding of the host strain, gene manipulation targets were predicted by omics analyses and applied for engineering C. glutamicum, which resulted in the development of an engineered strain capable of efficiently producing glutaric acid. Furthermore, the new glutaric acid exporter was discovered for the first time, which was used to further increase glutaric acid production through enhancing product excretion. Last but not least, culture conditions were optimized for high-level glutaric acid production. As a result, the final engineered strain was able to produce 105.3 g/L glutaric acid, the highest titer ever reported, in 69 hours by fed-batch fermentation.
Professor Sang Yup Lee said, “It is meaningful that we were able to develop a highly efficient glutaric acid producer capable of producing glutaric acid at the world’s highest titer without any byproducts from renewable carbon sources. This will further accelerate the bio-based production of valuable chemicals in pharmaceutical/medical/chemical industries.” This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation and funded by the Ministry of Science and ICT.
-Profile
Distinguished Professor Sang Yup Lee
leesy@kaist.ac.kr
http://mbel.kaist.ac.kr
Department of Chemical and Biomolecular Engineering
KAIST
2020.11.17 View 8119 -
 KAIST Showcases Healthcare Technologies at K-Hospital Fair 2020
KAIST Pavilion showcased its innovative medical and healthcare technologies and their advanced applications at the K-Hospital Fair 2020. Five KAIST research groups who teamed up for the Post-COVID-19 New Deal R&D Initiative Project participated in the fair held in Seoul last week.
The K-Hospital Fair is a yearly event organized by the Korean Hospital Association to present the latest research and practical innovations to help the medical industry better serve the patients. This year, 120 healthcare organizations participated in the fair and operated 320 booths.
At the fair, a research group led by Professor Il-Doo Kim from the Department of Materials Science and Engineering demonstrated the manufacturing process of orthogonal nanofibers used to develop their ‘recyclable nano-fiber filtered face mask’ introduced in March of this year. This mask has garnered immense international attention for maintaining its sturdy frame and filtering function even after being washed more than 20 times. Professor Kim is now extending his facilities for the mass production of this mask at his start-up company. While awaiting final approval from the Ministry of Food and Drug Safety to bring his product into the market, Professor Kim is developing other mask variations such as eco-friendly biodegradable masks and transparent masks to aid the hearing-impaired who rely on lip reading to communicate.
The team working under Professor Wonho Choe from the Department of Nuclear and Quantum Engineering presented two low-temperature plasma sterilizers for medical use, co-developed with Plasmapp, a start-up company founded by a KAIST alumnus. Their sterilizers are the first ones that can sterilize medical devices by diffusing hydrogen peroxide vapor into the pouch. They rapidly sterilize medical instruments and materials in just seven minutes without leaving toxic residue, while reducing sterilization time and costs by 90%.
Professor Hyung-Soon Park and his researchers from the Department of Mechanical Engineering introduced a smart protective suit ventilation system that features high cooling capacity and a slimmed-down design. For comfortable use, the suit is equipped with a technique that monitors its inner temperature and humidity and automatically controls its inner circulation accordingly. The group also presented a new system that helps a person in a contaminated suit undress without coming into contact with the contaminated outer part of the suit.
Professor Jong Chul Ye's group from the Department of Bio and Brain Engineering demonstrated AI software that can quickly diagnose an infectious disease based on chest X-ray imaging. The technique compares the differences in the severity of pneumonia in individual patients to distinguish whether their conditions fall under viral pneumonia including COVID-19, bacterial pneumonia, tuberculosis, other diseases, or normal conditions. The AI software visualizes the basis of its reasoning for each of the suspected diseases and provides them as information that can be utilized by medical personnel.
Finally, researchers of Professor Ki-Hun Jeong’s team from the Department of Bio and Brain Engineering demonstrated their ultra-high-speed sub-miniature molecular diagnostic system for the on-site diagnosis of diseases. The existing Polymerase Chain Reaction (PCR) diagnostic usually takes from 30 minutes to an hour to provide results, but their new technique using an LED light source can present results within just three minutes and it is expected to be used actively for on-site diagnosis.
Professor Choongsik Bae, the Director of the Post-COVID-19 New Deal R&D Initiative Project, said, “KAIST will build a healthy relationship amongst researchers, enterprises, and hospitals to contribute to the end of COVID-19 and build a new paradigm of Korean disease prevention and control.”
KAIST launched the Post-COVID-19 New Deal R&D Initiative in July with the support of the Ministry of Science and ICT of Korea. This unit was created to overcome the pandemic crisis by using science and technology, and to contribute to economic development by creating a new antiviral drug industry. The unit is comprised of 464 KAIST members including professors, researchers, and students as well as 503 professionals from enterprises, hospitals, and research centers.
(END)
2020.10.26 View 16016
KAIST Showcases Healthcare Technologies at K-Hospital Fair 2020
KAIST Pavilion showcased its innovative medical and healthcare technologies and their advanced applications at the K-Hospital Fair 2020. Five KAIST research groups who teamed up for the Post-COVID-19 New Deal R&D Initiative Project participated in the fair held in Seoul last week.
The K-Hospital Fair is a yearly event organized by the Korean Hospital Association to present the latest research and practical innovations to help the medical industry better serve the patients. This year, 120 healthcare organizations participated in the fair and operated 320 booths.
At the fair, a research group led by Professor Il-Doo Kim from the Department of Materials Science and Engineering demonstrated the manufacturing process of orthogonal nanofibers used to develop their ‘recyclable nano-fiber filtered face mask’ introduced in March of this year. This mask has garnered immense international attention for maintaining its sturdy frame and filtering function even after being washed more than 20 times. Professor Kim is now extending his facilities for the mass production of this mask at his start-up company. While awaiting final approval from the Ministry of Food and Drug Safety to bring his product into the market, Professor Kim is developing other mask variations such as eco-friendly biodegradable masks and transparent masks to aid the hearing-impaired who rely on lip reading to communicate.
The team working under Professor Wonho Choe from the Department of Nuclear and Quantum Engineering presented two low-temperature plasma sterilizers for medical use, co-developed with Plasmapp, a start-up company founded by a KAIST alumnus. Their sterilizers are the first ones that can sterilize medical devices by diffusing hydrogen peroxide vapor into the pouch. They rapidly sterilize medical instruments and materials in just seven minutes without leaving toxic residue, while reducing sterilization time and costs by 90%.
Professor Hyung-Soon Park and his researchers from the Department of Mechanical Engineering introduced a smart protective suit ventilation system that features high cooling capacity and a slimmed-down design. For comfortable use, the suit is equipped with a technique that monitors its inner temperature and humidity and automatically controls its inner circulation accordingly. The group also presented a new system that helps a person in a contaminated suit undress without coming into contact with the contaminated outer part of the suit.
Professor Jong Chul Ye's group from the Department of Bio and Brain Engineering demonstrated AI software that can quickly diagnose an infectious disease based on chest X-ray imaging. The technique compares the differences in the severity of pneumonia in individual patients to distinguish whether their conditions fall under viral pneumonia including COVID-19, bacterial pneumonia, tuberculosis, other diseases, or normal conditions. The AI software visualizes the basis of its reasoning for each of the suspected diseases and provides them as information that can be utilized by medical personnel.
Finally, researchers of Professor Ki-Hun Jeong’s team from the Department of Bio and Brain Engineering demonstrated their ultra-high-speed sub-miniature molecular diagnostic system for the on-site diagnosis of diseases. The existing Polymerase Chain Reaction (PCR) diagnostic usually takes from 30 minutes to an hour to provide results, but their new technique using an LED light source can present results within just three minutes and it is expected to be used actively for on-site diagnosis.
Professor Choongsik Bae, the Director of the Post-COVID-19 New Deal R&D Initiative Project, said, “KAIST will build a healthy relationship amongst researchers, enterprises, and hospitals to contribute to the end of COVID-19 and build a new paradigm of Korean disease prevention and control.”
KAIST launched the Post-COVID-19 New Deal R&D Initiative in July with the support of the Ministry of Science and ICT of Korea. This unit was created to overcome the pandemic crisis by using science and technology, and to contribute to economic development by creating a new antiviral drug industry. The unit is comprised of 464 KAIST members including professors, researchers, and students as well as 503 professionals from enterprises, hospitals, and research centers.
(END)
2020.10.26 View 16016 -
 Professor Won-Ki Cho Selected as the 2020 SUHF Young Investigator
Professor Won-Ki Cho from the Department of Biological Sciences was named one of three recipients of the 2020 Suh Kyung-Bae Science Foundation (SUHF) Young Investigator Award.
The SUHF is a non-profit organization established in 2016 and funded by a personal donation of 300 billion KRW in shares from Chairman and CEO Kyung-Bae Suh of the Amorepacific Group. The primary purpose of the foundation is to serve as a platform to nurture and provide comprehensive long-term support for creative and passionate young Korean scientists committed to pursuing research in the field of life sciences. The SUHF selects three to five scientists through an open recruiting process every year and grants each scientist a maximum of 2.5 billion KRW over a period of up to five years.
Since January this year, the foundation received 67 research proposals from scientists across the nation, especially from those who had less than five years of experience as professors, and selected the three recipients.
Professor Cho proposed research on how to observe the interactions between nuclear structures and constantly-changing chromatin monomers in four dimensions through ultra-high-resolution imaging of single living cells. This proposal was recognized as one that could help us better understand the process of transcription regulation, which remains a long-standing question in biology.
The other awards were given to Professor Soung-hun Roh of Seoul National University and Professor Joo-Hyeon Lee of the University of Cambridge.
With these three new awardees, a total of 17 scientists have been named SUHF Young Investigators to date, and the funding to support these scientists now totals 42.5 billion KRW.
Professor Inkyung Jung and Professor Ki-Jun Yoon from the Department of Biological Sciences, and Professor Young Seok Ju and Professor Jeong Ho Lee from the Graduate School of Medical Science and Engineering are the four previous winners from KAIST in the years 2017 through 2019.
(END)
2020.10.15 View 14948
Professor Won-Ki Cho Selected as the 2020 SUHF Young Investigator
Professor Won-Ki Cho from the Department of Biological Sciences was named one of three recipients of the 2020 Suh Kyung-Bae Science Foundation (SUHF) Young Investigator Award.
The SUHF is a non-profit organization established in 2016 and funded by a personal donation of 300 billion KRW in shares from Chairman and CEO Kyung-Bae Suh of the Amorepacific Group. The primary purpose of the foundation is to serve as a platform to nurture and provide comprehensive long-term support for creative and passionate young Korean scientists committed to pursuing research in the field of life sciences. The SUHF selects three to five scientists through an open recruiting process every year and grants each scientist a maximum of 2.5 billion KRW over a period of up to five years.
Since January this year, the foundation received 67 research proposals from scientists across the nation, especially from those who had less than five years of experience as professors, and selected the three recipients.
Professor Cho proposed research on how to observe the interactions between nuclear structures and constantly-changing chromatin monomers in four dimensions through ultra-high-resolution imaging of single living cells. This proposal was recognized as one that could help us better understand the process of transcription regulation, which remains a long-standing question in biology.
The other awards were given to Professor Soung-hun Roh of Seoul National University and Professor Joo-Hyeon Lee of the University of Cambridge.
With these three new awardees, a total of 17 scientists have been named SUHF Young Investigators to date, and the funding to support these scientists now totals 42.5 billion KRW.
Professor Inkyung Jung and Professor Ki-Jun Yoon from the Department of Biological Sciences, and Professor Young Seok Ju and Professor Jeong Ho Lee from the Graduate School of Medical Science and Engineering are the four previous winners from KAIST in the years 2017 through 2019.
(END)
2020.10.15 View 14948 -
 E. coli Engineered to Grow on CO₂ and Formic Acid as Sole Carbon Sources
- An E. coli strain that can grow to a relatively high cell density solely on CO₂ and formic acid was developed by employing metabolic engineering. -
Most biorefinery processes have relied on the use of biomass as a raw material for the production of chemicals and materials. Even though the use of CO₂ as a carbon source in biorefineries is desirable, it has not been possible to make common microbial strains such as E. coli grow on CO₂.
Now, a metabolic engineering research group at KAIST has developed a strategy to grow an E. coli strain to higher cell density solely on CO₂ and formic acid. Formic acid is a one carbon carboxylic acid, and can be easily produced from CO₂ using a variety of methods. Since it is easier to store and transport than CO₂, formic acid can be considered a good liquid-form alternative of CO₂.
With support from the C1 Gas Refinery R&D Center and the Ministry of Science and ICT, a research team led by Distinguished Professor Sang Yup Lee stepped up their work to develop an engineered E. coli strain capable of growing up to 11-fold higher cell density than those previously reported, using CO₂ and formic acid as sole carbon sources. This work was published in Nature Microbiology on September 28.
Despite the recent reports by several research groups on the development of E. coli strains capable of growing on CO₂ and formic acid, the maximum cell growth remained too low (optical density of around 1) and thus the production of chemicals from CO₂ and formic acid has been far from realized.
The team previously reported the reconstruction of the tetrahydrofolate cycle and reverse glycine cleavage pathway to construct an engineered E. coli strain that can sustain growth on CO₂ and formic acid. To further enhance the growth, the research team introduced the previously designed synthetic CO₂ and formic acid assimilation pathway, and two formate dehydrogenases.
Metabolic fluxes were also fine-tuned, the gluconeogenic flux enhanced, and the levels of cytochrome bo3 and bd-I ubiquinol oxidase for ATP generation were optimized. This engineered E. coli strain was able to grow to a relatively high OD600 of 7~11, showing promise as a platform strain growing solely on CO₂ and formic acid.
Professor Lee said, “We engineered E. coli that can grow to a higher cell density only using CO₂ and formic acid. We think that this is an important step forward, but this is not the end. The engineered strain we developed still needs further engineering so that it can grow faster to a much higher density.”
Professor Lee’s team is continuing to develop such a strain. “In the future, we would be delighted to see the production of chemicals from an engineered E. coli strain using CO₂ and formic acid as sole carbon sources,” he added.
-Profile:Distinguished Professor Sang Yup Leehttp://mbel.kaist.ac.krDepartment of Chemical and Biomolecular EngineeringKAIST
2020.09.29 View 12543
E. coli Engineered to Grow on CO₂ and Formic Acid as Sole Carbon Sources
- An E. coli strain that can grow to a relatively high cell density solely on CO₂ and formic acid was developed by employing metabolic engineering. -
Most biorefinery processes have relied on the use of biomass as a raw material for the production of chemicals and materials. Even though the use of CO₂ as a carbon source in biorefineries is desirable, it has not been possible to make common microbial strains such as E. coli grow on CO₂.
Now, a metabolic engineering research group at KAIST has developed a strategy to grow an E. coli strain to higher cell density solely on CO₂ and formic acid. Formic acid is a one carbon carboxylic acid, and can be easily produced from CO₂ using a variety of methods. Since it is easier to store and transport than CO₂, formic acid can be considered a good liquid-form alternative of CO₂.
With support from the C1 Gas Refinery R&D Center and the Ministry of Science and ICT, a research team led by Distinguished Professor Sang Yup Lee stepped up their work to develop an engineered E. coli strain capable of growing up to 11-fold higher cell density than those previously reported, using CO₂ and formic acid as sole carbon sources. This work was published in Nature Microbiology on September 28.
Despite the recent reports by several research groups on the development of E. coli strains capable of growing on CO₂ and formic acid, the maximum cell growth remained too low (optical density of around 1) and thus the production of chemicals from CO₂ and formic acid has been far from realized.
The team previously reported the reconstruction of the tetrahydrofolate cycle and reverse glycine cleavage pathway to construct an engineered E. coli strain that can sustain growth on CO₂ and formic acid. To further enhance the growth, the research team introduced the previously designed synthetic CO₂ and formic acid assimilation pathway, and two formate dehydrogenases.
Metabolic fluxes were also fine-tuned, the gluconeogenic flux enhanced, and the levels of cytochrome bo3 and bd-I ubiquinol oxidase for ATP generation were optimized. This engineered E. coli strain was able to grow to a relatively high OD600 of 7~11, showing promise as a platform strain growing solely on CO₂ and formic acid.
Professor Lee said, “We engineered E. coli that can grow to a higher cell density only using CO₂ and formic acid. We think that this is an important step forward, but this is not the end. The engineered strain we developed still needs further engineering so that it can grow faster to a much higher density.”
Professor Lee’s team is continuing to develop such a strain. “In the future, we would be delighted to see the production of chemicals from an engineered E. coli strain using CO₂ and formic acid as sole carbon sources,” he added.
-Profile:Distinguished Professor Sang Yup Leehttp://mbel.kaist.ac.krDepartment of Chemical and Biomolecular EngineeringKAIST
2020.09.29 View 12543 -
 Deep Learning Helps Explore the Structural and Strategic Bases of Autism
Psychiatrists typically diagnose autism spectrum disorders (ASD) by observing a person’s behavior and by leaning on the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), widely considered the “bible” of mental health diagnosis.
However, there are substantial differences amongst individuals on the spectrum and a great deal remains unknown by science about the causes of autism, or even what autism is. As a result, an accurate diagnosis of ASD and a prognosis prediction for patients can be extremely difficult.
But what if artificial intelligence (AI) could help? Deep learning, a type of AI, deploys artificial neural networks based on the human brain to recognize patterns in a way that is akin to, and in some cases can surpass, human ability. The technique, or rather suite of techniques, has enjoyed remarkable success in recent years in fields as diverse as voice recognition, translation, autonomous vehicles, and drug discovery.
A group of researchers from KAIST in collaboration with the Yonsei University College of Medicine has applied these deep learning techniques to autism diagnosis. Their findings were published on August 14 in the journal IEEE Access.
Magnetic resonance imaging (MRI) scans of brains of people known to have autism have been used by researchers and clinicians to try to identify structures of the brain they believed were associated with ASD. These researchers have achieved considerable success in identifying abnormal grey and white matter volume and irregularities in cerebral cortex activation and connections as being associated with the condition.
These findings have subsequently been deployed in studies attempting more consistent diagnoses of patients than has been achieved via psychiatrist observations during counseling sessions. While such studies have reported high levels of diagnostic accuracy, the number of participants in these studies has been small, often under 50, and diagnostic performance drops markedly when applied to large sample sizes or on datasets that include people from a wide variety of populations and locations.
“There was something as to what defines autism that human researchers and clinicians must have been overlooking,” said Keun-Ah Cheon, one of the two corresponding authors and a professor in Department of Child and Adolescent Psychiatry at Severance Hospital of the Yonsei University College of Medicine.
“And humans poring over thousands of MRI scans won’t be able to pick up on what we’ve been missing,” she continued. “But we thought AI might be able to.”
So the team applied five different categories of deep learning models to an open-source dataset of more than 1,000 MRI scans from the Autism Brain Imaging Data Exchange (ABIDE) initiative, which has collected brain imaging data from laboratories around the world, and to a smaller, but higher-resolution MRI image dataset (84 images) taken from the Child Psychiatric Clinic at Severance Hospital, Yonsei University College of Medicine. In both cases, the researchers used both structural MRIs (examining the anatomy of the brain) and functional MRIs (examining brain activity in different regions).
The models allowed the team to explore the structural bases of ASD brain region by brain region, focusing in particular on many structures below the cerebral cortex, including the basal ganglia, which are involved in motor function (movement) as well as learning and memory.
Crucially, these specific types of deep learning models also offered up possible explanations of how the AI had come up with its rationale for these findings.
“Understanding the way that the AI has classified these brain structures and dynamics is extremely important,” said Sang Wan Lee, the other corresponding author and an associate professor at KAIST. “It’s no good if a doctor can tell a patient that the computer says they have autism, but not be able to say why the computer knows that.”
The deep learning models were also able to describe how much a particular aspect contributed to ASD, an analysis tool that can assist psychiatric physicians during the diagnosis process to identify the severity of the autism.
“Doctors should be able to use this to offer a personalized diagnosis for patients, including a prognosis of how the condition could develop,” Lee said.
“Artificial intelligence is not going to put psychiatrists out of a job,” he explained. “But using AI as a tool should enable doctors to better understand and diagnose complex disorders than they could do on their own.”
-ProfileProfessor Sang Wan LeeDepartment of Bio and Brain EngineeringLaboratory for Brain and Machine Intelligence https://aibrain.kaist.ac.kr/
KAIST
2020.09.23 View 12438
Deep Learning Helps Explore the Structural and Strategic Bases of Autism
Psychiatrists typically diagnose autism spectrum disorders (ASD) by observing a person’s behavior and by leaning on the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), widely considered the “bible” of mental health diagnosis.
However, there are substantial differences amongst individuals on the spectrum and a great deal remains unknown by science about the causes of autism, or even what autism is. As a result, an accurate diagnosis of ASD and a prognosis prediction for patients can be extremely difficult.
But what if artificial intelligence (AI) could help? Deep learning, a type of AI, deploys artificial neural networks based on the human brain to recognize patterns in a way that is akin to, and in some cases can surpass, human ability. The technique, or rather suite of techniques, has enjoyed remarkable success in recent years in fields as diverse as voice recognition, translation, autonomous vehicles, and drug discovery.
A group of researchers from KAIST in collaboration with the Yonsei University College of Medicine has applied these deep learning techniques to autism diagnosis. Their findings were published on August 14 in the journal IEEE Access.
Magnetic resonance imaging (MRI) scans of brains of people known to have autism have been used by researchers and clinicians to try to identify structures of the brain they believed were associated with ASD. These researchers have achieved considerable success in identifying abnormal grey and white matter volume and irregularities in cerebral cortex activation and connections as being associated with the condition.
These findings have subsequently been deployed in studies attempting more consistent diagnoses of patients than has been achieved via psychiatrist observations during counseling sessions. While such studies have reported high levels of diagnostic accuracy, the number of participants in these studies has been small, often under 50, and diagnostic performance drops markedly when applied to large sample sizes or on datasets that include people from a wide variety of populations and locations.
“There was something as to what defines autism that human researchers and clinicians must have been overlooking,” said Keun-Ah Cheon, one of the two corresponding authors and a professor in Department of Child and Adolescent Psychiatry at Severance Hospital of the Yonsei University College of Medicine.
“And humans poring over thousands of MRI scans won’t be able to pick up on what we’ve been missing,” she continued. “But we thought AI might be able to.”
So the team applied five different categories of deep learning models to an open-source dataset of more than 1,000 MRI scans from the Autism Brain Imaging Data Exchange (ABIDE) initiative, which has collected brain imaging data from laboratories around the world, and to a smaller, but higher-resolution MRI image dataset (84 images) taken from the Child Psychiatric Clinic at Severance Hospital, Yonsei University College of Medicine. In both cases, the researchers used both structural MRIs (examining the anatomy of the brain) and functional MRIs (examining brain activity in different regions).
The models allowed the team to explore the structural bases of ASD brain region by brain region, focusing in particular on many structures below the cerebral cortex, including the basal ganglia, which are involved in motor function (movement) as well as learning and memory.
Crucially, these specific types of deep learning models also offered up possible explanations of how the AI had come up with its rationale for these findings.
“Understanding the way that the AI has classified these brain structures and dynamics is extremely important,” said Sang Wan Lee, the other corresponding author and an associate professor at KAIST. “It’s no good if a doctor can tell a patient that the computer says they have autism, but not be able to say why the computer knows that.”
The deep learning models were also able to describe how much a particular aspect contributed to ASD, an analysis tool that can assist psychiatric physicians during the diagnosis process to identify the severity of the autism.
“Doctors should be able to use this to offer a personalized diagnosis for patients, including a prognosis of how the condition could develop,” Lee said.
“Artificial intelligence is not going to put psychiatrists out of a job,” he explained. “But using AI as a tool should enable doctors to better understand and diagnose complex disorders than they could do on their own.”
-ProfileProfessor Sang Wan LeeDepartment of Bio and Brain EngineeringLaboratory for Brain and Machine Intelligence https://aibrain.kaist.ac.kr/
KAIST
2020.09.23 View 12438 -
 Before Eyes Open, They Get Ready to See
- Spontaneous retinal waves can generate long-range horizontal connectivity in visual cortex. -
A KAIST research team’s computational simulations demonstrated that the waves of spontaneous neural activity in the retinas of still-closed eyes in mammals develop long-range horizontal connections in the visual cortex during early developmental stages.
This new finding featured in the August 19 edition of Journal of Neuroscience as a cover article has resolved a long-standing puzzle for understanding visual neuroscience regarding the early organization of functional architectures in the mammalian visual cortex before eye-opening, especially the long-range horizontal connectivity known as “feature-specific” circuitry.
To prepare the animal to see when its eyes open, neural circuits in the brain’s visual system must begin developing earlier. However, the proper development of many brain regions involved in vision generally requires sensory input through the eyes.
In the primary visual cortex of the higher mammalian taxa, cortical neurons of similar functional tuning to a visual feature are linked together by long-range horizontal circuits that play a crucial role in visual information processing.
Surprisingly, these long-range horizontal connections in the primary visual cortex of higher mammals emerge before the onset of sensory experience, and the mechanism underlying this phenomenon has remained elusive.
To investigate this mechanism, a group of researchers led by Professor Se-Bum Paik from the Department of Bio and Brain Engineering at KAIST implemented computational simulations of early visual pathways using data obtained from the retinal circuits in young animals before eye-opening, including cats, monkeys, and mice.
From these simulations, the researchers found that spontaneous waves propagating in ON and OFF retinal mosaics can initialize the wiring of long-range horizontal connections by selectively co-activating cortical neurons of similar functional tuning, whereas equivalent random activities cannot induce such organizations.
The simulations also showed that emerged long-range horizontal connections can induce the patterned cortical activities, matching the topography of underlying functional maps even in salt-and-pepper type organizations observed in rodents. This result implies that the model developed by Professor Paik and his group can provide a universal principle for the developmental mechanism of long-range horizontal connections in both higher mammals as well as rodents.
Professor Paik said, “Our model provides a deeper understanding of how the functional architectures in the visual cortex can originate from the spatial organization of the periphery, without sensory experience during early developmental periods.”
He continued, “We believe that our findings will be of great interest to scientists working in a wide range of fields such as neuroscience, vision science, and developmental biology.”
This work was supported by the National Research Foundation of Korea (NRF). Undergraduate student Jinwoo Kim participated in this research project and presented the findings as the lead author as part of the Undergraduate Research Participation (URP) Program at KAIST.
Figures and image credit: Professor Se-Bum Paik, KAIST
Image usage restrictions: News organizations may use or redistribute these figures and image, with proper attribution, as part of news coverage of this paper only.
Publication:
Jinwoo Kim, Min Song, and Se-Bum Paik. (2020). Spontaneous retinal waves generate long-range horizontal connectivity in visual cortex. Journal of Neuroscience, Available online athttps://www.jneurosci.org/content/early/2020/07/17/JNEUROSCI.0649-20.2020
Profile: Se-Bum Paik
Assistant Professor
sbpaik@kaist.ac.kr
http://vs.kaist.ac.kr/
VSNN Laboratory
Department of Bio and Brain Engineering
Program of Brain and Cognitive Engineering
http://kaist.ac.kr
Korea Advanced Institute of Science and Technology (KAIST) Daejeon, Republic of Korea
Profile: Jinwoo Kim
Undergraduate Student
bugkjw@kaist.ac.kr
Department of Bio and Brain Engineering, KAIST
Profile: Min Song
Ph.D. Candidate
night@kaist.ac.kr
Program of Brain and Cognitive Engineering, KAIST
(END)
2020.08.25 View 14579
Before Eyes Open, They Get Ready to See
- Spontaneous retinal waves can generate long-range horizontal connectivity in visual cortex. -
A KAIST research team’s computational simulations demonstrated that the waves of spontaneous neural activity in the retinas of still-closed eyes in mammals develop long-range horizontal connections in the visual cortex during early developmental stages.
This new finding featured in the August 19 edition of Journal of Neuroscience as a cover article has resolved a long-standing puzzle for understanding visual neuroscience regarding the early organization of functional architectures in the mammalian visual cortex before eye-opening, especially the long-range horizontal connectivity known as “feature-specific” circuitry.
To prepare the animal to see when its eyes open, neural circuits in the brain’s visual system must begin developing earlier. However, the proper development of many brain regions involved in vision generally requires sensory input through the eyes.
In the primary visual cortex of the higher mammalian taxa, cortical neurons of similar functional tuning to a visual feature are linked together by long-range horizontal circuits that play a crucial role in visual information processing.
Surprisingly, these long-range horizontal connections in the primary visual cortex of higher mammals emerge before the onset of sensory experience, and the mechanism underlying this phenomenon has remained elusive.
To investigate this mechanism, a group of researchers led by Professor Se-Bum Paik from the Department of Bio and Brain Engineering at KAIST implemented computational simulations of early visual pathways using data obtained from the retinal circuits in young animals before eye-opening, including cats, monkeys, and mice.
From these simulations, the researchers found that spontaneous waves propagating in ON and OFF retinal mosaics can initialize the wiring of long-range horizontal connections by selectively co-activating cortical neurons of similar functional tuning, whereas equivalent random activities cannot induce such organizations.
The simulations also showed that emerged long-range horizontal connections can induce the patterned cortical activities, matching the topography of underlying functional maps even in salt-and-pepper type organizations observed in rodents. This result implies that the model developed by Professor Paik and his group can provide a universal principle for the developmental mechanism of long-range horizontal connections in both higher mammals as well as rodents.
Professor Paik said, “Our model provides a deeper understanding of how the functional architectures in the visual cortex can originate from the spatial organization of the periphery, without sensory experience during early developmental periods.”
He continued, “We believe that our findings will be of great interest to scientists working in a wide range of fields such as neuroscience, vision science, and developmental biology.”
This work was supported by the National Research Foundation of Korea (NRF). Undergraduate student Jinwoo Kim participated in this research project and presented the findings as the lead author as part of the Undergraduate Research Participation (URP) Program at KAIST.
Figures and image credit: Professor Se-Bum Paik, KAIST
Image usage restrictions: News organizations may use or redistribute these figures and image, with proper attribution, as part of news coverage of this paper only.
Publication:
Jinwoo Kim, Min Song, and Se-Bum Paik. (2020). Spontaneous retinal waves generate long-range horizontal connectivity in visual cortex. Journal of Neuroscience, Available online athttps://www.jneurosci.org/content/early/2020/07/17/JNEUROSCI.0649-20.2020
Profile: Se-Bum Paik
Assistant Professor
sbpaik@kaist.ac.kr
http://vs.kaist.ac.kr/
VSNN Laboratory
Department of Bio and Brain Engineering
Program of Brain and Cognitive Engineering
http://kaist.ac.kr
Korea Advanced Institute of Science and Technology (KAIST) Daejeon, Republic of Korea
Profile: Jinwoo Kim
Undergraduate Student
bugkjw@kaist.ac.kr
Department of Bio and Brain Engineering, KAIST
Profile: Min Song
Ph.D. Candidate
night@kaist.ac.kr
Program of Brain and Cognitive Engineering, KAIST
(END)
2020.08.25 View 14579 -
 Sulfur-Containing Polymer Generates High Refractive Index and Transparency
Transparent polymer thin film with refractive index exceeding 1.9 to serve as new platform materials for high-end optical device applications
Researchers reported a novel technology enhancing the high transparency of refractive polymer film via a one-step vapor deposition process. The sulfur-containing polymer (SCP) film produced by Professor Sung Gap Im’s research team at KAIST’s Department of Chemical and Biomolecular Engineering has exhibited excellent environmental stability and chemical resistance, which is highly desirable for its application in long-term optical device applications. The high refractive index exceeding 1.9 while being fully transparent in the entire visible range will help expand the applications of optoelectronic devices.
The refractive index is a ratio of the speed of light in a vacuum to the phase velocity of light in a material, used as a measure of how much the path of light is bent when passing through a material. With the miniaturization of various optical parts used in mobile devices and imaging, demand has been rapidly growing for high refractive index transparent materials that induce more light refraction with a thin film.
As polymers have outstanding physical properties and can be easily processed in various forms, they are widely used in a variety of applications such as plastic eyeglass lenses. However, there have been very few polymers developed so far with a refractive index exceeding 1.75, and existing high refractive index polymers require costly materials and complicated manufacturing processes.
Above all, core technologies for producing such materials have been dominated by Japanese companies, causing long-standing challenges for Korean manufacturers. Securing a stable supply of high-performance, high refractive index materials is crucial for the production of optical devices that are lighter, more affordable, and can be freely manipulated.
The research team successfully manufactured a whole new polymer thin film material with a refractive index exceeding 1.9 and excellent transparency, using just a one-step chemical reaction. The SCP film showed outstanding optical transparency across the entire visible light region, presumably due to the uniformly dispersed, short-segment polysulfide chains, which is a distinct feature unachievable in polymerizations with molten sulfur.
The team focused on the fact that elemental sulfur is easily sublimated to produce a high refractive index polymer by polymerizing the vaporized sulfur with a variety of substances. This method suppresses the formation of overly long S-S chains while achieving outstanding thermal stability in high sulfur concentrations and generating transparent non-crystalline polymers across the entire visible spectrum.
Due to the characteristics of the vapor phase process, the high refractive index thin film can be coated not just on silicon wafers or glass substrates, but on a wide range of textured surfaces as well. We believe this thin film polymer is the first to have achieved an ultrahigh refractive index exceeding 1.9.
Professor Im said, “This high-performance polymer film can be created in a simple one-step manner, which is highly advantageous in the synthesis of SCPs with a high refractive index. This will serve as a platform material for future high-end optical device applications.”
This study, in collaboration with research teams from Seoul National University and Kyung Hee University, was reported in Science Advances. (Title: One-Step Vapor-Phase Synthesis of Transparent High-Refractive Index Sulfur-Containing Polymers)
This research was supported by the Ministry of Science and ICT’s Global Frontier Project (Center for Advanced Soft-Electronics), Leading Research Center Support Program (Wearable Platform Materials Technology Center), and Basic Science Research Program (Advanced Research Project).
2020.08.04 View 10565
Sulfur-Containing Polymer Generates High Refractive Index and Transparency
Transparent polymer thin film with refractive index exceeding 1.9 to serve as new platform materials for high-end optical device applications
Researchers reported a novel technology enhancing the high transparency of refractive polymer film via a one-step vapor deposition process. The sulfur-containing polymer (SCP) film produced by Professor Sung Gap Im’s research team at KAIST’s Department of Chemical and Biomolecular Engineering has exhibited excellent environmental stability and chemical resistance, which is highly desirable for its application in long-term optical device applications. The high refractive index exceeding 1.9 while being fully transparent in the entire visible range will help expand the applications of optoelectronic devices.
The refractive index is a ratio of the speed of light in a vacuum to the phase velocity of light in a material, used as a measure of how much the path of light is bent when passing through a material. With the miniaturization of various optical parts used in mobile devices and imaging, demand has been rapidly growing for high refractive index transparent materials that induce more light refraction with a thin film.
As polymers have outstanding physical properties and can be easily processed in various forms, they are widely used in a variety of applications such as plastic eyeglass lenses. However, there have been very few polymers developed so far with a refractive index exceeding 1.75, and existing high refractive index polymers require costly materials and complicated manufacturing processes.
Above all, core technologies for producing such materials have been dominated by Japanese companies, causing long-standing challenges for Korean manufacturers. Securing a stable supply of high-performance, high refractive index materials is crucial for the production of optical devices that are lighter, more affordable, and can be freely manipulated.
The research team successfully manufactured a whole new polymer thin film material with a refractive index exceeding 1.9 and excellent transparency, using just a one-step chemical reaction. The SCP film showed outstanding optical transparency across the entire visible light region, presumably due to the uniformly dispersed, short-segment polysulfide chains, which is a distinct feature unachievable in polymerizations with molten sulfur.
The team focused on the fact that elemental sulfur is easily sublimated to produce a high refractive index polymer by polymerizing the vaporized sulfur with a variety of substances. This method suppresses the formation of overly long S-S chains while achieving outstanding thermal stability in high sulfur concentrations and generating transparent non-crystalline polymers across the entire visible spectrum.
Due to the characteristics of the vapor phase process, the high refractive index thin film can be coated not just on silicon wafers or glass substrates, but on a wide range of textured surfaces as well. We believe this thin film polymer is the first to have achieved an ultrahigh refractive index exceeding 1.9.
Professor Im said, “This high-performance polymer film can be created in a simple one-step manner, which is highly advantageous in the synthesis of SCPs with a high refractive index. This will serve as a platform material for future high-end optical device applications.”
This study, in collaboration with research teams from Seoul National University and Kyung Hee University, was reported in Science Advances. (Title: One-Step Vapor-Phase Synthesis of Transparent High-Refractive Index Sulfur-Containing Polymers)
This research was supported by the Ministry of Science and ICT’s Global Frontier Project (Center for Advanced Soft-Electronics), Leading Research Center Support Program (Wearable Platform Materials Technology Center), and Basic Science Research Program (Advanced Research Project).
2020.08.04 View 10565