CT
-
 KAIST Develops Neuromorphic Semiconductor Chip that Learns and Corrects Itself
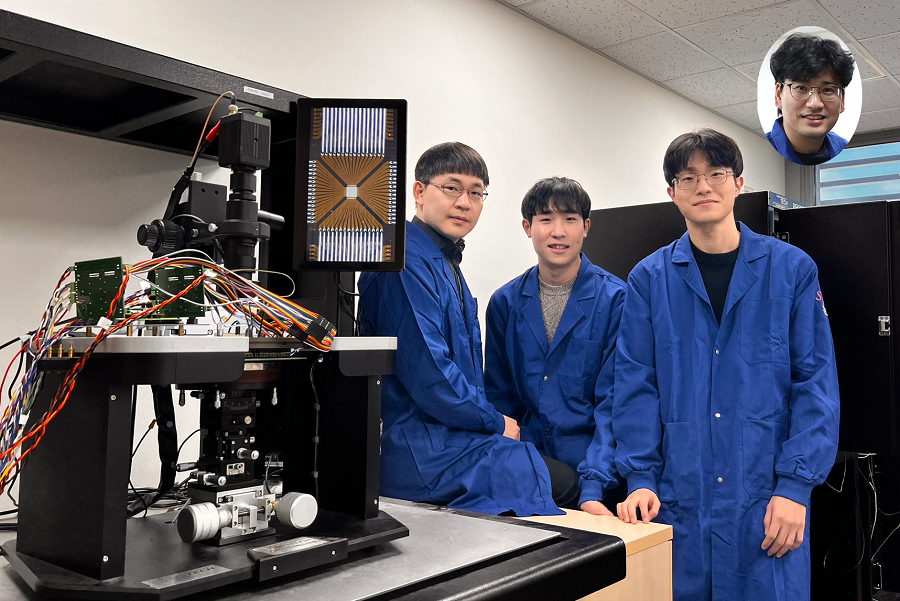
< Photo. The research team of the School of Electrical Engineering posed by the newly deveoped processor. (From center to the right) Professor Young-Gyu Yoon, Integrated Master's and Doctoral Program Students Seungjae Han and Hakcheon Jeong and Professor Shinhyun Choi >
- Professor Shinhyun Choi and Professor Young-Gyu Yoon’s Joint Research Team from the School of Electrical Engineering developed a computing chip that can learn, correct errors, and process AI tasks
- Equipping a computing chip with high-reliability memristor devices with self-error correction functions for real-time learning and image processing
Existing computer systems have separate data processing and storage devices, making them inefficient for processing complex data like AI. A KAIST research team has developed a memristor-based integrated system similar to the way our brain processes information. It is now ready for application in various devices including smart security cameras, allowing them to recognize suspicious activity immediately without having to rely on remote cloud servers, and medical devices with which it can help analyze health data in real time.
KAIST (President Kwang Hyung Lee) announced on the 17th of January that the joint research team of Professor Shinhyun Choi and Professor Young-Gyu Yoon of the School of Electrical Engineering has developed a next-generation neuromorphic semiconductor-based ultra-small computing chip that can learn and correct errors on its own.
< Figure 1. Scanning electron microscope (SEM) image of a computing chip equipped with a highly reliable selector-less 32×32 memristor crossbar array (left). Hardware system developed for real-time artificial intelligence implementation (right). >
What is special about this computing chip is that it can learn and correct errors that occur due to non-ideal characteristics that were difficult to solve in existing neuromorphic devices. For example, when processing a video stream, the chip learns to automatically separate a moving object from the background, and it becomes better at this task over time.
This self-learning ability has been proven by achieving accuracy comparable to ideal computer simulations in real-time image processing. The research team's main achievement is that it has completed a system that is both reliable and practical, beyond the development of brain-like components.
The research team has developed the world's first memristor-based integrated system that can adapt to immediate environmental changes, and has presented an innovative solution that overcomes the limitations of existing technology.
< Figure 2. Background and foreground separation results of an image containing non-ideal characteristics of memristor devices (left). Real-time image separation results through on-device learning using the memristor computing chip developed by our research team (right). >
At the heart of this innovation is a next-generation semiconductor device called a memristor*. The variable resistance characteristics of this device can replace the role of synapses in neural networks, and by utilizing it, data storage and computation can be performed simultaneously, just like our brain cells.
*Memristor: A compound word of memory and resistor, next-generation electrical device whose resistance value is determined by the amount and direction of charge that has flowed between the two terminals in the past.
The research team designed a highly reliable memristor that can precisely control resistance changes and developed an efficient system that excludes complex compensation processes through self-learning. This study is significant in that it experimentally verified the commercialization possibility of a next-generation neuromorphic semiconductor-based integrated system that supports real-time learning and inference.
This technology will revolutionize the way artificial intelligence is used in everyday devices, allowing AI tasks to be processed locally without relying on remote cloud servers, making them faster, more privacy-protected, and more energy-efficient.
“This system is like a smart workspace where everything is within arm’s reach instead of having to go back and forth between desks and file cabinets,” explained KAIST researchers Hakcheon Jeong and Seungjae Han, who led the development of this technology. “This is similar to the way our brain processes information, where everything is processed efficiently at once at one spot.”
The research was conducted with Hakcheon Jeong and Seungjae Han, the students of Integrated Master's and Doctoral Program at KAIST School of Electrical Engineering being the co-first authors, the results of which was published online in the international academic journal, Nature Electronics, on January 8, 2025.
*Paper title: Self-supervised video processing with self-calibration on an analogue computing platform based on a selector-less memristor array ( https://doi.org/10.1038/s41928-024-01318-6 )
This research was supported by the Next-Generation Intelligent Semiconductor Technology Development Project, Excellent New Researcher Project and PIM AI Semiconductor Core Technology Development Project of the National Research Foundation of Korea, and the Electronics and Telecommunications Research Institute Research and Development Support Project of the Institute of Information & communications Technology Planning & Evaluation.
2025.01.17 View 7431
KAIST Develops Neuromorphic Semiconductor Chip that Learns and Corrects Itself
< Photo. The research team of the School of Electrical Engineering posed by the newly deveoped processor. (From center to the right) Professor Young-Gyu Yoon, Integrated Master's and Doctoral Program Students Seungjae Han and Hakcheon Jeong and Professor Shinhyun Choi >
- Professor Shinhyun Choi and Professor Young-Gyu Yoon’s Joint Research Team from the School of Electrical Engineering developed a computing chip that can learn, correct errors, and process AI tasks
- Equipping a computing chip with high-reliability memristor devices with self-error correction functions for real-time learning and image processing
Existing computer systems have separate data processing and storage devices, making them inefficient for processing complex data like AI. A KAIST research team has developed a memristor-based integrated system similar to the way our brain processes information. It is now ready for application in various devices including smart security cameras, allowing them to recognize suspicious activity immediately without having to rely on remote cloud servers, and medical devices with which it can help analyze health data in real time.
KAIST (President Kwang Hyung Lee) announced on the 17th of January that the joint research team of Professor Shinhyun Choi and Professor Young-Gyu Yoon of the School of Electrical Engineering has developed a next-generation neuromorphic semiconductor-based ultra-small computing chip that can learn and correct errors on its own.
< Figure 1. Scanning electron microscope (SEM) image of a computing chip equipped with a highly reliable selector-less 32×32 memristor crossbar array (left). Hardware system developed for real-time artificial intelligence implementation (right). >
What is special about this computing chip is that it can learn and correct errors that occur due to non-ideal characteristics that were difficult to solve in existing neuromorphic devices. For example, when processing a video stream, the chip learns to automatically separate a moving object from the background, and it becomes better at this task over time.
This self-learning ability has been proven by achieving accuracy comparable to ideal computer simulations in real-time image processing. The research team's main achievement is that it has completed a system that is both reliable and practical, beyond the development of brain-like components.
The research team has developed the world's first memristor-based integrated system that can adapt to immediate environmental changes, and has presented an innovative solution that overcomes the limitations of existing technology.
< Figure 2. Background and foreground separation results of an image containing non-ideal characteristics of memristor devices (left). Real-time image separation results through on-device learning using the memristor computing chip developed by our research team (right). >
At the heart of this innovation is a next-generation semiconductor device called a memristor*. The variable resistance characteristics of this device can replace the role of synapses in neural networks, and by utilizing it, data storage and computation can be performed simultaneously, just like our brain cells.
*Memristor: A compound word of memory and resistor, next-generation electrical device whose resistance value is determined by the amount and direction of charge that has flowed between the two terminals in the past.
The research team designed a highly reliable memristor that can precisely control resistance changes and developed an efficient system that excludes complex compensation processes through self-learning. This study is significant in that it experimentally verified the commercialization possibility of a next-generation neuromorphic semiconductor-based integrated system that supports real-time learning and inference.
This technology will revolutionize the way artificial intelligence is used in everyday devices, allowing AI tasks to be processed locally without relying on remote cloud servers, making them faster, more privacy-protected, and more energy-efficient.
“This system is like a smart workspace where everything is within arm’s reach instead of having to go back and forth between desks and file cabinets,” explained KAIST researchers Hakcheon Jeong and Seungjae Han, who led the development of this technology. “This is similar to the way our brain processes information, where everything is processed efficiently at once at one spot.”
The research was conducted with Hakcheon Jeong and Seungjae Han, the students of Integrated Master's and Doctoral Program at KAIST School of Electrical Engineering being the co-first authors, the results of which was published online in the international academic journal, Nature Electronics, on January 8, 2025.
*Paper title: Self-supervised video processing with self-calibration on an analogue computing platform based on a selector-less memristor array ( https://doi.org/10.1038/s41928-024-01318-6 )
This research was supported by the Next-Generation Intelligent Semiconductor Technology Development Project, Excellent New Researcher Project and PIM AI Semiconductor Core Technology Development Project of the National Research Foundation of Korea, and the Electronics and Telecommunications Research Institute Research and Development Support Project of the Institute of Information & communications Technology Planning & Evaluation.
2025.01.17 View 7431 -
 KAIST Develops Insect-Eye-Inspired Camera Capturing 9,120 Frames Per Second
< (From left) Bio and Brain Engineering PhD Student Jae-Myeong Kwon, Professor Ki-Hun Jeong, PhD Student Hyun-Kyung Kim, PhD Student Young-Gil Cha, and Professor Min H. Kim of the School of Computing >
The compound eyes of insects can detect fast-moving objects in parallel and, in low-light conditions, enhance sensitivity by integrating signals over time to determine motion. Inspired by these biological mechanisms, KAIST researchers have successfully developed a low-cost, high-speed camera that overcomes the limitations of frame rate and sensitivity faced by conventional high-speed cameras.
KAIST (represented by President Kwang Hyung Lee) announced on the 16th of January that a research team led by Professors Ki-Hun Jeong (Department of Bio and Brain Engineering) and Min H. Kim (School of Computing) has developed a novel bio-inspired camera capable of ultra-high-speed imaging with high sensitivity by mimicking the visual structure of insect eyes.
High-quality imaging under high-speed and low-light conditions is a critical challenge in many applications. While conventional high-speed cameras excel in capturing fast motion, their sensitivity decreases as frame rates increase because the time available to collect light is reduced.
To address this issue, the research team adopted an approach similar to insect vision, utilizing multiple optical channels and temporal summation. Unlike traditional monocular camera systems, the bio-inspired camera employs a compound-eye-like structure that allows for the parallel acquisition of frames from different time intervals.
< Figure 1. (A) Vision in a fast-eyed insect. Reflected light from swiftly moving objects sequentially stimulates the photoreceptors along the individual optical channels called ommatidia, of which the visual signals are separately and parallelly processed via the lamina and medulla. Each neural response is temporally summed to enhance the visual signals. The parallel processing and temporal summation allow fast and low-light imaging in dim light. (B) High-speed and high-sensitivity microlens array camera (HS-MAC). A rolling shutter image sensor is utilized to simultaneously acquire multiple frames by channel division, and temporal summation is performed in parallel to realize high speed and sensitivity even in a low-light environment. In addition, the frame components of a single fragmented array image are stitched into a single blurred frame, which is subsequently deblurred by compressive image reconstruction. >
During this process, light is accumulated over overlapping time periods for each frame, increasing the signal-to-noise ratio. The researchers demonstrated that their bio-inspired camera could capture objects up to 40 times dimmer than those detectable by conventional high-speed cameras.
The team also introduced a "channel-splitting" technique to significantly enhance the camera's speed, achieving frame rates thousands of times faster than those supported by the image sensors used in packaging. Additionally, a "compressed image restoration" algorithm was employed to eliminate blur caused by frame integration and reconstruct sharp images.
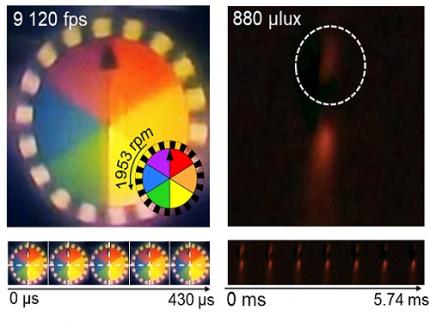
The resulting bio-inspired camera is less than one millimeter thick and extremely compact, capable of capturing 9,120 frames per second while providing clear images in low-light conditions.
< Figure 2. A high-speed, high-sensitivity biomimetic camera packaged in an image sensor. It is made small enough to fit on a finger, with a thickness of less than 1 mm. >
The research team plans to extend this technology to develop advanced image processing algorithms for 3D imaging and super-resolution imaging, aiming for applications in biomedical imaging, mobile devices, and various other camera technologies.
Hyun-Kyung Kim, a doctoral student in the Department of Bio and Brain Engineering at KAIST and the study's first author, stated, “We have experimentally validated that the insect-eye-inspired camera delivers outstanding performance in high-speed and low-light imaging despite its small size. This camera opens up possibilities for diverse applications in portable camera systems, security surveillance, and medical imaging.”
< Figure 3. Rotating plate and flame captured using the high-speed, high-sensitivity biomimetic camera. The rotating plate at 1,950 rpm was accurately captured at 9,120 fps. In addition, the pinch-off of the flame with a faint intensity of 880 µlux was accurately captured at 1,020 fps. >
This research was published in the international journal Science Advances in January 2025 (Paper Title: “Biologically-inspired microlens array camera for high-speed and high-sensitivity imaging”).
DOI: https://doi.org/10.1126/sciadv.ads3389
This study was supported by the Korea Research Institute for Defense Technology Planning and Advancement (KRIT) of the Defense Acquisition Program Administration (DAPA), the Ministry of Science and ICT, and the Ministry of Trade, Industry and Energy (MOTIE).
2025.01.16 View 7363
KAIST Develops Insect-Eye-Inspired Camera Capturing 9,120 Frames Per Second
< (From left) Bio and Brain Engineering PhD Student Jae-Myeong Kwon, Professor Ki-Hun Jeong, PhD Student Hyun-Kyung Kim, PhD Student Young-Gil Cha, and Professor Min H. Kim of the School of Computing >
The compound eyes of insects can detect fast-moving objects in parallel and, in low-light conditions, enhance sensitivity by integrating signals over time to determine motion. Inspired by these biological mechanisms, KAIST researchers have successfully developed a low-cost, high-speed camera that overcomes the limitations of frame rate and sensitivity faced by conventional high-speed cameras.
KAIST (represented by President Kwang Hyung Lee) announced on the 16th of January that a research team led by Professors Ki-Hun Jeong (Department of Bio and Brain Engineering) and Min H. Kim (School of Computing) has developed a novel bio-inspired camera capable of ultra-high-speed imaging with high sensitivity by mimicking the visual structure of insect eyes.
High-quality imaging under high-speed and low-light conditions is a critical challenge in many applications. While conventional high-speed cameras excel in capturing fast motion, their sensitivity decreases as frame rates increase because the time available to collect light is reduced.
To address this issue, the research team adopted an approach similar to insect vision, utilizing multiple optical channels and temporal summation. Unlike traditional monocular camera systems, the bio-inspired camera employs a compound-eye-like structure that allows for the parallel acquisition of frames from different time intervals.
< Figure 1. (A) Vision in a fast-eyed insect. Reflected light from swiftly moving objects sequentially stimulates the photoreceptors along the individual optical channels called ommatidia, of which the visual signals are separately and parallelly processed via the lamina and medulla. Each neural response is temporally summed to enhance the visual signals. The parallel processing and temporal summation allow fast and low-light imaging in dim light. (B) High-speed and high-sensitivity microlens array camera (HS-MAC). A rolling shutter image sensor is utilized to simultaneously acquire multiple frames by channel division, and temporal summation is performed in parallel to realize high speed and sensitivity even in a low-light environment. In addition, the frame components of a single fragmented array image are stitched into a single blurred frame, which is subsequently deblurred by compressive image reconstruction. >
During this process, light is accumulated over overlapping time periods for each frame, increasing the signal-to-noise ratio. The researchers demonstrated that their bio-inspired camera could capture objects up to 40 times dimmer than those detectable by conventional high-speed cameras.
The team also introduced a "channel-splitting" technique to significantly enhance the camera's speed, achieving frame rates thousands of times faster than those supported by the image sensors used in packaging. Additionally, a "compressed image restoration" algorithm was employed to eliminate blur caused by frame integration and reconstruct sharp images.
The resulting bio-inspired camera is less than one millimeter thick and extremely compact, capable of capturing 9,120 frames per second while providing clear images in low-light conditions.
< Figure 2. A high-speed, high-sensitivity biomimetic camera packaged in an image sensor. It is made small enough to fit on a finger, with a thickness of less than 1 mm. >
The research team plans to extend this technology to develop advanced image processing algorithms for 3D imaging and super-resolution imaging, aiming for applications in biomedical imaging, mobile devices, and various other camera technologies.
Hyun-Kyung Kim, a doctoral student in the Department of Bio and Brain Engineering at KAIST and the study's first author, stated, “We have experimentally validated that the insect-eye-inspired camera delivers outstanding performance in high-speed and low-light imaging despite its small size. This camera opens up possibilities for diverse applications in portable camera systems, security surveillance, and medical imaging.”
< Figure 3. Rotating plate and flame captured using the high-speed, high-sensitivity biomimetic camera. The rotating plate at 1,950 rpm was accurately captured at 9,120 fps. In addition, the pinch-off of the flame with a faint intensity of 880 µlux was accurately captured at 1,020 fps. >
This research was published in the international journal Science Advances in January 2025 (Paper Title: “Biologically-inspired microlens array camera for high-speed and high-sensitivity imaging”).
DOI: https://doi.org/10.1126/sciadv.ads3389
This study was supported by the Korea Research Institute for Defense Technology Planning and Advancement (KRIT) of the Defense Acquisition Program Administration (DAPA), the Ministry of Science and ICT, and the Ministry of Trade, Industry and Energy (MOTIE).
2025.01.16 View 7363 -
 KAIST Alumni Association to Honor Alumni of the Year Award Winners
Photo 1. Photo of the KAIST Alumni of the Year Award Recipients
(From left) UST President Lee-whan Kim, CEO Han Chung of iThree Systems Co., Ltd., CEO Dong Myung Kim of LG Energy Solution Co., Ltd., and Professor Hyun Myung of the School of Electrical Engineering at KAIST
KAIST (President Kwang Hyung Lee) announced on Monday, the 13th of January that the Alumni Association (President Yun-Tae Lee) has selected its Alumni of the Year.
This year’s honorees are: ▴ President Lee-whan Kim of the Korea National University of Science and Technology (UST), ▴ CEO Han Chung of i3 Systems, ▴ CEO Dong Myung Kim of LG Energy Solution, and ▴ Professor Hyun Myung of the School of Electrical Engineering at KAIST.
The honorees were selected based on their achievements over the past year, and the award ceremony will be held at the 2025 KAIST Alumni Association New Year’s Gathering to be held at the L Tower in Seoul at 5 PM on Friday the 17th.
The KAIST Alumni of the Year Award is an award presented by the Alumni Association to alumni who have contributed to the development of the country and the society or have brought honor to their alma mater through outstanding academic achievements and community service. Since its establishment in 1992, 126 recipients have been awarded.
Lee-whan Kim (Master's graduate of Mechanical Engineering, 82), the President of the Korea National University of Science and Technology (UST), established a leading foundation for national science and technology policy and strategy, and played a leading role in innovating national science and technology capabilities through the advancement of the national research and development system and the advancement of science and technology personnel training. In particular, he played a pivotal role in the establishment of UST and the Korea Science Academy (KSA), and greatly contributed to establishing a foundation for the training and utilization of science and technology personnel.
Han Chung (Master's graduate of Electrical Engineering, 91, with Ph.D. degree in 96), the CEO of i3 Systems, is a first-generation researcher in the field of domestic infrared detectors. He developed military detectors for over 30 years and founded i3 Systems, a specialized infrared detector company, in 1998. Currently, he supplies more than 80% of the infrared detectors used by the Korean military, and has also achieved export results to over 20 countries.
Dong Myung Kim (Master's graduate of Materials Science and Engineering, 94, with Ph.D. degree in 98) the CEO of LG Energy Solution Co., Ltd. has led innovation in the battery field with his ceaseless exploration and challenging spirit, and is known as an authority in the secondary battery industry. He played a leading role in establishing K-Battery as a global leader, strengthened the country's future industrial competitiveness, and greatly contributed to the development of science and technology.
Hyun Myung (Bachelor's graduate of Electrical Engineering, 92, with Master's degree in 94, and Ph.D. degree in 98) a Professor of Electrical Engineering, KAIST, won first place in the world at the Quadruped Robot Challenge (QRC) hosted by the IEEE’s International Conference on Robotics and Automation (ICRA) 2023 with the 'DreamWaQ' system, an AI walking technology based on deep reinforcement learning that utilizes non-video sensory technologies. He contributed to enhancing the competitiveness of the domestic robot industry by developing his own fully autonomous walking technology that recognizes the environment around the robot and finds the optimal path.
Yun-Tae Lee, the 27th president of the KAIST Alumni Association, said, “KAIST alumni have been the driving force behind the growth of industries in all walks of life by continuously conducting research and development in the field of advanced science and technology for a long time,” and added, “I am very proud of the KAIST alumni award recipients who are leading science and technology on the world stage beyond Korea, and I sincerely thank them for their efforts and achievements.”
2025.01.15 View 5347
KAIST Alumni Association to Honor Alumni of the Year Award Winners
Photo 1. Photo of the KAIST Alumni of the Year Award Recipients
(From left) UST President Lee-whan Kim, CEO Han Chung of iThree Systems Co., Ltd., CEO Dong Myung Kim of LG Energy Solution Co., Ltd., and Professor Hyun Myung of the School of Electrical Engineering at KAIST
KAIST (President Kwang Hyung Lee) announced on Monday, the 13th of January that the Alumni Association (President Yun-Tae Lee) has selected its Alumni of the Year.
This year’s honorees are: ▴ President Lee-whan Kim of the Korea National University of Science and Technology (UST), ▴ CEO Han Chung of i3 Systems, ▴ CEO Dong Myung Kim of LG Energy Solution, and ▴ Professor Hyun Myung of the School of Electrical Engineering at KAIST.
The honorees were selected based on their achievements over the past year, and the award ceremony will be held at the 2025 KAIST Alumni Association New Year’s Gathering to be held at the L Tower in Seoul at 5 PM on Friday the 17th.
The KAIST Alumni of the Year Award is an award presented by the Alumni Association to alumni who have contributed to the development of the country and the society or have brought honor to their alma mater through outstanding academic achievements and community service. Since its establishment in 1992, 126 recipients have been awarded.
Lee-whan Kim (Master's graduate of Mechanical Engineering, 82), the President of the Korea National University of Science and Technology (UST), established a leading foundation for national science and technology policy and strategy, and played a leading role in innovating national science and technology capabilities through the advancement of the national research and development system and the advancement of science and technology personnel training. In particular, he played a pivotal role in the establishment of UST and the Korea Science Academy (KSA), and greatly contributed to establishing a foundation for the training and utilization of science and technology personnel.
Han Chung (Master's graduate of Electrical Engineering, 91, with Ph.D. degree in 96), the CEO of i3 Systems, is a first-generation researcher in the field of domestic infrared detectors. He developed military detectors for over 30 years and founded i3 Systems, a specialized infrared detector company, in 1998. Currently, he supplies more than 80% of the infrared detectors used by the Korean military, and has also achieved export results to over 20 countries.
Dong Myung Kim (Master's graduate of Materials Science and Engineering, 94, with Ph.D. degree in 98) the CEO of LG Energy Solution Co., Ltd. has led innovation in the battery field with his ceaseless exploration and challenging spirit, and is known as an authority in the secondary battery industry. He played a leading role in establishing K-Battery as a global leader, strengthened the country's future industrial competitiveness, and greatly contributed to the development of science and technology.
Hyun Myung (Bachelor's graduate of Electrical Engineering, 92, with Master's degree in 94, and Ph.D. degree in 98) a Professor of Electrical Engineering, KAIST, won first place in the world at the Quadruped Robot Challenge (QRC) hosted by the IEEE’s International Conference on Robotics and Automation (ICRA) 2023 with the 'DreamWaQ' system, an AI walking technology based on deep reinforcement learning that utilizes non-video sensory technologies. He contributed to enhancing the competitiveness of the domestic robot industry by developing his own fully autonomous walking technology that recognizes the environment around the robot and finds the optimal path.
Yun-Tae Lee, the 27th president of the KAIST Alumni Association, said, “KAIST alumni have been the driving force behind the growth of industries in all walks of life by continuously conducting research and development in the field of advanced science and technology for a long time,” and added, “I am very proud of the KAIST alumni award recipients who are leading science and technology on the world stage beyond Korea, and I sincerely thank them for their efforts and achievements.”
2025.01.15 View 5347 -
 KAIST Research Team Develops Stretchable Microelectrodes Array for Organoid Signal Monitoring
< Photo 1. (From top left) Professor Hyunjoo J. Lee, Dr. Mi-Young Son, Dr. Mi-Ok Lee(In the front row from left) Doctoral student Kiup Kim, Doctoral student Youngsun Lee >
On January 14th, the KAIST research team led by Professor Hyunjoo J. Lee from the School of Electrical Engineering in collaboration with Dr. Mi-Young Son and Dr. Mi-Ok Lee at Korea Research Institute of Bioscience and Biotechnology (KRIBB) announced the development of a highly stretchable microelectrode array (sMEA) designed for non-invasive electrophysiological signal measurement of organoids.
Organoids* are highly promising models for human biology and are expected to replace many animal experiments. Their potential applications include disease modeling, drug screening, and personalized medicine as they closely mimic the structure and function of humans.
*Organoids: three-dimensional in vitro tissue models derived from human stem cells
Despite these advantages, existing organoid research has primarily focused on genetic analysis, with limited studies on organoid functionality. For effective drug evaluation and precise biological research, technology that preserves the three-dimensional structure of organoids while enabling real-time monitoring of their functions is needed. However, it’s challenging to provide non-invasive ways to evaluate the functionalities without incurring damage to the tissues. This challenge is particularly significant for electrophysiological signal measurement in cardiac and brain organoids since the sensor needs to be in direct contact with organoids of varying size and irregular shape. Achieving tight contact between electrodes and the external surface of the organoids without damaging the organoids has been a persistent challenge.
< Figure 1. Schematic image of highly stretchable MEA (sMEA) with protruding microelectrodes. >
The KAIST research team developed a highly stretchable microelectrode array with a unique serpentine structure that contacts the surface of organoids in a highly conformal fashion. They successfully demonstrated real-time measurement and analysis of electrophysiological signals from two types of electrogenic organoids (heart and brain). By employing a micro-electromechanical system (MEMS)-based process, the team fabricated the serpentine-structured microelectrode array and used an electrochemical deposition process to develop PEDOT:PSS-based protruding microelectrodes. These innovations demonstrated exceptional stretchability and close surface adherence to various organoid sizes. The protruding microelectrodes improved contact between organoids and the electrodes, ensuring stable and reliable electrophysiological signal measurements with high signal-to-noise ratios (SNR).
< Figure 2. Conceptual illustration, optical image, and fluorescence images of an organoid captured by the sMEA with protruding microelectrodes.>
Using this technology, the team successfully monitored and analyzed electrophysiological signals from cardiac spheroids of various sizes, revealing three-dimensional signal propagation patterns and identifying changes in signal characteristics according to size. They also measured electrophysiological signals in midbrain organoids, demonstrating the versatility of the technology. Additionally, they monitored signal modulations induced by various drugs, showcasing the potential of this technology for drug screening applications.
< Figure 3. SNR improvement effect by protruding PEDOT:PSS microelectrodes. >
Prof. Hyunjoo Jenny Lee stated, “By integrating MEMS technology and electrochemical deposition techniques, we successfully developed a stretchable microelectrode array adaptable to organoids of diverse sizes and shapes. The high practicality is a major advantage of this system since the fabrication is based on semiconductor fabrication with high volume production, reliability, and accuracy. This technology that enables in situ, real-time analysis of states and functionalities of organoids will be a game changer in high-through drug screening.”
This study led by Ph.D. candidate Kiup Kim from KAIST and Ph.D. candidate Youngsun Lee from KRIBB, with significant contributions from Dr. Kwang Bo Jung, was published online on December 15, 2024 in Advanced Materials (IF: 27.4).
< Figure 4. Drug screening using cardiac spheroids and midbrain organoids.>
This research was supported by a grant from 3D-TissueChip Based Drug Discovery Platform Technology Development Program (No. 20009209) funded by the Ministry of Trade, Industry & Energy (MOTIE, Korea), by the Commercialization Promotion Agency for R&D Outcomes (COMPA) funded by the Ministry of Science and ICT (MSIT) (RS-2024-00415902), by the K-Brain Project of the National Research Foundation (NRF) funded by the Korean government (MSIT) (RS-2023-00262568), by BK21 FOUR (Connected AI Education & Research Program for Industry and Society Innovation, KAIST EE, No. 4120200113769), and by Korea Research Institute of Bioscience and Biotechnology (KRIBB) Research Initiative Program (KGM4722432).
2025.01.14 View 4015
KAIST Research Team Develops Stretchable Microelectrodes Array for Organoid Signal Monitoring
< Photo 1. (From top left) Professor Hyunjoo J. Lee, Dr. Mi-Young Son, Dr. Mi-Ok Lee(In the front row from left) Doctoral student Kiup Kim, Doctoral student Youngsun Lee >
On January 14th, the KAIST research team led by Professor Hyunjoo J. Lee from the School of Electrical Engineering in collaboration with Dr. Mi-Young Son and Dr. Mi-Ok Lee at Korea Research Institute of Bioscience and Biotechnology (KRIBB) announced the development of a highly stretchable microelectrode array (sMEA) designed for non-invasive electrophysiological signal measurement of organoids.
Organoids* are highly promising models for human biology and are expected to replace many animal experiments. Their potential applications include disease modeling, drug screening, and personalized medicine as they closely mimic the structure and function of humans.
*Organoids: three-dimensional in vitro tissue models derived from human stem cells
Despite these advantages, existing organoid research has primarily focused on genetic analysis, with limited studies on organoid functionality. For effective drug evaluation and precise biological research, technology that preserves the three-dimensional structure of organoids while enabling real-time monitoring of their functions is needed. However, it’s challenging to provide non-invasive ways to evaluate the functionalities without incurring damage to the tissues. This challenge is particularly significant for electrophysiological signal measurement in cardiac and brain organoids since the sensor needs to be in direct contact with organoids of varying size and irregular shape. Achieving tight contact between electrodes and the external surface of the organoids without damaging the organoids has been a persistent challenge.
< Figure 1. Schematic image of highly stretchable MEA (sMEA) with protruding microelectrodes. >
The KAIST research team developed a highly stretchable microelectrode array with a unique serpentine structure that contacts the surface of organoids in a highly conformal fashion. They successfully demonstrated real-time measurement and analysis of electrophysiological signals from two types of electrogenic organoids (heart and brain). By employing a micro-electromechanical system (MEMS)-based process, the team fabricated the serpentine-structured microelectrode array and used an electrochemical deposition process to develop PEDOT:PSS-based protruding microelectrodes. These innovations demonstrated exceptional stretchability and close surface adherence to various organoid sizes. The protruding microelectrodes improved contact between organoids and the electrodes, ensuring stable and reliable electrophysiological signal measurements with high signal-to-noise ratios (SNR).
< Figure 2. Conceptual illustration, optical image, and fluorescence images of an organoid captured by the sMEA with protruding microelectrodes.>
Using this technology, the team successfully monitored and analyzed electrophysiological signals from cardiac spheroids of various sizes, revealing three-dimensional signal propagation patterns and identifying changes in signal characteristics according to size. They also measured electrophysiological signals in midbrain organoids, demonstrating the versatility of the technology. Additionally, they monitored signal modulations induced by various drugs, showcasing the potential of this technology for drug screening applications.
< Figure 3. SNR improvement effect by protruding PEDOT:PSS microelectrodes. >
Prof. Hyunjoo Jenny Lee stated, “By integrating MEMS technology and electrochemical deposition techniques, we successfully developed a stretchable microelectrode array adaptable to organoids of diverse sizes and shapes. The high practicality is a major advantage of this system since the fabrication is based on semiconductor fabrication with high volume production, reliability, and accuracy. This technology that enables in situ, real-time analysis of states and functionalities of organoids will be a game changer in high-through drug screening.”
This study led by Ph.D. candidate Kiup Kim from KAIST and Ph.D. candidate Youngsun Lee from KRIBB, with significant contributions from Dr. Kwang Bo Jung, was published online on December 15, 2024 in Advanced Materials (IF: 27.4).
< Figure 4. Drug screening using cardiac spheroids and midbrain organoids.>
This research was supported by a grant from 3D-TissueChip Based Drug Discovery Platform Technology Development Program (No. 20009209) funded by the Ministry of Trade, Industry & Energy (MOTIE, Korea), by the Commercialization Promotion Agency for R&D Outcomes (COMPA) funded by the Ministry of Science and ICT (MSIT) (RS-2024-00415902), by the K-Brain Project of the National Research Foundation (NRF) funded by the Korean government (MSIT) (RS-2023-00262568), by BK21 FOUR (Connected AI Education & Research Program for Industry and Society Innovation, KAIST EE, No. 4120200113769), and by Korea Research Institute of Bioscience and Biotechnology (KRIBB) Research Initiative Program (KGM4722432).
2025.01.14 View 4015 -
 KAIST Develops CamBio - a New Biotemplating Method
- Professor Jae-Byum Chang and Professor Yeon Sik Jung’s joint research team of the Department of Materials Science and Engineering developed a highly tunable bio-templating method “CamBio” that makes use of intracellular protein structures
- Substrate performance improvement of up to 230% demonstrated via surface-enhanced Raman spectroscopy (SERS)
- Expected to have price competitiveness over bio-templating method as it expands the range of biological samples
- Expected to expand the range of application of nanostructure synthesis technology by utilizing various biological structures
< Photo 1. (From left) Professor Yeon Sik Jung, Ph.D. candidate Dae-Hyeon Song, Professor Jae-Byum Chang, and (from top right) Dr. Chang Woo Song and Dr. Seunghee H. Cho of the Department of Materials Science and Engineering >
Biological structures have complex characteristics that are difficult to replicate artificially, but biotemplating methods* that directly utilize these biological structures have been used in various fields of application. The KAIST research team succeeded in utilizing previously unusable biological structures and expanding the areas in which biotemplate methods can be applied.
*Biotemplating: A method of using biotemplates as a mold to create functional structural materials, utilizing the functions of these biological structures, from viruses to the tissues and organs that make up our bodies
KAIST (President Kwang Hyung Lee) announced on the 10th that a joint research team of Professors Jae-Byum Chang and Professor Yeon Sik Jung of the Department of Materials Science and Engineering developed a biotemplating method that utilizes specific intracellular proteins in biological samples and has high tunability.
Existing biotemplate methods mainly utilize only the external surface of biological samples or have limitations in utilizing the structure-function correlation of various biological structures due to limited dimensions and sample sizes, making it difficult to create functional nanostructures.
To solve this problem, the research team studied a way to utilize various biological structures within the cells while retaining high tunability.
< Figure 1. CamBio utilizing microtubules, a intracellular protein structure. The silver nanoparticle chains synthesized along the microtubules that span the entire cell interior can be observed through an electron microscope, and it is shown that this can be used as a successful SERS substrate. >
As a result of the research, the team developed the “Conversion to advanced materials via labeled Biostructure”, shortened as “CamBio”, which enables the selective synthesis of nanostructures with various characteristics and sizes from specific protein structures composed of diverse proteins within biological specimens.
The CamBio method secures high tunability of functional nanostructures that can be manufactured from biological samples by merging various manufacturing and biological technologies.
Through the technology of repeatedly attaching antibodies, arranging cells in a certain shape, and thinly slicing tissue, the functional nanostructures made with CamBio showed improved performance on the surface-enhanced Raman spectroscopy (SERS)* substrate used for material detection.
*Surface-enhanced Raman spectroscopy (SERS): A technology that can detect very small amounts of substances using light, based on the principle that specific substances react to light and amplifies signals on surfaces of metals such as gold or silver.
The research team found that the nanoparticle chains made using the intracellular protein structures through the process of repeated labeling with antibodies allowed easier control, and improved SERS performance by up to 230%.
In addition, the research team expanded from utilizing the structures inside cells to obtaining samples of muscle tissues inside meat using a cryostat and successfully producing a substrate with periodic bands made of metal particles by performing the CamBio process. This method of producing a substrate not only allows large-scale production using biological samples, but also shows that it is a cost-effective method.
< Figure 2. A method for securing tunability using CamBio at the cell level. Examples of controlling characteristics by integrating iterative labeling and cell pattering techniques with CamBio are shown. >
The CamBio developed by the research team is expected to be used as a way to solve problems faced by various research fields as it is to expand the range of bio-samples that can be produced for various usage.
The first author, Dae-Hyeon Song, a Ph.D. candidate of KAIST Department of Materials Science and Engineering said, “Through CamBio, we have comprehensively accumulated biotemplating methods that can utilize more diverse protein structures,” and “If combined with the state-of-the-art biological technologies such as gene editing and 3D bioprinting and new material synthesis technologies, biostructures can be utilized in various fields of application.”
< Figure 3. A method for securing tunability using CamBio at the tissue level. In order to utilize proteins inside muscle tissue, the frozen tissue sectioning technology is combined, and through this, a substrate with a periodic nanoparticle band pattern is successfully produced, and it is shown that large-area acquisition of samples and price competitiveness can be achieved. >
This study, in which the Ph.D. candidate Dae-Hyeon Song along with Dr. Chang Woo Song, and Dr. Seunghee H. Cho of the same department participated as the first authors, was published online in the international academic journal, Advanced Science, on November 13th, 2024.
(Paper title: Highly Tunable, Nanomaterial-Functionalized Structural Templating of Intracellular Protein Structures Within Biological Species) https://doi.org/10.1002/advs.202406492
This study was conducted with a combination of support from various programs including the National Convergence Research of Scientific Challenges (National Research Foundation of Korea (NRF) 2024), Engineering Reseach Center (ERC) (Wearable Platform Materials Technology Center, NRF 2023), ERC (Global Bio-integrated Materials Center, NRF 2024), and the National Advanced Program for Biological Research Resources (Bioimaging Data Curation Center, NRF 2024) funded by Ministry of Science and ICT.
2025.01.10 View 4933
KAIST Develops CamBio - a New Biotemplating Method
- Professor Jae-Byum Chang and Professor Yeon Sik Jung’s joint research team of the Department of Materials Science and Engineering developed a highly tunable bio-templating method “CamBio” that makes use of intracellular protein structures
- Substrate performance improvement of up to 230% demonstrated via surface-enhanced Raman spectroscopy (SERS)
- Expected to have price competitiveness over bio-templating method as it expands the range of biological samples
- Expected to expand the range of application of nanostructure synthesis technology by utilizing various biological structures
< Photo 1. (From left) Professor Yeon Sik Jung, Ph.D. candidate Dae-Hyeon Song, Professor Jae-Byum Chang, and (from top right) Dr. Chang Woo Song and Dr. Seunghee H. Cho of the Department of Materials Science and Engineering >
Biological structures have complex characteristics that are difficult to replicate artificially, but biotemplating methods* that directly utilize these biological structures have been used in various fields of application. The KAIST research team succeeded in utilizing previously unusable biological structures and expanding the areas in which biotemplate methods can be applied.
*Biotemplating: A method of using biotemplates as a mold to create functional structural materials, utilizing the functions of these biological structures, from viruses to the tissues and organs that make up our bodies
KAIST (President Kwang Hyung Lee) announced on the 10th that a joint research team of Professors Jae-Byum Chang and Professor Yeon Sik Jung of the Department of Materials Science and Engineering developed a biotemplating method that utilizes specific intracellular proteins in biological samples and has high tunability.
Existing biotemplate methods mainly utilize only the external surface of biological samples or have limitations in utilizing the structure-function correlation of various biological structures due to limited dimensions and sample sizes, making it difficult to create functional nanostructures.
To solve this problem, the research team studied a way to utilize various biological structures within the cells while retaining high tunability.
< Figure 1. CamBio utilizing microtubules, a intracellular protein structure. The silver nanoparticle chains synthesized along the microtubules that span the entire cell interior can be observed through an electron microscope, and it is shown that this can be used as a successful SERS substrate. >
As a result of the research, the team developed the “Conversion to advanced materials via labeled Biostructure”, shortened as “CamBio”, which enables the selective synthesis of nanostructures with various characteristics and sizes from specific protein structures composed of diverse proteins within biological specimens.
The CamBio method secures high tunability of functional nanostructures that can be manufactured from biological samples by merging various manufacturing and biological technologies.
Through the technology of repeatedly attaching antibodies, arranging cells in a certain shape, and thinly slicing tissue, the functional nanostructures made with CamBio showed improved performance on the surface-enhanced Raman spectroscopy (SERS)* substrate used for material detection.
*Surface-enhanced Raman spectroscopy (SERS): A technology that can detect very small amounts of substances using light, based on the principle that specific substances react to light and amplifies signals on surfaces of metals such as gold or silver.
The research team found that the nanoparticle chains made using the intracellular protein structures through the process of repeated labeling with antibodies allowed easier control, and improved SERS performance by up to 230%.
In addition, the research team expanded from utilizing the structures inside cells to obtaining samples of muscle tissues inside meat using a cryostat and successfully producing a substrate with periodic bands made of metal particles by performing the CamBio process. This method of producing a substrate not only allows large-scale production using biological samples, but also shows that it is a cost-effective method.
< Figure 2. A method for securing tunability using CamBio at the cell level. Examples of controlling characteristics by integrating iterative labeling and cell pattering techniques with CamBio are shown. >
The CamBio developed by the research team is expected to be used as a way to solve problems faced by various research fields as it is to expand the range of bio-samples that can be produced for various usage.
The first author, Dae-Hyeon Song, a Ph.D. candidate of KAIST Department of Materials Science and Engineering said, “Through CamBio, we have comprehensively accumulated biotemplating methods that can utilize more diverse protein structures,” and “If combined with the state-of-the-art biological technologies such as gene editing and 3D bioprinting and new material synthesis technologies, biostructures can be utilized in various fields of application.”
< Figure 3. A method for securing tunability using CamBio at the tissue level. In order to utilize proteins inside muscle tissue, the frozen tissue sectioning technology is combined, and through this, a substrate with a periodic nanoparticle band pattern is successfully produced, and it is shown that large-area acquisition of samples and price competitiveness can be achieved. >
This study, in which the Ph.D. candidate Dae-Hyeon Song along with Dr. Chang Woo Song, and Dr. Seunghee H. Cho of the same department participated as the first authors, was published online in the international academic journal, Advanced Science, on November 13th, 2024.
(Paper title: Highly Tunable, Nanomaterial-Functionalized Structural Templating of Intracellular Protein Structures Within Biological Species) https://doi.org/10.1002/advs.202406492
This study was conducted with a combination of support from various programs including the National Convergence Research of Scientific Challenges (National Research Foundation of Korea (NRF) 2024), Engineering Reseach Center (ERC) (Wearable Platform Materials Technology Center, NRF 2023), ERC (Global Bio-integrated Materials Center, NRF 2024), and the National Advanced Program for Biological Research Resources (Bioimaging Data Curation Center, NRF 2024) funded by Ministry of Science and ICT.
2025.01.10 View 4933 -
 “Cross-Generation Collaborative Labs” for Semiconductor, Chemistry, and Computer Science Opened
< Photo of Professor Hoi-Jun Yoo (center) of the School of Electrical Engineering at the signboard unveiling ceremony >
KAIST held a ceremony to mark the opening of three additional ‘Cross-Generation Collaborative Labs’ on the morning of January 7th, 2025.
The “Next-Generation AI Semiconductor System Lab” by Professor Hoi-Jun Yoo of the School of Electrical Engineering, the “Molecular Spectroscopy and Chemical Dynamics Lab” by Professor Sang Kyu Kim of the Department of Chemistry, and the “Advanced Data Computing Lab” by Professor Sue Bok Moon of the School of Computer Science are the three new labs given the honored titled of the “Cross-Generation Collaborative Lab”.
The Cross-Generation Collaborative Lab is KAIST’s unique system that was set up to facilitate the collaboration between retiring professors and junior professors to continue the achievements and know-how the elders have accumulated over their academic career. Since its introduction in 2018, nine labs have been named to be the Cross-Generation Labs, and this year’s new addition brings the total up to twelve.
The ‘Next-Generation AI Semiconductor System Lab’ led by Professor Hoi-Jun Yoo will be operated by Professor Joo-Young Kim of the same school.
Professor Hoi-Jun Yoo is a world-renowned scholar with outstanding research achievements in the field of on-device AI semiconductor design. Professor Joo-Young Kim is an up-and-coming researcher studying large language models and design of AI semiconductors for server computers, and is currently researching technologies to design PIM (Processing-in-Memory), a core technology in the field of AI semiconductors.
Their research goal is to systematically collaborate and transfer next-generation AI semiconductor design technology, including brain-mimicking AI algorithms such as deep neural networks and generative AI, to integrate core technologies, and to maximize the usability of R&D outputs, thereby further solidifying the position of Korean AI semiconductor companies in the global market.
Professor Hoi-Jun Yoo said, “I believe that, we will be able to present a development direction of for the next-generation AI semiconductors industries at home and abroad through collaborative research and play a key role in transferring and expanding global leadership.”
< Professor Sang Kyu Kim of the Department of Chemistry (middle), at the signboard unveiling ceremony for his laboratory >
The “Molecular Spectroscopy and Chemical Dynamics Laboratory”, where Professor Sang Kyu Kim of the Department of Chemistry is in charge, will be operated by Professor Tae Kyu Kim of the same department, and another professor in the field of spectroscopy and dynamics will join in the future.
Professor Sang Kyu Kim has secured technologies for developing unique experimental equipment based on ultrashort lasers and supersonic molecular beams, and is a world leader who has been creatively pioneering new fields of experimental physical chemistry.
The research goal is to describe chemical reactions and verify from a quantum mechanical perspective and introduce new theories and technologies to pursue a complete understanding of the principles of chemical reactions. In addition, the accompanying basic scientific knowledge will be applied to the design of new materials.
Professor Sang Kyu Kim said, “I am very happy to be able to pass on the research infrastructure to the next generation through this system, and I will continue to nurture it to grow into a world-class research lab through trans-generational collaborative research.”
< Photo of Professor Sue Bok Moon (center) at the signboard unveiling ceremony by the School of Computing >
Lastly, the “Advanced Data Computing Lab” led by Professor Sue Bok Moon is joined by Professor Mee Young Cha of the same school and Professor Wonjae Lee of the Graduate School of Culture Technology.
Professor Sue Bok Moon showed the infinite possibilities of large-scale data-based social network research through Cyworld, YouTube, and Twitter, and had a great influence on related fields beyond the field of computer science.
Professor Mee Young Cha is a data scientist who analyzes difficult social issues such as misinformation, poverty, and disaster detection using big data-based AI. She is the first Korean to be recognized for her achievements as the director of the Max Planck Institute in Germany, a world-class basic science research institute. Therefore, there is high expectation for synergy effects from overseas collaborative research and technology transfer and sharing among the participating professors of the collaborative research lab. Professor Wonjae Lee is researching dynamic interaction analysis between science and technology using structural topic models.
They plan to conduct research aimed at improving the analysis and understanding of negative influences occurring online, and in particular, developing a hateful precursor detection model using emotions and morality to preemptively block hateful expressions.
Professor Sue Bok Moon said, “Through this collaborative research lab, we will play a key role in conducting in-depth collaborative research on unexpected negative influences in the AI era so that we can have a high level of competitiveness worldwide.”
The ceremonies for the unveiling of the new Cross-Generation Collaborative Lab signboard were held in front of each lab from 10:00 AM on the 7th, in the attendance of President Kwang Hyung Lee, Senior Vice President for Research Sang Yup Lee, and other key officials of KAIST and the new staff members to join the laboratories.
2025.01.07 View 4883
“Cross-Generation Collaborative Labs” for Semiconductor, Chemistry, and Computer Science Opened
< Photo of Professor Hoi-Jun Yoo (center) of the School of Electrical Engineering at the signboard unveiling ceremony >
KAIST held a ceremony to mark the opening of three additional ‘Cross-Generation Collaborative Labs’ on the morning of January 7th, 2025.
The “Next-Generation AI Semiconductor System Lab” by Professor Hoi-Jun Yoo of the School of Electrical Engineering, the “Molecular Spectroscopy and Chemical Dynamics Lab” by Professor Sang Kyu Kim of the Department of Chemistry, and the “Advanced Data Computing Lab” by Professor Sue Bok Moon of the School of Computer Science are the three new labs given the honored titled of the “Cross-Generation Collaborative Lab”.
The Cross-Generation Collaborative Lab is KAIST’s unique system that was set up to facilitate the collaboration between retiring professors and junior professors to continue the achievements and know-how the elders have accumulated over their academic career. Since its introduction in 2018, nine labs have been named to be the Cross-Generation Labs, and this year’s new addition brings the total up to twelve.
The ‘Next-Generation AI Semiconductor System Lab’ led by Professor Hoi-Jun Yoo will be operated by Professor Joo-Young Kim of the same school.
Professor Hoi-Jun Yoo is a world-renowned scholar with outstanding research achievements in the field of on-device AI semiconductor design. Professor Joo-Young Kim is an up-and-coming researcher studying large language models and design of AI semiconductors for server computers, and is currently researching technologies to design PIM (Processing-in-Memory), a core technology in the field of AI semiconductors.
Their research goal is to systematically collaborate and transfer next-generation AI semiconductor design technology, including brain-mimicking AI algorithms such as deep neural networks and generative AI, to integrate core technologies, and to maximize the usability of R&D outputs, thereby further solidifying the position of Korean AI semiconductor companies in the global market.
Professor Hoi-Jun Yoo said, “I believe that, we will be able to present a development direction of for the next-generation AI semiconductors industries at home and abroad through collaborative research and play a key role in transferring and expanding global leadership.”
< Professor Sang Kyu Kim of the Department of Chemistry (middle), at the signboard unveiling ceremony for his laboratory >
The “Molecular Spectroscopy and Chemical Dynamics Laboratory”, where Professor Sang Kyu Kim of the Department of Chemistry is in charge, will be operated by Professor Tae Kyu Kim of the same department, and another professor in the field of spectroscopy and dynamics will join in the future.
Professor Sang Kyu Kim has secured technologies for developing unique experimental equipment based on ultrashort lasers and supersonic molecular beams, and is a world leader who has been creatively pioneering new fields of experimental physical chemistry.
The research goal is to describe chemical reactions and verify from a quantum mechanical perspective and introduce new theories and technologies to pursue a complete understanding of the principles of chemical reactions. In addition, the accompanying basic scientific knowledge will be applied to the design of new materials.
Professor Sang Kyu Kim said, “I am very happy to be able to pass on the research infrastructure to the next generation through this system, and I will continue to nurture it to grow into a world-class research lab through trans-generational collaborative research.”
< Photo of Professor Sue Bok Moon (center) at the signboard unveiling ceremony by the School of Computing >
Lastly, the “Advanced Data Computing Lab” led by Professor Sue Bok Moon is joined by Professor Mee Young Cha of the same school and Professor Wonjae Lee of the Graduate School of Culture Technology.
Professor Sue Bok Moon showed the infinite possibilities of large-scale data-based social network research through Cyworld, YouTube, and Twitter, and had a great influence on related fields beyond the field of computer science.
Professor Mee Young Cha is a data scientist who analyzes difficult social issues such as misinformation, poverty, and disaster detection using big data-based AI. She is the first Korean to be recognized for her achievements as the director of the Max Planck Institute in Germany, a world-class basic science research institute. Therefore, there is high expectation for synergy effects from overseas collaborative research and technology transfer and sharing among the participating professors of the collaborative research lab. Professor Wonjae Lee is researching dynamic interaction analysis between science and technology using structural topic models.
They plan to conduct research aimed at improving the analysis and understanding of negative influences occurring online, and in particular, developing a hateful precursor detection model using emotions and morality to preemptively block hateful expressions.
Professor Sue Bok Moon said, “Through this collaborative research lab, we will play a key role in conducting in-depth collaborative research on unexpected negative influences in the AI era so that we can have a high level of competitiveness worldwide.”
The ceremonies for the unveiling of the new Cross-Generation Collaborative Lab signboard were held in front of each lab from 10:00 AM on the 7th, in the attendance of President Kwang Hyung Lee, Senior Vice President for Research Sang Yup Lee, and other key officials of KAIST and the new staff members to join the laboratories.
2025.01.07 View 4883 -
 KAIST Wins CES 2025 Innovation Award, Showcasing Innovative Technologies
KAIST will showcase innovative technologies at the world’s largest technology fair, the Consumer Electronics Show (CES 2025). In addition, KAIST startups VIRNECT Inc., Standard Energy Inc., A2US Inc., and Panmnesia, Inc. won the 2025 CES Innovation Awards.
< Image 1. 3D-Graphical Profile of CES 2025 KAIST Exhibition Booth >
KAIST (President Kwang-Hyung Lee) announced on the 31st that it will operate a 140㎡ standalone booth at CES Eureka Park, which will be held in Las Vegas, USA from January 7th to 10th next year, to showcase KAIST's innovative technologies to global companies and investors.
KAIST startups VIRNECT, Standard Energy, A2US, and Panmnesia, Inc. won the 2025 CES Innovation Awards. ▴VIRNECT won the Innovation Award in the ‘Industrial Equipment and Machinery’ category for ‘VisionX’, an AI-based smart glass for industrial sites; ▴Standard Energy Co., Ltd. won the Innovation Award in the ‘Smart City’ category for developing the world’s first vanadium-ion battery; ▴A2US won the Innovation Award in the ‘Environment & Energy’ category for its portable air purifier that eliminates bacteria, odors, and fine dust in the air with just water droplets; ▴Panmnesia, Inc. won the Innovation Award in the ‘Computer Peripherals and Accessories’ category for its ‘CXL-based GPU Memory Expansion Kit’ that can drastically reduce the cost of building AI infrastructure.
< Image 2. (From left on the top row) VIRNECT, Standard Energy, (From left on the bottom row) A2US, Panmnesia, Inc. >
This exhibition will feature 15 startups that are standing out in cutting-edge technologies such as artificial intelligence (AI), robotics, mobility, and sustainability. In particular, AI-based deep tech startups in various industries such as logistics, architecture, and medicine will take up half of the total, showcasing the companies’ innovative AI technologies.
Polyphenol Factory Co.,Ltd introduces ‘Grabity’, a hair loss shampoo launched domestically, which applies the patented ingredient ‘LiftMax 308™’ that forms an instantaneous protective layer on the hair during the shampooing process. A real-time demonstration will be held at this exhibition hall so that visitors can experience the effects of the ingredient directly, and plans to enter the global market starting with the launch on Amazon in the US in January 2025.
VIRNECT will present ‘VisionX’, a prototype that won the Innovation Award this time. The product provides a chatbot AI through an AI voice interface, and has a function that allows users to check the status of the equipment in real time through conversations with the AI and receive troubleshooting guidance through voice conversations, so users can experience it directly at the KAIST Hall.
‘Standard Energy’ plans to exhibit ‘Energy Tile’, an indoor ESS that utilizes the world’s first vanadium ion battery (hereinafter referred to as VIB). VIB is absolutely safe from fire and has high installation flexibility, so it can be applied to smart cities and AI data centers.
‘A2US’ is the only company in the world that has hydroxyl radical water production technology, and won the Innovation Award for its first product, an air purifier. In the future, it is expected to be widely commercialized in air and water purification, smart farms, food tech, and semiconductor cleaning using safe and environmentally friendly hydroxyl radical water.
Panmnesia, Inc. won the CES Innovation Award for its GPU memory expansion solution equipped with its CXL 3.1 IP. By connecting a memory expansion device using Panmnesia’s CXL IP, the GPU’s memory capacity can be expanded to the terabyte level. Following the Innovation Award for ‘CXL-equipped AI Accelerator’ at CES 2024 last year, it is the only company to have won the Innovation Award for its AI-oriented CXL solution for two consecutive years.
In addition, technologies from a total of 15 companies will be introduced, including ▴Omelet ▴NEXTWAVE ▴Planby Technologies ▴Cosmo Bee ▴ImpactAI ▴Roen Surgical ▴DIDEN Roboticss ▴Autopedia ▴OAQ ▴HydroXpand ▴BOOKEND ▴Sterri.
On the central stage of the KAIST Hall, KAIST students selected as CES Student Supporters will conduct interviews with participating companies and promote the companies' innovative technologies and solutions. On the 8th, from 5 PM to 7 PM, a KAIST NIGHT event will be held where pre-invited investors and participating companies can network.
Keon Jae Lee, the head of the Institute of Technology Value Creation, said, “Through CES 2025, we will showcase innovative technologies and solutions from startups based on KAIST’s deep science and deep tech, and lead commercialization in cutting-edge technology fields such as AI, robotics, mobility, and environment/energy. KAIST plans to further promote technology commercialization by supporting the growth and marketing of innovative startups through the Institute of Technology Value Creation and by strengthening global networks and expanding cooperation opportunities.”
2024.12.31 View 6357
KAIST Wins CES 2025 Innovation Award, Showcasing Innovative Technologies
KAIST will showcase innovative technologies at the world’s largest technology fair, the Consumer Electronics Show (CES 2025). In addition, KAIST startups VIRNECT Inc., Standard Energy Inc., A2US Inc., and Panmnesia, Inc. won the 2025 CES Innovation Awards.
< Image 1. 3D-Graphical Profile of CES 2025 KAIST Exhibition Booth >
KAIST (President Kwang-Hyung Lee) announced on the 31st that it will operate a 140㎡ standalone booth at CES Eureka Park, which will be held in Las Vegas, USA from January 7th to 10th next year, to showcase KAIST's innovative technologies to global companies and investors.
KAIST startups VIRNECT, Standard Energy, A2US, and Panmnesia, Inc. won the 2025 CES Innovation Awards. ▴VIRNECT won the Innovation Award in the ‘Industrial Equipment and Machinery’ category for ‘VisionX’, an AI-based smart glass for industrial sites; ▴Standard Energy Co., Ltd. won the Innovation Award in the ‘Smart City’ category for developing the world’s first vanadium-ion battery; ▴A2US won the Innovation Award in the ‘Environment & Energy’ category for its portable air purifier that eliminates bacteria, odors, and fine dust in the air with just water droplets; ▴Panmnesia, Inc. won the Innovation Award in the ‘Computer Peripherals and Accessories’ category for its ‘CXL-based GPU Memory Expansion Kit’ that can drastically reduce the cost of building AI infrastructure.
< Image 2. (From left on the top row) VIRNECT, Standard Energy, (From left on the bottom row) A2US, Panmnesia, Inc. >
This exhibition will feature 15 startups that are standing out in cutting-edge technologies such as artificial intelligence (AI), robotics, mobility, and sustainability. In particular, AI-based deep tech startups in various industries such as logistics, architecture, and medicine will take up half of the total, showcasing the companies’ innovative AI technologies.
Polyphenol Factory Co.,Ltd introduces ‘Grabity’, a hair loss shampoo launched domestically, which applies the patented ingredient ‘LiftMax 308™’ that forms an instantaneous protective layer on the hair during the shampooing process. A real-time demonstration will be held at this exhibition hall so that visitors can experience the effects of the ingredient directly, and plans to enter the global market starting with the launch on Amazon in the US in January 2025.
VIRNECT will present ‘VisionX’, a prototype that won the Innovation Award this time. The product provides a chatbot AI through an AI voice interface, and has a function that allows users to check the status of the equipment in real time through conversations with the AI and receive troubleshooting guidance through voice conversations, so users can experience it directly at the KAIST Hall.
‘Standard Energy’ plans to exhibit ‘Energy Tile’, an indoor ESS that utilizes the world’s first vanadium ion battery (hereinafter referred to as VIB). VIB is absolutely safe from fire and has high installation flexibility, so it can be applied to smart cities and AI data centers.
‘A2US’ is the only company in the world that has hydroxyl radical water production technology, and won the Innovation Award for its first product, an air purifier. In the future, it is expected to be widely commercialized in air and water purification, smart farms, food tech, and semiconductor cleaning using safe and environmentally friendly hydroxyl radical water.
Panmnesia, Inc. won the CES Innovation Award for its GPU memory expansion solution equipped with its CXL 3.1 IP. By connecting a memory expansion device using Panmnesia’s CXL IP, the GPU’s memory capacity can be expanded to the terabyte level. Following the Innovation Award for ‘CXL-equipped AI Accelerator’ at CES 2024 last year, it is the only company to have won the Innovation Award for its AI-oriented CXL solution for two consecutive years.
In addition, technologies from a total of 15 companies will be introduced, including ▴Omelet ▴NEXTWAVE ▴Planby Technologies ▴Cosmo Bee ▴ImpactAI ▴Roen Surgical ▴DIDEN Roboticss ▴Autopedia ▴OAQ ▴HydroXpand ▴BOOKEND ▴Sterri.
On the central stage of the KAIST Hall, KAIST students selected as CES Student Supporters will conduct interviews with participating companies and promote the companies' innovative technologies and solutions. On the 8th, from 5 PM to 7 PM, a KAIST NIGHT event will be held where pre-invited investors and participating companies can network.
Keon Jae Lee, the head of the Institute of Technology Value Creation, said, “Through CES 2025, we will showcase innovative technologies and solutions from startups based on KAIST’s deep science and deep tech, and lead commercialization in cutting-edge technology fields such as AI, robotics, mobility, and environment/energy. KAIST plans to further promote technology commercialization by supporting the growth and marketing of innovative startups through the Institute of Technology Value Creation and by strengthening global networks and expanding cooperation opportunities.”
2024.12.31 View 6357 -
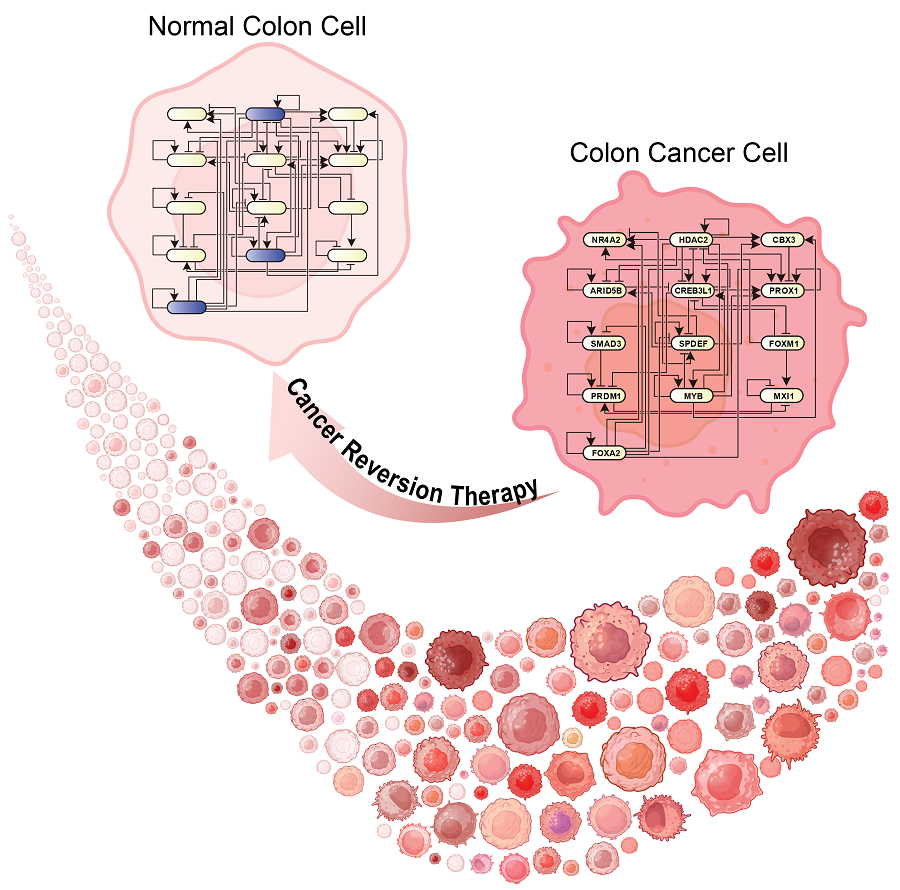 KAIST Develops Foundational Technology to Revert Cancer Cells to Normal Cells
Despite the development of numerous cancer treatment technologies, the common goal of current cancer therapies is to eliminate cancer cells. This approach, however, faces fundamental limitations, including cancer cells developing resistance and returning, as well as severe side effects from the destruction of healthy cells.
< (From top left) Bio and Brain Engineering PhD candidates Juhee Kim, Jeong-Ryeol Gong, Chun-Kyung Lee, and Hoon-Min Kim posed for a group photo with Professor Kwang-Hyun Cho >
KAIST (represented by President Kwang Hyung Lee) announced on the 20th of December that a research team led by Professor Kwang-Hyun Cho from the Department of Bio and Brain Engineering has developed a groundbreaking technology that can treat colon cancer by converting cancer cells into a state resembling normal colon cells without killing them, thus avoiding side effects.
The research team focused on the observation that during the oncogenesis process, normal cells regress along their differentiation trajectory. Building on this insight, they developed a technology to create a digital twin of the gene network associated with the differentiation trajectory of normal cells.
< Figure 1. Technology for creating a digital twin of a gene network from single-cell transcriptome data of a normal cell differentiation trajectory. Professor Kwang-Hyun Cho's research team developed a digital twin creation technology that precisely observes the dynamics of gene regulatory relationships during the process of normal cells differentiating along a differentiation trajectory and analyzes the relationships among key genes to build a mathematical model that can be simulated (A-F). In addition, they developed a technology to discover key regulatory factors that control the differentiation trajectory of normal cells by simulating and analyzing this digital twin. >
< Figure 2. Digital twin simulation simulating the differentiation trajectory of normal colon cells. The dynamics of single-cell transcriptome data for the differentiation trajectory of normal colon cells were analyzed (A) and a digital twin of the gene network was developed representing the regulatory relationships of key genes in this differentiation trajectory (B). The simulation results of the digital twin confirm that it readily reproduces the dynamics of single-cell transcriptome data (C, D). >
Through simulation analysis, the team systematically identified master molecular switches that induce normal cell differentiation. When these switches were applied to colon cancer cells, the cancer cells reverted to a normal-like state, a result confirmed through molecular and cellular experiments as well as animal studies.
< Figure 3. Discovery of top-level key control factors that induce differentiation of normal colon cells. By applying control factor discovery technology to the digital twin model, three genes, HDAC2, FOXA2, and MYB, were discovered as key control factors that induce differentiation of normal colon cells (A, B). The results of simulation analysis of the regulatory effects of the discovered control factors through the digital twin confirmed that they could induce complete differentiation of colon cells (C). >
< Figure 4. Verification of the effect of the key control factors discovered using colon cancer cells and animal experiments on the reversibility of colon cancer. The key control factors of the normal colon cell differentiation trajectory discovered through digital twin simulation analysis were applied to actual colon cancer cells and colon cancer mouse animal models to experimentally verify the effect of cancer reversibility. The key control factors significantly reduced the proliferation of three colon cancer cell lines (A), and this was confirmed in the same way in animal models (B-D). >
This research demonstrates that cancer cell reversion can be systematically achieved by analyzing and utilizing the digital twin of the cancer cell gene network, rather than relying on serendipitous discoveries. The findings hold significant promise for developing reversible cancer therapies that can be applied to various types of cancer.
< Figure 5. The change in overall gene expression was confirmed through the regulation of the identified key regulatory factors, which converted the state of colon cancer cells to that of normal colon cells. The transcriptomes of colon cancer tissues and normal colon tissues from more than 400 colon cancer patients were compared with the transcriptomes of colon cancer cell lines and reversible colon cancer cell lines, respectively. The comparison results confirmed that the regulation of the identified key regulatory factors converted all three colon cancer cell lines to a state similar to the transcriptome expression of normal colon tissues. >
Professor Kwang-Hyun Cho remarked, "The fact that cancer cells can be converted back to normal cells is an astonishing phenomenon. This study proves that such reversion can be systematically induced."
He further emphasized, "This research introduces the novel concept of reversible cancer therapy by reverting cancer cells to normal cells. It also develops foundational technology for identifying targets for cancer reversion through the systematic analysis of normal cell differentiation trajectories."
This research included contributions from Jeong-Ryeol Gong, Chun-Kyung Lee, Hoon-Min Kim, Juhee Kim, and Jaeog Jeon, and was published in the online edition of the international journal Advanced Science by Wiley on December 11. (Title: “Control of Cellular Differentiation Trajectories for Cancer Reversion”) DOI: https://doi.org/10.1002/advs.202402132
< Figure 6. Schematic diagram of the research results. Professor Kwang-Hyun Cho's research team developed a source technology to systematically discover key control factors that can induce reversibility of colon cancer cells through a systems biology approach and a digital twin simulation analysis of the differentiation trajectory of normal colon cells, and verified the effects of reversion on actual colon cancer through molecular cell experiments and animal experiments. >
The study was supported by the Ministry of Science and ICT and the National Research Foundation of Korea through the Mid-Career Researcher Program and Basic Research Laboratory Program. The research findings have been transferred to BioRevert Inc., where they will be used for the development of practical cancer reversion therapies.
2024.12.23 View 101676
KAIST Develops Foundational Technology to Revert Cancer Cells to Normal Cells
Despite the development of numerous cancer treatment technologies, the common goal of current cancer therapies is to eliminate cancer cells. This approach, however, faces fundamental limitations, including cancer cells developing resistance and returning, as well as severe side effects from the destruction of healthy cells.
< (From top left) Bio and Brain Engineering PhD candidates Juhee Kim, Jeong-Ryeol Gong, Chun-Kyung Lee, and Hoon-Min Kim posed for a group photo with Professor Kwang-Hyun Cho >
KAIST (represented by President Kwang Hyung Lee) announced on the 20th of December that a research team led by Professor Kwang-Hyun Cho from the Department of Bio and Brain Engineering has developed a groundbreaking technology that can treat colon cancer by converting cancer cells into a state resembling normal colon cells without killing them, thus avoiding side effects.
The research team focused on the observation that during the oncogenesis process, normal cells regress along their differentiation trajectory. Building on this insight, they developed a technology to create a digital twin of the gene network associated with the differentiation trajectory of normal cells.
< Figure 1. Technology for creating a digital twin of a gene network from single-cell transcriptome data of a normal cell differentiation trajectory. Professor Kwang-Hyun Cho's research team developed a digital twin creation technology that precisely observes the dynamics of gene regulatory relationships during the process of normal cells differentiating along a differentiation trajectory and analyzes the relationships among key genes to build a mathematical model that can be simulated (A-F). In addition, they developed a technology to discover key regulatory factors that control the differentiation trajectory of normal cells by simulating and analyzing this digital twin. >
< Figure 2. Digital twin simulation simulating the differentiation trajectory of normal colon cells. The dynamics of single-cell transcriptome data for the differentiation trajectory of normal colon cells were analyzed (A) and a digital twin of the gene network was developed representing the regulatory relationships of key genes in this differentiation trajectory (B). The simulation results of the digital twin confirm that it readily reproduces the dynamics of single-cell transcriptome data (C, D). >
Through simulation analysis, the team systematically identified master molecular switches that induce normal cell differentiation. When these switches were applied to colon cancer cells, the cancer cells reverted to a normal-like state, a result confirmed through molecular and cellular experiments as well as animal studies.
< Figure 3. Discovery of top-level key control factors that induce differentiation of normal colon cells. By applying control factor discovery technology to the digital twin model, three genes, HDAC2, FOXA2, and MYB, were discovered as key control factors that induce differentiation of normal colon cells (A, B). The results of simulation analysis of the regulatory effects of the discovered control factors through the digital twin confirmed that they could induce complete differentiation of colon cells (C). >
< Figure 4. Verification of the effect of the key control factors discovered using colon cancer cells and animal experiments on the reversibility of colon cancer. The key control factors of the normal colon cell differentiation trajectory discovered through digital twin simulation analysis were applied to actual colon cancer cells and colon cancer mouse animal models to experimentally verify the effect of cancer reversibility. The key control factors significantly reduced the proliferation of three colon cancer cell lines (A), and this was confirmed in the same way in animal models (B-D). >
This research demonstrates that cancer cell reversion can be systematically achieved by analyzing and utilizing the digital twin of the cancer cell gene network, rather than relying on serendipitous discoveries. The findings hold significant promise for developing reversible cancer therapies that can be applied to various types of cancer.
< Figure 5. The change in overall gene expression was confirmed through the regulation of the identified key regulatory factors, which converted the state of colon cancer cells to that of normal colon cells. The transcriptomes of colon cancer tissues and normal colon tissues from more than 400 colon cancer patients were compared with the transcriptomes of colon cancer cell lines and reversible colon cancer cell lines, respectively. The comparison results confirmed that the regulation of the identified key regulatory factors converted all three colon cancer cell lines to a state similar to the transcriptome expression of normal colon tissues. >
Professor Kwang-Hyun Cho remarked, "The fact that cancer cells can be converted back to normal cells is an astonishing phenomenon. This study proves that such reversion can be systematically induced."
He further emphasized, "This research introduces the novel concept of reversible cancer therapy by reverting cancer cells to normal cells. It also develops foundational technology for identifying targets for cancer reversion through the systematic analysis of normal cell differentiation trajectories."
This research included contributions from Jeong-Ryeol Gong, Chun-Kyung Lee, Hoon-Min Kim, Juhee Kim, and Jaeog Jeon, and was published in the online edition of the international journal Advanced Science by Wiley on December 11. (Title: “Control of Cellular Differentiation Trajectories for Cancer Reversion”) DOI: https://doi.org/10.1002/advs.202402132
< Figure 6. Schematic diagram of the research results. Professor Kwang-Hyun Cho's research team developed a source technology to systematically discover key control factors that can induce reversibility of colon cancer cells through a systems biology approach and a digital twin simulation analysis of the differentiation trajectory of normal colon cells, and verified the effects of reversion on actual colon cancer through molecular cell experiments and animal experiments. >
The study was supported by the Ministry of Science and ICT and the National Research Foundation of Korea through the Mid-Career Researcher Program and Basic Research Laboratory Program. The research findings have been transferred to BioRevert Inc., where they will be used for the development of practical cancer reversion therapies.
2024.12.23 View 101676 -
 KAIST Extends Lithium Metal Battery Lifespan by 750% Using Water
Lithium metal, a next-generation anode material, has been highlighted for overcoming the performance limitations of commercial batteries. However, issues inherent to lithium metal have caused shortened battery lifespans and increased fire risks. KAIST researchers have achieved a world-class breakthrough by extending the lifespan of lithium metal anodes by approximately 750% only using water.
KAIST (represented by President Kwang Hyung Lee) announced on the 2nd of December that Professor Il-Doo Kim from the Department of Materials Science and Engineering, in collaboration with Professor Jiyoung Lee from Ajou University, successfully stabilized lithium growth and significantly enhanced the lifespan of next-generation lithium metal batteries using eco-friendly hollow nanofibers as protective layers.
Conventional protective layer technologies, which involve applying a surface coating onto lithium metal in order to create an artificial interface with the electrolyte, have relied on toxic processes and expensive materials, with limited improvements in the lifespan of lithium metal anodes.
< Figure 1. Schematic illustration of the fabrication process of the newly developed protective membrane by eco-friendly electrospinning process using water >
To address these limitations, Professor Kim’s team proposed a hollow nanofiber protective layer capable of controlling lithium-ion growth through both physical and chemical means. This protective layer was manufactured through an environmentally friendly electrospinning process* using guar gum** extracted from plants as the primary material and utilizing water as the sole solvent.
*Electrospinning process: A method where polymer solutions are subjected to an electric field, producing continuous fibers with diameters ranging from tens of nanometers to several micrometers.
**Guar gum: A natural polymer extracted from guar beans, composed mainly of monosaccharides. Its oxidized functional groups regulate interactions with lithium ions.
< Figure 2. Physical and chemical control of Lithium dendrite by the newly developed protective membrane >
The nanofiber protective layer effectively controlled reversible chemical reactions between the electrolyte and lithium ions. The hollow spaces within the fibers suppressed the random accumulation of lithium ions on the metal surface, stabilizing the interface between the lithium metal surface and the electrolyte.
< Figure 3. Performance of Lithium metal battery full cells with the newly developed protective membrane >
As a result, the lithium metal anodes with this protective layer demonstrated approximately a 750% increase in lifespan compared to conventional lithium metal anodes. The battery retained 93.3% of its capacity even after 300 charge-discharge cycles, achieving world-class performance.
The researchers also verified that this natural protective layer decomposes entirely within about a month in soil, proving its eco-friendly nature throughout the manufacturing and disposal process.
< Figure 4. Excellent decomposition rate of the newly developed protective membrane >
Professor Il-Doo Kim explained, “By leveraging both physical and chemical protective functions, we were able to guide reversible reactions between lithium metal and the electrolyte more effectively and suppress dendrite growth, resulting in lithium metal anodes with unprecedented lifespan characteristics.”
He added, “As the environmental burden caused by battery production and disposal becomes a pressing issue due to surging battery demand, this water-based manufacturing method with biodegradable properties will significantly contribute to the commercialization of next-generation eco-friendly batteries.”
This study was led by Dr. Jiyoung Lee (now a professor in the Department of Chemical Engineering at Ajou University) and Dr. Hyunsub Song (currently at Samsung Electronics), both graduates of KAIST’s Department of Materials Science and Engineering. The findings were published as a front cover article in Advanced Materials, Volume 36, Issue 47, on November 21.
(Paper title: “Overcoming Chemical and Mechanical Instabilities in Lithium Metal Anodes with Sustainable and Eco-Friendly Artificial SEI Layer”)
The research was supported by the KAIST-LG Energy Solution Frontier Research Lab (FRL), the Alchemist Project funded by the Ministry of Trade, Industry and Energy, and the Top-Tier Research Support Program from the Ministry of Science and ICT.
2024.12.12 View 6560
KAIST Extends Lithium Metal Battery Lifespan by 750% Using Water
Lithium metal, a next-generation anode material, has been highlighted for overcoming the performance limitations of commercial batteries. However, issues inherent to lithium metal have caused shortened battery lifespans and increased fire risks. KAIST researchers have achieved a world-class breakthrough by extending the lifespan of lithium metal anodes by approximately 750% only using water.
KAIST (represented by President Kwang Hyung Lee) announced on the 2nd of December that Professor Il-Doo Kim from the Department of Materials Science and Engineering, in collaboration with Professor Jiyoung Lee from Ajou University, successfully stabilized lithium growth and significantly enhanced the lifespan of next-generation lithium metal batteries using eco-friendly hollow nanofibers as protective layers.
Conventional protective layer technologies, which involve applying a surface coating onto lithium metal in order to create an artificial interface with the electrolyte, have relied on toxic processes and expensive materials, with limited improvements in the lifespan of lithium metal anodes.
< Figure 1. Schematic illustration of the fabrication process of the newly developed protective membrane by eco-friendly electrospinning process using water >
To address these limitations, Professor Kim’s team proposed a hollow nanofiber protective layer capable of controlling lithium-ion growth through both physical and chemical means. This protective layer was manufactured through an environmentally friendly electrospinning process* using guar gum** extracted from plants as the primary material and utilizing water as the sole solvent.
*Electrospinning process: A method where polymer solutions are subjected to an electric field, producing continuous fibers with diameters ranging from tens of nanometers to several micrometers.
**Guar gum: A natural polymer extracted from guar beans, composed mainly of monosaccharides. Its oxidized functional groups regulate interactions with lithium ions.
< Figure 2. Physical and chemical control of Lithium dendrite by the newly developed protective membrane >
The nanofiber protective layer effectively controlled reversible chemical reactions between the electrolyte and lithium ions. The hollow spaces within the fibers suppressed the random accumulation of lithium ions on the metal surface, stabilizing the interface between the lithium metal surface and the electrolyte.
< Figure 3. Performance of Lithium metal battery full cells with the newly developed protective membrane >
As a result, the lithium metal anodes with this protective layer demonstrated approximately a 750% increase in lifespan compared to conventional lithium metal anodes. The battery retained 93.3% of its capacity even after 300 charge-discharge cycles, achieving world-class performance.
The researchers also verified that this natural protective layer decomposes entirely within about a month in soil, proving its eco-friendly nature throughout the manufacturing and disposal process.
< Figure 4. Excellent decomposition rate of the newly developed protective membrane >
Professor Il-Doo Kim explained, “By leveraging both physical and chemical protective functions, we were able to guide reversible reactions between lithium metal and the electrolyte more effectively and suppress dendrite growth, resulting in lithium metal anodes with unprecedented lifespan characteristics.”
He added, “As the environmental burden caused by battery production and disposal becomes a pressing issue due to surging battery demand, this water-based manufacturing method with biodegradable properties will significantly contribute to the commercialization of next-generation eco-friendly batteries.”
This study was led by Dr. Jiyoung Lee (now a professor in the Department of Chemical Engineering at Ajou University) and Dr. Hyunsub Song (currently at Samsung Electronics), both graduates of KAIST’s Department of Materials Science and Engineering. The findings were published as a front cover article in Advanced Materials, Volume 36, Issue 47, on November 21.
(Paper title: “Overcoming Chemical and Mechanical Instabilities in Lithium Metal Anodes with Sustainable and Eco-Friendly Artificial SEI Layer”)
The research was supported by the KAIST-LG Energy Solution Frontier Research Lab (FRL), the Alchemist Project funded by the Ministry of Trade, Industry and Energy, and the Top-Tier Research Support Program from the Ministry of Science and ICT.
2024.12.12 View 6560 -
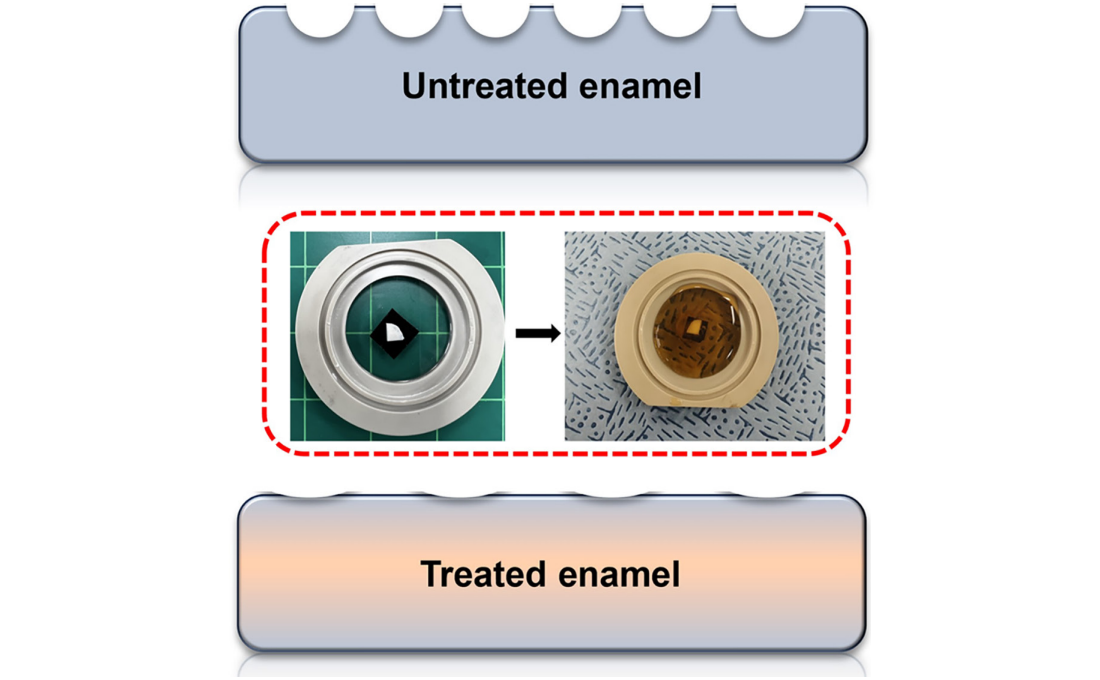 KAIST Scientifically Identifies a Method to Prevent Dental Erosion from Carbonated Drinks
A Korean research team, which had previously visualized and scientifically proven the harmful effects of carbonated drinks like cola on dental health using nanotechnology, has now identified a mechanism for an effective method to prevent tooth damage caused by these beverages.
KAIST (represented by President Kwang Hyung Lee) announced on the 5th of December that a team led by Professor Seungbum Hong from the Department of Materials Science and Engineering, in collaboration with Seoul National University's School of Dentistry (Departments of Pediatric Dentistry and Oral Microbiology) and Professor Hye Ryung Byon’s research team from the Department of Chemistry, has revealed through nanotechnology that silver diamine fluoride (SDF)* forms a fluoride-containing protective layer on the tooth surface, effectively inhibiting cola-induced erosion.
*SDF (Silver Diamine Fluoride): A dental agent primarily used for the treatment and prevention of tooth decay. SDF strengthens carious lesions, suppresses bacterial growth, and halts the progression of cavities.
The team analyzed the surface morphology and mechanical properties of tooth enamel on a nanoscale using atomic force microscopy (AFM). They also examined the chemical properties of the nano-film formed by SDF treatment using X-ray photoelectron spectroscopy (XPS)* and Fourier-transform infrared spectroscopy (FTIR)*.
*XPS (X-ray Photoelectron Spectroscopy): A powerful surface analysis technique used to investigate the chemical composition and electronic structure of materials.
*FTIR (Fourier-Transform Infrared Spectroscopy): An analytical method that identifies the molecular structure and composition of materials by analyzing how they absorb or transmit infrared light.
The findings showed significant differences in surface roughness and elastic modulus between teeth exposed to cola with and without SDF treatment. Teeth treated with SDF exhibited minimal changes in surface roughness due to erosion (from 64 nm to 70 nm) and maintained a high elastic modulus (from 215 GPa to 205 GPa).
This was attributed to the formation of a fluoroapatite* layer by SDF, which acted as a protective shield.
*Fluoroapatite: A phosphate mineral with the chemical formula Ca₅(PO₄)₃F (calcium fluoro-phosphate). It can occur naturally or be synthesized biologically/artificially and plays a crucial role in strengthening teeth and bones.
< Figure 1. Schematic of the workflow. Surface morphology and mechanical properties of untreated and treated silver diamine fluoride (SDF) treated enamel exposed to cola were analyzed over time using atomic force microscopy (AFM). >
Professor Young J. Kim from Seoul National University's Department of Pediatric Dentistry noted, "This technology could be applied to prevent dental erosion and strengthen teeth for both children and adults. It is a cost-effective and accessible dental treatment."
< Figure 2. Changes in surface roughness and elastic modulus according to time of exposure to cola for SDF untreated and treated teeth. After 1 hour, the surface roughness of the SDF untreated teeth rapidly became rougher from 83 nm to 287 nm and the elastic modulus weakened from 125 GPa to 13 GPa, whereas the surface roughness of the SDF treated teeth changed only slightly from 64 nm to 70 nm and the elastic modulus barely changed from 215 GPa to 205 GPa, maintaining a similar state to the initial state. >
Professor Seungbum Hong emphasized, "Dental health significantly impacts quality of life. This research offers an effective non-invasive method to prevent early dental erosion, moving beyond traditional surgical treatments. By simply applying SDF, dental erosion can be prevented and enamel strengthened, potentially reducing pain and costs associated with treatment."
This study, led by the first author Aditi Saha, a PhD student in KAIST’s Department of Materials Science and Engineering, was published in the international journal Biomaterials Research on November 7 under the title "Nanoscale Study on Noninvasive Prevention of Dental Erosion of Enamel by Silver Diamine Fluoride". The research was supported by the National Research Foundation of Korea.
2024.12.11 View 5073
KAIST Scientifically Identifies a Method to Prevent Dental Erosion from Carbonated Drinks
A Korean research team, which had previously visualized and scientifically proven the harmful effects of carbonated drinks like cola on dental health using nanotechnology, has now identified a mechanism for an effective method to prevent tooth damage caused by these beverages.
KAIST (represented by President Kwang Hyung Lee) announced on the 5th of December that a team led by Professor Seungbum Hong from the Department of Materials Science and Engineering, in collaboration with Seoul National University's School of Dentistry (Departments of Pediatric Dentistry and Oral Microbiology) and Professor Hye Ryung Byon’s research team from the Department of Chemistry, has revealed through nanotechnology that silver diamine fluoride (SDF)* forms a fluoride-containing protective layer on the tooth surface, effectively inhibiting cola-induced erosion.
*SDF (Silver Diamine Fluoride): A dental agent primarily used for the treatment and prevention of tooth decay. SDF strengthens carious lesions, suppresses bacterial growth, and halts the progression of cavities.
The team analyzed the surface morphology and mechanical properties of tooth enamel on a nanoscale using atomic force microscopy (AFM). They also examined the chemical properties of the nano-film formed by SDF treatment using X-ray photoelectron spectroscopy (XPS)* and Fourier-transform infrared spectroscopy (FTIR)*.
*XPS (X-ray Photoelectron Spectroscopy): A powerful surface analysis technique used to investigate the chemical composition and electronic structure of materials.
*FTIR (Fourier-Transform Infrared Spectroscopy): An analytical method that identifies the molecular structure and composition of materials by analyzing how they absorb or transmit infrared light.
The findings showed significant differences in surface roughness and elastic modulus between teeth exposed to cola with and without SDF treatment. Teeth treated with SDF exhibited minimal changes in surface roughness due to erosion (from 64 nm to 70 nm) and maintained a high elastic modulus (from 215 GPa to 205 GPa).
This was attributed to the formation of a fluoroapatite* layer by SDF, which acted as a protective shield.
*Fluoroapatite: A phosphate mineral with the chemical formula Ca₅(PO₄)₃F (calcium fluoro-phosphate). It can occur naturally or be synthesized biologically/artificially and plays a crucial role in strengthening teeth and bones.
< Figure 1. Schematic of the workflow. Surface morphology and mechanical properties of untreated and treated silver diamine fluoride (SDF) treated enamel exposed to cola were analyzed over time using atomic force microscopy (AFM). >
Professor Young J. Kim from Seoul National University's Department of Pediatric Dentistry noted, "This technology could be applied to prevent dental erosion and strengthen teeth for both children and adults. It is a cost-effective and accessible dental treatment."
< Figure 2. Changes in surface roughness and elastic modulus according to time of exposure to cola for SDF untreated and treated teeth. After 1 hour, the surface roughness of the SDF untreated teeth rapidly became rougher from 83 nm to 287 nm and the elastic modulus weakened from 125 GPa to 13 GPa, whereas the surface roughness of the SDF treated teeth changed only slightly from 64 nm to 70 nm and the elastic modulus barely changed from 215 GPa to 205 GPa, maintaining a similar state to the initial state. >
Professor Seungbum Hong emphasized, "Dental health significantly impacts quality of life. This research offers an effective non-invasive method to prevent early dental erosion, moving beyond traditional surgical treatments. By simply applying SDF, dental erosion can be prevented and enamel strengthened, potentially reducing pain and costs associated with treatment."
This study, led by the first author Aditi Saha, a PhD student in KAIST’s Department of Materials Science and Engineering, was published in the international journal Biomaterials Research on November 7 under the title "Nanoscale Study on Noninvasive Prevention of Dental Erosion of Enamel by Silver Diamine Fluoride". The research was supported by the National Research Foundation of Korea.
2024.12.11 View 5073 -
 KAIST Develops a Multifunctional Structural Battery Capable of Energy Storage and Load Support
Structural batteries are used in industries such as eco-friendly, energy-based automobiles, mobility, and aerospace, and they must simultaneously meet the requirements of high energy density for energy storage and high load-bearing capacity. Conventional structural battery technology has struggled to enhance both functions concurrently. However, KAIST researchers have succeeded in developing foundational technology to address this issue.
< Photo 1. (From left) Professor Seong Su Kim, PhD candidates Sangyoon Bae and Su Hyun Lim of the Department of Mechanical Engineering >
< Photo 2. (From left) Professor Seong Su Kim and Master's Graduate Mohamad A. Raja of KAIST Department of Mechanical Engineering >
KAIST (represented by President Kwang Hyung Lee) announced on the 19th of November that Professor Seong Su Kim's team from the Department of Mechanical Engineering has developed a thin, uniform, high-density, multifunctional structural carbon fiber composite battery* capable of supporting loads, and that is free from fire risks while offering high energy density.
*Multifunctional structural batteries: Refers to the ability of each material in the composite to simultaneously serve as a load-bearing structure and an energy storage element.
Early structural batteries involved embedding commercial lithium-ion batteries into layered composite materials. These batteries suffered from low integration of their mechanical and electrochemical properties, leading to challenges in material processing, assembly, and design optimization, making commercialization difficult.
To overcome these challenges, Professor Kim's team explored the concept of "energy-storing composite materials," focusing on interface and curing properties, which are critical in traditional composite design. This led to the development of high-density multifunctional structural carbon fiber composite batteries that maximize multifunctionality.
The team analyzed the curing mechanisms of epoxy resin, known for its strong mechanical properties, combined with ionic liquid and carbonate electrolyte-based solid polymer electrolytes. By controlling temperature and pressure, they were able to optimize the curing process.
The newly developed structural battery was manufactured through vacuum compression molding, increasing the volume fraction of carbon fibers—serving as both electrodes and current collectors—by over 160% compared to previous carbon-fiber-based batteries.
This greatly increased the contact area between electrodes and electrolytes, resulting in a high-density structural battery with improved electrochemical performance. Furthermore, the team effectively controlled air bubbles within the structural battery during the curing process, simultaneously enhancing the battery's mechanical properties.
Professor Seong Su Kim, the lead researcher, explained, “We proposed a framework for designing solid polymer electrolytes, a core material for high-stiffness, ultra-thin structural batteries, from both material and structural perspectives. These material-based structural batteries can serve as internal components in cars, drones, airplanes, and robots, significantly extending their operating time with a single charge. This represents a foundational technology for next-generation multifunctional energy storage applications.”
< Figure 2. Supplementary cover of ACS Applied Materials & Interfaces >
Mohamad A. Raja, a master’s graduate of KAIST’s Department of Mechanical Engineering, participated as the first author of this research, which was published in the prestigious journal ACS Applied Materials & Interfaces on September 10. The paper was recognized for its excellence and selected as a supplementary cover article. (Paper title: “Thin, Uniform, and Highly Packed Multifunctional Structural Carbon Fiber Composite Battery Lamina Informed by Solid Polymer Electrolyte Cure Kinetics.” https://doi.org/10.1021/acsami.4c08698)
This research was supported by the National Research Foundation of Korea’s Mid-Career Researcher Program and the National Semiconductor Research Laboratory Development Program.
2024.11.27 View 5823
KAIST Develops a Multifunctional Structural Battery Capable of Energy Storage and Load Support
Structural batteries are used in industries such as eco-friendly, energy-based automobiles, mobility, and aerospace, and they must simultaneously meet the requirements of high energy density for energy storage and high load-bearing capacity. Conventional structural battery technology has struggled to enhance both functions concurrently. However, KAIST researchers have succeeded in developing foundational technology to address this issue.
< Photo 1. (From left) Professor Seong Su Kim, PhD candidates Sangyoon Bae and Su Hyun Lim of the Department of Mechanical Engineering >
< Photo 2. (From left) Professor Seong Su Kim and Master's Graduate Mohamad A. Raja of KAIST Department of Mechanical Engineering >
KAIST (represented by President Kwang Hyung Lee) announced on the 19th of November that Professor Seong Su Kim's team from the Department of Mechanical Engineering has developed a thin, uniform, high-density, multifunctional structural carbon fiber composite battery* capable of supporting loads, and that is free from fire risks while offering high energy density.
*Multifunctional structural batteries: Refers to the ability of each material in the composite to simultaneously serve as a load-bearing structure and an energy storage element.
Early structural batteries involved embedding commercial lithium-ion batteries into layered composite materials. These batteries suffered from low integration of their mechanical and electrochemical properties, leading to challenges in material processing, assembly, and design optimization, making commercialization difficult.
To overcome these challenges, Professor Kim's team explored the concept of "energy-storing composite materials," focusing on interface and curing properties, which are critical in traditional composite design. This led to the development of high-density multifunctional structural carbon fiber composite batteries that maximize multifunctionality.
The team analyzed the curing mechanisms of epoxy resin, known for its strong mechanical properties, combined with ionic liquid and carbonate electrolyte-based solid polymer electrolytes. By controlling temperature and pressure, they were able to optimize the curing process.
The newly developed structural battery was manufactured through vacuum compression molding, increasing the volume fraction of carbon fibers—serving as both electrodes and current collectors—by over 160% compared to previous carbon-fiber-based batteries.
This greatly increased the contact area between electrodes and electrolytes, resulting in a high-density structural battery with improved electrochemical performance. Furthermore, the team effectively controlled air bubbles within the structural battery during the curing process, simultaneously enhancing the battery's mechanical properties.
Professor Seong Su Kim, the lead researcher, explained, “We proposed a framework for designing solid polymer electrolytes, a core material for high-stiffness, ultra-thin structural batteries, from both material and structural perspectives. These material-based structural batteries can serve as internal components in cars, drones, airplanes, and robots, significantly extending their operating time with a single charge. This represents a foundational technology for next-generation multifunctional energy storage applications.”
< Figure 2. Supplementary cover of ACS Applied Materials & Interfaces >
Mohamad A. Raja, a master’s graduate of KAIST’s Department of Mechanical Engineering, participated as the first author of this research, which was published in the prestigious journal ACS Applied Materials & Interfaces on September 10. The paper was recognized for its excellence and selected as a supplementary cover article. (Paper title: “Thin, Uniform, and Highly Packed Multifunctional Structural Carbon Fiber Composite Battery Lamina Informed by Solid Polymer Electrolyte Cure Kinetics.” https://doi.org/10.1021/acsami.4c08698)
This research was supported by the National Research Foundation of Korea’s Mid-Career Researcher Program and the National Semiconductor Research Laboratory Development Program.
2024.11.27 View 5823 -
 KAIST Researchers Suggest an Extraordinary Alternative to Petroleum-based PET - Bacteria!
< (From left) Dr. Cindy Pricilia, Ph.D. Candidate Cheon Woo Moon, Distinguished Professor Sang Yup Lee >
Currently, the world is suffering from environmental problems caused by plastic waste. The KAIST research team has succeeded in producing a microbial-based plastic that is biodegradable and can replace existing PET bottles, making it a hot topic.
Our university announced on the 7th of November that the research team of Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering has succeeded in developing a microbial strain that efficiently produces pseudoaromatic polyester monomer to replace polyethylene terephthalate (PET) using systems metabolic engineering.
Pseudoaromatic dicarboxylic acids have better physical properties and higher biodegradability than aromatic polyester (PET) when synthesized as polymers, and are attracting attention as an eco-friendly monomer* that can be synthesized into polymers. The production of pseudoaromatic dicarboxylic acids through chemical methods has the problems of low yield and selectivity, complex reaction conditions, and the generation of hazardous waste.
*Monomer: A material for making polymers, which is used to synthesize polymers by polymerizing monomers together
< Figure. Overview of pseudoaromatic dicarboxylic acid production using metabolically engineered C. glutamicum. >
To solve this problem, Professor Sang Yup Lee's research team used metabolic engineering to develop a microbial strain that efficiently produces five types of pseudoaromatic dicarboxylic acids, including 2-pyrone-4,6-dicarboxylic acid and four types of pyridine dicarboxylic acids (2,3-, 2,4-, 2,5-, 2,6-pyridine dicarboxylic acids), in Corynebacterium, a bacterium mainly used for amino acid production.
The research team used metabolic engineering techniques to build a platform microbial strain that enhances the metabolic flow of protocatechuic acid, which is used as a precursor for several pseudoaromatic dicarboxylic acids, and prevents the loss of precursors.
Based on this, the genetic manipulation target was discovered through transcriptome analysis, producing 76.17 g/L of 2-pyrone-4,6-dicarboxylic acid, and by newly discovering and constructing three types of pyridine dicarboxylic acid production metabolic pathways, successfully producing 2.79 g/L of 2,3-pyridine dicarboxylic acid, 0.49 g/L of 2,4-pyridine dicarboxylic acid, and 1.42 g/L of 2,5-pyridine dicarboxylic acid.
In addition, the research team confirmed the production of 15.01 g/L through the construction and reinforcement of the 2,6-pyridine dicarboxylic acid biosynthesis pathway, successfully producing a total of five similar aromatic dicarboxylic acids with high efficiency.
In conclusion, the team succeeded in producing 2,4-, 2,5-, and 2,6-pyridine dicarboxylic acids at the world's highest concentration. In particular, 2,4-, 2,5-pyridine dicarboxylic acid achieved production on the scale of g/L, which was previously produced in extremely small amounts (mg/L).
Based on this study, it is expected that it will be applied to various polyester production industrial processes, and it is also expected that it will be actively utilized in research on the production of similar aromatic polyesters.
Professor Sang Yup Lee, the corresponding author, said, “The significance lies in the fact that we have developed an eco-friendly technology that efficiently produces similar aromatic polyester monomers based on microorganisms,” and “This study will help the microorganism-based bio-monomer industry replace the petrochemical-based chemical industry in the future.”
The results of this study were published in the international academic journal, the Proceedings of the National Academy of Sciences of United States of America (PNAS) on October 30th.
※ Paper title: Metabolic engineering of Corynebacterium glutamicum for the production of pyrone and pyridine dicarboxylic acids
※ Author information: Jae Sung Cho (co-first author), Zi Wei Luo (co-first author), Cheon Woo Moon (co-first author), Cindy Prabowo (co-author), Sang Yup Lee (corresponding author) - a total of 5 people
This study was conducted with the support of the Development of Next-generation Biorefinery Platform Technologies for Leading Bio-based Chemicals Industry Project and the Development of Platform Technologies of Microbial Cell Factories for the Next-generation Biorefineries Project (Project leader: Professor Sang Yup Lee) from the National Research Foundation supported by the Ministry of Science and Technology and ICT of Korea.
2024.11.08 View 8983
KAIST Researchers Suggest an Extraordinary Alternative to Petroleum-based PET - Bacteria!
< (From left) Dr. Cindy Pricilia, Ph.D. Candidate Cheon Woo Moon, Distinguished Professor Sang Yup Lee >
Currently, the world is suffering from environmental problems caused by plastic waste. The KAIST research team has succeeded in producing a microbial-based plastic that is biodegradable and can replace existing PET bottles, making it a hot topic.
Our university announced on the 7th of November that the research team of Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering has succeeded in developing a microbial strain that efficiently produces pseudoaromatic polyester monomer to replace polyethylene terephthalate (PET) using systems metabolic engineering.
Pseudoaromatic dicarboxylic acids have better physical properties and higher biodegradability than aromatic polyester (PET) when synthesized as polymers, and are attracting attention as an eco-friendly monomer* that can be synthesized into polymers. The production of pseudoaromatic dicarboxylic acids through chemical methods has the problems of low yield and selectivity, complex reaction conditions, and the generation of hazardous waste.
*Monomer: A material for making polymers, which is used to synthesize polymers by polymerizing monomers together
< Figure. Overview of pseudoaromatic dicarboxylic acid production using metabolically engineered C. glutamicum. >
To solve this problem, Professor Sang Yup Lee's research team used metabolic engineering to develop a microbial strain that efficiently produces five types of pseudoaromatic dicarboxylic acids, including 2-pyrone-4,6-dicarboxylic acid and four types of pyridine dicarboxylic acids (2,3-, 2,4-, 2,5-, 2,6-pyridine dicarboxylic acids), in Corynebacterium, a bacterium mainly used for amino acid production.
The research team used metabolic engineering techniques to build a platform microbial strain that enhances the metabolic flow of protocatechuic acid, which is used as a precursor for several pseudoaromatic dicarboxylic acids, and prevents the loss of precursors.
Based on this, the genetic manipulation target was discovered through transcriptome analysis, producing 76.17 g/L of 2-pyrone-4,6-dicarboxylic acid, and by newly discovering and constructing three types of pyridine dicarboxylic acid production metabolic pathways, successfully producing 2.79 g/L of 2,3-pyridine dicarboxylic acid, 0.49 g/L of 2,4-pyridine dicarboxylic acid, and 1.42 g/L of 2,5-pyridine dicarboxylic acid.
In addition, the research team confirmed the production of 15.01 g/L through the construction and reinforcement of the 2,6-pyridine dicarboxylic acid biosynthesis pathway, successfully producing a total of five similar aromatic dicarboxylic acids with high efficiency.
In conclusion, the team succeeded in producing 2,4-, 2,5-, and 2,6-pyridine dicarboxylic acids at the world's highest concentration. In particular, 2,4-, 2,5-pyridine dicarboxylic acid achieved production on the scale of g/L, which was previously produced in extremely small amounts (mg/L).
Based on this study, it is expected that it will be applied to various polyester production industrial processes, and it is also expected that it will be actively utilized in research on the production of similar aromatic polyesters.
Professor Sang Yup Lee, the corresponding author, said, “The significance lies in the fact that we have developed an eco-friendly technology that efficiently produces similar aromatic polyester monomers based on microorganisms,” and “This study will help the microorganism-based bio-monomer industry replace the petrochemical-based chemical industry in the future.”
The results of this study were published in the international academic journal, the Proceedings of the National Academy of Sciences of United States of America (PNAS) on October 30th.
※ Paper title: Metabolic engineering of Corynebacterium glutamicum for the production of pyrone and pyridine dicarboxylic acids
※ Author information: Jae Sung Cho (co-first author), Zi Wei Luo (co-first author), Cheon Woo Moon (co-first author), Cindy Prabowo (co-author), Sang Yup Lee (corresponding author) - a total of 5 people
This study was conducted with the support of the Development of Next-generation Biorefinery Platform Technologies for Leading Bio-based Chemicals Industry Project and the Development of Platform Technologies of Microbial Cell Factories for the Next-generation Biorefineries Project (Project leader: Professor Sang Yup Lee) from the National Research Foundation supported by the Ministry of Science and Technology and ICT of Korea.
2024.11.08 View 8983