mechanism
-
 Now You Can See Floral Scents!
Optical interferometry visualizes how often lilies emit volatile organic compounds
Have you ever thought about when flowers emit their scents?
KAIST mechanical engineers and biological scientists directly visualized how often a lily releases a floral scent using a laser interferometry method. These measurement results can provide new insights for understanding and further exploring the biosynthesis and emission mechanisms of floral volatiles.
Why is it important to know this? It is well known that the fragrance of flowers affects their interactions with pollinators, microorganisms, and florivores. For instance, many flowering plants can tune their scent emission rates when pollinators are active for pollination. Petunias and the wild tobacco Nicotiana attenuata emit floral scents at night to attract night-active pollinators. Thus, visualizing scent emissions can help us understand the ecological evolution of plant-pollinator interactions.
Many groups have been trying to develop methods for scent analysis. Mass spectrometry has been one widely used method for investigating the fragrance of flowers. Although mass spectrometry reveals the quality and quantity of floral scents, it is impossible to directly measure the releasing frequency. A laser-based gas detection system and a smartphone-based detection system using chemo-responsive dyes have also been used to measure volatile organic compounds (VOCs) in real-time, but it is still hard to measure the time-dependent emission rate of floral scents.
However, the KAIST research team co-led by Professor Hyoungsoo Kim from the Department of Mechanical Engineering and Professor Sang-Gyu Kim from the Department of Biological Sciences measured a refractive index difference between the vapor of the VOCs of lilies and the air to measure the emission frequency. The floral scent vapor was detected and the refractive index of air was 1.0 while that of the major floral scent of a linalool lily was 1.46.
Professor Hyoungsoo Kim said, “We expect this technology to be further applicable to various industrial sectors such as developing it to detect hazardous substances in a space.” The research team also plans to identify the DNA mechanism that controls floral scent secretion.
The current work entitled “Real-time visualization of scent accumulation reveals the frequency of floral scent emissions” was published in ‘Frontiers in Plant Science’ on April 18, 2022. (https://doi.org/10.3389/fpls.2022.835305).
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF-2021R1A2C2007835), the Rural Development Administration (PJ016403), and the KAIST-funded Global Singularity Research PREP-Program.
-Publication:H. Kim, G. Lee, J. Song, and S.-G. Kim, "Real-time visualization of scent accumulation reveals the frequency of floral scent emissions," Frontiers in Plant Science 18, 835305 (2022) (https://doi.org/10.3389/fpls.2022.835305)
-Profile:Professor Hyoungsoo Kimhttp://fil.kaist.ac.kr
@MadeInH on TwitterDepartment of Mechanical EngineeringKAIST
Professor Sang-Gyu Kimhttps://sites.google.com/view/kimlab/home Department of Biological SciencesKAIST
2022.05.25 View 9826
Now You Can See Floral Scents!
Optical interferometry visualizes how often lilies emit volatile organic compounds
Have you ever thought about when flowers emit their scents?
KAIST mechanical engineers and biological scientists directly visualized how often a lily releases a floral scent using a laser interferometry method. These measurement results can provide new insights for understanding and further exploring the biosynthesis and emission mechanisms of floral volatiles.
Why is it important to know this? It is well known that the fragrance of flowers affects their interactions with pollinators, microorganisms, and florivores. For instance, many flowering plants can tune their scent emission rates when pollinators are active for pollination. Petunias and the wild tobacco Nicotiana attenuata emit floral scents at night to attract night-active pollinators. Thus, visualizing scent emissions can help us understand the ecological evolution of plant-pollinator interactions.
Many groups have been trying to develop methods for scent analysis. Mass spectrometry has been one widely used method for investigating the fragrance of flowers. Although mass spectrometry reveals the quality and quantity of floral scents, it is impossible to directly measure the releasing frequency. A laser-based gas detection system and a smartphone-based detection system using chemo-responsive dyes have also been used to measure volatile organic compounds (VOCs) in real-time, but it is still hard to measure the time-dependent emission rate of floral scents.
However, the KAIST research team co-led by Professor Hyoungsoo Kim from the Department of Mechanical Engineering and Professor Sang-Gyu Kim from the Department of Biological Sciences measured a refractive index difference between the vapor of the VOCs of lilies and the air to measure the emission frequency. The floral scent vapor was detected and the refractive index of air was 1.0 while that of the major floral scent of a linalool lily was 1.46.
Professor Hyoungsoo Kim said, “We expect this technology to be further applicable to various industrial sectors such as developing it to detect hazardous substances in a space.” The research team also plans to identify the DNA mechanism that controls floral scent secretion.
The current work entitled “Real-time visualization of scent accumulation reveals the frequency of floral scent emissions” was published in ‘Frontiers in Plant Science’ on April 18, 2022. (https://doi.org/10.3389/fpls.2022.835305).
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF-2021R1A2C2007835), the Rural Development Administration (PJ016403), and the KAIST-funded Global Singularity Research PREP-Program.
-Publication:H. Kim, G. Lee, J. Song, and S.-G. Kim, "Real-time visualization of scent accumulation reveals the frequency of floral scent emissions," Frontiers in Plant Science 18, 835305 (2022) (https://doi.org/10.3389/fpls.2022.835305)
-Profile:Professor Hyoungsoo Kimhttp://fil.kaist.ac.kr
@MadeInH on TwitterDepartment of Mechanical EngineeringKAIST
Professor Sang-Gyu Kimhttps://sites.google.com/view/kimlab/home Department of Biological SciencesKAIST
2022.05.25 View 9826 -
 How Stingrays Became the Most Efficient Swimmers in Nature
Study shows the hydrodynamic benefits of protruding eyes and mouth in a self-propelled flexible stingray
With their compressed bodies and flexible pectoral fins, stingrays have evolved to become one of nature’s most efficient swimmers. Scientists have long wondered about the role played by their protruding eyes and mouths, which one might expect to be hydrodynamic disadvantages.
Professor Hyung Jin Sung and his colleagues have discovered how such features on simulated stingrays affect a range of forces involved in propulsion, such as pressure and vorticity. Despite what one might expect, their research team found these protruding features actually help streamline the stingrays.
‘The influence of the 3D protruding eyes and mouth on a self-propelled flexible stingray and its underlying hydrodynamic mechanism are not yet fully understood,” said Professor Sung. “In the present study, the hydrodynamic benefit of protruding eyes and mouth was explored for the first time, revealing their hydrodynamic role.”
To illustrate the complex interplay between hydrodynamic forces, the researchers set to work creating a computer model of a self-propelled flexible plate. They clamped the front end of the model and then forced it to mimic the up-and-down harmonic oscillations stingrays use to propel themselves.
To re-create the effect of the eyes and mouth on the surrounding water, the team simulated multiple rigid plates on the model. They compared this model to one without eyes and a mouth using a technique called the penalty immersed boundary method.
“Managing random fish swimming and isolating the desired purpose of the measurements from numerous factors was difficult,” Sung said. “To overcome these limitations, the penalty immersed boundary method was adopted to find the hydrodynamic benefits of the protruding eyes and mouth.”
The team discovered that the eyes and mouth generated a vortex of flow in the forward-backward , which increased negative pressure at the simulated animal’s front, and a side-to-side vortex that increased the pressure difference above and below the stingray. The result was increased thrust and accelerated cruising.
Further analysis showed that the eyes and mouth increased overall propulsion efficiency by more than 20.5% and 10.6%, respectively.
Researchers hope their work, driven by curiosity, further stokes interest in exploring fluid phenomena in nature. They are hoping to find ways to adapt this for next-generation water vehicle designs based more closely on marine animals.
This study was supported by the National Research Foundation of Korea and the State Scholar Fund from the China Scholarship Council.
-ProfileProfessor Hyung Jin SungDepartment of Mechanical EngineeringKAIST
-PublicationHyung Jin Sung, Qian Mao, Ziazhen Zhao, Yingzheng Liu, “Hydrodynamic benefits of protruding eyes and mouth in a self-propelled flexible stingray,” Aug.31, 2021, Physics of Fluids
(https://doi.org/10.1063/5.0061287)
-News release from the American Institute of Physics, Aug.31, 2021
2021.09.06 View 7895
How Stingrays Became the Most Efficient Swimmers in Nature
Study shows the hydrodynamic benefits of protruding eyes and mouth in a self-propelled flexible stingray
With their compressed bodies and flexible pectoral fins, stingrays have evolved to become one of nature’s most efficient swimmers. Scientists have long wondered about the role played by their protruding eyes and mouths, which one might expect to be hydrodynamic disadvantages.
Professor Hyung Jin Sung and his colleagues have discovered how such features on simulated stingrays affect a range of forces involved in propulsion, such as pressure and vorticity. Despite what one might expect, their research team found these protruding features actually help streamline the stingrays.
‘The influence of the 3D protruding eyes and mouth on a self-propelled flexible stingray and its underlying hydrodynamic mechanism are not yet fully understood,” said Professor Sung. “In the present study, the hydrodynamic benefit of protruding eyes and mouth was explored for the first time, revealing their hydrodynamic role.”
To illustrate the complex interplay between hydrodynamic forces, the researchers set to work creating a computer model of a self-propelled flexible plate. They clamped the front end of the model and then forced it to mimic the up-and-down harmonic oscillations stingrays use to propel themselves.
To re-create the effect of the eyes and mouth on the surrounding water, the team simulated multiple rigid plates on the model. They compared this model to one without eyes and a mouth using a technique called the penalty immersed boundary method.
“Managing random fish swimming and isolating the desired purpose of the measurements from numerous factors was difficult,” Sung said. “To overcome these limitations, the penalty immersed boundary method was adopted to find the hydrodynamic benefits of the protruding eyes and mouth.”
The team discovered that the eyes and mouth generated a vortex of flow in the forward-backward , which increased negative pressure at the simulated animal’s front, and a side-to-side vortex that increased the pressure difference above and below the stingray. The result was increased thrust and accelerated cruising.
Further analysis showed that the eyes and mouth increased overall propulsion efficiency by more than 20.5% and 10.6%, respectively.
Researchers hope their work, driven by curiosity, further stokes interest in exploring fluid phenomena in nature. They are hoping to find ways to adapt this for next-generation water vehicle designs based more closely on marine animals.
This study was supported by the National Research Foundation of Korea and the State Scholar Fund from the China Scholarship Council.
-ProfileProfessor Hyung Jin SungDepartment of Mechanical EngineeringKAIST
-PublicationHyung Jin Sung, Qian Mao, Ziazhen Zhao, Yingzheng Liu, “Hydrodynamic benefits of protruding eyes and mouth in a self-propelled flexible stingray,” Aug.31, 2021, Physics of Fluids
(https://doi.org/10.1063/5.0061287)
-News release from the American Institute of Physics, Aug.31, 2021
2021.09.06 View 7895 -
 Professor Ki-Jun Yoon selected as the 2019 SUHF Young Investigator
< Professor Ki-Jun Yoon >
Professor Ki-Jun Yoon from the Department of Biological Sciences was named one of four recipients of the 2019 Suh Kyung-Bae Science Foundation (SUHF) Young Investigator Awards.
The SUHF is a non-profit organization established in 2016 and funded by a personal donation of 300 billion KRW in shares from Chairman and CEO Kyung-Bae Suh of the Amorepacific Group. The primary purpose of the foundation is to serve as a platform to nurture and provide comprehensive long-term support for creative and passionate young Korean scientists committed to pursuing research in the field of life sciences. The SUHF selects three to five scientists through an open recruiting process every year, and grants each scientist a maximum of 2.5 billion KRW over a period of up to five years.
Since January this year, the foundation received 83 research proposals from scientists across the nation, especially from those who had less than five years of experience as professors, and selected the four recipients, including Professor Yoon.
Professor Yoon was recognized for his contributions to the advancement of research on how post-transcriptional mechanisms may modulate stem cell properties. His research project involves deciphering the molecular mechanisms controlling RNA metabolism in neural stem cells during normal development, and how alterations in RNA regulatory programs lead to human brain disorders.
< (From left) Professor Joo-Hong Park, Professor Yuree Lee, Chairman and CEO Kyung-Bae Suh, Professor Eunjung Lee, Professor Ki-Jun Yoon, ⓒ Amorepacific Group >
The other awards were given to Professor Joo-Hong Park and Professor Yuree Lee of Seoul National University, and Professor Eunjung Lee of Boston Children's Hospital and Harvard Medical School.
The awards ceremony was held on September 18 at the Amorepacific Headquarters in Seoul.
With these four new awardees, a total of 14 scientists have been named as SUHF Young Investigators to date.
(END)
2019.09.23 View 9598
Professor Ki-Jun Yoon selected as the 2019 SUHF Young Investigator
< Professor Ki-Jun Yoon >
Professor Ki-Jun Yoon from the Department of Biological Sciences was named one of four recipients of the 2019 Suh Kyung-Bae Science Foundation (SUHF) Young Investigator Awards.
The SUHF is a non-profit organization established in 2016 and funded by a personal donation of 300 billion KRW in shares from Chairman and CEO Kyung-Bae Suh of the Amorepacific Group. The primary purpose of the foundation is to serve as a platform to nurture and provide comprehensive long-term support for creative and passionate young Korean scientists committed to pursuing research in the field of life sciences. The SUHF selects three to five scientists through an open recruiting process every year, and grants each scientist a maximum of 2.5 billion KRW over a period of up to five years.
Since January this year, the foundation received 83 research proposals from scientists across the nation, especially from those who had less than five years of experience as professors, and selected the four recipients, including Professor Yoon.
Professor Yoon was recognized for his contributions to the advancement of research on how post-transcriptional mechanisms may modulate stem cell properties. His research project involves deciphering the molecular mechanisms controlling RNA metabolism in neural stem cells during normal development, and how alterations in RNA regulatory programs lead to human brain disorders.
< (From left) Professor Joo-Hong Park, Professor Yuree Lee, Chairman and CEO Kyung-Bae Suh, Professor Eunjung Lee, Professor Ki-Jun Yoon, ⓒ Amorepacific Group >
The other awards were given to Professor Joo-Hong Park and Professor Yuree Lee of Seoul National University, and Professor Eunjung Lee of Boston Children's Hospital and Harvard Medical School.
The awards ceremony was held on September 18 at the Amorepacific Headquarters in Seoul.
With these four new awardees, a total of 14 scientists have been named as SUHF Young Investigators to date.
(END)
2019.09.23 View 9598 -
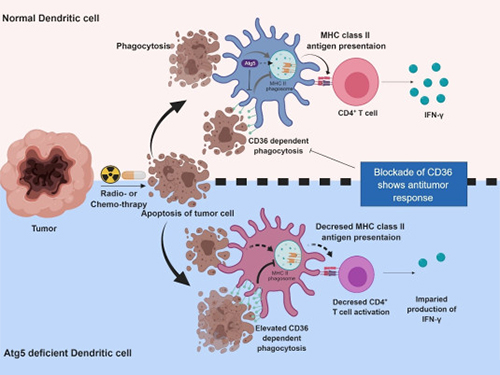 Autophagy in Dendritic Cells Helps Anticancer Activity
Autophagy contributes to the homeostasis of a cell and recently another function of autophagy has been reported. A KAIST research team found that the autophagy of dendritic cells supports T-cell anticancer activity.
Autophagy is a process of maintaining cell homeostasis by removing cellular waste and damaged cellular organelles; nevertheless, its role in the presentation of phagocytized tumor-associated antigens remains vague.
Meanwhile, dendritic cells are the ones that recognize pathogens or cancer antigens, and induce immune responses in T cells. When cancer cells are killed by radiation or an anticancer drug, dendritic cells absorb and remove them and present antigens on their surface to transfer them to T-cells.
Professor Heung Kyu Lee from the Graduate School of Medical Science and Engineering and his team found that the autophagy of dendritic cells plays a key role in T-cell activation and they proposed the principles of enhancing anti-cancer effects.
Their experiments showed that T-cell activation of dendritic cells as well as anticancer immune response dropped when there is a deficiency of Atg5 (autophagy-related) in dendritic cells.
Interestingly, Atg5-deficient dendritic cells significantly elevated receptor CD36 on the surface of the cells, which increased the phagocytosis of apoptotic tumor cells yet restricted the activation of T-cells.
At this time, when introducing antibodies into the system in order to block the receptor CD36, the anti-tumor T-cell response increased substantially while tumor growth declined.
Professor Lee said, “This study allowed us to explore the role of autophagy in the anti-cancer immune response of T-cells. We look forward to developing targeted anti-cancer therapies using the receptor CD36.”
This research was published in Autophagy (10.1080/15548627.2019.1596493) on March 22, 2019.
Figure 1.Mechanism of autophagy in dendritic cells
Figure 2. A role of autophagy in dendritic cells
2019.05.13 View 51447
Autophagy in Dendritic Cells Helps Anticancer Activity
Autophagy contributes to the homeostasis of a cell and recently another function of autophagy has been reported. A KAIST research team found that the autophagy of dendritic cells supports T-cell anticancer activity.
Autophagy is a process of maintaining cell homeostasis by removing cellular waste and damaged cellular organelles; nevertheless, its role in the presentation of phagocytized tumor-associated antigens remains vague.
Meanwhile, dendritic cells are the ones that recognize pathogens or cancer antigens, and induce immune responses in T cells. When cancer cells are killed by radiation or an anticancer drug, dendritic cells absorb and remove them and present antigens on their surface to transfer them to T-cells.
Professor Heung Kyu Lee from the Graduate School of Medical Science and Engineering and his team found that the autophagy of dendritic cells plays a key role in T-cell activation and they proposed the principles of enhancing anti-cancer effects.
Their experiments showed that T-cell activation of dendritic cells as well as anticancer immune response dropped when there is a deficiency of Atg5 (autophagy-related) in dendritic cells.
Interestingly, Atg5-deficient dendritic cells significantly elevated receptor CD36 on the surface of the cells, which increased the phagocytosis of apoptotic tumor cells yet restricted the activation of T-cells.
At this time, when introducing antibodies into the system in order to block the receptor CD36, the anti-tumor T-cell response increased substantially while tumor growth declined.
Professor Lee said, “This study allowed us to explore the role of autophagy in the anti-cancer immune response of T-cells. We look forward to developing targeted anti-cancer therapies using the receptor CD36.”
This research was published in Autophagy (10.1080/15548627.2019.1596493) on March 22, 2019.
Figure 1.Mechanism of autophagy in dendritic cells
Figure 2. A role of autophagy in dendritic cells
2019.05.13 View 51447 -
 Cellular Mechanism for Severe Viral Hepatitis Identified
(Professor Shin(left) and Professor Jung)
KAIST medical scientists identified a cellular mechanism causing inflammatory changes in regulatory T cells that can lead to severe viral hepatitis. Research on this mechanism will help further understand the nature of various inflammatory diseases and lead to the development of relevant clinical treatments.
It is known that activated immune cells of patients with viral hepatitis destroy hepatocyte, but its regulatory mechanism has not yet been described.
Regulatory T cells inhibit activation of other immune cells and thus are important for homeostasis of the immune system. However, recent studies contradictorily show that immune inhibitory functions of regulatory T cells weaken in inflammatory conditions and the cells secrete inflammatory cytokines in response. Meanwhile, such a phenomenon was not observed in viral hepatitis including types A, B and C.
The team focused on changes in regulatory T cells in patients with viral hepatitis and discovered that regulatory T cells undergo inflammatory changes to secrete inflammatory cytokines (protein secreted by immune cells) called TNF. They also proved regulatory T cells that secrete TNF contribute to the progression of viral hepatitis.
The team confirmed that regulatory T cells of acute hepatitis A patients have reduced immune-inhibitory functions. Instead, their regulatory T cells secrete TNF. Through this research, the team identified a molecular mechanism for changes in regulatory T cells and identified the transcription factor regulating the process. Furthermore, the team found similar changes to be also present in hepatitis B and C patients.
A KAIST immunology research team led by Professors Eui-Cheol Shin and Min Kyung Jung at the Graduate School of Medical Science & Engineering conducted this translational research with teams from Chungnam National University and Yonsei University to identify the mechanism in humans, instead of using animal models. The research was described in Gastroenterology last December.
Professor Shin said, “This is the first research on regulatory T cells that contributes to hepatocyte damage in viral hepatitis.” He continued, “It is significant for identifying the cells and the molecules that can be used as effective treatment targets for viral hepatitis in the future. This research was funded by the Samsung Science and Technology Foundation.
(Figure1: Treg cells from acute hepatitis A (AHA) patients produce tumor necrosis factor (TNF) andhave reduced suppressive activity. These changes are due to a decrease in FoxP3 transcription factor and an increase in RORγt transcription factor. TNF-producing Treg cells are associated with severe liver injury in AHA patients.)
(Figure 2: A higher proportion of Treg cells from patients with acute hepatitis A, compared with healthy controls, produced TNF upon stimulation with anti-CD3 and anti-CD2. This study reports the presence and the significance of TNF-producing Treg cells for the first time in human patients.)
2018.01.18 View 8883
Cellular Mechanism for Severe Viral Hepatitis Identified
(Professor Shin(left) and Professor Jung)
KAIST medical scientists identified a cellular mechanism causing inflammatory changes in regulatory T cells that can lead to severe viral hepatitis. Research on this mechanism will help further understand the nature of various inflammatory diseases and lead to the development of relevant clinical treatments.
It is known that activated immune cells of patients with viral hepatitis destroy hepatocyte, but its regulatory mechanism has not yet been described.
Regulatory T cells inhibit activation of other immune cells and thus are important for homeostasis of the immune system. However, recent studies contradictorily show that immune inhibitory functions of regulatory T cells weaken in inflammatory conditions and the cells secrete inflammatory cytokines in response. Meanwhile, such a phenomenon was not observed in viral hepatitis including types A, B and C.
The team focused on changes in regulatory T cells in patients with viral hepatitis and discovered that regulatory T cells undergo inflammatory changes to secrete inflammatory cytokines (protein secreted by immune cells) called TNF. They also proved regulatory T cells that secrete TNF contribute to the progression of viral hepatitis.
The team confirmed that regulatory T cells of acute hepatitis A patients have reduced immune-inhibitory functions. Instead, their regulatory T cells secrete TNF. Through this research, the team identified a molecular mechanism for changes in regulatory T cells and identified the transcription factor regulating the process. Furthermore, the team found similar changes to be also present in hepatitis B and C patients.
A KAIST immunology research team led by Professors Eui-Cheol Shin and Min Kyung Jung at the Graduate School of Medical Science & Engineering conducted this translational research with teams from Chungnam National University and Yonsei University to identify the mechanism in humans, instead of using animal models. The research was described in Gastroenterology last December.
Professor Shin said, “This is the first research on regulatory T cells that contributes to hepatocyte damage in viral hepatitis.” He continued, “It is significant for identifying the cells and the molecules that can be used as effective treatment targets for viral hepatitis in the future. This research was funded by the Samsung Science and Technology Foundation.
(Figure1: Treg cells from acute hepatitis A (AHA) patients produce tumor necrosis factor (TNF) andhave reduced suppressive activity. These changes are due to a decrease in FoxP3 transcription factor and an increase in RORγt transcription factor. TNF-producing Treg cells are associated with severe liver injury in AHA patients.)
(Figure 2: A higher proportion of Treg cells from patients with acute hepatitis A, compared with healthy controls, produced TNF upon stimulation with anti-CD3 and anti-CD2. This study reports the presence and the significance of TNF-producing Treg cells for the first time in human patients.)
2018.01.18 View 8883 -
 Professor Suk-Bok Chang receives 14th Korea Science Award in the field of Chemistry
Professor Suk-Bok Chang from the Department of Chemistry at KAIST received the “2013 Korea Science Award” in chemistry hosted by the National Research Foundation and the Ministry of Science, ICT, and Future Planning, Republic of Korea.
The Korea Science Award is a presidential award of Korea, which was first established in 1987 to recognize research excellence in natural science. Three scientists are selected for the award in every other year.
Professor Chang primarily researches the catalyzing mechanism of carbon-hydrogen bonds in organic molecules. He has succeeded in making great progress in the field of organic chemistry especially in developing a new type of transition metal catalytic behavior that can be applied to low-reactivity compounds.
Hydrocarbons are abundant in nature, but its unreactive nature in ambient conditions makes it unsuitable as reactant for compound synthesis. In addition, the mechanism behind transition metal catalyzed carbon-hydrogen bond synthesis has not been proven sufficiently.
The prediction that fossil fuels will be depleted before the end of the century makes hydrocarbon synthesis an extremely important matter.
The need for an effective hydrocarbon synthesis method inspired Professor Chang to pursue research in the transition metal catalysis method and to develop a catalytic system that would allow efficient synthesis even in ambient conditions.
Professor Chang has been the lead researcher for the Institute for Basic Science’s “molecule catalysis reaction research team” since December 2012 and has been carrying out this research in KAIST.
2014.01.27 View 11667
Professor Suk-Bok Chang receives 14th Korea Science Award in the field of Chemistry
Professor Suk-Bok Chang from the Department of Chemistry at KAIST received the “2013 Korea Science Award” in chemistry hosted by the National Research Foundation and the Ministry of Science, ICT, and Future Planning, Republic of Korea.
The Korea Science Award is a presidential award of Korea, which was first established in 1987 to recognize research excellence in natural science. Three scientists are selected for the award in every other year.
Professor Chang primarily researches the catalyzing mechanism of carbon-hydrogen bonds in organic molecules. He has succeeded in making great progress in the field of organic chemistry especially in developing a new type of transition metal catalytic behavior that can be applied to low-reactivity compounds.
Hydrocarbons are abundant in nature, but its unreactive nature in ambient conditions makes it unsuitable as reactant for compound synthesis. In addition, the mechanism behind transition metal catalyzed carbon-hydrogen bond synthesis has not been proven sufficiently.
The prediction that fossil fuels will be depleted before the end of the century makes hydrocarbon synthesis an extremely important matter.
The need for an effective hydrocarbon synthesis method inspired Professor Chang to pursue research in the transition metal catalysis method and to develop a catalytic system that would allow efficient synthesis even in ambient conditions.
Professor Chang has been the lead researcher for the Institute for Basic Science’s “molecule catalysis reaction research team” since December 2012 and has been carrying out this research in KAIST.
2014.01.27 View 11667 -
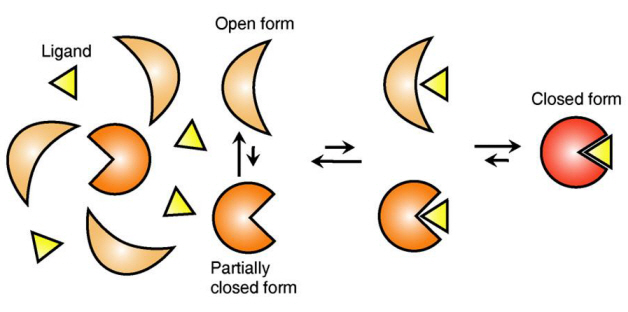 Ligand Recognition Mechanism of Protein Identified
Professor Hak-Sung Kim
-“Solved the 50 year old mystery of how protein recognises and binds to ligands”
- Exciting potential for understanding life phenomena and the further development of highly effective therapeutic agent development
KAIST’s Biological Science Department’s Professor Hak-Sung Kim, working in collaboration with Professor Sung-Chul Hong of Department of Physics, Seoul National University, has identified the mechanism of how the protein recognizes and binds to ligands within the human body.
The research findings were published in the online edition of Nature Chemical Biology (March 18), which is the most prestigious journal in the field of life science.
Since the research identified the mechanism, of which protein recognises and binds to ligands, it will take an essential role in understanding complex life phenomenon by understanding regulatory function of protein.
Also, ligand recognition of proteins is closely related to the cause of various diseases. Therefore the research team hopes to contribute to the development of highly effective treatments.
Ligands, well-known examples include nucleic acid and proteins, form the structure of an organism or are essential constituents with special functions such as information signalling.
In particular, the most important role of protein is recognising and binding to a particular ligand and hence regulating and maintaining life phenomena. The abnormal occurrence of an error in recognition of ligands may lead to various diseases.
The research team focused on the repetition of change in protein structure from the most stable “open form” to a relatively unstable “partially closed form”.
Professor Kim’s team analysed the change in protein structure when binding to a ligand on a molecular level in real time to explain the ligand recognition mechanism.
The research findings showed that ligands prefer the most stable protein structure. The team was the first in the world to identify that ligands alter protein structure to the most stable, the lowest energy level, when it binds to the protein.
In addition, the team found that ligands bind to unstable partially-closed forms to change protein structure.
The existing models to explain ligand recognition mechanism of protein are “Induced Custom Model”, which involves change in protein structure in binding to ligands, and the “Structure Selection Model”, which argues that ligands select and recognise only the best protein structure out of many. The academic world considers that the team’s research findings have perfectly proved the models through experiments for the first time in the world.
Professor Kim explained, “In the presence of ligands, there exists a phenomenon where the speed of altering protein structure is changed. This phenomenon is analysed on a molecular level to prove ligand recognition mechanism of protein for the first time”. He also said, “The 50-year old mystery, that existed only as a hypothesis on biology textbooks and was thought never to be solved, has been confirmed through experiments for the first time.”
Figure 1: Proteins, with open and partially open form, recognising and binding to ligands.
Figure 2: Ligands temporarily bind to a stable protein structure, open form, which changes into the most stable structure, closed form. In addition, binding to partially closed form also changes protein structure to closed form.
2013.04.01 View 12550
Ligand Recognition Mechanism of Protein Identified
Professor Hak-Sung Kim
-“Solved the 50 year old mystery of how protein recognises and binds to ligands”
- Exciting potential for understanding life phenomena and the further development of highly effective therapeutic agent development
KAIST’s Biological Science Department’s Professor Hak-Sung Kim, working in collaboration with Professor Sung-Chul Hong of Department of Physics, Seoul National University, has identified the mechanism of how the protein recognizes and binds to ligands within the human body.
The research findings were published in the online edition of Nature Chemical Biology (March 18), which is the most prestigious journal in the field of life science.
Since the research identified the mechanism, of which protein recognises and binds to ligands, it will take an essential role in understanding complex life phenomenon by understanding regulatory function of protein.
Also, ligand recognition of proteins is closely related to the cause of various diseases. Therefore the research team hopes to contribute to the development of highly effective treatments.
Ligands, well-known examples include nucleic acid and proteins, form the structure of an organism or are essential constituents with special functions such as information signalling.
In particular, the most important role of protein is recognising and binding to a particular ligand and hence regulating and maintaining life phenomena. The abnormal occurrence of an error in recognition of ligands may lead to various diseases.
The research team focused on the repetition of change in protein structure from the most stable “open form” to a relatively unstable “partially closed form”.
Professor Kim’s team analysed the change in protein structure when binding to a ligand on a molecular level in real time to explain the ligand recognition mechanism.
The research findings showed that ligands prefer the most stable protein structure. The team was the first in the world to identify that ligands alter protein structure to the most stable, the lowest energy level, when it binds to the protein.
In addition, the team found that ligands bind to unstable partially-closed forms to change protein structure.
The existing models to explain ligand recognition mechanism of protein are “Induced Custom Model”, which involves change in protein structure in binding to ligands, and the “Structure Selection Model”, which argues that ligands select and recognise only the best protein structure out of many. The academic world considers that the team’s research findings have perfectly proved the models through experiments for the first time in the world.
Professor Kim explained, “In the presence of ligands, there exists a phenomenon where the speed of altering protein structure is changed. This phenomenon is analysed on a molecular level to prove ligand recognition mechanism of protein for the first time”. He also said, “The 50-year old mystery, that existed only as a hypothesis on biology textbooks and was thought never to be solved, has been confirmed through experiments for the first time.”
Figure 1: Proteins, with open and partially open form, recognising and binding to ligands.
Figure 2: Ligands temporarily bind to a stable protein structure, open form, which changes into the most stable structure, closed form. In addition, binding to partially closed form also changes protein structure to closed form.
2013.04.01 View 12550 -
 'Scientist-Engineer of the Month' for December: Professor Choi Joon Ho
Professor Choi Joon Ho (department of Biological Sciences) was made ‘Scientist-Engineer of December’ for his discovery of new gene (twenty-four) that helps biorhythm and proving that this gene helps control biorhythm.
Professor Choi published 100 dissertations over the past 25 years and made significant advancements in the field of molecular virus and neurobiology.
In 1995 Professor Choi uncovered the fact that the NS3 protein in C type hepatitis function as RNA helicase thereby opening the path to developing a cure for C type hepatitis; this is an international patent with Chiron corporation. The result was published in Biochemical and Biophysical Research Communications Journal and was the most domestically referred to dissertation in biological sciences in 1999.
In addition Professor Choi published in Nature magazine in 1999, a dissertation that uncovered the fact that the DNA of papillomar virus has another protein (hSNF5) that direct it apart from ordinary proteins.
In 2000~2005 Professor Choi published many dissertations in journals like Immunity, Cancer Research, Molecular and Cellular Biology, Oncogene, Journal of Virology, and etc.
Professor Choi screened over 10,000 species of pomace fly mutations and discovered the twenty-four gene that affects the biorhythm of pomace flies. He analyzed this gene further and found a new function that was different from known biorhythm mechanisms.
This research allowed a better understanding of biological clock of pomace flies and therefore was another step towards better understanding the control mechanism of human biological clock.
2012.01.31 View 10341
'Scientist-Engineer of the Month' for December: Professor Choi Joon Ho
Professor Choi Joon Ho (department of Biological Sciences) was made ‘Scientist-Engineer of December’ for his discovery of new gene (twenty-four) that helps biorhythm and proving that this gene helps control biorhythm.
Professor Choi published 100 dissertations over the past 25 years and made significant advancements in the field of molecular virus and neurobiology.
In 1995 Professor Choi uncovered the fact that the NS3 protein in C type hepatitis function as RNA helicase thereby opening the path to developing a cure for C type hepatitis; this is an international patent with Chiron corporation. The result was published in Biochemical and Biophysical Research Communications Journal and was the most domestically referred to dissertation in biological sciences in 1999.
In addition Professor Choi published in Nature magazine in 1999, a dissertation that uncovered the fact that the DNA of papillomar virus has another protein (hSNF5) that direct it apart from ordinary proteins.
In 2000~2005 Professor Choi published many dissertations in journals like Immunity, Cancer Research, Molecular and Cellular Biology, Oncogene, Journal of Virology, and etc.
Professor Choi screened over 10,000 species of pomace fly mutations and discovered the twenty-four gene that affects the biorhythm of pomace flies. He analyzed this gene further and found a new function that was different from known biorhythm mechanisms.
This research allowed a better understanding of biological clock of pomace flies and therefore was another step towards better understanding the control mechanism of human biological clock.
2012.01.31 View 10341 -
 New Bio-Clock gene and its function found
The Ministry of Education, Science and Technology announced that a Korean research team has found a new gene responsible for maintaining the bio-clock (twenty-four) and its mechanism.
Twnety-four was led by Professor Choi Joon Ho and Dr. Lee Jong Bin of KAIST (department of Biology) and was a joint operation with Professor Ravi Allada and Dr.Lim Jeong Hoon of Northwestern University (department of neurobiology) and the result was published in ‘Nature’ magazine.
The research team experimented with transformed small fruit flies for 4 years and found that there was an undiscovered gene that deals with the bio rhythm in the brain which they named ‘twenty-four’.
The understanding with genes prior to twenty-four was that these genes regulate biorhythm in the transcription phase (DNA to mRNA). Twenty-four operates in the step after transcription when the ribosome creates proteins. Especially twenty-four has a great effect on the ‘period protein’ which acts as a sub-atomic clock that regulates the rhythm and life of each cell.
The experiment was innovational in that it was able to scientifically prove the function of the protein produced by the gene.
The result is expected to help solve the problems associated with sleep disorders, jetlags, eating rhythms, bio rhythms, etc.
The name twenty-four was the fact that a day, a cycle, is 24 hours long and the gene’s serial numbers CG4857 adds up to twenty four.
2011.02.23 View 13341
New Bio-Clock gene and its function found
The Ministry of Education, Science and Technology announced that a Korean research team has found a new gene responsible for maintaining the bio-clock (twenty-four) and its mechanism.
Twnety-four was led by Professor Choi Joon Ho and Dr. Lee Jong Bin of KAIST (department of Biology) and was a joint operation with Professor Ravi Allada and Dr.Lim Jeong Hoon of Northwestern University (department of neurobiology) and the result was published in ‘Nature’ magazine.
The research team experimented with transformed small fruit flies for 4 years and found that there was an undiscovered gene that deals with the bio rhythm in the brain which they named ‘twenty-four’.
The understanding with genes prior to twenty-four was that these genes regulate biorhythm in the transcription phase (DNA to mRNA). Twenty-four operates in the step after transcription when the ribosome creates proteins. Especially twenty-four has a great effect on the ‘period protein’ which acts as a sub-atomic clock that regulates the rhythm and life of each cell.
The experiment was innovational in that it was able to scientifically prove the function of the protein produced by the gene.
The result is expected to help solve the problems associated with sleep disorders, jetlags, eating rhythms, bio rhythms, etc.
The name twenty-four was the fact that a day, a cycle, is 24 hours long and the gene’s serial numbers CG4857 adds up to twenty four.
2011.02.23 View 13341 -
 Scaling Laws between Population and Facility Densities Found
A research team led by Prof. Ha-Woong Jeong of the Department of Physics, KAIST, has found a positive correlation between facilities and population densities, university authorities said on Tuesday (Sept. 2). The research was conducted in the cooperation with a research team of Prof. Beom-Jun Kim at Sungkyunkwan University.
The researchers investigated the ideal relation between the population and the facilities within the framework of an economic mechanism governing microdynamics.
In previous studies based on the global optimization of facility positions in minimizing the overall travel distance between people and facilities, the relation between population and facilities should follow a simple law. The new empirical analysis, however, determined that the law is not a fixed value but spreads in a broad range depending on facility types.
To explain this discrepancy, the researchers proposed a model based on economic mechanism that mimics the competitive balance between the profit of the facilities and the social opportunity cost for population.
The results were published in the Proceedings of the National Academy of Sciences of the United States on Aug. 25.
2009.09.04 View 14404
Scaling Laws between Population and Facility Densities Found
A research team led by Prof. Ha-Woong Jeong of the Department of Physics, KAIST, has found a positive correlation between facilities and population densities, university authorities said on Tuesday (Sept. 2). The research was conducted in the cooperation with a research team of Prof. Beom-Jun Kim at Sungkyunkwan University.
The researchers investigated the ideal relation between the population and the facilities within the framework of an economic mechanism governing microdynamics.
In previous studies based on the global optimization of facility positions in minimizing the overall travel distance between people and facilities, the relation between population and facilities should follow a simple law. The new empirical analysis, however, determined that the law is not a fixed value but spreads in a broad range depending on facility types.
To explain this discrepancy, the researchers proposed a model based on economic mechanism that mimics the competitive balance between the profit of the facilities and the social opportunity cost for population.
The results were published in the Proceedings of the National Academy of Sciences of the United States on Aug. 25.
2009.09.04 View 14404 -
 Researchers Find Mechanism of Tumor Suppressor Genes
By Kim Tae-gyu. Staff ReporterTHE Korea Times 02-06-2004
Korean scientists continue to break new ground in fighting cancer as domestic researchers examined the mechanism of a gene which can help detect and treat various sorts of cancer.
Korea Advanced Institute of Science and Technology (KAIST) Prof. Lim Dae-sik on Thursday said his team uncovered the mechanism of RASSF1A (Ras Association Domain Family 1 A), or tumor suppressor genes, for the first time in the world.
The gene was widely considered to play an important role in reducing the proliferation of cancer cells, but its exact function and processes have remained unknown up to now.
It is the second cancer-related breakthrough by Koreans in a week after Korea Institute of Science and Technology (KIST) Prof. Chung Hesson unveiled the oral anti-cancer drug.
``Cancer results from the failed management of cell cycles due to things like radiation. After a two-year intensive study, we found out how RASSF1A governs the cell cycle,"" Lim said.
Lim added cancer is caused by abnormal cells, which continue to grow and divide out of control unlike normal cells, which die over time. Cancer cells develop into malignant tumors, eventually inflicting damaging effect on the human body.
As a result, a lack of the RASSF1A indicates a high possibility of cancer and injection of it into cells is believed to help cure the deadly disease, according to Lim.
Dr. Song Min-sup, who took charge of the research, said the findings will especially pave the way for the detection and treatment of lung cancer.
``The dearth of RASSF1A was reported mostly in the case of lung cancer. The new findings will provide insight into the diagnosis and cure of lung cancer from its early stages,"" Song explained.
Lung cancer is a very elusive disease because it doesn"t cause symptoms in its infancy. When symptoms do occur, usually it is too late.
``We expect commercial detection kits or drugs for lung cancer in around five years after pre-clinic experimentation and two-phase clinic trials,"" Song expected.
Details of the study is available in the scientific journal Nature Cell Biology in its February edition.
voc200@koreatimes.co.kr
2004.03.15 View 18665
Researchers Find Mechanism of Tumor Suppressor Genes
By Kim Tae-gyu. Staff ReporterTHE Korea Times 02-06-2004
Korean scientists continue to break new ground in fighting cancer as domestic researchers examined the mechanism of a gene which can help detect and treat various sorts of cancer.
Korea Advanced Institute of Science and Technology (KAIST) Prof. Lim Dae-sik on Thursday said his team uncovered the mechanism of RASSF1A (Ras Association Domain Family 1 A), or tumor suppressor genes, for the first time in the world.
The gene was widely considered to play an important role in reducing the proliferation of cancer cells, but its exact function and processes have remained unknown up to now.
It is the second cancer-related breakthrough by Koreans in a week after Korea Institute of Science and Technology (KIST) Prof. Chung Hesson unveiled the oral anti-cancer drug.
``Cancer results from the failed management of cell cycles due to things like radiation. After a two-year intensive study, we found out how RASSF1A governs the cell cycle,"" Lim said.
Lim added cancer is caused by abnormal cells, which continue to grow and divide out of control unlike normal cells, which die over time. Cancer cells develop into malignant tumors, eventually inflicting damaging effect on the human body.
As a result, a lack of the RASSF1A indicates a high possibility of cancer and injection of it into cells is believed to help cure the deadly disease, according to Lim.
Dr. Song Min-sup, who took charge of the research, said the findings will especially pave the way for the detection and treatment of lung cancer.
``The dearth of RASSF1A was reported mostly in the case of lung cancer. The new findings will provide insight into the diagnosis and cure of lung cancer from its early stages,"" Song explained.
Lung cancer is a very elusive disease because it doesn"t cause symptoms in its infancy. When symptoms do occur, usually it is too late.
``We expect commercial detection kits or drugs for lung cancer in around five years after pre-clinic experimentation and two-phase clinic trials,"" Song expected.
Details of the study is available in the scientific journal Nature Cell Biology in its February edition.
voc200@koreatimes.co.kr
2004.03.15 View 18665