Science
-
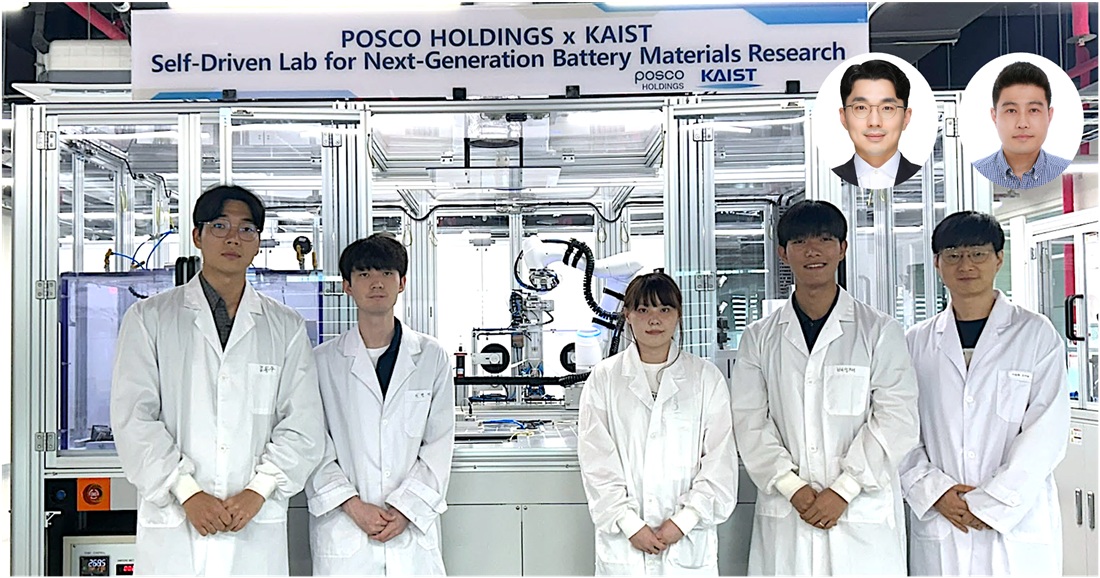 Material Innovation Realized with Robotic Arms and AI, Without Human Researchers
<(From Left) M.S candidate Dongwoo Kim from KAIST, Ph.D candidate Hyun-Gi Lee from KAIST, Intern Yeham Kang from KAIST, M.S candidate Seongjae Bae from KAIST, Professor Dong-Hwa Seo from KAIST, (From top right, from left) Senior Researcher Inchul Park from POSCO Holdings, Senior Researcher Jung Woo Park, senior researcher from POSCO Holdings>
A joint research team from industry and academia in Korea has successfully developed an autonomous lab that uses AI and automation to create new cathode materials for secondary batteries. This system operates without human intervention, drastically reducing researcher labor and cutting the material discovery period by 93%.
* Autonomous Lab: A platform that autonomously designs, conducts, and analyzes experiments to find the optimal material.
KAIST (President Kwang Hyung Lee) announced on the 3rd of August that the research team led by Professor Dong-Hwa Seo of the Department of Materials Science and Engineering, in collaboration with the team of LIB Materials Research Center in Energy Materials R&D Laboratories at POSCO Holdings' POSCO N.EX.T Hub (Director Ki Soo Kim), built the lab to explore cathode materials using AI and automation technology.
Developing secondary battery cathode materials is a labor-intensive and time-consuming process for skilled researchers. It involves extensive exploration of various compositions and experimental variables through weighing, transporting, mixing, sintering*, and analyzing samples.
* Sintering: A process in which powder particles are heated to form a single solid mass through thermal activation.
The research team's autonomous lab combines an automated system with an AI model. The system handles all experimental steps—weighing, mixing, pelletizing, sintering, and analysis—without human interference. The AI model then interprets the data, learns from it, and selects the best candidates for the next experiment.
<Figure 1. Outline of the Anode Material Autonomous Exploration Laboratory>
To increase efficiency, the team designed the automation system with separate modules for each process, which are managed by a central robotic arm. This modular approach reduces the system's reliance on the robotic arm.
The team also significantly improved the synthesis speed by using a new high-speed sintering method, which is 50 times faster than the conventional low-speed method. This allows the autonomous lab to acquire 12 times more material data compared to traditional, researcher-led experiments.
<Figure 2. Synthesis of Cathode Material Using a High-Speed Sintering Device>
The vast amount of data collected is automatically interpreted by the AI model to extract information such as synthesized phases and impurity ratios. This data is systematically stored to create a high-quality database, which then serves as training data for an optimization AI model. This creates a closed-loop experimental system that recommends the next cathode composition and synthesis conditions for the automated system.
* Closed-loop experimental system: A system that independently performs all experimental processes without researcher intervention.
Operating this intelligent automation system 24 hours a day can secure more than 12 times the experimental data and shorten material discovery time by 93%. For a project requiring 500 experiments, the system can complete the work in about 6 days, whereas a traditional researcher-led approach would take 84 days.
During development, POSCO Holdings team managed the overall project planning, reviewed the platform design, and co-developed the partial module design and AI-based experimental model. The KAIST team, led by Professor Dong-hwa Seo, was responsible for the actual system implementation and operation, including platform design, module fabrication, algorithm creation, and system verification and improvement.
Professor Dong-Hwa Seo of KAIST stated that this system is a solution to the decrease in research personnel due to the low birth rate in Korea. He expects it will enhance global competitiveness by accelerating secondary battery material development through the acquisition of high-quality data.
<Figure 3. Exterior View (Side) of the Cathode Material Autonomous Exploration Laboratory>
POSCO N.EX.T Hub plans to apply an upgraded version of this autonomous lab to its own research facilities after 2026 to dramatically speed up next-generation secondary battery material development. They are planning further developments to enhance the system's stability and scalability, and hope this industry-academia collaboration will serve as a model for using innovative technology in real-world R&D.
<Figure 4. Exterior View (Front) of the Cathode Material Autonomous Exploration Laboratory>
The research was spearheaded by Ph.D. student Hyun-Gi Lee, along with master's students Seongjae Bae and Dongwoo Kim from Professor Dong-Hwa Seo’s lab at KAIST. Senior researchers Jung Woo Park and Inchul Park from LIB Materials Research Center of POSCO N.EX.T Hub's Energy Materials R&D Laboratories (Director Jeongjin Hong) also participated.
2025.08.06 View 261
Material Innovation Realized with Robotic Arms and AI, Without Human Researchers
<(From Left) M.S candidate Dongwoo Kim from KAIST, Ph.D candidate Hyun-Gi Lee from KAIST, Intern Yeham Kang from KAIST, M.S candidate Seongjae Bae from KAIST, Professor Dong-Hwa Seo from KAIST, (From top right, from left) Senior Researcher Inchul Park from POSCO Holdings, Senior Researcher Jung Woo Park, senior researcher from POSCO Holdings>
A joint research team from industry and academia in Korea has successfully developed an autonomous lab that uses AI and automation to create new cathode materials for secondary batteries. This system operates without human intervention, drastically reducing researcher labor and cutting the material discovery period by 93%.
* Autonomous Lab: A platform that autonomously designs, conducts, and analyzes experiments to find the optimal material.
KAIST (President Kwang Hyung Lee) announced on the 3rd of August that the research team led by Professor Dong-Hwa Seo of the Department of Materials Science and Engineering, in collaboration with the team of LIB Materials Research Center in Energy Materials R&D Laboratories at POSCO Holdings' POSCO N.EX.T Hub (Director Ki Soo Kim), built the lab to explore cathode materials using AI and automation technology.
Developing secondary battery cathode materials is a labor-intensive and time-consuming process for skilled researchers. It involves extensive exploration of various compositions and experimental variables through weighing, transporting, mixing, sintering*, and analyzing samples.
* Sintering: A process in which powder particles are heated to form a single solid mass through thermal activation.
The research team's autonomous lab combines an automated system with an AI model. The system handles all experimental steps—weighing, mixing, pelletizing, sintering, and analysis—without human interference. The AI model then interprets the data, learns from it, and selects the best candidates for the next experiment.
<Figure 1. Outline of the Anode Material Autonomous Exploration Laboratory>
To increase efficiency, the team designed the automation system with separate modules for each process, which are managed by a central robotic arm. This modular approach reduces the system's reliance on the robotic arm.
The team also significantly improved the synthesis speed by using a new high-speed sintering method, which is 50 times faster than the conventional low-speed method. This allows the autonomous lab to acquire 12 times more material data compared to traditional, researcher-led experiments.
<Figure 2. Synthesis of Cathode Material Using a High-Speed Sintering Device>
The vast amount of data collected is automatically interpreted by the AI model to extract information such as synthesized phases and impurity ratios. This data is systematically stored to create a high-quality database, which then serves as training data for an optimization AI model. This creates a closed-loop experimental system that recommends the next cathode composition and synthesis conditions for the automated system.
* Closed-loop experimental system: A system that independently performs all experimental processes without researcher intervention.
Operating this intelligent automation system 24 hours a day can secure more than 12 times the experimental data and shorten material discovery time by 93%. For a project requiring 500 experiments, the system can complete the work in about 6 days, whereas a traditional researcher-led approach would take 84 days.
During development, POSCO Holdings team managed the overall project planning, reviewed the platform design, and co-developed the partial module design and AI-based experimental model. The KAIST team, led by Professor Dong-hwa Seo, was responsible for the actual system implementation and operation, including platform design, module fabrication, algorithm creation, and system verification and improvement.
Professor Dong-Hwa Seo of KAIST stated that this system is a solution to the decrease in research personnel due to the low birth rate in Korea. He expects it will enhance global competitiveness by accelerating secondary battery material development through the acquisition of high-quality data.
<Figure 3. Exterior View (Side) of the Cathode Material Autonomous Exploration Laboratory>
POSCO N.EX.T Hub plans to apply an upgraded version of this autonomous lab to its own research facilities after 2026 to dramatically speed up next-generation secondary battery material development. They are planning further developments to enhance the system's stability and scalability, and hope this industry-academia collaboration will serve as a model for using innovative technology in real-world R&D.
<Figure 4. Exterior View (Front) of the Cathode Material Autonomous Exploration Laboratory>
The research was spearheaded by Ph.D. student Hyun-Gi Lee, along with master's students Seongjae Bae and Dongwoo Kim from Professor Dong-Hwa Seo’s lab at KAIST. Senior researchers Jung Woo Park and Inchul Park from LIB Materials Research Center of POSCO N.EX.T Hub's Energy Materials R&D Laboratories (Director Jeongjin Hong) also participated.
2025.08.06 View 261 -
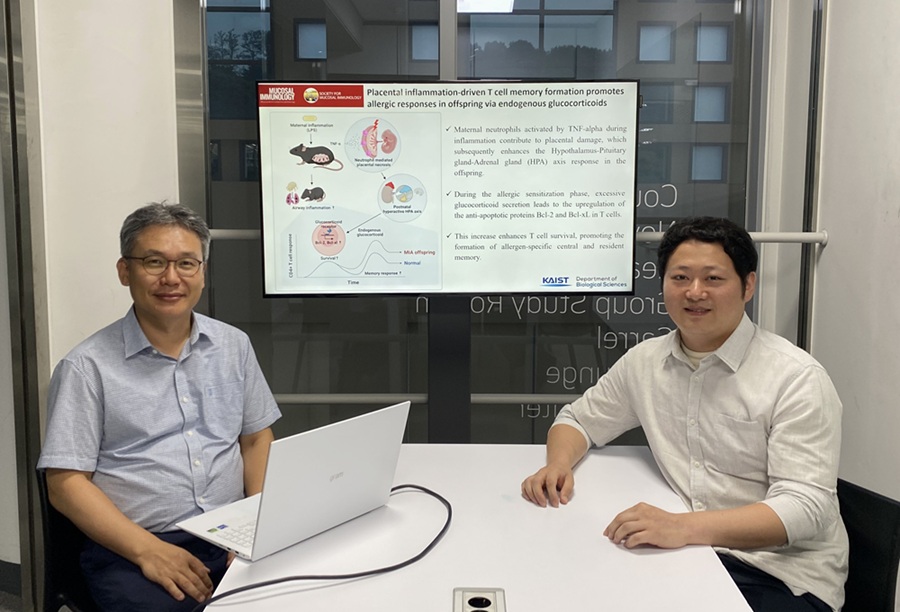 KAIST Reveals Placental Inflammation as the Cause of Allergies such as Pediatric Asthma
<(From left)Professor Heung-kyu Lee from the Department of Biological Sciences, Dr.Myeong Seung Kwon from the Graduate School of Medical Science>
It is already well-known that when a mother experiences inflammation during pregnancy, her child is more likely to develop allergic diseases. Recently, a KAIST research team became the first in the world to discover that inflammation within the placenta affects the fetus's immune system, leading to the child exhibiting excessive allergic reactions after birth. This study presents a new possibility for the early prediction and prevention of allergic diseases such as pediatric asthma.
KAIST (President Kwang Hyung Lee) announced on the 4th of August that a research team led by Professor Heung-kyu Lee from the Department of Biological Sciences found that inflammation occurring during pregnancy affects the fetus's stress response regulation system through the placenta. As a result, the survival and memory differentiation of T cells (key cells in the adaptive immune system) increase, which can lead to stronger allergic reactions in the child after birth.
The research team proved this through experiments on mice that had excessive inflammation induced during pregnancy. First, they injected the toxin component 'LPS (lipopolysaccharide),' a substance known to be a representative material that induces an inflammatory response in the immune system, into the mice to cause an inflammatory response in their bodies, which also caused inflammation in the placenta.
It was confirmed that the placental tissue, due to the inflammatory response, increased a signaling substance called 'Tumor Necrosis Factor-alpha (TNF-α),' and this substance activated immune cells called 'neutrophils*', causing inflammatory damage to the placenta. *Neutrophils: The most abundant type of white blood cells in our bodies (40-75%), playing an important role in innate immunity and killing invading bacteria and fungi.
This damage modulated postnatal offspring stress response, leading to a large secretion of stress hormone (glucocorticoid). As a result, the offspring's T cells, which are responsible for immune memory, survived longer and had stronger memory functions.
In particular, the memory T cells created through this process caused excessive allergic reactions when repeatedly exposed to antigens after birth. Specifically, when house dust mite 'allergens' were exposed to the airways of mice, a strong eosinophilic inflammatory response and excessive immune activation were observed, with an increase in immune cells important for allergy and asthma reactions.
Professor Heung Kyu Lee stated, "This study is the first in the world to identify how a mother's inflammatory response during pregnancy affects the fetus's allergic immune system through the placenta." He added, "This will be an important scientific basis for developing biomarkers for early prediction and establishing prevention strategies for pediatric allergic diseases."
The first author of this study is Dr. Myeong Seung Kwon from the KAIST Graduate School of Medical Science (currently a clinical fellow of gynecological oncology at Konyang University Hospital's Department of Obstetrics and Gynecology), and the research results were published in the authoritative journal in the field of mucosal immunology, 'Mucosal Immunology,' on July 1st. ※ Paper Title: Placental inflammation-driven T cell memory formation promotes allergic responses in offspring via endogenous glucocorticoids ※ DOI: https://doi.org/10.1016/j.mucimm.2025.06.006
This research was conducted as part of the Basic Science Research Program and the Bio-Medical Technology Development Program supported by the Ministry of Science and ICT and the National Research Foundation of Korea.
2025.08.03 View 208
KAIST Reveals Placental Inflammation as the Cause of Allergies such as Pediatric Asthma
<(From left)Professor Heung-kyu Lee from the Department of Biological Sciences, Dr.Myeong Seung Kwon from the Graduate School of Medical Science>
It is already well-known that when a mother experiences inflammation during pregnancy, her child is more likely to develop allergic diseases. Recently, a KAIST research team became the first in the world to discover that inflammation within the placenta affects the fetus's immune system, leading to the child exhibiting excessive allergic reactions after birth. This study presents a new possibility for the early prediction and prevention of allergic diseases such as pediatric asthma.
KAIST (President Kwang Hyung Lee) announced on the 4th of August that a research team led by Professor Heung-kyu Lee from the Department of Biological Sciences found that inflammation occurring during pregnancy affects the fetus's stress response regulation system through the placenta. As a result, the survival and memory differentiation of T cells (key cells in the adaptive immune system) increase, which can lead to stronger allergic reactions in the child after birth.
The research team proved this through experiments on mice that had excessive inflammation induced during pregnancy. First, they injected the toxin component 'LPS (lipopolysaccharide),' a substance known to be a representative material that induces an inflammatory response in the immune system, into the mice to cause an inflammatory response in their bodies, which also caused inflammation in the placenta.
It was confirmed that the placental tissue, due to the inflammatory response, increased a signaling substance called 'Tumor Necrosis Factor-alpha (TNF-α),' and this substance activated immune cells called 'neutrophils*', causing inflammatory damage to the placenta. *Neutrophils: The most abundant type of white blood cells in our bodies (40-75%), playing an important role in innate immunity and killing invading bacteria and fungi.
This damage modulated postnatal offspring stress response, leading to a large secretion of stress hormone (glucocorticoid). As a result, the offspring's T cells, which are responsible for immune memory, survived longer and had stronger memory functions.
In particular, the memory T cells created through this process caused excessive allergic reactions when repeatedly exposed to antigens after birth. Specifically, when house dust mite 'allergens' were exposed to the airways of mice, a strong eosinophilic inflammatory response and excessive immune activation were observed, with an increase in immune cells important for allergy and asthma reactions.
Professor Heung Kyu Lee stated, "This study is the first in the world to identify how a mother's inflammatory response during pregnancy affects the fetus's allergic immune system through the placenta." He added, "This will be an important scientific basis for developing biomarkers for early prediction and establishing prevention strategies for pediatric allergic diseases."
The first author of this study is Dr. Myeong Seung Kwon from the KAIST Graduate School of Medical Science (currently a clinical fellow of gynecological oncology at Konyang University Hospital's Department of Obstetrics and Gynecology), and the research results were published in the authoritative journal in the field of mucosal immunology, 'Mucosal Immunology,' on July 1st. ※ Paper Title: Placental inflammation-driven T cell memory formation promotes allergic responses in offspring via endogenous glucocorticoids ※ DOI: https://doi.org/10.1016/j.mucimm.2025.06.006
This research was conducted as part of the Basic Science Research Program and the Bio-Medical Technology Development Program supported by the Ministry of Science and ICT and the National Research Foundation of Korea.
2025.08.03 View 208 -
 Better Sleep, Better Life — KAIST’s Sleep Algorithm Comes to Samsung Galaxy Watches
<Professor Jae Kyoung Kim of KAIST's Department of Mathematical Sciences>
Did you know that over 80% of people worldwide have irregular sleep habits? These sleep issues don’t just leave us feeling tired — they affect our health, focus, and quality of life. Now, a new sleep algorithm developed by a team of Korean researchers is aiming to change that. And it’s available on Samsung Galaxy smartwatches around the world, including the newly launched Galaxy Watch8 series.
The personalized sleep guide, created by Professor Jae Kyoung Kim’s research team at KAIST and the Institute for Basic Science (IBS), doesn’t just tell you how long you slept. It actually recommends the best time for you to go to bed — helping you build healthy sleep habits and feel more refreshed every day.
What makes it special? Unlike most sleep features that focus only on the past (“You slept six hours last night”), this algorithm looks ahead. Using mathematical models and your body’s circadian rhythm, it suggests a personalized “sleep window” — like “Going to bed between 11:10 PM and 11:40 PM is ideal for you tonight.”
“It’s kind of like a weather forecast,” said Professor Kim. “Instead of just telling you what happened yesterday, it helps you prepare for tomorrow — so you can sleep better and feel better.”
<Conceptual Diagram of a Smart Sleep Algorithm>
The algorithm was developed over three years by a small team of mathematicians, not professional app developers. “We faced a lot of challenges trying to turn our research into a real product,” Kim admitted. “People kept asking us when they could try the algorithm, and we always felt bad that we couldn’t release it properly. Now, thanks to the support of KAIST’s Technology Commercialization Center and our partnership with Samsung, our work will finally reach people around the world.”
The academic world is paying attention, too. Professor Kim’s presentation on the algorithm was selected for the Hot Topics session at SLEEP 2025, the world’s largest sleep conference held in the U.S., and will also be featured at World Sleep 2025 in Singapore this fall.
Professor Kim is also working with Professor Eun Yeon Joo’s team at Samsung Medical Center to develop even more advanced sleep recommendation technology. Together, they created “SLEEPS,” an algorithm that predicts sleep disorders (available at sleep-math.com). Meanwhile, development continues on their own sleep app — with the hope of bringing math-powered sleep science into more people’s everyday lives.
Professor Kim is a world-renowned expert in mathematical biology. In 2025, he became the first Korean scientist to give a keynote speech at the SIAM Annual Meeting, and the first Korean to join the editorial board of SIAM Review, one of the most prestigious journals in applied mathematics. His work shows how basic science and mathematics can lead to real solutions that help people live healthier, better lives.
2025.07.28 View 487
Better Sleep, Better Life — KAIST’s Sleep Algorithm Comes to Samsung Galaxy Watches
<Professor Jae Kyoung Kim of KAIST's Department of Mathematical Sciences>
Did you know that over 80% of people worldwide have irregular sleep habits? These sleep issues don’t just leave us feeling tired — they affect our health, focus, and quality of life. Now, a new sleep algorithm developed by a team of Korean researchers is aiming to change that. And it’s available on Samsung Galaxy smartwatches around the world, including the newly launched Galaxy Watch8 series.
The personalized sleep guide, created by Professor Jae Kyoung Kim’s research team at KAIST and the Institute for Basic Science (IBS), doesn’t just tell you how long you slept. It actually recommends the best time for you to go to bed — helping you build healthy sleep habits and feel more refreshed every day.
What makes it special? Unlike most sleep features that focus only on the past (“You slept six hours last night”), this algorithm looks ahead. Using mathematical models and your body’s circadian rhythm, it suggests a personalized “sleep window” — like “Going to bed between 11:10 PM and 11:40 PM is ideal for you tonight.”
“It’s kind of like a weather forecast,” said Professor Kim. “Instead of just telling you what happened yesterday, it helps you prepare for tomorrow — so you can sleep better and feel better.”
<Conceptual Diagram of a Smart Sleep Algorithm>
The algorithm was developed over three years by a small team of mathematicians, not professional app developers. “We faced a lot of challenges trying to turn our research into a real product,” Kim admitted. “People kept asking us when they could try the algorithm, and we always felt bad that we couldn’t release it properly. Now, thanks to the support of KAIST’s Technology Commercialization Center and our partnership with Samsung, our work will finally reach people around the world.”
The academic world is paying attention, too. Professor Kim’s presentation on the algorithm was selected for the Hot Topics session at SLEEP 2025, the world’s largest sleep conference held in the U.S., and will also be featured at World Sleep 2025 in Singapore this fall.
Professor Kim is also working with Professor Eun Yeon Joo’s team at Samsung Medical Center to develop even more advanced sleep recommendation technology. Together, they created “SLEEPS,” an algorithm that predicts sleep disorders (available at sleep-math.com). Meanwhile, development continues on their own sleep app — with the hope of bringing math-powered sleep science into more people’s everyday lives.
Professor Kim is a world-renowned expert in mathematical biology. In 2025, he became the first Korean scientist to give a keynote speech at the SIAM Annual Meeting, and the first Korean to join the editorial board of SIAM Review, one of the most prestigious journals in applied mathematics. His work shows how basic science and mathematics can lead to real solutions that help people live healthier, better lives.
2025.07.28 View 487 -
 Why Do Plants Attack Themselves? The Secret of Genetic Conflict Revealed
<Professor Ji-Joon Song of the KAIST Department of Biological Sciences>
Plants, with their unique immune systems, sometimes launch 'autoimmune responses' by mistakenly identifying their own protein structures as pathogens. In particular, 'hybrid necrosis,' a phenomenon where descendant plants fail to grow healthily and perish after cross-breeding different varieties, has long been a difficult challenge for botanists and agricultural researchers. In response, an international research team has successfully elucidated the mechanism inducing plant autoimmune responses and proposed a novel strategy for cultivar improvement that can predict and avoid these reactions.
Professor Ji-Joon Song's research team at KAIST, in collaboration with teams from the National University of Singapore (NUS) and the University of Oxford, announced on the 21st of July that they have elucidated the structure and function of the 'DM3' protein complex, which triggers plant autoimmune responses, using cryo-electron microscopy (Cryo-EM) technology.
This research is drawing attention because it identifies defects in protein structure as the cause of hybrid necrosis, which occurs due to an abnormal reaction of immune receptors during cross-breeding between plant hybrids.
This protein (DM3) is originally an enzyme involved in the plant's immune response, but problems arise when the structure of the DM3 protein is damaged in a specific protein combination called 'DANGEROUS MIX (DM)'.
Notably, one variant of DM3, the 'DM3Col-0' variant, forms a stable complex with six proteins and is recognized as normal, thus not triggering an immune response. In contrast, another 'DM3Hh-0' variant has improper binding between its six proteins, causing the plant to recognize it as an 'abnormal state' and trigger an immune alarm, leading to autoimmunity.
The research team visualized this structure using atomic-resolution cryo-electron microscopy (Cryo-EM) and revealed that the immune-inducing ability is not due to the enzymatic function of the DM3 protein, but rather to 'differences in protein binding affinity.'
<Figure 1. Mechanism of Plant Autoimmunity Triggered by the Collapse of the DM3 Protein Complex>
This demonstrates that plants can initiate an immune response by recognizing not only 'external pathogens' but also 'internal protein structures' when they undergo abnormal changes, treating them as if they were pathogens.
The study shows how sensitively the plant immune system changes and triggers autoimmune responses when genes are mixed and protein structures change during the cross-breeding of different plant varieties. It significantly advanced the understanding of genetic incompatibility that can occur during natural cross-breeding and cultivar improvement processes.
Dr. Gijeong Kim, the co-first author, stated, "Through international research collaboration, we presented a new perspective on understanding the plant immune system by leveraging the autoimmune phenomenon, completing a high-quality study that encompasses structural biochemistry, genetics, and cell biological experiments."
Professor Ji-Joon Song of the KAIST Department of Biological Sciences, who led the research, said, "The fact that the immune system can detect not only external pathogens but also structural abnormalities in its own proteins will set a new standard for plant biotechnology and crop breeding strategies. Cryo-electron microscopy-based structural analysis will be an important tool for understanding the essence of gene interactions."
This research, with Professor Ji-Joon Song and Professor Eunyoung Chae of the University of Oxford as co-corresponding authors, Dr. Gijeong Kim (currently a postdoctoral researcher at the University of Zurich) and Dr. Wei-Lin Wan of the National University of Singapore as co-first authors, and Ph.D candidate Nayun Kim, as the second author, was published on July 17th in Molecular Cell, a sister journal of the international academic journal Cell.
This research was supported by the KAIST Grand Challenge 30 project.
Article Title: Structural determinants of DANGEROUS MIX 3, an alpha/beta hydrolase that triggers NLR-mediated genetic incompatibility in plants DOI: https://doi.org/10.1016/j.molcel.2025.06.021
2025.07.21 View 508
Why Do Plants Attack Themselves? The Secret of Genetic Conflict Revealed
<Professor Ji-Joon Song of the KAIST Department of Biological Sciences>
Plants, with their unique immune systems, sometimes launch 'autoimmune responses' by mistakenly identifying their own protein structures as pathogens. In particular, 'hybrid necrosis,' a phenomenon where descendant plants fail to grow healthily and perish after cross-breeding different varieties, has long been a difficult challenge for botanists and agricultural researchers. In response, an international research team has successfully elucidated the mechanism inducing plant autoimmune responses and proposed a novel strategy for cultivar improvement that can predict and avoid these reactions.
Professor Ji-Joon Song's research team at KAIST, in collaboration with teams from the National University of Singapore (NUS) and the University of Oxford, announced on the 21st of July that they have elucidated the structure and function of the 'DM3' protein complex, which triggers plant autoimmune responses, using cryo-electron microscopy (Cryo-EM) technology.
This research is drawing attention because it identifies defects in protein structure as the cause of hybrid necrosis, which occurs due to an abnormal reaction of immune receptors during cross-breeding between plant hybrids.
This protein (DM3) is originally an enzyme involved in the plant's immune response, but problems arise when the structure of the DM3 protein is damaged in a specific protein combination called 'DANGEROUS MIX (DM)'.
Notably, one variant of DM3, the 'DM3Col-0' variant, forms a stable complex with six proteins and is recognized as normal, thus not triggering an immune response. In contrast, another 'DM3Hh-0' variant has improper binding between its six proteins, causing the plant to recognize it as an 'abnormal state' and trigger an immune alarm, leading to autoimmunity.
The research team visualized this structure using atomic-resolution cryo-electron microscopy (Cryo-EM) and revealed that the immune-inducing ability is not due to the enzymatic function of the DM3 protein, but rather to 'differences in protein binding affinity.'
<Figure 1. Mechanism of Plant Autoimmunity Triggered by the Collapse of the DM3 Protein Complex>
This demonstrates that plants can initiate an immune response by recognizing not only 'external pathogens' but also 'internal protein structures' when they undergo abnormal changes, treating them as if they were pathogens.
The study shows how sensitively the plant immune system changes and triggers autoimmune responses when genes are mixed and protein structures change during the cross-breeding of different plant varieties. It significantly advanced the understanding of genetic incompatibility that can occur during natural cross-breeding and cultivar improvement processes.
Dr. Gijeong Kim, the co-first author, stated, "Through international research collaboration, we presented a new perspective on understanding the plant immune system by leveraging the autoimmune phenomenon, completing a high-quality study that encompasses structural biochemistry, genetics, and cell biological experiments."
Professor Ji-Joon Song of the KAIST Department of Biological Sciences, who led the research, said, "The fact that the immune system can detect not only external pathogens but also structural abnormalities in its own proteins will set a new standard for plant biotechnology and crop breeding strategies. Cryo-electron microscopy-based structural analysis will be an important tool for understanding the essence of gene interactions."
This research, with Professor Ji-Joon Song and Professor Eunyoung Chae of the University of Oxford as co-corresponding authors, Dr. Gijeong Kim (currently a postdoctoral researcher at the University of Zurich) and Dr. Wei-Lin Wan of the National University of Singapore as co-first authors, and Ph.D candidate Nayun Kim, as the second author, was published on July 17th in Molecular Cell, a sister journal of the international academic journal Cell.
This research was supported by the KAIST Grand Challenge 30 project.
Article Title: Structural determinants of DANGEROUS MIX 3, an alpha/beta hydrolase that triggers NLR-mediated genetic incompatibility in plants DOI: https://doi.org/10.1016/j.molcel.2025.06.021
2025.07.21 View 508 -
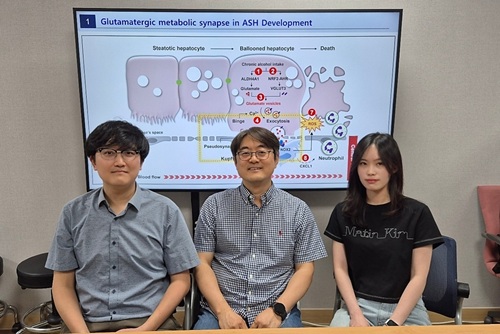 KAIST reveals for the first time the mechanism by which alcohol triggers liver inflammation
<(From left)Dr. Keungmo Yang, Professor Won-Il Jeong, Ph.D candidate Kyurae Kim>
Excessive alcohol consumption causes alcoholic liver disease, and about 20% of these cases progress to alcohol-associated steatohepatitis (ASH), which can lead to liver cirrhosis and liver failure. Early diagnosis and treatment are therefore extremely important. A KAIST research team has identified a new molecular mechanism in which alcohol-damaged liver cells increase reactive oxygen species (ROS), leading to cell death and inflammatory responses. In addition, they discovered that Kupffer cells, immune cells residing in the liver, act as a “dual-function regulator” that can either promote or suppress inflammation through interactions with liver cells.
KAIST (President Kwang-Hyung Lee) announced on the 17th that a research team led by Professor Won-Il Jeong from the Graduate School of Medical Science and Engineering, in collaboration with Professor Won Kim’s team at Seoul National University Boramae Medical Center, has uncovered the molecular pathway of liver damage and inflammation caused by alcohol consumption. This finding offers new clues for the diagnosis and treatment of alcohol-associated liver disease (ALD).
Professor Won-Il Jeong’s research team found that during chronic alcohol intake, expression of the vesicular glutamate transporter VGLUT3 increases, leading to glutamate accumulation in hepatocytes. Subsequent binge drinking causes rapid changes in intracellular calcium levels, which then triggers glutamate* secretion. The secreted glutamate stimulates the glutamate receptor mGluR5 on liver-resident macrophages (Kupffer cells), which induces ROS production and activates a pathological pathway resulting in hepatocyte death and inflammation.
*Glutamate: A type of amino acid involved in intercellular signaling, protein synthesis, and energy metabolism in various tissues including the brain and liver. In excess, it can cause overexcitation and death of nerve cells.
Glutamate accumulation in perivenous hepatocytes through vesicular glutamate transporter 3 after 2-week EtOH intake and its release by binge drinking>
A particularly groundbreaking aspect of this study is that damaged hepatocytes and Kupffer cells can form a "pseudosynapse"—a structure similar to a synapse which is previously thought to occur only in the brain—enabling them to exchange signals. This is the first time such a phenomenon has been identified in the liver.
This pseudosynapse forms when hepatocytes expand (ballooning) due to alcohol, becoming physically attached to Kupffer cells. Simply put, the damaged hepatocytes don’t just die—they send distress signals to nearby immune cells, prompting a response.
This discovery proposes a new paradigm: even in peripheral organs, direct structural contact between cells can allow signal transmission. It also shows that damaged hepatocytes can actively stimulate macrophages and induce regeneration through cell death, revealing the liver’s “autonomous recovery function.”
The team also confirmed in animal models that genetic or pharmacological inhibition of VGLUT3, mGluR5, or the ROS-producing enzyme NOX2 reduces alcohol-induced liver damage. They also confirmed that the same mechanism observed in animal models was present in human patients with ALD by analyzing blood and liver tissue samples.
Professor Won-Il Jeong of KAIST said, “These findings may serve as new molecular targets for early diagnosis and treatment of ASH in the future.”
This study was jointly led by Dr. Keungmo Yang (now at Yeouido St. Mary’s Hospital) and Kyurae Kim, a doctoral candidate at KAIST, who served as co–first authors. It was conducted in collaboration with Professor Won Kim’s team at Seoul National University Boramae Medical Center and was published in the journal Nature Communications on July 1.
※ Article Title: Binge drinking triggers VGLUT3-mediated glutamate secretion and subsequent hepatic inflammation by activating mGluR5/NOX2 in Kupffer cells ※ DOI: https://doi.org/10.1038/s41467-025-60820-3
This study was supported by the Ministry of Science and ICT through the National Research Foundation of Korea's Global Leader Program, Mid-Career Researcher Program, and the Bio & Medical Technology Development Program.
2025.07.17 View 643
KAIST reveals for the first time the mechanism by which alcohol triggers liver inflammation
<(From left)Dr. Keungmo Yang, Professor Won-Il Jeong, Ph.D candidate Kyurae Kim>
Excessive alcohol consumption causes alcoholic liver disease, and about 20% of these cases progress to alcohol-associated steatohepatitis (ASH), which can lead to liver cirrhosis and liver failure. Early diagnosis and treatment are therefore extremely important. A KAIST research team has identified a new molecular mechanism in which alcohol-damaged liver cells increase reactive oxygen species (ROS), leading to cell death and inflammatory responses. In addition, they discovered that Kupffer cells, immune cells residing in the liver, act as a “dual-function regulator” that can either promote or suppress inflammation through interactions with liver cells.
KAIST (President Kwang-Hyung Lee) announced on the 17th that a research team led by Professor Won-Il Jeong from the Graduate School of Medical Science and Engineering, in collaboration with Professor Won Kim’s team at Seoul National University Boramae Medical Center, has uncovered the molecular pathway of liver damage and inflammation caused by alcohol consumption. This finding offers new clues for the diagnosis and treatment of alcohol-associated liver disease (ALD).
Professor Won-Il Jeong’s research team found that during chronic alcohol intake, expression of the vesicular glutamate transporter VGLUT3 increases, leading to glutamate accumulation in hepatocytes. Subsequent binge drinking causes rapid changes in intracellular calcium levels, which then triggers glutamate* secretion. The secreted glutamate stimulates the glutamate receptor mGluR5 on liver-resident macrophages (Kupffer cells), which induces ROS production and activates a pathological pathway resulting in hepatocyte death and inflammation.
*Glutamate: A type of amino acid involved in intercellular signaling, protein synthesis, and energy metabolism in various tissues including the brain and liver. In excess, it can cause overexcitation and death of nerve cells.
Glutamate accumulation in perivenous hepatocytes through vesicular glutamate transporter 3 after 2-week EtOH intake and its release by binge drinking>
A particularly groundbreaking aspect of this study is that damaged hepatocytes and Kupffer cells can form a "pseudosynapse"—a structure similar to a synapse which is previously thought to occur only in the brain—enabling them to exchange signals. This is the first time such a phenomenon has been identified in the liver.
This pseudosynapse forms when hepatocytes expand (ballooning) due to alcohol, becoming physically attached to Kupffer cells. Simply put, the damaged hepatocytes don’t just die—they send distress signals to nearby immune cells, prompting a response.
This discovery proposes a new paradigm: even in peripheral organs, direct structural contact between cells can allow signal transmission. It also shows that damaged hepatocytes can actively stimulate macrophages and induce regeneration through cell death, revealing the liver’s “autonomous recovery function.”
The team also confirmed in animal models that genetic or pharmacological inhibition of VGLUT3, mGluR5, or the ROS-producing enzyme NOX2 reduces alcohol-induced liver damage. They also confirmed that the same mechanism observed in animal models was present in human patients with ALD by analyzing blood and liver tissue samples.
Professor Won-Il Jeong of KAIST said, “These findings may serve as new molecular targets for early diagnosis and treatment of ASH in the future.”
This study was jointly led by Dr. Keungmo Yang (now at Yeouido St. Mary’s Hospital) and Kyurae Kim, a doctoral candidate at KAIST, who served as co–first authors. It was conducted in collaboration with Professor Won Kim’s team at Seoul National University Boramae Medical Center and was published in the journal Nature Communications on July 1.
※ Article Title: Binge drinking triggers VGLUT3-mediated glutamate secretion and subsequent hepatic inflammation by activating mGluR5/NOX2 in Kupffer cells ※ DOI: https://doi.org/10.1038/s41467-025-60820-3
This study was supported by the Ministry of Science and ICT through the National Research Foundation of Korea's Global Leader Program, Mid-Career Researcher Program, and the Bio & Medical Technology Development Program.
2025.07.17 View 643 -
 KAIST Ushers in Era of Predicting ‘Optimal Alloys’ Using AI, Without High-Temperature Experiments
<Picture1.(From Left) Prof. Seungbum Hong, Ph.D candidate Youngwoo Choi>
Steel alloys used in automobiles and machinery parts are typically manufactured through a melting process at high temperatures. The phenomenon where the components remain unchanged during melting is called “congruent melting.” KAIST researchers have now addressed this process—traditionally only possible through high-temperature experiments—using artificial intelligence (AI). This study draws attention as it proposes a new direction for future alloy development by predicting in advance how well alloy components will mix during melting, a long-standing challenge in the field.
KAIST (President Kwang Hyung Lee) announced on the 14th of July that Professor Seungbum Hong’s research team from the Department of Materials Science and Engineering, in international collaboration with Professor Chris Wolverton’s group at Northwestern University, has developed a high-accuracy machine learning model that predicts whether alloy components will remain stable during melting. This was achieved using formation energy data derived from Density Functional Theory (DFT)* calculations.
*Density Functional Theory (DFT): A computational quantum mechanical method used to investigate the electronic structure of many-body systems, especially atoms, molecules, and solids, based on electron density.
The research team combined formation energy values obtained via DFT with experimental melting reaction data to train a machine learning model on 4,536 binary compounds.
Among the various machine learning algorithms tested, the XGBoost-based classification model demonstrated the highest accuracy in predicting whether alloys would mix well, achieving a prediction accuracy of approximately 82.5%. The team also applied the Shapley value method* to analyze the key features of the model. One major finding was that sharp changes in the slope of the formation energy curve (referred to as “convex hull sharpness”) were the most significant factor. A steep slope indicates a composition with energetically favorable (i.e., stable) formation.
*Shapley value: An explainability method in AI used to determine how much each feature contributed to a prediction.
The most notable significance of this study is that it predicts alloy melting behavior without performing high-temperature experiments. This is especially useful for materials such as high-entropy alloys or ultra-heat-resistant alloys, which are difficult to handle experimentally. The approach could also be extended to the design of complex multi-component alloy systems in the future.
Furthermore, the physical indicators identified by the AI model showed high consistency with actual experimental results on how well alloys mix and remain stable. This suggests that the model could be broadly applied to the development of various metal materials and the prediction of structural stability.
Professor Seungbum Hong of KAIST stated, “This research demonstrates how data-driven predictive materials development is possible by integrating computational methods, experimental data, and machine learning—departing from the traditional experience-based alloy design.” He added, “In the future, by incorporating state-of-the-art AI techniques such as generative models and reinforcement learning, we could enter an era where completely new alloys are designed automatically.”
<Model performance and feature importance analysis for predicting melting congruency. (a) SHAP summary plot showing the impact of individual features on model predictions. (b) Confusion matrix illustrating the model’s classification performance. (c) Receiver operating characteristic (ROC) curve with an AUC (area under the curve) score of 0.87, indicating a strong classification performance.>
Ph.D. candidate Youngwoo Choi, from the Department of Materials Science and Engineering at KAIST, participated as the first author. The study was published in the May issue of APL Machine Learning, a prestigious journal in the field of machine learning published by the American Institute of Physics, and was selected as a “Featured Article.”
※ Paper title: Machine learning-based melting congruency prediction of binary compounds using density functional theory-calculated formation energy ※ DOI: 10.1063/5.0247514
This research was supported by the Ministry of Science and ICT and the National Research Foundation of Korea.
2025.07.14 View 467
KAIST Ushers in Era of Predicting ‘Optimal Alloys’ Using AI, Without High-Temperature Experiments
<Picture1.(From Left) Prof. Seungbum Hong, Ph.D candidate Youngwoo Choi>
Steel alloys used in automobiles and machinery parts are typically manufactured through a melting process at high temperatures. The phenomenon where the components remain unchanged during melting is called “congruent melting.” KAIST researchers have now addressed this process—traditionally only possible through high-temperature experiments—using artificial intelligence (AI). This study draws attention as it proposes a new direction for future alloy development by predicting in advance how well alloy components will mix during melting, a long-standing challenge in the field.
KAIST (President Kwang Hyung Lee) announced on the 14th of July that Professor Seungbum Hong’s research team from the Department of Materials Science and Engineering, in international collaboration with Professor Chris Wolverton’s group at Northwestern University, has developed a high-accuracy machine learning model that predicts whether alloy components will remain stable during melting. This was achieved using formation energy data derived from Density Functional Theory (DFT)* calculations.
*Density Functional Theory (DFT): A computational quantum mechanical method used to investigate the electronic structure of many-body systems, especially atoms, molecules, and solids, based on electron density.
The research team combined formation energy values obtained via DFT with experimental melting reaction data to train a machine learning model on 4,536 binary compounds.
Among the various machine learning algorithms tested, the XGBoost-based classification model demonstrated the highest accuracy in predicting whether alloys would mix well, achieving a prediction accuracy of approximately 82.5%. The team also applied the Shapley value method* to analyze the key features of the model. One major finding was that sharp changes in the slope of the formation energy curve (referred to as “convex hull sharpness”) were the most significant factor. A steep slope indicates a composition with energetically favorable (i.e., stable) formation.
*Shapley value: An explainability method in AI used to determine how much each feature contributed to a prediction.
The most notable significance of this study is that it predicts alloy melting behavior without performing high-temperature experiments. This is especially useful for materials such as high-entropy alloys or ultra-heat-resistant alloys, which are difficult to handle experimentally. The approach could also be extended to the design of complex multi-component alloy systems in the future.
Furthermore, the physical indicators identified by the AI model showed high consistency with actual experimental results on how well alloys mix and remain stable. This suggests that the model could be broadly applied to the development of various metal materials and the prediction of structural stability.
Professor Seungbum Hong of KAIST stated, “This research demonstrates how data-driven predictive materials development is possible by integrating computational methods, experimental data, and machine learning—departing from the traditional experience-based alloy design.” He added, “In the future, by incorporating state-of-the-art AI techniques such as generative models and reinforcement learning, we could enter an era where completely new alloys are designed automatically.”
<Model performance and feature importance analysis for predicting melting congruency. (a) SHAP summary plot showing the impact of individual features on model predictions. (b) Confusion matrix illustrating the model’s classification performance. (c) Receiver operating characteristic (ROC) curve with an AUC (area under the curve) score of 0.87, indicating a strong classification performance.>
Ph.D. candidate Youngwoo Choi, from the Department of Materials Science and Engineering at KAIST, participated as the first author. The study was published in the May issue of APL Machine Learning, a prestigious journal in the field of machine learning published by the American Institute of Physics, and was selected as a “Featured Article.”
※ Paper title: Machine learning-based melting congruency prediction of binary compounds using density functional theory-calculated formation energy ※ DOI: 10.1063/5.0247514
This research was supported by the Ministry of Science and ICT and the National Research Foundation of Korea.
2025.07.14 View 467 -
 KAIST Shows That the Brain Can Distinguish Glucose: Clues to Treat Obesity and Diabetes
<(From left)Prof. Greg S.B Suh, Dr. Jieun Kim, Dr. Shinhye Kim, Researcher Wongyo Jeong)
“How does our brain distinguish glucose from the many nutrients absorbed in the gut?” Starting with this question, a KAIST research team has demonstrated that the brain can selectively recognize specific nutrients—particularly glucose—beyond simply detecting total calorie content. This study is expected to offer a new paradigm for appetite control and the treatment of metabolic diseases.
On the 9th, KAIST (President Kwang Hyung Lee) announced that Professor Greg S.B. Suh’s team in the Department of Biological Sciences, in collaboration with Professor Young-Gyun Park’s team (BarNeuro), Professor Seung-Hee Lee’s team (Department of Biological Sciences), and the Albert Einstein College of Medicine in New York, had identified the existence of a gut-brain circuit that allows animals in a hungry state to selectively detect and prefer glucose in the gut.
Organisms derive energy from various nutrients including sugars, proteins, and fats. Previous studies have shown that total caloric information in the gut suppresses hunger neurons in the hypothalamus to regulate appetite. However, the existence of a gut-brain circuit that specifically responds to glucose and corresponding brain cells had not been demonstrated until now.
In this study, the team successfully identified a “gut-brain circuit” that senses glucose—essential for brain function—and regulates food intake behavior for required nutrients.
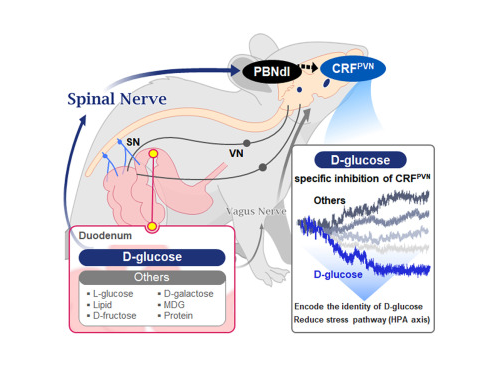
They further proved, for the first time, that this circuit responds within seconds to not only hunger or external stimuli but also to specific caloric nutrients directly introduced into the small intestine, particularly D-glucose, through the activity of “CRF neurons*” in the brain’s hypothalamus.
*CRF neurons: These neurons secrete corticotropin-releasing factor (CRF) in the hypothalamus and are central to the hypothalamic-pituitary-adrenal (HPA) axis, the body’s core physiological system for responding to stress. CRF neurons are known to regulate neuroendocrine balance in response to stress stimuli.
Using optogenetics to precisely track neural activity in real time, the researchers injected various nutrients—D-glucose, L-glucose, amino acids, and fats—directly into the small intestines of mice and observed the results.
They discovered that among the CRF neurons located in the paraventricular nucleus (PVN)* of the hypothalamus, only those specific to D-glucose showed selective responses. These neurons did not respond—or showed inverse reactions—to other sugars or to proteins and fats. This is the first demonstration that single neurons in the brain can guide nutrient-specific responses depending on gut nutrient influx.
*PVN (Paraventricular Nucleus): A key nucleus within the hypothalamus responsible for maintaining bodily homeostasis.
The team also revealed that glucose-sensing signals in the small intestine are transmitted via the spinal cord to the dorsolateral parabrachial nucleus (PBNdl) of the brain, and from there to CRF neurons in the PVN. In contrast, signals for amino acids and fats are transmitted to the brain through the vagus nerve, a different pathway.
In optogenetic inhibition experiments, suppressing CRF neurons in fasting mice eliminated their preference for glucose, proving that this circuit is essential for glucose-specific nutrient preference.
This study was inspired by Professor Suh’s earlier research at NYU using fruit flies, where he identified “DH44 neurons” that selectively detect glucose and sugar in the gut. Based on the hypothesis that hypothalamic neurons in mammals would show similar functional responses to glucose, the current study was launched.
To test this hypothesis, Dr. Jineun Kim (KAIST Ph.D. graduate, now at Caltech) demonstrated during her doctoral research that hungry mice preferred glucose among various intragastrically infused nutrients and that CRF neurons exhibited rapid and specific responses.
Along with Wongyo Jung (KAIST B.S. graduate, now Ph.D. student at Caltech), they modeled and experimentally confirmed the critical role of CRF neurons. Dr. Shinhye Kim, through collaboration, revealed that specific spinal neurons play a key role in conveying intestinal nutrient information to the brain.
Dr. Jineun Kim and Dr. Shinhye Kim said, “This study started from a simple but fundamental question—‘How does the brain distinguish glucose from various nutrients absorbed in the gut?’ We have shown that spinal-based gut-brain circuits play a central role in energy metabolism and homeostasis by transmitting specific gut nutrient signals to the brain.”
Professor Suh added, “By identifying a gut-brain pathway specialized for glucose, this research offers a new therapeutic target for metabolic diseases such as obesity and diabetes. Our future research will explore similar circuits for sensing other essential nutrients like amino acids and fats and their interaction mechanisms.”
Ph.D. student Jineun Kim, Dr. Shinhye Kim, and student Wongyo Jung (co-first authors) contributed to this study, which was published online in the international journal Neuron on June 20, 2025.
※ Paper Title: Encoding the glucose identity by discrete hypothalamic neurons via the gut-brain axis ※ DOI: https://doi.org/10.1016/j.neuron.2025.05.024
This study was supported by the Samsung Science & Technology Foundation, the National Research Foundation of Korea (NRF) Leader Research Program, the POSCO Cheongam Science Fellowship, the Asan Foundation Biomedical Science Scholarship, the Institute for Basic Science (IBS), and the KAIST KAIX program.
2025.07.09 View 762
KAIST Shows That the Brain Can Distinguish Glucose: Clues to Treat Obesity and Diabetes
<(From left)Prof. Greg S.B Suh, Dr. Jieun Kim, Dr. Shinhye Kim, Researcher Wongyo Jeong)
“How does our brain distinguish glucose from the many nutrients absorbed in the gut?” Starting with this question, a KAIST research team has demonstrated that the brain can selectively recognize specific nutrients—particularly glucose—beyond simply detecting total calorie content. This study is expected to offer a new paradigm for appetite control and the treatment of metabolic diseases.
On the 9th, KAIST (President Kwang Hyung Lee) announced that Professor Greg S.B. Suh’s team in the Department of Biological Sciences, in collaboration with Professor Young-Gyun Park’s team (BarNeuro), Professor Seung-Hee Lee’s team (Department of Biological Sciences), and the Albert Einstein College of Medicine in New York, had identified the existence of a gut-brain circuit that allows animals in a hungry state to selectively detect and prefer glucose in the gut.
Organisms derive energy from various nutrients including sugars, proteins, and fats. Previous studies have shown that total caloric information in the gut suppresses hunger neurons in the hypothalamus to regulate appetite. However, the existence of a gut-brain circuit that specifically responds to glucose and corresponding brain cells had not been demonstrated until now.
In this study, the team successfully identified a “gut-brain circuit” that senses glucose—essential for brain function—and regulates food intake behavior for required nutrients.
They further proved, for the first time, that this circuit responds within seconds to not only hunger or external stimuli but also to specific caloric nutrients directly introduced into the small intestine, particularly D-glucose, through the activity of “CRF neurons*” in the brain’s hypothalamus.
*CRF neurons: These neurons secrete corticotropin-releasing factor (CRF) in the hypothalamus and are central to the hypothalamic-pituitary-adrenal (HPA) axis, the body’s core physiological system for responding to stress. CRF neurons are known to regulate neuroendocrine balance in response to stress stimuli.
Using optogenetics to precisely track neural activity in real time, the researchers injected various nutrients—D-glucose, L-glucose, amino acids, and fats—directly into the small intestines of mice and observed the results.
They discovered that among the CRF neurons located in the paraventricular nucleus (PVN)* of the hypothalamus, only those specific to D-glucose showed selective responses. These neurons did not respond—or showed inverse reactions—to other sugars or to proteins and fats. This is the first demonstration that single neurons in the brain can guide nutrient-specific responses depending on gut nutrient influx.
*PVN (Paraventricular Nucleus): A key nucleus within the hypothalamus responsible for maintaining bodily homeostasis.
The team also revealed that glucose-sensing signals in the small intestine are transmitted via the spinal cord to the dorsolateral parabrachial nucleus (PBNdl) of the brain, and from there to CRF neurons in the PVN. In contrast, signals for amino acids and fats are transmitted to the brain through the vagus nerve, a different pathway.
In optogenetic inhibition experiments, suppressing CRF neurons in fasting mice eliminated their preference for glucose, proving that this circuit is essential for glucose-specific nutrient preference.
This study was inspired by Professor Suh’s earlier research at NYU using fruit flies, where he identified “DH44 neurons” that selectively detect glucose and sugar in the gut. Based on the hypothesis that hypothalamic neurons in mammals would show similar functional responses to glucose, the current study was launched.
To test this hypothesis, Dr. Jineun Kim (KAIST Ph.D. graduate, now at Caltech) demonstrated during her doctoral research that hungry mice preferred glucose among various intragastrically infused nutrients and that CRF neurons exhibited rapid and specific responses.
Along with Wongyo Jung (KAIST B.S. graduate, now Ph.D. student at Caltech), they modeled and experimentally confirmed the critical role of CRF neurons. Dr. Shinhye Kim, through collaboration, revealed that specific spinal neurons play a key role in conveying intestinal nutrient information to the brain.
Dr. Jineun Kim and Dr. Shinhye Kim said, “This study started from a simple but fundamental question—‘How does the brain distinguish glucose from various nutrients absorbed in the gut?’ We have shown that spinal-based gut-brain circuits play a central role in energy metabolism and homeostasis by transmitting specific gut nutrient signals to the brain.”
Professor Suh added, “By identifying a gut-brain pathway specialized for glucose, this research offers a new therapeutic target for metabolic diseases such as obesity and diabetes. Our future research will explore similar circuits for sensing other essential nutrients like amino acids and fats and their interaction mechanisms.”
Ph.D. student Jineun Kim, Dr. Shinhye Kim, and student Wongyo Jung (co-first authors) contributed to this study, which was published online in the international journal Neuron on June 20, 2025.
※ Paper Title: Encoding the glucose identity by discrete hypothalamic neurons via the gut-brain axis ※ DOI: https://doi.org/10.1016/j.neuron.2025.05.024
This study was supported by the Samsung Science & Technology Foundation, the National Research Foundation of Korea (NRF) Leader Research Program, the POSCO Cheongam Science Fellowship, the Asan Foundation Biomedical Science Scholarship, the Institute for Basic Science (IBS), and the KAIST KAIX program.
2025.07.09 View 762 -
 KAIST Develops Novel Candidiasis Treatment Overcoming Side Effects and Resistance
<(From left) Ph. D Candidate Ju Yeon Chung, Prof.Hyun Jung Chung, Ph.D candidate Seungju Yang, Ph.D candidate Ayoung Park, Dr. Yoon-Kyoung Hong from Asan Medical Center, Prof. Yong Pil Chong, Dr. Eunhee Jeon>
Candida, a type of fungus, which can spread throughout the body via the bloodstream, leading to organ damage and sepsis. Recently, the incidence of candidiasis has surged due to the increase in immunosuppressive therapies, medical implants, and transplantation. Korean researchers have successfully developed a next-generation treatment that, unlike existing antifungals, selectively acts only on Candida, achieving both high therapeutic efficacy and low side effects simultaneously.
KAIST (President Kwang Hyung Lee) announced on the 8th that a research team led by Professor Hyun-Jung Chung of the Department of Biological Sciences, in collaboration with Professor Yong Pil Jeong's team at Asan Medical Center, developed a gene-based nanotherapy (FTNx) that simultaneously inhibits two key enzymes in the Candida cell wall.
Current antifungal drugs for Candida have low target selectivity, which can affect human cells. Furthermore, their therapeutic efficacy is gradually decreasing due to the emergence of new resistant strains. Especially for immunocompromised patients, the infection progresses rapidly and has a poor prognosis, making the development of new treatments to overcome the limitations of existing therapies urgent.
The developed treatment can be administered systemically, and by combining gene suppression technology with nanomaterial technology, it effectively overcomes the structural limitations of existing compound-based drugs and successfully achieves selective treatment against only Candida.
The research team created a gold nanoparticle-based complex loaded with short DNA fragments called antisense oligonucleotides (ASO), which simultaneously target two crucial enzymes—β-1,3-glucan synthase (FKS1) and chitin synthase (CHS3)—important for forming the cell wall of the Candida fungus.
By applying a surface coating technology that binds to a specific glycolipid structure (a structure combining sugar and fat) on the Candida cell wall, a targeted delivery device was implemented. This successfully achieved a precise targeting effect, ensuring the complex is not delivered to human cells at all but acts selectively only on Candida.
<Figure 1: Overview of antifungal therapy design and experimental approach>
This complex, after entering Candida cells, cleaves the mRNA produced by the FKS1 and CHS3 genes, thereby inhibiting translation and simultaneously blocking the synthesis of cell wall components β-1,3-glucan and chitin. As a result, the
Candida cell wall loses its structural stability and collapses, suppressing bacterial survival and proliferation.
In fact, experiments using a systemic candidiasis model in mice confirmed the therapeutic effect: a significant reduction in
Candida count in the organs, normalization of immune responses, and a notable increase in survival rates were observed in the treated group.
Professor Hyun-Jung Chung, who led the research, stated, "This study presents a method to overcome the issues of human toxicity and drug resistance spread with existing treatments, marking an important turning point by demonstrating the applicability of gene therapy for systemic infections". She added, "We plan to continue research on optimizing administration methods and verifying toxicity for future clinical application."
This research involved Ju Yeon Chung and Yoon-Kyoung Hong as co-first authors , and was published in the international journal 'Nature Communications' on July 1st.
Paper Title: Effective treatment of systemic candidiasis by synergistic targeting of cell wall synthesis
DOI: 10.1038/s41467-025-60684-7
This research was supported by the Ministry of Health and Welfare and the National Research Foundation of Korea.
2025.07.08 View 703
KAIST Develops Novel Candidiasis Treatment Overcoming Side Effects and Resistance
<(From left) Ph. D Candidate Ju Yeon Chung, Prof.Hyun Jung Chung, Ph.D candidate Seungju Yang, Ph.D candidate Ayoung Park, Dr. Yoon-Kyoung Hong from Asan Medical Center, Prof. Yong Pil Chong, Dr. Eunhee Jeon>
Candida, a type of fungus, which can spread throughout the body via the bloodstream, leading to organ damage and sepsis. Recently, the incidence of candidiasis has surged due to the increase in immunosuppressive therapies, medical implants, and transplantation. Korean researchers have successfully developed a next-generation treatment that, unlike existing antifungals, selectively acts only on Candida, achieving both high therapeutic efficacy and low side effects simultaneously.
KAIST (President Kwang Hyung Lee) announced on the 8th that a research team led by Professor Hyun-Jung Chung of the Department of Biological Sciences, in collaboration with Professor Yong Pil Jeong's team at Asan Medical Center, developed a gene-based nanotherapy (FTNx) that simultaneously inhibits two key enzymes in the Candida cell wall.
Current antifungal drugs for Candida have low target selectivity, which can affect human cells. Furthermore, their therapeutic efficacy is gradually decreasing due to the emergence of new resistant strains. Especially for immunocompromised patients, the infection progresses rapidly and has a poor prognosis, making the development of new treatments to overcome the limitations of existing therapies urgent.
The developed treatment can be administered systemically, and by combining gene suppression technology with nanomaterial technology, it effectively overcomes the structural limitations of existing compound-based drugs and successfully achieves selective treatment against only Candida.
The research team created a gold nanoparticle-based complex loaded with short DNA fragments called antisense oligonucleotides (ASO), which simultaneously target two crucial enzymes—β-1,3-glucan synthase (FKS1) and chitin synthase (CHS3)—important for forming the cell wall of the Candida fungus.
By applying a surface coating technology that binds to a specific glycolipid structure (a structure combining sugar and fat) on the Candida cell wall, a targeted delivery device was implemented. This successfully achieved a precise targeting effect, ensuring the complex is not delivered to human cells at all but acts selectively only on Candida.
<Figure 1: Overview of antifungal therapy design and experimental approach>
This complex, after entering Candida cells, cleaves the mRNA produced by the FKS1 and CHS3 genes, thereby inhibiting translation and simultaneously blocking the synthesis of cell wall components β-1,3-glucan and chitin. As a result, the
Candida cell wall loses its structural stability and collapses, suppressing bacterial survival and proliferation.
In fact, experiments using a systemic candidiasis model in mice confirmed the therapeutic effect: a significant reduction in
Candida count in the organs, normalization of immune responses, and a notable increase in survival rates were observed in the treated group.
Professor Hyun-Jung Chung, who led the research, stated, "This study presents a method to overcome the issues of human toxicity and drug resistance spread with existing treatments, marking an important turning point by demonstrating the applicability of gene therapy for systemic infections". She added, "We plan to continue research on optimizing administration methods and verifying toxicity for future clinical application."
This research involved Ju Yeon Chung and Yoon-Kyoung Hong as co-first authors , and was published in the international journal 'Nature Communications' on July 1st.
Paper Title: Effective treatment of systemic candidiasis by synergistic targeting of cell wall synthesis
DOI: 10.1038/s41467-025-60684-7
This research was supported by the Ministry of Health and Welfare and the National Research Foundation of Korea.
2025.07.08 View 703 -
 Professor Moon-Jeong Choi Appointed as an Advisor for the ITU's 'AI for Good Global Summit'
Professor Moon-Jeong Choi from KAIST’s Graduate School of Science and Technology Policy has been appointed as an advisor for "Innovate for Impact" at the AI for Good Global Summit, organized by the International Telecommunication Union (ITU), a specialized agency of the United Nations (UN).
The ITU is the UN's oldest specialized agency in the field of information and communication technology (ICT) and serves as a crucial body for coordinating global ICT policies and standards.
This advisory committee was formed to explore global cooperation strategies for realizing the social value of Artificial Intelligence (AI) and promoting sustainable development. Experts from around the world are participating as committee members, with Professor Choi being the sole Korean representative.
<Moon-Jeong Choi from KAIST’s Graduate School of Science and Technology Policy>
The AI for Good Global Summit is taking place in Geneva, Switzerland from July 8 to 11. It is organized by the ITU in collaboration with approximately 40 other UN-affiliated organizations. The summit aims to address global challenges facing humanity through the use of AI technology, focusing on key agenda items such as identifying AI application cases, discussing international policies and technical standards, and strengthening global partnerships.
As an "Innovate for Impact" advisor, Professor Choi will evaluate AI application cases from various countries, participating in case analyses primarily focused on public interest and social impact. The summit will move beyond discussions of technical performance to focus on how AI can contribute to the public good, with diverse case studies from around the world being debated. Notably, during a policy panel discussion at the summit, Professor Choi will discuss policy frameworks for AI transparency, inclusivity, and fairness under the theme of "Responsible AI Development."
Professor Choi commented, "I believe the social impact of technology mirrors the values and systems of each nation. As a society's core values permeate technology, the way AI is developed and used varies significantly from country to country. These differences lead to diverse manifestations of AI's impact on society." She further emphasized, "Korea's vision of becoming an AI powerhouse should not merely be about technological superiority, but rather about enhancing social capital through human-centered AI and realizing communal values that enable us to live together."
Professor Moon-Jeong Choi currently serves as the Dean of the Graduate School of Science and Technology Policy. She is also an external director for the National Information Society Agency (2023-present) and chair of the Korea-OECD Digital Society Initiative (2024-present).
For more information about the AI for Good Global Summit, please visit the official website: https://aiforgood.itu.int.
2025.07.08 View 542
Professor Moon-Jeong Choi Appointed as an Advisor for the ITU's 'AI for Good Global Summit'
Professor Moon-Jeong Choi from KAIST’s Graduate School of Science and Technology Policy has been appointed as an advisor for "Innovate for Impact" at the AI for Good Global Summit, organized by the International Telecommunication Union (ITU), a specialized agency of the United Nations (UN).
The ITU is the UN's oldest specialized agency in the field of information and communication technology (ICT) and serves as a crucial body for coordinating global ICT policies and standards.
This advisory committee was formed to explore global cooperation strategies for realizing the social value of Artificial Intelligence (AI) and promoting sustainable development. Experts from around the world are participating as committee members, with Professor Choi being the sole Korean representative.
<Moon-Jeong Choi from KAIST’s Graduate School of Science and Technology Policy>
The AI for Good Global Summit is taking place in Geneva, Switzerland from July 8 to 11. It is organized by the ITU in collaboration with approximately 40 other UN-affiliated organizations. The summit aims to address global challenges facing humanity through the use of AI technology, focusing on key agenda items such as identifying AI application cases, discussing international policies and technical standards, and strengthening global partnerships.
As an "Innovate for Impact" advisor, Professor Choi will evaluate AI application cases from various countries, participating in case analyses primarily focused on public interest and social impact. The summit will move beyond discussions of technical performance to focus on how AI can contribute to the public good, with diverse case studies from around the world being debated. Notably, during a policy panel discussion at the summit, Professor Choi will discuss policy frameworks for AI transparency, inclusivity, and fairness under the theme of "Responsible AI Development."
Professor Choi commented, "I believe the social impact of technology mirrors the values and systems of each nation. As a society's core values permeate technology, the way AI is developed and used varies significantly from country to country. These differences lead to diverse manifestations of AI's impact on society." She further emphasized, "Korea's vision of becoming an AI powerhouse should not merely be about technological superiority, but rather about enhancing social capital through human-centered AI and realizing communal values that enable us to live together."
Professor Moon-Jeong Choi currently serves as the Dean of the Graduate School of Science and Technology Policy. She is also an external director for the National Information Society Agency (2023-present) and chair of the Korea-OECD Digital Society Initiative (2024-present).
For more information about the AI for Good Global Summit, please visit the official website: https://aiforgood.itu.int.
2025.07.08 View 542 -
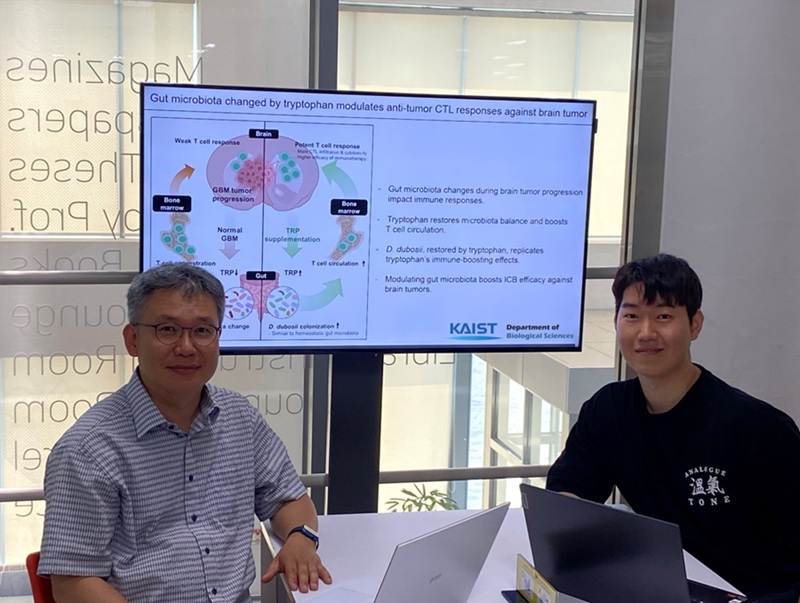 KAIST Enhances Immunotherapy for Difficult-to-Treat Brain Tumors with Gut Microbiota
< Photo 1.(From left) Prof. Heung Kyu Lee, Department of Biological Sciences,
and Dr. Hyeon Cheol Kim>
Advanced treatments, known as immunotherapies that activate T cells—our body's immune cells—to eliminate cancer cells, have shown limited efficacy as standalone therapies for glioblastoma, the most lethal form of brain tumor. This is due to their minimal response to glioblastoma and high resistance to treatment.
Now, a KAIST research team has now demonstrated a new therapeutic strategy that can enhance the efficacy of immunotherapy for brain tumors by utilizing gut microbes and their metabolites. This also opens up possibilities for developing microbiome-based immunotherapy supplements in the future.
KAIST (President Kwang Hyung Lee) announced on July 1 that a research team led by Professor Heung Kyu Lee of the Department of Biological Sciences discovered and demonstrated a method to significantly improve the efficiency of glioblastoma immunotherapy by focusing on changes in the gut microbial ecosystem.
The research team noted that as glioblastoma progresses, the concentration of ‘tryptophan’, an important amino acid in the gut, sharply decreases, leading to changes in the gut microbial ecosystem. They discovered that by supplementing tryptophan to restore microbial diversity, specific beneficial strains activate CD8 T cells (a type of immune cell) and induce their infiltration into tumor tissues. Through a mouse model of glioblastoma, the research team confirmed that tryptophan supplementation enhanced the response of cancer-attacking T cells (especially CD8 T cells), leading to their increased migration to tumor sites such as lymph nodes and the brain.
In this process, they also revealed that ‘Duncaniella dubosii’, a beneficial commensal bacterium present in the gut, plays a crucial role. This bacterium helped T cells effectively redistribute within the body, and survival rates significantly improved when used in combination with immunotherapy (anti-PD-1).
Furthermore, it was demonstrated that even when this commensal bacterium was administered alone to germ-free mice (mice without any commensal microbes), the survival rate for glioblastoma increased. This is because the bacterium utilizes tryptophan to regulate the gut environment, and the metabolites produced in this process strengthen the ability of CD8 T cells to attack cancer cells.
Professor Heung Kyu Lee explained, "This research is a meaningful achievement, showing that even in intractable brain tumors where immune checkpoint inhibitors had no effect, a combined strategy utilizing gut microbes can significantly enhance treatment response."
Dr. Hyeon Cheol Kim of KAIST (currently a postdoctoral researcher at the Institute for Biological Sciences) participated as the first author. The research findings were published online in Cell Reports, an international journal in the life sciences, on June 26.
This research was conducted as part of the Basic Research Program and Bio & Medical Technology Development Program supported by the Ministry of Science and ICT and the National Research Foundation of Korea.
※Paper Title: Gut microbiota dysbiosis induced by brain tumor modulates the efficacy of immunotherapy
※DOI: https://doi.org/10.1016/j.celrep.2025.115825
2025.07.02 View 1418
KAIST Enhances Immunotherapy for Difficult-to-Treat Brain Tumors with Gut Microbiota
< Photo 1.(From left) Prof. Heung Kyu Lee, Department of Biological Sciences,
and Dr. Hyeon Cheol Kim>
Advanced treatments, known as immunotherapies that activate T cells—our body's immune cells—to eliminate cancer cells, have shown limited efficacy as standalone therapies for glioblastoma, the most lethal form of brain tumor. This is due to their minimal response to glioblastoma and high resistance to treatment.
Now, a KAIST research team has now demonstrated a new therapeutic strategy that can enhance the efficacy of immunotherapy for brain tumors by utilizing gut microbes and their metabolites. This also opens up possibilities for developing microbiome-based immunotherapy supplements in the future.
KAIST (President Kwang Hyung Lee) announced on July 1 that a research team led by Professor Heung Kyu Lee of the Department of Biological Sciences discovered and demonstrated a method to significantly improve the efficiency of glioblastoma immunotherapy by focusing on changes in the gut microbial ecosystem.
The research team noted that as glioblastoma progresses, the concentration of ‘tryptophan’, an important amino acid in the gut, sharply decreases, leading to changes in the gut microbial ecosystem. They discovered that by supplementing tryptophan to restore microbial diversity, specific beneficial strains activate CD8 T cells (a type of immune cell) and induce their infiltration into tumor tissues. Through a mouse model of glioblastoma, the research team confirmed that tryptophan supplementation enhanced the response of cancer-attacking T cells (especially CD8 T cells), leading to their increased migration to tumor sites such as lymph nodes and the brain.
In this process, they also revealed that ‘Duncaniella dubosii’, a beneficial commensal bacterium present in the gut, plays a crucial role. This bacterium helped T cells effectively redistribute within the body, and survival rates significantly improved when used in combination with immunotherapy (anti-PD-1).
Furthermore, it was demonstrated that even when this commensal bacterium was administered alone to germ-free mice (mice without any commensal microbes), the survival rate for glioblastoma increased. This is because the bacterium utilizes tryptophan to regulate the gut environment, and the metabolites produced in this process strengthen the ability of CD8 T cells to attack cancer cells.
Professor Heung Kyu Lee explained, "This research is a meaningful achievement, showing that even in intractable brain tumors where immune checkpoint inhibitors had no effect, a combined strategy utilizing gut microbes can significantly enhance treatment response."
Dr. Hyeon Cheol Kim of KAIST (currently a postdoctoral researcher at the Institute for Biological Sciences) participated as the first author. The research findings were published online in Cell Reports, an international journal in the life sciences, on June 26.
This research was conducted as part of the Basic Research Program and Bio & Medical Technology Development Program supported by the Ministry of Science and ICT and the National Research Foundation of Korea.
※Paper Title: Gut microbiota dysbiosis induced by brain tumor modulates the efficacy of immunotherapy
※DOI: https://doi.org/10.1016/j.celrep.2025.115825
2025.07.02 View 1418 -
 New and Highly Efficient Recycling Technology to Turn Used Tires into Raw Materials for Rubber and Nylon
< (From left) Kyungmin Choi (MS-Ph.D. integrated course, Department of Chemistry), Dr. Beomsoon Park, Professor Soon Hyeok Hong, Dr. Kyoungil Cho >
Approximately 1.5 billions of tires are discarded globally every year, and this is identified as one of the major causes of serious environmental pollution. The research team at the Department of Chemistry at KAIST has achieved a breakthrough by selectively converting waste tires into high-purity cyclic alkenes, valuable chemical building blocks used in the production of rubber and nylon fibers. This advance marks a new milestone in chemical recycling technology for waste tires.
The team, led by Professor Soon Hyeok Hong, has developed a dual-catalyst-based reaction system that overcomes the long-standing challenges associated with recycling vulcanized rubber materials.
Tires are composed of complex blends of synthetic and natural rubber, and their physical strength and durability are reinforced with additives such as silica, carbon black, and antioxidants. In particular, cross-linking between rubber chains is formed through the vulcanization process, giving them a structure resistant to heat and pressure, which is one of the main reasons why chemical recycling of waste tires is difficult.
Until now, waste tire recycling has mainly relied on pyrolysis or mechanical recycling methods. The pyrolysis method is a technology that decomposes polymer chains at high temperatures of 350-800°C to convert them into fuel oil, but it clearly has limitations such as high energy consumption, low selectivity, and the production of low-quality hydrocarbon mixtures.
To solve these problems, the research team developed a method to convert waste rubber into useful chemicals using dual catalysis. The first catalyst helps to break down rubber molecules by changing their bonding structure, and the second catalyst creates cyclic compounds through a ring-closing reaction.
This process shows high selectivity of up to 92% and a yield of 82%. The produced cyclopentene can be recycled into rubber, and cyclohexene can be used as a raw material for nylon fibers, making them industrially very valuable.
The research team successfully applied the developed system to discarded waste tires, achieving selective conversion into high-purity cyclic alkenes. Unlike the existing pyrolysis method, this is evaluated as a new turning point in the field of waste tire recycling as it can produce high-value chemicals through low-temperature precision catalytic reactions.
In addition, this catalytic platform is compatible with a wide range of synthetic and waste rubbers, positioning it as a promising foundation for scalable, circular solutions in the polymer and materials industries.
< Figure 1. Development of a Catalytic Method for Chemical Recycling of Waste Rubber >
Professor Hong stated, "This research offers an innovative solution for the chemical recycling of waste tires. We aim to develop next-generation high-efficiency catalysts and lay the groundwork for commercialization to enhance economic feasibility. Ultimately, our goal is to contribute to solving the broader waste plastic problem through fundamental chemistry."
This research, in which Beomsoon Park, Kyoungil Cho, and Kyungmin Choi participated, was supported by the National Research Foundation of Korea and was published online in the internationally renowned academic journal ‘Chem’ on June 18th.
※Paper Title: Catalytic and Selective Chemical Recycling of Post-Consumer Rubbers into Cycloalkenes
※DOI: 10.1016/j.chempr.2025.102625
2025.06.26 View 2481
New and Highly Efficient Recycling Technology to Turn Used Tires into Raw Materials for Rubber and Nylon
< (From left) Kyungmin Choi (MS-Ph.D. integrated course, Department of Chemistry), Dr. Beomsoon Park, Professor Soon Hyeok Hong, Dr. Kyoungil Cho >
Approximately 1.5 billions of tires are discarded globally every year, and this is identified as one of the major causes of serious environmental pollution. The research team at the Department of Chemistry at KAIST has achieved a breakthrough by selectively converting waste tires into high-purity cyclic alkenes, valuable chemical building blocks used in the production of rubber and nylon fibers. This advance marks a new milestone in chemical recycling technology for waste tires.
The team, led by Professor Soon Hyeok Hong, has developed a dual-catalyst-based reaction system that overcomes the long-standing challenges associated with recycling vulcanized rubber materials.
Tires are composed of complex blends of synthetic and natural rubber, and their physical strength and durability are reinforced with additives such as silica, carbon black, and antioxidants. In particular, cross-linking between rubber chains is formed through the vulcanization process, giving them a structure resistant to heat and pressure, which is one of the main reasons why chemical recycling of waste tires is difficult.
Until now, waste tire recycling has mainly relied on pyrolysis or mechanical recycling methods. The pyrolysis method is a technology that decomposes polymer chains at high temperatures of 350-800°C to convert them into fuel oil, but it clearly has limitations such as high energy consumption, low selectivity, and the production of low-quality hydrocarbon mixtures.
To solve these problems, the research team developed a method to convert waste rubber into useful chemicals using dual catalysis. The first catalyst helps to break down rubber molecules by changing their bonding structure, and the second catalyst creates cyclic compounds through a ring-closing reaction.
This process shows high selectivity of up to 92% and a yield of 82%. The produced cyclopentene can be recycled into rubber, and cyclohexene can be used as a raw material for nylon fibers, making them industrially very valuable.
The research team successfully applied the developed system to discarded waste tires, achieving selective conversion into high-purity cyclic alkenes. Unlike the existing pyrolysis method, this is evaluated as a new turning point in the field of waste tire recycling as it can produce high-value chemicals through low-temperature precision catalytic reactions.
In addition, this catalytic platform is compatible with a wide range of synthetic and waste rubbers, positioning it as a promising foundation for scalable, circular solutions in the polymer and materials industries.
< Figure 1. Development of a Catalytic Method for Chemical Recycling of Waste Rubber >
Professor Hong stated, "This research offers an innovative solution for the chemical recycling of waste tires. We aim to develop next-generation high-efficiency catalysts and lay the groundwork for commercialization to enhance economic feasibility. Ultimately, our goal is to contribute to solving the broader waste plastic problem through fundamental chemistry."
This research, in which Beomsoon Park, Kyoungil Cho, and Kyungmin Choi participated, was supported by the National Research Foundation of Korea and was published online in the internationally renowned academic journal ‘Chem’ on June 18th.
※Paper Title: Catalytic and Selective Chemical Recycling of Post-Consumer Rubbers into Cycloalkenes
※DOI: 10.1016/j.chempr.2025.102625
2025.06.26 View 2481 -
 Simultaneous Analysis of 21 Chemical Reactions... AI to Transform New Drug Development
< Photo 1. (From left) Professor Hyunwoo Kim and students Donghun Kim and Gyeongseon Choi in the Integrated M.S./Ph.D. program of the Department of Chemistry >
Thalidomide, a drug once used to alleviate morning sickness in pregnant women, exhibits distinct properties due to its optical isomers* in the body: one isomer has a sedative effect, while the other causes severe side effects like birth defects. As this example illustrates, precise organic synthesis techniques, which selectively synthesize only the desired optical isomer, are crucial in new drug development. Overcoming the traditional methods that struggled with simultaneously analyzing multiple reactants, our research team has developed the world's first technology to precisely analyze 21 types of reactants simultaneously. This breakthrough is expected to make a significant contribution to new drug development utilizing AI and robots.
*Optical Isomers: A pair of molecules with the same chemical formula that are mirror images of each other and cannot be superimposed due to their asymmetric structure. This is analogous to a left and right hand, which are similar in form but cannot be perfectly overlaid.
KAIST's Professor Hyunwoo Kim's research team in the Department of Chemistry announced on the 16th that they have developed an innovative optical isomer analysis technology suitable for the era of AI-driven autonomous synthesis*. This research is the world's first technology to precisely analyze asymmetric catalytic reactions involving multiple reactants simultaneously using high-resolution fluorine nuclear magnetic resonance spectroscopy (19F NMR). It is expected to make groundbreaking contributions to various fields, including new drug development and catalyst optimization.
*AI-driven Autonomous Synthesis: An advanced technology that automates and optimizes chemical substance synthesis processes using artificial intelligence (AI). It is gaining attention as a core element for realizing automated and intelligent research environments in future laboratories. AI predicts and adjusts experimental conditions, interprets results, and designs subsequent experiments independently, minimizing human intervention in repetitive experiments and significantly increasing research efficiency and innovativeness.
Currently, while autonomous synthesis systems can automate everything from reaction design to execution, reaction analysis still relies on individual processing using traditional equipment. This leads to slower speeds and bottlenecks, making it unsuitable for high-speed repetitive experiments.
Furthermore, multi-substrate simultaneous screening techniques proposed in the 1990s garnered attention as a strategy to maximize reaction analysis efficiency. However, limitations of existing chromatography-based analysis methods restricted the number of applicable substrates. In asymmetric synthesis reactions, which selectively synthesize only the desired optical isomer, simultaneously analyzing more than 10 types of substrates was nearly impossible.
< Figure 1. Conventional organic reaction evaluation methods follow a process of deriving optimal reaction conditions using a single substrate, then expanding the substrate scope one by one under those conditions, leaving potential reaction areas unexplored. To overcome this, high-throughput screening is introduced to broadly explore catalyst reactivity for various substrates. When combined with multi-substrate screening, this approach allows for a much broader and more systematic understanding of reaction scope and trends. >
To overcome these limitations, the research team developed a 19F NMR-based multi-substrate simultaneous screening technology. This method involves performing asymmetric catalytic reactions with multiple reactants in a single reaction vessel, introducing a fluorine functional group into the products, and then applying their self-developed chiral cobalt reagent to clearly quantify all optical isomers using 19F NMR.
Utilizing the excellent resolution and sensitivity of 19F NMR, the research team successfully performed asymmetric synthesis reactions of 21 substrates simultaneously in a single reaction vessel and quantitatively measured the product yield and optical isomer ratio without any separate purification steps.
Professor Hyunwoo Kim stated, "While anyone can perform asymmetric synthesis reactions with multiple substrates in one reactor, accurately analyzing all the products has been a challenging problem to solve until now. We expect that achieving world-class multi-substrate screening analysis technology will greatly contribute to enhancing the analytical capabilities of AI-driven autonomous synthesis platforms."
< Figure 2. A method for analyzing multi-substrate asymmetric catalytic reactions, where different substrates react simultaneously in a single reactor, using fluorine nuclear magnetic resonance has been implemented. By utilizing the characteristics of fluorine nuclear magnetic resonance, which has a clean background signal and a wide chemical shift range, the reactivity of each substrate can be quantitatively analyzed. It is also shown that the optical activity of all reactants can be simultaneously measured using a cobalt metal complex. >
He further added, "This research provides a technology that can rapidly verify the efficiency and selectivity of asymmetric catalytic reactions essential for new drug development, and it is expected to be utilized as a core analytical tool for AI-driven autonomous research."
< Figure 3. It can be seen that in a multi-substrate reductive amination reaction using a total of 21 substrates, the yield and optical activity of the reactants according to the catalyst system were simultaneously measured using a fluorine nuclear magnetic resonance-based analysis platform. The yield of each reactant is indicated by color saturation, and the optical activity by numbers. >
Donghun Kim (first author, Integrated M.S./Ph.D. program) and Gyeongseon Choi (second author, Integrated M.S./Ph.D. program) from the KAIST Department of Chemistry participated in this research. The study was published online in the Journal of the American Chemical Society on May 27, 2025.※ Paper Title: One-pot Multisubstrate Screening for Asymmetric Catalysis Enabled by 19F NMR-based Simultaneous Chiral Analysis※ DOI: 10.1021/jacs.5c03446
This research was supported by the National Research Foundation of Korea's Mid-Career Researcher Program, the Asymmetric Catalytic Reaction Design Center, and the KAIST KC30 Project.
< Figure 4. Conceptual diagram of performing multi-substrate screening reactions and utilizing fluorine nuclear magnetic resonance spectroscopy. >
2025.06.16 View 2945
Simultaneous Analysis of 21 Chemical Reactions... AI to Transform New Drug Development
< Photo 1. (From left) Professor Hyunwoo Kim and students Donghun Kim and Gyeongseon Choi in the Integrated M.S./Ph.D. program of the Department of Chemistry >
Thalidomide, a drug once used to alleviate morning sickness in pregnant women, exhibits distinct properties due to its optical isomers* in the body: one isomer has a sedative effect, while the other causes severe side effects like birth defects. As this example illustrates, precise organic synthesis techniques, which selectively synthesize only the desired optical isomer, are crucial in new drug development. Overcoming the traditional methods that struggled with simultaneously analyzing multiple reactants, our research team has developed the world's first technology to precisely analyze 21 types of reactants simultaneously. This breakthrough is expected to make a significant contribution to new drug development utilizing AI and robots.
*Optical Isomers: A pair of molecules with the same chemical formula that are mirror images of each other and cannot be superimposed due to their asymmetric structure. This is analogous to a left and right hand, which are similar in form but cannot be perfectly overlaid.
KAIST's Professor Hyunwoo Kim's research team in the Department of Chemistry announced on the 16th that they have developed an innovative optical isomer analysis technology suitable for the era of AI-driven autonomous synthesis*. This research is the world's first technology to precisely analyze asymmetric catalytic reactions involving multiple reactants simultaneously using high-resolution fluorine nuclear magnetic resonance spectroscopy (19F NMR). It is expected to make groundbreaking contributions to various fields, including new drug development and catalyst optimization.
*AI-driven Autonomous Synthesis: An advanced technology that automates and optimizes chemical substance synthesis processes using artificial intelligence (AI). It is gaining attention as a core element for realizing automated and intelligent research environments in future laboratories. AI predicts and adjusts experimental conditions, interprets results, and designs subsequent experiments independently, minimizing human intervention in repetitive experiments and significantly increasing research efficiency and innovativeness.
Currently, while autonomous synthesis systems can automate everything from reaction design to execution, reaction analysis still relies on individual processing using traditional equipment. This leads to slower speeds and bottlenecks, making it unsuitable for high-speed repetitive experiments.
Furthermore, multi-substrate simultaneous screening techniques proposed in the 1990s garnered attention as a strategy to maximize reaction analysis efficiency. However, limitations of existing chromatography-based analysis methods restricted the number of applicable substrates. In asymmetric synthesis reactions, which selectively synthesize only the desired optical isomer, simultaneously analyzing more than 10 types of substrates was nearly impossible.
< Figure 1. Conventional organic reaction evaluation methods follow a process of deriving optimal reaction conditions using a single substrate, then expanding the substrate scope one by one under those conditions, leaving potential reaction areas unexplored. To overcome this, high-throughput screening is introduced to broadly explore catalyst reactivity for various substrates. When combined with multi-substrate screening, this approach allows for a much broader and more systematic understanding of reaction scope and trends. >
To overcome these limitations, the research team developed a 19F NMR-based multi-substrate simultaneous screening technology. This method involves performing asymmetric catalytic reactions with multiple reactants in a single reaction vessel, introducing a fluorine functional group into the products, and then applying their self-developed chiral cobalt reagent to clearly quantify all optical isomers using 19F NMR.
Utilizing the excellent resolution and sensitivity of 19F NMR, the research team successfully performed asymmetric synthesis reactions of 21 substrates simultaneously in a single reaction vessel and quantitatively measured the product yield and optical isomer ratio without any separate purification steps.
Professor Hyunwoo Kim stated, "While anyone can perform asymmetric synthesis reactions with multiple substrates in one reactor, accurately analyzing all the products has been a challenging problem to solve until now. We expect that achieving world-class multi-substrate screening analysis technology will greatly contribute to enhancing the analytical capabilities of AI-driven autonomous synthesis platforms."
< Figure 2. A method for analyzing multi-substrate asymmetric catalytic reactions, where different substrates react simultaneously in a single reactor, using fluorine nuclear magnetic resonance has been implemented. By utilizing the characteristics of fluorine nuclear magnetic resonance, which has a clean background signal and a wide chemical shift range, the reactivity of each substrate can be quantitatively analyzed. It is also shown that the optical activity of all reactants can be simultaneously measured using a cobalt metal complex. >
He further added, "This research provides a technology that can rapidly verify the efficiency and selectivity of asymmetric catalytic reactions essential for new drug development, and it is expected to be utilized as a core analytical tool for AI-driven autonomous research."
< Figure 3. It can be seen that in a multi-substrate reductive amination reaction using a total of 21 substrates, the yield and optical activity of the reactants according to the catalyst system were simultaneously measured using a fluorine nuclear magnetic resonance-based analysis platform. The yield of each reactant is indicated by color saturation, and the optical activity by numbers. >
Donghun Kim (first author, Integrated M.S./Ph.D. program) and Gyeongseon Choi (second author, Integrated M.S./Ph.D. program) from the KAIST Department of Chemistry participated in this research. The study was published online in the Journal of the American Chemical Society on May 27, 2025.※ Paper Title: One-pot Multisubstrate Screening for Asymmetric Catalysis Enabled by 19F NMR-based Simultaneous Chiral Analysis※ DOI: 10.1021/jacs.5c03446
This research was supported by the National Research Foundation of Korea's Mid-Career Researcher Program, the Asymmetric Catalytic Reaction Design Center, and the KAIST KC30 Project.
< Figure 4. Conceptual diagram of performing multi-substrate screening reactions and utilizing fluorine nuclear magnetic resonance spectroscopy. >
2025.06.16 View 2945