Chem
-
 Affordable Genetic Diagnostic Technique for Target DNA Analysis Developed
Professor Hyun-Gyu Park of the Department of Chemical and Biomolecular Engineering at KAIST has developed a technique to analyze various target DNAs using an aptamer, a DNA fragment that can recognize and bind to a specific protein or enzyme. This technique will allow the development of affordable genetic diagnoses for new bacteria or virus, such as Middle Ease Respiratory Syndrome (MERS). The research findings were published in the June issue of Chemical Communications, issued by the Royal Society of Chemistry in the United Kingdom. The paper was selected as a lead article of the journal.
The existing genetic diagnosis technique, based on molecular beacon probes, requires a new beacon probe whenever a target DNA mutates. As a result, it was costly to analyze various target DNA fragments. To address this problem, Professor Park’s team designed an aptamer that binds and deactivates DNA polymerase. The technique was used in reverse, so that the aptemer did not bind to the polymerase, maintaining its activated state, only if the target DNA was present. These probes are called TagMan probes.
The controlled activation and deactivation of DNA polymerase enables nucleic acid to elongate or dwindle, making it possible to measure fluorescence signals coming from TaqMan probes. This same probe can be used to detect various target DNAs, leading to the development of a new and sensitive genetic diagnostic technique.
Unlike the existing molecular beacon probe technique which requires a new probe for every target DNA, this new technique uses the same fluorescent TaqMan probe, which is cheaper and easier to detect a number of different target nucleic acid fragments. The application of this technique will make the process of identifying and detecting foreign DNAs from pathogens such as virus and bacteria more affordable and simple.
Professor Park said, “This technique will enable us to develop simpler diagnostic kits for new pathogens, such as MERS, allowing a faster response to various diseases. Our technology can also be applied widely in the field of genetic diagnostics.”
Picture: A schematic image of target nucleic acid extracted through the activation and deactivation of DNA polymerase
2015.07.31 View 10801
Affordable Genetic Diagnostic Technique for Target DNA Analysis Developed
Professor Hyun-Gyu Park of the Department of Chemical and Biomolecular Engineering at KAIST has developed a technique to analyze various target DNAs using an aptamer, a DNA fragment that can recognize and bind to a specific protein or enzyme. This technique will allow the development of affordable genetic diagnoses for new bacteria or virus, such as Middle Ease Respiratory Syndrome (MERS). The research findings were published in the June issue of Chemical Communications, issued by the Royal Society of Chemistry in the United Kingdom. The paper was selected as a lead article of the journal.
The existing genetic diagnosis technique, based on molecular beacon probes, requires a new beacon probe whenever a target DNA mutates. As a result, it was costly to analyze various target DNA fragments. To address this problem, Professor Park’s team designed an aptamer that binds and deactivates DNA polymerase. The technique was used in reverse, so that the aptemer did not bind to the polymerase, maintaining its activated state, only if the target DNA was present. These probes are called TagMan probes.
The controlled activation and deactivation of DNA polymerase enables nucleic acid to elongate or dwindle, making it possible to measure fluorescence signals coming from TaqMan probes. This same probe can be used to detect various target DNAs, leading to the development of a new and sensitive genetic diagnostic technique.
Unlike the existing molecular beacon probe technique which requires a new probe for every target DNA, this new technique uses the same fluorescent TaqMan probe, which is cheaper and easier to detect a number of different target nucleic acid fragments. The application of this technique will make the process of identifying and detecting foreign DNAs from pathogens such as virus and bacteria more affordable and simple.
Professor Park said, “This technique will enable us to develop simpler diagnostic kits for new pathogens, such as MERS, allowing a faster response to various diseases. Our technology can also be applied widely in the field of genetic diagnostics.”
Picture: A schematic image of target nucleic acid extracted through the activation and deactivation of DNA polymerase
2015.07.31 View 10801 -
 3D Plasmon Antenna Capable of Focusing Light into Few Nanometers
Professors Myung-Ki Kim and Yong-Hee Lee, both of the Physics Department at KAIST, and their research teams have developed a three dimensional (3D) gap-plasmon antenna which can focus light into a space a few nanometers wide. Their research findings were published in the June 10th issue of Nano Letters.
Focusing light into a point-like space is an active research field with many applications. However, concentrating light into a smaller space than its wavelength is often hindered by diffraction. To tackle this problem, many researchers have utilized the plasmonic phenomenon of a metal where light can be confined to a greater extent by overcoming the diffraction limit.
Many researchers have focused on developing a two dimensional (2D) plasmon antenna and were able to focus a light under 5 nanometers wide. However, this 2D antenna revealed a challenge: the light disperses to the opposite end regardless of how small its beam was focused. To solve this difficulty, a 3D structure had to be employed to maximize the light's intensity.
Adopting the proximal focused-ion-beam milling technology, the KAIST research team developed a 3D four nanometer wide gap-plasmon antenna. By squeezing the photons into a 3D nano space of 4 x 10 x 10 nm3 size, the researchers were able to increase the intensity of light by 400,000 times stronger than that of the incident light. Capitalizing on the enhanced intensity of light within the antenna, they intensified the second-harmonic signal and verified that the light was focused in the nano gap by scanning cathodoluminescent images.
The researchers anticipate that this technology will improve the speed of data transfer and processing up to the level of a terahertz (one trillion times per second) and to enlarge the storage volume per unit area on hard disks by 100 times. In addition, high definition images of submolecule size can be taken with actual light, instead of with an electron microscope, while improving the semiconductor process to a smaller size of few nanometers.
Professor Kim said, “A simple yet ingenious idea has shifted the research paradigm from 2D gap-plasmon antennas to 3D antennas. This technology will see numerous applications including in the field of information technology, data storage, imaging medical science, and semiconductor processes.”
The research was sponsored by the National Research Foundation of Korea.
Figure 1: 3D Gap-Plasmon Antenna Structure and Simulation Results
Figure 2 – Constructed 3D Gap-Plasmon Antenna Structure
Figure 3 – Amplified Second Harmonic Signal Generation and Light Focused in the Nano Gap
2015.06.24 View 10790
3D Plasmon Antenna Capable of Focusing Light into Few Nanometers
Professors Myung-Ki Kim and Yong-Hee Lee, both of the Physics Department at KAIST, and their research teams have developed a three dimensional (3D) gap-plasmon antenna which can focus light into a space a few nanometers wide. Their research findings were published in the June 10th issue of Nano Letters.
Focusing light into a point-like space is an active research field with many applications. However, concentrating light into a smaller space than its wavelength is often hindered by diffraction. To tackle this problem, many researchers have utilized the plasmonic phenomenon of a metal where light can be confined to a greater extent by overcoming the diffraction limit.
Many researchers have focused on developing a two dimensional (2D) plasmon antenna and were able to focus a light under 5 nanometers wide. However, this 2D antenna revealed a challenge: the light disperses to the opposite end regardless of how small its beam was focused. To solve this difficulty, a 3D structure had to be employed to maximize the light's intensity.
Adopting the proximal focused-ion-beam milling technology, the KAIST research team developed a 3D four nanometer wide gap-plasmon antenna. By squeezing the photons into a 3D nano space of 4 x 10 x 10 nm3 size, the researchers were able to increase the intensity of light by 400,000 times stronger than that of the incident light. Capitalizing on the enhanced intensity of light within the antenna, they intensified the second-harmonic signal and verified that the light was focused in the nano gap by scanning cathodoluminescent images.
The researchers anticipate that this technology will improve the speed of data transfer and processing up to the level of a terahertz (one trillion times per second) and to enlarge the storage volume per unit area on hard disks by 100 times. In addition, high definition images of submolecule size can be taken with actual light, instead of with an electron microscope, while improving the semiconductor process to a smaller size of few nanometers.
Professor Kim said, “A simple yet ingenious idea has shifted the research paradigm from 2D gap-plasmon antennas to 3D antennas. This technology will see numerous applications including in the field of information technology, data storage, imaging medical science, and semiconductor processes.”
The research was sponsored by the National Research Foundation of Korea.
Figure 1: 3D Gap-Plasmon Antenna Structure and Simulation Results
Figure 2 – Constructed 3D Gap-Plasmon Antenna Structure
Figure 3 – Amplified Second Harmonic Signal Generation and Light Focused in the Nano Gap
2015.06.24 View 10790 -
 KAIST Team Develops Flexible PRAM
Phase change random access memory (PRAM) is one of the strongest candidates for next-generation nonvolatile memory for flexible and wearable electronics. In order to be used as a core memory for flexible devices, the most important issue is reducing high operating current. The effective solution is to decrease cell size in sub-micron region as in commercialized conventional PRAM. However, the scaling to nano-dimension on flexible substrates is extremely difficult due to soft nature and photolithographic limits on plastics, thus practical flexible PRAM has not been realized yet.
Recently, a team led by Professors Keon Jae Lee and Yeon Sik Jung of the Department of Materials Science and Engineering at KAIST has developed the first flexible PRAM enabled by self-assembled block copolymer (BCP) silica nanostructures with an ultralow current operation (below one quarter of conventional PRAM without BCP) on plastic substrates. BCP is the mixture of two different polymer materials, which can easily create self-ordered arrays of sub-20 nm features through simple spin-coating and plasma treatments. BCP silica nanostructures successfully lowered the contact area by localizing the volume change of phase-change materials and thus resulted in significant power reduction. Furthermore, the ultrathin silicon-based diodes were integrated with phase-change memories (PCM) to suppress the inter-cell interference, which demonstrated random access capability for flexible and wearable electronics. Their work was published in the March issue of ACS Nano: "Flexible One Diode-One Phase Change Memory Array Enabled by Block Copolymer Self-Assembly."
Another way to achieve ultralow-powered PRAM is to utilize self-structured conductive filaments (CF) instead of the resistor-type conventional heater. The self-structured CF nanoheater originated from unipolar memristor can generate strong heat toward phase-change materials due to high current density through the nanofilament. This ground-breaking methodology shows that sub-10 nm filament heater, without using expensive and non-compatible nanolithography, achieved nanoscale switching volume of phase change materials, resulted in the PCM writing current of below 20 uA, the lowest value among top-down PCM devices. This achievement was published in the June online issue of ACS Nano: "Self-Structured Conductive Filament Nanoheater for Chalcogenide Phase Transition." In addition, due to self-structured low-power technology compatible to plastics, the research team has recently succeeded in fabricating a flexible PRAM on wearable substrates.
Professor Lee said, "The demonstration of low power PRAM on plastics is one of the most important issues for next-generation wearable and flexible non-volatile memory. Our innovative and simple methodology represents the strong potential for commercializing flexible PRAM."
In addition, he wrote a review paper regarding the nanotechnology-based electronic devices in the June online issue of Advanced Materials entitled "Performance Enhancement of Electronic and Energy Devices via Block Copolymer Self-Assembly."
Picture Caption:
Low-power nonvolatile PRAM for flexible and wearable memories enabled by (a) self-assembled BCP silica nanostructures and (b) self-structured conductive filament nanoheater.
2015.06.15 View 15828
KAIST Team Develops Flexible PRAM
Phase change random access memory (PRAM) is one of the strongest candidates for next-generation nonvolatile memory for flexible and wearable electronics. In order to be used as a core memory for flexible devices, the most important issue is reducing high operating current. The effective solution is to decrease cell size in sub-micron region as in commercialized conventional PRAM. However, the scaling to nano-dimension on flexible substrates is extremely difficult due to soft nature and photolithographic limits on plastics, thus practical flexible PRAM has not been realized yet.
Recently, a team led by Professors Keon Jae Lee and Yeon Sik Jung of the Department of Materials Science and Engineering at KAIST has developed the first flexible PRAM enabled by self-assembled block copolymer (BCP) silica nanostructures with an ultralow current operation (below one quarter of conventional PRAM without BCP) on plastic substrates. BCP is the mixture of two different polymer materials, which can easily create self-ordered arrays of sub-20 nm features through simple spin-coating and plasma treatments. BCP silica nanostructures successfully lowered the contact area by localizing the volume change of phase-change materials and thus resulted in significant power reduction. Furthermore, the ultrathin silicon-based diodes were integrated with phase-change memories (PCM) to suppress the inter-cell interference, which demonstrated random access capability for flexible and wearable electronics. Their work was published in the March issue of ACS Nano: "Flexible One Diode-One Phase Change Memory Array Enabled by Block Copolymer Self-Assembly."
Another way to achieve ultralow-powered PRAM is to utilize self-structured conductive filaments (CF) instead of the resistor-type conventional heater. The self-structured CF nanoheater originated from unipolar memristor can generate strong heat toward phase-change materials due to high current density through the nanofilament. This ground-breaking methodology shows that sub-10 nm filament heater, without using expensive and non-compatible nanolithography, achieved nanoscale switching volume of phase change materials, resulted in the PCM writing current of below 20 uA, the lowest value among top-down PCM devices. This achievement was published in the June online issue of ACS Nano: "Self-Structured Conductive Filament Nanoheater for Chalcogenide Phase Transition." In addition, due to self-structured low-power technology compatible to plastics, the research team has recently succeeded in fabricating a flexible PRAM on wearable substrates.
Professor Lee said, "The demonstration of low power PRAM on plastics is one of the most important issues for next-generation wearable and flexible non-volatile memory. Our innovative and simple methodology represents the strong potential for commercializing flexible PRAM."
In addition, he wrote a review paper regarding the nanotechnology-based electronic devices in the June online issue of Advanced Materials entitled "Performance Enhancement of Electronic and Energy Devices via Block Copolymer Self-Assembly."
Picture Caption:
Low-power nonvolatile PRAM for flexible and wearable memories enabled by (a) self-assembled BCP silica nanostructures and (b) self-structured conductive filament nanoheater.
2015.06.15 View 15828 -
 Anti-Cancer Therapy Delivering Drug to an Entire Tumor Developed
KAIST’s Department of Bio and Brain Engineering Professor Ji-Ho Park and his team successfully developed a new highly efficacious anti-cancer nanotechnology by delivering anti-cancer drugs uniformly to an entire tumor. Their research results were published in Nano Letters online on March 31, 2015.
To treat inoperable tumors, anti-cancer medicine is commonly used. However, efficient drug delivery to tumor cells is often difficult, treating an entire tumor with drugs even more so.
Using the existing drug delivery systems, including nanotechnology, a drug can be delivered only to tumor cells near blood vessels, leaving cells at the heart of a tumor intact. Since most drugs are injected into the bloodstream, tumor recurrence post medication is frequent.
Therefore, the team used liposomes that can fuse to the cell membrane and enter the cell. Once inside liposomes the drug can travel into the bloodstream, enter tumor cells near blood vessels, where they are loaded to exosomes, which are naturally occurring nanoparticles in the body. Since exosomes can travel between cells, the drug can be delivered efficiently into inner cells of the tumor.
Exosomes, which are secreted by cells that exist in the tumor microenvironment, is known to have an important role in tumor progression and metastasis since they transfer biological materials between cells. The research team started the investigation recognizing the possibility of delivering the anti-cancer drug to the entire tumor using exosomes.
The team injected the light-sensitive anti-cancer drug using their new delivery technique into experimental mice. The researchers applied light to the tumor site to activate the anti-cancer treatment and analyzed a tissue sample. They observed the effects of the anti-cancer drug in the entire tumor tissue.
The team’s results establish a ground-breaking foothold in drug delivery technology development that can be tailored to specific diseases by understanding its microenvironment. The work paves the way to more effective drug delivery systems for many chronic diseases, including cancer tumors that were difficult to treat due to the inability to penetrate deep into the tissue.
The team is currently conducting experiments with other anti-cancer drugs, which are being developed by pharmaceutical companies, using their tumor-penetrating drug delivery nanotechnology, to identify its effects on malignant tumors.
Professor Park said, “This research is the first to apply biological nanoparticles, exosomes that are continuously secreted and can transfer materials to neighboring cells, to deliver drugs directly to the heart of tumor.”
Picture: Incorporation of hydrophilic and hydrophobic compounds into membrane vesicles by engineering the parental cells via synthetic liposomes.
2015.04.07 View 12679
Anti-Cancer Therapy Delivering Drug to an Entire Tumor Developed
KAIST’s Department of Bio and Brain Engineering Professor Ji-Ho Park and his team successfully developed a new highly efficacious anti-cancer nanotechnology by delivering anti-cancer drugs uniformly to an entire tumor. Their research results were published in Nano Letters online on March 31, 2015.
To treat inoperable tumors, anti-cancer medicine is commonly used. However, efficient drug delivery to tumor cells is often difficult, treating an entire tumor with drugs even more so.
Using the existing drug delivery systems, including nanotechnology, a drug can be delivered only to tumor cells near blood vessels, leaving cells at the heart of a tumor intact. Since most drugs are injected into the bloodstream, tumor recurrence post medication is frequent.
Therefore, the team used liposomes that can fuse to the cell membrane and enter the cell. Once inside liposomes the drug can travel into the bloodstream, enter tumor cells near blood vessels, where they are loaded to exosomes, which are naturally occurring nanoparticles in the body. Since exosomes can travel between cells, the drug can be delivered efficiently into inner cells of the tumor.
Exosomes, which are secreted by cells that exist in the tumor microenvironment, is known to have an important role in tumor progression and metastasis since they transfer biological materials between cells. The research team started the investigation recognizing the possibility of delivering the anti-cancer drug to the entire tumor using exosomes.
The team injected the light-sensitive anti-cancer drug using their new delivery technique into experimental mice. The researchers applied light to the tumor site to activate the anti-cancer treatment and analyzed a tissue sample. They observed the effects of the anti-cancer drug in the entire tumor tissue.
The team’s results establish a ground-breaking foothold in drug delivery technology development that can be tailored to specific diseases by understanding its microenvironment. The work paves the way to more effective drug delivery systems for many chronic diseases, including cancer tumors that were difficult to treat due to the inability to penetrate deep into the tissue.
The team is currently conducting experiments with other anti-cancer drugs, which are being developed by pharmaceutical companies, using their tumor-penetrating drug delivery nanotechnology, to identify its effects on malignant tumors.
Professor Park said, “This research is the first to apply biological nanoparticles, exosomes that are continuously secreted and can transfer materials to neighboring cells, to deliver drugs directly to the heart of tumor.”
Picture: Incorporation of hydrophilic and hydrophobic compounds into membrane vesicles by engineering the parental cells via synthetic liposomes.
2015.04.07 View 12679 -
 Polymers with Highly Improved Light-transformation Efficiency
A joint Korean research team, led by Professor Bum-Joon Kim of the Department of Chemical and Biomolecular Engineering at KAIST and Professor Young-Woo Han of the Department of Nanofusion Engineering at Pusan National University, has developed a new type of electrically-conductive polymer for solar batteries with an improved light-transformation efficiency of up to 5%. The team considers it a viable replacement for existing plastic batteries for solar power which is viewed as the energy source of the future.
Polymer solar cells have greater structural stability and heat resistance compared to fullerene organic solar cells. However, they have lower light-transformation efficiency—below 4%—compared to 10% of the latter. The low efficiency is due to the failure of blending among the polymers that compose the active layer of the cell. This phenomenon deters the formation and movement of electrons and thus lowers light-transformation efficiency.
By manipulating the structure and concentration of conductive polymers, the team was able to effectively increase the polymer blending and increase light-transformation efficiency. The team was able to maximize the efficiency up to 6% which is the highest reported ratio.
Professor Kim said, “This research demonstrates that conductive polymer plastics can be used widely for solar cells and batteries for mobile devices.”
The research findings were published in the February 18th issue of the Journal of the American Chemical Society (JACS).
Picture: Flexible Solar Cell Polymer Developed by the Research Team
2015.04.05 View 11797
Polymers with Highly Improved Light-transformation Efficiency
A joint Korean research team, led by Professor Bum-Joon Kim of the Department of Chemical and Biomolecular Engineering at KAIST and Professor Young-Woo Han of the Department of Nanofusion Engineering at Pusan National University, has developed a new type of electrically-conductive polymer for solar batteries with an improved light-transformation efficiency of up to 5%. The team considers it a viable replacement for existing plastic batteries for solar power which is viewed as the energy source of the future.
Polymer solar cells have greater structural stability and heat resistance compared to fullerene organic solar cells. However, they have lower light-transformation efficiency—below 4%—compared to 10% of the latter. The low efficiency is due to the failure of blending among the polymers that compose the active layer of the cell. This phenomenon deters the formation and movement of electrons and thus lowers light-transformation efficiency.
By manipulating the structure and concentration of conductive polymers, the team was able to effectively increase the polymer blending and increase light-transformation efficiency. The team was able to maximize the efficiency up to 6% which is the highest reported ratio.
Professor Kim said, “This research demonstrates that conductive polymer plastics can be used widely for solar cells and batteries for mobile devices.”
The research findings were published in the February 18th issue of the Journal of the American Chemical Society (JACS).
Picture: Flexible Solar Cell Polymer Developed by the Research Team
2015.04.05 View 11797 -
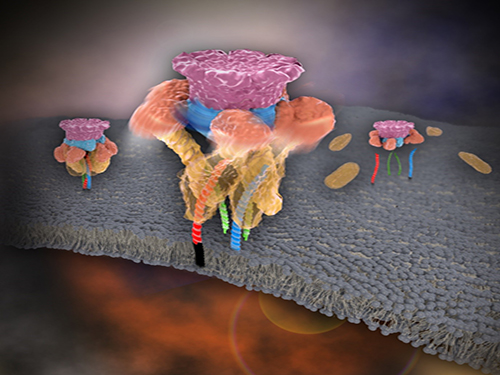 Mystery in Membrane Traffic How NSF Disassembles Single SNAR Complex Solved
KAIST researchers discovered that the protein N-ethylmaleimide-sensitive factor (NSF) unravels a single SNARE complex using one round ATP turnover by tearing the complex with a single burst, contradicting a previous theory that it unwinds in a processive manner.
In 2013, James E. Rothman, Randy W. Schekman, and Thomas C. Südhof won the Nobel Prize in Physiology or Medicine for their discoveries of molecular machineries for vesicle trafficking, a major transport system in cells for maintaining cellular processes. Vesicle traffic acts as a kind of “home-delivery service” in cells. Vesicles package and deliver materials such as proteins and hormones from one cell organelle to another. Then it releases its contents by fusing with the target organelle’s membrane. One example of vesicle traffic is in neuronal communications, where neurotransmitters are released from a neuron. Some of the key proteins for vesicle traffic discovered by the Nobel Prize winners were N-ethylmaleimide-sensitive factor (NSF), alpha-soluble NSF attachment protein (α-SNAP), and soluble SNAP receptors (SNAREs).
SNARE proteins are known as the minimal machinery for membrane fusion. To induce membrane fusion, the proteins combine to form a SNARE complex in a four helical bundle, and NSF and α-SNAP disassemble the SNARE complex for reuse. In particular, NSF can bind an energy source molecule, adenosine triphosphate (ATP), and the ATP-bound NSF develops internal tension via cleavage of ATP. This process is used to exert great force on SNARE complexes, eventually pulling them apart. However, although about 30 years have passed since the Nobel Prize winners’ discovery, how NSF/α-SNAP disassembled the SNARE complex remained a mystery to scientists due to a lack in methodology.
In a recent issue of Science, published on March 27, 2015, a research team, led by Tae-Young Yoon of the Department of Physics at the Korea Advanced Institute of Science and Technology (KAIST) and Reinhard Jahn of the Department of Neurobiology of the Max-Planck-Institute for Biophysical Chemistry, reports that NSF/α-SNAP disassemble a single SNARE complex using various single-molecule biophysical methods that allow them to monitor and manipulate individual protein complexes.
“We have learned that NSF releases energy in a burst within 20 milliseconds to “tear” the SNARE complex apart in a one-step global unfolding reaction, which is immediately followed by the release of SNARE proteins,” said Yoon.
Previously, it was believed that NSF disassembled a SNARE complex by unwinding it in a processive manner. Also, largely unexplained was how many cycles of ATP hydrolysis were required and how these cycles were connected to the disassembly of the SNARE complex.
Yoon added, “From our research, we found that NSF requires hydrolysis of ATPs that were already bound before it attached to the SNAREs—which means that only one round of an ATP turnover is sufficient for SNARE complex disassembly. Moreover, this is possible because NSF pulls a SNARE complex apart by building up the energy from individual ATPs and releasing it at once, yielding a “spring-loaded” mechanism.”
NSF is a member of the ATPases associated with various cellular activities family (AAA+ ATPase), which is essential for many cellular functions such as DNA replication and protein degradation, membrane fusion, microtubule severing, peroxisome biogenesis, signal transduction, and the regulation of gene expression. This research has added valuable new insights and hints for studying AAA+ ATPase proteins, which are crucial for various living beings.
The title of the research paper is “Spring-loaded unraveling of a single SNARE complex by NSF in one round of ATP turnover.” (DOI: 10.1126/science.aaa5267)
Youtube Link: https://www.youtube.com/watch?v=FqTSYHtyHWE&feature=youtu.be
Picture 1. Working model of how NSF/α-SNAP disassemble a single SNARE complex
Picture 2. After neurotransmitter release, NSF disassembles a single SNARE complex using a single round of ATP turnover in a single burst reaction.
2015.03.28 View 11416
Mystery in Membrane Traffic How NSF Disassembles Single SNAR Complex Solved
KAIST researchers discovered that the protein N-ethylmaleimide-sensitive factor (NSF) unravels a single SNARE complex using one round ATP turnover by tearing the complex with a single burst, contradicting a previous theory that it unwinds in a processive manner.
In 2013, James E. Rothman, Randy W. Schekman, and Thomas C. Südhof won the Nobel Prize in Physiology or Medicine for their discoveries of molecular machineries for vesicle trafficking, a major transport system in cells for maintaining cellular processes. Vesicle traffic acts as a kind of “home-delivery service” in cells. Vesicles package and deliver materials such as proteins and hormones from one cell organelle to another. Then it releases its contents by fusing with the target organelle’s membrane. One example of vesicle traffic is in neuronal communications, where neurotransmitters are released from a neuron. Some of the key proteins for vesicle traffic discovered by the Nobel Prize winners were N-ethylmaleimide-sensitive factor (NSF), alpha-soluble NSF attachment protein (α-SNAP), and soluble SNAP receptors (SNAREs).
SNARE proteins are known as the minimal machinery for membrane fusion. To induce membrane fusion, the proteins combine to form a SNARE complex in a four helical bundle, and NSF and α-SNAP disassemble the SNARE complex for reuse. In particular, NSF can bind an energy source molecule, adenosine triphosphate (ATP), and the ATP-bound NSF develops internal tension via cleavage of ATP. This process is used to exert great force on SNARE complexes, eventually pulling them apart. However, although about 30 years have passed since the Nobel Prize winners’ discovery, how NSF/α-SNAP disassembled the SNARE complex remained a mystery to scientists due to a lack in methodology.
In a recent issue of Science, published on March 27, 2015, a research team, led by Tae-Young Yoon of the Department of Physics at the Korea Advanced Institute of Science and Technology (KAIST) and Reinhard Jahn of the Department of Neurobiology of the Max-Planck-Institute for Biophysical Chemistry, reports that NSF/α-SNAP disassemble a single SNARE complex using various single-molecule biophysical methods that allow them to monitor and manipulate individual protein complexes.
“We have learned that NSF releases energy in a burst within 20 milliseconds to “tear” the SNARE complex apart in a one-step global unfolding reaction, which is immediately followed by the release of SNARE proteins,” said Yoon.
Previously, it was believed that NSF disassembled a SNARE complex by unwinding it in a processive manner. Also, largely unexplained was how many cycles of ATP hydrolysis were required and how these cycles were connected to the disassembly of the SNARE complex.
Yoon added, “From our research, we found that NSF requires hydrolysis of ATPs that were already bound before it attached to the SNAREs—which means that only one round of an ATP turnover is sufficient for SNARE complex disassembly. Moreover, this is possible because NSF pulls a SNARE complex apart by building up the energy from individual ATPs and releasing it at once, yielding a “spring-loaded” mechanism.”
NSF is a member of the ATPases associated with various cellular activities family (AAA+ ATPase), which is essential for many cellular functions such as DNA replication and protein degradation, membrane fusion, microtubule severing, peroxisome biogenesis, signal transduction, and the regulation of gene expression. This research has added valuable new insights and hints for studying AAA+ ATPase proteins, which are crucial for various living beings.
The title of the research paper is “Spring-loaded unraveling of a single SNARE complex by NSF in one round of ATP turnover.” (DOI: 10.1126/science.aaa5267)
Youtube Link: https://www.youtube.com/watch?v=FqTSYHtyHWE&feature=youtu.be
Picture 1. Working model of how NSF/α-SNAP disassemble a single SNARE complex
Picture 2. After neurotransmitter release, NSF disassembles a single SNARE complex using a single round of ATP turnover in a single burst reaction.
2015.03.28 View 11416 -
 KAIST Develops a Credit-Card-Thick Flexible Lithium Ion Battery
Since the battery can be charged wirelessly, useful applications are expected including medical patches and smart cards.
Professor Jang Wook Choi at KAIST’s Graduate School of Energy, Environment, Water, and Sustainability (EEWS) and Dr. Jae Yong Song at the Korea Research Institute of Standards and Science jointly led research to invent a flexible lithium ion battery that is thinner than a credit card and can be charged wirelessly.
Their research findings were published online in Nano Letters on March 6, 2015. Lithium ion batteries are widely used today in various electronics including mobile devices and electronic cars. Researchers said that their work could help accelerate the development of flexible and wearable electronics.
Conventional lithium ion batteries are manufactured based on a layering technology, stacking up anodes, separating films, and cathodes like a sandwich, which makes it difficult to reduce their thickness. In addition, friction arises between layers, making the batteries impossible to bend. The coating films of electrodes easily come off, which contributes to the batteries’ poor performance.
The research team abandoned the existing production technology. Instead, they removed the separating films, layered the cathodes and anodes collinearly on a plane, and created a partition between electrodes to eliminate potential problems, such as short circuits and voltage dips, commonly present in lithium ion batteries.
After more than five thousand consecutive flexing experiments, the research team confirmed the possibility of a more flexible electrode structure while maintaining the battery performance comparable to the level of current lithium ion batteries.
Flexible batteries can be applied to integrated smart cards, cosmetic and medical patches, and skin adhesive sensors that can control a computer with voice commands or gesture as seen in the movie “Iron Man.”
Moreover, the team has successfully developed wireless-charging technology using electromagnetic induction and solar batteries.
They are currently developing a mass production process to combine this planar battery technology and printing, to ultimately create a new paradigm to print semiconductors and batteries using 3D printers.
Professor Choi said, “This new technology will contribute to diversifying patch functions as it is applicable to power various adhesive medical patches.”
Picture 1: Medical patch (left) and flexible secondary battery (right)
Picture 2: Diagram of flexible battery
Picture 3: Smart card embedding flexible battery
2015.03.24 View 13282
KAIST Develops a Credit-Card-Thick Flexible Lithium Ion Battery
Since the battery can be charged wirelessly, useful applications are expected including medical patches and smart cards.
Professor Jang Wook Choi at KAIST’s Graduate School of Energy, Environment, Water, and Sustainability (EEWS) and Dr. Jae Yong Song at the Korea Research Institute of Standards and Science jointly led research to invent a flexible lithium ion battery that is thinner than a credit card and can be charged wirelessly.
Their research findings were published online in Nano Letters on March 6, 2015. Lithium ion batteries are widely used today in various electronics including mobile devices and electronic cars. Researchers said that their work could help accelerate the development of flexible and wearable electronics.
Conventional lithium ion batteries are manufactured based on a layering technology, stacking up anodes, separating films, and cathodes like a sandwich, which makes it difficult to reduce their thickness. In addition, friction arises between layers, making the batteries impossible to bend. The coating films of electrodes easily come off, which contributes to the batteries’ poor performance.
The research team abandoned the existing production technology. Instead, they removed the separating films, layered the cathodes and anodes collinearly on a plane, and created a partition between electrodes to eliminate potential problems, such as short circuits and voltage dips, commonly present in lithium ion batteries.
After more than five thousand consecutive flexing experiments, the research team confirmed the possibility of a more flexible electrode structure while maintaining the battery performance comparable to the level of current lithium ion batteries.
Flexible batteries can be applied to integrated smart cards, cosmetic and medical patches, and skin adhesive sensors that can control a computer with voice commands or gesture as seen in the movie “Iron Man.”
Moreover, the team has successfully developed wireless-charging technology using electromagnetic induction and solar batteries.
They are currently developing a mass production process to combine this planar battery technology and printing, to ultimately create a new paradigm to print semiconductors and batteries using 3D printers.
Professor Choi said, “This new technology will contribute to diversifying patch functions as it is applicable to power various adhesive medical patches.”
Picture 1: Medical patch (left) and flexible secondary battery (right)
Picture 2: Diagram of flexible battery
Picture 3: Smart card embedding flexible battery
2015.03.24 View 13282 -
 Light Driven Drug-Enzyme Reaction Catalytic Platform Developed
Low Cost Dye Used, Hope for Future Development of High Value Medicinal Products to Treat Cardiovascular Disease and Gastric Ulcers
A KAIST research team from the Departments of Materials Science and Engineering and of Chemical and Biomolecular Engineering, led respectively by Professors Chan Beum Park and Ki Jun Jeong, has developed a new reaction platform to induce drug-enzyme reaction using light.
The research results were published in the journal Angewandte Chemie, International Edition, as the back cover on 12 January 2015.
Applications of this technology may enable production of high value products such as medicine for cardiovascular disease and gastric ulcers, for example Omeprazole, using an inexpensive dye.
Cytochrome P450 is an enzyme involved in oxidative response which has an important role in drug and hormone metabolism in organisms. It is known to be responsible for metabolism of 75% of drugs in humans and is considered a fundamental factor in new drug development.
To activate cytochrome P450, the enzyme must receive an electron by reducing the enzyme. In addition, NADPH (a coenzyme) needs to be present. However, since NADPH is expensive, the use of cytochrome P450 was limited to the laboratory and has not yet been commercialized.
The research team used photosensitizer eosin Y instead of NADPH to develop “Whole Cell Photo-Biocatalysis” in bacteria E. coli. By exposing inexpensive eosin Y to light, cytochrome P450 reaction was catalyzed to produce the expensive metabolic material.
Professor Park said, “This research enabled industrial application of cytochrome P450 enzyme, which was previous limited.” He continued, “This technology will help greatly in producing high value medical products using cytochrome P450 enzyme.”
The research was funded by the National Research Foundation of Korea and KAIST's High Risk High Return Project (HRHRP).
Figure 1: Mimetic Diagram of Electron Transfer from Light to Cytochrome P450 Enzyme via Eosin Y, EY
Figure 2: The back cover of Angewandte Chemie published on 12 January 2015, showing the research results
2015.01.26 View 11218
Light Driven Drug-Enzyme Reaction Catalytic Platform Developed
Low Cost Dye Used, Hope for Future Development of High Value Medicinal Products to Treat Cardiovascular Disease and Gastric Ulcers
A KAIST research team from the Departments of Materials Science and Engineering and of Chemical and Biomolecular Engineering, led respectively by Professors Chan Beum Park and Ki Jun Jeong, has developed a new reaction platform to induce drug-enzyme reaction using light.
The research results were published in the journal Angewandte Chemie, International Edition, as the back cover on 12 January 2015.
Applications of this technology may enable production of high value products such as medicine for cardiovascular disease and gastric ulcers, for example Omeprazole, using an inexpensive dye.
Cytochrome P450 is an enzyme involved in oxidative response which has an important role in drug and hormone metabolism in organisms. It is known to be responsible for metabolism of 75% of drugs in humans and is considered a fundamental factor in new drug development.
To activate cytochrome P450, the enzyme must receive an electron by reducing the enzyme. In addition, NADPH (a coenzyme) needs to be present. However, since NADPH is expensive, the use of cytochrome P450 was limited to the laboratory and has not yet been commercialized.
The research team used photosensitizer eosin Y instead of NADPH to develop “Whole Cell Photo-Biocatalysis” in bacteria E. coli. By exposing inexpensive eosin Y to light, cytochrome P450 reaction was catalyzed to produce the expensive metabolic material.
Professor Park said, “This research enabled industrial application of cytochrome P450 enzyme, which was previous limited.” He continued, “This technology will help greatly in producing high value medical products using cytochrome P450 enzyme.”
The research was funded by the National Research Foundation of Korea and KAIST's High Risk High Return Project (HRHRP).
Figure 1: Mimetic Diagram of Electron Transfer from Light to Cytochrome P450 Enzyme via Eosin Y, EY
Figure 2: The back cover of Angewandte Chemie published on 12 January 2015, showing the research results
2015.01.26 View 11218 -
 KAIST Announces the Recipients of Distinguished Alumni Awards
The KAIST Alumni Association (KAA) announced four “Proud KAIST Alumni” awards recipients for the year 2014: Sung-Wook Park, the Chief Executive Officer and President of SK Hynix; Seung Ho Shin, the President of Kangwon National University; Kew-Ho Lee, the President of the Korea Research Institute of Chemical Technology; and Mun-Kee Choi, the former Minister of Science, ICT and Future Planning of the Republic of Korea. The award ceremony took place during the 2015 KAA’s New Year's ceremony on January 17, 2015 at the Palace Hotel in Seoul.
Sung-Wook Park (M.S. ’82 and Ph.D. ’88, Department of Materials Science and Engineering), the Chief Executive Officer and President of SK Hynix, has worked as an expert in the field of memory semi-conductors for the past 30 years. He developed innovative technology and improved production efficiency, enabling the Korean semi-conductor industry to become a global leader.
Seung Ho Shin (M.S. ’79 and Ph.D. ’87, Department of Physics), the President of Kangwon National University (KNU), worked in the field of optical information processing, producing excellent research achievements and teaching the next generation of scientists. As the president of KNU, he has set an exemplary leadership in higher education.
Kew-Ho Lee (M.S. ’75, Department of Chemistry), the President of the Korea Research Institute of Chemical Technology, pioneered the field of separation film production which contributed greatly to Korean technological developments. He led several domestic and international societies to facilitate dynamic exchanges between industry and academia and with the international community.
Mun-Kee Choi (M.S. ’76, Department of Industrial and Systems Engineering), the former Minister of Science, ICT and Future Planning, the Republic of Korea, is a great contributor to the information and communications technology in Korea, working as a leader in the field of broadband integrated service digital network. He is also an educator for gifted students in science and technology, and a manager of the Electronics and Telecommunications Research Institute.
The Alumni Association established the “Proud KAIST Alumni Awards” in 1992 to recognize its alumni’s outstanding contributions to Korea and KAIST.
Pictured from left to right, Sung-Wook Park (the Chief Executive Officer and President of SK Hynix), Seung Ho Shin (the President of Kangwon National University), Kew-Ho Lee (the President of the Korea Research Institute of Chemical Technology), and Mun-Kee Choi (the former Minister of Science, ICT and Future Planning)
2015.01.19 View 16095
KAIST Announces the Recipients of Distinguished Alumni Awards
The KAIST Alumni Association (KAA) announced four “Proud KAIST Alumni” awards recipients for the year 2014: Sung-Wook Park, the Chief Executive Officer and President of SK Hynix; Seung Ho Shin, the President of Kangwon National University; Kew-Ho Lee, the President of the Korea Research Institute of Chemical Technology; and Mun-Kee Choi, the former Minister of Science, ICT and Future Planning of the Republic of Korea. The award ceremony took place during the 2015 KAA’s New Year's ceremony on January 17, 2015 at the Palace Hotel in Seoul.
Sung-Wook Park (M.S. ’82 and Ph.D. ’88, Department of Materials Science and Engineering), the Chief Executive Officer and President of SK Hynix, has worked as an expert in the field of memory semi-conductors for the past 30 years. He developed innovative technology and improved production efficiency, enabling the Korean semi-conductor industry to become a global leader.
Seung Ho Shin (M.S. ’79 and Ph.D. ’87, Department of Physics), the President of Kangwon National University (KNU), worked in the field of optical information processing, producing excellent research achievements and teaching the next generation of scientists. As the president of KNU, he has set an exemplary leadership in higher education.
Kew-Ho Lee (M.S. ’75, Department of Chemistry), the President of the Korea Research Institute of Chemical Technology, pioneered the field of separation film production which contributed greatly to Korean technological developments. He led several domestic and international societies to facilitate dynamic exchanges between industry and academia and with the international community.
Mun-Kee Choi (M.S. ’76, Department of Industrial and Systems Engineering), the former Minister of Science, ICT and Future Planning, the Republic of Korea, is a great contributor to the information and communications technology in Korea, working as a leader in the field of broadband integrated service digital network. He is also an educator for gifted students in science and technology, and a manager of the Electronics and Telecommunications Research Institute.
The Alumni Association established the “Proud KAIST Alumni Awards” in 1992 to recognize its alumni’s outstanding contributions to Korea and KAIST.
Pictured from left to right, Sung-Wook Park (the Chief Executive Officer and President of SK Hynix), Seung Ho Shin (the President of Kangwon National University), Kew-Ho Lee (the President of the Korea Research Institute of Chemical Technology), and Mun-Kee Choi (the former Minister of Science, ICT and Future Planning)
2015.01.19 View 16095 -
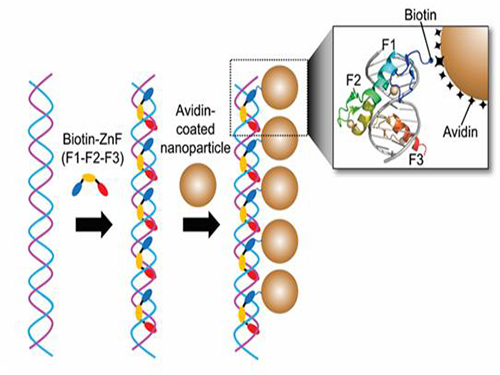 Nanoparticle Cluster Manufacturing Technique Using DNA Binding Protein Developed
Professor Hak-Sung Kim of the Department of Biological Sciences at KAIST and Yiseul Ryu, a doctoral candidate, used the Zinc Finger protein that specifically binds to target DNA sequence to develop a new manufacturing technique for size-controllable magnetic Nanoparticle Clusters (NPCs). Their research results were published in Angewandte Chemie International Edition online on 25 November 2014.
NPCs are structures consisting of magnetic nanoparticles, gold nanoparticles, and quantum dots, each of which are smaller than 100 nm (10-9m). NPCs have a distinctive property of collectivity not seen in single nanoparticles.
Specifically NPCS differ in physical and optical properties such as Plasmon coupling absorbance, energy transfers between particles, electron transfers, and conductivity. Therefore, NPCs can be employed in biological and medical research as well as the development of nanoelectric and nanoplasmon devices.
To make use of these novel properties, the size and the composition of the cluster must be exquisitely controlled. However, previous techniques relied on chemical binding which required complex steps, making it difficult to control the size and composition of NPCs.
Professor Kim’s team used Zinc Finger, a DNA binding protein, to develop a NPCs manufacturing technique to create clusters of the desired size easily. The Zinc Finger protein contains a zinc ion and specifically recognizes DNA sequence upon binding, which allows the exquisite control of the size and the cluster composition. The technique is also bio-friendly.
Professor Kim’s team created linear structure of different sizes of NPCs using Zinc Finger proteins and three DNA sequences of different lengths. The NPCs they produced confirmed their ability to control the size and structure of the cluster by using different DNA lengths.
The NPCs showed tripled T2 relaxation rates compared to the existing MRI contrast media (Feridex) and effectively transported to targeted cells. The research findings show the potential use of NPCs in biological and medical fields such as MRI contrast media, fluorescence imaging, and drug transport.
The research used the specific binding property of protein and DNA to develop a new method to create an inorganic nanoparticle’s supramolecular assembly. The technique can be used and applied extensively in other nanoparticles for future research in diagnosis, imaging, and drug and gene delivery.
Figure 1. A Mimetic Diagram of NPCs Manufacturing Technique Using DNA Binding Protein Zinc Finger
Figure 2. Transmission Electron Microscopy Images showing different sizes of NPCs depending on the length of the DNA
2014.12.04 View 14061
Nanoparticle Cluster Manufacturing Technique Using DNA Binding Protein Developed
Professor Hak-Sung Kim of the Department of Biological Sciences at KAIST and Yiseul Ryu, a doctoral candidate, used the Zinc Finger protein that specifically binds to target DNA sequence to develop a new manufacturing technique for size-controllable magnetic Nanoparticle Clusters (NPCs). Their research results were published in Angewandte Chemie International Edition online on 25 November 2014.
NPCs are structures consisting of magnetic nanoparticles, gold nanoparticles, and quantum dots, each of which are smaller than 100 nm (10-9m). NPCs have a distinctive property of collectivity not seen in single nanoparticles.
Specifically NPCS differ in physical and optical properties such as Plasmon coupling absorbance, energy transfers between particles, electron transfers, and conductivity. Therefore, NPCs can be employed in biological and medical research as well as the development of nanoelectric and nanoplasmon devices.
To make use of these novel properties, the size and the composition of the cluster must be exquisitely controlled. However, previous techniques relied on chemical binding which required complex steps, making it difficult to control the size and composition of NPCs.
Professor Kim’s team used Zinc Finger, a DNA binding protein, to develop a NPCs manufacturing technique to create clusters of the desired size easily. The Zinc Finger protein contains a zinc ion and specifically recognizes DNA sequence upon binding, which allows the exquisite control of the size and the cluster composition. The technique is also bio-friendly.
Professor Kim’s team created linear structure of different sizes of NPCs using Zinc Finger proteins and three DNA sequences of different lengths. The NPCs they produced confirmed their ability to control the size and structure of the cluster by using different DNA lengths.
The NPCs showed tripled T2 relaxation rates compared to the existing MRI contrast media (Feridex) and effectively transported to targeted cells. The research findings show the potential use of NPCs in biological and medical fields such as MRI contrast media, fluorescence imaging, and drug transport.
The research used the specific binding property of protein and DNA to develop a new method to create an inorganic nanoparticle’s supramolecular assembly. The technique can be used and applied extensively in other nanoparticles for future research in diagnosis, imaging, and drug and gene delivery.
Figure 1. A Mimetic Diagram of NPCs Manufacturing Technique Using DNA Binding Protein Zinc Finger
Figure 2. Transmission Electron Microscopy Images showing different sizes of NPCs depending on the length of the DNA
2014.12.04 View 14061 -
 Elsevier Selects a KAIST Graduate's Paper as the Top Cited Papers in 2011-2012
Dr. Myung-Won Seo, a graduate from the Department of Chemical and Bimolecular Engineering at KAIST, published a paper in January 2011 in Chemical Engineering Journal, which was entitled “Solid Circulation and Loop-seal Characteristics of a Dual Circulating Fluidized Bed: Experiments and CFD Simulation.” His paper was selected by Elsevier as the Top Cited Papers of 2011-2012. The Chemical Engineering Journal is a renowned peer-reviewed journal issued by Elsevier.
Dr. Seo published another paper, “CFD Simulation with Experiments in a Dual Circulating Fluidized Bed Gasifier,” in January 2012 in Computers & Chemical Engineering, which was also selected as the Most Downloaded Papers in 2012-2013.
Dr. Seo graduated with a doctoral degree from KAIST in 2011. He is currently working at the Clean Fuel Laboratory, the Korea Institute of Energy Research, Daejeon, as a researcher. His research areas are coal gasification, upgrading, and liquefaction, as well as energy and chemical production from low-grade fuels such as biomass and wastes.
2014.11.24 View 10574
Elsevier Selects a KAIST Graduate's Paper as the Top Cited Papers in 2011-2012
Dr. Myung-Won Seo, a graduate from the Department of Chemical and Bimolecular Engineering at KAIST, published a paper in January 2011 in Chemical Engineering Journal, which was entitled “Solid Circulation and Loop-seal Characteristics of a Dual Circulating Fluidized Bed: Experiments and CFD Simulation.” His paper was selected by Elsevier as the Top Cited Papers of 2011-2012. The Chemical Engineering Journal is a renowned peer-reviewed journal issued by Elsevier.
Dr. Seo published another paper, “CFD Simulation with Experiments in a Dual Circulating Fluidized Bed Gasifier,” in January 2012 in Computers & Chemical Engineering, which was also selected as the Most Downloaded Papers in 2012-2013.
Dr. Seo graduated with a doctoral degree from KAIST in 2011. He is currently working at the Clean Fuel Laboratory, the Korea Institute of Energy Research, Daejeon, as a researcher. His research areas are coal gasification, upgrading, and liquefaction, as well as energy and chemical production from low-grade fuels such as biomass and wastes.
2014.11.24 View 10574 -
 Eggshell-like Cell Encapsulation and Degradation Technology Developed
Some bacteria form endospores on cell walls to protect their DNA in case of nutrient deficiency. When an endospore meets a suitable environment for survival, the cell can revert to the original state from which it can reproduce.
The technique that can artificially control such phenomenon was developed by an international team of researchers. At first, a cell is wrapped and preserved like an egg. When the cell is needed, the technique allows the endospore to decompose while it is alive. Future applications for this technique include cell-based biosensor, cell therapy, and biocatalyst processes.
Professors Insung Choi and Younghoon Lee from the Department of Chemistry at KAIST as well as and Professor Frank Caruso from the University of Melbourne developed this technique which permits a cell to stay alive by coating it with film on a nanometer scale and then to be decomposed while it is alive.
The research finding was published in the November 10th issue of Angewandte Chemie International Edition as the lead article.
Cell encapsulation allows researchers to capture a cell in a tight capsule while it is alive. It is highly recognized in cell-based applications where the control of cell stability and cell-division is the biggest issue.
Traditional cell encapsulation methods utilized organic film or inorganic capsules that are made of organic film moldings. Although these films tightly closed around the cell, because they were not easily decomposable, it was difficult to apply the method.
The research team succeeded in encapsulating each cell in a metal-polyphenol film by mixing tannic acid and iron ion solution with yeast cells.
Usually extracted from oak barks or grape peels, tannic acid is a natural substance. It forms a metal-polyphenol film within ten seconds when it meets iron ions due to its high affinity with cells. Cells encapsulated with this film presented high survival rates. Since the film forms quickly in a simple manner, it was possible to obtain large amount of encapsulated cells.
The research team also found that the metal-polyphenol film was stable in neutral pH, but is easily degradable under a weak acidic condition. Using this property, they were able to control cell division by restoring the cell to its pre-encapsulated state at a desired moment.
Protecting the cell from the external environment like an egg shell, the metal-polyphenol film protected the cell against foreign conditions such as lytic enzymes, extended exposure to UV radiation, and silver nanoparticles. The research indicated that the encapsulated cells had a high survival rate even under extreme environments.
Professor Lee said that “not only the cells remain alive during the encapsulation stage, but also they can be protected under extreme environment.” He added, “This is an advanced cell encapsulation technology that allows controlling cell-division of those cells through responsive shell degradation on-demand.”
Professor Choi commented, “Although the cell encapsulation technology is still in its infancy, as the technology matures the application of cell-manipulation technology will be actualized.” He highlighted that “it will serve as a breakthrough to problems faced by cell-based applications.”
Sponsored by the Ministry of Science, ICT and Future Planning and the National Research Foundation of Korea, the research was led by two Master’s candidates, Ji Hun Park and Kyung Hwan Kim, under the joint guidance of research professors from KAIST and the University of Melbourne.
Figure 1: Lead article of Angewandte Chemie
Background: Shows a live native yeast (in green) encapsulated in a metal-polyphenol film (in red) illustrating the vitality of the yeast
Front: A native yeast at each encapsulation stage
Pictured on the bottom left is a cell prior to encapsulation. Following the red arrow, the native yeast is in purple to show metal-polyphenol film formed around the cell. The cell after the green arrow is a visualization of the degradation of the film in weak acidic condition.
Figure 2: A mimetic diagram of cell encapsulation with a metal-polyphenol film
Top: A native yeast before encapsulation
Middle: A native yeast encapsulated with Tannic Acid-Fe (III) Nanoshell – cell-division of the encapsulated cell is controlled by pH and the shell is protected against silver nanoparticle, lytic enzyme, and UV-C
Bottom: Shell degradation on-demand depending on pH
2014.11.18 View 11053
Eggshell-like Cell Encapsulation and Degradation Technology Developed
Some bacteria form endospores on cell walls to protect their DNA in case of nutrient deficiency. When an endospore meets a suitable environment for survival, the cell can revert to the original state from which it can reproduce.
The technique that can artificially control such phenomenon was developed by an international team of researchers. At first, a cell is wrapped and preserved like an egg. When the cell is needed, the technique allows the endospore to decompose while it is alive. Future applications for this technique include cell-based biosensor, cell therapy, and biocatalyst processes.
Professors Insung Choi and Younghoon Lee from the Department of Chemistry at KAIST as well as and Professor Frank Caruso from the University of Melbourne developed this technique which permits a cell to stay alive by coating it with film on a nanometer scale and then to be decomposed while it is alive.
The research finding was published in the November 10th issue of Angewandte Chemie International Edition as the lead article.
Cell encapsulation allows researchers to capture a cell in a tight capsule while it is alive. It is highly recognized in cell-based applications where the control of cell stability and cell-division is the biggest issue.
Traditional cell encapsulation methods utilized organic film or inorganic capsules that are made of organic film moldings. Although these films tightly closed around the cell, because they were not easily decomposable, it was difficult to apply the method.
The research team succeeded in encapsulating each cell in a metal-polyphenol film by mixing tannic acid and iron ion solution with yeast cells.
Usually extracted from oak barks or grape peels, tannic acid is a natural substance. It forms a metal-polyphenol film within ten seconds when it meets iron ions due to its high affinity with cells. Cells encapsulated with this film presented high survival rates. Since the film forms quickly in a simple manner, it was possible to obtain large amount of encapsulated cells.
The research team also found that the metal-polyphenol film was stable in neutral pH, but is easily degradable under a weak acidic condition. Using this property, they were able to control cell division by restoring the cell to its pre-encapsulated state at a desired moment.
Protecting the cell from the external environment like an egg shell, the metal-polyphenol film protected the cell against foreign conditions such as lytic enzymes, extended exposure to UV radiation, and silver nanoparticles. The research indicated that the encapsulated cells had a high survival rate even under extreme environments.
Professor Lee said that “not only the cells remain alive during the encapsulation stage, but also they can be protected under extreme environment.” He added, “This is an advanced cell encapsulation technology that allows controlling cell-division of those cells through responsive shell degradation on-demand.”
Professor Choi commented, “Although the cell encapsulation technology is still in its infancy, as the technology matures the application of cell-manipulation technology will be actualized.” He highlighted that “it will serve as a breakthrough to problems faced by cell-based applications.”
Sponsored by the Ministry of Science, ICT and Future Planning and the National Research Foundation of Korea, the research was led by two Master’s candidates, Ji Hun Park and Kyung Hwan Kim, under the joint guidance of research professors from KAIST and the University of Melbourne.
Figure 1: Lead article of Angewandte Chemie
Background: Shows a live native yeast (in green) encapsulated in a metal-polyphenol film (in red) illustrating the vitality of the yeast
Front: A native yeast at each encapsulation stage
Pictured on the bottom left is a cell prior to encapsulation. Following the red arrow, the native yeast is in purple to show metal-polyphenol film formed around the cell. The cell after the green arrow is a visualization of the degradation of the film in weak acidic condition.
Figure 2: A mimetic diagram of cell encapsulation with a metal-polyphenol film
Top: A native yeast before encapsulation
Middle: A native yeast encapsulated with Tannic Acid-Fe (III) Nanoshell – cell-division of the encapsulated cell is controlled by pH and the shell is protected against silver nanoparticle, lytic enzyme, and UV-C
Bottom: Shell degradation on-demand depending on pH
2014.11.18 View 11053