CHI
-
 Machine Learning-Based Algorithm to Speed up DNA Sequencing
The algorithm presents the first full-fledged, short-read alignment software that leverages learned indices for solving the exact match search problem for efficient seeding
The human genome consists of a complete set of DNA, which is about 6.4 billion letters long. Because of its size, reading the whole genome sequence at once is challenging. So scientists use DNA sequencers to produce hundreds of millions of DNA sequence fragments, or short reads, up to 300 letters long. Then the DNA sequencer assembles all the short reads like a giant jigsaw puzzle to reconstruct the entire genome sequence. Even with very fast computers, this job can take hours to complete.
A research team at KAIST has achieved up to 3.45x faster speeds by developing the first short-read alignment software that uses a recent advance in machine-learning called a learned index.
The research team reported their findings on March 7, 2022 in the journal Bioinformatics. The software has been released as open source and can be found on github (https://github.com/kaist-ina/BWA-MEME).
Next-generation sequencing (NGS) is a state-of-the-art DNA sequencing method. Projects are underway with the goal of producing genome sequencing at population scale. Modern NGS hardware is capable of generating billions of short reads in a single run. Then the short reads have to be aligned with the reference DNA sequence. With large-scale DNA sequencing operations running hundreds of next-generation sequences, the need for an efficient short read alignment tool has become even more critical. Accelerating the DNA sequence alignment would be a step toward achieving the goal of population-scale sequencing. However, existing algorithms are limited in their performance because of their frequent memory accesses.
BWA-MEM2 is a popular short-read alignment software package currently used to sequence the DNA. However, it has its limitations. The state-of-the-art alignment has two phases – seeding and extending. During the seeding phase, searches find exact matches of short reads in the reference DNA sequence. During the extending phase, the short reads from the seeding phase are extended. In the current process, bottlenecks occur in the seeding phase. Finding the exact matches slows the process.
The researchers set out to solve the problem of accelerating the DNA sequence alignment. To speed the process, they applied machine learning techniques to create an algorithmic improvement. Their algorithm, BWA-MEME (BWA-MEM emulated) leverages learned indices to solve the exact match search problem. The original software compared one character at a time for an exact match search. The team’s new algorithm achieves up to 3.45x faster speeds in seeding throughput over BWA-MEM2 by reducing the number of instructions by 4.60x and memory accesses by 8.77x. “Through this study, it has been shown that full genome big data analysis can be performed faster and less costly than conventional methods by applying machine learning technology,” said Professor Dongsu Han from the School of Electrical Engineering at KAIST.
The researchers’ ultimate goal was to develop efficient software that scientists from academia and industry could use on a daily basis for analyzing big data in genomics. “With the recent advances in artificial intelligence and machine learning, we see so many opportunities for designing better software for genomic data analysis. The potential is there for accelerating existing analysis as well as enabling new types of analysis, and our goal is to develop such software,” added Han.
Whole genome sequencing has traditionally been used for discovering genomic mutations and identifying the root causes of diseases, which leads to the discovery and development of new drugs and cures. There could be many potential applications. Whole genome sequencing is used not only for research, but also for clinical purposes. “The science and technology for analyzing genomic data is making rapid progress to make it more accessible for scientists and patients. This will enhance our understanding about diseases and develop a better cure for patients of various diseases.”
The research was funded by the National Research Foundation of the Korean government’s Ministry of Science and ICT.
-PublicationYoungmok Jung, Dongsu Han, “BWA-MEME:BWA-MEM emulated with a machine learning approach,” Bioinformatics, Volume 38, Issue 9, May 2022
(https://doi.org/10.1093/bioinformatics/btac137)
-ProfileProfessor Dongsu HanSchool of Electrical EngineeringKAIST
2022.05.10 View 10456
Machine Learning-Based Algorithm to Speed up DNA Sequencing
The algorithm presents the first full-fledged, short-read alignment software that leverages learned indices for solving the exact match search problem for efficient seeding
The human genome consists of a complete set of DNA, which is about 6.4 billion letters long. Because of its size, reading the whole genome sequence at once is challenging. So scientists use DNA sequencers to produce hundreds of millions of DNA sequence fragments, or short reads, up to 300 letters long. Then the DNA sequencer assembles all the short reads like a giant jigsaw puzzle to reconstruct the entire genome sequence. Even with very fast computers, this job can take hours to complete.
A research team at KAIST has achieved up to 3.45x faster speeds by developing the first short-read alignment software that uses a recent advance in machine-learning called a learned index.
The research team reported their findings on March 7, 2022 in the journal Bioinformatics. The software has been released as open source and can be found on github (https://github.com/kaist-ina/BWA-MEME).
Next-generation sequencing (NGS) is a state-of-the-art DNA sequencing method. Projects are underway with the goal of producing genome sequencing at population scale. Modern NGS hardware is capable of generating billions of short reads in a single run. Then the short reads have to be aligned with the reference DNA sequence. With large-scale DNA sequencing operations running hundreds of next-generation sequences, the need for an efficient short read alignment tool has become even more critical. Accelerating the DNA sequence alignment would be a step toward achieving the goal of population-scale sequencing. However, existing algorithms are limited in their performance because of their frequent memory accesses.
BWA-MEM2 is a popular short-read alignment software package currently used to sequence the DNA. However, it has its limitations. The state-of-the-art alignment has two phases – seeding and extending. During the seeding phase, searches find exact matches of short reads in the reference DNA sequence. During the extending phase, the short reads from the seeding phase are extended. In the current process, bottlenecks occur in the seeding phase. Finding the exact matches slows the process.
The researchers set out to solve the problem of accelerating the DNA sequence alignment. To speed the process, they applied machine learning techniques to create an algorithmic improvement. Their algorithm, BWA-MEME (BWA-MEM emulated) leverages learned indices to solve the exact match search problem. The original software compared one character at a time for an exact match search. The team’s new algorithm achieves up to 3.45x faster speeds in seeding throughput over BWA-MEM2 by reducing the number of instructions by 4.60x and memory accesses by 8.77x. “Through this study, it has been shown that full genome big data analysis can be performed faster and less costly than conventional methods by applying machine learning technology,” said Professor Dongsu Han from the School of Electrical Engineering at KAIST.
The researchers’ ultimate goal was to develop efficient software that scientists from academia and industry could use on a daily basis for analyzing big data in genomics. “With the recent advances in artificial intelligence and machine learning, we see so many opportunities for designing better software for genomic data analysis. The potential is there for accelerating existing analysis as well as enabling new types of analysis, and our goal is to develop such software,” added Han.
Whole genome sequencing has traditionally been used for discovering genomic mutations and identifying the root causes of diseases, which leads to the discovery and development of new drugs and cures. There could be many potential applications. Whole genome sequencing is used not only for research, but also for clinical purposes. “The science and technology for analyzing genomic data is making rapid progress to make it more accessible for scientists and patients. This will enhance our understanding about diseases and develop a better cure for patients of various diseases.”
The research was funded by the National Research Foundation of the Korean government’s Ministry of Science and ICT.
-PublicationYoungmok Jung, Dongsu Han, “BWA-MEME:BWA-MEM emulated with a machine learning approach,” Bioinformatics, Volume 38, Issue 9, May 2022
(https://doi.org/10.1093/bioinformatics/btac137)
-ProfileProfessor Dongsu HanSchool of Electrical EngineeringKAIST
2022.05.10 View 10456 -
 LightPC Presents a Resilient System Using Only Non-Volatile Memory
Lightweight Persistence Centric System (LightPC) ensures both data and execution persistence for energy-efficient full system persistence
A KAIST research team has developed hardware and software technology that ensures both data and execution persistence. The Lightweight Persistence Centric System (LightPC) makes the systems resilient against power failures by utilizing only non-volatile memory as the main memory.
“We mounted non-volatile memory on a system board prototype and created an operating system to verify the effectiveness of LightPC,” said Professor Myoungsoo Jung. The team confirmed that LightPC validated its execution while powering up and down in the middle of execution, showing up to eight times more memory, 4.3 times faster application execution, and 73% lower power consumption compared to traditional systems.
Professor Jung said that LightPC can be utilized in a variety of fields such as data centers and high-performance computing to provide large-capacity memory, high performance, low power consumption, and service reliability.
In general, power failures on legacy systems can lead to the loss of data stored in the DRAM-based main memory. Unlike volatile memory such as DRAM, non-volatile memory can retain its data without power. Although non-volatile memory has the characteristics of lower power consumption and larger capacity than DRAM, non-volatile memory is typically used for the task of secondary storage due to its lower write performance. For this reason, nonvolatile memory is often used with DRAM. However, modern systems employing non-volatile memory-based main memory experience unexpected performance degradation due to the complicated memory microarchitecture.
To enable both data and execution persistent in legacy systems, it is necessary to transfer the data from the volatile memory to the non-volatile memory. Checkpointing is one possible solution. It periodically transfers the data in preparation for a sudden power failure. While this technology is essential for ensuring high mobility and reliability for users, checkpointing also has fatal drawbacks. It takes additional time and power to move data and requires a data recovery process as well as restarting the system.
In order to address these issues, the research team developed a processor and memory controller to raise the performance of non-volatile memory-only memory. LightPC matches the performance of DRAM by minimizing the internal volatile memory components from non-volatile memory, exposing the non-volatile memory (PRAM) media to the host, and increasing parallelism to service on-the-fly requests as soon as possible.
The team also presented operating system technology that quickly makes execution states of running processes persistent without the need for a checkpointing process. The operating system prevents all modifications to execution states and data by keeping all program executions idle before transferring data in order to support consistency within a period much shorter than the standard power hold-up time of about 16 minutes. For consistency, when the power is recovered, the computer almost immediately revives itself and re-executes all the offline processes immediately without the need for a boot process.
The researchers will present their work (LightPC: Hardware and Software Co-Design for Energy-Efficient Full System Persistence) at the International Symposium on Computer Architecture (ISCA) 2022 in New York in June. More information is available at the CAMELab website (http://camelab.org).
-Profile: Professor Myoungsoo Jung Computer Architecture and Memory Systems Laboratory (CAMEL)http://camelab.org School of Electrical EngineeringKAIST
2022.04.25 View 24130
LightPC Presents a Resilient System Using Only Non-Volatile Memory
Lightweight Persistence Centric System (LightPC) ensures both data and execution persistence for energy-efficient full system persistence
A KAIST research team has developed hardware and software technology that ensures both data and execution persistence. The Lightweight Persistence Centric System (LightPC) makes the systems resilient against power failures by utilizing only non-volatile memory as the main memory.
“We mounted non-volatile memory on a system board prototype and created an operating system to verify the effectiveness of LightPC,” said Professor Myoungsoo Jung. The team confirmed that LightPC validated its execution while powering up and down in the middle of execution, showing up to eight times more memory, 4.3 times faster application execution, and 73% lower power consumption compared to traditional systems.
Professor Jung said that LightPC can be utilized in a variety of fields such as data centers and high-performance computing to provide large-capacity memory, high performance, low power consumption, and service reliability.
In general, power failures on legacy systems can lead to the loss of data stored in the DRAM-based main memory. Unlike volatile memory such as DRAM, non-volatile memory can retain its data without power. Although non-volatile memory has the characteristics of lower power consumption and larger capacity than DRAM, non-volatile memory is typically used for the task of secondary storage due to its lower write performance. For this reason, nonvolatile memory is often used with DRAM. However, modern systems employing non-volatile memory-based main memory experience unexpected performance degradation due to the complicated memory microarchitecture.
To enable both data and execution persistent in legacy systems, it is necessary to transfer the data from the volatile memory to the non-volatile memory. Checkpointing is one possible solution. It periodically transfers the data in preparation for a sudden power failure. While this technology is essential for ensuring high mobility and reliability for users, checkpointing also has fatal drawbacks. It takes additional time and power to move data and requires a data recovery process as well as restarting the system.
In order to address these issues, the research team developed a processor and memory controller to raise the performance of non-volatile memory-only memory. LightPC matches the performance of DRAM by minimizing the internal volatile memory components from non-volatile memory, exposing the non-volatile memory (PRAM) media to the host, and increasing parallelism to service on-the-fly requests as soon as possible.
The team also presented operating system technology that quickly makes execution states of running processes persistent without the need for a checkpointing process. The operating system prevents all modifications to execution states and data by keeping all program executions idle before transferring data in order to support consistency within a period much shorter than the standard power hold-up time of about 16 minutes. For consistency, when the power is recovered, the computer almost immediately revives itself and re-executes all the offline processes immediately without the need for a boot process.
The researchers will present their work (LightPC: Hardware and Software Co-Design for Energy-Efficient Full System Persistence) at the International Symposium on Computer Architecture (ISCA) 2022 in New York in June. More information is available at the CAMELab website (http://camelab.org).
-Profile: Professor Myoungsoo Jung Computer Architecture and Memory Systems Laboratory (CAMEL)http://camelab.org School of Electrical EngineeringKAIST
2022.04.25 View 24130 -
 Mathematicians Identify a Key Source of Cell-to-Cell Variability in Cell Signaling
Systematic inferences identify a major source of heterogeneity in cell signaling dynamics
Why do genetically identical cells respond differently to the same external stimuli, such as antibiotics? This long-standing mystery has been solved by KAIST and IBS mathematicians who have developed a new framework for analyzing cell responses to some stimuli. The team found that the cell-to-cell variability in antibiotic stress response increases as the effective length of the cell signaling pathway (i.e., the number of rate-limiting steps) increases. This finding could identify more effective chemotherapies to overcome the fractional killing of cancer cells caused by cell-to-cell variability.
Cells in the human body contain signal transduction systems that respond to various external stimuli such as antibiotics and changes in osmotic pressure. When an external stimulus is detected, various biochemical reactions occur sequentially. This leads to the expression of relevant genes, allowing the cells to respond to the perturbed external environment. Furthermore, signal transduction leads to a drug response (e.g., antibiotic resistance genes are expressed when antibiotic drugs are given).
However, even when the same external stimuli are detected, the responses of individual cells are greatly heterogeneous. This leads to the emergence of persister cells that are highly resistant to drugs. To identify potential sources of this cell-to cell variability, many studies have been conducted. However, most of the intermediate signal transduction reactions are unobservable with current experimental techniques.
A group of researchers including Dae Wook Kim and Hyukpyo Hong and led by Professor Jae Kyoung Kim from the KAIST Department of Mathematical Sciences and IBS Biomedical Mathematics Group solved the mystery by exploiting queueing theory and Bayesian inference methodology. They proposed a queueing process that describes the signal transduction system in cells. Based on this, they developed Bayesian inference computational software using MBI (the Moment-based Bayesian Inference method). This enables the analysis of the signal transduction system without a direct observation of the intermediate steps. This study was published in Science Advances.
By analyzing experimental data from Escherichia coli using MBI, the research team found that cell-to-cell variability increases as the number of rate-limiting steps in the signaling pathway increases.
The rate-limiting steps denote the slowest steps (i.e., bottlenecks) in sequential biochemical reaction steps composing cell signaling pathways and thus dominates most of the signaling time. As the number of the rate-limiting steps increases, the intensity of the transduced signal becomes greatly heterogeneous even in a population of genetically identical cells. This finding is expected to provide a new paradigm for studying the heterogeneous antibiotic resistance of cells, which is a big challenge in cancer medicine.
Professor Kim said, “As a mathematician, I am excited to help advance the understanding of cell-to-cell variability in response to external stimuli. I hope this finding facilitates the development of more effective chemotherapies.”
This work was supported by the Samsung Science and Technology Foundation, the National Research Foundation of Korea, and the Institute for Basic Science.
-Publication:Dae Wook Kim, Hyukpyo Hong, and Jae Kyoung Kim (2022) “Systematic inference identifies a major source of heterogeneity in cell signaling dynamics: the rate-limiting step number,”Science Advances March 18, 2022 (DOI: 10.1126/sciadv.abl4598)
-Profile:Professor Jae Kyoung Kimhttp://mathsci.kaist.ac.kr/~jaekkim
jaekkim@kaist.ac.kr@umichkim on TwitterDepartment of Mathematical SciencesKAIST
2022.03.29 View 11483
Mathematicians Identify a Key Source of Cell-to-Cell Variability in Cell Signaling
Systematic inferences identify a major source of heterogeneity in cell signaling dynamics
Why do genetically identical cells respond differently to the same external stimuli, such as antibiotics? This long-standing mystery has been solved by KAIST and IBS mathematicians who have developed a new framework for analyzing cell responses to some stimuli. The team found that the cell-to-cell variability in antibiotic stress response increases as the effective length of the cell signaling pathway (i.e., the number of rate-limiting steps) increases. This finding could identify more effective chemotherapies to overcome the fractional killing of cancer cells caused by cell-to-cell variability.
Cells in the human body contain signal transduction systems that respond to various external stimuli such as antibiotics and changes in osmotic pressure. When an external stimulus is detected, various biochemical reactions occur sequentially. This leads to the expression of relevant genes, allowing the cells to respond to the perturbed external environment. Furthermore, signal transduction leads to a drug response (e.g., antibiotic resistance genes are expressed when antibiotic drugs are given).
However, even when the same external stimuli are detected, the responses of individual cells are greatly heterogeneous. This leads to the emergence of persister cells that are highly resistant to drugs. To identify potential sources of this cell-to cell variability, many studies have been conducted. However, most of the intermediate signal transduction reactions are unobservable with current experimental techniques.
A group of researchers including Dae Wook Kim and Hyukpyo Hong and led by Professor Jae Kyoung Kim from the KAIST Department of Mathematical Sciences and IBS Biomedical Mathematics Group solved the mystery by exploiting queueing theory and Bayesian inference methodology. They proposed a queueing process that describes the signal transduction system in cells. Based on this, they developed Bayesian inference computational software using MBI (the Moment-based Bayesian Inference method). This enables the analysis of the signal transduction system without a direct observation of the intermediate steps. This study was published in Science Advances.
By analyzing experimental data from Escherichia coli using MBI, the research team found that cell-to-cell variability increases as the number of rate-limiting steps in the signaling pathway increases.
The rate-limiting steps denote the slowest steps (i.e., bottlenecks) in sequential biochemical reaction steps composing cell signaling pathways and thus dominates most of the signaling time. As the number of the rate-limiting steps increases, the intensity of the transduced signal becomes greatly heterogeneous even in a population of genetically identical cells. This finding is expected to provide a new paradigm for studying the heterogeneous antibiotic resistance of cells, which is a big challenge in cancer medicine.
Professor Kim said, “As a mathematician, I am excited to help advance the understanding of cell-to-cell variability in response to external stimuli. I hope this finding facilitates the development of more effective chemotherapies.”
This work was supported by the Samsung Science and Technology Foundation, the National Research Foundation of Korea, and the Institute for Basic Science.
-Publication:Dae Wook Kim, Hyukpyo Hong, and Jae Kyoung Kim (2022) “Systematic inference identifies a major source of heterogeneity in cell signaling dynamics: the rate-limiting step number,”Science Advances March 18, 2022 (DOI: 10.1126/sciadv.abl4598)
-Profile:Professor Jae Kyoung Kimhttp://mathsci.kaist.ac.kr/~jaekkim
jaekkim@kaist.ac.kr@umichkim on TwitterDepartment of Mathematical SciencesKAIST
2022.03.29 View 11483 -
 Decoding Brain Signals to Control a Robotic Arm
Advanced brain-machine interface system successfully interprets arm movement directions from neural signals in the brain
Researchers have developed a mind-reading system for decoding neural signals from the brain during arm movement. The method, described in the journal Applied Soft Computing, can be used by a person to control a robotic arm through a brain-machine interface (BMI).
A BMI is a device that translates nerve signals into commands to control a machine, such as a computer or a robotic limb. There are two main techniques for monitoring neural signals in BMIs: electroencephalography (EEG) and electrocorticography (ECoG).
The EEG exhibits signals from electrodes on the surface of the scalp and is widely employed because it is non-invasive, relatively cheap, safe and easy to use. However, the EEG has low spatial resolution and detects irrelevant neural signals, which makes it difficult to interpret the intentions of individuals from the EEG.
On the other hand, the ECoG is an invasive method that involves placing electrodes directly on the surface of the cerebral cortex below the scalp. Compared with the EEG, the ECoG can monitor neural signals with much higher spatial resolution and less background noise. However, this technique has several drawbacks.
“The ECoG is primarily used to find potential sources of epileptic seizures, meaning the electrodes are placed in different locations for different patients and may not be in the optimal regions of the brain for detecting sensory and movement signals,” explained Professor Jaeseung Jeong, a brain scientist at KAIST. “This inconsistency makes it difficult to decode brain signals to predict movements.”
To overcome these problems, Professor Jeong’s team developed a new method for decoding ECoG neural signals during arm movement. The system is based on a machine-learning system for analysing and predicting neural signals called an ‘echo-state network’ and a mathematical probability model called the Gaussian distribution.
In the study, the researchers recorded ECoG signals from four individuals with epilepsy while they were performing a reach-and-grasp task. Because the ECoG electrodes were placed according to the potential sources of each patient’s epileptic seizures, only 22% to 44% of the electrodes were located in the regions of the brain responsible for controlling movement.
During the movement task, the participants were given visual cues, either by placing a real tennis ball in front of them, or via a virtual reality headset showing a clip of a human arm reaching forward in first-person view. They were asked to reach forward, grasp an object, then return their hand and release the object, while wearing motion sensors on their wrists and fingers. In a second task, they were instructed to imagine reaching forward without moving their arms.
The researchers monitored the signals from the ECoG electrodes during real and imaginary arm movements, and tested whether the new system could predict the direction of this movement from the neural signals. They found that the novel decoder successfully classified arm movements in 24 directions in three-dimensional space, both in the real and virtual tasks, and that the results were at least five times more accurate than chance. They also used a computer simulation to show that the novel ECoG decoder could control the movements of a robotic arm.
Overall, the results suggest that the new machine learning-based BCI system successfully used ECoG signals to interpret the direction of the intended movements. The next steps will be to improve the accuracy and efficiency of the decoder. In the future, it could be used in a real-time BMI device to help people with movement or sensory impairments.
This research was supported by the KAIST Global Singularity Research Program of 2021, Brain Research Program of the National Research Foundation of Korea funded by the Ministry of Science, ICT, and Future Planning, and the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education.
-PublicationHoon-Hee Kim, Jaeseung Jeong, “An electrocorticographic decoder for arm movement for brain-machine interface using an echo state network and Gaussian readout,” Applied SoftComputing online December 31, 2021 (doi.org/10.1016/j.asoc.2021.108393)
-ProfileProfessor Jaeseung JeongDepartment of Bio and Brain EngineeringCollege of EngineeringKAIST
2022.03.18 View 13523
Decoding Brain Signals to Control a Robotic Arm
Advanced brain-machine interface system successfully interprets arm movement directions from neural signals in the brain
Researchers have developed a mind-reading system for decoding neural signals from the brain during arm movement. The method, described in the journal Applied Soft Computing, can be used by a person to control a robotic arm through a brain-machine interface (BMI).
A BMI is a device that translates nerve signals into commands to control a machine, such as a computer or a robotic limb. There are two main techniques for monitoring neural signals in BMIs: electroencephalography (EEG) and electrocorticography (ECoG).
The EEG exhibits signals from electrodes on the surface of the scalp and is widely employed because it is non-invasive, relatively cheap, safe and easy to use. However, the EEG has low spatial resolution and detects irrelevant neural signals, which makes it difficult to interpret the intentions of individuals from the EEG.
On the other hand, the ECoG is an invasive method that involves placing electrodes directly on the surface of the cerebral cortex below the scalp. Compared with the EEG, the ECoG can monitor neural signals with much higher spatial resolution and less background noise. However, this technique has several drawbacks.
“The ECoG is primarily used to find potential sources of epileptic seizures, meaning the electrodes are placed in different locations for different patients and may not be in the optimal regions of the brain for detecting sensory and movement signals,” explained Professor Jaeseung Jeong, a brain scientist at KAIST. “This inconsistency makes it difficult to decode brain signals to predict movements.”
To overcome these problems, Professor Jeong’s team developed a new method for decoding ECoG neural signals during arm movement. The system is based on a machine-learning system for analysing and predicting neural signals called an ‘echo-state network’ and a mathematical probability model called the Gaussian distribution.
In the study, the researchers recorded ECoG signals from four individuals with epilepsy while they were performing a reach-and-grasp task. Because the ECoG electrodes were placed according to the potential sources of each patient’s epileptic seizures, only 22% to 44% of the electrodes were located in the regions of the brain responsible for controlling movement.
During the movement task, the participants were given visual cues, either by placing a real tennis ball in front of them, or via a virtual reality headset showing a clip of a human arm reaching forward in first-person view. They were asked to reach forward, grasp an object, then return their hand and release the object, while wearing motion sensors on their wrists and fingers. In a second task, they were instructed to imagine reaching forward without moving their arms.
The researchers monitored the signals from the ECoG electrodes during real and imaginary arm movements, and tested whether the new system could predict the direction of this movement from the neural signals. They found that the novel decoder successfully classified arm movements in 24 directions in three-dimensional space, both in the real and virtual tasks, and that the results were at least five times more accurate than chance. They also used a computer simulation to show that the novel ECoG decoder could control the movements of a robotic arm.
Overall, the results suggest that the new machine learning-based BCI system successfully used ECoG signals to interpret the direction of the intended movements. The next steps will be to improve the accuracy and efficiency of the decoder. In the future, it could be used in a real-time BMI device to help people with movement or sensory impairments.
This research was supported by the KAIST Global Singularity Research Program of 2021, Brain Research Program of the National Research Foundation of Korea funded by the Ministry of Science, ICT, and Future Planning, and the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education.
-PublicationHoon-Hee Kim, Jaeseung Jeong, “An electrocorticographic decoder for arm movement for brain-machine interface using an echo state network and Gaussian readout,” Applied SoftComputing online December 31, 2021 (doi.org/10.1016/j.asoc.2021.108393)
-ProfileProfessor Jaeseung JeongDepartment of Bio and Brain EngineeringCollege of EngineeringKAIST
2022.03.18 View 13523 -
 CXL-Based Memory Disaggregation Technology Opens Up a New Direction for Big Data Solution Frameworks
A KAIST team’s compute express link (CXL) provides new insights on memory disaggregation and ensures direct access and high-performance capabilities
A team from the Computer Architecture and Memory Systems Laboratory (CAMEL) at KAIST presented a new compute express link (CXL) solution whose directly accessible, and high-performance memory disaggregation opens new directions for big data memory processing. Professor Myoungsoo Jung said the team’s technology significantly improves performance compared to existing remote direct memory access (RDMA)-based memory disaggregation.
CXL is a peripheral component interconnect-express (PCIe)-based new dynamic multi-protocol made for efficiently utilizing memory devices and accelerators. Many enterprise data centers and memory vendors are paying attention to it as the next-generation multi-protocol for the era of big data.
Emerging big data applications such as machine learning, graph analytics, and in-memory databases require large memory capacities. However, scaling out the memory capacity via a prior memory interface like double data rate (DDR) is limited by the number of the central processing units (CPUs) and memory controllers. Therefore, memory disaggregation, which allows connecting a host to another host’s memory or memory nodes, has appeared.
RDMA is a way that a host can directly access another host’s memory via InfiniBand, the commonly used network protocol in data centers. Nowadays, most existing memory disaggregation technologies employ RDMA to get a large memory capacity. As a result, a host can share another host’s memory by transferring the data between local and remote memory.
Although RDMA-based memory disaggregation provides a large memory capacity to a host, two critical problems exist. First, scaling out the memory still needs an extra CPU to be added. Since passive memory such as dynamic random-access memory (DRAM), cannot operate by itself, it should be controlled by the CPU. Second, redundant data copies and software fabric interventions for RDMA-based memory disaggregation cause longer access latency. For example, remote memory access latency in RDMA-based memory disaggregation is multiple orders of magnitude longer than local memory access.
To address these issues, Professor Jung’s team developed the CXL-based memory disaggregation framework, including CXL-enabled customized CPUs, CXL devices, CXL switches, and CXL-aware operating system modules. The team’s CXL device is a pure passive and directly accessible memory node that contains multiple DRAM dual inline memory modules (DIMMs) and a CXL memory controller. Since the CXL memory controller supports the memory in the CXL device, a host can utilize the memory node without processor or software intervention. The team’s CXL switch enables scaling out a host’s memory capacity by hierarchically connecting multiple CXL devices to the CXL switch allowing more than hundreds of devices. Atop the switches and devices, the team’s CXL-enabled operating system removes redundant data copy and protocol conversion exhibited by conventional RDMA, which can significantly decrease access latency to the memory nodes.
In a test comparing loading 64B (cacheline) data from memory pooling devices, CXL-based memory disaggregation showed 8.2 times higher data load performance than RDMA-based memory disaggregation and even similar performance to local DRAM memory. In the team’s evaluations for a big data benchmark such as a machine learning-based test, CXL-based memory disaggregation technology also showed a maximum of 3.7 times higher performance than prior RDMA-based memory disaggregation technologies.
“Escaping from the conventional RDMA-based memory disaggregation, our CXL-based memory disaggregation framework can provide high scalability and performance for diverse datacenters and cloud service infrastructures,” said Professor Jung. He went on to stress, “Our CXL-based memory disaggregation research will bring about a new paradigm for memory solutions that will lead the era of big data.”
-Profile: Professor Myoungsoo Jung Computer Architecture and Memory Systems Laboratory (CAMEL)http://camelab.org School of Electrical EngineeringKAIST
2022.03.16 View 24155
CXL-Based Memory Disaggregation Technology Opens Up a New Direction for Big Data Solution Frameworks
A KAIST team’s compute express link (CXL) provides new insights on memory disaggregation and ensures direct access and high-performance capabilities
A team from the Computer Architecture and Memory Systems Laboratory (CAMEL) at KAIST presented a new compute express link (CXL) solution whose directly accessible, and high-performance memory disaggregation opens new directions for big data memory processing. Professor Myoungsoo Jung said the team’s technology significantly improves performance compared to existing remote direct memory access (RDMA)-based memory disaggregation.
CXL is a peripheral component interconnect-express (PCIe)-based new dynamic multi-protocol made for efficiently utilizing memory devices and accelerators. Many enterprise data centers and memory vendors are paying attention to it as the next-generation multi-protocol for the era of big data.
Emerging big data applications such as machine learning, graph analytics, and in-memory databases require large memory capacities. However, scaling out the memory capacity via a prior memory interface like double data rate (DDR) is limited by the number of the central processing units (CPUs) and memory controllers. Therefore, memory disaggregation, which allows connecting a host to another host’s memory or memory nodes, has appeared.
RDMA is a way that a host can directly access another host’s memory via InfiniBand, the commonly used network protocol in data centers. Nowadays, most existing memory disaggregation technologies employ RDMA to get a large memory capacity. As a result, a host can share another host’s memory by transferring the data between local and remote memory.
Although RDMA-based memory disaggregation provides a large memory capacity to a host, two critical problems exist. First, scaling out the memory still needs an extra CPU to be added. Since passive memory such as dynamic random-access memory (DRAM), cannot operate by itself, it should be controlled by the CPU. Second, redundant data copies and software fabric interventions for RDMA-based memory disaggregation cause longer access latency. For example, remote memory access latency in RDMA-based memory disaggregation is multiple orders of magnitude longer than local memory access.
To address these issues, Professor Jung’s team developed the CXL-based memory disaggregation framework, including CXL-enabled customized CPUs, CXL devices, CXL switches, and CXL-aware operating system modules. The team’s CXL device is a pure passive and directly accessible memory node that contains multiple DRAM dual inline memory modules (DIMMs) and a CXL memory controller. Since the CXL memory controller supports the memory in the CXL device, a host can utilize the memory node without processor or software intervention. The team’s CXL switch enables scaling out a host’s memory capacity by hierarchically connecting multiple CXL devices to the CXL switch allowing more than hundreds of devices. Atop the switches and devices, the team’s CXL-enabled operating system removes redundant data copy and protocol conversion exhibited by conventional RDMA, which can significantly decrease access latency to the memory nodes.
In a test comparing loading 64B (cacheline) data from memory pooling devices, CXL-based memory disaggregation showed 8.2 times higher data load performance than RDMA-based memory disaggregation and even similar performance to local DRAM memory. In the team’s evaluations for a big data benchmark such as a machine learning-based test, CXL-based memory disaggregation technology also showed a maximum of 3.7 times higher performance than prior RDMA-based memory disaggregation technologies.
“Escaping from the conventional RDMA-based memory disaggregation, our CXL-based memory disaggregation framework can provide high scalability and performance for diverse datacenters and cloud service infrastructures,” said Professor Jung. He went on to stress, “Our CXL-based memory disaggregation research will bring about a new paradigm for memory solutions that will lead the era of big data.”
-Profile: Professor Myoungsoo Jung Computer Architecture and Memory Systems Laboratory (CAMEL)http://camelab.org School of Electrical EngineeringKAIST
2022.03.16 View 24155 -
 KAA Recognizes 4 Distinguished Alumni of the Year
The KAIST Alumni Association (KAA) recognized four distinguished alumni of the year during a ceremony on February 25 in Seoul. The four Distinguished Alumni Awardees are Distinguished Professor Sukbok Chang from the KAIST Department of Chemistry, Hyunshil Ahn, head of the AI Economy Institute and an editorial writer at The Korea Economic Daily, CEO Hwan-ho Sung of PSTech, and President Hark Kyu Park of Samsung Electronics.
Distinguished Professor Sukbok Chang who received his MS from the Department of Chemistry in 1985 has been a pioneer in the novel field of ‘carbon-hydrogen bond activation reactions’. He has significantly contributed to raising Korea’s international reputation in natural sciences and received the Kyungam Academic Award in 2013, the 14th Korea Science Award in 2015, the 1st Science and Technology Prize of Korea Toray in 2018, and the Best Scientist/Engineer Award Korea in 2019. Furthermore, he was named as a Highly Cited Researcher who ranked in the top 1% of citations by field and publication year in the Web of Science citation index for seven consecutive years from 2015 to 2021, demonstrating his leadership as a global scholar.
Hyunshil Ahn, a graduate of the School of Business and Technology Management with an MS in 1985 and a PhD in 1987, was appointed as the first head of the AI Economy Institute when The Korea Economic Daily was the first Korean media outlet to establish an AI economy lab. He has contributed to creating new roles for the press and media in the 4th industrial revolution, and added to the popularization of AI technology through regulation reform and consulting on industrial policies.
PSTech CEO Hwan-ho Sung is a graduate of the School of Electrical Engineering where he received an MS in 1988 and a PhD in EMBA in 2008. He has run the electronics company PSTech for over 20 years and successfully localized the production of power equipment, which previously depended on foreign technology. His development of the world’s first power equipment that can be applied to new industries including semiconductors and displays was recognized through this award.
Samsung Electronics President Hark Kyu Park graduated from the School of Business and Technology Management with an MS in 1986. He not only enhanced Korea’s national competitiveness by expanding the semiconductor industry, but also established contract-based semiconductor departments at Korean universities including KAIST, Sungkyunkwan University, Yonsei University, and Postech, and semiconductor track courses at KAIST, Sogang University, Seoul National University, and Postech to nurture professional talents. He also led the national semiconductor coexistence system by leading private sector-government-academia collaborations to strengthen competence in semiconductors, and continues to make unconditional investments in strong small businesses.
KAA President Chilhee Chung said, “Thanks to our alumni contributing at the highest levels of our society, the name of our alma mater shines brighter. As role models for our younger alumni, I hope greater honours will follow our awardees in the future.”
2022.03.03 View 8860
KAA Recognizes 4 Distinguished Alumni of the Year
The KAIST Alumni Association (KAA) recognized four distinguished alumni of the year during a ceremony on February 25 in Seoul. The four Distinguished Alumni Awardees are Distinguished Professor Sukbok Chang from the KAIST Department of Chemistry, Hyunshil Ahn, head of the AI Economy Institute and an editorial writer at The Korea Economic Daily, CEO Hwan-ho Sung of PSTech, and President Hark Kyu Park of Samsung Electronics.
Distinguished Professor Sukbok Chang who received his MS from the Department of Chemistry in 1985 has been a pioneer in the novel field of ‘carbon-hydrogen bond activation reactions’. He has significantly contributed to raising Korea’s international reputation in natural sciences and received the Kyungam Academic Award in 2013, the 14th Korea Science Award in 2015, the 1st Science and Technology Prize of Korea Toray in 2018, and the Best Scientist/Engineer Award Korea in 2019. Furthermore, he was named as a Highly Cited Researcher who ranked in the top 1% of citations by field and publication year in the Web of Science citation index for seven consecutive years from 2015 to 2021, demonstrating his leadership as a global scholar.
Hyunshil Ahn, a graduate of the School of Business and Technology Management with an MS in 1985 and a PhD in 1987, was appointed as the first head of the AI Economy Institute when The Korea Economic Daily was the first Korean media outlet to establish an AI economy lab. He has contributed to creating new roles for the press and media in the 4th industrial revolution, and added to the popularization of AI technology through regulation reform and consulting on industrial policies.
PSTech CEO Hwan-ho Sung is a graduate of the School of Electrical Engineering where he received an MS in 1988 and a PhD in EMBA in 2008. He has run the electronics company PSTech for over 20 years and successfully localized the production of power equipment, which previously depended on foreign technology. His development of the world’s first power equipment that can be applied to new industries including semiconductors and displays was recognized through this award.
Samsung Electronics President Hark Kyu Park graduated from the School of Business and Technology Management with an MS in 1986. He not only enhanced Korea’s national competitiveness by expanding the semiconductor industry, but also established contract-based semiconductor departments at Korean universities including KAIST, Sungkyunkwan University, Yonsei University, and Postech, and semiconductor track courses at KAIST, Sogang University, Seoul National University, and Postech to nurture professional talents. He also led the national semiconductor coexistence system by leading private sector-government-academia collaborations to strengthen competence in semiconductors, and continues to make unconditional investments in strong small businesses.
KAA President Chilhee Chung said, “Thanks to our alumni contributing at the highest levels of our society, the name of our alma mater shines brighter. As role models for our younger alumni, I hope greater honours will follow our awardees in the future.”
2022.03.03 View 8860 -
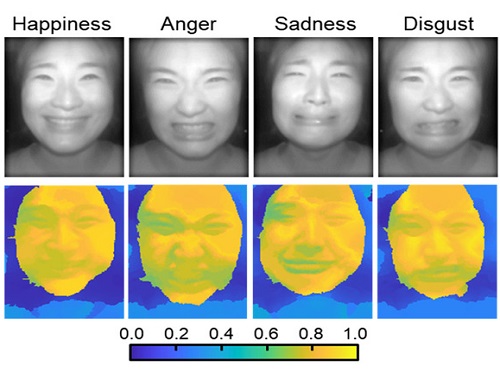 AI Light-Field Camera Reads 3D Facial Expressions
Machine-learned, light-field camera reads facial expressions from high-contrast illumination invariant 3D facial images
A joint research team led by Professors Ki-Hun Jeong and Doheon Lee from the KAIST Department of Bio and Brain Engineering reported the development of a technique for facial expression detection by merging near-infrared light-field camera techniques with artificial intelligence (AI) technology.
Unlike a conventional camera, the light-field camera contains micro-lens arrays in front of the image sensor, which makes the camera small enough to fit into a smart phone, while allowing it to acquire the spatial and directional information of the light with a single shot. The technique has received attention as it can reconstruct images in a variety of ways including multi-views, refocusing, and 3D image acquisition, giving rise to many potential applications.
However, the optical crosstalk between shadows caused by external light sources in the environment and the micro-lens has limited existing light-field cameras from being able to provide accurate image contrast and 3D reconstruction.
The joint research team applied a vertical-cavity surface-emitting laser (VCSEL) in the near-IR range to stabilize the accuracy of 3D image reconstruction that previously depended on environmental light. When an external light source is shone on a face at 0-, 30-, and 60-degree angles, the light field camera reduces 54% of image reconstruction errors. Additionally, by inserting a light-absorbing layer for visible and near-IR wavelengths between the micro-lens arrays, the team could minimize optical crosstalk while increasing the image contrast by 2.1 times.
Through this technique, the team could overcome the limitations of existing light-field cameras and was able to develop their NIR-based light-field camera (NIR-LFC), optimized for the 3D image reconstruction of facial expressions. Using the NIR-LFC, the team acquired high-quality 3D reconstruction images of facial expressions expressing various emotions regardless of the lighting conditions of the surrounding environment.
The facial expressions in the acquired 3D images were distinguished through machine learning with an average of 85% accuracy – a statistically significant figure compared to when 2D images were used. Furthermore, by calculating the interdependency of distance information that varies with facial expression in 3D images, the team could identify the information a light-field camera utilizes to distinguish human expressions.
Professor Ki-Hun Jeong said, “The sub-miniature light-field camera developed by the research team has the potential to become the new platform to quantitatively analyze the facial expressions and emotions of humans.” To highlight the significance of this research, he added, “It could be applied in various fields including mobile healthcare, field diagnosis, social cognition, and human-machine interactions.”
This research was published in Advanced Intelligent Systems online on December 16, under the title, “Machine-Learned Light-field Camera that Reads Facial Expression from High-Contrast and Illumination Invariant 3D Facial Images.” This research was funded by the Ministry of Science and ICT and the Ministry of Trade, Industry and Energy.
-Publication“Machine-learned light-field camera that reads fascial expression from high-contrast and illumination invariant 3D facial images,” Sang-In Bae, Sangyeon Lee, Jae-Myeong Kwon, Hyun-Kyung Kim. Kyung-Won Jang, Doheon Lee, Ki-Hun Jeong, Advanced Intelligent Systems, December 16, 2021 (doi.org/10.1002/aisy.202100182)
ProfileProfessor Ki-Hun JeongBiophotonic LaboratoryDepartment of Bio and Brain EngineeringKAIST
Professor Doheon LeeDepartment of Bio and Brain EngineeringKAIST
2022.01.21 View 13876
AI Light-Field Camera Reads 3D Facial Expressions
Machine-learned, light-field camera reads facial expressions from high-contrast illumination invariant 3D facial images
A joint research team led by Professors Ki-Hun Jeong and Doheon Lee from the KAIST Department of Bio and Brain Engineering reported the development of a technique for facial expression detection by merging near-infrared light-field camera techniques with artificial intelligence (AI) technology.
Unlike a conventional camera, the light-field camera contains micro-lens arrays in front of the image sensor, which makes the camera small enough to fit into a smart phone, while allowing it to acquire the spatial and directional information of the light with a single shot. The technique has received attention as it can reconstruct images in a variety of ways including multi-views, refocusing, and 3D image acquisition, giving rise to many potential applications.
However, the optical crosstalk between shadows caused by external light sources in the environment and the micro-lens has limited existing light-field cameras from being able to provide accurate image contrast and 3D reconstruction.
The joint research team applied a vertical-cavity surface-emitting laser (VCSEL) in the near-IR range to stabilize the accuracy of 3D image reconstruction that previously depended on environmental light. When an external light source is shone on a face at 0-, 30-, and 60-degree angles, the light field camera reduces 54% of image reconstruction errors. Additionally, by inserting a light-absorbing layer for visible and near-IR wavelengths between the micro-lens arrays, the team could minimize optical crosstalk while increasing the image contrast by 2.1 times.
Through this technique, the team could overcome the limitations of existing light-field cameras and was able to develop their NIR-based light-field camera (NIR-LFC), optimized for the 3D image reconstruction of facial expressions. Using the NIR-LFC, the team acquired high-quality 3D reconstruction images of facial expressions expressing various emotions regardless of the lighting conditions of the surrounding environment.
The facial expressions in the acquired 3D images were distinguished through machine learning with an average of 85% accuracy – a statistically significant figure compared to when 2D images were used. Furthermore, by calculating the interdependency of distance information that varies with facial expression in 3D images, the team could identify the information a light-field camera utilizes to distinguish human expressions.
Professor Ki-Hun Jeong said, “The sub-miniature light-field camera developed by the research team has the potential to become the new platform to quantitatively analyze the facial expressions and emotions of humans.” To highlight the significance of this research, he added, “It could be applied in various fields including mobile healthcare, field diagnosis, social cognition, and human-machine interactions.”
This research was published in Advanced Intelligent Systems online on December 16, under the title, “Machine-Learned Light-field Camera that Reads Facial Expression from High-Contrast and Illumination Invariant 3D Facial Images.” This research was funded by the Ministry of Science and ICT and the Ministry of Trade, Industry and Energy.
-Publication“Machine-learned light-field camera that reads fascial expression from high-contrast and illumination invariant 3D facial images,” Sang-In Bae, Sangyeon Lee, Jae-Myeong Kwon, Hyun-Kyung Kim. Kyung-Won Jang, Doheon Lee, Ki-Hun Jeong, Advanced Intelligent Systems, December 16, 2021 (doi.org/10.1002/aisy.202100182)
ProfileProfessor Ki-Hun JeongBiophotonic LaboratoryDepartment of Bio and Brain EngineeringKAIST
Professor Doheon LeeDepartment of Bio and Brain EngineeringKAIST
2022.01.21 View 13876 -
 KAIST ISPI Releases Report on the Global AI Innovation Landscape
Providing key insights for building a successful AI ecosystem
The KAIST Innovation Strategy and Policy Institute (ISPI) has launched a report on the global innovation landscape of artificial intelligence in collaboration with Clarivate Plc. The report shows that AI has become a key technology and that cross-industry learning is an important AI innovation. It also stresses that the quality of innovation, not volume, is a critical success factor in technological competitiveness.
Key findings of the report include:
• Neural networks and machine learning have been unrivaled in terms of scale and growth (more than 46%), and most other AI technologies show a growth rate of more than 20%.
• Although Mainland China has shown the highest growth rate in terms of AI inventions, the influence of Chinese AI is relatively low. In contrast, the United States holds a leading position in AI-related inventions in terms of both quantity and influence.
• The U.S. and Canada have built an industry-oriented AI technology development ecosystem through organic cooperation with both academia and the Government. Mainland China and South Korea, by contrast, have a government-driven AI technology development ecosystem with relatively low qualitative outputs from the sector.
• The U.S., the U.K., and Canada have a relatively high proportion of inventions in robotics and autonomous control, whereas in Mainland China and South Korea, machine learning and neural networks are making progress. Each country/region produces high-quality inventions in their predominant AI fields, while the U.S. has produced high-impact inventions in almost all AI fields.
“The driving forces in building a sustainable AI innovation ecosystem are important national strategies. A country’s future AI capabilities will be determined by how quickly and robustly it develops its own AI ecosystem and how well it transforms the existing industry with AI technologies. Countries that build a successful AI ecosystem have the potential to accelerate growth while absorbing the AI capabilities of other countries. AI talents are already moving to countries with excellent AI ecosystems,” said Director of the ISPI Wonjoon Kim.
“AI, together with other high-tech IT technologies including big data and the Internet of Things are accelerating the digital transformation by leading an intelligent hyper-connected society and enabling the convergence of technology and business. With the rapid growth of AI innovation, AI applications are also expanding in various ways across industries and in our lives,” added Justin Kim, Special Advisor at the ISPI and a co-author of the report.
2021.12.21 View 9498
KAIST ISPI Releases Report on the Global AI Innovation Landscape
Providing key insights for building a successful AI ecosystem
The KAIST Innovation Strategy and Policy Institute (ISPI) has launched a report on the global innovation landscape of artificial intelligence in collaboration with Clarivate Plc. The report shows that AI has become a key technology and that cross-industry learning is an important AI innovation. It also stresses that the quality of innovation, not volume, is a critical success factor in technological competitiveness.
Key findings of the report include:
• Neural networks and machine learning have been unrivaled in terms of scale and growth (more than 46%), and most other AI technologies show a growth rate of more than 20%.
• Although Mainland China has shown the highest growth rate in terms of AI inventions, the influence of Chinese AI is relatively low. In contrast, the United States holds a leading position in AI-related inventions in terms of both quantity and influence.
• The U.S. and Canada have built an industry-oriented AI technology development ecosystem through organic cooperation with both academia and the Government. Mainland China and South Korea, by contrast, have a government-driven AI technology development ecosystem with relatively low qualitative outputs from the sector.
• The U.S., the U.K., and Canada have a relatively high proportion of inventions in robotics and autonomous control, whereas in Mainland China and South Korea, machine learning and neural networks are making progress. Each country/region produces high-quality inventions in their predominant AI fields, while the U.S. has produced high-impact inventions in almost all AI fields.
“The driving forces in building a sustainable AI innovation ecosystem are important national strategies. A country’s future AI capabilities will be determined by how quickly and robustly it develops its own AI ecosystem and how well it transforms the existing industry with AI technologies. Countries that build a successful AI ecosystem have the potential to accelerate growth while absorbing the AI capabilities of other countries. AI talents are already moving to countries with excellent AI ecosystems,” said Director of the ISPI Wonjoon Kim.
“AI, together with other high-tech IT technologies including big data and the Internet of Things are accelerating the digital transformation by leading an intelligent hyper-connected society and enabling the convergence of technology and business. With the rapid growth of AI innovation, AI applications are also expanding in various ways across industries and in our lives,” added Justin Kim, Special Advisor at the ISPI and a co-author of the report.
2021.12.21 View 9498 -
 New Chiral Nanostructures to Extend the Material Platform
Researchers observed a wide window of chiroptical activity from nanomaterials
A research team transferred chirality from the molecular scale to a microscale to extend material platforms and applications. The optical activity from this novel chiral material encompasses to short-wave infrared region.
This platform could serve as a powerful strategy for hierarchical chirality transfer through self-assembly, generating broad optical activity and providing immense applications including bio, telecommunication, and imaging technique. This is the first observation of such a wide window of chiroptical activity from nanomaterials.
“We synthesized chiral copper sulfides using cysteine, as the stabilizer, and transferring the chirality from molecular to the microscale through self-assembly,” explained Professor Jihyeon Yeom from the Department of Materials Science and Engineering, who led the research. The result was reported in ACS Nano on September 14.
Chiral nanomaterials provide a rich platform for versatile applications. Tuning the wavelength of polarization rotation maxima in the broad range is a promising candidate for infrared neural stimulation, imaging, and nanothermometry. However, the majority of previously developed chiral nanomaterials revealed the optical activity in a relatively shorter wavelength range, not in short-wave infrared.
To achieve chiroptical activity in the short-wave infrared region, materials should be in sub-micrometer dimensions, which are compatible with the wavelength of short-wave infrared region light for strong light-matter interaction. They also should have the optical property of short-wave infrared region absorption while forming a structure with chirality.
Professor Yeom’s team induced self-assembly of the chiral nanoparticles by controlling the attraction and repulsion forces between the building block nanoparticles. During this process, molecular chirality of cysteine was transferred to the nanoscale chirality of nanoparticles, and then transferred to the micrometer scale chirality of nanoflowers with 1.5-2 2 μm dimensions formed by the self-assembly.
“We will work to expand the wavelength range of chiroptical activity to the short-wave infrared region, thus reshaping our daily lives in the form of a bio-barcode that can store vast amount of information under the skin,” said Professor Yeom.
This study was funded by the Ministry of Science and ICT, the Ministry of Health and Welfare, the Ministry of Food and Drug Safety, the National Research Foundation of Korea,the KAIST URP Program, the KAIST Creative Challenging Research Program, Samsung and POSCO Science Fellowship.
-PublicationKi Hyun Park, Junyoung Kwon, Uichang Jeong, Ji-Young Kim, Nicholas A.Kotov, Jihyeon Yeom, “Broad Chrioptical Activity from Ultraviolet to Short-Wave Infrared by Chirality Transfer from Molecular to Micrometer Scale," September 14, 2021 ACS Nano (https://doi.org/10.1021/acsnano.1c05888)
-ProfileProfessor Jihyeon YeomNovel Nanomaterials for New Platforms LaboratoryDepartment of Materials Science and EngineeringKAIST
2021.10.22 View 11535
New Chiral Nanostructures to Extend the Material Platform
Researchers observed a wide window of chiroptical activity from nanomaterials
A research team transferred chirality from the molecular scale to a microscale to extend material platforms and applications. The optical activity from this novel chiral material encompasses to short-wave infrared region.
This platform could serve as a powerful strategy for hierarchical chirality transfer through self-assembly, generating broad optical activity and providing immense applications including bio, telecommunication, and imaging technique. This is the first observation of such a wide window of chiroptical activity from nanomaterials.
“We synthesized chiral copper sulfides using cysteine, as the stabilizer, and transferring the chirality from molecular to the microscale through self-assembly,” explained Professor Jihyeon Yeom from the Department of Materials Science and Engineering, who led the research. The result was reported in ACS Nano on September 14.
Chiral nanomaterials provide a rich platform for versatile applications. Tuning the wavelength of polarization rotation maxima in the broad range is a promising candidate for infrared neural stimulation, imaging, and nanothermometry. However, the majority of previously developed chiral nanomaterials revealed the optical activity in a relatively shorter wavelength range, not in short-wave infrared.
To achieve chiroptical activity in the short-wave infrared region, materials should be in sub-micrometer dimensions, which are compatible with the wavelength of short-wave infrared region light for strong light-matter interaction. They also should have the optical property of short-wave infrared region absorption while forming a structure with chirality.
Professor Yeom’s team induced self-assembly of the chiral nanoparticles by controlling the attraction and repulsion forces between the building block nanoparticles. During this process, molecular chirality of cysteine was transferred to the nanoscale chirality of nanoparticles, and then transferred to the micrometer scale chirality of nanoflowers with 1.5-2 2 μm dimensions formed by the self-assembly.
“We will work to expand the wavelength range of chiroptical activity to the short-wave infrared region, thus reshaping our daily lives in the form of a bio-barcode that can store vast amount of information under the skin,” said Professor Yeom.
This study was funded by the Ministry of Science and ICT, the Ministry of Health and Welfare, the Ministry of Food and Drug Safety, the National Research Foundation of Korea,the KAIST URP Program, the KAIST Creative Challenging Research Program, Samsung and POSCO Science Fellowship.
-PublicationKi Hyun Park, Junyoung Kwon, Uichang Jeong, Ji-Young Kim, Nicholas A.Kotov, Jihyeon Yeom, “Broad Chrioptical Activity from Ultraviolet to Short-Wave Infrared by Chirality Transfer from Molecular to Micrometer Scale," September 14, 2021 ACS Nano (https://doi.org/10.1021/acsnano.1c05888)
-ProfileProfessor Jihyeon YeomNovel Nanomaterials for New Platforms LaboratoryDepartment of Materials Science and EngineeringKAIST
2021.10.22 View 11535 -
 Deep Learning Framework to Enable Material Design in Unseen Domain
Researchers propose a deep neural network-based forward design space exploration using active transfer learning and data augmentation
A new study proposed a deep neural network-based forward design approach that enables an efficient search for superior materials far beyond the domain of the initial training set. This approach compensates for the weak predictive power of neural networks on an unseen domain through gradual updates of the neural network with active transfer learning and data augmentation methods.
Professor Seungwha Ryu believes that this study will help address a variety of optimization problems that have an astronomical number of possible design configurations. For the grid composite optimization problem, the proposed framework was able to provide excellent designs close to the global optima, even with the addition of a very small dataset corresponding to less than 0.5% of the initial training data-set size. This study was reported in npj Computational Materials last month.
“We wanted to mitigate the limitation of the neural network, weak predictive power beyond the training set domain for the material or structure design,” said Professor Ryu from the Department of Mechanical Engineering.
Neural network-based generative models have been actively investigated as an inverse design method for finding novel materials in a vast design space. However, the applicability of conventional generative models is limited because they cannot access data outside the range of training sets. Advanced generative models that were devised to overcome this limitation also suffer from weak predictive power for the unseen domain.
Professor Ryu’s team, in collaboration with researchers from Professor Grace Gu’s group at UC Berkeley, devised a design method that simultaneously expands the domain using the strong predictive power of a deep neural network and searches for the optimal design by repetitively performing three key steps.
First, it searches for few candidates with improved properties located close to the training set via genetic algorithms, by mixing superior designs within the training set. Then, it checks to see if the candidates really have improved properties, and expands the training set by duplicating the validated designs via a data augmentation method. Finally, they can expand the reliable prediction domain by updating the neural network with the new superior designs via transfer learning. Because the expansion proceeds along relatively narrow but correct routes toward the optimal design (depicted in the schematic of Fig. 1), the framework enables an efficient search.
As a data-hungry method, a deep neural network model tends to have reliable predictive power only within and near the domain of the training set. When the optimal configuration of materials and structures lies far beyond the initial training set, which frequently is the case, neural network-based design methods suffer from weak predictive power and become inefficient.
Researchers expect that the framework will be applicable for a wide range of optimization problems in other science and engineering disciplines with astronomically large design space, because it provides an efficient way of gradually expanding the reliable prediction domain toward the target design while avoiding the risk of being stuck in local minima. Especially, being a less-data-hungry method, design problems in which data generation is time-consuming and expensive will benefit most from this new framework.
The research team is currently applying the optimization framework for the design task of metamaterial structures, segmented thermoelectric generators, and optimal sensor distributions. “From these sets of on-going studies, we expect to better recognize the pros and cons, and the potential of the suggested algorithm. Ultimately, we want to devise more efficient machine learning-based design approaches,” explained Professor Ryu.This study was funded by the National Research Foundation of Korea and the KAIST Global Singularity Research Project.
-Publication
Yongtae Kim, Youngsoo, Charles Yang, Kundo Park, Grace X. Gu, and Seunghwa Ryu, “Deep learning framework for material design space exploration using active transfer learning and data augmentation,” npj Computational Materials (https://doi.org/10.1038/s41524-021-00609-2)
-Profile
Professor Seunghwa Ryu
Mechanics & Materials Modeling Lab
Department of Mechanical Engineering
KAIST
2021.09.29 View 13501
Deep Learning Framework to Enable Material Design in Unseen Domain
Researchers propose a deep neural network-based forward design space exploration using active transfer learning and data augmentation
A new study proposed a deep neural network-based forward design approach that enables an efficient search for superior materials far beyond the domain of the initial training set. This approach compensates for the weak predictive power of neural networks on an unseen domain through gradual updates of the neural network with active transfer learning and data augmentation methods.
Professor Seungwha Ryu believes that this study will help address a variety of optimization problems that have an astronomical number of possible design configurations. For the grid composite optimization problem, the proposed framework was able to provide excellent designs close to the global optima, even with the addition of a very small dataset corresponding to less than 0.5% of the initial training data-set size. This study was reported in npj Computational Materials last month.
“We wanted to mitigate the limitation of the neural network, weak predictive power beyond the training set domain for the material or structure design,” said Professor Ryu from the Department of Mechanical Engineering.
Neural network-based generative models have been actively investigated as an inverse design method for finding novel materials in a vast design space. However, the applicability of conventional generative models is limited because they cannot access data outside the range of training sets. Advanced generative models that were devised to overcome this limitation also suffer from weak predictive power for the unseen domain.
Professor Ryu’s team, in collaboration with researchers from Professor Grace Gu’s group at UC Berkeley, devised a design method that simultaneously expands the domain using the strong predictive power of a deep neural network and searches for the optimal design by repetitively performing three key steps.
First, it searches for few candidates with improved properties located close to the training set via genetic algorithms, by mixing superior designs within the training set. Then, it checks to see if the candidates really have improved properties, and expands the training set by duplicating the validated designs via a data augmentation method. Finally, they can expand the reliable prediction domain by updating the neural network with the new superior designs via transfer learning. Because the expansion proceeds along relatively narrow but correct routes toward the optimal design (depicted in the schematic of Fig. 1), the framework enables an efficient search.
As a data-hungry method, a deep neural network model tends to have reliable predictive power only within and near the domain of the training set. When the optimal configuration of materials and structures lies far beyond the initial training set, which frequently is the case, neural network-based design methods suffer from weak predictive power and become inefficient.
Researchers expect that the framework will be applicable for a wide range of optimization problems in other science and engineering disciplines with astronomically large design space, because it provides an efficient way of gradually expanding the reliable prediction domain toward the target design while avoiding the risk of being stuck in local minima. Especially, being a less-data-hungry method, design problems in which data generation is time-consuming and expensive will benefit most from this new framework.
The research team is currently applying the optimization framework for the design task of metamaterial structures, segmented thermoelectric generators, and optimal sensor distributions. “From these sets of on-going studies, we expect to better recognize the pros and cons, and the potential of the suggested algorithm. Ultimately, we want to devise more efficient machine learning-based design approaches,” explained Professor Ryu.This study was funded by the National Research Foundation of Korea and the KAIST Global Singularity Research Project.
-Publication
Yongtae Kim, Youngsoo, Charles Yang, Kundo Park, Grace X. Gu, and Seunghwa Ryu, “Deep learning framework for material design space exploration using active transfer learning and data augmentation,” npj Computational Materials (https://doi.org/10.1038/s41524-021-00609-2)
-Profile
Professor Seunghwa Ryu
Mechanics & Materials Modeling Lab
Department of Mechanical Engineering
KAIST
2021.09.29 View 13501 -
 Prof. Changho Suh Named the 2021 James L. Massey Awardee
Professor Changho Suh from the School of Electrical Engineering was named the recipient of the 2021 James L.Massey Award. The award recognizes outstanding achievement in research and teaching by young scholars in the information theory community. The award is named in honor of James L. Massey, who was an internationally acclaimed pioneer in digital communications and revered teacher and mentor to communications engineers.
Professor Suh is a recipient of numerous awards, including the 2021 James L. Massey Research & Teaching Award for Young Scholars from the IEEE Information Theory Society, the 2019 AFOSR Grant, the 2019 Google Education Grant, the 2018 IEIE/IEEE Joint Award, the 2015 IEIE Haedong Young Engineer Award, the 2013 IEEE Communications Society Stephen O. Rice Prize, the 2011 David J. Sakrison Memorial Prize (the best dissertation award in UC Berkeley EECS), the 2009 IEEE ISIT Best Student Paper Award, the 2020 LINKGENESIS Best Teacher Award (the campus-wide Grand Prize in Teaching), and the four Departmental Teaching Awards (2013, 2019, 2020, 2021).
Dr. Suh is an IEEE Information Theory Society Distinguished Lecturer, the General Chair of the Inaugural IEEE East Asian School of Information Theory, and a Member of the Young Korean Academy of Science and Technology. He is also an Associate Editor of Machine Learning for the IEEE Transactions on Information Theory, the Editor for the IEEE Information Theory Newsletter, a Column Editor for IEEE BITS the Information Theory Magazine, an Area Chair of NeurIPS 2021, and on the Senior Program Committee of IJCAI 2019–2021.
2021.07.27 View 9507
Prof. Changho Suh Named the 2021 James L. Massey Awardee
Professor Changho Suh from the School of Electrical Engineering was named the recipient of the 2021 James L.Massey Award. The award recognizes outstanding achievement in research and teaching by young scholars in the information theory community. The award is named in honor of James L. Massey, who was an internationally acclaimed pioneer in digital communications and revered teacher and mentor to communications engineers.
Professor Suh is a recipient of numerous awards, including the 2021 James L. Massey Research & Teaching Award for Young Scholars from the IEEE Information Theory Society, the 2019 AFOSR Grant, the 2019 Google Education Grant, the 2018 IEIE/IEEE Joint Award, the 2015 IEIE Haedong Young Engineer Award, the 2013 IEEE Communications Society Stephen O. Rice Prize, the 2011 David J. Sakrison Memorial Prize (the best dissertation award in UC Berkeley EECS), the 2009 IEEE ISIT Best Student Paper Award, the 2020 LINKGENESIS Best Teacher Award (the campus-wide Grand Prize in Teaching), and the four Departmental Teaching Awards (2013, 2019, 2020, 2021).
Dr. Suh is an IEEE Information Theory Society Distinguished Lecturer, the General Chair of the Inaugural IEEE East Asian School of Information Theory, and a Member of the Young Korean Academy of Science and Technology. He is also an Associate Editor of Machine Learning for the IEEE Transactions on Information Theory, the Editor for the IEEE Information Theory Newsletter, a Column Editor for IEEE BITS the Information Theory Magazine, an Area Chair of NeurIPS 2021, and on the Senior Program Committee of IJCAI 2019–2021.
2021.07.27 View 9507 -
 Hydrogel-Based Flexible Brain-Machine Interface
The interface is easy to insert into the body when dry, but behaves ‘stealthily’ inside the brain when wet
Professor Seongjun Park’s research team and collaborators revealed a newly developed hydrogel-based flexible brain-machine interface. To study the structure of the brain or to identify and treat neurological diseases, it is crucial to develop an interface that can stimulate the brain and detect its signals in real time. However, existing neural interfaces are mechanically and chemically different from real brain tissue. This causes foreign body response and forms an insulating layer (glial scar) around the interface, which shortens its lifespan.
To solve this problem, the research team developed a ‘brain-mimicking interface’ by inserting a custom-made multifunctional fiber bundle into the hydrogel body. The device is composed not only of an optical fiber that controls specific nerve cells with light in order to perform optogenetic procedures, but it also has an electrode bundle to read brain signals and a microfluidic channel to deliver drugs to the brain.
The interface is easy to insert into the body when dry, as hydrogels become solid. But once in the body, the hydrogel will quickly absorb body fluids and resemble the properties of its surrounding tissues, thereby minimizing foreign body response.
The research team applied the device on animal models, and showed that it was possible to detect neural signals for up to six months, which is far beyond what had been previously recorded. It was also possible to conduct long-term optogenetic and behavioral experiments on freely moving mice with a significant reduction in foreign body responses such as glial and immunological activation compared to existing devices.
“This research is significant in that it was the first to utilize a hydrogel as part of a multifunctional neural interface probe, which increased its lifespan dramatically,” said Professor Park. “With our discovery, we look forward to advancements in research on neurological disorders like Alzheimer’s or Parkinson’s disease that require long-term observation.”
The research was published in Nature Communications on June 8, 2021. (Title: Adaptive and multifunctional hydrogel hybrid probes for long-term sensing and modulation of neural activity) The study was conducted jointly with an MIT research team composed of Professor Polina Anikeeva, Professor Xuanhe Zhao, and Dr. Hyunwoo Yook.
This research was supported by the National Research Foundation (NRF) grant for emerging research, Korea Medical Device Development Fund, KK-JRC Smart Project, KAIST Global Initiative Program, and Post-AI Project.
-PublicationPark, S., Yuk, H., Zhao, R. et al. Adaptive and multifunctional hydrogel hybrid probes for long-term sensing and modulation of neural activity. Nat Commun 12, 3435 (2021). https://doi.org/10.1038/s41467-021-23802-9
-ProfileProfessor Seongjun ParkBio and Neural Interfaces LaboratoryDepartment of Bio and Brain EngineeringKAIST
2021.07.13 View 13016
Hydrogel-Based Flexible Brain-Machine Interface
The interface is easy to insert into the body when dry, but behaves ‘stealthily’ inside the brain when wet
Professor Seongjun Park’s research team and collaborators revealed a newly developed hydrogel-based flexible brain-machine interface. To study the structure of the brain or to identify and treat neurological diseases, it is crucial to develop an interface that can stimulate the brain and detect its signals in real time. However, existing neural interfaces are mechanically and chemically different from real brain tissue. This causes foreign body response and forms an insulating layer (glial scar) around the interface, which shortens its lifespan.
To solve this problem, the research team developed a ‘brain-mimicking interface’ by inserting a custom-made multifunctional fiber bundle into the hydrogel body. The device is composed not only of an optical fiber that controls specific nerve cells with light in order to perform optogenetic procedures, but it also has an electrode bundle to read brain signals and a microfluidic channel to deliver drugs to the brain.
The interface is easy to insert into the body when dry, as hydrogels become solid. But once in the body, the hydrogel will quickly absorb body fluids and resemble the properties of its surrounding tissues, thereby minimizing foreign body response.
The research team applied the device on animal models, and showed that it was possible to detect neural signals for up to six months, which is far beyond what had been previously recorded. It was also possible to conduct long-term optogenetic and behavioral experiments on freely moving mice with a significant reduction in foreign body responses such as glial and immunological activation compared to existing devices.
“This research is significant in that it was the first to utilize a hydrogel as part of a multifunctional neural interface probe, which increased its lifespan dramatically,” said Professor Park. “With our discovery, we look forward to advancements in research on neurological disorders like Alzheimer’s or Parkinson’s disease that require long-term observation.”
The research was published in Nature Communications on June 8, 2021. (Title: Adaptive and multifunctional hydrogel hybrid probes for long-term sensing and modulation of neural activity) The study was conducted jointly with an MIT research team composed of Professor Polina Anikeeva, Professor Xuanhe Zhao, and Dr. Hyunwoo Yook.
This research was supported by the National Research Foundation (NRF) grant for emerging research, Korea Medical Device Development Fund, KK-JRC Smart Project, KAIST Global Initiative Program, and Post-AI Project.
-PublicationPark, S., Yuk, H., Zhao, R. et al. Adaptive and multifunctional hydrogel hybrid probes for long-term sensing and modulation of neural activity. Nat Commun 12, 3435 (2021). https://doi.org/10.1038/s41467-021-23802-9
-ProfileProfessor Seongjun ParkBio and Neural Interfaces LaboratoryDepartment of Bio and Brain EngineeringKAIST
2021.07.13 View 13016