Science
-
 Professor Won-Ki Cho Selected as the 2020 SUHF Young Investigator
Professor Won-Ki Cho from the Department of Biological Sciences was named one of three recipients of the 2020 Suh Kyung-Bae Science Foundation (SUHF) Young Investigator Award.
The SUHF is a non-profit organization established in 2016 and funded by a personal donation of 300 billion KRW in shares from Chairman and CEO Kyung-Bae Suh of the Amorepacific Group. The primary purpose of the foundation is to serve as a platform to nurture and provide comprehensive long-term support for creative and passionate young Korean scientists committed to pursuing research in the field of life sciences. The SUHF selects three to five scientists through an open recruiting process every year and grants each scientist a maximum of 2.5 billion KRW over a period of up to five years.
Since January this year, the foundation received 67 research proposals from scientists across the nation, especially from those who had less than five years of experience as professors, and selected the three recipients.
Professor Cho proposed research on how to observe the interactions between nuclear structures and constantly-changing chromatin monomers in four dimensions through ultra-high-resolution imaging of single living cells. This proposal was recognized as one that could help us better understand the process of transcription regulation, which remains a long-standing question in biology.
The other awards were given to Professor Soung-hun Roh of Seoul National University and Professor Joo-Hyeon Lee of the University of Cambridge.
With these three new awardees, a total of 17 scientists have been named SUHF Young Investigators to date, and the funding to support these scientists now totals 42.5 billion KRW.
Professor Inkyung Jung and Professor Ki-Jun Yoon from the Department of Biological Sciences, and Professor Young Seok Ju and Professor Jeong Ho Lee from the Graduate School of Medical Science and Engineering are the four previous winners from KAIST in the years 2017 through 2019.
(END)
2020.10.15 View 14965
Professor Won-Ki Cho Selected as the 2020 SUHF Young Investigator
Professor Won-Ki Cho from the Department of Biological Sciences was named one of three recipients of the 2020 Suh Kyung-Bae Science Foundation (SUHF) Young Investigator Award.
The SUHF is a non-profit organization established in 2016 and funded by a personal donation of 300 billion KRW in shares from Chairman and CEO Kyung-Bae Suh of the Amorepacific Group. The primary purpose of the foundation is to serve as a platform to nurture and provide comprehensive long-term support for creative and passionate young Korean scientists committed to pursuing research in the field of life sciences. The SUHF selects three to five scientists through an open recruiting process every year and grants each scientist a maximum of 2.5 billion KRW over a period of up to five years.
Since January this year, the foundation received 67 research proposals from scientists across the nation, especially from those who had less than five years of experience as professors, and selected the three recipients.
Professor Cho proposed research on how to observe the interactions between nuclear structures and constantly-changing chromatin monomers in four dimensions through ultra-high-resolution imaging of single living cells. This proposal was recognized as one that could help us better understand the process of transcription regulation, which remains a long-standing question in biology.
The other awards were given to Professor Soung-hun Roh of Seoul National University and Professor Joo-Hyeon Lee of the University of Cambridge.
With these three new awardees, a total of 17 scientists have been named SUHF Young Investigators to date, and the funding to support these scientists now totals 42.5 billion KRW.
Professor Inkyung Jung and Professor Ki-Jun Yoon from the Department of Biological Sciences, and Professor Young Seok Ju and Professor Jeong Ho Lee from the Graduate School of Medical Science and Engineering are the four previous winners from KAIST in the years 2017 through 2019.
(END)
2020.10.15 View 14965 -
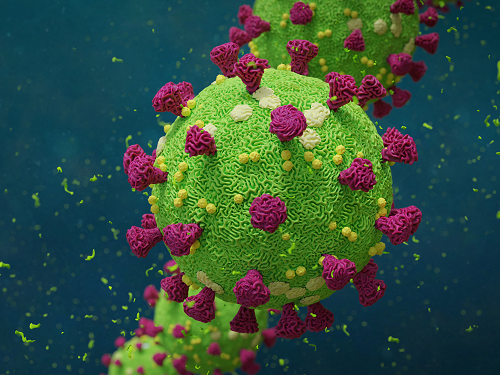 Biomarker Predicts Who Will Have Severe COVID-19
- Airway cell analyses showing an activated immune axis could pinpoint the COVID-19 patients who will most benefit from targeted therapies.-
KAIST researchers have identified key markers that could help pinpoint patients who are bound to get a severe reaction to COVID-19 infection. This would help doctors provide the right treatments at the right time, potentially saving lives. The findings were published in the journal Frontiers in Immunology on August 28.
People’s immune systems react differently to infection with SARS-CoV-2, the virus that causes COVID-19, ranging from mild to severe, life-threatening responses.
To understand the differences in responses, Professor Heung Kyu Lee and PhD candidate Jang Hyun Park from the Graduate School of Medical Science and Engineering at KAIST analysed ribonucleic acid (RNA) sequencing data extracted from individual airway cells of healthy controls and of mildly and severely ill patients with COVID-19. The data was available in a public database previously published by a group of Chinese researchers.
“Our analyses identified an association between immune cells called neutrophils and special cell receptors that bind to the steroid hormone glucocorticoid,” Professor Lee explained. “This finding could be used as a biomarker for predicting disease severity in patients and thus selecting a targeted therapy that can help treat them at an appropriate time,” he added.
Severe illness in COVID-19 is associated with an exaggerated immune response that leads to excessive airway-damaging inflammation. This condition, known as acute respiratory distress syndrome (ARDS), accounts for 70% of deaths in fatal COVID-19 infections.
Scientists already know that this excessive inflammation involves heightened neutrophil recruitment to the airways, but the detailed mechanisms of this reaction are still unclear.
Lee and Park’s analyses found that a group of immune cells called myeloid cells produced excess amounts of neutrophil-recruiting chemicals in severely ill patients, including a cytokine called tumour necrosis factor (TNF) and a chemokine called CXCL8.
Further RNA analyses of neutrophils in severely ill patients showed they were less able to recruit very important T cells needed for attacking the virus. At the same time, the neutrophils produced too many extracellular molecules that normally trap pathogens, but damage airway cells when produced in excess.
The researchers additionally found that the airway cells in severely ill patients were not expressing enough glucocorticoid receptors. This was correlated with increased CXCL8 expression and neutrophil recruitment.
Glucocorticoids, like the well-known drug dexamethasone, are anti-inflammatory agents that could play a role in treating COVID-19. However, using them in early or mild forms of the infection could suppress the necessary immune reactions to combat the virus. But if airway damage has already happened in more severe cases, glucocorticoid treatment would be ineffective.
Knowing who to give this treatment to and when is really important. COVID-19 patients showing reduced glucocorticoid receptor expression, increased CXCL8 expression, and excess neutrophil recruitment to the airways could benefit from treatment with glucocorticoids to prevent airway damage. Further research is needed, however, to confirm the relationship between glucocorticoids and neutrophil inflammation at the protein level.
“Our study could serve as a springboard towards more accurate and reliable COVID-19 treatments,” Professor Lee said.
This work was supported by the National Research Foundation of Korea, and Mobile Clinic Module Project funded by KAIST.
Figure. Low glucocorticoid receptor (GR) expression led to excessive inflammation and lung damage by neutrophils through enhancing the expression of CXCL8 and other cytokines.
Image credit: Professor Heung Kyu Lee, KAIST. Created with Biorender.com.
Image usage restrictions: News organizations may use or redistribute these figures and image, with proper attribution, as part of news coverage of this paper only.
-Publication:
Jang Hyun Park, and Heung Kyu Lee. (2020). Re-analysis of Single Cell Transcriptome Reveals That the NR3C1-CXCL8-Neutrophil Axis Determines the Severity of COVID-19. Frontiers in Immunology, Available online at https://doi.org/10.3389/fimmu.2020.02145
-Profile: Heung Kyu Lee
Associate Professor
heungkyu.lee@kaist.ac.kr
https://www.heungkyulee.kaist.ac.kr/
Laboratory of Host Defenses
Graduate School of Medical Science and Engineering (GSMSE)
The Center for Epidemic Preparedness at KAIST Institute
http://kaist.ac.kr
Korea Advanced Institute of Science and Technology (KAIST)
Daejeon, Republic of Korea
Profile: Jang Hyun Park
PhD Candidate
janghyun.park@kaist.ac.kr
GSMSE, KAIST
2020.09.17 View 18131
Biomarker Predicts Who Will Have Severe COVID-19
- Airway cell analyses showing an activated immune axis could pinpoint the COVID-19 patients who will most benefit from targeted therapies.-
KAIST researchers have identified key markers that could help pinpoint patients who are bound to get a severe reaction to COVID-19 infection. This would help doctors provide the right treatments at the right time, potentially saving lives. The findings were published in the journal Frontiers in Immunology on August 28.
People’s immune systems react differently to infection with SARS-CoV-2, the virus that causes COVID-19, ranging from mild to severe, life-threatening responses.
To understand the differences in responses, Professor Heung Kyu Lee and PhD candidate Jang Hyun Park from the Graduate School of Medical Science and Engineering at KAIST analysed ribonucleic acid (RNA) sequencing data extracted from individual airway cells of healthy controls and of mildly and severely ill patients with COVID-19. The data was available in a public database previously published by a group of Chinese researchers.
“Our analyses identified an association between immune cells called neutrophils and special cell receptors that bind to the steroid hormone glucocorticoid,” Professor Lee explained. “This finding could be used as a biomarker for predicting disease severity in patients and thus selecting a targeted therapy that can help treat them at an appropriate time,” he added.
Severe illness in COVID-19 is associated with an exaggerated immune response that leads to excessive airway-damaging inflammation. This condition, known as acute respiratory distress syndrome (ARDS), accounts for 70% of deaths in fatal COVID-19 infections.
Scientists already know that this excessive inflammation involves heightened neutrophil recruitment to the airways, but the detailed mechanisms of this reaction are still unclear.
Lee and Park’s analyses found that a group of immune cells called myeloid cells produced excess amounts of neutrophil-recruiting chemicals in severely ill patients, including a cytokine called tumour necrosis factor (TNF) and a chemokine called CXCL8.
Further RNA analyses of neutrophils in severely ill patients showed they were less able to recruit very important T cells needed for attacking the virus. At the same time, the neutrophils produced too many extracellular molecules that normally trap pathogens, but damage airway cells when produced in excess.
The researchers additionally found that the airway cells in severely ill patients were not expressing enough glucocorticoid receptors. This was correlated with increased CXCL8 expression and neutrophil recruitment.
Glucocorticoids, like the well-known drug dexamethasone, are anti-inflammatory agents that could play a role in treating COVID-19. However, using them in early or mild forms of the infection could suppress the necessary immune reactions to combat the virus. But if airway damage has already happened in more severe cases, glucocorticoid treatment would be ineffective.
Knowing who to give this treatment to and when is really important. COVID-19 patients showing reduced glucocorticoid receptor expression, increased CXCL8 expression, and excess neutrophil recruitment to the airways could benefit from treatment with glucocorticoids to prevent airway damage. Further research is needed, however, to confirm the relationship between glucocorticoids and neutrophil inflammation at the protein level.
“Our study could serve as a springboard towards more accurate and reliable COVID-19 treatments,” Professor Lee said.
This work was supported by the National Research Foundation of Korea, and Mobile Clinic Module Project funded by KAIST.
Figure. Low glucocorticoid receptor (GR) expression led to excessive inflammation and lung damage by neutrophils through enhancing the expression of CXCL8 and other cytokines.
Image credit: Professor Heung Kyu Lee, KAIST. Created with Biorender.com.
Image usage restrictions: News organizations may use or redistribute these figures and image, with proper attribution, as part of news coverage of this paper only.
-Publication:
Jang Hyun Park, and Heung Kyu Lee. (2020). Re-analysis of Single Cell Transcriptome Reveals That the NR3C1-CXCL8-Neutrophil Axis Determines the Severity of COVID-19. Frontiers in Immunology, Available online at https://doi.org/10.3389/fimmu.2020.02145
-Profile: Heung Kyu Lee
Associate Professor
heungkyu.lee@kaist.ac.kr
https://www.heungkyulee.kaist.ac.kr/
Laboratory of Host Defenses
Graduate School of Medical Science and Engineering (GSMSE)
The Center for Epidemic Preparedness at KAIST Institute
http://kaist.ac.kr
Korea Advanced Institute of Science and Technology (KAIST)
Daejeon, Republic of Korea
Profile: Jang Hyun Park
PhD Candidate
janghyun.park@kaist.ac.kr
GSMSE, KAIST
2020.09.17 View 18131 -
 Microscopy Approach Poised to Offer New Insights into Liver Diseases
Researchers have developed a new way to visualize the progression of nonalcoholic fatty liver disease (NAFLD) in mouse models of the disease. The new microscopy method provides a high-resolution 3D view that could lead to important new insights into NAFLD, a condition in which too much fat is stored in the liver.
“It is estimated that a quarter of the adult global population has NAFLD, yet an effective treatment strategy has not been found,” said professor Pilhan Kim from the Graduate School of Medical Science and Engineering at KAIST. “NAFLD is associated with obesity and type 2 diabetes and can sometimes progress to liver failure in serious case.”
In the Optical Society (OSA) journal Biomedical Optics Express, Professor Kim and colleagues reported their new imaging technique and showed that it can be used to observe how tiny droplets of fat, or lipids, accumulate in the liver cells of living mice over time.
“It has been challenging to find a treatment strategy for NAFLD because most studies examine excised liver tissue that represents just one timepoint in disease progression,” said Professor Kim. “Our technique can capture details of lipid accumulation over time, providing a highly useful research tool for identifying the multiple parameters that likely contribute to the disease and could be targeted with treatment.”
Capturing the dynamics of NAFLD in living mouse models of the disease requires the ability to observe quickly changing interactions of biological components in intact tissue in real-time. To accomplish this, the researchers developed a custom intravital confocal and two-photon microscopy system that acquires images of multiple fluorescent labels at video-rate with cellular resolution.
“With video-rate imaging capability, the continuous movement of liver tissue in live mice due to breathing and heart beating could be tracked in real time and precisely compensated,” said Professor Kim. “This provided motion-artifact free high-resolution images of cellular and sub-cellular sized individual lipid droplets.”
The key to fast imaging was a polygonal mirror that rotated at more than 240 miles per hour to provide extremely fast laser scanning. The researchers also incorporated four different lasers and four high-sensitivity optical detectors into the setup so that they could acquire multi-color images to capture different color fluorescent probes used to label the lipid droplets and microvasculature in the livers of live mice.
“Our approach can capture real-time changes in cell behavior and morphology, vascular structure and function, and the spatiotemporal localization of biological components while directly visualizing of lipid droplet development in NAFLD progression,” said Professor Kim. “It also allows the analysis of the highly complex behaviors of various immune cells as NAFLD progresses.”
The researchers demonstrated their approach by using it to observe the development and spatial distribution of lipid droplets in individual mice with NAFLD induced by a methionine and choline-deficient diet. Next, they plan to use it to study how the liver microenvironment changes during NAFLD progression by imaging the same mouse over time. They also want to use their microscope technique to visualize various immune cells and lipid droplets to better understand the complex liver microenvironment in NAFLD progression.
2020.08.21 View 10585
Microscopy Approach Poised to Offer New Insights into Liver Diseases
Researchers have developed a new way to visualize the progression of nonalcoholic fatty liver disease (NAFLD) in mouse models of the disease. The new microscopy method provides a high-resolution 3D view that could lead to important new insights into NAFLD, a condition in which too much fat is stored in the liver.
“It is estimated that a quarter of the adult global population has NAFLD, yet an effective treatment strategy has not been found,” said professor Pilhan Kim from the Graduate School of Medical Science and Engineering at KAIST. “NAFLD is associated with obesity and type 2 diabetes and can sometimes progress to liver failure in serious case.”
In the Optical Society (OSA) journal Biomedical Optics Express, Professor Kim and colleagues reported their new imaging technique and showed that it can be used to observe how tiny droplets of fat, or lipids, accumulate in the liver cells of living mice over time.
“It has been challenging to find a treatment strategy for NAFLD because most studies examine excised liver tissue that represents just one timepoint in disease progression,” said Professor Kim. “Our technique can capture details of lipid accumulation over time, providing a highly useful research tool for identifying the multiple parameters that likely contribute to the disease and could be targeted with treatment.”
Capturing the dynamics of NAFLD in living mouse models of the disease requires the ability to observe quickly changing interactions of biological components in intact tissue in real-time. To accomplish this, the researchers developed a custom intravital confocal and two-photon microscopy system that acquires images of multiple fluorescent labels at video-rate with cellular resolution.
“With video-rate imaging capability, the continuous movement of liver tissue in live mice due to breathing and heart beating could be tracked in real time and precisely compensated,” said Professor Kim. “This provided motion-artifact free high-resolution images of cellular and sub-cellular sized individual lipid droplets.”
The key to fast imaging was a polygonal mirror that rotated at more than 240 miles per hour to provide extremely fast laser scanning. The researchers also incorporated four different lasers and four high-sensitivity optical detectors into the setup so that they could acquire multi-color images to capture different color fluorescent probes used to label the lipid droplets and microvasculature in the livers of live mice.
“Our approach can capture real-time changes in cell behavior and morphology, vascular structure and function, and the spatiotemporal localization of biological components while directly visualizing of lipid droplet development in NAFLD progression,” said Professor Kim. “It also allows the analysis of the highly complex behaviors of various immune cells as NAFLD progresses.”
The researchers demonstrated their approach by using it to observe the development and spatial distribution of lipid droplets in individual mice with NAFLD induced by a methionine and choline-deficient diet. Next, they plan to use it to study how the liver microenvironment changes during NAFLD progression by imaging the same mouse over time. They also want to use their microscope technique to visualize various immune cells and lipid droplets to better understand the complex liver microenvironment in NAFLD progression.
2020.08.21 View 10585 -
 Tinkering with Roundworm Proteins Offers Hope for Anti-aging Drugs
- The somatic nuclear protein kinase VRK-1 increases the worm’s lifespan through AMPK activation, and this mechanism can be applied to promoting human longevity, the study reveals. -
KAIST researchers have been able to dial up and down creatures’ lifespans by altering the activity of proteins found in roundworm cells that tell them to convert sugar into energy when their cellular energy is running low. Humans also have these proteins, offering up the intriguing possibilities for developing longevity-promoting drugs. These new findings were published on July 1 in Science Advances.
The roundworm Caenorhabditis elegans (C. elegans), a millimeter-long nematode commonly used in lab testing, enjoyed a boost in its lifespan when researchers tinkered with a couple of proteins involved in monitoring the energy use by its cells.
The proteins VRK-1 and AMPK work in tandem in roundworm cells, with the former telling the latter to get to work by sticking a phosphate molecule, composed of one phosphorus and four oxygen atoms, on it. In turn, AMPK’s role is to monitor energy levels in cells, when cellular energy is running low. In essence, VRK-1 regulates AMPK, and AMPK regulates the cellular energy status.
Using a range of different biological research tools, including introducing foreign genes into the worm, a group of researchers led by Professor Seung-Jae V. Lee from the Department of Biological Sciences at KAIST were able to dial up and down the activity of the gene that tells cells to produce the VRK-1 protein. This gene has remained pretty much unchanged throughout evolution. Most complex organisms have this same gene, including humans.
Lead author of the study Sangsoon Park and his colleagues confirmed that the overexpression, or increased production, of the VRK-1 protein boosted the lifespan of the C. elegans, which normally lives just two to three weeks, and the inhibition of VRK-1 production reduced its lifespan.
The research team found that the activity of the VRK-1-to-AMPK cellular-energy monitoring process is increased in low cellular energy status by reduced mitochondrial respiration, the set of metabolic chemical reactions that make use of the oxygen the worm breathes to convert macronutrients from food into the energy “currency” that cells spend to do everything they need to do.
It is already known that mitochondria, the energy-producing engine rooms in cells, play a crucial role in aging, and declines in the functioning of mitochondria are associated with age-related diseases. At the same time, the mild inhibition of mitochondrial respiration has been shown to promote longevity in a range of species, including flies and mammals.
When the research team performed similar tinkering with cultured human cells, they found they could also replicate this ramping up and down of the VRK-1-to-AMPK process that occurs in roundworms.
“This raises the intriguing possibility that VRK-1 also functions as a factor in governing human longevity, and so perhaps we can start developing longevity-promoting drugs that alter the activity of VRK-1,” explained Professor Lee.
At the very least, the research points us in an interesting direction for investigating new therapeutic strategies to combat metabolic disorders by targeting the modulation of VRK-1. Metabolic disorders involve the disruption of chemical reactions in the body, including diseases of the mitochondria.
But before metabolic disorder therapeutics or longevity drugs can be contemplated by scientists, further research still needs to be carried out to better understand how VRK-1 works to activate AMPK, as well as figure out the precise mechanics of how AMPK controls cellular energy.
This work was supported by the National Research Foundation (NRF), and the Ministry of Science and ICT (MSIT) of Korea.
Image credit: Seung-Jae V. LEE, KAIST.
Image usage restrictions: News organizations may use or redistribute this image, with proper attribution, as part of news coverage of this paper only.
Publication:
Park, S., et al. (2020) ‘VRK-1 extends life span by activation of AMPK via phosphorylation’. Science Advances, Volume 6. No. 27, eaaw7824. Available online at https://doi.org/10.1126/sciadv.aaw7824
Profile: Seung-Jae V. Lee, Ph.D.
Professor
seungjaevlee@kaist.ac.kr
https://sites.google.com/view/mgakaist
Molecular Genetics of Aging Laboratory
Department of Biological Sciences
Korea Advanced Institute of Science and Technology (KAIST)
https://www.kaist.ac.krDaejeon 34141, Korea
(END)
2020.07.31 View 13309
Tinkering with Roundworm Proteins Offers Hope for Anti-aging Drugs
- The somatic nuclear protein kinase VRK-1 increases the worm’s lifespan through AMPK activation, and this mechanism can be applied to promoting human longevity, the study reveals. -
KAIST researchers have been able to dial up and down creatures’ lifespans by altering the activity of proteins found in roundworm cells that tell them to convert sugar into energy when their cellular energy is running low. Humans also have these proteins, offering up the intriguing possibilities for developing longevity-promoting drugs. These new findings were published on July 1 in Science Advances.
The roundworm Caenorhabditis elegans (C. elegans), a millimeter-long nematode commonly used in lab testing, enjoyed a boost in its lifespan when researchers tinkered with a couple of proteins involved in monitoring the energy use by its cells.
The proteins VRK-1 and AMPK work in tandem in roundworm cells, with the former telling the latter to get to work by sticking a phosphate molecule, composed of one phosphorus and four oxygen atoms, on it. In turn, AMPK’s role is to monitor energy levels in cells, when cellular energy is running low. In essence, VRK-1 regulates AMPK, and AMPK regulates the cellular energy status.
Using a range of different biological research tools, including introducing foreign genes into the worm, a group of researchers led by Professor Seung-Jae V. Lee from the Department of Biological Sciences at KAIST were able to dial up and down the activity of the gene that tells cells to produce the VRK-1 protein. This gene has remained pretty much unchanged throughout evolution. Most complex organisms have this same gene, including humans.
Lead author of the study Sangsoon Park and his colleagues confirmed that the overexpression, or increased production, of the VRK-1 protein boosted the lifespan of the C. elegans, which normally lives just two to three weeks, and the inhibition of VRK-1 production reduced its lifespan.
The research team found that the activity of the VRK-1-to-AMPK cellular-energy monitoring process is increased in low cellular energy status by reduced mitochondrial respiration, the set of metabolic chemical reactions that make use of the oxygen the worm breathes to convert macronutrients from food into the energy “currency” that cells spend to do everything they need to do.
It is already known that mitochondria, the energy-producing engine rooms in cells, play a crucial role in aging, and declines in the functioning of mitochondria are associated with age-related diseases. At the same time, the mild inhibition of mitochondrial respiration has been shown to promote longevity in a range of species, including flies and mammals.
When the research team performed similar tinkering with cultured human cells, they found they could also replicate this ramping up and down of the VRK-1-to-AMPK process that occurs in roundworms.
“This raises the intriguing possibility that VRK-1 also functions as a factor in governing human longevity, and so perhaps we can start developing longevity-promoting drugs that alter the activity of VRK-1,” explained Professor Lee.
At the very least, the research points us in an interesting direction for investigating new therapeutic strategies to combat metabolic disorders by targeting the modulation of VRK-1. Metabolic disorders involve the disruption of chemical reactions in the body, including diseases of the mitochondria.
But before metabolic disorder therapeutics or longevity drugs can be contemplated by scientists, further research still needs to be carried out to better understand how VRK-1 works to activate AMPK, as well as figure out the precise mechanics of how AMPK controls cellular energy.
This work was supported by the National Research Foundation (NRF), and the Ministry of Science and ICT (MSIT) of Korea.
Image credit: Seung-Jae V. LEE, KAIST.
Image usage restrictions: News organizations may use or redistribute this image, with proper attribution, as part of news coverage of this paper only.
Publication:
Park, S., et al. (2020) ‘VRK-1 extends life span by activation of AMPK via phosphorylation’. Science Advances, Volume 6. No. 27, eaaw7824. Available online at https://doi.org/10.1126/sciadv.aaw7824
Profile: Seung-Jae V. Lee, Ph.D.
Professor
seungjaevlee@kaist.ac.kr
https://sites.google.com/view/mgakaist
Molecular Genetics of Aging Laboratory
Department of Biological Sciences
Korea Advanced Institute of Science and Technology (KAIST)
https://www.kaist.ac.krDaejeon 34141, Korea
(END)
2020.07.31 View 13309 -
 Study Finds Interferon Triggers Inflammation in Severe COVID-19
KAIST medical scientists and their colleagues confirmed that the type I interferon response plays a pivotal role in exacerbating inflammation in severe COVID-19 cases. Severe COVID-19 has been shown to be caused by a hyper-inflammatory response. Particularly, inflammatory cytokines secreted by classical monocytes and macrophages are believed to play a crucial role in the severe progression of COVID-19.
A new single-cell RNA sequencing analysis of more than 59,000 cells from three different patient cohorts provided a detailed look at patients’ immune responses in severe cases of COVID-19. The results suggest that patients with severe cases of COVID-19 experience increased regulation of the type I interferon (IFN-I) inflammation-triggering pathway, a signature that the researchers also observed in patients hospitalized with severe cases of influenza.
Their findings suggest that anti-inflammatory treatment strategies for COVID-19 should also be aimed toward the IFN-I signaling pathway, in addition to targeting inflammatory molecules such as TNF, IL-1, and IL-6, which have been associated with COVID-19.
The research team under Professor Eui-Cheol Shin from the Graduate School of Medical Science and Engineering sequenced the RNA from a total of 59,572 blood cells obtained from four healthy donors, eight patients with mild or severe COVID-19, and five patients with severe influenza.
By comparison, patients with severe cases of influenza showed increased expression of various IFN-stimulated genes, but did not experience TNF/IL-1 responses as seen in COVID-19 patients. Unlike the flu cohort, patients in the severe COVID-19 cohort exhibited the IFN-I signature concurrently with TNF/IL-1-driven inflammation – a combination also not seen in patients with milder cases of COVID-19.
Their result, along with past mouse studies that highlight how the timing of IFN-I expression is critical to determining the outcome of SARS, support targeting IFN-I as a potential treatment strategy for severe COVID-19.
Professor Shin said, “This research provides insights for designing therapeutic options for COVID-19 by investigating very closely how the immune cells of COVDI-19 patients develop. We will continue to conduct research on novel therapeutic immune mechanisms and target therapeutic anti-inflammatory medication to improve the survival of severe COVID-19 patients.”
This study, conducted in collaboration with Severance Hospital at Yonsei University, Asan Medical Center, and Chungbuk National University, was featured in Science Immunology on July 10. This work was funded by Samsung Science and Technology Foundation and SUHF Fellowship.
-PublicationScience Immunology 10 Jul 2020:Vol. 5, Issue 49, eabd1554DOI: 10.1126/sciimmunol.abd1554
-ProfileProfessorEui-Cheol ShinGraduate School of Medical Science and EngineeringLaboratory of Immunology & Infectious Diseases (http://liid.kaist.ac.kr/)euicheols@kaist.ac.krKAIST
2020.07.14 View 9586
Study Finds Interferon Triggers Inflammation in Severe COVID-19
KAIST medical scientists and their colleagues confirmed that the type I interferon response plays a pivotal role in exacerbating inflammation in severe COVID-19 cases. Severe COVID-19 has been shown to be caused by a hyper-inflammatory response. Particularly, inflammatory cytokines secreted by classical monocytes and macrophages are believed to play a crucial role in the severe progression of COVID-19.
A new single-cell RNA sequencing analysis of more than 59,000 cells from three different patient cohorts provided a detailed look at patients’ immune responses in severe cases of COVID-19. The results suggest that patients with severe cases of COVID-19 experience increased regulation of the type I interferon (IFN-I) inflammation-triggering pathway, a signature that the researchers also observed in patients hospitalized with severe cases of influenza.
Their findings suggest that anti-inflammatory treatment strategies for COVID-19 should also be aimed toward the IFN-I signaling pathway, in addition to targeting inflammatory molecules such as TNF, IL-1, and IL-6, which have been associated with COVID-19.
The research team under Professor Eui-Cheol Shin from the Graduate School of Medical Science and Engineering sequenced the RNA from a total of 59,572 blood cells obtained from four healthy donors, eight patients with mild or severe COVID-19, and five patients with severe influenza.
By comparison, patients with severe cases of influenza showed increased expression of various IFN-stimulated genes, but did not experience TNF/IL-1 responses as seen in COVID-19 patients. Unlike the flu cohort, patients in the severe COVID-19 cohort exhibited the IFN-I signature concurrently with TNF/IL-1-driven inflammation – a combination also not seen in patients with milder cases of COVID-19.
Their result, along with past mouse studies that highlight how the timing of IFN-I expression is critical to determining the outcome of SARS, support targeting IFN-I as a potential treatment strategy for severe COVID-19.
Professor Shin said, “This research provides insights for designing therapeutic options for COVID-19 by investigating very closely how the immune cells of COVDI-19 patients develop. We will continue to conduct research on novel therapeutic immune mechanisms and target therapeutic anti-inflammatory medication to improve the survival of severe COVID-19 patients.”
This study, conducted in collaboration with Severance Hospital at Yonsei University, Asan Medical Center, and Chungbuk National University, was featured in Science Immunology on July 10. This work was funded by Samsung Science and Technology Foundation and SUHF Fellowship.
-PublicationScience Immunology 10 Jul 2020:Vol. 5, Issue 49, eabd1554DOI: 10.1126/sciimmunol.abd1554
-ProfileProfessorEui-Cheol ShinGraduate School of Medical Science and EngineeringLaboratory of Immunology & Infectious Diseases (http://liid.kaist.ac.kr/)euicheols@kaist.ac.krKAIST
2020.07.14 View 9586 -
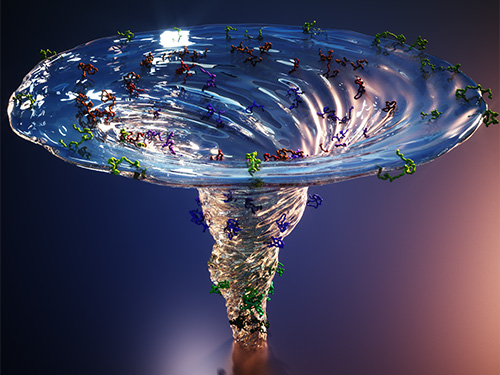 X-ray Scattering Shines Light on Protein Folding
- Multiple forms of a non-functional, unfolded protein follow different pathways and timelines to reach its folded, functional state, a study reveals. -
KAIST researchers have used an X-ray method to track how proteins fold, which could improve computer simulations of this process, with implications for understanding diseases and improving drug discovery. Their findings were reported in the Proceedings of the National Academy of Sciences of the United States of America (PNAS) on June 30.
When proteins are translated from their DNA codes, they quickly transform from a non-functional, unfolded state into their folded, functional state. Problems in folding can lead to diseases like Alzheimer’s and Parkinson’s.
“Protein folding is one of the most important biological processes, as it forms the functioning 3D protein structure,” explained the physical chemist Hyotcherl Ihee of the Department of Chemistry at KAIST. Dr. Tae Wu Kim, the lead author of this research from Ihee’s group, added, “Understanding the mechanisms of protein folding is important, and could pave the way for disease study and drug development.”
Ihee’s team developed an approach using an X-ray scattering technique to uncover how the protein cytochrome c folds from its initial unfolded state. This protein is composed of a chain of 104 amino acids with an iron-containing heme molecule. It is often used for protein folding studies.
The researchers placed the protein in a solution and shined ultraviolet light on it. This process provides electrons to cytochrome c, reducing the iron within it from the ferric to the ferrous form, which initiates folding. As this was happening, the researchers beamed X-rays at very short intervals onto the sample. The X-rays scattered off all the atomic pairs in the sample and a detector continuously recorded the X-ray scattering patterns. The X-ray scattering patterns provided direct information regarding the 3D protein structure and the changes made in these patterns over time showed real-time motion of the protein during the folding process.
The team found cytochrome c proteins initially exist in a wide variety of unfolded states. Once the folding process is triggered, they stop by a group of intermediates within 31.6 microseconds, and then those intermediates follow different pathways with different folding times to reach an energetically stable folded state.
“We don’t know if this diversity in folding paths can be generalized to other proteins,” Ihee confessed. He continued, “However, we believe that our approach can be used to study other protein folding systems.”
Ihee hopes this approach can improve the accuracy of models that simulate protein interactions by including information on their unstructured states. These simulations are important as they can help identify barriers to proper folding and predict a protein’s folded state given its amino acid sequence. Ultimately, the models could help clarify how some diseases develop and how drugs interact with various protein structures.
Ihee’s group collaborated with Professor Young Min Rhee at the KAIST Department of Chemistry, and this work was supported by the National Research Foundation of Korea (NRF) and the Institute for Basic Science (IBS).
Figure. The scientists found that non-functional unfolded forms of the protein cytochrome c follow different pathways and timelines to reach a stable functional folded state.
Publications:
Kim, T. W., et al. (2020) ‘Protein folding from heterogeneous unfolded state revealed by time-resolved X-ray solution scattering’. PNAS. Volume 117. Issue 26. Page 14996-15005. Available online at https://doi.org/10.1073/pnas.1913442117
Profile: Hyotcherl Ihee, Ph.D.
Professor
hyotcherl.ihee@kaist.ac.kr
http://time.kaist.ac.kr/
Ihee Laboratory
Department of Chemistry
KAIST
https://www.kaist.ac.kr
Daejeon 34141, Korea
Profile: Young Min Rhee, Ph.D.
Professor
ymrhee@kaist.ac.kr
http://singlet.kaist.ac.kr
Rhee Research Group
Department of Chemistry
KAIST
https://www.kaist.ac.kr
Daejeon 34141, Korea
(END)
2020.07.09 View 16866
X-ray Scattering Shines Light on Protein Folding
- Multiple forms of a non-functional, unfolded protein follow different pathways and timelines to reach its folded, functional state, a study reveals. -
KAIST researchers have used an X-ray method to track how proteins fold, which could improve computer simulations of this process, with implications for understanding diseases and improving drug discovery. Their findings were reported in the Proceedings of the National Academy of Sciences of the United States of America (PNAS) on June 30.
When proteins are translated from their DNA codes, they quickly transform from a non-functional, unfolded state into their folded, functional state. Problems in folding can lead to diseases like Alzheimer’s and Parkinson’s.
“Protein folding is one of the most important biological processes, as it forms the functioning 3D protein structure,” explained the physical chemist Hyotcherl Ihee of the Department of Chemistry at KAIST. Dr. Tae Wu Kim, the lead author of this research from Ihee’s group, added, “Understanding the mechanisms of protein folding is important, and could pave the way for disease study and drug development.”
Ihee’s team developed an approach using an X-ray scattering technique to uncover how the protein cytochrome c folds from its initial unfolded state. This protein is composed of a chain of 104 amino acids with an iron-containing heme molecule. It is often used for protein folding studies.
The researchers placed the protein in a solution and shined ultraviolet light on it. This process provides electrons to cytochrome c, reducing the iron within it from the ferric to the ferrous form, which initiates folding. As this was happening, the researchers beamed X-rays at very short intervals onto the sample. The X-rays scattered off all the atomic pairs in the sample and a detector continuously recorded the X-ray scattering patterns. The X-ray scattering patterns provided direct information regarding the 3D protein structure and the changes made in these patterns over time showed real-time motion of the protein during the folding process.
The team found cytochrome c proteins initially exist in a wide variety of unfolded states. Once the folding process is triggered, they stop by a group of intermediates within 31.6 microseconds, and then those intermediates follow different pathways with different folding times to reach an energetically stable folded state.
“We don’t know if this diversity in folding paths can be generalized to other proteins,” Ihee confessed. He continued, “However, we believe that our approach can be used to study other protein folding systems.”
Ihee hopes this approach can improve the accuracy of models that simulate protein interactions by including information on their unstructured states. These simulations are important as they can help identify barriers to proper folding and predict a protein’s folded state given its amino acid sequence. Ultimately, the models could help clarify how some diseases develop and how drugs interact with various protein structures.
Ihee’s group collaborated with Professor Young Min Rhee at the KAIST Department of Chemistry, and this work was supported by the National Research Foundation of Korea (NRF) and the Institute for Basic Science (IBS).
Figure. The scientists found that non-functional unfolded forms of the protein cytochrome c follow different pathways and timelines to reach a stable functional folded state.
Publications:
Kim, T. W., et al. (2020) ‘Protein folding from heterogeneous unfolded state revealed by time-resolved X-ray solution scattering’. PNAS. Volume 117. Issue 26. Page 14996-15005. Available online at https://doi.org/10.1073/pnas.1913442117
Profile: Hyotcherl Ihee, Ph.D.
Professor
hyotcherl.ihee@kaist.ac.kr
http://time.kaist.ac.kr/
Ihee Laboratory
Department of Chemistry
KAIST
https://www.kaist.ac.kr
Daejeon 34141, Korea
Profile: Young Min Rhee, Ph.D.
Professor
ymrhee@kaist.ac.kr
http://singlet.kaist.ac.kr
Rhee Research Group
Department of Chemistry
KAIST
https://www.kaist.ac.kr
Daejeon 34141, Korea
(END)
2020.07.09 View 16866 -
 Professor J.H. Lee Wins the Innovators in Science Award
Professor Jeong Ho Lee from the Graduate School of Medical Science and Engineering won the Early-Career Scientist Award of the 2020 Innovators in Science Award. The New York Academy of Sciences administers the award in partnership with Takeda Pharmaceutical Company.
The Innovators in Science Award grants two prizes of US $200,000 each year: one to an Early-Career Scientist and the other to a well-established Senior Scientist who have distinguished themselves for the creative thinking and impact of their rare disease research. The Senior Scientist Awardee is Dr. Adrian R. Krainer, at Cold Spring Harbor Laboratory whose research focused on the mechanisms and control of RNA splicing.
Prof. Lee is recognized for his research investigating genetic mutations in stem cells in the brain that result in rare developmental brain disorders. He was the first to identify the causes of intractable epilepsies and has identified the genes responsible for several developmental brain disorders, including focal cortical dysplasia, Joubert syndrome—a disorder characterized by an underdevelopment of the brainstem—and hemimegaloencephaly, which is the abnormal enlargement of one side of the brain.
“It is a great honor to be recognized by a jury of such globally respected scientists whom I greatly admire,” said Prof. Lee. “More importantly, this award validates research into brain somatic mutations as an important area of exploration to help patients suffering from devastating and untreatable neurological disorders.”
Prof. Lee also is the Director of the National Creative Research Initiative Center for Brain Somatic Mutations, and Co-founder and Chief Technology Officer of SoVarGen, a biopharmaceutical company aiming to discover novel therapeutics and diagnosis for intractable central nervous system (CNS) diseases caused by low-level somatic mutation.
The Innovators in Science Award is a limited submission competition in which research universities, academic institutions, government or non-profit institutions, or equivalent from around the globe with a well-established record of scientific excellence are invited to nominate their most promising Early-Career Scientists and their most outstanding Senior Scientists working in one of four selected therapeutic fields of neuroscience, gastroenterology, oncology, and regenerative medicine. The 2020 Winners will be honored at the virtual Innovators in Science Award Ceremony and Symposium in October 2020.
2020.07.09 View 11102
Professor J.H. Lee Wins the Innovators in Science Award
Professor Jeong Ho Lee from the Graduate School of Medical Science and Engineering won the Early-Career Scientist Award of the 2020 Innovators in Science Award. The New York Academy of Sciences administers the award in partnership with Takeda Pharmaceutical Company.
The Innovators in Science Award grants two prizes of US $200,000 each year: one to an Early-Career Scientist and the other to a well-established Senior Scientist who have distinguished themselves for the creative thinking and impact of their rare disease research. The Senior Scientist Awardee is Dr. Adrian R. Krainer, at Cold Spring Harbor Laboratory whose research focused on the mechanisms and control of RNA splicing.
Prof. Lee is recognized for his research investigating genetic mutations in stem cells in the brain that result in rare developmental brain disorders. He was the first to identify the causes of intractable epilepsies and has identified the genes responsible for several developmental brain disorders, including focal cortical dysplasia, Joubert syndrome—a disorder characterized by an underdevelopment of the brainstem—and hemimegaloencephaly, which is the abnormal enlargement of one side of the brain.
“It is a great honor to be recognized by a jury of such globally respected scientists whom I greatly admire,” said Prof. Lee. “More importantly, this award validates research into brain somatic mutations as an important area of exploration to help patients suffering from devastating and untreatable neurological disorders.”
Prof. Lee also is the Director of the National Creative Research Initiative Center for Brain Somatic Mutations, and Co-founder and Chief Technology Officer of SoVarGen, a biopharmaceutical company aiming to discover novel therapeutics and diagnosis for intractable central nervous system (CNS) diseases caused by low-level somatic mutation.
The Innovators in Science Award is a limited submission competition in which research universities, academic institutions, government or non-profit institutions, or equivalent from around the globe with a well-established record of scientific excellence are invited to nominate their most promising Early-Career Scientists and their most outstanding Senior Scientists working in one of four selected therapeutic fields of neuroscience, gastroenterology, oncology, and regenerative medicine. The 2020 Winners will be honored at the virtual Innovators in Science Award Ceremony and Symposium in October 2020.
2020.07.09 View 11102 -
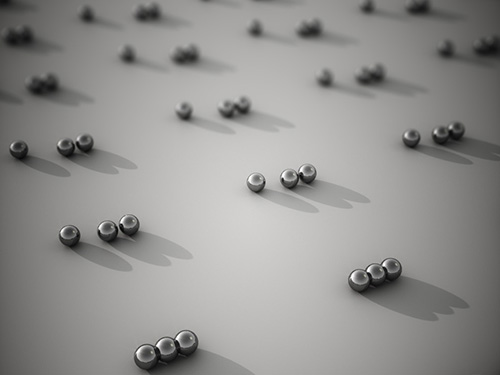 Every Moment of Ultrafast Chemical Bonding Now Captured on Film
- The emerging moment of bond formation, two separate bonding steps, and subsequent vibrational motions were visualized. -
< Emergence of molecular vibrations and the evolution to covalent bonds observed in the research. Video Credit: KEK IMSS >
A team of South Korean researchers led by Professor Hyotcherl Ihee from the Department of Chemistry at KAIST reported the direct observation of the birthing moment of chemical bonds by tracking real-time atomic positions in the molecule. Professor Ihee, who also serves as Associate Director of the Center for Nanomaterials and Chemical Reactions at the Institute for Basic Science (IBS), conducted this study in collaboration with scientists at the Institute of Materials Structure Science of High Energy Accelerator Research Organization (KEK IMSS, Japan), RIKEN (Japan), and Pohang Accelerator Laboratory (PAL, South Korea). This work was published in Nature on June 24.
Targeted cancer drugs work by striking a tight bond between cancer cell and specific molecular targets that are involved in the growth and spread of cancer. Detailed images of such chemical bonding sites or pathways can provide key information necessary for maximizing the efficacy of oncogene treatments. However, atomic movements in a molecule have never been captured in the middle of the action, not even for an extremely simple molecule such as a triatomic molecule, made of only three atoms.
Professor Ihee's group and their international collaborators finally succeeded in capturing the ongoing reaction process of the chemical bond formation in the gold trimer. "The femtosecond-resolution images revealed that such molecular events took place in two separate stages, not simultaneously as previously assumed," says Professor Ihee, the corresponding author of the study. "The atoms in the gold trimer complex atoms remain in motion even after the chemical bonding is complete. The distance between the atoms increased and decreased periodically, exhibiting the molecular vibration. These visualized molecular vibrations allowed us to name the characteristic motion of each observed vibrational mode." adds Professor Ihee.
Atoms move extremely fast at a scale of femtosecond (fs) ― quadrillionths (or millionths of a billionth) of a second. Its movement is minute in the level of angstrom equal to one ten-billionth of a meter. They are especially elusive during the transition state where reaction intermediates are transitioning from reactants to products in a flash. The KAIST-IBS research team made this experimentally challenging task possible by using femtosecond x-ray liquidography (solution scattering). This experimental technique combines laser photolysis and x-ray scattering techniques. When a laser pulse strikes the sample, X-rays scatter and initiate the chemical bond formation reaction in the gold trimer complex. Femtosecond x-ray pulses obtained from a special light source called an x-ray free-electron laser (XFEL) were used to interrogate the bond-forming process. The experiments were performed at two XFEL facilities (4th generation linear accelerator) that are PAL-XFEL in South Korea and SACLA in Japan, and this study was conducted in collaboration with researchers from KEK IMSS, PAL, RIKEN, and the Japan Synchrotron Radiation Research Institute (JASRI).
Scattered waves from each atom interfere with each other and thus their x-ray scattering images are characterized by specific travel directions. The KAIST-IBS research team traced real-time positions of the three gold atoms over time by analyzing x-ray scattering images, which are determined by a three-dimensional structure of a molecule. Structural changes in the molecule complex resulted in multiple characteristic scattering images over time. When a molecule is excited by a laser pulse, multiple vibrational quantum states are simultaneously excited. The superposition of several excited vibrational quantum states is called a wave packet. The researchers tracked the wave packet in three-dimensional nuclear coordinates and found that the first half round of chemical bonding was formed within 35 fs after photoexcitation. The second half of the reaction followed within 360 fs to complete the entire reaction dynamics.
They also accurately illustrated molecular vibration motions in both temporal- and spatial-wise. This is quite a remarkable feat considering that such an ultrafast speed and a minute length of motion are quite challenging conditions for acquiring precise experimental data.
In this study, the KAIST-IBS research team improved upon their 2015 study published by Nature. In the previous study in 2015, the speed of the x-ray camera (time resolution) was limited to 500 fs, and the molecular structure had already changed to be linear with two chemical bonds within 500 fs. In this study, the progress of the bond formation and bent-to-linear structural transformation could be observed in real time, thanks to the improvement time resolution down to 100 fs. Thereby, the asynchronous bond formation mechanism in which two chemical bonds are formed in 35 fs and 360 fs, respectively, and the bent-to-linear transformation completed in 335 fs were visualized. In short, in addition to observing the beginning and end of chemical reactions, they reported every moment of the intermediate, ongoing rearrangement of nuclear configurations with dramatically improved experimental and analytical methods.
They will push this method of 'real-time tracking of atomic positions in a molecule and molecular vibration using femtosecond x-ray scattering' to reveal the mechanisms of organic and inorganic catalytic reactions and reactions involving proteins in the human body. "By directly tracking the molecular vibrations and real-time positions of all atoms in a molecule in the middle of reaction, we will be able to uncover mechanisms of various unknown organic and inorganic catalytic reactions and biochemical reactions," notes Dr. Jong Goo Kim, the lead author of the study.
Publications:
Kim, J. G., et al. (2020) ‘Mapping the emergence of molecular vibrations mediating bond formation’. Nature. Volume 582. Page 520-524. Available online at https://doi.org/10.1038/s41586-020-2417-3
Profile: Hyotcherl Ihee, Ph.D.
Professor
hyotcherl.ihee@kaist.ac.kr
http://time.kaist.ac.kr/
Ihee Laboratory
Department of Chemistry
KAIST
https://www.kaist.ac.kr
Daejeon 34141, Korea
(END)
2020.06.24 View 19718
Every Moment of Ultrafast Chemical Bonding Now Captured on Film
- The emerging moment of bond formation, two separate bonding steps, and subsequent vibrational motions were visualized. -
< Emergence of molecular vibrations and the evolution to covalent bonds observed in the research. Video Credit: KEK IMSS >
A team of South Korean researchers led by Professor Hyotcherl Ihee from the Department of Chemistry at KAIST reported the direct observation of the birthing moment of chemical bonds by tracking real-time atomic positions in the molecule. Professor Ihee, who also serves as Associate Director of the Center for Nanomaterials and Chemical Reactions at the Institute for Basic Science (IBS), conducted this study in collaboration with scientists at the Institute of Materials Structure Science of High Energy Accelerator Research Organization (KEK IMSS, Japan), RIKEN (Japan), and Pohang Accelerator Laboratory (PAL, South Korea). This work was published in Nature on June 24.
Targeted cancer drugs work by striking a tight bond between cancer cell and specific molecular targets that are involved in the growth and spread of cancer. Detailed images of such chemical bonding sites or pathways can provide key information necessary for maximizing the efficacy of oncogene treatments. However, atomic movements in a molecule have never been captured in the middle of the action, not even for an extremely simple molecule such as a triatomic molecule, made of only three atoms.
Professor Ihee's group and their international collaborators finally succeeded in capturing the ongoing reaction process of the chemical bond formation in the gold trimer. "The femtosecond-resolution images revealed that such molecular events took place in two separate stages, not simultaneously as previously assumed," says Professor Ihee, the corresponding author of the study. "The atoms in the gold trimer complex atoms remain in motion even after the chemical bonding is complete. The distance between the atoms increased and decreased periodically, exhibiting the molecular vibration. These visualized molecular vibrations allowed us to name the characteristic motion of each observed vibrational mode." adds Professor Ihee.
Atoms move extremely fast at a scale of femtosecond (fs) ― quadrillionths (or millionths of a billionth) of a second. Its movement is minute in the level of angstrom equal to one ten-billionth of a meter. They are especially elusive during the transition state where reaction intermediates are transitioning from reactants to products in a flash. The KAIST-IBS research team made this experimentally challenging task possible by using femtosecond x-ray liquidography (solution scattering). This experimental technique combines laser photolysis and x-ray scattering techniques. When a laser pulse strikes the sample, X-rays scatter and initiate the chemical bond formation reaction in the gold trimer complex. Femtosecond x-ray pulses obtained from a special light source called an x-ray free-electron laser (XFEL) were used to interrogate the bond-forming process. The experiments were performed at two XFEL facilities (4th generation linear accelerator) that are PAL-XFEL in South Korea and SACLA in Japan, and this study was conducted in collaboration with researchers from KEK IMSS, PAL, RIKEN, and the Japan Synchrotron Radiation Research Institute (JASRI).
Scattered waves from each atom interfere with each other and thus their x-ray scattering images are characterized by specific travel directions. The KAIST-IBS research team traced real-time positions of the three gold atoms over time by analyzing x-ray scattering images, which are determined by a three-dimensional structure of a molecule. Structural changes in the molecule complex resulted in multiple characteristic scattering images over time. When a molecule is excited by a laser pulse, multiple vibrational quantum states are simultaneously excited. The superposition of several excited vibrational quantum states is called a wave packet. The researchers tracked the wave packet in three-dimensional nuclear coordinates and found that the first half round of chemical bonding was formed within 35 fs after photoexcitation. The second half of the reaction followed within 360 fs to complete the entire reaction dynamics.
They also accurately illustrated molecular vibration motions in both temporal- and spatial-wise. This is quite a remarkable feat considering that such an ultrafast speed and a minute length of motion are quite challenging conditions for acquiring precise experimental data.
In this study, the KAIST-IBS research team improved upon their 2015 study published by Nature. In the previous study in 2015, the speed of the x-ray camera (time resolution) was limited to 500 fs, and the molecular structure had already changed to be linear with two chemical bonds within 500 fs. In this study, the progress of the bond formation and bent-to-linear structural transformation could be observed in real time, thanks to the improvement time resolution down to 100 fs. Thereby, the asynchronous bond formation mechanism in which two chemical bonds are formed in 35 fs and 360 fs, respectively, and the bent-to-linear transformation completed in 335 fs were visualized. In short, in addition to observing the beginning and end of chemical reactions, they reported every moment of the intermediate, ongoing rearrangement of nuclear configurations with dramatically improved experimental and analytical methods.
They will push this method of 'real-time tracking of atomic positions in a molecule and molecular vibration using femtosecond x-ray scattering' to reveal the mechanisms of organic and inorganic catalytic reactions and reactions involving proteins in the human body. "By directly tracking the molecular vibrations and real-time positions of all atoms in a molecule in the middle of reaction, we will be able to uncover mechanisms of various unknown organic and inorganic catalytic reactions and biochemical reactions," notes Dr. Jong Goo Kim, the lead author of the study.
Publications:
Kim, J. G., et al. (2020) ‘Mapping the emergence of molecular vibrations mediating bond formation’. Nature. Volume 582. Page 520-524. Available online at https://doi.org/10.1038/s41586-020-2417-3
Profile: Hyotcherl Ihee, Ph.D.
Professor
hyotcherl.ihee@kaist.ac.kr
http://time.kaist.ac.kr/
Ihee Laboratory
Department of Chemistry
KAIST
https://www.kaist.ac.kr
Daejeon 34141, Korea
(END)
2020.06.24 View 19718 -
 Highly Efficient Charge-to-Spin Interconversion in Graphene Heterostructures
Researchers present a new route for designing a graphene-based active spintronic component
KAIST physicists described a route to design the energy-efficient generation, manipulation and detection of spin currents using nonmagnetic two-dimensional materials. The research team, led by Professor Sungjae Cho, observed highly efficient charge-to-spin interconversion via the gate-tunable Rashba-Edelstien effect (REE) in graphene heterostructures.
This research paves the way for the application of graphene as an active spintronic component for generating, controlling, and detecting spin current without ferromagnetic electrodes or magnetic fields.
Graphene is a promising spintronic component owing to its long spin diffusion length. However, its small spin-orbit coupling limits the potential of graphene in spintronic applications since graphene cannot be used to generate, control, or detect spin current.
“We successfully increased the spin-orbit coupling of graphene by stacking graphene on top of 2H-TaS2, which is one of the transition metal dichalcogenide materials with the largest spin-orbit coupling. Graphene now can be used to generate, control, and detect spin current,” Professor Cho said.
The Rashba-Edelstein effect is a physical mechanism that enables charge current-to-spin current interconversion by spin-dependent band structure induced by the Rashba effect, a momentum-dependent splitting of spin bands in low-dimensional condensed matter systems.
Professor Cho’s group demonstrated the gate-tunable Rashba-Edelstein effect in a multilayer graphene for the first time. The Rahsba-Edelstein effect allows the two-dimensional conduction electrons of graphene to be magnetized by an applied charge current and form a spin current. Furthermore, as the Fermi level of graphene, tuned by gate voltage, moves from the valence to conduction band, the spin current generated by graphene reversed its spin direction.
This spin reversal is useful in the design of low-power-consumption transistors utilizing spins in that it provides the carrier “On” state with spin up holes (or spin down electrons) and the "Off" state with zero net spin polarization at so called “charge neutrality point” where numbers of electrons and holes are equal.
“Our work is the first demonstration of charge-to-spin interconversion in a metallic TMD (transition-metal dichalcogenides) and graphene heterostructure with a spin polarization state controlled by a gate. We expect that the all-electrical spin-switching effect and the reversal of non-equilibrium spin polarization by the application of gate voltage is applicable for the energy-efficient generation and manipulation of spin currents using nonmagnetic van der Waals materials,” explained Professor Cho.
This study (https://pubs.acs.org/doi/10.1021/acsnano.0c01037) was supported by the National Research Foundation of Korea.
Publication:
Lijun Li, Jin Zhang, Gyuho Myeong, Wongil Shin, Hongsik Lim, Boram Kim, Seungho Kim, Taehyeok Jin, Stuart Cavill, Beom Seo Kim, Changyoung Kim, Johannes Lischner, Aires Ferreira, and Sungjae Cho, Gate-Tunable Reversible Rashba−Edelstein Effect in a Few-Layer Graphene/2H-TaS2 Heterostructure at Room Temperature. ACS Nano 2020. Link to download the paper: https://pubs.acs.org/doi/10.1021/acsnano.0c01037
Profile:
Professor Sungjae Cho, PhD
sungjae.cho@kaist.ac.kr
http://qtak.kaist.ac.kr
Department of Physics
Korea Advanced Institute of Science and Technology (KAIST)
https://www.kaist.ac.kr
Daejeon 34141, Korea
2020.05.18 View 11177
Highly Efficient Charge-to-Spin Interconversion in Graphene Heterostructures
Researchers present a new route for designing a graphene-based active spintronic component
KAIST physicists described a route to design the energy-efficient generation, manipulation and detection of spin currents using nonmagnetic two-dimensional materials. The research team, led by Professor Sungjae Cho, observed highly efficient charge-to-spin interconversion via the gate-tunable Rashba-Edelstien effect (REE) in graphene heterostructures.
This research paves the way for the application of graphene as an active spintronic component for generating, controlling, and detecting spin current without ferromagnetic electrodes or magnetic fields.
Graphene is a promising spintronic component owing to its long spin diffusion length. However, its small spin-orbit coupling limits the potential of graphene in spintronic applications since graphene cannot be used to generate, control, or detect spin current.
“We successfully increased the spin-orbit coupling of graphene by stacking graphene on top of 2H-TaS2, which is one of the transition metal dichalcogenide materials with the largest spin-orbit coupling. Graphene now can be used to generate, control, and detect spin current,” Professor Cho said.
The Rashba-Edelstein effect is a physical mechanism that enables charge current-to-spin current interconversion by spin-dependent band structure induced by the Rashba effect, a momentum-dependent splitting of spin bands in low-dimensional condensed matter systems.
Professor Cho’s group demonstrated the gate-tunable Rashba-Edelstein effect in a multilayer graphene for the first time. The Rahsba-Edelstein effect allows the two-dimensional conduction electrons of graphene to be magnetized by an applied charge current and form a spin current. Furthermore, as the Fermi level of graphene, tuned by gate voltage, moves from the valence to conduction band, the spin current generated by graphene reversed its spin direction.
This spin reversal is useful in the design of low-power-consumption transistors utilizing spins in that it provides the carrier “On” state with spin up holes (or spin down electrons) and the "Off" state with zero net spin polarization at so called “charge neutrality point” where numbers of electrons and holes are equal.
“Our work is the first demonstration of charge-to-spin interconversion in a metallic TMD (transition-metal dichalcogenides) and graphene heterostructure with a spin polarization state controlled by a gate. We expect that the all-electrical spin-switching effect and the reversal of non-equilibrium spin polarization by the application of gate voltage is applicable for the energy-efficient generation and manipulation of spin currents using nonmagnetic van der Waals materials,” explained Professor Cho.
This study (https://pubs.acs.org/doi/10.1021/acsnano.0c01037) was supported by the National Research Foundation of Korea.
Publication:
Lijun Li, Jin Zhang, Gyuho Myeong, Wongil Shin, Hongsik Lim, Boram Kim, Seungho Kim, Taehyeok Jin, Stuart Cavill, Beom Seo Kim, Changyoung Kim, Johannes Lischner, Aires Ferreira, and Sungjae Cho, Gate-Tunable Reversible Rashba−Edelstein Effect in a Few-Layer Graphene/2H-TaS2 Heterostructure at Room Temperature. ACS Nano 2020. Link to download the paper: https://pubs.acs.org/doi/10.1021/acsnano.0c01037
Profile:
Professor Sungjae Cho, PhD
sungjae.cho@kaist.ac.kr
http://qtak.kaist.ac.kr
Department of Physics
Korea Advanced Institute of Science and Technology (KAIST)
https://www.kaist.ac.kr
Daejeon 34141, Korea
2020.05.18 View 11177 -
 Simple Molecular Reagents to Treat Alzheimer’s Disease
- Researchers report minimalistic principles for designing small molecules with multiple reactivities against dementia. -
Sometimes the most complex problems actually have very simple solutions. A group of South Korean researchers reported an efficient and effective redox-based strategy for incorporating multiple functions into simple molecular reagents against neurodegenerative disorders. The team developed redox-active aromatic molecular reagents with a simple structural composition that can simultaneously target and modulate various pathogenic factors in complex neurodegenerative disorders such as Alzheimer’s disease.
Alzheimer’s disease is one of the most prevalent neurodegenerative disorders, affecting one in ten people over the age of 65. Early-onset dementia also increasingly affects younger people.
A number of pathogenic elements such as reactive oxygen species, amyloid-beta, and metal ions have been suggested as potential causes of Alzheimer’s disease. Each element itself can lead to Alzheimer’s disease, but interactions between them may also aggravate the patient’s condition or interfere with the appropriate clinical care.
For example, when interacting with amyloid-beta, metal ions foster the aggregation and accumulation of amyloid-beta peptides that can induce oxidative stress and toxicity in the brain and lead to neurodegeneration.
Because these pathogenic factors of Alzheimer’s disease are intertwined, developing therapeutic agents that are capable of simultaneously regulating metal ion dyshomeostasis, amyloid-beta agglutination, and oxidative stress responses remains a key to halting the progression of the disease.
A research team led by Professor Mi Hee Lim from the Department of Chemistry at KAIST demonstrated the feasibility of structure-mechanism-based molecular design for controlling a molecule’s chemical reactivity toward the various pathological factors of Alzheimer’s disease by tuning the redox properties of the molecule.
This study, featured as the ‘ACS Editors’ Choice’ in the May 6th issue of the Journal of the American Chemical Society (JACS), was conducted in conjunction with KAIST Professor Mu-Hyun Baik’s group and Professor Joo-Young Lee’s group at the Asan Medical Center.
Professor Lim and her collaborators rationally designed and generated 10 compact aromatic molecules presenting a range of redox potentials by adjusting the electronic distribution of the phenyl, phenylene, or pyridyl moiety to impart redox-dependent reactivities against the multiple pathogenic factors in Alzheimer’s disease.
During the team’s biochemical and biophysical studies, these designed molecular reagents displayed redox-dependent reactivities against numerous desirable targets that are associated with Alzheimer’s disease such as free radicals, metal-free amyloid-beta, and metal-bound amyloid-beta.
Further mechanistic results revealed that the redox properties of these designed molecular reagents were essential for their function. The team demonstrated that these reagents engaged in oxidative reactions with metal-free and metal-bound amyloid-beta and led to chemical modifications. The products of such oxidative transformations were observed to form covalent adducts with amyloid-beta and alter its aggregation.
Moreover, the administration of the most promising candidate molecule significantly attenuated the amyloid pathology in the brains of Alzheimer’s disease transgenic mice and improved their cognitive defects.
Professor Lim said, “This strategy is straightforward, time-saving, and cost-effective, and its effect is significant. We are excited to help enable the advancement of new therapeutic agents for neurodegenerative disorders, which can improve the lives of so many patients.”
This work was supported by the National Research Foundation (NRF) of Korea, the Institute for Basic Science (IBS), and the Asan Institute for Life Sciences.
Image credit: Professor Mi Hee Lim, KAIST
Image usage restrictions: News organizations may use or redistribute this image, with proper attribution, as part of the news coverage of this paper only.
Publication:
Kim, M. et al. (2020) ‘Minimalistic Principles for Designing Small Molecules with Multiple Reactivities against Pathological Factors in Dementia.’ Journal of the American Chemical Society (JACS), Volume 142, Issue 18, pp.8183-8193. Available online at https://doi.org/10.1021/jacs.9b13100
Profile:
Mi Hee Lim
Professor
miheelim@kaist.ac.kr
http://sites.google.com/site/miheelimlab
Lim Laboratory
Department of Chemistry
KAIST
Profile:
Mu-Hyun Baik
Professor
mbaik2805@kaist.ac.kr
https://baik-laboratory.com/
Baik Laboratory
Department of Chemistry
KAIST
Profile:
Joo-Yong Lee
Professor
jlee@amc.seoul.kr
Asan Institute for Life Sciences
Asan Medical Center
(END)
2020.05.11 View 17538
Simple Molecular Reagents to Treat Alzheimer’s Disease
- Researchers report minimalistic principles for designing small molecules with multiple reactivities against dementia. -
Sometimes the most complex problems actually have very simple solutions. A group of South Korean researchers reported an efficient and effective redox-based strategy for incorporating multiple functions into simple molecular reagents against neurodegenerative disorders. The team developed redox-active aromatic molecular reagents with a simple structural composition that can simultaneously target and modulate various pathogenic factors in complex neurodegenerative disorders such as Alzheimer’s disease.
Alzheimer’s disease is one of the most prevalent neurodegenerative disorders, affecting one in ten people over the age of 65. Early-onset dementia also increasingly affects younger people.
A number of pathogenic elements such as reactive oxygen species, amyloid-beta, and metal ions have been suggested as potential causes of Alzheimer’s disease. Each element itself can lead to Alzheimer’s disease, but interactions between them may also aggravate the patient’s condition or interfere with the appropriate clinical care.
For example, when interacting with amyloid-beta, metal ions foster the aggregation and accumulation of amyloid-beta peptides that can induce oxidative stress and toxicity in the brain and lead to neurodegeneration.
Because these pathogenic factors of Alzheimer’s disease are intertwined, developing therapeutic agents that are capable of simultaneously regulating metal ion dyshomeostasis, amyloid-beta agglutination, and oxidative stress responses remains a key to halting the progression of the disease.
A research team led by Professor Mi Hee Lim from the Department of Chemistry at KAIST demonstrated the feasibility of structure-mechanism-based molecular design for controlling a molecule’s chemical reactivity toward the various pathological factors of Alzheimer’s disease by tuning the redox properties of the molecule.
This study, featured as the ‘ACS Editors’ Choice’ in the May 6th issue of the Journal of the American Chemical Society (JACS), was conducted in conjunction with KAIST Professor Mu-Hyun Baik’s group and Professor Joo-Young Lee’s group at the Asan Medical Center.
Professor Lim and her collaborators rationally designed and generated 10 compact aromatic molecules presenting a range of redox potentials by adjusting the electronic distribution of the phenyl, phenylene, or pyridyl moiety to impart redox-dependent reactivities against the multiple pathogenic factors in Alzheimer’s disease.
During the team’s biochemical and biophysical studies, these designed molecular reagents displayed redox-dependent reactivities against numerous desirable targets that are associated with Alzheimer’s disease such as free radicals, metal-free amyloid-beta, and metal-bound amyloid-beta.
Further mechanistic results revealed that the redox properties of these designed molecular reagents were essential for their function. The team demonstrated that these reagents engaged in oxidative reactions with metal-free and metal-bound amyloid-beta and led to chemical modifications. The products of such oxidative transformations were observed to form covalent adducts with amyloid-beta and alter its aggregation.
Moreover, the administration of the most promising candidate molecule significantly attenuated the amyloid pathology in the brains of Alzheimer’s disease transgenic mice and improved their cognitive defects.
Professor Lim said, “This strategy is straightforward, time-saving, and cost-effective, and its effect is significant. We are excited to help enable the advancement of new therapeutic agents for neurodegenerative disorders, which can improve the lives of so many patients.”
This work was supported by the National Research Foundation (NRF) of Korea, the Institute for Basic Science (IBS), and the Asan Institute for Life Sciences.
Image credit: Professor Mi Hee Lim, KAIST
Image usage restrictions: News organizations may use or redistribute this image, with proper attribution, as part of the news coverage of this paper only.
Publication:
Kim, M. et al. (2020) ‘Minimalistic Principles for Designing Small Molecules with Multiple Reactivities against Pathological Factors in Dementia.’ Journal of the American Chemical Society (JACS), Volume 142, Issue 18, pp.8183-8193. Available online at https://doi.org/10.1021/jacs.9b13100
Profile:
Mi Hee Lim
Professor
miheelim@kaist.ac.kr
http://sites.google.com/site/miheelimlab
Lim Laboratory
Department of Chemistry
KAIST
Profile:
Mu-Hyun Baik
Professor
mbaik2805@kaist.ac.kr
https://baik-laboratory.com/
Baik Laboratory
Department of Chemistry
KAIST
Profile:
Joo-Yong Lee
Professor
jlee@amc.seoul.kr
Asan Institute for Life Sciences
Asan Medical Center
(END)
2020.05.11 View 17538 -
 Breastfeeding Helps Prevent Mothers from Developing Diabetes after Childbirth
A team of South Korean researchers found that lactation can lower the incidence and reduce the risk of maternal postpartum diabetes. The researchers identified that lactation increases the mass and function of pancreatic beta cells through serotonin production. The team suggested that sustained improvements in pancreatic beta cells, which can last for years even after the cessation of lactation, improve mothers’ metabolic health in addition to providing health benefits for infants.
Pregnancy imposes a substantial metabolic burden on women through weight gain and increased insulin resistance. Various other factors, including a history of gestational diabetes, maternal age, and obesity, further affect women’s risk of progressing to diabetes after delivery, and the risk of postpartum diabetes increases more in women who have had gestational diabetes and/or repeated deliveries.
Diabetes-related complications include damage to blood vessels, which can lead to cardiovascular and cerebrovascular diseases such as heart attack and stroke, and problems with the nerves, eyes, kidneys, and many more. Since diabetes can pose a serious threat to mothers’ metabolic health, the management of maternal metabolic risk factors is important, especially in the peripartum period. Previous epidemiological studies have reported that lactation reduces the risk of postpartum diabetes, but the mechanisms underlying this benefit have remained elusive.
The study, published in Science Translational Medicine on April 29, explains the biology underpinning this observation on the beneficial effects of lactation. Professor Hail Kim from the Graduate School of Medical Science and Engineering at KAIST led and jointly conducted the study in conjunction with researchers from the Seoul National University Bundang Hospital (SNUBH) and Chungnam National University (CNU) in Korea, and the University of California, San Francisco (UCSF) in the US.
In their study, the team observed that the milk-secreting hormone ‘prolactin’ in lactating mothers not only promotes milk production, but also plays a major role in stimulating insulin-secreting pancreatic beta cells that regulate blood glucose in the body.
The researchers also found that ‘serotonin’, known as a chemical that contributes to wellbeing and happiness, is produced in pancreatic beta cells during lactation. Serotonin in pancreatic beta cells act as an antioxidant and reduce oxidative stress, making mothers’ beta cells healthier. Serotonin also induces the proliferation of beta cells, thereby increasing the beta cell mass and helping maintain proper glucose levels.
The research team conducted follow-up examinations on a total of 174 postpartum women, 85 lactated and 99 non-lactated, at two months postpartum and annually thereafter for at least three years. The results demonstrated that mothers who had undergone lactation improved pancreatic beta cell mass and function, and showed improved glucose homeostasis with approximately 20mg/dL lower glucose levels, thereby reducing the risk of postpartum diabetes in women. Surprisingly, this beneficial effect was maintained after the cessation of lactation, for more than three years after delivery.
Professor Kim said, “We are happy to prove that lactation benefits female metabolic health by improving beta cell mass and function as well as glycemic control.”
“Our future studies on the modulation of the molecular serotonergic pathway in accordance with the management of maternal metabolic risk factors may lead to new therapeutics to help prevent mothers from developing metabolic disorders,” he added.
This work was supported by grants from the National Research Foundation (NRF) and the National Research Council of Science and Technology (NST) of Korea, the National Institutes of Health (NIH), the Larry L. Hillblom Foundation, and the Health Fellowship Foundation.
Image credit: Professor Hail Kim, KAIST
Image usage restrictions: News organizations may use or redistribute this image, with proper attribution, as part of news coverage of this paper only.
Publication:
Moon, J. H et al. (2020) ‘Lactation improves pancreatic β cell mass and function through serotonin production.’ Science Translational Medicine, 12, eaay0455. Available online at https://doi.org/10.1126/scitranslmed.aay0455
Profile: Hail Kim, MD, PhD
hailkim@kaist.edu
Associate Professor
Graduate School of Medical Science and Engineering (GSMSE)
Korea Advanced Institute of Science and Technology (KAIST)
Profile: Hak Chul Jang, MD, PhD
janghak@snu.ac.kr
Professor
Division of Endocrinology and Metabolism
Seoul National University Bundang Hospital (SNUBH)
President
Korean Diabetes Association
Profile: Joon Ho Moon, MD, PhD
moonjoonho@gmail.com
Clinical Fellow
Division of Endocrinology and Metabolism
SNUBH
Profile: Hyeongseok Kim, MD, PhD
hskim85kor@gmail.com
Assistant Professor
Chungnam National University (CNU)
Profile: Professor Michael S. German, MD
Michael.German@ucsf.edu
Professor
Diabetes Center
University of California, San Francisco (UCSF)
(END)
2020.04.29 View 21711
Breastfeeding Helps Prevent Mothers from Developing Diabetes after Childbirth
A team of South Korean researchers found that lactation can lower the incidence and reduce the risk of maternal postpartum diabetes. The researchers identified that lactation increases the mass and function of pancreatic beta cells through serotonin production. The team suggested that sustained improvements in pancreatic beta cells, which can last for years even after the cessation of lactation, improve mothers’ metabolic health in addition to providing health benefits for infants.
Pregnancy imposes a substantial metabolic burden on women through weight gain and increased insulin resistance. Various other factors, including a history of gestational diabetes, maternal age, and obesity, further affect women’s risk of progressing to diabetes after delivery, and the risk of postpartum diabetes increases more in women who have had gestational diabetes and/or repeated deliveries.
Diabetes-related complications include damage to blood vessels, which can lead to cardiovascular and cerebrovascular diseases such as heart attack and stroke, and problems with the nerves, eyes, kidneys, and many more. Since diabetes can pose a serious threat to mothers’ metabolic health, the management of maternal metabolic risk factors is important, especially in the peripartum period. Previous epidemiological studies have reported that lactation reduces the risk of postpartum diabetes, but the mechanisms underlying this benefit have remained elusive.
The study, published in Science Translational Medicine on April 29, explains the biology underpinning this observation on the beneficial effects of lactation. Professor Hail Kim from the Graduate School of Medical Science and Engineering at KAIST led and jointly conducted the study in conjunction with researchers from the Seoul National University Bundang Hospital (SNUBH) and Chungnam National University (CNU) in Korea, and the University of California, San Francisco (UCSF) in the US.
In their study, the team observed that the milk-secreting hormone ‘prolactin’ in lactating mothers not only promotes milk production, but also plays a major role in stimulating insulin-secreting pancreatic beta cells that regulate blood glucose in the body.
The researchers also found that ‘serotonin’, known as a chemical that contributes to wellbeing and happiness, is produced in pancreatic beta cells during lactation. Serotonin in pancreatic beta cells act as an antioxidant and reduce oxidative stress, making mothers’ beta cells healthier. Serotonin also induces the proliferation of beta cells, thereby increasing the beta cell mass and helping maintain proper glucose levels.
The research team conducted follow-up examinations on a total of 174 postpartum women, 85 lactated and 99 non-lactated, at two months postpartum and annually thereafter for at least three years. The results demonstrated that mothers who had undergone lactation improved pancreatic beta cell mass and function, and showed improved glucose homeostasis with approximately 20mg/dL lower glucose levels, thereby reducing the risk of postpartum diabetes in women. Surprisingly, this beneficial effect was maintained after the cessation of lactation, for more than three years after delivery.
Professor Kim said, “We are happy to prove that lactation benefits female metabolic health by improving beta cell mass and function as well as glycemic control.”
“Our future studies on the modulation of the molecular serotonergic pathway in accordance with the management of maternal metabolic risk factors may lead to new therapeutics to help prevent mothers from developing metabolic disorders,” he added.
This work was supported by grants from the National Research Foundation (NRF) and the National Research Council of Science and Technology (NST) of Korea, the National Institutes of Health (NIH), the Larry L. Hillblom Foundation, and the Health Fellowship Foundation.
Image credit: Professor Hail Kim, KAIST
Image usage restrictions: News organizations may use or redistribute this image, with proper attribution, as part of news coverage of this paper only.
Publication:
Moon, J. H et al. (2020) ‘Lactation improves pancreatic β cell mass and function through serotonin production.’ Science Translational Medicine, 12, eaay0455. Available online at https://doi.org/10.1126/scitranslmed.aay0455
Profile: Hail Kim, MD, PhD
hailkim@kaist.edu
Associate Professor
Graduate School of Medical Science and Engineering (GSMSE)
Korea Advanced Institute of Science and Technology (KAIST)
Profile: Hak Chul Jang, MD, PhD
janghak@snu.ac.kr
Professor
Division of Endocrinology and Metabolism
Seoul National University Bundang Hospital (SNUBH)
President
Korean Diabetes Association
Profile: Joon Ho Moon, MD, PhD
moonjoonho@gmail.com
Clinical Fellow
Division of Endocrinology and Metabolism
SNUBH
Profile: Hyeongseok Kim, MD, PhD
hskim85kor@gmail.com
Assistant Professor
Chungnam National University (CNU)
Profile: Professor Michael S. German, MD
Michael.German@ucsf.edu
Professor
Diabetes Center
University of California, San Francisco (UCSF)
(END)
2020.04.29 View 21711 -
 A Study Finds Neuropeptide Somatostatin Enhances Visual Processing
Researchers have confirmed that neuropeptide somatostatin can improve cognitive function in the brain. A research group of Professor Seung-Hee Lee from the Department of Biological Sciences at KAIST found that the application of neuropeptide somatostatin improves visual processing and cognitive behaviors by reducing excitatory inputs to parvalbumin-positive interneurons in the cortex.
This study, reported at Science Advances on April 22nd (EST), sheds a new light on the therapeutics of neurodegenerative diseases. According to a recent study in Korea, one in ten seniors over 65 is experiencing dementia-related symptoms in their daily lives such like memory loss, cognitive decline, and motion function disorders. Professor Lee believes that somatostatin treatment can be directly applied to the recovery of cognitive functions in Alzheimer’s disease patients.
Professor Lee started this study noting the fact that the level of somatostatin expression was dramatically decreased in the cerebral cortex and cerebrospinal fluid of Alzheimer’s disease patients
Somatostatin-expressing neurons in the cortex are known to exert the dendritic inhibition of pyramidal neurons via GABAergic transmission. Previous studies focused on their inhibitory effects on cortical circuits, but somatostatin-expressing neurons can co-release somatostatin upon activation. Despite the abundant expression of somatostatin and its receptors in the cerebral cortex, it was not known if somatostatin could modulate cognitive processing in the cortex.
The research team demonstrated that the somatostatin treatment into the cerebral cortex could enhance visual processing and cognitive behaviors in mice. The research team combined behaviors, in vivo and in vitro electrophysiology, and electron microscopy techniques to reveal how the activation of somatostatin receptors in vivo enhanced the ability of visual recognition in animals. Interestingly, somatostatin release can reduce excitatory synaptic transmission to another subtype of GABAergic interneurons, parvalbumin (PV)-expressing neurons.
As somatostatin is a stable and safe neuropeptide expressed naturally in the mammalian brain, it was safe to be injected into the cortex and cerebrospinal fluid, showing a potential application to drug development for curing cognitive disorders in humans.
Professor Lee said, “Our research confirmed the key role of the neuropeptide SST in modulating cortical function and enhancing cognitive ability in the mammalian brain. I hope new drugs can be developed based on the function of somatostatin to treat cognitive disabilities in many patients suffering from neurological disorders.”
This study was supported by the National Research Foundation of Korea.
Publication:
Song, Y. H et al. (2020) ‘Somatostatin enhances visual processing and perception by suppressing excitatory inputs to parvalbumin-positive interneurons in V1’, Science Advances, 6(17). Available online at https://doi.org/10.1126/sciadv.aaz0517
Profile:
Seung-Hee Lee
Associate Professor
shlee1@kaist.ac.kr
https://sites.google.com/site/leelab2013/
Sensory Processing Lab (SPL)
Department of Biological Sciences (BIO)
Korea Advanced Institute of Science and Technology (KAIST)
Profile:
You-Hyang Song
Researcher (Ph.D.)
dbgidtm17@kaist.ac.kr
SPL, KAIST BIO
Profile:
Yang-Sun Hwang
Researcher (M.S.)
hys940129@kaist.ac.kr
SPL, KAIST BIO
(END)
2020.04.23 View 14603
A Study Finds Neuropeptide Somatostatin Enhances Visual Processing
Researchers have confirmed that neuropeptide somatostatin can improve cognitive function in the brain. A research group of Professor Seung-Hee Lee from the Department of Biological Sciences at KAIST found that the application of neuropeptide somatostatin improves visual processing and cognitive behaviors by reducing excitatory inputs to parvalbumin-positive interneurons in the cortex.
This study, reported at Science Advances on April 22nd (EST), sheds a new light on the therapeutics of neurodegenerative diseases. According to a recent study in Korea, one in ten seniors over 65 is experiencing dementia-related symptoms in their daily lives such like memory loss, cognitive decline, and motion function disorders. Professor Lee believes that somatostatin treatment can be directly applied to the recovery of cognitive functions in Alzheimer’s disease patients.
Professor Lee started this study noting the fact that the level of somatostatin expression was dramatically decreased in the cerebral cortex and cerebrospinal fluid of Alzheimer’s disease patients
Somatostatin-expressing neurons in the cortex are known to exert the dendritic inhibition of pyramidal neurons via GABAergic transmission. Previous studies focused on their inhibitory effects on cortical circuits, but somatostatin-expressing neurons can co-release somatostatin upon activation. Despite the abundant expression of somatostatin and its receptors in the cerebral cortex, it was not known if somatostatin could modulate cognitive processing in the cortex.
The research team demonstrated that the somatostatin treatment into the cerebral cortex could enhance visual processing and cognitive behaviors in mice. The research team combined behaviors, in vivo and in vitro electrophysiology, and electron microscopy techniques to reveal how the activation of somatostatin receptors in vivo enhanced the ability of visual recognition in animals. Interestingly, somatostatin release can reduce excitatory synaptic transmission to another subtype of GABAergic interneurons, parvalbumin (PV)-expressing neurons.
As somatostatin is a stable and safe neuropeptide expressed naturally in the mammalian brain, it was safe to be injected into the cortex and cerebrospinal fluid, showing a potential application to drug development for curing cognitive disorders in humans.
Professor Lee said, “Our research confirmed the key role of the neuropeptide SST in modulating cortical function and enhancing cognitive ability in the mammalian brain. I hope new drugs can be developed based on the function of somatostatin to treat cognitive disabilities in many patients suffering from neurological disorders.”
This study was supported by the National Research Foundation of Korea.
Publication:
Song, Y. H et al. (2020) ‘Somatostatin enhances visual processing and perception by suppressing excitatory inputs to parvalbumin-positive interneurons in V1’, Science Advances, 6(17). Available online at https://doi.org/10.1126/sciadv.aaz0517
Profile:
Seung-Hee Lee
Associate Professor
shlee1@kaist.ac.kr
https://sites.google.com/site/leelab2013/
Sensory Processing Lab (SPL)
Department of Biological Sciences (BIO)
Korea Advanced Institute of Science and Technology (KAIST)
Profile:
You-Hyang Song
Researcher (Ph.D.)
dbgidtm17@kaist.ac.kr
SPL, KAIST BIO
Profile:
Yang-Sun Hwang
Researcher (M.S.)
hys940129@kaist.ac.kr
SPL, KAIST BIO
(END)
2020.04.23 View 14603