Electron+Microscopy
-
 High-Performance Sodium Ion Batteries Using Copper Sulfide
(Prof.Yuk and his two PhD candidates Parks)
Researchers presented a new strategy for extending sodium ion batteries’ cyclability using copper sulfide as the electrode material. This strategy has led to high-performance conversion reactions and is expected to advance the commercialization of sodium ion batteries as they emerge as an alternative to lithium ion batteries.
Professor Jong Min Yuk’s team confirmed the stable sodium storage mechanism using copper sulfide, a superior electrode material that is pulverization-tolerant and induces capacity recovery. Their findings suggest that when employing copper sulfide, sodium ion batteries will have a lifetime of more than five years with one charge per a day. Even better, copper sulfide, composed of abundant natural materials such as copper and sulfur, has better cost competitiveness than lithium ion batteries, which use lithium and cobalt.
Intercalation-type materials such as graphite, which serve as commercialized anode materials in lithium ion batteries, have not been viable for high-capacity sodium storage due to their insufficient interlayer spacing. Thus, conversion and alloying reactions type materials have been explored to meet higher capacity in the anode part. However, those materials generally bring up large volume expansions and abrupt crystallographic changes, which lead to severe capacity degradation.
The team confirmed that semi-coherent phase interfaces and grain boundaries in conversion reactions played key roles in enabling pulverization-tolerant conversion reactions and capacity recovery, respectively.
Most of conversion and alloying reactions type battery materials usually experience severe capacity degradations due to having completely different crystal structures and large volume expansion before and after the reactions. However, copper sulfides underwent a gradual crystallographic change to make the semi-coherent interfaces, which eventually prevented the pulverization of particles. Based on this unique mechanism, the team confirmed that copper sulfide exhibits a high capacity and high cycling stability regardless of its size and morphology.
Professor Yuk said, “Sodium ion batteries employing copper sulfide can advance sodium ion batteries, which could contribute to the development of low-cost energy storage systems and address the micro-dust issue”
This study was posted in Advanced Science on April 26 online and selected as the inside back cover for June issue.
(Figure: Schematic model demonstrating grain boundaries and phase interfaces formations.)
2019.07.15 View 29143
High-Performance Sodium Ion Batteries Using Copper Sulfide
(Prof.Yuk and his two PhD candidates Parks)
Researchers presented a new strategy for extending sodium ion batteries’ cyclability using copper sulfide as the electrode material. This strategy has led to high-performance conversion reactions and is expected to advance the commercialization of sodium ion batteries as they emerge as an alternative to lithium ion batteries.
Professor Jong Min Yuk’s team confirmed the stable sodium storage mechanism using copper sulfide, a superior electrode material that is pulverization-tolerant and induces capacity recovery. Their findings suggest that when employing copper sulfide, sodium ion batteries will have a lifetime of more than five years with one charge per a day. Even better, copper sulfide, composed of abundant natural materials such as copper and sulfur, has better cost competitiveness than lithium ion batteries, which use lithium and cobalt.
Intercalation-type materials such as graphite, which serve as commercialized anode materials in lithium ion batteries, have not been viable for high-capacity sodium storage due to their insufficient interlayer spacing. Thus, conversion and alloying reactions type materials have been explored to meet higher capacity in the anode part. However, those materials generally bring up large volume expansions and abrupt crystallographic changes, which lead to severe capacity degradation.
The team confirmed that semi-coherent phase interfaces and grain boundaries in conversion reactions played key roles in enabling pulverization-tolerant conversion reactions and capacity recovery, respectively.
Most of conversion and alloying reactions type battery materials usually experience severe capacity degradations due to having completely different crystal structures and large volume expansion before and after the reactions. However, copper sulfides underwent a gradual crystallographic change to make the semi-coherent interfaces, which eventually prevented the pulverization of particles. Based on this unique mechanism, the team confirmed that copper sulfide exhibits a high capacity and high cycling stability regardless of its size and morphology.
Professor Yuk said, “Sodium ion batteries employing copper sulfide can advance sodium ion batteries, which could contribute to the development of low-cost energy storage systems and address the micro-dust issue”
This study was posted in Advanced Science on April 26 online and selected as the inside back cover for June issue.
(Figure: Schematic model demonstrating grain boundaries and phase interfaces formations.)
2019.07.15 View 29143 -
 Professor Lee Jeong Yong Receives 2012 'KAISTian of the Year' Award
Professor Lee Jeong Yong (Department of Material Science and Engineering) received the 2012 ‘KAISTian of the Year’ Award.
Professor Lee had successfully developed a technique that allowed the observation and analysis of liquid in atomic scale.
The technique is expected to have great impact on nano-material synthesis in solution, explaining electrode and electrolyte reaction, liquid and catalysis reaction research, and etc. and was therefore named as the best experimental accomplishment in KAIST in 2012.
Professor Lee and his team’s finding has been published in the April edition of Science magazine and has had attracted the attention of the world. In addition, BBC News, and Science & Environment reported on the findings as their respective top articles.
The optical microscope is incapable of atomic scale observation and the electron microscopes are capable but because of the vacuum state all liquids undergo evaporation making it impossible to observe liquids in an atomic scale.
Professor Lee’s team wrapped the liquid with a layer of grapheme to prevent evaporation and successfully observed real time the platinum growth process in solution.
Professor Lee’s findings were introduced as an example of exemplar research case in the Presidential address for ‘Science Day’ in April.
2013.01.22 View 10447
Professor Lee Jeong Yong Receives 2012 'KAISTian of the Year' Award
Professor Lee Jeong Yong (Department of Material Science and Engineering) received the 2012 ‘KAISTian of the Year’ Award.
Professor Lee had successfully developed a technique that allowed the observation and analysis of liquid in atomic scale.
The technique is expected to have great impact on nano-material synthesis in solution, explaining electrode and electrolyte reaction, liquid and catalysis reaction research, and etc. and was therefore named as the best experimental accomplishment in KAIST in 2012.
Professor Lee and his team’s finding has been published in the April edition of Science magazine and has had attracted the attention of the world. In addition, BBC News, and Science & Environment reported on the findings as their respective top articles.
The optical microscope is incapable of atomic scale observation and the electron microscopes are capable but because of the vacuum state all liquids undergo evaporation making it impossible to observe liquids in an atomic scale.
Professor Lee’s team wrapped the liquid with a layer of grapheme to prevent evaporation and successfully observed real time the platinum growth process in solution.
Professor Lee’s findings were introduced as an example of exemplar research case in the Presidential address for ‘Science Day’ in April.
2013.01.22 View 10447 -
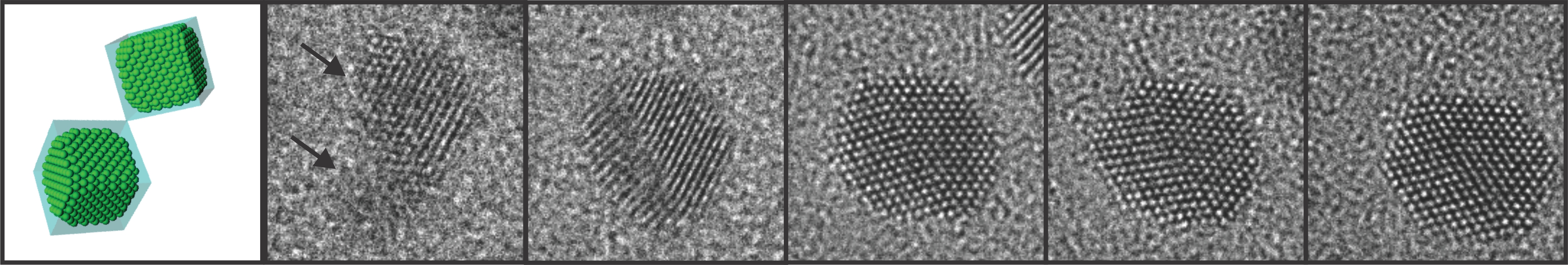 High-resolution Atomic Imaging of Specimens in Liquid Observed by Transmission Electron Microscopes Using Graphene Liquid Cells
Looking into specimens in liquid at the atomic level to understand nanoscale processes so far regarded as impossible to witnessThe Korea Advanced Institute of Science and Technology (KAIST) announced that a research team from the Department of Materials Science and Engineering has developed a technology that enables scientists and engineers to observe processes occurring in liquid media on the smallest possible scale which is less than a nanometer.
Professor Jeong Yong Lee and Researcher Jong Min Yuk, in collaboration with Professors Paul Alivisatos’s and Alex Zettl’s groups at the University of California, Berkeley, succeeded in making a graphene liquid cell or capsule, confining an ultra-thin liquid film between layers of graphene, for real-time and in situ imagining of nanoscale processes in fluids with atomic-level resolution by a transmission electron microscope (TEM). Their research was published in the April 6, 2012 issue of Science. (http://www.sciencemag.org/content/336/6077/61.abstract)
The graphene liquid cell (GLC) is composed of two sheets of graphene sandwiched to create a sealed chamber where a platinum growth solution is encapsulated in the form of a thin slice. Each graphene layer has a thickness of one carbon atom, the thinnest membrane that has ever been used to fabricate a liquid cell required for TEM.
The research team peered inside the GLC to observe the growth and dynamics of platinum nanocrystals in solution as they coalesced into a larger size, during which the graphene membrane with the encapsulated liquid remained intact. The researchers from KAIST and the UC Berkeley identified important features in the ongoing process of the nanocrystals’ coalescence and their expansion through coalescence to form certain shapes by imaging the phenomena with atomic-level resolution.
Professor Lee said,
“It has now become possible for scientists to observe what is happening in liquids on an atomic level under transmission electron microscopes.”
Researcher Yuk, one of the first authors of the paper, explained his research work.
“This research will promote other fields of study related to materials in a fluid stage including physical, chemical, and biological phenomena at the atomic level and promises numerous applications in the future. Pending further studies on liquid microscopy, the full application of a graphene-liquid-cell (GLC) TEM to biological samples is yet to be confirmed. Nonetheless, the GLC is the most effective technique developed today to sustain the natural state of fluid samples or species suspended in the liquid for a TEM imaging.”
The transmission electron microscope (TEM), first introduced in the 1930s, produces images at a significantly higher resolution than light microscopes, allowing users to examine the smallest level of physical, chemical, and biological phenomena. Observations by TEM with atomic resolution, however, have been limited to solid and/or frozen samples, and thus it has previously been impossible to study the real time fluid dynamics of liquid phases.
TEM imaging is performed in a high vacuum chamber in which a thin slice of the imaged sample is situated, and an electron beam passes through the slice to create an image. In this process, a liquid medium, unlike solid or frozen samples, evaporates, making it difficult to observe under TEM.
Attempts to produce a liquid capsule have thus far been made with electron-transparent membranes of such materials as silicon nitride or silicon oxide; such liquid capsules are relatively thick (tens to one hundred nanometers), however, resulting in poor electron transmittance with a reduced resolution of only a few nanometers. Silicon nitride is 25 nanometers thick, whereas graphene is only 0.34 nanometers.
Graphene, most commonly found in bulk graphite, is the thinnest material made out of carbon atoms. It has unique properties such as mechanical tensile strength, high flexibility, impermeability to small molecules, and high electrical conductivity. Graphene is an excellent material to hold micro- and nanoscopic objects for observation in a transmission electron microscope by minimizing scattering of the electron beam that irradiates a liquid sample while reducing charging and heating effects.
###
Figure 1. Schematic illustration of graphene liquid cells. Sandwiched two sheets of graphene encapsulate a platinum growth solution.
Figure 2. In-situ TEM observation of nanocrystal growth and shape evolution. TEM images of platinum nanocrystal coalescence and their faceting in the growth solution.
2012.04.23 View 13093
High-resolution Atomic Imaging of Specimens in Liquid Observed by Transmission Electron Microscopes Using Graphene Liquid Cells
Looking into specimens in liquid at the atomic level to understand nanoscale processes so far regarded as impossible to witnessThe Korea Advanced Institute of Science and Technology (KAIST) announced that a research team from the Department of Materials Science and Engineering has developed a technology that enables scientists and engineers to observe processes occurring in liquid media on the smallest possible scale which is less than a nanometer.
Professor Jeong Yong Lee and Researcher Jong Min Yuk, in collaboration with Professors Paul Alivisatos’s and Alex Zettl’s groups at the University of California, Berkeley, succeeded in making a graphene liquid cell or capsule, confining an ultra-thin liquid film between layers of graphene, for real-time and in situ imagining of nanoscale processes in fluids with atomic-level resolution by a transmission electron microscope (TEM). Their research was published in the April 6, 2012 issue of Science. (http://www.sciencemag.org/content/336/6077/61.abstract)
The graphene liquid cell (GLC) is composed of two sheets of graphene sandwiched to create a sealed chamber where a platinum growth solution is encapsulated in the form of a thin slice. Each graphene layer has a thickness of one carbon atom, the thinnest membrane that has ever been used to fabricate a liquid cell required for TEM.
The research team peered inside the GLC to observe the growth and dynamics of platinum nanocrystals in solution as they coalesced into a larger size, during which the graphene membrane with the encapsulated liquid remained intact. The researchers from KAIST and the UC Berkeley identified important features in the ongoing process of the nanocrystals’ coalescence and their expansion through coalescence to form certain shapes by imaging the phenomena with atomic-level resolution.
Professor Lee said,
“It has now become possible for scientists to observe what is happening in liquids on an atomic level under transmission electron microscopes.”
Researcher Yuk, one of the first authors of the paper, explained his research work.
“This research will promote other fields of study related to materials in a fluid stage including physical, chemical, and biological phenomena at the atomic level and promises numerous applications in the future. Pending further studies on liquid microscopy, the full application of a graphene-liquid-cell (GLC) TEM to biological samples is yet to be confirmed. Nonetheless, the GLC is the most effective technique developed today to sustain the natural state of fluid samples or species suspended in the liquid for a TEM imaging.”
The transmission electron microscope (TEM), first introduced in the 1930s, produces images at a significantly higher resolution than light microscopes, allowing users to examine the smallest level of physical, chemical, and biological phenomena. Observations by TEM with atomic resolution, however, have been limited to solid and/or frozen samples, and thus it has previously been impossible to study the real time fluid dynamics of liquid phases.
TEM imaging is performed in a high vacuum chamber in which a thin slice of the imaged sample is situated, and an electron beam passes through the slice to create an image. In this process, a liquid medium, unlike solid or frozen samples, evaporates, making it difficult to observe under TEM.
Attempts to produce a liquid capsule have thus far been made with electron-transparent membranes of such materials as silicon nitride or silicon oxide; such liquid capsules are relatively thick (tens to one hundred nanometers), however, resulting in poor electron transmittance with a reduced resolution of only a few nanometers. Silicon nitride is 25 nanometers thick, whereas graphene is only 0.34 nanometers.
Graphene, most commonly found in bulk graphite, is the thinnest material made out of carbon atoms. It has unique properties such as mechanical tensile strength, high flexibility, impermeability to small molecules, and high electrical conductivity. Graphene is an excellent material to hold micro- and nanoscopic objects for observation in a transmission electron microscope by minimizing scattering of the electron beam that irradiates a liquid sample while reducing charging and heating effects.
###
Figure 1. Schematic illustration of graphene liquid cells. Sandwiched two sheets of graphene encapsulate a platinum growth solution.
Figure 2. In-situ TEM observation of nanocrystal growth and shape evolution. TEM images of platinum nanocrystal coalescence and their faceting in the growth solution.
2012.04.23 View 13093