AR
-
 Professor Lik-Hang Lee Offers Metaverse Course for Hong Kong Productivity Council
Professor Lik-Hang Lee from the Department of Industrial System Engineering will offer a metaverse course in partnership with the Hong Kong Productivity Council (HKPC) from the Spring 2022 semester to Hong Kong-based professionals.
“The Metaverse Course for Professionals” aims to nurture world-class talents of the metaverse in response to surging demand for virtual worlds and virtual-physical blended environments. The HKPC’s R&D scientists, consultants, software engineers, and related professionals will attend the course. They will receive a professional certificate on managing and developing metaverse skills upon the completion of this intensive course.
The course will provide essential skills and knowledge about the parallel virtual universe and how to leverage digitalization and industrialization in the metaverse era. The course includes comprehensive modules, such as designing and implementing virtual-physical blended environments, metaverse technology and ecosystems, immersive smart cities, token economies, and intelligent industrialization in the metaverse era.
Professor Lee believes in the decades to come that we will see rising numbers of virtual worlds in cyberspace known as the ‘Immersive Internet’ that will be characterized by high levels of immersiveness, user interactivity, and user-machine collaborations.
“Consumers in virtual worlds will create novel content as well as personalized products and services, becoming as catalyst for ‘hyperpersonalization’ in the next industrial revolution,” he said.
Professor Lee said he will continue offering world-class education related to the metaverse to students in KAIST and professionals from various industrial sectors, as his Augmented Reality and Media Lab will focus on a variety of metaverse topics such as metaverse campuses and industrial metaverses.
The HKPC has worked to address innovative solutions for Hong Kong industries and enterprises since 1967, helping them achieve optimized resource utilization, effectiveness, and cost reduction as well as enhanced productivity and competitiveness in both local and international markets. The HKPC has advocated for facilitating Hong Kong’s reindustrialization powered by Industry 4.0 and e-commerce 4.0 with a strong emphasis on R&D, IoT, AI, digital manufacturing.
The Augmented Reality and Media Lab led by Professor Lee will continue its close partnerships with HKPC and its other partners to help build the epicentre of the metaverse in the region. Furthermore, the lab will fully leverage its well-established research niches in user-centric, virtual-physical cyberspace (https://www.lhlee.com/projects-8 ) to serve upcoming projects related to industrial metaverses, which aligns with the departmental focus on smart factories and artificial intelligence.
2022.04.06 View 9470
Professor Lik-Hang Lee Offers Metaverse Course for Hong Kong Productivity Council
Professor Lik-Hang Lee from the Department of Industrial System Engineering will offer a metaverse course in partnership with the Hong Kong Productivity Council (HKPC) from the Spring 2022 semester to Hong Kong-based professionals.
“The Metaverse Course for Professionals” aims to nurture world-class talents of the metaverse in response to surging demand for virtual worlds and virtual-physical blended environments. The HKPC’s R&D scientists, consultants, software engineers, and related professionals will attend the course. They will receive a professional certificate on managing and developing metaverse skills upon the completion of this intensive course.
The course will provide essential skills and knowledge about the parallel virtual universe and how to leverage digitalization and industrialization in the metaverse era. The course includes comprehensive modules, such as designing and implementing virtual-physical blended environments, metaverse technology and ecosystems, immersive smart cities, token economies, and intelligent industrialization in the metaverse era.
Professor Lee believes in the decades to come that we will see rising numbers of virtual worlds in cyberspace known as the ‘Immersive Internet’ that will be characterized by high levels of immersiveness, user interactivity, and user-machine collaborations.
“Consumers in virtual worlds will create novel content as well as personalized products and services, becoming as catalyst for ‘hyperpersonalization’ in the next industrial revolution,” he said.
Professor Lee said he will continue offering world-class education related to the metaverse to students in KAIST and professionals from various industrial sectors, as his Augmented Reality and Media Lab will focus on a variety of metaverse topics such as metaverse campuses and industrial metaverses.
The HKPC has worked to address innovative solutions for Hong Kong industries and enterprises since 1967, helping them achieve optimized resource utilization, effectiveness, and cost reduction as well as enhanced productivity and competitiveness in both local and international markets. The HKPC has advocated for facilitating Hong Kong’s reindustrialization powered by Industry 4.0 and e-commerce 4.0 with a strong emphasis on R&D, IoT, AI, digital manufacturing.
The Augmented Reality and Media Lab led by Professor Lee will continue its close partnerships with HKPC and its other partners to help build the epicentre of the metaverse in the region. Furthermore, the lab will fully leverage its well-established research niches in user-centric, virtual-physical cyberspace (https://www.lhlee.com/projects-8 ) to serve upcoming projects related to industrial metaverses, which aligns with the departmental focus on smart factories and artificial intelligence.
2022.04.06 View 9470 -
 Distinguished Professor Sukbok Chang Named the 2022 Ho-Am Laureate
Distinguished Professor Sukbok Chang from the Department of Chemistry was named the awardee of the Ho-Am Prize in the fields of chemistry and life sciences. The award has recognized the most distinguished scholars, individuals, and organizations in physics and mathematics, chemistry and life sciences, engineering, medicine, arts, and community service in honor of the late founder of Samsung Group Byong-Chul Lee, whose penname is Ho-Am. The awards ceremony will be held on May 31 and awardees will receive 300 million KRW in prize money.
Professor Chang became the fourth KAIST Ho-Am laureate following Distinguished Professor Sang Yup Lee in engineering in 2014, Distinguished Professor Jun Ho Oh in engineering in 2016, and Distinguished Professor Gou Young Koh in medicine in 2018.
Professor Chang is a renowned chemist who has made pioneering research in the area of transition metal catalysis for organic transformations. Professor Chang is also one of the Highly Cited Researchers who rank in the top 1% of citations by field and publication year in the Web of Science citation index. He has made the list seven years in a row from 2016.
Professor Chang has developed a range of new and impactful C-H bond functionalization reactions. By using his approaches, value-added molecules can be readily produced from chemical feedstocks, representatively hydrocarbons and (hetero)arenes. His research team elucidated fundamental key mechanistic aspects in the course of the essential C-H bond activation process of unreactive starting materials. He was able to utilize the obtained mechanistic understanding for the subsequent catalyst design to develop more efficient and highly (stereo)selective catalytic reactions.
Among the numerous contributions he made, the design of new mechanistic approaches toward metal nitrenoid transfers are of especially high impact to the chemical community. Indeed, a series of important transition metal catalyst systems were developed by Professor Chang to enable the direct and selective C-H amidation of unreactive organic compounds, thereby producing aminated compounds that have important applicability in synthetic, medicinal, and materials science. He has also pioneered in the area of asymmetric C-H amination chemistry by creatively devising various types of chiral transition metal catalyst systems, and his team proved for the first time that chiral lactam compounds can be obtained at an excellent level of stereoselectivity.
Another significant contribution of Professor. Chang was the introduction of dioxazolones as a robust but highly reactive source of acyl nitrenoids for the catalytic C-H amidation reactions, and this reagent is now broadly utilized in synthetic chemistry worldwide.
Professor Chang also leads a research group in the Center for Catalytic Hydrocarbon Functionalizations at the Institute for Basic Science.
2022.04.06 View 9095
Distinguished Professor Sukbok Chang Named the 2022 Ho-Am Laureate
Distinguished Professor Sukbok Chang from the Department of Chemistry was named the awardee of the Ho-Am Prize in the fields of chemistry and life sciences. The award has recognized the most distinguished scholars, individuals, and organizations in physics and mathematics, chemistry and life sciences, engineering, medicine, arts, and community service in honor of the late founder of Samsung Group Byong-Chul Lee, whose penname is Ho-Am. The awards ceremony will be held on May 31 and awardees will receive 300 million KRW in prize money.
Professor Chang became the fourth KAIST Ho-Am laureate following Distinguished Professor Sang Yup Lee in engineering in 2014, Distinguished Professor Jun Ho Oh in engineering in 2016, and Distinguished Professor Gou Young Koh in medicine in 2018.
Professor Chang is a renowned chemist who has made pioneering research in the area of transition metal catalysis for organic transformations. Professor Chang is also one of the Highly Cited Researchers who rank in the top 1% of citations by field and publication year in the Web of Science citation index. He has made the list seven years in a row from 2016.
Professor Chang has developed a range of new and impactful C-H bond functionalization reactions. By using his approaches, value-added molecules can be readily produced from chemical feedstocks, representatively hydrocarbons and (hetero)arenes. His research team elucidated fundamental key mechanistic aspects in the course of the essential C-H bond activation process of unreactive starting materials. He was able to utilize the obtained mechanistic understanding for the subsequent catalyst design to develop more efficient and highly (stereo)selective catalytic reactions.
Among the numerous contributions he made, the design of new mechanistic approaches toward metal nitrenoid transfers are of especially high impact to the chemical community. Indeed, a series of important transition metal catalyst systems were developed by Professor Chang to enable the direct and selective C-H amidation of unreactive organic compounds, thereby producing aminated compounds that have important applicability in synthetic, medicinal, and materials science. He has also pioneered in the area of asymmetric C-H amination chemistry by creatively devising various types of chiral transition metal catalyst systems, and his team proved for the first time that chiral lactam compounds can be obtained at an excellent level of stereoselectivity.
Another significant contribution of Professor. Chang was the introduction of dioxazolones as a robust but highly reactive source of acyl nitrenoids for the catalytic C-H amidation reactions, and this reagent is now broadly utilized in synthetic chemistry worldwide.
Professor Chang also leads a research group in the Center for Catalytic Hydrocarbon Functionalizations at the Institute for Basic Science.
2022.04.06 View 9095 -
 Mathematicians Identify a Key Source of Cell-to-Cell Variability in Cell Signaling
Systematic inferences identify a major source of heterogeneity in cell signaling dynamics
Why do genetically identical cells respond differently to the same external stimuli, such as antibiotics? This long-standing mystery has been solved by KAIST and IBS mathematicians who have developed a new framework for analyzing cell responses to some stimuli. The team found that the cell-to-cell variability in antibiotic stress response increases as the effective length of the cell signaling pathway (i.e., the number of rate-limiting steps) increases. This finding could identify more effective chemotherapies to overcome the fractional killing of cancer cells caused by cell-to-cell variability.
Cells in the human body contain signal transduction systems that respond to various external stimuli such as antibiotics and changes in osmotic pressure. When an external stimulus is detected, various biochemical reactions occur sequentially. This leads to the expression of relevant genes, allowing the cells to respond to the perturbed external environment. Furthermore, signal transduction leads to a drug response (e.g., antibiotic resistance genes are expressed when antibiotic drugs are given).
However, even when the same external stimuli are detected, the responses of individual cells are greatly heterogeneous. This leads to the emergence of persister cells that are highly resistant to drugs. To identify potential sources of this cell-to cell variability, many studies have been conducted. However, most of the intermediate signal transduction reactions are unobservable with current experimental techniques.
A group of researchers including Dae Wook Kim and Hyukpyo Hong and led by Professor Jae Kyoung Kim from the KAIST Department of Mathematical Sciences and IBS Biomedical Mathematics Group solved the mystery by exploiting queueing theory and Bayesian inference methodology. They proposed a queueing process that describes the signal transduction system in cells. Based on this, they developed Bayesian inference computational software using MBI (the Moment-based Bayesian Inference method). This enables the analysis of the signal transduction system without a direct observation of the intermediate steps. This study was published in Science Advances.
By analyzing experimental data from Escherichia coli using MBI, the research team found that cell-to-cell variability increases as the number of rate-limiting steps in the signaling pathway increases.
The rate-limiting steps denote the slowest steps (i.e., bottlenecks) in sequential biochemical reaction steps composing cell signaling pathways and thus dominates most of the signaling time. As the number of the rate-limiting steps increases, the intensity of the transduced signal becomes greatly heterogeneous even in a population of genetically identical cells. This finding is expected to provide a new paradigm for studying the heterogeneous antibiotic resistance of cells, which is a big challenge in cancer medicine.
Professor Kim said, “As a mathematician, I am excited to help advance the understanding of cell-to-cell variability in response to external stimuli. I hope this finding facilitates the development of more effective chemotherapies.”
This work was supported by the Samsung Science and Technology Foundation, the National Research Foundation of Korea, and the Institute for Basic Science.
-Publication:Dae Wook Kim, Hyukpyo Hong, and Jae Kyoung Kim (2022) “Systematic inference identifies a major source of heterogeneity in cell signaling dynamics: the rate-limiting step number,”Science Advances March 18, 2022 (DOI: 10.1126/sciadv.abl4598)
-Profile:Professor Jae Kyoung Kimhttp://mathsci.kaist.ac.kr/~jaekkim
jaekkim@kaist.ac.kr@umichkim on TwitterDepartment of Mathematical SciencesKAIST
2022.03.29 View 11631
Mathematicians Identify a Key Source of Cell-to-Cell Variability in Cell Signaling
Systematic inferences identify a major source of heterogeneity in cell signaling dynamics
Why do genetically identical cells respond differently to the same external stimuli, such as antibiotics? This long-standing mystery has been solved by KAIST and IBS mathematicians who have developed a new framework for analyzing cell responses to some stimuli. The team found that the cell-to-cell variability in antibiotic stress response increases as the effective length of the cell signaling pathway (i.e., the number of rate-limiting steps) increases. This finding could identify more effective chemotherapies to overcome the fractional killing of cancer cells caused by cell-to-cell variability.
Cells in the human body contain signal transduction systems that respond to various external stimuli such as antibiotics and changes in osmotic pressure. When an external stimulus is detected, various biochemical reactions occur sequentially. This leads to the expression of relevant genes, allowing the cells to respond to the perturbed external environment. Furthermore, signal transduction leads to a drug response (e.g., antibiotic resistance genes are expressed when antibiotic drugs are given).
However, even when the same external stimuli are detected, the responses of individual cells are greatly heterogeneous. This leads to the emergence of persister cells that are highly resistant to drugs. To identify potential sources of this cell-to cell variability, many studies have been conducted. However, most of the intermediate signal transduction reactions are unobservable with current experimental techniques.
A group of researchers including Dae Wook Kim and Hyukpyo Hong and led by Professor Jae Kyoung Kim from the KAIST Department of Mathematical Sciences and IBS Biomedical Mathematics Group solved the mystery by exploiting queueing theory and Bayesian inference methodology. They proposed a queueing process that describes the signal transduction system in cells. Based on this, they developed Bayesian inference computational software using MBI (the Moment-based Bayesian Inference method). This enables the analysis of the signal transduction system without a direct observation of the intermediate steps. This study was published in Science Advances.
By analyzing experimental data from Escherichia coli using MBI, the research team found that cell-to-cell variability increases as the number of rate-limiting steps in the signaling pathway increases.
The rate-limiting steps denote the slowest steps (i.e., bottlenecks) in sequential biochemical reaction steps composing cell signaling pathways and thus dominates most of the signaling time. As the number of the rate-limiting steps increases, the intensity of the transduced signal becomes greatly heterogeneous even in a population of genetically identical cells. This finding is expected to provide a new paradigm for studying the heterogeneous antibiotic resistance of cells, which is a big challenge in cancer medicine.
Professor Kim said, “As a mathematician, I am excited to help advance the understanding of cell-to-cell variability in response to external stimuli. I hope this finding facilitates the development of more effective chemotherapies.”
This work was supported by the Samsung Science and Technology Foundation, the National Research Foundation of Korea, and the Institute for Basic Science.
-Publication:Dae Wook Kim, Hyukpyo Hong, and Jae Kyoung Kim (2022) “Systematic inference identifies a major source of heterogeneity in cell signaling dynamics: the rate-limiting step number,”Science Advances March 18, 2022 (DOI: 10.1126/sciadv.abl4598)
-Profile:Professor Jae Kyoung Kimhttp://mathsci.kaist.ac.kr/~jaekkim
jaekkim@kaist.ac.kr@umichkim on TwitterDepartment of Mathematical SciencesKAIST
2022.03.29 View 11631 -
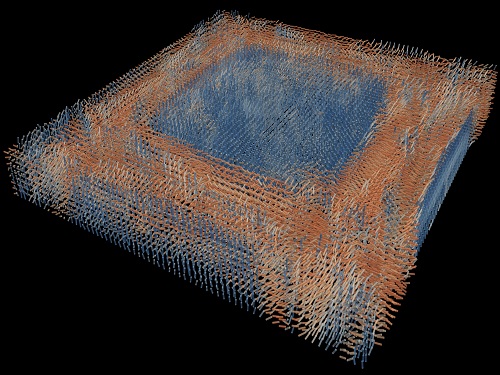 Tomographic Measurement of Dielectric Tensors
Dielectric tensor tomography allows the direct measurement of the 3D dielectric tensors of optically anisotropic structures
A research team reported the direct measurement of dielectric tensors of anisotropic structures including the spatial variations of principal refractive indices and directors. The group also demonstrated quantitative tomographic measurements of various nematic liquid-crystal structures and their fast 3D nonequilibrium dynamics using a 3D label-free tomographic method. The method was described in Nature Materials.
Light-matter interactions are described by the dielectric tensor. Despite their importance in basic science and applications, it has not been possible to measure 3D dielectric tensors directly. The main challenge was due to the vectorial nature of light scattering from a 3D anisotropic structure. Previous approaches only addressed 3D anisotropic information indirectly and were limited to two-dimensional, qualitative, strict sample conditions or assumptions.
The research team developed a method enabling the tomographic reconstruction of 3D dielectric tensors without any preparation or assumptions. A sample is illuminated with a laser beam with various angles and circularly polarization states. Then, the light fields scattered from a sample are holographically measured and converted into vectorial diffraction components. Finally, by inversely solving a vectorial wave equation, the 3D dielectric tensor is reconstructed.
Professor YongKeun Park said, “There were a greater number of unknowns in direct measuring than with the conventional approach. We applied our approach to measure additional holographic images by slightly tilting the incident angle.”
He said that the slightly tilted illumination provides an additional orthogonal polarization, which makes the underdetermined problem become the determined problem. “Although scattered fields are dependent on the illumination angle, the Fourier differentiation theorem enables the extraction of the same dielectric tensor for the slightly tilted illumination,” Professor Park added.
His team’s method was validated by reconstructing well-known liquid crystal (LC) structures, including the twisted nematic, hybrid aligned nematic, radial, and bipolar configurations. Furthermore, the research team demonstrated the experimental measurements of the non-equilibrium dynamics of annihilating, nucleating, and merging LC droplets, and the LC polymer network with repeating 3D topological defects.
“This is the first experimental measurement of non-equilibrium dynamics and 3D topological defects in LC structures in a label-free manner. Our method enables the exploration of inaccessible nematic structures and interactions in non-equilibrium dynamics,” first author Dr. Seungwoo Shin explained.
-PublicationSeungwoo Shin, Jonghee Eun, Sang Seok Lee, Changjae Lee, Herve Hugonnet, Dong Ki Yoon, Shin-Hyun Kim, Jongwoo Jeong, YongKeun Park, “Tomographic Measurement ofDielectric Tensors at Optical Frequency,” Nature Materials March 02, 2022 (https://doi.org/10/1038/s41563-022-01202-8)
-ProfileProfessor YongKeun ParkBiomedical Optics Laboratory (http://bmol.kaist.ac.kr)Department of PhysicsCollege of Natural SciencesKAIST
2022.03.22 View 9668
Tomographic Measurement of Dielectric Tensors
Dielectric tensor tomography allows the direct measurement of the 3D dielectric tensors of optically anisotropic structures
A research team reported the direct measurement of dielectric tensors of anisotropic structures including the spatial variations of principal refractive indices and directors. The group also demonstrated quantitative tomographic measurements of various nematic liquid-crystal structures and their fast 3D nonequilibrium dynamics using a 3D label-free tomographic method. The method was described in Nature Materials.
Light-matter interactions are described by the dielectric tensor. Despite their importance in basic science and applications, it has not been possible to measure 3D dielectric tensors directly. The main challenge was due to the vectorial nature of light scattering from a 3D anisotropic structure. Previous approaches only addressed 3D anisotropic information indirectly and were limited to two-dimensional, qualitative, strict sample conditions or assumptions.
The research team developed a method enabling the tomographic reconstruction of 3D dielectric tensors without any preparation or assumptions. A sample is illuminated with a laser beam with various angles and circularly polarization states. Then, the light fields scattered from a sample are holographically measured and converted into vectorial diffraction components. Finally, by inversely solving a vectorial wave equation, the 3D dielectric tensor is reconstructed.
Professor YongKeun Park said, “There were a greater number of unknowns in direct measuring than with the conventional approach. We applied our approach to measure additional holographic images by slightly tilting the incident angle.”
He said that the slightly tilted illumination provides an additional orthogonal polarization, which makes the underdetermined problem become the determined problem. “Although scattered fields are dependent on the illumination angle, the Fourier differentiation theorem enables the extraction of the same dielectric tensor for the slightly tilted illumination,” Professor Park added.
His team’s method was validated by reconstructing well-known liquid crystal (LC) structures, including the twisted nematic, hybrid aligned nematic, radial, and bipolar configurations. Furthermore, the research team demonstrated the experimental measurements of the non-equilibrium dynamics of annihilating, nucleating, and merging LC droplets, and the LC polymer network with repeating 3D topological defects.
“This is the first experimental measurement of non-equilibrium dynamics and 3D topological defects in LC structures in a label-free manner. Our method enables the exploration of inaccessible nematic structures and interactions in non-equilibrium dynamics,” first author Dr. Seungwoo Shin explained.
-PublicationSeungwoo Shin, Jonghee Eun, Sang Seok Lee, Changjae Lee, Herve Hugonnet, Dong Ki Yoon, Shin-Hyun Kim, Jongwoo Jeong, YongKeun Park, “Tomographic Measurement ofDielectric Tensors at Optical Frequency,” Nature Materials March 02, 2022 (https://doi.org/10/1038/s41563-022-01202-8)
-ProfileProfessor YongKeun ParkBiomedical Optics Laboratory (http://bmol.kaist.ac.kr)Department of PhysicsCollege of Natural SciencesKAIST
2022.03.22 View 9668 -
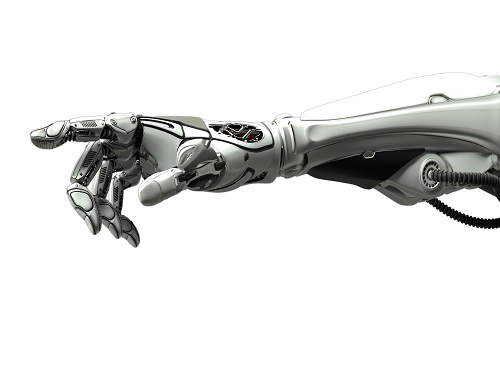 Decoding Brain Signals to Control a Robotic Arm
Advanced brain-machine interface system successfully interprets arm movement directions from neural signals in the brain
Researchers have developed a mind-reading system for decoding neural signals from the brain during arm movement. The method, described in the journal Applied Soft Computing, can be used by a person to control a robotic arm through a brain-machine interface (BMI).
A BMI is a device that translates nerve signals into commands to control a machine, such as a computer or a robotic limb. There are two main techniques for monitoring neural signals in BMIs: electroencephalography (EEG) and electrocorticography (ECoG).
The EEG exhibits signals from electrodes on the surface of the scalp and is widely employed because it is non-invasive, relatively cheap, safe and easy to use. However, the EEG has low spatial resolution and detects irrelevant neural signals, which makes it difficult to interpret the intentions of individuals from the EEG.
On the other hand, the ECoG is an invasive method that involves placing electrodes directly on the surface of the cerebral cortex below the scalp. Compared with the EEG, the ECoG can monitor neural signals with much higher spatial resolution and less background noise. However, this technique has several drawbacks.
“The ECoG is primarily used to find potential sources of epileptic seizures, meaning the electrodes are placed in different locations for different patients and may not be in the optimal regions of the brain for detecting sensory and movement signals,” explained Professor Jaeseung Jeong, a brain scientist at KAIST. “This inconsistency makes it difficult to decode brain signals to predict movements.”
To overcome these problems, Professor Jeong’s team developed a new method for decoding ECoG neural signals during arm movement. The system is based on a machine-learning system for analysing and predicting neural signals called an ‘echo-state network’ and a mathematical probability model called the Gaussian distribution.
In the study, the researchers recorded ECoG signals from four individuals with epilepsy while they were performing a reach-and-grasp task. Because the ECoG electrodes were placed according to the potential sources of each patient’s epileptic seizures, only 22% to 44% of the electrodes were located in the regions of the brain responsible for controlling movement.
During the movement task, the participants were given visual cues, either by placing a real tennis ball in front of them, or via a virtual reality headset showing a clip of a human arm reaching forward in first-person view. They were asked to reach forward, grasp an object, then return their hand and release the object, while wearing motion sensors on their wrists and fingers. In a second task, they were instructed to imagine reaching forward without moving their arms.
The researchers monitored the signals from the ECoG electrodes during real and imaginary arm movements, and tested whether the new system could predict the direction of this movement from the neural signals. They found that the novel decoder successfully classified arm movements in 24 directions in three-dimensional space, both in the real and virtual tasks, and that the results were at least five times more accurate than chance. They also used a computer simulation to show that the novel ECoG decoder could control the movements of a robotic arm.
Overall, the results suggest that the new machine learning-based BCI system successfully used ECoG signals to interpret the direction of the intended movements. The next steps will be to improve the accuracy and efficiency of the decoder. In the future, it could be used in a real-time BMI device to help people with movement or sensory impairments.
This research was supported by the KAIST Global Singularity Research Program of 2021, Brain Research Program of the National Research Foundation of Korea funded by the Ministry of Science, ICT, and Future Planning, and the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education.
-PublicationHoon-Hee Kim, Jaeseung Jeong, “An electrocorticographic decoder for arm movement for brain-machine interface using an echo state network and Gaussian readout,” Applied SoftComputing online December 31, 2021 (doi.org/10.1016/j.asoc.2021.108393)
-ProfileProfessor Jaeseung JeongDepartment of Bio and Brain EngineeringCollege of EngineeringKAIST
2022.03.18 View 13688
Decoding Brain Signals to Control a Robotic Arm
Advanced brain-machine interface system successfully interprets arm movement directions from neural signals in the brain
Researchers have developed a mind-reading system for decoding neural signals from the brain during arm movement. The method, described in the journal Applied Soft Computing, can be used by a person to control a robotic arm through a brain-machine interface (BMI).
A BMI is a device that translates nerve signals into commands to control a machine, such as a computer or a robotic limb. There are two main techniques for monitoring neural signals in BMIs: electroencephalography (EEG) and electrocorticography (ECoG).
The EEG exhibits signals from electrodes on the surface of the scalp and is widely employed because it is non-invasive, relatively cheap, safe and easy to use. However, the EEG has low spatial resolution and detects irrelevant neural signals, which makes it difficult to interpret the intentions of individuals from the EEG.
On the other hand, the ECoG is an invasive method that involves placing electrodes directly on the surface of the cerebral cortex below the scalp. Compared with the EEG, the ECoG can monitor neural signals with much higher spatial resolution and less background noise. However, this technique has several drawbacks.
“The ECoG is primarily used to find potential sources of epileptic seizures, meaning the electrodes are placed in different locations for different patients and may not be in the optimal regions of the brain for detecting sensory and movement signals,” explained Professor Jaeseung Jeong, a brain scientist at KAIST. “This inconsistency makes it difficult to decode brain signals to predict movements.”
To overcome these problems, Professor Jeong’s team developed a new method for decoding ECoG neural signals during arm movement. The system is based on a machine-learning system for analysing and predicting neural signals called an ‘echo-state network’ and a mathematical probability model called the Gaussian distribution.
In the study, the researchers recorded ECoG signals from four individuals with epilepsy while they were performing a reach-and-grasp task. Because the ECoG electrodes were placed according to the potential sources of each patient’s epileptic seizures, only 22% to 44% of the electrodes were located in the regions of the brain responsible for controlling movement.
During the movement task, the participants were given visual cues, either by placing a real tennis ball in front of them, or via a virtual reality headset showing a clip of a human arm reaching forward in first-person view. They were asked to reach forward, grasp an object, then return their hand and release the object, while wearing motion sensors on their wrists and fingers. In a second task, they were instructed to imagine reaching forward without moving their arms.
The researchers monitored the signals from the ECoG electrodes during real and imaginary arm movements, and tested whether the new system could predict the direction of this movement from the neural signals. They found that the novel decoder successfully classified arm movements in 24 directions in three-dimensional space, both in the real and virtual tasks, and that the results were at least five times more accurate than chance. They also used a computer simulation to show that the novel ECoG decoder could control the movements of a robotic arm.
Overall, the results suggest that the new machine learning-based BCI system successfully used ECoG signals to interpret the direction of the intended movements. The next steps will be to improve the accuracy and efficiency of the decoder. In the future, it could be used in a real-time BMI device to help people with movement or sensory impairments.
This research was supported by the KAIST Global Singularity Research Program of 2021, Brain Research Program of the National Research Foundation of Korea funded by the Ministry of Science, ICT, and Future Planning, and the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education.
-PublicationHoon-Hee Kim, Jaeseung Jeong, “An electrocorticographic decoder for arm movement for brain-machine interface using an echo state network and Gaussian readout,” Applied SoftComputing online December 31, 2021 (doi.org/10.1016/j.asoc.2021.108393)
-ProfileProfessor Jaeseung JeongDepartment of Bio and Brain EngineeringCollege of EngineeringKAIST
2022.03.18 View 13688 -
 'Fingerprint' Machine Learning Technique Identifies Different Bacteria in Seconds
A synergistic combination of surface-enhanced Raman spectroscopy and deep learning serves as an effective platform for separation-free detection of bacteria in arbitrary media
Bacterial identification can take hours and often longer, precious time when diagnosing infections and selecting appropriate treatments. There may be a quicker, more accurate process according to researchers at KAIST. By teaching a deep learning algorithm to identify the “fingerprint” spectra of the molecular components of various bacteria, the researchers could classify various bacteria in different media with accuracies of up to 98%.
Their results were made available online on Jan. 18 in Biosensors and Bioelectronics, ahead of publication in the journal’s April issue.
Bacteria-induced illnesses, those caused by direct bacterial infection or by exposure to bacterial toxins, can induce painful symptoms and even lead to death, so the rapid detection of bacteria is crucial to prevent the intake of contaminated foods and to diagnose infections from clinical samples, such as urine. “By using surface-enhanced Raman spectroscopy (SERS) analysis boosted with a newly proposed deep learning model, we demonstrated a markedly simple, fast, and effective route to classify the signals of two common bacteria and their resident media without any separation procedures,” said Professor Sungho Jo from the School of Computing.
Raman spectroscopy sends light through a sample to see how it scatters. The results reveal structural information about the sample — the spectral fingerprint — allowing researchers to identify its molecules. The surface-enhanced version places sample cells on noble metal nanostructures that help amplify the sample’s signals.
However, it is challenging to obtain consistent and clear spectra of bacteria due to numerous overlapping peak sources, such as proteins in cell walls. “Moreover, strong signals of surrounding media are also enhanced to overwhelm target signals, requiring time-consuming and tedious bacterial separation steps,” said Professor Yeon Sik Jung from the Department of Materials Science and Engineering.
To parse through the noisy signals, the researchers implemented an artificial intelligence method called deep learning that can hierarchically extract certain features of the spectral information to classify data. They specifically designed their model, named the dual-branch wide-kernel network (DualWKNet), to efficiently learn the correlation between spectral features. Such an ability is critical for analyzing one-dimensional spectral data, according to Professor Jo.
“Despite having interfering signals or noise from the media, which make the general shapes of different bacterial spectra and their residing media signals look similar, high classification accuracies of bacterial types and their media were achieved,” Professor Jo said, explaining that DualWKNet allowed the team to identify key peaks in each class that were almost indiscernible in individual spectra, enhancing the classification accuracies. “Ultimately, with the use of DualWKNet replacing the bacteria and media separation steps, our method dramatically reduces analysis time.”
The researchers plan to use their platform to study more bacteria and media types, using the information to build a training data library of various bacterial types in additional media to reduce the collection and detection times for new samples.
“We developed a meaningful universal platform for rapid bacterial detection with the collaboration between SERS and deep learning,” Professor Jo said. “We hope to extend the use of our deep learning-based SERS analysis platform to detect numerous types of bacteria in additional media that are important for food or clinical analysis, such as blood.”
The National R&D Program, through a National Research Foundation of Korea grant funded by the Ministry of Science and ICT, supported this research.
-PublicationEojin Rho, Minjoon Kim, Seunghee H. Cho, Bongjae Choi, Hyungjoon Park, Hanhwi Jang, Yeon Sik Jung, Sungho Jo, “Separation-free bacterial identification in arbitrary media via deepneural network-based SERS analysis,” Biosensors and Bioelectronics online January 18, 2022 (doi.org/10.1016/j.bios.2022.113991)
-ProfileProfessor Yeon Sik JungDepartment of Materials Science and EngineeringKAIST
Professor Sungho JoSchool of ComputingKAIST
2022.03.04 View 23210
'Fingerprint' Machine Learning Technique Identifies Different Bacteria in Seconds
A synergistic combination of surface-enhanced Raman spectroscopy and deep learning serves as an effective platform for separation-free detection of bacteria in arbitrary media
Bacterial identification can take hours and often longer, precious time when diagnosing infections and selecting appropriate treatments. There may be a quicker, more accurate process according to researchers at KAIST. By teaching a deep learning algorithm to identify the “fingerprint” spectra of the molecular components of various bacteria, the researchers could classify various bacteria in different media with accuracies of up to 98%.
Their results were made available online on Jan. 18 in Biosensors and Bioelectronics, ahead of publication in the journal’s April issue.
Bacteria-induced illnesses, those caused by direct bacterial infection or by exposure to bacterial toxins, can induce painful symptoms and even lead to death, so the rapid detection of bacteria is crucial to prevent the intake of contaminated foods and to diagnose infections from clinical samples, such as urine. “By using surface-enhanced Raman spectroscopy (SERS) analysis boosted with a newly proposed deep learning model, we demonstrated a markedly simple, fast, and effective route to classify the signals of two common bacteria and their resident media without any separation procedures,” said Professor Sungho Jo from the School of Computing.
Raman spectroscopy sends light through a sample to see how it scatters. The results reveal structural information about the sample — the spectral fingerprint — allowing researchers to identify its molecules. The surface-enhanced version places sample cells on noble metal nanostructures that help amplify the sample’s signals.
However, it is challenging to obtain consistent and clear spectra of bacteria due to numerous overlapping peak sources, such as proteins in cell walls. “Moreover, strong signals of surrounding media are also enhanced to overwhelm target signals, requiring time-consuming and tedious bacterial separation steps,” said Professor Yeon Sik Jung from the Department of Materials Science and Engineering.
To parse through the noisy signals, the researchers implemented an artificial intelligence method called deep learning that can hierarchically extract certain features of the spectral information to classify data. They specifically designed their model, named the dual-branch wide-kernel network (DualWKNet), to efficiently learn the correlation between spectral features. Such an ability is critical for analyzing one-dimensional spectral data, according to Professor Jo.
“Despite having interfering signals or noise from the media, which make the general shapes of different bacterial spectra and their residing media signals look similar, high classification accuracies of bacterial types and their media were achieved,” Professor Jo said, explaining that DualWKNet allowed the team to identify key peaks in each class that were almost indiscernible in individual spectra, enhancing the classification accuracies. “Ultimately, with the use of DualWKNet replacing the bacteria and media separation steps, our method dramatically reduces analysis time.”
The researchers plan to use their platform to study more bacteria and media types, using the information to build a training data library of various bacterial types in additional media to reduce the collection and detection times for new samples.
“We developed a meaningful universal platform for rapid bacterial detection with the collaboration between SERS and deep learning,” Professor Jo said. “We hope to extend the use of our deep learning-based SERS analysis platform to detect numerous types of bacteria in additional media that are important for food or clinical analysis, such as blood.”
The National R&D Program, through a National Research Foundation of Korea grant funded by the Ministry of Science and ICT, supported this research.
-PublicationEojin Rho, Minjoon Kim, Seunghee H. Cho, Bongjae Choi, Hyungjoon Park, Hanhwi Jang, Yeon Sik Jung, Sungho Jo, “Separation-free bacterial identification in arbitrary media via deepneural network-based SERS analysis,” Biosensors and Bioelectronics online January 18, 2022 (doi.org/10.1016/j.bios.2022.113991)
-ProfileProfessor Yeon Sik JungDepartment of Materials Science and EngineeringKAIST
Professor Sungho JoSchool of ComputingKAIST
2022.03.04 View 23210 -
 Scientist Discover How Circadian Rhythm Can Be Both Strong and Flexible
Study reveals that master and slave oscillators function via different molecular mechanisms
From tiny fruit flies to human beings, all animals on Earth maintain their daily rhythms based on their internal circadian clock. The circadian clock enables organisms to undergo rhythmic changes in behavior and physiology based on a 24-hour circadian cycle. For example, our own biological clock tells our brain to release melatonin, a sleep-inducing hormone, at night time.
The discovery of the molecular mechanism of the circadian clock was bestowed the Nobel Prize in Physiology or Medicine 2017. From what we know, no one centralized clock is responsible for our circadian cycles. Instead, it operates in a hierarchical network where there are “master pacemaker” and “slave oscillator”.
The master pacemaker receives various input signals from the environment such as light. The master then drives the slave oscillator that regulates various outputs such as sleep, feeding, and metabolism. Despite the different roles of the pacemaker neurons, they are known to share common molecular mechanisms that are well conserved in all lifeforms. For example, interlocked systems of multiple transcriptional-translational feedback loops (TTFLs) composed of core clock proteins have been deeply studied in fruit flies.
However, there is still much that we need to learn about our own biological clock. The hierarchically-organized nature of master and slave clock neurons leads to a prevailing belief that they share an identical molecular clockwork. At the same time, the different roles they serve in regulating bodily rhythms also raise the question of whether they might function under different molecular clockworks.
Research team led by Professor Kim Jae Kyoung from the Department of Mathematical Sciences, a chief investigator at the Biomedical Mathematics Group at the Institute for Basic Science, used a combination of mathematical and experimental approaches using fruit flies to answer this question. The team found that the master clock and the slave clock operate via different molecular mechanisms.
In both master and slave neurons of fruit flies, a circadian rhythm-related protein called PER is produced and degraded at different rates depending on the time of the day. Previously, the team found that the master clock neuron (sLNvs) and the slave clock neuron (DN1ps) have different profiles of PER in wild-type and Clk-Δ mutant Drosophila. This hinted that there might be a potential difference in molecular clockworks between the master and slave clock neurons.
However, due to the complexity of the molecular clockwork, it was challenging to identify the source of such differences. Thus, the team developed a mathematical model describing the molecular clockworks of the master and slave clocks. Then, all possible molecular differences between the master and slave clock neurons were systematically investigated by using computer simulations. The model predicted that PER is more efficiently produced and then rapidly degraded in the master clock compared to the slave clock neurons. This prediction was then confirmed by the follow-up experiments using animal.
Then, why do the master clock neurons have such different molecular properties from the slave clock neurons? To answer this question, the research team again used the combination of mathematical model simulation and experiments. It was found that the faster rate of synthesis of PER in the master clock neurons allows them to generate synchronized rhythms with a high level of amplitude. Generation of such a strong rhythm with high amplitude is critical to delivering clear signals to slave clock neurons.
However, such strong rhythms would typically be unfavorable when it comes to adapting to environmental changes. These include natural causes such as different daylight hours across summer and winter seasons, up to more extreme artificial cases such as jet lag that occurs after international travel. Thanks to the distinct property of the master clock neurons, it is able to undergo phase dispersion when the standard light-dark cycle is disrupted, drastically reducing the level of PER. The master clock neurons can then easily adapt to the new diurnal cycle. Our master pacemaker’s plasticity explains how we can quickly adjust to the new time zones after international flights after just a brief period of jet lag.
It is hoped that the findings of this study can have future clinical implications when it comes to treating various disorders that affect our circadian rhythm. Professor Kim notes, “When the circadian clock loses its robustness and flexibility, the circadian rhythms sleep disorders can occur. As this study identifies the molecular mechanism that generates robustness and flexibility of the circadian clock, it can facilitate the identification of the cause of and treatment strategy for the circadian rhythm sleep disorders.” This work was supported by the Human Frontier Science Program.
-PublicationEui Min Jeong, Miri Kwon, Eunjoo Cho, Sang Hyuk Lee, Hyun Kim, Eun Young Kim, and Jae Kyoung Kim, “Systematic modeling-driven experiments identify distinct molecularclockworks underlying hierarchically organized pacemaker neurons,” February 22, 2022, Proceedings of the National Academy of Sciences of the United States of America
-ProfileProfessor Jae Kyoung KimDepartment of Mathematical SciencesKAIST
2022.02.23 View 11656
Scientist Discover How Circadian Rhythm Can Be Both Strong and Flexible
Study reveals that master and slave oscillators function via different molecular mechanisms
From tiny fruit flies to human beings, all animals on Earth maintain their daily rhythms based on their internal circadian clock. The circadian clock enables organisms to undergo rhythmic changes in behavior and physiology based on a 24-hour circadian cycle. For example, our own biological clock tells our brain to release melatonin, a sleep-inducing hormone, at night time.
The discovery of the molecular mechanism of the circadian clock was bestowed the Nobel Prize in Physiology or Medicine 2017. From what we know, no one centralized clock is responsible for our circadian cycles. Instead, it operates in a hierarchical network where there are “master pacemaker” and “slave oscillator”.
The master pacemaker receives various input signals from the environment such as light. The master then drives the slave oscillator that regulates various outputs such as sleep, feeding, and metabolism. Despite the different roles of the pacemaker neurons, they are known to share common molecular mechanisms that are well conserved in all lifeforms. For example, interlocked systems of multiple transcriptional-translational feedback loops (TTFLs) composed of core clock proteins have been deeply studied in fruit flies.
However, there is still much that we need to learn about our own biological clock. The hierarchically-organized nature of master and slave clock neurons leads to a prevailing belief that they share an identical molecular clockwork. At the same time, the different roles they serve in regulating bodily rhythms also raise the question of whether they might function under different molecular clockworks.
Research team led by Professor Kim Jae Kyoung from the Department of Mathematical Sciences, a chief investigator at the Biomedical Mathematics Group at the Institute for Basic Science, used a combination of mathematical and experimental approaches using fruit flies to answer this question. The team found that the master clock and the slave clock operate via different molecular mechanisms.
In both master and slave neurons of fruit flies, a circadian rhythm-related protein called PER is produced and degraded at different rates depending on the time of the day. Previously, the team found that the master clock neuron (sLNvs) and the slave clock neuron (DN1ps) have different profiles of PER in wild-type and Clk-Δ mutant Drosophila. This hinted that there might be a potential difference in molecular clockworks between the master and slave clock neurons.
However, due to the complexity of the molecular clockwork, it was challenging to identify the source of such differences. Thus, the team developed a mathematical model describing the molecular clockworks of the master and slave clocks. Then, all possible molecular differences between the master and slave clock neurons were systematically investigated by using computer simulations. The model predicted that PER is more efficiently produced and then rapidly degraded in the master clock compared to the slave clock neurons. This prediction was then confirmed by the follow-up experiments using animal.
Then, why do the master clock neurons have such different molecular properties from the slave clock neurons? To answer this question, the research team again used the combination of mathematical model simulation and experiments. It was found that the faster rate of synthesis of PER in the master clock neurons allows them to generate synchronized rhythms with a high level of amplitude. Generation of such a strong rhythm with high amplitude is critical to delivering clear signals to slave clock neurons.
However, such strong rhythms would typically be unfavorable when it comes to adapting to environmental changes. These include natural causes such as different daylight hours across summer and winter seasons, up to more extreme artificial cases such as jet lag that occurs after international travel. Thanks to the distinct property of the master clock neurons, it is able to undergo phase dispersion when the standard light-dark cycle is disrupted, drastically reducing the level of PER. The master clock neurons can then easily adapt to the new diurnal cycle. Our master pacemaker’s plasticity explains how we can quickly adjust to the new time zones after international flights after just a brief period of jet lag.
It is hoped that the findings of this study can have future clinical implications when it comes to treating various disorders that affect our circadian rhythm. Professor Kim notes, “When the circadian clock loses its robustness and flexibility, the circadian rhythms sleep disorders can occur. As this study identifies the molecular mechanism that generates robustness and flexibility of the circadian clock, it can facilitate the identification of the cause of and treatment strategy for the circadian rhythm sleep disorders.” This work was supported by the Human Frontier Science Program.
-PublicationEui Min Jeong, Miri Kwon, Eunjoo Cho, Sang Hyuk Lee, Hyun Kim, Eun Young Kim, and Jae Kyoung Kim, “Systematic modeling-driven experiments identify distinct molecularclockworks underlying hierarchically organized pacemaker neurons,” February 22, 2022, Proceedings of the National Academy of Sciences of the United States of America
-ProfileProfessor Jae Kyoung KimDepartment of Mathematical SciencesKAIST
2022.02.23 View 11656 -
 A Mathematical Model Shows High Viral Transmissions Reduce the Progression Rates for Severe Covid-19
The model suggests a clue as to when a pandemic will turn into an endemic
A mathematical model demonstrated that high transmission rates among highly vaccinated populations of COVID-19 ultimately reduce the numbers of severe cases. This model suggests a clue as to when this pandemic will turn into an endemic.
With the future of the pandemic remaining uncertain, a research team of mathematicians and medical scientists analyzed a mathematical model that may predict how the changing transmission rate of COVID-19 would affect the settlement process of the virus as a mild respiratory virus.
The team led by Professor Jae Kyoung Kim from the Department of Mathematical Science and Professor Eui-Cheol Shin from the Graduate School of Medical Science and Engineering used a new approach by dividing the human immune responses to SARS-CoV-2 into a shorter-term neutralizing antibody response and a longer-term T-cell immune response, and applying them each to a mathematical model. Additionally, the analysis was based on the fact that although breakthrough infection may occur frequently, the immune response of the patient will be boosted after recovery from each breakthrough infection.
The results showed that in an environment with a high vaccination rate, although COVID-19 cases may rise temporarily when the transmission rate increases, the ratio of critical cases would ultimately decline, thereby decreasing the total number of critical cases and in fact settling COVID-19 as a mild respiratory disease more quickly.
Conditions in which the number of cases may spike include relaxing social distancing measures or the rise of variants with higher transmission rates like the Omicron variant. This research did not take the less virulent characteristic of the Omicron variant into account but focused on the results of its high transmission rate, thereby predicting what may happen in the process of the endemic transition of COVID-19.
The research team pointed out the limitations of their mathematical model, such as the lack of consideration for age or patients with underlying diseases, and explained that the results of this study must be applied with care when compared against high-risk groups. Additionally, as medical systems may collapse when the number of cases rises sharply, this study must be interpreted with prudence and applied accordingly. The research team therefore emphasized that for policies that encourage a step-wise return to normality to succeed, the sustainable maintenance of public health systems is indispensable.
Professor Kim said, “We have drawn a counter-intuitive conclusion amid the unpredictable pandemic through an adequate mathematical model,” asserting the importance of applying mathematical models to medical research.
Professor Shin said, “Although the Omicron variant has become the dominant strain and the number of cases is rising rapidly in South Korea, it is important to use scientific approaches to predict the future and apply them to policies rather than fearing the current situation.”
The results of the research were published on medRxiv.org on February 11, under the title “Increasing viral transmission paradoxically reduces progression rates to severe COVID-19 during endemic transition.”
This research was funded by the Institute of Basic Science, the Korea Health Industry Development Institute, and the National Research Foundation of Korea.
-PublicationHyukpyo Hong, Ji Yun Noh, Hyojung Lee, Sunhwa Choi, Boseung Choi, Jae Kyung Kim, Eui-Cheol Shin, “Increasing viral transmission paradoxically reduces progression rates to
severe COVID-19 during endemic transition,” medRxiv, February 9, 2022 (doi.org/10.1101/2022.02.09.22270633)
-ProfileProfessor Jae Kyung KimDepartment of Mathematical SciencesKAIST
Professor Eui-Cheol ShinGraduate School of Medical Science and EngineeringKAIST
2022.02.22 View 11982
A Mathematical Model Shows High Viral Transmissions Reduce the Progression Rates for Severe Covid-19
The model suggests a clue as to when a pandemic will turn into an endemic
A mathematical model demonstrated that high transmission rates among highly vaccinated populations of COVID-19 ultimately reduce the numbers of severe cases. This model suggests a clue as to when this pandemic will turn into an endemic.
With the future of the pandemic remaining uncertain, a research team of mathematicians and medical scientists analyzed a mathematical model that may predict how the changing transmission rate of COVID-19 would affect the settlement process of the virus as a mild respiratory virus.
The team led by Professor Jae Kyoung Kim from the Department of Mathematical Science and Professor Eui-Cheol Shin from the Graduate School of Medical Science and Engineering used a new approach by dividing the human immune responses to SARS-CoV-2 into a shorter-term neutralizing antibody response and a longer-term T-cell immune response, and applying them each to a mathematical model. Additionally, the analysis was based on the fact that although breakthrough infection may occur frequently, the immune response of the patient will be boosted after recovery from each breakthrough infection.
The results showed that in an environment with a high vaccination rate, although COVID-19 cases may rise temporarily when the transmission rate increases, the ratio of critical cases would ultimately decline, thereby decreasing the total number of critical cases and in fact settling COVID-19 as a mild respiratory disease more quickly.
Conditions in which the number of cases may spike include relaxing social distancing measures or the rise of variants with higher transmission rates like the Omicron variant. This research did not take the less virulent characteristic of the Omicron variant into account but focused on the results of its high transmission rate, thereby predicting what may happen in the process of the endemic transition of COVID-19.
The research team pointed out the limitations of their mathematical model, such as the lack of consideration for age or patients with underlying diseases, and explained that the results of this study must be applied with care when compared against high-risk groups. Additionally, as medical systems may collapse when the number of cases rises sharply, this study must be interpreted with prudence and applied accordingly. The research team therefore emphasized that for policies that encourage a step-wise return to normality to succeed, the sustainable maintenance of public health systems is indispensable.
Professor Kim said, “We have drawn a counter-intuitive conclusion amid the unpredictable pandemic through an adequate mathematical model,” asserting the importance of applying mathematical models to medical research.
Professor Shin said, “Although the Omicron variant has become the dominant strain and the number of cases is rising rapidly in South Korea, it is important to use scientific approaches to predict the future and apply them to policies rather than fearing the current situation.”
The results of the research were published on medRxiv.org on February 11, under the title “Increasing viral transmission paradoxically reduces progression rates to severe COVID-19 during endemic transition.”
This research was funded by the Institute of Basic Science, the Korea Health Industry Development Institute, and the National Research Foundation of Korea.
-PublicationHyukpyo Hong, Ji Yun Noh, Hyojung Lee, Sunhwa Choi, Boseung Choi, Jae Kyung Kim, Eui-Cheol Shin, “Increasing viral transmission paradoxically reduces progression rates to
severe COVID-19 during endemic transition,” medRxiv, February 9, 2022 (doi.org/10.1101/2022.02.09.22270633)
-ProfileProfessor Jae Kyung KimDepartment of Mathematical SciencesKAIST
Professor Eui-Cheol ShinGraduate School of Medical Science and EngineeringKAIST
2022.02.22 View 11982 -
 Five Projects Ranked in the Top 100 for National R&D Excellence
Five KAIST research projects were selected as the 2021 Top 100 for National R&D Excellence by the Ministry of Science and ICT and the Korea Institute of Science & Technology Evaluation and Planning.
The five projects are:-The development of E. coli that proliferates with only formic acid and carbon dioxide by Distinguished Professor Sang Yup Lee from the Department of Chemical and Biomolecular Engineering
-An original reverse aging technology that restores an old human skin cell into a younger one by Professor Kwang-Hyun Cho from the Department of Bio and Brain Engineering-The development of next-generation high-efficiency perovskite-silicon tandem solar cells by Professor Byungha Shin from the Department of Materials Science and Engineering-Research on the effects of ultrafine dust in the atmosphere has on energy consumption by Professor Jiyong Eom from the School of Business and Technology Management-Research on a molecular trigger that controls the phase transformation of bio materials by Professor Myungchul Kim from the Department of Bio and Brain Engineering
Started in 2006, an Evaluation Committee composed of experts in industries, universities, and research institutes has made the preliminary selections of the most outstanding research projects based on their significance as a scientific and technological development and their socioeconomic effects. The finalists went through an open public evaluation. The final 100 studies are from six fields: 18 from mechanics & materials, 26 from biology & marine sciences, 19 from ICT & electronics, 10 from interdisciplinary research, and nine from natural science and infrastructure.
The selected 100 studies will receive a certificate and an award plaque from the minister of MSIT as well as additional points for business and institutional evaluations according to appropriate regulations, and the selected researchers will be strongly recommended as candidates for national meritorious awards.
In particular, to help the 100 selected research projects become more accessible for the general public, their main contents will be provided in a free e-book ‘The Top 100 for National R&D Excellence of 2021’ that will be available from online booksellers.
2022.02.17 View 12061
Five Projects Ranked in the Top 100 for National R&D Excellence
Five KAIST research projects were selected as the 2021 Top 100 for National R&D Excellence by the Ministry of Science and ICT and the Korea Institute of Science & Technology Evaluation and Planning.
The five projects are:-The development of E. coli that proliferates with only formic acid and carbon dioxide by Distinguished Professor Sang Yup Lee from the Department of Chemical and Biomolecular Engineering
-An original reverse aging technology that restores an old human skin cell into a younger one by Professor Kwang-Hyun Cho from the Department of Bio and Brain Engineering-The development of next-generation high-efficiency perovskite-silicon tandem solar cells by Professor Byungha Shin from the Department of Materials Science and Engineering-Research on the effects of ultrafine dust in the atmosphere has on energy consumption by Professor Jiyong Eom from the School of Business and Technology Management-Research on a molecular trigger that controls the phase transformation of bio materials by Professor Myungchul Kim from the Department of Bio and Brain Engineering
Started in 2006, an Evaluation Committee composed of experts in industries, universities, and research institutes has made the preliminary selections of the most outstanding research projects based on their significance as a scientific and technological development and their socioeconomic effects. The finalists went through an open public evaluation. The final 100 studies are from six fields: 18 from mechanics & materials, 26 from biology & marine sciences, 19 from ICT & electronics, 10 from interdisciplinary research, and nine from natural science and infrastructure.
The selected 100 studies will receive a certificate and an award plaque from the minister of MSIT as well as additional points for business and institutional evaluations according to appropriate regulations, and the selected researchers will be strongly recommended as candidates for national meritorious awards.
In particular, to help the 100 selected research projects become more accessible for the general public, their main contents will be provided in a free e-book ‘The Top 100 for National R&D Excellence of 2021’ that will be available from online booksellers.
2022.02.17 View 12061 -
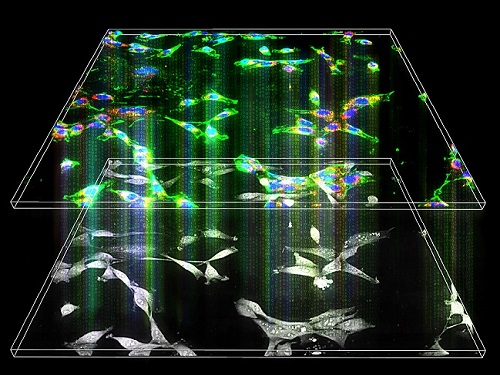 Label-Free Multiplexed Microtomography of Endogenous Subcellular Dynamics Using Deep Learning
AI-based holographic microscopy allows molecular imaging without introducing exogenous labeling agents
A research team upgraded the 3D microtomography observing dynamics of label-free live cells in multiplexed fluorescence imaging. The AI-powered 3D holotomographic microscopy extracts various molecular information from live unlabeled biological cells in real time without exogenous labeling or staining agents.
Professor YongKeum Park’s team and the startup Tomocube encoded 3D refractive index tomograms using the refractive index as a means of measurement. Then they decoded the information with a deep learning-based model that infers multiple 3D fluorescence tomograms from the refractive index measurements of the corresponding subcellular targets, thereby achieving multiplexed micro tomography. This study was reported in Nature Cell Biology online on December 7, 2021.
Fluorescence microscopy is the most widely used optical microscopy technique due to its high biochemical specificity. However, it needs to genetically manipulate or to stain cells with fluorescent labels in order to express fluorescent proteins. These labeling processes inevitably affect the intrinsic physiology of cells. It also has challenges in long-term measuring due to photobleaching and phototoxicity. The overlapped spectra of multiplexed fluorescence signals also hinder the viewing of various structures at the same time. More critically, it took several hours to observe the cells after preparing them.
3D holographic microscopy, also known as holotomography, is providing new ways to quantitatively image live cells without pretreatments such as staining. Holotomography can accurately and quickly measure the morphological and structural information of cells, but only provides limited biochemical and molecular information.
The 'AI microscope' created in this process takes advantage of the features of both holographic microscopy and fluorescence microscopy. That is, a specific image from a fluorescence microscope can be obtained without a fluorescent label. Therefore, the microscope can observe many types of cellular structures in their natural state in 3D and at the same time as fast as one millisecond, and long-term measurements over several days are also possible.
The Tomocube-KAIST team showed that fluorescence images can be directly and precisely predicted from holotomographic images in various cells and conditions. Using the quantitative relationship between the spatial distribution of the refractive index found by AI and the major structures in cells, it was possible to decipher the spatial distribution of the refractive index. And surprisingly, it confirmed that this relationship is constant regardless of cell type.
Professor Park said, “We were able to develop a new concept microscope that combines the advantages of several microscopes with the multidisciplinary research of AI, optics, and biology. It will be immediately applicable for new types of cells not included in the existing data and is expected to be widely applicable for various biological and medical research.”
When comparing the molecular image information extracted by AI with the molecular image information physically obtained by fluorescence staining in 3D space, it showed a 97% or more conformity, which is a level that is difficult to distinguish with the naked eye.
“Compared to the sub-60% accuracy of the fluorescence information extracted from the model developed by the Google AI team, it showed significantly higher performance,” Professor Park added.
This work was supported by the KAIST Up program, the BK21+ program, Tomocube, the National Research Foundation of Korea, and the Ministry of Science and ICT, and the Ministry of Health & Welfare.
-Publication
Hyun-seok Min, Won-Do Heo, YongKeun Park, et al. “Label-free multiplexed microtomography of endogenous subcellular dynamics using generalizable deep learning,” Nature Cell Biology (doi.org/10.1038/s41556-021-00802-x) published online December 07 2021.
-Profile
Professor YongKeun Park
Biomedical Optics Laboratory
Department of Physics
KAIST
2022.02.09 View 12762
Label-Free Multiplexed Microtomography of Endogenous Subcellular Dynamics Using Deep Learning
AI-based holographic microscopy allows molecular imaging without introducing exogenous labeling agents
A research team upgraded the 3D microtomography observing dynamics of label-free live cells in multiplexed fluorescence imaging. The AI-powered 3D holotomographic microscopy extracts various molecular information from live unlabeled biological cells in real time without exogenous labeling or staining agents.
Professor YongKeum Park’s team and the startup Tomocube encoded 3D refractive index tomograms using the refractive index as a means of measurement. Then they decoded the information with a deep learning-based model that infers multiple 3D fluorescence tomograms from the refractive index measurements of the corresponding subcellular targets, thereby achieving multiplexed micro tomography. This study was reported in Nature Cell Biology online on December 7, 2021.
Fluorescence microscopy is the most widely used optical microscopy technique due to its high biochemical specificity. However, it needs to genetically manipulate or to stain cells with fluorescent labels in order to express fluorescent proteins. These labeling processes inevitably affect the intrinsic physiology of cells. It also has challenges in long-term measuring due to photobleaching and phototoxicity. The overlapped spectra of multiplexed fluorescence signals also hinder the viewing of various structures at the same time. More critically, it took several hours to observe the cells after preparing them.
3D holographic microscopy, also known as holotomography, is providing new ways to quantitatively image live cells without pretreatments such as staining. Holotomography can accurately and quickly measure the morphological and structural information of cells, but only provides limited biochemical and molecular information.
The 'AI microscope' created in this process takes advantage of the features of both holographic microscopy and fluorescence microscopy. That is, a specific image from a fluorescence microscope can be obtained without a fluorescent label. Therefore, the microscope can observe many types of cellular structures in their natural state in 3D and at the same time as fast as one millisecond, and long-term measurements over several days are also possible.
The Tomocube-KAIST team showed that fluorescence images can be directly and precisely predicted from holotomographic images in various cells and conditions. Using the quantitative relationship between the spatial distribution of the refractive index found by AI and the major structures in cells, it was possible to decipher the spatial distribution of the refractive index. And surprisingly, it confirmed that this relationship is constant regardless of cell type.
Professor Park said, “We were able to develop a new concept microscope that combines the advantages of several microscopes with the multidisciplinary research of AI, optics, and biology. It will be immediately applicable for new types of cells not included in the existing data and is expected to be widely applicable for various biological and medical research.”
When comparing the molecular image information extracted by AI with the molecular image information physically obtained by fluorescence staining in 3D space, it showed a 97% or more conformity, which is a level that is difficult to distinguish with the naked eye.
“Compared to the sub-60% accuracy of the fluorescence information extracted from the model developed by the Google AI team, it showed significantly higher performance,” Professor Park added.
This work was supported by the KAIST Up program, the BK21+ program, Tomocube, the National Research Foundation of Korea, and the Ministry of Science and ICT, and the Ministry of Health & Welfare.
-Publication
Hyun-seok Min, Won-Do Heo, YongKeun Park, et al. “Label-free multiplexed microtomography of endogenous subcellular dynamics using generalizable deep learning,” Nature Cell Biology (doi.org/10.1038/s41556-021-00802-x) published online December 07 2021.
-Profile
Professor YongKeun Park
Biomedical Optics Laboratory
Department of Physics
KAIST
2022.02.09 View 12762 -
 Thermal Superconductor Lab Becomes the 7th Cross-Generation Collaborative Lab
The Thermal Superconductor Lab led by Senior Professor Sung Jin Kim from the Department of Mechanical Engineering will team up with Junior Professor Youngsuk Nam to develop next-generation superconductors. The two professor team was selected as the 7th Cross-Generation Collaborative Lab last week and will sustain the academic legacy of Professor Kim’s three decades of research on superconductors. The team will continue to develop thin, next-generation superconductors that carry super thermal conductivity using phase transition control technology and thin film packaging.
Thin-filmed, next-generation superconductors can be used in various high-temperature flexible electronic devices. The superconductors built inside of the semiconductor device packages will also be used for managing the low-powered but high-performance temperatures of semiconductor and electronic equipment.
Professor Kim said, “I am very pleased that my research, know-how, and knowledge from over 30 years of work will continue through the Cross-Generation Collaborative Lab system with Professor Nam. We will spare no effort to advance superconductor technology and play a part in KAIST leading global technology fields.” Junior Professor Nam also stressed that the team is excited to continue its research on crucial technology for managing the temperatures of semiconductors and other electronic equipment.
KAIST started this innovative research system in 2018, and in 2021 it established the steering committee to select new labs based on: originality, differentiation, and excellence; academic, social, economic impact; the urgency of cross-generation research; the senior professor’s academic excellence and international reputation; and the senior professor’s research vision. Selected labs receive 500 million KRW in research funding over five years.
2022.01.27 View 7360
Thermal Superconductor Lab Becomes the 7th Cross-Generation Collaborative Lab
The Thermal Superconductor Lab led by Senior Professor Sung Jin Kim from the Department of Mechanical Engineering will team up with Junior Professor Youngsuk Nam to develop next-generation superconductors. The two professor team was selected as the 7th Cross-Generation Collaborative Lab last week and will sustain the academic legacy of Professor Kim’s three decades of research on superconductors. The team will continue to develop thin, next-generation superconductors that carry super thermal conductivity using phase transition control technology and thin film packaging.
Thin-filmed, next-generation superconductors can be used in various high-temperature flexible electronic devices. The superconductors built inside of the semiconductor device packages will also be used for managing the low-powered but high-performance temperatures of semiconductor and electronic equipment.
Professor Kim said, “I am very pleased that my research, know-how, and knowledge from over 30 years of work will continue through the Cross-Generation Collaborative Lab system with Professor Nam. We will spare no effort to advance superconductor technology and play a part in KAIST leading global technology fields.” Junior Professor Nam also stressed that the team is excited to continue its research on crucial technology for managing the temperatures of semiconductors and other electronic equipment.
KAIST started this innovative research system in 2018, and in 2021 it established the steering committee to select new labs based on: originality, differentiation, and excellence; academic, social, economic impact; the urgency of cross-generation research; the senior professor’s academic excellence and international reputation; and the senior professor’s research vision. Selected labs receive 500 million KRW in research funding over five years.
2022.01.27 View 7360 -
 Eco-Friendly Micro-Supercapacitors Using Fallen Leaves
Green micro-supercapacitors on a single leaf could easily be applied in wearable electronics, smart houses, and IoTs
A KAIST research team has developed graphene-inorganic-hybrid micro-supercapacitors made of fallen leaves using femtosecond laser direct writing. The rapid development of wearable electronics requires breakthrough innovations in flexible energy storage devices in which micro-supercapacitors have drawn a great deal of interest due to their high power density, long lifetimes, and short charging times. Recently, there has been an enormous increase in waste batteries owing to the growing demand and the shortened replacement cycle in consumer electronics. The safety and environmental issues involved in the collection, recycling, and processing of such waste batteries are creating a number of challenges.
Forests cover about 30 percent of the Earth’s surface and produce a huge amount of fallen leaves. This naturally occurring biomass comes in large quantities and is completely biodegradable, which makes it an attractive sustainable resource. Nevertheless, if the fallen leaves are left neglected instead of being used efficiently, they can contribute to fire hazards, air pollution, and global warming.
To solve both problems at once, a research team led by Professor Young-Jin Kim from the Department of Mechanical Engineering and Dr. Hana Yoon from the Korea Institute of Energy Research developed a novel technology that can create 3D porous graphene microelectrodes with high electrical conductivity by irradiating femtosecond laser pulses on the leaves in ambient air. This one-step fabrication does not require any additional materials or pre-treatment.
They showed that this technique could quickly and easily produce porous graphene electrodes at a low price, and demonstrated potential applications by fabricating graphene micro-supercapacitors to power an LED and an electronic watch. These results open up a new possibility for the mass production of flexible and green graphene-based electronic devices.
Professor Young-Jin Kim said, “Leaves create forest biomass that comes in unmanageable quantities, so using them for next-generation energy storage devices makes it possible for us to reuse waste resources, thereby establishing a virtuous cycle.”
This research was published in Advanced Functional Materials last month and was sponsored by the Ministry of Agriculture Food and Rural Affairs, the Korea Forest Service, and the Korea Institute of Energy Research.
-Publication
Truong-Son Dinh Le, Yeong A. Lee, Han Ku Nam, Kyu Yeon Jang, Dongwook Yang, Byunggi Kim, Kanghoon Yim, Seung Woo Kim, Hana Yoon, and Young-jin Kim, “Green Flexible Graphene-Inorganic-Hybrid Micro-Supercapacitors Made of Fallen Leaves Enabled by Ultrafast Laser Pulses," December 05, 2021, Advanced Functional Materials (doi.org/10.1002/adfm.202107768)
-ProfileProfessor Young-Jin KimUltra-Precision Metrology and Manufacturing (UPM2) LaboratoryDepartment of Mechanical EngineeringKAIST
2022.01.27 View 14043
Eco-Friendly Micro-Supercapacitors Using Fallen Leaves
Green micro-supercapacitors on a single leaf could easily be applied in wearable electronics, smart houses, and IoTs
A KAIST research team has developed graphene-inorganic-hybrid micro-supercapacitors made of fallen leaves using femtosecond laser direct writing. The rapid development of wearable electronics requires breakthrough innovations in flexible energy storage devices in which micro-supercapacitors have drawn a great deal of interest due to their high power density, long lifetimes, and short charging times. Recently, there has been an enormous increase in waste batteries owing to the growing demand and the shortened replacement cycle in consumer electronics. The safety and environmental issues involved in the collection, recycling, and processing of such waste batteries are creating a number of challenges.
Forests cover about 30 percent of the Earth’s surface and produce a huge amount of fallen leaves. This naturally occurring biomass comes in large quantities and is completely biodegradable, which makes it an attractive sustainable resource. Nevertheless, if the fallen leaves are left neglected instead of being used efficiently, they can contribute to fire hazards, air pollution, and global warming.
To solve both problems at once, a research team led by Professor Young-Jin Kim from the Department of Mechanical Engineering and Dr. Hana Yoon from the Korea Institute of Energy Research developed a novel technology that can create 3D porous graphene microelectrodes with high electrical conductivity by irradiating femtosecond laser pulses on the leaves in ambient air. This one-step fabrication does not require any additional materials or pre-treatment.
They showed that this technique could quickly and easily produce porous graphene electrodes at a low price, and demonstrated potential applications by fabricating graphene micro-supercapacitors to power an LED and an electronic watch. These results open up a new possibility for the mass production of flexible and green graphene-based electronic devices.
Professor Young-Jin Kim said, “Leaves create forest biomass that comes in unmanageable quantities, so using them for next-generation energy storage devices makes it possible for us to reuse waste resources, thereby establishing a virtuous cycle.”
This research was published in Advanced Functional Materials last month and was sponsored by the Ministry of Agriculture Food and Rural Affairs, the Korea Forest Service, and the Korea Institute of Energy Research.
-Publication
Truong-Son Dinh Le, Yeong A. Lee, Han Ku Nam, Kyu Yeon Jang, Dongwook Yang, Byunggi Kim, Kanghoon Yim, Seung Woo Kim, Hana Yoon, and Young-jin Kim, “Green Flexible Graphene-Inorganic-Hybrid Micro-Supercapacitors Made of Fallen Leaves Enabled by Ultrafast Laser Pulses," December 05, 2021, Advanced Functional Materials (doi.org/10.1002/adfm.202107768)
-ProfileProfessor Young-Jin KimUltra-Precision Metrology and Manufacturing (UPM2) LaboratoryDepartment of Mechanical EngineeringKAIST
2022.01.27 View 14043