research

(The team of Professor Chang, far right, at the Department of Chemistry)
KAIST chemists have designed a novel strategy to synthesize ring-shaped cyclic molecules, highly sought-after by pharmaceutical and chemical industries, and known as gamma-lactams. This study describes how these five-membered rings can be prepared from inexpensive and readily available feedstock hydrocarbons, as well as from complex organic molecules, such as amino acids and steroids.
Gamma-lactams find several applications in medicinal, synthetic, and material chemistry. For example, they are included in a large number of pharmaceutically active compounds with antibiotic, anti-inflammatory, and anti-tumoral functions. This research was published in Science on March 2.
Conversion of hydrocarbons into nitrogen-containing compounds is an important area of research, where the challenge lies in breaking strong carbon-hydrogen (C−H) bonds, and converting them into carbon-nitrogen (C–N) bonds in a controlled fashion. For this reason, hydrocarbons are difficult to use as starting materials, albeit the fact that they exist in large quantities in nature.
Over the last 35 years, chemists have found ways of converting simple hydrocarbons into nitrogen-containing rings, such as indoles or pyrrolidines, but gamma-lactams proved impossible to prepare using the same approaches. Researchers hypothesized that such failure was due to alternative chemical pathways that steer the reaction away from the wanted rings: The reaction intermediate (carbonylnitrene) quickly breaks down into unsought products. Using computer models of the desired and undesired reaction pathways, the team found a strategy to completely shut down the latter in order to obtain the longed-for gamma-lactams. For the first time, these four carbons and one nitrogen cyclic molecules were obtained directly from simple feedstock chemicals.
Led by Professor Chang Sukbok at the Department of Chemistry, the team designed the winning reaction with the help of computer simulations that analyze the reaction mechanisms and calculate the energy required for the reaction to take place. According to such computer predictions, the reaction could follow three pathways, leading to the formation of either the desired gamma-lactam, an unwanted product (isocyanate), or the degradation of the catalyst caused by the substrate reacting with the catalyst backbone. Combining experimental observations and detailed computer simulations, the team designed an iridium-based catalyst, highly selective for the gamma-lactam formation. In this way, the two undesired pathways were systematically shut down, leaving the formation of the nitrogen-containing ring as the only possible outcome. Professor Chang is also in charge of the Center for Catalytic Hydrocarbon Functionalizations at the Institute for Basic Science (IBS).
“With this work we offer a brand new solution to a long-standing challenge and demonstrate the power of what we call mechanism-based reaction development,” explains Professor Baik Mu-Hyun, a corresponding author of the study.
Beyond using cheap feedstock hydrocarbons as substrates, the team was also successful in converting amino acids, steroids, and other bio-relevant molecules into gamma-lactams, which might find a variety of applications as plant insecticide, drugs against parasitic worms, or anti-aging agents. This new synthetic technology gives much easier access to these complicated molecules and will enable the development of potential drugs in a much shorter amount of time at a lower cost.
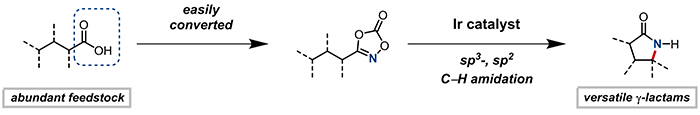
Figure 1: Selective amidation reaction using newly designed iridium (Ir) catalysts. Abundant in nature Hydrocarbons are used as substrates to synthesize nitrogen-containing ring, called gamma-lactams.
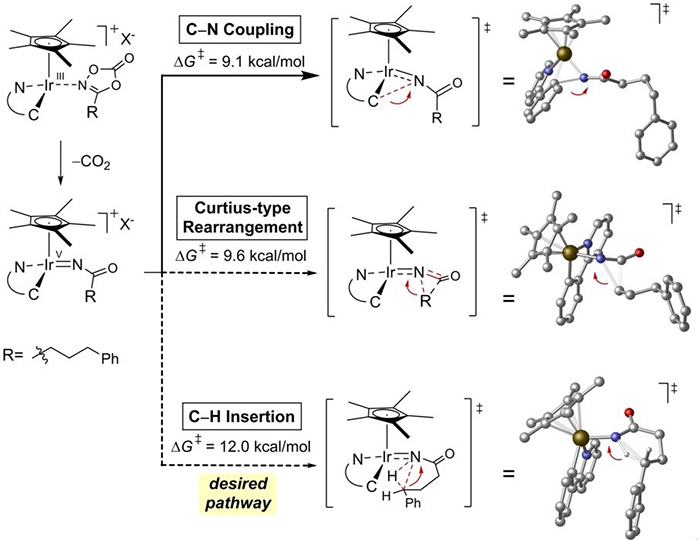
Figure 2: Three possible reaction pathways and energy barriers predicted by computational chemistry. The scientists developed new iridium-based catalysts that are highly selective for the C–H insertion pathway which leads to the desired gamma-lactam molecules.
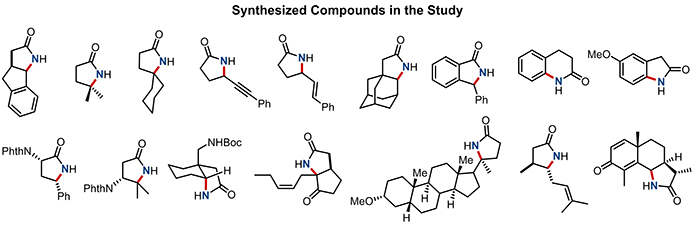
Figure 3: Interesting gamma-lactams derived from natural and unnatural amino acids, steroids, etc., which may be used to protect plants against insects, fight parasitic worms, or as anti-aging agents.
-
research A Novel Material for Transparent and Flexible Displays
(Research team led by Professor Sang Youl Kim from the Department of Chemistry) The next generation of flexible and transparent displays will require a high-performing and flexible polymeric material that has the optical and thermal properties of glass. The material must be transparent to visible light and have a low coefficient of thermal expansion (CTE). Unfortunately, such a polymeric material has not been available. A KAIST research team has succeeded in making a new polymeric mat
2019-01-24 -
research Photonic Capsules for Injectable Laser Resonators
A KAIST research group presented photonic capsules for injectable laser resonators using microfluidic technology. The capsule’s diameter is comparable to a human hair and stable in gas and liquid media, so it is injectable into any target volume. The research group headed by Professor Shin-Hyun Kim in the Department of Chemical and Biomolecular Engineering applied an interesting optical property from nature. Professor Kim, who has dived deep into photonic materials research inspired
2018-07-05 -
research Structural Insight into the Molecular Mechanism of PET Degradation
A KAIST metabolic engineering research team has newly suggested a molecular mechanism showing superior degradability of poly ethylene terephthalate (PET). This is the first report to simultaneously determine the 3D crystal structure of Ideonella sakaiensis PETase and develop the new variant with enhanced PET degradation. Recently, diverse research projects are working to address the non-degradability of materials. A poly ethylene terephthalate (PET)-degrading bacterium called Ideonella sa
2018-01-31 -
research Study Identifies the Novel Molecular Signal for Triggering Septic Shock
Professor Seyun Kim’s team at the Department of Biological Sciences reported the mechanism by which cellular signaling transduction networks are precisely controlled in mediating innate immune responses, such as sepsis, by the enzyme IPMK (Inositol polyphosphate multikinase) which is essential for inositol biosynthesis metabolism. In collaboration with Professor Hyun Seong Roh at Seoul National University, the study’s first author, Eunha Kim, a Ph.D. candidate in Department of
2017-05-11 -
research Expanding the Genetic Code of Mus Musculus
Professor Hee-Sung Park of the Department of Chemistry, who garnered attention for his novel strategy of installing authentic post-translational modifications into recombinant proteins, expanded his research portfolio to another level. Professor Park’s team was the first to report the generation of a mouse strain with an expanded genetic code, allowing site-specific incorporation of unnatural amino acids. Professor Park published the research on the new chemical biology method for achiev
2017-03-27