Nature
-
 Solutal Marangoni Flows of Miscible Liquid Drive Transport without Surface Contamination
(Professor Hyoungsoo Kim, Department of Mechanical Engineering, KAIST)
A research team led by Hyoungsoo Kim, a professor of Mechanical Engineering at KAIST, succeeded in quantifying the phenomenon called, the Marangoni effect, which occurs at the interface between alcohol and water. It is expected that this finding will be a valuable resource used for effectively removing impurities from a surface fluid without any contamination, and developing materials that can replace surfactants.
This research, co-conducted with a research team led by Professor Howard A. Stone at Princeton University, was published online in Nature Physics on July 31.
The Marangoni effect, also known as tears of wine, is generated when two fluids having a different surface tension meet, causing finite mixing, spreading time and length scale. Typically, people believe that infinitely miscible liquids immediately mix together; however, it is not always true according to this paper.
The typical surface tension of alcohol is three times lower than that of water, and this different surface tension generates the Marangoni-driven convection flow at the interface of the two liquids. In addition, there is a certain amount of time required for them to mix.
This phenomenon has been discussed many times since it was discovered in early the 20th century, yet there was a limit to quantifying and explaining it.
Professor Kim, considering the mixing and spreading mechanism, used various flow visualization techniques and equipment for capturing high speed images in his experiment.
Through the flow visualization methods, the team succeeded in quantifying and explaining the complex, physicochemical phenomenon generated between water and alcohol. Moreover, they developed a theoretical model to predict the physicochemical hydrodynamic phenomena.
The theoretical model can predict the speed of Marangoni-driven convection flow, the area of a drop of alcohol and the time required to develop the flow field. Hence, this model can map out types of materials (e.g., alcohol) and the volume of a drop of liquid as applicable to target a specific situation.
Moreover, the research team believes that the interfacial flow enables the driving of bulk flows and that it can be a source of technology for effectively delivering drugs and removing impurities from a surface of substance without causing secondary contamination.
Above all, the results show a possibility for replacing surfactant with alcohol as a material used for delivering drugs. In the case of the drug delivery, some drugs are encapsulated with a surfactant in order to be effectively transported in vivo; however, the surfactant accumulates in the body, which can cause various side effects, such as heart disease. Therefore, using new materials like alcohol for drug delivery will contribute to preventing the side effects caused by the surfactant.
“The surfactant is used for delivering drugs, but it is difficult to be expelled from the body. This will cause various side effects, such as heart diseases in asthmatic patients,” said Professor Kim. “I hope that using new materials, like alcohol, will free people from these side effects.”
(Marangoni-driven convection flow generated at the interface between water and alcohol, and the flow visualization results)
- A drop of alcohol on a water surface
- Comparison of mixing structures on the surface
- Marangoni mixing flow under the free surface
2017.08.18 View 9623
Solutal Marangoni Flows of Miscible Liquid Drive Transport without Surface Contamination
(Professor Hyoungsoo Kim, Department of Mechanical Engineering, KAIST)
A research team led by Hyoungsoo Kim, a professor of Mechanical Engineering at KAIST, succeeded in quantifying the phenomenon called, the Marangoni effect, which occurs at the interface between alcohol and water. It is expected that this finding will be a valuable resource used for effectively removing impurities from a surface fluid without any contamination, and developing materials that can replace surfactants.
This research, co-conducted with a research team led by Professor Howard A. Stone at Princeton University, was published online in Nature Physics on July 31.
The Marangoni effect, also known as tears of wine, is generated when two fluids having a different surface tension meet, causing finite mixing, spreading time and length scale. Typically, people believe that infinitely miscible liquids immediately mix together; however, it is not always true according to this paper.
The typical surface tension of alcohol is three times lower than that of water, and this different surface tension generates the Marangoni-driven convection flow at the interface of the two liquids. In addition, there is a certain amount of time required for them to mix.
This phenomenon has been discussed many times since it was discovered in early the 20th century, yet there was a limit to quantifying and explaining it.
Professor Kim, considering the mixing and spreading mechanism, used various flow visualization techniques and equipment for capturing high speed images in his experiment.
Through the flow visualization methods, the team succeeded in quantifying and explaining the complex, physicochemical phenomenon generated between water and alcohol. Moreover, they developed a theoretical model to predict the physicochemical hydrodynamic phenomena.
The theoretical model can predict the speed of Marangoni-driven convection flow, the area of a drop of alcohol and the time required to develop the flow field. Hence, this model can map out types of materials (e.g., alcohol) and the volume of a drop of liquid as applicable to target a specific situation.
Moreover, the research team believes that the interfacial flow enables the driving of bulk flows and that it can be a source of technology for effectively delivering drugs and removing impurities from a surface of substance without causing secondary contamination.
Above all, the results show a possibility for replacing surfactant with alcohol as a material used for delivering drugs. In the case of the drug delivery, some drugs are encapsulated with a surfactant in order to be effectively transported in vivo; however, the surfactant accumulates in the body, which can cause various side effects, such as heart disease. Therefore, using new materials like alcohol for drug delivery will contribute to preventing the side effects caused by the surfactant.
“The surfactant is used for delivering drugs, but it is difficult to be expelled from the body. This will cause various side effects, such as heart diseases in asthmatic patients,” said Professor Kim. “I hope that using new materials, like alcohol, will free people from these side effects.”
(Marangoni-driven convection flow generated at the interface between water and alcohol, and the flow visualization results)
- A drop of alcohol on a water surface
- Comparison of mixing structures on the surface
- Marangoni mixing flow under the free surface
2017.08.18 View 9623 -
 Material-Independent Nanocoating Antimicrobial Spray Significantly Extends the Shelf Life of Produce
The edible coating on produce has drawn a great deal of attention in the food and agricultural industry. It could not only prolong postharvest shelf life of produce against external changes in the environment but also provide additional nutrients to be useful for human health. However, most versions of the coating have had intrinsic limitations in their practical application. First, highly specific interactions between coating materials and target surfaces are required for a stable and durable coating. Even further, the coating of bulk substrates, such as fruits, is time consuming or is not achievable in the conventional solution-based coating. In this respect, material-independent and rapid coating strategies are highly demanded.
The research team led by Professor Insung Choi of the Department of Chemistry developed a sprayable nanocoating technique using plant-derived polyphenol that can be applied to any surface. This new nanocoating process can be completed in seconds to form nanometer-thick films, allowing for the coating of commodity goods, such as shoe insoles and fruits, in a controlled fashion. For example, spray-coated mandarin oranges and strawberries show significantly-prolonged postharvest shelf life, suggesting the practical potential in edible coatings of perishable produce.
The technology has been patented and is currently being commercialized for widespread use as a means of preserving produce. The research results have recently been published in Scientific Reports on Aug 1.
Polyphenols, a metabolite of photosynthesis, possess several hydroxyl groups and are found in a large number of plants showing excellent antioxidant properties. They have been widely used as a nontoxic food additive and are known to exhibit antibacterial, as well as potential anti-carcinogenic capabilities. Polyphenols can also be used with iron ions, which are naturally found in the body, to form an adhesive complex, which has been used in leather tanning, ink, etc.
The research team combined these chemical properties of polyphenol-iron complexes with spray techniques to develop their nanocoating technology. Compared to conventional immersion coating methods, which dip substrates in specialized coating solutions, this spray technique can coat the select areas more quickly. The spray also prevents cross contamination, which is a big concern for immersion methods. The research team has showcased the spray’s ability to coat a variety of different materials, including metals, plastics, glass, as well as textile fabrics. The polyphenol complex has been used to form antifogging films on corrective lenses, as well as antifungal treatments for shoe soles, demonstrating the versatility of their technique.
Furthermore, the spray has been used to coat produce with a naturally antibacterial, edible film. The coatings significantly improved the shelf life of tangerines and strawberries, preserving freshness beyond 28 days and 58 hours, respectively. (Uncoated fruit decomposed and became moldy under the same conditions). See the image below.
a –I, II: Uncoated and coated tangerines incubated for 14 and 28 days in daily-life settings
b –I: Uncoated and coated strawberries incubated for 58 hours in daily-life settings
b –II: Statistical investigation of the resulting edibility.
Professor Choi said, “Nanocoating technologies are still in their infancy, but they have untapped potential for exciting applications. As we have shown, nanocoatings can be easily adapted for several different uses, and the creative combination of existing nanomaterials and coating methods can synergize to unlock this potential.”
2017.08.10 View 10513
Material-Independent Nanocoating Antimicrobial Spray Significantly Extends the Shelf Life of Produce
The edible coating on produce has drawn a great deal of attention in the food and agricultural industry. It could not only prolong postharvest shelf life of produce against external changes in the environment but also provide additional nutrients to be useful for human health. However, most versions of the coating have had intrinsic limitations in their practical application. First, highly specific interactions between coating materials and target surfaces are required for a stable and durable coating. Even further, the coating of bulk substrates, such as fruits, is time consuming or is not achievable in the conventional solution-based coating. In this respect, material-independent and rapid coating strategies are highly demanded.
The research team led by Professor Insung Choi of the Department of Chemistry developed a sprayable nanocoating technique using plant-derived polyphenol that can be applied to any surface. This new nanocoating process can be completed in seconds to form nanometer-thick films, allowing for the coating of commodity goods, such as shoe insoles and fruits, in a controlled fashion. For example, spray-coated mandarin oranges and strawberries show significantly-prolonged postharvest shelf life, suggesting the practical potential in edible coatings of perishable produce.
The technology has been patented and is currently being commercialized for widespread use as a means of preserving produce. The research results have recently been published in Scientific Reports on Aug 1.
Polyphenols, a metabolite of photosynthesis, possess several hydroxyl groups and are found in a large number of plants showing excellent antioxidant properties. They have been widely used as a nontoxic food additive and are known to exhibit antibacterial, as well as potential anti-carcinogenic capabilities. Polyphenols can also be used with iron ions, which are naturally found in the body, to form an adhesive complex, which has been used in leather tanning, ink, etc.
The research team combined these chemical properties of polyphenol-iron complexes with spray techniques to develop their nanocoating technology. Compared to conventional immersion coating methods, which dip substrates in specialized coating solutions, this spray technique can coat the select areas more quickly. The spray also prevents cross contamination, which is a big concern for immersion methods. The research team has showcased the spray’s ability to coat a variety of different materials, including metals, plastics, glass, as well as textile fabrics. The polyphenol complex has been used to form antifogging films on corrective lenses, as well as antifungal treatments for shoe soles, demonstrating the versatility of their technique.
Furthermore, the spray has been used to coat produce with a naturally antibacterial, edible film. The coatings significantly improved the shelf life of tangerines and strawberries, preserving freshness beyond 28 days and 58 hours, respectively. (Uncoated fruit decomposed and became moldy under the same conditions). See the image below.
a –I, II: Uncoated and coated tangerines incubated for 14 and 28 days in daily-life settings
b –I: Uncoated and coated strawberries incubated for 58 hours in daily-life settings
b –II: Statistical investigation of the resulting edibility.
Professor Choi said, “Nanocoating technologies are still in their infancy, but they have untapped potential for exciting applications. As we have shown, nanocoatings can be easily adapted for several different uses, and the creative combination of existing nanomaterials and coating methods can synergize to unlock this potential.”
2017.08.10 View 10513 -
 Cooperative Tumor Cell Membrane-Targeted Phototherapy
A KAIST research team led by Professor Ji-Ho Park in the Bio and Brain Engineering Department at KAIST developed a technology for the effective treatment of cancer by delivering synthetic receptors throughout tumor tissue. The study, led by Ph.D. candidate Heegon Kim, was published online in Nature Communications on June 19.
Cancer targeted therapy generally refers to therapy targeting specific molecules that are involved in the growth and generation of cancer. The targeted delivery of therapeutics using targeting agents such as antibodies or nanomaterials has improved the precision and safety of cancer therapy.
However, the paucity and heterogeneity of identified molecular targets within tumors have resulted in poor and uneven distribution of targeted agents, thus compromising treatment outcomes.
To solve this problem, the team constructed a cooperative targeting system in which synthetic and biological nanocomponents participate together in the tumor cell membrane-selective localization of synthetic receptors to amplify the subsequent targeting of therapeutics. Here, synthetic and biological nanocomponents refer to liposomes and extracellular vesicles, respectively.
The synthetic receptors are first delivered selectively to tumor cell membranes in the perivascular region using liposomes. By hitchhiking with extracellular vesicles secreted by the cells, the synthetic receptors are transferred to neighboring cells and further spread throughout the tumor tissues where the molecular targets are limited.
Hitchhiking extracellular vesicles for delivery of synthetic receptors was possible since extracellular vesicles, such as exosomes, mediate intercellular communications by transferring various biological components such as lipids, cytosolic proteins, and RNA through a membrane fusion process. They also play a supportive role in promoting tumor progression in that tumor-derived extracellular vesicles deliver oncogenic signals to normal host cells.
The team showed that this tumor cell membrane-targeted delivery of synthetic receptors led to a uniform distribution of synthetic receptors throughout a tumor and subsequently led to enhanced phototherapeutic efficacy of the targeted photosensitizer.
Professor Park said, “The cooperative tumor targeting system is expected to be applied in treating various diseases that are hard to target.”
The research was funded by the Basic Science Research Program through the National Research Foundation funded by the Ministry of Science, ICT & Future Planning, and the National R&D Program for Cancer Control funded by the Ministry for Health and Welfare.
(Ph.D. candidates Hee Gon Kim (left) and Chanhee Oh)
Figure 1. A schematic of a cooperative tumor targeting system via delivery of synthetic receptors.
Figure 2. A confocal microscopic image of a tumor section after cooperative targeting by synthetic receptor delivery. Green and magenta represent vessels and therapeutic agents inside a tumor respectively.
2017.07.07 View 12866
Cooperative Tumor Cell Membrane-Targeted Phototherapy
A KAIST research team led by Professor Ji-Ho Park in the Bio and Brain Engineering Department at KAIST developed a technology for the effective treatment of cancer by delivering synthetic receptors throughout tumor tissue. The study, led by Ph.D. candidate Heegon Kim, was published online in Nature Communications on June 19.
Cancer targeted therapy generally refers to therapy targeting specific molecules that are involved in the growth and generation of cancer. The targeted delivery of therapeutics using targeting agents such as antibodies or nanomaterials has improved the precision and safety of cancer therapy.
However, the paucity and heterogeneity of identified molecular targets within tumors have resulted in poor and uneven distribution of targeted agents, thus compromising treatment outcomes.
To solve this problem, the team constructed a cooperative targeting system in which synthetic and biological nanocomponents participate together in the tumor cell membrane-selective localization of synthetic receptors to amplify the subsequent targeting of therapeutics. Here, synthetic and biological nanocomponents refer to liposomes and extracellular vesicles, respectively.
The synthetic receptors are first delivered selectively to tumor cell membranes in the perivascular region using liposomes. By hitchhiking with extracellular vesicles secreted by the cells, the synthetic receptors are transferred to neighboring cells and further spread throughout the tumor tissues where the molecular targets are limited.
Hitchhiking extracellular vesicles for delivery of synthetic receptors was possible since extracellular vesicles, such as exosomes, mediate intercellular communications by transferring various biological components such as lipids, cytosolic proteins, and RNA through a membrane fusion process. They also play a supportive role in promoting tumor progression in that tumor-derived extracellular vesicles deliver oncogenic signals to normal host cells.
The team showed that this tumor cell membrane-targeted delivery of synthetic receptors led to a uniform distribution of synthetic receptors throughout a tumor and subsequently led to enhanced phototherapeutic efficacy of the targeted photosensitizer.
Professor Park said, “The cooperative tumor targeting system is expected to be applied in treating various diseases that are hard to target.”
The research was funded by the Basic Science Research Program through the National Research Foundation funded by the Ministry of Science, ICT & Future Planning, and the National R&D Program for Cancer Control funded by the Ministry for Health and Welfare.
(Ph.D. candidates Hee Gon Kim (left) and Chanhee Oh)
Figure 1. A schematic of a cooperative tumor targeting system via delivery of synthetic receptors.
Figure 2. A confocal microscopic image of a tumor section after cooperative targeting by synthetic receptor delivery. Green and magenta represent vessels and therapeutic agents inside a tumor respectively.
2017.07.07 View 12866 -
 Bio-based p-Xylene Oxidation into Terephthalic Acid by Engineered E.coli
KAIST researchers have established an efficient biocatalytic system to produce terephthalic acid (TPA) from p-xylene (pX). It will allow this industrially important bulk chemical to be made available in a more environmentally-friendly manner.
The research team developed metabolically engineered Escherichia coli (E.coli) to biologically transform pX into TPA, a chemical necessary in the manufacturing of polyethylene terephthalate (PET). This biocatalysis system represents a greener and more efficient alternative to the traditional chemical methods for TPA production. This research, headed by Distinguished Professor Sang Yup Lee, was published in Nature Communications on May 31.
The research team utilized a metabolic engineering and synthetic biology approach to develop a recombinant microorganism that can oxidize pX into TPA using microbial fermentation. TPA is a globally important chemical commodity for manufacturing PET. It can be applied to manufacture plastic bottles, clothing fibers, films, and many other products. Currently, TPA is produced from pX oxidation through an industrially well-known chemical process (with a typical TPA yield of over 95 mol%), which shows, however, such drawbacks as intensive energy requirements at high temperatures and pressure, usage of heavy metal catalysts, and the unavoidable byproduct formation of 4-carboxybenzaldehyde.
The research team designed and constructed a synthetic metabolic pathway by incorporating the upper xylene degradation pathway of Pseudomonas putida F1 and the lower p-toluene sulfonate pathway of Comamonas testosteroni T-2, which successfully produced TPA from pX in small-scale cultures, with the formation of p-toluate (pTA) as the major byproduct. The team further optimized the pathway gene expression levels by using a synthetic biology toolkit, which gave the final engineered E. coli strain showing increased TPA production and the complete elimination of the byproduct.
Using this best-performing strain, the team designed an elegant two-phase (aqueous/organic) fermentation system for TPA production on a larger scale, where pX was supplied in the organic phase. Through a number of optimization steps, the team ultimately achieved production of 13.3 g TPA from 8.8 g pX, which represented an extraordinary yield of 97 mol%.
The team has developed a microbial biotechnology application which is reportedly the first successful example of the bio-based production of TPA from pX by the microbial fermentation of engineered E. coli. This bio-based TPA technology presents several advantages such as ambient reaction temperature and pressure, no use of heavy metals or other toxic chemicals, the removable of byproduct formation, and it is 100% environmentally compatible.
Professor Lee said, “We presented promising biotechnology for producing large amounts of the commodity chemical TPA, which is used for PET manufacturing, through metabolically engineered gut bacterium. Our research is meaningful in that it demonstrates the feasibility of the biotechnological production of bulk chemicals, and if reproducible when up-scaled, it will represent a breakthrough in hydrocarbon bioconversions.”
Ph.D. candidate Zi Wei Luo is the first author of this research (DOI:10.1038/ncomms15689).The research was supported by the Intelligent Synthetic Biology Center through the Global Frontier Project (2011-0031963) of the Ministry of Science, ICT & Future Planning through the National Research Foundation of Korea.
Figure: Biotransformation of pX into TPA by engineered E. coli.
This schematic diagram shows the overall conceptualization of how metabolically engineered E. coli produced TPA from pX. The engineered E. coli was developed through reconstituting a synthetic metabolic pathway for pX conversion to TPA and optimized for increased TPA yield and byproduct elimination. Two-phase partitioning fermentation system was developed for demonstrating the feasibility of large-scale production of TPA from pX using the engineered E. coli strains, where pX was supplied in the organic phase and TPA was produced in the aqueous phase.
2017.06.05 View 13486
Bio-based p-Xylene Oxidation into Terephthalic Acid by Engineered E.coli
KAIST researchers have established an efficient biocatalytic system to produce terephthalic acid (TPA) from p-xylene (pX). It will allow this industrially important bulk chemical to be made available in a more environmentally-friendly manner.
The research team developed metabolically engineered Escherichia coli (E.coli) to biologically transform pX into TPA, a chemical necessary in the manufacturing of polyethylene terephthalate (PET). This biocatalysis system represents a greener and more efficient alternative to the traditional chemical methods for TPA production. This research, headed by Distinguished Professor Sang Yup Lee, was published in Nature Communications on May 31.
The research team utilized a metabolic engineering and synthetic biology approach to develop a recombinant microorganism that can oxidize pX into TPA using microbial fermentation. TPA is a globally important chemical commodity for manufacturing PET. It can be applied to manufacture plastic bottles, clothing fibers, films, and many other products. Currently, TPA is produced from pX oxidation through an industrially well-known chemical process (with a typical TPA yield of over 95 mol%), which shows, however, such drawbacks as intensive energy requirements at high temperatures and pressure, usage of heavy metal catalysts, and the unavoidable byproduct formation of 4-carboxybenzaldehyde.
The research team designed and constructed a synthetic metabolic pathway by incorporating the upper xylene degradation pathway of Pseudomonas putida F1 and the lower p-toluene sulfonate pathway of Comamonas testosteroni T-2, which successfully produced TPA from pX in small-scale cultures, with the formation of p-toluate (pTA) as the major byproduct. The team further optimized the pathway gene expression levels by using a synthetic biology toolkit, which gave the final engineered E. coli strain showing increased TPA production and the complete elimination of the byproduct.
Using this best-performing strain, the team designed an elegant two-phase (aqueous/organic) fermentation system for TPA production on a larger scale, where pX was supplied in the organic phase. Through a number of optimization steps, the team ultimately achieved production of 13.3 g TPA from 8.8 g pX, which represented an extraordinary yield of 97 mol%.
The team has developed a microbial biotechnology application which is reportedly the first successful example of the bio-based production of TPA from pX by the microbial fermentation of engineered E. coli. This bio-based TPA technology presents several advantages such as ambient reaction temperature and pressure, no use of heavy metals or other toxic chemicals, the removable of byproduct formation, and it is 100% environmentally compatible.
Professor Lee said, “We presented promising biotechnology for producing large amounts of the commodity chemical TPA, which is used for PET manufacturing, through metabolically engineered gut bacterium. Our research is meaningful in that it demonstrates the feasibility of the biotechnological production of bulk chemicals, and if reproducible when up-scaled, it will represent a breakthrough in hydrocarbon bioconversions.”
Ph.D. candidate Zi Wei Luo is the first author of this research (DOI:10.1038/ncomms15689).The research was supported by the Intelligent Synthetic Biology Center through the Global Frontier Project (2011-0031963) of the Ministry of Science, ICT & Future Planning through the National Research Foundation of Korea.
Figure: Biotransformation of pX into TPA by engineered E. coli.
This schematic diagram shows the overall conceptualization of how metabolically engineered E. coli produced TPA from pX. The engineered E. coli was developed through reconstituting a synthetic metabolic pathway for pX conversion to TPA and optimized for increased TPA yield and byproduct elimination. Two-phase partitioning fermentation system was developed for demonstrating the feasibility of large-scale production of TPA from pX using the engineered E. coli strains, where pX was supplied in the organic phase and TPA was produced in the aqueous phase.
2017.06.05 View 13486 -
 Observation of the Phase Transition of Liquid Crystal Defects
KAIST researchers observed the phase transition of topological defects formed by liquid crystal (LC) materials for the first time.
The phase transition of topological defects, which was also the theme of the Nobel Prize for Physics in 2016, can be difficult to understand for a layperson but it needs to be studied to understand the mysteries of the universe or the underlying physics of skyrmions, which have intrinsic topological defects.
If the galaxy is taken as an example in the universe, it is difficult to observe the topological defects because the system is too large to observe some changes over a limited period of time. In the case of defect structures formed by LC molecules, they are not only a suitable size to observe with an optical microscope, but also the time period in which the phase transition of a defect occurring can be directly observed over a few seconds, which can be extended to a few minutes. The defect structures formed by LC material have radial, circular, or spiral shapes centering on a singularity (defect core), like the singularity that was already introduced in the famous movie "Interstellar,” which is the center point of black hole.
In general, LC materials are mainly used in liquid crystal displays (LCDs) and optical sensors because it is easy to control their specific orientation and they have fast response characteristics and huge anisotropic optical properties. It is advantageous in terms of the performance of LCDs that the defects of the LC materials are minimized. The research team led by Professor Dong Ki Yoon in the Graduate School of Nanoscience and Technology did not simply minimize such defects but actively tried to use the LC defects as building blocks to make micro- and nanostructures for the patterning applications. During these efforts, they found the way to directly study the phase transition of topological defects under in-situ conditions.
Considering the LC material from the viewpoint of a device like a LCD, robustness is important. Therefore, the LC material is injected through the capillary phenomenon between a rigid two-glass plate and the orientation of the LCs can be followed by the surface anchoring condition of the glass substrate. However, in this conventional case, it is difficult to observe the phase transition of the LC defect due to this strong surface anchoring force induced by the solid substrate.
In order to solve this problem, the research team designed a platform, in which the movement of the LC molecules was not restricted, by forming a thin film of LC material on water, which is like oil floating on water. For this, a droplet of LC material was dripped onto water and spread to form a thin film. The topological defects formed under this circumstance could show the thermal phase transition when the temperature was changed. In addition, this approach can trace back the morphology of the original defect structure from the sequential changes during the temperature changes, which can give hints to the study of the formation of topological defects in the cosmos or skyrmions.
Prof. Yoon said, “The study of LC crystal defects itself has been extensively studied by physicists and mathematicians for about 100 years. However, this is the first time that we have observed the phase transition of LC defects directly.” He also added, "Korea is leading in the LCD industry, but our basic research on LCs is not at the world's research level."
The first author of this study is Dr. Min-Jun Gimand supported by a grant from the National Research Foundation (NRF) and funded by the Korean Government (MSIP). The research result was published on May 30, 2017 in Nature Communications.
Figure 1. The phase transition of the LC topological defect on cooling.
Figure 2. Polarizing optical microscopy images of topological defects depending on the strength of the director field. (a,b,e) Convergent director field arrangements of LC molecules and corresponding schematic images; (c,d,f) Divergent director field arrangements of LC molecules and corresponding schematic images.
2017.06.02 View 11826
Observation of the Phase Transition of Liquid Crystal Defects
KAIST researchers observed the phase transition of topological defects formed by liquid crystal (LC) materials for the first time.
The phase transition of topological defects, which was also the theme of the Nobel Prize for Physics in 2016, can be difficult to understand for a layperson but it needs to be studied to understand the mysteries of the universe or the underlying physics of skyrmions, which have intrinsic topological defects.
If the galaxy is taken as an example in the universe, it is difficult to observe the topological defects because the system is too large to observe some changes over a limited period of time. In the case of defect structures formed by LC molecules, they are not only a suitable size to observe with an optical microscope, but also the time period in which the phase transition of a defect occurring can be directly observed over a few seconds, which can be extended to a few minutes. The defect structures formed by LC material have radial, circular, or spiral shapes centering on a singularity (defect core), like the singularity that was already introduced in the famous movie "Interstellar,” which is the center point of black hole.
In general, LC materials are mainly used in liquid crystal displays (LCDs) and optical sensors because it is easy to control their specific orientation and they have fast response characteristics and huge anisotropic optical properties. It is advantageous in terms of the performance of LCDs that the defects of the LC materials are minimized. The research team led by Professor Dong Ki Yoon in the Graduate School of Nanoscience and Technology did not simply minimize such defects but actively tried to use the LC defects as building blocks to make micro- and nanostructures for the patterning applications. During these efforts, they found the way to directly study the phase transition of topological defects under in-situ conditions.
Considering the LC material from the viewpoint of a device like a LCD, robustness is important. Therefore, the LC material is injected through the capillary phenomenon between a rigid two-glass plate and the orientation of the LCs can be followed by the surface anchoring condition of the glass substrate. However, in this conventional case, it is difficult to observe the phase transition of the LC defect due to this strong surface anchoring force induced by the solid substrate.
In order to solve this problem, the research team designed a platform, in which the movement of the LC molecules was not restricted, by forming a thin film of LC material on water, which is like oil floating on water. For this, a droplet of LC material was dripped onto water and spread to form a thin film. The topological defects formed under this circumstance could show the thermal phase transition when the temperature was changed. In addition, this approach can trace back the morphology of the original defect structure from the sequential changes during the temperature changes, which can give hints to the study of the formation of topological defects in the cosmos or skyrmions.
Prof. Yoon said, “The study of LC crystal defects itself has been extensively studied by physicists and mathematicians for about 100 years. However, this is the first time that we have observed the phase transition of LC defects directly.” He also added, "Korea is leading in the LCD industry, but our basic research on LCs is not at the world's research level."
The first author of this study is Dr. Min-Jun Gimand supported by a grant from the National Research Foundation (NRF) and funded by the Korean Government (MSIP). The research result was published on May 30, 2017 in Nature Communications.
Figure 1. The phase transition of the LC topological defect on cooling.
Figure 2. Polarizing optical microscopy images of topological defects depending on the strength of the director field. (a,b,e) Convergent director field arrangements of LC molecules and corresponding schematic images; (c,d,f) Divergent director field arrangements of LC molecules and corresponding schematic images.
2017.06.02 View 11826 -
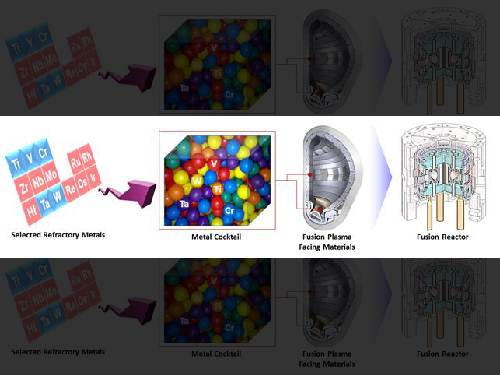 Extreme Materials for Fusion with Metal Cocktail
The research team under Professor Ryu Ho-jin of the Department of Nuclear and Quantum Engineering has developed a new material for facing fusion plasma environments using metal powder mixing technology. This technology is expected to extend the range of materials that can be designed for use in extreme environments such as in fusion power generators.
The durability of the tokamak vessel, which holds high-temperature plasma, is very important to create fusion power reactors, which are expected to be a future energy source. Currently, high-melting-point metals, such as tungsten, are considered plasma-facing materials to protect the tokamak vessel. However, high-energy thermal shocks, plasma ions, and neutrons are fatal to the plasma-facing material during high temperature fusion plasma operation. Therefore, it is necessary to develop new high-performance materials.
The ITER project, in which seven countries including the United States, the EU, and Korea participate jointly, is constructing a nuclear fusion experimental reactor in France with the goal of achieving the first plasma in 2025 and deuterium-tritium fusion operation in 2035. In Korea, the KSTAR tokamak at the National Fusion Research Institute has succeeded in maintaining high-performance plasma for 70 seconds.
Researchers in Europe, the United States, and China, who are leading the research on fusion plasma-facing materials, are studying the improvement of physical properties by adding a small amount of metal elements to tungsten. However, Professor Ryu’s team reported that by mixing various metals’ powders, including tungsten, they have succeeded in producing a new material that has twice the hardness and strength of tungsten. The difference in the atomic sizes of the well-mixed elements in the alloy is very significant because it makes it difficult to deform the alloy.
The team will continue its research to find alloying compositions that optimize mechanical properties as well as thermal conductivity, plasma interactions, neutron irradiation embrittlement, tritium absorption, and high-temperature oxidation properties.
Professor Ryu said, "Fusion plasma-facing materials are exposed to extreme environments and no metal is capable of withstanding thermal shock, plasma, and neutron damage simultaneously. As a result of this research, attempts to develop complex metallic materials for nuclear fusion and nuclear power are expected to become more active around the world. "
Ph.D. candidate Owais Ahmed Waseem is the first author of this project. The research is supported by the Ministry of Science, ICT and Future Planning, the Korea Research Foundation's Fusion Basic Research project, and the Engineering Research Center. The results were published in 'Scientific Report' on May 16.
Figure 1. Tungsten-based high strengh alloy sample
Figure 2. Fusion plasma facing material development by powder processing of refractory elements
2017.05.26 View 11105
Extreme Materials for Fusion with Metal Cocktail
The research team under Professor Ryu Ho-jin of the Department of Nuclear and Quantum Engineering has developed a new material for facing fusion plasma environments using metal powder mixing technology. This technology is expected to extend the range of materials that can be designed for use in extreme environments such as in fusion power generators.
The durability of the tokamak vessel, which holds high-temperature plasma, is very important to create fusion power reactors, which are expected to be a future energy source. Currently, high-melting-point metals, such as tungsten, are considered plasma-facing materials to protect the tokamak vessel. However, high-energy thermal shocks, plasma ions, and neutrons are fatal to the plasma-facing material during high temperature fusion plasma operation. Therefore, it is necessary to develop new high-performance materials.
The ITER project, in which seven countries including the United States, the EU, and Korea participate jointly, is constructing a nuclear fusion experimental reactor in France with the goal of achieving the first plasma in 2025 and deuterium-tritium fusion operation in 2035. In Korea, the KSTAR tokamak at the National Fusion Research Institute has succeeded in maintaining high-performance plasma for 70 seconds.
Researchers in Europe, the United States, and China, who are leading the research on fusion plasma-facing materials, are studying the improvement of physical properties by adding a small amount of metal elements to tungsten. However, Professor Ryu’s team reported that by mixing various metals’ powders, including tungsten, they have succeeded in producing a new material that has twice the hardness and strength of tungsten. The difference in the atomic sizes of the well-mixed elements in the alloy is very significant because it makes it difficult to deform the alloy.
The team will continue its research to find alloying compositions that optimize mechanical properties as well as thermal conductivity, plasma interactions, neutron irradiation embrittlement, tritium absorption, and high-temperature oxidation properties.
Professor Ryu said, "Fusion plasma-facing materials are exposed to extreme environments and no metal is capable of withstanding thermal shock, plasma, and neutron damage simultaneously. As a result of this research, attempts to develop complex metallic materials for nuclear fusion and nuclear power are expected to become more active around the world. "
Ph.D. candidate Owais Ahmed Waseem is the first author of this project. The research is supported by the Ministry of Science, ICT and Future Planning, the Korea Research Foundation's Fusion Basic Research project, and the Engineering Research Center. The results were published in 'Scientific Report' on May 16.
Figure 1. Tungsten-based high strengh alloy sample
Figure 2. Fusion plasma facing material development by powder processing of refractory elements
2017.05.26 View 11105 -
 Controlling 3D Behavior of Biological Cells Using Laser Holographic Techniques
A research team led by Professor YongKeun Park of the Physics Department at KAIST has developed an optical manipulation technique that can freely control the position, orientation, and shape of microscopic samples having complex shapes. The study has been published online in Nature Communications on May 22.
Conventional optical manipulation techniques called “optical tweezers,” have been used as an invaluable tool for exerting micro-scale force on microscopic particles and manipulating three-dimensional (3-D) positions of particles. Optical tweezers employ a tightly-focused laser whose beam diameter is smaller than one micrometer (1/100 of hair thickness), which can generate attractive force on neighboring microscopic particles moving toward the beam focus. Controlling the positions of the beam focus enabled researchers to hold the particles and move them freely to other locations so they coined the name “optical tweezers,” and have been widely used in various fields of physical and biological studies.
So far, most experiments using optical tweezers have been conducted for trapping spherical particles because physical principles can easily predict optical forces and the responding motion of microspheres. For trapping objects having complicated shapes, however, conventional optical tweezers induce unstable motion of such particles, and controllable orientation of such objects is limited, which hinder controlling the 3-D motion of microscopic objects having complex shapes such as living cells.
The research team has developed a new optical manipulation technique that can trap complex objects of arbitrary shapes. This technique first measures 3-D structures of an object in real time using a 3-D holographic microscope, which shares the same physical principle of X-Ray CT imaging. Based on the measured 3-D shape of the object, the researchers precisely calculates the shape of light that can stably control the object. When the shape of light is the same as the shape of the object, the energy of the object is minimized, which provides the stable trapping of the object having the complicated shape.
Moreover, by controlling the shape of light to have various positions, directions, and shapes of objects, it is possible to freely control the 3-D motion of the object and make the object have a desired shape. This process resembles the generation of a mold for casting a statue having desired shape so the researchers coined the name of the present technique “tomographic mold for optical trapping (TOMOTRAP).” The team succeeded in trapping individual human red blood cells stably, rotating them with desired orientations, folding them in an L-shape, and assembling two red blood cells together to form a new structure. In addition, colon cancer cells having a complex structure could be stably trapped and rotated at desired orientations. All of which have been difficult to be realized by the conventional optical techniques.
Professor Park said, “Our technique has the advantage of controlling the 3-D motion of complex shaped objects without knowing prior information about their shape and optical characteristics, and can be applied in various fields including physics, optics, nanotechnology, and medical science.”
Dr. Kyoohyun Kim, the lead author of this paper, noted that this technique can induce controlled deformation of biological cells with desired shapes. “This approach can be also applied to real-time monitoring of surgical prognosis of cellular-level surgeries for capturing and deforming cells as well as subcellular organelles,” added Kim.
Figure 1. Concept of optical manipulation techniques
Figure 2. Experimental setup
Figure 3. Research results
2017.05.25 View 9982
Controlling 3D Behavior of Biological Cells Using Laser Holographic Techniques
A research team led by Professor YongKeun Park of the Physics Department at KAIST has developed an optical manipulation technique that can freely control the position, orientation, and shape of microscopic samples having complex shapes. The study has been published online in Nature Communications on May 22.
Conventional optical manipulation techniques called “optical tweezers,” have been used as an invaluable tool for exerting micro-scale force on microscopic particles and manipulating three-dimensional (3-D) positions of particles. Optical tweezers employ a tightly-focused laser whose beam diameter is smaller than one micrometer (1/100 of hair thickness), which can generate attractive force on neighboring microscopic particles moving toward the beam focus. Controlling the positions of the beam focus enabled researchers to hold the particles and move them freely to other locations so they coined the name “optical tweezers,” and have been widely used in various fields of physical and biological studies.
So far, most experiments using optical tweezers have been conducted for trapping spherical particles because physical principles can easily predict optical forces and the responding motion of microspheres. For trapping objects having complicated shapes, however, conventional optical tweezers induce unstable motion of such particles, and controllable orientation of such objects is limited, which hinder controlling the 3-D motion of microscopic objects having complex shapes such as living cells.
The research team has developed a new optical manipulation technique that can trap complex objects of arbitrary shapes. This technique first measures 3-D structures of an object in real time using a 3-D holographic microscope, which shares the same physical principle of X-Ray CT imaging. Based on the measured 3-D shape of the object, the researchers precisely calculates the shape of light that can stably control the object. When the shape of light is the same as the shape of the object, the energy of the object is minimized, which provides the stable trapping of the object having the complicated shape.
Moreover, by controlling the shape of light to have various positions, directions, and shapes of objects, it is possible to freely control the 3-D motion of the object and make the object have a desired shape. This process resembles the generation of a mold for casting a statue having desired shape so the researchers coined the name of the present technique “tomographic mold for optical trapping (TOMOTRAP).” The team succeeded in trapping individual human red blood cells stably, rotating them with desired orientations, folding them in an L-shape, and assembling two red blood cells together to form a new structure. In addition, colon cancer cells having a complex structure could be stably trapped and rotated at desired orientations. All of which have been difficult to be realized by the conventional optical techniques.
Professor Park said, “Our technique has the advantage of controlling the 3-D motion of complex shaped objects without knowing prior information about their shape and optical characteristics, and can be applied in various fields including physics, optics, nanotechnology, and medical science.”
Dr. Kyoohyun Kim, the lead author of this paper, noted that this technique can induce controlled deformation of biological cells with desired shapes. “This approach can be also applied to real-time monitoring of surgical prognosis of cellular-level surgeries for capturing and deforming cells as well as subcellular organelles,” added Kim.
Figure 1. Concept of optical manipulation techniques
Figure 2. Experimental setup
Figure 3. Research results
2017.05.25 View 9982 -
 Processable High Internal Phase Pickering Emulsion Using Depletion Attraction
Professor Siyoung Choi’s research team from the KAIST Department of Chemical & Biomolecular Engineering used physical force to successfully produce a stable emulsion.
Emulsions, commonly known as cosmetic products, refer to stably dispersed structures of oil droplets in water (or water droplets in oil). Pickering emulsions refer to emulsions stabilized using solid particles, instead of detergent. Traditionally, it is said that water and oil do not mix. Until recently, detergent was added to mix oil and water for dispersion. Emulsions have traditionally been produced using this technique and are currently used for products such as mayonnaise, sun block, and lotion.
On the other hand, Pickering emulsions have been used after stabilization of chemical treatments on solid particle surfaces to enhance adsorption power. However, there were limitations in its application, since the treatment process is complex and its applicable range remains limited. Instead of chemical treatment on Pickering emulsion surfaces, the research team mixed small macromolecules a few nanometer in size with larger solid particles (tens of nanometers to a few micrometers). This induced depletion force was used to successfully stabilize the emulsion.
Depletion force refers to the force a large number of small particles induces to aggregate the bigger particles, in order to secure free space for themselves. In short, the force induces an attraction between larger particles. Until now, depletion force could only be applied to solids and solid particles. However, the research team used macromolecules and large particles such as solid particles and oil droplets to show the applicability of depletion force between solids and liquids. By introducing macromolecules that act as smaller particles, hydrophilic solid particles enhanced the adsorption of solid particles to the oil droplet surface, while preventing dissociation from the particle surface, resulting in the maintenance of a stable state.
The research team confirmed the possibility of the simple production of various porous macromolecular materials using stable Pickering emulsions. Such porous macromolecules are expected to be applicable in separation film, systems engineering, drug delivery, and sensors, given their large surface area.
Professor KyuHan Kim, the first author said, “Until now, depletion force has only been used between solid colloid particles. This research has scientific significance since it is the first example of using depletion force between solid particles and liquid droplets.”
Professor Choi said, “Beyond its academic significance, this technology could contribute to industries and national competitiveness.” He continued, “Since this technology uses physical force, not chemical, to produce stable emulsion, it can be used regardless of the type of solid particle and macromolecule. Further, it could be used in customized porous material production for special purposes.”
The research was published in Nature Communications online on February 1. In particular, this research is significant since an undergraduate student, Subeen Kim, participated in the project as a second author through the KAIST Undergraduate Research Program (URP). This research was funded by the National Research Foundation of Korea.
(Figure 1: Images of the inner structure of porous macromolecules produced using the new technology)
(Figure 2: Images showing the measurement of rheological properties of Pickering emulsions and system processability)
(Figure 3: Images showing a stable Pickering emulsion system)
2017.04.19 View 10410
Processable High Internal Phase Pickering Emulsion Using Depletion Attraction
Professor Siyoung Choi’s research team from the KAIST Department of Chemical & Biomolecular Engineering used physical force to successfully produce a stable emulsion.
Emulsions, commonly known as cosmetic products, refer to stably dispersed structures of oil droplets in water (or water droplets in oil). Pickering emulsions refer to emulsions stabilized using solid particles, instead of detergent. Traditionally, it is said that water and oil do not mix. Until recently, detergent was added to mix oil and water for dispersion. Emulsions have traditionally been produced using this technique and are currently used for products such as mayonnaise, sun block, and lotion.
On the other hand, Pickering emulsions have been used after stabilization of chemical treatments on solid particle surfaces to enhance adsorption power. However, there were limitations in its application, since the treatment process is complex and its applicable range remains limited. Instead of chemical treatment on Pickering emulsion surfaces, the research team mixed small macromolecules a few nanometer in size with larger solid particles (tens of nanometers to a few micrometers). This induced depletion force was used to successfully stabilize the emulsion.
Depletion force refers to the force a large number of small particles induces to aggregate the bigger particles, in order to secure free space for themselves. In short, the force induces an attraction between larger particles. Until now, depletion force could only be applied to solids and solid particles. However, the research team used macromolecules and large particles such as solid particles and oil droplets to show the applicability of depletion force between solids and liquids. By introducing macromolecules that act as smaller particles, hydrophilic solid particles enhanced the adsorption of solid particles to the oil droplet surface, while preventing dissociation from the particle surface, resulting in the maintenance of a stable state.
The research team confirmed the possibility of the simple production of various porous macromolecular materials using stable Pickering emulsions. Such porous macromolecules are expected to be applicable in separation film, systems engineering, drug delivery, and sensors, given their large surface area.
Professor KyuHan Kim, the first author said, “Until now, depletion force has only been used between solid colloid particles. This research has scientific significance since it is the first example of using depletion force between solid particles and liquid droplets.”
Professor Choi said, “Beyond its academic significance, this technology could contribute to industries and national competitiveness.” He continued, “Since this technology uses physical force, not chemical, to produce stable emulsion, it can be used regardless of the type of solid particle and macromolecule. Further, it could be used in customized porous material production for special purposes.”
The research was published in Nature Communications online on February 1. In particular, this research is significant since an undergraduate student, Subeen Kim, participated in the project as a second author through the KAIST Undergraduate Research Program (URP). This research was funded by the National Research Foundation of Korea.
(Figure 1: Images of the inner structure of porous macromolecules produced using the new technology)
(Figure 2: Images showing the measurement of rheological properties of Pickering emulsions and system processability)
(Figure 3: Images showing a stable Pickering emulsion system)
2017.04.19 View 10410 -
 Tactile Sensor for Robot Skin Advanced by KAIST Team
The joint research team of Professors Jung Kim and Inkyu Park from the Department of Mechanical Engineering developed a tactile sensor that can act as skin for robots using silicon and carbon materials. This technology produced a sensor that can absorb shock and distinguish various forms of touch, and it is hoped to be used as robot skin in the future.
Skin serves an important role as the largest organ of the human body. As well as protecting major organs from external shock, skin also measures and distinguishes delicate tactile information and transfer it to the nervous system.
Current robotic sensory technology allows robots to have visual and auditory systems at nearly similar levels to human capacity, but there are limitations in tactile sensors that can detect changes in the environment throughout the body. To apply skin with similar functions as humans to robots, it is essential to develop skin sensor technology with high flexibility and high shock absorption. Another limitation for developing robot skin was connecting numerous sensors all over the body using electric wiring.
To overcome this problem, the research team combined silicon and carbon nanotubes (CNT) to produce a composite, which was then used in combination with a medical imaging technique called electrical impedance tomography (EIT). This led to technology that can distinguish various forms of force over a large area without electrical wiring.
The sensing material can distinguish the location and the size of various forms by touch, and thus can be applied to robot skin that can absorb shock as well as serves as a 3D computer interface and tactile sensor. It can withstand strong force such as a hammer strike, and can be re-used even after partial damage to the sensor by filling and hardening the damaged region with composite. Further, the sensor can be made by filling a 3D shape frame with silicon-nanotube composite. Using this technology, new forms of computer interaces can be developed with both curbed and flat surfaces.
This research was conducted through a collaboration between Professor Park, an expert in nanostructures and sensors, and Professor Kim, an expert in bio-robotics. Hence, the technology is likely to be applied in real products.
Professor Kim said, “Flexible tactile sensors can not only be directly adhered to the body, but they also provides information on modified states in multiple dimensions”. He continued, “This technology will contribute to the soft robot industry in the areas of robot skin and the field of wearable medical appliances.”
Professor Park said, “This technology implemented a next-generation user interface through the integration of functional nano-composite material and computer tomography.”
This research was published in Scientific Reports, a sister journal of Nature, online on January 25. This research was conducted as joint research by first author Hyo-Sang Lee, as well as Donguk Kwon and Ji-seung Cho, and was funded by the Ministry of Science, ICT and Future Planning.
(Fiigrue 1: Robotic hand responding to resistance via a connection with the developed tactile sensor)
(Figure 2: Manufacturing process for pressure-resistant composite using silicon rubber and carbon nanotubes)
(Figure 3: Computer interface using pressure-resistant composite)
2017.04.17 View 14146
Tactile Sensor for Robot Skin Advanced by KAIST Team
The joint research team of Professors Jung Kim and Inkyu Park from the Department of Mechanical Engineering developed a tactile sensor that can act as skin for robots using silicon and carbon materials. This technology produced a sensor that can absorb shock and distinguish various forms of touch, and it is hoped to be used as robot skin in the future.
Skin serves an important role as the largest organ of the human body. As well as protecting major organs from external shock, skin also measures and distinguishes delicate tactile information and transfer it to the nervous system.
Current robotic sensory technology allows robots to have visual and auditory systems at nearly similar levels to human capacity, but there are limitations in tactile sensors that can detect changes in the environment throughout the body. To apply skin with similar functions as humans to robots, it is essential to develop skin sensor technology with high flexibility and high shock absorption. Another limitation for developing robot skin was connecting numerous sensors all over the body using electric wiring.
To overcome this problem, the research team combined silicon and carbon nanotubes (CNT) to produce a composite, which was then used in combination with a medical imaging technique called electrical impedance tomography (EIT). This led to technology that can distinguish various forms of force over a large area without electrical wiring.
The sensing material can distinguish the location and the size of various forms by touch, and thus can be applied to robot skin that can absorb shock as well as serves as a 3D computer interface and tactile sensor. It can withstand strong force such as a hammer strike, and can be re-used even after partial damage to the sensor by filling and hardening the damaged region with composite. Further, the sensor can be made by filling a 3D shape frame with silicon-nanotube composite. Using this technology, new forms of computer interaces can be developed with both curbed and flat surfaces.
This research was conducted through a collaboration between Professor Park, an expert in nanostructures and sensors, and Professor Kim, an expert in bio-robotics. Hence, the technology is likely to be applied in real products.
Professor Kim said, “Flexible tactile sensors can not only be directly adhered to the body, but they also provides information on modified states in multiple dimensions”. He continued, “This technology will contribute to the soft robot industry in the areas of robot skin and the field of wearable medical appliances.”
Professor Park said, “This technology implemented a next-generation user interface through the integration of functional nano-composite material and computer tomography.”
This research was published in Scientific Reports, a sister journal of Nature, online on January 25. This research was conducted as joint research by first author Hyo-Sang Lee, as well as Donguk Kwon and Ji-seung Cho, and was funded by the Ministry of Science, ICT and Future Planning.
(Fiigrue 1: Robotic hand responding to resistance via a connection with the developed tactile sensor)
(Figure 2: Manufacturing process for pressure-resistant composite using silicon rubber and carbon nanotubes)
(Figure 3: Computer interface using pressure-resistant composite)
2017.04.17 View 14146 -
 Expanding the Genetic Code of Mus Musculus
Professor Hee-Sung Park of the Department of Chemistry, who garnered attention for his novel strategy of installing authentic post-translational modifications into recombinant proteins, expanded his research portfolio to another level. Professor Park’s team was the first to report the generation of a mouse strain with an expanded genetic code, allowing site-specific incorporation of unnatural amino acids.
Professor Park published the research on the new chemical biology method for achieving selective chemical modifications in proteins in Science last September. The research team, this time in collaboration with Professor Chan Bae Park of the Department of Physiology at the Ajou University School of Medicine, demonstrated temporal and spatial control of protein acetylation in various organs of the transgenic mouse using a recombinant green fluorescent protein as a model protein. This research was published in the online edition of Nature Communications on February 21.
This approach enables the rapid onset of position-specific acetylation of a target protein at any developmental stage, facilitating temporal and spatial control of protein acetylation in various organs of the transgenic mouse. Such temporal and spatial control of protein acetylation will be of prime importance for investigating many essential biological processes and human diseases at the tissue and organism level.
Almost all human proteins, the products of about 25,000 genes, are known to undergo various post-translational modifications during and after synthesis. Post-translation modifications regulate the function of cellular proteins, playing a key role in many essential processes such as delivering signals and body growth. However, the unusual protein modifications, aroused from genetic and/or environmental factors, trigger severe diseases including cancer, dementia, and diabetes.
The team inserted transgenes into the mouse genome to allocate the site-specific addition of unnatural amino acids. The researchers inserted a modified version of lysine into the house mice, which allowed for the control of the acetylation. They used recombinant green fluorescent proteins from transgenic house mice as models for control of the acetylation.
The team was also able to regulate the acetylation of specific temporal and spatial frames in the mice, restraining the abnormality in proteins to certain organs such as the liver and kidneys. The research team said the strategy will provide a powerful tool for systematic in vivo study of cellular proteins in the most commonly used mammalian model organisms for human physiology and disease. Professor Park said, “This method can be easily extended to generate a wide range of custom-made transgenic mouse strains for further investigating diverse proteins of interest.” He added, “This method can be further extended to generate a wide range of custom-made transgenic mouse strains, opening a new paradigm for investigating anti-cancer and cerebral disease treatments.
This work was supported by grants from KAIST Systems Healthcare and the Medicinal Bioconvergence Research Center and the Intelligent Synthetic Biology Center of the Global Frontier Project funded by the Ministry of Science, ICT & Future Planning and the Ministry of Food and Drug Safety.
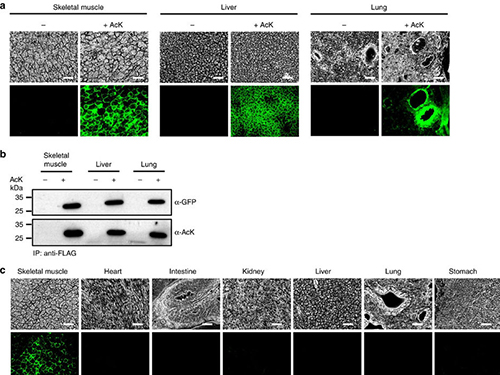
(Figure:Temporal and spatial control of in vivo protein acetylation)
(a) Temporal expression of acetylated GFPuv in the AcK-GFPamber mouse. The expression of GFPuv in skeletal muscle, liver, and lung tissues was detected only in the AcK-injected mouse. Scale bar, 200 µm. (b) Western blotting of anti-FLAG-immunoprecipitated proteins from tissues of the AcK-GFPamber mouse. Acetylated GFPuv was produced after AcK injection. (c) Spatial expression of acetylated GFPuv in the AcK-GFPamber mouse. Acetylated GFPuv was observed only in skeletal muscle when AcK was directly delivered to the tissues. Sacle bar, 200 µm.
2017.03.27 View 11197
Expanding the Genetic Code of Mus Musculus
Professor Hee-Sung Park of the Department of Chemistry, who garnered attention for his novel strategy of installing authentic post-translational modifications into recombinant proteins, expanded his research portfolio to another level. Professor Park’s team was the first to report the generation of a mouse strain with an expanded genetic code, allowing site-specific incorporation of unnatural amino acids.
Professor Park published the research on the new chemical biology method for achieving selective chemical modifications in proteins in Science last September. The research team, this time in collaboration with Professor Chan Bae Park of the Department of Physiology at the Ajou University School of Medicine, demonstrated temporal and spatial control of protein acetylation in various organs of the transgenic mouse using a recombinant green fluorescent protein as a model protein. This research was published in the online edition of Nature Communications on February 21.
This approach enables the rapid onset of position-specific acetylation of a target protein at any developmental stage, facilitating temporal and spatial control of protein acetylation in various organs of the transgenic mouse. Such temporal and spatial control of protein acetylation will be of prime importance for investigating many essential biological processes and human diseases at the tissue and organism level.
Almost all human proteins, the products of about 25,000 genes, are known to undergo various post-translational modifications during and after synthesis. Post-translation modifications regulate the function of cellular proteins, playing a key role in many essential processes such as delivering signals and body growth. However, the unusual protein modifications, aroused from genetic and/or environmental factors, trigger severe diseases including cancer, dementia, and diabetes.
The team inserted transgenes into the mouse genome to allocate the site-specific addition of unnatural amino acids. The researchers inserted a modified version of lysine into the house mice, which allowed for the control of the acetylation. They used recombinant green fluorescent proteins from transgenic house mice as models for control of the acetylation.
The team was also able to regulate the acetylation of specific temporal and spatial frames in the mice, restraining the abnormality in proteins to certain organs such as the liver and kidneys. The research team said the strategy will provide a powerful tool for systematic in vivo study of cellular proteins in the most commonly used mammalian model organisms for human physiology and disease. Professor Park said, “This method can be easily extended to generate a wide range of custom-made transgenic mouse strains for further investigating diverse proteins of interest.” He added, “This method can be further extended to generate a wide range of custom-made transgenic mouse strains, opening a new paradigm for investigating anti-cancer and cerebral disease treatments.
This work was supported by grants from KAIST Systems Healthcare and the Medicinal Bioconvergence Research Center and the Intelligent Synthetic Biology Center of the Global Frontier Project funded by the Ministry of Science, ICT & Future Planning and the Ministry of Food and Drug Safety.
(Figure:Temporal and spatial control of in vivo protein acetylation)
(a) Temporal expression of acetylated GFPuv in the AcK-GFPamber mouse. The expression of GFPuv in skeletal muscle, liver, and lung tissues was detected only in the AcK-injected mouse. Scale bar, 200 µm. (b) Western blotting of anti-FLAG-immunoprecipitated proteins from tissues of the AcK-GFPamber mouse. Acetylated GFPuv was produced after AcK injection. (c) Spatial expression of acetylated GFPuv in the AcK-GFPamber mouse. Acetylated GFPuv was observed only in skeletal muscle when AcK was directly delivered to the tissues. Sacle bar, 200 µm.
2017.03.27 View 11197 -
 A New Approach to 3D Holographic Displays Greatly Improves the Image Quality
With the addition of holographic diffusers or frosted glasses to wavefront modulators, KAIST researchers offer a simple and practical solution to significantly enhance the performance of 3D dynamic holographic displays by 2,600 times.
The potential applications of three-dimensional (3D) digital holograms are enormous. In addition to arts and entertainment, various fields including biomedical imaging, scientific visualization, engineering design, and displays could benefit from this technology. For example, creating full-sized organs for 3D analysis by doctors could be helpful, but it remained a challenge owing to the limitation of hologram-generation techniques.
A research team led by Professor YongKeun Park of the Physics Department at KAIST has come up with a solution and developed a 3D holographic display that performs more than 2,600 times better than existing 3D holographic displays. This study is expected to improve the limited size and viewing angle of 3D images, which were a major problem of the current holographic displays. The study was published online in Nature Photonics on January 23, 2017.
3D holograms, which often appear in science fiction films, are a familiar technology to the public, but holograms in movies are created with computer graphic effects. Methods for creating true 3D holograms are still being studied in the laboratory. For example, due to the difficulty of generating real 3D images, recent virtual reality (VR) and augmented reality (AR) devices project two different two-dimensional (2D) images onto a viewer to induce optical illusions.
To create a 3D hologram that can be viewed without special equipment such as 3D glasses, the wavefront of light must be controlled using wavefront modulators such as spatial light modulators (SLMs) and deformable mirrors (DMs). A wavefront modulator is an optical manipulation device that can control the direction of light propagation.
However, the biggest limitation to using these modulators as 3D displays is the number of pixels. The large number of pixels that are packed into high-resolution displays developed in recent years are suitable for a 2D image, and the amount of information contained in those pixels cannot produce a 3D image. For this reason, a 3D image that can be made with existing wavefront modulator technology is 1 cm in size with a narrow viewing angle of 3 degrees, which is far from practicable.
As an alternative, KAIST researchers used a DM and added two successive holographic diffusers to scatter light. By scattering light in many directions, this allows for a wider viewing angle and larger image, but results in volume speckle fields, which are caused by the interference of multiple scattered light. Random volume speckle fields cannot be used to display 3D images.
To fix the problem, the researchers employed a wavefront-shaping technique to control the fields. As a result, they succeeded in producing an enhanced 3D holographic image with a viewing angle of 35 degrees in a volume of 2 cm in length, width, and height. This yielded a performance that was about 2,600 times stronger than the original image definition generated when they used a DM without a diffuser.
Professor Park said, “Scattering light has previously been believed to interfere with the recognition of objects, but we have demonstrated that current 3D displays can be improved significantly with an increased viewing angle and image size by properly controlling the scattered light.”
Hyeonseung Yu, who is the lead author of this research article and a doctoral candidate in the Department of Physics, KAIST, noted that this technology signals a good start to develop a practical model for dynamic 3D hologram displays that can be enjoyed without the need for special eyeglasses. “This approach can also be applied to AR and VR technology to enhance the image resolution and viewing angles,” added Yu.
The research paper is entitled “Ultrahigh-definition Dynamic 3D Holographic Display by Active Control of Volume Speckle Fields.”
Figure 1. Concept of Scattering Display
The size and viewing angle of 3D images can be simultaneously increased when a scattering medium (diffuser) is introduced. By controlling the wavefront impinging on the scattering medium, the desired 3D hologram is generated.
Figure 2. Experimental Setup
The optical set-up consists of a deformable mirror and the scattering medium with two successive holographic diffusers. A high-numerical-aperture imaging unit mounted on a three-axis motorized translational system is utilized for wavefront optimization and imaging.
Figure 3. 3D Images Projected
This picture shows 3D images in a volume of 2 cm × 2 cm × 2 cm with a viewing angle of 35 degrees using one of the wavefront modulators, a digital micromirror device (DMD).
Figure 4. Artist’s Rendition of the Proposed Concept
A dynamic 3D hologram of a face is displayed.
2017.02.01 View 14839
A New Approach to 3D Holographic Displays Greatly Improves the Image Quality
With the addition of holographic diffusers or frosted glasses to wavefront modulators, KAIST researchers offer a simple and practical solution to significantly enhance the performance of 3D dynamic holographic displays by 2,600 times.
The potential applications of three-dimensional (3D) digital holograms are enormous. In addition to arts and entertainment, various fields including biomedical imaging, scientific visualization, engineering design, and displays could benefit from this technology. For example, creating full-sized organs for 3D analysis by doctors could be helpful, but it remained a challenge owing to the limitation of hologram-generation techniques.
A research team led by Professor YongKeun Park of the Physics Department at KAIST has come up with a solution and developed a 3D holographic display that performs more than 2,600 times better than existing 3D holographic displays. This study is expected to improve the limited size and viewing angle of 3D images, which were a major problem of the current holographic displays. The study was published online in Nature Photonics on January 23, 2017.
3D holograms, which often appear in science fiction films, are a familiar technology to the public, but holograms in movies are created with computer graphic effects. Methods for creating true 3D holograms are still being studied in the laboratory. For example, due to the difficulty of generating real 3D images, recent virtual reality (VR) and augmented reality (AR) devices project two different two-dimensional (2D) images onto a viewer to induce optical illusions.
To create a 3D hologram that can be viewed without special equipment such as 3D glasses, the wavefront of light must be controlled using wavefront modulators such as spatial light modulators (SLMs) and deformable mirrors (DMs). A wavefront modulator is an optical manipulation device that can control the direction of light propagation.
However, the biggest limitation to using these modulators as 3D displays is the number of pixels. The large number of pixels that are packed into high-resolution displays developed in recent years are suitable for a 2D image, and the amount of information contained in those pixels cannot produce a 3D image. For this reason, a 3D image that can be made with existing wavefront modulator technology is 1 cm in size with a narrow viewing angle of 3 degrees, which is far from practicable.
As an alternative, KAIST researchers used a DM and added two successive holographic diffusers to scatter light. By scattering light in many directions, this allows for a wider viewing angle and larger image, but results in volume speckle fields, which are caused by the interference of multiple scattered light. Random volume speckle fields cannot be used to display 3D images.
To fix the problem, the researchers employed a wavefront-shaping technique to control the fields. As a result, they succeeded in producing an enhanced 3D holographic image with a viewing angle of 35 degrees in a volume of 2 cm in length, width, and height. This yielded a performance that was about 2,600 times stronger than the original image definition generated when they used a DM without a diffuser.
Professor Park said, “Scattering light has previously been believed to interfere with the recognition of objects, but we have demonstrated that current 3D displays can be improved significantly with an increased viewing angle and image size by properly controlling the scattered light.”
Hyeonseung Yu, who is the lead author of this research article and a doctoral candidate in the Department of Physics, KAIST, noted that this technology signals a good start to develop a practical model for dynamic 3D hologram displays that can be enjoyed without the need for special eyeglasses. “This approach can also be applied to AR and VR technology to enhance the image resolution and viewing angles,” added Yu.
The research paper is entitled “Ultrahigh-definition Dynamic 3D Holographic Display by Active Control of Volume Speckle Fields.”
Figure 1. Concept of Scattering Display
The size and viewing angle of 3D images can be simultaneously increased when a scattering medium (diffuser) is introduced. By controlling the wavefront impinging on the scattering medium, the desired 3D hologram is generated.
Figure 2. Experimental Setup
The optical set-up consists of a deformable mirror and the scattering medium with two successive holographic diffusers. A high-numerical-aperture imaging unit mounted on a three-axis motorized translational system is utilized for wavefront optimization and imaging.
Figure 3. 3D Images Projected
This picture shows 3D images in a volume of 2 cm × 2 cm × 2 cm with a viewing angle of 35 degrees using one of the wavefront modulators, a digital micromirror device (DMD).
Figure 4. Artist’s Rendition of the Proposed Concept
A dynamic 3D hologram of a face is displayed.
2017.02.01 View 14839 -
 Adsorbent That Can Selectively Remove Water Contaminants
Professor Cafer T. Yavuz and his team at the Graduate School of Energy, Environment, Water, and Sustainability (EEWS) have developed an adsorbent that can selectively capture soluble organic contaminants in water.
This water treatment adsorbent is a fluorine-based nanoporous polymer that can selectively remove water-soluble micromolecules. It has the added advantage of being cheap and easily synthesized, while also being renewable.
The results of this research have been published online in Nature Communication on November 10, 2016. The research paper is titled “Charge-specific Size-dependent Separation of Water-soluble Organic Molecules by Fluorinated Nanoporous Networks.” (DOI: 10.1038/ncomms13377)
Water pollution is accelerating as a result of global industrial development and warming. As new materials are produced and applied in the agricultural and industrial sectors, the types of contaminants expelled as sewage and waste water are also becoming diverse.
Chemicals such as dyes and pesticides can be especially harmful because they are made up of small and highly soluble organic particles that cannot be completely removed during the water treatment process, ultimately ending up in our drinking water.
The current conventional water treatment systems utilize processes such as activated carbon, ozonolysis, and reverse osmosis membrane. These processes, however, are designed to remove larger organic molecules with lower solubility, thus removal of very small molecules with high solubility is difficult. In addition, these micromolecules tend to be charged, therefore are less easily separated in aqueous form.
The research team aimed to remove these small molecules using a new adsorbent technology.
In order to remove aqueous organic molecular contaminants, the team needed an adsorbent that can adsorb micro-sized molecules. It also needed to introduce a chemical function that would allow it to selectively adsorb molecules, and lastly, the adsorbent needed to be structurally stable as it would be used underwater.
The team subsequently developed an adsorbent of fluorine-based porous organic polymer that met all the conditions listed above. By controlling the size of the pores, this adsorbent is able to selectively adsorb aqueous micromolecules of less than 1-2 nm in size.
In addition, in order to separate specific contaminants, there should be a chemical functionality, such as the ability to strongly interact with the target material. Fluorine, the most electronegative atom, interacts strongly with charged soluble organic molecules.
The research team incorporated fluorine into an adsorbent, enabling it to separate charged organic molecules up to 8 times faster than neutral molecules.
The adsorbent developed by Professor Yavuz’s team has wide industrial applications. It can be used in batch-adsorption tests, as well as in column separation for size- and charge-specific adsorption.
Professor Yavuz stated that “the charge-selective properties displayed by fluorine has the potential to be applied in desalination or water treatment processes using membranes."
This paper was first-authored by Dr. Jeehye Byun, and the research was funded by KAIST’s High Risk High Return Program and the Ministry of Science, ICT and Future Planning of Korea’s Mid-Career Researcher Program, as well as its Technology Development Program to Solve Climate Change.
Figure 1. Diagram conceptualizing the process of charge- and size-specific separation by the fluorine-based porous polymer adsorbent
Figure 2. Difference in absorbance before and after using a porous fluorine polymer column to separate organic molecules
Figure 3. Adsorption properties of a fluorine polymer according to the charge and size of organic molecules
2017.01.17 View 11641
Adsorbent That Can Selectively Remove Water Contaminants
Professor Cafer T. Yavuz and his team at the Graduate School of Energy, Environment, Water, and Sustainability (EEWS) have developed an adsorbent that can selectively capture soluble organic contaminants in water.
This water treatment adsorbent is a fluorine-based nanoporous polymer that can selectively remove water-soluble micromolecules. It has the added advantage of being cheap and easily synthesized, while also being renewable.
The results of this research have been published online in Nature Communication on November 10, 2016. The research paper is titled “Charge-specific Size-dependent Separation of Water-soluble Organic Molecules by Fluorinated Nanoporous Networks.” (DOI: 10.1038/ncomms13377)
Water pollution is accelerating as a result of global industrial development and warming. As new materials are produced and applied in the agricultural and industrial sectors, the types of contaminants expelled as sewage and waste water are also becoming diverse.
Chemicals such as dyes and pesticides can be especially harmful because they are made up of small and highly soluble organic particles that cannot be completely removed during the water treatment process, ultimately ending up in our drinking water.
The current conventional water treatment systems utilize processes such as activated carbon, ozonolysis, and reverse osmosis membrane. These processes, however, are designed to remove larger organic molecules with lower solubility, thus removal of very small molecules with high solubility is difficult. In addition, these micromolecules tend to be charged, therefore are less easily separated in aqueous form.
The research team aimed to remove these small molecules using a new adsorbent technology.
In order to remove aqueous organic molecular contaminants, the team needed an adsorbent that can adsorb micro-sized molecules. It also needed to introduce a chemical function that would allow it to selectively adsorb molecules, and lastly, the adsorbent needed to be structurally stable as it would be used underwater.
The team subsequently developed an adsorbent of fluorine-based porous organic polymer that met all the conditions listed above. By controlling the size of the pores, this adsorbent is able to selectively adsorb aqueous micromolecules of less than 1-2 nm in size.
In addition, in order to separate specific contaminants, there should be a chemical functionality, such as the ability to strongly interact with the target material. Fluorine, the most electronegative atom, interacts strongly with charged soluble organic molecules.
The research team incorporated fluorine into an adsorbent, enabling it to separate charged organic molecules up to 8 times faster than neutral molecules.
The adsorbent developed by Professor Yavuz’s team has wide industrial applications. It can be used in batch-adsorption tests, as well as in column separation for size- and charge-specific adsorption.
Professor Yavuz stated that “the charge-selective properties displayed by fluorine has the potential to be applied in desalination or water treatment processes using membranes."
This paper was first-authored by Dr. Jeehye Byun, and the research was funded by KAIST’s High Risk High Return Program and the Ministry of Science, ICT and Future Planning of Korea’s Mid-Career Researcher Program, as well as its Technology Development Program to Solve Climate Change.
Figure 1. Diagram conceptualizing the process of charge- and size-specific separation by the fluorine-based porous polymer adsorbent
Figure 2. Difference in absorbance before and after using a porous fluorine polymer column to separate organic molecules
Figure 3. Adsorption properties of a fluorine polymer according to the charge and size of organic molecules
2017.01.17 View 11641