SCIE
-
 Former Minister of Science and Technology Woo Sik Kim Elected as New Chairman of Board of Trustees
Dr. Woo Sik Kim, former Minister of Science and Technology and Deputy Prime Minister, was elected as the new chairman of the KAIST Board of Trustees on March 26. Dr. Kim will succeed Chairman Jang-Mu Lee, whose three-year term expired last month.
Dr. Kim is a chemical engineering professor who spent most of his academic career at Yonsei University from 1968. In 2000, he held the office of president of Yonsei University for four years before moving to the Presidential Office of President Roh Moo-Hyun as his chief of staff in 2004. After serving in the Blue House for two years, he served as the Minister of Science and Technology from 2006 to 2008.
An emeritus fellow of the National Academy of Engineering of Korea (NAEK), Chairman Kim also taught at KAIST as an invited distinguished professor from 2008 to 2010. He is currently the chairman of the Creativity Engineering Institute (CEI).
(END)
2020.04.06 View 15669
Former Minister of Science and Technology Woo Sik Kim Elected as New Chairman of Board of Trustees
Dr. Woo Sik Kim, former Minister of Science and Technology and Deputy Prime Minister, was elected as the new chairman of the KAIST Board of Trustees on March 26. Dr. Kim will succeed Chairman Jang-Mu Lee, whose three-year term expired last month.
Dr. Kim is a chemical engineering professor who spent most of his academic career at Yonsei University from 1968. In 2000, he held the office of president of Yonsei University for four years before moving to the Presidential Office of President Roh Moo-Hyun as his chief of staff in 2004. After serving in the Blue House for two years, he served as the Minister of Science and Technology from 2006 to 2008.
An emeritus fellow of the National Academy of Engineering of Korea (NAEK), Chairman Kim also taught at KAIST as an invited distinguished professor from 2008 to 2010. He is currently the chairman of the Creativity Engineering Institute (CEI).
(END)
2020.04.06 View 15669 -
 A Global Campaign of ‘Facts before Rumors’ on COVID-19 Launched
- A KAIST data scientist group responds to facts and rumors on COVID-19 for global awareness of the pandemic. -
Like the novel coronavirus, rumors have no borders. The world is fighting to contain the pandemic, but we also have to deal with the appalling spread of an infodemic that is as contagious as the virus. This infodemic, a pandemic of false information, is bringing chaos and extreme fear to the general public.
Professor Meeyoung Cha’s group at the School of Computing started a global campaign called ‘Facts before Rumors,’ to prevent the spread of false information from crossing borders. She explained, “We saw many rumors that had already been fact-checked long before in China and South Korea now begin to circulate in other countries, sometimes leading to detrimental results. We launched an official campaign, Facts before Rumors, to deliver COVID-19-related facts to countries where the number of cases is now increasing.” She released the first set of facts on March 26 via her Twitter account @nekozzang.
Professor Cha, a data scientist who has focused on detecting global fake news, is now part of the COVID-19 AI Task Force at the Global Strategy Institute at KAIST. She is also leading the Data Science Group at the Institute for Basic Science (IBS) as Chief Investigator.
Her research group worked in collaboration with the College of Nursing at Ewha Woman’s University to identify 15 claims about COVID-19 that circulated on social networks (SNS) and among the general public. The team fact-checked these claims based on information from the WHO and CDCs of Korea and the US. The research group is now working on translating the list of claims into Portuguese, Spanish, Persian, Chinese, Amharic, Hindi, and Vietnamese. Delivering facts before rumors, the team says, will help contain the disease and prevent any harm caused by misinformation.
The pandemic, which spread in China and South Korea before arriving in Europe and the US, is now moving into South America, Africa, and Southeast Asia. “We would like to play a part in preventing the further spread of the disease with the provision of only scientifically vetted, truthful facts,” said the team.
For this campaign, Professor Cha’s team investigated more than 200 rumored claims on COVID-19 in China during the early days of the pandemic. These claims spread in different levels: while some were only relevant locally or in larger regions of China, others propagated in Asia and are now spreading to countries that are currently most affected by the disease.
For example, the false claim which publicized that ‘Fireworks can help tame the virus in the air’ only spread in China. Other claims such as ‘Eating garlic helps people overcome the disease’ or ‘Gargling with salt water prevents the contraction of the disease,’ spread around the world even after being proved groundless.
The team noted, however, that the times at which these claims propagate are different from one country to another. “This opens up an opportunity to debunk rumors in some countries, even before they start to emerge,” said Professor Cha.
Kun-Woo Kim, a master’s candidate in the Department of Industrial Design who joined this campaign and designed the Facts before Rumors chart also expressed his hope that this campaign will help reduce the number of victims. He added, “I am very grateful to our scientists who quickly responded to the Fact Check in these challenging times.”
2020.03.27 View 15344
A Global Campaign of ‘Facts before Rumors’ on COVID-19 Launched
- A KAIST data scientist group responds to facts and rumors on COVID-19 for global awareness of the pandemic. -
Like the novel coronavirus, rumors have no borders. The world is fighting to contain the pandemic, but we also have to deal with the appalling spread of an infodemic that is as contagious as the virus. This infodemic, a pandemic of false information, is bringing chaos and extreme fear to the general public.
Professor Meeyoung Cha’s group at the School of Computing started a global campaign called ‘Facts before Rumors,’ to prevent the spread of false information from crossing borders. She explained, “We saw many rumors that had already been fact-checked long before in China and South Korea now begin to circulate in other countries, sometimes leading to detrimental results. We launched an official campaign, Facts before Rumors, to deliver COVID-19-related facts to countries where the number of cases is now increasing.” She released the first set of facts on March 26 via her Twitter account @nekozzang.
Professor Cha, a data scientist who has focused on detecting global fake news, is now part of the COVID-19 AI Task Force at the Global Strategy Institute at KAIST. She is also leading the Data Science Group at the Institute for Basic Science (IBS) as Chief Investigator.
Her research group worked in collaboration with the College of Nursing at Ewha Woman’s University to identify 15 claims about COVID-19 that circulated on social networks (SNS) and among the general public. The team fact-checked these claims based on information from the WHO and CDCs of Korea and the US. The research group is now working on translating the list of claims into Portuguese, Spanish, Persian, Chinese, Amharic, Hindi, and Vietnamese. Delivering facts before rumors, the team says, will help contain the disease and prevent any harm caused by misinformation.
The pandemic, which spread in China and South Korea before arriving in Europe and the US, is now moving into South America, Africa, and Southeast Asia. “We would like to play a part in preventing the further spread of the disease with the provision of only scientifically vetted, truthful facts,” said the team.
For this campaign, Professor Cha’s team investigated more than 200 rumored claims on COVID-19 in China during the early days of the pandemic. These claims spread in different levels: while some were only relevant locally or in larger regions of China, others propagated in Asia and are now spreading to countries that are currently most affected by the disease.
For example, the false claim which publicized that ‘Fireworks can help tame the virus in the air’ only spread in China. Other claims such as ‘Eating garlic helps people overcome the disease’ or ‘Gargling with salt water prevents the contraction of the disease,’ spread around the world even after being proved groundless.
The team noted, however, that the times at which these claims propagate are different from one country to another. “This opens up an opportunity to debunk rumors in some countries, even before they start to emerge,” said Professor Cha.
Kun-Woo Kim, a master’s candidate in the Department of Industrial Design who joined this campaign and designed the Facts before Rumors chart also expressed his hope that this campaign will help reduce the number of victims. He added, “I am very grateful to our scientists who quickly responded to the Fact Check in these challenging times.”
2020.03.27 View 15344 -
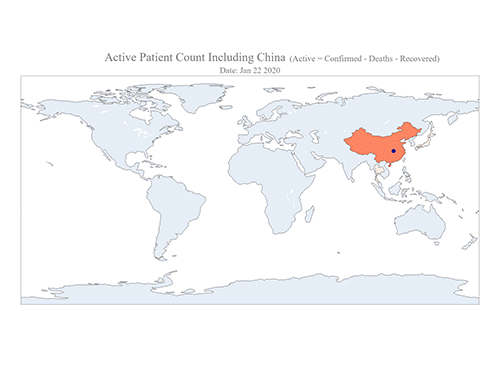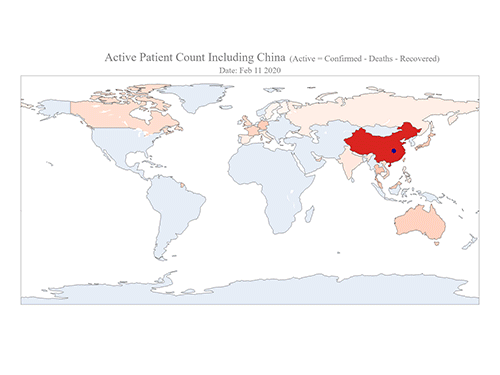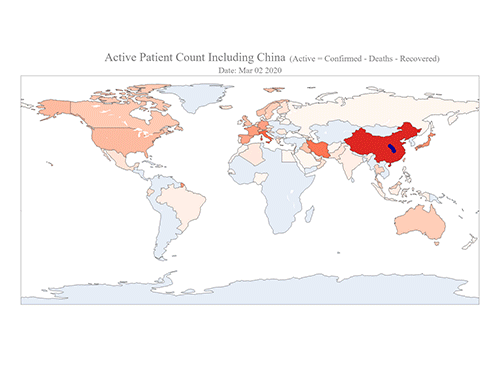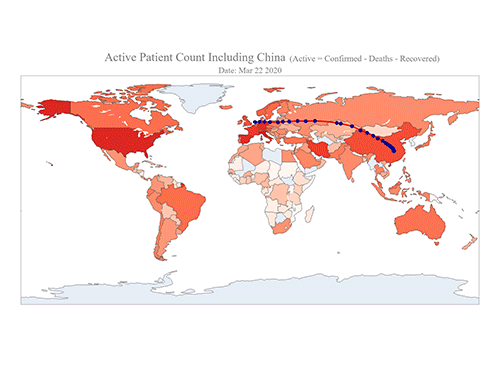 COVID-19 Map Shows How the Global Pandemic Moves
- A School of Computing team facilitated the data from COVID-19 to show the global spread of the virus. -
The COVID-19 map made by KAIST data scientists shows where and how the virus is spreading from China, reportedly the epicenter of the disease.
Professor Meeyoung Cha from the School of Computing and her group facilitated data based on the number of confirmed cases from January 22 to March 22 to analyze the trends of this global epidemic. The statistics include the number of confirmed cases, recoveries, and deaths across major continents based on the number of confirmed case data during that period.
The moving dot on the map strikingly shows how the confirmed cases are moving across the globe. According to their statistics, the centroid of the disease starts from near Wuhan in China and moved to Korea, then through the European region via Italy and Iran.
The data is collected by a graduate student from the School of Computing, Geng Sun, who started the process during the time he was quarantined since coming back from his home in China. An undergraduate colleague of Geng's, Gabriel Camilo Lima who made the map, is now working remotely from his home in Brazil since all undergraduate students were required to move out of the dormitory last week. The university closed all undergraduate housing and advised the undergraduate students to go back home in a preventive measure to stop the virus from spreading across the campus.
Gabriel said he calculated the centroid of all confirmed cases up to a given day. He explained, “I weighed each coordinate by the number of cases in that region and country and calculated an approximate center of gravity.”
“The Earth is round, so the shortest path from Asia to Europe is often through Russia. In early March, the center of gravity of new cases was moving from Asia to Europe. Therefore, the centroid is moving to the west and goes through Russia, even though Russia has not reported many cases,” he added.
Professor Cha, who is also responsible for the Data Science Group at the Institute for Basic Science (IBS) as the Chief Investigator, said their group will continue to update the map using public data at https://ds.ibs.re.kr/index.php/covid-19/.
(END)
2020.03.27 View 14765
COVID-19 Map Shows How the Global Pandemic Moves
- A School of Computing team facilitated the data from COVID-19 to show the global spread of the virus. -
The COVID-19 map made by KAIST data scientists shows where and how the virus is spreading from China, reportedly the epicenter of the disease.
Professor Meeyoung Cha from the School of Computing and her group facilitated data based on the number of confirmed cases from January 22 to March 22 to analyze the trends of this global epidemic. The statistics include the number of confirmed cases, recoveries, and deaths across major continents based on the number of confirmed case data during that period.
The moving dot on the map strikingly shows how the confirmed cases are moving across the globe. According to their statistics, the centroid of the disease starts from near Wuhan in China and moved to Korea, then through the European region via Italy and Iran.
The data is collected by a graduate student from the School of Computing, Geng Sun, who started the process during the time he was quarantined since coming back from his home in China. An undergraduate colleague of Geng's, Gabriel Camilo Lima who made the map, is now working remotely from his home in Brazil since all undergraduate students were required to move out of the dormitory last week. The university closed all undergraduate housing and advised the undergraduate students to go back home in a preventive measure to stop the virus from spreading across the campus.
Gabriel said he calculated the centroid of all confirmed cases up to a given day. He explained, “I weighed each coordinate by the number of cases in that region and country and calculated an approximate center of gravity.”
“The Earth is round, so the shortest path from Asia to Europe is often through Russia. In early March, the center of gravity of new cases was moving from Asia to Europe. Therefore, the centroid is moving to the west and goes through Russia, even though Russia has not reported many cases,” he added.
Professor Cha, who is also responsible for the Data Science Group at the Institute for Basic Science (IBS) as the Chief Investigator, said their group will continue to update the map using public data at https://ds.ibs.re.kr/index.php/covid-19/.
(END)
2020.03.27 View 14765 -
 Highly Efficient and Stable Double Layer Solar Cell Developed
Solar cells convert light into energy, but they can be inefficient and vulnerable to the environment, degrading with, ironically, too much light or other factors, including moisture and low temperature. An international research team has developed a new type of solar cell that can both withstand environmental hazards and is 26.7% efficient in power conversion.
They published their results on March 26 in Science.
The researchers, led by Byungha Shin, a professor from the Department of Materials Science and Engineering at KAIST, focused on developing a new class of light-absorbing material, called a wide bandgap perovskite. The material has a highly effective crystal structure that can process the power needs, but it can become problematic when exposed to environmental hazards, such as moisture. Researchers have made some progress increasing the efficiency of solar cells based on perovskite, but the material has greater potential than what was previously achieved.
To achieve better performance, Shin and his team built a double layer solar cell, called tandem, in which two or more light absorbers are stacked together to better utilize solar energy. To use perovskite in these tandem devices, the scientists modified the material’s optical property, which allows it to absorb a wider range of solar energy. Without the adjustment, the material is not as useful in achieving high performing tandem solar cells. The modification of the optical property of perovskite, however, comes with a penalty — the material becomes hugely vulnerable to the environment, in particular, to light.
To counteract the wide bandgap perovskite’s delicate nature, the researchers engineered combinations of molecules composing a two-dimensional layer in the perovskite, stabilizing the solar cells.
“We developed a high-quality wide bandgap perovskite material and, in combination with silicon solar cells, achieved world-class perovskite-silicon tandem cells,” Shin said.
The development was only possible due to the engineering method, in which the mixing ratio of the molecules building the two-dimensional layer are carefully controlled. In this case, the perovskite material not only improved efficiency of the resulting solar cell but also gained durability, retaining 80% of its initial power conversion capability even after 1,000 hours of continuous illumination. This is the first time such a high efficiency has been achieved with a wide bandgap perovskite single layer alone, according to Shin.
“Such high-efficiency wide bandgap perovskite is an essential technology for achieving ultra-high efficiency of perovskite-silicon tandem (double layer) solar cells,” Shin said. “The results also show the importance of bandgap matching of upper and lower cells in these tandem solar cells.”
The researchers, having stabilized the wide bandgap perovskite material, are now focused on developing even more efficient tandem solar cells that are expected to have more than 30% of power conversion efficiency, something that no one has achieved yet,
“Our ultimate goal is to develop ultra-high-efficiency tandem solar cells that contribute to the increase of shared solar energy among all energy sources,” Shin said. “We want to contribute to making the planet healthier.”
This work was supported by the National Research Foundation of Korea, the Korea Institute of Energy Technology Evaluation and Planning, the Ministry of Trade Industry and Energy of Korea, and the U.S. Department of Energy.
Other contributors include Daehan Kim, Jekyung Kim, Passarut Boonmongkolras, Seong Ryul Pae and Minkyu Kim, all of whom affiliated with the Department of Materials Science and Engineering at KAIST. Other authors include Byron W. Larson, Sean P. Dunfield, Chuanxiao Xiao, Jinhui Tong, Fei Zhang, Joseph J. Berry, Kai Zhu and Dong Hoe Kim, all of who are affiliated with the National Renewable Energy Laboratory in Colorado. Dunfield is also affiliated with the Materials Science and Engineering Program at the University of Colorado; Berry is also affiliated with the Department of Physics and the Renewable and Sustainable Energy Institute at the University of Colorado Boulder; and Kim is also affiliated with the Department of Nanotechnology and Advanced Materials Engineering at Sejong University. Hee Joon Jung and Vinayak Dravid of the Department of Materials Science and Engineering at Northwestern University; Ik Jae Park, Su Geun Ji and Jin Young Kim of the Department of Materials Science and Engineering at Seoul National University; and Seok Beom Kang of the Department of Nanotechnology and Advanced Materials Engineering of Sejong University also contributed.
Image credit: Professor Byungha Shin, KAIST
Image usage restrictions: News organizations may use or redistribute this image, with proper attribution, as part of news coverage of this paper only.
Publication: Kim et al. (2020) “Efficient, stable silicon tandem cells enabled by anion-engineered wide band gap perovskites”. Science. Available online at https://doi.org/10.1126/science.aba3433
Profile:
Byungha Shin
Professor
byungha@kaist.ac.kr
http://energymatlab.kaist.ac.kr/
Department of Materials Science and Engineering
KAIST
Profile:
Daehan Kim
Ph.D. Candidate
zxzx4592@kaist.ac.kr
http://energymatlab.kaist.ac.kr/
Department of Materials Science and Engineering
KAIST
(END)
2020.03.27 View 24736
Highly Efficient and Stable Double Layer Solar Cell Developed
Solar cells convert light into energy, but they can be inefficient and vulnerable to the environment, degrading with, ironically, too much light or other factors, including moisture and low temperature. An international research team has developed a new type of solar cell that can both withstand environmental hazards and is 26.7% efficient in power conversion.
They published their results on March 26 in Science.
The researchers, led by Byungha Shin, a professor from the Department of Materials Science and Engineering at KAIST, focused on developing a new class of light-absorbing material, called a wide bandgap perovskite. The material has a highly effective crystal structure that can process the power needs, but it can become problematic when exposed to environmental hazards, such as moisture. Researchers have made some progress increasing the efficiency of solar cells based on perovskite, but the material has greater potential than what was previously achieved.
To achieve better performance, Shin and his team built a double layer solar cell, called tandem, in which two or more light absorbers are stacked together to better utilize solar energy. To use perovskite in these tandem devices, the scientists modified the material’s optical property, which allows it to absorb a wider range of solar energy. Without the adjustment, the material is not as useful in achieving high performing tandem solar cells. The modification of the optical property of perovskite, however, comes with a penalty — the material becomes hugely vulnerable to the environment, in particular, to light.
To counteract the wide bandgap perovskite’s delicate nature, the researchers engineered combinations of molecules composing a two-dimensional layer in the perovskite, stabilizing the solar cells.
“We developed a high-quality wide bandgap perovskite material and, in combination with silicon solar cells, achieved world-class perovskite-silicon tandem cells,” Shin said.
The development was only possible due to the engineering method, in which the mixing ratio of the molecules building the two-dimensional layer are carefully controlled. In this case, the perovskite material not only improved efficiency of the resulting solar cell but also gained durability, retaining 80% of its initial power conversion capability even after 1,000 hours of continuous illumination. This is the first time such a high efficiency has been achieved with a wide bandgap perovskite single layer alone, according to Shin.
“Such high-efficiency wide bandgap perovskite is an essential technology for achieving ultra-high efficiency of perovskite-silicon tandem (double layer) solar cells,” Shin said. “The results also show the importance of bandgap matching of upper and lower cells in these tandem solar cells.”
The researchers, having stabilized the wide bandgap perovskite material, are now focused on developing even more efficient tandem solar cells that are expected to have more than 30% of power conversion efficiency, something that no one has achieved yet,
“Our ultimate goal is to develop ultra-high-efficiency tandem solar cells that contribute to the increase of shared solar energy among all energy sources,” Shin said. “We want to contribute to making the planet healthier.”
This work was supported by the National Research Foundation of Korea, the Korea Institute of Energy Technology Evaluation and Planning, the Ministry of Trade Industry and Energy of Korea, and the U.S. Department of Energy.
Other contributors include Daehan Kim, Jekyung Kim, Passarut Boonmongkolras, Seong Ryul Pae and Minkyu Kim, all of whom affiliated with the Department of Materials Science and Engineering at KAIST. Other authors include Byron W. Larson, Sean P. Dunfield, Chuanxiao Xiao, Jinhui Tong, Fei Zhang, Joseph J. Berry, Kai Zhu and Dong Hoe Kim, all of who are affiliated with the National Renewable Energy Laboratory in Colorado. Dunfield is also affiliated with the Materials Science and Engineering Program at the University of Colorado; Berry is also affiliated with the Department of Physics and the Renewable and Sustainable Energy Institute at the University of Colorado Boulder; and Kim is also affiliated with the Department of Nanotechnology and Advanced Materials Engineering at Sejong University. Hee Joon Jung and Vinayak Dravid of the Department of Materials Science and Engineering at Northwestern University; Ik Jae Park, Su Geun Ji and Jin Young Kim of the Department of Materials Science and Engineering at Seoul National University; and Seok Beom Kang of the Department of Nanotechnology and Advanced Materials Engineering of Sejong University also contributed.
Image credit: Professor Byungha Shin, KAIST
Image usage restrictions: News organizations may use or redistribute this image, with proper attribution, as part of news coverage of this paper only.
Publication: Kim et al. (2020) “Efficient, stable silicon tandem cells enabled by anion-engineered wide band gap perovskites”. Science. Available online at https://doi.org/10.1126/science.aba3433
Profile:
Byungha Shin
Professor
byungha@kaist.ac.kr
http://energymatlab.kaist.ac.kr/
Department of Materials Science and Engineering
KAIST
Profile:
Daehan Kim
Ph.D. Candidate
zxzx4592@kaist.ac.kr
http://energymatlab.kaist.ac.kr/
Department of Materials Science and Engineering
KAIST
(END)
2020.03.27 View 24736 -
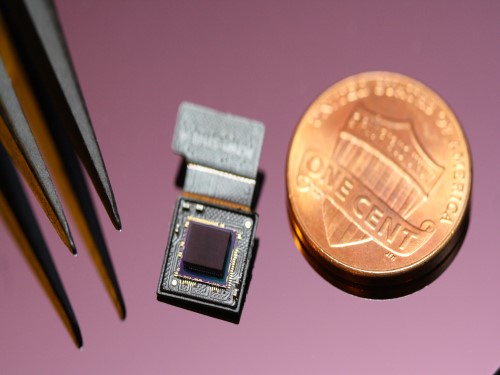 Ultrathin but Fully Packaged High-Resolution Camera
- Biologically inspired ultrathin arrayed camera captures super-resolution images. -
The unique structures of biological vision systems in nature inspired scientists to design ultracompact imaging systems. A research group led by Professor Ki-Hun Jeong have made an ultracompact camera that captures high-contrast and high-resolution images. Fully packaged with micro-optical elements such as inverted micro-lenses, multilayered pinhole arrays, and gap spacers on the image sensor, the camera boasts a total track length of 740 μm and a field of view of 73°.
Inspired by the eye structures of the paper wasp species Xenos peckii, the research team completely suppressed optical noise between micro-lenses while reducing camera thickness. The camera has successfully demonstrated high-contrast clear array images acquired from tiny micro lenses. To further enhance the image quality of the captured image, the team combined the arrayed images into one image through super-resolution imaging.
An insect’s compound eye has superior visual characteristics, such as a wide viewing angle, high motion sensitivity, and a large depth of field while maintaining a small volume of visual structure with a small focal length. Among them, the eyes of Xenos peckii and an endoparasite found on paper wasps have hundreds of photoreceptors in a single lens unlike conventional compound eyes. In particular, the eye structures of an adult Xenos peckii exhibit hundreds of photoreceptors on an individual eyelet and offer engineering inspiration for ultrathin cameras or imaging applications because they have higher visual acuity than other compound eyes.
For instance, Xenos peckii’s eye-inspired cameras provide a 50 times higher spatial resolution than those based on arthropod eyes. In addition, the effective image resolution of the Xenos peckii’s eye can be further improved using the image overlaps between neighboring eyelets. This unique structure offers higher visual resolution than other insect eyes.
The team achieved high-contrast and super-resolution imaging through a novel arrayed design of micro-optical elements comprising multilayered aperture arrays and inverted micro-lens arrays directly stacked over an image sensor. This optical component was integrated with a complementary metal oxide semiconductor image sensor.
This is first demonstration of super-resolution imaging which acquires a single integrated image with high contrast and high resolving power reconstructed from high-contrast array images. It is expected that this ultrathin arrayed camera can be applied for further developing mobile devices, advanced surveillance vehicles, and endoscopes.
Professor Jeong said, “This research has led to technological advances in imaging technology. We will continue to strive to make significant impacts on multidisciplinary research projects in the fields of microtechnology and nanotechnology, seeking inspiration from natural photonic structures.”
This work was featured in Light Science & Applications last month and was supported by the National Research Foundation (NRF) of and the Ministry of Health and Welfare (MOHW) of Korea.
Image credit: Professor Ki-Hun Jeong, KAIST
Image usage restrictions: News organizations may use or redistribute this image, with proper attribution, as part of news coverage of this paper only.
Publication:
Kisoo Kim, Kyung-Won Jang, Jae-Kwan Ryu, and Ki-Hun Jeong. (2020) “Biologically inspired ultrathin arrayed camera for high-contrast and high-resolution imaging”. Light Science & Applications. Volume 9. Article 28. Available online at https://doi.org/10.1038/s41377-020-0261-8
Profile:
Ki-Hun Jeong
Professor
kjeong@kaist.ac.kr
http://biophotonics.kaist.ac.kr/
Department of Bio and Brain Engineering
KAIST
Profile:
Kisoo Kim
Ph.D. Candidate
kisoo.kim1@kaist.ac.kr
http://biophotonics.kaist.ac.kr/
Department of Bio and Brain Engineering
KAIST
(END)
2020.03.23 View 22571
Ultrathin but Fully Packaged High-Resolution Camera
- Biologically inspired ultrathin arrayed camera captures super-resolution images. -
The unique structures of biological vision systems in nature inspired scientists to design ultracompact imaging systems. A research group led by Professor Ki-Hun Jeong have made an ultracompact camera that captures high-contrast and high-resolution images. Fully packaged with micro-optical elements such as inverted micro-lenses, multilayered pinhole arrays, and gap spacers on the image sensor, the camera boasts a total track length of 740 μm and a field of view of 73°.
Inspired by the eye structures of the paper wasp species Xenos peckii, the research team completely suppressed optical noise between micro-lenses while reducing camera thickness. The camera has successfully demonstrated high-contrast clear array images acquired from tiny micro lenses. To further enhance the image quality of the captured image, the team combined the arrayed images into one image through super-resolution imaging.
An insect’s compound eye has superior visual characteristics, such as a wide viewing angle, high motion sensitivity, and a large depth of field while maintaining a small volume of visual structure with a small focal length. Among them, the eyes of Xenos peckii and an endoparasite found on paper wasps have hundreds of photoreceptors in a single lens unlike conventional compound eyes. In particular, the eye structures of an adult Xenos peckii exhibit hundreds of photoreceptors on an individual eyelet and offer engineering inspiration for ultrathin cameras or imaging applications because they have higher visual acuity than other compound eyes.
For instance, Xenos peckii’s eye-inspired cameras provide a 50 times higher spatial resolution than those based on arthropod eyes. In addition, the effective image resolution of the Xenos peckii’s eye can be further improved using the image overlaps between neighboring eyelets. This unique structure offers higher visual resolution than other insect eyes.
The team achieved high-contrast and super-resolution imaging through a novel arrayed design of micro-optical elements comprising multilayered aperture arrays and inverted micro-lens arrays directly stacked over an image sensor. This optical component was integrated with a complementary metal oxide semiconductor image sensor.
This is first demonstration of super-resolution imaging which acquires a single integrated image with high contrast and high resolving power reconstructed from high-contrast array images. It is expected that this ultrathin arrayed camera can be applied for further developing mobile devices, advanced surveillance vehicles, and endoscopes.
Professor Jeong said, “This research has led to technological advances in imaging technology. We will continue to strive to make significant impacts on multidisciplinary research projects in the fields of microtechnology and nanotechnology, seeking inspiration from natural photonic structures.”
This work was featured in Light Science & Applications last month and was supported by the National Research Foundation (NRF) of and the Ministry of Health and Welfare (MOHW) of Korea.
Image credit: Professor Ki-Hun Jeong, KAIST
Image usage restrictions: News organizations may use or redistribute this image, with proper attribution, as part of news coverage of this paper only.
Publication:
Kisoo Kim, Kyung-Won Jang, Jae-Kwan Ryu, and Ki-Hun Jeong. (2020) “Biologically inspired ultrathin arrayed camera for high-contrast and high-resolution imaging”. Light Science & Applications. Volume 9. Article 28. Available online at https://doi.org/10.1038/s41377-020-0261-8
Profile:
Ki-Hun Jeong
Professor
kjeong@kaist.ac.kr
http://biophotonics.kaist.ac.kr/
Department of Bio and Brain Engineering
KAIST
Profile:
Kisoo Kim
Ph.D. Candidate
kisoo.kim1@kaist.ac.kr
http://biophotonics.kaist.ac.kr/
Department of Bio and Brain Engineering
KAIST
(END)
2020.03.23 View 22571 -
 3D Hierarchically Porous Nanostructured Catalyst Helps Efficiently Reduce CO2
- This new catalyst will bring CO2 one step closer to serving as a sustainable energy source. -
KAIST researchers developed a three-dimensional (3D) hierarchically porous nanostructured catalyst with carbon dioxide (CO2) to carbon monoxide (CO) conversion rate up to 3.96 times higher than that of conventional nanoporous gold catalysts. This new catalyst helps overcome the existing limitations of the mass transport that has been a major cause of decreases in the CO2 conversion rate, holding a strong promise for the large-scale and cost-effective electrochemical conversion of CO2 into useful chemicals.
As CO2 emissions increase and fossil fuels deplete globally, reducing and converting CO2 to clean energy electrochemically has attracted a great deal of attention as a promising technology. Especially due to the fact that the CO2 reduction reaction occurs competitively with hydrogen evolution reactions (HER) at similar redox potentials, the development of an efficient electrocatalyst for selective and robust CO2 reduction reactions has remained a key technological issue.
Gold (Au) is one of the most commonly used catalysts in CO2 reduction reactions, but the high cost and scarcity of Au pose obstacles for mass commercial applications. The development of nanostructures has been extensively studied as a potential approach to improving the selectivity for target products and maximizing the number of active stable sites, thus enhancing the energy efficiency.
However, the nanopores of the previously reported complex nanostructures were easily blocked by gaseous CO bubbles during aqueous reactions. The CO bubbles hindered mass transport of the reactants through the electrolyte, resulting in low CO2 conversion rates.
In the study published in the Proceedings of the National Academy of Sciences of the USA (PNAS) on March 4, a research group at KAIST led by Professor Seokwoo Jeon and Professor Jihun Oh from the Department of Materials Science and Engineering designed a 3D hierarchically porous Au nanostructure with two different sizes of macropores and nanopores. The team used proximity-field nanopatterning (PnP) and electroplating techniques that are effective for fabricating the 3D well-ordered nanostructures.
The proposed nanostructure, comprised of interconnected macroporous channels 200 to 300 nanometers (nm) wide and 10 nm nanopores, induces efficient mass transport through the interconnected macroporous channels as well as high selectivity by producing highly active stable sites from numerous nanopores.
As a result, its electrodes show a high CO selectivity of 85.8% at a low overpotential of 0.264 V and efficient mass activity that is up to 3.96 times higher than that of de-alloyed nanoporous Au electrodes.
“These results are expected to solve the problem of mass transfer in the field of similar electrochemical reactions and can be applied to a wide range of green energy applications for the efficient utilization of electrocatalysts,” said the researchers.
This work was supported by the National Research Foundation (NRF) of Korea.
Image credit: Professor Seokwoo Jeon and Professor Jihun Oh, KAIST
Image usage restrictions: News organizations may use or redistribute this image, with proper attribution, as part of news coverage of this paper only.
Publication:
Hyun et al. (2020) Hierarchically porous Au nanostructures with interconnected channels for efficient mass transport in electrocatalytic CO2 reduction. Proceedings of the National Academy of Sciences of the USA (PNAS). Available online at https://doi.org/10.1073/pnas.1918837117
Profile:
Seokwoo Jeon, PhD
Professor
jeon39@kaist.ac.kr
http://fdml.kaist.ac.kr
Department of Materials Science and Engineering (MSE)
https://www.kaist.ac.kr
Korea Advanced Institute of Science and Technology (KAIST)Daejeon, Republic of Korea
Profile:
Jihun Oh, PhD
Associate Professor
jihun.oh@kaist.ac.kr
http://les.kaist.ac.kr
Department of Materials Science and Engineering (MSE)
Department of Energy, Environment, Water and Sustainability (EEWS)
KAIST
Profile:
Gayea Hyun
PhD Candidate
cldywkd93@kaist.ac.kr
http://fdml.kaist.ac.kr
Flexible Devices and Metamaterials Laboratory (FDML)
Department of Materials Science and Engineering (MSE)
KAIST
Profile:
Jun Tae Song, PhD
Assistant Professor
song.juntae@cstf.kyushu-u.ac.jp
http://www.cstf.kyushu-u.ac.jp/~ishihara-lab/
Department of Applied Chemistry
https://www.kyushu-u.ac.jp
Kyushu UniversityFukuoka, Japan
(END)
2020.03.13 View 20988
3D Hierarchically Porous Nanostructured Catalyst Helps Efficiently Reduce CO2
- This new catalyst will bring CO2 one step closer to serving as a sustainable energy source. -
KAIST researchers developed a three-dimensional (3D) hierarchically porous nanostructured catalyst with carbon dioxide (CO2) to carbon monoxide (CO) conversion rate up to 3.96 times higher than that of conventional nanoporous gold catalysts. This new catalyst helps overcome the existing limitations of the mass transport that has been a major cause of decreases in the CO2 conversion rate, holding a strong promise for the large-scale and cost-effective electrochemical conversion of CO2 into useful chemicals.
As CO2 emissions increase and fossil fuels deplete globally, reducing and converting CO2 to clean energy electrochemically has attracted a great deal of attention as a promising technology. Especially due to the fact that the CO2 reduction reaction occurs competitively with hydrogen evolution reactions (HER) at similar redox potentials, the development of an efficient electrocatalyst for selective and robust CO2 reduction reactions has remained a key technological issue.
Gold (Au) is one of the most commonly used catalysts in CO2 reduction reactions, but the high cost and scarcity of Au pose obstacles for mass commercial applications. The development of nanostructures has been extensively studied as a potential approach to improving the selectivity for target products and maximizing the number of active stable sites, thus enhancing the energy efficiency.
However, the nanopores of the previously reported complex nanostructures were easily blocked by gaseous CO bubbles during aqueous reactions. The CO bubbles hindered mass transport of the reactants through the electrolyte, resulting in low CO2 conversion rates.
In the study published in the Proceedings of the National Academy of Sciences of the USA (PNAS) on March 4, a research group at KAIST led by Professor Seokwoo Jeon and Professor Jihun Oh from the Department of Materials Science and Engineering designed a 3D hierarchically porous Au nanostructure with two different sizes of macropores and nanopores. The team used proximity-field nanopatterning (PnP) and electroplating techniques that are effective for fabricating the 3D well-ordered nanostructures.
The proposed nanostructure, comprised of interconnected macroporous channels 200 to 300 nanometers (nm) wide and 10 nm nanopores, induces efficient mass transport through the interconnected macroporous channels as well as high selectivity by producing highly active stable sites from numerous nanopores.
As a result, its electrodes show a high CO selectivity of 85.8% at a low overpotential of 0.264 V and efficient mass activity that is up to 3.96 times higher than that of de-alloyed nanoporous Au electrodes.
“These results are expected to solve the problem of mass transfer in the field of similar electrochemical reactions and can be applied to a wide range of green energy applications for the efficient utilization of electrocatalysts,” said the researchers.
This work was supported by the National Research Foundation (NRF) of Korea.
Image credit: Professor Seokwoo Jeon and Professor Jihun Oh, KAIST
Image usage restrictions: News organizations may use or redistribute this image, with proper attribution, as part of news coverage of this paper only.
Publication:
Hyun et al. (2020) Hierarchically porous Au nanostructures with interconnected channels for efficient mass transport in electrocatalytic CO2 reduction. Proceedings of the National Academy of Sciences of the USA (PNAS). Available online at https://doi.org/10.1073/pnas.1918837117
Profile:
Seokwoo Jeon, PhD
Professor
jeon39@kaist.ac.kr
http://fdml.kaist.ac.kr
Department of Materials Science and Engineering (MSE)
https://www.kaist.ac.kr
Korea Advanced Institute of Science and Technology (KAIST)Daejeon, Republic of Korea
Profile:
Jihun Oh, PhD
Associate Professor
jihun.oh@kaist.ac.kr
http://les.kaist.ac.kr
Department of Materials Science and Engineering (MSE)
Department of Energy, Environment, Water and Sustainability (EEWS)
KAIST
Profile:
Gayea Hyun
PhD Candidate
cldywkd93@kaist.ac.kr
http://fdml.kaist.ac.kr
Flexible Devices and Metamaterials Laboratory (FDML)
Department of Materials Science and Engineering (MSE)
KAIST
Profile:
Jun Tae Song, PhD
Assistant Professor
song.juntae@cstf.kyushu-u.ac.jp
http://www.cstf.kyushu-u.ac.jp/~ishihara-lab/
Department of Applied Chemistry
https://www.kyushu-u.ac.jp
Kyushu UniversityFukuoka, Japan
(END)
2020.03.13 View 20988 -
 Scientists Observe the Elusive Kondo Screening Cloud
Scientists ended a 50-year quest by directly observing a quantum phenomenon
An international research group of Professor Heung-Sun Sim has ended a 50-year quest by directly observing a quantum phenomenon known as a Kondo screening cloud. This research, published in Nature on March 11, opens a novel way to engineer spin screening and entanglement. According to the research, the cloud can mediate interactions between distant spins confined in quantum dots, which is a necessary protocol for semiconductor spin-based quantum information processing. This spin-spin interaction mediated by the Kondo cloud is unique since both its strength and sign (two spins favor either parallel or anti-parallel configuration) are electrically tunable, while conventional schemes cannot reverse the sign.
This phenomenon, which is important for many physical phenomena such as dilute magnetic impurities and spin glasses, is essentially a cloud that masks magnetic impurities in a material. It was known to exist but its spatial extension had never been observed, creating controversy over whether such an extension actually existed.
Magnetism arises from a property of electrons known as spin, meaning that they have angular momentum aligned in one of either two directions, conventionally known as up and down. However, due to a phenomenon known as the Kondo effect, the spins of conduction electrons—the electrons that flow freely in a material—become entangled with a localized magnetic impurity, and effectively screen it. The strength of this spin coupling, calibrated as a temperature, is known as the Kondo temperature.
The size of the cloud is another important parameter for a material containing multiple magnetic impurities because the spins in the cloud couple with one another and mediate the coupling between magnetic impurities when the clouds overlap. This happens in various materials such as Kondo lattices, spin glasses, and high temperature superconductors.
Although the Kondo effect for a single magnetic impurity is now a text-book subject in many-body physics, detection of its key object, the Kondo cloud and its length, has remained elusive despite many attempts during the past five decades. Experiments using nuclear magnetic resonance or scanning tunneling microscopy, two common methods for understanding the structure of matter, have either shown no signature of the cloud, or demonstrated a signature only at a very short distance, less than 1 nanometer, so much shorter than the predicted cloud size, which was in the micron range.
In the present study, the authors observed a Kondo screening cloud formed by an impurity defined as a localized electron spin in a quantum dot—a type of “artificial atom”—coupled to quasi-one-dimensional conduction electrons, and then used an interferometer to measure changes in the Kondo temperature, allowing them to investigate the presence of a cloud at the interferometer end.
Essentially, they slightly perturbed the conduction electrons at a location away from the quantum dot using an electrostatic gate. The wave of conducting electrons scattered by this perturbation returned back to the quantum dot and interfered with itself. This is similar to how a wave on a water surface being scattered by a wall forms a stripe pattern. The Kondo cloud is a quantum mechanical object which acts to preserve the wave nature of electrons inside the cloud.
Even though there is no direct electrostatic influence of the perturbation on the quantum dot, this interference modifies the Kondo signature measured by electron conductance through the quantum dot if the perturbation is present inside the cloud. In the study, the researchers found that the length as well as the shape of the cloud is universally scaled by the inverse of the Kondo temperature, and that the cloud’s size and shape were in good agreement with theoretical calculations.
Professor Sim at the Department of Physics proposed the method for detecting the Kondo cloud in the co-research with the RIKEN Center for Emergent Matter Science, the City University of Hong Kong, the University of Tokyo, and Ruhr University Bochum in Germany.
Professor Sim said, “The observed spin cloud is a micrometer-size object that has quantum mechanical wave nature and entanglement. This is why the spin cloud has not been observed despite a long search. It is remarkable in a fundamental and technical point of view that such a large quantum object can now be created, controlled, and detected.
Dr. Michihisa Yamamoto of the RIKEN Center for Emergent Matter Science also said, “It is very satisfying to have been able to obtain real space image of the Kondo cloud, as it is a real breakthrough for understanding various systems containing multiple magnetic impurities. The size of the Kondo cloud in semiconductors was found to be much larger than the typical size of semiconductor devices.”
Publication:
Borzenets et al. (2020) Observation of the Kondo screening cloud. Nature, 579. pp.210-213. Available online at https://doi.org/10.1038/s41586-020-2058-6
Profile:
Heung-Sun Sim, PhD
Professor
hssim@kaist.ac.kr
https://qet.kaist.ac.kr/
Quantum Electron Correlation & Transport Theory Group (QECT Lab)
https://qc.kaist.ac.kr/index.php/group1/
Center for Quantum Coherence In COndensed Matter
Department of Physics
https://www.kaist.ac.kr
Korea Advanced Institute of Science and Technology (KAIST)
Daejeon, Republic of Korea
2020.03.13 View 19276
Scientists Observe the Elusive Kondo Screening Cloud
Scientists ended a 50-year quest by directly observing a quantum phenomenon
An international research group of Professor Heung-Sun Sim has ended a 50-year quest by directly observing a quantum phenomenon known as a Kondo screening cloud. This research, published in Nature on March 11, opens a novel way to engineer spin screening and entanglement. According to the research, the cloud can mediate interactions between distant spins confined in quantum dots, which is a necessary protocol for semiconductor spin-based quantum information processing. This spin-spin interaction mediated by the Kondo cloud is unique since both its strength and sign (two spins favor either parallel or anti-parallel configuration) are electrically tunable, while conventional schemes cannot reverse the sign.
This phenomenon, which is important for many physical phenomena such as dilute magnetic impurities and spin glasses, is essentially a cloud that masks magnetic impurities in a material. It was known to exist but its spatial extension had never been observed, creating controversy over whether such an extension actually existed.
Magnetism arises from a property of electrons known as spin, meaning that they have angular momentum aligned in one of either two directions, conventionally known as up and down. However, due to a phenomenon known as the Kondo effect, the spins of conduction electrons—the electrons that flow freely in a material—become entangled with a localized magnetic impurity, and effectively screen it. The strength of this spin coupling, calibrated as a temperature, is known as the Kondo temperature.
The size of the cloud is another important parameter for a material containing multiple magnetic impurities because the spins in the cloud couple with one another and mediate the coupling between magnetic impurities when the clouds overlap. This happens in various materials such as Kondo lattices, spin glasses, and high temperature superconductors.
Although the Kondo effect for a single magnetic impurity is now a text-book subject in many-body physics, detection of its key object, the Kondo cloud and its length, has remained elusive despite many attempts during the past five decades. Experiments using nuclear magnetic resonance or scanning tunneling microscopy, two common methods for understanding the structure of matter, have either shown no signature of the cloud, or demonstrated a signature only at a very short distance, less than 1 nanometer, so much shorter than the predicted cloud size, which was in the micron range.
In the present study, the authors observed a Kondo screening cloud formed by an impurity defined as a localized electron spin in a quantum dot—a type of “artificial atom”—coupled to quasi-one-dimensional conduction electrons, and then used an interferometer to measure changes in the Kondo temperature, allowing them to investigate the presence of a cloud at the interferometer end.
Essentially, they slightly perturbed the conduction electrons at a location away from the quantum dot using an electrostatic gate. The wave of conducting electrons scattered by this perturbation returned back to the quantum dot and interfered with itself. This is similar to how a wave on a water surface being scattered by a wall forms a stripe pattern. The Kondo cloud is a quantum mechanical object which acts to preserve the wave nature of electrons inside the cloud.
Even though there is no direct electrostatic influence of the perturbation on the quantum dot, this interference modifies the Kondo signature measured by electron conductance through the quantum dot if the perturbation is present inside the cloud. In the study, the researchers found that the length as well as the shape of the cloud is universally scaled by the inverse of the Kondo temperature, and that the cloud’s size and shape were in good agreement with theoretical calculations.
Professor Sim at the Department of Physics proposed the method for detecting the Kondo cloud in the co-research with the RIKEN Center for Emergent Matter Science, the City University of Hong Kong, the University of Tokyo, and Ruhr University Bochum in Germany.
Professor Sim said, “The observed spin cloud is a micrometer-size object that has quantum mechanical wave nature and entanglement. This is why the spin cloud has not been observed despite a long search. It is remarkable in a fundamental and technical point of view that such a large quantum object can now be created, controlled, and detected.
Dr. Michihisa Yamamoto of the RIKEN Center for Emergent Matter Science also said, “It is very satisfying to have been able to obtain real space image of the Kondo cloud, as it is a real breakthrough for understanding various systems containing multiple magnetic impurities. The size of the Kondo cloud in semiconductors was found to be much larger than the typical size of semiconductor devices.”
Publication:
Borzenets et al. (2020) Observation of the Kondo screening cloud. Nature, 579. pp.210-213. Available online at https://doi.org/10.1038/s41586-020-2058-6
Profile:
Heung-Sun Sim, PhD
Professor
hssim@kaist.ac.kr
https://qet.kaist.ac.kr/
Quantum Electron Correlation & Transport Theory Group (QECT Lab)
https://qc.kaist.ac.kr/index.php/group1/
Center for Quantum Coherence In COndensed Matter
Department of Physics
https://www.kaist.ac.kr
Korea Advanced Institute of Science and Technology (KAIST)
Daejeon, Republic of Korea
2020.03.13 View 19276 -
 A Single Biological Factor Predicts Distinct Cortical Organizations across Mammalian Species
-A KAIST team’s mathematical sampling model shows that retino-cortical mapping is a prime determinant in the topography of cortical organization.-
Researchers have explained how visual cortexes develop uniquely across the brains of different mammalian species. A KAIST research team led by Professor Se-Bum Paik from the Department of Bio and Brain Engineering has identified a single biological factor, the retino-cortical mapping ratio, that predicts distinct cortical organizations across mammalian species.
This new finding has resolved a long-standing puzzle in understanding visual neuroscience regarding the origin of functional architectures in the visual cortex. The study published in Cell Reports on March 10 demonstrates that the evolutionary variation of biological parameters may induce the development of distinct functional circuits in the visual cortex, even without species-specific developmental mechanisms.
In the primary visual cortex (V1) of mammals, neural tuning to visual stimulus orientation is organized into one of two distinct topographic patterns across species. While primates have columnar orientation maps, a salt-and-pepper type organization is observed in rodents.
For decades, this sharp contrast between cortical organizations has spawned fundamental questions about the origin of functional architectures in the V1. However, it remained unknown whether these patterns reflect disparate developmental mechanisms across mammalian taxa, or simply originate from variations in biological parameters under a universal development process.
To identify a determinant predicting distinct cortical organizations, Professor Paik and his researchers Jaeson Jang and Min Song examined the exact condition that generates columnar and salt-and-pepper organizations, respectively. Next, they applied a mathematical model to investigate how the topographic information of the underlying retinal mosaics pattern could be differently mapped onto a cortical space, depending on the mapping condition.
The research team proved that the retino-cortical feedforwarding mapping ratio appeared to be correlated to the cortical organization of each species. In the model simulations, the team found that distinct cortical circuitries can arise from different V1 areas and retinal ganglion cell (RGC) mosaic sizes. The team’s mathematical sampling model shows that retino-cortical mapping is a prime determinant in the topography of cortical organization, and this prediction was confirmed by neural parameter analysis of the data from eight phylogenetically distinct mammalian species.
Furthermore, the researchers proved that the Nyquist sampling theorem explains this parametric division of cortical organization with high accuracy. They showed that a mathematical model predicts that the organization of cortical orientation tuning makes a sharp transition around the Nyquist sampling frequency, explaining why cortical organizations can be observed in either columnar or salt-and-pepper organizations, but not in intermediates between these two stages.
Professor Paik said, “Our findings make a significant impact for understanding the origin of functional architectures in the visual cortex of the brain, and will provide a broad conceptual advancement as well as advanced insights into the mechanism underlying neural development in evolutionarily divergent species.”
He continued, “We believe that our findings will be of great interest to scientists working in a wide range of fields such as neuroscience, vision science, and developmental biology.”
This work was supported by the National Research Foundation of Korea (NRF).
Image credit: Professor Se-Bum Paik, KAIST
Image usage restrictions: News organizations may use or redistribute this image, with proper attribution, as part of news coverage of this paper only.
Publication:
Jaeson Jang, Min Song, and Se-Bum Paik. (2020). Retino-cortical mapping ratio predicts columnar and salt-and-pepper organization in mammalian visual cortex. Cell Reports. Volume 30. Issue 10. pp. 3270-3279. Available online at https://doi.org/10.1016/j.celrep.2020.02.038
Profile:
Se-Bum Paik
Assistant Professor
sbpaik@kaist.ac.kr
http://vs.kaist.ac.kr/
VSNN Laboratory
Department of Bio and Brain Engineering
Program of Brain and Cognitive Engineering
http://kaist.ac.kr
Korea Advanced Institute of Science and Technology (KAIST)
Daejeon, Republic of Korea
Profile:
Jaeson Jang
Ph.D. Candidate
jaesonjang@kaist.ac.kr
Department of Bio and Brain Engineering, KAIST
Profile:
Min Song
Ph.D. Candidate
night@kaist.ac.kr
Program of Brain and Cognitive Engineering, KAIST
(END)
2020.03.11 View 16887
A Single Biological Factor Predicts Distinct Cortical Organizations across Mammalian Species
-A KAIST team’s mathematical sampling model shows that retino-cortical mapping is a prime determinant in the topography of cortical organization.-
Researchers have explained how visual cortexes develop uniquely across the brains of different mammalian species. A KAIST research team led by Professor Se-Bum Paik from the Department of Bio and Brain Engineering has identified a single biological factor, the retino-cortical mapping ratio, that predicts distinct cortical organizations across mammalian species.
This new finding has resolved a long-standing puzzle in understanding visual neuroscience regarding the origin of functional architectures in the visual cortex. The study published in Cell Reports on March 10 demonstrates that the evolutionary variation of biological parameters may induce the development of distinct functional circuits in the visual cortex, even without species-specific developmental mechanisms.
In the primary visual cortex (V1) of mammals, neural tuning to visual stimulus orientation is organized into one of two distinct topographic patterns across species. While primates have columnar orientation maps, a salt-and-pepper type organization is observed in rodents.
For decades, this sharp contrast between cortical organizations has spawned fundamental questions about the origin of functional architectures in the V1. However, it remained unknown whether these patterns reflect disparate developmental mechanisms across mammalian taxa, or simply originate from variations in biological parameters under a universal development process.
To identify a determinant predicting distinct cortical organizations, Professor Paik and his researchers Jaeson Jang and Min Song examined the exact condition that generates columnar and salt-and-pepper organizations, respectively. Next, they applied a mathematical model to investigate how the topographic information of the underlying retinal mosaics pattern could be differently mapped onto a cortical space, depending on the mapping condition.
The research team proved that the retino-cortical feedforwarding mapping ratio appeared to be correlated to the cortical organization of each species. In the model simulations, the team found that distinct cortical circuitries can arise from different V1 areas and retinal ganglion cell (RGC) mosaic sizes. The team’s mathematical sampling model shows that retino-cortical mapping is a prime determinant in the topography of cortical organization, and this prediction was confirmed by neural parameter analysis of the data from eight phylogenetically distinct mammalian species.
Furthermore, the researchers proved that the Nyquist sampling theorem explains this parametric division of cortical organization with high accuracy. They showed that a mathematical model predicts that the organization of cortical orientation tuning makes a sharp transition around the Nyquist sampling frequency, explaining why cortical organizations can be observed in either columnar or salt-and-pepper organizations, but not in intermediates between these two stages.
Professor Paik said, “Our findings make a significant impact for understanding the origin of functional architectures in the visual cortex of the brain, and will provide a broad conceptual advancement as well as advanced insights into the mechanism underlying neural development in evolutionarily divergent species.”
He continued, “We believe that our findings will be of great interest to scientists working in a wide range of fields such as neuroscience, vision science, and developmental biology.”
This work was supported by the National Research Foundation of Korea (NRF).
Image credit: Professor Se-Bum Paik, KAIST
Image usage restrictions: News organizations may use or redistribute this image, with proper attribution, as part of news coverage of this paper only.
Publication:
Jaeson Jang, Min Song, and Se-Bum Paik. (2020). Retino-cortical mapping ratio predicts columnar and salt-and-pepper organization in mammalian visual cortex. Cell Reports. Volume 30. Issue 10. pp. 3270-3279. Available online at https://doi.org/10.1016/j.celrep.2020.02.038
Profile:
Se-Bum Paik
Assistant Professor
sbpaik@kaist.ac.kr
http://vs.kaist.ac.kr/
VSNN Laboratory
Department of Bio and Brain Engineering
Program of Brain and Cognitive Engineering
http://kaist.ac.kr
Korea Advanced Institute of Science and Technology (KAIST)
Daejeon, Republic of Korea
Profile:
Jaeson Jang
Ph.D. Candidate
jaesonjang@kaist.ac.kr
Department of Bio and Brain Engineering, KAIST
Profile:
Min Song
Ph.D. Candidate
night@kaist.ac.kr
Program of Brain and Cognitive Engineering, KAIST
(END)
2020.03.11 View 16887 -
 New Catalyst Recycles Greenhouse Gases into Fuel and Hydrogen Gas
< Professor Cafer T. Yavuz (left), PhD Candidate Youngdong Song (center), and Researcher Sreerangappa Ramesh (right) >
Scientists have taken a major step toward a circular carbon economy by developing a long-lasting, economical catalyst that recycles greenhouse gases into ingredients that can be used in fuel, hydrogen gas, and other chemicals. The results could be revolutionary in the effort to reverse global warming, according to the researchers. The study was published on February 14 in Science.
“We set out to develop an effective catalyst that can convert large amounts of the greenhouse gases carbon dioxide and methane without failure,” said Cafer T. Yavuz, paper author and associate professor of chemical and biomolecular engineering and of chemistry at KAIST.
The catalyst, made from inexpensive and abundant nickel, magnesium, and molybdenum, initiates and speeds up the rate of reaction that converts carbon dioxide and methane into hydrogen gas. It can work efficiently for more than a month.
This conversion is called ‘dry reforming’, where harmful gases, such as carbon dioxide, are processed to produce more useful chemicals that could be refined for use in fuel, plastics, or even pharmaceuticals. It is an effective process, but it previously required rare and expensive metals such as platinum and rhodium to induce a brief and inefficient chemical reaction.
Other researchers had previously proposed nickel as a more economical solution, but carbon byproducts would build up and the surface nanoparticles would bind together on the cheaper metal, fundamentally changing the composition and geometry of the catalyst and rendering it useless.
“The difficulty arises from the lack of control on scores of active sites over the bulky catalysts surfaces because any refinement procedures attempted also change the nature of the catalyst itself,” Yavuz said.
The researchers produced nickel-molybdenum nanoparticles under a reductive environment in the presence of a single crystalline magnesium oxide. As the ingredients were heated under reactive gas, the nanoparticles moved on the pristine crystal surface seeking anchoring points. The resulting activated catalyst sealed its own high-energy active sites and permanently fixed the location of the nanoparticles — meaning that the nickel-based catalyst will not have a carbon build up, nor will the surface particles bind to one another.
“It took us almost a year to understand the underlying mechanism,” said first author Youngdong Song, a graduate student in the Department of Chemical and Biomolecular Engineering at KAIST. “Once we studied all the chemical events in detail, we were shocked.”
The researchers dubbed the catalyst Nanocatalysts on Single Crystal Edges (NOSCE). The magnesium-oxide nanopowder comes from a finely structured form of magnesium oxide, where the molecules bind continuously to the edge. There are no breaks or defects in the surface, allowing for uniform and predictable reactions.
“Our study solves a number of challenges the catalyst community faces,” Yavuz said. “We believe the NOSCE mechanism will improve other inefficient catalytic reactions and provide even further savings of greenhouse gas emissions.”
This work was supported, in part, by the Saudi-Aramco-KAIST CO2 Management Center and the National Research Foundation of Korea.
Other contributors include Ercan Ozdemir, Sreerangappa Ramesh, Aldiar Adishev, and Saravanan Subramanian, all of whom are affiliated with the Graduate School of Energy, Environment, Water and Sustainability at KAIST; Aadesh Harale, Mohammed Albuali, Bandar Abdullah Fadhel, and Aqil Jamal, all of whom are with the Research and Development Center in Saudi Arabia; and Dohyun Moon and Sun Hee Choi, both of whom are with the Pohang Accelerator Laboratory in Korea. Ozdemir is also affiliated with the Institute of Nanotechnology at the Gebze Technical University in Turkey; Fadhel and Jamal are also affiliated with the Saudi-Armco-KAIST CO2 Management Center in Korea.
<Newly developed catalyst that recycles greenhouse gases into ingredients that can be used in fuel, hydrogen gas and other chemicals.>
Publication:
Song et al. (2020) Dry reforming of methane by stable Ni–Mo nanocatalysts on single-crystalline MgO. Science, Vol. 367, Issue 6479, pp. 777-781. Available online at http://dx.doi.org/10.1126/science.aav2412
Profile: Prof. Cafer T. Yavuz, MA, PhD
yavuz@kaist.ac.kr
http://yavuz.kaist.ac.kr/
Associate Professor
Oxide and Organic Nanomaterials for the Environment (ONE) Laboratory
Graduate School of Energy, Environment, Water and Sustainability (EEWS)
Korea Advanced Institute of Science and Technology (KAIST)
http://kaist.ac.kr
Daejeon, Republic of Korea
Profile: Youngdong Song ydsong88@kaist.ac.kr
Ph.D. Candidate
Department of Chemical and Biomolecular Engineering
Korea Advanced Institute of Science and Technology (KAIST)
http://kaist.ac.kr
Daejeon, Republic of Korea
(END)
2020.02.17 View 20386
New Catalyst Recycles Greenhouse Gases into Fuel and Hydrogen Gas
< Professor Cafer T. Yavuz (left), PhD Candidate Youngdong Song (center), and Researcher Sreerangappa Ramesh (right) >
Scientists have taken a major step toward a circular carbon economy by developing a long-lasting, economical catalyst that recycles greenhouse gases into ingredients that can be used in fuel, hydrogen gas, and other chemicals. The results could be revolutionary in the effort to reverse global warming, according to the researchers. The study was published on February 14 in Science.
“We set out to develop an effective catalyst that can convert large amounts of the greenhouse gases carbon dioxide and methane without failure,” said Cafer T. Yavuz, paper author and associate professor of chemical and biomolecular engineering and of chemistry at KAIST.
The catalyst, made from inexpensive and abundant nickel, magnesium, and molybdenum, initiates and speeds up the rate of reaction that converts carbon dioxide and methane into hydrogen gas. It can work efficiently for more than a month.
This conversion is called ‘dry reforming’, where harmful gases, such as carbon dioxide, are processed to produce more useful chemicals that could be refined for use in fuel, plastics, or even pharmaceuticals. It is an effective process, but it previously required rare and expensive metals such as platinum and rhodium to induce a brief and inefficient chemical reaction.
Other researchers had previously proposed nickel as a more economical solution, but carbon byproducts would build up and the surface nanoparticles would bind together on the cheaper metal, fundamentally changing the composition and geometry of the catalyst and rendering it useless.
“The difficulty arises from the lack of control on scores of active sites over the bulky catalysts surfaces because any refinement procedures attempted also change the nature of the catalyst itself,” Yavuz said.
The researchers produced nickel-molybdenum nanoparticles under a reductive environment in the presence of a single crystalline magnesium oxide. As the ingredients were heated under reactive gas, the nanoparticles moved on the pristine crystal surface seeking anchoring points. The resulting activated catalyst sealed its own high-energy active sites and permanently fixed the location of the nanoparticles — meaning that the nickel-based catalyst will not have a carbon build up, nor will the surface particles bind to one another.
“It took us almost a year to understand the underlying mechanism,” said first author Youngdong Song, a graduate student in the Department of Chemical and Biomolecular Engineering at KAIST. “Once we studied all the chemical events in detail, we were shocked.”
The researchers dubbed the catalyst Nanocatalysts on Single Crystal Edges (NOSCE). The magnesium-oxide nanopowder comes from a finely structured form of magnesium oxide, where the molecules bind continuously to the edge. There are no breaks or defects in the surface, allowing for uniform and predictable reactions.
“Our study solves a number of challenges the catalyst community faces,” Yavuz said. “We believe the NOSCE mechanism will improve other inefficient catalytic reactions and provide even further savings of greenhouse gas emissions.”
This work was supported, in part, by the Saudi-Aramco-KAIST CO2 Management Center and the National Research Foundation of Korea.
Other contributors include Ercan Ozdemir, Sreerangappa Ramesh, Aldiar Adishev, and Saravanan Subramanian, all of whom are affiliated with the Graduate School of Energy, Environment, Water and Sustainability at KAIST; Aadesh Harale, Mohammed Albuali, Bandar Abdullah Fadhel, and Aqil Jamal, all of whom are with the Research and Development Center in Saudi Arabia; and Dohyun Moon and Sun Hee Choi, both of whom are with the Pohang Accelerator Laboratory in Korea. Ozdemir is also affiliated with the Institute of Nanotechnology at the Gebze Technical University in Turkey; Fadhel and Jamal are also affiliated with the Saudi-Armco-KAIST CO2 Management Center in Korea.
<Newly developed catalyst that recycles greenhouse gases into ingredients that can be used in fuel, hydrogen gas and other chemicals.>
Publication:
Song et al. (2020) Dry reforming of methane by stable Ni–Mo nanocatalysts on single-crystalline MgO. Science, Vol. 367, Issue 6479, pp. 777-781. Available online at http://dx.doi.org/10.1126/science.aav2412
Profile: Prof. Cafer T. Yavuz, MA, PhD
yavuz@kaist.ac.kr
http://yavuz.kaist.ac.kr/
Associate Professor
Oxide and Organic Nanomaterials for the Environment (ONE) Laboratory
Graduate School of Energy, Environment, Water and Sustainability (EEWS)
Korea Advanced Institute of Science and Technology (KAIST)
http://kaist.ac.kr
Daejeon, Republic of Korea
Profile: Youngdong Song ydsong88@kaist.ac.kr
Ph.D. Candidate
Department of Chemical and Biomolecular Engineering
Korea Advanced Institute of Science and Technology (KAIST)
http://kaist.ac.kr
Daejeon, Republic of Korea
(END)
2020.02.17 View 20386 -
 What Fuels a “Domino Effect” in Cancer Drug Resistance?
KAIST researchers have identified mechanisms that relay prior acquired resistance to the first-line chemotherapy to the second-line targeted therapy, fueling a “domino effect” in cancer drug resistance. Their study featured in the February 7 edition of Science Advances suggests a new strategy for improving the second-line setting of cancer treatment for patients who showed resistance to anti-cancer drugs.
Resistance to cancer drugs is often managed in the clinic by chemotherapy and targeted therapy. Unlike chemotherapy that works by repressing fast-proliferating cells, targeted therapy blocks a single oncogenic pathway to halt tumor growth. In many cases, targeted therapy is engaged as a maintenance therapy or employed in the second-line after front-line chemotherapy.
A team of researchers led by Professor Yoosik Kim from the Department of Chemical and Biomolecular Engineering and the KAIST Institute for Health Science and Technology (KIHST) has discovered an unexpected resistance signature that occurs between chemotherapy and targeted therapy. The team further identified a set of integrated mechanisms that promotes this kind of sequential therapy resistance.
“There have been multiple clinical accounts reflecting that targeted therapies tend to be least successful in patients who have exhausted all standard treatments,” said the first author of the paper Mark Borris D. Aldonza. He continued, “These accounts ignited our hypothesis that failed responses to some chemotherapies might speed up the evolution of resistance to other drugs, particularly those with specific targets.”
Aldonza and his colleagues extracted large amounts of drug-resistance information from the open-source database the Genomics of Drug Sensitivity in Cancer (GDSC), which contains thousands of drug response data entries from various human cancer cell lines. Their big data analysis revealed that cancer cell lines resistant to chemotherapies classified as anti-mitotic drugs (AMDs), toxins that inhibit overacting cell division, are also resistant to a class of targeted therapies called epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs).
In all of the cancer types analyzed, more than 84 percent of those resistant to AMDs, representatively ‘paclitaxel’, were also resistant to at least nine EGFR-TKIs. In lung, pancreatic, and breast cancers where paclitaxel is often used as a first-line, standard-of-care regimen, greater than 92 percent showed resistance to EGFR-TKIs. Professor Kim said, “It is surprising to see that such collateral resistance can occur specifically between two chemically different classes of drugs.”
To figure out how failed responses to paclitaxel leads to resistance to EGFR-TKIs, the team validated co-resistance signatures that they found in the database by generating and analyzing a subset of slow-doubling, paclitaxel-resistant cancer models called ‘persisters’.
The results demonstrated that paclitaxel-resistant cancers remodel their stress response by first becoming more stem cell-like, evolving the ability to self-renew to adapt to more stressful conditions like drug exposures. More surprisingly, when the researchers characterized the metabolic state of the cells, EGFR-TKI persisters derived from paclitaxel-resistant cancer cells showed high dependencies to energy-producing processes such as glycolysis and glutaminolysis.
“We found that, without an energy stimulus like glucose, these cells transform to becoming more senescent, a characteristic of cells that have arrested cell division. However, this senescence is controlled by stem cell factors, which the paclitaxel-resistant cancers use to escape from this arrested state given a favorable condition to re-grow,” said Aldonza.
Professor Kim explained, “Before this research, there was no reason to expect that acquiring the cancer stem cell phenotype that dramatically leads to a cascade of changes in cellular states affecting metabolism and cell death is linked with drug-specific sequential resistance between two classes of therapies.”
He added, “The expansion of our work to other working models of drug resistance in a much more clinically-relevant setting, perhaps in clinical trials, will take on increasing importance, as sequential treatment strategies will continue to be adapted to various forms of anti-cancer therapy regimens.”
This study was supported by the Basic Science Research Program of the National Research Foundation of Korea (NRF-2016R1C1B2009886), and the KAIST Future Systems Healthcare Project (KAISTHEALTHCARE42) funded by the Korean Ministry of Science and ICT (MSIT). Undergraduate student Aldonza participated in this research project and presented the findings as the lead author as part of the Undergraduate Research Participation (URP) Program at KAIST.
< Figure 1. Schematic overview of the study. >
< Figure 2. Big data analysis revealing co-resistance signatures between classes of anti-cancer drugs. >
Publication:
Aldonza et al. (2020) Prior acquired resistance to paclitaxel relays diverse EGFR-targeted therapy persistence mechanisms. Science Advances, Vol. 6, No. 6, eaav7416. Available online at http://dx.doi.org/10.1126/sciadv.aav7416
Profile: Prof. Yoosik Kim, MA, PhD
ysyoosik@kaist.ac.kr
https://qcbio.kaist.ac.kr/
Assistant Professor
Bio Network Analysis Laboratory
Department of Chemical and Biomolecular Engineering
Korea Advanced Institute of Science and Technology (KAIST)
http://kaist.ac.kr
Daejeon, Republic of Korea
Profile: Mark Borris D. Aldonza
borris@kaist.ac.kr
Undergraduate Student
Department of Biological Sciences
Korea Advanced Institute of Science and Technology (KAIST)
http://kaist.ac.kr
Daejeon, Republic of Korea
(END)
2020.02.10 View 18512
What Fuels a “Domino Effect” in Cancer Drug Resistance?
KAIST researchers have identified mechanisms that relay prior acquired resistance to the first-line chemotherapy to the second-line targeted therapy, fueling a “domino effect” in cancer drug resistance. Their study featured in the February 7 edition of Science Advances suggests a new strategy for improving the second-line setting of cancer treatment for patients who showed resistance to anti-cancer drugs.
Resistance to cancer drugs is often managed in the clinic by chemotherapy and targeted therapy. Unlike chemotherapy that works by repressing fast-proliferating cells, targeted therapy blocks a single oncogenic pathway to halt tumor growth. In many cases, targeted therapy is engaged as a maintenance therapy or employed in the second-line after front-line chemotherapy.
A team of researchers led by Professor Yoosik Kim from the Department of Chemical and Biomolecular Engineering and the KAIST Institute for Health Science and Technology (KIHST) has discovered an unexpected resistance signature that occurs between chemotherapy and targeted therapy. The team further identified a set of integrated mechanisms that promotes this kind of sequential therapy resistance.
“There have been multiple clinical accounts reflecting that targeted therapies tend to be least successful in patients who have exhausted all standard treatments,” said the first author of the paper Mark Borris D. Aldonza. He continued, “These accounts ignited our hypothesis that failed responses to some chemotherapies might speed up the evolution of resistance to other drugs, particularly those with specific targets.”
Aldonza and his colleagues extracted large amounts of drug-resistance information from the open-source database the Genomics of Drug Sensitivity in Cancer (GDSC), which contains thousands of drug response data entries from various human cancer cell lines. Their big data analysis revealed that cancer cell lines resistant to chemotherapies classified as anti-mitotic drugs (AMDs), toxins that inhibit overacting cell division, are also resistant to a class of targeted therapies called epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs).
In all of the cancer types analyzed, more than 84 percent of those resistant to AMDs, representatively ‘paclitaxel’, were also resistant to at least nine EGFR-TKIs. In lung, pancreatic, and breast cancers where paclitaxel is often used as a first-line, standard-of-care regimen, greater than 92 percent showed resistance to EGFR-TKIs. Professor Kim said, “It is surprising to see that such collateral resistance can occur specifically between two chemically different classes of drugs.”
To figure out how failed responses to paclitaxel leads to resistance to EGFR-TKIs, the team validated co-resistance signatures that they found in the database by generating and analyzing a subset of slow-doubling, paclitaxel-resistant cancer models called ‘persisters’.
The results demonstrated that paclitaxel-resistant cancers remodel their stress response by first becoming more stem cell-like, evolving the ability to self-renew to adapt to more stressful conditions like drug exposures. More surprisingly, when the researchers characterized the metabolic state of the cells, EGFR-TKI persisters derived from paclitaxel-resistant cancer cells showed high dependencies to energy-producing processes such as glycolysis and glutaminolysis.
“We found that, without an energy stimulus like glucose, these cells transform to becoming more senescent, a characteristic of cells that have arrested cell division. However, this senescence is controlled by stem cell factors, which the paclitaxel-resistant cancers use to escape from this arrested state given a favorable condition to re-grow,” said Aldonza.
Professor Kim explained, “Before this research, there was no reason to expect that acquiring the cancer stem cell phenotype that dramatically leads to a cascade of changes in cellular states affecting metabolism and cell death is linked with drug-specific sequential resistance between two classes of therapies.”
He added, “The expansion of our work to other working models of drug resistance in a much more clinically-relevant setting, perhaps in clinical trials, will take on increasing importance, as sequential treatment strategies will continue to be adapted to various forms of anti-cancer therapy regimens.”
This study was supported by the Basic Science Research Program of the National Research Foundation of Korea (NRF-2016R1C1B2009886), and the KAIST Future Systems Healthcare Project (KAISTHEALTHCARE42) funded by the Korean Ministry of Science and ICT (MSIT). Undergraduate student Aldonza participated in this research project and presented the findings as the lead author as part of the Undergraduate Research Participation (URP) Program at KAIST.
< Figure 1. Schematic overview of the study. >
< Figure 2. Big data analysis revealing co-resistance signatures between classes of anti-cancer drugs. >
Publication:
Aldonza et al. (2020) Prior acquired resistance to paclitaxel relays diverse EGFR-targeted therapy persistence mechanisms. Science Advances, Vol. 6, No. 6, eaav7416. Available online at http://dx.doi.org/10.1126/sciadv.aav7416
Profile: Prof. Yoosik Kim, MA, PhD
ysyoosik@kaist.ac.kr
https://qcbio.kaist.ac.kr/
Assistant Professor
Bio Network Analysis Laboratory
Department of Chemical and Biomolecular Engineering
Korea Advanced Institute of Science and Technology (KAIST)
http://kaist.ac.kr
Daejeon, Republic of Korea
Profile: Mark Borris D. Aldonza
borris@kaist.ac.kr
Undergraduate Student
Department of Biological Sciences
Korea Advanced Institute of Science and Technology (KAIST)
http://kaist.ac.kr
Daejeon, Republic of Korea
(END)
2020.02.10 View 18512 -
 Professor Meeyoung Cha, First Young Information Scientist Awardee from KAIST
< Professor Meeyoung Cha (Left) >
Professor Meeyoung Cha from the School of Computing was awarded the fourth Young Information Scientist Award by the Korean Institute of Information Scientists and Engineers (KIISE) last month. Professor Cha is the first from KAIST to win the prize since its establishment under the auspices of the WWW 2014 organizing committee.
The Young Information Scientist Award is awarded to scientists under the age of 40 who have contributed to the development of information science and excelled in research and development in their field.
Professor Cha played a leading role as a young information scientist, having been cited more than 13,000 times in other research papers on fake news detection, lightweight and robust representation of economic scales from satellite imagery, and the development of insomnia detection models.
Reputed as a promising researcher, Professor Cha has been selected as the chief investigator at the Institute for Basic Science (IBS) since January 2019 where she has been operating the Data Science Group under the Center for Mathematical and Computational Sciences.
Professor Cha was also invited as a keynote speaker to the Conference on Empirical Methods in Natural Language Processing (EMNLP), an international academic conference held in Hong Kong in November 2019, where she gave a lecture on “Current Challenges in Computational Social Science” for 1,900 attendees.
(END)
2019.12.31 View 4161
Professor Meeyoung Cha, First Young Information Scientist Awardee from KAIST
< Professor Meeyoung Cha (Left) >
Professor Meeyoung Cha from the School of Computing was awarded the fourth Young Information Scientist Award by the Korean Institute of Information Scientists and Engineers (KIISE) last month. Professor Cha is the first from KAIST to win the prize since its establishment under the auspices of the WWW 2014 organizing committee.
The Young Information Scientist Award is awarded to scientists under the age of 40 who have contributed to the development of information science and excelled in research and development in their field.
Professor Cha played a leading role as a young information scientist, having been cited more than 13,000 times in other research papers on fake news detection, lightweight and robust representation of economic scales from satellite imagery, and the development of insomnia detection models.
Reputed as a promising researcher, Professor Cha has been selected as the chief investigator at the Institute for Basic Science (IBS) since January 2019 where she has been operating the Data Science Group under the Center for Mathematical and Computational Sciences.
Professor Cha was also invited as a keynote speaker to the Conference on Empirical Methods in Natural Language Processing (EMNLP), an international academic conference held in Hong Kong in November 2019, where she gave a lecture on “Current Challenges in Computational Social Science” for 1,900 attendees.
(END)
2019.12.31 View 4161 -
 Professor Sung Yong Kim Elected as the Chair of PICES MONITOR
< Professor Sung Yong Kim >
Professor Sung Yong Kim from the Department of Mechanical Engineering was elected as the chair of the Technical Committee on Monitoring (MONITOR) of the North Pacific Marine Science Organization (PICES).
PICES is an intergovernmental marine science organization that was established in 1992 through a collaboration between six North Pacific nations including South Korea, Russia, the United States, Japan, China, and Canada to exchange and discuss research on the Pacific waters. Its headquarters is located in Canada and the organization consists of seven affiliated maritime science and marine technology committees.
Professor Kim was elected as the chair of the technical committee that focuses on monitoring and will be part of the Science Board as an ex-officio member. His term will last three years from November 2019.
Professor Kim was recognized for his academic excellence, expertise, and leadership among oceanographers both domestically and internationally.
Professor Kim will also participate as an academia civilian committee member of the Maritime and Fisheries Science and Technology Committee under the Korean Ministry of Oceans and Fisheries for two years from December 18, 2019.
He stated, “I will give my full efforts to broaden Korean oceanography research by participating in maritime leadership positions at home and abroad, and help South Korea become a maritime powerhouse.”
(END)
2019.12.22 View 12418
Professor Sung Yong Kim Elected as the Chair of PICES MONITOR
< Professor Sung Yong Kim >
Professor Sung Yong Kim from the Department of Mechanical Engineering was elected as the chair of the Technical Committee on Monitoring (MONITOR) of the North Pacific Marine Science Organization (PICES).
PICES is an intergovernmental marine science organization that was established in 1992 through a collaboration between six North Pacific nations including South Korea, Russia, the United States, Japan, China, and Canada to exchange and discuss research on the Pacific waters. Its headquarters is located in Canada and the organization consists of seven affiliated maritime science and marine technology committees.
Professor Kim was elected as the chair of the technical committee that focuses on monitoring and will be part of the Science Board as an ex-officio member. His term will last three years from November 2019.
Professor Kim was recognized for his academic excellence, expertise, and leadership among oceanographers both domestically and internationally.
Professor Kim will also participate as an academia civilian committee member of the Maritime and Fisheries Science and Technology Committee under the Korean Ministry of Oceans and Fisheries for two years from December 18, 2019.
He stated, “I will give my full efforts to broaden Korean oceanography research by participating in maritime leadership positions at home and abroad, and help South Korea become a maritime powerhouse.”
(END)
2019.12.22 View 12418