MBEL
-
 Prof. Sang Yup Lee Elected as a Foreign Member of the Royal Society
Vice President for Research Distinguished Professor Sang Yup Lee was elected as a foreign member of the Royal Society in the UK. On May 6, the Society announced the list of distinguished new 52 fellows and 10 foreign members who achieved exceptional contributions to science. Professor Lee and Professor V. Narry Kim from Seoul National University are the first foreign members ever elected from Korea.
The Royal Society, established in 1660, is one of the most prestigious national science academies and a fellowship of 1,600 of the world’s most eminent scientists. From Newton to Darwin, Einstein, Hawking, and beyond, pioneers and paragons in their fields are elected by their peers. To date, there are 280 Nobel prize winners among the fellows.
Distinguished Professor Lee from the Department of Chemical and Biomolecular Engineering at KAIST is one of the Highly Cited Researchers (HCRs) who pioneered systems metabolic engineering and developed various micro-organisms for producing a wide range of fuels, chemicals, materials, and natural compounds.
His seminal scholarship and research career have already been recognized worldwide. He is the first Korean ever elected into the National Academy of Inventors (NAI) in the US and one of 13 scholars elected as an International Member of both the National Academy of Sciences (NAS) and the National Academy of Engineering (NAE) in the US. With this fellowship, he added one more accolade of being the first non-US and British Commonwealth scientist elected into the three most prestigious science academies: the NAS, the NAE, and the Royal Society.
2021.05.07 View 14038
Prof. Sang Yup Lee Elected as a Foreign Member of the Royal Society
Vice President for Research Distinguished Professor Sang Yup Lee was elected as a foreign member of the Royal Society in the UK. On May 6, the Society announced the list of distinguished new 52 fellows and 10 foreign members who achieved exceptional contributions to science. Professor Lee and Professor V. Narry Kim from Seoul National University are the first foreign members ever elected from Korea.
The Royal Society, established in 1660, is one of the most prestigious national science academies and a fellowship of 1,600 of the world’s most eminent scientists. From Newton to Darwin, Einstein, Hawking, and beyond, pioneers and paragons in their fields are elected by their peers. To date, there are 280 Nobel prize winners among the fellows.
Distinguished Professor Lee from the Department of Chemical and Biomolecular Engineering at KAIST is one of the Highly Cited Researchers (HCRs) who pioneered systems metabolic engineering and developed various micro-organisms for producing a wide range of fuels, chemicals, materials, and natural compounds.
His seminal scholarship and research career have already been recognized worldwide. He is the first Korean ever elected into the National Academy of Inventors (NAI) in the US and one of 13 scholars elected as an International Member of both the National Academy of Sciences (NAS) and the National Academy of Engineering (NAE) in the US. With this fellowship, he added one more accolade of being the first non-US and British Commonwealth scientist elected into the three most prestigious science academies: the NAS, the NAE, and the Royal Society.
2021.05.07 View 14038 -
 Distinguished Professor Sang Yup Lee Honored with Charles D. Scott Award
Vice President for Research Sang Yup Lee received the 2021 Charles D. Scott Award from the Society for Industrial Microbiology and Biotechnology. Distinguished Professor Lee from the Department of Chemical and Biomolecular Engineering at KAIST is the first Asian awardee.
The Charles D. Scott Award, initiated in 1995, recognizes individuals who have made significant contributions to enable and further the use of biotechnology to produce fuels and chemicals. The award is named in honor of Dr. Charles D. Scott, who founded the Symposium on Biomaterials, Fuels, and Chemicals and chaired the conference for its first ten years.
Professor Lee has pioneered systems metabolic engineering and developed various micro-organisms capable of producing a wide range of fuels, chemicals, materials, and natural compounds, many of them for the first time. Some of the breakthroughs include the microbial production of gasoline, diacids, diamines, PLA and PLGA polymers, and several natural products.
More recently, his team has developed a microbial strain capable of the mass production of succinic acid, a monomer for manufacturing polyester, with the highest production efficiency to date, as well as a Corynebacterium glutamicum strain capable of producing high-level glutaric acid. They also engineered for the first time a bacterium capable of producing carminic acid, a natural red colorant that is widely used for food and cosmetics.
Professor Lee is one of the Highly Cited Researchers (HCR), ranked in the top 1% by citations in their field by Clarivate Analytics for four consecutive years from 2017. He is the first Korean fellow ever elected into the National Academy of Inventors in the US and one of 13 scholars elected as an International Member of both the National Academy of Sciences and the National Academy of Engineering in the USA.
The awards ceremony will take place during the Symposium on Biomaterials, Fuels, and Chemicals held online from April 26.
2021.04.27 View 11827
Distinguished Professor Sang Yup Lee Honored with Charles D. Scott Award
Vice President for Research Sang Yup Lee received the 2021 Charles D. Scott Award from the Society for Industrial Microbiology and Biotechnology. Distinguished Professor Lee from the Department of Chemical and Biomolecular Engineering at KAIST is the first Asian awardee.
The Charles D. Scott Award, initiated in 1995, recognizes individuals who have made significant contributions to enable and further the use of biotechnology to produce fuels and chemicals. The award is named in honor of Dr. Charles D. Scott, who founded the Symposium on Biomaterials, Fuels, and Chemicals and chaired the conference for its first ten years.
Professor Lee has pioneered systems metabolic engineering and developed various micro-organisms capable of producing a wide range of fuels, chemicals, materials, and natural compounds, many of them for the first time. Some of the breakthroughs include the microbial production of gasoline, diacids, diamines, PLA and PLGA polymers, and several natural products.
More recently, his team has developed a microbial strain capable of the mass production of succinic acid, a monomer for manufacturing polyester, with the highest production efficiency to date, as well as a Corynebacterium glutamicum strain capable of producing high-level glutaric acid. They also engineered for the first time a bacterium capable of producing carminic acid, a natural red colorant that is widely used for food and cosmetics.
Professor Lee is one of the Highly Cited Researchers (HCR), ranked in the top 1% by citations in their field by Clarivate Analytics for four consecutive years from 2017. He is the first Korean fellow ever elected into the National Academy of Inventors in the US and one of 13 scholars elected as an International Member of both the National Academy of Sciences and the National Academy of Engineering in the USA.
The awards ceremony will take place during the Symposium on Biomaterials, Fuels, and Chemicals held online from April 26.
2021.04.27 View 11827 -
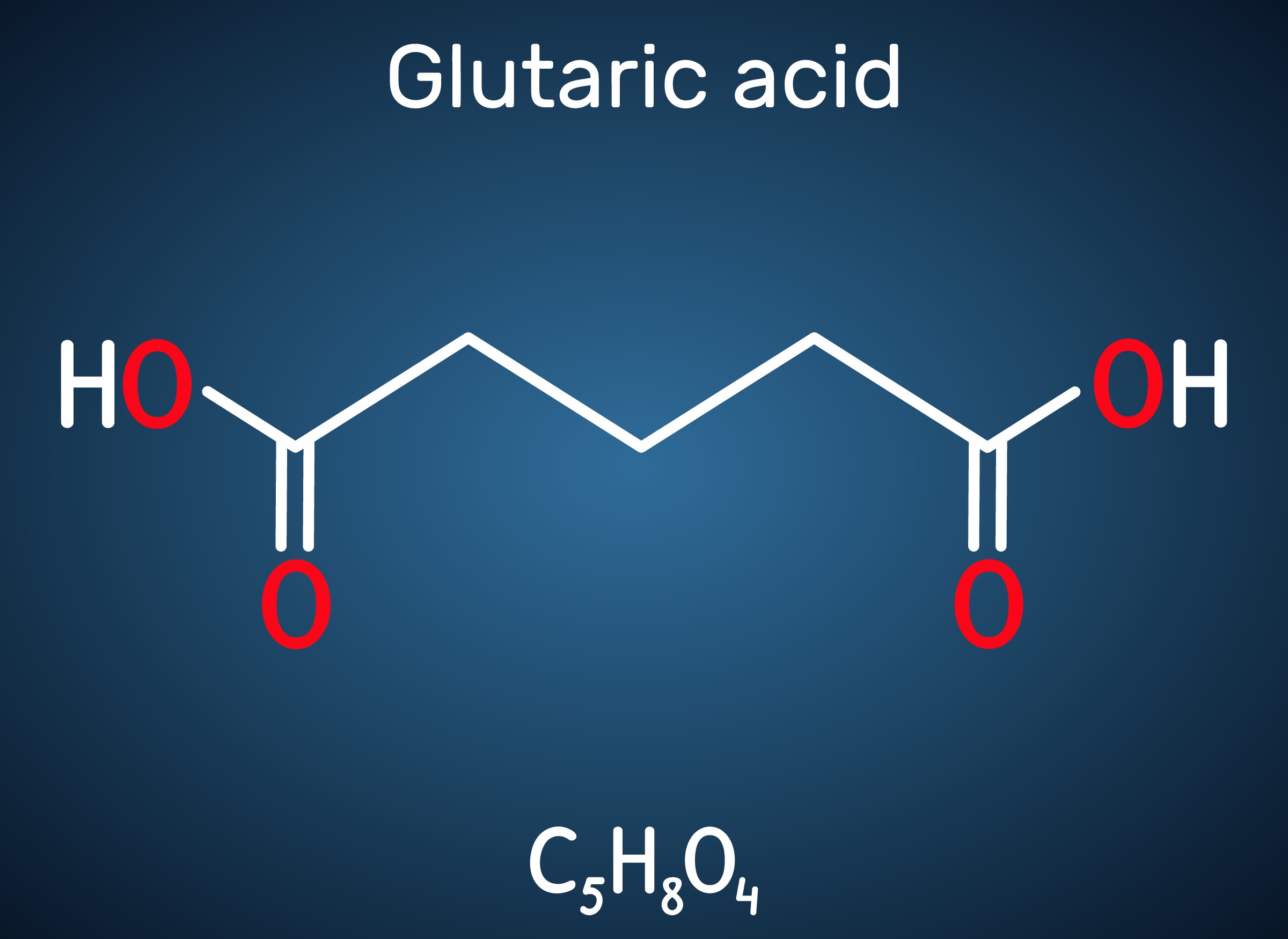 Engineered C. glutamicum Strain Capable of Producing High-Level Glutaric Acid from Glucose
An engineered C. glutamicum strain that can produce the world’s highest titer of glutaric acid was developed by employing systems metabolic engineering strategies
A metabolic engineering research group at KAIST has developed an engineered Corynebacterium glutamicum strain capable of producing high-level glutaric acid without byproducts from glucose. This new strategy will be useful for developing engineered micro-organisms for the bio-based production of value-added chemicals.
Glutaric acid, also known as pentanedioic acid, is a carboxylic acid that is widely used for various applications including the production of polyesters, polyamides, polyurethanes, glutaric anhydride, 1,5-pentanediol, and 5-hydroxyvaleric acid. Glutaric acid has been produced using various petroleum-based chemical methods, relying on non-renewable and toxic starting materials. Thus, various approaches have been taken to biologically produce glutaric acid from renewable resources. Previously, the development of the first glutaric acid producing Escherichia coli by introducing Pseudomonas putida genes was reported by a research group from KAIST, but the titer was low. Glutaric acid production by metabolically engineered Corynebacterium glutamicum has also been reported in several studies, but further improvements in glutaric acid production seemed possible since C. glutamicum has the capability of producing more than 130 g/L of L-lysine.
A research group comprised of Taehee Han, Gi Bae Kim, and Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering addressed this issue. Their research paper “Glutaric acid production by systems metabolic engineering of an L-lysine-overproducing Corynebacterium glutamicum” was published online in PNAS on November 16, 2020.
This research reports the development of a metabolically engineered C. glutamicum strain capable of efficiently producing glutaric acid, starting from an L-lysine overproducer. The following novel strategies and approaches to achieve high-level glutaric acid production were employed. First, metabolic pathways in C. glutamicum were reconstituted for glutaric acid production by introducing P. putida genes. Then, multi-omics analyses including genome, transcriptome, and fluxome were conducted to understand the phenotype of the L-lysine overproducer strain. In addition to systematic understanding of the host strain, gene manipulation targets were predicted by omics analyses and applied for engineering C. glutamicum, which resulted in the development of an engineered strain capable of efficiently producing glutaric acid. Furthermore, the new glutaric acid exporter was discovered for the first time, which was used to further increase glutaric acid production through enhancing product excretion. Last but not least, culture conditions were optimized for high-level glutaric acid production. As a result, the final engineered strain was able to produce 105.3 g/L glutaric acid, the highest titer ever reported, in 69 hours by fed-batch fermentation.
Professor Sang Yup Lee said, “It is meaningful that we were able to develop a highly efficient glutaric acid producer capable of producing glutaric acid at the world’s highest titer without any byproducts from renewable carbon sources. This will further accelerate the bio-based production of valuable chemicals in pharmaceutical/medical/chemical industries.” This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation and funded by the Ministry of Science and ICT.
-Profile
Distinguished Professor Sang Yup Lee
leesy@kaist.ac.kr
http://mbel.kaist.ac.kr
Department of Chemical and Biomolecular Engineering
KAIST
2020.11.17 View 8765
Engineered C. glutamicum Strain Capable of Producing High-Level Glutaric Acid from Glucose
An engineered C. glutamicum strain that can produce the world’s highest titer of glutaric acid was developed by employing systems metabolic engineering strategies
A metabolic engineering research group at KAIST has developed an engineered Corynebacterium glutamicum strain capable of producing high-level glutaric acid without byproducts from glucose. This new strategy will be useful for developing engineered micro-organisms for the bio-based production of value-added chemicals.
Glutaric acid, also known as pentanedioic acid, is a carboxylic acid that is widely used for various applications including the production of polyesters, polyamides, polyurethanes, glutaric anhydride, 1,5-pentanediol, and 5-hydroxyvaleric acid. Glutaric acid has been produced using various petroleum-based chemical methods, relying on non-renewable and toxic starting materials. Thus, various approaches have been taken to biologically produce glutaric acid from renewable resources. Previously, the development of the first glutaric acid producing Escherichia coli by introducing Pseudomonas putida genes was reported by a research group from KAIST, but the titer was low. Glutaric acid production by metabolically engineered Corynebacterium glutamicum has also been reported in several studies, but further improvements in glutaric acid production seemed possible since C. glutamicum has the capability of producing more than 130 g/L of L-lysine.
A research group comprised of Taehee Han, Gi Bae Kim, and Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering addressed this issue. Their research paper “Glutaric acid production by systems metabolic engineering of an L-lysine-overproducing Corynebacterium glutamicum” was published online in PNAS on November 16, 2020.
This research reports the development of a metabolically engineered C. glutamicum strain capable of efficiently producing glutaric acid, starting from an L-lysine overproducer. The following novel strategies and approaches to achieve high-level glutaric acid production were employed. First, metabolic pathways in C. glutamicum were reconstituted for glutaric acid production by introducing P. putida genes. Then, multi-omics analyses including genome, transcriptome, and fluxome were conducted to understand the phenotype of the L-lysine overproducer strain. In addition to systematic understanding of the host strain, gene manipulation targets were predicted by omics analyses and applied for engineering C. glutamicum, which resulted in the development of an engineered strain capable of efficiently producing glutaric acid. Furthermore, the new glutaric acid exporter was discovered for the first time, which was used to further increase glutaric acid production through enhancing product excretion. Last but not least, culture conditions were optimized for high-level glutaric acid production. As a result, the final engineered strain was able to produce 105.3 g/L glutaric acid, the highest titer ever reported, in 69 hours by fed-batch fermentation.
Professor Sang Yup Lee said, “It is meaningful that we were able to develop a highly efficient glutaric acid producer capable of producing glutaric acid at the world’s highest titer without any byproducts from renewable carbon sources. This will further accelerate the bio-based production of valuable chemicals in pharmaceutical/medical/chemical industries.” This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation and funded by the Ministry of Science and ICT.
-Profile
Distinguished Professor Sang Yup Lee
leesy@kaist.ac.kr
http://mbel.kaist.ac.kr
Department of Chemical and Biomolecular Engineering
KAIST
2020.11.17 View 8765 -
 E. coli Engineered to Grow on CO₂ and Formic Acid as Sole Carbon Sources
- An E. coli strain that can grow to a relatively high cell density solely on CO₂ and formic acid was developed by employing metabolic engineering. -
Most biorefinery processes have relied on the use of biomass as a raw material for the production of chemicals and materials. Even though the use of CO₂ as a carbon source in biorefineries is desirable, it has not been possible to make common microbial strains such as E. coli grow on CO₂.
Now, a metabolic engineering research group at KAIST has developed a strategy to grow an E. coli strain to higher cell density solely on CO₂ and formic acid. Formic acid is a one carbon carboxylic acid, and can be easily produced from CO₂ using a variety of methods. Since it is easier to store and transport than CO₂, formic acid can be considered a good liquid-form alternative of CO₂.
With support from the C1 Gas Refinery R&D Center and the Ministry of Science and ICT, a research team led by Distinguished Professor Sang Yup Lee stepped up their work to develop an engineered E. coli strain capable of growing up to 11-fold higher cell density than those previously reported, using CO₂ and formic acid as sole carbon sources. This work was published in Nature Microbiology on September 28.
Despite the recent reports by several research groups on the development of E. coli strains capable of growing on CO₂ and formic acid, the maximum cell growth remained too low (optical density of around 1) and thus the production of chemicals from CO₂ and formic acid has been far from realized.
The team previously reported the reconstruction of the tetrahydrofolate cycle and reverse glycine cleavage pathway to construct an engineered E. coli strain that can sustain growth on CO₂ and formic acid. To further enhance the growth, the research team introduced the previously designed synthetic CO₂ and formic acid assimilation pathway, and two formate dehydrogenases.
Metabolic fluxes were also fine-tuned, the gluconeogenic flux enhanced, and the levels of cytochrome bo3 and bd-I ubiquinol oxidase for ATP generation were optimized. This engineered E. coli strain was able to grow to a relatively high OD600 of 7~11, showing promise as a platform strain growing solely on CO₂ and formic acid.
Professor Lee said, “We engineered E. coli that can grow to a higher cell density only using CO₂ and formic acid. We think that this is an important step forward, but this is not the end. The engineered strain we developed still needs further engineering so that it can grow faster to a much higher density.”
Professor Lee’s team is continuing to develop such a strain. “In the future, we would be delighted to see the production of chemicals from an engineered E. coli strain using CO₂ and formic acid as sole carbon sources,” he added.
-Profile:Distinguished Professor Sang Yup Leehttp://mbel.kaist.ac.krDepartment of Chemical and Biomolecular EngineeringKAIST
2020.09.29 View 13336
E. coli Engineered to Grow on CO₂ and Formic Acid as Sole Carbon Sources
- An E. coli strain that can grow to a relatively high cell density solely on CO₂ and formic acid was developed by employing metabolic engineering. -
Most biorefinery processes have relied on the use of biomass as a raw material for the production of chemicals and materials. Even though the use of CO₂ as a carbon source in biorefineries is desirable, it has not been possible to make common microbial strains such as E. coli grow on CO₂.
Now, a metabolic engineering research group at KAIST has developed a strategy to grow an E. coli strain to higher cell density solely on CO₂ and formic acid. Formic acid is a one carbon carboxylic acid, and can be easily produced from CO₂ using a variety of methods. Since it is easier to store and transport than CO₂, formic acid can be considered a good liquid-form alternative of CO₂.
With support from the C1 Gas Refinery R&D Center and the Ministry of Science and ICT, a research team led by Distinguished Professor Sang Yup Lee stepped up their work to develop an engineered E. coli strain capable of growing up to 11-fold higher cell density than those previously reported, using CO₂ and formic acid as sole carbon sources. This work was published in Nature Microbiology on September 28.
Despite the recent reports by several research groups on the development of E. coli strains capable of growing on CO₂ and formic acid, the maximum cell growth remained too low (optical density of around 1) and thus the production of chemicals from CO₂ and formic acid has been far from realized.
The team previously reported the reconstruction of the tetrahydrofolate cycle and reverse glycine cleavage pathway to construct an engineered E. coli strain that can sustain growth on CO₂ and formic acid. To further enhance the growth, the research team introduced the previously designed synthetic CO₂ and formic acid assimilation pathway, and two formate dehydrogenases.
Metabolic fluxes were also fine-tuned, the gluconeogenic flux enhanced, and the levels of cytochrome bo3 and bd-I ubiquinol oxidase for ATP generation were optimized. This engineered E. coli strain was able to grow to a relatively high OD600 of 7~11, showing promise as a platform strain growing solely on CO₂ and formic acid.
Professor Lee said, “We engineered E. coli that can grow to a higher cell density only using CO₂ and formic acid. We think that this is an important step forward, but this is not the end. The engineered strain we developed still needs further engineering so that it can grow faster to a much higher density.”
Professor Lee’s team is continuing to develop such a strain. “In the future, we would be delighted to see the production of chemicals from an engineered E. coli strain using CO₂ and formic acid as sole carbon sources,” he added.
-Profile:Distinguished Professor Sang Yup Leehttp://mbel.kaist.ac.krDepartment of Chemical and Biomolecular EngineeringKAIST
2020.09.29 View 13336 -
 Efficiently Producing Fatty Acids and Biofuels from Glucose
Researchers have presented a new strategy for efficiently producing fatty acids and biofuels that can transform glucose and oleaginous microorganisms into microbial diesel fuel, with one-step direct fermentative production.
The newly developed strain, created by Distinguished Professor Sang Yup Lee and his team, showed the highest efficiency in producing fatty acids and biodiesels ever reported. It will be expected to serve as a new platform to sustainably produce a wide array of fatty acid-based products from glucose and other carbon substrates.
Fossil fuels, which have long been energy resources for our daily lives, are now facing serious challenges: depletion of their reserves and their role in global warming. The production of sustainable bio-based renewable energy has emerged as an essential alternative and many studies to replace fossil fuels are underway. One of the representative examples is biodiesel. Currently, it is mainly being produced through the transesterification of vegetable oils or animal fats.
The research team engineered oleaginous microorganisms, Rhodococcus opacus, to produce fatty acids and their derivatives that can be used as biodiesel from glucose, one of the most abundant and cheap sugars derived from non-edible biomass.
Professor Lee’s team has already engineered Escherichia coli to produce short-chain hydrocarbons, which can be used as gasoline (published in Nature as the cover paper in 2013). However, the production efficiency of the short-chain hydrocarbons using E. coli (0.58 g/L) fell short of the levels required for commercialization.
To overcome these issues, the team employed oil-accumulating Rhodococcus opacus as a host strain in this study. First, the team optimized the cultivation conditions of Rhodococcus opacus to maximize the accumulation of oil (triacylglycerol), which serves as a precursor for the biosynthesis of fatty acids and their derivatives. Then, they systematically analyzed the metabolism of the strain and redesigned it to enable higher levels of fatty acids and two kinds of fatty acid-derived biodiesels (fatty acid ethyl esters and long-chain hydrocarbons) to be produced.
They found that the resulting strains produced 50.2, 21.3, and 5.2 g/L of fatty acids, fatty acid ethyl esters, and long-chain hydrocarbons, respectively. These are all the highest concentrations ever reported by microbial fermentations. It is expected that these strains can contribute to the future industrialization of microbial-based biodiesel production.
“This technology creates fatty acids and biodiesel with high efficiency by utilizing lignocellulose, one of the most abundant resources on the Earth, without depending on fossil fuels and vegetable or animal oils. This will provide new opportunities for oil and petroleum industries, which have long relied on fossil fuels, to turn to sustainable and eco-friendly biotechnologies,” said Professor Lee.
This paper titled “Engineering of an oleaginous bacterium for the production of fatty acids and fuels” was published in Nature Chemical Biology on June 17.
This work was supported by the Technology Development Program to Solve Climate Changes on Systems Metabolic Engineering for Biorefineries from the Ministry of Science and ICT through the National Research Foundation (NRF) of Korea (NRF-2012M1A2A2026556 and NRF-2012M1A2A2026557).
(Figure: Metabolic engineering for the production of free fatty acids (FFAs), fatty acid ethyl esters (FAEEs), and long-chain hydrocarbons (LCHCs) in Rhodococcus opacus PD630. Researchers have presented a new strategy for efficiently producing fatty acids and biofuels that can transform glucose and oleaginous microorganisms into microbial diesel fuel, with one-step direct fermentative production.)
# # #
Source:
Hye Mi Kim, Tong Un Chae, So Young Choi, Won Jun Kim and Sang Yup Lee. Engineering of an oleaginous bacterium for the production of fatty acids and fuels. Nature Chemical Biology ( https://www.nature.com/nchembio/ ) DOI: 10.1038/s41589-019-0295-5
Profile
Dr. Sang Yup Lee
leesy@kaist.ac.kr
Distinguished Professor at the Department of Chemical and Biomolecular Engineering
KAIST
2019.06.19 View 52756
Efficiently Producing Fatty Acids and Biofuels from Glucose
Researchers have presented a new strategy for efficiently producing fatty acids and biofuels that can transform glucose and oleaginous microorganisms into microbial diesel fuel, with one-step direct fermentative production.
The newly developed strain, created by Distinguished Professor Sang Yup Lee and his team, showed the highest efficiency in producing fatty acids and biodiesels ever reported. It will be expected to serve as a new platform to sustainably produce a wide array of fatty acid-based products from glucose and other carbon substrates.
Fossil fuels, which have long been energy resources for our daily lives, are now facing serious challenges: depletion of their reserves and their role in global warming. The production of sustainable bio-based renewable energy has emerged as an essential alternative and many studies to replace fossil fuels are underway. One of the representative examples is biodiesel. Currently, it is mainly being produced through the transesterification of vegetable oils or animal fats.
The research team engineered oleaginous microorganisms, Rhodococcus opacus, to produce fatty acids and their derivatives that can be used as biodiesel from glucose, one of the most abundant and cheap sugars derived from non-edible biomass.
Professor Lee’s team has already engineered Escherichia coli to produce short-chain hydrocarbons, which can be used as gasoline (published in Nature as the cover paper in 2013). However, the production efficiency of the short-chain hydrocarbons using E. coli (0.58 g/L) fell short of the levels required for commercialization.
To overcome these issues, the team employed oil-accumulating Rhodococcus opacus as a host strain in this study. First, the team optimized the cultivation conditions of Rhodococcus opacus to maximize the accumulation of oil (triacylglycerol), which serves as a precursor for the biosynthesis of fatty acids and their derivatives. Then, they systematically analyzed the metabolism of the strain and redesigned it to enable higher levels of fatty acids and two kinds of fatty acid-derived biodiesels (fatty acid ethyl esters and long-chain hydrocarbons) to be produced.
They found that the resulting strains produced 50.2, 21.3, and 5.2 g/L of fatty acids, fatty acid ethyl esters, and long-chain hydrocarbons, respectively. These are all the highest concentrations ever reported by microbial fermentations. It is expected that these strains can contribute to the future industrialization of microbial-based biodiesel production.
“This technology creates fatty acids and biodiesel with high efficiency by utilizing lignocellulose, one of the most abundant resources on the Earth, without depending on fossil fuels and vegetable or animal oils. This will provide new opportunities for oil and petroleum industries, which have long relied on fossil fuels, to turn to sustainable and eco-friendly biotechnologies,” said Professor Lee.
This paper titled “Engineering of an oleaginous bacterium for the production of fatty acids and fuels” was published in Nature Chemical Biology on June 17.
This work was supported by the Technology Development Program to Solve Climate Changes on Systems Metabolic Engineering for Biorefineries from the Ministry of Science and ICT through the National Research Foundation (NRF) of Korea (NRF-2012M1A2A2026556 and NRF-2012M1A2A2026557).
(Figure: Metabolic engineering for the production of free fatty acids (FFAs), fatty acid ethyl esters (FAEEs), and long-chain hydrocarbons (LCHCs) in Rhodococcus opacus PD630. Researchers have presented a new strategy for efficiently producing fatty acids and biofuels that can transform glucose and oleaginous microorganisms into microbial diesel fuel, with one-step direct fermentative production.)
# # #
Source:
Hye Mi Kim, Tong Un Chae, So Young Choi, Won Jun Kim and Sang Yup Lee. Engineering of an oleaginous bacterium for the production of fatty acids and fuels. Nature Chemical Biology ( https://www.nature.com/nchembio/ ) DOI: 10.1038/s41589-019-0295-5
Profile
Dr. Sang Yup Lee
leesy@kaist.ac.kr
Distinguished Professor at the Department of Chemical and Biomolecular Engineering
KAIST
2019.06.19 View 52756 -
 Engineered Microbial Production of Grape Flavoring
(Image 1: Engineered bacteria that produce grape flavoring.)
Researchers report a microbial method for producing an artificial grape flavor. Methyl anthranilate (MANT) is a common grape flavoring and odorant compound currently produced through a petroleum-based process that uses large volumes of toxic acid catalysts.
Professor Sang-Yup Lee’s team at the Department of Chemical and Biomolecular Engineering demonstrated production of MANT, a naturally occurring compound, via engineered bacteria. The authors engineered strains of Escherichia coli and Corynebacetrium glutamicum to produce MANT through a plant-based engineered metabolic pathway.
The authors tuned the bacterial metabolic pathway by optimizing the levels of AAMT1, the key enzyme in the process. To maximize production of MANT, the authors tested six strategies, including increasing the supply of a precursor compound and enhancing the availability of a co-substrate. The most productive strategy proved to be a two-phase extractive culture, in which MANT was extracted into a solvent. This strategy produced MANT on the scale of 4.47 to 5.74 grams per liter, a significant amount, considering that engineered microbes produce most natural products at a scale of milligrams or micrograms per liter.
According to the authors, the results suggest that MANT and other related molecules produced through industrial processes can be produced at scale by engineered microbes in a manner that would allow them to be marketed as natural one, instead of artificial one.
This study, featured at the Proceeding of the National Academy of Sciences of the USA on May 13, was supported by the Technology Development Program to Solve Climate Changes on Systems Metabolic Engineering for Biorefineries from the Ministry of Science and ICT.
(Image 2. Overview of the strategies applied for the microbial production of grape flavoring.)
2019.05.15 View 58655
Engineered Microbial Production of Grape Flavoring
(Image 1: Engineered bacteria that produce grape flavoring.)
Researchers report a microbial method for producing an artificial grape flavor. Methyl anthranilate (MANT) is a common grape flavoring and odorant compound currently produced through a petroleum-based process that uses large volumes of toxic acid catalysts.
Professor Sang-Yup Lee’s team at the Department of Chemical and Biomolecular Engineering demonstrated production of MANT, a naturally occurring compound, via engineered bacteria. The authors engineered strains of Escherichia coli and Corynebacetrium glutamicum to produce MANT through a plant-based engineered metabolic pathway.
The authors tuned the bacterial metabolic pathway by optimizing the levels of AAMT1, the key enzyme in the process. To maximize production of MANT, the authors tested six strategies, including increasing the supply of a precursor compound and enhancing the availability of a co-substrate. The most productive strategy proved to be a two-phase extractive culture, in which MANT was extracted into a solvent. This strategy produced MANT on the scale of 4.47 to 5.74 grams per liter, a significant amount, considering that engineered microbes produce most natural products at a scale of milligrams or micrograms per liter.
According to the authors, the results suggest that MANT and other related molecules produced through industrial processes can be produced at scale by engineered microbes in a manner that would allow them to be marketed as natural one, instead of artificial one.
This study, featured at the Proceeding of the National Academy of Sciences of the USA on May 13, was supported by the Technology Development Program to Solve Climate Changes on Systems Metabolic Engineering for Biorefineries from the Ministry of Science and ICT.
(Image 2. Overview of the strategies applied for the microbial production of grape flavoring.)
2019.05.15 View 58655 -
 Cross-Generation Collaborative Labs Open
KAIST opened two cross-generation collaborative labs last month. This novel approach will pair up senior and junior faculty members for sustaining research and academic achievements even after the senior researcher retires.
This is one of the Vision 2031 innovation initiatives established to extend the spectrum of knowledge and research competitiveness. The selected labs will be funded for five years and the funding will be extended if necessary. KAIST will continue to select new labs every year.
A five-member selection committee including the Nobel Laureates Professor Klaus Von Klitzing at the Max-Planck Institute for Solid State Research and Dr. Kurt Wüthrich from ETH Zürich selected the first two labs with senior-junior pairs in March.
(Two renowned scholars' Cross-Generation Collaborative Labs which opened last month.
Distinguished Professor Lee's lab (above) andChair Professor Sung's lab)
Both labs are run by world-renowned scholars: the Systems Metabolic Engineering and Systems Healthcare Laboratory headed by Distinguished Professor Sang-Yup Lee in the Department of Chemical and Biomolecular Engineering and the Acousto-Microfluidics Research Center for Next-Generation Healthcare led by Chair Professor Hyung Jin Sung in the Department of Mechanical Engineering.
Distinguished Professor Lee will be teamed up with Professor Hyun Uk Kim, and their lab aims to mass produce new eco-friendly chemical materials as well as higher-value-added materials which will be used for medicine. The new platform technologies created in the lab are expected to provide information which will benefit human healthcare.
Meanwhile, the Acousto-Microfluidics Research Center for Next-Generation Healthcare will team up with Professors Hyoungsoo Kim and Yeunwoo Cho under Chair Professor Sung. The lab will conduct research on controlling fluids and objects exquisitely on a micro-nano scale by using high-frequency acoustic waves. The lab plans to develop a next-generation healthcare platform for customized diagnoses as well as disease treatment.
KAIST President Sung-Chul Shin, who introduced this novel idea in his research innovation initiative, said that he hopes the Cross-Generation Collaborative Labs will contribute to honoring senior scholars’ research legacies and passing knowledge down to junior researchers in order to further develop their academic achievements. He said, “I sincerely hope the labs will make numerous research breakthroughs in the very near future.”
2018.05.03 View 14671
Cross-Generation Collaborative Labs Open
KAIST opened two cross-generation collaborative labs last month. This novel approach will pair up senior and junior faculty members for sustaining research and academic achievements even after the senior researcher retires.
This is one of the Vision 2031 innovation initiatives established to extend the spectrum of knowledge and research competitiveness. The selected labs will be funded for five years and the funding will be extended if necessary. KAIST will continue to select new labs every year.
A five-member selection committee including the Nobel Laureates Professor Klaus Von Klitzing at the Max-Planck Institute for Solid State Research and Dr. Kurt Wüthrich from ETH Zürich selected the first two labs with senior-junior pairs in March.
(Two renowned scholars' Cross-Generation Collaborative Labs which opened last month.
Distinguished Professor Lee's lab (above) andChair Professor Sung's lab)
Both labs are run by world-renowned scholars: the Systems Metabolic Engineering and Systems Healthcare Laboratory headed by Distinguished Professor Sang-Yup Lee in the Department of Chemical and Biomolecular Engineering and the Acousto-Microfluidics Research Center for Next-Generation Healthcare led by Chair Professor Hyung Jin Sung in the Department of Mechanical Engineering.
Distinguished Professor Lee will be teamed up with Professor Hyun Uk Kim, and their lab aims to mass produce new eco-friendly chemical materials as well as higher-value-added materials which will be used for medicine. The new platform technologies created in the lab are expected to provide information which will benefit human healthcare.
Meanwhile, the Acousto-Microfluidics Research Center for Next-Generation Healthcare will team up with Professors Hyoungsoo Kim and Yeunwoo Cho under Chair Professor Sung. The lab will conduct research on controlling fluids and objects exquisitely on a micro-nano scale by using high-frequency acoustic waves. The lab plans to develop a next-generation healthcare platform for customized diagnoses as well as disease treatment.
KAIST President Sung-Chul Shin, who introduced this novel idea in his research innovation initiative, said that he hopes the Cross-Generation Collaborative Labs will contribute to honoring senior scholars’ research legacies and passing knowledge down to junior researchers in order to further develop their academic achievements. He said, “I sincerely hope the labs will make numerous research breakthroughs in the very near future.”
2018.05.03 View 14671 -
 KAIST and Hanwha Chemical Agree on Research Collaboration
KAIST signed a memorandum of understanding (MOU) with Hanwha Chemical Co., Ltd., a Korean chemical and auto manufacturer, on November 2, 2015 to establish a research center on campus.
The research center, which will be named “KAIST-Hanwha Chemical Future Technology Research Center,” will implement joint research projects for five years beginning from 2016 to develop innovative, green technologies that will help the Korean chemical industry boost its global competitiveness and to nurture top researchers and engineers in chemical engineering.
The research center will lead the development of next-generation petrochemical materials and manufacturing technology and the establishment of pure high-refining processes which are more energy-efficient and environmentally friendly. KAIST and Hanwha will strive to secure new technologies that have the greatest commercialization potential in the global market. They will also establish a scholarship fund for 15 KAIST doctoral students in the Department of Chemical and Biomolecular Engineering.
Many professors from the Chemical and Biomolecular Engineering Department including Distinguished Professor Sang Yup Lee, who was listed in the Top 20 Translational Researchers of 2014 by Nature Biotechnology this year, and Professor Hyunjoo Lee who received the Woman Scholar award at the 2015 World Chemistry Conference, will work at the research center.
Professor Lee, the head of the research center, said, “Collaborating with Hanwha will give us a strong basis for our efforts to carry out original research and train the best researchers in the field.”
Chang-Bum Kim, the Chief Executive Officer (CEO) of Hanwha Chemical, said,
“We hope our collaborations with KAIST will go beyond the typical industry and university cooperation. The two organizations will indeed jointly operate the research center, and this will become a new model for industry and university cooperation. We expect that the research center will play a crucial role in the development of new products and technologies to grow the Korean chemical industry.”
In the photo, President Steve Kang of KAIST (fourth from left) and CEO Chang-Bum Kim of Hanwha Chemical (fifth from left) hold the MOU together.
2015.11.01 View 14298
KAIST and Hanwha Chemical Agree on Research Collaboration
KAIST signed a memorandum of understanding (MOU) with Hanwha Chemical Co., Ltd., a Korean chemical and auto manufacturer, on November 2, 2015 to establish a research center on campus.
The research center, which will be named “KAIST-Hanwha Chemical Future Technology Research Center,” will implement joint research projects for five years beginning from 2016 to develop innovative, green technologies that will help the Korean chemical industry boost its global competitiveness and to nurture top researchers and engineers in chemical engineering.
The research center will lead the development of next-generation petrochemical materials and manufacturing technology and the establishment of pure high-refining processes which are more energy-efficient and environmentally friendly. KAIST and Hanwha will strive to secure new technologies that have the greatest commercialization potential in the global market. They will also establish a scholarship fund for 15 KAIST doctoral students in the Department of Chemical and Biomolecular Engineering.
Many professors from the Chemical and Biomolecular Engineering Department including Distinguished Professor Sang Yup Lee, who was listed in the Top 20 Translational Researchers of 2014 by Nature Biotechnology this year, and Professor Hyunjoo Lee who received the Woman Scholar award at the 2015 World Chemistry Conference, will work at the research center.
Professor Lee, the head of the research center, said, “Collaborating with Hanwha will give us a strong basis for our efforts to carry out original research and train the best researchers in the field.”
Chang-Bum Kim, the Chief Executive Officer (CEO) of Hanwha Chemical, said,
“We hope our collaborations with KAIST will go beyond the typical industry and university cooperation. The two organizations will indeed jointly operate the research center, and this will become a new model for industry and university cooperation. We expect that the research center will play a crucial role in the development of new products and technologies to grow the Korean chemical industry.”
In the photo, President Steve Kang of KAIST (fourth from left) and CEO Chang-Bum Kim of Hanwha Chemical (fifth from left) hold the MOU together.
2015.11.01 View 14298