plastic
-
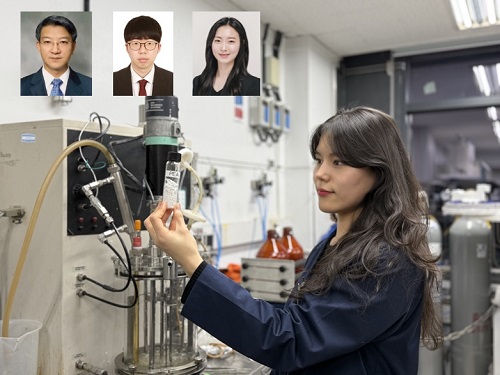 KAIST Develops Eco-Friendly, Nylon-Like Plastic Using Microorganisms
Poly(ester amide) amide is a next-generation material that combines the advantages of PET (polyester) and nylon (polyamide), two widely used plastics. However, it could only be produced from fossil fuels, which posed environmental concerns. Using microorganisms, KAIST researchers have successfully developed a new bio-based plastic to replace conventional plastic.
KAIST (represented by President Kwang Hyung Lee) announced on the 20th of March that a research team led by Distinguished Professor Sang Yup Lee from the Department of Chemical and Biomolecular Engineering has developed microbial strains through systems metabolic engineering to produce various eco-friendly, bio-based poly(ester amide)s. The team collaborated with researchers from the Korea Research Institute of Chemical Technology (KRICT, President Young-Kook Lee) to analyze and confirm the properties of the resulting plastic.
Professor Sang Yup Lee’s research team designed new metabolic pathways that do not naturally exist in microorganisms, and developed a platform microbial strain capable of producing nine different types of poly(ester amide)s, including poly(3-hydroxybutyrate-ran-3-aminopropionate) and poly(3-hydroxybutyrate-ran-4-aminobutyrate).
Using glucose derived from abundant biomass sources such as waste wood and weeds, the team successfully produced poly(ester amide)s in an eco-friendly manner. The researchers also confirmed the potential for industrial-scale production by demonstrating high production efficiency (54.57 g/L) using fed-batch fermentation of the engineered strain.
In collaboration with researchers Haemin Jeong and Jihoon Shin from KRICT, the KAIST team analyzed the properties of the bio-based plastic and found that it exhibited characteristics similar to high-density polyethylene (HDPE). This means the new plastic is not only eco-friendly but also strong and durable enough to replace conventional plastics.
The engineered strains and strategies developed in this study are expected to be useful not only for producing various poly(ester amide)s but also for constructing metabolic pathways for the biosynthesis of other types of polymers.
Professor Sang Yup Lee stated, “This study is the first to demonstrate the possibility of producing poly(ester amide)s (plastics) through a renewable bio-based chemical process rather than relying on the petroleum-based chemical industry. We plan to further enhance the production yield and efficiency through continued research.”
The study was published online on March 17 in the international journal Nature Chemical Biology.
·Title: Biosynthesis of poly(ester amide)s in engineered Escherichia coli
·DOI: 10.1038/s41589-025-01842-2
·Authors: A total of seven authors including Tong Un Chae (KAIST, first author), So Young Choi (KAIST, second author), Da-Hee Ahn (KAIST, third author), Woo Dae Jang (KAIST, fourth author), Haemin Jeong (KRICT, fifth author), Jihoon Shin (KRICT, sixth author), and Sang Yup Lee (KAIST, corresponding author).
This research was supported by the Ministry of Science and ICT (MSIT) under the Eco-Friendly Chemical Technology Development Project as part of the "Next-Generation Biorefinery Technology Development to Lead the Bio-Chemical Industry" initiative (project led by Distinguished Professor Sang Yup Lee at KAIST).
2025.03.24 View 4663
KAIST Develops Eco-Friendly, Nylon-Like Plastic Using Microorganisms
Poly(ester amide) amide is a next-generation material that combines the advantages of PET (polyester) and nylon (polyamide), two widely used plastics. However, it could only be produced from fossil fuels, which posed environmental concerns. Using microorganisms, KAIST researchers have successfully developed a new bio-based plastic to replace conventional plastic.
KAIST (represented by President Kwang Hyung Lee) announced on the 20th of March that a research team led by Distinguished Professor Sang Yup Lee from the Department of Chemical and Biomolecular Engineering has developed microbial strains through systems metabolic engineering to produce various eco-friendly, bio-based poly(ester amide)s. The team collaborated with researchers from the Korea Research Institute of Chemical Technology (KRICT, President Young-Kook Lee) to analyze and confirm the properties of the resulting plastic.
Professor Sang Yup Lee’s research team designed new metabolic pathways that do not naturally exist in microorganisms, and developed a platform microbial strain capable of producing nine different types of poly(ester amide)s, including poly(3-hydroxybutyrate-ran-3-aminopropionate) and poly(3-hydroxybutyrate-ran-4-aminobutyrate).
Using glucose derived from abundant biomass sources such as waste wood and weeds, the team successfully produced poly(ester amide)s in an eco-friendly manner. The researchers also confirmed the potential for industrial-scale production by demonstrating high production efficiency (54.57 g/L) using fed-batch fermentation of the engineered strain.
In collaboration with researchers Haemin Jeong and Jihoon Shin from KRICT, the KAIST team analyzed the properties of the bio-based plastic and found that it exhibited characteristics similar to high-density polyethylene (HDPE). This means the new plastic is not only eco-friendly but also strong and durable enough to replace conventional plastics.
The engineered strains and strategies developed in this study are expected to be useful not only for producing various poly(ester amide)s but also for constructing metabolic pathways for the biosynthesis of other types of polymers.
Professor Sang Yup Lee stated, “This study is the first to demonstrate the possibility of producing poly(ester amide)s (plastics) through a renewable bio-based chemical process rather than relying on the petroleum-based chemical industry. We plan to further enhance the production yield and efficiency through continued research.”
The study was published online on March 17 in the international journal Nature Chemical Biology.
·Title: Biosynthesis of poly(ester amide)s in engineered Escherichia coli
·DOI: 10.1038/s41589-025-01842-2
·Authors: A total of seven authors including Tong Un Chae (KAIST, first author), So Young Choi (KAIST, second author), Da-Hee Ahn (KAIST, third author), Woo Dae Jang (KAIST, fourth author), Haemin Jeong (KRICT, fifth author), Jihoon Shin (KRICT, sixth author), and Sang Yup Lee (KAIST, corresponding author).
This research was supported by the Ministry of Science and ICT (MSIT) under the Eco-Friendly Chemical Technology Development Project as part of the "Next-Generation Biorefinery Technology Development to Lead the Bio-Chemical Industry" initiative (project led by Distinguished Professor Sang Yup Lee at KAIST).
2025.03.24 View 4663 -
 Formosa Group of Taiwan to Establish Bio R&D Center at KAIST Investing 12.5 M USD
KAIST (President Kwang-Hyung Lee) announced on February 17th that it signed an agreement for cooperation in the bio-medical field with Formosa Group, one of the three largest companies in Taiwan.
< Formosa Group Chairman Sandy Wang and KAIST President Kwang-Hyung Lee at the signing ceremony >
Formosa Group Executive Committee member and Chairman Sandy Wang, who leads the group's bio and eco-friendly energy sectors, decided to establish a bio-medical research center within KAIST and invest approximately KRW 18 billion or more over 5 years. In addition, to commercialize the research results, KAIST and Formosa Group will establish a joint venture in Korea with KAIST Holdings, a KAIST-funded company.
The cooperation between the two organizations began in early 2023 when KAIST signed a comprehensive exchange and cooperation agreement (MOU) with Ming Chi University of Science and Technology (明志科技大學), Chang Gung University (長庚大學), and Chang Gung Memorial Hospital (長庚記念醫院), which are established and supported by Formosa Group. Afterwards, Chairman Sandy Wang visited KAIST in May 2024 and signed a more specific business agreement (MOA).
KAIST Holdings is a holding company established by KAIST, a government-funded organization, to attract investment and conduct business, and will pursue the establishment of a joint venture with a 50:50 equity structure in cooperation with Formosa Group. KAIST Holdings will invest KAIST’s intellectual property rights, and Formosa Group will invest a corresponding amount of funds.
The KAIST-Formosa joint venture will provide research funds to the KAIST-Formosa Bio-Medical Research Center to be established in the future, secure the right to implement the intellectual property rights generated, and promote full-scale business.
The KAIST-Formosa Bio-Medical Research Center will establish a ‘brain organoid bank’ created by obtaining tissues from hundreds of patients with degenerative brain diseases, thereby securing high-dimensional data that will reveal the fundamental causes of aging and disease. It is expected that KAIST’s world-class artificial intelligence technology will analyze large-scale patient data to find the causes of aging and disease.
Through this business, it is expected that by 2030, five years from now, it will discover more than 10 types of intractable brain disease treatments and expand to more than 20 businesses, including human cell-centered diagnostics and preclinical businesses, and secure infrastructure and intellectual property rights that can create value worth approximately KRW 250 billion.
The Chang Gung Memorial Hospital in Taiwan has 10,000 beds and handles 35,000 patients per day, and systematically accumulates patient tissue and clinical data. Chang Gung Memorial Hospital will differentiate the tissues of patients with degenerative brain diseases and send them to the KAIST-Formosa Bio-Medical Research Center, which will then produce brain organoids to be used for disease research and new drug development. This will allow the world’s largest patient tissue data bank to be established.
Dean Daesoo Kim of the College of Life Science and Bioengineering at KAIST said, “This collaboration between KAIST and Formosa Group is a new research collaboration model that goes beyond joint research to establish a joint venture and global commercialization of developed technologies, and it is significant in that it can serve as an opportunity to promote biomedical research and development.”
With this agreement, KAIST, which has been promoting the KAIST Advanced Regenerative Medicine Engineering Center in Osong K-Bio Square, has secured a practical global partner.
< Representatives of the Formosa Group and KAIST >
KAIST’s Senior Vice President for Planning and Budget, Professor Kyung-Soo Kim emphasized, “KAIST has made great efforts to secure an edge in state-of-the-art biomedical fields such as stem cells and gene editing technology, by attracting the world’s best experts and discovering global cooperation partners, and these results can ultimately be linked to the Osong K-Bio Square project.”
SVP Kim then predicted, “In particular, the practical cooperation with Taiwan’s best Formosa Chang Gung Memorial Hospital, which has abundant clinical experience in stem cell treatment, will be an important axis of KAIST’s bio innovation strategy.”
Formosa Chairman Sandy Wang emphasized that this investment and cooperation is built on trust in KAIST’s R&D capabilities and the passion of its researchers. And added that through this, the Formosa Group will practice corporate social responsibility and take an important first step together with KAIST to protect the welfare and health of humanity. She also went on the say that she expects to see the cooperation expanded to various fields such as mobility and semiconductors based on the successes begotten from the cooperation in the bio field.
KAIST President Kwang-Hyung Lee said, “I evaluate this agreement as one of the most important events that will spearhead KAIST into overseas biotechnology stages,” and added, “I expect that this cooperation will be an opportunity for Taiwan and Korea, both of which have IT industry-centered structures, to create new growth engines in the bio industry.” Meanwhile, Formosa Group is a company founded by Chairman Sandy Wang’s father, Chairman Yung-Ching Wang. It is the world’s No. 1 plastic PVC producer and is leading core industries of the Taiwanese economy, including semiconductors, steel, heavy industry, bio, and batteries. Chairman Yung-Ching Wang was respected by the Taiwanese people for his exemplary return of wealth to society under the belief that the companies and assets he founded “belong to the people.”
2025.02.17 View 3746
Formosa Group of Taiwan to Establish Bio R&D Center at KAIST Investing 12.5 M USD
KAIST (President Kwang-Hyung Lee) announced on February 17th that it signed an agreement for cooperation in the bio-medical field with Formosa Group, one of the three largest companies in Taiwan.
< Formosa Group Chairman Sandy Wang and KAIST President Kwang-Hyung Lee at the signing ceremony >
Formosa Group Executive Committee member and Chairman Sandy Wang, who leads the group's bio and eco-friendly energy sectors, decided to establish a bio-medical research center within KAIST and invest approximately KRW 18 billion or more over 5 years. In addition, to commercialize the research results, KAIST and Formosa Group will establish a joint venture in Korea with KAIST Holdings, a KAIST-funded company.
The cooperation between the two organizations began in early 2023 when KAIST signed a comprehensive exchange and cooperation agreement (MOU) with Ming Chi University of Science and Technology (明志科技大學), Chang Gung University (長庚大學), and Chang Gung Memorial Hospital (長庚記念醫院), which are established and supported by Formosa Group. Afterwards, Chairman Sandy Wang visited KAIST in May 2024 and signed a more specific business agreement (MOA).
KAIST Holdings is a holding company established by KAIST, a government-funded organization, to attract investment and conduct business, and will pursue the establishment of a joint venture with a 50:50 equity structure in cooperation with Formosa Group. KAIST Holdings will invest KAIST’s intellectual property rights, and Formosa Group will invest a corresponding amount of funds.
The KAIST-Formosa joint venture will provide research funds to the KAIST-Formosa Bio-Medical Research Center to be established in the future, secure the right to implement the intellectual property rights generated, and promote full-scale business.
The KAIST-Formosa Bio-Medical Research Center will establish a ‘brain organoid bank’ created by obtaining tissues from hundreds of patients with degenerative brain diseases, thereby securing high-dimensional data that will reveal the fundamental causes of aging and disease. It is expected that KAIST’s world-class artificial intelligence technology will analyze large-scale patient data to find the causes of aging and disease.
Through this business, it is expected that by 2030, five years from now, it will discover more than 10 types of intractable brain disease treatments and expand to more than 20 businesses, including human cell-centered diagnostics and preclinical businesses, and secure infrastructure and intellectual property rights that can create value worth approximately KRW 250 billion.
The Chang Gung Memorial Hospital in Taiwan has 10,000 beds and handles 35,000 patients per day, and systematically accumulates patient tissue and clinical data. Chang Gung Memorial Hospital will differentiate the tissues of patients with degenerative brain diseases and send them to the KAIST-Formosa Bio-Medical Research Center, which will then produce brain organoids to be used for disease research and new drug development. This will allow the world’s largest patient tissue data bank to be established.
Dean Daesoo Kim of the College of Life Science and Bioengineering at KAIST said, “This collaboration between KAIST and Formosa Group is a new research collaboration model that goes beyond joint research to establish a joint venture and global commercialization of developed technologies, and it is significant in that it can serve as an opportunity to promote biomedical research and development.”
With this agreement, KAIST, which has been promoting the KAIST Advanced Regenerative Medicine Engineering Center in Osong K-Bio Square, has secured a practical global partner.
< Representatives of the Formosa Group and KAIST >
KAIST’s Senior Vice President for Planning and Budget, Professor Kyung-Soo Kim emphasized, “KAIST has made great efforts to secure an edge in state-of-the-art biomedical fields such as stem cells and gene editing technology, by attracting the world’s best experts and discovering global cooperation partners, and these results can ultimately be linked to the Osong K-Bio Square project.”
SVP Kim then predicted, “In particular, the practical cooperation with Taiwan’s best Formosa Chang Gung Memorial Hospital, which has abundant clinical experience in stem cell treatment, will be an important axis of KAIST’s bio innovation strategy.”
Formosa Chairman Sandy Wang emphasized that this investment and cooperation is built on trust in KAIST’s R&D capabilities and the passion of its researchers. And added that through this, the Formosa Group will practice corporate social responsibility and take an important first step together with KAIST to protect the welfare and health of humanity. She also went on the say that she expects to see the cooperation expanded to various fields such as mobility and semiconductors based on the successes begotten from the cooperation in the bio field.
KAIST President Kwang-Hyung Lee said, “I evaluate this agreement as one of the most important events that will spearhead KAIST into overseas biotechnology stages,” and added, “I expect that this cooperation will be an opportunity for Taiwan and Korea, both of which have IT industry-centered structures, to create new growth engines in the bio industry.” Meanwhile, Formosa Group is a company founded by Chairman Sandy Wang’s father, Chairman Yung-Ching Wang. It is the world’s No. 1 plastic PVC producer and is leading core industries of the Taiwanese economy, including semiconductors, steel, heavy industry, bio, and batteries. Chairman Yung-Ching Wang was respected by the Taiwanese people for his exemplary return of wealth to society under the belief that the companies and assets he founded “belong to the people.”
2025.02.17 View 3746 -
 Novel High-performance and Sustainable Paper Coating Material created by KAIST-Yonsei University Research Team to reduce microplastic pollution
What if there is a biodegradable packaging material with high performance without leaving microplastics?
Plastic pollution presents a global challenge that must be solved. In particular, packaging accounts for 30-50% of the total plastic consumption. While paper packaging is eco-friendly, it lacks crucial functionalities like moisture resistance and strength. Traditional coating materials exacerbate plastic pollution, prompting the need for sustainable alternatives.
Polyethylene (PE) and ethylene vinyl alcohol (EVOH) are typically used as coating materials to improve the low barrier properties of paper packaging, but these substances do not decompose and worsen microplastic pollution when disposed of in the natural environment. In response to this problem, packaging materials made from bio-based substances and biodegradable plastics have been developed, but in most cases, as the packaging performance improves, the biodegradability diminishes rapidly.
KAIST announced that a joint research team led by Professor Jaewook Myung of the Department of Civil and Environmental Engineering, Professor Hanseul Yang of the Department of Life Sciences, and Professor Jongcheol Seo of the Department of Packaging and Logistics <Figure 4. Back cover art of Green Chemistry journal of the latest volume, describing the boric acid cross-linked poly(vinyl alcohol) coated paper featuring marine biodegradability, biocompatibility, high barrier properties, and robustness developed through this study.>
at Yonsei University tackled the challenge of balancing packaging performance and sustainability. They successfully developed a sustainable, marine biodegradable, high-performance paper coating material.
* Biodegradable plastic: A plastic that can be decomposed by microorganisms in natural environments such as soil and ocean or artificial conditions such as industrial composting and anaerobic digestion by microorganisms.
*Microplastics: Tiny pieces of plastic less than 5 mm, produced during the decomposition of bulk plastic materials. Microplastics can persist in the sea for more than decades, causing severe marine pollution.
The team utilized boric acid-crosslinked poly(vinyl alcohol) (PVA), a biodegradable plastic, to coat the paper, thereby enhancing its biodegradability, barrier properties, and strength. The resulting coated paper exhibited superior performance compared to conventional plastics, with excellent barrier properties and physical strength, even in humid conditions.
<Figure 1. (a) Chemical structure of boric acid-crosslinked poly(vinyl alcohol) coating on paper, (b-c) Oxygen and water vapor barrier properties, (d-f) Tensile strength in dry and moist conditions. OTR: Oxygen transmission rate, WVTR: Water vapor transmission rate.>
The team also conducted an in-depth examination of biodegradation and biocompatibility to systematically evaluate the sustainability of the newly developed coated paper. Biodegradation was assessed by simulating the marine environment, known for its challenging biodegradability conditions. The team employed a respiratory system-based bioreactor to measure the degree of carbon mineralization into carbon dioxide. After 111 days of biodegradation, it was found that the coated papers achieved 59-82% biodegradation depending on the coating component. The phenomenon in which marine bacteria are decomposing the coating material was captured through a scanning electron microscope. In addition, in vitro biocompatibility was confirmed through human embryonic kidney and mouse embryonic fibroblast cells, as well as high in-vivo biocompatibility of the coated paper was verified through mouse experiments.
Through this study, the joint research team proposed a coating strategy that can improve packaging performance while upholding sustainability to address the drawbacks of paper packaging. The boric acid-crosslinked PVA-coated paper eliminates the need for artificial composting conditions or sewage treatment facilities. Being biodegradable in natural environments and characterized by low toxicity, this newly coated paper does not exacerbate environmental pollution when accidentally discarded. Thus, it presents a sustainable substitute for plastic packaging materials.
<Figure 2. (a) Normal paper and boric acid-crosslinked poly(vinyl alcohol) coated paper, (b) Biodegradation of the coated paper by marine bacteria, (c) Result of cytotoxicity test using human embryonic kidney and mouse embryonic fibroblast cells. (d) Vital organs after one-month exposure of the coated papers to mice.>
Professor Jaewook Myung at KAIST, who led the sustainability study of coated paper, said, "The development of a marine biodegradable high-performance paper coating is the result of combining the innovative technologies of three leading research teams in each field." He said, “We will continue to develop sustainable materials with excellent performance.” Meanwhile, Professor Jongchul Seo of Yonsei University, who led the research on the development of high-performance paper coating, mentioned, “Through this research, we have developed a sustainable paper packaging technology that can replace non-degradable plastic packaging, and we expect the research outcome will be applied in industry,”.
<Figure 3. End-of-life scenario of papers coated by BA-crosslinked PVA in the marine environment. The coated papers potentially be disintegrated by marine microorganisms and ocean waves and tides. The depolymerization of PVA coating and paper is then mediated by extracellular depolymerases such as oxidases and cellulases, after which the small subunits (oligomers and monomers) are assimilated by microbial cells. The carbon components in the coated papers are ultimately mineralized into CO2, posing no harm in the ocean.>
The work was published in Green Chemistry and Food Chemistry journals. This study was conducted with the support of the Korea Research Foundation and the Korea Institute for Agriculture, Food and Rural Affairs Technology Planning and Evaluation, etc.
*Title of paper published in Green Chemistry: Boric acid-crosslinked poly(vinyl alcohol): biodegradable, biocompatible, robust, and high-barrier paper coating
※ Selected as the article for the back cover of the journal .
- Authors: Shinhyeong Choe, Seulki You, Kitae Park, Youngju Kim, Jehee Park, Yongjun Cho, Jongchul Seo, Hanseul Yang, and Jaewook Myung)
- Date: April 17, 2024
- DOI: 10.1039/D4GC00618F
*Title of paper published in Food Chemistry: Effect of epichlorohydrin treatment on the coating process and performance of high-barrier paper packaging
- Authors: Kitae Park, Shinhyeong Choe, Kambiz Sadeghi, Pradeep Kumar Panda, Jaewook Myung, Dowan Kim, and Jongchul Seo
- Date: February 19, 2024
- DOI: 10.1016/j.foodchem.2024.138772
<Figure 4. Back cover art of Green Chemistry journal of the latest volume, describing the boric acid cross-linked poly(vinyl alcohol) coated paper featuring marine biodegradability, biocompatibility, high barrier properties, and robustness developed through this study.>
2024.05.22 View 8074
Novel High-performance and Sustainable Paper Coating Material created by KAIST-Yonsei University Research Team to reduce microplastic pollution
What if there is a biodegradable packaging material with high performance without leaving microplastics?
Plastic pollution presents a global challenge that must be solved. In particular, packaging accounts for 30-50% of the total plastic consumption. While paper packaging is eco-friendly, it lacks crucial functionalities like moisture resistance and strength. Traditional coating materials exacerbate plastic pollution, prompting the need for sustainable alternatives.
Polyethylene (PE) and ethylene vinyl alcohol (EVOH) are typically used as coating materials to improve the low barrier properties of paper packaging, but these substances do not decompose and worsen microplastic pollution when disposed of in the natural environment. In response to this problem, packaging materials made from bio-based substances and biodegradable plastics have been developed, but in most cases, as the packaging performance improves, the biodegradability diminishes rapidly.
KAIST announced that a joint research team led by Professor Jaewook Myung of the Department of Civil and Environmental Engineering, Professor Hanseul Yang of the Department of Life Sciences, and Professor Jongcheol Seo of the Department of Packaging and Logistics <Figure 4. Back cover art of Green Chemistry journal of the latest volume, describing the boric acid cross-linked poly(vinyl alcohol) coated paper featuring marine biodegradability, biocompatibility, high barrier properties, and robustness developed through this study.>
at Yonsei University tackled the challenge of balancing packaging performance and sustainability. They successfully developed a sustainable, marine biodegradable, high-performance paper coating material.
* Biodegradable plastic: A plastic that can be decomposed by microorganisms in natural environments such as soil and ocean or artificial conditions such as industrial composting and anaerobic digestion by microorganisms.
*Microplastics: Tiny pieces of plastic less than 5 mm, produced during the decomposition of bulk plastic materials. Microplastics can persist in the sea for more than decades, causing severe marine pollution.
The team utilized boric acid-crosslinked poly(vinyl alcohol) (PVA), a biodegradable plastic, to coat the paper, thereby enhancing its biodegradability, barrier properties, and strength. The resulting coated paper exhibited superior performance compared to conventional plastics, with excellent barrier properties and physical strength, even in humid conditions.
<Figure 1. (a) Chemical structure of boric acid-crosslinked poly(vinyl alcohol) coating on paper, (b-c) Oxygen and water vapor barrier properties, (d-f) Tensile strength in dry and moist conditions. OTR: Oxygen transmission rate, WVTR: Water vapor transmission rate.>
The team also conducted an in-depth examination of biodegradation and biocompatibility to systematically evaluate the sustainability of the newly developed coated paper. Biodegradation was assessed by simulating the marine environment, known for its challenging biodegradability conditions. The team employed a respiratory system-based bioreactor to measure the degree of carbon mineralization into carbon dioxide. After 111 days of biodegradation, it was found that the coated papers achieved 59-82% biodegradation depending on the coating component. The phenomenon in which marine bacteria are decomposing the coating material was captured through a scanning electron microscope. In addition, in vitro biocompatibility was confirmed through human embryonic kidney and mouse embryonic fibroblast cells, as well as high in-vivo biocompatibility of the coated paper was verified through mouse experiments.
Through this study, the joint research team proposed a coating strategy that can improve packaging performance while upholding sustainability to address the drawbacks of paper packaging. The boric acid-crosslinked PVA-coated paper eliminates the need for artificial composting conditions or sewage treatment facilities. Being biodegradable in natural environments and characterized by low toxicity, this newly coated paper does not exacerbate environmental pollution when accidentally discarded. Thus, it presents a sustainable substitute for plastic packaging materials.
<Figure 2. (a) Normal paper and boric acid-crosslinked poly(vinyl alcohol) coated paper, (b) Biodegradation of the coated paper by marine bacteria, (c) Result of cytotoxicity test using human embryonic kidney and mouse embryonic fibroblast cells. (d) Vital organs after one-month exposure of the coated papers to mice.>
Professor Jaewook Myung at KAIST, who led the sustainability study of coated paper, said, "The development of a marine biodegradable high-performance paper coating is the result of combining the innovative technologies of three leading research teams in each field." He said, “We will continue to develop sustainable materials with excellent performance.” Meanwhile, Professor Jongchul Seo of Yonsei University, who led the research on the development of high-performance paper coating, mentioned, “Through this research, we have developed a sustainable paper packaging technology that can replace non-degradable plastic packaging, and we expect the research outcome will be applied in industry,”.
<Figure 3. End-of-life scenario of papers coated by BA-crosslinked PVA in the marine environment. The coated papers potentially be disintegrated by marine microorganisms and ocean waves and tides. The depolymerization of PVA coating and paper is then mediated by extracellular depolymerases such as oxidases and cellulases, after which the small subunits (oligomers and monomers) are assimilated by microbial cells. The carbon components in the coated papers are ultimately mineralized into CO2, posing no harm in the ocean.>
The work was published in Green Chemistry and Food Chemistry journals. This study was conducted with the support of the Korea Research Foundation and the Korea Institute for Agriculture, Food and Rural Affairs Technology Planning and Evaluation, etc.
*Title of paper published in Green Chemistry: Boric acid-crosslinked poly(vinyl alcohol): biodegradable, biocompatible, robust, and high-barrier paper coating
※ Selected as the article for the back cover of the journal .
- Authors: Shinhyeong Choe, Seulki You, Kitae Park, Youngju Kim, Jehee Park, Yongjun Cho, Jongchul Seo, Hanseul Yang, and Jaewook Myung)
- Date: April 17, 2024
- DOI: 10.1039/D4GC00618F
*Title of paper published in Food Chemistry: Effect of epichlorohydrin treatment on the coating process and performance of high-barrier paper packaging
- Authors: Kitae Park, Shinhyeong Choe, Kambiz Sadeghi, Pradeep Kumar Panda, Jaewook Myung, Dowan Kim, and Jongchul Seo
- Date: February 19, 2024
- DOI: 10.1016/j.foodchem.2024.138772
<Figure 4. Back cover art of Green Chemistry journal of the latest volume, describing the boric acid cross-linked poly(vinyl alcohol) coated paper featuring marine biodegradability, biocompatibility, high barrier properties, and robustness developed through this study.>
2024.05.22 View 8074 -
 KAIST begins full-scale cooperation with Taiwan’s Formosa Group
< (From left) Senior Vice President for Planning and Budget Kyung-Soo Kim, and Professor Minee Choi of the Department of Brain and Cognitive Sciences of KAIST along with Chairman of Formosa Group Sandy Wang and KAIST President Kwang-Hyung Lee, and Dean Daesoo Kim of KAIST College of Life Science and Bioengineering >
KAIST is pursuing cooperation in the fields of advanced biotechnology and eco-friendly energy with Formosa Plastics Group, one of Taiwan's three largest companies.
To this end, Chairman Sandy Wang, a member of Formosa Group's standing committee and leader of the group's bio and eco-friendly energy sector, will visit KAIST on the 13th of this month. This is the first time that the owner of Formosa Group has made an official visit to KAIST.
Cooperation between the two institutions began last March when our university signed a memorandum of understanding on comprehensive exchange and cooperation with Ming Chi University of Science and Technology (明志科技大學), Chang Gung University(長庚大學), and Chang Gung Memorial Hospital(長庚記念醫院), three of many institutions established and supported by Formosa Group.
Based on this, Chairman Sandy Wang, who visits our university to promote more exchanges and cooperation, talked about ‘the education of children and corporate social return and practice of his father, Chairman Yung-Ching Wang,’ through a special lecture for the school leadership as a part of the Monthly Lecture on KAIST’s Leadership Innovation Day.
She then visited KAIST's research and engineering facilities related to Taiwan's future industries, such as advanced biotechnology and eco-friendly energy, and discussed global industry-academic cooperation plans. In the future, the two organizations plan to appoint adjunct professors and promote practical global cooperation, including joint student guidance and research cooperation. We plan to pursue effective mid- to long-term cooperation, such as conducting battery application research with the KAIST Next-Generation ESS Research Center and opening a graduate program specialized in stem cell and gene editing technology in connection with Chang Gung University and Chang Gung Memorial Hospital. The newly established cooperative relationship will also promote Formosa Group's investment and cooperation with KAIST's outstanding venture companies related to bio and eco-friendly energy to lay the foundation for innovative industrial cooperation between Taiwan and Korea.
President Kwang-Hyung Lee said, “The Formosa Group has a global network, so we regard it to be a key partner that will position KAIST’s bio and engineering technology in the global stages.” He also said, “With Chairman Sandy Wang’s visit, Taiwan is emerging as a global economic powerhouse,” and added, “We expect to continue our close cooperative relationship with the company.”
Formosa Group is a company founded by the late Chairman Yung-Ching Wang, the father of Chairman Sandy Wang. As the world's No. 1 plastic PVC producer, it is leading the core industries of Taiwan's economy, including semiconductors, steel, heavy industry, bio, and batteries. Chairman Yung-Ching Wang was respected by the Taiwanese people by setting an example of returning his wealth to society under the belief that the companies and assets he built ‘belonged to the people.’ Chang Gung University, Chang Gung Memorial Hospital, and Ming Chi University of Technology, which are pursuing cooperation with our university, were also established as part of the social contribution promoted by Chairman Yung-Ching Wang and are receiving financial support from Formosa Group.
2024.05.09 View 6546
KAIST begins full-scale cooperation with Taiwan’s Formosa Group
< (From left) Senior Vice President for Planning and Budget Kyung-Soo Kim, and Professor Minee Choi of the Department of Brain and Cognitive Sciences of KAIST along with Chairman of Formosa Group Sandy Wang and KAIST President Kwang-Hyung Lee, and Dean Daesoo Kim of KAIST College of Life Science and Bioengineering >
KAIST is pursuing cooperation in the fields of advanced biotechnology and eco-friendly energy with Formosa Plastics Group, one of Taiwan's three largest companies.
To this end, Chairman Sandy Wang, a member of Formosa Group's standing committee and leader of the group's bio and eco-friendly energy sector, will visit KAIST on the 13th of this month. This is the first time that the owner of Formosa Group has made an official visit to KAIST.
Cooperation between the two institutions began last March when our university signed a memorandum of understanding on comprehensive exchange and cooperation with Ming Chi University of Science and Technology (明志科技大學), Chang Gung University(長庚大學), and Chang Gung Memorial Hospital(長庚記念醫院), three of many institutions established and supported by Formosa Group.
Based on this, Chairman Sandy Wang, who visits our university to promote more exchanges and cooperation, talked about ‘the education of children and corporate social return and practice of his father, Chairman Yung-Ching Wang,’ through a special lecture for the school leadership as a part of the Monthly Lecture on KAIST’s Leadership Innovation Day.
She then visited KAIST's research and engineering facilities related to Taiwan's future industries, such as advanced biotechnology and eco-friendly energy, and discussed global industry-academic cooperation plans. In the future, the two organizations plan to appoint adjunct professors and promote practical global cooperation, including joint student guidance and research cooperation. We plan to pursue effective mid- to long-term cooperation, such as conducting battery application research with the KAIST Next-Generation ESS Research Center and opening a graduate program specialized in stem cell and gene editing technology in connection with Chang Gung University and Chang Gung Memorial Hospital. The newly established cooperative relationship will also promote Formosa Group's investment and cooperation with KAIST's outstanding venture companies related to bio and eco-friendly energy to lay the foundation for innovative industrial cooperation between Taiwan and Korea.
President Kwang-Hyung Lee said, “The Formosa Group has a global network, so we regard it to be a key partner that will position KAIST’s bio and engineering technology in the global stages.” He also said, “With Chairman Sandy Wang’s visit, Taiwan is emerging as a global economic powerhouse,” and added, “We expect to continue our close cooperative relationship with the company.”
Formosa Group is a company founded by the late Chairman Yung-Ching Wang, the father of Chairman Sandy Wang. As the world's No. 1 plastic PVC producer, it is leading the core industries of Taiwan's economy, including semiconductors, steel, heavy industry, bio, and batteries. Chairman Yung-Ching Wang was respected by the Taiwanese people by setting an example of returning his wealth to society under the belief that the companies and assets he built ‘belonged to the people.’ Chang Gung University, Chang Gung Memorial Hospital, and Ming Chi University of Technology, which are pursuing cooperation with our university, were also established as part of the social contribution promoted by Chairman Yung-Ching Wang and are receiving financial support from Formosa Group.
2024.05.09 View 6546 -
 KAIST introduces eco-friendly technologies for plastic production and biodegradation
- A research team under Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering published a paper in Nature Microbiology on the overview and trends of plastic production and degradation technology using microorganisms.
- Eco-friendly and sustainable plastic production and degradation technology using microorganisms as a core technology to achieve a plastic circular economy was presented.
Plastic is one of the important materials in modern society, with approximately 460 million tons produced annually and with expected production reaching approximately 1.23 billion tons in 2060. However, since 1950, plastic waste totaling more than 6.3 billion tons has been generated, and it is believed that more than 140 million tons of plastic waste has accumulated in the aquatic environment. Recently, the seriousness of microplastic pollution has emerged, not only posing a risk to the marine ecosystem and human health, but also worsening global warming by inhibiting the activity of marine plankton, which play an important role in lowering the Earth's carbon dioxide concentration.
KAIST President Kwang-Hyung Lee announced on December 11 that a research team under Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering had published a paper titled 'Sustainable production and degradation of plastics using microbes', which covers the latest technologies for producing plastics and processing waste plastics in an eco-friendly manner using microorganisms.
As the international community moves to solve this plastic problem, various efforts are being made, including 175 countries participating to conclude a legally binding agreement with the goal of ending plastic pollution by 2024. Various technologies are being developed for sustainable plastic production and processing, and among them, biotechnology using microorganisms is attracting attention.
Microorganisms have the ability to naturally produce or decompose certain compounds, and this ability is maximized through biotechnologies such as metabolic engineering and enzyme engineering to produce plastics from renewable biomass resources instead of fossil raw materials and to decompose waste plastics.
Accordingly, the research team comprehensively analyzed the latest microorganism-based technologies for the sustainable production and decomposition of plastics and presented how they actually contribute to solving the plastic problem. Based on this, they presented limitations, prospects, and research directions of the technologies for achieving a circular economy for plastics.
Microorganism-based technologies for various plastics range from widely used synthetic plastics such as polyethylene (PE) to promising bioplastics such as natural polymers derived from microorganisms (polyhydroxyalkanoate (PHA)) that are completely biodegradable in the natural environment and do not pose a risk of microplastic generation. Commercialization statuses and latest technologies were also discussed. In addition, the technology to decompose these plastics using microorganisms and their enzymes and the upcycling technology to convert them into other useful compounds after decomposition were introduced, highlighting the competitiveness and potential of technology using microorganisms.
First author So Young Choi, a research assistant professor in the Department of Chemical and Biomolecular Engineering at KAIST, said, “In the future, we will be able to easily find eco-friendly plastics made using microorganisms all around us,” and corresponding author Distinguished Professor Sang Yup Lee said, “Plastic can be made more sustainable. It is important to use plastics responsibly to protect the environment and simultaneously achieve economic and social development through the new plastics industry, and we look forward to the improved performance of microbial metabolic engineering technology.”
This paper was published on November 30th in the online edition of Nature Microbiology.
※ Paper Title : Sustainable production and degradation of plastics using microbes
Authors: So Young Choi, Youngjoon Lee, Hye Eun Yu, In Jin Cho, Minju Kang & Sang Yup Lee
2023.12.11 View 7202
KAIST introduces eco-friendly technologies for plastic production and biodegradation
- A research team under Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering published a paper in Nature Microbiology on the overview and trends of plastic production and degradation technology using microorganisms.
- Eco-friendly and sustainable plastic production and degradation technology using microorganisms as a core technology to achieve a plastic circular economy was presented.
Plastic is one of the important materials in modern society, with approximately 460 million tons produced annually and with expected production reaching approximately 1.23 billion tons in 2060. However, since 1950, plastic waste totaling more than 6.3 billion tons has been generated, and it is believed that more than 140 million tons of plastic waste has accumulated in the aquatic environment. Recently, the seriousness of microplastic pollution has emerged, not only posing a risk to the marine ecosystem and human health, but also worsening global warming by inhibiting the activity of marine plankton, which play an important role in lowering the Earth's carbon dioxide concentration.
KAIST President Kwang-Hyung Lee announced on December 11 that a research team under Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering had published a paper titled 'Sustainable production and degradation of plastics using microbes', which covers the latest technologies for producing plastics and processing waste plastics in an eco-friendly manner using microorganisms.
As the international community moves to solve this plastic problem, various efforts are being made, including 175 countries participating to conclude a legally binding agreement with the goal of ending plastic pollution by 2024. Various technologies are being developed for sustainable plastic production and processing, and among them, biotechnology using microorganisms is attracting attention.
Microorganisms have the ability to naturally produce or decompose certain compounds, and this ability is maximized through biotechnologies such as metabolic engineering and enzyme engineering to produce plastics from renewable biomass resources instead of fossil raw materials and to decompose waste plastics.
Accordingly, the research team comprehensively analyzed the latest microorganism-based technologies for the sustainable production and decomposition of plastics and presented how they actually contribute to solving the plastic problem. Based on this, they presented limitations, prospects, and research directions of the technologies for achieving a circular economy for plastics.
Microorganism-based technologies for various plastics range from widely used synthetic plastics such as polyethylene (PE) to promising bioplastics such as natural polymers derived from microorganisms (polyhydroxyalkanoate (PHA)) that are completely biodegradable in the natural environment and do not pose a risk of microplastic generation. Commercialization statuses and latest technologies were also discussed. In addition, the technology to decompose these plastics using microorganisms and their enzymes and the upcycling technology to convert them into other useful compounds after decomposition were introduced, highlighting the competitiveness and potential of technology using microorganisms.
First author So Young Choi, a research assistant professor in the Department of Chemical and Biomolecular Engineering at KAIST, said, “In the future, we will be able to easily find eco-friendly plastics made using microorganisms all around us,” and corresponding author Distinguished Professor Sang Yup Lee said, “Plastic can be made more sustainable. It is important to use plastics responsibly to protect the environment and simultaneously achieve economic and social development through the new plastics industry, and we look forward to the improved performance of microbial metabolic engineering technology.”
This paper was published on November 30th in the online edition of Nature Microbiology.
※ Paper Title : Sustainable production and degradation of plastics using microbes
Authors: So Young Choi, Youngjoon Lee, Hye Eun Yu, In Jin Cho, Minju Kang & Sang Yup Lee
2023.12.11 View 7202 -
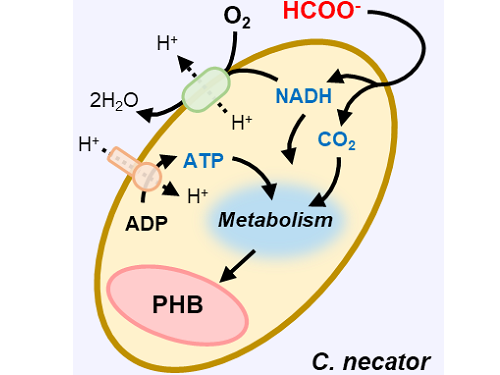 A biohybrid system to extract 20 times more bioplastic from CO2 developed by KAIST researchers
As the issues surrounding global climate change intensify, more attention and determined efforts are required to re-grasp the issue as a state of “crisis” and respond to it properly. Among the various methods of recycling CO2, the electrochemical CO2 conversion technology is a technology that can convert CO2 into useful chemical substances using electrical energy. Since it is easy to operate facilities and can use the electricity from renewable sources like the solar cells or the wind power, it has received a lot of attention as an eco-friendly technology can contribute to reducing greenhouse gases and achieve carbon neutrality.
KAIST (President Kwang Hyung Lee) announced on the 30th that the joint research team led by Professor Hyunjoo Lee and Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering succeeded in developing a technology that produces bioplastics from CO2 with high efficiency by developing a hybrid system that interlinked the electrochemical CO2 conversion and microbial bio conversion methods together. The results of the research, which showed the world's highest productivity by more than 20 times compared to similar systems, were published online on March 27th in the "Proceedings of the National Academy of Sciences (PNAS)".
※ Paper title: Biohybrid CO2 electrolysis for the direct synthesis of polyesters from CO2
※ Author information: Jinkyu Lim (currently at Stanford Linear Accelerator Center, co-first author), So Young Choi (KAIST, co-first author), Jae Won Lee (KAIST, co-first author), Hyunjoo Lee (KAIST, corresponding author), Sang Yup Lee (KAIST, corresponding author)
For the efficient conversion of CO2, high-efficiency electrode catalysts and systems are actively being developed. As conversion products, only compounds containing one or up to three carbon atoms are produced on a limited basis. Compounds of one carbon, such as CO, formic acid, and ethylene, are produced with relatively high efficiency. Liquid compounds of several carbons, such as ethanol, acetic acid, and propanol, can also be produced by these systems, but due to the nature of the chemical reaction that requires more electrons, there are limitations involving the conversion efficiency and the product selection.
Accordingly, a joint research team led by Professor Hyunjoo Lee and Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering at KAIST developed a technology to produce bioplastics from CO2 by linking electrochemical conversion technology with bioconversion method that uses microorganisms.
This electrochemical-bio hybrid system is in the form of having an electrolyzer, in which electrochemical conversion reactions occur, connected to a fermenter, in which microorganisms are cultured. When CO2 is converted to formic acid in the electrolyzer, and it is fed into the fermenter in which the microbes like the Cupriavidus necator, in this case, consumes the carbon source to produce polyhydroxyalkanoate (PHA), a microbial-derived bioplastic.
According to the research results of the existing hybrid concepts, there was a disadvantage of having low productivity or stopping at a non-continuous process due to problems of low efficiency of the electrolysis and irregular results arising from the culturing conditions of the microbes.
In order to overcome these problems, the joint research team made formic acid with a gas diffusion electrode using gaseous CO2. In addition, the team developed a 'physiologically compatible catholyte' that can be used as a culture medium for microorganisms as well as an electrolyte that allows the electrolysis to occur sufficiently without inhibiting the growth of microorganisms, without having to have a additional separation and purification process, which allowed the acide to be supplied directly to microorganisms.
Through this, the electrolyte solution containing formic acid made from CO2 enters the fermentation tank, is used for microbial culture, and enters the electrolyzer to be circulated, maximizing the utilization of the electrolyte solution and remaining formic acid. In addition, a filter was installed to ensure that only the electrolyte solution with any and all microorganisms that can affect the electrosis filtered out is supplied back to the electrolyzer, and that the microorganisms exist only in the fermenter, designing the two system to work well together with utmost efficiency.
Through the developed hybrid system, the produced bioplastic, poly-3-hydroxybutyrate (PHB), of up to 83% of the cell dry weight was produced from CO2, which produced 1.38g of PHB from a 4 cm2 electrode, which is the world's first gram(g) level production and is more than 20 times more productive than previous research. In addition, the hybrid system is expected to be applied to various industrial processes in the future as it shows promises of the continuous culture system.
The corresponding authors, Professor Hyunjoo Lee and Distinguished Professor Sang Yup Lee noted that “The results of this research are technologies that can be applied to the production of various chemical substances as well as bioplastics, and are expected to be used as key parts needed in achieving carbon neutrality in the future.”
This research was received and performed with the supports from the CO2 Reduction Catalyst and Energy Device Technology Development Project, the Heterogeneous Atomic Catalyst Control Project, and the Next-generation Biorefinery Source Technology Development Project to lead the Biochemical Industry of the Oil-replacement Eco-friendly Chemical Technology Development Program by the Ministry of Science and ICT.
Figure 1. Schematic diagram and photo of the biohybrid CO2 electrolysis system.
(A) A conceptual scheme and (B) a photograph of the biohybrid CO2 electrolysis system. (C) A detailed scheme of reaction inside the system. Gaseous CO2 was converted to formate in the electrolyzer, and the formate was converted to PHB by the cells in the fermenter. The catholyte was developed so that it is compatible with both CO2 electrolysis and fermentation and was continuously circulated.
2023.03.30 View 11717
A biohybrid system to extract 20 times more bioplastic from CO2 developed by KAIST researchers
As the issues surrounding global climate change intensify, more attention and determined efforts are required to re-grasp the issue as a state of “crisis” and respond to it properly. Among the various methods of recycling CO2, the electrochemical CO2 conversion technology is a technology that can convert CO2 into useful chemical substances using electrical energy. Since it is easy to operate facilities and can use the electricity from renewable sources like the solar cells or the wind power, it has received a lot of attention as an eco-friendly technology can contribute to reducing greenhouse gases and achieve carbon neutrality.
KAIST (President Kwang Hyung Lee) announced on the 30th that the joint research team led by Professor Hyunjoo Lee and Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering succeeded in developing a technology that produces bioplastics from CO2 with high efficiency by developing a hybrid system that interlinked the electrochemical CO2 conversion and microbial bio conversion methods together. The results of the research, which showed the world's highest productivity by more than 20 times compared to similar systems, were published online on March 27th in the "Proceedings of the National Academy of Sciences (PNAS)".
※ Paper title: Biohybrid CO2 electrolysis for the direct synthesis of polyesters from CO2
※ Author information: Jinkyu Lim (currently at Stanford Linear Accelerator Center, co-first author), So Young Choi (KAIST, co-first author), Jae Won Lee (KAIST, co-first author), Hyunjoo Lee (KAIST, corresponding author), Sang Yup Lee (KAIST, corresponding author)
For the efficient conversion of CO2, high-efficiency electrode catalysts and systems are actively being developed. As conversion products, only compounds containing one or up to three carbon atoms are produced on a limited basis. Compounds of one carbon, such as CO, formic acid, and ethylene, are produced with relatively high efficiency. Liquid compounds of several carbons, such as ethanol, acetic acid, and propanol, can also be produced by these systems, but due to the nature of the chemical reaction that requires more electrons, there are limitations involving the conversion efficiency and the product selection.
Accordingly, a joint research team led by Professor Hyunjoo Lee and Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering at KAIST developed a technology to produce bioplastics from CO2 by linking electrochemical conversion technology with bioconversion method that uses microorganisms.
This electrochemical-bio hybrid system is in the form of having an electrolyzer, in which electrochemical conversion reactions occur, connected to a fermenter, in which microorganisms are cultured. When CO2 is converted to formic acid in the electrolyzer, and it is fed into the fermenter in which the microbes like the Cupriavidus necator, in this case, consumes the carbon source to produce polyhydroxyalkanoate (PHA), a microbial-derived bioplastic.
According to the research results of the existing hybrid concepts, there was a disadvantage of having low productivity or stopping at a non-continuous process due to problems of low efficiency of the electrolysis and irregular results arising from the culturing conditions of the microbes.
In order to overcome these problems, the joint research team made formic acid with a gas diffusion electrode using gaseous CO2. In addition, the team developed a 'physiologically compatible catholyte' that can be used as a culture medium for microorganisms as well as an electrolyte that allows the electrolysis to occur sufficiently without inhibiting the growth of microorganisms, without having to have a additional separation and purification process, which allowed the acide to be supplied directly to microorganisms.
Through this, the electrolyte solution containing formic acid made from CO2 enters the fermentation tank, is used for microbial culture, and enters the electrolyzer to be circulated, maximizing the utilization of the electrolyte solution and remaining formic acid. In addition, a filter was installed to ensure that only the electrolyte solution with any and all microorganisms that can affect the electrosis filtered out is supplied back to the electrolyzer, and that the microorganisms exist only in the fermenter, designing the two system to work well together with utmost efficiency.
Through the developed hybrid system, the produced bioplastic, poly-3-hydroxybutyrate (PHB), of up to 83% of the cell dry weight was produced from CO2, which produced 1.38g of PHB from a 4 cm2 electrode, which is the world's first gram(g) level production and is more than 20 times more productive than previous research. In addition, the hybrid system is expected to be applied to various industrial processes in the future as it shows promises of the continuous culture system.
The corresponding authors, Professor Hyunjoo Lee and Distinguished Professor Sang Yup Lee noted that “The results of this research are technologies that can be applied to the production of various chemical substances as well as bioplastics, and are expected to be used as key parts needed in achieving carbon neutrality in the future.”
This research was received and performed with the supports from the CO2 Reduction Catalyst and Energy Device Technology Development Project, the Heterogeneous Atomic Catalyst Control Project, and the Next-generation Biorefinery Source Technology Development Project to lead the Biochemical Industry of the Oil-replacement Eco-friendly Chemical Technology Development Program by the Ministry of Science and ICT.
Figure 1. Schematic diagram and photo of the biohybrid CO2 electrolysis system.
(A) A conceptual scheme and (B) a photograph of the biohybrid CO2 electrolysis system. (C) A detailed scheme of reaction inside the system. Gaseous CO2 was converted to formate in the electrolyzer, and the formate was converted to PHB by the cells in the fermenter. The catholyte was developed so that it is compatible with both CO2 electrolysis and fermentation and was continuously circulated.
2023.03.30 View 11717 -
 Enhanced Natural Gas Storage to Help Reduce Global Warming
< Professor Atilhan (left) and Professor Yavuz (right) >
Researchers have designed plastic-based materials that can store natural gas more effectively. These new materials can not only make large-scale, cost-effective, and safe natural gas storage possible, but further hold a strong promise for combating global warming.
Natural gas (predominantly methane) is a clean energy alternative. It is stored by compression, liquefaction, or adsorption. Among these, adsorbed natural gas (ANG) storage is a more efficient, cheaper, and safer alternative to conventional compressed natural gas (CNG) and liquefied natural gas (LNG) storage approaches that have drawbacks such as low storage efficiency, high costs, and safety concerns. However, developing adsorptive materials that can more fully exploit the advantages of ANG storage has remained a challenging task.
A KAIST research team led by Professor Cafer T. Yavuz from the Graduate School of Energy, Environment, Water, and Sustainability (EEWS), in collaboration with Professor Mert Atilhan’s group from Texas A&M University, synthesized 29 unique porous polymeric structures with inherent flexibility, and tested their methane gas uptake capacity at high pressures. These porous polymers had varying synthetic complexities, porosities, and morphologies, and the researchers subjected each porous polymer to pure methane gas under various conditions to study the ANG performances.
Of these 29 distinct chemical structures, COP-150 was particularly noteworthy as it achieved a high deliverable gravimetric methane working capacity when cycled between 5 and 100 bar at 273 K, which is 98% of the total uptake capacity. This result surpassed the target set by the United States Department of Energy (US DOE).
COP-150 is the first ever structure to fulfil both the gravimetric and volumetric requirements of the US DOE for successful vehicular use, and the total cost to produce the COP-150 adsorbent was only 1 USD per kilogram.
COP-150 can be produced using freely available and easily accessible plastic materials, and moreover, its synthesis takes place at room temperature, open to the air, and no previous purification of the chemicals is required. The pressure-triggered flexible structure of COP-150 is also advantageous in terms of the total working capacity of deliverable methane for real applications.
The research team believed that the increased pressure flexes the network structure of COP-150 showing “swelling” behavior, and suggested that the flexibility provides rapid desorption and thermal management, while the hydrophobicity and the nature of the covalently bonded framework allow these promising materials to tolerate harsh conditions.
This swelling mechanism of expansion-contraction solves two other major issues, the team noted. Firstly, when using adsorbents based on such a mechanism, unsafe pressure spikes that may occur due to temperature swings can be eliminated. In addition, contamination can also be minimized, since the adsorbent remains contracted when no gas is stored.
Professor Yavuz said, “We envision a whole host of new designs and mechanisms to be developed based on our concept. Since natural gas is a much cleaner fuel than coal and petroleum, new developments in this realm will help switching to the use of less polluting fuels.”
Professor Atilhan agreed the most important impact of their research is on the environment. “Using natural gas more than coal and petroleum will significantly reduce greenhouse gas emissions. We believe, one day, we might see vehicles equipped with our materials that are run by a cleaner natural gas fuel,” he added.
This study, reported in Nature Energy on July 8, was supported by National Research Foundation of Korea (NRF) grants ( NRF-2016R1A2B4011027, NRF-2017M3A7B4042140, and NRF-2017M3A7B4042235).
< Suggested chemical structure of COP-150 >
< Initial ingredients (left) and final product (right) of COP-150 synthesis >
< Comparison of highest reported volumetric working capacities >
(END)
2019.08.09 View 28107
Enhanced Natural Gas Storage to Help Reduce Global Warming
< Professor Atilhan (left) and Professor Yavuz (right) >
Researchers have designed plastic-based materials that can store natural gas more effectively. These new materials can not only make large-scale, cost-effective, and safe natural gas storage possible, but further hold a strong promise for combating global warming.
Natural gas (predominantly methane) is a clean energy alternative. It is stored by compression, liquefaction, or adsorption. Among these, adsorbed natural gas (ANG) storage is a more efficient, cheaper, and safer alternative to conventional compressed natural gas (CNG) and liquefied natural gas (LNG) storage approaches that have drawbacks such as low storage efficiency, high costs, and safety concerns. However, developing adsorptive materials that can more fully exploit the advantages of ANG storage has remained a challenging task.
A KAIST research team led by Professor Cafer T. Yavuz from the Graduate School of Energy, Environment, Water, and Sustainability (EEWS), in collaboration with Professor Mert Atilhan’s group from Texas A&M University, synthesized 29 unique porous polymeric structures with inherent flexibility, and tested their methane gas uptake capacity at high pressures. These porous polymers had varying synthetic complexities, porosities, and morphologies, and the researchers subjected each porous polymer to pure methane gas under various conditions to study the ANG performances.
Of these 29 distinct chemical structures, COP-150 was particularly noteworthy as it achieved a high deliverable gravimetric methane working capacity when cycled between 5 and 100 bar at 273 K, which is 98% of the total uptake capacity. This result surpassed the target set by the United States Department of Energy (US DOE).
COP-150 is the first ever structure to fulfil both the gravimetric and volumetric requirements of the US DOE for successful vehicular use, and the total cost to produce the COP-150 adsorbent was only 1 USD per kilogram.
COP-150 can be produced using freely available and easily accessible plastic materials, and moreover, its synthesis takes place at room temperature, open to the air, and no previous purification of the chemicals is required. The pressure-triggered flexible structure of COP-150 is also advantageous in terms of the total working capacity of deliverable methane for real applications.
The research team believed that the increased pressure flexes the network structure of COP-150 showing “swelling” behavior, and suggested that the flexibility provides rapid desorption and thermal management, while the hydrophobicity and the nature of the covalently bonded framework allow these promising materials to tolerate harsh conditions.
This swelling mechanism of expansion-contraction solves two other major issues, the team noted. Firstly, when using adsorbents based on such a mechanism, unsafe pressure spikes that may occur due to temperature swings can be eliminated. In addition, contamination can also be minimized, since the adsorbent remains contracted when no gas is stored.
Professor Yavuz said, “We envision a whole host of new designs and mechanisms to be developed based on our concept. Since natural gas is a much cleaner fuel than coal and petroleum, new developments in this realm will help switching to the use of less polluting fuels.”
Professor Atilhan agreed the most important impact of their research is on the environment. “Using natural gas more than coal and petroleum will significantly reduce greenhouse gas emissions. We believe, one day, we might see vehicles equipped with our materials that are run by a cleaner natural gas fuel,” he added.
This study, reported in Nature Energy on July 8, was supported by National Research Foundation of Korea (NRF) grants ( NRF-2016R1A2B4011027, NRF-2017M3A7B4042140, and NRF-2017M3A7B4042235).
< Suggested chemical structure of COP-150 >
< Initial ingredients (left) and final product (right) of COP-150 synthesis >
< Comparison of highest reported volumetric working capacities >
(END)
2019.08.09 View 28107 -
 Mystery of Biological Plastic Synthesis Machinery Unveiled
Plastics and other polymers are used every day. These polymers are mostly made from fossil resources by refining petrochemicals. On the other hand, many microorganisms naturally synthesize polyesters known as polyhydroxyalkanoates (PHAs) as distinct granules inside cells.
PHAs are a family of microbial polyesters that have attracted much attention as biodegradable and biocompatible plastics and elastomers that can substitute petrochemical counterparts. There have been numerous papers and patents on gene cloning and metabolic engineering of PHA biosynthetic machineries, biochemical studies, and production of PHAs; simple Google search with “polyhydroxyalkanoates” yielded returns of 223,000 document pages. PHAs have always been considered amazing examples of biological polymer synthesis. It is astounding to see PHAs of 500 kDa to sometimes as high as 10,000 kDa can be synthesized in vivo by PHA synthase, the key polymerizing enzyme in PHA biosynthesis. They have attracted great interest in determining the crystal structure of PHA synthase over the last 30 years, but unfortunately without success. Thus, the characteristics and molecular mechanisms of PHA synthase were under a dark veil.
In two papers published back-to-back in Biotechnology Journal online on November 30, 2016, a Korean research team led by Professor Kyung-Jin Kim at Kyungpook National University and Distinguished Professor Sang Yup Lee at the Korea Advanced Institute of Science and Technology (KAIST) described the crystal structure of PHA synthase from Ralstonia eutropha, the best studied bacterium for PHA production, and reported the structural basis for the detailed molecular mechanisms of PHA biosynthesis. The crystal structure has been deposited to Protein Data Bank in February 2016. After deciphering the crystal structure of the catalytic domain of PHA synthase, in addition to other structural studies on whole enzyme and related proteins, the research team also performed experiments to elucidate the mechanisms of the enzyme reaction, validating detailed structures, enzyme engineering, and also N-terminal domain studies among others.
Through several biochemical studies based on crystal structure, the authors show that PHA synthase exists as a dimer and is divided into two distinct domains, the N-terminal domain (RePhaC1ND) and the C-terminal domain (RePhaC1CD). The RePhaC1CD catalyzes the polymerization reaction via a non-processive ping-pong mechanism using a Cys-His-Asp catalytic triad. The two catalytic sites of the RePhaC1CD dimer are positioned 33.4 Å apart, suggesting that the polymerization reaction occurs independently at each site. This study also presents the structure-based mechanisms for substrate specificities of various PHA synthases from different classes.
Professor Sang Yup Lee, who has worked on this topic for more than 20 years, said,
“The results and information presented in these two papers have long been awaited not only in the PHA community, but also metabolic engineering, bacteriology/microbiology, and in general biological sciences communities. The structural information on PHA synthase together with the recently deciphered reaction mechanisms will be valuable for understanding the detailed mechanisms of biosynthesizing this important energy/redox storage material, and also for the rational engineering of PHA synthases to produce designer bioplastics from various monomers more efficiently.”
Indeed, these two papers published in Biotechnology Journal finally reveal the 30-year mystery of machinery of biological polyester synthesis, and will serve as the essential compass in creating designer and more efficient bioplastic machineries.
References:
Jieun Kim, Yeo-Jin Kim, So Young Choi, Sang Yup Lee and Kyung-Jin Kim. “Crystal structure of Ralstonia eutropha polyhydroxyalkanoate synthase C-terminal domain and reaction mechanisms” Biotechnology Journal DOI: 10.1002/biot.201600648
http://onlinelibrary.wiley.com/doi/10.1002/biot.201600648/abstract
Yeo-Jin Kim, So Young Choi, Jieun Kim, Kyeong Sik Jin, Sang Yup Lee and Kyung-Jin Kim. “Structure and function of the N-terminal domain of Ralstonia eutropha polyhydroxyalkanoate synthase, and the proposed structure and mechanisms of the whole enzyme” Biotechnology Journal DOI: 10.1002/biot.201600649
http://onlinelibrary.wiley.com/doi/10.1002/biot.201600649/abstract
2016.12.02 View 11267
Mystery of Biological Plastic Synthesis Machinery Unveiled
Plastics and other polymers are used every day. These polymers are mostly made from fossil resources by refining petrochemicals. On the other hand, many microorganisms naturally synthesize polyesters known as polyhydroxyalkanoates (PHAs) as distinct granules inside cells.
PHAs are a family of microbial polyesters that have attracted much attention as biodegradable and biocompatible plastics and elastomers that can substitute petrochemical counterparts. There have been numerous papers and patents on gene cloning and metabolic engineering of PHA biosynthetic machineries, biochemical studies, and production of PHAs; simple Google search with “polyhydroxyalkanoates” yielded returns of 223,000 document pages. PHAs have always been considered amazing examples of biological polymer synthesis. It is astounding to see PHAs of 500 kDa to sometimes as high as 10,000 kDa can be synthesized in vivo by PHA synthase, the key polymerizing enzyme in PHA biosynthesis. They have attracted great interest in determining the crystal structure of PHA synthase over the last 30 years, but unfortunately without success. Thus, the characteristics and molecular mechanisms of PHA synthase were under a dark veil.
In two papers published back-to-back in Biotechnology Journal online on November 30, 2016, a Korean research team led by Professor Kyung-Jin Kim at Kyungpook National University and Distinguished Professor Sang Yup Lee at the Korea Advanced Institute of Science and Technology (KAIST) described the crystal structure of PHA synthase from Ralstonia eutropha, the best studied bacterium for PHA production, and reported the structural basis for the detailed molecular mechanisms of PHA biosynthesis. The crystal structure has been deposited to Protein Data Bank in February 2016. After deciphering the crystal structure of the catalytic domain of PHA synthase, in addition to other structural studies on whole enzyme and related proteins, the research team also performed experiments to elucidate the mechanisms of the enzyme reaction, validating detailed structures, enzyme engineering, and also N-terminal domain studies among others.
Through several biochemical studies based on crystal structure, the authors show that PHA synthase exists as a dimer and is divided into two distinct domains, the N-terminal domain (RePhaC1ND) and the C-terminal domain (RePhaC1CD). The RePhaC1CD catalyzes the polymerization reaction via a non-processive ping-pong mechanism using a Cys-His-Asp catalytic triad. The two catalytic sites of the RePhaC1CD dimer are positioned 33.4 Å apart, suggesting that the polymerization reaction occurs independently at each site. This study also presents the structure-based mechanisms for substrate specificities of various PHA synthases from different classes.
Professor Sang Yup Lee, who has worked on this topic for more than 20 years, said,
“The results and information presented in these two papers have long been awaited not only in the PHA community, but also metabolic engineering, bacteriology/microbiology, and in general biological sciences communities. The structural information on PHA synthase together with the recently deciphered reaction mechanisms will be valuable for understanding the detailed mechanisms of biosynthesizing this important energy/redox storage material, and also for the rational engineering of PHA synthases to produce designer bioplastics from various monomers more efficiently.”
Indeed, these two papers published in Biotechnology Journal finally reveal the 30-year mystery of machinery of biological polyester synthesis, and will serve as the essential compass in creating designer and more efficient bioplastic machineries.
References:
Jieun Kim, Yeo-Jin Kim, So Young Choi, Sang Yup Lee and Kyung-Jin Kim. “Crystal structure of Ralstonia eutropha polyhydroxyalkanoate synthase C-terminal domain and reaction mechanisms” Biotechnology Journal DOI: 10.1002/biot.201600648
http://onlinelibrary.wiley.com/doi/10.1002/biot.201600648/abstract
Yeo-Jin Kim, So Young Choi, Jieun Kim, Kyeong Sik Jin, Sang Yup Lee and Kyung-Jin Kim. “Structure and function of the N-terminal domain of Ralstonia eutropha polyhydroxyalkanoate synthase, and the proposed structure and mechanisms of the whole enzyme” Biotechnology Journal DOI: 10.1002/biot.201600649
http://onlinelibrary.wiley.com/doi/10.1002/biot.201600649/abstract
2016.12.02 View 11267 -
 Technology for Non-Breaking Smartphone Display Developed
High-strength plastic display has been developed by applying a glass-fiber fabric.
“Will bring about innovation to the field by replacing glass substrates”
It is now possible to manufacture non-breaking smartphone display. Heavy glass substrates of large-screen televisions will be replaced with light plastic films.
Professor Choon Sup Yoon from KAIST’s Department of Physics and KAIST Institute for Information Technology Convergence has developed the technology for high-strength plastic substrates to replace glass displays.
The plastic substrate created by Professor Yoon and his research team have greatly enhanced needed properties of heat resistance, transparency, flexibility, inner chemical capability, and tensile strength. Although the material retains flexibility as a native advantage of plastic film, its tensile strength is three times greater than that of normal glass, which is a degree similar to tempered glass. In addition, Professor Yoon’s substrate is as colorless and transparent as glass and resists heat up to 450℃, while its thermal expansivity is only 10% to 20% of existing plastics.
Glass substrates are currently used in practically every display such as mobile phone screens, televisions, and computer monitors for having smooth surface and satisfying basic conditions for display substrates. However, as glass substrates are heavy and easily broken, researchers studied colorless and transparent plastic polyimide films to replace glass substrates for their excellent thermal and chemical stability.
Nonetheless, colorless and transparent polyimide films do not have sufficient heat resistance and mechanical solidity. To resolve this problem, polyimide films are impregnated with glass-fiber fabrics, but it was far from commercialization as the impregnation exacerbates the roughness of surface and light transmittance. The roughness of the surface increases as the solvent evaporates in the impregnation process, resulting in surface roughness of around 0.4μm. The downturn in light transmittance is due to light scattering effect by the discording refractive index of polyimide film and glass-fiber fabric.
Professor Yoon’s research team resolved these issues by tuning the refractive indices of transparent polyimide film and glass-fiber fabric up to four decimal places, and by developing the technology of flattening the film’s surface roughness to a few nanometers. As a result, the research team achieved heat expansivity of 11ppm/℃, surface roughness of 0.9nm, tensile strength of 250MPa, bending curvature radius of 2mm, and light transmittance at 90% with a 110μm-thick glass-fiber fabric impregnated transparent polyimide film substrate.
“The developed substrate can not only replace the traditional glass substrate but also be applied as flexible display substrate,” said Professor Yoon in prospect, “it will bring about technological innovation in display industry as it can fundamentally resolve the issue of shattering mobile phone displays, reduce the weight and thickness of large-area televisions, and apply Roll to Roll process in display manufacture.”
Supported by the Ministry of Knowledge Economy for five years, the technology has applied for 3 patents and is in discussion for technology transfer with related business.
Figure 1. The according (left) and discording (right) refractive indices of glass-fiber fabric and polyimide film. The characters on the left are sharp and clear, but the characters on the right appear foggy.
Figure 2. Picture of the developed glass-fiber fabric
2013.06.09 View 10012
Technology for Non-Breaking Smartphone Display Developed
High-strength plastic display has been developed by applying a glass-fiber fabric.
“Will bring about innovation to the field by replacing glass substrates”
It is now possible to manufacture non-breaking smartphone display. Heavy glass substrates of large-screen televisions will be replaced with light plastic films.
Professor Choon Sup Yoon from KAIST’s Department of Physics and KAIST Institute for Information Technology Convergence has developed the technology for high-strength plastic substrates to replace glass displays.
The plastic substrate created by Professor Yoon and his research team have greatly enhanced needed properties of heat resistance, transparency, flexibility, inner chemical capability, and tensile strength. Although the material retains flexibility as a native advantage of plastic film, its tensile strength is three times greater than that of normal glass, which is a degree similar to tempered glass. In addition, Professor Yoon’s substrate is as colorless and transparent as glass and resists heat up to 450℃, while its thermal expansivity is only 10% to 20% of existing plastics.
Glass substrates are currently used in practically every display such as mobile phone screens, televisions, and computer monitors for having smooth surface and satisfying basic conditions for display substrates. However, as glass substrates are heavy and easily broken, researchers studied colorless and transparent plastic polyimide films to replace glass substrates for their excellent thermal and chemical stability.
Nonetheless, colorless and transparent polyimide films do not have sufficient heat resistance and mechanical solidity. To resolve this problem, polyimide films are impregnated with glass-fiber fabrics, but it was far from commercialization as the impregnation exacerbates the roughness of surface and light transmittance. The roughness of the surface increases as the solvent evaporates in the impregnation process, resulting in surface roughness of around 0.4μm. The downturn in light transmittance is due to light scattering effect by the discording refractive index of polyimide film and glass-fiber fabric.
Professor Yoon’s research team resolved these issues by tuning the refractive indices of transparent polyimide film and glass-fiber fabric up to four decimal places, and by developing the technology of flattening the film’s surface roughness to a few nanometers. As a result, the research team achieved heat expansivity of 11ppm/℃, surface roughness of 0.9nm, tensile strength of 250MPa, bending curvature radius of 2mm, and light transmittance at 90% with a 110μm-thick glass-fiber fabric impregnated transparent polyimide film substrate.
“The developed substrate can not only replace the traditional glass substrate but also be applied as flexible display substrate,” said Professor Yoon in prospect, “it will bring about technological innovation in display industry as it can fundamentally resolve the issue of shattering mobile phone displays, reduce the weight and thickness of large-area televisions, and apply Roll to Roll process in display manufacture.”
Supported by the Ministry of Knowledge Economy for five years, the technology has applied for 3 patents and is in discussion for technology transfer with related business.
Figure 1. The according (left) and discording (right) refractive indices of glass-fiber fabric and polyimide film. The characters on the left are sharp and clear, but the characters on the right appear foggy.
Figure 2. Picture of the developed glass-fiber fabric
2013.06.09 View 10012 -
 President Nam Pyo Suh receives Honorary Doctorate from Bilkent University, Turkey
President of KAIST Nam Pyo Suh received an Honorary Doctorate from Turkey’s Bilkent University on June 13th, 2012.
Bilkent University revealed that it is President Suh’s invention of a plastic manufacture process used all over the world and the combination of academic achievements like the creation of the axiomatic design theory that merits the Honorary Doctorate.
After the presentation ceremony, President Suh gave a lecture to professors and students at Bilkent University on the "University of the Future: Changing Education Paradigm."
Bilkent University is located in Ankara, the capital of Turkey and was established in 1984, which is largely regarded as Turkey’s best private university. It ranked 32 out of 50 universities in Times Higher Educations’ 100 Under 50 List of World’s Best New Universities.
2012.06.18 View 9891
President Nam Pyo Suh receives Honorary Doctorate from Bilkent University, Turkey
President of KAIST Nam Pyo Suh received an Honorary Doctorate from Turkey’s Bilkent University on June 13th, 2012.
Bilkent University revealed that it is President Suh’s invention of a plastic manufacture process used all over the world and the combination of academic achievements like the creation of the axiomatic design theory that merits the Honorary Doctorate.
After the presentation ceremony, President Suh gave a lecture to professors and students at Bilkent University on the "University of the Future: Changing Education Paradigm."
Bilkent University is located in Ankara, the capital of Turkey and was established in 1984, which is largely regarded as Turkey’s best private university. It ranked 32 out of 50 universities in Times Higher Educations’ 100 Under 50 List of World’s Best New Universities.
2012.06.18 View 9891 -
 KAIST paves the way to commercialize flexible display screens
Source: IDTechEX, Feb. 28, 2011
KAIST paves the way to commercialize flexible display screens
28 Feb 2011
Transparent plastic and glass cloths, which have a limited thermal expansion needed for the production of flexible display screens and solar power cells, were developed by researchers at KAIST (Korea Advance Institute of Science & Technology).
The research, led by KAIST"s Professor Byoung-Soo Bae, was funded by the Engineering Research Center under the initiative of the Ministry of Education, Science and Technology and the National Research Foundation. The research result was printed as the cover paper of "Advanced Materials".
Professor Bae"s team developed a hybrid material with the same properties as fiber glass. With the material, they created a transparent, plastic film sheet resistant to heat. Transparent plastic film sheets were used by researchers all over the world to develop devices such as flexible displays or solar power cells that can be fit into various living spaces. However, plastic films are heat sensitive and tend to expand as temperature increases, thereby making it difficult to produce displays or solar power cells.
The new transparent, plastic film screen shows that heat expansion index (13ppm/oC) similar to that of glass fiber (9ppm/oC) due to the presence of glass fibers; its heat resistance allows to be used for displays and solar power cells over 250oC.
Professor Bae"s team succeeded in producing a flexible thin plastic film available for use in LCD or AMOLED screens and thin solar power cells.
Professor Bae commented, "Not only the newly developed plastic film has superior qualities, compared to the old models, but also it is cheap to produce, potentially bringing forward the day when flexible displays and solar panels become commonplace. With the cooperation of various industries, research institutes and universities, we will strive to improve the existing design and develop it further."
http://www.printedelectronicsworld.com/articles/kaist_paves_the_way_to_commercialize_flexible_display_screens_00003144.asp?sessionid=1
2011.03.01 View 16063
KAIST paves the way to commercialize flexible display screens
Source: IDTechEX, Feb. 28, 2011
KAIST paves the way to commercialize flexible display screens
28 Feb 2011
Transparent plastic and glass cloths, which have a limited thermal expansion needed for the production of flexible display screens and solar power cells, were developed by researchers at KAIST (Korea Advance Institute of Science & Technology).
The research, led by KAIST"s Professor Byoung-Soo Bae, was funded by the Engineering Research Center under the initiative of the Ministry of Education, Science and Technology and the National Research Foundation. The research result was printed as the cover paper of "Advanced Materials".
Professor Bae"s team developed a hybrid material with the same properties as fiber glass. With the material, they created a transparent, plastic film sheet resistant to heat. Transparent plastic film sheets were used by researchers all over the world to develop devices such as flexible displays or solar power cells that can be fit into various living spaces. However, plastic films are heat sensitive and tend to expand as temperature increases, thereby making it difficult to produce displays or solar power cells.
The new transparent, plastic film screen shows that heat expansion index (13ppm/oC) similar to that of glass fiber (9ppm/oC) due to the presence of glass fibers; its heat resistance allows to be used for displays and solar power cells over 250oC.
Professor Bae"s team succeeded in producing a flexible thin plastic film available for use in LCD or AMOLED screens and thin solar power cells.
Professor Bae commented, "Not only the newly developed plastic film has superior qualities, compared to the old models, but also it is cheap to produce, potentially bringing forward the day when flexible displays and solar panels become commonplace. With the cooperation of various industries, research institutes and universities, we will strive to improve the existing design and develop it further."
http://www.printedelectronicsworld.com/articles/kaist_paves_the_way_to_commercialize_flexible_display_screens_00003144.asp?sessionid=1
2011.03.01 View 16063 -
 KAIST developed a plastic film board less sensitive to heat.
The research result was made the cover of magazine, Advanced Materials and is accredited to paving the way to commercialize flexible display screens and solar power cells.
Transparent plastic and glass cloths, which have a limited thermal expansion needed for the production of flexible display screens and solar power cells, were developed by Korean researchers.
The research, led by KAIST’s Professor Byoung-Soo Bae, was funded by the Engineering Research Center under the initiative of the Ministry of Education, Science and Technology and the National Research Foundation. The research result was printed as the cover paper of ‘Advanced Materials’ which is the leading magazine in the field of materials science.
Professor Bae’s team developed a hybrid material with the same properties as fiber glass. With the material, they created a transparent, plastic film sheet resistant to heat. Transparent plastic film sheets were used by researchers all over the world to develop devices such as flexible displays or solar power cells that can be fit into various living spaces. However, plastic films are heat sensitive and tend to expand as temperature increases, thereby making it difficult to produce displays or solar power cells.
The new transparent, plastic film screen shows that heat expansion index (13ppm/oC) similar to that of glass fiber (9ppm/oC) due to the presence of glass fibers; its heat resistance allows to be used for displays and solar power cells over 250oC.
Professor Bae’s team succeeded in producing a flexible thin plastic film available for use in LCD or AMOLED screens and thin solar power cells.
Professor Bae commented, “Not only the newly developed plastic film has superior qualities, compared to the old models, but also it is cheap to produce, potentially bringing forward the day when flexible displays and solar panels become commonplace. With the cooperation of various industries, research institutes and universities, we will strive to improve the existing design and develop it further.”
2011.01.05 View 16800
KAIST developed a plastic film board less sensitive to heat.
The research result was made the cover of magazine, Advanced Materials and is accredited to paving the way to commercialize flexible display screens and solar power cells.
Transparent plastic and glass cloths, which have a limited thermal expansion needed for the production of flexible display screens and solar power cells, were developed by Korean researchers.
The research, led by KAIST’s Professor Byoung-Soo Bae, was funded by the Engineering Research Center under the initiative of the Ministry of Education, Science and Technology and the National Research Foundation. The research result was printed as the cover paper of ‘Advanced Materials’ which is the leading magazine in the field of materials science.
Professor Bae’s team developed a hybrid material with the same properties as fiber glass. With the material, they created a transparent, plastic film sheet resistant to heat. Transparent plastic film sheets were used by researchers all over the world to develop devices such as flexible displays or solar power cells that can be fit into various living spaces. However, plastic films are heat sensitive and tend to expand as temperature increases, thereby making it difficult to produce displays or solar power cells.
The new transparent, plastic film screen shows that heat expansion index (13ppm/oC) similar to that of glass fiber (9ppm/oC) due to the presence of glass fibers; its heat resistance allows to be used for displays and solar power cells over 250oC.
Professor Bae’s team succeeded in producing a flexible thin plastic film available for use in LCD or AMOLED screens and thin solar power cells.
Professor Bae commented, “Not only the newly developed plastic film has superior qualities, compared to the old models, but also it is cheap to produce, potentially bringing forward the day when flexible displays and solar panels become commonplace. With the cooperation of various industries, research institutes and universities, we will strive to improve the existing design and develop it further.”
2011.01.05 View 16800