ICA
-
 Success in Real-Time Observation of the Formation Process of Topological Solitons, a Core Technology for Next-Generation Information Transfer
< From left) Geonhyeong Park (Ph.D. Candidate), Yun-Seok Choi (Ph.D.), Professor Dong Ki Yoon, and Changjae Lee (Ph.D. Candidate) of the Department of Chemistry >
Professor Dong Ki Yoon's research team in the Department of Chemistry at KAIST announced on the 11th that they have succeeded in controlling the formation of topological solitons in a regular, large-area manner through the self-assembly of chiral liquid crystal materials and observing their formation process in real-time.
A soliton refers to a phenomenon where a specific wave persists without dissipating through interaction with its surroundings. In particular, even when a wave is transmitted over long distances, it retains its unique information until it reaches the desired destination. Therefore, in today's digital society, which is susceptible to hacking, solitons are highly anticipated to be the core of future communication due to their inherent high stability. Furthermore, topological solitons created using organic liquid crystal molecules are expected to be utilized as next-generation anti-counterfeiting devices and memory elements due to their unique spin directionality.
Professor Yoon's team specifically revealed the formation process of topological solitons in this study, which had not been observable in real-time under mild conditions such as room temperature until now. This was made possible by using self-assembling chiral liquid crystal materials in a confined space created by air pillars.
This research, in which Geonhyeong Park (Ph.D. Candidate, Department of Chemistry) and Dr. Ahram Suh participated as co-first authors, and Dr. Yun-Seok Choi and Changjae Lee (Ph.D. Candidate) from the same group also participated, was published online in the international journal 'Advanced Materials' on June 5th and is scheduled to be featured as the back cover of the July issue. (Paper title: "Fabrication of Arrays of Topological Solitons in Patterned Chiral Liquid Crystals for Real-Time Observation of Morphogenesis")
< Figure 1. Schematic diagram of the research>
< Figure 2. Real-time observation of topological soliton formation using liquid crystals>
In this study, Professor Yoon's team implemented topological soliton structures at approximately 30 degrees Celsius, similar to room temperature, using chiral (asymmetric) liquid crystal materials instead of the conventional liquid crystal molecules widely used as core materials in liquid crystal displays (LCDs). Generally, complex equipment is required to control the formation of topological solitons, and their formation time is very short, which has hindered research into their formation process until now.
To achieve regular formation and control of topological solitons formed by chiral liquid crystal molecules, Professor Yoon's team precisely controlled a combination of vertical alignment layers, which can orient molecules vertically, and air pillars. Specifically, they prepared concave patterns based on circular silicon material, several micrometers (one-millionth of a meter) in size, coated with a vertical alignment layer, and a glass substrate. By adjusting the gap to several micrometers and injecting chiral liquid crystal material, air pillars were spontaneously formed on the concave patterns. Subsequently, the liquid crystal molecules were vertically aligned on all substrates, inevitably causing regular distortions between the substrates, and between the substrate and the air pillars, thus developing a system where chiral molecular structures, i.e., topological solitons, could be formed.
The key to the formation and control of topological solitons lies in controlling the thermal phase transition to occur regularly as desired when cooling from the isotropic phase temperature (approximately 40 degrees Celsius) to the liquid crystal phase temperature (approximately 30 degrees Celsius), where the liquid crystal material near the air pillars is cooler than the liquid crystal material between the glass substrate and the silicon patterned parts. This is consistent with the everyday wisdom of eating steamed eggs from a 'Ttukbaegi' (earthen pot) by starting from the relatively cooler part exposed to the air (near the air pillars) rather than the hot pot part (silicon or glass substrate part).
Through real-time analysis, the research team elucidated that topological defects are formed by the naturally formed air pillars through controlled thermal phase transition, and topological solitons are formed only at the locations of these defects. This analysis technique has the potential for application in various fields, including the interpretation of topological soliton formation found in other physical phenomena such as skyrmion particles in electromagnetism.
< Figure 3. Snapshots during the formation process of regularly arranged topological solitons>
Professor Dong Ki Yoon stated, "General topological solitons are known to be highly stable, capable only of generation or annihilation. Through the results of this research, we can understand the formation process of solitons in more detail, and they can be used as spintronics application technology, considered a next-generation semiconductor device for storing and recording information."
This research was conducted in collaboration with Professor Ivan Smalyukh's laboratory at the University of Colorado, Department of Physics, and was supported by the Multiscale Chiral Structures Research Center and strategic projects of the National Research Foundation of Korea under the Ministry of Science and ICT.
2022.07.11 View 1592
Success in Real-Time Observation of the Formation Process of Topological Solitons, a Core Technology for Next-Generation Information Transfer
< From left) Geonhyeong Park (Ph.D. Candidate), Yun-Seok Choi (Ph.D.), Professor Dong Ki Yoon, and Changjae Lee (Ph.D. Candidate) of the Department of Chemistry >
Professor Dong Ki Yoon's research team in the Department of Chemistry at KAIST announced on the 11th that they have succeeded in controlling the formation of topological solitons in a regular, large-area manner through the self-assembly of chiral liquid crystal materials and observing their formation process in real-time.
A soliton refers to a phenomenon where a specific wave persists without dissipating through interaction with its surroundings. In particular, even when a wave is transmitted over long distances, it retains its unique information until it reaches the desired destination. Therefore, in today's digital society, which is susceptible to hacking, solitons are highly anticipated to be the core of future communication due to their inherent high stability. Furthermore, topological solitons created using organic liquid crystal molecules are expected to be utilized as next-generation anti-counterfeiting devices and memory elements due to their unique spin directionality.
Professor Yoon's team specifically revealed the formation process of topological solitons in this study, which had not been observable in real-time under mild conditions such as room temperature until now. This was made possible by using self-assembling chiral liquid crystal materials in a confined space created by air pillars.
This research, in which Geonhyeong Park (Ph.D. Candidate, Department of Chemistry) and Dr. Ahram Suh participated as co-first authors, and Dr. Yun-Seok Choi and Changjae Lee (Ph.D. Candidate) from the same group also participated, was published online in the international journal 'Advanced Materials' on June 5th and is scheduled to be featured as the back cover of the July issue. (Paper title: "Fabrication of Arrays of Topological Solitons in Patterned Chiral Liquid Crystals for Real-Time Observation of Morphogenesis")
< Figure 1. Schematic diagram of the research>
< Figure 2. Real-time observation of topological soliton formation using liquid crystals>
In this study, Professor Yoon's team implemented topological soliton structures at approximately 30 degrees Celsius, similar to room temperature, using chiral (asymmetric) liquid crystal materials instead of the conventional liquid crystal molecules widely used as core materials in liquid crystal displays (LCDs). Generally, complex equipment is required to control the formation of topological solitons, and their formation time is very short, which has hindered research into their formation process until now.
To achieve regular formation and control of topological solitons formed by chiral liquid crystal molecules, Professor Yoon's team precisely controlled a combination of vertical alignment layers, which can orient molecules vertically, and air pillars. Specifically, they prepared concave patterns based on circular silicon material, several micrometers (one-millionth of a meter) in size, coated with a vertical alignment layer, and a glass substrate. By adjusting the gap to several micrometers and injecting chiral liquid crystal material, air pillars were spontaneously formed on the concave patterns. Subsequently, the liquid crystal molecules were vertically aligned on all substrates, inevitably causing regular distortions between the substrates, and between the substrate and the air pillars, thus developing a system where chiral molecular structures, i.e., topological solitons, could be formed.
The key to the formation and control of topological solitons lies in controlling the thermal phase transition to occur regularly as desired when cooling from the isotropic phase temperature (approximately 40 degrees Celsius) to the liquid crystal phase temperature (approximately 30 degrees Celsius), where the liquid crystal material near the air pillars is cooler than the liquid crystal material between the glass substrate and the silicon patterned parts. This is consistent with the everyday wisdom of eating steamed eggs from a 'Ttukbaegi' (earthen pot) by starting from the relatively cooler part exposed to the air (near the air pillars) rather than the hot pot part (silicon or glass substrate part).
Through real-time analysis, the research team elucidated that topological defects are formed by the naturally formed air pillars through controlled thermal phase transition, and topological solitons are formed only at the locations of these defects. This analysis technique has the potential for application in various fields, including the interpretation of topological soliton formation found in other physical phenomena such as skyrmion particles in electromagnetism.
< Figure 3. Snapshots during the formation process of regularly arranged topological solitons>
Professor Dong Ki Yoon stated, "General topological solitons are known to be highly stable, capable only of generation or annihilation. Through the results of this research, we can understand the formation process of solitons in more detail, and they can be used as spintronics application technology, considered a next-generation semiconductor device for storing and recording information."
This research was conducted in collaboration with Professor Ivan Smalyukh's laboratory at the University of Colorado, Department of Physics, and was supported by the Multiscale Chiral Structures Research Center and strategic projects of the National Research Foundation of Korea under the Ministry of Science and ICT.
2022.07.11 View 1592 -
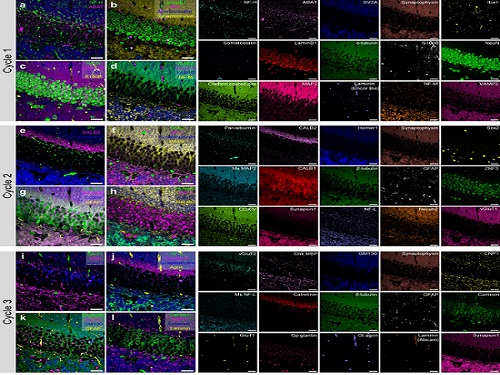 PICASSO Technique Drives Biological Molecules into Technicolor
The new imaging approach brings current imaging colors from four to more than 15 for mapping overlapping proteins
Pablo Picasso’s surreal cubist artistic style shifted common features into unrecognizable scenes, but a new imaging approach bearing his namesake may elucidate the most complicated subject: the brain. Employing artificial intelligence to clarify spectral color blending of tiny molecules used to stain specific proteins and other items of research interest, the PICASSO technique, allows researchers to use more than 15 colors to image and parse our overlapping proteins.
The PICASSO developers, based in Korea, published their approach on May 5 in Nature Communications.
Fluorophores — the staining molecules — emit specific colors when excited by a light, but if more than four fluorophores are used, their emitted colors overlap and blend. Researchers previously developed techniques to correct this spectral overlap by precisely defining the matrix of mixed and unmixed images. This measurement depends on reference spectra, found by identifying clear images of only one fluorophore-stained specimen or of multiple, identically prepared specimens that only contain a single fluorophore each.
“Such reference spectra measurement could be complicated to perform in highly heterogeneous specimens, such as the brain, due to the highly varied emission spectra of fluorophores depending on the subregions from which the spectra were measured,” said co-corresponding author Young-Gyu Yoon, professor in the School of Electrical Engineering at KAIST. He explained that the subregions would each need their own spectra reference measurements, making for an inefficient, time-consuming process. “To address this problem, we developed an approach that does not require reference spectra measurements.”
The approach is the “Process of ultra-multiplexed Imaging of biomolecules viA the unmixing of the Signals of Spectrally Overlapping fluorophores,” also known as PICASSO. Ultra-multiplexed imaging refers to visualizing the numerous individual components of a unit. Like a cinema multiplex in which each theater plays a different movie, each protein in a cell has a different role. By staining with fluorophores, researchers can begin to understand those roles.
“We devised a strategy based on information theory; unmixing is performed by iteratively minimizing the mutual information between mixed images,” said co-corresponding author Jae-Byum Chang, professor in the Department of Materials Science and Engineering, KAIST. “This allows us to get away with the assumption that the spatial distribution of different proteins is mutually exclusive and enables accurate information unmixing.”
To demonstrate PICASSO’s capabilities, the researchers applied the technique to imaging a mouse brain. With a single round of staining, they performed 15-color multiplexed imaging of a mouse brain. Although small, mouse brains are still complex, multifaceted organs that can take significant resources to map. According to the researchers, PICASSO can improve the capabilities of other imaging techniques and allow for the use of even more fluorophore colors.
Using one such imaging technique in combination with PICASSO, the team achieved 45-color multiplexed imaging of the mouse brain in only three staining and imaging cycles, according to Yoon.
“PICASSO is a versatile tool for the multiplexed biomolecule imaging of cultured cells, tissue slices and clinical specimens,” Chang said. “We anticipate that PICASSO will be useful for a broad range of applications for which biomolecules’ spatial information is important. One such application the tool would be useful for is revealing the cellular heterogeneities of tumor microenvironments, especially the heterogeneous populations of immune cells, which are closely related to cancer prognoses and the efficacy of cancer therapies.”
The Samsung Research Funding & Incubation Center for Future Technology supported this work. Spectral imaging was performed at the Korea Basic Science Institute Western Seoul Center.
-PublicationJunyoung Seo, Yeonbo Sim, Jeewon Kim, Hyunwoo Kim, In Cho, Hoyeon Nam, Yong-Gyu Yoon, Jae-Byum Chang, “PICASSO allows ultra-multiplexed fluorescence imaging of spatiallyoverlapping proteins without reference spectra measurements,” May 5, Nature Communications (doi.org/10.1038/s41467-022-30168-z)
-ProfileProfessor Jae-Byum ChangDepartment of Materials Science and EngineeringCollege of EngineeringKAIST
Professor Young-Gyu YoonSchool of Electrical EngineeringCollege of EngineeringKAIST
2022.06.22 View 12757
PICASSO Technique Drives Biological Molecules into Technicolor
The new imaging approach brings current imaging colors from four to more than 15 for mapping overlapping proteins
Pablo Picasso’s surreal cubist artistic style shifted common features into unrecognizable scenes, but a new imaging approach bearing his namesake may elucidate the most complicated subject: the brain. Employing artificial intelligence to clarify spectral color blending of tiny molecules used to stain specific proteins and other items of research interest, the PICASSO technique, allows researchers to use more than 15 colors to image and parse our overlapping proteins.
The PICASSO developers, based in Korea, published their approach on May 5 in Nature Communications.
Fluorophores — the staining molecules — emit specific colors when excited by a light, but if more than four fluorophores are used, their emitted colors overlap and blend. Researchers previously developed techniques to correct this spectral overlap by precisely defining the matrix of mixed and unmixed images. This measurement depends on reference spectra, found by identifying clear images of only one fluorophore-stained specimen or of multiple, identically prepared specimens that only contain a single fluorophore each.
“Such reference spectra measurement could be complicated to perform in highly heterogeneous specimens, such as the brain, due to the highly varied emission spectra of fluorophores depending on the subregions from which the spectra were measured,” said co-corresponding author Young-Gyu Yoon, professor in the School of Electrical Engineering at KAIST. He explained that the subregions would each need their own spectra reference measurements, making for an inefficient, time-consuming process. “To address this problem, we developed an approach that does not require reference spectra measurements.”
The approach is the “Process of ultra-multiplexed Imaging of biomolecules viA the unmixing of the Signals of Spectrally Overlapping fluorophores,” also known as PICASSO. Ultra-multiplexed imaging refers to visualizing the numerous individual components of a unit. Like a cinema multiplex in which each theater plays a different movie, each protein in a cell has a different role. By staining with fluorophores, researchers can begin to understand those roles.
“We devised a strategy based on information theory; unmixing is performed by iteratively minimizing the mutual information between mixed images,” said co-corresponding author Jae-Byum Chang, professor in the Department of Materials Science and Engineering, KAIST. “This allows us to get away with the assumption that the spatial distribution of different proteins is mutually exclusive and enables accurate information unmixing.”
To demonstrate PICASSO’s capabilities, the researchers applied the technique to imaging a mouse brain. With a single round of staining, they performed 15-color multiplexed imaging of a mouse brain. Although small, mouse brains are still complex, multifaceted organs that can take significant resources to map. According to the researchers, PICASSO can improve the capabilities of other imaging techniques and allow for the use of even more fluorophore colors.
Using one such imaging technique in combination with PICASSO, the team achieved 45-color multiplexed imaging of the mouse brain in only three staining and imaging cycles, according to Yoon.
“PICASSO is a versatile tool for the multiplexed biomolecule imaging of cultured cells, tissue slices and clinical specimens,” Chang said. “We anticipate that PICASSO will be useful for a broad range of applications for which biomolecules’ spatial information is important. One such application the tool would be useful for is revealing the cellular heterogeneities of tumor microenvironments, especially the heterogeneous populations of immune cells, which are closely related to cancer prognoses and the efficacy of cancer therapies.”
The Samsung Research Funding & Incubation Center for Future Technology supported this work. Spectral imaging was performed at the Korea Basic Science Institute Western Seoul Center.
-PublicationJunyoung Seo, Yeonbo Sim, Jeewon Kim, Hyunwoo Kim, In Cho, Hoyeon Nam, Yong-Gyu Yoon, Jae-Byum Chang, “PICASSO allows ultra-multiplexed fluorescence imaging of spatiallyoverlapping proteins without reference spectra measurements,” May 5, Nature Communications (doi.org/10.1038/s41467-022-30168-z)
-ProfileProfessor Jae-Byum ChangDepartment of Materials Science and EngineeringCollege of EngineeringKAIST
Professor Young-Gyu YoonSchool of Electrical EngineeringCollege of EngineeringKAIST
2022.06.22 View 12757 -
 Now You Can See Floral Scents!
Optical interferometry visualizes how often lilies emit volatile organic compounds
Have you ever thought about when flowers emit their scents?
KAIST mechanical engineers and biological scientists directly visualized how often a lily releases a floral scent using a laser interferometry method. These measurement results can provide new insights for understanding and further exploring the biosynthesis and emission mechanisms of floral volatiles.
Why is it important to know this? It is well known that the fragrance of flowers affects their interactions with pollinators, microorganisms, and florivores. For instance, many flowering plants can tune their scent emission rates when pollinators are active for pollination. Petunias and the wild tobacco Nicotiana attenuata emit floral scents at night to attract night-active pollinators. Thus, visualizing scent emissions can help us understand the ecological evolution of plant-pollinator interactions.
Many groups have been trying to develop methods for scent analysis. Mass spectrometry has been one widely used method for investigating the fragrance of flowers. Although mass spectrometry reveals the quality and quantity of floral scents, it is impossible to directly measure the releasing frequency. A laser-based gas detection system and a smartphone-based detection system using chemo-responsive dyes have also been used to measure volatile organic compounds (VOCs) in real-time, but it is still hard to measure the time-dependent emission rate of floral scents.
However, the KAIST research team co-led by Professor Hyoungsoo Kim from the Department of Mechanical Engineering and Professor Sang-Gyu Kim from the Department of Biological Sciences measured a refractive index difference between the vapor of the VOCs of lilies and the air to measure the emission frequency. The floral scent vapor was detected and the refractive index of air was 1.0 while that of the major floral scent of a linalool lily was 1.46.
Professor Hyoungsoo Kim said, “We expect this technology to be further applicable to various industrial sectors such as developing it to detect hazardous substances in a space.” The research team also plans to identify the DNA mechanism that controls floral scent secretion.
The current work entitled “Real-time visualization of scent accumulation reveals the frequency of floral scent emissions” was published in ‘Frontiers in Plant Science’ on April 18, 2022. (https://doi.org/10.3389/fpls.2022.835305).
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF-2021R1A2C2007835), the Rural Development Administration (PJ016403), and the KAIST-funded Global Singularity Research PREP-Program.
-Publication:H. Kim, G. Lee, J. Song, and S.-G. Kim, "Real-time visualization of scent accumulation reveals the frequency of floral scent emissions," Frontiers in Plant Science 18, 835305 (2022) (https://doi.org/10.3389/fpls.2022.835305)
-Profile:Professor Hyoungsoo Kimhttp://fil.kaist.ac.kr
@MadeInH on TwitterDepartment of Mechanical EngineeringKAIST
Professor Sang-Gyu Kimhttps://sites.google.com/view/kimlab/home Department of Biological SciencesKAIST
2022.05.25 View 12244
Now You Can See Floral Scents!
Optical interferometry visualizes how often lilies emit volatile organic compounds
Have you ever thought about when flowers emit their scents?
KAIST mechanical engineers and biological scientists directly visualized how often a lily releases a floral scent using a laser interferometry method. These measurement results can provide new insights for understanding and further exploring the biosynthesis and emission mechanisms of floral volatiles.
Why is it important to know this? It is well known that the fragrance of flowers affects their interactions with pollinators, microorganisms, and florivores. For instance, many flowering plants can tune their scent emission rates when pollinators are active for pollination. Petunias and the wild tobacco Nicotiana attenuata emit floral scents at night to attract night-active pollinators. Thus, visualizing scent emissions can help us understand the ecological evolution of plant-pollinator interactions.
Many groups have been trying to develop methods for scent analysis. Mass spectrometry has been one widely used method for investigating the fragrance of flowers. Although mass spectrometry reveals the quality and quantity of floral scents, it is impossible to directly measure the releasing frequency. A laser-based gas detection system and a smartphone-based detection system using chemo-responsive dyes have also been used to measure volatile organic compounds (VOCs) in real-time, but it is still hard to measure the time-dependent emission rate of floral scents.
However, the KAIST research team co-led by Professor Hyoungsoo Kim from the Department of Mechanical Engineering and Professor Sang-Gyu Kim from the Department of Biological Sciences measured a refractive index difference between the vapor of the VOCs of lilies and the air to measure the emission frequency. The floral scent vapor was detected and the refractive index of air was 1.0 while that of the major floral scent of a linalool lily was 1.46.
Professor Hyoungsoo Kim said, “We expect this technology to be further applicable to various industrial sectors such as developing it to detect hazardous substances in a space.” The research team also plans to identify the DNA mechanism that controls floral scent secretion.
The current work entitled “Real-time visualization of scent accumulation reveals the frequency of floral scent emissions” was published in ‘Frontiers in Plant Science’ on April 18, 2022. (https://doi.org/10.3389/fpls.2022.835305).
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF-2021R1A2C2007835), the Rural Development Administration (PJ016403), and the KAIST-funded Global Singularity Research PREP-Program.
-Publication:H. Kim, G. Lee, J. Song, and S.-G. Kim, "Real-time visualization of scent accumulation reveals the frequency of floral scent emissions," Frontiers in Plant Science 18, 835305 (2022) (https://doi.org/10.3389/fpls.2022.835305)
-Profile:Professor Hyoungsoo Kimhttp://fil.kaist.ac.kr
@MadeInH on TwitterDepartment of Mechanical EngineeringKAIST
Professor Sang-Gyu Kimhttps://sites.google.com/view/kimlab/home Department of Biological SciencesKAIST
2022.05.25 View 12244 -
 VP Sang Yup Lee Receives Honorary Doctorate from DTU
Vice President for Research, Distinguished Professor Sang Yup Lee at the Department of Chemical & Biomolecular Engineering, was awarded an honorary doctorate from the Technical University of Denmark (DTU) during the DTU Commemoration Day 2022 on April 29. The event drew distinguished guests, students, and faculty including HRH The Crown Prince Frederik Andre Henrik Christian and DTU President Anders Bjarklev.
Professor Lee was recognized for his exceptional scholarship in the field of systems metabolic engineering, which led to the development of microcell factories capable of producing a wide range of fuels, chemicals, materials, and natural compounds, many for the first time.
Professor Lee said in his acceptance speech that KAIST’s continued partnership with DTU in the field of biotechnology will lead to significant contributions in the global efforts to respond to climate change and promote green growth.
DTU CPO and CSO Dina Petronovic Nielson, who heads DTU Biosustain, also lauded Professor Lee saying, “It is not only a great honor for Professor Lee to be induced at DTU but also great honor for DTU to have him.”
Professor Lee also gave commemorative lectures at DTU Biosustain in Lingby and the Bio Innovation Research Institute at the Novo Nordisk Foundation in Copenhagen while in Denmark.
DTU, one of the leading science and technology universities in Europe, has been awarding honorary doctorates since 1921, including to Nobel laureate in chemistry Professor Frances Arnold at Caltech. Professor Lee is the first Korean to receive an honorary doctorate from DTU.
2022.05.03 View 13369
VP Sang Yup Lee Receives Honorary Doctorate from DTU
Vice President for Research, Distinguished Professor Sang Yup Lee at the Department of Chemical & Biomolecular Engineering, was awarded an honorary doctorate from the Technical University of Denmark (DTU) during the DTU Commemoration Day 2022 on April 29. The event drew distinguished guests, students, and faculty including HRH The Crown Prince Frederik Andre Henrik Christian and DTU President Anders Bjarklev.
Professor Lee was recognized for his exceptional scholarship in the field of systems metabolic engineering, which led to the development of microcell factories capable of producing a wide range of fuels, chemicals, materials, and natural compounds, many for the first time.
Professor Lee said in his acceptance speech that KAIST’s continued partnership with DTU in the field of biotechnology will lead to significant contributions in the global efforts to respond to climate change and promote green growth.
DTU CPO and CSO Dina Petronovic Nielson, who heads DTU Biosustain, also lauded Professor Lee saying, “It is not only a great honor for Professor Lee to be induced at DTU but also great honor for DTU to have him.”
Professor Lee also gave commemorative lectures at DTU Biosustain in Lingby and the Bio Innovation Research Institute at the Novo Nordisk Foundation in Copenhagen while in Denmark.
DTU, one of the leading science and technology universities in Europe, has been awarding honorary doctorates since 1921, including to Nobel laureate in chemistry Professor Frances Arnold at Caltech. Professor Lee is the first Korean to receive an honorary doctorate from DTU.
2022.05.03 View 13369 -
 A New Strategy for Active Metasurface Design Provides a Full 360° Phase Tunable Metasurface
The new strategy displays an unprecedented upper limit of dynamic phase modulation with no significant variations in optical amplitude
An international team of researchers led by Professor Min Seok Jang of KAIST and Professor Victor W. Brar of the University of Wisconsin-Madison has demonstrated a widely applicable methodology enabling a full 360° active phase modulation for metasurfaces while maintaining significant levels of uniform light amplitude. This strategy can be fundamentally applied to any spectral region with any structures and resonances that fit the bill.
Metasurfaces are optical components with specialized functionalities indispensable for real-life applications ranging from LIDAR and spectroscopy to futuristic technologies such as invisibility cloaks and holograms. They are known for their compact and micro/nano-sized nature, which enables them to be integrated into electronic computerized systems with sizes that are ever decreasing as predicted by Moore’s law.
In order to allow for such innovations, metasurfaces must be capable of manipulating the impinging light, doing so by manipulating either the light’s amplitude or phase (or both) and emitting it back out. However, dynamically modulating the phase with the full circle range has been a notoriously difficult task, with very few works managing to do so by sacrificing a substantial amount of amplitude control.
Challenged by these limitations, the team proposed a general methodology that enables metasurfaces to implement a dynamic phase modulation with the complete 360° phase range, all the while uniformly maintaining significant levels of amplitude.
The underlying reason for the difficulty achieving such a feat is that there is a fundamental trade-off regarding dynamically controlling the optical phase of light. Metasurfaces generally perform such a function through optical resonances, an excitation of electrons inside the metasurface structure that harmonically oscillate together with the incident light. In order to be able to modulate through the entire range of 0-360°, the optical resonance frequency (the center of the spectrum) must be tuned by a large amount while the linewidth (the width of the spectrum) is kept to a minimum. However, to electrically tune the optical resonance frequency of the metasurface on demand, there needs to be a controllable influx and outflux of electrons into the metasurface and this inevitably leads to a larger linewidth of the aforementioned optical resonance.
The problem is further compounded by the fact that the phase and the amplitude of optical resonances are closely correlated in a complex, non-linear fashion, making it very difficult to hold substantial control over the amplitude while changing the phase.
The team’s work circumvented both problems by using two optical resonances, each with specifically designated properties. One resonance provides the decoupling between the phase and amplitude so that the phase is able to be tuned while significant and uniform levels of amplitude are maintained, as well as providing a narrow linewidth.
The other resonance provides the capability of being sufficiently tuned to a large degree so that the complete full circle range of phase modulation is achievable. The quintessence of the work is then to combine the different properties of the two resonances through a phenomenon called avoided crossing, so that the interactions between the two resonances lead to an amalgamation of the desired traits that achieves and even surpasses the full 360° phase modulation with uniform amplitude.
Professor Jang said, “Our research proposes a new methodology in dynamic phase modulation that breaks through the conventional limits and trade-offs, while being broadly applicable in diverse types of metasurfaces. We hope that this idea helps researchers implement and realize many key applications of metasurfaces, such as LIDAR and holograms, so that the nanophotonics industry keeps growing and provides a brighter technological future.”
The research paper authored by Ju Young Kim and Juho Park, et al., and titled "Full 2π Tunable Phase Modulation Using Avoided Crossing of Resonances" was published in Nature Communications on April 19. The research was funded by the Samsung Research Funding & Incubation Center of Samsung Electronics.
-Publication:Ju Young Kim, Juho Park, Gregory R. Holdman, Jacob T. Heiden, Shinho Kim, Victor W. Brar, and Min Seok Jang, “Full 2π Tunable Phase Modulation Using Avoided Crossing ofResonances” Nature Communications on April 19 (2022). doi.org/10.1038/s41467-022-29721-7
-ProfileProfessor Min Seok JangSchool of Electrical EngineeringKAIST
2022.05.02 View 9793
A New Strategy for Active Metasurface Design Provides a Full 360° Phase Tunable Metasurface
The new strategy displays an unprecedented upper limit of dynamic phase modulation with no significant variations in optical amplitude
An international team of researchers led by Professor Min Seok Jang of KAIST and Professor Victor W. Brar of the University of Wisconsin-Madison has demonstrated a widely applicable methodology enabling a full 360° active phase modulation for metasurfaces while maintaining significant levels of uniform light amplitude. This strategy can be fundamentally applied to any spectral region with any structures and resonances that fit the bill.
Metasurfaces are optical components with specialized functionalities indispensable for real-life applications ranging from LIDAR and spectroscopy to futuristic technologies such as invisibility cloaks and holograms. They are known for their compact and micro/nano-sized nature, which enables them to be integrated into electronic computerized systems with sizes that are ever decreasing as predicted by Moore’s law.
In order to allow for such innovations, metasurfaces must be capable of manipulating the impinging light, doing so by manipulating either the light’s amplitude or phase (or both) and emitting it back out. However, dynamically modulating the phase with the full circle range has been a notoriously difficult task, with very few works managing to do so by sacrificing a substantial amount of amplitude control.
Challenged by these limitations, the team proposed a general methodology that enables metasurfaces to implement a dynamic phase modulation with the complete 360° phase range, all the while uniformly maintaining significant levels of amplitude.
The underlying reason for the difficulty achieving such a feat is that there is a fundamental trade-off regarding dynamically controlling the optical phase of light. Metasurfaces generally perform such a function through optical resonances, an excitation of electrons inside the metasurface structure that harmonically oscillate together with the incident light. In order to be able to modulate through the entire range of 0-360°, the optical resonance frequency (the center of the spectrum) must be tuned by a large amount while the linewidth (the width of the spectrum) is kept to a minimum. However, to electrically tune the optical resonance frequency of the metasurface on demand, there needs to be a controllable influx and outflux of electrons into the metasurface and this inevitably leads to a larger linewidth of the aforementioned optical resonance.
The problem is further compounded by the fact that the phase and the amplitude of optical resonances are closely correlated in a complex, non-linear fashion, making it very difficult to hold substantial control over the amplitude while changing the phase.
The team’s work circumvented both problems by using two optical resonances, each with specifically designated properties. One resonance provides the decoupling between the phase and amplitude so that the phase is able to be tuned while significant and uniform levels of amplitude are maintained, as well as providing a narrow linewidth.
The other resonance provides the capability of being sufficiently tuned to a large degree so that the complete full circle range of phase modulation is achievable. The quintessence of the work is then to combine the different properties of the two resonances through a phenomenon called avoided crossing, so that the interactions between the two resonances lead to an amalgamation of the desired traits that achieves and even surpasses the full 360° phase modulation with uniform amplitude.
Professor Jang said, “Our research proposes a new methodology in dynamic phase modulation that breaks through the conventional limits and trade-offs, while being broadly applicable in diverse types of metasurfaces. We hope that this idea helps researchers implement and realize many key applications of metasurfaces, such as LIDAR and holograms, so that the nanophotonics industry keeps growing and provides a brighter technological future.”
The research paper authored by Ju Young Kim and Juho Park, et al., and titled "Full 2π Tunable Phase Modulation Using Avoided Crossing of Resonances" was published in Nature Communications on April 19. The research was funded by the Samsung Research Funding & Incubation Center of Samsung Electronics.
-Publication:Ju Young Kim, Juho Park, Gregory R. Holdman, Jacob T. Heiden, Shinho Kim, Victor W. Brar, and Min Seok Jang, “Full 2π Tunable Phase Modulation Using Avoided Crossing ofResonances” Nature Communications on April 19 (2022). doi.org/10.1038/s41467-022-29721-7
-ProfileProfessor Min Seok JangSchool of Electrical EngineeringKAIST
2022.05.02 View 9793 -
 Professor Hyunjoo Jenny Lee to Co-Chair IEEE MEMS 2025
Professor Hyunjoo Jenny Lee from the School of Electrical Engineering has been appointed General Chair of the 38th IEEE MEMS 2025 (International Conference on Micro Electro Mechanical Systems). Professor Lee, who is 40, is the conference’s youngest General Chair to date and will work jointly with Professor Sheng-Shian Li of Taiwan’s National Tsing Hua University as co-chairs in 2025.
IEEE MEMS is a top-tier international conference on microelectromechanical systems and it serves as a core academic showcase for MEMS research and technology in areas such as microsensors and actuators.
With over 800 MEMS paper submissions each year, the conference only accepts and publishes about 250 of them after a rigorous review process recognized for its world-class prestige. Of all the submissions, fewer than 10% are chosen for oral presentations.
2022.04.18 View 9410
Professor Hyunjoo Jenny Lee to Co-Chair IEEE MEMS 2025
Professor Hyunjoo Jenny Lee from the School of Electrical Engineering has been appointed General Chair of the 38th IEEE MEMS 2025 (International Conference on Micro Electro Mechanical Systems). Professor Lee, who is 40, is the conference’s youngest General Chair to date and will work jointly with Professor Sheng-Shian Li of Taiwan’s National Tsing Hua University as co-chairs in 2025.
IEEE MEMS is a top-tier international conference on microelectromechanical systems and it serves as a core academic showcase for MEMS research and technology in areas such as microsensors and actuators.
With over 800 MEMS paper submissions each year, the conference only accepts and publishes about 250 of them after a rigorous review process recognized for its world-class prestige. Of all the submissions, fewer than 10% are chosen for oral presentations.
2022.04.18 View 9410 -
 Mathematicians Identify a Key Source of Cell-to-Cell Variability in Cell Signaling
Systematic inferences identify a major source of heterogeneity in cell signaling dynamics
Why do genetically identical cells respond differently to the same external stimuli, such as antibiotics? This long-standing mystery has been solved by KAIST and IBS mathematicians who have developed a new framework for analyzing cell responses to some stimuli. The team found that the cell-to-cell variability in antibiotic stress response increases as the effective length of the cell signaling pathway (i.e., the number of rate-limiting steps) increases. This finding could identify more effective chemotherapies to overcome the fractional killing of cancer cells caused by cell-to-cell variability.
Cells in the human body contain signal transduction systems that respond to various external stimuli such as antibiotics and changes in osmotic pressure. When an external stimulus is detected, various biochemical reactions occur sequentially. This leads to the expression of relevant genes, allowing the cells to respond to the perturbed external environment. Furthermore, signal transduction leads to a drug response (e.g., antibiotic resistance genes are expressed when antibiotic drugs are given).
However, even when the same external stimuli are detected, the responses of individual cells are greatly heterogeneous. This leads to the emergence of persister cells that are highly resistant to drugs. To identify potential sources of this cell-to cell variability, many studies have been conducted. However, most of the intermediate signal transduction reactions are unobservable with current experimental techniques.
A group of researchers including Dae Wook Kim and Hyukpyo Hong and led by Professor Jae Kyoung Kim from the KAIST Department of Mathematical Sciences and IBS Biomedical Mathematics Group solved the mystery by exploiting queueing theory and Bayesian inference methodology. They proposed a queueing process that describes the signal transduction system in cells. Based on this, they developed Bayesian inference computational software using MBI (the Moment-based Bayesian Inference method). This enables the analysis of the signal transduction system without a direct observation of the intermediate steps. This study was published in Science Advances.
By analyzing experimental data from Escherichia coli using MBI, the research team found that cell-to-cell variability increases as the number of rate-limiting steps in the signaling pathway increases.
The rate-limiting steps denote the slowest steps (i.e., bottlenecks) in sequential biochemical reaction steps composing cell signaling pathways and thus dominates most of the signaling time. As the number of the rate-limiting steps increases, the intensity of the transduced signal becomes greatly heterogeneous even in a population of genetically identical cells. This finding is expected to provide a new paradigm for studying the heterogeneous antibiotic resistance of cells, which is a big challenge in cancer medicine.
Professor Kim said, “As a mathematician, I am excited to help advance the understanding of cell-to-cell variability in response to external stimuli. I hope this finding facilitates the development of more effective chemotherapies.”
This work was supported by the Samsung Science and Technology Foundation, the National Research Foundation of Korea, and the Institute for Basic Science.
-Publication:Dae Wook Kim, Hyukpyo Hong, and Jae Kyoung Kim (2022) “Systematic inference identifies a major source of heterogeneity in cell signaling dynamics: the rate-limiting step number,”Science Advances March 18, 2022 (DOI: 10.1126/sciadv.abl4598)
-Profile:Professor Jae Kyoung Kimhttp://mathsci.kaist.ac.kr/~jaekkim
jaekkim@kaist.ac.kr@umichkim on TwitterDepartment of Mathematical SciencesKAIST
2022.03.29 View 12801
Mathematicians Identify a Key Source of Cell-to-Cell Variability in Cell Signaling
Systematic inferences identify a major source of heterogeneity in cell signaling dynamics
Why do genetically identical cells respond differently to the same external stimuli, such as antibiotics? This long-standing mystery has been solved by KAIST and IBS mathematicians who have developed a new framework for analyzing cell responses to some stimuli. The team found that the cell-to-cell variability in antibiotic stress response increases as the effective length of the cell signaling pathway (i.e., the number of rate-limiting steps) increases. This finding could identify more effective chemotherapies to overcome the fractional killing of cancer cells caused by cell-to-cell variability.
Cells in the human body contain signal transduction systems that respond to various external stimuli such as antibiotics and changes in osmotic pressure. When an external stimulus is detected, various biochemical reactions occur sequentially. This leads to the expression of relevant genes, allowing the cells to respond to the perturbed external environment. Furthermore, signal transduction leads to a drug response (e.g., antibiotic resistance genes are expressed when antibiotic drugs are given).
However, even when the same external stimuli are detected, the responses of individual cells are greatly heterogeneous. This leads to the emergence of persister cells that are highly resistant to drugs. To identify potential sources of this cell-to cell variability, many studies have been conducted. However, most of the intermediate signal transduction reactions are unobservable with current experimental techniques.
A group of researchers including Dae Wook Kim and Hyukpyo Hong and led by Professor Jae Kyoung Kim from the KAIST Department of Mathematical Sciences and IBS Biomedical Mathematics Group solved the mystery by exploiting queueing theory and Bayesian inference methodology. They proposed a queueing process that describes the signal transduction system in cells. Based on this, they developed Bayesian inference computational software using MBI (the Moment-based Bayesian Inference method). This enables the analysis of the signal transduction system without a direct observation of the intermediate steps. This study was published in Science Advances.
By analyzing experimental data from Escherichia coli using MBI, the research team found that cell-to-cell variability increases as the number of rate-limiting steps in the signaling pathway increases.
The rate-limiting steps denote the slowest steps (i.e., bottlenecks) in sequential biochemical reaction steps composing cell signaling pathways and thus dominates most of the signaling time. As the number of the rate-limiting steps increases, the intensity of the transduced signal becomes greatly heterogeneous even in a population of genetically identical cells. This finding is expected to provide a new paradigm for studying the heterogeneous antibiotic resistance of cells, which is a big challenge in cancer medicine.
Professor Kim said, “As a mathematician, I am excited to help advance the understanding of cell-to-cell variability in response to external stimuli. I hope this finding facilitates the development of more effective chemotherapies.”
This work was supported by the Samsung Science and Technology Foundation, the National Research Foundation of Korea, and the Institute for Basic Science.
-Publication:Dae Wook Kim, Hyukpyo Hong, and Jae Kyoung Kim (2022) “Systematic inference identifies a major source of heterogeneity in cell signaling dynamics: the rate-limiting step number,”Science Advances March 18, 2022 (DOI: 10.1126/sciadv.abl4598)
-Profile:Professor Jae Kyoung Kimhttp://mathsci.kaist.ac.kr/~jaekkim
jaekkim@kaist.ac.kr@umichkim on TwitterDepartment of Mathematical SciencesKAIST
2022.03.29 View 12801 -
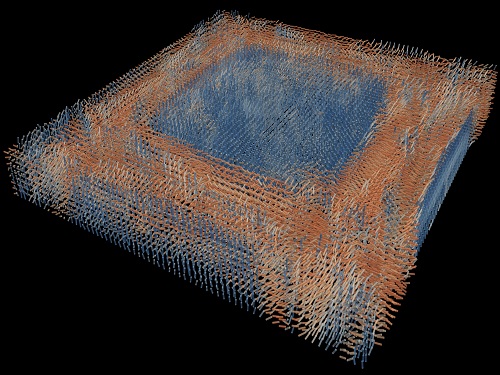 Tomographic Measurement of Dielectric Tensors
Dielectric tensor tomography allows the direct measurement of the 3D dielectric tensors of optically anisotropic structures
A research team reported the direct measurement of dielectric tensors of anisotropic structures including the spatial variations of principal refractive indices and directors. The group also demonstrated quantitative tomographic measurements of various nematic liquid-crystal structures and their fast 3D nonequilibrium dynamics using a 3D label-free tomographic method. The method was described in Nature Materials.
Light-matter interactions are described by the dielectric tensor. Despite their importance in basic science and applications, it has not been possible to measure 3D dielectric tensors directly. The main challenge was due to the vectorial nature of light scattering from a 3D anisotropic structure. Previous approaches only addressed 3D anisotropic information indirectly and were limited to two-dimensional, qualitative, strict sample conditions or assumptions.
The research team developed a method enabling the tomographic reconstruction of 3D dielectric tensors without any preparation or assumptions. A sample is illuminated with a laser beam with various angles and circularly polarization states. Then, the light fields scattered from a sample are holographically measured and converted into vectorial diffraction components. Finally, by inversely solving a vectorial wave equation, the 3D dielectric tensor is reconstructed.
Professor YongKeun Park said, “There were a greater number of unknowns in direct measuring than with the conventional approach. We applied our approach to measure additional holographic images by slightly tilting the incident angle.”
He said that the slightly tilted illumination provides an additional orthogonal polarization, which makes the underdetermined problem become the determined problem. “Although scattered fields are dependent on the illumination angle, the Fourier differentiation theorem enables the extraction of the same dielectric tensor for the slightly tilted illumination,” Professor Park added.
His team’s method was validated by reconstructing well-known liquid crystal (LC) structures, including the twisted nematic, hybrid aligned nematic, radial, and bipolar configurations. Furthermore, the research team demonstrated the experimental measurements of the non-equilibrium dynamics of annihilating, nucleating, and merging LC droplets, and the LC polymer network with repeating 3D topological defects.
“This is the first experimental measurement of non-equilibrium dynamics and 3D topological defects in LC structures in a label-free manner. Our method enables the exploration of inaccessible nematic structures and interactions in non-equilibrium dynamics,” first author Dr. Seungwoo Shin explained.
-PublicationSeungwoo Shin, Jonghee Eun, Sang Seok Lee, Changjae Lee, Herve Hugonnet, Dong Ki Yoon, Shin-Hyun Kim, Jongwoo Jeong, YongKeun Park, “Tomographic Measurement ofDielectric Tensors at Optical Frequency,” Nature Materials March 02, 2022 (https://doi.org/10/1038/s41563-022-01202-8)
-ProfileProfessor YongKeun ParkBiomedical Optics Laboratory (http://bmol.kaist.ac.kr)Department of PhysicsCollege of Natural SciencesKAIST
2022.03.22 View 10559
Tomographic Measurement of Dielectric Tensors
Dielectric tensor tomography allows the direct measurement of the 3D dielectric tensors of optically anisotropic structures
A research team reported the direct measurement of dielectric tensors of anisotropic structures including the spatial variations of principal refractive indices and directors. The group also demonstrated quantitative tomographic measurements of various nematic liquid-crystal structures and their fast 3D nonequilibrium dynamics using a 3D label-free tomographic method. The method was described in Nature Materials.
Light-matter interactions are described by the dielectric tensor. Despite their importance in basic science and applications, it has not been possible to measure 3D dielectric tensors directly. The main challenge was due to the vectorial nature of light scattering from a 3D anisotropic structure. Previous approaches only addressed 3D anisotropic information indirectly and were limited to two-dimensional, qualitative, strict sample conditions or assumptions.
The research team developed a method enabling the tomographic reconstruction of 3D dielectric tensors without any preparation or assumptions. A sample is illuminated with a laser beam with various angles and circularly polarization states. Then, the light fields scattered from a sample are holographically measured and converted into vectorial diffraction components. Finally, by inversely solving a vectorial wave equation, the 3D dielectric tensor is reconstructed.
Professor YongKeun Park said, “There were a greater number of unknowns in direct measuring than with the conventional approach. We applied our approach to measure additional holographic images by slightly tilting the incident angle.”
He said that the slightly tilted illumination provides an additional orthogonal polarization, which makes the underdetermined problem become the determined problem. “Although scattered fields are dependent on the illumination angle, the Fourier differentiation theorem enables the extraction of the same dielectric tensor for the slightly tilted illumination,” Professor Park added.
His team’s method was validated by reconstructing well-known liquid crystal (LC) structures, including the twisted nematic, hybrid aligned nematic, radial, and bipolar configurations. Furthermore, the research team demonstrated the experimental measurements of the non-equilibrium dynamics of annihilating, nucleating, and merging LC droplets, and the LC polymer network with repeating 3D topological defects.
“This is the first experimental measurement of non-equilibrium dynamics and 3D topological defects in LC structures in a label-free manner. Our method enables the exploration of inaccessible nematic structures and interactions in non-equilibrium dynamics,” first author Dr. Seungwoo Shin explained.
-PublicationSeungwoo Shin, Jonghee Eun, Sang Seok Lee, Changjae Lee, Herve Hugonnet, Dong Ki Yoon, Shin-Hyun Kim, Jongwoo Jeong, YongKeun Park, “Tomographic Measurement ofDielectric Tensors at Optical Frequency,” Nature Materials March 02, 2022 (https://doi.org/10/1038/s41563-022-01202-8)
-ProfileProfessor YongKeun ParkBiomedical Optics Laboratory (http://bmol.kaist.ac.kr)Department of PhysicsCollege of Natural SciencesKAIST
2022.03.22 View 10559 -
 Scientist Discover How Circadian Rhythm Can Be Both Strong and Flexible
Study reveals that master and slave oscillators function via different molecular mechanisms
From tiny fruit flies to human beings, all animals on Earth maintain their daily rhythms based on their internal circadian clock. The circadian clock enables organisms to undergo rhythmic changes in behavior and physiology based on a 24-hour circadian cycle. For example, our own biological clock tells our brain to release melatonin, a sleep-inducing hormone, at night time.
The discovery of the molecular mechanism of the circadian clock was bestowed the Nobel Prize in Physiology or Medicine 2017. From what we know, no one centralized clock is responsible for our circadian cycles. Instead, it operates in a hierarchical network where there are “master pacemaker” and “slave oscillator”.
The master pacemaker receives various input signals from the environment such as light. The master then drives the slave oscillator that regulates various outputs such as sleep, feeding, and metabolism. Despite the different roles of the pacemaker neurons, they are known to share common molecular mechanisms that are well conserved in all lifeforms. For example, interlocked systems of multiple transcriptional-translational feedback loops (TTFLs) composed of core clock proteins have been deeply studied in fruit flies.
However, there is still much that we need to learn about our own biological clock. The hierarchically-organized nature of master and slave clock neurons leads to a prevailing belief that they share an identical molecular clockwork. At the same time, the different roles they serve in regulating bodily rhythms also raise the question of whether they might function under different molecular clockworks.
Research team led by Professor Kim Jae Kyoung from the Department of Mathematical Sciences, a chief investigator at the Biomedical Mathematics Group at the Institute for Basic Science, used a combination of mathematical and experimental approaches using fruit flies to answer this question. The team found that the master clock and the slave clock operate via different molecular mechanisms.
In both master and slave neurons of fruit flies, a circadian rhythm-related protein called PER is produced and degraded at different rates depending on the time of the day. Previously, the team found that the master clock neuron (sLNvs) and the slave clock neuron (DN1ps) have different profiles of PER in wild-type and Clk-Δ mutant Drosophila. This hinted that there might be a potential difference in molecular clockworks between the master and slave clock neurons.
However, due to the complexity of the molecular clockwork, it was challenging to identify the source of such differences. Thus, the team developed a mathematical model describing the molecular clockworks of the master and slave clocks. Then, all possible molecular differences between the master and slave clock neurons were systematically investigated by using computer simulations. The model predicted that PER is more efficiently produced and then rapidly degraded in the master clock compared to the slave clock neurons. This prediction was then confirmed by the follow-up experiments using animal.
Then, why do the master clock neurons have such different molecular properties from the slave clock neurons? To answer this question, the research team again used the combination of mathematical model simulation and experiments. It was found that the faster rate of synthesis of PER in the master clock neurons allows them to generate synchronized rhythms with a high level of amplitude. Generation of such a strong rhythm with high amplitude is critical to delivering clear signals to slave clock neurons.
However, such strong rhythms would typically be unfavorable when it comes to adapting to environmental changes. These include natural causes such as different daylight hours across summer and winter seasons, up to more extreme artificial cases such as jet lag that occurs after international travel. Thanks to the distinct property of the master clock neurons, it is able to undergo phase dispersion when the standard light-dark cycle is disrupted, drastically reducing the level of PER. The master clock neurons can then easily adapt to the new diurnal cycle. Our master pacemaker’s plasticity explains how we can quickly adjust to the new time zones after international flights after just a brief period of jet lag.
It is hoped that the findings of this study can have future clinical implications when it comes to treating various disorders that affect our circadian rhythm. Professor Kim notes, “When the circadian clock loses its robustness and flexibility, the circadian rhythms sleep disorders can occur. As this study identifies the molecular mechanism that generates robustness and flexibility of the circadian clock, it can facilitate the identification of the cause of and treatment strategy for the circadian rhythm sleep disorders.” This work was supported by the Human Frontier Science Program.
-PublicationEui Min Jeong, Miri Kwon, Eunjoo Cho, Sang Hyuk Lee, Hyun Kim, Eun Young Kim, and Jae Kyoung Kim, “Systematic modeling-driven experiments identify distinct molecularclockworks underlying hierarchically organized pacemaker neurons,” February 22, 2022, Proceedings of the National Academy of Sciences of the United States of America
-ProfileProfessor Jae Kyoung KimDepartment of Mathematical SciencesKAIST
2022.02.23 View 12844
Scientist Discover How Circadian Rhythm Can Be Both Strong and Flexible
Study reveals that master and slave oscillators function via different molecular mechanisms
From tiny fruit flies to human beings, all animals on Earth maintain their daily rhythms based on their internal circadian clock. The circadian clock enables organisms to undergo rhythmic changes in behavior and physiology based on a 24-hour circadian cycle. For example, our own biological clock tells our brain to release melatonin, a sleep-inducing hormone, at night time.
The discovery of the molecular mechanism of the circadian clock was bestowed the Nobel Prize in Physiology or Medicine 2017. From what we know, no one centralized clock is responsible for our circadian cycles. Instead, it operates in a hierarchical network where there are “master pacemaker” and “slave oscillator”.
The master pacemaker receives various input signals from the environment such as light. The master then drives the slave oscillator that regulates various outputs such as sleep, feeding, and metabolism. Despite the different roles of the pacemaker neurons, they are known to share common molecular mechanisms that are well conserved in all lifeforms. For example, interlocked systems of multiple transcriptional-translational feedback loops (TTFLs) composed of core clock proteins have been deeply studied in fruit flies.
However, there is still much that we need to learn about our own biological clock. The hierarchically-organized nature of master and slave clock neurons leads to a prevailing belief that they share an identical molecular clockwork. At the same time, the different roles they serve in regulating bodily rhythms also raise the question of whether they might function under different molecular clockworks.
Research team led by Professor Kim Jae Kyoung from the Department of Mathematical Sciences, a chief investigator at the Biomedical Mathematics Group at the Institute for Basic Science, used a combination of mathematical and experimental approaches using fruit flies to answer this question. The team found that the master clock and the slave clock operate via different molecular mechanisms.
In both master and slave neurons of fruit flies, a circadian rhythm-related protein called PER is produced and degraded at different rates depending on the time of the day. Previously, the team found that the master clock neuron (sLNvs) and the slave clock neuron (DN1ps) have different profiles of PER in wild-type and Clk-Δ mutant Drosophila. This hinted that there might be a potential difference in molecular clockworks between the master and slave clock neurons.
However, due to the complexity of the molecular clockwork, it was challenging to identify the source of such differences. Thus, the team developed a mathematical model describing the molecular clockworks of the master and slave clocks. Then, all possible molecular differences between the master and slave clock neurons were systematically investigated by using computer simulations. The model predicted that PER is more efficiently produced and then rapidly degraded in the master clock compared to the slave clock neurons. This prediction was then confirmed by the follow-up experiments using animal.
Then, why do the master clock neurons have such different molecular properties from the slave clock neurons? To answer this question, the research team again used the combination of mathematical model simulation and experiments. It was found that the faster rate of synthesis of PER in the master clock neurons allows them to generate synchronized rhythms with a high level of amplitude. Generation of such a strong rhythm with high amplitude is critical to delivering clear signals to slave clock neurons.
However, such strong rhythms would typically be unfavorable when it comes to adapting to environmental changes. These include natural causes such as different daylight hours across summer and winter seasons, up to more extreme artificial cases such as jet lag that occurs after international travel. Thanks to the distinct property of the master clock neurons, it is able to undergo phase dispersion when the standard light-dark cycle is disrupted, drastically reducing the level of PER. The master clock neurons can then easily adapt to the new diurnal cycle. Our master pacemaker’s plasticity explains how we can quickly adjust to the new time zones after international flights after just a brief period of jet lag.
It is hoped that the findings of this study can have future clinical implications when it comes to treating various disorders that affect our circadian rhythm. Professor Kim notes, “When the circadian clock loses its robustness and flexibility, the circadian rhythms sleep disorders can occur. As this study identifies the molecular mechanism that generates robustness and flexibility of the circadian clock, it can facilitate the identification of the cause of and treatment strategy for the circadian rhythm sleep disorders.” This work was supported by the Human Frontier Science Program.
-PublicationEui Min Jeong, Miri Kwon, Eunjoo Cho, Sang Hyuk Lee, Hyun Kim, Eun Young Kim, and Jae Kyoung Kim, “Systematic modeling-driven experiments identify distinct molecularclockworks underlying hierarchically organized pacemaker neurons,” February 22, 2022, Proceedings of the National Academy of Sciences of the United States of America
-ProfileProfessor Jae Kyoung KimDepartment of Mathematical SciencesKAIST
2022.02.23 View 12844 -
 President Lee Presents Plans to Nurture Next-Generation Talents
President Lee stressed that nurturing medical scientists, semiconductor R&D personnel, startup entrepreneurs, and global innovators are key missions he will continue to pursue during a news conference
KAIST President Kwang Hyung Lee said that nurturing medical scientists, semiconductor R&D personnel, startup entrepreneurs, and global innovators are key missions he will continue to pursue during an online news conference marking the 1st anniversary of him becoming the president on February 15.
He said that nurturing physician-scientists is the most critical mission for KAIST to help the nation create a new growth engine. He said KAIST will help the nation drive the bio-industry and provide medical science resources for the nation’s health sector. To this end, he said that KAIST will open its Medical Science and Technology School by 2026.
“We plan to expand the current Graduate School of Medical Science and Engineering into a new Medical Science and Technology School that will focus entirely on a condensed MD-PhD course converging the fields of AI, bio, and physics,” he said.
The school aims to foster medical scientists whose research results will eventually be commercialized. He said that the university is now discussing revisions to related laws and regulations with the government and other universities.
To supply human resources to the semiconductor industry, President Lee said the university will add a campus in Pyongtaek City that will serve as an advanced convergence research hub in the field of next generation semiconductors in collaboration with Samsung Electronics and the city of Pyongtaek. The three-stage opening plan projected the final opening of the campus by 2036. During the first stage, which will be completed by 2026, it will construct the campus infrastructure in Pyongtaek city where Samsung Semiconductors runs two massive semiconductor complexes. By 2031, it plans to launch the open research platform including a future cities research center and future vehicles research center. The campus will open the global industrial collaboration cluster hub by 2036.
In the global arena, President Lee said he is working to open the New York campus with stakeholders in the United States. He announced the plan last December that was endorsed by New York-based entrepreneur Hee-Nam Bae, the chairman of Big Continent Inc. President Lee and Chairman Lee signed an MOU for the funding to open the campus in New York.
“We are discussing how to facilitate the plan and best accommodate the interests and potential of our students. Many ideas and plans are on the table and we think it will take longer than expected to finalize the plan,” explained President Lee.
However, he added that the basic idea is to offer art tech and health technology programs as well as an AI-based finance MBA at the New York campus, in addition to it serving as the startup accelerator of KAIST.
President Lee stressed the importance of technology commercialization when successfully launching KAIST Holdings last month to help spinoffs of KAIST labs accelerate their end results. He said that KAIST Holdings will build a virtuous supporting system to commercialize the technology startups coming from KAIST.
“We plan to list at least 10 KAIST startups on the KOSDAQ and two on the NASDAQ by 2031. KAIST Holdings also aims to nurture companies valued at a total of one billion KRW and earn 100 billion KRW in technology fees by 2031.
2022.02.17 View 14047
President Lee Presents Plans to Nurture Next-Generation Talents
President Lee stressed that nurturing medical scientists, semiconductor R&D personnel, startup entrepreneurs, and global innovators are key missions he will continue to pursue during a news conference
KAIST President Kwang Hyung Lee said that nurturing medical scientists, semiconductor R&D personnel, startup entrepreneurs, and global innovators are key missions he will continue to pursue during an online news conference marking the 1st anniversary of him becoming the president on February 15.
He said that nurturing physician-scientists is the most critical mission for KAIST to help the nation create a new growth engine. He said KAIST will help the nation drive the bio-industry and provide medical science resources for the nation’s health sector. To this end, he said that KAIST will open its Medical Science and Technology School by 2026.
“We plan to expand the current Graduate School of Medical Science and Engineering into a new Medical Science and Technology School that will focus entirely on a condensed MD-PhD course converging the fields of AI, bio, and physics,” he said.
The school aims to foster medical scientists whose research results will eventually be commercialized. He said that the university is now discussing revisions to related laws and regulations with the government and other universities.
To supply human resources to the semiconductor industry, President Lee said the university will add a campus in Pyongtaek City that will serve as an advanced convergence research hub in the field of next generation semiconductors in collaboration with Samsung Electronics and the city of Pyongtaek. The three-stage opening plan projected the final opening of the campus by 2036. During the first stage, which will be completed by 2026, it will construct the campus infrastructure in Pyongtaek city where Samsung Semiconductors runs two massive semiconductor complexes. By 2031, it plans to launch the open research platform including a future cities research center and future vehicles research center. The campus will open the global industrial collaboration cluster hub by 2036.
In the global arena, President Lee said he is working to open the New York campus with stakeholders in the United States. He announced the plan last December that was endorsed by New York-based entrepreneur Hee-Nam Bae, the chairman of Big Continent Inc. President Lee and Chairman Lee signed an MOU for the funding to open the campus in New York.
“We are discussing how to facilitate the plan and best accommodate the interests and potential of our students. Many ideas and plans are on the table and we think it will take longer than expected to finalize the plan,” explained President Lee.
However, he added that the basic idea is to offer art tech and health technology programs as well as an AI-based finance MBA at the New York campus, in addition to it serving as the startup accelerator of KAIST.
President Lee stressed the importance of technology commercialization when successfully launching KAIST Holdings last month to help spinoffs of KAIST labs accelerate their end results. He said that KAIST Holdings will build a virtuous supporting system to commercialize the technology startups coming from KAIST.
“We plan to list at least 10 KAIST startups on the KOSDAQ and two on the NASDAQ by 2031. KAIST Holdings also aims to nurture companies valued at a total of one billion KRW and earn 100 billion KRW in technology fees by 2031.
2022.02.17 View 14047 -
 Team KAIST Makes Its Presence Felt in the Self-Driving Tech Industry
Team KAIST finishes 4th at the inaugural CES Autonomous Racing Competition
Team KAIST led by Professor Hyunchul Shim and Unmanned Systems Research Group (USRG) placed fourth in an autonomous race car competition in Las Vegas last week, making its presence felt in the self-driving automotive tech industry.
Team KAIST, beat its first competitor, Auburn University, with speeds of up to 131 mph at the Autonomous Challenge at CES held at the Las Vegas Motor Speedway. However, the team failed to advance to the final round when it lost to PoliMOVE, comprised of the Polytechnic University of Milan and the University of Alabama, the final winner of the $150,000 USD race.
A total of eight teams competed in the self-driving race. The race was conducted as a single elimination tournament consisting of multiple rounds of matches. Two cars took turns playing the role of defender and attacker, and each car attempted to outpace the other until one of them was unable to complete the mission.
Each team designed the algorithm to control its racecar, the Dallara-built AV-21, which can reach a speed of up to 173 mph, and make it safely drive around the track at high speeds without crashing into the other.
The event is the CES version of the Indy Autonomous Challenge, a competition that took place for the first time in October last year to encourage university students from around the world to develop complicated software for autonomous driving and advance relevant technologies. Team KAIST placed 4th at the Indy Autonomous Challenge, which qualified it to participate in this race.
“The technical level of the CES race is much higher than last October’s and we had a very tough race. We advanced to the semifinals for two consecutive races. I think our autonomous vehicle technology is proving itself to the world,” said Professor Shim.
Professor Shim’s research group has been working on the development of autonomous aerial and ground vehicles for the past 12 years. A self-driving car developed by the lab was certified by the South Korean government to run on public roads.
The vehicle the team used cost more than 1 million USD to build. Many of the other teams had to repair their vehicle more than once due to accidents and had to spend a lot on repairs. “We are the only one who did not have any accidents, and this is a testament to our technological prowess,” said Professor Shim.
He said the financial funding to purchase pricy parts and equipment for the racecar is always a challenge given the very tight research budget and absence of corporate sponsorships.
However, Professor Shim and his research group plan to participate in the next race in September and in the 2023 CES race.
“I think we need more systemic and proactive research and support systems to earn better results but there is nothing better than the group of passionate students who are taking part in this project with us,” Shim added.
2022.01.12 View 13372
Team KAIST Makes Its Presence Felt in the Self-Driving Tech Industry
Team KAIST finishes 4th at the inaugural CES Autonomous Racing Competition
Team KAIST led by Professor Hyunchul Shim and Unmanned Systems Research Group (USRG) placed fourth in an autonomous race car competition in Las Vegas last week, making its presence felt in the self-driving automotive tech industry.
Team KAIST, beat its first competitor, Auburn University, with speeds of up to 131 mph at the Autonomous Challenge at CES held at the Las Vegas Motor Speedway. However, the team failed to advance to the final round when it lost to PoliMOVE, comprised of the Polytechnic University of Milan and the University of Alabama, the final winner of the $150,000 USD race.
A total of eight teams competed in the self-driving race. The race was conducted as a single elimination tournament consisting of multiple rounds of matches. Two cars took turns playing the role of defender and attacker, and each car attempted to outpace the other until one of them was unable to complete the mission.
Each team designed the algorithm to control its racecar, the Dallara-built AV-21, which can reach a speed of up to 173 mph, and make it safely drive around the track at high speeds without crashing into the other.
The event is the CES version of the Indy Autonomous Challenge, a competition that took place for the first time in October last year to encourage university students from around the world to develop complicated software for autonomous driving and advance relevant technologies. Team KAIST placed 4th at the Indy Autonomous Challenge, which qualified it to participate in this race.
“The technical level of the CES race is much higher than last October’s and we had a very tough race. We advanced to the semifinals for two consecutive races. I think our autonomous vehicle technology is proving itself to the world,” said Professor Shim.
Professor Shim’s research group has been working on the development of autonomous aerial and ground vehicles for the past 12 years. A self-driving car developed by the lab was certified by the South Korean government to run on public roads.
The vehicle the team used cost more than 1 million USD to build. Many of the other teams had to repair their vehicle more than once due to accidents and had to spend a lot on repairs. “We are the only one who did not have any accidents, and this is a testament to our technological prowess,” said Professor Shim.
He said the financial funding to purchase pricy parts and equipment for the racecar is always a challenge given the very tight research budget and absence of corporate sponsorships.
However, Professor Shim and his research group plan to participate in the next race in September and in the 2023 CES race.
“I think we need more systemic and proactive research and support systems to earn better results but there is nothing better than the group of passionate students who are taking part in this project with us,” Shim added.
2022.01.12 View 13372 -
 AI Weather Forecasting Research Center Opens
The Kim Jaechul Graduate School of AI in collaboration with the National Institute of Meteorological Sciences (NIMS) under the National Meteorological Administration launched the AI Weather Forecasting Research Center last month.
The KAIST AI Weather Forecasting Research Center headed by Professor Seyoung Yoon was established with funding from from the AlphaWeather Development Research Project of the National Institute of Meteorological Sciences. KAIST was finally selected asas the project facilitator.
AlphaWeather is an AI system that utilizes and analyzes approximately approximately 150,000 ,000 pieces of weather information per hour to help weather forecasters produce accurate weather forecasts. The research center is composed of three research teams with the following goals: (a) developdevelop AI technology for precipitation nowcasting, (b) developdevelop AI technology for accelerating physical process-based numerical models, and (c) develop dAI technology for supporting weather forecasters. The teams consist of 15 staff member members from NIMS and 61 researchers from the Kim Jaechul Graduate School of AI at KAIST.
The research center is developing an AI algorithm for precipitation nowcasting (with up to six hours of lead time), which uses satellite images, radar reflectivity, and data collected from weather stations. It is also developing an AI algorithm for correcting biases in the prediction results from multiple numerical models. Finally, it is Finally, it is developing AI technology that supports weather forecasters by standardizing and automating repetitive manual processes. After verification, the the results obtained will be used by by the Korean National Weather Service as a next-generation forecasting/special-reporting system intelligence engine from 2026.
2022.01.10 View 8224
AI Weather Forecasting Research Center Opens
The Kim Jaechul Graduate School of AI in collaboration with the National Institute of Meteorological Sciences (NIMS) under the National Meteorological Administration launched the AI Weather Forecasting Research Center last month.
The KAIST AI Weather Forecasting Research Center headed by Professor Seyoung Yoon was established with funding from from the AlphaWeather Development Research Project of the National Institute of Meteorological Sciences. KAIST was finally selected asas the project facilitator.
AlphaWeather is an AI system that utilizes and analyzes approximately approximately 150,000 ,000 pieces of weather information per hour to help weather forecasters produce accurate weather forecasts. The research center is composed of three research teams with the following goals: (a) developdevelop AI technology for precipitation nowcasting, (b) developdevelop AI technology for accelerating physical process-based numerical models, and (c) develop dAI technology for supporting weather forecasters. The teams consist of 15 staff member members from NIMS and 61 researchers from the Kim Jaechul Graduate School of AI at KAIST.
The research center is developing an AI algorithm for precipitation nowcasting (with up to six hours of lead time), which uses satellite images, radar reflectivity, and data collected from weather stations. It is also developing an AI algorithm for correcting biases in the prediction results from multiple numerical models. Finally, it is Finally, it is developing AI technology that supports weather forecasters by standardizing and automating repetitive manual processes. After verification, the the results obtained will be used by by the Korean National Weather Service as a next-generation forecasting/special-reporting system intelligence engine from 2026.
2022.01.10 View 8224