Hepatology
-
 Discovery of an Optimal Drug Combination: Overcoming Resistance to Targeted Drugs for Liver Cancer
A KAIST research team presented a novel method for improving medication treatment for liver cancer using Systems Biology, combining research from information technology and the life sciences. Professor Kwang-Hyun Cho in the Department of Bio and Brain Engineering at KAIST conducted the research in collaboration with Professor Jung-Hwan Yoon in the Department of Internal Medicine at Seoul National University Hospital. This research was published in Hepatology in September 2017 (available online from August 24, 2017).
Liver cancer is the fifth and seventh most common cancer found in men and women throughout the world, which places it second in the cause of cancer deaths. In particular, Korea has 28.4 deaths from liver cancer per 100,000 persons, the highest death rate among OECD countries and twice that of Japan.
Each year in Korea, 16,000 people get liver cancer on average, yet the five-year survival rate stands below 12%. According to the National Cancer Information Center, lung cancer (17,399) took the highest portion of cancer-related deaths, followed by liver cancer (11,311) based on last year data.
Liver cancer is known to carry the highest social cost in comparison to other cancers and it causes the highest fatality in earlier age groups (40s-50s). In that sense, it is necessary to develop a new treatment that mitigates side effects yet elevates the survival rate.
There are ways in which liver cancer can be cured, such as surgery, embolization, and medication treatments; however, the options become limited for curing progressive cancer, a stage in which surgical methods cannot be executed.
Among anticancer medications, Sorafenib, a drug known for enhancing the survival rate of cancer patients, is a unique drug allowed for use as a targeted anticancer medication for progressive liver cancer patients. Its sales reached more than ten billion KRW annually in Korea, but its efficacy works on only about 20% of the treated patients. Also, acquired resistance to Sorafenib is emerging. Additionally, the action mechanism and resistance mechanism of Sorafenib is only vaguely identified.Although Sorafenib only extends the survival rate of terminal cancer patients less than three months on average, it is widely being used because drugs developed by global pharmaceutical companies failed to outperform its effectiveness.
Professor Cho’s research team analyzed the expression changes of genes in cell lines in response to Sorafenib in order to identify the effect and the resistance mechanism of Sorafenib.
As a result, the team discovered the resistance mechanism of Sorafenib using Systems Biology analysis. By combining computer simulations and biological experiments, it was revealed that protein disulfide isomerase (PDI) plays a crucial role in the resistance mechanism of Sorafenib and that its efficacy can be improved significantly by blocking PDI.
The research team used mice in the experiment and discovered the synergic effect of PDI inhibition with Sorafenib for reducing liver cancer cells, known as hepatocellular carcinoma. Also, more PDIs are shown in tissue from patients who possess a resistance to Sorafenib. From these findings, the team could identify the possibility of its clinical applications. The team also confirmed these findings from clinical data through a retrospective cohort study.
“Molecules that play an important role in cell lines are mostly put under complex regulation. For this reason, the existing biological research has a fundamental limitations for discovering its underlying principles,” Professor Cho said. “This research is a representative case of overcoming this limitation of traditional life science research by using a Systems Biology approach, combining IT and life science. It suggests the possibility of developing a new method that overcomes drug resistance with a network analysis of the targeted drug action mechanism of cancer.”
The research was supported by the National Research Foundation of Korea (NRF) and funded by the Ministry of Science and ICT.
(Figure 1. Simulation results from cellular experiments using hepatocellular carcinoma)
(Figure 2. Network analysis and computer simulation by using the endoplasmic reticulum (ER) stress network)
(Figure 3. ER stress network model)
2017.08.30 View 13857
Discovery of an Optimal Drug Combination: Overcoming Resistance to Targeted Drugs for Liver Cancer
A KAIST research team presented a novel method for improving medication treatment for liver cancer using Systems Biology, combining research from information technology and the life sciences. Professor Kwang-Hyun Cho in the Department of Bio and Brain Engineering at KAIST conducted the research in collaboration with Professor Jung-Hwan Yoon in the Department of Internal Medicine at Seoul National University Hospital. This research was published in Hepatology in September 2017 (available online from August 24, 2017).
Liver cancer is the fifth and seventh most common cancer found in men and women throughout the world, which places it second in the cause of cancer deaths. In particular, Korea has 28.4 deaths from liver cancer per 100,000 persons, the highest death rate among OECD countries and twice that of Japan.
Each year in Korea, 16,000 people get liver cancer on average, yet the five-year survival rate stands below 12%. According to the National Cancer Information Center, lung cancer (17,399) took the highest portion of cancer-related deaths, followed by liver cancer (11,311) based on last year data.
Liver cancer is known to carry the highest social cost in comparison to other cancers and it causes the highest fatality in earlier age groups (40s-50s). In that sense, it is necessary to develop a new treatment that mitigates side effects yet elevates the survival rate.
There are ways in which liver cancer can be cured, such as surgery, embolization, and medication treatments; however, the options become limited for curing progressive cancer, a stage in which surgical methods cannot be executed.
Among anticancer medications, Sorafenib, a drug known for enhancing the survival rate of cancer patients, is a unique drug allowed for use as a targeted anticancer medication for progressive liver cancer patients. Its sales reached more than ten billion KRW annually in Korea, but its efficacy works on only about 20% of the treated patients. Also, acquired resistance to Sorafenib is emerging. Additionally, the action mechanism and resistance mechanism of Sorafenib is only vaguely identified.Although Sorafenib only extends the survival rate of terminal cancer patients less than three months on average, it is widely being used because drugs developed by global pharmaceutical companies failed to outperform its effectiveness.
Professor Cho’s research team analyzed the expression changes of genes in cell lines in response to Sorafenib in order to identify the effect and the resistance mechanism of Sorafenib.
As a result, the team discovered the resistance mechanism of Sorafenib using Systems Biology analysis. By combining computer simulations and biological experiments, it was revealed that protein disulfide isomerase (PDI) plays a crucial role in the resistance mechanism of Sorafenib and that its efficacy can be improved significantly by blocking PDI.
The research team used mice in the experiment and discovered the synergic effect of PDI inhibition with Sorafenib for reducing liver cancer cells, known as hepatocellular carcinoma. Also, more PDIs are shown in tissue from patients who possess a resistance to Sorafenib. From these findings, the team could identify the possibility of its clinical applications. The team also confirmed these findings from clinical data through a retrospective cohort study.
“Molecules that play an important role in cell lines are mostly put under complex regulation. For this reason, the existing biological research has a fundamental limitations for discovering its underlying principles,” Professor Cho said. “This research is a representative case of overcoming this limitation of traditional life science research by using a Systems Biology approach, combining IT and life science. It suggests the possibility of developing a new method that overcomes drug resistance with a network analysis of the targeted drug action mechanism of cancer.”
The research was supported by the National Research Foundation of Korea (NRF) and funded by the Ministry of Science and ICT.
(Figure 1. Simulation results from cellular experiments using hepatocellular carcinoma)
(Figure 2. Network analysis and computer simulation by using the endoplasmic reticulum (ER) stress network)
(Figure 3. ER stress network model)
2017.08.30 View 13857 -
 Regulatory T Cells Influence Liver Damage of Hepatitis A Patients
Liver damage becomes more severe with the decrease of regulatory T cells
“This research will aid the development of hepatitis A targeted drug,” said a KAIST researcher.
The KAIST Graduate School of Medical Science and Engineering’s Professor Eui-Cheol Shin and his research team have identified the mechanism, explaining how the regulatory T cells are responsible for the body’s immune system and how they have induced liver damage of hepatitis A patients.
The research results were published online in the July 9th edition of ‘Gut,’ the world’s most prominent journal in the field of gastroenterology.
Hepatitis A is an acute form of hepatitis caused by hepatitis A virus. The virus spreads through oral contact and enters the body via digestive organs.
Regulatory T cells play an important role in maintaining the homeostasis of the body’s immune system by inhibiting the activation of other immune cells. In the case of chronic viral infections, regulatory T cells are known to contribute to the duration of the infection, weakening the immune response to virus infections. However, there has been no information on what roles the regulatory T cells perform in the case of acute viral infections.
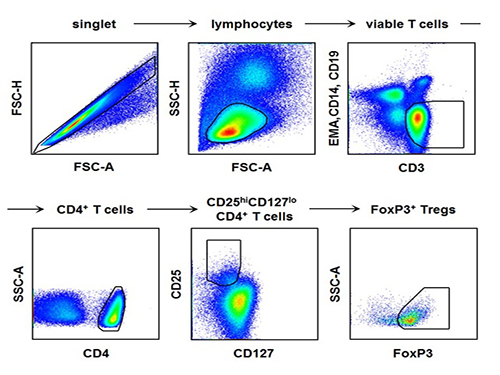
The research team used the fluorescence flow cytometry technique to determine the number and characteristics of a variety of immune cells, including regulatory T cells, in the blood of hepatitis A patients.
Consequently, the researchers confirmed that the decrease in the regulatory T cells immune inhibitory ability was consistent with a significant reduction in the number of regulatory T cells in the blood of hepatitis A patients. Furthermore, it was identified that the more noticeable decrease of regulatory T cells led to the occurrence of a more severe liver injury.
The analysis of hepatitis A patient’s blood proved that the cause of the decrease in the number and function of regulatory T cells was the increased expression of cell surface protein ‘Fas,’ which induces cell death.
Professor Shin said, “This study is the first case which proposes the mechanism for clinical aspects in not only hepatitis A, but also acute virus infection.” He added on the future prospect of the research that: “In the future, we can prevent tissue damage by inhibiting cell death of regulatory T cells for severe acute viral infections that do not have an effective treatment for the virus itself.”
[Picture]
The picture shows the process of fluorescence flow cytometry technique to study regulatory T cell in the blood of hepatitis A patients.
2014.08.11 View 11750
Regulatory T Cells Influence Liver Damage of Hepatitis A Patients
Liver damage becomes more severe with the decrease of regulatory T cells
“This research will aid the development of hepatitis A targeted drug,” said a KAIST researcher.
The KAIST Graduate School of Medical Science and Engineering’s Professor Eui-Cheol Shin and his research team have identified the mechanism, explaining how the regulatory T cells are responsible for the body’s immune system and how they have induced liver damage of hepatitis A patients.
The research results were published online in the July 9th edition of ‘Gut,’ the world’s most prominent journal in the field of gastroenterology.
Hepatitis A is an acute form of hepatitis caused by hepatitis A virus. The virus spreads through oral contact and enters the body via digestive organs.
Regulatory T cells play an important role in maintaining the homeostasis of the body’s immune system by inhibiting the activation of other immune cells. In the case of chronic viral infections, regulatory T cells are known to contribute to the duration of the infection, weakening the immune response to virus infections. However, there has been no information on what roles the regulatory T cells perform in the case of acute viral infections.
The research team used the fluorescence flow cytometry technique to determine the number and characteristics of a variety of immune cells, including regulatory T cells, in the blood of hepatitis A patients.
Consequently, the researchers confirmed that the decrease in the regulatory T cells immune inhibitory ability was consistent with a significant reduction in the number of regulatory T cells in the blood of hepatitis A patients. Furthermore, it was identified that the more noticeable decrease of regulatory T cells led to the occurrence of a more severe liver injury.
The analysis of hepatitis A patient’s blood proved that the cause of the decrease in the number and function of regulatory T cells was the increased expression of cell surface protein ‘Fas,’ which induces cell death.
Professor Shin said, “This study is the first case which proposes the mechanism for clinical aspects in not only hepatitis A, but also acute virus infection.” He added on the future prospect of the research that: “In the future, we can prevent tissue damage by inhibiting cell death of regulatory T cells for severe acute viral infections that do not have an effective treatment for the virus itself.”
[Picture]
The picture shows the process of fluorescence flow cytometry technique to study regulatory T cell in the blood of hepatitis A patients.
2014.08.11 View 11750