department+of+bio+and+brain+engineering
-
 KAIST to support the Genetic Donguibogam Research Project for global market entry of a new natural drug produced by Green Cross Corporation HS
In the wake of the spread of the Middle East Respiratory Syndrome (MERS), sales of immune-enhancing products in Korea such as red and white ginseng have risen dramatically. Ginseng is one of Korea’s major health supplement it exports, but due to the lack of precise scientific knowledge of its mechanism, sales of ginseng account for less than 2% of the global market share.
The Genetic Donguibogam Research Project represents a group of research initiatives to study genes and environmental factors that contribute to diseases and to discover alternative treatments through Eastern medicine. The project is being led by KAIST’s Department of Bio & Brain Engineering Professor Do-Heon Lee.
Professor Lee and Chief Executive Officer Young-Hyo Yoo of Green Cross Corporation HS, a Korean pharmaceutical company, signed a memorandum of understanding (MOU), as well as a non-disclosure agreement (NDA) to develop a naturally derived drug with an enhanced ginsenoside, pharmacological compounds of ginseng, for the global market entry of BST204 on June 10, 2015.
Donguibogam is the traditional Korean source for the principles and practice of Eastern medicine, which was compiled by the royal physician Heo Jun and first published in 1613 during the Joseon Dynasty of Korea.
Cooperating with Green Cross Co., HS, KAIST researchers will use a multi-component, multi-target (MCMT)-based development platform to produce the new natural drug, BST204. This cooperation is expected to assist the entry of the drug into the European market.
Green Cross Co., HS has applied a bio-conversion technique to ginseng to develop BST204, which is a drug with enhanced active constituent of aginsenosides. The drug is the first produced by any Korean pharmaceutical company to complete the first phase of clinical trials in Germany and is about to start the second phase of trials.
Professor Do-Heon Lee, the Director of the project said, “Genetic Donguibogam Research Project seeks to create new innovative healthcare material for the future using integrated fundamental technologies such as virtual human body computer modelling and multi-omics to explain the mechanism in which natural ingredients affect the human body.” He continued, “Especially, by employing the virtual human body computer modelling, we can develop an innovative new technology that will greatly assist Korean pharmaceutical industry and make it the platform technology in entering global markets.”
Young-Hyo Yoo, the CEO of Green Cross Co., HS, said, “For a new naturally derived drug to be acknowledged in the global market, such as Europe and the US, its mechanism, as well as its effectiveness and safety, should be proven. However, it is difficult and costly to explain the mechanism in which the complex composition of a natural substance influences the body. Innovative technology is needed to solve this problem.”
Professor Do-Heon Lee (left in the picture), the Director of Genetic Donguibogam Research Project, stands abreast Young-Hyo Yoo (right in the picture), the CEO of Green Cross Co., HS.
2015.06.10 View 7731
KAIST to support the Genetic Donguibogam Research Project for global market entry of a new natural drug produced by Green Cross Corporation HS
In the wake of the spread of the Middle East Respiratory Syndrome (MERS), sales of immune-enhancing products in Korea such as red and white ginseng have risen dramatically. Ginseng is one of Korea’s major health supplement it exports, but due to the lack of precise scientific knowledge of its mechanism, sales of ginseng account for less than 2% of the global market share.
The Genetic Donguibogam Research Project represents a group of research initiatives to study genes and environmental factors that contribute to diseases and to discover alternative treatments through Eastern medicine. The project is being led by KAIST’s Department of Bio & Brain Engineering Professor Do-Heon Lee.
Professor Lee and Chief Executive Officer Young-Hyo Yoo of Green Cross Corporation HS, a Korean pharmaceutical company, signed a memorandum of understanding (MOU), as well as a non-disclosure agreement (NDA) to develop a naturally derived drug with an enhanced ginsenoside, pharmacological compounds of ginseng, for the global market entry of BST204 on June 10, 2015.
Donguibogam is the traditional Korean source for the principles and practice of Eastern medicine, which was compiled by the royal physician Heo Jun and first published in 1613 during the Joseon Dynasty of Korea.
Cooperating with Green Cross Co., HS, KAIST researchers will use a multi-component, multi-target (MCMT)-based development platform to produce the new natural drug, BST204. This cooperation is expected to assist the entry of the drug into the European market.
Green Cross Co., HS has applied a bio-conversion technique to ginseng to develop BST204, which is a drug with enhanced active constituent of aginsenosides. The drug is the first produced by any Korean pharmaceutical company to complete the first phase of clinical trials in Germany and is about to start the second phase of trials.
Professor Do-Heon Lee, the Director of the project said, “Genetic Donguibogam Research Project seeks to create new innovative healthcare material for the future using integrated fundamental technologies such as virtual human body computer modelling and multi-omics to explain the mechanism in which natural ingredients affect the human body.” He continued, “Especially, by employing the virtual human body computer modelling, we can develop an innovative new technology that will greatly assist Korean pharmaceutical industry and make it the platform technology in entering global markets.”
Young-Hyo Yoo, the CEO of Green Cross Co., HS, said, “For a new naturally derived drug to be acknowledged in the global market, such as Europe and the US, its mechanism, as well as its effectiveness and safety, should be proven. However, it is difficult and costly to explain the mechanism in which the complex composition of a natural substance influences the body. Innovative technology is needed to solve this problem.”
Professor Do-Heon Lee (left in the picture), the Director of Genetic Donguibogam Research Project, stands abreast Young-Hyo Yoo (right in the picture), the CEO of Green Cross Co., HS.
2015.06.10 View 7731 -
 Anti-Cancer Therapy Delivering Drug to an Entire Tumor Developed
KAIST’s Department of Bio and Brain Engineering Professor Ji-Ho Park and his team successfully developed a new highly efficacious anti-cancer nanotechnology by delivering anti-cancer drugs uniformly to an entire tumor. Their research results were published in Nano Letters online on March 31, 2015.
To treat inoperable tumors, anti-cancer medicine is commonly used. However, efficient drug delivery to tumor cells is often difficult, treating an entire tumor with drugs even more so.
Using the existing drug delivery systems, including nanotechnology, a drug can be delivered only to tumor cells near blood vessels, leaving cells at the heart of a tumor intact. Since most drugs are injected into the bloodstream, tumor recurrence post medication is frequent.
Therefore, the team used liposomes that can fuse to the cell membrane and enter the cell. Once inside liposomes the drug can travel into the bloodstream, enter tumor cells near blood vessels, where they are loaded to exosomes, which are naturally occurring nanoparticles in the body. Since exosomes can travel between cells, the drug can be delivered efficiently into inner cells of the tumor.
Exosomes, which are secreted by cells that exist in the tumor microenvironment, is known to have an important role in tumor progression and metastasis since they transfer biological materials between cells. The research team started the investigation recognizing the possibility of delivering the anti-cancer drug to the entire tumor using exosomes.
The team injected the light-sensitive anti-cancer drug using their new delivery technique into experimental mice. The researchers applied light to the tumor site to activate the anti-cancer treatment and analyzed a tissue sample. They observed the effects of the anti-cancer drug in the entire tumor tissue.
The team’s results establish a ground-breaking foothold in drug delivery technology development that can be tailored to specific diseases by understanding its microenvironment. The work paves the way to more effective drug delivery systems for many chronic diseases, including cancer tumors that were difficult to treat due to the inability to penetrate deep into the tissue.
The team is currently conducting experiments with other anti-cancer drugs, which are being developed by pharmaceutical companies, using their tumor-penetrating drug delivery nanotechnology, to identify its effects on malignant tumors.
Professor Park said, “This research is the first to apply biological nanoparticles, exosomes that are continuously secreted and can transfer materials to neighboring cells, to deliver drugs directly to the heart of tumor.”
Picture: Incorporation of hydrophilic and hydrophobic compounds into membrane vesicles by engineering the parental cells via synthetic liposomes.
2015.04.07 View 11091
Anti-Cancer Therapy Delivering Drug to an Entire Tumor Developed
KAIST’s Department of Bio and Brain Engineering Professor Ji-Ho Park and his team successfully developed a new highly efficacious anti-cancer nanotechnology by delivering anti-cancer drugs uniformly to an entire tumor. Their research results were published in Nano Letters online on March 31, 2015.
To treat inoperable tumors, anti-cancer medicine is commonly used. However, efficient drug delivery to tumor cells is often difficult, treating an entire tumor with drugs even more so.
Using the existing drug delivery systems, including nanotechnology, a drug can be delivered only to tumor cells near blood vessels, leaving cells at the heart of a tumor intact. Since most drugs are injected into the bloodstream, tumor recurrence post medication is frequent.
Therefore, the team used liposomes that can fuse to the cell membrane and enter the cell. Once inside liposomes the drug can travel into the bloodstream, enter tumor cells near blood vessels, where they are loaded to exosomes, which are naturally occurring nanoparticles in the body. Since exosomes can travel between cells, the drug can be delivered efficiently into inner cells of the tumor.
Exosomes, which are secreted by cells that exist in the tumor microenvironment, is known to have an important role in tumor progression and metastasis since they transfer biological materials between cells. The research team started the investigation recognizing the possibility of delivering the anti-cancer drug to the entire tumor using exosomes.
The team injected the light-sensitive anti-cancer drug using their new delivery technique into experimental mice. The researchers applied light to the tumor site to activate the anti-cancer treatment and analyzed a tissue sample. They observed the effects of the anti-cancer drug in the entire tumor tissue.
The team’s results establish a ground-breaking foothold in drug delivery technology development that can be tailored to specific diseases by understanding its microenvironment. The work paves the way to more effective drug delivery systems for many chronic diseases, including cancer tumors that were difficult to treat due to the inability to penetrate deep into the tissue.
The team is currently conducting experiments with other anti-cancer drugs, which are being developed by pharmaceutical companies, using their tumor-penetrating drug delivery nanotechnology, to identify its effects on malignant tumors.
Professor Park said, “This research is the first to apply biological nanoparticles, exosomes that are continuously secreted and can transfer materials to neighboring cells, to deliver drugs directly to the heart of tumor.”
Picture: Incorporation of hydrophilic and hydrophobic compounds into membrane vesicles by engineering the parental cells via synthetic liposomes.
2015.04.07 View 11091 -
 A Key Signal Transduction Pathway Switch in Cardiomyocyte Identified
A KAIST research team has identified the fundamental principle in deciding the fate of cardiomyocyte or heart muscle cells. They have determined that it depends on the degree of stimulus in β-adrenergic receptor signal transduction pathway in the cardiomyocyte to control cells' survival or death. The findings, the team hopes, can be used to treat various heart diseases including heart failure.
The research was led by KAIST Department of Bio and Brain Engineering Chair Professor Kwang-Hyun Cho and conducted by Dr. Sung-Young Shin (lead author) and Ph.D. candidates Ho-Sung Lee and Joon-Hyuk Kang. The research was conducted jointly with GIST (Gwangju Institute of Science and Technology) Department of Biological Sciences Professor Do-Han Kim’s team. The research was supported by the Ministry of Science, ICT and Future Planning, Republic of Korea, and the National Research Foundation of Korea. The paper was published in Nature Communications on December 17, 2014 with the title, “The switching role of β-adrenergic receptor signalling in cell survival or death decision of cardiomyocytes.”
The β-adrenergic receptor signal transduction pathway can promote cell survival (mediated by β2 receptors), but also can result in cell death by inducing toxin (mediated by β1 receptors) that leads to various heart diseases including heart failure. Past attempts to identify the fundamental principle in the fate determining process of cardiomyocyte based on β-adrenergic receptor signalling concluded without much success.
The β-adrenergic receptor is a type of protein on the cell membrane of cardiomyocyte (heart muscle cell) that when stimulated by neurohormones such as epinephrine or norepinephrine would transduce signals making the cardiomyocyte contract faster and stronger.
The research team used large-scale computer simulation analysis and systems biology to identify ERK* and ICER** signal transduction pathways mediated by a feed-forward circuit as a key molecular switch that decides between cell survival and death.
Weak β-adrenergic receptor stimulations activate ERK signal transduction pathway, increasing Bcl-2*** protein expression to promote cardiomyocyte survival. On the other hand, strong β-adrenergic receptor stimulations activate ICER signal transduction pathway, reducing Bcl-2 protein expression to promote cardiomyocyte death.
Researchers used a systems biology approach to identify the mechanism of B-blocker****, a common drug prescribed for heart failure. When cardiomyocyte is treated with β1 inhibitor, strong stimulation on β-adrenergic receptor increases Bcl-2 expression, improving the chance of cardiomyocyte survival, a cell protection effect.
Professor Kwang-Hyun Cho said, “This research used systems biology, an integrated, convergence research of IT (information technology) and BT (biotechnology), to successfully identify the mechanism in deciding the fate of cardiomyocytes based on the β-adrenergic receptor signal transduction pathway for the first time. I am hopeful that this research will enable the control of cardiomyocyte survival and death to treat various heart diseases including heart failure.”
Professor Cho’s team was the first to pioneer a new field of systems biology, especially concerning the complex signal transduction network involved in diseases. Their research is focused on modelling, analyzing simulations, and experimentally proving signal pathways. Professor Cho has published 140 articles in international journals including Cell, Science, and Nature.
* ERK (Extracellular signal-regulated kinases): Signal transduction molecule involved in cell survival
** ICER (Inducible cAMP early repressor): Signal transduction molecule involved in cell death
*** Bcl-2 (B-cell lymphoma 2): Key signal transduction molecule involved in promotion of cell survival
**** β-blocker: Drug that acts as β-adrenergic receptor inhibitor known to slow the progression of heart failure, hence used most commonly in medicine.
Picture: A schematic diagram for the β-AR signalling network
2015.01.05 View 12268
A Key Signal Transduction Pathway Switch in Cardiomyocyte Identified
A KAIST research team has identified the fundamental principle in deciding the fate of cardiomyocyte or heart muscle cells. They have determined that it depends on the degree of stimulus in β-adrenergic receptor signal transduction pathway in the cardiomyocyte to control cells' survival or death. The findings, the team hopes, can be used to treat various heart diseases including heart failure.
The research was led by KAIST Department of Bio and Brain Engineering Chair Professor Kwang-Hyun Cho and conducted by Dr. Sung-Young Shin (lead author) and Ph.D. candidates Ho-Sung Lee and Joon-Hyuk Kang. The research was conducted jointly with GIST (Gwangju Institute of Science and Technology) Department of Biological Sciences Professor Do-Han Kim’s team. The research was supported by the Ministry of Science, ICT and Future Planning, Republic of Korea, and the National Research Foundation of Korea. The paper was published in Nature Communications on December 17, 2014 with the title, “The switching role of β-adrenergic receptor signalling in cell survival or death decision of cardiomyocytes.”
The β-adrenergic receptor signal transduction pathway can promote cell survival (mediated by β2 receptors), but also can result in cell death by inducing toxin (mediated by β1 receptors) that leads to various heart diseases including heart failure. Past attempts to identify the fundamental principle in the fate determining process of cardiomyocyte based on β-adrenergic receptor signalling concluded without much success.
The β-adrenergic receptor is a type of protein on the cell membrane of cardiomyocyte (heart muscle cell) that when stimulated by neurohormones such as epinephrine or norepinephrine would transduce signals making the cardiomyocyte contract faster and stronger.
The research team used large-scale computer simulation analysis and systems biology to identify ERK* and ICER** signal transduction pathways mediated by a feed-forward circuit as a key molecular switch that decides between cell survival and death.
Weak β-adrenergic receptor stimulations activate ERK signal transduction pathway, increasing Bcl-2*** protein expression to promote cardiomyocyte survival. On the other hand, strong β-adrenergic receptor stimulations activate ICER signal transduction pathway, reducing Bcl-2 protein expression to promote cardiomyocyte death.
Researchers used a systems biology approach to identify the mechanism of B-blocker****, a common drug prescribed for heart failure. When cardiomyocyte is treated with β1 inhibitor, strong stimulation on β-adrenergic receptor increases Bcl-2 expression, improving the chance of cardiomyocyte survival, a cell protection effect.
Professor Kwang-Hyun Cho said, “This research used systems biology, an integrated, convergence research of IT (information technology) and BT (biotechnology), to successfully identify the mechanism in deciding the fate of cardiomyocytes based on the β-adrenergic receptor signal transduction pathway for the first time. I am hopeful that this research will enable the control of cardiomyocyte survival and death to treat various heart diseases including heart failure.”
Professor Cho’s team was the first to pioneer a new field of systems biology, especially concerning the complex signal transduction network involved in diseases. Their research is focused on modelling, analyzing simulations, and experimentally proving signal pathways. Professor Cho has published 140 articles in international journals including Cell, Science, and Nature.
* ERK (Extracellular signal-regulated kinases): Signal transduction molecule involved in cell survival
** ICER (Inducible cAMP early repressor): Signal transduction molecule involved in cell death
*** Bcl-2 (B-cell lymphoma 2): Key signal transduction molecule involved in promotion of cell survival
**** β-blocker: Drug that acts as β-adrenergic receptor inhibitor known to slow the progression of heart failure, hence used most commonly in medicine.
Picture: A schematic diagram for the β-AR signalling network
2015.01.05 View 12268 -
 KAIST Researchers Develops Sensor That Reads Emotional States of Users
A piloerection monitoring sensor attached on the skin
The American Institute of Physics distributed a press release dated June 24, 2014 on a research paper written by a KAIST research team, which was published in its journal entitled Applied Physics Letters (APL). APL features concise, up-to-date reports in significant new findings in applied physics.
According to the release, “KAIST researchers have developed a flexible, wearable 20 mm x 20 mm polymer sensor that can directly measure the degree and occurrence on the skin of goose bumps, which is caused by sudden changes in body temperature or emotional states.”
The lead researcher was Professor Young-Ho Cho from the Department of Bio and Brain Engineering at KAIST.
If you would like to read the press release, please go to the link below:
American Institute of Physics, June 24, 2014
“New technology: The goose bump sensor”
http://www.eurekalert.org/pub_releases/2014-06/aiop-ntt062314.php
2014.06.26 View 8222
KAIST Researchers Develops Sensor That Reads Emotional States of Users
A piloerection monitoring sensor attached on the skin
The American Institute of Physics distributed a press release dated June 24, 2014 on a research paper written by a KAIST research team, which was published in its journal entitled Applied Physics Letters (APL). APL features concise, up-to-date reports in significant new findings in applied physics.
According to the release, “KAIST researchers have developed a flexible, wearable 20 mm x 20 mm polymer sensor that can directly measure the degree and occurrence on the skin of goose bumps, which is caused by sudden changes in body temperature or emotional states.”
The lead researcher was Professor Young-Ho Cho from the Department of Bio and Brain Engineering at KAIST.
If you would like to read the press release, please go to the link below:
American Institute of Physics, June 24, 2014
“New technology: The goose bump sensor”
http://www.eurekalert.org/pub_releases/2014-06/aiop-ntt062314.php
2014.06.26 View 8222 -
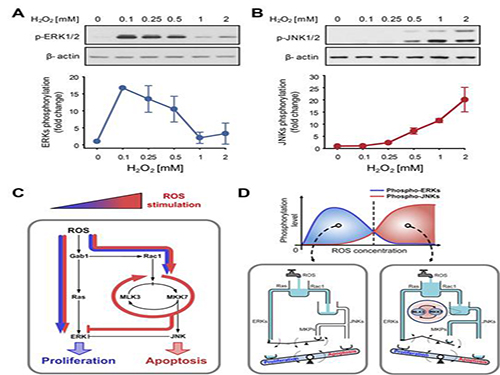 A mechanism for how reactive oxygen species cause cell responses studied
A research team led by Professor Kwang-Hyun Cho of the Department of Biology and Brain Engineering, KAIST, and Dr. Gi-Sun Kwon of the Korea Research Institute of Bioscience and Biotechnology succeeded in proving the mechanism behind the determination of cell life in relation to reactive oxygen species.
The results of the venture were published in the June 3rd edition of Science Signaling. The title of the research paper is “MLK3 is part of a feedback mechanism that regulates different cellular responses to reactive oxygen species.”
The research team discovered that the molecular switch that determines the division of apoptosis of a cell was based on MLK3 feedback mechanism. MLK stands for mixed-lineage kinase.
Under sufficient stress, the mechanism instructs the cell to undergo the division but in an overly stressful environment, the mechanism stops the cell division and instead, induces apoptosis. This discovery is expected to be a breakthrough in illnesses related to the concentration of the reactive oxygen species (ROS).
At low concentration of ROS, the protein associated with cell division, ERK (extracellular-signal-regulated kinase), is activated while as the ROS concentration increases, JNK (c-Jun N-terminal protein kinases), responsible for apoptosis, becomes activated. Furthermore, through computer simulation analysis and mathematical modeling, in tandem with molecular cell biology experiments, the MLK3 based feedback mechanism was the fundamental molecular switch that determines the balance between ERK and JNK, and ultimately the cell’s responses.
Professor Cho commented that “the contradicting cell responses to ROS had remained a mystery, but with the system biology, an approach in which information technology and biotechnology converge, such riddles can be resolved. We expect that the proven mechanism will be used to overcome aging or cancer growth as a result of ROS in the near future.”
Picture shows the process of identifying cell responses caused by reactive oxygen species.
2014.06.13 View 8779
A mechanism for how reactive oxygen species cause cell responses studied
A research team led by Professor Kwang-Hyun Cho of the Department of Biology and Brain Engineering, KAIST, and Dr. Gi-Sun Kwon of the Korea Research Institute of Bioscience and Biotechnology succeeded in proving the mechanism behind the determination of cell life in relation to reactive oxygen species.
The results of the venture were published in the June 3rd edition of Science Signaling. The title of the research paper is “MLK3 is part of a feedback mechanism that regulates different cellular responses to reactive oxygen species.”
The research team discovered that the molecular switch that determines the division of apoptosis of a cell was based on MLK3 feedback mechanism. MLK stands for mixed-lineage kinase.
Under sufficient stress, the mechanism instructs the cell to undergo the division but in an overly stressful environment, the mechanism stops the cell division and instead, induces apoptosis. This discovery is expected to be a breakthrough in illnesses related to the concentration of the reactive oxygen species (ROS).
At low concentration of ROS, the protein associated with cell division, ERK (extracellular-signal-regulated kinase), is activated while as the ROS concentration increases, JNK (c-Jun N-terminal protein kinases), responsible for apoptosis, becomes activated. Furthermore, through computer simulation analysis and mathematical modeling, in tandem with molecular cell biology experiments, the MLK3 based feedback mechanism was the fundamental molecular switch that determines the balance between ERK and JNK, and ultimately the cell’s responses.
Professor Cho commented that “the contradicting cell responses to ROS had remained a mystery, but with the system biology, an approach in which information technology and biotechnology converge, such riddles can be resolved. We expect that the proven mechanism will be used to overcome aging or cancer growth as a result of ROS in the near future.”
Picture shows the process of identifying cell responses caused by reactive oxygen species.
2014.06.13 View 8779 -
 A Molecular Switch Controlling Self-Assembly of Protein Nanotubes Discovered
International collaborative research among South Korea, United States, and Israel research institutionsThe key to the treatment of cancer and brain disease mechanism The molecular switch that controls the self-assembly structure of the protein nanotubes, which plays crucial role in cell division and intracellular transport of materials, has been discovered. KAIST Bio and Brain Engineering Department’s Professor Myeong-Cheol Choi and Professor Chae-Yeon Song conducted the research, in collaboration with the University of California in Santa Barbara, U.S., and Hebrew University in Israel. The findings of the research were published in Nature Materials on the 19th. Microtubules are tube shaped and composed of protein that plays a key role in cell division, cytoskeleton, and intercellular material transport and is only 25nm in diameter (1/100,000 thickness of a human hair). Conventionally, cancer treatment focused on disrupting the formation of microtubules to suppress the division of cancer cells. In addition Alzheimer’s is known to be caused by the diminishing of structural integrity of microtubules responsible for intercellular material transport which leads to failure in signal transfer. The research team utilized synchrotron x-ray scattering and transmission electron microscope to analyze the self assemble structure of protein nanotubes to subnanometer accuracy. As a result, the microtubules were found to assemble into 25nm thickness tubules by stacking protein blocks 4 x 5 x 8nm in dimension. In the process, the research team discovered the molecular switch that controls the shape of these protein blocks. In addition the research team was successful in creating a new protein tube structure. Professor Choi commented that they were successful in introducing a new paradigm that suggests the possibility of controlling the complex biological functions of human’s biological system with the simple use of physical principles. He commented further that it is anticipated that the findings will allow for the application of bio nanotubes in engineering and that this is a small step in finding the mechanism behind cancer treatment and neural diseases.
2014.02.03 View 9787
A Molecular Switch Controlling Self-Assembly of Protein Nanotubes Discovered
International collaborative research among South Korea, United States, and Israel research institutionsThe key to the treatment of cancer and brain disease mechanism The molecular switch that controls the self-assembly structure of the protein nanotubes, which plays crucial role in cell division and intracellular transport of materials, has been discovered. KAIST Bio and Brain Engineering Department’s Professor Myeong-Cheol Choi and Professor Chae-Yeon Song conducted the research, in collaboration with the University of California in Santa Barbara, U.S., and Hebrew University in Israel. The findings of the research were published in Nature Materials on the 19th. Microtubules are tube shaped and composed of protein that plays a key role in cell division, cytoskeleton, and intercellular material transport and is only 25nm in diameter (1/100,000 thickness of a human hair). Conventionally, cancer treatment focused on disrupting the formation of microtubules to suppress the division of cancer cells. In addition Alzheimer’s is known to be caused by the diminishing of structural integrity of microtubules responsible for intercellular material transport which leads to failure in signal transfer. The research team utilized synchrotron x-ray scattering and transmission electron microscope to analyze the self assemble structure of protein nanotubes to subnanometer accuracy. As a result, the microtubules were found to assemble into 25nm thickness tubules by stacking protein blocks 4 x 5 x 8nm in dimension. In the process, the research team discovered the molecular switch that controls the shape of these protein blocks. In addition the research team was successful in creating a new protein tube structure. Professor Choi commented that they were successful in introducing a new paradigm that suggests the possibility of controlling the complex biological functions of human’s biological system with the simple use of physical principles. He commented further that it is anticipated that the findings will allow for the application of bio nanotubes in engineering and that this is a small step in finding the mechanism behind cancer treatment and neural diseases.
2014.02.03 View 9787 -
 Two Dimensions of Value: Dopamine Neurons Represent Reward but not Aversiveness
Professor Christopher D. Fiorillo of the Bio & Brain Engineering (http://ineuron.kaist.ac.kr/web/home.html) at KAIST published a research paper in the August 2 issue of Science. The title of the paper is “Two Dimensions of Value: Dopamine Neurons Represent Reward but not Aversiveness.” The following is an introduction of his research work:
To make decisions, we need to estimate the value of sensory stimuli and motor actions, their “goodness” and “badness.” We can imagine that good and bad are two ends of a single continuum, or dimension, of value. This would be analogous to the single dimension of light intensity, which ranges from dark on one end to bright light on the other, with many shades of gray in between. Past models of behavior and learning have been based on a single continuum of value, and it has been proposed that a particular group of neurons (brain cells) that use dopamine as a neurotransmitter (chemical messenger) represent the single dimension of value, signaling both good and bad.
The experiments reported here show that dopamine neurons are sensitive to the value of reward but not punishment (like the aversiveness of a bitter taste). This demonstrates that reward and aversiveness are represented as two discrete dimensions (or categories) in the brain. “Reward” refers to the category of good things (food, water, sex, money, etc.), and “punishment” to the category of bad things (stimuli associated with harm to the body and that cause pain or other unpleasant sensations or emotions).
Rather than having one neurotransmitter (dopamine) to represent a single dimension of value, the present results imply the existence of four neurotransmitters to represent two dimensions of value. Dopamine signals evidence for reward (“gains”) and some other neurotransmitter presumably signals evidence against reward (“losses”). Likewise, there should be a neurotransmitter for evidence of danger and another for evidence of safety. It is interesting that there are three other neurotransmitters that are analogous to dopamine in many respects (serotonin, norepinephrine, and acetylcholine), and it is possible that they could represent the other three value signals.
For the research article, please visit: http://www.sciencemag.org/content/341/6145/546.abstract
For the Science 2nd issue, please visit: http://www.sciencemag.org/content/current#ResearchArticles
Illustration of Value Dimension
2013.08.08 View 7481
Two Dimensions of Value: Dopamine Neurons Represent Reward but not Aversiveness
Professor Christopher D. Fiorillo of the Bio & Brain Engineering (http://ineuron.kaist.ac.kr/web/home.html) at KAIST published a research paper in the August 2 issue of Science. The title of the paper is “Two Dimensions of Value: Dopamine Neurons Represent Reward but not Aversiveness.” The following is an introduction of his research work:
To make decisions, we need to estimate the value of sensory stimuli and motor actions, their “goodness” and “badness.” We can imagine that good and bad are two ends of a single continuum, or dimension, of value. This would be analogous to the single dimension of light intensity, which ranges from dark on one end to bright light on the other, with many shades of gray in between. Past models of behavior and learning have been based on a single continuum of value, and it has been proposed that a particular group of neurons (brain cells) that use dopamine as a neurotransmitter (chemical messenger) represent the single dimension of value, signaling both good and bad.
The experiments reported here show that dopamine neurons are sensitive to the value of reward but not punishment (like the aversiveness of a bitter taste). This demonstrates that reward and aversiveness are represented as two discrete dimensions (or categories) in the brain. “Reward” refers to the category of good things (food, water, sex, money, etc.), and “punishment” to the category of bad things (stimuli associated with harm to the body and that cause pain or other unpleasant sensations or emotions).
Rather than having one neurotransmitter (dopamine) to represent a single dimension of value, the present results imply the existence of four neurotransmitters to represent two dimensions of value. Dopamine signals evidence for reward (“gains”) and some other neurotransmitter presumably signals evidence against reward (“losses”). Likewise, there should be a neurotransmitter for evidence of danger and another for evidence of safety. It is interesting that there are three other neurotransmitters that are analogous to dopamine in many respects (serotonin, norepinephrine, and acetylcholine), and it is possible that they could represent the other three value signals.
For the research article, please visit: http://www.sciencemag.org/content/341/6145/546.abstract
For the Science 2nd issue, please visit: http://www.sciencemag.org/content/current#ResearchArticles
Illustration of Value Dimension
2013.08.08 View 7481 -
 Firefly inspired high efficiency LED technology developed
A firefly inspired, high efficiency self-illuminating LED has been developed.
Professor Jeong Gi Hoon (Department of Bio and Brain Engineering) mimicked the nanostructure of the external layer of the illumination organ of a firefly and succeeded in fabricating high illumination efficiency LED lenses.
Conventional lenses required expensive anti-reflection coating. The developed lenses utilize the bio-inspired nanostructure on the surface of the lenses themselves to reduce the reflectivity of the lenses thereby decreasing production costs.
The developed antireflection nanostructure is expected to be applied to various digital devices and lighting fixtures.
Antireflective structures have been applied in various fields in order to enhance light efficiency However these structures have been limited to flat surfaces and therefore was difficult to implement to curved surfaces like LED lenses.
Professor Jeong’s team solved this problem by using three dimensional micro molding processes.
The team fabricated the nanostructure by forming a single nanoparticle layer on the silicon oxide and performing dry etching. On this nanostructure PDMS was poured and manipulated to fabricate a lens structure similar to that of a firefly.
The fabricated lens showed similar efficiency as conventional antireflection coating.
2012.11.29 View 7875
Firefly inspired high efficiency LED technology developed
A firefly inspired, high efficiency self-illuminating LED has been developed.
Professor Jeong Gi Hoon (Department of Bio and Brain Engineering) mimicked the nanostructure of the external layer of the illumination organ of a firefly and succeeded in fabricating high illumination efficiency LED lenses.
Conventional lenses required expensive anti-reflection coating. The developed lenses utilize the bio-inspired nanostructure on the surface of the lenses themselves to reduce the reflectivity of the lenses thereby decreasing production costs.
The developed antireflection nanostructure is expected to be applied to various digital devices and lighting fixtures.
Antireflective structures have been applied in various fields in order to enhance light efficiency However these structures have been limited to flat surfaces and therefore was difficult to implement to curved surfaces like LED lenses.
Professor Jeong’s team solved this problem by using three dimensional micro molding processes.
The team fabricated the nanostructure by forming a single nanoparticle layer on the silicon oxide and performing dry etching. On this nanostructure PDMS was poured and manipulated to fabricate a lens structure similar to that of a firefly.
The fabricated lens showed similar efficiency as conventional antireflection coating.
2012.11.29 View 7875 -
 Systems biology demystifies the resistance mechanism of targeted cancer medication
Korean researchers have found the fundamental resistance mechanism of the MEK inhibitor, a recently highlighted chemotherapy method, laying the foundation for future research on overcoming cancer drug resistance and improving cancer survival rates. This research is meaningful because it was conducted through systems biology, a fusion of IT and biotechnology.
The research was conducted by Professor Gwang hyun Cho’s team from the Department of Biology at KAIST and was supported by the Ministry of Education, Science and Technology and the National Research Foundation of Korea.
The research was published as the cover paper for the June edition of the Journal of Molecular Cell Biology (Title: The cross regulation between ERK and PI3K signaling pathways determines the tumoricidal efficacy of MEK inhibitor).
Targeted anticancer medication targets certain molecules in the signaling pathway of the tumor cell and not only has fewer side effects than pre-existing anticancer medication, but also has high clinical efficacy. The technology also allows the creation of personalized medication and has been widely praised by scientists worldwide.
However, resistances to the targeted medication have often been found before or during the clinical stage, eventually causing the medications to fail to reach the drug development stage. Moreover, even if the drug is effective, the survival rate is low and the redevelopment rate is high.
An active pathway in most tumor cells is the ERK (Extracellular signal-regulated kinases) signaling pathway. This pathway is especially important in the development of skin cancer or thyroid cancer, which are developed by the mutation of the BRAF gene inside the path. In these cases, the MEK (Extracellular signal-regulated kinases) inhibitor is an effective treatment because it targets the pathway itself. However, the built-up resistance to the inhibitor commonly leads to the redevelopment of cancer.
Professor Cho’s research team used large scale computer simulations to analyze the fundamental resistance mechanism of the MEK inhibitor and used molecular cell biological experiments as well as bio-imaging* techniques to verify the results.
* Bio-imaging: Checking biological phenomena at the cellular and molecular levels using imagery
The research team used different mutational variables, which revealed that the use of the MEK inhibitor reduced the transmission of the ERK signal but led to the activation of another signaling pathway (the PI3K signaling pathway), reducing the effectiveness of the medication. Professor Cho’s team also found that this response originated from the complex interaction between the signaling matter as well as the feedback network structure, suggesting that the mix of the MEK inhibitor with other drugs could improve the effects of the targeted anticancer medication.
Professor Cho stated that this research was the first of its kind to examine the drug resistivity against the MEK inhibitor at the systematic dimension and showed how the effects of drugs on the signaling pathways of cells could be predicted using computer simulation. It also showed how basic research on signaling networks can be applied to clinical drug use, successfully suggesting a new research platform on overcoming resistance to targeting medication using its fundamental mechanism.
2012.07.06 View 10491
Systems biology demystifies the resistance mechanism of targeted cancer medication
Korean researchers have found the fundamental resistance mechanism of the MEK inhibitor, a recently highlighted chemotherapy method, laying the foundation for future research on overcoming cancer drug resistance and improving cancer survival rates. This research is meaningful because it was conducted through systems biology, a fusion of IT and biotechnology.
The research was conducted by Professor Gwang hyun Cho’s team from the Department of Biology at KAIST and was supported by the Ministry of Education, Science and Technology and the National Research Foundation of Korea.
The research was published as the cover paper for the June edition of the Journal of Molecular Cell Biology (Title: The cross regulation between ERK and PI3K signaling pathways determines the tumoricidal efficacy of MEK inhibitor).
Targeted anticancer medication targets certain molecules in the signaling pathway of the tumor cell and not only has fewer side effects than pre-existing anticancer medication, but also has high clinical efficacy. The technology also allows the creation of personalized medication and has been widely praised by scientists worldwide.
However, resistances to the targeted medication have often been found before or during the clinical stage, eventually causing the medications to fail to reach the drug development stage. Moreover, even if the drug is effective, the survival rate is low and the redevelopment rate is high.
An active pathway in most tumor cells is the ERK (Extracellular signal-regulated kinases) signaling pathway. This pathway is especially important in the development of skin cancer or thyroid cancer, which are developed by the mutation of the BRAF gene inside the path. In these cases, the MEK (Extracellular signal-regulated kinases) inhibitor is an effective treatment because it targets the pathway itself. However, the built-up resistance to the inhibitor commonly leads to the redevelopment of cancer.
Professor Cho’s research team used large scale computer simulations to analyze the fundamental resistance mechanism of the MEK inhibitor and used molecular cell biological experiments as well as bio-imaging* techniques to verify the results.
* Bio-imaging: Checking biological phenomena at the cellular and molecular levels using imagery
The research team used different mutational variables, which revealed that the use of the MEK inhibitor reduced the transmission of the ERK signal but led to the activation of another signaling pathway (the PI3K signaling pathway), reducing the effectiveness of the medication. Professor Cho’s team also found that this response originated from the complex interaction between the signaling matter as well as the feedback network structure, suggesting that the mix of the MEK inhibitor with other drugs could improve the effects of the targeted anticancer medication.
Professor Cho stated that this research was the first of its kind to examine the drug resistivity against the MEK inhibitor at the systematic dimension and showed how the effects of drugs on the signaling pathways of cells could be predicted using computer simulation. It also showed how basic research on signaling networks can be applied to clinical drug use, successfully suggesting a new research platform on overcoming resistance to targeting medication using its fundamental mechanism.
2012.07.06 View 10491 -
 The output of terahertz waves enhanced by KAIST team
KAIST researchers have greatly improved the output of terahertz waves, the blue ocean of the optics world. This technology is expected to be applied to portable X-ray cameras, small bio-diagnostic systems, and in many other devices.
Professor Ki-Hun Jeong"s research team from the Department of Bio and Brain Engineering used optical nano-antenna technology to increase the output of terahertz waves by three times.
Terahertz waves are electromagnetic waves with frequencies between 100GHz to 30THz. They are produced when a femtosecond (10^-15 s) pulse laser is shone on a semiconductor substrate with photoconduction antennas, causing a photocurrent pulse of one picosecond (10^-12 s). Their long wavelengths, in comparison to visible light and infrared rays, give terahertz waves a high penetration power with less energy than X-rays, making them less harmful to humans.
These qualities allow us to see through objects, just as X-rays do, but because terahertz waves absorb certain frequencies, we can detect hidden explosives or drugs, which was not possible with X-rays. We can even identify fake drugs. Furthermore, using the spectral information, we can analyze a material"s innate qualities without chemical processing, making it possible to identify skin diseases without harming the body. However, the output was not sufficient to be used in biosensors and other applications.
Prof. Jeong"s team added optical nano-antennas, made from gold nano-rods, in between the photoconduction antennas and optimized the structure. This resulted in nanoplasmonic resonance in the photoconduction substrate, increasing the degree of integration of the photocurrent pulse and resulting in a three times larger output.
Hence, it is not only possible to see through objects more clearly, but it is also possible to analyze components without a biopsy.
Professor Jeong explained, "This technology, coupled with the miniaturization of terahertz devices, can be applied to endoscopes to detect early epithelial cancer" and that he will focus on creating and commercializing these biosensor systems.
This research was published in the March issue of the international nanotechnology journal ACS Nano and was funded by the Korea Evaluation Institute of Industrial Technology and the National Research Foundation of Korea.
Figure: Mimetic diagram of a THz generator with nano-antennas
2012.04.29 View 11281
The output of terahertz waves enhanced by KAIST team
KAIST researchers have greatly improved the output of terahertz waves, the blue ocean of the optics world. This technology is expected to be applied to portable X-ray cameras, small bio-diagnostic systems, and in many other devices.
Professor Ki-Hun Jeong"s research team from the Department of Bio and Brain Engineering used optical nano-antenna technology to increase the output of terahertz waves by three times.
Terahertz waves are electromagnetic waves with frequencies between 100GHz to 30THz. They are produced when a femtosecond (10^-15 s) pulse laser is shone on a semiconductor substrate with photoconduction antennas, causing a photocurrent pulse of one picosecond (10^-12 s). Their long wavelengths, in comparison to visible light and infrared rays, give terahertz waves a high penetration power with less energy than X-rays, making them less harmful to humans.
These qualities allow us to see through objects, just as X-rays do, but because terahertz waves absorb certain frequencies, we can detect hidden explosives or drugs, which was not possible with X-rays. We can even identify fake drugs. Furthermore, using the spectral information, we can analyze a material"s innate qualities without chemical processing, making it possible to identify skin diseases without harming the body. However, the output was not sufficient to be used in biosensors and other applications.
Prof. Jeong"s team added optical nano-antennas, made from gold nano-rods, in between the photoconduction antennas and optimized the structure. This resulted in nanoplasmonic resonance in the photoconduction substrate, increasing the degree of integration of the photocurrent pulse and resulting in a three times larger output.
Hence, it is not only possible to see through objects more clearly, but it is also possible to analyze components without a biopsy.
Professor Jeong explained, "This technology, coupled with the miniaturization of terahertz devices, can be applied to endoscopes to detect early epithelial cancer" and that he will focus on creating and commercializing these biosensor systems.
This research was published in the March issue of the international nanotechnology journal ACS Nano and was funded by the Korea Evaluation Institute of Industrial Technology and the National Research Foundation of Korea.
Figure: Mimetic diagram of a THz generator with nano-antennas
2012.04.29 View 11281