epilepsy
-
 Professor J.H. Lee Wins the Innovators in Science Award
Professor Jeong Ho Lee from the Graduate School of Medical Science and Engineering won the Early-Career Scientist Award of the 2020 Innovators in Science Award. The New York Academy of Sciences administers the award in partnership with Takeda Pharmaceutical Company.
The Innovators in Science Award grants two prizes of US $200,000 each year: one to an Early-Career Scientist and the other to a well-established Senior Scientist who have distinguished themselves for the creative thinking and impact of their rare disease research. The Senior Scientist Awardee is Dr. Adrian R. Krainer, at Cold Spring Harbor Laboratory whose research focused on the mechanisms and control of RNA splicing.
Prof. Lee is recognized for his research investigating genetic mutations in stem cells in the brain that result in rare developmental brain disorders. He was the first to identify the causes of intractable epilepsies and has identified the genes responsible for several developmental brain disorders, including focal cortical dysplasia, Joubert syndrome—a disorder characterized by an underdevelopment of the brainstem—and hemimegaloencephaly, which is the abnormal enlargement of one side of the brain.
“It is a great honor to be recognized by a jury of such globally respected scientists whom I greatly admire,” said Prof. Lee. “More importantly, this award validates research into brain somatic mutations as an important area of exploration to help patients suffering from devastating and untreatable neurological disorders.”
Prof. Lee also is the Director of the National Creative Research Initiative Center for Brain Somatic Mutations, and Co-founder and Chief Technology Officer of SoVarGen, a biopharmaceutical company aiming to discover novel therapeutics and diagnosis for intractable central nervous system (CNS) diseases caused by low-level somatic mutation.
The Innovators in Science Award is a limited submission competition in which research universities, academic institutions, government or non-profit institutions, or equivalent from around the globe with a well-established record of scientific excellence are invited to nominate their most promising Early-Career Scientists and their most outstanding Senior Scientists working in one of four selected therapeutic fields of neuroscience, gastroenterology, oncology, and regenerative medicine. The 2020 Winners will be honored at the virtual Innovators in Science Award Ceremony and Symposium in October 2020.
2020.07.09 View 11871
Professor J.H. Lee Wins the Innovators in Science Award
Professor Jeong Ho Lee from the Graduate School of Medical Science and Engineering won the Early-Career Scientist Award of the 2020 Innovators in Science Award. The New York Academy of Sciences administers the award in partnership with Takeda Pharmaceutical Company.
The Innovators in Science Award grants two prizes of US $200,000 each year: one to an Early-Career Scientist and the other to a well-established Senior Scientist who have distinguished themselves for the creative thinking and impact of their rare disease research. The Senior Scientist Awardee is Dr. Adrian R. Krainer, at Cold Spring Harbor Laboratory whose research focused on the mechanisms and control of RNA splicing.
Prof. Lee is recognized for his research investigating genetic mutations in stem cells in the brain that result in rare developmental brain disorders. He was the first to identify the causes of intractable epilepsies and has identified the genes responsible for several developmental brain disorders, including focal cortical dysplasia, Joubert syndrome—a disorder characterized by an underdevelopment of the brainstem—and hemimegaloencephaly, which is the abnormal enlargement of one side of the brain.
“It is a great honor to be recognized by a jury of such globally respected scientists whom I greatly admire,” said Prof. Lee. “More importantly, this award validates research into brain somatic mutations as an important area of exploration to help patients suffering from devastating and untreatable neurological disorders.”
Prof. Lee also is the Director of the National Creative Research Initiative Center for Brain Somatic Mutations, and Co-founder and Chief Technology Officer of SoVarGen, a biopharmaceutical company aiming to discover novel therapeutics and diagnosis for intractable central nervous system (CNS) diseases caused by low-level somatic mutation.
The Innovators in Science Award is a limited submission competition in which research universities, academic institutions, government or non-profit institutions, or equivalent from around the globe with a well-established record of scientific excellence are invited to nominate their most promising Early-Career Scientists and their most outstanding Senior Scientists working in one of four selected therapeutic fields of neuroscience, gastroenterology, oncology, and regenerative medicine. The 2020 Winners will be honored at the virtual Innovators in Science Award Ceremony and Symposium in October 2020.
2020.07.09 View 11871 -
 Professor Jeong Ho Lee Receives the 2015 Pediatric Epilepsies Research Award
The award identifies leading scientists worldwide and funds their cutting-edge research in epilepsy.
The Citizen United for Research in Epilepsy (CURE) announced on September 7, 2015, that Jeong Ho Lee, a professor of the Graduate School of Medical Science and Engineering at KAIST, will be awarded the 2015 Pediatric Epilepsies Research Award.
The Pediatric Epilepsies Research Award is given annually to a researcher who has conducted novel, innovative research projects that address severe, intractable pediatric epilepsies as well as collaborative, interdisciplinary projects that explore new approaches to find a treatment for pediatric epilepsies.
Lee was recognized for his leading study in the field of intractable epilepsy. He is the first Korean who has ever received this award, securing a research grant of USD 250,000 for two years.
Lee has conducted research on brain somatic mutations as the novel cause of childhood intractable epilepsy. Pediatric epilepsies account for approximately 70% of all cases of epilepsy.
Established in 1998, CURE is a non-profit American organization based in Chicago, Illinois, which is committed to funding research and various initiatives that will lead to breakthroughs to cure epilepsy.
Since its inception, CURE has been at the forefront of epilepsy research, raising more than USD 32 million to support researchers and scientists worldwide. It has also awarded more than 180 cutting-edge projects in 13 countries.
2015.09.09 View 12342
Professor Jeong Ho Lee Receives the 2015 Pediatric Epilepsies Research Award
The award identifies leading scientists worldwide and funds their cutting-edge research in epilepsy.
The Citizen United for Research in Epilepsy (CURE) announced on September 7, 2015, that Jeong Ho Lee, a professor of the Graduate School of Medical Science and Engineering at KAIST, will be awarded the 2015 Pediatric Epilepsies Research Award.
The Pediatric Epilepsies Research Award is given annually to a researcher who has conducted novel, innovative research projects that address severe, intractable pediatric epilepsies as well as collaborative, interdisciplinary projects that explore new approaches to find a treatment for pediatric epilepsies.
Lee was recognized for his leading study in the field of intractable epilepsy. He is the first Korean who has ever received this award, securing a research grant of USD 250,000 for two years.
Lee has conducted research on brain somatic mutations as the novel cause of childhood intractable epilepsy. Pediatric epilepsies account for approximately 70% of all cases of epilepsy.
Established in 1998, CURE is a non-profit American organization based in Chicago, Illinois, which is committed to funding research and various initiatives that will lead to breakthroughs to cure epilepsy.
Since its inception, CURE has been at the forefront of epilepsy research, raising more than USD 32 million to support researchers and scientists worldwide. It has also awarded more than 180 cutting-edge projects in 13 countries.
2015.09.09 View 12342 -
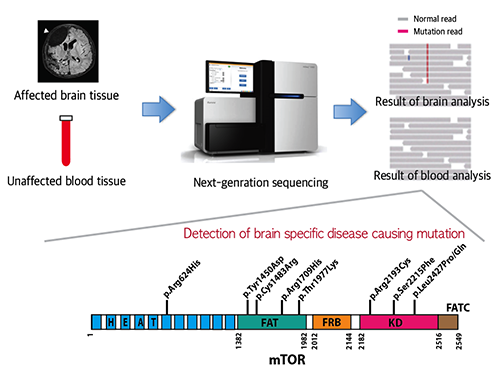 Mutations Occurring Only in Brain Responsible for Intractable Epilepsy Identified
KAIST researchers have discovered that brain somatic mutations in MTOR gene induce intractable epilepsy and suggest a precision medicine to treat epileptic seizures.
Epilepsy is a brain disorder which afflicts more than 50 million people worldwide. Many epilepsy patients can control their symptoms through medication, but about 30% suffer from intractable epilepsy and are unable to manage the disease with drugs. Intractable epilepsy causes multiple seizures, permanent mental, physical, and developmental disabilities, and even death. Therefore, surgical removal of the affected area from the brain has been practiced as a treatment for patients with medically refractory seizures, but this too fails to provide a complete solution because only 60% of the patients who undergo surgery are rendered free of seizures.
A Korean research team led by Professor Jeong Ho Lee of the Graduate School of Medical Science and Engineering at the Korea Advanced Institute of Science and Technology (KAIST) and Professor Dong-Seok Kim of Epilepsy Research Center at Yonsei University College of Medicine has recently identified brain somatic mutations in the gene of mechanistic target of rapamycin (MTOR) as the cause of focal cortical dysplasia type II (FCDII), one of the most important and common inducers to intractable epilepsy, particularly in children. They propose a targeted therapy to lessen epileptic seizures by suppressing the activation of mTOR kinase, a signaling protein in the brain. Their research results were published online in Nature Medicine on March 23, 2015.
FCDII contributes to the abnormal developments of the cerebral cortex, ranging from cortical disruption to severe forms of cortical dyslamination, balloon cells, and dysplastic neurons. The research team studied 77 FCDII patients with intractable epilepsy who had received a surgery to remove the affected regions from the brain. The researchers used various deep sequencing technologies to conduct comparative DNA analysis of the samples obtained from the patients’ brain and blood, or saliva. They reported that about 16% of the studied patients had somatic mutations in their brain. Such mutations, however, did not take place in their blood or saliva DNA.
Professor Jeong Ho Lee of KAIST said, “This is an important finding. Unlike our previous belief that genetic mutations causing intractable epilepsy exist anywhere in the human body including blood, specific gene mutations incurred only in the brain can lead to intractable epilepsy. From our animal models, we could see how a small fraction of mutations carrying neurons in the brain could affect its entire function.”
The research team recapitulated the pathogenesis of intractable epilepsy by inducing the focal cortical expression of mutated mTOR in the mouse brain via electroporation method and observed as the mouse develop epileptic symptoms. They then treated these mice with the drug called “rapamycin” to inhibit the activity of mTOR protein and observed that it suppressed the development of epileptic seizures with cytomegalic neurons.
“Our study offers the first evidence that brain-somatic activating mutations in MTOR cause FCDII and identifies mTOR as a treatment target for intractable epilepsy,” said co-author Dr. Dong-Seok Kim, a neurosurgeon at Yonsei Medical Center with the country’s largest surgical experiences in treating patients with this condition.
The research paper is titled “Brain somatic mutations in MTOR cause focal cortical dysplasia type II leading to intractable epilepsy.” (Digital Object Identifier #: 10.1038/nm.3824)
Picture 1: A schematic image to show how to detect brain specific mutation using next-generation sequencing technology with blood-brain paired sample. Simple comparison of non-overlapping mutations between affected and unaffected tissues is able to detect brain specific mutations.
Picture 2: A schematic image to show how to generate focal cortical dysplasia mouse model. This mouse model open the new window of drug screening for seizure patients.
Picture 3: Targeted medicine can rescue the focal cortical dysplasia symptoms including cytomegalic neuron & intractable epilepsy.
2015.03.25 View 15597
Mutations Occurring Only in Brain Responsible for Intractable Epilepsy Identified
KAIST researchers have discovered that brain somatic mutations in MTOR gene induce intractable epilepsy and suggest a precision medicine to treat epileptic seizures.
Epilepsy is a brain disorder which afflicts more than 50 million people worldwide. Many epilepsy patients can control their symptoms through medication, but about 30% suffer from intractable epilepsy and are unable to manage the disease with drugs. Intractable epilepsy causes multiple seizures, permanent mental, physical, and developmental disabilities, and even death. Therefore, surgical removal of the affected area from the brain has been practiced as a treatment for patients with medically refractory seizures, but this too fails to provide a complete solution because only 60% of the patients who undergo surgery are rendered free of seizures.
A Korean research team led by Professor Jeong Ho Lee of the Graduate School of Medical Science and Engineering at the Korea Advanced Institute of Science and Technology (KAIST) and Professor Dong-Seok Kim of Epilepsy Research Center at Yonsei University College of Medicine has recently identified brain somatic mutations in the gene of mechanistic target of rapamycin (MTOR) as the cause of focal cortical dysplasia type II (FCDII), one of the most important and common inducers to intractable epilepsy, particularly in children. They propose a targeted therapy to lessen epileptic seizures by suppressing the activation of mTOR kinase, a signaling protein in the brain. Their research results were published online in Nature Medicine on March 23, 2015.
FCDII contributes to the abnormal developments of the cerebral cortex, ranging from cortical disruption to severe forms of cortical dyslamination, balloon cells, and dysplastic neurons. The research team studied 77 FCDII patients with intractable epilepsy who had received a surgery to remove the affected regions from the brain. The researchers used various deep sequencing technologies to conduct comparative DNA analysis of the samples obtained from the patients’ brain and blood, or saliva. They reported that about 16% of the studied patients had somatic mutations in their brain. Such mutations, however, did not take place in their blood or saliva DNA.
Professor Jeong Ho Lee of KAIST said, “This is an important finding. Unlike our previous belief that genetic mutations causing intractable epilepsy exist anywhere in the human body including blood, specific gene mutations incurred only in the brain can lead to intractable epilepsy. From our animal models, we could see how a small fraction of mutations carrying neurons in the brain could affect its entire function.”
The research team recapitulated the pathogenesis of intractable epilepsy by inducing the focal cortical expression of mutated mTOR in the mouse brain via electroporation method and observed as the mouse develop epileptic symptoms. They then treated these mice with the drug called “rapamycin” to inhibit the activity of mTOR protein and observed that it suppressed the development of epileptic seizures with cytomegalic neurons.
“Our study offers the first evidence that brain-somatic activating mutations in MTOR cause FCDII and identifies mTOR as a treatment target for intractable epilepsy,” said co-author Dr. Dong-Seok Kim, a neurosurgeon at Yonsei Medical Center with the country’s largest surgical experiences in treating patients with this condition.
The research paper is titled “Brain somatic mutations in MTOR cause focal cortical dysplasia type II leading to intractable epilepsy.” (Digital Object Identifier #: 10.1038/nm.3824)
Picture 1: A schematic image to show how to detect brain specific mutation using next-generation sequencing technology with blood-brain paired sample. Simple comparison of non-overlapping mutations between affected and unaffected tissues is able to detect brain specific mutations.
Picture 2: A schematic image to show how to generate focal cortical dysplasia mouse model. This mouse model open the new window of drug screening for seizure patients.
Picture 3: Targeted medicine can rescue the focal cortical dysplasia symptoms including cytomegalic neuron & intractable epilepsy.
2015.03.25 View 15597