Gou+Young+Koh
-
 Newly Identified Meningeal Lymphatic Vessels Answers the Key Questions on Brain Clearance
(Figure: Schematic images of location and features of meningeal lymphatic vessels and their changes associated with ageing.)
Just see what happens when your neighborhood’s waste disposal system is out of service. Not only do the piles of trash stink but they can indeed hinder the area’s normal functioning. That is also the case when the brain’s waste management is on the blink.
The buildup of toxic proteins in the brain causes a massive damage to the nerves, leading to cognitive dysfunction and increased probability of developing neurodegenerative disorders such as Alzheimer's disease. Though the brain drains its waste via the cerebrospinal fluid (CSF), little has been understood about an accurate route for the brain’s cleansing mechanism.
Medical scientists led by Professor Gou Young Koh at the Graduate School of Medical Science and Engineering have reported the basal side of the skull as the major route, so called “hotspot” for CSF drainage.
They found that basal meningeal lymphatic vessels (mLVs) function as the main plumbing pipes for CSF. They confirmed macromolecules in the CSF mainly runs through the basal mLVs. Notably, the team also revealed that the brain’s major drainage system, specifically basal mLVs are impaired with aging. Their findings have been reported in the journal Nature on July 24.
Throughout our body, excess fluids and waste products are removed from tissues via lymphatic vessels. It was only recently discovered that the brain also has a lymphatic drainage system. mLVs are supposed to carry waste from the brain tissue fluid and the CSF down the deep cervical lymph nodes for disposal. Still scientist are left with one perplexing question — where is the main exit for the CSF? Though mLVs in the upper part of the skull (dorsal meningeal lymphatic vessels) were reported as the brain’s clearance pathways in 2014, no substantial drainage mechanism was observed in that section.
“As a hidden exit for CSF, we looked into the mLVs trapped within complex structures at the base of the skull,” says Dr. Ji Hoon Ahn, the first author of this study. The researchers used several techniques to characterize the basal mLVs in detail. They used a genetically engineered lymphatic-reporter mouse model to visualize mLVs under a fluorescence microscope. By performing a careful examination of the mice skull, they found distinctive features of basal mLVs that make them suitable for CSF uptake and drainage. Just like typical functional lymphatic vessels, basal mLVs are found to have abundant lymphatic vessel branches with finger-like protrusions. Additionally, valves inside the basal mLVs allow the flow to go in one direction. In particular, they found that the basal mLVs are closely located to the CSF. Dr. Hyunsoo Cho, the first author of this study explains, “All up, it seemed a solid case that basal mLVs are the brain’s main clearance pathways.
The researchers verified such specialized morphologic characteristics of basal mLVs indeed facilitate the CSF uptake and drainage. Using CSF contrast-enhanced magnetic resonance imaging in a rat model, they found that CSF is drained preferentially through the basal mLVs. They also utilized a lymphatic-reporter mouse model and discovered that fluorescence-tagged tracer injected into the brain itself or the CSF is cleared mainly through the basal mLVs. Jun-Hee Kim, the first author of this study notes, “We literally saw that the brain clearance mechanism utilizing basal outflow route to exit the skull.
It has long been suggested that CSF turnover and drainage declines with ageing. However, alteration of mLVs associated with ageing is poorly understood. In this study, the researchers observed changes of mLVs in young (3-month-old) and aged (24~27-months-old) mice. They found that the structure of the basal mLVs and their lymphatic valves in aged mice become severely flawed, thus hampering CSF clearance. The corresponding author of this study, Dr. Koh says, “By characterizing the precise route for fluids leaving the brain, this study improves our understanding on how waste is cleared from the brain. Our findings also provide further insights into the role of impaired CSF clearance in the development of age-related neurodegenerative diseases.”
Many current therapies for Alzheimer’s disease target abnormally accumulated proteins, such as beta-amyloid. By mapping out a precise route for the brain’s waste clearance system, this study may be able to help find ways to improve the brain’s cleansing function. Such breakthrough might become quite a sensational strategy for eliminating the buildup of aging-related toxic proteins. “It definitely warrants more extensive investigation of mLVs in patients with age-related neurodegenerative disease such as Alzheimer’s disease prior to clinical investigation,” adds Professor Koh.
2019.07.25 View 33633
Newly Identified Meningeal Lymphatic Vessels Answers the Key Questions on Brain Clearance
(Figure: Schematic images of location and features of meningeal lymphatic vessels and their changes associated with ageing.)
Just see what happens when your neighborhood’s waste disposal system is out of service. Not only do the piles of trash stink but they can indeed hinder the area’s normal functioning. That is also the case when the brain’s waste management is on the blink.
The buildup of toxic proteins in the brain causes a massive damage to the nerves, leading to cognitive dysfunction and increased probability of developing neurodegenerative disorders such as Alzheimer's disease. Though the brain drains its waste via the cerebrospinal fluid (CSF), little has been understood about an accurate route for the brain’s cleansing mechanism.
Medical scientists led by Professor Gou Young Koh at the Graduate School of Medical Science and Engineering have reported the basal side of the skull as the major route, so called “hotspot” for CSF drainage.
They found that basal meningeal lymphatic vessels (mLVs) function as the main plumbing pipes for CSF. They confirmed macromolecules in the CSF mainly runs through the basal mLVs. Notably, the team also revealed that the brain’s major drainage system, specifically basal mLVs are impaired with aging. Their findings have been reported in the journal Nature on July 24.
Throughout our body, excess fluids and waste products are removed from tissues via lymphatic vessels. It was only recently discovered that the brain also has a lymphatic drainage system. mLVs are supposed to carry waste from the brain tissue fluid and the CSF down the deep cervical lymph nodes for disposal. Still scientist are left with one perplexing question — where is the main exit for the CSF? Though mLVs in the upper part of the skull (dorsal meningeal lymphatic vessels) were reported as the brain’s clearance pathways in 2014, no substantial drainage mechanism was observed in that section.
“As a hidden exit for CSF, we looked into the mLVs trapped within complex structures at the base of the skull,” says Dr. Ji Hoon Ahn, the first author of this study. The researchers used several techniques to characterize the basal mLVs in detail. They used a genetically engineered lymphatic-reporter mouse model to visualize mLVs under a fluorescence microscope. By performing a careful examination of the mice skull, they found distinctive features of basal mLVs that make them suitable for CSF uptake and drainage. Just like typical functional lymphatic vessels, basal mLVs are found to have abundant lymphatic vessel branches with finger-like protrusions. Additionally, valves inside the basal mLVs allow the flow to go in one direction. In particular, they found that the basal mLVs are closely located to the CSF. Dr. Hyunsoo Cho, the first author of this study explains, “All up, it seemed a solid case that basal mLVs are the brain’s main clearance pathways.
The researchers verified such specialized morphologic characteristics of basal mLVs indeed facilitate the CSF uptake and drainage. Using CSF contrast-enhanced magnetic resonance imaging in a rat model, they found that CSF is drained preferentially through the basal mLVs. They also utilized a lymphatic-reporter mouse model and discovered that fluorescence-tagged tracer injected into the brain itself or the CSF is cleared mainly through the basal mLVs. Jun-Hee Kim, the first author of this study notes, “We literally saw that the brain clearance mechanism utilizing basal outflow route to exit the skull.
It has long been suggested that CSF turnover and drainage declines with ageing. However, alteration of mLVs associated with ageing is poorly understood. In this study, the researchers observed changes of mLVs in young (3-month-old) and aged (24~27-months-old) mice. They found that the structure of the basal mLVs and their lymphatic valves in aged mice become severely flawed, thus hampering CSF clearance. The corresponding author of this study, Dr. Koh says, “By characterizing the precise route for fluids leaving the brain, this study improves our understanding on how waste is cleared from the brain. Our findings also provide further insights into the role of impaired CSF clearance in the development of age-related neurodegenerative diseases.”
Many current therapies for Alzheimer’s disease target abnormally accumulated proteins, such as beta-amyloid. By mapping out a precise route for the brain’s waste clearance system, this study may be able to help find ways to improve the brain’s cleansing function. Such breakthrough might become quite a sensational strategy for eliminating the buildup of aging-related toxic proteins. “It definitely warrants more extensive investigation of mLVs in patients with age-related neurodegenerative disease such as Alzheimer’s disease prior to clinical investigation,” adds Professor Koh.
2019.07.25 View 33633 -
 Distinguished Professor Koh Donates His Ho-Am Prize Money
(From left: Distinguished Professor Gou Young Koh and KAIST President Sung-Chul Shin)
Distinguished Professor Gou Young Koh from the Graduate School of Medical Science and Engineering donated one hundred million KRW to KAIST that he received for winning the Ho-Am Prize.
Professor Koh, who is widely renowned for angiogenesis, was appointed as the 2018 laureate of the 28th Ho-Am Prize for demonstrating the effective reduction of tumor progression and metastasis via tumor vessel normalization.
He made the donation to the Graduate School of Medical Science and Engineering, where he conducted his research. “As a basic medical scientist, it is my great honor to receive this prize for the recognition of my research outcome. I will give impetus to research for continuous development,” Professor Koh said.
Professor Koh also received the 5th Asan Award in Medicine in 2012 and the 7th Kyung-Ahm Award in 2011. He was also the awardee of the 17th Wunsch Medical Award. He has donated cash prizes to the school every time he is awarded.
KAIST President Sung-Chul Shin said, “I would like to express my gratitude to the professor for his generous donation to the school. It will be a great help fostering outstanding medical scientists.
Professor Koh received his MD-PhD from the Medical School of Chonbuk National University. After finishing his post-doctoral program at Cornell University and Indiana State University, he was appointed as a professor at Chonbuk National University and POSTECH. Currently, he holds the position of distinguished professor at KAIST and director of the IBS Center for Vascular Research.
2018.06.20 View 6372
Distinguished Professor Koh Donates His Ho-Am Prize Money
(From left: Distinguished Professor Gou Young Koh and KAIST President Sung-Chul Shin)
Distinguished Professor Gou Young Koh from the Graduate School of Medical Science and Engineering donated one hundred million KRW to KAIST that he received for winning the Ho-Am Prize.
Professor Koh, who is widely renowned for angiogenesis, was appointed as the 2018 laureate of the 28th Ho-Am Prize for demonstrating the effective reduction of tumor progression and metastasis via tumor vessel normalization.
He made the donation to the Graduate School of Medical Science and Engineering, where he conducted his research. “As a basic medical scientist, it is my great honor to receive this prize for the recognition of my research outcome. I will give impetus to research for continuous development,” Professor Koh said.
Professor Koh also received the 5th Asan Award in Medicine in 2012 and the 7th Kyung-Ahm Award in 2011. He was also the awardee of the 17th Wunsch Medical Award. He has donated cash prizes to the school every time he is awarded.
KAIST President Sung-Chul Shin said, “I would like to express my gratitude to the professor for his generous donation to the school. It will be a great help fostering outstanding medical scientists.
Professor Koh received his MD-PhD from the Medical School of Chonbuk National University. After finishing his post-doctoral program at Cornell University and Indiana State University, he was appointed as a professor at Chonbuk National University and POSTECH. Currently, he holds the position of distinguished professor at KAIST and director of the IBS Center for Vascular Research.
2018.06.20 View 6372 -
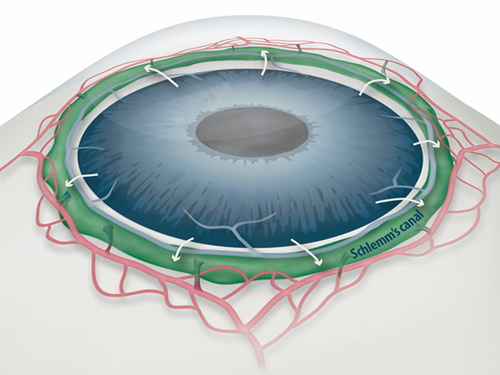 Draining Eyes Clogged with Glaucoma
Professor Gou Young Koh in the Graduate School of Medical Science and Engineering and his team have identified a new mechanism involved in the development and progression of glaucoma, and found a potential therapeutic option to treat it. Glaucoma is the second cause of irreversible blindness, after cataracts. It affects about 3.5% of the world population aged 40 to 80.
Professor Koh also serves as the director of the Center for Vascular Research at the Institute for Basic Science. The IBS said the study, published in the Journal of Clinical Investigation, is expected to help the development of therapies to treat primary open-angle glaucoma (POAG), which counts for three quarters of all glaucoma patients.
One of the most important risk factors for glaucoma is the increased pressure inside the eye. A liquid called aqueous humor is constantly produced and drained out from the eye. It transports nutrients and inflates the eye giving it a roughly spherical shape. However, if this fluid cannot flow out of the eye chambers freely, an increase in intraocular pressure can damage the optic nerve, leading to vision loss. The precise mechanism of elevated resistance to aqueous humor outflow remains unclear, and although the current treatments for glaucoma tackle the production and outflow of aqueous humor, their outcomes are still poor.
A component of the eye that plays a fundamental role in draining out the aqueous humor is Schlemm's canal. It collects the aqueous humor and mediates its transfer from the eye chambers to blood circulation. The cells on the walls of the canal, endothelial cells, ship the liquid from the inner to the outer side in “packages”, called vacuoles. As the shape and number of the vacuoles reflects the outflow performance, several giant vacuoles are expected in the normal outflow process.
The team explained how imbalances in Schlemm's canal significantly increase the risk of glaucoma. They showed that an important regulator for canal functionality is the angiopoietin-Tie2 system. Angiopoietins, such as Ang1 and Ang2, are proteins important for the growth of new blood vessels and Tie2 is the receptor that binds them. It is known that the angiopoietin-Tie2 system plays a role in Schlemm’s canal formation, as Tie2 mutations or angiopoietin absence result in congenital glaucoma. However, this study clarified that it is also critically important during adulthood.
The researchers reported that adult mice deficient in Tie2 suffer from an elevated intraocular pressure, retinal neuronal damage and partial visual impairment. Moreover, they had a markedly decreased number of giant vacuoles inside Schlemm’s canal endothelial cells, which indicate a poor aqueous humor drainage.
The scientists also investigated if and how this process changes in older mice, as aging is a major risk factor for glaucoma, and showed that aged mice experience reduced levels of giant vacuoles, Tie2, Ang1, and Ang2, as well as other proteins connected with the angiopoietin-Tie2 pathway, like Prox1.
To test whether Tie2 activation could shift the situation, the researchers tested the antibody ABTAA (Ang2-binding and Tie2-activating antibody). They injected it in one eye of mice, while the other eye of the same mice functioned as the negative control. After one week, levels of Tie2 and Prox1, number and diameter of giant vacuoles in Schlemm’s canals increased in the ABTAA-treated eyes compared to control eyes. The researchers observed a similar outcome with decreased intraocular pressure when ABTAA was injected to the eyes of mice suffering from POAG with regressed Schlemm’s canals, indicating that this antibody might be considered as a therapeutic option.
"Slow development of glaucoma treatments is partly due to the poor understanding of the underlying pathogenesis," said Professor Koh, the corresponding author of the study. "We hope that identifying the critical role of the angiopoietin-Tie2 system in adult Schlemm’s canals will bring a significant boost in the development of therapeutics."
Figure 1: Schlemm's canal position inside the eye.
Schlemm's canal (green) plays a fundamental role in draining the aqueous humor (white arrows) from the anterior chamber of the eye to blood circulation. If the aqueous humor is not able to flow out freely, elevated intraocular pressure damages the optical nerve causing glaucoma and eventually blindness.
Figure 2: Electron microscope images reveal how the aqueous humor is packaged in vacuoles (arrowheads) inside the cells forming the walls of Schlemm's canal.
Aging and glaucoma cause the number and size of giant vacuoles to decrease, meaning that the aqueous humor outflow is compromised. The images compare the giant vacuoles in Schlemm's canals of a healthy mouse (top) and a mouse lacking Tie2 (bottom)
Figure 3: The Ang2-binding and Tie2-activating antibody (ABTAA) rejuvenates the eye of aged mice and rescues them from glaucoma.
Aging causes a reduction of the protein Tie2, a risk factor for increased intraocular pressure and glaucoma. In this experiment, one eye of mice lacking Ang1 and Ang2 was injected with the premixed ABTAA and Ang2, while the other eye was used as negative control. The researchers observed an increase in the area of Schlemm’s canal, together with higher levels of Tie2 (red) and lower intraocular pressure, suggesting that ABTAA restores the canal's functionality. The image includes the transcription factor Prox1 (green) and CD144 (blue), a protein present at the junctions between cells that form the wall of the canal. The angiopoietin-Tie2 system and Prox1 are linked by a vicious circle: the less Tie2 and Ang2, the less Prox1, leading to Schlemm's canal damage, increase in intraocular pressure, and acceleration of glaucoma progression.
2017.09.19 View 7875
Draining Eyes Clogged with Glaucoma
Professor Gou Young Koh in the Graduate School of Medical Science and Engineering and his team have identified a new mechanism involved in the development and progression of glaucoma, and found a potential therapeutic option to treat it. Glaucoma is the second cause of irreversible blindness, after cataracts. It affects about 3.5% of the world population aged 40 to 80.
Professor Koh also serves as the director of the Center for Vascular Research at the Institute for Basic Science. The IBS said the study, published in the Journal of Clinical Investigation, is expected to help the development of therapies to treat primary open-angle glaucoma (POAG), which counts for three quarters of all glaucoma patients.
One of the most important risk factors for glaucoma is the increased pressure inside the eye. A liquid called aqueous humor is constantly produced and drained out from the eye. It transports nutrients and inflates the eye giving it a roughly spherical shape. However, if this fluid cannot flow out of the eye chambers freely, an increase in intraocular pressure can damage the optic nerve, leading to vision loss. The precise mechanism of elevated resistance to aqueous humor outflow remains unclear, and although the current treatments for glaucoma tackle the production and outflow of aqueous humor, their outcomes are still poor.
A component of the eye that plays a fundamental role in draining out the aqueous humor is Schlemm's canal. It collects the aqueous humor and mediates its transfer from the eye chambers to blood circulation. The cells on the walls of the canal, endothelial cells, ship the liquid from the inner to the outer side in “packages”, called vacuoles. As the shape and number of the vacuoles reflects the outflow performance, several giant vacuoles are expected in the normal outflow process.
The team explained how imbalances in Schlemm's canal significantly increase the risk of glaucoma. They showed that an important regulator for canal functionality is the angiopoietin-Tie2 system. Angiopoietins, such as Ang1 and Ang2, are proteins important for the growth of new blood vessels and Tie2 is the receptor that binds them. It is known that the angiopoietin-Tie2 system plays a role in Schlemm’s canal formation, as Tie2 mutations or angiopoietin absence result in congenital glaucoma. However, this study clarified that it is also critically important during adulthood.
The researchers reported that adult mice deficient in Tie2 suffer from an elevated intraocular pressure, retinal neuronal damage and partial visual impairment. Moreover, they had a markedly decreased number of giant vacuoles inside Schlemm’s canal endothelial cells, which indicate a poor aqueous humor drainage.
The scientists also investigated if and how this process changes in older mice, as aging is a major risk factor for glaucoma, and showed that aged mice experience reduced levels of giant vacuoles, Tie2, Ang1, and Ang2, as well as other proteins connected with the angiopoietin-Tie2 pathway, like Prox1.
To test whether Tie2 activation could shift the situation, the researchers tested the antibody ABTAA (Ang2-binding and Tie2-activating antibody). They injected it in one eye of mice, while the other eye of the same mice functioned as the negative control. After one week, levels of Tie2 and Prox1, number and diameter of giant vacuoles in Schlemm’s canals increased in the ABTAA-treated eyes compared to control eyes. The researchers observed a similar outcome with decreased intraocular pressure when ABTAA was injected to the eyes of mice suffering from POAG with regressed Schlemm’s canals, indicating that this antibody might be considered as a therapeutic option.
"Slow development of glaucoma treatments is partly due to the poor understanding of the underlying pathogenesis," said Professor Koh, the corresponding author of the study. "We hope that identifying the critical role of the angiopoietin-Tie2 system in adult Schlemm’s canals will bring a significant boost in the development of therapeutics."
Figure 1: Schlemm's canal position inside the eye.
Schlemm's canal (green) plays a fundamental role in draining the aqueous humor (white arrows) from the anterior chamber of the eye to blood circulation. If the aqueous humor is not able to flow out freely, elevated intraocular pressure damages the optical nerve causing glaucoma and eventually blindness.
Figure 2: Electron microscope images reveal how the aqueous humor is packaged in vacuoles (arrowheads) inside the cells forming the walls of Schlemm's canal.
Aging and glaucoma cause the number and size of giant vacuoles to decrease, meaning that the aqueous humor outflow is compromised. The images compare the giant vacuoles in Schlemm's canals of a healthy mouse (top) and a mouse lacking Tie2 (bottom)
Figure 3: The Ang2-binding and Tie2-activating antibody (ABTAA) rejuvenates the eye of aged mice and rescues them from glaucoma.
Aging causes a reduction of the protein Tie2, a risk factor for increased intraocular pressure and glaucoma. In this experiment, one eye of mice lacking Ang1 and Ang2 was injected with the premixed ABTAA and Ang2, while the other eye was used as negative control. The researchers observed an increase in the area of Schlemm’s canal, together with higher levels of Tie2 (red) and lower intraocular pressure, suggesting that ABTAA restores the canal's functionality. The image includes the transcription factor Prox1 (green) and CD144 (blue), a protein present at the junctions between cells that form the wall of the canal. The angiopoietin-Tie2 system and Prox1 are linked by a vicious circle: the less Tie2 and Ang2, the less Prox1, leading to Schlemm's canal damage, increase in intraocular pressure, and acceleration of glaucoma progression.
2017.09.19 View 7875