Blood
-
 AI-Driven Wearable Blood Pressure Sensor for Continuous Health Monitoring – Published in Nature Reviews Cardiology
A KAIST research team led by Professor Keon Jae Lee has proposed an innovative theoretical framework and research strategies for AI-based wearable blood pressure sensors, paving the way for continuous and non-invasive cardiovascular monitoring.
Hypertension is a leading chronic disease affecting over a billion people worldwide and is a major risk factor for severe cardiovascular conditions such as myocardial infarction, stroke, and heart failure. Traditional blood pressure measurement relies on intermittent, cuff-based methods, which fail to capture real-time fluctuations and present challenges in continuous patient monitoring.
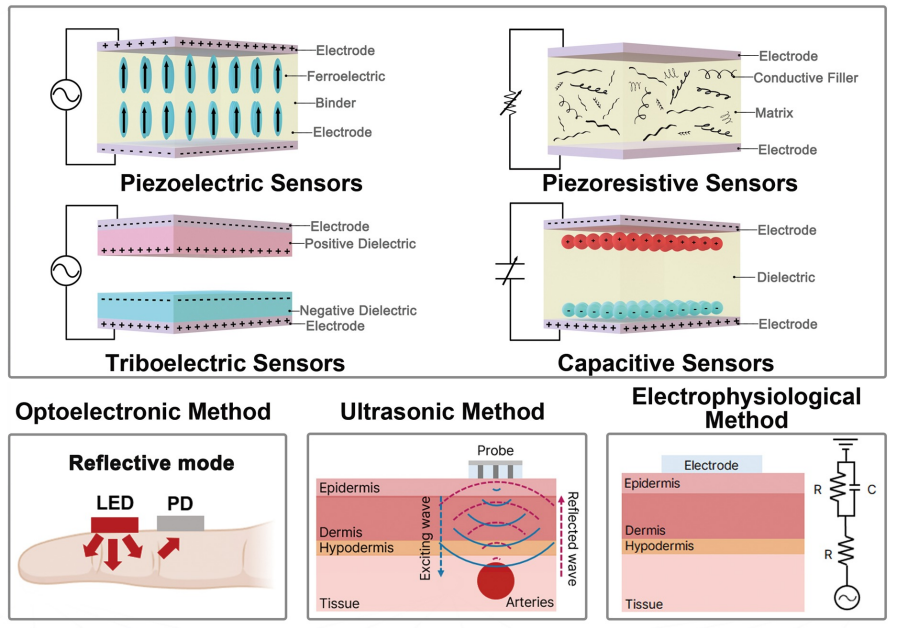
Wearable blood pressure sensors offer a non-invasive solution for continuous blood pressure monitoring, enabling real-time tracking and personalized cardiovascular health management. However, current technologies lack the accuracy and reliability required for medical applications, limiting their practical use. To address these challenges, advancements in high-sensitivity sensor technology and AI signal processing algorithms are essential.
Building on their previous study in Advanced Materials (doi.org/10.1002/adma.202301627), which validated the clinical feasibility of flexible piezoelectric blood pressure sensors, Professor Lee’s team conducted an in-depth review of the latest advancements in cuffless wearable sensors, focusing on key technical and clinical challenges. Their review highlights clinical aspects of clinical implementation, real-time data transmission, signal quality degradation, and AI algorithm accuracy.
Professor Keon Jae Lee said, “This paper systematically demonstrates the feasibility of medical-grade wearable blood pressure sensors, overcoming what was previously considered an insurmountable challenge. We propose theoretical strategies to address technical barriers, opening new possibilities for future innovations in this field. With continued advancements, we expect these sensors to gain trust and be commercialized soon, significantly improving quality of life.”
This review entitled “Wearable blood pressure sensors for cardiovascular monitoring and machine learning algorithms for blood pressure estimation” was published in the February 18 issue of Nature Reviews Cardiology (Impact Factor: 41.7). (doi.org/10.1038/s41569-025-01127-0)
< Figure 1. Overview of wearable blood pressure sensor technologies for cardiovascular health care >
[Reference] Min S. et al., (2025) “Wearable blood pressure sensors for
cardiovascular monitoring and machine learning algorithms for blood pressure estimation.” Nature Reviews Cardiology
(doi.org/10.1038/s41569-025-01127-0)
[Main Author] Seongwook Min (Korea Advanced Institute of Science and Technology), Jaehun An (Korea Advanced Institute of Science and Technology), Jae Hee Lee (Northwestern University),
* Contact email : Professor Keon Jae Lee (keonlee@kaist.ac.kr)
2025.03.04 View 5320
AI-Driven Wearable Blood Pressure Sensor for Continuous Health Monitoring – Published in Nature Reviews Cardiology
A KAIST research team led by Professor Keon Jae Lee has proposed an innovative theoretical framework and research strategies for AI-based wearable blood pressure sensors, paving the way for continuous and non-invasive cardiovascular monitoring.
Hypertension is a leading chronic disease affecting over a billion people worldwide and is a major risk factor for severe cardiovascular conditions such as myocardial infarction, stroke, and heart failure. Traditional blood pressure measurement relies on intermittent, cuff-based methods, which fail to capture real-time fluctuations and present challenges in continuous patient monitoring.
Wearable blood pressure sensors offer a non-invasive solution for continuous blood pressure monitoring, enabling real-time tracking and personalized cardiovascular health management. However, current technologies lack the accuracy and reliability required for medical applications, limiting their practical use. To address these challenges, advancements in high-sensitivity sensor technology and AI signal processing algorithms are essential.
Building on their previous study in Advanced Materials (doi.org/10.1002/adma.202301627), which validated the clinical feasibility of flexible piezoelectric blood pressure sensors, Professor Lee’s team conducted an in-depth review of the latest advancements in cuffless wearable sensors, focusing on key technical and clinical challenges. Their review highlights clinical aspects of clinical implementation, real-time data transmission, signal quality degradation, and AI algorithm accuracy.
Professor Keon Jae Lee said, “This paper systematically demonstrates the feasibility of medical-grade wearable blood pressure sensors, overcoming what was previously considered an insurmountable challenge. We propose theoretical strategies to address technical barriers, opening new possibilities for future innovations in this field. With continued advancements, we expect these sensors to gain trust and be commercialized soon, significantly improving quality of life.”
This review entitled “Wearable blood pressure sensors for cardiovascular monitoring and machine learning algorithms for blood pressure estimation” was published in the February 18 issue of Nature Reviews Cardiology (Impact Factor: 41.7). (doi.org/10.1038/s41569-025-01127-0)
< Figure 1. Overview of wearable blood pressure sensor technologies for cardiovascular health care >
[Reference] Min S. et al., (2025) “Wearable blood pressure sensors for
cardiovascular monitoring and machine learning algorithms for blood pressure estimation.” Nature Reviews Cardiology
(doi.org/10.1038/s41569-025-01127-0)
[Main Author] Seongwook Min (Korea Advanced Institute of Science and Technology), Jaehun An (Korea Advanced Institute of Science and Technology), Jae Hee Lee (Northwestern University),
* Contact email : Professor Keon Jae Lee (keonlee@kaist.ac.kr)
2025.03.04 View 5320 -
 KAIST Team Develops Highly-Sensitive Wearable Piezoelectric Blood Pressure Sensor for Continuous Health Monitoring
- A collaborative research team led by KAIST Professor Keon Jae Lee verifies the accuracy of the highly-sensitive sensor through clinical trials
- Commercialization of the watch and patch-type sensor is in progress
A KAIST research team led by Professor Keon Jae Lee from the Department of Materials Science and Engineering and the College of Medicine of the Catholic University of Korea has developed a highly sensitive, wearable piezoelectric blood pressure sensor.
Blood pressure is a critical indicator for assessing general health and predicting stroke or heart failure. In particular, cardiovascular disease is the leading cause of global death, therefore, periodic measurement of blood pressure is crucial for personal healthcare.
Recently, there has been a growing interest in healthcare devices for continuous blood pressure monitoring. Although smart watches using LED-based photoplethysmography (PPG) technology have been on market, these devices have been limited by the accuracy constraints of optical sensors, making it hard to meet the international standards of automatic sphygmomanometers.
Professor Lee’s team has developed the wearable piezoelectric blood pressure sensor by transferring a highly sensitive, inorganic piezoelectric membrane from bulk sapphire substrates to flexible substrates. Ultrathin piezoelectric sensors with a thickness of several micrometers (one hundredth of the human hair) exhibit conformal contact with the skin to successfully collect accurate blood pressure from the subtle pulsation of the blood vessels.
Clinical trial at the St. Mary’s Hospital of the Catholic University validated the accuracy of blood pressure sensor at par with international standard with errors within ±5 mmHg and a standard deviation under 8 mmHg for both systolic and diastolic blood pressure. In addition, the research team successfully embedded the sensor on a watch-type product to enable continuous monitoring of blood pressure.
Prof. Keon Jae Lee said, “Major target of our healthcare devices is hypertensive patients for their daily medical check-up. We plan to develop a comfortable patch-type sensor to monitor blood pressure during sleep and have a start-up company commercialize these watch and patch-type products soon.”
This result titled “Clinical validation of wearable piezoelectric blood pressure sensor for health monitoring” was published in the online issue of Advanced Materials on March 24th, 2023. (DOI: 10.1002/adma.202301627)
Figure 1. Schematic illustration of the overall concept for a wearable piezoelectric blood pressure sensor (WPBPS).
Figure 2. Wearable piezoelectric blood pressure sensor (WPBPS) mounted on a watch (a) Schematic design of the WPBPS-embedded wristwatch. (b) Block diagram of the wireless communication circuit, which filters, amplifies, and transmits wireless data to portable devices. (c) Pulse waveforms transmitted from the wristwatch to the portable device by the wireless communication circuit. The inset shows a photograph of monitoring a user’s beat-to-beat pulses and their corresponding BP values in real time using the developed WPBPS-mounted wristwatch.
2023.04.17 View 11050
KAIST Team Develops Highly-Sensitive Wearable Piezoelectric Blood Pressure Sensor for Continuous Health Monitoring
- A collaborative research team led by KAIST Professor Keon Jae Lee verifies the accuracy of the highly-sensitive sensor through clinical trials
- Commercialization of the watch and patch-type sensor is in progress
A KAIST research team led by Professor Keon Jae Lee from the Department of Materials Science and Engineering and the College of Medicine of the Catholic University of Korea has developed a highly sensitive, wearable piezoelectric blood pressure sensor.
Blood pressure is a critical indicator for assessing general health and predicting stroke or heart failure. In particular, cardiovascular disease is the leading cause of global death, therefore, periodic measurement of blood pressure is crucial for personal healthcare.
Recently, there has been a growing interest in healthcare devices for continuous blood pressure monitoring. Although smart watches using LED-based photoplethysmography (PPG) technology have been on market, these devices have been limited by the accuracy constraints of optical sensors, making it hard to meet the international standards of automatic sphygmomanometers.
Professor Lee’s team has developed the wearable piezoelectric blood pressure sensor by transferring a highly sensitive, inorganic piezoelectric membrane from bulk sapphire substrates to flexible substrates. Ultrathin piezoelectric sensors with a thickness of several micrometers (one hundredth of the human hair) exhibit conformal contact with the skin to successfully collect accurate blood pressure from the subtle pulsation of the blood vessels.
Clinical trial at the St. Mary’s Hospital of the Catholic University validated the accuracy of blood pressure sensor at par with international standard with errors within ±5 mmHg and a standard deviation under 8 mmHg for both systolic and diastolic blood pressure. In addition, the research team successfully embedded the sensor on a watch-type product to enable continuous monitoring of blood pressure.
Prof. Keon Jae Lee said, “Major target of our healthcare devices is hypertensive patients for their daily medical check-up. We plan to develop a comfortable patch-type sensor to monitor blood pressure during sleep and have a start-up company commercialize these watch and patch-type products soon.”
This result titled “Clinical validation of wearable piezoelectric blood pressure sensor for health monitoring” was published in the online issue of Advanced Materials on March 24th, 2023. (DOI: 10.1002/adma.202301627)
Figure 1. Schematic illustration of the overall concept for a wearable piezoelectric blood pressure sensor (WPBPS).
Figure 2. Wearable piezoelectric blood pressure sensor (WPBPS) mounted on a watch (a) Schematic design of the WPBPS-embedded wristwatch. (b) Block diagram of the wireless communication circuit, which filters, amplifies, and transmits wireless data to portable devices. (c) Pulse waveforms transmitted from the wristwatch to the portable device by the wireless communication circuit. The inset shows a photograph of monitoring a user’s beat-to-beat pulses and their corresponding BP values in real time using the developed WPBPS-mounted wristwatch.
2023.04.17 View 11050 -
 Identification of How Chemotherapy Drug Works Could Deliver Personalized Cancer Treatment
The chemotherapy drug decitabine is commonly used to treat patients with blood cancers, but its response rate is somewhat low. Researchers have now identified why this is the case, opening the door to more personalized cancer therapies for those with these types of cancers, and perhaps further afield.
Researchers have identified the genetic and molecular mechanisms within cells that make the chemotherapy drug decitabine—used to treat patients with myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) —work for some patients but not others. The findings should assist clinicians in developing more patient-specific treatment strategies.
The findings were published in the Proceedings of the National Academies of Science on March 30.
The chemotherapy drug decitabine, also known by its brand name Dacogen, works by modifying our DNA that in turn switches on genes that stop the cancer cells from growing and replicating. However, decitabine’s response rate is somewhat low (showing improvement in just 30-35% of patients), which leaves something of a mystery as to why it works well for some patients but not for others. To find out why this happens, researchers from the KAIST investigated the molecular mediators that are involved with regulating the effects of the drug.
Decitabine works to activate the production of endogenous retroviruses (ERVs), which in turn induces an immune response. ERVs are viruses that long ago inserted dormant copies of themselves into the human genome. Decitabine in essence, ‘reactivates’ these viral elements and produces double-stranded RNAs (dsRNAs) that the immune system views as a foreign body.
“However, the mechanisms involved in this process, in particular how production and transport of these ERV dsRNAs were regulated within the cell were understudied,” said corresponding author Yoosik Kim, professor in the Department of Chemical and Biomolecular Engineering at KAIST.
“So to explain why decitabine works in some patients but not others, we investigated what these molecular mechanisms were,” added Kim.
To do so, the researchers used image-based RNA interference (RNAi) screening. This is a relatively new technique in which specific sequences within a genome are knocked out of action or “downregulated.” Large-scale screening, which can be performed in cultured cells or within live organisms, works to investigate the function of different genes. The KAIST researchers collaborated with the Institut Pasteur Korea to analyze the effect of downregulating genes that recognize ERV dsRNAs and could be involved in the cellular response to decitabine.
From these initial screening results, they performed an even more detailed downregulation screening analysis. Through the screening, they were able to identify two particular gene sequences involved in the production of an RNA-binding protein called Staufen1 and the production of a strand of RNA that does not in turn produce any proteins called TINCR that play a key regulatory role in response to the drug. Staufen1 binds directly to dsRNAs and stabilizes them in concert with the TINCR.
If a patient is not producing sufficient Staufen1 and TINCR, then the dsRNA viral mimics quickly degrade before the immune system can spot them. And, crucially for cancer therapy, this means that patients with lower expression (activation) of these sequences will show inferior response to decitabine. Indeed, the researchers confirmed that MDS/AML patients with low Staufen1 and TINCR expression did not benefit from decitabine therapy.
“We can now isolate patients who will not benefit from the therapy and direct them to a different type of therapy,” said first author Yongsuk Ku. “This serves as an important step toward developing a patient-specific treatment cancer strategy.”
As the researchers used patient samples taken from bone marrow, the next step will be to try to develop a testing method that can identify the problem from just blood samples, which are much easier to acquire from patients.
The team plans to investigate if the analysis can be extended to patients with solid tumors in addition to those with blood cancers.
-Profile
Professor Yoosik Kim
https://qcbio.kaist.ac.kr/
Department of Chemical and Biomolecular Engineering
KAIST
-Publication
Noncanonical immune response to the inhibition of DNA methylation by Staufen1 via stabilization of endogenous retrovirus RNAs, PNAS
2021.05.24 View 12822
Identification of How Chemotherapy Drug Works Could Deliver Personalized Cancer Treatment
The chemotherapy drug decitabine is commonly used to treat patients with blood cancers, but its response rate is somewhat low. Researchers have now identified why this is the case, opening the door to more personalized cancer therapies for those with these types of cancers, and perhaps further afield.
Researchers have identified the genetic and molecular mechanisms within cells that make the chemotherapy drug decitabine—used to treat patients with myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) —work for some patients but not others. The findings should assist clinicians in developing more patient-specific treatment strategies.
The findings were published in the Proceedings of the National Academies of Science on March 30.
The chemotherapy drug decitabine, also known by its brand name Dacogen, works by modifying our DNA that in turn switches on genes that stop the cancer cells from growing and replicating. However, decitabine’s response rate is somewhat low (showing improvement in just 30-35% of patients), which leaves something of a mystery as to why it works well for some patients but not for others. To find out why this happens, researchers from the KAIST investigated the molecular mediators that are involved with regulating the effects of the drug.
Decitabine works to activate the production of endogenous retroviruses (ERVs), which in turn induces an immune response. ERVs are viruses that long ago inserted dormant copies of themselves into the human genome. Decitabine in essence, ‘reactivates’ these viral elements and produces double-stranded RNAs (dsRNAs) that the immune system views as a foreign body.
“However, the mechanisms involved in this process, in particular how production and transport of these ERV dsRNAs were regulated within the cell were understudied,” said corresponding author Yoosik Kim, professor in the Department of Chemical and Biomolecular Engineering at KAIST.
“So to explain why decitabine works in some patients but not others, we investigated what these molecular mechanisms were,” added Kim.
To do so, the researchers used image-based RNA interference (RNAi) screening. This is a relatively new technique in which specific sequences within a genome are knocked out of action or “downregulated.” Large-scale screening, which can be performed in cultured cells or within live organisms, works to investigate the function of different genes. The KAIST researchers collaborated with the Institut Pasteur Korea to analyze the effect of downregulating genes that recognize ERV dsRNAs and could be involved in the cellular response to decitabine.
From these initial screening results, they performed an even more detailed downregulation screening analysis. Through the screening, they were able to identify two particular gene sequences involved in the production of an RNA-binding protein called Staufen1 and the production of a strand of RNA that does not in turn produce any proteins called TINCR that play a key regulatory role in response to the drug. Staufen1 binds directly to dsRNAs and stabilizes them in concert with the TINCR.
If a patient is not producing sufficient Staufen1 and TINCR, then the dsRNA viral mimics quickly degrade before the immune system can spot them. And, crucially for cancer therapy, this means that patients with lower expression (activation) of these sequences will show inferior response to decitabine. Indeed, the researchers confirmed that MDS/AML patients with low Staufen1 and TINCR expression did not benefit from decitabine therapy.
“We can now isolate patients who will not benefit from the therapy and direct them to a different type of therapy,” said first author Yongsuk Ku. “This serves as an important step toward developing a patient-specific treatment cancer strategy.”
As the researchers used patient samples taken from bone marrow, the next step will be to try to develop a testing method that can identify the problem from just blood samples, which are much easier to acquire from patients.
The team plans to investigate if the analysis can be extended to patients with solid tumors in addition to those with blood cancers.
-Profile
Professor Yoosik Kim
https://qcbio.kaist.ac.kr/
Department of Chemical and Biomolecular Engineering
KAIST
-Publication
Noncanonical immune response to the inhibition of DNA methylation by Staufen1 via stabilization of endogenous retrovirus RNAs, PNAS
2021.05.24 View 12822 -
 Professor Ko Kyu Young Appointed as a Distinguished Professor at KAIST
Professor Ko Kyu Young of the Graduate School of Medical Sciences was appointed as the Distinguished Professor at KAIST.
Professor Ko is famous internationally for his work on the catalyst for blood vessel growth COMP-ANG1, and also for his research on blood vessel growth and lymph duct growth control.
Professor Ko developed the Double Anti-Angiogenic Protein (DAAP) which effectively restricts the blood vessels from growing, opening a new approach to curing caner. The paper was published in ‘Cancer Cell’ as the cover paper (2010 August 17th edition) and is widely recognized as the marker that sums up the new paradigm of cure for cancer.
In addition, his work on explaining how the new antigen interacts with the T-lymphocyte during a vaccination lead to the possibility of the increase of the efficiency of vaccination. The result of the research was published as the cover paper in ‘Immunity’ magazine.
As is obvious to see his work with blood vessel growth and lymph duct growth and control is being published in major scientific journals. In addition he is continuously invited to international conferences as guest speakers and leader, effectively leading the field. As a result, he was appointed as the editor of ‘Blood’ magazine, the world’s best journal in the field of hematology and received ‘2010 KAISTian of the Year’ Award.
The title Distinguished Professor is appointed to those who have made world-class research results and educational results and actively lead their respective field. They are provided with extra incentives and can even continue on with the professorship after retirement.
It is only limited to 3% of the professors at KAIST and has to be someone recommended by the President, Vice-President, and the Deans of department and their worthiness is scrutinized by a foreign expert.
2011.03.25 View 13638
Professor Ko Kyu Young Appointed as a Distinguished Professor at KAIST
Professor Ko Kyu Young of the Graduate School of Medical Sciences was appointed as the Distinguished Professor at KAIST.
Professor Ko is famous internationally for his work on the catalyst for blood vessel growth COMP-ANG1, and also for his research on blood vessel growth and lymph duct growth control.
Professor Ko developed the Double Anti-Angiogenic Protein (DAAP) which effectively restricts the blood vessels from growing, opening a new approach to curing caner. The paper was published in ‘Cancer Cell’ as the cover paper (2010 August 17th edition) and is widely recognized as the marker that sums up the new paradigm of cure for cancer.
In addition, his work on explaining how the new antigen interacts with the T-lymphocyte during a vaccination lead to the possibility of the increase of the efficiency of vaccination. The result of the research was published as the cover paper in ‘Immunity’ magazine.
As is obvious to see his work with blood vessel growth and lymph duct growth and control is being published in major scientific journals. In addition he is continuously invited to international conferences as guest speakers and leader, effectively leading the field. As a result, he was appointed as the editor of ‘Blood’ magazine, the world’s best journal in the field of hematology and received ‘2010 KAISTian of the Year’ Award.
The title Distinguished Professor is appointed to those who have made world-class research results and educational results and actively lead their respective field. They are provided with extra incentives and can even continue on with the professorship after retirement.
It is only limited to 3% of the professors at KAIST and has to be someone recommended by the President, Vice-President, and the Deans of department and their worthiness is scrutinized by a foreign expert.
2011.03.25 View 13638 -
 Success in differentiating Functional Vascular Progenitor Cells (VPC)
KAIST’s Professor Han Yong Man successfully differentiated vascular progenitor cells from human embryonic stem cells and reversed differentiated stem cells.
The research went beyond the current method of synthesis of embryonic body or mice cell ball culture and used the careful alteration of signal transmission system of the human embryonic stem cells to differentiate the formation of vascular progenitor cells.
The team controlled the MEK/ERK and BMP signal transmission system that serves an important role in the self replication of human embryonic stem cells and successfully differentiated 20% of the cells experimented on to vascular progenitor cells.
The vascular progenitor cells produced with such a method successfully differentiated into cells forming the endodermis of the blood vessel, vascular smooth muscle cells and hematopoietic cells in an environment outside of the human body and also successfully differentiated into blood vessels in nude mice.
In addition, the vascular progenitor cell derived from human embryonic cells successfully formed blood vessels or secreted vascular growth factors and increased the blood flow and the necrosis of blood vessels when injected into an animal with limb ischemic illness.
The research was funded by the Ministry of Education, Science and Technology, 21st Century Frontier Research and Development Institution’s Cell Application Research Department and Professor Ko Kyu Young (KAIST), Professor Choi Chul Hee (KAIST), Professor Jeong Hyung Min (Cha Medical School) and Doctor Jo Lee Sook (Researcher in Korea Bio Engineering Institute) participated in it.
The results of the research was published as the cover paper of the September edition of “Blood (IF:10.55)”, the American Blood Journal and has been patented domestically and has finished registration of foreign PCT.
The results of the experiment opened the possibility of providing a patient specific cure using stem cells in the field of blood vessel illness.
2011.01.18 View 17829
Success in differentiating Functional Vascular Progenitor Cells (VPC)
KAIST’s Professor Han Yong Man successfully differentiated vascular progenitor cells from human embryonic stem cells and reversed differentiated stem cells.
The research went beyond the current method of synthesis of embryonic body or mice cell ball culture and used the careful alteration of signal transmission system of the human embryonic stem cells to differentiate the formation of vascular progenitor cells.
The team controlled the MEK/ERK and BMP signal transmission system that serves an important role in the self replication of human embryonic stem cells and successfully differentiated 20% of the cells experimented on to vascular progenitor cells.
The vascular progenitor cells produced with such a method successfully differentiated into cells forming the endodermis of the blood vessel, vascular smooth muscle cells and hematopoietic cells in an environment outside of the human body and also successfully differentiated into blood vessels in nude mice.
In addition, the vascular progenitor cell derived from human embryonic cells successfully formed blood vessels or secreted vascular growth factors and increased the blood flow and the necrosis of blood vessels when injected into an animal with limb ischemic illness.
The research was funded by the Ministry of Education, Science and Technology, 21st Century Frontier Research and Development Institution’s Cell Application Research Department and Professor Ko Kyu Young (KAIST), Professor Choi Chul Hee (KAIST), Professor Jeong Hyung Min (Cha Medical School) and Doctor Jo Lee Sook (Researcher in Korea Bio Engineering Institute) participated in it.
The results of the research was published as the cover paper of the September edition of “Blood (IF:10.55)”, the American Blood Journal and has been patented domestically and has finished registration of foreign PCT.
The results of the experiment opened the possibility of providing a patient specific cure using stem cells in the field of blood vessel illness.
2011.01.18 View 17829 -
 KAIST Research Team Identified Promising New Source to Obtain Stem Cells
KAIST Research Team Identified Promising New Source to Obtain Stem Cells
A research team at KAIST led by Professor Gou-Young Koh, M.D. and Ph.D., of the Department of Biological Sciences, has found evidence that fat tissue, known as adipose tissue, may be a promising new source of valuable and easy-to-obtain regenerative cells called hematopoietic stem and progenitor cells (HSPCs).
HSPCs are adult stem cells that have the ability to generate and develop into many different kinds of cells. They are now used to repair damaged tissues and are being studied for their potential to treat a vast array of chronic and degenerative conditions such as leukemia. Mostly found in bone marrow but with a limited quantity, HSPCs are hard to cultivate in vitro, thus becoming an obstacle to use them for research and therapeutic purposes.
Within the adipose tissue is a special cell population known as the stromal vascular fraction (SVF), which share similar properties to those in the bone marrow. Cells in the bone marrow and SVF have the ability to differentiate into several cell types. In addition, both adipose and bone marrow offer similar environments for optimal stem cell growth and reproduction.
Given the fact that adipose and bone marrow tissues share similar properties, Dr. Koh and his team conducted a research, injecting granulocyte colony-stimulating factor (G-CSF), a growth hormone used to encourage the development of stem cells, into an adipose tissue of a mouse whose bone marrow is damaged. As a result, the team has found that the SVF derived from adipose tissue contains functional HSPCs capable of generating hematopoietic (blood-forming) cells to repair the damaged bone morrow.
The Ministry of Education, Science and Technology nominated the KAIST research as one of its sponsoring 21st Century Frontier R&D Programs. Director Dong-Wook Kim of Stem Cell Research Center (SCRS) that oversees the KAIST team expressed a possibility to use the adipose tissue as an alternative source to obtain stem cells for regeneration medicine.
Dr. Koh also said, “It’s been a well known method to extract HSPCs from the bone morrow or blood, but it’s the first time to identify adipose tissue, before considered useless, as a new possible supplier for functional and transplantable HSPCs.”
The study results have received an important recognition from the academia—the American Society of Hematology published the research as a main article in its official journal, Blood, for the February 4th, 2010 issue, which is the most citied peer-reviewed publication in the field.
2010.02.05 View 15074
KAIST Research Team Identified Promising New Source to Obtain Stem Cells
KAIST Research Team Identified Promising New Source to Obtain Stem Cells
A research team at KAIST led by Professor Gou-Young Koh, M.D. and Ph.D., of the Department of Biological Sciences, has found evidence that fat tissue, known as adipose tissue, may be a promising new source of valuable and easy-to-obtain regenerative cells called hematopoietic stem and progenitor cells (HSPCs).
HSPCs are adult stem cells that have the ability to generate and develop into many different kinds of cells. They are now used to repair damaged tissues and are being studied for their potential to treat a vast array of chronic and degenerative conditions such as leukemia. Mostly found in bone marrow but with a limited quantity, HSPCs are hard to cultivate in vitro, thus becoming an obstacle to use them for research and therapeutic purposes.
Within the adipose tissue is a special cell population known as the stromal vascular fraction (SVF), which share similar properties to those in the bone marrow. Cells in the bone marrow and SVF have the ability to differentiate into several cell types. In addition, both adipose and bone marrow offer similar environments for optimal stem cell growth and reproduction.
Given the fact that adipose and bone marrow tissues share similar properties, Dr. Koh and his team conducted a research, injecting granulocyte colony-stimulating factor (G-CSF), a growth hormone used to encourage the development of stem cells, into an adipose tissue of a mouse whose bone marrow is damaged. As a result, the team has found that the SVF derived from adipose tissue contains functional HSPCs capable of generating hematopoietic (blood-forming) cells to repair the damaged bone morrow.
The Ministry of Education, Science and Technology nominated the KAIST research as one of its sponsoring 21st Century Frontier R&D Programs. Director Dong-Wook Kim of Stem Cell Research Center (SCRS) that oversees the KAIST team expressed a possibility to use the adipose tissue as an alternative source to obtain stem cells for regeneration medicine.
Dr. Koh also said, “It’s been a well known method to extract HSPCs from the bone morrow or blood, but it’s the first time to identify adipose tissue, before considered useless, as a new possible supplier for functional and transplantable HSPCs.”
The study results have received an important recognition from the academia—the American Society of Hematology published the research as a main article in its official journal, Blood, for the February 4th, 2010 issue, which is the most citied peer-reviewed publication in the field.
2010.02.05 View 15074