IES
-
 KAIST research team develops a cheap and safe redox flow battery
Redox flow batteries, one of the potential replacements for the widely used lithium-ion secondary batteries, can be utilized as new and renewable energy as well as for energy storage systems (ESS) thanks to their low cost, low flammability, and long lifetime of over 20 years. Since the price of vanadium, the most widely used active material for redox flow batteries, has been rising in recent years, scientists have been actively searching for redox materials to replace it.
On March 23, a joint research team led by Professors Hye Ryung Byon and Mu-Hyun Baik from the KAIST Department of Chemistry, and Professor Jongcheol Seo from the POSTECH Department of Chemistry announced that they had developed a highly soluble and stable organic redox-active molecule for use in aqueous redox flow batteries.
The research team focused on developing aqueous redox flow batteries by redesigning an organic molecule. It is possible to control the solubility and electrochemical redox potential of organic molecules by engineering their design, which makes them a promising active material candidate with possibly higher energy storage capabilities than vanadium. Most organic redox-active molecules have low solubilities or have slow chemical stability during redox reactions. Low solubility means low energy storage capacity and low chemical stability leads to reduced cycle performance. For this research, the team chose naphthalene diimide (NDI) as their active molecule. Until now, there was little research done on NDI despite its high chemical stability, as it shows low solubility in aqueous electrolyte solutions.
Although NDI molecules are almost insoluble in water, the research team tethered four ammonium functionalities and achieved a solubility as high as 1.5M* in water. In addition, they confirmed that when a 1M solution of NDI was used in neutral redox flow batteries for 500 cycles, 98% of its capacity was maintained. This means 0.004% capacity decay per cycle, and only 2% of its capacity would be lost if the battery were to be operated for 45 days.
Furthermore, the developed NDI molecule can save two electrons per molecule, and the team proved that 2M of electrons could be stored in every 1M of NDI solution used. For reference, vanadium used in vanadium redox flow batteries, which require a highly concentrated sulfuric acid solution, has a solubility of about 1.6M and can only hold one electron per molecule, meaning it can store a total of 1.6M of electrons. Therefore, the newly developed NDI active molecule shows a higher storage capacity compared to existing vanadium devices.
*1M (mol/L): 6.022 x 1023 active molecules are present in 1L of solution
This paper, written by co-first authors Research Professor Vikram Singh, and Ph.D. candidates Seongyeon Kwon and Yunseop Choi, was published in the online version of Advanced Materials on February 7 under the title, Controlling π-π interactions of highly soluble naphthalene diimide derivatives for neutral pH aqueous redox flow batteries. Ph.D. Candidate Yelim Yi and Professor Mi Hee Lee’s team from the KAIST Department of Chemistry also contributed to the study by conducting electron paramagnetic resonance analyses.
Professor Hye Ryung Byon said, “We have demonstrated the principles of molecular design by modifying an existing organic active molecule with low solubility and utilizing it as an active molecule for redox flow batteries. We have also shown that during a redox reaction, we can use molecular interactions to suppress the chemical reactivity of radically formed molecules.”
She added, “Should this be used later for aqueous redox flow batteries, along with its high energy density and high solubility, it would also have the advantage of being available for use in neutral pH electrolytes. Vanadium redox flow batteries currently use acidic solutions, which cause corrosion, and we expect our molecule to solve this issue. Since existing lithium ion-based ESS are flammable, we must develop safer and cheaper next-generation ESS, and our research has shown great promise in addressing this.”
This research was funded by Samsung Research Funding & Incubation Center, the Institute for Basic Science, and the National Research Foundation.
Figure 1. (a) Structures of various NDI molecules. (b) Solubility of NDI molecules in water (black bars) and aqueous electrolytes including KCl electrolyte (blue bars). (c–d) Structural changes of the molecules as the developed NDI molecule stores two electrons. (c) Illustration of cluster combination and separation of NDI molecules developed during redox reaction and (d) Snapshot of the MD simulation. NDI molecules prepared from the left, formation of bimolecular sieve and tetramolecular sieve clusters after the first reductive reaction, and a single molecule with a three-dimensional structure after the second reduction.
Figure 2. Performance results of an aqueous redox flow battery using 1M of the developed NDI molecule as the cathode electrolyte and 3.1M of ammonium iodine as the anode electrolyte. Using 1.5 M KCl solution. (a) A schematic diagram of a redox flow battery. (b) Voltage-capacity graph according to cycle in a redox flow battery. (c) Graphs of capacity and coulombs, voltage, and energy efficiency maintained at 500 cycles.
2023.04.03 View 7663
KAIST research team develops a cheap and safe redox flow battery
Redox flow batteries, one of the potential replacements for the widely used lithium-ion secondary batteries, can be utilized as new and renewable energy as well as for energy storage systems (ESS) thanks to their low cost, low flammability, and long lifetime of over 20 years. Since the price of vanadium, the most widely used active material for redox flow batteries, has been rising in recent years, scientists have been actively searching for redox materials to replace it.
On March 23, a joint research team led by Professors Hye Ryung Byon and Mu-Hyun Baik from the KAIST Department of Chemistry, and Professor Jongcheol Seo from the POSTECH Department of Chemistry announced that they had developed a highly soluble and stable organic redox-active molecule for use in aqueous redox flow batteries.
The research team focused on developing aqueous redox flow batteries by redesigning an organic molecule. It is possible to control the solubility and electrochemical redox potential of organic molecules by engineering their design, which makes them a promising active material candidate with possibly higher energy storage capabilities than vanadium. Most organic redox-active molecules have low solubilities or have slow chemical stability during redox reactions. Low solubility means low energy storage capacity and low chemical stability leads to reduced cycle performance. For this research, the team chose naphthalene diimide (NDI) as their active molecule. Until now, there was little research done on NDI despite its high chemical stability, as it shows low solubility in aqueous electrolyte solutions.
Although NDI molecules are almost insoluble in water, the research team tethered four ammonium functionalities and achieved a solubility as high as 1.5M* in water. In addition, they confirmed that when a 1M solution of NDI was used in neutral redox flow batteries for 500 cycles, 98% of its capacity was maintained. This means 0.004% capacity decay per cycle, and only 2% of its capacity would be lost if the battery were to be operated for 45 days.
Furthermore, the developed NDI molecule can save two electrons per molecule, and the team proved that 2M of electrons could be stored in every 1M of NDI solution used. For reference, vanadium used in vanadium redox flow batteries, which require a highly concentrated sulfuric acid solution, has a solubility of about 1.6M and can only hold one electron per molecule, meaning it can store a total of 1.6M of electrons. Therefore, the newly developed NDI active molecule shows a higher storage capacity compared to existing vanadium devices.
*1M (mol/L): 6.022 x 1023 active molecules are present in 1L of solution
This paper, written by co-first authors Research Professor Vikram Singh, and Ph.D. candidates Seongyeon Kwon and Yunseop Choi, was published in the online version of Advanced Materials on February 7 under the title, Controlling π-π interactions of highly soluble naphthalene diimide derivatives for neutral pH aqueous redox flow batteries. Ph.D. Candidate Yelim Yi and Professor Mi Hee Lee’s team from the KAIST Department of Chemistry also contributed to the study by conducting electron paramagnetic resonance analyses.
Professor Hye Ryung Byon said, “We have demonstrated the principles of molecular design by modifying an existing organic active molecule with low solubility and utilizing it as an active molecule for redox flow batteries. We have also shown that during a redox reaction, we can use molecular interactions to suppress the chemical reactivity of radically formed molecules.”
She added, “Should this be used later for aqueous redox flow batteries, along with its high energy density and high solubility, it would also have the advantage of being available for use in neutral pH electrolytes. Vanadium redox flow batteries currently use acidic solutions, which cause corrosion, and we expect our molecule to solve this issue. Since existing lithium ion-based ESS are flammable, we must develop safer and cheaper next-generation ESS, and our research has shown great promise in addressing this.”
This research was funded by Samsung Research Funding & Incubation Center, the Institute for Basic Science, and the National Research Foundation.
Figure 1. (a) Structures of various NDI molecules. (b) Solubility of NDI molecules in water (black bars) and aqueous electrolytes including KCl electrolyte (blue bars). (c–d) Structural changes of the molecules as the developed NDI molecule stores two electrons. (c) Illustration of cluster combination and separation of NDI molecules developed during redox reaction and (d) Snapshot of the MD simulation. NDI molecules prepared from the left, formation of bimolecular sieve and tetramolecular sieve clusters after the first reductive reaction, and a single molecule with a three-dimensional structure after the second reduction.
Figure 2. Performance results of an aqueous redox flow battery using 1M of the developed NDI molecule as the cathode electrolyte and 3.1M of ammonium iodine as the anode electrolyte. Using 1.5 M KCl solution. (a) A schematic diagram of a redox flow battery. (b) Voltage-capacity graph according to cycle in a redox flow battery. (c) Graphs of capacity and coulombs, voltage, and energy efficiency maintained at 500 cycles.
2023.04.03 View 7663 -
 Overview of the 30-year history of metabolic engineering
< Distinguished Professor Sang Yup Lee from the Department of Chemical and Biomolecular Engineering at KAIST >
A research team comprised of Gi Bae Kim, Dr. So Young Choi, Dr. In Jin Cho, Da-Hee Ahn, and Distinguished Professor Sang Yup Lee from the Department of Chemical and Biomolecular Engineering at KAIST reported the 30-year history of metabolic engineering, highlighting examples of recent progress in the field and contributions to sustainability and health. Their paper “Metabolic engineering for sustainability and health” was published online in the 40th anniversary special issue of Trends in Biotechnology on January 10, 2023.
Metabolic engineering, a discipline of engineering that modifies cell phenotypes through molecular and genetic-level manipulations to improve cellular activities, has been studied since the early 1990s, and has progressed significantly over the past 30 years. In particular, metabolic engineering has enabled the engineering of microorganisms for the development of microbial cell factories capable of efficiently producing chemicals and materials as well as degrading recalcitrant contaminants.
This review article revisited how metabolic engineering has advanced over the past 30 years, from the advent of genetic engineering techniques such as recombinant DNA technologies to recent breakthroughs in systems metabolic engineering and data science aided by artificial intelligence. The research team highlighted momentous events and achievements in metabolic engineering, providing both trends and future directions in the field. Metabolic engineering’s contributions to bio-based sustainable chemicals and clean energy, health, and bioremediation were also reviewed. Finally, the research team shared their perspectives on the future challenges impacting metabolic engineering than must be overcome in order to achieve advancements in sustainability and health.
Distinguished Professor Sang Yup Lee said, “Replacing fossil resource-based chemical processes with bio-based sustainable processes for the production of chemicals, fuels, and materials using metabolic engineering has become our essential task for the future. By looking back on the 30+ years of metabolic engineering, we aimed to highlight the contributions of metabolic engineering to achieve sustainability and good health.” He added, “Metabolic engineering will play an increasingly important role as a key solution to the climate crisis, environmental pollution, food and energy shortages, and health problems in aging societies.”
< Figure: Metabolic Engineering Timeline >
2023.01.25 View 11564
Overview of the 30-year history of metabolic engineering
< Distinguished Professor Sang Yup Lee from the Department of Chemical and Biomolecular Engineering at KAIST >
A research team comprised of Gi Bae Kim, Dr. So Young Choi, Dr. In Jin Cho, Da-Hee Ahn, and Distinguished Professor Sang Yup Lee from the Department of Chemical and Biomolecular Engineering at KAIST reported the 30-year history of metabolic engineering, highlighting examples of recent progress in the field and contributions to sustainability and health. Their paper “Metabolic engineering for sustainability and health” was published online in the 40th anniversary special issue of Trends in Biotechnology on January 10, 2023.
Metabolic engineering, a discipline of engineering that modifies cell phenotypes through molecular and genetic-level manipulations to improve cellular activities, has been studied since the early 1990s, and has progressed significantly over the past 30 years. In particular, metabolic engineering has enabled the engineering of microorganisms for the development of microbial cell factories capable of efficiently producing chemicals and materials as well as degrading recalcitrant contaminants.
This review article revisited how metabolic engineering has advanced over the past 30 years, from the advent of genetic engineering techniques such as recombinant DNA technologies to recent breakthroughs in systems metabolic engineering and data science aided by artificial intelligence. The research team highlighted momentous events and achievements in metabolic engineering, providing both trends and future directions in the field. Metabolic engineering’s contributions to bio-based sustainable chemicals and clean energy, health, and bioremediation were also reviewed. Finally, the research team shared their perspectives on the future challenges impacting metabolic engineering than must be overcome in order to achieve advancements in sustainability and health.
Distinguished Professor Sang Yup Lee said, “Replacing fossil resource-based chemical processes with bio-based sustainable processes for the production of chemicals, fuels, and materials using metabolic engineering has become our essential task for the future. By looking back on the 30+ years of metabolic engineering, we aimed to highlight the contributions of metabolic engineering to achieve sustainability and good health.” He added, “Metabolic engineering will play an increasingly important role as a key solution to the climate crisis, environmental pollution, food and energy shortages, and health problems in aging societies.”
< Figure: Metabolic Engineering Timeline >
2023.01.25 View 11564 -
 A KAIST Research Team Develops Diesel Reforming Catalyst Enabling Hydrogen Production for Future Mobile Fuel Cells
This catalyst capability allowing stable hydrogen production from commercial diesel is expected to be applied in mobile fuel cell systems in the future hydrogen economy
On August 16, a joint research team led by Professors Joongmyeon Bae and Kang Taek Lee of KAIST’s Department of Mechanical Engineering and Dr. Chan-Woo Lee of Korea Institute of Energy Research (KIER) announced the successful development of a highly active and durable reforming catalyst allowing hydrogen production from commercial diesel.
Fuel reforming is a hydrogen production technique that extracts hydrogen from hydrocarbons through catalytic reactions. Diesel, being a liquid fuel, has a high storage density for hydrogen and is easy to transport and store. There have therefore been continuous research efforts to apply hydrogel supply systems using diesel reformation in mobile fuel cells, such as for auxiliary power in heavy trucks or air-independent propulsion (AIP) systems in submarines.
However, diesel is a mixture of high hydrocarbons including long-chained paraffin, double-bonded olefin, and aromatic hydrocarbons with benzene groups, and it requires a highly active catalyst to effectively break them down. In addition, the catalyst must be extremely durable against caulking and sintering, as they are often the main causes of catalyst degradation. Such challenges have limited the use of diesel reformation technologies to date.
The joint research team successfully developed a highly active and durable diesel reforming catalyst through elution (a heat treatment method used to uniformly grow active metals retained in an oxide support as ions in the form of metal nanoparticles), forming alloy nanoparticles. The design was based on the fact that eluted nanoparticles strongly interact with the support, allowing a high degree of dispersion at high temperatures, and that producing an alloy from dissimilar metals can increase the performance of catalysts through a synergistic effect.
The research team introduced a solution combustion synthesis method to produce a multi-component catalyst with a trace amount of platinum (Pt) and ruthenium (Ru) penetrated into a ceria (CeO2) lattice, which is a structure commonly used as a support for catalysts in redox reactions. When exposed to a diesel reforming reaction environment, the catalyst induces Pt-Ru alloy nanoparticle formation upon Pt and Ru elution onto the support surface.
In addition to the catalyst analysis, the research team also succeeded in characterizing the behaviour of active metal elution and alloy formation from an energetic perspective using a density functional theory-based calculation. In a performance comparison test between the Pt-Ru alloy catalyst against existing single-metal catalysts, the reforming activity was shown to have improved, as it showed a 100% fuel conversion rate even at a low temperature (600oC, compared to the original 800oC). In a long-term durability test (800oC, 200 hours), the catalyst showed commercial stability by successfully producing hydrogen from commercial diesel without performance degradation.
The study was conducted by Ph.D. candidate Jaemyung Lee of KAIST’s Department of Mechanical Engineering as the first author. Ph.D. candidate Changho Yeon of KIER, Dr. Jiwoo Oh of KAIST’s Department of Mechanical Engineering, Dr. Gwangwoo Han of KIER, Ph.D. candidate Jeong Do Yoo of KAIST’s Department of Mechanical Engineering, and Dr. Hyung Joong Yun of the Korea Basic Science Institute contributed as co-authors. Dr. Chan-Woo Lee of KIER and Professors Kang Taek Lee and Joongmyeon Bae of KAIST’s Department of Mechanical Engineering contributed as corresponding authors. The research was published in the online version of Applied Catalysis B: Environmental (IF 24.319, JCR 0.93%) on June 17, under the title “Highly Active and Stable Catalyst with Exsolved PtRu Alloy Nanoparticles for Hydrogen Production via Commercial Diesel Reforming”.
Professor Joongmyeon Bae said, “The fact that hydrogen can be stably produced from commercial diesel makes this a very meaningful achievement, and we look forward to this technology contributing to the active introduction of mobile fuel cell systems in the early hydrogen economy.” He added, “Our approach to catalyst design may be applied not only to reforming reactions, but also in various other fields.”
This research was supported by the National Research Foundation of Korea through funding from the Ministry of Science, ICT and Future Planning.
Figure. Schematic diagram of high-performance diesel reforming catalyst with eluted platinum-ruthenium alloy nanoparticles and long-term durability verification experiment results for commercial diesel reforming reaction
2022.09.07 View 15243
A KAIST Research Team Develops Diesel Reforming Catalyst Enabling Hydrogen Production for Future Mobile Fuel Cells
This catalyst capability allowing stable hydrogen production from commercial diesel is expected to be applied in mobile fuel cell systems in the future hydrogen economy
On August 16, a joint research team led by Professors Joongmyeon Bae and Kang Taek Lee of KAIST’s Department of Mechanical Engineering and Dr. Chan-Woo Lee of Korea Institute of Energy Research (KIER) announced the successful development of a highly active and durable reforming catalyst allowing hydrogen production from commercial diesel.
Fuel reforming is a hydrogen production technique that extracts hydrogen from hydrocarbons through catalytic reactions. Diesel, being a liquid fuel, has a high storage density for hydrogen and is easy to transport and store. There have therefore been continuous research efforts to apply hydrogel supply systems using diesel reformation in mobile fuel cells, such as for auxiliary power in heavy trucks or air-independent propulsion (AIP) systems in submarines.
However, diesel is a mixture of high hydrocarbons including long-chained paraffin, double-bonded olefin, and aromatic hydrocarbons with benzene groups, and it requires a highly active catalyst to effectively break them down. In addition, the catalyst must be extremely durable against caulking and sintering, as they are often the main causes of catalyst degradation. Such challenges have limited the use of diesel reformation technologies to date.
The joint research team successfully developed a highly active and durable diesel reforming catalyst through elution (a heat treatment method used to uniformly grow active metals retained in an oxide support as ions in the form of metal nanoparticles), forming alloy nanoparticles. The design was based on the fact that eluted nanoparticles strongly interact with the support, allowing a high degree of dispersion at high temperatures, and that producing an alloy from dissimilar metals can increase the performance of catalysts through a synergistic effect.
The research team introduced a solution combustion synthesis method to produce a multi-component catalyst with a trace amount of platinum (Pt) and ruthenium (Ru) penetrated into a ceria (CeO2) lattice, which is a structure commonly used as a support for catalysts in redox reactions. When exposed to a diesel reforming reaction environment, the catalyst induces Pt-Ru alloy nanoparticle formation upon Pt and Ru elution onto the support surface.
In addition to the catalyst analysis, the research team also succeeded in characterizing the behaviour of active metal elution and alloy formation from an energetic perspective using a density functional theory-based calculation. In a performance comparison test between the Pt-Ru alloy catalyst against existing single-metal catalysts, the reforming activity was shown to have improved, as it showed a 100% fuel conversion rate even at a low temperature (600oC, compared to the original 800oC). In a long-term durability test (800oC, 200 hours), the catalyst showed commercial stability by successfully producing hydrogen from commercial diesel without performance degradation.
The study was conducted by Ph.D. candidate Jaemyung Lee of KAIST’s Department of Mechanical Engineering as the first author. Ph.D. candidate Changho Yeon of KIER, Dr. Jiwoo Oh of KAIST’s Department of Mechanical Engineering, Dr. Gwangwoo Han of KIER, Ph.D. candidate Jeong Do Yoo of KAIST’s Department of Mechanical Engineering, and Dr. Hyung Joong Yun of the Korea Basic Science Institute contributed as co-authors. Dr. Chan-Woo Lee of KIER and Professors Kang Taek Lee and Joongmyeon Bae of KAIST’s Department of Mechanical Engineering contributed as corresponding authors. The research was published in the online version of Applied Catalysis B: Environmental (IF 24.319, JCR 0.93%) on June 17, under the title “Highly Active and Stable Catalyst with Exsolved PtRu Alloy Nanoparticles for Hydrogen Production via Commercial Diesel Reforming”.
Professor Joongmyeon Bae said, “The fact that hydrogen can be stably produced from commercial diesel makes this a very meaningful achievement, and we look forward to this technology contributing to the active introduction of mobile fuel cell systems in the early hydrogen economy.” He added, “Our approach to catalyst design may be applied not only to reforming reactions, but also in various other fields.”
This research was supported by the National Research Foundation of Korea through funding from the Ministry of Science, ICT and Future Planning.
Figure. Schematic diagram of high-performance diesel reforming catalyst with eluted platinum-ruthenium alloy nanoparticles and long-term durability verification experiment results for commercial diesel reforming reaction
2022.09.07 View 15243 -
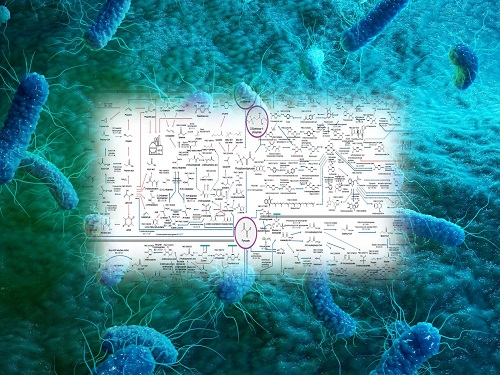 Interactive Map of Metabolical Synthesis of Chemicals
An interactive map that compiled the chemicals produced by biological, chemical and combined reactions has been distributed on the web
- A team led by Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering, organized and distributed an all-inclusive listing of chemical substances that can be synthesized using microorganisms
- It is expected to be used by researchers around the world as it enables easy assessment of the synthetic pathway through the web.
A research team comprised of Woo Dae Jang, Gi Bae Kim, and Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering at KAIST reported an interactive metabolic map of bio-based chemicals. Their research paper “An interactive metabolic map of bio-based chemicals” was published online in Trends in Biotechnology on August 10, 2022.
As a response to rapid climate change and environmental pollution, research on the production of petrochemical products using microorganisms is receiving attention as a sustainable alternative to existing methods of productions. In order to synthesize various chemical substances, materials, and fuel using microorganisms, it is necessary to first construct the biosynthetic pathway toward desired product by exploration and discovery and introduce them into microorganisms. In addition, in order to efficiently synthesize various chemical substances, it is sometimes necessary to employ chemical methods along with bioengineering methods using microorganisms at the same time. For the production of non-native chemicals, novel pathways are designed by recruiting enzymes from heterologous sources or employing enzymes designed though rational engineering, directed evolution, or ab initio design.
The research team had completed a map of chemicals which compiled all available pathways of biological and/or chemical reactions that lead to the production of various bio-based chemicals back in 2019 and published the map in Nature Catalysis. The map was distributed in the form of a poster to industries and academia so that the synthesis paths of bio-based chemicals could be checked at a glance.
The research team has expanded the bio-based chemicals map this time in the form of an interactive map on the web so that anyone with internet access can quickly explore efficient paths to synthesize desired products. The web-based map provides interactive visual tools to allow interactive visualization, exploration, and analysis of complex networks of biological and/or chemical reactions toward the desired products. In addition, the reported paper also discusses the production of natural compounds that are used for diverse purposes such as food and medicine, which will help designing novel pathways through similar approaches or by exploiting the promiscuity of enzymes described in the map. The published bio-based chemicals map is also available at http://systemsbiotech.co.kr.
The co-first authors, Dr. Woo Dae Jang and Ph.D. student Gi Bae Kim, said, “We conducted this study to address the demand for updating the previously distributed chemicals map and enhancing its versatility.” “The map is expected to be utilized in a variety of research and in efforts to set strategies and prospects for chemical production incorporating bio and chemical methods that are detailed in the map.”
Distinguished Professor Sang Yup Lee said, “The interactive bio-based chemicals map is expected to help design and optimization of the metabolic pathways for the biosynthesis of target chemicals together with the strategies of chemical conversions, serving as a blueprint for developing further ideas on the production of desired chemicals through biological and/or chemical reactions.”
The interactive metabolic map of bio-based chemicals.
2022.08.11 View 16472
Interactive Map of Metabolical Synthesis of Chemicals
An interactive map that compiled the chemicals produced by biological, chemical and combined reactions has been distributed on the web
- A team led by Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering, organized and distributed an all-inclusive listing of chemical substances that can be synthesized using microorganisms
- It is expected to be used by researchers around the world as it enables easy assessment of the synthetic pathway through the web.
A research team comprised of Woo Dae Jang, Gi Bae Kim, and Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering at KAIST reported an interactive metabolic map of bio-based chemicals. Their research paper “An interactive metabolic map of bio-based chemicals” was published online in Trends in Biotechnology on August 10, 2022.
As a response to rapid climate change and environmental pollution, research on the production of petrochemical products using microorganisms is receiving attention as a sustainable alternative to existing methods of productions. In order to synthesize various chemical substances, materials, and fuel using microorganisms, it is necessary to first construct the biosynthetic pathway toward desired product by exploration and discovery and introduce them into microorganisms. In addition, in order to efficiently synthesize various chemical substances, it is sometimes necessary to employ chemical methods along with bioengineering methods using microorganisms at the same time. For the production of non-native chemicals, novel pathways are designed by recruiting enzymes from heterologous sources or employing enzymes designed though rational engineering, directed evolution, or ab initio design.
The research team had completed a map of chemicals which compiled all available pathways of biological and/or chemical reactions that lead to the production of various bio-based chemicals back in 2019 and published the map in Nature Catalysis. The map was distributed in the form of a poster to industries and academia so that the synthesis paths of bio-based chemicals could be checked at a glance.
The research team has expanded the bio-based chemicals map this time in the form of an interactive map on the web so that anyone with internet access can quickly explore efficient paths to synthesize desired products. The web-based map provides interactive visual tools to allow interactive visualization, exploration, and analysis of complex networks of biological and/or chemical reactions toward the desired products. In addition, the reported paper also discusses the production of natural compounds that are used for diverse purposes such as food and medicine, which will help designing novel pathways through similar approaches or by exploiting the promiscuity of enzymes described in the map. The published bio-based chemicals map is also available at http://systemsbiotech.co.kr.
The co-first authors, Dr. Woo Dae Jang and Ph.D. student Gi Bae Kim, said, “We conducted this study to address the demand for updating the previously distributed chemicals map and enhancing its versatility.” “The map is expected to be utilized in a variety of research and in efforts to set strategies and prospects for chemical production incorporating bio and chemical methods that are detailed in the map.”
Distinguished Professor Sang Yup Lee said, “The interactive bio-based chemicals map is expected to help design and optimization of the metabolic pathways for the biosynthesis of target chemicals together with the strategies of chemical conversions, serving as a blueprint for developing further ideas on the production of desired chemicals through biological and/or chemical reactions.”
The interactive metabolic map of bio-based chemicals.
2022.08.11 View 16472 -
 The 1st Global Entrepreneurship Summer Camp bridges KAIST and Silicon Valley, US
Twenty KAIST students gave a go at selling their business ideas to investors at Silicon Valley on the “Pitch Day” at 2022 Global Entrepreneurship Summer Camp.
From Tuesday, June 21 to Monday, July 4, 2022, KAIST held the first Global Entrepreneurship Summer Camp (GESC).
The 2022 GESC, which was organized in collaboration with Stanford Technology Ventures Program (STVP), KOTRA Silicon Valley IT Center, and KAIST Alumni at Silicon Valley, was a pilot program that offered opportunities of experiencing and learning about the cases of startup companies in Silicon Valley and a chance to expand businesses to Silicon Valley through networking.
Twenty KAIST students, including pre-startup entrepreneurs and students interested in global entrepreneurship with less than one year of business experience were selected. The first week of the program was organized by Startup KAIST while the second week program was organized by the Center for Global Strategies and Planning (GSP) at KAIST in collaboration with the Stanford Technology Venture Program (STVP), KAIST Alumni at Silicon Valley, and KOTRA at Silicon Valley.
Dr. Mo-Yun Lei Fong, the Executive Director of STVP, said, “The program offered an opportunity for us to realize our vision of empowering aspiring entrepreneurs to become global citizens who create and scale responsible innovation. By collaborating with KAIST and offering entrepreneurial insights to Korean students, we are able to have a positive impact on a global scale.” Mo added, “The program also enabled STVP to build bridges, learn from the students, and refine our culturally relevant curriculum by understanding Korean culture and ideas.”
On the “Pitch Day” on July 1, following a special talk by Dr. Chong-Moon Lee, the Chairman of AmBex Venture Partners, the students presented their team business ideas such as an AI-assisted, noise-canceling pillow devised for better sleep, a metaverse dating application, an XR virtual conferencing system, and an AI language tutoring application to the entice global investors’ curiosity. The invited investors, majorly based in Silicon Valley, commented that all the presentation was very exciting, and the level of pitches was beyond the expectation considering that the students have given only two weeks.
Ms. Seunghee Lee of the team “Bored KAIST Yacht Club”, which was awarded the first prize, explained, “our item, called ‘Meta-Everland’, is a service that offers real-time dating experiences similar to off-line dates. The GESC taught me that anybody can launch a startup as long as they are willing. Developing a business model from ideation and taking it to the actual pitching was challenging, but it was a very thrilling experience at the same time.” Lee added, “Most importantly, over the course of the program and the final pitch, I found out that an interesting idea can attract investors interest even at a very early stage of the launching.”
Mr. Byunghoon Hwang, a student who attended the program said, “Having learned the thoughts and attitudes the people at the front line of Silicon Valley, my views on career and launching of a start-up have been expanded a lot.”
Ms. Marina Mondragon, another attendee at the program, also said that the program was very meaningful because she was able to learn the difference between the ecosystem for the new start-up businesses at Korea and at Silicon Valley through her talks with the CEOs at Silicon Valley.
The program was co-organized by the Center for Global Strategies and Planning at KAIST International Office and Startup of KAIST. Dr. Man-Sung Yim, the Associate Vice President for KAIST International Office, who guided students in Silicon Valley, said, “I believe the GESC program broadened the views and entrepreneurial mindset of students. After joining this program, students stepped forward to become a founder of startups.” In addition, Dr. Young-Tae Kim, the Associate Vice President of the Institute for Startup KAIST, addressed “Startup KAIST will support business items founded via the program through various other programs in order to enhance their competitiveness in the global market.”
The GSP and Startup KAIST will continuously revamp the program by selecting distinguished fellows to join the program and coming up with innovative startup items.
Profile:
Sooa Lee, Ph.D.
Research Assistant Professor
slee900@kaist.ac.kr
Center for Global Strategies and Planning
Office of Global Initiatives
KAIST International Office
https://io.kaist.ac.kr
Korea Advanced Institute of Science and Technology (KAIST)Daejeon, Republic of Korea
2022.07.05 View 13654
The 1st Global Entrepreneurship Summer Camp bridges KAIST and Silicon Valley, US
Twenty KAIST students gave a go at selling their business ideas to investors at Silicon Valley on the “Pitch Day” at 2022 Global Entrepreneurship Summer Camp.
From Tuesday, June 21 to Monday, July 4, 2022, KAIST held the first Global Entrepreneurship Summer Camp (GESC).
The 2022 GESC, which was organized in collaboration with Stanford Technology Ventures Program (STVP), KOTRA Silicon Valley IT Center, and KAIST Alumni at Silicon Valley, was a pilot program that offered opportunities of experiencing and learning about the cases of startup companies in Silicon Valley and a chance to expand businesses to Silicon Valley through networking.
Twenty KAIST students, including pre-startup entrepreneurs and students interested in global entrepreneurship with less than one year of business experience were selected. The first week of the program was organized by Startup KAIST while the second week program was organized by the Center for Global Strategies and Planning (GSP) at KAIST in collaboration with the Stanford Technology Venture Program (STVP), KAIST Alumni at Silicon Valley, and KOTRA at Silicon Valley.
Dr. Mo-Yun Lei Fong, the Executive Director of STVP, said, “The program offered an opportunity for us to realize our vision of empowering aspiring entrepreneurs to become global citizens who create and scale responsible innovation. By collaborating with KAIST and offering entrepreneurial insights to Korean students, we are able to have a positive impact on a global scale.” Mo added, “The program also enabled STVP to build bridges, learn from the students, and refine our culturally relevant curriculum by understanding Korean culture and ideas.”
On the “Pitch Day” on July 1, following a special talk by Dr. Chong-Moon Lee, the Chairman of AmBex Venture Partners, the students presented their team business ideas such as an AI-assisted, noise-canceling pillow devised for better sleep, a metaverse dating application, an XR virtual conferencing system, and an AI language tutoring application to the entice global investors’ curiosity. The invited investors, majorly based in Silicon Valley, commented that all the presentation was very exciting, and the level of pitches was beyond the expectation considering that the students have given only two weeks.
Ms. Seunghee Lee of the team “Bored KAIST Yacht Club”, which was awarded the first prize, explained, “our item, called ‘Meta-Everland’, is a service that offers real-time dating experiences similar to off-line dates. The GESC taught me that anybody can launch a startup as long as they are willing. Developing a business model from ideation and taking it to the actual pitching was challenging, but it was a very thrilling experience at the same time.” Lee added, “Most importantly, over the course of the program and the final pitch, I found out that an interesting idea can attract investors interest even at a very early stage of the launching.”
Mr. Byunghoon Hwang, a student who attended the program said, “Having learned the thoughts and attitudes the people at the front line of Silicon Valley, my views on career and launching of a start-up have been expanded a lot.”
Ms. Marina Mondragon, another attendee at the program, also said that the program was very meaningful because she was able to learn the difference between the ecosystem for the new start-up businesses at Korea and at Silicon Valley through her talks with the CEOs at Silicon Valley.
The program was co-organized by the Center for Global Strategies and Planning at KAIST International Office and Startup of KAIST. Dr. Man-Sung Yim, the Associate Vice President for KAIST International Office, who guided students in Silicon Valley, said, “I believe the GESC program broadened the views and entrepreneurial mindset of students. After joining this program, students stepped forward to become a founder of startups.” In addition, Dr. Young-Tae Kim, the Associate Vice President of the Institute for Startup KAIST, addressed “Startup KAIST will support business items founded via the program through various other programs in order to enhance their competitiveness in the global market.”
The GSP and Startup KAIST will continuously revamp the program by selecting distinguished fellows to join the program and coming up with innovative startup items.
Profile:
Sooa Lee, Ph.D.
Research Assistant Professor
slee900@kaist.ac.kr
Center for Global Strategies and Planning
Office of Global Initiatives
KAIST International Office
https://io.kaist.ac.kr
Korea Advanced Institute of Science and Technology (KAIST)Daejeon, Republic of Korea
2022.07.05 View 13654 -
 Professor Sang Su Lee’s Team Wins Seven iF Design Awards 2022
Professor Sang Su Lee from the Department of Industrial Design and his team’s five apps made in collaboration with NH Investment and Securities won iF Design Awards in the fields of UI, UX, service design, product design, and communication. These apps are now offered as NH Investment and Securities mobile applications.
The iF Design Awards recognize top quality creativity in product design, communication, packaging, service design and concepts, and architecture and interior design, as well as user experience (UX) and interface for digital media (UI).
In the field of UI, ‘Gretell’ is a mobile stock investment app service designed by Lee and his team to support investors struggling to learn about investing by archiving personalized information. Gretell provides investment information including news and reports. Users learn, evaluate, and leave comments. This shows both quantitative and qualitative indications, leading to rational decision-making. Other user’s comments are shared to reduce confirmation bias. Through this process, Gretell helps users who are impulsive or easily swayed by others’ opinions to grow as independent investors.
‘Bright’ is another app created by Lee’s team. It helps people exercise their rights as shareholders. As the need to exercise shareholders’ rights increases, many people are frustrated that investors with a small number of shares don’t have a lot of power. Bright provides a space for shareholders to share their opinions and brings people together so that individuals can be more proactive as shareholders. The Integrated Power of Attorney System (IPAS) expands the chances for shareholders to exercise their rights and allows users to submit proposals that can be communicated during the general meeting. Bright fosters influential shareholders, responsible companies, and a healthy society.
For communications, ‘Rewind’ is a stock information services app that visualizes past stock charts through sentiment analysis. Existing services focus on numbers, while Rewind takes a qualitative approach. Rewind analyzes public sentiment toward each event by collecting opinions on social media and then visualizes them chronologically along with the stock chart. Rewind allows users to review stock market movements and record their thoughts. Users can gain their own insights into current events in the stock market and make wiser investment decisions. The intuitive color gradient design provides a pleasant and simplified information experience.
In the area of interfaces for digital media and service design, ‘Groo’ is a green bond investing service app that helps users participate in green investment though investing in green bonds that support green projects for environmental improvement. Not restricted to trading bonds, Groo joins users in the holistic experience of green investing, from taking an interest in environmental issues to confirming the impact of the investment.
Next, ‘Modu’ is a story-based empathy expression training game for children with intellectual disabilities. Modu was developed to support emotion recognition and empathy behavior training in children with mild intellectual disabilities (MID) and borderline intellectual functioning (BIF).
Finally, the diving VR device for neutral buoyancy training, ‘Blow-yancy’, also made winner’s list. The device mimics scuba diving training without having to go into the water, therefore beginner divers are able getting feeling of diving while remaining perfectly safe and not harming any corals. It is expected that the device will be able to help protect at-risk underwater ecosystems.
2022.05.10 View 9771
Professor Sang Su Lee’s Team Wins Seven iF Design Awards 2022
Professor Sang Su Lee from the Department of Industrial Design and his team’s five apps made in collaboration with NH Investment and Securities won iF Design Awards in the fields of UI, UX, service design, product design, and communication. These apps are now offered as NH Investment and Securities mobile applications.
The iF Design Awards recognize top quality creativity in product design, communication, packaging, service design and concepts, and architecture and interior design, as well as user experience (UX) and interface for digital media (UI).
In the field of UI, ‘Gretell’ is a mobile stock investment app service designed by Lee and his team to support investors struggling to learn about investing by archiving personalized information. Gretell provides investment information including news and reports. Users learn, evaluate, and leave comments. This shows both quantitative and qualitative indications, leading to rational decision-making. Other user’s comments are shared to reduce confirmation bias. Through this process, Gretell helps users who are impulsive or easily swayed by others’ opinions to grow as independent investors.
‘Bright’ is another app created by Lee’s team. It helps people exercise their rights as shareholders. As the need to exercise shareholders’ rights increases, many people are frustrated that investors with a small number of shares don’t have a lot of power. Bright provides a space for shareholders to share their opinions and brings people together so that individuals can be more proactive as shareholders. The Integrated Power of Attorney System (IPAS) expands the chances for shareholders to exercise their rights and allows users to submit proposals that can be communicated during the general meeting. Bright fosters influential shareholders, responsible companies, and a healthy society.
For communications, ‘Rewind’ is a stock information services app that visualizes past stock charts through sentiment analysis. Existing services focus on numbers, while Rewind takes a qualitative approach. Rewind analyzes public sentiment toward each event by collecting opinions on social media and then visualizes them chronologically along with the stock chart. Rewind allows users to review stock market movements and record their thoughts. Users can gain their own insights into current events in the stock market and make wiser investment decisions. The intuitive color gradient design provides a pleasant and simplified information experience.
In the area of interfaces for digital media and service design, ‘Groo’ is a green bond investing service app that helps users participate in green investment though investing in green bonds that support green projects for environmental improvement. Not restricted to trading bonds, Groo joins users in the holistic experience of green investing, from taking an interest in environmental issues to confirming the impact of the investment.
Next, ‘Modu’ is a story-based empathy expression training game for children with intellectual disabilities. Modu was developed to support emotion recognition and empathy behavior training in children with mild intellectual disabilities (MID) and borderline intellectual functioning (BIF).
Finally, the diving VR device for neutral buoyancy training, ‘Blow-yancy’, also made winner’s list. The device mimics scuba diving training without having to go into the water, therefore beginner divers are able getting feeling of diving while remaining perfectly safe and not harming any corals. It is expected that the device will be able to help protect at-risk underwater ecosystems.
2022.05.10 View 9771 -
 A Study Shows Reactive Electrolyte Additives Improve Lithium Metal Battery Performance
Stable electrode-electrolyte interfaces constructed by fluorine- and nitrogen-donating ionic additives provide an opportunity to improve high-performance lithium metal batteries
A research team showed that electrolyte additives increase the lifetime of lithium metal batteries and remarkably improve the performance of fast charging and discharging. Professor Nam-Soon Choi’s team from the Department of Chemical and Biomolecular Engineering at KAIST hierarchized the solid electrolyte interphase to make a dual-layer structure and showed groundbreaking run times for lithium metal batteries.
The team applied two electrolyte additives that have different reduction and adsorption properties to improve the functionality of the dual-layer solid electrolyte interphase. In addition, the team has confirmed that the structural stability of the nickel-rich cathode was achieved through the formation of a thin protective layer on the cathode. This study was reported in Energy Storage Materials.
Securing high-energy-density lithium metal batteries with a long lifespan and fast charging performance is vital for realizing their ubiquitous use as superior power sources for electric vehicles. Lithium metal batteries comprise a lithium metal anode that delivers 10 times higher capacity than the graphite anodes in lithium-ion batteries. Therefore, lithium metal is an indispensable anode material for realizing high-energy rechargeable batteries. However, undesirable reactions among the electrolytes with lithium metal anodes can reduce the power and this remains an impediment to achieving a longer battery lifespan. Previous studies only focused on the formation of the solid electrolyte interphase on the surface of the lithium metal anode.
The team designed a way to create a dual-layer solid electrolyte interphase to resolve the instability of the lithium metal anode by using electrolyte additives, depending on their electron accepting ability and adsorption tendencies. This hierarchical structure of the solid electrolyte interphase on the lithium metal anode has the potential to be further applied to lithium-alloy anodes, lithium storage structures, and anode-free technology to meet market expectations for electrolyte technology.
The batteries with lithium metal anodes and nickel-rich cathodes represented 80.9% of the initial capacity after 600 cycles and achieved a high Coulombic efficiency of 99.94%. These remarkable results contributed to the development of protective dual-layer solid electrolyte interphase technology for lithium metal anodes.
Professor Choi said that the research suggests a new direction for the development of electrolyte additives to regulate the unstable lithium metal anode-electrolyte interface, the biggest hurdle in research on lithium metal batteries.
She added that anode-free secondary battery technology is expected to be a game changer in the secondary battery market and electrolyte additive technology will contribute to the enhancement of anode-free secondary batteries through the stabilization of lithium metal anodes.
This research was funded by the Technology Development Program to Solve Climate Change of the National Research Foundation in Korea funded by the Ministry of Science, ICT & Future Planning and the Technology Innovation Program funded by the Ministry of Trade, Industry & Energy, and Hyundai Motor Company.
- PublicationSaehun Kim, Sung O Park, Min-Young Lee, Jeong-A Lee, Imanuel Kristanto, Tae Kyung Lee, Daeyeon Hwang, Juyoung Kim, Tae-Ung Wi, Hyun-Wook Lee, Sang Kyu Kwak, and NamSoon Choi, “Stable electrode-electrolyte interfaces constructed by fluorine- and nitrogen-donating ionic additives for high-performance lithium metal batteries,” Energy Storage Materials,45, 1-13 (2022), (doi: https://doi.org/10.1016/j.ensm.2021.10.031)
- ProfileProfessor Nam-Soon ChoiEnergy Materials LaboratoryDepartment of Chemical and Biomolecular EngineeringKAIST
2021.12.16 View 10398
A Study Shows Reactive Electrolyte Additives Improve Lithium Metal Battery Performance
Stable electrode-electrolyte interfaces constructed by fluorine- and nitrogen-donating ionic additives provide an opportunity to improve high-performance lithium metal batteries
A research team showed that electrolyte additives increase the lifetime of lithium metal batteries and remarkably improve the performance of fast charging and discharging. Professor Nam-Soon Choi’s team from the Department of Chemical and Biomolecular Engineering at KAIST hierarchized the solid electrolyte interphase to make a dual-layer structure and showed groundbreaking run times for lithium metal batteries.
The team applied two electrolyte additives that have different reduction and adsorption properties to improve the functionality of the dual-layer solid electrolyte interphase. In addition, the team has confirmed that the structural stability of the nickel-rich cathode was achieved through the formation of a thin protective layer on the cathode. This study was reported in Energy Storage Materials.
Securing high-energy-density lithium metal batteries with a long lifespan and fast charging performance is vital for realizing their ubiquitous use as superior power sources for electric vehicles. Lithium metal batteries comprise a lithium metal anode that delivers 10 times higher capacity than the graphite anodes in lithium-ion batteries. Therefore, lithium metal is an indispensable anode material for realizing high-energy rechargeable batteries. However, undesirable reactions among the electrolytes with lithium metal anodes can reduce the power and this remains an impediment to achieving a longer battery lifespan. Previous studies only focused on the formation of the solid electrolyte interphase on the surface of the lithium metal anode.
The team designed a way to create a dual-layer solid electrolyte interphase to resolve the instability of the lithium metal anode by using electrolyte additives, depending on their electron accepting ability and adsorption tendencies. This hierarchical structure of the solid electrolyte interphase on the lithium metal anode has the potential to be further applied to lithium-alloy anodes, lithium storage structures, and anode-free technology to meet market expectations for electrolyte technology.
The batteries with lithium metal anodes and nickel-rich cathodes represented 80.9% of the initial capacity after 600 cycles and achieved a high Coulombic efficiency of 99.94%. These remarkable results contributed to the development of protective dual-layer solid electrolyte interphase technology for lithium metal anodes.
Professor Choi said that the research suggests a new direction for the development of electrolyte additives to regulate the unstable lithium metal anode-electrolyte interface, the biggest hurdle in research on lithium metal batteries.
She added that anode-free secondary battery technology is expected to be a game changer in the secondary battery market and electrolyte additive technology will contribute to the enhancement of anode-free secondary batteries through the stabilization of lithium metal anodes.
This research was funded by the Technology Development Program to Solve Climate Change of the National Research Foundation in Korea funded by the Ministry of Science, ICT & Future Planning and the Technology Innovation Program funded by the Ministry of Trade, Industry & Energy, and Hyundai Motor Company.
- PublicationSaehun Kim, Sung O Park, Min-Young Lee, Jeong-A Lee, Imanuel Kristanto, Tae Kyung Lee, Daeyeon Hwang, Juyoung Kim, Tae-Ung Wi, Hyun-Wook Lee, Sang Kyu Kwak, and NamSoon Choi, “Stable electrode-electrolyte interfaces constructed by fluorine- and nitrogen-donating ionic additives for high-performance lithium metal batteries,” Energy Storage Materials,45, 1-13 (2022), (doi: https://doi.org/10.1016/j.ensm.2021.10.031)
- ProfileProfessor Nam-Soon ChoiEnergy Materials LaboratoryDepartment of Chemical and Biomolecular EngineeringKAIST
2021.12.16 View 10398 -
 Nanoscale Self-Assembling Salt-Crystal ‘Origami’ Balls Envelop Liquids
Mechanical engineers have devised a ‘crystal capillary origami’ technique where salt crystals spontaneously encapsulate liquid droplets
Researchers have developed a technique whereby they can spontaneously encapsulate microscopic droplets of water and oil emulsion in a tiny sphere made of salt crystals—sort of like a minute, self-constructing origami soccer ball filled with liquid. The process, which they are calling ‘crystal capillary origami,’ could be used in a range of fields from more precise drug delivery to nanoscale medical devices.The technique is described in a paper appearing in the journal Nanoscale on September 21.
Capillary action, or ‘capillarity,’ will be familiar to most people as the way that water or other liquids can move up narrow tubes or other porous materials seemingly in defiance of gravity (for example within the vascular systems of plants, or even more simply, the drawing up of paint between the hairs of a paintbrush). This effect is due to the forces of cohesion (the tendency of a liquid’s molecules to stick together), which results in surface tension, and adhesion (their tendency to stick to the surface of other substances). The strength of the capillarity depends on the chemistry of the liquid, the chemistry of the porous material, and on the other forces acting on them both. For example, a liquid with lower surface tension than water would not be able to hold up a water strider insect.
Less well known is a related phenomenon, elasto-capillarity, that takes advantage of the relationship between capillarity and the elasticity of a very tiny flat sheet of a solid material. In certain circumstances, the capillary forces can overcome the elastic bending resistance of the sheet.
This relationship can be exploited to create ‘capillary origami,’ or three-dimensional structures. When a liquid droplet is placed on the flat sheet, the latter can spontaneously encapsulate the former due to surface tension. Capillary origami can take on other forms including wrinkling, buckling, or self-folding into other shapes. The specific geometrical shape that the 3D capillary origami structure ends up taking is determined by both the chemistry of the flat sheet and that of the liquid, and by carefully designing the shape and size of the sheet.
There is one big problem with these small devices, however. “These conventional self-assembled origami structures cannot be completely spherical and will always have discontinuous boundaries, or what you might call ‘edges,’ as a result of the original two-dimensional shape of the sheet,” said Kwangseok Park, a lead researcher on the project. He added, “These edges could turn out to be future defects with the potential for failure in the face of increased stress.” Non-spherical particles are also known to be more disadvantageous than spherical particles in terms of cellular uptake.
Professor Hyoungsoo Kim from the Department of Mechanical Engineering explained, “This is why researchers have long been on the hunt for substances that could produce a fully spherical capillary origami structure.”
The authors of the study have demonstrated such an origami sphere for the first time. They showed how instead of a flat sheet, the growth of salt-crystals can perform capillary origami action in a similar manner. What they call ‘crystal capillary origami’ spontaneously constructs a smooth spherical shell capsule from these same surface tension effects, but now the spontaneous encapsulation of a liquid is determined by the elasto-capillary conditions of growing crystals.
Here, the term ‘salt’ refers to a compound of one positively charged ion and another negatively charged. Table salt, or sodium chloride, is just one example of a salt. The researchers used four other salts: calcium propionate, sodium salicylate, calcium nitrate tetrahydrate, and sodium bicarbonate to envelop a water-oil emulsion. Normally, a salt such as sodium chloride has a cubical crystal structure, but these four salts form plate-like structures as crystallites or ‘grains’ (the microscopic shape that forms when a crystal first starts to grow) instead. These plates then self-assemble into perfect spheres.
Using scanning electron microscopy and X-ray diffraction analysis, they investigated the mechanism of such formation and concluded that it was ‘Laplace pressure’ that drives the crystallite plates to cover the emulsion surface. Laplace pressure describes the pressure difference between the interior and exterior of a curved surface caused by the surface tension at the interface between the two substances, in this case between the salt water and the oil.
The researchers hope that these self-assembling nanostructures can be used for encapsulation applications in a range of sectors, from the food industry and cosmetics to drug delivery and even tiny medical devices.
-Publication
Kwangseok Park, Hyoungsoo Kim “Crystal capillary origami capsule with self-assembled nanostructure,” Nanoscale, 13(35), 14656-14665 (DOI: 10.1039/d1nr02456f)
-Profile
Professor Hyoungsoo Kim
Fluid and Interface Laboratory
http://fil.kaist.ac.kr
Department of Mechanical Engineering
KAIST
2021.11.04 View 10739
Nanoscale Self-Assembling Salt-Crystal ‘Origami’ Balls Envelop Liquids
Mechanical engineers have devised a ‘crystal capillary origami’ technique where salt crystals spontaneously encapsulate liquid droplets
Researchers have developed a technique whereby they can spontaneously encapsulate microscopic droplets of water and oil emulsion in a tiny sphere made of salt crystals—sort of like a minute, self-constructing origami soccer ball filled with liquid. The process, which they are calling ‘crystal capillary origami,’ could be used in a range of fields from more precise drug delivery to nanoscale medical devices.The technique is described in a paper appearing in the journal Nanoscale on September 21.
Capillary action, or ‘capillarity,’ will be familiar to most people as the way that water or other liquids can move up narrow tubes or other porous materials seemingly in defiance of gravity (for example within the vascular systems of plants, or even more simply, the drawing up of paint between the hairs of a paintbrush). This effect is due to the forces of cohesion (the tendency of a liquid’s molecules to stick together), which results in surface tension, and adhesion (their tendency to stick to the surface of other substances). The strength of the capillarity depends on the chemistry of the liquid, the chemistry of the porous material, and on the other forces acting on them both. For example, a liquid with lower surface tension than water would not be able to hold up a water strider insect.
Less well known is a related phenomenon, elasto-capillarity, that takes advantage of the relationship between capillarity and the elasticity of a very tiny flat sheet of a solid material. In certain circumstances, the capillary forces can overcome the elastic bending resistance of the sheet.
This relationship can be exploited to create ‘capillary origami,’ or three-dimensional structures. When a liquid droplet is placed on the flat sheet, the latter can spontaneously encapsulate the former due to surface tension. Capillary origami can take on other forms including wrinkling, buckling, or self-folding into other shapes. The specific geometrical shape that the 3D capillary origami structure ends up taking is determined by both the chemistry of the flat sheet and that of the liquid, and by carefully designing the shape and size of the sheet.
There is one big problem with these small devices, however. “These conventional self-assembled origami structures cannot be completely spherical and will always have discontinuous boundaries, or what you might call ‘edges,’ as a result of the original two-dimensional shape of the sheet,” said Kwangseok Park, a lead researcher on the project. He added, “These edges could turn out to be future defects with the potential for failure in the face of increased stress.” Non-spherical particles are also known to be more disadvantageous than spherical particles in terms of cellular uptake.
Professor Hyoungsoo Kim from the Department of Mechanical Engineering explained, “This is why researchers have long been on the hunt for substances that could produce a fully spherical capillary origami structure.”
The authors of the study have demonstrated such an origami sphere for the first time. They showed how instead of a flat sheet, the growth of salt-crystals can perform capillary origami action in a similar manner. What they call ‘crystal capillary origami’ spontaneously constructs a smooth spherical shell capsule from these same surface tension effects, but now the spontaneous encapsulation of a liquid is determined by the elasto-capillary conditions of growing crystals.
Here, the term ‘salt’ refers to a compound of one positively charged ion and another negatively charged. Table salt, or sodium chloride, is just one example of a salt. The researchers used four other salts: calcium propionate, sodium salicylate, calcium nitrate tetrahydrate, and sodium bicarbonate to envelop a water-oil emulsion. Normally, a salt such as sodium chloride has a cubical crystal structure, but these four salts form plate-like structures as crystallites or ‘grains’ (the microscopic shape that forms when a crystal first starts to grow) instead. These plates then self-assemble into perfect spheres.
Using scanning electron microscopy and X-ray diffraction analysis, they investigated the mechanism of such formation and concluded that it was ‘Laplace pressure’ that drives the crystallite plates to cover the emulsion surface. Laplace pressure describes the pressure difference between the interior and exterior of a curved surface caused by the surface tension at the interface between the two substances, in this case between the salt water and the oil.
The researchers hope that these self-assembling nanostructures can be used for encapsulation applications in a range of sectors, from the food industry and cosmetics to drug delivery and even tiny medical devices.
-Publication
Kwangseok Park, Hyoungsoo Kim “Crystal capillary origami capsule with self-assembled nanostructure,” Nanoscale, 13(35), 14656-14665 (DOI: 10.1039/d1nr02456f)
-Profile
Professor Hyoungsoo Kim
Fluid and Interface Laboratory
http://fil.kaist.ac.kr
Department of Mechanical Engineering
KAIST
2021.11.04 View 10739 -
 Diva Sumi Jo to Join the KAIST Faculty
Visiting Distinguished Professor Jo will enrich KAIST’s scholarship and inspire futuristic art and technology research
Soprano Sumi Jo will join the KAIST faculty from the spring 2022 semester. Named as a visiting distinguished professor in the Graduate School of Culture Technology, she will give special leadership lectures. Her tenure will be through September 2024.
Jo joined the appointment ceremony held online at KAIST on October 14 from Portugal and expressed her high expectations for teaching KAIST students from next year. “I am very grateful for this opportunity to meet students at KAIST, the birthplace of advanced science and technology in Korea,” she said.
KAIST President Kwang Hyung Lee, who has stressed the importance of humanities and the arts in convergence studies of science and technology, lauded her joining the faculty as a big asset who will enrich KAIST’s scholarship. “Soprano Sumi Jo rose to stardom on the global music stage with her unrivaled talent and effort. I truly believe her experience and passion will inspire our students to expand their horizon of thought and knowledge,” said President Lee.
Distinguished Professor Jo will also participate in convergence research at the Graduate School of Culture Technology with KAIST professors and many other experts. The Sumi Jo Performing Arts Research Center at the Graduate School of Culture Technology will conduct research on the converging of imaging and audio processing technologies that will enhance virtual artists’ performances. Distinguished Professor Jo explained, “The world is changing so fast. I look forward to working on culture technology research at KAIST that will raise our life quality.”
Professor Juhan Nam from the Graduate School of Culture Technology said, “We look forward to working closely with her and her team to develop research themes that envision futuristic art combined with technology such as the metaverse and non-fungible tokens (NFTs).
Coloratura soprano Jo was born in Seoul and educated at Seoul National University and the Conservatorio Santa Cecilia in Italy. Among her teachers were Carolo Bergonzi and Giasnnelas Borelli. Following her graduation from the Conservatorio Santa Cecilia in 1985, she swept major international competitions in Seoul and Europe. In 1986, she was unanimously awarded the first prize in the Carlo Alberto Cappelli International Competition in Verona which is open only to the first-prize winners of major competitions.
Since her debut in the role of Gilda in Verdi’s Rigolleto in Italy in 1986, she has performed on the world's biggest stages along with noted maestros such as Herbert von Karajan, Georg Solti, Zubin Mehta, and James Levine. Distinguished Professor Jo, one of the most sought-after sopranos in the world, released more than 40 albums.
2021.10.15 View 6479
Diva Sumi Jo to Join the KAIST Faculty
Visiting Distinguished Professor Jo will enrich KAIST’s scholarship and inspire futuristic art and technology research
Soprano Sumi Jo will join the KAIST faculty from the spring 2022 semester. Named as a visiting distinguished professor in the Graduate School of Culture Technology, she will give special leadership lectures. Her tenure will be through September 2024.
Jo joined the appointment ceremony held online at KAIST on October 14 from Portugal and expressed her high expectations for teaching KAIST students from next year. “I am very grateful for this opportunity to meet students at KAIST, the birthplace of advanced science and technology in Korea,” she said.
KAIST President Kwang Hyung Lee, who has stressed the importance of humanities and the arts in convergence studies of science and technology, lauded her joining the faculty as a big asset who will enrich KAIST’s scholarship. “Soprano Sumi Jo rose to stardom on the global music stage with her unrivaled talent and effort. I truly believe her experience and passion will inspire our students to expand their horizon of thought and knowledge,” said President Lee.
Distinguished Professor Jo will also participate in convergence research at the Graduate School of Culture Technology with KAIST professors and many other experts. The Sumi Jo Performing Arts Research Center at the Graduate School of Culture Technology will conduct research on the converging of imaging and audio processing technologies that will enhance virtual artists’ performances. Distinguished Professor Jo explained, “The world is changing so fast. I look forward to working on culture technology research at KAIST that will raise our life quality.”
Professor Juhan Nam from the Graduate School of Culture Technology said, “We look forward to working closely with her and her team to develop research themes that envision futuristic art combined with technology such as the metaverse and non-fungible tokens (NFTs).
Coloratura soprano Jo was born in Seoul and educated at Seoul National University and the Conservatorio Santa Cecilia in Italy. Among her teachers were Carolo Bergonzi and Giasnnelas Borelli. Following her graduation from the Conservatorio Santa Cecilia in 1985, she swept major international competitions in Seoul and Europe. In 1986, she was unanimously awarded the first prize in the Carlo Alberto Cappelli International Competition in Verona which is open only to the first-prize winners of major competitions.
Since her debut in the role of Gilda in Verdi’s Rigolleto in Italy in 1986, she has performed on the world's biggest stages along with noted maestros such as Herbert von Karajan, Georg Solti, Zubin Mehta, and James Levine. Distinguished Professor Jo, one of the most sought-after sopranos in the world, released more than 40 albums.
2021.10.15 View 6479 -
 Quantum Laser Turns Energy Loss into Gain
A new laser that generates quantum particles can recycle lost energy for highly efficient, low threshold laser applications
Scientists at KAIST have fabricated a laser system that generates highly interactive quantum particles at room temperature. Their findings, published in the journal Nature Photonics, could lead to a single microcavity laser system that requires lower threshold energy as its energy loss increases.
The system, developed by KAIST physicist Yong-Hoon Cho and colleagues, involves shining light through a single hexagonal-shaped microcavity treated with a loss-modulated silicon nitride substrate. The system design leads to the generation of a polariton laser at room temperature, which is exciting because this usually requires cryogenic temperatures.
The researchers found another unique and counter-intuitive feature of this design. Normally, energy is lost during laser operation. But in this system, as energy loss increased, the amount of energy needed to induce lasing decreased. Exploiting this phenomenon could lead to the development of high efficiency, low threshold lasers for future quantum optical devices.
“This system applies a concept of quantum physics known as parity-time reversal symmetry,” explains Professor Cho. “This is an important platform that allows energy loss to be used as gain. It can be used to reduce laser threshold energy for classical optical devices and sensors, as well as quantum devices and controlling the direction of light.”
The key is the design and materials. The hexagonal microcavity divides light particles into two different modes: one that passes through the upward-facing triangle of the hexagon and another that passes through its downward-facing triangle. Both modes of light particles have the same energy and path but don’t interact with each other.
However, the light particles do interact with other particles called excitons, provided by the hexagonal microcavity, which is made of semiconductors. This interaction leads to the generation of new quantum particles called polaritons that then interact with each other to generate the polariton laser. By controlling the degree of loss between the microcavity and the semiconductor substrate, an intriguing phenomenon arises, with the threshold energy becoming smaller as energy loss increases. This research was supported by the Samsung Science and Technology Foundation and Korea’s National Research Foundation.
-PublicationSong,H.G, Choi, M, Woo, K.Y. Yong-Hoon Cho Room-temperature polaritonic non-Hermitian system with single microcavityNature Photonics (https://doi.org/10.1038/s41566-021-00820-z)
-ProfileProfessor Yong-Hoon ChoQuantum & Nanobio Photonics Laboratoryhttp://qnp.kaist.ac.kr/
Department of PhysicsKAIST
2021.07.07 View 11555
Quantum Laser Turns Energy Loss into Gain
A new laser that generates quantum particles can recycle lost energy for highly efficient, low threshold laser applications
Scientists at KAIST have fabricated a laser system that generates highly interactive quantum particles at room temperature. Their findings, published in the journal Nature Photonics, could lead to a single microcavity laser system that requires lower threshold energy as its energy loss increases.
The system, developed by KAIST physicist Yong-Hoon Cho and colleagues, involves shining light through a single hexagonal-shaped microcavity treated with a loss-modulated silicon nitride substrate. The system design leads to the generation of a polariton laser at room temperature, which is exciting because this usually requires cryogenic temperatures.
The researchers found another unique and counter-intuitive feature of this design. Normally, energy is lost during laser operation. But in this system, as energy loss increased, the amount of energy needed to induce lasing decreased. Exploiting this phenomenon could lead to the development of high efficiency, low threshold lasers for future quantum optical devices.
“This system applies a concept of quantum physics known as parity-time reversal symmetry,” explains Professor Cho. “This is an important platform that allows energy loss to be used as gain. It can be used to reduce laser threshold energy for classical optical devices and sensors, as well as quantum devices and controlling the direction of light.”
The key is the design and materials. The hexagonal microcavity divides light particles into two different modes: one that passes through the upward-facing triangle of the hexagon and another that passes through its downward-facing triangle. Both modes of light particles have the same energy and path but don’t interact with each other.
However, the light particles do interact with other particles called excitons, provided by the hexagonal microcavity, which is made of semiconductors. This interaction leads to the generation of new quantum particles called polaritons that then interact with each other to generate the polariton laser. By controlling the degree of loss between the microcavity and the semiconductor substrate, an intriguing phenomenon arises, with the threshold energy becoming smaller as energy loss increases. This research was supported by the Samsung Science and Technology Foundation and Korea’s National Research Foundation.
-PublicationSong,H.G, Choi, M, Woo, K.Y. Yong-Hoon Cho Room-temperature polaritonic non-Hermitian system with single microcavityNature Photonics (https://doi.org/10.1038/s41566-021-00820-z)
-ProfileProfessor Yong-Hoon ChoQuantum & Nanobio Photonics Laboratoryhttp://qnp.kaist.ac.kr/
Department of PhysicsKAIST
2021.07.07 View 11555 -
 Professor Poong Hyun Seong Elected INSC Chair
Professor Emeritus Poong Hyun Seong from the Department of Nuclear and Quantum Engineering was elected as the Chairman of the International Nuclear Societies Council (INSC). His two-year term began on January 1.
The INSC is an organization made up of nuclear societies all over the world, representing more than 80,000 nuclear professionals. The INSC founded in 1990 acts as a global forum to establish common goals of nuclear power usage, delivering the views and ideas of professionals throughout their regional societies.
The INSC has advocated for nuclear power to be deemed an indispensable clean energy resources that can mitigate the climate change. The council has engaged in public awareness and publicity activities promoting the advantages of nuclear energy for developing next-generation power plants such as small nuclear reactors, local heating system, seawater desalination, and fair production of energy.
Professor Seong is a globally renowned scholar in the fields of nuclear instrumentation control and human factor engineering. He retired last year after 30-year career at KAIST. He took on leadership roles in the Korea Nuclear Society and served as a member of the Korea Nuclear Safety and Security Commission as well as Atomic Energy Commission. A fellow at the America Nuclear Society, Professor Seong served as the first vice chair of the INSC and he received the Don Miller Award in 2019. The award established in 2009 by the American Nuclear Society in honor of former ANS President Don Miller is given to an individual who has made a significant contribution to the advancement of nuclear instrumentation and control of human-machine interfaces.
He led the leadership role to help the Korean government steered into efficient and reasonable energy policymaking. More recently, as the Korean government decided to abandon nuclear energy, he actively opposed the government’s pivot. Professor Seong said, “Advanced countries like the US, UK, France, and Japan push forward the production of renewable energy by driving nuclear power plant under their pledges toward carbon neutrality by 2050. However, we are very concerned about the government’s policy shift to decrease the number of nuclear power plants while increasing the fossil fuel usage. I don’t think we can realize carbon neutrality by 2050 with the current policy.”
(END)
2021.01.13 View 7752
Professor Poong Hyun Seong Elected INSC Chair
Professor Emeritus Poong Hyun Seong from the Department of Nuclear and Quantum Engineering was elected as the Chairman of the International Nuclear Societies Council (INSC). His two-year term began on January 1.
The INSC is an organization made up of nuclear societies all over the world, representing more than 80,000 nuclear professionals. The INSC founded in 1990 acts as a global forum to establish common goals of nuclear power usage, delivering the views and ideas of professionals throughout their regional societies.
The INSC has advocated for nuclear power to be deemed an indispensable clean energy resources that can mitigate the climate change. The council has engaged in public awareness and publicity activities promoting the advantages of nuclear energy for developing next-generation power plants such as small nuclear reactors, local heating system, seawater desalination, and fair production of energy.
Professor Seong is a globally renowned scholar in the fields of nuclear instrumentation control and human factor engineering. He retired last year after 30-year career at KAIST. He took on leadership roles in the Korea Nuclear Society and served as a member of the Korea Nuclear Safety and Security Commission as well as Atomic Energy Commission. A fellow at the America Nuclear Society, Professor Seong served as the first vice chair of the INSC and he received the Don Miller Award in 2019. The award established in 2009 by the American Nuclear Society in honor of former ANS President Don Miller is given to an individual who has made a significant contribution to the advancement of nuclear instrumentation and control of human-machine interfaces.
He led the leadership role to help the Korean government steered into efficient and reasonable energy policymaking. More recently, as the Korean government decided to abandon nuclear energy, he actively opposed the government’s pivot. Professor Seong said, “Advanced countries like the US, UK, France, and Japan push forward the production of renewable energy by driving nuclear power plant under their pledges toward carbon neutrality by 2050. However, we are very concerned about the government’s policy shift to decrease the number of nuclear power plants while increasing the fossil fuel usage. I don’t think we can realize carbon neutrality by 2050 with the current policy.”
(END)
2021.01.13 View 7752 -
 Researchers Report Longest-lived Aqueous Flow Batteries
New technology to overcome the life limit of next-generation water-cell batteries
A research team led by Professor Hee-Tak Kim from the Department of Chemical and Biomolecular Engineering has developed water-based zinc/bromine redox flow batteries (ZBBs) with the best life expectancy among all the redox flow batteries reported by identifying and solving the deterioration issue with zinc electrodes.
Professor Kim, head of the Advanced Battery Center at KAIST's Nano-fusion Research Institute, said, "We presented a new technology to overcome the life limit of next-generation water-cell batteries. Not only is it cheaper than conventional lithium-ion batteries, but it can contribute to the expansion of renewable energy and the safe supply of energy storage systems that can run with more than 80 percent energy efficiency."
ZBBs were found to have stable life spans of more than 5,000 cycles, even at a high current density of 100 mA/cm2. It was also confirmed that it represented the highest output and life expectancy compared to Redox flow batteries (RFBs) reported worldwide, which use other redox couples such as zinc-bromine, zinc-iodine, zinc-iron, and vanadium.
Recently, more attention has been focused on energy storage system (ESS) that can improve energy utilization efficiency by storing new and late-night power in large quantities and supplying it to the grid if necessary to supplement the intermittent nature of renewable energy and meet peak power demand.
However, lithium-ion batteries (LIBs), which are currently the core technology of ESSs, have been criticized for not being suitable for ESSs, which store large amounts of electricity due to their inherent risk of ignition and fire. In fact, a total of 33 cases of ESSs using LIBs in Korea had fire accidents, and 35% of all ESS facilities were shut down. This is estimated to have resulted in more than 700 billion won in losses.
As a result, water-based RFBs have drawn great attention. In particular, ZBBs that use ultra-low-cost bromide (ZnBr2) as an active material have been developed for ESSs since the 1970s, with their advantages of high cell voltage, high energy density, and low price compared to other RFBs. Until now, however, the commercialization of ZBBs has been delayed due to the short life span caused by the zinc electrodes. In particular, the uneven "dendrite" growth behavior of zinc metals during the charging and discharging process leads to internal short circuits in the battery which shorten its life.
The research team noted that self-aggregation occurs through the surface diffusion of zinc nuclei on the carbon electrode surface with low surface energy, and determined that self-aggregation was the main cause of zinc dendrite formation through quantum mechanics-based computer simulations and transmission electron microscopy. Furthermore, it was found that the surface diffusion of the zinc nuclei was inhibited in certain carbon fault structures so that dendrites were not produced.
Single vacancy defect, where one carbon atom is removed, exchanges zinc nuclei and electrons, and is strongly coupled, thus inhibiting surface diffusion and enabling uniform nuclear production/growth. The research team applied carbon electrodes with high density fault structure to ZBBs, achieving life characteristics of more than 5,000 cycles at a high charge current density (100 mA/cm2), which is 30 times that of LIBs.
This ESS technology, which can supply eco-friendly electric energy such as renewable energy to the private sector through technology that can drive safe and cheap redox flow batteries for long life, is expected to draw attention once again.
Publication:
Ju-Hyuk Lee, Riyul Kim, Soohyun Kim, Jiyun Heo, Hyeokjin Kwon, Jung Hoon Yang, and Hee-Tak Kim. 2020. Dendrite-free Zn electrodeposition triggered by interatomic orbital hybridization of Zn and single vacancy carbon defects for aqueous Zn-based flow batteries. Energy and Environmental Science, 2020, 13, 2839-2848.
Link to download the full-text paper:http://xlink.rsc.org/?DOI=D0EE00723D
Profile: Prof. Hee-Tak Kimheetak.kim@kaist.ac.krhttp://eed.kaist.ac.krAssociate ProfessorDepartment of Chemical & Biomolecular EngineeringKAIST
2020.12.16 View 14802
Researchers Report Longest-lived Aqueous Flow Batteries
New technology to overcome the life limit of next-generation water-cell batteries
A research team led by Professor Hee-Tak Kim from the Department of Chemical and Biomolecular Engineering has developed water-based zinc/bromine redox flow batteries (ZBBs) with the best life expectancy among all the redox flow batteries reported by identifying and solving the deterioration issue with zinc electrodes.
Professor Kim, head of the Advanced Battery Center at KAIST's Nano-fusion Research Institute, said, "We presented a new technology to overcome the life limit of next-generation water-cell batteries. Not only is it cheaper than conventional lithium-ion batteries, but it can contribute to the expansion of renewable energy and the safe supply of energy storage systems that can run with more than 80 percent energy efficiency."
ZBBs were found to have stable life spans of more than 5,000 cycles, even at a high current density of 100 mA/cm2. It was also confirmed that it represented the highest output and life expectancy compared to Redox flow batteries (RFBs) reported worldwide, which use other redox couples such as zinc-bromine, zinc-iodine, zinc-iron, and vanadium.
Recently, more attention has been focused on energy storage system (ESS) that can improve energy utilization efficiency by storing new and late-night power in large quantities and supplying it to the grid if necessary to supplement the intermittent nature of renewable energy and meet peak power demand.
However, lithium-ion batteries (LIBs), which are currently the core technology of ESSs, have been criticized for not being suitable for ESSs, which store large amounts of electricity due to their inherent risk of ignition and fire. In fact, a total of 33 cases of ESSs using LIBs in Korea had fire accidents, and 35% of all ESS facilities were shut down. This is estimated to have resulted in more than 700 billion won in losses.
As a result, water-based RFBs have drawn great attention. In particular, ZBBs that use ultra-low-cost bromide (ZnBr2) as an active material have been developed for ESSs since the 1970s, with their advantages of high cell voltage, high energy density, and low price compared to other RFBs. Until now, however, the commercialization of ZBBs has been delayed due to the short life span caused by the zinc electrodes. In particular, the uneven "dendrite" growth behavior of zinc metals during the charging and discharging process leads to internal short circuits in the battery which shorten its life.
The research team noted that self-aggregation occurs through the surface diffusion of zinc nuclei on the carbon electrode surface with low surface energy, and determined that self-aggregation was the main cause of zinc dendrite formation through quantum mechanics-based computer simulations and transmission electron microscopy. Furthermore, it was found that the surface diffusion of the zinc nuclei was inhibited in certain carbon fault structures so that dendrites were not produced.
Single vacancy defect, where one carbon atom is removed, exchanges zinc nuclei and electrons, and is strongly coupled, thus inhibiting surface diffusion and enabling uniform nuclear production/growth. The research team applied carbon electrodes with high density fault structure to ZBBs, achieving life characteristics of more than 5,000 cycles at a high charge current density (100 mA/cm2), which is 30 times that of LIBs.
This ESS technology, which can supply eco-friendly electric energy such as renewable energy to the private sector through technology that can drive safe and cheap redox flow batteries for long life, is expected to draw attention once again.
Publication:
Ju-Hyuk Lee, Riyul Kim, Soohyun Kim, Jiyun Heo, Hyeokjin Kwon, Jung Hoon Yang, and Hee-Tak Kim. 2020. Dendrite-free Zn electrodeposition triggered by interatomic orbital hybridization of Zn and single vacancy carbon defects for aqueous Zn-based flow batteries. Energy and Environmental Science, 2020, 13, 2839-2848.
Link to download the full-text paper:http://xlink.rsc.org/?DOI=D0EE00723D
Profile: Prof. Hee-Tak Kimheetak.kim@kaist.ac.krhttp://eed.kaist.ac.krAssociate ProfessorDepartment of Chemical & Biomolecular EngineeringKAIST
2020.12.16 View 14802