bio
-
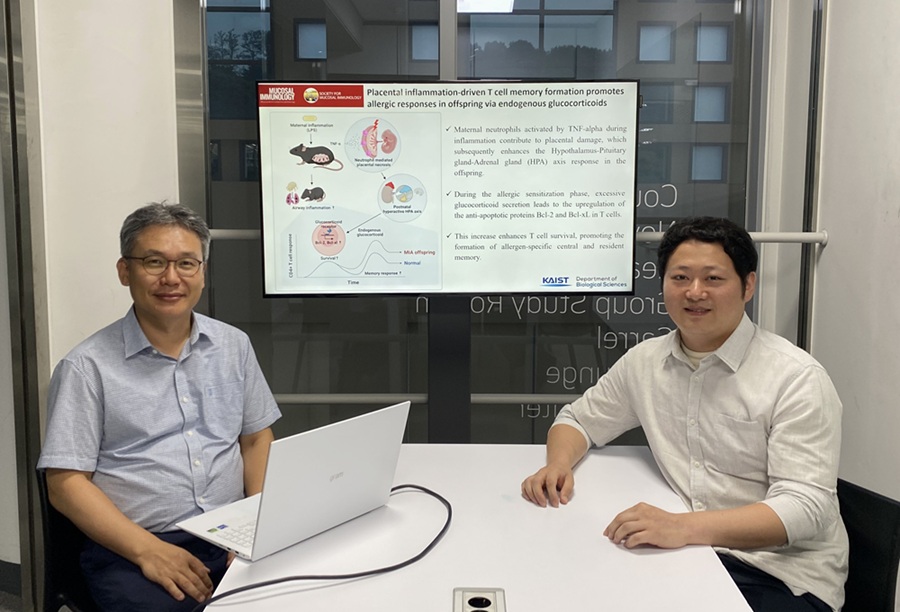 KAIST Reveals Placental Inflammation as the Cause of Allergies such as Pediatric Asthma
<(From left)Professor Heung-kyu Lee from the Department of Biological Sciences, Dr.Myeong Seung Kwon from the Graduate School of Medical Science>
It is already well-known that when a mother experiences inflammation during pregnancy, her child is more likely to develop allergic diseases. Recently, a KAIST research team became the first in the world to discover that inflammation within the placenta affects the fetus's immune system, leading to the child exhibiting excessive allergic reactions after birth. This study presents a new possibility for the early prediction and prevention of allergic diseases such as pediatric asthma.
KAIST (President Kwang Hyung Lee) announced on the 4th of August that a research team led by Professor Heung-kyu Lee from the Department of Biological Sciences found that inflammation occurring during pregnancy affects the fetus's stress response regulation system through the placenta. As a result, the survival and memory differentiation of T cells (key cells in the adaptive immune system) increase, which can lead to stronger allergic reactions in the child after birth.
The research team proved this through experiments on mice that had excessive inflammation induced during pregnancy. First, they injected the toxin component 'LPS (lipopolysaccharide),' a substance known to be a representative material that induces an inflammatory response in the immune system, into the mice to cause an inflammatory response in their bodies, which also caused inflammation in the placenta.
It was confirmed that the placental tissue, due to the inflammatory response, increased a signaling substance called 'Tumor Necrosis Factor-alpha (TNF-α),' and this substance activated immune cells called 'neutrophils*', causing inflammatory damage to the placenta. *Neutrophils: The most abundant type of white blood cells in our bodies (40-75%), playing an important role in innate immunity and killing invading bacteria and fungi.
This damage modulated postnatal offspring stress response, leading to a large secretion of stress hormone (glucocorticoid). As a result, the offspring's T cells, which are responsible for immune memory, survived longer and had stronger memory functions.
In particular, the memory T cells created through this process caused excessive allergic reactions when repeatedly exposed to antigens after birth. Specifically, when house dust mite 'allergens' were exposed to the airways of mice, a strong eosinophilic inflammatory response and excessive immune activation were observed, with an increase in immune cells important for allergy and asthma reactions.
Professor Heung Kyu Lee stated, "This study is the first in the world to identify how a mother's inflammatory response during pregnancy affects the fetus's allergic immune system through the placenta." He added, "This will be an important scientific basis for developing biomarkers for early prediction and establishing prevention strategies for pediatric allergic diseases."
The first author of this study is Dr. Myeong Seung Kwon from the KAIST Graduate School of Medical Science (currently a clinical fellow of gynecological oncology at Konyang University Hospital's Department of Obstetrics and Gynecology), and the research results were published in the authoritative journal in the field of mucosal immunology, 'Mucosal Immunology,' on July 1st. ※ Paper Title: Placental inflammation-driven T cell memory formation promotes allergic responses in offspring via endogenous glucocorticoids ※ DOI: https://doi.org/10.1016/j.mucimm.2025.06.006
This research was conducted as part of the Basic Science Research Program and the Bio-Medical Technology Development Program supported by the Ministry of Science and ICT and the National Research Foundation of Korea.
2025.08.03 View 187
KAIST Reveals Placental Inflammation as the Cause of Allergies such as Pediatric Asthma
<(From left)Professor Heung-kyu Lee from the Department of Biological Sciences, Dr.Myeong Seung Kwon from the Graduate School of Medical Science>
It is already well-known that when a mother experiences inflammation during pregnancy, her child is more likely to develop allergic diseases. Recently, a KAIST research team became the first in the world to discover that inflammation within the placenta affects the fetus's immune system, leading to the child exhibiting excessive allergic reactions after birth. This study presents a new possibility for the early prediction and prevention of allergic diseases such as pediatric asthma.
KAIST (President Kwang Hyung Lee) announced on the 4th of August that a research team led by Professor Heung-kyu Lee from the Department of Biological Sciences found that inflammation occurring during pregnancy affects the fetus's stress response regulation system through the placenta. As a result, the survival and memory differentiation of T cells (key cells in the adaptive immune system) increase, which can lead to stronger allergic reactions in the child after birth.
The research team proved this through experiments on mice that had excessive inflammation induced during pregnancy. First, they injected the toxin component 'LPS (lipopolysaccharide),' a substance known to be a representative material that induces an inflammatory response in the immune system, into the mice to cause an inflammatory response in their bodies, which also caused inflammation in the placenta.
It was confirmed that the placental tissue, due to the inflammatory response, increased a signaling substance called 'Tumor Necrosis Factor-alpha (TNF-α),' and this substance activated immune cells called 'neutrophils*', causing inflammatory damage to the placenta. *Neutrophils: The most abundant type of white blood cells in our bodies (40-75%), playing an important role in innate immunity and killing invading bacteria and fungi.
This damage modulated postnatal offspring stress response, leading to a large secretion of stress hormone (glucocorticoid). As a result, the offspring's T cells, which are responsible for immune memory, survived longer and had stronger memory functions.
In particular, the memory T cells created through this process caused excessive allergic reactions when repeatedly exposed to antigens after birth. Specifically, when house dust mite 'allergens' were exposed to the airways of mice, a strong eosinophilic inflammatory response and excessive immune activation were observed, with an increase in immune cells important for allergy and asthma reactions.
Professor Heung Kyu Lee stated, "This study is the first in the world to identify how a mother's inflammatory response during pregnancy affects the fetus's allergic immune system through the placenta." He added, "This will be an important scientific basis for developing biomarkers for early prediction and establishing prevention strategies for pediatric allergic diseases."
The first author of this study is Dr. Myeong Seung Kwon from the KAIST Graduate School of Medical Science (currently a clinical fellow of gynecological oncology at Konyang University Hospital's Department of Obstetrics and Gynecology), and the research results were published in the authoritative journal in the field of mucosal immunology, 'Mucosal Immunology,' on July 1st. ※ Paper Title: Placental inflammation-driven T cell memory formation promotes allergic responses in offspring via endogenous glucocorticoids ※ DOI: https://doi.org/10.1016/j.mucimm.2025.06.006
This research was conducted as part of the Basic Science Research Program and the Bio-Medical Technology Development Program supported by the Ministry of Science and ICT and the National Research Foundation of Korea.
2025.08.03 View 187 -
 KAIST Enables On-Site Disease Diagnosis in Just 3 Minutes... Nanozyme Reaction Selectivity Improved 38-Fold
<(From Left) Professor Jinwoo Lee, Ph.D candidate Seonhye Park and Ph.D candidate Daeeun Choi from Chemical & Biomolecular Engineering>
To enable early diagnosis of acute illnesses and effective management of chronic conditions, point-of-care testing (POCT) technology—diagnostics conducted near the patient—is drawing global attention. The key to POCT lies in enzymes that recognize and react precisely with specific substances. However, traditional natural enzymes are expensive and unstable, and nanozymes (enzyme-mimicking catalysts) have suffered from low reaction selectivity. Now, a Korean research team has developed a high-sensitivity sensor platform that achieves 38 times higher selectivity than existing nanozymes and allows disease diagnostics visible to the naked eye within just 3 minutes.
On the 28th, KAIST (President Kwang Hyung Lee) announced that Professor Jinwoo Lee’s research team from the Department of Chemical & Biomolecular Engineering, in collaboration with teams led by Professor Jeong Woo Han at Seoul National University and Professor Moon Il Kim at Gachon University, has developed a new single-atom catalyst that selectively performs only peroxidase-like reactions while maintaining high reaction efficiency.
Using bodily fluids such as blood, urine, or saliva, this diagnostic platform enables test results to be read within minutes even outside hospital settings—greatly improving medical accessibility and ensuring timely treatment. The key lies in the visual detection of biomarkers (disease indicators) through color changes triggered by enzyme reactions. However, natural enzymes are expensive and easily degraded in diagnostic environments, limiting their storage and distribution.
To address this, inorganic nanozyme materials have been developed as substitutes. Yet, they typically lack selectivity—when hydrogen peroxide is used as a substrate, the same catalyst triggers both peroxidase-like reactions (which cause color change) and catalase-like reactions (which remove the substrate), reducing diagnostic signal accuracy.
To control catalyst selectivity at the atomic level, the researchers used an innovative structural design: attaching chlorine (Cl) ligands in a three-dimensional configuration to the central ruthenium (Ru) atom to fine-tune its chemical properties. This enabled them to isolate only the desired diagnostic signal.
<Figure1. The catalyst in this study (ruthenium single-atom catalyst) exhibits peroxidase-like activity with selectivity akin to natural enzymes through three-dimensional directional ligand coordination. Due to the absence of competing catalase activity, selective peroxidase-like reactions proceed under biomimetic conditions. In contrast, conventional single-atom catalysts with active sites arranged on planar surfaces exhibit dual functionality depending on pH. Under neutral conditions, their catalase activity leads to hydrogen peroxide depletion, hindering accurate detection. The catalyst in this study eliminates such interference, enabling direct detection of biomarkers through coupled reactions with oxidases without the need for cumbersome steps like buffer replacement. The ability to simultaneously detect multiple target substances under biomimetic conditions demonstrates the practicality of ruthenium single-atom catalysts for on-site diagnostics>
Experimental results showed that the new catalyst achieved over 38-fold improvement in selectivity compared to existing nanozymes, with significantly increased sensitivity and speed in detecting hydrogen peroxide. Even in near-physiological conditions (pH 6.0), the catalyst maintained its performance, proving its applicability in real-world diagnostics.
By incorporating the catalyst and oxidase into a paper-based sensor, the team created a system that could simultaneously detect four key biomarkers related to health: glucose, lactate, cholesterol, and choline—all with a simple color change.
This platform is broadly applicable across various disease diagnostics and can deliver results within 3 minutes without complex instruments or pH adjustments. The findings show that diagnostic performance can be dramatically improved without changing the platform itself, but rather by engineering the catalyst structure.
<Figure 2.(a) Schematic diagram of the paper sensor (Zone 1: glucose oxidase immobilized; Zone 2: lactate oxidase immobilized; Zone 3: choline oxidase immobilized; Zone 4: cholesterol oxidase immobilized; Zone 5: no oxidase enzyme). (b) Single biomarker (single disease indicator) detection using the ruthenium single‑atom catalyst–based paper sensor.(c) Multiple biomarker (multiple disease indicator) detection using the ruthenium single‑atom catalyst–based paper sensor>
Professor Jinwoo Lee of KAIST commented, “This study is significant in that it simultaneously achieves enzyme-level selectivity and reactivity by structurally designing single-atom catalysts.” He added that “the structure–function-based catalyst design strategy can be extended to the development of various metal-based catalysts and other reaction domains where selectivity is critical.”
Seonhye Park and Daeeun Choi, both Ph.D. candidates at KAIST, are co-first authors. The research was published on July 6, 2025, in the prestigious journal Advanced Materials
-Title: Breaking the Selectivity Barrier of Single-Atom Nanozymes Through Out-of-Plane Ligand Coordinatio
- Authors: Seonhye Park (KAIST, co–first author), Daeeun Choi (KAIST, co–first author), Kyu In Shim (SNU, co–first author), Phuong Thy Nguyen (Gachon Univ., co–first author), Seongbeen Kim (KAIST), Seung Yeop Yi (KAIST), Moon Il Kim (Gachon Univ., corresponding author), Jeong Woo Han (SNU, corresponding author), Jinwoo Lee (KAIST, corresponding author
-DOI: https://doi.org/10.1002/adma.202506480
This research was supported by the Ministry of Science and ICT and the National Research Foundation of Korea (NRF).
2025.07.29 View 392
KAIST Enables On-Site Disease Diagnosis in Just 3 Minutes... Nanozyme Reaction Selectivity Improved 38-Fold
<(From Left) Professor Jinwoo Lee, Ph.D candidate Seonhye Park and Ph.D candidate Daeeun Choi from Chemical & Biomolecular Engineering>
To enable early diagnosis of acute illnesses and effective management of chronic conditions, point-of-care testing (POCT) technology—diagnostics conducted near the patient—is drawing global attention. The key to POCT lies in enzymes that recognize and react precisely with specific substances. However, traditional natural enzymes are expensive and unstable, and nanozymes (enzyme-mimicking catalysts) have suffered from low reaction selectivity. Now, a Korean research team has developed a high-sensitivity sensor platform that achieves 38 times higher selectivity than existing nanozymes and allows disease diagnostics visible to the naked eye within just 3 minutes.
On the 28th, KAIST (President Kwang Hyung Lee) announced that Professor Jinwoo Lee’s research team from the Department of Chemical & Biomolecular Engineering, in collaboration with teams led by Professor Jeong Woo Han at Seoul National University and Professor Moon Il Kim at Gachon University, has developed a new single-atom catalyst that selectively performs only peroxidase-like reactions while maintaining high reaction efficiency.
Using bodily fluids such as blood, urine, or saliva, this diagnostic platform enables test results to be read within minutes even outside hospital settings—greatly improving medical accessibility and ensuring timely treatment. The key lies in the visual detection of biomarkers (disease indicators) through color changes triggered by enzyme reactions. However, natural enzymes are expensive and easily degraded in diagnostic environments, limiting their storage and distribution.
To address this, inorganic nanozyme materials have been developed as substitutes. Yet, they typically lack selectivity—when hydrogen peroxide is used as a substrate, the same catalyst triggers both peroxidase-like reactions (which cause color change) and catalase-like reactions (which remove the substrate), reducing diagnostic signal accuracy.
To control catalyst selectivity at the atomic level, the researchers used an innovative structural design: attaching chlorine (Cl) ligands in a three-dimensional configuration to the central ruthenium (Ru) atom to fine-tune its chemical properties. This enabled them to isolate only the desired diagnostic signal.
<Figure1. The catalyst in this study (ruthenium single-atom catalyst) exhibits peroxidase-like activity with selectivity akin to natural enzymes through three-dimensional directional ligand coordination. Due to the absence of competing catalase activity, selective peroxidase-like reactions proceed under biomimetic conditions. In contrast, conventional single-atom catalysts with active sites arranged on planar surfaces exhibit dual functionality depending on pH. Under neutral conditions, their catalase activity leads to hydrogen peroxide depletion, hindering accurate detection. The catalyst in this study eliminates such interference, enabling direct detection of biomarkers through coupled reactions with oxidases without the need for cumbersome steps like buffer replacement. The ability to simultaneously detect multiple target substances under biomimetic conditions demonstrates the practicality of ruthenium single-atom catalysts for on-site diagnostics>
Experimental results showed that the new catalyst achieved over 38-fold improvement in selectivity compared to existing nanozymes, with significantly increased sensitivity and speed in detecting hydrogen peroxide. Even in near-physiological conditions (pH 6.0), the catalyst maintained its performance, proving its applicability in real-world diagnostics.
By incorporating the catalyst and oxidase into a paper-based sensor, the team created a system that could simultaneously detect four key biomarkers related to health: glucose, lactate, cholesterol, and choline—all with a simple color change.
This platform is broadly applicable across various disease diagnostics and can deliver results within 3 minutes without complex instruments or pH adjustments. The findings show that diagnostic performance can be dramatically improved without changing the platform itself, but rather by engineering the catalyst structure.
<Figure 2.(a) Schematic diagram of the paper sensor (Zone 1: glucose oxidase immobilized; Zone 2: lactate oxidase immobilized; Zone 3: choline oxidase immobilized; Zone 4: cholesterol oxidase immobilized; Zone 5: no oxidase enzyme). (b) Single biomarker (single disease indicator) detection using the ruthenium single‑atom catalyst–based paper sensor.(c) Multiple biomarker (multiple disease indicator) detection using the ruthenium single‑atom catalyst–based paper sensor>
Professor Jinwoo Lee of KAIST commented, “This study is significant in that it simultaneously achieves enzyme-level selectivity and reactivity by structurally designing single-atom catalysts.” He added that “the structure–function-based catalyst design strategy can be extended to the development of various metal-based catalysts and other reaction domains where selectivity is critical.”
Seonhye Park and Daeeun Choi, both Ph.D. candidates at KAIST, are co-first authors. The research was published on July 6, 2025, in the prestigious journal Advanced Materials
-Title: Breaking the Selectivity Barrier of Single-Atom Nanozymes Through Out-of-Plane Ligand Coordinatio
- Authors: Seonhye Park (KAIST, co–first author), Daeeun Choi (KAIST, co–first author), Kyu In Shim (SNU, co–first author), Phuong Thy Nguyen (Gachon Univ., co–first author), Seongbeen Kim (KAIST), Seung Yeop Yi (KAIST), Moon Il Kim (Gachon Univ., corresponding author), Jeong Woo Han (SNU, corresponding author), Jinwoo Lee (KAIST, corresponding author
-DOI: https://doi.org/10.1002/adma.202506480
This research was supported by the Ministry of Science and ICT and the National Research Foundation of Korea (NRF).
2025.07.29 View 392 -
 KAIST Team Develops Optogenetic Platform for Spatiotemporal Control of Protein and mRNA Storage and Release
<Dr. Chaeyeon Lee, Professor Won Do Heo from Department of Biological Sciences>
A KAIST research team led by Professor Won Do Heo (Department of Biological Sciences) has developed an optogenetic platform, RELISR (REversible LIght-induced Store and Release), that enables precise spatiotemporal control over the storage and release of proteins and mRNAs in living cells and animals.
Traditional optogenetic condensate systems have been limited by their reliance on non-specific multivalent interactions, which can lead to unintended sequestration or release of endogenous molecules. RELISR overcomes these limitations by employing highly specific protein–protein (nanobody–antigen) and protein–RNA (MCP–MS2) interactions, enabling the selective and reversible compartmentalization of target proteins or mRNAs within engineered, membrane-less condensates.
In the dark, RELISR stably sequesters target molecules within condensates, physically isolating them from the cellular environment. Upon blue light stimulation, the condensates rapidly dissolve, releasing the stored proteins or mRNAs, which immediately regain their cellular functions or translational competency. This allows for reversible and rapid modulation of molecular activities in response to optical cues.
< Figure 1. Overview of the Artificial Condensate System (RELISR). The artificial condensate system, RELISR, includes "Protein-RELISR" for storing proteins and "mRNA-RELISR" for storing mRNA. These artificial condensates can be disassembled by blue light irradiation and reassembled in a dark state>
The research team demonstrated that RELISR enables temporal and spatial regulation of protein activity and mRNA translation in various cell types, including cultured neurons and mouse liver tissue. Comparative studies showed that RELISR provides more robust and reversible control of translation than previous systems based on spatial translocation.
While previous optogenetic systems such as LARIAT (Lee et al., Nature Methods, 2014) and mRNA-LARIAT (Kim et al., Nat. Cell Biol., 2019) enabled the selective sequestration of proteins or mRNAs into membrane-less condensates in response to light, they were primarily limited to the trapping phase. The RELISR platform introduced in this study establishes a new paradigm by enabling both the targeted storage of proteins and mRNAs and their rapid, light-triggered release. This approach allows researchers to not only confine molecular function on demand, but also to restore activity with precise temporal control.
< Figure 2. Cell shape change using the artificial condensate system (RELISR). A target protein, Vav2, which contributes to cell shape, was stored within the artificial condensate and then released after light irradiation. This release activated the target protein Vav2, causing a change in cell shape. It was confirmed that the storage, release, and activation of various proteins were effectively achieved>
Professor Heo stated, “RELISR is a versatile optogenetic tool that enables the precise control of protein and mRNA function at defined times and locations in living systems. We anticipate this platform will be broadly applicable for studies of cell signaling, neural circuits, and therapeutic development. Furthermore, the combination of RELISR with genome editing or tissue-targeted delivery could further expand its utility for molecular medicine.”
< Figure 3. Expression of a target mRNA using the artificial condensate system (RELISR) in mice. The genetic material for the artificial condensate system, RELISR, was injected into a living mouse. Using this system, a target mRNA was stored within the mouse's liver. Upon light irradiation, the mRNA was released, which induced the translation of a luminescent protein>
This research was conducted by first author Dr. Chaeyeon Lee, under the supervision of Professor Heo, with contributions from Dr. Daseuli Yu (co-corresponding author) and Professor YongKeun Park (co-corresponding author, Department of Physics), whose group performed quantitative imaging analyses of biophysical changes induced by RELISR in cells.
The findings were published in Nature Communications (July 7, 2025; DOI: 10.1038/s41467-025-61322-y). This work was supported by the Samsung Future Technology Foundation and the National Research Foundation of Korea.
2025.07.23 View 319
KAIST Team Develops Optogenetic Platform for Spatiotemporal Control of Protein and mRNA Storage and Release
<Dr. Chaeyeon Lee, Professor Won Do Heo from Department of Biological Sciences>
A KAIST research team led by Professor Won Do Heo (Department of Biological Sciences) has developed an optogenetic platform, RELISR (REversible LIght-induced Store and Release), that enables precise spatiotemporal control over the storage and release of proteins and mRNAs in living cells and animals.
Traditional optogenetic condensate systems have been limited by their reliance on non-specific multivalent interactions, which can lead to unintended sequestration or release of endogenous molecules. RELISR overcomes these limitations by employing highly specific protein–protein (nanobody–antigen) and protein–RNA (MCP–MS2) interactions, enabling the selective and reversible compartmentalization of target proteins or mRNAs within engineered, membrane-less condensates.
In the dark, RELISR stably sequesters target molecules within condensates, physically isolating them from the cellular environment. Upon blue light stimulation, the condensates rapidly dissolve, releasing the stored proteins or mRNAs, which immediately regain their cellular functions or translational competency. This allows for reversible and rapid modulation of molecular activities in response to optical cues.
< Figure 1. Overview of the Artificial Condensate System (RELISR). The artificial condensate system, RELISR, includes "Protein-RELISR" for storing proteins and "mRNA-RELISR" for storing mRNA. These artificial condensates can be disassembled by blue light irradiation and reassembled in a dark state>
The research team demonstrated that RELISR enables temporal and spatial regulation of protein activity and mRNA translation in various cell types, including cultured neurons and mouse liver tissue. Comparative studies showed that RELISR provides more robust and reversible control of translation than previous systems based on spatial translocation.
While previous optogenetic systems such as LARIAT (Lee et al., Nature Methods, 2014) and mRNA-LARIAT (Kim et al., Nat. Cell Biol., 2019) enabled the selective sequestration of proteins or mRNAs into membrane-less condensates in response to light, they were primarily limited to the trapping phase. The RELISR platform introduced in this study establishes a new paradigm by enabling both the targeted storage of proteins and mRNAs and their rapid, light-triggered release. This approach allows researchers to not only confine molecular function on demand, but also to restore activity with precise temporal control.
< Figure 2. Cell shape change using the artificial condensate system (RELISR). A target protein, Vav2, which contributes to cell shape, was stored within the artificial condensate and then released after light irradiation. This release activated the target protein Vav2, causing a change in cell shape. It was confirmed that the storage, release, and activation of various proteins were effectively achieved>
Professor Heo stated, “RELISR is a versatile optogenetic tool that enables the precise control of protein and mRNA function at defined times and locations in living systems. We anticipate this platform will be broadly applicable for studies of cell signaling, neural circuits, and therapeutic development. Furthermore, the combination of RELISR with genome editing or tissue-targeted delivery could further expand its utility for molecular medicine.”
< Figure 3. Expression of a target mRNA using the artificial condensate system (RELISR) in mice. The genetic material for the artificial condensate system, RELISR, was injected into a living mouse. Using this system, a target mRNA was stored within the mouse's liver. Upon light irradiation, the mRNA was released, which induced the translation of a luminescent protein>
This research was conducted by first author Dr. Chaeyeon Lee, under the supervision of Professor Heo, with contributions from Dr. Daseuli Yu (co-corresponding author) and Professor YongKeun Park (co-corresponding author, Department of Physics), whose group performed quantitative imaging analyses of biophysical changes induced by RELISR in cells.
The findings were published in Nature Communications (July 7, 2025; DOI: 10.1038/s41467-025-61322-y). This work was supported by the Samsung Future Technology Foundation and the National Research Foundation of Korea.
2025.07.23 View 319 -
 KAIST Successfully Implements 3D Brain-Mimicking Platform with 6x Higher Precision
<(From left) Dr. Dongjo Yoon, Professor Je-Kyun Park from the Department of Bio and Brain Engineering, (upper right) Professor Yoonkey Nam, Dr. Soo Jee Kim>
Existing three-dimensional (3D) neuronal culture technology has limitations in brain research due to the difficulty of precisely replicating the brain's complex multilayered structure and the lack of a platform that can simultaneously analyze both structure and function. A KAIST research team has successfully developed an integrated platform that can implement brain-like layered neuronal structures using 3D printing technology and precisely measure neuronal activity within them.
KAIST (President Kwang Hyung Lee) announced on the 16th of July that a joint research team led by Professors Je-Kyun Park and Yoonkey Nam from the Department of Bio and Brain Engineering has developed an integrated platform capable of fabricating high-resolution 3D multilayer neuronal networks using low-viscosity natural hydrogels with mechanical properties similar to brain tissue, and simultaneously analyzing their structural and functional connectivity.
Conventional bioprinting technology uses high-viscosity bioinks for structural stability, but this limits neuronal proliferation and neurite growth. Conversely, neural cell-friendly low-viscosity hydrogels are difficult to precisely pattern, leading to a fundamental trade-off between structural stability and biological function. The research team completed a sophisticated and stable brain-mimicking platform by combining three key technologies that enable the precise creation of brain structure with dilute gels, accurate alignment between layers, and simultaneous observation of neuronal activity.
The three core technologies are: ▲ 'Capillary Pinning Effect' technology, which enables the dilute gel (hydrogel) to adhere firmly to a stainless steel mesh (micromesh) to prevent it from flowing, thereby reproducing brain structures with six times greater precision (resolution of 500 μm or less) than conventional methods; ▲ the '3D Printing Aligner,' a cylindrical design that ensures the printed layers are precisely stacked without misalignment, guaranteeing the accurate assembly of multilayer structures and stable integration with microelectrode chips; and ▲ 'Dual-mode Analysis System' technology, which simultaneously measures electrical signals from below and observes cell activity with light (calcium imaging) from above, allowing for the simultaneous verification of the functional operation of interlayer connections through multiple methods.
< Figure 1. Platform integrating brain-structure-mimicking neural network model construction and functional measurement technology>
The research team successfully implemented a three-layered mini-brain structure using 3D printing with a fibrin hydrogel, which has elastic properties similar to those of the brain, and experimentally verified the process of actual neural cells transmitting and receiving signals within it.
Cortical neurons were placed in the upper and lower layers, while the middle layer was left empty but designed to allow neurons to penetrate and connect through it. Electrical signals were measured from the lower layer using a microsensor (electrode chip), and cell activity was observed from the upper layer using light (calcium imaging). The results showed that when electrical stimulation was applied, neural cells in both upper and lower layers responded simultaneously. When a synapse-blocking agent (synaptic blocker) was introduced, the response decreased, proving that the neural cells were genuinely connected and transmitting signals.
Professor Je-Kyun Park of KAIST explained, "This research is a joint development achievement of an integrated platform that can simultaneously reproduce the complex multilayered structure and function of brain tissue. Compared to existing technologies where signal measurement was impossible for more than 14 days, this platform maintains a stable microelectrode chip interface for over 27 days, allowing the real-time analysis of structure-function relationships. It can be utilized in various brain research fields such as neurological disease modeling, brain function research, neurotoxicity assessment, and neuroprotective drug screening in the future."
The research, in which Dr. Soo Jee Kim and Dr. Dongjo Yoon from KAIST's Department of Bio and Brain Engineering participated as co-first authors, was published online in the international journal 'Biosensors and Bioelectronics' on June 11, 2025.
※Paper: Hybrid biofabrication of multilayered 3D neuronal networks with structural and functional interlayer connectivity
※DOI: https://doi.org/10.1016/j.bios.2025.117688
2025.07.16 View 551
KAIST Successfully Implements 3D Brain-Mimicking Platform with 6x Higher Precision
<(From left) Dr. Dongjo Yoon, Professor Je-Kyun Park from the Department of Bio and Brain Engineering, (upper right) Professor Yoonkey Nam, Dr. Soo Jee Kim>
Existing three-dimensional (3D) neuronal culture technology has limitations in brain research due to the difficulty of precisely replicating the brain's complex multilayered structure and the lack of a platform that can simultaneously analyze both structure and function. A KAIST research team has successfully developed an integrated platform that can implement brain-like layered neuronal structures using 3D printing technology and precisely measure neuronal activity within them.
KAIST (President Kwang Hyung Lee) announced on the 16th of July that a joint research team led by Professors Je-Kyun Park and Yoonkey Nam from the Department of Bio and Brain Engineering has developed an integrated platform capable of fabricating high-resolution 3D multilayer neuronal networks using low-viscosity natural hydrogels with mechanical properties similar to brain tissue, and simultaneously analyzing their structural and functional connectivity.
Conventional bioprinting technology uses high-viscosity bioinks for structural stability, but this limits neuronal proliferation and neurite growth. Conversely, neural cell-friendly low-viscosity hydrogels are difficult to precisely pattern, leading to a fundamental trade-off between structural stability and biological function. The research team completed a sophisticated and stable brain-mimicking platform by combining three key technologies that enable the precise creation of brain structure with dilute gels, accurate alignment between layers, and simultaneous observation of neuronal activity.
The three core technologies are: ▲ 'Capillary Pinning Effect' technology, which enables the dilute gel (hydrogel) to adhere firmly to a stainless steel mesh (micromesh) to prevent it from flowing, thereby reproducing brain structures with six times greater precision (resolution of 500 μm or less) than conventional methods; ▲ the '3D Printing Aligner,' a cylindrical design that ensures the printed layers are precisely stacked without misalignment, guaranteeing the accurate assembly of multilayer structures and stable integration with microelectrode chips; and ▲ 'Dual-mode Analysis System' technology, which simultaneously measures electrical signals from below and observes cell activity with light (calcium imaging) from above, allowing for the simultaneous verification of the functional operation of interlayer connections through multiple methods.
< Figure 1. Platform integrating brain-structure-mimicking neural network model construction and functional measurement technology>
The research team successfully implemented a three-layered mini-brain structure using 3D printing with a fibrin hydrogel, which has elastic properties similar to those of the brain, and experimentally verified the process of actual neural cells transmitting and receiving signals within it.
Cortical neurons were placed in the upper and lower layers, while the middle layer was left empty but designed to allow neurons to penetrate and connect through it. Electrical signals were measured from the lower layer using a microsensor (electrode chip), and cell activity was observed from the upper layer using light (calcium imaging). The results showed that when electrical stimulation was applied, neural cells in both upper and lower layers responded simultaneously. When a synapse-blocking agent (synaptic blocker) was introduced, the response decreased, proving that the neural cells were genuinely connected and transmitting signals.
Professor Je-Kyun Park of KAIST explained, "This research is a joint development achievement of an integrated platform that can simultaneously reproduce the complex multilayered structure and function of brain tissue. Compared to existing technologies where signal measurement was impossible for more than 14 days, this platform maintains a stable microelectrode chip interface for over 27 days, allowing the real-time analysis of structure-function relationships. It can be utilized in various brain research fields such as neurological disease modeling, brain function research, neurotoxicity assessment, and neuroprotective drug screening in the future."
The research, in which Dr. Soo Jee Kim and Dr. Dongjo Yoon from KAIST's Department of Bio and Brain Engineering participated as co-first authors, was published online in the international journal 'Biosensors and Bioelectronics' on June 11, 2025.
※Paper: Hybrid biofabrication of multilayered 3D neuronal networks with structural and functional interlayer connectivity
※DOI: https://doi.org/10.1016/j.bios.2025.117688
2025.07.16 View 551 -
 KAIST Develops Novel Candidiasis Treatment Overcoming Side Effects and Resistance
<(From left) Ph. D Candidate Ju Yeon Chung, Prof.Hyun Jung Chung, Ph.D candidate Seungju Yang, Ph.D candidate Ayoung Park, Dr. Yoon-Kyoung Hong from Asan Medical Center, Prof. Yong Pil Chong, Dr. Eunhee Jeon>
Candida, a type of fungus, which can spread throughout the body via the bloodstream, leading to organ damage and sepsis. Recently, the incidence of candidiasis has surged due to the increase in immunosuppressive therapies, medical implants, and transplantation. Korean researchers have successfully developed a next-generation treatment that, unlike existing antifungals, selectively acts only on Candida, achieving both high therapeutic efficacy and low side effects simultaneously.
KAIST (President Kwang Hyung Lee) announced on the 8th that a research team led by Professor Hyun-Jung Chung of the Department of Biological Sciences, in collaboration with Professor Yong Pil Jeong's team at Asan Medical Center, developed a gene-based nanotherapy (FTNx) that simultaneously inhibits two key enzymes in the Candida cell wall.
Current antifungal drugs for Candida have low target selectivity, which can affect human cells. Furthermore, their therapeutic efficacy is gradually decreasing due to the emergence of new resistant strains. Especially for immunocompromised patients, the infection progresses rapidly and has a poor prognosis, making the development of new treatments to overcome the limitations of existing therapies urgent.
The developed treatment can be administered systemically, and by combining gene suppression technology with nanomaterial technology, it effectively overcomes the structural limitations of existing compound-based drugs and successfully achieves selective treatment against only Candida.
The research team created a gold nanoparticle-based complex loaded with short DNA fragments called antisense oligonucleotides (ASO), which simultaneously target two crucial enzymes—β-1,3-glucan synthase (FKS1) and chitin synthase (CHS3)—important for forming the cell wall of the Candida fungus.
By applying a surface coating technology that binds to a specific glycolipid structure (a structure combining sugar and fat) on the Candida cell wall, a targeted delivery device was implemented. This successfully achieved a precise targeting effect, ensuring the complex is not delivered to human cells at all but acts selectively only on Candida.
<Figure 1: Overview of antifungal therapy design and experimental approach>
This complex, after entering Candida cells, cleaves the mRNA produced by the FKS1 and CHS3 genes, thereby inhibiting translation and simultaneously blocking the synthesis of cell wall components β-1,3-glucan and chitin. As a result, the
Candida cell wall loses its structural stability and collapses, suppressing bacterial survival and proliferation.
In fact, experiments using a systemic candidiasis model in mice confirmed the therapeutic effect: a significant reduction in
Candida count in the organs, normalization of immune responses, and a notable increase in survival rates were observed in the treated group.
Professor Hyun-Jung Chung, who led the research, stated, "This study presents a method to overcome the issues of human toxicity and drug resistance spread with existing treatments, marking an important turning point by demonstrating the applicability of gene therapy for systemic infections". She added, "We plan to continue research on optimizing administration methods and verifying toxicity for future clinical application."
This research involved Ju Yeon Chung and Yoon-Kyoung Hong as co-first authors , and was published in the international journal 'Nature Communications' on July 1st.
Paper Title: Effective treatment of systemic candidiasis by synergistic targeting of cell wall synthesis
DOI: 10.1038/s41467-025-60684-7
This research was supported by the Ministry of Health and Welfare and the National Research Foundation of Korea.
2025.07.08 View 681
KAIST Develops Novel Candidiasis Treatment Overcoming Side Effects and Resistance
<(From left) Ph. D Candidate Ju Yeon Chung, Prof.Hyun Jung Chung, Ph.D candidate Seungju Yang, Ph.D candidate Ayoung Park, Dr. Yoon-Kyoung Hong from Asan Medical Center, Prof. Yong Pil Chong, Dr. Eunhee Jeon>
Candida, a type of fungus, which can spread throughout the body via the bloodstream, leading to organ damage and sepsis. Recently, the incidence of candidiasis has surged due to the increase in immunosuppressive therapies, medical implants, and transplantation. Korean researchers have successfully developed a next-generation treatment that, unlike existing antifungals, selectively acts only on Candida, achieving both high therapeutic efficacy and low side effects simultaneously.
KAIST (President Kwang Hyung Lee) announced on the 8th that a research team led by Professor Hyun-Jung Chung of the Department of Biological Sciences, in collaboration with Professor Yong Pil Jeong's team at Asan Medical Center, developed a gene-based nanotherapy (FTNx) that simultaneously inhibits two key enzymes in the Candida cell wall.
Current antifungal drugs for Candida have low target selectivity, which can affect human cells. Furthermore, their therapeutic efficacy is gradually decreasing due to the emergence of new resistant strains. Especially for immunocompromised patients, the infection progresses rapidly and has a poor prognosis, making the development of new treatments to overcome the limitations of existing therapies urgent.
The developed treatment can be administered systemically, and by combining gene suppression technology with nanomaterial technology, it effectively overcomes the structural limitations of existing compound-based drugs and successfully achieves selective treatment against only Candida.
The research team created a gold nanoparticle-based complex loaded with short DNA fragments called antisense oligonucleotides (ASO), which simultaneously target two crucial enzymes—β-1,3-glucan synthase (FKS1) and chitin synthase (CHS3)—important for forming the cell wall of the Candida fungus.
By applying a surface coating technology that binds to a specific glycolipid structure (a structure combining sugar and fat) on the Candida cell wall, a targeted delivery device was implemented. This successfully achieved a precise targeting effect, ensuring the complex is not delivered to human cells at all but acts selectively only on Candida.
<Figure 1: Overview of antifungal therapy design and experimental approach>
This complex, after entering Candida cells, cleaves the mRNA produced by the FKS1 and CHS3 genes, thereby inhibiting translation and simultaneously blocking the synthesis of cell wall components β-1,3-glucan and chitin. As a result, the
Candida cell wall loses its structural stability and collapses, suppressing bacterial survival and proliferation.
In fact, experiments using a systemic candidiasis model in mice confirmed the therapeutic effect: a significant reduction in
Candida count in the organs, normalization of immune responses, and a notable increase in survival rates were observed in the treated group.
Professor Hyun-Jung Chung, who led the research, stated, "This study presents a method to overcome the issues of human toxicity and drug resistance spread with existing treatments, marking an important turning point by demonstrating the applicability of gene therapy for systemic infections". She added, "We plan to continue research on optimizing administration methods and verifying toxicity for future clinical application."
This research involved Ju Yeon Chung and Yoon-Kyoung Hong as co-first authors , and was published in the international journal 'Nature Communications' on July 1st.
Paper Title: Effective treatment of systemic candidiasis by synergistic targeting of cell wall synthesis
DOI: 10.1038/s41467-025-60684-7
This research was supported by the Ministry of Health and Welfare and the National Research Foundation of Korea.
2025.07.08 View 681 -
 KAIST Presents a Breakthrough in Overcoming Drug Resistance in Cancer – Hope for Treating Intractable Diseases like Diabetes
<(From the left) Prof. Hyun Uk Kim, Ph.D candiate Hae Deok Jung, Ph.D candidate Jina Lim, Prof.Yoosik Kim from the Department of Chemical and Biomolecular Engineering>
One of the biggest obstacles in cancer treatment is drug resistance in cancer cells. Conventional efforts have focused on identifying new drug targets to eliminate these resistant cells, but such approaches can often lead to even stronger resistance. Now, researchers at KAIST have developed a computational framework to predict key metabolic genes that can re-sensitize resistant cancer cells to treatment. This technique holds promise not only for a variety of cancer therapies but also for treating metabolic diseases such as diabetes.
On the 7th of July, KAIST (President Kwang Hyung Lee) announced that a research team led by Professors Hyun Uk Kim and Yoosik Kim from the Department of Chemical and Biomolecular Engineering had developed a computational framework that predicts metabolic gene targets to re-sensitize drug-resistant breast cancer cells. This was achieved using a metabolic network model capable of simulating human metabolism.
Focusing on metabolic alterations—key characteristics in the formation of drug resistance—the researchers developed a metabolism-based approach to identify gene targets that could enhance drug responsiveness by regulating the metabolism of drug-resistant breast cancer cells.
< Computational framework that can identify metabolic gene targets to revert the metabolic state of the drug-resistant cells to that of the drug-sensitive parental cells>
The team first constructed cell-specific metabolic network models by integrating proteomic data obtained from two different types of drug-resistant MCF7 breast cancer cell lines: one resistant to doxorubicin and the other to paclitaxel. They then performed gene knockout simulations* on all of the metabolic genes and analyzed the results.
*Gene knockout simulation: A computational method to predict changes in a biological network by virtually removing specific genes.
As a result, they discovered that suppressing certain genes could make previously resistant cancer cells responsive to anticancer drugs again. Specifically, they identified GOT1 as a target in doxorubicin-resistant cells, GPI in paclitaxel-resistant cells, and SLC1A5 as a common target for both drugs.
The predictions were experimentally validated by suppressing proteins encoded by these genes, which led to the re-sensitization of the drug-resistant cancer cells.
Furthermore, consistent re-sensitization effects were also observed when the same proteins were inhibited in other types of breast cancer cells that had developed resistance to the same drugs.
Professor Yoosik Kim remarked, “Cellular metabolism plays a crucial role in various intractable diseases including infectious and degenerative conditions. This new technology, which predicts metabolic regulation switches, can serve as a foundational tool not only for treating drug-resistant breast cancer but also for a wide range of diseases that currently lack effective therapies.”
Professor Hyun Uk Kim, who led the study, emphasized, “The significance of this research lies in our ability to accurately predict key metabolic genes that can make resistant cancer cells responsive to treatment again—using only computer simulations and minimal experimental data. This framework can be widely applied to discover new therapeutic targets in various cancers and metabolic diseases.”
The study, in which Ph.D. candidates JinA Lim and Hae Deok Jung from KAIST participated as co-first authors, was published online on June 25 in Proceedings of the National Academy of Sciences (PNAS), a leading multidisciplinary journal that covers top-tier research in life sciences, physics, engineering, and social sciences.
※ Title: Genome-scale knockout simulation and clustering analysis of drug-resistant breast cancer cells reveal drug sensitization targets ※ DOI: https://doi.org/10.1073/pnas.2425384122 ※ Authors: JinA Lim (KAIST, co-first author), Hae Deok Jung (KAIST, co-first author), Han Suk Ryu (Seoul National University Hospital, corresponding author), Yoosik Kim (KAIST, corresponding author), Hyun Uk Kim (KAIST, corresponding author), and five others.
This research was supported by the Ministry of Science and ICT through the National Research Foundation of Korea, and the Electronics and Telecommunications Research Institute (ETRI).
2025.07.08 View 1093
KAIST Presents a Breakthrough in Overcoming Drug Resistance in Cancer – Hope for Treating Intractable Diseases like Diabetes
<(From the left) Prof. Hyun Uk Kim, Ph.D candiate Hae Deok Jung, Ph.D candidate Jina Lim, Prof.Yoosik Kim from the Department of Chemical and Biomolecular Engineering>
One of the biggest obstacles in cancer treatment is drug resistance in cancer cells. Conventional efforts have focused on identifying new drug targets to eliminate these resistant cells, but such approaches can often lead to even stronger resistance. Now, researchers at KAIST have developed a computational framework to predict key metabolic genes that can re-sensitize resistant cancer cells to treatment. This technique holds promise not only for a variety of cancer therapies but also for treating metabolic diseases such as diabetes.
On the 7th of July, KAIST (President Kwang Hyung Lee) announced that a research team led by Professors Hyun Uk Kim and Yoosik Kim from the Department of Chemical and Biomolecular Engineering had developed a computational framework that predicts metabolic gene targets to re-sensitize drug-resistant breast cancer cells. This was achieved using a metabolic network model capable of simulating human metabolism.
Focusing on metabolic alterations—key characteristics in the formation of drug resistance—the researchers developed a metabolism-based approach to identify gene targets that could enhance drug responsiveness by regulating the metabolism of drug-resistant breast cancer cells.
< Computational framework that can identify metabolic gene targets to revert the metabolic state of the drug-resistant cells to that of the drug-sensitive parental cells>
The team first constructed cell-specific metabolic network models by integrating proteomic data obtained from two different types of drug-resistant MCF7 breast cancer cell lines: one resistant to doxorubicin and the other to paclitaxel. They then performed gene knockout simulations* on all of the metabolic genes and analyzed the results.
*Gene knockout simulation: A computational method to predict changes in a biological network by virtually removing specific genes.
As a result, they discovered that suppressing certain genes could make previously resistant cancer cells responsive to anticancer drugs again. Specifically, they identified GOT1 as a target in doxorubicin-resistant cells, GPI in paclitaxel-resistant cells, and SLC1A5 as a common target for both drugs.
The predictions were experimentally validated by suppressing proteins encoded by these genes, which led to the re-sensitization of the drug-resistant cancer cells.
Furthermore, consistent re-sensitization effects were also observed when the same proteins were inhibited in other types of breast cancer cells that had developed resistance to the same drugs.
Professor Yoosik Kim remarked, “Cellular metabolism plays a crucial role in various intractable diseases including infectious and degenerative conditions. This new technology, which predicts metabolic regulation switches, can serve as a foundational tool not only for treating drug-resistant breast cancer but also for a wide range of diseases that currently lack effective therapies.”
Professor Hyun Uk Kim, who led the study, emphasized, “The significance of this research lies in our ability to accurately predict key metabolic genes that can make resistant cancer cells responsive to treatment again—using only computer simulations and minimal experimental data. This framework can be widely applied to discover new therapeutic targets in various cancers and metabolic diseases.”
The study, in which Ph.D. candidates JinA Lim and Hae Deok Jung from KAIST participated as co-first authors, was published online on June 25 in Proceedings of the National Academy of Sciences (PNAS), a leading multidisciplinary journal that covers top-tier research in life sciences, physics, engineering, and social sciences.
※ Title: Genome-scale knockout simulation and clustering analysis of drug-resistant breast cancer cells reveal drug sensitization targets ※ DOI: https://doi.org/10.1073/pnas.2425384122 ※ Authors: JinA Lim (KAIST, co-first author), Hae Deok Jung (KAIST, co-first author), Han Suk Ryu (Seoul National University Hospital, corresponding author), Yoosik Kim (KAIST, corresponding author), Hyun Uk Kim (KAIST, corresponding author), and five others.
This research was supported by the Ministry of Science and ICT through the National Research Foundation of Korea, and the Electronics and Telecommunications Research Institute (ETRI).
2025.07.08 View 1093 -
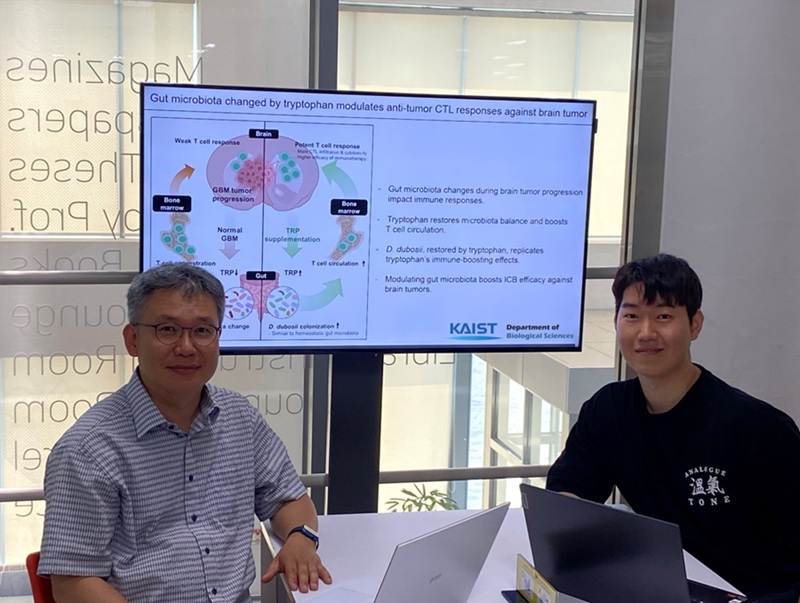 KAIST Enhances Immunotherapy for Difficult-to-Treat Brain Tumors with Gut Microbiota
< Photo 1.(From left) Prof. Heung Kyu Lee, Department of Biological Sciences,
and Dr. Hyeon Cheol Kim>
Advanced treatments, known as immunotherapies that activate T cells—our body's immune cells—to eliminate cancer cells, have shown limited efficacy as standalone therapies for glioblastoma, the most lethal form of brain tumor. This is due to their minimal response to glioblastoma and high resistance to treatment.
Now, a KAIST research team has now demonstrated a new therapeutic strategy that can enhance the efficacy of immunotherapy for brain tumors by utilizing gut microbes and their metabolites. This also opens up possibilities for developing microbiome-based immunotherapy supplements in the future.
KAIST (President Kwang Hyung Lee) announced on July 1 that a research team led by Professor Heung Kyu Lee of the Department of Biological Sciences discovered and demonstrated a method to significantly improve the efficiency of glioblastoma immunotherapy by focusing on changes in the gut microbial ecosystem.
The research team noted that as glioblastoma progresses, the concentration of ‘tryptophan’, an important amino acid in the gut, sharply decreases, leading to changes in the gut microbial ecosystem. They discovered that by supplementing tryptophan to restore microbial diversity, specific beneficial strains activate CD8 T cells (a type of immune cell) and induce their infiltration into tumor tissues. Through a mouse model of glioblastoma, the research team confirmed that tryptophan supplementation enhanced the response of cancer-attacking T cells (especially CD8 T cells), leading to their increased migration to tumor sites such as lymph nodes and the brain.
In this process, they also revealed that ‘Duncaniella dubosii’, a beneficial commensal bacterium present in the gut, plays a crucial role. This bacterium helped T cells effectively redistribute within the body, and survival rates significantly improved when used in combination with immunotherapy (anti-PD-1).
Furthermore, it was demonstrated that even when this commensal bacterium was administered alone to germ-free mice (mice without any commensal microbes), the survival rate for glioblastoma increased. This is because the bacterium utilizes tryptophan to regulate the gut environment, and the metabolites produced in this process strengthen the ability of CD8 T cells to attack cancer cells.
Professor Heung Kyu Lee explained, "This research is a meaningful achievement, showing that even in intractable brain tumors where immune checkpoint inhibitors had no effect, a combined strategy utilizing gut microbes can significantly enhance treatment response."
Dr. Hyeon Cheol Kim of KAIST (currently a postdoctoral researcher at the Institute for Biological Sciences) participated as the first author. The research findings were published online in Cell Reports, an international journal in the life sciences, on June 26.
This research was conducted as part of the Basic Research Program and Bio & Medical Technology Development Program supported by the Ministry of Science and ICT and the National Research Foundation of Korea.
※Paper Title: Gut microbiota dysbiosis induced by brain tumor modulates the efficacy of immunotherapy
※DOI: https://doi.org/10.1016/j.celrep.2025.115825
2025.07.02 View 1388
KAIST Enhances Immunotherapy for Difficult-to-Treat Brain Tumors with Gut Microbiota
< Photo 1.(From left) Prof. Heung Kyu Lee, Department of Biological Sciences,
and Dr. Hyeon Cheol Kim>
Advanced treatments, known as immunotherapies that activate T cells—our body's immune cells—to eliminate cancer cells, have shown limited efficacy as standalone therapies for glioblastoma, the most lethal form of brain tumor. This is due to their minimal response to glioblastoma and high resistance to treatment.
Now, a KAIST research team has now demonstrated a new therapeutic strategy that can enhance the efficacy of immunotherapy for brain tumors by utilizing gut microbes and their metabolites. This also opens up possibilities for developing microbiome-based immunotherapy supplements in the future.
KAIST (President Kwang Hyung Lee) announced on July 1 that a research team led by Professor Heung Kyu Lee of the Department of Biological Sciences discovered and demonstrated a method to significantly improve the efficiency of glioblastoma immunotherapy by focusing on changes in the gut microbial ecosystem.
The research team noted that as glioblastoma progresses, the concentration of ‘tryptophan’, an important amino acid in the gut, sharply decreases, leading to changes in the gut microbial ecosystem. They discovered that by supplementing tryptophan to restore microbial diversity, specific beneficial strains activate CD8 T cells (a type of immune cell) and induce their infiltration into tumor tissues. Through a mouse model of glioblastoma, the research team confirmed that tryptophan supplementation enhanced the response of cancer-attacking T cells (especially CD8 T cells), leading to their increased migration to tumor sites such as lymph nodes and the brain.
In this process, they also revealed that ‘Duncaniella dubosii’, a beneficial commensal bacterium present in the gut, plays a crucial role. This bacterium helped T cells effectively redistribute within the body, and survival rates significantly improved when used in combination with immunotherapy (anti-PD-1).
Furthermore, it was demonstrated that even when this commensal bacterium was administered alone to germ-free mice (mice without any commensal microbes), the survival rate for glioblastoma increased. This is because the bacterium utilizes tryptophan to regulate the gut environment, and the metabolites produced in this process strengthen the ability of CD8 T cells to attack cancer cells.
Professor Heung Kyu Lee explained, "This research is a meaningful achievement, showing that even in intractable brain tumors where immune checkpoint inhibitors had no effect, a combined strategy utilizing gut microbes can significantly enhance treatment response."
Dr. Hyeon Cheol Kim of KAIST (currently a postdoctoral researcher at the Institute for Biological Sciences) participated as the first author. The research findings were published online in Cell Reports, an international journal in the life sciences, on June 26.
This research was conducted as part of the Basic Research Program and Bio & Medical Technology Development Program supported by the Ministry of Science and ICT and the National Research Foundation of Korea.
※Paper Title: Gut microbiota dysbiosis induced by brain tumor modulates the efficacy of immunotherapy
※DOI: https://doi.org/10.1016/j.celrep.2025.115825
2025.07.02 View 1388 -
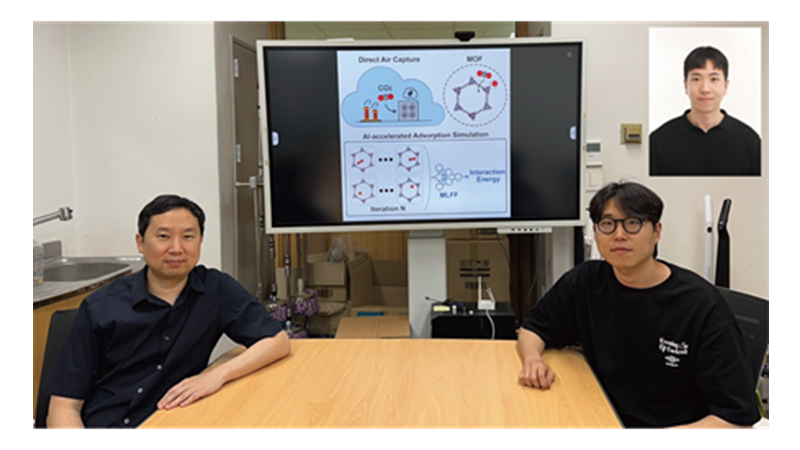 KAIST Develops AI to Easily Find Promising Materials That Capture Only CO₂
< Photo 1. (From left) Professor Jihan Kim, Ph.D. candidate Yunsung Lim and Dr. Hyunsoo Park of the Department of Chemical and Biomolecular Engineering >
In order to help prevent the climate crisis, actively reducing already-emitted CO₂ is essential. Accordingly, direct air capture (DAC) — a technology that directly extracts only CO₂ from the air — is gaining attention. However, effectively capturing pure CO₂ is not easy due to water vapor (H₂O) present in the air. KAIST researchers have successfully used AI-driven machine learning techniques to identify the most promising CO₂-capturing materials among metal-organic frameworks (MOFs), a key class of materials studied for this technology.
KAIST (President Kwang Hyung Lee) announced on the 29th of June that a research team led by Professor Jihan Kim from the Department of Chemical and Biomolecular Engineering, in collaboration with a team at Imperial College London, has developed a machine-learning-based simulation method that can quickly and accurately screen MOFs best suited for atmospheric CO₂ capture.
< Figure 1. Concept diagram of Direct Air Capture (DAC) technology and carbon capture using Metal-Organic Frameworks (MOFs). MOFs are promising porous materials capable of capturing carbon dioxide from the atmosphere, drawing attention as a core material for DAC technology. >
To overcome the difficulty of discovering high-performance materials due to the complexity of structures and the limitations of predicting intermolecular interactions, the research team developed a machine learning force field (MLFF) capable of precisely predicting the interactions between CO₂, water (H₂O), and MOFs. This new method enables calculations of MOF adsorption properties with quantum-mechanics-level accuracy at vastly faster speeds than before.
Using this system, the team screened over 8,000 experimentally synthesized MOF structures, identifying more than 100 promising candidates for CO₂ capture. Notably, this included new candidates that had not been uncovered by traditional force-field-based simulations. The team also analyzed the relationships between MOF chemical structure and adsorption performance, proposing seven key chemical features that will help in designing new materials for DAC.
< Figure 2. Concept diagram of adsorption simulation using Machine Learning Force Field (MLFF). The developed MLFF is applicable to various MOF structures and allows for precise calculation of adsorption properties by predicting interaction energies during repetitive Widom insertion simulations. It is characterized by simultaneously achieving high accuracy and low computational cost compared to conventional classical force fields. >
This research is recognized as a significant advance in the DAC field, greatly enhancing materials design and simulation by precisely predicting MOF-CO₂ and MOF-H₂O interactions.
The results of this research, with Ph.D. candidate Yunsung Lim and Dr. Hyunsoo Park of KAIST as co-first authors, were published in the international academic journal Matter on June 12.
※Paper Title: Accelerating CO₂ direct air capture screening for metal–organic frameworks with a transferable machine learning force field
※DOI: 10.1016/j.matt.2025.102203
This research was supported by the Saudi Aramco-KAIST CO₂ Management Center and the Ministry of Science and ICT's Global C.L.E.A.N. Project.
2025.06.29 View 1437
KAIST Develops AI to Easily Find Promising Materials That Capture Only CO₂
< Photo 1. (From left) Professor Jihan Kim, Ph.D. candidate Yunsung Lim and Dr. Hyunsoo Park of the Department of Chemical and Biomolecular Engineering >
In order to help prevent the climate crisis, actively reducing already-emitted CO₂ is essential. Accordingly, direct air capture (DAC) — a technology that directly extracts only CO₂ from the air — is gaining attention. However, effectively capturing pure CO₂ is not easy due to water vapor (H₂O) present in the air. KAIST researchers have successfully used AI-driven machine learning techniques to identify the most promising CO₂-capturing materials among metal-organic frameworks (MOFs), a key class of materials studied for this technology.
KAIST (President Kwang Hyung Lee) announced on the 29th of June that a research team led by Professor Jihan Kim from the Department of Chemical and Biomolecular Engineering, in collaboration with a team at Imperial College London, has developed a machine-learning-based simulation method that can quickly and accurately screen MOFs best suited for atmospheric CO₂ capture.
< Figure 1. Concept diagram of Direct Air Capture (DAC) technology and carbon capture using Metal-Organic Frameworks (MOFs). MOFs are promising porous materials capable of capturing carbon dioxide from the atmosphere, drawing attention as a core material for DAC technology. >
To overcome the difficulty of discovering high-performance materials due to the complexity of structures and the limitations of predicting intermolecular interactions, the research team developed a machine learning force field (MLFF) capable of precisely predicting the interactions between CO₂, water (H₂O), and MOFs. This new method enables calculations of MOF adsorption properties with quantum-mechanics-level accuracy at vastly faster speeds than before.
Using this system, the team screened over 8,000 experimentally synthesized MOF structures, identifying more than 100 promising candidates for CO₂ capture. Notably, this included new candidates that had not been uncovered by traditional force-field-based simulations. The team also analyzed the relationships between MOF chemical structure and adsorption performance, proposing seven key chemical features that will help in designing new materials for DAC.
< Figure 2. Concept diagram of adsorption simulation using Machine Learning Force Field (MLFF). The developed MLFF is applicable to various MOF structures and allows for precise calculation of adsorption properties by predicting interaction energies during repetitive Widom insertion simulations. It is characterized by simultaneously achieving high accuracy and low computational cost compared to conventional classical force fields. >
This research is recognized as a significant advance in the DAC field, greatly enhancing materials design and simulation by precisely predicting MOF-CO₂ and MOF-H₂O interactions.
The results of this research, with Ph.D. candidate Yunsung Lim and Dr. Hyunsoo Park of KAIST as co-first authors, were published in the international academic journal Matter on June 12.
※Paper Title: Accelerating CO₂ direct air capture screening for metal–organic frameworks with a transferable machine learning force field
※DOI: 10.1016/j.matt.2025.102203
This research was supported by the Saudi Aramco-KAIST CO₂ Management Center and the Ministry of Science and ICT's Global C.L.E.A.N. Project.
2025.06.29 View 1437 -
 Distinguished Professor Sang Yup Lee Wins 2025 Global Metabolic Engineering Award
< Distinguished Professor Sang Yup Lee (Senior Vice President for Research) from the Department of Chemical & Biomolecular Engineering >
KAIST announced on the 20th that Professor Sang Yup Lee, who serves as the Vice President for Research and a Distinguished Professor at our university, has been awarded the '2025 Gregory N. Stephanopoulos Award for Metabolic Engineering' by the International Metabolic Engineering Society (IMES). Professor Lee delivered his award lecture at the 16th Metabolic Engineering Conference (ME16), held in Copenhagen, Denmark, from June 15th to 19th.
This award was established through contributions from the American Institute of Chemical Engineers (AIChE) Foundation, as well as fellow colleagues and acquaintances, to honor the achievements of Dr. Gregory Stephanopoulos, widely recognized as one of the pioneers of metabolic engineering. Presented biennially, the award recognizes scientists who have successfully commercialized fundamental research in metabolic engineering or have made outstanding contributions to the quantitative analysis, design, and modeling of metabolic pathways.
Professor Sang Yup Lee boasts an impressive record of over 770 journal papers and more than 860 patents. His groundbreaking research in metabolic engineering and biochemical engineering is highly acclaimed globally.
Throughout his 31 years as a professor at KAIST, Professor Lee has developed various metabolic engineering-based technologies and strategies. These advancements have been transferred to industries, facilitating the production of bulk chemicals, polymers, natural products, pharmaceuticals, and health functional foods. He has also founded companies and actively engages in advisory roles with various enterprises.
The International Metabolic Engineering Society (IMES) defines metabolic engineering as the manipulation of metabolic pathways in microorganisms or cells to produce useful substances (such as pharmaceuticals, biofuels, and chemical products). It utilizes tools like systems biology, synthetic biology, and computational modeling with the aim of enhancing the economic viability and sustainability of bio-based processes.
Furthermore, Professor Lee previously received the Merck Metabolic Engineering Award, a prominent international award in the field, in 2008. In 2018, he was honored with the Eni Award, often referred to as the Nobel Prize in energy, presented by the President of Italy.
Professor Sang Yup Lee remarked, "Metabolic engineering is a discipline that leads the current and future of biotechnology. It is a tremendous honor to receive this meaningful award at a time when the transition to a bio-based economy is accelerating. Together with my students and fellow researchers, we have generated numerous patents and transferred technologies to industry, and also established startups in the fields of biofuels, wound healing, and cosmetics. I will continue to pursue research that encompasses both fundamental research and technological commercialization."
The 'International Metabolic Engineering Society (IMES)' is a specialized society under the American Institute of Chemical Engineers. Its mission is to enable the production of various bio-based products, including pharmaceuticals, food additives, chemicals, and fuels, through metabolic engineering. The society hosts the Metabolic Engineering Conference biennially, offering researchers opportunities for knowledge exchange and collaboration.
2025.06.20 View 2143
Distinguished Professor Sang Yup Lee Wins 2025 Global Metabolic Engineering Award
< Distinguished Professor Sang Yup Lee (Senior Vice President for Research) from the Department of Chemical & Biomolecular Engineering >
KAIST announced on the 20th that Professor Sang Yup Lee, who serves as the Vice President for Research and a Distinguished Professor at our university, has been awarded the '2025 Gregory N. Stephanopoulos Award for Metabolic Engineering' by the International Metabolic Engineering Society (IMES). Professor Lee delivered his award lecture at the 16th Metabolic Engineering Conference (ME16), held in Copenhagen, Denmark, from June 15th to 19th.
This award was established through contributions from the American Institute of Chemical Engineers (AIChE) Foundation, as well as fellow colleagues and acquaintances, to honor the achievements of Dr. Gregory Stephanopoulos, widely recognized as one of the pioneers of metabolic engineering. Presented biennially, the award recognizes scientists who have successfully commercialized fundamental research in metabolic engineering or have made outstanding contributions to the quantitative analysis, design, and modeling of metabolic pathways.
Professor Sang Yup Lee boasts an impressive record of over 770 journal papers and more than 860 patents. His groundbreaking research in metabolic engineering and biochemical engineering is highly acclaimed globally.
Throughout his 31 years as a professor at KAIST, Professor Lee has developed various metabolic engineering-based technologies and strategies. These advancements have been transferred to industries, facilitating the production of bulk chemicals, polymers, natural products, pharmaceuticals, and health functional foods. He has also founded companies and actively engages in advisory roles with various enterprises.
The International Metabolic Engineering Society (IMES) defines metabolic engineering as the manipulation of metabolic pathways in microorganisms or cells to produce useful substances (such as pharmaceuticals, biofuels, and chemical products). It utilizes tools like systems biology, synthetic biology, and computational modeling with the aim of enhancing the economic viability and sustainability of bio-based processes.
Furthermore, Professor Lee previously received the Merck Metabolic Engineering Award, a prominent international award in the field, in 2008. In 2018, he was honored with the Eni Award, often referred to as the Nobel Prize in energy, presented by the President of Italy.
Professor Sang Yup Lee remarked, "Metabolic engineering is a discipline that leads the current and future of biotechnology. It is a tremendous honor to receive this meaningful award at a time when the transition to a bio-based economy is accelerating. Together with my students and fellow researchers, we have generated numerous patents and transferred technologies to industry, and also established startups in the fields of biofuels, wound healing, and cosmetics. I will continue to pursue research that encompasses both fundamental research and technological commercialization."
The 'International Metabolic Engineering Society (IMES)' is a specialized society under the American Institute of Chemical Engineers. Its mission is to enable the production of various bio-based products, including pharmaceuticals, food additives, chemicals, and fuels, through metabolic engineering. The society hosts the Metabolic Engineering Conference biennially, offering researchers opportunities for knowledge exchange and collaboration.
2025.06.20 View 2143 -
 KAIST Develops Glare-Free, Heat-Blocking 'Smart Window'... Applicable to Buildings and Vehicles
• Professor Hong Chul Moon of the Department of Chemical and Biomolecular Engineering develops RECM, a next-generation smart window technology, expecting cooling energy savings and effective indoor thermal management.
• When using the developed RECM, a significantly superior temperature reduction effect is observed compared to conventional windows.
• With a 'pedestrian-friendly smart window' design that eliminates glare by suppressing external reflections, it is expected to be adapted in architectural structures, transportation, and more.
< (From left) First author Hoy Jung Jo, Professor Hong Chul Moon >
In the building sector, which accounts for approximately 40% of global energy consumption, heat ingress through windows has been identified as a primary cause of wasted heating and cooling energy. Our research team has successfully developed a 'pedestrian-friendly smart window' technology capable of not only reducing heating and cooling energy in urban buildings but also resolving the persistent issue of 'light pollution' in urban living.
On the 17th of June, Professor Hong Chul Moon's research team at KAIST's Department of Chemical and Biomolecular Engineering announced the development of a 'smart window technology' that allows users to control the light and heat entering through windows according to their intent, and effectively neutralize glare from external sources.
Recently, 'active smart window' technology, which enables free adjustment of light and heat based on user operation, has garnered significant attention. Unlike conventional windows that passively react to changes in temperature or light, this is a next-generation window system that can be controlled in real-time via electrical signals.
The next-generation smart window technology developed by the research team, RECM (Reversible Electrodeposition and Electrochromic Mirror), is a smart window system based on a single-structured *electrochromic device that can actively control the transmittance of visible light and near-infrared (heat).
*Electrochromic device: A device whose optical properties change in response to an electrical signal.
In particular, by effectively suppressing the glare phenomenon caused by external reflected light—a problem previously identified in traditional metal *deposition smart windows—through the combined application of electrochromic materials, a 'pedestrian-friendly smart window' suitable for building facades has been realized.
*Deposition: A process involving the electrochemical reaction to coat metal ions, such as Ag+, onto an electrode surface in solid form.
The RECM system developed in this study operates in three modes depending on voltage control.
Mode I (Transparent Mode) is advantageous for allowing sunlight to enter the indoor space during winter, as it transmits both light and heat like ordinary glass.
In Mode II (Colored Mode), *Prussian Blue (PB) and **DHV+• chemical species are formed through a redox (oxidation-reduction) reaction, causing the window to turn a deep blue color. In this state, light is absorbed, and only a portion of the heat is transmitted, allowing for privacy while enabling appropriate indoor temperature control.
*Prussian Blue: An electrochromic material that transitions between colorless and blue upon electrical stimulation.
**DHV+•: A radical state colored molecule generated upon electrical stimulation.
Mode III (Colored and Deposition Mode) involves the reduction and deposition of silver (Ag+) ions on the electrode surface, reflecting both light and heat. Concurrently, the colored material absorbs the reflected light, effectively blocking glare for external pedestrians.
The research team validated the practical indoor temperature reduction effect of the RECM technology through experiments utilizing a miniature model house. When a conventional glass window was installed, the indoor temperature rose to 58.7°C within 45 minutes. Conversely, when RECM was operated in Mode III, the temperature reached 31.5°C, demonstrating a temperature reduction effect of approximately 27.2°C.
Furthermore, since each state transition is achievable solely by electrical signals, it is regarded as an active smart technology capable of instantaneous response according to season, time, and intended use.
< Figure 1. Operation mechanism of the RECM smart window. The RECM system can switch among three states—transparent, colored, and colored & deposition—via electrical stimulation. At -1.6 V, DHV•+ and Prussian Blue (PB) are formed, blocking visible light to provide privacy protection and heat blocking. At -2.0 V, silver (Ag) is deposited on the electrode surface, reflecting light and heat, while DHV•+ and Prussian Blue absorb reflected light, effectively suppressing external glare. Through this mechanism, it functions as an active smart window that simultaneously controls light, heat, and glare. >
Professor Hong Chul Moon of KAIST, the corresponding author of this study, stated, "This research goes beyond existing smart window technologies limited to visible light control, presenting a truly smart window platform that comprehensively considers not only active indoor thermal control but also the visual safety of pedestrians." He added, "Various applications are anticipated, from urban buildings to vehicles and trains."
< Figure 2. Analysis of glare suppression effect of conventional reflective smart windows and RECM. This figure presents the results comparing the glare phenomenon occurring during silver (Ag) deposition between conventional reflective smart windows and RECM Mode III. Conventional reflective devices resulted in strong reflected light on the desk surface due to their high reflectivity. In contrast, RECM Mode III, where the colored material absorbed reflected light, showed a 33% reduction in reflected light intensity, and no reflected light was observed from outside. This highlights the RECM system's distinctiveness and practicality as a 'pedestrian-friendly smart window' optimized for dense urban environments, extending beyond just heat blocking. >
The findings of this research were published on June 13, 2025, in Volume 10, Issue 6 of 'ACS Energy Letters'. The listed authors for this publication are Hoy Jung Jo, Yeon Jae Jang, Hyeon-Don Kim, Kwang-Seop Kim, and Hong Chul Moon.
※ Paper Title: Glare-Free, Energy-Efficient Smart Windows: A Pedestrian-Friendly System with Dynamically Tunable Light and Heat Regulation
※ DOI: 10.1021/acsenergylett.5c00637
< Figure 3. Temperature reduction performance verification in a miniature model house. The actual heat blocking effect was evaluated by applying RECM devices to a model building. Under identical conditions, the indoor temperature with ordinary glass rose to 58.7°C, whereas with RECM in Mode III, it reached 31.5°C, demonstrating a maximum temperature reduction effect of 27.2°C. The indoor temperature difference was also visually confirmed through thermal images, which proves the potential for indoor temperature control in urban buildings. >
This research was supported by the Nano & Material Technology Development Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT and the internal research program of the Korea Institute of Machinery and Materials.
2025.06.20 View 4151
KAIST Develops Glare-Free, Heat-Blocking 'Smart Window'... Applicable to Buildings and Vehicles
• Professor Hong Chul Moon of the Department of Chemical and Biomolecular Engineering develops RECM, a next-generation smart window technology, expecting cooling energy savings and effective indoor thermal management.
• When using the developed RECM, a significantly superior temperature reduction effect is observed compared to conventional windows.
• With a 'pedestrian-friendly smart window' design that eliminates glare by suppressing external reflections, it is expected to be adapted in architectural structures, transportation, and more.
< (From left) First author Hoy Jung Jo, Professor Hong Chul Moon >
In the building sector, which accounts for approximately 40% of global energy consumption, heat ingress through windows has been identified as a primary cause of wasted heating and cooling energy. Our research team has successfully developed a 'pedestrian-friendly smart window' technology capable of not only reducing heating and cooling energy in urban buildings but also resolving the persistent issue of 'light pollution' in urban living.
On the 17th of June, Professor Hong Chul Moon's research team at KAIST's Department of Chemical and Biomolecular Engineering announced the development of a 'smart window technology' that allows users to control the light and heat entering through windows according to their intent, and effectively neutralize glare from external sources.
Recently, 'active smart window' technology, which enables free adjustment of light and heat based on user operation, has garnered significant attention. Unlike conventional windows that passively react to changes in temperature or light, this is a next-generation window system that can be controlled in real-time via electrical signals.
The next-generation smart window technology developed by the research team, RECM (Reversible Electrodeposition and Electrochromic Mirror), is a smart window system based on a single-structured *electrochromic device that can actively control the transmittance of visible light and near-infrared (heat).
*Electrochromic device: A device whose optical properties change in response to an electrical signal.
In particular, by effectively suppressing the glare phenomenon caused by external reflected light—a problem previously identified in traditional metal *deposition smart windows—through the combined application of electrochromic materials, a 'pedestrian-friendly smart window' suitable for building facades has been realized.
*Deposition: A process involving the electrochemical reaction to coat metal ions, such as Ag+, onto an electrode surface in solid form.
The RECM system developed in this study operates in three modes depending on voltage control.
Mode I (Transparent Mode) is advantageous for allowing sunlight to enter the indoor space during winter, as it transmits both light and heat like ordinary glass.
In Mode II (Colored Mode), *Prussian Blue (PB) and **DHV+• chemical species are formed through a redox (oxidation-reduction) reaction, causing the window to turn a deep blue color. In this state, light is absorbed, and only a portion of the heat is transmitted, allowing for privacy while enabling appropriate indoor temperature control.
*Prussian Blue: An electrochromic material that transitions between colorless and blue upon electrical stimulation.
**DHV+•: A radical state colored molecule generated upon electrical stimulation.
Mode III (Colored and Deposition Mode) involves the reduction and deposition of silver (Ag+) ions on the electrode surface, reflecting both light and heat. Concurrently, the colored material absorbs the reflected light, effectively blocking glare for external pedestrians.
The research team validated the practical indoor temperature reduction effect of the RECM technology through experiments utilizing a miniature model house. When a conventional glass window was installed, the indoor temperature rose to 58.7°C within 45 minutes. Conversely, when RECM was operated in Mode III, the temperature reached 31.5°C, demonstrating a temperature reduction effect of approximately 27.2°C.
Furthermore, since each state transition is achievable solely by electrical signals, it is regarded as an active smart technology capable of instantaneous response according to season, time, and intended use.
< Figure 1. Operation mechanism of the RECM smart window. The RECM system can switch among three states—transparent, colored, and colored & deposition—via electrical stimulation. At -1.6 V, DHV•+ and Prussian Blue (PB) are formed, blocking visible light to provide privacy protection and heat blocking. At -2.0 V, silver (Ag) is deposited on the electrode surface, reflecting light and heat, while DHV•+ and Prussian Blue absorb reflected light, effectively suppressing external glare. Through this mechanism, it functions as an active smart window that simultaneously controls light, heat, and glare. >
Professor Hong Chul Moon of KAIST, the corresponding author of this study, stated, "This research goes beyond existing smart window technologies limited to visible light control, presenting a truly smart window platform that comprehensively considers not only active indoor thermal control but also the visual safety of pedestrians." He added, "Various applications are anticipated, from urban buildings to vehicles and trains."
< Figure 2. Analysis of glare suppression effect of conventional reflective smart windows and RECM. This figure presents the results comparing the glare phenomenon occurring during silver (Ag) deposition between conventional reflective smart windows and RECM Mode III. Conventional reflective devices resulted in strong reflected light on the desk surface due to their high reflectivity. In contrast, RECM Mode III, where the colored material absorbed reflected light, showed a 33% reduction in reflected light intensity, and no reflected light was observed from outside. This highlights the RECM system's distinctiveness and practicality as a 'pedestrian-friendly smart window' optimized for dense urban environments, extending beyond just heat blocking. >
The findings of this research were published on June 13, 2025, in Volume 10, Issue 6 of 'ACS Energy Letters'. The listed authors for this publication are Hoy Jung Jo, Yeon Jae Jang, Hyeon-Don Kim, Kwang-Seop Kim, and Hong Chul Moon.
※ Paper Title: Glare-Free, Energy-Efficient Smart Windows: A Pedestrian-Friendly System with Dynamically Tunable Light and Heat Regulation
※ DOI: 10.1021/acsenergylett.5c00637
< Figure 3. Temperature reduction performance verification in a miniature model house. The actual heat blocking effect was evaluated by applying RECM devices to a model building. Under identical conditions, the indoor temperature with ordinary glass rose to 58.7°C, whereas with RECM in Mode III, it reached 31.5°C, demonstrating a maximum temperature reduction effect of 27.2°C. The indoor temperature difference was also visually confirmed through thermal images, which proves the potential for indoor temperature control in urban buildings. >
This research was supported by the Nano & Material Technology Development Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT and the internal research program of the Korea Institute of Machinery and Materials.
2025.06.20 View 4151 -
 High-Resolution Spectrometer that Fits into Smartphones Developed by KAIST Researchers
- Professor Mooseok Jang's research team at the Department of Bio and Brain Engineering develops an ultra-compact, high-resolution spectrometer using 'double-layer disordered metasurfaces' that generate unique random patterns depending on light's color.
- Unlike conventional dispersion-based spectrometers that were difficult to apply to portable devices, this new concept spectrometer technology achieves 1nm-level high resolution in a device smaller than 1cm, comparable in size to a fingernail.
- It can be utilized as a built-in spectrometer in smartphones and wearable devices in the future, and can be expanded to advanced optical technologies such as hyperspectral imaging and ultrafast imaging.
< Photo 1. (From left) Professor Mooseok Jang, Dong-gu Lee (Ph.D. candidate), Gookho Song (Ph.D. candidate) >
Color, as the way light's wavelength is perceived by the human eye, goes beyond a simple aesthetic element, containing important scientific information like a substance's composition or state. Spectrometers are optical devices that analyze material properties by decomposing light into its constituent wavelengths, and they are widely used in various scientific and industrial fields, including material analysis, chemical component detection, and life science research. Existing high-resolution spectrometers were large and complex, making them difficult for widespread daily use. However, thanks to the ultra-compact, high-resolution spectrometer developed by KAIST researchers, it is now expected that light's color information can be utilized even within smartphones or wearable devices.
KAIST (President Kwang Hyung Lee) announced on the 13th that Professor Mooseok Jang's research team at the Department of Bio and Brain Engineering has successfully developed a reconstruction-based spectrometer technology using double-layer disordered metasurfaces*.
*Double-layer disordered metasurface: An innovative optical device that complexly scatters light through two layers of disordered nanostructures, creating unique and predictable speckle patterns for each wavelength.
Existing high-resolution spectrometers have a large form factor, on the order of tens of centimeters, and require complex calibration processes to maintain accuracy. This fundamentally stems from the operating principle of traditional dispersive elements, such as gratings and prisms, which separate light wavelengths along the propagation direction, much like a rainbow separates colors. Consequently, despite the potential for light's color information to be widely useful in daily life, spectroscopic technology has been limited to laboratory or industrial manufacturing environments.
< Figure 1. Through a simple structure consisting of a double layer of disordered metasurfaces and an image sensor, it was shown that speckles of predictable spectral channels with high spectral resolution can be generated in a compact form factor. The high similarity between the measured and calculated speckles was used to solve the inverse problem and verify the ability to reconstruct the spectrum. >
The research team devised a method that departs from the conventional spectroscopic paradigm of using diffraction gratings or prisms, which establish a one-to-one correspondence between light's color information and its propagation direction, by utilizing designed disordered structures as optical components. In this process, they employed metasurfaces, which can freely control the light propagation process using structures tens to hundreds of nanometers in size, to accurately implement 'complex random patterns (speckle*)'.
*Speckle: An irregular pattern of light intensity created by the interference of multiple wavefronts of light.
Specifically, they developed a method that involves implementing a double-layer disordered metasurface to generate wavelength-specific speckle patterns and then reconstructing precise color information (wavelength) of the light from the random patterns measured by a camera.
As a result, they successfully developed a new concept spectrometer technology that can accurately measure light across a broad range of visible to infrared (440-1,300nm) with a high resolution of 1 nanometer (nm) in a device smaller than a fingernail (less than 1cm) using only a single image capture.
< Figure 2. A disordered metasurface is a metasurface with irregularly arranged structures ranging from tens to hundreds of nanometers in size. In a double-layer structure, a propagation space is placed between the two metasurfaces to control the output speckle with high degrees of freedom, thereby achieving a spectral resolution of 1 nm even in a form factor smaller than 1 cm. >
Dong-gu Lee, a lead author of this study, stated, "This technology is implemented in a way that is directly integrated with commercial image sensors, and we expect that it will enable easy acquisition and utilization of light's wavelength information in daily life when built into mobile devices in the future."
Professor Mooseok Jang said, "This technology overcomes the limitations of existing RGB three-color based machine vision fields, which only distinguish and recognize three color components (red, green, blue), and has diverse applications. We anticipate various applied research for this technology, which expands the horizon of laboratory-level technology to daily-level machine vision technology for applications such as food component analysis, crop health diagnosis, skin health measurement, environmental pollution detection, and bio/medical diagnostics." He added, "Furthermore, it can be extended to various advanced optical technologies such as hyperspectral imaging, which records wavelength and spatial information simultaneously with high resolution, 3D optical trapping technology, which precisely controls light of multiple wavelengths into desired forms, and ultrafast imaging technology, which captures phenomena occurring in very short periods."
This research was collaboratively led by Dong-gu Lee (Ph.D. candidate) and Gookho Song (Ph.D. candidate) from the KAIST Department of Bio and Brain Engineering as co-first authors, with Professor Mooseok Jang as the corresponding author. The findings were published online in the international journal Science Advances on May 28, 2025.* Paper Title: Reconstructive spectrometer using double-layer disordered metasurfaces* DOI: 10.1126/sciadv.adv2376
This research was supported by the Samsung Research Funding and Incubation Center of Samsung Electronics grant, the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT), and the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MSIT).
2025.06.13 View 2658
High-Resolution Spectrometer that Fits into Smartphones Developed by KAIST Researchers
- Professor Mooseok Jang's research team at the Department of Bio and Brain Engineering develops an ultra-compact, high-resolution spectrometer using 'double-layer disordered metasurfaces' that generate unique random patterns depending on light's color.
- Unlike conventional dispersion-based spectrometers that were difficult to apply to portable devices, this new concept spectrometer technology achieves 1nm-level high resolution in a device smaller than 1cm, comparable in size to a fingernail.
- It can be utilized as a built-in spectrometer in smartphones and wearable devices in the future, and can be expanded to advanced optical technologies such as hyperspectral imaging and ultrafast imaging.
< Photo 1. (From left) Professor Mooseok Jang, Dong-gu Lee (Ph.D. candidate), Gookho Song (Ph.D. candidate) >
Color, as the way light's wavelength is perceived by the human eye, goes beyond a simple aesthetic element, containing important scientific information like a substance's composition or state. Spectrometers are optical devices that analyze material properties by decomposing light into its constituent wavelengths, and they are widely used in various scientific and industrial fields, including material analysis, chemical component detection, and life science research. Existing high-resolution spectrometers were large and complex, making them difficult for widespread daily use. However, thanks to the ultra-compact, high-resolution spectrometer developed by KAIST researchers, it is now expected that light's color information can be utilized even within smartphones or wearable devices.
KAIST (President Kwang Hyung Lee) announced on the 13th that Professor Mooseok Jang's research team at the Department of Bio and Brain Engineering has successfully developed a reconstruction-based spectrometer technology using double-layer disordered metasurfaces*.
*Double-layer disordered metasurface: An innovative optical device that complexly scatters light through two layers of disordered nanostructures, creating unique and predictable speckle patterns for each wavelength.
Existing high-resolution spectrometers have a large form factor, on the order of tens of centimeters, and require complex calibration processes to maintain accuracy. This fundamentally stems from the operating principle of traditional dispersive elements, such as gratings and prisms, which separate light wavelengths along the propagation direction, much like a rainbow separates colors. Consequently, despite the potential for light's color information to be widely useful in daily life, spectroscopic technology has been limited to laboratory or industrial manufacturing environments.
< Figure 1. Through a simple structure consisting of a double layer of disordered metasurfaces and an image sensor, it was shown that speckles of predictable spectral channels with high spectral resolution can be generated in a compact form factor. The high similarity between the measured and calculated speckles was used to solve the inverse problem and verify the ability to reconstruct the spectrum. >
The research team devised a method that departs from the conventional spectroscopic paradigm of using diffraction gratings or prisms, which establish a one-to-one correspondence between light's color information and its propagation direction, by utilizing designed disordered structures as optical components. In this process, they employed metasurfaces, which can freely control the light propagation process using structures tens to hundreds of nanometers in size, to accurately implement 'complex random patterns (speckle*)'.
*Speckle: An irregular pattern of light intensity created by the interference of multiple wavefronts of light.
Specifically, they developed a method that involves implementing a double-layer disordered metasurface to generate wavelength-specific speckle patterns and then reconstructing precise color information (wavelength) of the light from the random patterns measured by a camera.
As a result, they successfully developed a new concept spectrometer technology that can accurately measure light across a broad range of visible to infrared (440-1,300nm) with a high resolution of 1 nanometer (nm) in a device smaller than a fingernail (less than 1cm) using only a single image capture.
< Figure 2. A disordered metasurface is a metasurface with irregularly arranged structures ranging from tens to hundreds of nanometers in size. In a double-layer structure, a propagation space is placed between the two metasurfaces to control the output speckle with high degrees of freedom, thereby achieving a spectral resolution of 1 nm even in a form factor smaller than 1 cm. >
Dong-gu Lee, a lead author of this study, stated, "This technology is implemented in a way that is directly integrated with commercial image sensors, and we expect that it will enable easy acquisition and utilization of light's wavelength information in daily life when built into mobile devices in the future."
Professor Mooseok Jang said, "This technology overcomes the limitations of existing RGB three-color based machine vision fields, which only distinguish and recognize three color components (red, green, blue), and has diverse applications. We anticipate various applied research for this technology, which expands the horizon of laboratory-level technology to daily-level machine vision technology for applications such as food component analysis, crop health diagnosis, skin health measurement, environmental pollution detection, and bio/medical diagnostics." He added, "Furthermore, it can be extended to various advanced optical technologies such as hyperspectral imaging, which records wavelength and spatial information simultaneously with high resolution, 3D optical trapping technology, which precisely controls light of multiple wavelengths into desired forms, and ultrafast imaging technology, which captures phenomena occurring in very short periods."
This research was collaboratively led by Dong-gu Lee (Ph.D. candidate) and Gookho Song (Ph.D. candidate) from the KAIST Department of Bio and Brain Engineering as co-first authors, with Professor Mooseok Jang as the corresponding author. The findings were published online in the international journal Science Advances on May 28, 2025.* Paper Title: Reconstructive spectrometer using double-layer disordered metasurfaces* DOI: 10.1126/sciadv.adv2376
This research was supported by the Samsung Research Funding and Incubation Center of Samsung Electronics grant, the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT), and the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MSIT).
2025.06.13 View 2658 -
 KAIST Successfully Develops High-Performance Water Electrolysis Without Platinum, Bringing Hydrogen Economy Closer
< Photo 1. (Front row, from left) Jeesoo Park (Ph.D. Candidate), Professor Hee-Tak Kim (Back row, from left) Kyunghwa Seok (Ph.D. Candidate), Dr. Gisu Doo, Euntaek Oh (Ph.D. Candidate) >
Hydrogen is gaining attention as a clean energy source that emits no carbon. Among various methods, water electrolysis, which splits water into hydrogen and oxygen using electricity, is recognized as an eco-friendly hydrogen production method. Specifically, proton exchange membrane water electrolysis (PEMWE) is considered a next-generation hydrogen production technology due to its ability to produce high-purity hydrogen at high pressure. However, existing PEMWE technology has faced limitations in commercialization due to its heavy reliance on expensive precious metal catalysts and coating materials. Korean researchers have now proposed a new solution to address these technical and economic bottlenecks.
KAIST (President Kwang Hyung Lee) announced on June 11th that a research team led by Professor Hee-Tak Kim of the Department of Chemical and Biomolecular Engineering, in a joint study with Dr. Gisu Doo of the Korea Institute of Energy Research (KIER, President Chang-keun Lee), has developed a next-generation water electrolysis technology that achieves high performance without the need for expensive platinum (Pt) coating.
The research team focused on the primary reason why 'iridium oxide (IrOx),' a highly active catalyst for water electrolysis electrodes, fails to perform optimally. They found that this is due to inefficient electron transfer and, for the first time in the world, demonstrated that performance can be maximized simply by controlling the catalyst particle size.
In this study, it was revealed that the reason iridium oxide catalysts do not exhibit excellent performance without platinum coating is due to 'electron transport resistance' that occurs at the interface between the catalyst, the ion conductor (hereinafter referred to as ionomer), and the Ti (titanium) substrate—core components inherently used together in water electrolysis electrodes.
Specifically, they identified that the 'pinch-off' phenomenon, where the electron pathway is blocked between the catalyst, ionomer, and titanium substrate, is the critical cause of reduced conductivity. The ionomer has properties close to an electron insulator, thereby hindering electron flow when it surrounds catalyst particles. Furthermore, when the ionomer comes into contact with the titanium substrate, an electron barrier forms on the surface oxide layer of the titanium substrate, significantly increasing resistance.
< Figure 1. Infographic related to electron transport resistance at the catalyst layer/diffusion layer interface >
To address this, the research team fabricated and compared catalysts of various particle sizes. Through single-cell evaluation and multiphysics simulations, they demonstrated, for the first time globally, that when iridium oxide catalyst particles with a size of 20 nanometers (nm) or larger are used, the ionomer mixed region decreases, ensuring an electron pathway and restoring conductivity.
Moreover, they successfully optimized the interfacial structure through precise design, simultaneously ensuring both reactivity and electron transport. This achievement demonstrated that the previously unavoidable trade-off between catalyst activity and conductivity can be overcome through meticulous interfacial design.
This breakthrough is expected to be a significant milestone not only for the development of high-performance catalyst materials but also for the future commercialization of proton exchange membrane water electrolysis systems that can achieve high efficiency while drastically reducing the amount of precious metals used.
Professor Hee-Tak Kim stated, "This research presents a new interface design strategy that can resolve the interfacial conductivity problem, which was a bottleneck in high-performance water electrolysis technology." He added, "By securing high performance even without expensive materials like platinum, it will be a stepping stone closer to realizing a hydrogen economy."
This research, with Jeesoo Park, a Ph.D. student from the Department of Chemical and Biomolecular Engineering at KAIST, as the first author, was published on June 7th in 'Energy & Environmental Science' (IF: 32.4, 2025), a leading international journal in the energy and environmental fields, and was recognized for its innovativeness and impact. (Paper title: On the interface electron transport problem of highly active IrOx catalysts, DOI: 10.1039/D4EE05816J).
This research was supported by the New and Renewable Energy Core Technology Development Project of the Ministry of Trade, Industry and Energy.
2025.06.11 View 3459
KAIST Successfully Develops High-Performance Water Electrolysis Without Platinum, Bringing Hydrogen Economy Closer
< Photo 1. (Front row, from left) Jeesoo Park (Ph.D. Candidate), Professor Hee-Tak Kim (Back row, from left) Kyunghwa Seok (Ph.D. Candidate), Dr. Gisu Doo, Euntaek Oh (Ph.D. Candidate) >
Hydrogen is gaining attention as a clean energy source that emits no carbon. Among various methods, water electrolysis, which splits water into hydrogen and oxygen using electricity, is recognized as an eco-friendly hydrogen production method. Specifically, proton exchange membrane water electrolysis (PEMWE) is considered a next-generation hydrogen production technology due to its ability to produce high-purity hydrogen at high pressure. However, existing PEMWE technology has faced limitations in commercialization due to its heavy reliance on expensive precious metal catalysts and coating materials. Korean researchers have now proposed a new solution to address these technical and economic bottlenecks.
KAIST (President Kwang Hyung Lee) announced on June 11th that a research team led by Professor Hee-Tak Kim of the Department of Chemical and Biomolecular Engineering, in a joint study with Dr. Gisu Doo of the Korea Institute of Energy Research (KIER, President Chang-keun Lee), has developed a next-generation water electrolysis technology that achieves high performance without the need for expensive platinum (Pt) coating.
The research team focused on the primary reason why 'iridium oxide (IrOx),' a highly active catalyst for water electrolysis electrodes, fails to perform optimally. They found that this is due to inefficient electron transfer and, for the first time in the world, demonstrated that performance can be maximized simply by controlling the catalyst particle size.
In this study, it was revealed that the reason iridium oxide catalysts do not exhibit excellent performance without platinum coating is due to 'electron transport resistance' that occurs at the interface between the catalyst, the ion conductor (hereinafter referred to as ionomer), and the Ti (titanium) substrate—core components inherently used together in water electrolysis electrodes.
Specifically, they identified that the 'pinch-off' phenomenon, where the electron pathway is blocked between the catalyst, ionomer, and titanium substrate, is the critical cause of reduced conductivity. The ionomer has properties close to an electron insulator, thereby hindering electron flow when it surrounds catalyst particles. Furthermore, when the ionomer comes into contact with the titanium substrate, an electron barrier forms on the surface oxide layer of the titanium substrate, significantly increasing resistance.
< Figure 1. Infographic related to electron transport resistance at the catalyst layer/diffusion layer interface >
To address this, the research team fabricated and compared catalysts of various particle sizes. Through single-cell evaluation and multiphysics simulations, they demonstrated, for the first time globally, that when iridium oxide catalyst particles with a size of 20 nanometers (nm) or larger are used, the ionomer mixed region decreases, ensuring an electron pathway and restoring conductivity.
Moreover, they successfully optimized the interfacial structure through precise design, simultaneously ensuring both reactivity and electron transport. This achievement demonstrated that the previously unavoidable trade-off between catalyst activity and conductivity can be overcome through meticulous interfacial design.
This breakthrough is expected to be a significant milestone not only for the development of high-performance catalyst materials but also for the future commercialization of proton exchange membrane water electrolysis systems that can achieve high efficiency while drastically reducing the amount of precious metals used.
Professor Hee-Tak Kim stated, "This research presents a new interface design strategy that can resolve the interfacial conductivity problem, which was a bottleneck in high-performance water electrolysis technology." He added, "By securing high performance even without expensive materials like platinum, it will be a stepping stone closer to realizing a hydrogen economy."
This research, with Jeesoo Park, a Ph.D. student from the Department of Chemical and Biomolecular Engineering at KAIST, as the first author, was published on June 7th in 'Energy & Environmental Science' (IF: 32.4, 2025), a leading international journal in the energy and environmental fields, and was recognized for its innovativeness and impact. (Paper title: On the interface electron transport problem of highly active IrOx catalysts, DOI: 10.1039/D4EE05816J).
This research was supported by the New and Renewable Energy Core Technology Development Project of the Ministry of Trade, Industry and Energy.
2025.06.11 View 3459