BT
-
 KAIST provides a comprehensive resource on microbial cell factories for sustainable chemical production
In silico analysis of five industrial microorganisms identifies optimal strains and metabolic engineering strategies for producing 235 valuable chemicals
Climate change and the depletion of fossil fuels have raised the global need for sustainable chemical production. In response to these environmental challenges, microbial cell factories are gaining attention as eco-friendly platforms for producing chemicals using renewable resources, while metabolic engineering technologies to enhance these cell factories are becoming crucial tools for maximizing production efficiency. However, difficulties in selecting suitable microbial strains and optimizing complex metabolic pathways continue to pose significant obstacles to practical industrial applications.
KAIST (President Kwang-Hyung Lee) announced on 27th of March that Distinguished Professor Sang Yup Lee’s research team in the Department of Chemical and Biomolecular Engineering comprehensively evaluated the production capabilities of various industrial microbial cell factories using in silico simulations and, based on these findings, identified the most suitable microbial strains for producing specific chemicals as well as optimal metabolic engineering strategies.
Previously, researchers attempted to determine the best strains and efficient metabolic engineering strategies among numerous microbial candidates through extensive biological experiments and meticulous verification processes. However, this approach required substantial time and costs. Recently, the introduction of genome-scale metabolic models (GEMs), which reconstruct the metabolic networks within an organism based on its entire genome information, has enabled systematic analysis of metabolic fluxes via computer simulations. This development offers a new way to overcome limitations of conventional experimental approaches, revolutionizing both strain selection and metabolic pathway design.
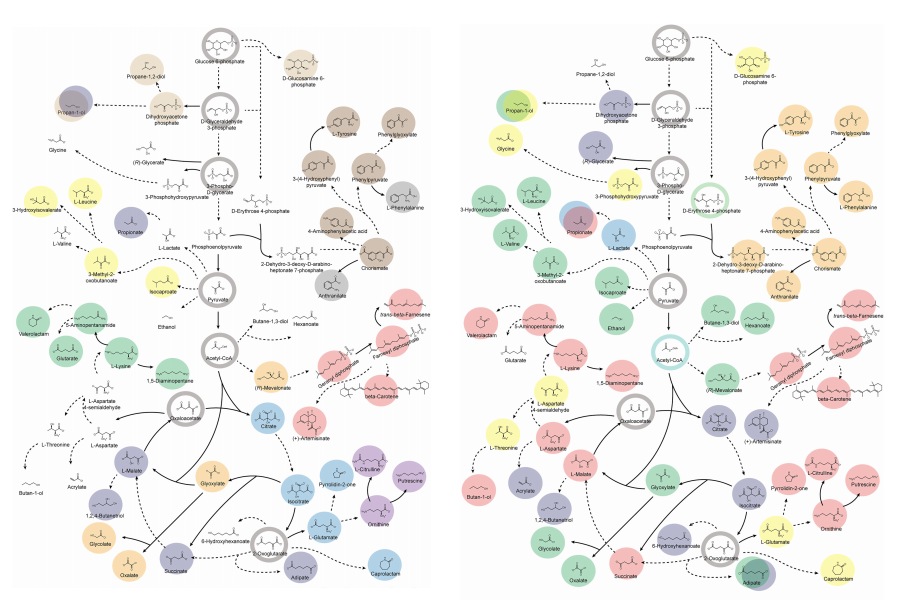
Accordingly, Professor Lee’s team at the Department of Chemical and Biomolecular Engineering, KAIST, evaluated the production capabilities of five representative industrial microorganisms—Escherichia coli, Saccharomyces cerevisiae, Bacillus subtilis, Corynebacterium glutamicum, and Pseudomonas putida—for 235 bio-based chemicals. Using GEMs, the researchers calculated both the maximum theoretical yields and the maximum achievable yields under industrial conditions for each chemical, thereby establishing criteria to identify the most suitable strains for each target compound.
< Figure 1. Outline of the strategy for improving microbial cell factories using a genome-scale metabolic model (GEM) >
The team specifically proposed strategies such as introducing heterologous enzyme reactions derived from other organisms and exchanging cofactors used by microbes to expand metabolic pathways. These strategies were shown to increase yields beyond the innate metabolic capacities of the microorganisms, resulting in higher production of industrially important chemicals such as mevalonic acid, propanol, fatty acids, and isoprenoids.
Moreover, by applying a computational approach to analyze metabolic fluxes in silico, the researchers suggested strategies for improving microbial strains to maximize the production of various chemicals. They quantitatively identified the relationships between specific enzyme reactions and target chemical production, as well as the relationships between enzymes and metabolites, determining which enzyme reactions should be up- or down-regulated. Through this, the team presented strategies not only to achieve high theoretical yields but also to maximize actual production capacities.
< Figure 2. Comparison of production routes and maximum yields of useful chemicals using representative industrial microorganisms >
Dr. Gi Bae Kim, the first author of this paper from the KAIST BioProcess Engineering Research Center, explained, “By introducing metabolic pathways derived from other organisms and exchanging cofactors, it is possible to design new microbial cell factories that surpass existing limitations. The strategies presented in this study will play a pivotal role in making microbial-based production processes more economical and efficient.” In addition, Distinguished Professor Sang Yup Lee noted, “This research serves as a key resource in the field of systems metabolic engineering, reducing difficulties in strain selection and pathway design, and enabling more efficient development of microbial cell factories. We expect it to greatly contribute to the future development of technologies for producing various eco-friendly chemicals, such as biofuels, bioplastics, and functional food materials.”
This research was conducted with the support from the Development of platform technologies of microbial cell factories for the next-generation biorefineries project and Development of advanced synthetic biology source technologies for leading the biomanufacturing industry project (Project Leader: Distinguished Professor Sang Yup Lee, KAIST) from National Research Foundation supported by the Korean Ministry of Science and ICT.
2025.03.27 View 3650
KAIST provides a comprehensive resource on microbial cell factories for sustainable chemical production
In silico analysis of five industrial microorganisms identifies optimal strains and metabolic engineering strategies for producing 235 valuable chemicals
Climate change and the depletion of fossil fuels have raised the global need for sustainable chemical production. In response to these environmental challenges, microbial cell factories are gaining attention as eco-friendly platforms for producing chemicals using renewable resources, while metabolic engineering technologies to enhance these cell factories are becoming crucial tools for maximizing production efficiency. However, difficulties in selecting suitable microbial strains and optimizing complex metabolic pathways continue to pose significant obstacles to practical industrial applications.
KAIST (President Kwang-Hyung Lee) announced on 27th of March that Distinguished Professor Sang Yup Lee’s research team in the Department of Chemical and Biomolecular Engineering comprehensively evaluated the production capabilities of various industrial microbial cell factories using in silico simulations and, based on these findings, identified the most suitable microbial strains for producing specific chemicals as well as optimal metabolic engineering strategies.
Previously, researchers attempted to determine the best strains and efficient metabolic engineering strategies among numerous microbial candidates through extensive biological experiments and meticulous verification processes. However, this approach required substantial time and costs. Recently, the introduction of genome-scale metabolic models (GEMs), which reconstruct the metabolic networks within an organism based on its entire genome information, has enabled systematic analysis of metabolic fluxes via computer simulations. This development offers a new way to overcome limitations of conventional experimental approaches, revolutionizing both strain selection and metabolic pathway design.
Accordingly, Professor Lee’s team at the Department of Chemical and Biomolecular Engineering, KAIST, evaluated the production capabilities of five representative industrial microorganisms—Escherichia coli, Saccharomyces cerevisiae, Bacillus subtilis, Corynebacterium glutamicum, and Pseudomonas putida—for 235 bio-based chemicals. Using GEMs, the researchers calculated both the maximum theoretical yields and the maximum achievable yields under industrial conditions for each chemical, thereby establishing criteria to identify the most suitable strains for each target compound.
< Figure 1. Outline of the strategy for improving microbial cell factories using a genome-scale metabolic model (GEM) >
The team specifically proposed strategies such as introducing heterologous enzyme reactions derived from other organisms and exchanging cofactors used by microbes to expand metabolic pathways. These strategies were shown to increase yields beyond the innate metabolic capacities of the microorganisms, resulting in higher production of industrially important chemicals such as mevalonic acid, propanol, fatty acids, and isoprenoids.
Moreover, by applying a computational approach to analyze metabolic fluxes in silico, the researchers suggested strategies for improving microbial strains to maximize the production of various chemicals. They quantitatively identified the relationships between specific enzyme reactions and target chemical production, as well as the relationships between enzymes and metabolites, determining which enzyme reactions should be up- or down-regulated. Through this, the team presented strategies not only to achieve high theoretical yields but also to maximize actual production capacities.
< Figure 2. Comparison of production routes and maximum yields of useful chemicals using representative industrial microorganisms >
Dr. Gi Bae Kim, the first author of this paper from the KAIST BioProcess Engineering Research Center, explained, “By introducing metabolic pathways derived from other organisms and exchanging cofactors, it is possible to design new microbial cell factories that surpass existing limitations. The strategies presented in this study will play a pivotal role in making microbial-based production processes more economical and efficient.” In addition, Distinguished Professor Sang Yup Lee noted, “This research serves as a key resource in the field of systems metabolic engineering, reducing difficulties in strain selection and pathway design, and enabling more efficient development of microbial cell factories. We expect it to greatly contribute to the future development of technologies for producing various eco-friendly chemicals, such as biofuels, bioplastics, and functional food materials.”
This research was conducted with the support from the Development of platform technologies of microbial cell factories for the next-generation biorefineries project and Development of advanced synthetic biology source technologies for leading the biomanufacturing industry project (Project Leader: Distinguished Professor Sang Yup Lee, KAIST) from National Research Foundation supported by the Korean Ministry of Science and ICT.
2025.03.27 View 3650 -
 Synthetic sRNAs to knockdown genes in medical and industrial bacteria
Bacteria are intimately involved in our daily lives. These microorganisms have been used in human history for food such as cheese, yogurt, and wine, In more recent years, through metabolic engineering, microorganisms been used extensively as microbial cell factories to manufacture plastics, feed for livestock, dietary supplements, and drugs. However, in addition to these bacteria that are beneficial to human lives, pathogens such as Pneumonia, Salmonella, and Staphylococcus that cause various infectious diseases are also ubiquitously present. It is important to be able to metabolically control these beneficial industrial bacteria for high value-added chemicals production and to manipulate harmful pathogens to suppress its pathogenic traits.
KAIST (President Kwang Hyung Lee) announced on the 10th that a research team led by Distinguished Professor Sang Yup Lee of the Department of Biochemical Engineering has developed a new sRNA tool that can effectively inhibit target genes in various bacteria, including both Gram-negative and Gram-positive bacteria. The research results were published online on April 24 in Nature Communications.
※ Thesis title: Targeted and high-throughput gene knockdown in diverse bacteria using synthetic sRNAs
※ Author information : Jae Sung Cho (co-1st), Dongsoo Yang (co-1st), Cindy Pricilia Surya Prabowo (co-author), Mohammad Rifqi Ghiffary (co-author), Taehee Han (co-author), Kyeong Rok Choi (co-author), Cheon Woo Moon (co-author), Hengrui Zhou (co-author), Jae Yong Ryu (co-author), Hyun Uk Kim (co-author) and Sang Yup Lee (corresponding author).
sRNA is an effective tool for synthesizing and regulating target genes in E. coli, but it has been difficult to apply to industrially useful Gram-positive bacteria such as Bacillus subtilis and Corynebacterium in addition to Gram-negative bacteria such as E. coli.
To address this issue, a research team led by Distinguished Professor Lee Sang Yup Lee of the Department of Chemical and Biomolecular Engineering at KAIST developed a new sRNA platform that can effectively suppress target genes in various bacteria, including both Gram-negative and positive bacteria. The research team surveyed thousands of microbial-derived sRNA systems in the microbial database, and eventually designated the sRNA system derived from 'Bacillus subtilis' that showed the highest gene knockdown efficiency, and designated it as “Broad-Host-Range sRNA”, or BHR-sRNA.
A similar well-known system is the CRISPR interference (CRISPRi) system, which is a modified CRISPR system that knocks down gene expression by suppressing the gene transcription process. However, the Cas9 protein in the CRISPRi system has a very high molecular weight, and there have been reports growth inhibition in bacteria. The BHR-sRNA system developed in this study did not affect bacterial growth while showing similar gene knockdown efficiencies to CRISPRi.
< Figure 1. a) Schematic illustration demonstrating the mechanism of syntetic sRNA b) Phylogenetic tree of the 16 Gram-negative and Gram-positive bacterial species tested for gene knockdown by the BHR-sRNA system. >
To validate the versatility of the BHR-sRNA system, 16 different gram-negative and gram-positive bacteria were selected and tested, where the BHR-sRNA system worked successfully in 15 of them. In addition, it was demonstrated that the gene knockdown capability was more effective than that of the existing E. coli-based sRNA system in 10 bacteria. The BHR-sRNA system proved to be a universal tool capable of effectively inhibiting gene expression in various bacteria.
In order to address the problem of antibiotic-resistant pathogens that have recently become more serious, the BHR-sRNA was demonstrated to suppress the pathogenicity by suppressing the gene producing the virulence factor. By using BHR-sRNA, biofilm formation, one of the factors resulting in antibiotic resistance, was inhibited by 73% in Staphylococcus epidermidis a pathogen that can cause hospital-acquired infections. Antibiotic resistance was also weakened by 58% in the pneumonia causing bacteria Klebsiella pneumoniae. In addition, BHR-sRNA was applied to industrial bacteria to develop microbial cell factories to produce high value-added chemicals with better production performance. Notably, superior industrial strains were constructed with the aid of BHR-sRNA to produce the following chemicals: valerolactam, a raw material for polyamide polymers, methyl-anthranilate, a grape-flavor food additive, and indigoidine, a blue-toned natural dye.
The BHR-sRNA developed through this study will help expedite the commercialization of bioprocesses to produce high value-added compounds and materials such as artificial meat, jet fuel, health supplements, pharmaceuticals, and plastics. It is also anticipated that to help eradicating antibiotic-resistant pathogens in preparation for another upcoming pandemic. “In the past, we could only develop new tools for gene knockdown for each bacterium, but now we have developed a tool that works for a variety of bacteria” said Distinguished Professor Sang Yup Lee.
This work was supported by the Development of Next-generation Biorefinery Platform Technologies for Leading Bio-based Chemicals Industry Project and the Development of Platform Technologies of Microbial Cell Factories for the Next-generation Biorefineries Project from NRF supported by the Korean MSIT.
2023.05.10 View 8486
Synthetic sRNAs to knockdown genes in medical and industrial bacteria
Bacteria are intimately involved in our daily lives. These microorganisms have been used in human history for food such as cheese, yogurt, and wine, In more recent years, through metabolic engineering, microorganisms been used extensively as microbial cell factories to manufacture plastics, feed for livestock, dietary supplements, and drugs. However, in addition to these bacteria that are beneficial to human lives, pathogens such as Pneumonia, Salmonella, and Staphylococcus that cause various infectious diseases are also ubiquitously present. It is important to be able to metabolically control these beneficial industrial bacteria for high value-added chemicals production and to manipulate harmful pathogens to suppress its pathogenic traits.
KAIST (President Kwang Hyung Lee) announced on the 10th that a research team led by Distinguished Professor Sang Yup Lee of the Department of Biochemical Engineering has developed a new sRNA tool that can effectively inhibit target genes in various bacteria, including both Gram-negative and Gram-positive bacteria. The research results were published online on April 24 in Nature Communications.
※ Thesis title: Targeted and high-throughput gene knockdown in diverse bacteria using synthetic sRNAs
※ Author information : Jae Sung Cho (co-1st), Dongsoo Yang (co-1st), Cindy Pricilia Surya Prabowo (co-author), Mohammad Rifqi Ghiffary (co-author), Taehee Han (co-author), Kyeong Rok Choi (co-author), Cheon Woo Moon (co-author), Hengrui Zhou (co-author), Jae Yong Ryu (co-author), Hyun Uk Kim (co-author) and Sang Yup Lee (corresponding author).
sRNA is an effective tool for synthesizing and regulating target genes in E. coli, but it has been difficult to apply to industrially useful Gram-positive bacteria such as Bacillus subtilis and Corynebacterium in addition to Gram-negative bacteria such as E. coli.
To address this issue, a research team led by Distinguished Professor Lee Sang Yup Lee of the Department of Chemical and Biomolecular Engineering at KAIST developed a new sRNA platform that can effectively suppress target genes in various bacteria, including both Gram-negative and positive bacteria. The research team surveyed thousands of microbial-derived sRNA systems in the microbial database, and eventually designated the sRNA system derived from 'Bacillus subtilis' that showed the highest gene knockdown efficiency, and designated it as “Broad-Host-Range sRNA”, or BHR-sRNA.
A similar well-known system is the CRISPR interference (CRISPRi) system, which is a modified CRISPR system that knocks down gene expression by suppressing the gene transcription process. However, the Cas9 protein in the CRISPRi system has a very high molecular weight, and there have been reports growth inhibition in bacteria. The BHR-sRNA system developed in this study did not affect bacterial growth while showing similar gene knockdown efficiencies to CRISPRi.
< Figure 1. a) Schematic illustration demonstrating the mechanism of syntetic sRNA b) Phylogenetic tree of the 16 Gram-negative and Gram-positive bacterial species tested for gene knockdown by the BHR-sRNA system. >
To validate the versatility of the BHR-sRNA system, 16 different gram-negative and gram-positive bacteria were selected and tested, where the BHR-sRNA system worked successfully in 15 of them. In addition, it was demonstrated that the gene knockdown capability was more effective than that of the existing E. coli-based sRNA system in 10 bacteria. The BHR-sRNA system proved to be a universal tool capable of effectively inhibiting gene expression in various bacteria.
In order to address the problem of antibiotic-resistant pathogens that have recently become more serious, the BHR-sRNA was demonstrated to suppress the pathogenicity by suppressing the gene producing the virulence factor. By using BHR-sRNA, biofilm formation, one of the factors resulting in antibiotic resistance, was inhibited by 73% in Staphylococcus epidermidis a pathogen that can cause hospital-acquired infections. Antibiotic resistance was also weakened by 58% in the pneumonia causing bacteria Klebsiella pneumoniae. In addition, BHR-sRNA was applied to industrial bacteria to develop microbial cell factories to produce high value-added chemicals with better production performance. Notably, superior industrial strains were constructed with the aid of BHR-sRNA to produce the following chemicals: valerolactam, a raw material for polyamide polymers, methyl-anthranilate, a grape-flavor food additive, and indigoidine, a blue-toned natural dye.
The BHR-sRNA developed through this study will help expedite the commercialization of bioprocesses to produce high value-added compounds and materials such as artificial meat, jet fuel, health supplements, pharmaceuticals, and plastics. It is also anticipated that to help eradicating antibiotic-resistant pathogens in preparation for another upcoming pandemic. “In the past, we could only develop new tools for gene knockdown for each bacterium, but now we have developed a tool that works for a variety of bacteria” said Distinguished Professor Sang Yup Lee.
This work was supported by the Development of Next-generation Biorefinery Platform Technologies for Leading Bio-based Chemicals Industry Project and the Development of Platform Technologies of Microbial Cell Factories for the Next-generation Biorefineries Project from NRF supported by the Korean MSIT.
2023.05.10 View 8486 -
 'Flying Drones for Rescue'
(Video Credit: ⓒNASA JPL)
< Team USRG and Professor Shim (second from the right) >
Having recently won the AI R&D Grand Challenge Competition in Korea, Team USRG (Unmanned System Research Group) led by Professor Hyunchul Shim from the School of Electrical Engineering is all geared up to take on their next challenges: the ‘Defense Advanced Research Projects Agency Subterranean Challenge (DARPA SubT Challenge)’ and ‘Lockheed Martin’s AlphaPilot Challenge’ next month.
Team USRG won the obstacle course race in the ‘2019 AI R&D Grand Challenge Competition’ on July 12. They managed to successfully dominate the challenging category of ‘control intelligence.’ Having to complete the obstacle course race solely using AI systems without any connection to the internet made it difficult for most of the eight participating teams to pass the third section of the race, and only Team USRG passed the long pipeline course during their attempt in the main event. They also demonstrated, after the main event, that their drone can navigate all of the checkpoints including landing on the “H” mark using deep learning.
Their drone flew through polls and pipes, and escaped from windows and mazes against strong winds, amid cheers and groans from the crowd gathered at the Korea Exhibition Center (KINTEX) in Goyang, Korea. The team was awarded three million KRW in prize money, and received a research grant worth six hundred million KRW from the Ministry of Science and ICT (MSIT).
“Being ranked first in the race for which we were never given a chance for a test flight means a lot to our team. Considering that we had no information on the exact size of the course in advance, this is a startling result,” said Professor Shim. “We will carry out further research with this funding, and compete once again with the improved AI and drone technology in the 2020 competition,” he added.
The AI R&D Grand Challenge Competition, which was first started in 2017, has been designed to promote AI research and development and expand its application to addressing high-risk technical challenges with significant socio-economic impact.
This year’s competition presented participants with a task where they had to develop AI software technology for drones to navigate themselves autonomously during complex disaster relief operations such as aid delivery.
Each team participated in one of the four tracks of the competition, and their drones were evaluated based on the criteria for each track. The divisions were broken up into intelligent context-awareness, intelligent character recognition, auditory intelligence, and control intelligence.
Team USRG’s technological prowess has been already well acclaimed among international peer groups. Teamed up with NASA JPL, Caltech, and MIT, they will compete in the subterranean mission during the ‘DARPA SubT Challenge’.
Team CoSTAR, as its name stands for, is working together to build ‘Collaborative SubTerranean Autonomous Resilient Robots.’ Professor Shim emphasized the role KAIST plays in Team CoSTAR as a leader in drone technology.
“I think when our drone technology will be added to our peers’ AI and robotics, Team CoSTAR will bring out unsurpassable synergy in completing the subterrestrial and planetary applications. I would like to follow the footprint of Hubo, the winning champion of the 2015 DARPA Robotics Challenge and even extend it to subterranean exploration,” he said.
These next generation autonomous subsurface explorers are now all optimizing the physical AI robot systems developed by Team CoSTAR. They will test their systems in more realistic field environments August 15 through 22 in Pittsburgh, USA. They have already received funding from DARPA for participating.
Team CoSTAR will compete in three consecutive yearly events starting this year, and the last event, planned for 2021, will put the team to the final test with courses that incorporate diverse challenges from all three events. Two million USD will be awarded to the winner after the final event, with additional prizes of up to 200,000 USD for self-funded teams.
Team USRG also ranked third in the recent Hyundai Motor Company’s ‘Autonomous Vehicle Competition’ and another challenge is on the horizon: Lockheed Martin’s ‘AlphaPilot Challenge’. In this event, the teams will be flying their drones through a series of racing gates, trying to beat the best human pilot. The challenge is hosted by Lockheed Martin, the world’s largest military contractor and the maker of the famed F-22 and F-35 stealth fighters, with the goal of stimulating the development of autonomous drones. Team USRG was selected from out of more than 400 teams from around the world and is preparing for a series of races this fall, beginning from the end of August.
Professor Shim said, “It is not easy to perform in a series of competitions in just a few months, but my students are smart, hardworking, and highly motivated. These events indeed demand a lot, but they really challenge the researchers to come up with technologies that work in the real world. This is the way robotics really should be.”
(END)
2019.07.26 View 14184
'Flying Drones for Rescue'
(Video Credit: ⓒNASA JPL)
< Team USRG and Professor Shim (second from the right) >
Having recently won the AI R&D Grand Challenge Competition in Korea, Team USRG (Unmanned System Research Group) led by Professor Hyunchul Shim from the School of Electrical Engineering is all geared up to take on their next challenges: the ‘Defense Advanced Research Projects Agency Subterranean Challenge (DARPA SubT Challenge)’ and ‘Lockheed Martin’s AlphaPilot Challenge’ next month.
Team USRG won the obstacle course race in the ‘2019 AI R&D Grand Challenge Competition’ on July 12. They managed to successfully dominate the challenging category of ‘control intelligence.’ Having to complete the obstacle course race solely using AI systems without any connection to the internet made it difficult for most of the eight participating teams to pass the third section of the race, and only Team USRG passed the long pipeline course during their attempt in the main event. They also demonstrated, after the main event, that their drone can navigate all of the checkpoints including landing on the “H” mark using deep learning.
Their drone flew through polls and pipes, and escaped from windows and mazes against strong winds, amid cheers and groans from the crowd gathered at the Korea Exhibition Center (KINTEX) in Goyang, Korea. The team was awarded three million KRW in prize money, and received a research grant worth six hundred million KRW from the Ministry of Science and ICT (MSIT).
“Being ranked first in the race for which we were never given a chance for a test flight means a lot to our team. Considering that we had no information on the exact size of the course in advance, this is a startling result,” said Professor Shim. “We will carry out further research with this funding, and compete once again with the improved AI and drone technology in the 2020 competition,” he added.
The AI R&D Grand Challenge Competition, which was first started in 2017, has been designed to promote AI research and development and expand its application to addressing high-risk technical challenges with significant socio-economic impact.
This year’s competition presented participants with a task where they had to develop AI software technology for drones to navigate themselves autonomously during complex disaster relief operations such as aid delivery.
Each team participated in one of the four tracks of the competition, and their drones were evaluated based on the criteria for each track. The divisions were broken up into intelligent context-awareness, intelligent character recognition, auditory intelligence, and control intelligence.
Team USRG’s technological prowess has been already well acclaimed among international peer groups. Teamed up with NASA JPL, Caltech, and MIT, they will compete in the subterranean mission during the ‘DARPA SubT Challenge’.
Team CoSTAR, as its name stands for, is working together to build ‘Collaborative SubTerranean Autonomous Resilient Robots.’ Professor Shim emphasized the role KAIST plays in Team CoSTAR as a leader in drone technology.
“I think when our drone technology will be added to our peers’ AI and robotics, Team CoSTAR will bring out unsurpassable synergy in completing the subterrestrial and planetary applications. I would like to follow the footprint of Hubo, the winning champion of the 2015 DARPA Robotics Challenge and even extend it to subterranean exploration,” he said.
These next generation autonomous subsurface explorers are now all optimizing the physical AI robot systems developed by Team CoSTAR. They will test their systems in more realistic field environments August 15 through 22 in Pittsburgh, USA. They have already received funding from DARPA for participating.
Team CoSTAR will compete in three consecutive yearly events starting this year, and the last event, planned for 2021, will put the team to the final test with courses that incorporate diverse challenges from all three events. Two million USD will be awarded to the winner after the final event, with additional prizes of up to 200,000 USD for self-funded teams.
Team USRG also ranked third in the recent Hyundai Motor Company’s ‘Autonomous Vehicle Competition’ and another challenge is on the horizon: Lockheed Martin’s ‘AlphaPilot Challenge’. In this event, the teams will be flying their drones through a series of racing gates, trying to beat the best human pilot. The challenge is hosted by Lockheed Martin, the world’s largest military contractor and the maker of the famed F-22 and F-35 stealth fighters, with the goal of stimulating the development of autonomous drones. Team USRG was selected from out of more than 400 teams from around the world and is preparing for a series of races this fall, beginning from the end of August.
Professor Shim said, “It is not easy to perform in a series of competitions in just a few months, but my students are smart, hardworking, and highly motivated. These events indeed demand a lot, but they really challenge the researchers to come up with technologies that work in the real world. This is the way robotics really should be.”
(END)
2019.07.26 View 14184 -
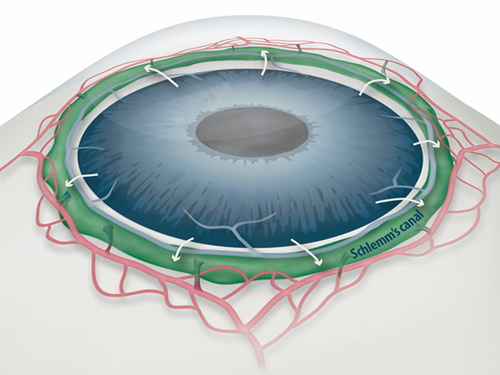 Draining Eyes Clogged with Glaucoma
Professor Gou Young Koh in the Graduate School of Medical Science and Engineering and his team have identified a new mechanism involved in the development and progression of glaucoma, and found a potential therapeutic option to treat it. Glaucoma is the second cause of irreversible blindness, after cataracts. It affects about 3.5% of the world population aged 40 to 80.
Professor Koh also serves as the director of the Center for Vascular Research at the Institute for Basic Science. The IBS said the study, published in the Journal of Clinical Investigation, is expected to help the development of therapies to treat primary open-angle glaucoma (POAG), which counts for three quarters of all glaucoma patients.
One of the most important risk factors for glaucoma is the increased pressure inside the eye. A liquid called aqueous humor is constantly produced and drained out from the eye. It transports nutrients and inflates the eye giving it a roughly spherical shape. However, if this fluid cannot flow out of the eye chambers freely, an increase in intraocular pressure can damage the optic nerve, leading to vision loss. The precise mechanism of elevated resistance to aqueous humor outflow remains unclear, and although the current treatments for glaucoma tackle the production and outflow of aqueous humor, their outcomes are still poor.
A component of the eye that plays a fundamental role in draining out the aqueous humor is Schlemm's canal. It collects the aqueous humor and mediates its transfer from the eye chambers to blood circulation. The cells on the walls of the canal, endothelial cells, ship the liquid from the inner to the outer side in “packages”, called vacuoles. As the shape and number of the vacuoles reflects the outflow performance, several giant vacuoles are expected in the normal outflow process.
The team explained how imbalances in Schlemm's canal significantly increase the risk of glaucoma. They showed that an important regulator for canal functionality is the angiopoietin-Tie2 system. Angiopoietins, such as Ang1 and Ang2, are proteins important for the growth of new blood vessels and Tie2 is the receptor that binds them. It is known that the angiopoietin-Tie2 system plays a role in Schlemm’s canal formation, as Tie2 mutations or angiopoietin absence result in congenital glaucoma. However, this study clarified that it is also critically important during adulthood.
The researchers reported that adult mice deficient in Tie2 suffer from an elevated intraocular pressure, retinal neuronal damage and partial visual impairment. Moreover, they had a markedly decreased number of giant vacuoles inside Schlemm’s canal endothelial cells, which indicate a poor aqueous humor drainage.
The scientists also investigated if and how this process changes in older mice, as aging is a major risk factor for glaucoma, and showed that aged mice experience reduced levels of giant vacuoles, Tie2, Ang1, and Ang2, as well as other proteins connected with the angiopoietin-Tie2 pathway, like Prox1.
To test whether Tie2 activation could shift the situation, the researchers tested the antibody ABTAA (Ang2-binding and Tie2-activating antibody). They injected it in one eye of mice, while the other eye of the same mice functioned as the negative control. After one week, levels of Tie2 and Prox1, number and diameter of giant vacuoles in Schlemm’s canals increased in the ABTAA-treated eyes compared to control eyes. The researchers observed a similar outcome with decreased intraocular pressure when ABTAA was injected to the eyes of mice suffering from POAG with regressed Schlemm’s canals, indicating that this antibody might be considered as a therapeutic option.
"Slow development of glaucoma treatments is partly due to the poor understanding of the underlying pathogenesis," said Professor Koh, the corresponding author of the study. "We hope that identifying the critical role of the angiopoietin-Tie2 system in adult Schlemm’s canals will bring a significant boost in the development of therapeutics."
Figure 1: Schlemm's canal position inside the eye.
Schlemm's canal (green) plays a fundamental role in draining the aqueous humor (white arrows) from the anterior chamber of the eye to blood circulation. If the aqueous humor is not able to flow out freely, elevated intraocular pressure damages the optical nerve causing glaucoma and eventually blindness.
Figure 2: Electron microscope images reveal how the aqueous humor is packaged in vacuoles (arrowheads) inside the cells forming the walls of Schlemm's canal.
Aging and glaucoma cause the number and size of giant vacuoles to decrease, meaning that the aqueous humor outflow is compromised. The images compare the giant vacuoles in Schlemm's canals of a healthy mouse (top) and a mouse lacking Tie2 (bottom)
Figure 3: The Ang2-binding and Tie2-activating antibody (ABTAA) rejuvenates the eye of aged mice and rescues them from glaucoma.
Aging causes a reduction of the protein Tie2, a risk factor for increased intraocular pressure and glaucoma. In this experiment, one eye of mice lacking Ang1 and Ang2 was injected with the premixed ABTAA and Ang2, while the other eye was used as negative control. The researchers observed an increase in the area of Schlemm’s canal, together with higher levels of Tie2 (red) and lower intraocular pressure, suggesting that ABTAA restores the canal's functionality. The image includes the transcription factor Prox1 (green) and CD144 (blue), a protein present at the junctions between cells that form the wall of the canal. The angiopoietin-Tie2 system and Prox1 are linked by a vicious circle: the less Tie2 and Ang2, the less Prox1, leading to Schlemm's canal damage, increase in intraocular pressure, and acceleration of glaucoma progression.
2017.09.19 View 7881
Draining Eyes Clogged with Glaucoma
Professor Gou Young Koh in the Graduate School of Medical Science and Engineering and his team have identified a new mechanism involved in the development and progression of glaucoma, and found a potential therapeutic option to treat it. Glaucoma is the second cause of irreversible blindness, after cataracts. It affects about 3.5% of the world population aged 40 to 80.
Professor Koh also serves as the director of the Center for Vascular Research at the Institute for Basic Science. The IBS said the study, published in the Journal of Clinical Investigation, is expected to help the development of therapies to treat primary open-angle glaucoma (POAG), which counts for three quarters of all glaucoma patients.
One of the most important risk factors for glaucoma is the increased pressure inside the eye. A liquid called aqueous humor is constantly produced and drained out from the eye. It transports nutrients and inflates the eye giving it a roughly spherical shape. However, if this fluid cannot flow out of the eye chambers freely, an increase in intraocular pressure can damage the optic nerve, leading to vision loss. The precise mechanism of elevated resistance to aqueous humor outflow remains unclear, and although the current treatments for glaucoma tackle the production and outflow of aqueous humor, their outcomes are still poor.
A component of the eye that plays a fundamental role in draining out the aqueous humor is Schlemm's canal. It collects the aqueous humor and mediates its transfer from the eye chambers to blood circulation. The cells on the walls of the canal, endothelial cells, ship the liquid from the inner to the outer side in “packages”, called vacuoles. As the shape and number of the vacuoles reflects the outflow performance, several giant vacuoles are expected in the normal outflow process.
The team explained how imbalances in Schlemm's canal significantly increase the risk of glaucoma. They showed that an important regulator for canal functionality is the angiopoietin-Tie2 system. Angiopoietins, such as Ang1 and Ang2, are proteins important for the growth of new blood vessels and Tie2 is the receptor that binds them. It is known that the angiopoietin-Tie2 system plays a role in Schlemm’s canal formation, as Tie2 mutations or angiopoietin absence result in congenital glaucoma. However, this study clarified that it is also critically important during adulthood.
The researchers reported that adult mice deficient in Tie2 suffer from an elevated intraocular pressure, retinal neuronal damage and partial visual impairment. Moreover, they had a markedly decreased number of giant vacuoles inside Schlemm’s canal endothelial cells, which indicate a poor aqueous humor drainage.
The scientists also investigated if and how this process changes in older mice, as aging is a major risk factor for glaucoma, and showed that aged mice experience reduced levels of giant vacuoles, Tie2, Ang1, and Ang2, as well as other proteins connected with the angiopoietin-Tie2 pathway, like Prox1.
To test whether Tie2 activation could shift the situation, the researchers tested the antibody ABTAA (Ang2-binding and Tie2-activating antibody). They injected it in one eye of mice, while the other eye of the same mice functioned as the negative control. After one week, levels of Tie2 and Prox1, number and diameter of giant vacuoles in Schlemm’s canals increased in the ABTAA-treated eyes compared to control eyes. The researchers observed a similar outcome with decreased intraocular pressure when ABTAA was injected to the eyes of mice suffering from POAG with regressed Schlemm’s canals, indicating that this antibody might be considered as a therapeutic option.
"Slow development of glaucoma treatments is partly due to the poor understanding of the underlying pathogenesis," said Professor Koh, the corresponding author of the study. "We hope that identifying the critical role of the angiopoietin-Tie2 system in adult Schlemm’s canals will bring a significant boost in the development of therapeutics."
Figure 1: Schlemm's canal position inside the eye.
Schlemm's canal (green) plays a fundamental role in draining the aqueous humor (white arrows) from the anterior chamber of the eye to blood circulation. If the aqueous humor is not able to flow out freely, elevated intraocular pressure damages the optical nerve causing glaucoma and eventually blindness.
Figure 2: Electron microscope images reveal how the aqueous humor is packaged in vacuoles (arrowheads) inside the cells forming the walls of Schlemm's canal.
Aging and glaucoma cause the number and size of giant vacuoles to decrease, meaning that the aqueous humor outflow is compromised. The images compare the giant vacuoles in Schlemm's canals of a healthy mouse (top) and a mouse lacking Tie2 (bottom)
Figure 3: The Ang2-binding and Tie2-activating antibody (ABTAA) rejuvenates the eye of aged mice and rescues them from glaucoma.
Aging causes a reduction of the protein Tie2, a risk factor for increased intraocular pressure and glaucoma. In this experiment, one eye of mice lacking Ang1 and Ang2 was injected with the premixed ABTAA and Ang2, while the other eye was used as negative control. The researchers observed an increase in the area of Schlemm’s canal, together with higher levels of Tie2 (red) and lower intraocular pressure, suggesting that ABTAA restores the canal's functionality. The image includes the transcription factor Prox1 (green) and CD144 (blue), a protein present at the junctions between cells that form the wall of the canal. The angiopoietin-Tie2 system and Prox1 are linked by a vicious circle: the less Tie2 and Ang2, the less Prox1, leading to Schlemm's canal damage, increase in intraocular pressure, and acceleration of glaucoma progression.
2017.09.19 View 7881 -
 New Building Endowed in Bio and Brain Engineering Department
An endowment from the former Chairman of Mirae Industries, Moon Soul Chung, was used to establish the Yang Bun Soon Building in the Bio and Brain Engineering Department at KAIST. The opening ceremony for the building took place on February 8 and was attended by President Sung-Mo Kang, KAIST administrators, faculty, and students.
The Yang Bun Soon Building, named after the wife of Chairman Chung, is a new addition to the Bio and Brain Engineering Department complex. The five-story building was erected next to the 11-story Chung Moon Soul Building, which was completed in 2003 using a portion of his first endowment to KAIST. Chairman Chung donated approximately 30 billion KRW for funding a convergence research for IT and BT in 2001.
The new building was completed with financing from Chung’s second endowment of 21.5 billion KRW in support of the fields of brain and cognitive sciences in 2014. The building will accommodate both lab facilities and lecture halls.
At the ceremony, President Kang thanked the Chungs for their continuing generosity to KAIST. He commended Chung for showing how entrepreneurs can fulfill their social responsibility by supporting Korea’s future through donations and support.
(Photo caption: Chung Moon Soul Building (left) and Yang Bun Soon Building(right))
2017.02.09 View 6713
New Building Endowed in Bio and Brain Engineering Department
An endowment from the former Chairman of Mirae Industries, Moon Soul Chung, was used to establish the Yang Bun Soon Building in the Bio and Brain Engineering Department at KAIST. The opening ceremony for the building took place on February 8 and was attended by President Sung-Mo Kang, KAIST administrators, faculty, and students.
The Yang Bun Soon Building, named after the wife of Chairman Chung, is a new addition to the Bio and Brain Engineering Department complex. The five-story building was erected next to the 11-story Chung Moon Soul Building, which was completed in 2003 using a portion of his first endowment to KAIST. Chairman Chung donated approximately 30 billion KRW for funding a convergence research for IT and BT in 2001.
The new building was completed with financing from Chung’s second endowment of 21.5 billion KRW in support of the fields of brain and cognitive sciences in 2014. The building will accommodate both lab facilities and lecture halls.
At the ceremony, President Kang thanked the Chungs for their continuing generosity to KAIST. He commended Chung for showing how entrepreneurs can fulfill their social responsibility by supporting Korea’s future through donations and support.
(Photo caption: Chung Moon Soul Building (left) and Yang Bun Soon Building(right))
2017.02.09 View 6713 -
 KAIST Research Team Identified Promising New Source to Obtain Stem Cells
KAIST Research Team Identified Promising New Source to Obtain Stem Cells
A research team at KAIST led by Professor Gou-Young Koh, M.D. and Ph.D., of the Department of Biological Sciences, has found evidence that fat tissue, known as adipose tissue, may be a promising new source of valuable and easy-to-obtain regenerative cells called hematopoietic stem and progenitor cells (HSPCs).
HSPCs are adult stem cells that have the ability to generate and develop into many different kinds of cells. They are now used to repair damaged tissues and are being studied for their potential to treat a vast array of chronic and degenerative conditions such as leukemia. Mostly found in bone marrow but with a limited quantity, HSPCs are hard to cultivate in vitro, thus becoming an obstacle to use them for research and therapeutic purposes.
Within the adipose tissue is a special cell population known as the stromal vascular fraction (SVF), which share similar properties to those in the bone marrow. Cells in the bone marrow and SVF have the ability to differentiate into several cell types. In addition, both adipose and bone marrow offer similar environments for optimal stem cell growth and reproduction.
Given the fact that adipose and bone marrow tissues share similar properties, Dr. Koh and his team conducted a research, injecting granulocyte colony-stimulating factor (G-CSF), a growth hormone used to encourage the development of stem cells, into an adipose tissue of a mouse whose bone marrow is damaged. As a result, the team has found that the SVF derived from adipose tissue contains functional HSPCs capable of generating hematopoietic (blood-forming) cells to repair the damaged bone morrow.
The Ministry of Education, Science and Technology nominated the KAIST research as one of its sponsoring 21st Century Frontier R&D Programs. Director Dong-Wook Kim of Stem Cell Research Center (SCRS) that oversees the KAIST team expressed a possibility to use the adipose tissue as an alternative source to obtain stem cells for regeneration medicine.
Dr. Koh also said, “It’s been a well known method to extract HSPCs from the bone morrow or blood, but it’s the first time to identify adipose tissue, before considered useless, as a new possible supplier for functional and transplantable HSPCs.”
The study results have received an important recognition from the academia—the American Society of Hematology published the research as a main article in its official journal, Blood, for the February 4th, 2010 issue, which is the most citied peer-reviewed publication in the field.
2010.02.05 View 14040
KAIST Research Team Identified Promising New Source to Obtain Stem Cells
KAIST Research Team Identified Promising New Source to Obtain Stem Cells
A research team at KAIST led by Professor Gou-Young Koh, M.D. and Ph.D., of the Department of Biological Sciences, has found evidence that fat tissue, known as adipose tissue, may be a promising new source of valuable and easy-to-obtain regenerative cells called hematopoietic stem and progenitor cells (HSPCs).
HSPCs are adult stem cells that have the ability to generate and develop into many different kinds of cells. They are now used to repair damaged tissues and are being studied for their potential to treat a vast array of chronic and degenerative conditions such as leukemia. Mostly found in bone marrow but with a limited quantity, HSPCs are hard to cultivate in vitro, thus becoming an obstacle to use them for research and therapeutic purposes.
Within the adipose tissue is a special cell population known as the stromal vascular fraction (SVF), which share similar properties to those in the bone marrow. Cells in the bone marrow and SVF have the ability to differentiate into several cell types. In addition, both adipose and bone marrow offer similar environments for optimal stem cell growth and reproduction.
Given the fact that adipose and bone marrow tissues share similar properties, Dr. Koh and his team conducted a research, injecting granulocyte colony-stimulating factor (G-CSF), a growth hormone used to encourage the development of stem cells, into an adipose tissue of a mouse whose bone marrow is damaged. As a result, the team has found that the SVF derived from adipose tissue contains functional HSPCs capable of generating hematopoietic (blood-forming) cells to repair the damaged bone morrow.
The Ministry of Education, Science and Technology nominated the KAIST research as one of its sponsoring 21st Century Frontier R&D Programs. Director Dong-Wook Kim of Stem Cell Research Center (SCRS) that oversees the KAIST team expressed a possibility to use the adipose tissue as an alternative source to obtain stem cells for regeneration medicine.
Dr. Koh also said, “It’s been a well known method to extract HSPCs from the bone morrow or blood, but it’s the first time to identify adipose tissue, before considered useless, as a new possible supplier for functional and transplantable HSPCs.”
The study results have received an important recognition from the academia—the American Society of Hematology published the research as a main article in its official journal, Blood, for the February 4th, 2010 issue, which is the most citied peer-reviewed publication in the field.
2010.02.05 View 14040