Intelligent+Soft-Materials+Lab
-
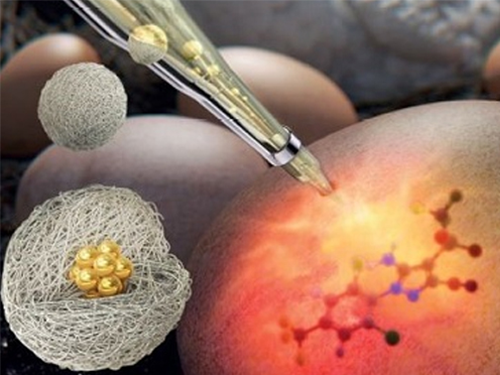 A Molecular Sensor for In-Situ Analysis of Complex Biological Fluids
A KAIST research group presented a molecular sensor with a microbead format for the rapid in-situ detection of harmful molecules in biological fluids or foods in a collaboration with a Korea Institute of Materials Science (KIMS) research group. As the sensor is designed to selectively concentrate charged small molecules and amplify the Raman signal, no time-consuming pretreatment of samples is required.
Raman spectra are commonly known as molecular fingerprints. However, their low intensity has restricted their use in molecular detection, especially for low concentrations. Raman signals can be dramatically amplified by locating the molecules on the surface of metal nanostructures where the electromagnetic field is strongly localized. However, it is still challenging to use Raman signals for the detection of small molecules dissolved in complex biological fluids. Adhesive proteins irreversibly adsorb on the metal surface, which prevents the access of small target molecules onto the metal surface. Therefore, it was a prerequisite to purify the samples before analysis. However, it takes a long time and is expensive.
A joint team from Professor Shin-Hyun Kim’s group in KAIST and Dr. Dong-Ho Kim’s group in KIMS has addressed the issue by encapsulating agglomerates of gold nanoparticles using a hydrogel. The hydrogel has three-dimensional network structures so that molecules smaller than the mesh are selectively permeable. Therefore, the hydrogel can exclude relatively large proteins, while allowing the infusion of small molecules. Therefore, the surface of gold nanoparticles remains intact against proteins, which accommodates small molecules. In particular, the charged hydrogel enables the concentration of oppositely-charged small molecules. That is, the purification is autonomously done by the materials, removing the need for time-consuming pretreatment. As a result, the Raman signal of small molecules can be selectively amplified in the absence of adhesive proteins.
Using the molecular sensors, the research team demonstrated the direct detection of fipronil sulfone dissolved in an egg without sample pretreatment. Recently, insecticide-contaminated eggs have spread in Europe, South Korea, and other countries, threatening health and causing social chaos. Fipronil is one of the most commonly used insecticides for veterinary medicine to combat fleas. The fipronil is absorbed through the chicken skin, from which a metabolite, fipronil sulfone, accumulates in the eggs. As the fipronil sulfone carries partial negative charges, it can be concentrated using positively-charged microgels while excluding adhesive proteins in eggs, such as ovalbumin, ovoglobulin, and ovomucoid. Therefore, the Raman spectrum of fipronil sulfone can be directly measured. The limit of direct detection of fipronil sulfone dissolved in an egg was measured at 0.05 ppm.
Professor Kim said, “The molecular sensors can be used not only for the direct detection of harmful molecules in foods but also for residual drugs or biomarkers in blood or urine.” Dr. Dong-Ho Kim said, “It will be possible to save time and cost as no sample treatment is required.”
This research was led by graduate student Dong Jae Kim and an article entitled “SERS-Active Charged Microgels for Size- and Charge-Selective Molecular Analysis of Complex Biological Samples” was published on October 4, 2018 in Small and featured on the inside cover of the journal.
Figure 1. Schematic illustrating the concentration of charged small molecules and the exclusion of large adhesive proteins using a charged hydrogel microbead containing an agglomerate of gold nanoparticles. The Raman signal of the small molecules is selectively amplified by the agglomerate.
Figure 2. Confocal laser scanning microscope images showing the concentration of oppositely charged molecules, where negatively-charged microgels are denoted by red circles and positively-charged microgels are denoted by blue circles. Green fluorescence originates from negatively-charged dye molecules and red fluorescence originates from positively-charged dye molecules.
Figure 3. Raman spectra measured from fipronil sulfone-spiked eggs, where the concentrations of fipronil sulfone are denoted; 0 ppm indicates no fipronil sulfone in the egg. The characteristic peaks of fipronil sulfone are denoted by the dotted lines.
2018.10.23 View 7272
A Molecular Sensor for In-Situ Analysis of Complex Biological Fluids
A KAIST research group presented a molecular sensor with a microbead format for the rapid in-situ detection of harmful molecules in biological fluids or foods in a collaboration with a Korea Institute of Materials Science (KIMS) research group. As the sensor is designed to selectively concentrate charged small molecules and amplify the Raman signal, no time-consuming pretreatment of samples is required.
Raman spectra are commonly known as molecular fingerprints. However, their low intensity has restricted their use in molecular detection, especially for low concentrations. Raman signals can be dramatically amplified by locating the molecules on the surface of metal nanostructures where the electromagnetic field is strongly localized. However, it is still challenging to use Raman signals for the detection of small molecules dissolved in complex biological fluids. Adhesive proteins irreversibly adsorb on the metal surface, which prevents the access of small target molecules onto the metal surface. Therefore, it was a prerequisite to purify the samples before analysis. However, it takes a long time and is expensive.
A joint team from Professor Shin-Hyun Kim’s group in KAIST and Dr. Dong-Ho Kim’s group in KIMS has addressed the issue by encapsulating agglomerates of gold nanoparticles using a hydrogel. The hydrogel has three-dimensional network structures so that molecules smaller than the mesh are selectively permeable. Therefore, the hydrogel can exclude relatively large proteins, while allowing the infusion of small molecules. Therefore, the surface of gold nanoparticles remains intact against proteins, which accommodates small molecules. In particular, the charged hydrogel enables the concentration of oppositely-charged small molecules. That is, the purification is autonomously done by the materials, removing the need for time-consuming pretreatment. As a result, the Raman signal of small molecules can be selectively amplified in the absence of adhesive proteins.
Using the molecular sensors, the research team demonstrated the direct detection of fipronil sulfone dissolved in an egg without sample pretreatment. Recently, insecticide-contaminated eggs have spread in Europe, South Korea, and other countries, threatening health and causing social chaos. Fipronil is one of the most commonly used insecticides for veterinary medicine to combat fleas. The fipronil is absorbed through the chicken skin, from which a metabolite, fipronil sulfone, accumulates in the eggs. As the fipronil sulfone carries partial negative charges, it can be concentrated using positively-charged microgels while excluding adhesive proteins in eggs, such as ovalbumin, ovoglobulin, and ovomucoid. Therefore, the Raman spectrum of fipronil sulfone can be directly measured. The limit of direct detection of fipronil sulfone dissolved in an egg was measured at 0.05 ppm.
Professor Kim said, “The molecular sensors can be used not only for the direct detection of harmful molecules in foods but also for residual drugs or biomarkers in blood or urine.” Dr. Dong-Ho Kim said, “It will be possible to save time and cost as no sample treatment is required.”
This research was led by graduate student Dong Jae Kim and an article entitled “SERS-Active Charged Microgels for Size- and Charge-Selective Molecular Analysis of Complex Biological Samples” was published on October 4, 2018 in Small and featured on the inside cover of the journal.
Figure 1. Schematic illustrating the concentration of charged small molecules and the exclusion of large adhesive proteins using a charged hydrogel microbead containing an agglomerate of gold nanoparticles. The Raman signal of the small molecules is selectively amplified by the agglomerate.
Figure 2. Confocal laser scanning microscope images showing the concentration of oppositely charged molecules, where negatively-charged microgels are denoted by red circles and positively-charged microgels are denoted by blue circles. Green fluorescence originates from negatively-charged dye molecules and red fluorescence originates from positively-charged dye molecules.
Figure 3. Raman spectra measured from fipronil sulfone-spiked eggs, where the concentrations of fipronil sulfone are denoted; 0 ppm indicates no fipronil sulfone in the egg. The characteristic peaks of fipronil sulfone are denoted by the dotted lines.
2018.10.23 View 7272 -
 Photonic Capsules for Injectable Laser Resonators
A KAIST research group presented photonic capsules for injectable laser resonators using microfluidic technology. The capsule’s diameter is comparable to a human hair and stable in gas and liquid media, so it is injectable into any target volume.
The research group headed by Professor Shin-Hyun Kim in the Department of Chemical and Biomolecular Engineering applied an interesting optical property from nature. Professor Kim, who has dived deep into photonic materials research inspired from nature such as the Morpho butterfly, used a trait of beetles this time.
Chrysina gloriosa, commonly known as the glorious beetle, shows a green color similar to leaves when illuminated by left-handed, circularly-polarized light while showing no color with right-handed, circularly-polarized light. This unique optical feature helps the beetles communicate with each other and protects them from predators.
The principle behind this interesting optical property of the beetles relies on helical nanostructures with left-handedness that are present on the shell of the beetles. The helical structures reflect a circularly-polarized light with the same handedness of the helix at the wavelength selected by the helical pitch through optical interference.
Such helical nanostructures can be artificially created using liquid crystals (LCs). LCs with a helical arrangement are referred to as cholesteric LCs (CLCs). The CLCs exhibit the polarization-dependent reflection of light in the same manner as the beetles and have been used for various photonic applications.
In particular, CLCs have been cast to a film format that serves as mirrorless laser resonators, unlike conventional lasing systems. However, the film-type CLCs are large in size and show unidirectional emission, which restricts the use of CLC resonators in microenvironments.
To overcome these limitations, Professor Kim’s group has encapsulated the CLCs with dual shells using microfluidic technology. The inner shell is a water layer that promotes the alignment of LC molecules and the outer shell is an elastic polymer layer that secures capsule stability and enables reversible mechanical deformation. The spherical symmetry of the capsules enables omnidirectional laser emissions. Moreover, laser intensity and lasing direction can be further controlled by deforming the capsules, while its wavelength remains tunable. This new type of CLC laser resonator is promising for laser treatments in various biomedical applications.
Professor Kim said, “The helical nanostructure used in the laser resonator resembles that of the shell of chrysina gloriosa. Humans learn from nature and engineer materials to create something unprecedented.”
This research was led by graduate student Sang Seok Lee and an article entitled “Wavelength-tunable and shape-reconfigurable photonic capsule resonators containing cholesteric liquid crystals” was published online on June 22, in Science Advances.
Figure 1. Chrysina gloriosa illuminated by left-handed (left panel) and right-handed (right panel) circularly-polarized lights.
(Image source: https://doi.org/10.1016/j.cub.2010.05.036 , permitted for reuse in news media)
Figure 2. Composition (left panel) and optical microscopy image (right panel) of the capsule-type laser resonator
2018.07.05 View 10404
Photonic Capsules for Injectable Laser Resonators
A KAIST research group presented photonic capsules for injectable laser resonators using microfluidic technology. The capsule’s diameter is comparable to a human hair and stable in gas and liquid media, so it is injectable into any target volume.
The research group headed by Professor Shin-Hyun Kim in the Department of Chemical and Biomolecular Engineering applied an interesting optical property from nature. Professor Kim, who has dived deep into photonic materials research inspired from nature such as the Morpho butterfly, used a trait of beetles this time.
Chrysina gloriosa, commonly known as the glorious beetle, shows a green color similar to leaves when illuminated by left-handed, circularly-polarized light while showing no color with right-handed, circularly-polarized light. This unique optical feature helps the beetles communicate with each other and protects them from predators.
The principle behind this interesting optical property of the beetles relies on helical nanostructures with left-handedness that are present on the shell of the beetles. The helical structures reflect a circularly-polarized light with the same handedness of the helix at the wavelength selected by the helical pitch through optical interference.
Such helical nanostructures can be artificially created using liquid crystals (LCs). LCs with a helical arrangement are referred to as cholesteric LCs (CLCs). The CLCs exhibit the polarization-dependent reflection of light in the same manner as the beetles and have been used for various photonic applications.
In particular, CLCs have been cast to a film format that serves as mirrorless laser resonators, unlike conventional lasing systems. However, the film-type CLCs are large in size and show unidirectional emission, which restricts the use of CLC resonators in microenvironments.
To overcome these limitations, Professor Kim’s group has encapsulated the CLCs with dual shells using microfluidic technology. The inner shell is a water layer that promotes the alignment of LC molecules and the outer shell is an elastic polymer layer that secures capsule stability and enables reversible mechanical deformation. The spherical symmetry of the capsules enables omnidirectional laser emissions. Moreover, laser intensity and lasing direction can be further controlled by deforming the capsules, while its wavelength remains tunable. This new type of CLC laser resonator is promising for laser treatments in various biomedical applications.
Professor Kim said, “The helical nanostructure used in the laser resonator resembles that of the shell of chrysina gloriosa. Humans learn from nature and engineer materials to create something unprecedented.”
This research was led by graduate student Sang Seok Lee and an article entitled “Wavelength-tunable and shape-reconfigurable photonic capsule resonators containing cholesteric liquid crystals” was published online on June 22, in Science Advances.
Figure 1. Chrysina gloriosa illuminated by left-handed (left panel) and right-handed (right panel) circularly-polarized lights.
(Image source: https://doi.org/10.1016/j.cub.2010.05.036 , permitted for reuse in news media)
Figure 2. Composition (left panel) and optical microscopy image (right panel) of the capsule-type laser resonator
2018.07.05 View 10404 -
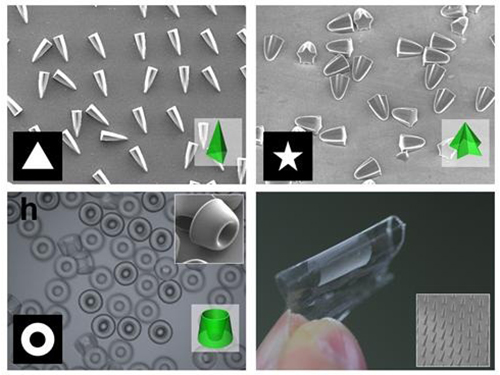 Novel Photolithographic Technology Enabling 3D Control over Functional Shapes of Microstructures
Professor Shin-Hyun Kim and his research team in the Department of Chemical and Biomolecular Engineering at KAIST have developed a novel photolithographic technology enabling control over the functional shapes of micropatterns using oxygen diffusion.
The research was published online in the March 13th issue of Nature Communications and was selected as a featured image for the journal.
Photolithography is a standard optical process for transferring micropatterns on to a substrate by exposing specific regions of the photoresist layer to ultraviolet (UV) light. It is used widely throughout industries that require micropatterns, especially in the semiconductor manufacturing industry.
Conventional photolithography relied on photomasks which protected certain regions of the substrate from the input UV light. Areas covered by the photomasks remain intact with the base layer while the areas exposed to the UV light are washed away, thus creating a micropattern. This technology was limited to a two-dimensional, disc-shaped design as the boundaries between the exposed and roofed regions are always in a parallel arrangement with the direction of the light.
Professor Kim’s research team discovered that: 1) the areas exposed to UV light lowered the concentration of oxygen and thus resulted in oxygen diffusion; and 2) manipulation of the diffusion speed and direction allowed control of the growth, shape and size of the polymers. Based on these findings, the team developed a new photolithographic technology that enabled the production of micropatterns with three-dimensional structures in various shapes and sizes.
Oxygen was considered an inhibitor during photopolymerization. Photoresist under UV light creates radicals which initialize a chemical reaction. These radicals are eliminated with the presence of oxygen and thus prevents the reaction. This suggests that the photoresist must be exposed to UV light for an extended time to completely remove oxygen for a chemical reaction to begin.
The research team, however, exploited the presence of oxygen. While the region affected by the UV light lowered oxygen concentration, the concentration in the untouched region remained unchanged. This difference in the concentrations caused a diffusion of oxygen to the region under UV light.
When the speed of the oxygen flow is slow, the diffusion occurs in parallel with the direction of the UV light. When fast, the diffusion process develops horizontally, outward from the area affected by the UV light. Professor Kim and his team proved this phenomenon both empirically and theoretically. Furthermore, by injecting an external oxygen source, the team was able to manipulate diffusion strength and direction, and thus control the shape and size of the polymer. The use of the polymerization inhibitors enabled and facilitated the fabrication of complex, three-dimensional micropatterns.
Professor Kim said, “While 3D printing is considered an innovative manufacturing technology, it cannot be used for mass-production of microscopic products. The new photolithographic technology will have a broad impact on both the academia and industry especially because existing, conventional photolithographic equipment can be used for the development of more complex micropatterns.” His newest technology will enhance the manufacturing process of three-dimensional polymers which were considered difficult to be commercialized.
The research was also dedicated to the late Professor Seung-Man Yang of the Department of Chemical and Biomolecular Engineering at KAIST. He was considered one of the greatest scholars in Korea in the field of hydrodynamics and colloids.
Picture 1: Featured Image of Nature Communications, March 2015
Picture 2: Polymers with various shapes and sizes produced with the new photolithographic technology developed by Professor Kim
2015.04.06 View 12232
Novel Photolithographic Technology Enabling 3D Control over Functional Shapes of Microstructures
Professor Shin-Hyun Kim and his research team in the Department of Chemical and Biomolecular Engineering at KAIST have developed a novel photolithographic technology enabling control over the functional shapes of micropatterns using oxygen diffusion.
The research was published online in the March 13th issue of Nature Communications and was selected as a featured image for the journal.
Photolithography is a standard optical process for transferring micropatterns on to a substrate by exposing specific regions of the photoresist layer to ultraviolet (UV) light. It is used widely throughout industries that require micropatterns, especially in the semiconductor manufacturing industry.
Conventional photolithography relied on photomasks which protected certain regions of the substrate from the input UV light. Areas covered by the photomasks remain intact with the base layer while the areas exposed to the UV light are washed away, thus creating a micropattern. This technology was limited to a two-dimensional, disc-shaped design as the boundaries between the exposed and roofed regions are always in a parallel arrangement with the direction of the light.
Professor Kim’s research team discovered that: 1) the areas exposed to UV light lowered the concentration of oxygen and thus resulted in oxygen diffusion; and 2) manipulation of the diffusion speed and direction allowed control of the growth, shape and size of the polymers. Based on these findings, the team developed a new photolithographic technology that enabled the production of micropatterns with three-dimensional structures in various shapes and sizes.
Oxygen was considered an inhibitor during photopolymerization. Photoresist under UV light creates radicals which initialize a chemical reaction. These radicals are eliminated with the presence of oxygen and thus prevents the reaction. This suggests that the photoresist must be exposed to UV light for an extended time to completely remove oxygen for a chemical reaction to begin.
The research team, however, exploited the presence of oxygen. While the region affected by the UV light lowered oxygen concentration, the concentration in the untouched region remained unchanged. This difference in the concentrations caused a diffusion of oxygen to the region under UV light.
When the speed of the oxygen flow is slow, the diffusion occurs in parallel with the direction of the UV light. When fast, the diffusion process develops horizontally, outward from the area affected by the UV light. Professor Kim and his team proved this phenomenon both empirically and theoretically. Furthermore, by injecting an external oxygen source, the team was able to manipulate diffusion strength and direction, and thus control the shape and size of the polymer. The use of the polymerization inhibitors enabled and facilitated the fabrication of complex, three-dimensional micropatterns.
Professor Kim said, “While 3D printing is considered an innovative manufacturing technology, it cannot be used for mass-production of microscopic products. The new photolithographic technology will have a broad impact on both the academia and industry especially because existing, conventional photolithographic equipment can be used for the development of more complex micropatterns.” His newest technology will enhance the manufacturing process of three-dimensional polymers which were considered difficult to be commercialized.
The research was also dedicated to the late Professor Seung-Man Yang of the Department of Chemical and Biomolecular Engineering at KAIST. He was considered one of the greatest scholars in Korea in the field of hydrodynamics and colloids.
Picture 1: Featured Image of Nature Communications, March 2015
Picture 2: Polymers with various shapes and sizes produced with the new photolithographic technology developed by Professor Kim
2015.04.06 View 12232