Cells
-
 KAIST Unveils New Possibilities for Treating Intractable Brain Tumors
< Photo 1. (From left) Professor Heung Kyu Lee, KAIST Department of Biological Sciences, and Dr. Keun Bon Ku >
Immunotherapy, which enhances the immune system's T cell response to eliminate cancer cells, has emerged as a key approach in cancer treatment. However, in the case of glioblastoma, an aggressive and treatment-resistant brain tumor, numerous clinical trials have failed to confirm their efficacy. Korean researchers have recently analyzed the mechanisms that cause T cell exhaustion, which is characterized by a loss of function or a weakened response following prolonged exposure to antigens in such intractable cancers, identifying key control factors in T cell activation and clarifying the mechanisms that enhance therapeutic effectiveness.
KAIST (represented by President Kwang Hyung Lee) announced on the 6th of November that Professor Heung Kyu Lee’s team from the Department of Biological Sciences, in collaboration with the Korea Research Institute of Chemical Technology (represented by President Young Kuk Lee), has confirmed improved survival rates in a glioblastoma mouse model. By removing the inhibitory Fc gamma receptor (FcγRIIB), the research team was able to restore the responsiveness of cytotoxic T cells to immune checkpoint inhibitors, leading to enhanced anticancer activity.
The research team examined the effect of FcγRIIB, an inhibitory receptor recently found in cytotoxic T cells, on tumor-infiltrating T cells and the therapeutic effectiveness of the anti-PD-1 immune checkpoint inhibitor.
< Figure 1. Study results on improved survival rate due to increased antitumor activity of anti-PD-1 treatment in inhibitory Fc gamma receptor(Fcgr2b) ablation mice with murine glioblastoma. >
Their findings showed that deleting FcγRIIB induced the increase of tumor antigen-specific memory T cells, which helps to suppress exhaustion, enhances stem-like qualities, and reactivates T cell-mediated antitumor immunity, particularly in response to anti-PD-1 treatment. Furthermore, FcγRIIB deletion led to an increase in antigen-specific memory T cells that maintained continuous infiltration into the tumor tissue.
This study presents a new therapeutic target for tumors unresponsive to immune checkpoint inhibitors and demonstrates that combining FcγRIIB inhibition with anti-PD-1 treatment can produce synergistic effects, potentially improving therapeutic outcomes for tumors like glioblastoma, which typically show resistance to anti-PD-1 therapy.
< Figure 2. Overview of the study on the enhanced response to anti-PD-1 therapy for glioblastoma brain tumors upon deletion of the inhibitory Fc gamma receptor (FcγRIIB) in tumor microenvironment. When the inhibitory Fc gamma receptor (FcγRIIB) of cytotoxic T cells is deleted, an increase in tumor-specific memory T cells (Ttsms) was observed. In addition, this T cell subset is identified as originating from the tumor-draining lymph nodes(TdLNs) and leads to persistent infiltration into the tumor tissue. Anti-PD-1 therapy leads to an increased anti-tumor immune response via Ttsms, which is confirmed by increased tumor cell toxicity and increased cell division and decreased cell de-migration indices. Ultimately, the increased cytotoxic T cell immune response leads to an increase in the survival rate of glioblastoma. >
Professor Heung Kyu Lee explained, "This study offers a way to overcome clinical failures in treating brain tumors with immune checkpoint therapy and opens possibilities for broader applications to other intractable cancers. It also highlights the potential of utilizing cytotoxic T cells for tumor cell therapy."
The study, led by Dr. Keun Bon Ku of KAIST (currently a senior researcher at the Korea Research Institute of Chemical Technology's Center for Infectious Disease Diagnosis and Prevention), along with Chae Won Kim, Yumin Kim, Byeong Hoon Kang, Jeongwoo La, In Kang, Won Hyung Park, Stephen Ahn, and Sung Ki Lee, was published online on October 26 in the Journal for ImmunoTherapy of Cancer, an international journal in tumor immunology and therapy from the Society for Immunotherapy of Cancer. (Paper title: “Inhibitory Fcγ receptor deletion enhances CD8 T cell stemness increasing anti-PD-1 therapy responsiveness against glioblastoma,” http://dx.doi.org/10.1136/jitc-2024-009449).
This research received support from the National Research Foundation of Korea, the Bio & Medical Technology Development Program, and the Samsung Science & Technology Foundation.
2024.11.15 View 5078
KAIST Unveils New Possibilities for Treating Intractable Brain Tumors
< Photo 1. (From left) Professor Heung Kyu Lee, KAIST Department of Biological Sciences, and Dr. Keun Bon Ku >
Immunotherapy, which enhances the immune system's T cell response to eliminate cancer cells, has emerged as a key approach in cancer treatment. However, in the case of glioblastoma, an aggressive and treatment-resistant brain tumor, numerous clinical trials have failed to confirm their efficacy. Korean researchers have recently analyzed the mechanisms that cause T cell exhaustion, which is characterized by a loss of function or a weakened response following prolonged exposure to antigens in such intractable cancers, identifying key control factors in T cell activation and clarifying the mechanisms that enhance therapeutic effectiveness.
KAIST (represented by President Kwang Hyung Lee) announced on the 6th of November that Professor Heung Kyu Lee’s team from the Department of Biological Sciences, in collaboration with the Korea Research Institute of Chemical Technology (represented by President Young Kuk Lee), has confirmed improved survival rates in a glioblastoma mouse model. By removing the inhibitory Fc gamma receptor (FcγRIIB), the research team was able to restore the responsiveness of cytotoxic T cells to immune checkpoint inhibitors, leading to enhanced anticancer activity.
The research team examined the effect of FcγRIIB, an inhibitory receptor recently found in cytotoxic T cells, on tumor-infiltrating T cells and the therapeutic effectiveness of the anti-PD-1 immune checkpoint inhibitor.
< Figure 1. Study results on improved survival rate due to increased antitumor activity of anti-PD-1 treatment in inhibitory Fc gamma receptor(Fcgr2b) ablation mice with murine glioblastoma. >
Their findings showed that deleting FcγRIIB induced the increase of tumor antigen-specific memory T cells, which helps to suppress exhaustion, enhances stem-like qualities, and reactivates T cell-mediated antitumor immunity, particularly in response to anti-PD-1 treatment. Furthermore, FcγRIIB deletion led to an increase in antigen-specific memory T cells that maintained continuous infiltration into the tumor tissue.
This study presents a new therapeutic target for tumors unresponsive to immune checkpoint inhibitors and demonstrates that combining FcγRIIB inhibition with anti-PD-1 treatment can produce synergistic effects, potentially improving therapeutic outcomes for tumors like glioblastoma, which typically show resistance to anti-PD-1 therapy.
< Figure 2. Overview of the study on the enhanced response to anti-PD-1 therapy for glioblastoma brain tumors upon deletion of the inhibitory Fc gamma receptor (FcγRIIB) in tumor microenvironment. When the inhibitory Fc gamma receptor (FcγRIIB) of cytotoxic T cells is deleted, an increase in tumor-specific memory T cells (Ttsms) was observed. In addition, this T cell subset is identified as originating from the tumor-draining lymph nodes(TdLNs) and leads to persistent infiltration into the tumor tissue. Anti-PD-1 therapy leads to an increased anti-tumor immune response via Ttsms, which is confirmed by increased tumor cell toxicity and increased cell division and decreased cell de-migration indices. Ultimately, the increased cytotoxic T cell immune response leads to an increase in the survival rate of glioblastoma. >
Professor Heung Kyu Lee explained, "This study offers a way to overcome clinical failures in treating brain tumors with immune checkpoint therapy and opens possibilities for broader applications to other intractable cancers. It also highlights the potential of utilizing cytotoxic T cells for tumor cell therapy."
The study, led by Dr. Keun Bon Ku of KAIST (currently a senior researcher at the Korea Research Institute of Chemical Technology's Center for Infectious Disease Diagnosis and Prevention), along with Chae Won Kim, Yumin Kim, Byeong Hoon Kang, Jeongwoo La, In Kang, Won Hyung Park, Stephen Ahn, and Sung Ki Lee, was published online on October 26 in the Journal for ImmunoTherapy of Cancer, an international journal in tumor immunology and therapy from the Society for Immunotherapy of Cancer. (Paper title: “Inhibitory Fcγ receptor deletion enhances CD8 T cell stemness increasing anti-PD-1 therapy responsiveness against glioblastoma,” http://dx.doi.org/10.1136/jitc-2024-009449).
This research received support from the National Research Foundation of Korea, the Bio & Medical Technology Development Program, and the Samsung Science & Technology Foundation.
2024.11.15 View 5078 -
 KAIST presents strategies for Holotomography in advanced bio research
Measuring and analyzing three-dimensional (3D) images of live cells and tissues is considered crucial in advanced fields of biology and medicine. Organoids, which are 3D structures that mimic organs, are particular examples that significantly benefits 3D live imaging. Organoids provide effective alternatives to animal testing in the drug development processes, and can rapidly determine personalized medicine. On the other hand, active researches are ongoing to utilize organoids for organ replacement.
< Figure 1. Schematic illustration of holotomography compared to X-ray CT. Similar to CT, they share the commonality of measuring the optical properties of an unlabeled specimen in three dimensions. Instead of X-rays, holotomography irradiates light in the visible range, and provides refractive index measurements of transparent specimens rather than absorptivity. While CT obtains three-dimensional information only through mechanical rotation of the irradiating light, holotomography can replace this by applying wavefront control technology in the visible range. >
Organelle-level observation of 3D biological specimens such as organoids and stem cell colonies without staining or preprocessing holds significant implications for both innovating basic research and bioindustrial applications related to regenerative medicine and bioindustrial applications.
Holotomography (HT) is a 3D optical microscopy that implements 3D reconstruction analogous to that of X-ray computed tomography (CT). Although HT and CT share a similar theoretical background, HT facilitates high-resolution examination inside cells and tissues, instead of the human body. HT obtains 3D images of cells and tissues at the organelle level without chemical or genetic labeling, thus overcomes various challenges of existing methods in bio research and industry. Its potential is highlighted in research fields where sample physiology must not be disrupted, such as regenerative medicine, personalized medicine, and infertility treatment.
< Figure 2. Label-free 3D imaging of diverse live cells. Time-lapse image of Hep3B cells illustrating subcellular morphology changes upon H2O2 treatment, followed by cellular recovery after returning to the regular cell culture medium. >
This paper introduces the advantages and broad applicability of HT to biomedical researchers, while presenting an overview of principles and future technical challenges to optical researchers. It showcases various cases of applying HT in studies such as 3D biology, regenerative medicine, and cancer research, as well as suggesting future optical development. Also, it categorizes HT based on the light source, to describe the principles, limitations, and improvements of each category in detail. Particularly, the paper addresses strategies for deepening cell and organoid studies by introducing artificial intelligence (AI) to HT.
Due to its potential to drive advanced bioindustry, HT is attracting interest and investment from universities and corporates worldwide. The KAIST research team has been leading this international field by developing core technologies and carrying out key application researches throughout the last decade.
< Figure 3. Various types of cells and organelles that make up the imaging barrier of a living intestinal organoid can be observed using holotomography. >
This paper, co-authored by Dr. Geon Kim from KAIST Research Center for Natural Sciences, Professor Ki-Jun Yoon's team from the Department of Biological Sciences, Director Bon-Kyoung Koo's team from the Institute for Basic Science (IBS) Center for Genome Engineering, and Dr. Seongsoo Lee's team from the Korea Basic Science Institute (KBSI), was published in 'Nature Reviews Methods Primers' on the 25th of July. This research was supported by the Leader Grant and Basic Science Research Program of the National Research Foundation, the Hologram Core Technology Development Grant of the Ministry of Science and ICT, the Nano and Material Technology Development Project, and the Health and Medical R&D Project of the Ministry of Health and Welfare.
2024.07.30 View 5444
KAIST presents strategies for Holotomography in advanced bio research
Measuring and analyzing three-dimensional (3D) images of live cells and tissues is considered crucial in advanced fields of biology and medicine. Organoids, which are 3D structures that mimic organs, are particular examples that significantly benefits 3D live imaging. Organoids provide effective alternatives to animal testing in the drug development processes, and can rapidly determine personalized medicine. On the other hand, active researches are ongoing to utilize organoids for organ replacement.
< Figure 1. Schematic illustration of holotomography compared to X-ray CT. Similar to CT, they share the commonality of measuring the optical properties of an unlabeled specimen in three dimensions. Instead of X-rays, holotomography irradiates light in the visible range, and provides refractive index measurements of transparent specimens rather than absorptivity. While CT obtains three-dimensional information only through mechanical rotation of the irradiating light, holotomography can replace this by applying wavefront control technology in the visible range. >
Organelle-level observation of 3D biological specimens such as organoids and stem cell colonies without staining or preprocessing holds significant implications for both innovating basic research and bioindustrial applications related to regenerative medicine and bioindustrial applications.
Holotomography (HT) is a 3D optical microscopy that implements 3D reconstruction analogous to that of X-ray computed tomography (CT). Although HT and CT share a similar theoretical background, HT facilitates high-resolution examination inside cells and tissues, instead of the human body. HT obtains 3D images of cells and tissues at the organelle level without chemical or genetic labeling, thus overcomes various challenges of existing methods in bio research and industry. Its potential is highlighted in research fields where sample physiology must not be disrupted, such as regenerative medicine, personalized medicine, and infertility treatment.
< Figure 2. Label-free 3D imaging of diverse live cells. Time-lapse image of Hep3B cells illustrating subcellular morphology changes upon H2O2 treatment, followed by cellular recovery after returning to the regular cell culture medium. >
This paper introduces the advantages and broad applicability of HT to biomedical researchers, while presenting an overview of principles and future technical challenges to optical researchers. It showcases various cases of applying HT in studies such as 3D biology, regenerative medicine, and cancer research, as well as suggesting future optical development. Also, it categorizes HT based on the light source, to describe the principles, limitations, and improvements of each category in detail. Particularly, the paper addresses strategies for deepening cell and organoid studies by introducing artificial intelligence (AI) to HT.
Due to its potential to drive advanced bioindustry, HT is attracting interest and investment from universities and corporates worldwide. The KAIST research team has been leading this international field by developing core technologies and carrying out key application researches throughout the last decade.
< Figure 3. Various types of cells and organelles that make up the imaging barrier of a living intestinal organoid can be observed using holotomography. >
This paper, co-authored by Dr. Geon Kim from KAIST Research Center for Natural Sciences, Professor Ki-Jun Yoon's team from the Department of Biological Sciences, Director Bon-Kyoung Koo's team from the Institute for Basic Science (IBS) Center for Genome Engineering, and Dr. Seongsoo Lee's team from the Korea Basic Science Institute (KBSI), was published in 'Nature Reviews Methods Primers' on the 25th of July. This research was supported by the Leader Grant and Basic Science Research Program of the National Research Foundation, the Hologram Core Technology Development Grant of the Ministry of Science and ICT, the Nano and Material Technology Development Project, and the Health and Medical R&D Project of the Ministry of Health and Welfare.
2024.07.30 View 5444 -
 KAIST presents a fundamental technology to remove metastatic traits from lung cancer cells
KAIST (President Kwang Hyung Lee) announced on January 30th that a research team led by Professor Kwang-Hyun Cho from the Department of Bio and Brain Engineering succeeded in using systems biology research to change the properties of carcinogenic cells in the lungs and eliminate both drug resistance and their ability to proliferate out to other areas of the body.
As the incidences of cancer increase within aging populations, cancer has become the most lethal disease threatening healthy life. Fatality rates are especially high when early detection does not happen in time and metastasis has occurred in various organs. In order to resolve this problem, a series of attempts were made to remove or lower the ability of cancer cells to spread, but they resulted in cancer cells in the intermediate state becoming more unstable and even more malignant, which created serious treatment challenges.
Professor Kwang-Hyun Cho's research team simulated various cancer cell states in the Epithelial-to-Mesenchymal Transition (EMT) of lung cancer cells, between epithelial cells without metastatic ability and mesenchymal cells with metastatic ability. A mathematical model of molecular network was established, and key regulators that could reverse the state of invasive and drug resistant mesenchymal cells back to the epithelial state were discovered through computer simulation analysis and molecular cell experiments. In particular, this process succeeded in properly reverting the mesenchymal lung cancer cells to a state where they were sensitive to chemotherapy treatment while avoiding the unstable EMT hybrid cell state in the middle process, which had remained a difficult problem.
The results of this research, in which KAIST Ph.D. student Namhee Kim, Dr. Chae Young Hwang, Researcher Taeyoung Kim, and Ph.D. student Hyunjin Kim participated, were published as an online paper in the international journal “Cancer Research” published by the American Association for Cancer Research (AACR) on January 30th. (Paper title: A cell fate reprogramming strategy reverses epithelial-to-mesenchymal transition of lung cancer cells while avoiding hybrid states)
Cells in an EMT hybrid state, which are caused by incomplete transitions during the EMT process in cancer cells, have the characteristics of both epithelial cells and mesenchymal cells, and are known to have high drug resistance and metastatic potential by acquiring high stem cell capacity. In particular, EMT is further enhanced through factors such as transforming growth factor-beta (TGF-β) secreted from the tumor microenvironment (TME) and, as a result, various cell states with high plasticity appear. Due to the complexity of EMT, it has been very difficult to completely reverse the transitional process of the mesenchymal cancer cells to an epithelial cell state in which metastatic ability and drug resistance are eliminated while avoiding the EMT hybrid cell state with high metastatic ability and drug resistance.
Professor Kwang-Hyun Cho's research team established a mathematical model of the gene regulation network that governs the complex process of EMT, and then applied large-scale computer simulation analysis and complex system network control technology to identify and verify 'p53', 'SMAD4', and 'ERK1' and 'ERK 2' (collectively ERKs) through molecular cell experiments as the three key molecular targets that can transform lung cancer cells in the mesenchymal cell state, reversed back to an epithelial cell state that no longer demonstrates the ability to metastasize, while avoiding the EMT hybrid cell state.
In particular, by analyzing the molecular regulatory mechanism of the complex EMT process at the system level, the key pathways were identified that were linked to the positive feedback that plays an important role in completely returning cancer cells to an epithelial cell state in which metastatic ability and drug resistance are removed.
This discovery is significant in that it proved that mesenchymal cells can be reverted to the state of epithelial cells under conditions where TGF-β stimulation are present, like they are in the actual environment where cancer tissue forms in the human body.
Abnormal EMT in cancer cells leads to various malignant traits such as the migration and invasion of cancer cells, changes in responsiveness to chemotherapy treatment, enhanced stem cell function, and the dissemination of cancer. In particular, the acquisition of the metastatic ability of cancer cells is a key determinant factor for the prognosis of cancer patients. The EMT reversal technology in lung cancer cells developed in this research is a new anti-cancer treatment strategy that reprograms cancer cells to eliminate their high plasticity and metastatic potential and increase their responsiveness to chemotherapy.
Professor Kwang-Hyun Cho said, "By succeeding in reversing the state of lung cancer cells that acquired high metastatic traits and resistance to drugs and reverting them to a treatable epithelial cell state with renewed sensitivity to chemotherapy, the research findings propose a new strategy for treatments that can improve the prognosis of cancer patients.”
Professor Kwang-Hyun Cho's research team was the first to present the principle of reversal treatment to revert cancer cells to normal cells, following through with the announcement of the results of their study that reverted colon cancer cells to normal colon cells in January of 2020, and also presenting successful re-programming research where the most malignant basal type breast cancer cells turned into less-malignant luminal type breast cancer cells that were treatable with hormonal therapies in January of 2022. This latest research result is the third in the development of reversal technology where lung cancer cells that had acquired metastatic traits returned to a state in which their metastatic ability was removed and drug sensitivity was enhanced.
This research was carried out with support from the Ministry of Science and ICT and the National Research Foundation of Korea's Basic Research in Science & Engineering Program for Mid-Career Researchers.
< Figure 1. Construction of the mathematical model of the regulatory network to represent the EMT phenotype based on the interaction between various molecules related to EMT.
(A) Professor Kwang-Hyun Cho's research team investigated numerous literatures and databases related to complex EMT, and based on comparative analysis of cell line data showing epithelial and mesenchymal cell conditions, they extracted key signaling pathways related to EMT and built a mathematical model of regulatory network (B) By comparing the results of computer simulation analysis and the molecular cell experiments, it was verified how well the constructed mathematical model simulated the actual cellular phenomena. >
< Figure 2. Understanding of various EMT phenotypes through large-scale computer simulation analysis and complex system network control technology.
(A) Through computer simulation analysis and experiments, Professor Kwang-Hyun Cho's research team found that complete control of EMT is impossible with single-molecule control alone. In particular, through comparison of the relative stability of attractors, it was revealed that the cell state exhibiting EMT hybrid characteristics has unstable properties. (B), (C) Based on these results, Prof. Cho’s team identified two feedbacks (positive feedback consisting of Snail-miR-34 and ZEB1-miR-200) that play an important role in avoiding the EMT hybrid state that appeared in the TGF-β-ON state. It was found through computer simulation analysis that the two feedbacks restore relatively high stability when the excavated p53 and SMAD4 are regulated. In addition, molecular cell experiments demonstrated that the expression levels of E-cad and ZEB1, which are representative phenotypic markers of EMT, changed similarly to the expression profile in the epithelial cell state, despite the TGF-β-ON state. >
< Figure 3. Complex molecular network analysis and discovery of reprogramming molecular targets for intact elimination of EMT hybrid features.
(A) Controlling the expression of p53 and SMAD4 in lung cancer cell lines was expected to overcome drug resistance, but contrary to expectations, chemotherapy responsiveness was not restored. (B) Professor Kwang-Hyun Cho's research team additionally analyzed computer simulations, genome data, and experimental results and found that high expression levels of TWIST1 and EPCAM were related to drug resistance. (C) Prof. Cho’s team identified three key molecular targets: p53, SMAD4 and ERK1 & ERK2. (D), (E) Furthermore, they identified a key pathway that plays an important role in completely reversing into epithelial cells while avoiding EMT hybrid characteristics, and confirmed through network analysis and attractor analysis that high stability of the key pathway was restored when the proposed molecular target was controlled. >
< Figure 4. Verification through experiments with lung cancer cell lines.
When p53 was activated and SMAD4 and ERK1/2 were inhibited in lung cancer cell lines, (A), (B) E-cad protein expression increased and ZEB1 protein expression decreased, and (C) mesenchymal cell status including TWIST1 and EPCAM and gene expression of markers related to stem cell potential characteristics were completely inhibited. In addition, (D) it was confirmed that resistance to chemotherapy treatment was also overcome as the cell state was reversed by the regulated target. >
< Figure 5. A schematic representation of the research results.
Prof. Cho’s research team identified key molecular regulatory pathways to avoid high plasticity formed by abnormal EMT of cancer cells and reverse it to an epithelial cell state through systems biology research. From this analysis, a reprogramming molecular target that can reverse the state of mesenchymal cells with acquired invasiveness and drug resistance to the state of epithelial cells with restored drug responsiveness was discovered.
For lung cancer cells, when a drug that enhances the expression of p53, one of the molecular targets discovered, and inhibits the expression of SMAD4 and ERK1 & ERK2 is administered, the molecular network of genes in the state of mesenchymal cells is modified, eventually eliminating metastatic ability and it is reprogrammed to turn into epithelial cells without the resistance to chemotherapy treatments. >
2023.01.30 View 18815
KAIST presents a fundamental technology to remove metastatic traits from lung cancer cells
KAIST (President Kwang Hyung Lee) announced on January 30th that a research team led by Professor Kwang-Hyun Cho from the Department of Bio and Brain Engineering succeeded in using systems biology research to change the properties of carcinogenic cells in the lungs and eliminate both drug resistance and their ability to proliferate out to other areas of the body.
As the incidences of cancer increase within aging populations, cancer has become the most lethal disease threatening healthy life. Fatality rates are especially high when early detection does not happen in time and metastasis has occurred in various organs. In order to resolve this problem, a series of attempts were made to remove or lower the ability of cancer cells to spread, but they resulted in cancer cells in the intermediate state becoming more unstable and even more malignant, which created serious treatment challenges.
Professor Kwang-Hyun Cho's research team simulated various cancer cell states in the Epithelial-to-Mesenchymal Transition (EMT) of lung cancer cells, between epithelial cells without metastatic ability and mesenchymal cells with metastatic ability. A mathematical model of molecular network was established, and key regulators that could reverse the state of invasive and drug resistant mesenchymal cells back to the epithelial state were discovered through computer simulation analysis and molecular cell experiments. In particular, this process succeeded in properly reverting the mesenchymal lung cancer cells to a state where they were sensitive to chemotherapy treatment while avoiding the unstable EMT hybrid cell state in the middle process, which had remained a difficult problem.
The results of this research, in which KAIST Ph.D. student Namhee Kim, Dr. Chae Young Hwang, Researcher Taeyoung Kim, and Ph.D. student Hyunjin Kim participated, were published as an online paper in the international journal “Cancer Research” published by the American Association for Cancer Research (AACR) on January 30th. (Paper title: A cell fate reprogramming strategy reverses epithelial-to-mesenchymal transition of lung cancer cells while avoiding hybrid states)
Cells in an EMT hybrid state, which are caused by incomplete transitions during the EMT process in cancer cells, have the characteristics of both epithelial cells and mesenchymal cells, and are known to have high drug resistance and metastatic potential by acquiring high stem cell capacity. In particular, EMT is further enhanced through factors such as transforming growth factor-beta (TGF-β) secreted from the tumor microenvironment (TME) and, as a result, various cell states with high plasticity appear. Due to the complexity of EMT, it has been very difficult to completely reverse the transitional process of the mesenchymal cancer cells to an epithelial cell state in which metastatic ability and drug resistance are eliminated while avoiding the EMT hybrid cell state with high metastatic ability and drug resistance.
Professor Kwang-Hyun Cho's research team established a mathematical model of the gene regulation network that governs the complex process of EMT, and then applied large-scale computer simulation analysis and complex system network control technology to identify and verify 'p53', 'SMAD4', and 'ERK1' and 'ERK 2' (collectively ERKs) through molecular cell experiments as the three key molecular targets that can transform lung cancer cells in the mesenchymal cell state, reversed back to an epithelial cell state that no longer demonstrates the ability to metastasize, while avoiding the EMT hybrid cell state.
In particular, by analyzing the molecular regulatory mechanism of the complex EMT process at the system level, the key pathways were identified that were linked to the positive feedback that plays an important role in completely returning cancer cells to an epithelial cell state in which metastatic ability and drug resistance are removed.
This discovery is significant in that it proved that mesenchymal cells can be reverted to the state of epithelial cells under conditions where TGF-β stimulation are present, like they are in the actual environment where cancer tissue forms in the human body.
Abnormal EMT in cancer cells leads to various malignant traits such as the migration and invasion of cancer cells, changes in responsiveness to chemotherapy treatment, enhanced stem cell function, and the dissemination of cancer. In particular, the acquisition of the metastatic ability of cancer cells is a key determinant factor for the prognosis of cancer patients. The EMT reversal technology in lung cancer cells developed in this research is a new anti-cancer treatment strategy that reprograms cancer cells to eliminate their high plasticity and metastatic potential and increase their responsiveness to chemotherapy.
Professor Kwang-Hyun Cho said, "By succeeding in reversing the state of lung cancer cells that acquired high metastatic traits and resistance to drugs and reverting them to a treatable epithelial cell state with renewed sensitivity to chemotherapy, the research findings propose a new strategy for treatments that can improve the prognosis of cancer patients.”
Professor Kwang-Hyun Cho's research team was the first to present the principle of reversal treatment to revert cancer cells to normal cells, following through with the announcement of the results of their study that reverted colon cancer cells to normal colon cells in January of 2020, and also presenting successful re-programming research where the most malignant basal type breast cancer cells turned into less-malignant luminal type breast cancer cells that were treatable with hormonal therapies in January of 2022. This latest research result is the third in the development of reversal technology where lung cancer cells that had acquired metastatic traits returned to a state in which their metastatic ability was removed and drug sensitivity was enhanced.
This research was carried out with support from the Ministry of Science and ICT and the National Research Foundation of Korea's Basic Research in Science & Engineering Program for Mid-Career Researchers.
< Figure 1. Construction of the mathematical model of the regulatory network to represent the EMT phenotype based on the interaction between various molecules related to EMT.
(A) Professor Kwang-Hyun Cho's research team investigated numerous literatures and databases related to complex EMT, and based on comparative analysis of cell line data showing epithelial and mesenchymal cell conditions, they extracted key signaling pathways related to EMT and built a mathematical model of regulatory network (B) By comparing the results of computer simulation analysis and the molecular cell experiments, it was verified how well the constructed mathematical model simulated the actual cellular phenomena. >
< Figure 2. Understanding of various EMT phenotypes through large-scale computer simulation analysis and complex system network control technology.
(A) Through computer simulation analysis and experiments, Professor Kwang-Hyun Cho's research team found that complete control of EMT is impossible with single-molecule control alone. In particular, through comparison of the relative stability of attractors, it was revealed that the cell state exhibiting EMT hybrid characteristics has unstable properties. (B), (C) Based on these results, Prof. Cho’s team identified two feedbacks (positive feedback consisting of Snail-miR-34 and ZEB1-miR-200) that play an important role in avoiding the EMT hybrid state that appeared in the TGF-β-ON state. It was found through computer simulation analysis that the two feedbacks restore relatively high stability when the excavated p53 and SMAD4 are regulated. In addition, molecular cell experiments demonstrated that the expression levels of E-cad and ZEB1, which are representative phenotypic markers of EMT, changed similarly to the expression profile in the epithelial cell state, despite the TGF-β-ON state. >
< Figure 3. Complex molecular network analysis and discovery of reprogramming molecular targets for intact elimination of EMT hybrid features.
(A) Controlling the expression of p53 and SMAD4 in lung cancer cell lines was expected to overcome drug resistance, but contrary to expectations, chemotherapy responsiveness was not restored. (B) Professor Kwang-Hyun Cho's research team additionally analyzed computer simulations, genome data, and experimental results and found that high expression levels of TWIST1 and EPCAM were related to drug resistance. (C) Prof. Cho’s team identified three key molecular targets: p53, SMAD4 and ERK1 & ERK2. (D), (E) Furthermore, they identified a key pathway that plays an important role in completely reversing into epithelial cells while avoiding EMT hybrid characteristics, and confirmed through network analysis and attractor analysis that high stability of the key pathway was restored when the proposed molecular target was controlled. >
< Figure 4. Verification through experiments with lung cancer cell lines.
When p53 was activated and SMAD4 and ERK1/2 were inhibited in lung cancer cell lines, (A), (B) E-cad protein expression increased and ZEB1 protein expression decreased, and (C) mesenchymal cell status including TWIST1 and EPCAM and gene expression of markers related to stem cell potential characteristics were completely inhibited. In addition, (D) it was confirmed that resistance to chemotherapy treatment was also overcome as the cell state was reversed by the regulated target. >
< Figure 5. A schematic representation of the research results.
Prof. Cho’s research team identified key molecular regulatory pathways to avoid high plasticity formed by abnormal EMT of cancer cells and reverse it to an epithelial cell state through systems biology research. From this analysis, a reprogramming molecular target that can reverse the state of mesenchymal cells with acquired invasiveness and drug resistance to the state of epithelial cells with restored drug responsiveness was discovered.
For lung cancer cells, when a drug that enhances the expression of p53, one of the molecular targets discovered, and inhibits the expression of SMAD4 and ERK1 & ERK2 is administered, the molecular network of genes in the state of mesenchymal cells is modified, eventually eliminating metastatic ability and it is reprogrammed to turn into epithelial cells without the resistance to chemotherapy treatments. >
2023.01.30 View 18815 -
 A KAIST Research Team Develops Diesel Reforming Catalyst Enabling Hydrogen Production for Future Mobile Fuel Cells
This catalyst capability allowing stable hydrogen production from commercial diesel is expected to be applied in mobile fuel cell systems in the future hydrogen economy
On August 16, a joint research team led by Professors Joongmyeon Bae and Kang Taek Lee of KAIST’s Department of Mechanical Engineering and Dr. Chan-Woo Lee of Korea Institute of Energy Research (KIER) announced the successful development of a highly active and durable reforming catalyst allowing hydrogen production from commercial diesel.
Fuel reforming is a hydrogen production technique that extracts hydrogen from hydrocarbons through catalytic reactions. Diesel, being a liquid fuel, has a high storage density for hydrogen and is easy to transport and store. There have therefore been continuous research efforts to apply hydrogel supply systems using diesel reformation in mobile fuel cells, such as for auxiliary power in heavy trucks or air-independent propulsion (AIP) systems in submarines.
However, diesel is a mixture of high hydrocarbons including long-chained paraffin, double-bonded olefin, and aromatic hydrocarbons with benzene groups, and it requires a highly active catalyst to effectively break them down. In addition, the catalyst must be extremely durable against caulking and sintering, as they are often the main causes of catalyst degradation. Such challenges have limited the use of diesel reformation technologies to date.
The joint research team successfully developed a highly active and durable diesel reforming catalyst through elution (a heat treatment method used to uniformly grow active metals retained in an oxide support as ions in the form of metal nanoparticles), forming alloy nanoparticles. The design was based on the fact that eluted nanoparticles strongly interact with the support, allowing a high degree of dispersion at high temperatures, and that producing an alloy from dissimilar metals can increase the performance of catalysts through a synergistic effect.
The research team introduced a solution combustion synthesis method to produce a multi-component catalyst with a trace amount of platinum (Pt) and ruthenium (Ru) penetrated into a ceria (CeO2) lattice, which is a structure commonly used as a support for catalysts in redox reactions. When exposed to a diesel reforming reaction environment, the catalyst induces Pt-Ru alloy nanoparticle formation upon Pt and Ru elution onto the support surface.
In addition to the catalyst analysis, the research team also succeeded in characterizing the behaviour of active metal elution and alloy formation from an energetic perspective using a density functional theory-based calculation. In a performance comparison test between the Pt-Ru alloy catalyst against existing single-metal catalysts, the reforming activity was shown to have improved, as it showed a 100% fuel conversion rate even at a low temperature (600oC, compared to the original 800oC). In a long-term durability test (800oC, 200 hours), the catalyst showed commercial stability by successfully producing hydrogen from commercial diesel without performance degradation.
The study was conducted by Ph.D. candidate Jaemyung Lee of KAIST’s Department of Mechanical Engineering as the first author. Ph.D. candidate Changho Yeon of KIER, Dr. Jiwoo Oh of KAIST’s Department of Mechanical Engineering, Dr. Gwangwoo Han of KIER, Ph.D. candidate Jeong Do Yoo of KAIST’s Department of Mechanical Engineering, and Dr. Hyung Joong Yun of the Korea Basic Science Institute contributed as co-authors. Dr. Chan-Woo Lee of KIER and Professors Kang Taek Lee and Joongmyeon Bae of KAIST’s Department of Mechanical Engineering contributed as corresponding authors. The research was published in the online version of Applied Catalysis B: Environmental (IF 24.319, JCR 0.93%) on June 17, under the title “Highly Active and Stable Catalyst with Exsolved PtRu Alloy Nanoparticles for Hydrogen Production via Commercial Diesel Reforming”.
Professor Joongmyeon Bae said, “The fact that hydrogen can be stably produced from commercial diesel makes this a very meaningful achievement, and we look forward to this technology contributing to the active introduction of mobile fuel cell systems in the early hydrogen economy.” He added, “Our approach to catalyst design may be applied not only to reforming reactions, but also in various other fields.”
This research was supported by the National Research Foundation of Korea through funding from the Ministry of Science, ICT and Future Planning.
Figure. Schematic diagram of high-performance diesel reforming catalyst with eluted platinum-ruthenium alloy nanoparticles and long-term durability verification experiment results for commercial diesel reforming reaction
2022.09.07 View 14963
A KAIST Research Team Develops Diesel Reforming Catalyst Enabling Hydrogen Production for Future Mobile Fuel Cells
This catalyst capability allowing stable hydrogen production from commercial diesel is expected to be applied in mobile fuel cell systems in the future hydrogen economy
On August 16, a joint research team led by Professors Joongmyeon Bae and Kang Taek Lee of KAIST’s Department of Mechanical Engineering and Dr. Chan-Woo Lee of Korea Institute of Energy Research (KIER) announced the successful development of a highly active and durable reforming catalyst allowing hydrogen production from commercial diesel.
Fuel reforming is a hydrogen production technique that extracts hydrogen from hydrocarbons through catalytic reactions. Diesel, being a liquid fuel, has a high storage density for hydrogen and is easy to transport and store. There have therefore been continuous research efforts to apply hydrogel supply systems using diesel reformation in mobile fuel cells, such as for auxiliary power in heavy trucks or air-independent propulsion (AIP) systems in submarines.
However, diesel is a mixture of high hydrocarbons including long-chained paraffin, double-bonded olefin, and aromatic hydrocarbons with benzene groups, and it requires a highly active catalyst to effectively break them down. In addition, the catalyst must be extremely durable against caulking and sintering, as they are often the main causes of catalyst degradation. Such challenges have limited the use of diesel reformation technologies to date.
The joint research team successfully developed a highly active and durable diesel reforming catalyst through elution (a heat treatment method used to uniformly grow active metals retained in an oxide support as ions in the form of metal nanoparticles), forming alloy nanoparticles. The design was based on the fact that eluted nanoparticles strongly interact with the support, allowing a high degree of dispersion at high temperatures, and that producing an alloy from dissimilar metals can increase the performance of catalysts through a synergistic effect.
The research team introduced a solution combustion synthesis method to produce a multi-component catalyst with a trace amount of platinum (Pt) and ruthenium (Ru) penetrated into a ceria (CeO2) lattice, which is a structure commonly used as a support for catalysts in redox reactions. When exposed to a diesel reforming reaction environment, the catalyst induces Pt-Ru alloy nanoparticle formation upon Pt and Ru elution onto the support surface.
In addition to the catalyst analysis, the research team also succeeded in characterizing the behaviour of active metal elution and alloy formation from an energetic perspective using a density functional theory-based calculation. In a performance comparison test between the Pt-Ru alloy catalyst against existing single-metal catalysts, the reforming activity was shown to have improved, as it showed a 100% fuel conversion rate even at a low temperature (600oC, compared to the original 800oC). In a long-term durability test (800oC, 200 hours), the catalyst showed commercial stability by successfully producing hydrogen from commercial diesel without performance degradation.
The study was conducted by Ph.D. candidate Jaemyung Lee of KAIST’s Department of Mechanical Engineering as the first author. Ph.D. candidate Changho Yeon of KIER, Dr. Jiwoo Oh of KAIST’s Department of Mechanical Engineering, Dr. Gwangwoo Han of KIER, Ph.D. candidate Jeong Do Yoo of KAIST’s Department of Mechanical Engineering, and Dr. Hyung Joong Yun of the Korea Basic Science Institute contributed as co-authors. Dr. Chan-Woo Lee of KIER and Professors Kang Taek Lee and Joongmyeon Bae of KAIST’s Department of Mechanical Engineering contributed as corresponding authors. The research was published in the online version of Applied Catalysis B: Environmental (IF 24.319, JCR 0.93%) on June 17, under the title “Highly Active and Stable Catalyst with Exsolved PtRu Alloy Nanoparticles for Hydrogen Production via Commercial Diesel Reforming”.
Professor Joongmyeon Bae said, “The fact that hydrogen can be stably produced from commercial diesel makes this a very meaningful achievement, and we look forward to this technology contributing to the active introduction of mobile fuel cell systems in the early hydrogen economy.” He added, “Our approach to catalyst design may be applied not only to reforming reactions, but also in various other fields.”
This research was supported by the National Research Foundation of Korea through funding from the Ministry of Science, ICT and Future Planning.
Figure. Schematic diagram of high-performance diesel reforming catalyst with eluted platinum-ruthenium alloy nanoparticles and long-term durability verification experiment results for commercial diesel reforming reaction
2022.09.07 View 14963 -
 PICASSO Technique Drives Biological Molecules into Technicolor
The new imaging approach brings current imaging colors from four to more than 15 for mapping overlapping proteins
Pablo Picasso’s surreal cubist artistic style shifted common features into unrecognizable scenes, but a new imaging approach bearing his namesake may elucidate the most complicated subject: the brain. Employing artificial intelligence to clarify spectral color blending of tiny molecules used to stain specific proteins and other items of research interest, the PICASSO technique, allows researchers to use more than 15 colors to image and parse our overlapping proteins.
The PICASSO developers, based in Korea, published their approach on May 5 in Nature Communications.
Fluorophores — the staining molecules — emit specific colors when excited by a light, but if more than four fluorophores are used, their emitted colors overlap and blend. Researchers previously developed techniques to correct this spectral overlap by precisely defining the matrix of mixed and unmixed images. This measurement depends on reference spectra, found by identifying clear images of only one fluorophore-stained specimen or of multiple, identically prepared specimens that only contain a single fluorophore each.
“Such reference spectra measurement could be complicated to perform in highly heterogeneous specimens, such as the brain, due to the highly varied emission spectra of fluorophores depending on the subregions from which the spectra were measured,” said co-corresponding author Young-Gyu Yoon, professor in the School of Electrical Engineering at KAIST. He explained that the subregions would each need their own spectra reference measurements, making for an inefficient, time-consuming process. “To address this problem, we developed an approach that does not require reference spectra measurements.”
The approach is the “Process of ultra-multiplexed Imaging of biomolecules viA the unmixing of the Signals of Spectrally Overlapping fluorophores,” also known as PICASSO. Ultra-multiplexed imaging refers to visualizing the numerous individual components of a unit. Like a cinema multiplex in which each theater plays a different movie, each protein in a cell has a different role. By staining with fluorophores, researchers can begin to understand those roles.
“We devised a strategy based on information theory; unmixing is performed by iteratively minimizing the mutual information between mixed images,” said co-corresponding author Jae-Byum Chang, professor in the Department of Materials Science and Engineering, KAIST. “This allows us to get away with the assumption that the spatial distribution of different proteins is mutually exclusive and enables accurate information unmixing.”
To demonstrate PICASSO’s capabilities, the researchers applied the technique to imaging a mouse brain. With a single round of staining, they performed 15-color multiplexed imaging of a mouse brain. Although small, mouse brains are still complex, multifaceted organs that can take significant resources to map. According to the researchers, PICASSO can improve the capabilities of other imaging techniques and allow for the use of even more fluorophore colors.
Using one such imaging technique in combination with PICASSO, the team achieved 45-color multiplexed imaging of the mouse brain in only three staining and imaging cycles, according to Yoon.
“PICASSO is a versatile tool for the multiplexed biomolecule imaging of cultured cells, tissue slices and clinical specimens,” Chang said. “We anticipate that PICASSO will be useful for a broad range of applications for which biomolecules’ spatial information is important. One such application the tool would be useful for is revealing the cellular heterogeneities of tumor microenvironments, especially the heterogeneous populations of immune cells, which are closely related to cancer prognoses and the efficacy of cancer therapies.”
The Samsung Research Funding & Incubation Center for Future Technology supported this work. Spectral imaging was performed at the Korea Basic Science Institute Western Seoul Center.
-PublicationJunyoung Seo, Yeonbo Sim, Jeewon Kim, Hyunwoo Kim, In Cho, Hoyeon Nam, Yong-Gyu Yoon, Jae-Byum Chang, “PICASSO allows ultra-multiplexed fluorescence imaging of spatiallyoverlapping proteins without reference spectra measurements,” May 5, Nature Communications (doi.org/10.1038/s41467-022-30168-z)
-ProfileProfessor Jae-Byum ChangDepartment of Materials Science and EngineeringCollege of EngineeringKAIST
Professor Young-Gyu YoonSchool of Electrical EngineeringCollege of EngineeringKAIST
2022.06.22 View 11226
PICASSO Technique Drives Biological Molecules into Technicolor
The new imaging approach brings current imaging colors from four to more than 15 for mapping overlapping proteins
Pablo Picasso’s surreal cubist artistic style shifted common features into unrecognizable scenes, but a new imaging approach bearing his namesake may elucidate the most complicated subject: the brain. Employing artificial intelligence to clarify spectral color blending of tiny molecules used to stain specific proteins and other items of research interest, the PICASSO technique, allows researchers to use more than 15 colors to image and parse our overlapping proteins.
The PICASSO developers, based in Korea, published their approach on May 5 in Nature Communications.
Fluorophores — the staining molecules — emit specific colors when excited by a light, but if more than four fluorophores are used, their emitted colors overlap and blend. Researchers previously developed techniques to correct this spectral overlap by precisely defining the matrix of mixed and unmixed images. This measurement depends on reference spectra, found by identifying clear images of only one fluorophore-stained specimen or of multiple, identically prepared specimens that only contain a single fluorophore each.
“Such reference spectra measurement could be complicated to perform in highly heterogeneous specimens, such as the brain, due to the highly varied emission spectra of fluorophores depending on the subregions from which the spectra were measured,” said co-corresponding author Young-Gyu Yoon, professor in the School of Electrical Engineering at KAIST. He explained that the subregions would each need their own spectra reference measurements, making for an inefficient, time-consuming process. “To address this problem, we developed an approach that does not require reference spectra measurements.”
The approach is the “Process of ultra-multiplexed Imaging of biomolecules viA the unmixing of the Signals of Spectrally Overlapping fluorophores,” also known as PICASSO. Ultra-multiplexed imaging refers to visualizing the numerous individual components of a unit. Like a cinema multiplex in which each theater plays a different movie, each protein in a cell has a different role. By staining with fluorophores, researchers can begin to understand those roles.
“We devised a strategy based on information theory; unmixing is performed by iteratively minimizing the mutual information between mixed images,” said co-corresponding author Jae-Byum Chang, professor in the Department of Materials Science and Engineering, KAIST. “This allows us to get away with the assumption that the spatial distribution of different proteins is mutually exclusive and enables accurate information unmixing.”
To demonstrate PICASSO’s capabilities, the researchers applied the technique to imaging a mouse brain. With a single round of staining, they performed 15-color multiplexed imaging of a mouse brain. Although small, mouse brains are still complex, multifaceted organs that can take significant resources to map. According to the researchers, PICASSO can improve the capabilities of other imaging techniques and allow for the use of even more fluorophore colors.
Using one such imaging technique in combination with PICASSO, the team achieved 45-color multiplexed imaging of the mouse brain in only three staining and imaging cycles, according to Yoon.
“PICASSO is a versatile tool for the multiplexed biomolecule imaging of cultured cells, tissue slices and clinical specimens,” Chang said. “We anticipate that PICASSO will be useful for a broad range of applications for which biomolecules’ spatial information is important. One such application the tool would be useful for is revealing the cellular heterogeneities of tumor microenvironments, especially the heterogeneous populations of immune cells, which are closely related to cancer prognoses and the efficacy of cancer therapies.”
The Samsung Research Funding & Incubation Center for Future Technology supported this work. Spectral imaging was performed at the Korea Basic Science Institute Western Seoul Center.
-PublicationJunyoung Seo, Yeonbo Sim, Jeewon Kim, Hyunwoo Kim, In Cho, Hoyeon Nam, Yong-Gyu Yoon, Jae-Byum Chang, “PICASSO allows ultra-multiplexed fluorescence imaging of spatiallyoverlapping proteins without reference spectra measurements,” May 5, Nature Communications (doi.org/10.1038/s41467-022-30168-z)
-ProfileProfessor Jae-Byum ChangDepartment of Materials Science and EngineeringCollege of EngineeringKAIST
Professor Young-Gyu YoonSchool of Electrical EngineeringCollege of EngineeringKAIST
2022.06.22 View 11226 -
 Five Projects Ranked in the Top 100 for National R&D Excellence
Five KAIST research projects were selected as the 2021 Top 100 for National R&D Excellence by the Ministry of Science and ICT and the Korea Institute of Science & Technology Evaluation and Planning.
The five projects are:-The development of E. coli that proliferates with only formic acid and carbon dioxide by Distinguished Professor Sang Yup Lee from the Department of Chemical and Biomolecular Engineering
-An original reverse aging technology that restores an old human skin cell into a younger one by Professor Kwang-Hyun Cho from the Department of Bio and Brain Engineering-The development of next-generation high-efficiency perovskite-silicon tandem solar cells by Professor Byungha Shin from the Department of Materials Science and Engineering-Research on the effects of ultrafine dust in the atmosphere has on energy consumption by Professor Jiyong Eom from the School of Business and Technology Management-Research on a molecular trigger that controls the phase transformation of bio materials by Professor Myungchul Kim from the Department of Bio and Brain Engineering
Started in 2006, an Evaluation Committee composed of experts in industries, universities, and research institutes has made the preliminary selections of the most outstanding research projects based on their significance as a scientific and technological development and their socioeconomic effects. The finalists went through an open public evaluation. The final 100 studies are from six fields: 18 from mechanics & materials, 26 from biology & marine sciences, 19 from ICT & electronics, 10 from interdisciplinary research, and nine from natural science and infrastructure.
The selected 100 studies will receive a certificate and an award plaque from the minister of MSIT as well as additional points for business and institutional evaluations according to appropriate regulations, and the selected researchers will be strongly recommended as candidates for national meritorious awards.
In particular, to help the 100 selected research projects become more accessible for the general public, their main contents will be provided in a free e-book ‘The Top 100 for National R&D Excellence of 2021’ that will be available from online booksellers.
2022.02.17 View 11726
Five Projects Ranked in the Top 100 for National R&D Excellence
Five KAIST research projects were selected as the 2021 Top 100 for National R&D Excellence by the Ministry of Science and ICT and the Korea Institute of Science & Technology Evaluation and Planning.
The five projects are:-The development of E. coli that proliferates with only formic acid and carbon dioxide by Distinguished Professor Sang Yup Lee from the Department of Chemical and Biomolecular Engineering
-An original reverse aging technology that restores an old human skin cell into a younger one by Professor Kwang-Hyun Cho from the Department of Bio and Brain Engineering-The development of next-generation high-efficiency perovskite-silicon tandem solar cells by Professor Byungha Shin from the Department of Materials Science and Engineering-Research on the effects of ultrafine dust in the atmosphere has on energy consumption by Professor Jiyong Eom from the School of Business and Technology Management-Research on a molecular trigger that controls the phase transformation of bio materials by Professor Myungchul Kim from the Department of Bio and Brain Engineering
Started in 2006, an Evaluation Committee composed of experts in industries, universities, and research institutes has made the preliminary selections of the most outstanding research projects based on their significance as a scientific and technological development and their socioeconomic effects. The finalists went through an open public evaluation. The final 100 studies are from six fields: 18 from mechanics & materials, 26 from biology & marine sciences, 19 from ICT & electronics, 10 from interdisciplinary research, and nine from natural science and infrastructure.
The selected 100 studies will receive a certificate and an award plaque from the minister of MSIT as well as additional points for business and institutional evaluations according to appropriate regulations, and the selected researchers will be strongly recommended as candidates for national meritorious awards.
In particular, to help the 100 selected research projects become more accessible for the general public, their main contents will be provided in a free e-book ‘The Top 100 for National R&D Excellence of 2021’ that will be available from online booksellers.
2022.02.17 View 11726 -
 Hydrogel-Based Flexible Brain-Machine Interface
The interface is easy to insert into the body when dry, but behaves ‘stealthily’ inside the brain when wet
Professor Seongjun Park’s research team and collaborators revealed a newly developed hydrogel-based flexible brain-machine interface. To study the structure of the brain or to identify and treat neurological diseases, it is crucial to develop an interface that can stimulate the brain and detect its signals in real time. However, existing neural interfaces are mechanically and chemically different from real brain tissue. This causes foreign body response and forms an insulating layer (glial scar) around the interface, which shortens its lifespan.
To solve this problem, the research team developed a ‘brain-mimicking interface’ by inserting a custom-made multifunctional fiber bundle into the hydrogel body. The device is composed not only of an optical fiber that controls specific nerve cells with light in order to perform optogenetic procedures, but it also has an electrode bundle to read brain signals and a microfluidic channel to deliver drugs to the brain.
The interface is easy to insert into the body when dry, as hydrogels become solid. But once in the body, the hydrogel will quickly absorb body fluids and resemble the properties of its surrounding tissues, thereby minimizing foreign body response.
The research team applied the device on animal models, and showed that it was possible to detect neural signals for up to six months, which is far beyond what had been previously recorded. It was also possible to conduct long-term optogenetic and behavioral experiments on freely moving mice with a significant reduction in foreign body responses such as glial and immunological activation compared to existing devices.
“This research is significant in that it was the first to utilize a hydrogel as part of a multifunctional neural interface probe, which increased its lifespan dramatically,” said Professor Park. “With our discovery, we look forward to advancements in research on neurological disorders like Alzheimer’s or Parkinson’s disease that require long-term observation.”
The research was published in Nature Communications on June 8, 2021. (Title: Adaptive and multifunctional hydrogel hybrid probes for long-term sensing and modulation of neural activity) The study was conducted jointly with an MIT research team composed of Professor Polina Anikeeva, Professor Xuanhe Zhao, and Dr. Hyunwoo Yook.
This research was supported by the National Research Foundation (NRF) grant for emerging research, Korea Medical Device Development Fund, KK-JRC Smart Project, KAIST Global Initiative Program, and Post-AI Project.
-PublicationPark, S., Yuk, H., Zhao, R. et al. Adaptive and multifunctional hydrogel hybrid probes for long-term sensing and modulation of neural activity. Nat Commun 12, 3435 (2021). https://doi.org/10.1038/s41467-021-23802-9
-ProfileProfessor Seongjun ParkBio and Neural Interfaces LaboratoryDepartment of Bio and Brain EngineeringKAIST
2021.07.13 View 12823
Hydrogel-Based Flexible Brain-Machine Interface
The interface is easy to insert into the body when dry, but behaves ‘stealthily’ inside the brain when wet
Professor Seongjun Park’s research team and collaborators revealed a newly developed hydrogel-based flexible brain-machine interface. To study the structure of the brain or to identify and treat neurological diseases, it is crucial to develop an interface that can stimulate the brain and detect its signals in real time. However, existing neural interfaces are mechanically and chemically different from real brain tissue. This causes foreign body response and forms an insulating layer (glial scar) around the interface, which shortens its lifespan.
To solve this problem, the research team developed a ‘brain-mimicking interface’ by inserting a custom-made multifunctional fiber bundle into the hydrogel body. The device is composed not only of an optical fiber that controls specific nerve cells with light in order to perform optogenetic procedures, but it also has an electrode bundle to read brain signals and a microfluidic channel to deliver drugs to the brain.
The interface is easy to insert into the body when dry, as hydrogels become solid. But once in the body, the hydrogel will quickly absorb body fluids and resemble the properties of its surrounding tissues, thereby minimizing foreign body response.
The research team applied the device on animal models, and showed that it was possible to detect neural signals for up to six months, which is far beyond what had been previously recorded. It was also possible to conduct long-term optogenetic and behavioral experiments on freely moving mice with a significant reduction in foreign body responses such as glial and immunological activation compared to existing devices.
“This research is significant in that it was the first to utilize a hydrogel as part of a multifunctional neural interface probe, which increased its lifespan dramatically,” said Professor Park. “With our discovery, we look forward to advancements in research on neurological disorders like Alzheimer’s or Parkinson’s disease that require long-term observation.”
The research was published in Nature Communications on June 8, 2021. (Title: Adaptive and multifunctional hydrogel hybrid probes for long-term sensing and modulation of neural activity) The study was conducted jointly with an MIT research team composed of Professor Polina Anikeeva, Professor Xuanhe Zhao, and Dr. Hyunwoo Yook.
This research was supported by the National Research Foundation (NRF) grant for emerging research, Korea Medical Device Development Fund, KK-JRC Smart Project, KAIST Global Initiative Program, and Post-AI Project.
-PublicationPark, S., Yuk, H., Zhao, R. et al. Adaptive and multifunctional hydrogel hybrid probes for long-term sensing and modulation of neural activity. Nat Commun 12, 3435 (2021). https://doi.org/10.1038/s41467-021-23802-9
-ProfileProfessor Seongjun ParkBio and Neural Interfaces LaboratoryDepartment of Bio and Brain EngineeringKAIST
2021.07.13 View 12823 -
 Study of T Cells from COVID-19 Convalescents Guides Vaccine Strategies
Researchers confirm that most COVID-19 patients in their convalescent stage carry stem cell-like memory T cells for months
A KAIST immunology research team found that most convalescent patients of COVID-19 develop and maintain T cell memory for over 10 months regardless of the severity of their symptoms. In addition, memory T cells proliferate rapidly after encountering their cognate antigen and accomplish their multifunctional roles. This study provides new insights for effective vaccine strategies against COVID-19, considering the self-renewal capacity and multipotency of memory T cells.
COVID-19 is a disease caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection. When patients recover from COVID-19, SARS-CoV-2-specific adaptive immune memory is developed. The adaptive immune system consists of two principal components: B cells that produce antibodies and T cells that eliminate infected cells. The current results suggest that the protective immune function of memory T cells will be implemented upon re-exposure to SARS-CoV-2.
Recently, the role of memory T cells against SARS-CoV-2 has been gaining attention as neutralizing antibodies wane after recovery. Although memory T cells cannot prevent the infection itself, they play a central role in preventing the severe progression of COVID-19. However, the longevity and functional maintenance of SARS-CoV-2-specific memory T cells remain unknown.
Professor Eui-Cheol Shin and his collaborators investigated the characteristics and functions of stem cell-like memory T cells, which are expected to play a crucial role in long-term immunity. Researchers analyzed the generation of stem cell-like memory T cells and multi-cytokine producing polyfunctional memory T cells, using cutting-edge immunological techniques.
This research is significant in that revealing the long-term immunity of COVID-19 convalescent patients provides an indicator regarding the long-term persistence of T cell immunity, one of the main goals of future vaccine development, as well as evaluating the long-term efficacy of currently available COVID-19 vaccines.
The research team is presently conducting a follow-up study to identify the memory T cell formation and functional characteristics of those who received COVID-19 vaccines, and to understand the immunological effect of COVID-19 vaccines by comparing the characteristics of memory T cells from vaccinated individuals with those of COVID-19 convalescent patients.
PhD candidate Jae Hyung Jung and Dr. Min-Seok Rha, a clinical fellow at Yonsei Severance Hospital, who led the study together explained, “Our analysis will enhance the understanding of COVID-19 immunity and establish an index for COVID-19 vaccine-induced memory T cells.”
“This study is the world’s longest longitudinal study on differentiation and functions of memory T cells among COVID-19 convalescent patients. The research on the temporal dynamics of immune responses has laid the groundwork for building a strategy for next-generation vaccine development,” Professor Shin added. This work was supported by the Samsung Science and Technology Foundation and KAIST, and was published in Nature Communications on June 30.
-Publication:
Jung, J.H., Rha, MS., Sa, M. et al. SARS-CoV-2-specific T cell memory is sustained in COVID-19 convalescent patients for 10 months with successful development of stem cell-like memory T cells. Nat Communications 12, 4043 (2021). https://doi.org/10.1038/s41467-021-24377-1
-Profile:
Professor Eui-Cheol Shin
Laboratory of Immunology & Infectious Diseases (http://liid.kaist.ac.kr/)
Graduate School of Medical Science and Engineering
KAIST
2021.07.05 View 14771
Study of T Cells from COVID-19 Convalescents Guides Vaccine Strategies
Researchers confirm that most COVID-19 patients in their convalescent stage carry stem cell-like memory T cells for months
A KAIST immunology research team found that most convalescent patients of COVID-19 develop and maintain T cell memory for over 10 months regardless of the severity of their symptoms. In addition, memory T cells proliferate rapidly after encountering their cognate antigen and accomplish their multifunctional roles. This study provides new insights for effective vaccine strategies against COVID-19, considering the self-renewal capacity and multipotency of memory T cells.
COVID-19 is a disease caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection. When patients recover from COVID-19, SARS-CoV-2-specific adaptive immune memory is developed. The adaptive immune system consists of two principal components: B cells that produce antibodies and T cells that eliminate infected cells. The current results suggest that the protective immune function of memory T cells will be implemented upon re-exposure to SARS-CoV-2.
Recently, the role of memory T cells against SARS-CoV-2 has been gaining attention as neutralizing antibodies wane after recovery. Although memory T cells cannot prevent the infection itself, they play a central role in preventing the severe progression of COVID-19. However, the longevity and functional maintenance of SARS-CoV-2-specific memory T cells remain unknown.
Professor Eui-Cheol Shin and his collaborators investigated the characteristics and functions of stem cell-like memory T cells, which are expected to play a crucial role in long-term immunity. Researchers analyzed the generation of stem cell-like memory T cells and multi-cytokine producing polyfunctional memory T cells, using cutting-edge immunological techniques.
This research is significant in that revealing the long-term immunity of COVID-19 convalescent patients provides an indicator regarding the long-term persistence of T cell immunity, one of the main goals of future vaccine development, as well as evaluating the long-term efficacy of currently available COVID-19 vaccines.
The research team is presently conducting a follow-up study to identify the memory T cell formation and functional characteristics of those who received COVID-19 vaccines, and to understand the immunological effect of COVID-19 vaccines by comparing the characteristics of memory T cells from vaccinated individuals with those of COVID-19 convalescent patients.
PhD candidate Jae Hyung Jung and Dr. Min-Seok Rha, a clinical fellow at Yonsei Severance Hospital, who led the study together explained, “Our analysis will enhance the understanding of COVID-19 immunity and establish an index for COVID-19 vaccine-induced memory T cells.”
“This study is the world’s longest longitudinal study on differentiation and functions of memory T cells among COVID-19 convalescent patients. The research on the temporal dynamics of immune responses has laid the groundwork for building a strategy for next-generation vaccine development,” Professor Shin added. This work was supported by the Samsung Science and Technology Foundation and KAIST, and was published in Nature Communications on June 30.
-Publication:
Jung, J.H., Rha, MS., Sa, M. et al. SARS-CoV-2-specific T cell memory is sustained in COVID-19 convalescent patients for 10 months with successful development of stem cell-like memory T cells. Nat Communications 12, 4043 (2021). https://doi.org/10.1038/s41467-021-24377-1
-Profile:
Professor Eui-Cheol Shin
Laboratory of Immunology & Infectious Diseases (http://liid.kaist.ac.kr/)
Graduate School of Medical Science and Engineering
KAIST
2021.07.05 View 14771 -
 Extremely Stable Perovskite Nanoparticles Films for Next-Generation Displays
Researchers have reported an extremely stable cross-linked perovskite nanoparticle that maintains a high photoluminescence quantum yield (PLQY) for 1.5 years in air and harsh liquid environments. This stable material’s design strategies, which addressed one of the most critical problems limiting their practical application, provide a breakthrough for the commercialization of perovskite nanoparticles in next-generation displays and bio-related applications.
According to the research team led by Professor Byeong-Soo Bae, their development can survive in severe environments such as water, various polar solvents, and high temperature with high humidity without additional encapsulation. This development is expected to enable perovskite nanoparticles to be applied to high color purity display applications as a practical color converting material. This result was published as the inside front cover article in Advanced Materials.
Perovskites, which consist of organics, metals, and halogen elements, have emerged as key elements in various optoelectronic applications. The power conversion efficiency of photovoltaic cells based on perovskites light absorbers has been rapidly increased. Perovskites are also great promise as a light emitter in display applications because of their low material cost, facile wavelength tunability, high (PLQY), very narrow emission band width, and wider color gamut than inorganic semiconducting nanocrystals and organic emitters. Thanks to these advantages, perovskites have been identified as a key color-converting material for next-generation high color-purity displays. In particular, perovskites are the only luminescence material that meets Rec. 2020 which is a new color standard in display industry.
However, perovskites are very unstable against heat, moisture, and light, which makes them almost impossible to use in practical applications. To solve these problems, many researchers have attempted to physically prevent perovskites from coming into contact with water molecules by passivating the perovskite grain and nanoparticle surfaces with organic ligands or inorganic shell materials, or by fabricating perovskite-polymer nanocomposites. These methods require complex processes and have limited stability in ambient air and water. Furthermore, stable perovskite nanoparticles in the various chemical environments and high temperatures with high humidity have not been reported yet.
The research team in collaboration with Seoul National University develops siloxane-encapsulated perovskite nanoparticle composite films. Here, perovskite nanoparticles are chemically crosslinked with thermally stable siloxane molecules, thereby significantly improving the stability of the perovskite nanoparticles without the need for any additional protecting layer.
Siloxane-encapsulated perovskite nanoparticle composite films exhibited a high PLQY (> 70%) value, which can be maintained over 600 days in water, various chemicals (alcohol, strong acidic and basic solutions), and high temperatures with high humidity (85℃/85%). The research team investigated the mechanisms impacting the chemical crosslinking and water molecule-induced stabilization of perovskite nanoparticles through various photo-physical analysis and density-functional theory calculation.
The research team confirmed that displays based on their siloxane-perovskite nanoparticle composite films exhibited higher PLQY and a wider color gamut than those of Cd-based quantum dots and demonstrated perfect color converting properties on commercial mobile phone screens. Unlike what was commonly believed in the halide perovskite field, the composite films showed excellent bio-compatibility because the siloxane matrix prevents the toxicity of Pb in perovskite nanoparticle.
By using this technology, the instability of perovskite materials, which is the biggest challenge for practical applications, is greatly improved through simple encapsulation method.
“Perovskite nanoparticle is the only photoluminescent material that can meet the next generation display color standard. Nevertheless, there has been reluctant to commercialize it due to its moisture vulnerability. The newly developed siloxane encapsulation technology will trigger more research on perovskite nanoparticles as color conversion materials and will accelerate early commercialization,” Professor Bae said.
This work was supported by the Wearable Platform Materials Technology Center (WMC) of the Engineering Research Center (ERC) Project, and the Leadership Research Program funded by the National Research Foundation of Korea.
-Publication:
Junho Jang, Young-Hoon Kim, Sunjoon Park, Dongsuk Yoo, Hyunjin Cho, Jinhyeong Jang, Han Beom Jeong, Hyunhwan Lee, Jong Min Yuk, Chan Beum Park, Duk Young Jeon, Yong-Hyun Kim, Byeong-Soo Bae, and Tae-Woo Lee. “Extremely Stable Luminescent Crosslinked Perovskite Nanoparticles under Harsh Environments over 1.5 Years” Advanced Materials, 2020, 2005255. https://doi.org/10.1002/adma.202005255.
Link to download the full-text paper:
https://onlinelibrary.wiley.com/doi/10.1002/adma.202005255
-Profile: Prof. Byeong-Soo Bae (Corresponding author)
bsbae@kaist.ac.kr
Lab. of Optical Materials & Coating
Department of Materials Science and Engineering
Korea Advanced Institute of Science and Technology (KAIST)
2020.12.29 View 14995
Extremely Stable Perovskite Nanoparticles Films for Next-Generation Displays
Researchers have reported an extremely stable cross-linked perovskite nanoparticle that maintains a high photoluminescence quantum yield (PLQY) for 1.5 years in air and harsh liquid environments. This stable material’s design strategies, which addressed one of the most critical problems limiting their practical application, provide a breakthrough for the commercialization of perovskite nanoparticles in next-generation displays and bio-related applications.
According to the research team led by Professor Byeong-Soo Bae, their development can survive in severe environments such as water, various polar solvents, and high temperature with high humidity without additional encapsulation. This development is expected to enable perovskite nanoparticles to be applied to high color purity display applications as a practical color converting material. This result was published as the inside front cover article in Advanced Materials.
Perovskites, which consist of organics, metals, and halogen elements, have emerged as key elements in various optoelectronic applications. The power conversion efficiency of photovoltaic cells based on perovskites light absorbers has been rapidly increased. Perovskites are also great promise as a light emitter in display applications because of their low material cost, facile wavelength tunability, high (PLQY), very narrow emission band width, and wider color gamut than inorganic semiconducting nanocrystals and organic emitters. Thanks to these advantages, perovskites have been identified as a key color-converting material for next-generation high color-purity displays. In particular, perovskites are the only luminescence material that meets Rec. 2020 which is a new color standard in display industry.
However, perovskites are very unstable against heat, moisture, and light, which makes them almost impossible to use in practical applications. To solve these problems, many researchers have attempted to physically prevent perovskites from coming into contact with water molecules by passivating the perovskite grain and nanoparticle surfaces with organic ligands or inorganic shell materials, or by fabricating perovskite-polymer nanocomposites. These methods require complex processes and have limited stability in ambient air and water. Furthermore, stable perovskite nanoparticles in the various chemical environments and high temperatures with high humidity have not been reported yet.
The research team in collaboration with Seoul National University develops siloxane-encapsulated perovskite nanoparticle composite films. Here, perovskite nanoparticles are chemically crosslinked with thermally stable siloxane molecules, thereby significantly improving the stability of the perovskite nanoparticles without the need for any additional protecting layer.
Siloxane-encapsulated perovskite nanoparticle composite films exhibited a high PLQY (> 70%) value, which can be maintained over 600 days in water, various chemicals (alcohol, strong acidic and basic solutions), and high temperatures with high humidity (85℃/85%). The research team investigated the mechanisms impacting the chemical crosslinking and water molecule-induced stabilization of perovskite nanoparticles through various photo-physical analysis and density-functional theory calculation.
The research team confirmed that displays based on their siloxane-perovskite nanoparticle composite films exhibited higher PLQY and a wider color gamut than those of Cd-based quantum dots and demonstrated perfect color converting properties on commercial mobile phone screens. Unlike what was commonly believed in the halide perovskite field, the composite films showed excellent bio-compatibility because the siloxane matrix prevents the toxicity of Pb in perovskite nanoparticle.
By using this technology, the instability of perovskite materials, which is the biggest challenge for practical applications, is greatly improved through simple encapsulation method.
“Perovskite nanoparticle is the only photoluminescent material that can meet the next generation display color standard. Nevertheless, there has been reluctant to commercialize it due to its moisture vulnerability. The newly developed siloxane encapsulation technology will trigger more research on perovskite nanoparticles as color conversion materials and will accelerate early commercialization,” Professor Bae said.
This work was supported by the Wearable Platform Materials Technology Center (WMC) of the Engineering Research Center (ERC) Project, and the Leadership Research Program funded by the National Research Foundation of Korea.
-Publication:
Junho Jang, Young-Hoon Kim, Sunjoon Park, Dongsuk Yoo, Hyunjin Cho, Jinhyeong Jang, Han Beom Jeong, Hyunhwan Lee, Jong Min Yuk, Chan Beum Park, Duk Young Jeon, Yong-Hyun Kim, Byeong-Soo Bae, and Tae-Woo Lee. “Extremely Stable Luminescent Crosslinked Perovskite Nanoparticles under Harsh Environments over 1.5 Years” Advanced Materials, 2020, 2005255. https://doi.org/10.1002/adma.202005255.
Link to download the full-text paper:
https://onlinelibrary.wiley.com/doi/10.1002/adma.202005255
-Profile: Prof. Byeong-Soo Bae (Corresponding author)
bsbae@kaist.ac.kr
Lab. of Optical Materials & Coating
Department of Materials Science and Engineering
Korea Advanced Institute of Science and Technology (KAIST)
2020.12.29 View 14995 -
 Simulations Open a New Way to Reverse Cell Aging
Turning off a newly identified enzyme could reverse a natural aging process in cells.
Research findings by a KAIST team provide insight into the complex mechanism of cellular senescence and present a potential therapeutic strategy for reducing age-related diseases associated with the accumulation of senescent cells.
Simulations that model molecular interactions have identified an enzyme that could be targeted to reverse a natural aging process called cellular senescence. The findings were validated with laboratory experiments on skin cells and skin equivalent tissues, and published in the Proceedings of the National Academy of Sciences (PNAS).
“Our research opens the door for a new generation that perceives aging as a reversible biological phenomenon,” says Professor Kwang-Hyun Cho of the Department of Bio and Brain engineering at the Korea Advanced Institute of Science and Technology (KAIST), who led the research with colleagues from KAIST and Amorepacific Corporation in Korea.
Cells respond to a variety of factors, such as oxidative stress, DNA damage, and shortening of the telomeres capping the ends of chromosomes, by entering a stable and persistent exit from the cell cycle. This process, called cellular senescence, is important, as it prevents damaged cells from proliferating and turning into cancer cells. But it is also a natural process that contributes to aging and age-related diseases. Recent research has shown that cellular senescence can be reversed. But the laboratory approaches used thus far also impair tissue regeneration or have the potential to trigger malignant transformations.
Professor Cho and his colleagues used an innovative strategy to identify molecules that could be targeted for reversing cellular senescence. The team pooled together information from the literature and databases about the molecular processes involved in cellular senescence. To this, they added results from their own research on the molecular processes involved in the proliferation, quiescence (a non-dividing cell that can re-enter the cell cycle) and senescence of skin fibroblasts, a cell type well known for repairing wounds. Using algorithms, they developed a model that simulates the interactions between these molecules. Their analyses allowed them to predict which molecules could be targeted to reverse cell senescence.
They then investigated one of the molecules, an enzyme called PDK1, in incubated senescent skin fibroblasts and three-dimensional skin equivalent tissue models. They found that blocking PDK1 led to the inhibition of two downstream signalling molecules, which in turn restored the cells’ ability to enter back into the cell cycle. Notably, the cells retained their capacity to regenerate wounded skin without proliferating in a way that could lead to malignant transformation.
The scientists recommend investigations are next done in organs and organisms to determine the full effect of PDK1 inhibition. Since the gene that codes for PDK1 is overexpressed in some cancers, the scientists expect that inhibiting it will have both anti-aging and anti-cancer effects.
-Profile
Professor Kwang-Hyun Cho
Laboratory for Systems Biology and Bio-Inspired Engineering
http://sbie.kaist.ac.kr
Department of Bio and Brain Engineering
KAIST
2020.11.26 View 14047
Simulations Open a New Way to Reverse Cell Aging
Turning off a newly identified enzyme could reverse a natural aging process in cells.
Research findings by a KAIST team provide insight into the complex mechanism of cellular senescence and present a potential therapeutic strategy for reducing age-related diseases associated with the accumulation of senescent cells.
Simulations that model molecular interactions have identified an enzyme that could be targeted to reverse a natural aging process called cellular senescence. The findings were validated with laboratory experiments on skin cells and skin equivalent tissues, and published in the Proceedings of the National Academy of Sciences (PNAS).
“Our research opens the door for a new generation that perceives aging as a reversible biological phenomenon,” says Professor Kwang-Hyun Cho of the Department of Bio and Brain engineering at the Korea Advanced Institute of Science and Technology (KAIST), who led the research with colleagues from KAIST and Amorepacific Corporation in Korea.
Cells respond to a variety of factors, such as oxidative stress, DNA damage, and shortening of the telomeres capping the ends of chromosomes, by entering a stable and persistent exit from the cell cycle. This process, called cellular senescence, is important, as it prevents damaged cells from proliferating and turning into cancer cells. But it is also a natural process that contributes to aging and age-related diseases. Recent research has shown that cellular senescence can be reversed. But the laboratory approaches used thus far also impair tissue regeneration or have the potential to trigger malignant transformations.
Professor Cho and his colleagues used an innovative strategy to identify molecules that could be targeted for reversing cellular senescence. The team pooled together information from the literature and databases about the molecular processes involved in cellular senescence. To this, they added results from their own research on the molecular processes involved in the proliferation, quiescence (a non-dividing cell that can re-enter the cell cycle) and senescence of skin fibroblasts, a cell type well known for repairing wounds. Using algorithms, they developed a model that simulates the interactions between these molecules. Their analyses allowed them to predict which molecules could be targeted to reverse cell senescence.
They then investigated one of the molecules, an enzyme called PDK1, in incubated senescent skin fibroblasts and three-dimensional skin equivalent tissue models. They found that blocking PDK1 led to the inhibition of two downstream signalling molecules, which in turn restored the cells’ ability to enter back into the cell cycle. Notably, the cells retained their capacity to regenerate wounded skin without proliferating in a way that could lead to malignant transformation.
The scientists recommend investigations are next done in organs and organisms to determine the full effect of PDK1 inhibition. Since the gene that codes for PDK1 is overexpressed in some cancers, the scientists expect that inhibiting it will have both anti-aging and anti-cancer effects.
-Profile
Professor Kwang-Hyun Cho
Laboratory for Systems Biology and Bio-Inspired Engineering
http://sbie.kaist.ac.kr
Department of Bio and Brain Engineering
KAIST
2020.11.26 View 14047 -
 Researchers Describe a Mechanism Inducing Self-Killing of Cancer Cells
(Professor Kim (left) and lead author Lee)
Researchers have described a new mechanism which induces the self-killing of cancer cells by perturbing ion homeostasis. A research team from the Department of Biochemical Engineering has developed helical polypeptide potassium ionophores that lead to the onset of programmed cell death. The ionophores increase the active oxygen concentration to stress endoplasmic reticulum to the point of cellular death.
The electrochemical gradient between extracellular and intracellular conditions plays an important role in cell growth and metabolism. When a cell’s ion homeostasis is disturbed, critical functions accelerating the activation of apoptosis are inhibited in the cell.
Although ionophores have been intensively used as an ion homeostasis disturber, the mechanisms of cell death have been unclear and the bio-applicability has been limited. In the study featured at Advanced Science, the team presented an alpha helical peptide-based anticancer agent that is capable of transporting potassium ions with water solubility. The cationic, hydrophilic, and potassium ionic groups were combined at the end of the peptide side chain to provide both ion transport and hydrophilic properties.
These peptide-based ionophores reduce the intracellular potassium concentration and at the same time increase the intracellular calcium concentration. Increased intracellular calcium concentrations produce intracellular reactive oxygen species, causing endoplasmic reticulum stress, and ultimately leading to apoptosis.
Anticancer effects were evaluated using tumor-bearing mice to confirm the therapeutic effect, even in animal models. It was found that tumor growth was strongly inhibited by endoplasmic stress-mediated apoptosis.
Lead author Dr. Dae-Yong Lee said, “A peptide-based ionophore is more effective than conventional chemotherapeutic agents because it induces apoptosis via elevated reactive oxygen species levels. Professor Yeu-Chun Kim said he expects this new mechanism to be widely used as a new chemotherapeutic strategy. This research was funded by the National Research Foundation.
2019.08.28 View 21737
Researchers Describe a Mechanism Inducing Self-Killing of Cancer Cells
(Professor Kim (left) and lead author Lee)
Researchers have described a new mechanism which induces the self-killing of cancer cells by perturbing ion homeostasis. A research team from the Department of Biochemical Engineering has developed helical polypeptide potassium ionophores that lead to the onset of programmed cell death. The ionophores increase the active oxygen concentration to stress endoplasmic reticulum to the point of cellular death.
The electrochemical gradient between extracellular and intracellular conditions plays an important role in cell growth and metabolism. When a cell’s ion homeostasis is disturbed, critical functions accelerating the activation of apoptosis are inhibited in the cell.
Although ionophores have been intensively used as an ion homeostasis disturber, the mechanisms of cell death have been unclear and the bio-applicability has been limited. In the study featured at Advanced Science, the team presented an alpha helical peptide-based anticancer agent that is capable of transporting potassium ions with water solubility. The cationic, hydrophilic, and potassium ionic groups were combined at the end of the peptide side chain to provide both ion transport and hydrophilic properties.
These peptide-based ionophores reduce the intracellular potassium concentration and at the same time increase the intracellular calcium concentration. Increased intracellular calcium concentrations produce intracellular reactive oxygen species, causing endoplasmic reticulum stress, and ultimately leading to apoptosis.
Anticancer effects were evaluated using tumor-bearing mice to confirm the therapeutic effect, even in animal models. It was found that tumor growth was strongly inhibited by endoplasmic stress-mediated apoptosis.
Lead author Dr. Dae-Yong Lee said, “A peptide-based ionophore is more effective than conventional chemotherapeutic agents because it induces apoptosis via elevated reactive oxygen species levels. Professor Yeu-Chun Kim said he expects this new mechanism to be widely used as a new chemotherapeutic strategy. This research was funded by the National Research Foundation.
2019.08.28 View 21737 -
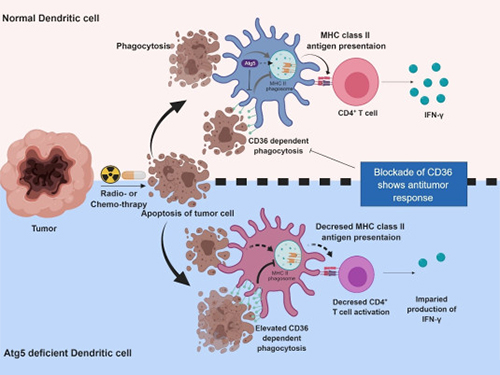 Autophagy in Dendritic Cells Helps Anticancer Activity
Autophagy contributes to the homeostasis of a cell and recently another function of autophagy has been reported. A KAIST research team found that the autophagy of dendritic cells supports T-cell anticancer activity.
Autophagy is a process of maintaining cell homeostasis by removing cellular waste and damaged cellular organelles; nevertheless, its role in the presentation of phagocytized tumor-associated antigens remains vague.
Meanwhile, dendritic cells are the ones that recognize pathogens or cancer antigens, and induce immune responses in T cells. When cancer cells are killed by radiation or an anticancer drug, dendritic cells absorb and remove them and present antigens on their surface to transfer them to T-cells.
Professor Heung Kyu Lee from the Graduate School of Medical Science and Engineering and his team found that the autophagy of dendritic cells plays a key role in T-cell activation and they proposed the principles of enhancing anti-cancer effects.
Their experiments showed that T-cell activation of dendritic cells as well as anticancer immune response dropped when there is a deficiency of Atg5 (autophagy-related) in dendritic cells.
Interestingly, Atg5-deficient dendritic cells significantly elevated receptor CD36 on the surface of the cells, which increased the phagocytosis of apoptotic tumor cells yet restricted the activation of T-cells.
At this time, when introducing antibodies into the system in order to block the receptor CD36, the anti-tumor T-cell response increased substantially while tumor growth declined.
Professor Lee said, “This study allowed us to explore the role of autophagy in the anti-cancer immune response of T-cells. We look forward to developing targeted anti-cancer therapies using the receptor CD36.”
This research was published in Autophagy (10.1080/15548627.2019.1596493) on March 22, 2019.
Figure 1.Mechanism of autophagy in dendritic cells
Figure 2. A role of autophagy in dendritic cells
2019.05.13 View 51839
Autophagy in Dendritic Cells Helps Anticancer Activity
Autophagy contributes to the homeostasis of a cell and recently another function of autophagy has been reported. A KAIST research team found that the autophagy of dendritic cells supports T-cell anticancer activity.
Autophagy is a process of maintaining cell homeostasis by removing cellular waste and damaged cellular organelles; nevertheless, its role in the presentation of phagocytized tumor-associated antigens remains vague.
Meanwhile, dendritic cells are the ones that recognize pathogens or cancer antigens, and induce immune responses in T cells. When cancer cells are killed by radiation or an anticancer drug, dendritic cells absorb and remove them and present antigens on their surface to transfer them to T-cells.
Professor Heung Kyu Lee from the Graduate School of Medical Science and Engineering and his team found that the autophagy of dendritic cells plays a key role in T-cell activation and they proposed the principles of enhancing anti-cancer effects.
Their experiments showed that T-cell activation of dendritic cells as well as anticancer immune response dropped when there is a deficiency of Atg5 (autophagy-related) in dendritic cells.
Interestingly, Atg5-deficient dendritic cells significantly elevated receptor CD36 on the surface of the cells, which increased the phagocytosis of apoptotic tumor cells yet restricted the activation of T-cells.
At this time, when introducing antibodies into the system in order to block the receptor CD36, the anti-tumor T-cell response increased substantially while tumor growth declined.
Professor Lee said, “This study allowed us to explore the role of autophagy in the anti-cancer immune response of T-cells. We look forward to developing targeted anti-cancer therapies using the receptor CD36.”
This research was published in Autophagy (10.1080/15548627.2019.1596493) on March 22, 2019.
Figure 1.Mechanism of autophagy in dendritic cells
Figure 2. A role of autophagy in dendritic cells
2019.05.13 View 51839