-
 KAIST Seals the Deal for Kenya KAIST Project
KAIST will participate in Kenya’s strategic economic development plan under the provision of a turnkey-based science and technology education consultancy for the establishment of the Kenya Advanced Institute of Science and Technology (Kenya KAIST).KAIST signed the contract on November 30 with the Konza Technopolis Development Authority to establish Kenya KAIST. Korea Eximbank will offer a 95 million USD loan to the Kenyan government for this project. The project will include the educational and architectural design and construction of Kenya KAIST. The campus will be constructed in the Konza Techno City nearby Nairobi by 2021, with the first batch of 200 graduate students starting classes in 2022. KAIST, in consortium with Samwoo and Sunjin architecture and engineering companies, will take the lead of the three-year project, with the kick-off ceremony planned at the end of next January in Nairobi. The Kenyan government plans to transform Kenya into a middle-income country under Vision 2030 through promoting science, technology, and innovation for national economic growth. Nicknamed Africa’s Silicon Savannah, Konza Techno City is a strategic science and technology hub to realize this vision. To this end, the medium-term plan set a goal to provide specialized research and training in various leading-edge engineering and advanced science fields.In the two-phase evaluation of the consultancy bidding, KAIST won preferred bidder status in the technical proposal evaluation, outbidding three other Korean consortia. Invited to the financial proposal bidding, the KAIST consortium successfully completed month-long contract negotiations with Kenya last week.KAIST will develop academic curricula for six initial departments (Mechanical Engineering, Electrical/Electronic Engineering, ICT Engineering, Chemical Engineering, Civil Engineering, and Agricultural Biotechnology), which will lay the ground work for engineering research and education in Kenya to meet emerging socioeconomic demands. In addition, KAIST will provide the education of basic sciences of math, physics, chemistry, and biology for students.It is also notable that the Kenyan government asked to develop an industry-academy cooperation program in Konza Techno City. It reflects the growing industrial needs of Kenya KAIST, which will be located in the center of the Konza Technopolis. It is anticipated that the technopolis will create 16,675 jobs in the medium term and over 200,000 after completion, positioning Kenya as an ICT hub within the region.KAIST also shares a similar history of establishment with Kenya KAIST, as it will be built with a foreign loan. KAIST, created by the Korean government in 1971 to drive the economic engine through advancement of science and technology with a six-million USD loan from USAID, has now become a donor institution that hands down science and technology education systems including the construction of campuses to underdeveloped countries.The successful case of KAIST has been benchmarked by many countries for years. For instance, KAIST set up the curriculum of the nuclear engineering program at the Khalifa University of Science and Technology in UAE in 2010. In China, Chongqing University of Technology is running its electrical engineering and computer science programs based on the educational systems and curricula offered by KAIST from 2015. In October, KAIST also signed an MOU with the Prince Mohammad Bin Salman College of Cyber Security, AI, and Advanced Technologies in Saudi Arabia to provide the undergraduate program for robotics.Among all these programs benchmarking KAIST, Kenya KAIST clearly stands out, for it is carrying out a turnkey-based project that encompasses every aspect of institution building ranging from educational curriculum development to campus construction and supervision.President Sung-Chul Shin is extremely excited about finalizing the deal, remarking, “It is of great significance that KAIST’s successful development model has carved out a unique path to becoming a global leading university that will benefit other countries. In only a half century, we have transitioned from a receiver to a donor institution, as the country itself has done.”“KAIST will spare no effort for Kenya KAIST to become a successful science and technology university that will play a crucial role in Kenya’s national development. I believe Kenya KAIST will be an exemplary case of an ODA (Official Development Assistance) project based on the development of science and technology to benefit underdeveloped countries,” he added.
2018.12.03 View 11967
KAIST Seals the Deal for Kenya KAIST Project
KAIST will participate in Kenya’s strategic economic development plan under the provision of a turnkey-based science and technology education consultancy for the establishment of the Kenya Advanced Institute of Science and Technology (Kenya KAIST).KAIST signed the contract on November 30 with the Konza Technopolis Development Authority to establish Kenya KAIST. Korea Eximbank will offer a 95 million USD loan to the Kenyan government for this project. The project will include the educational and architectural design and construction of Kenya KAIST. The campus will be constructed in the Konza Techno City nearby Nairobi by 2021, with the first batch of 200 graduate students starting classes in 2022. KAIST, in consortium with Samwoo and Sunjin architecture and engineering companies, will take the lead of the three-year project, with the kick-off ceremony planned at the end of next January in Nairobi. The Kenyan government plans to transform Kenya into a middle-income country under Vision 2030 through promoting science, technology, and innovation for national economic growth. Nicknamed Africa’s Silicon Savannah, Konza Techno City is a strategic science and technology hub to realize this vision. To this end, the medium-term plan set a goal to provide specialized research and training in various leading-edge engineering and advanced science fields.In the two-phase evaluation of the consultancy bidding, KAIST won preferred bidder status in the technical proposal evaluation, outbidding three other Korean consortia. Invited to the financial proposal bidding, the KAIST consortium successfully completed month-long contract negotiations with Kenya last week.KAIST will develop academic curricula for six initial departments (Mechanical Engineering, Electrical/Electronic Engineering, ICT Engineering, Chemical Engineering, Civil Engineering, and Agricultural Biotechnology), which will lay the ground work for engineering research and education in Kenya to meet emerging socioeconomic demands. In addition, KAIST will provide the education of basic sciences of math, physics, chemistry, and biology for students.It is also notable that the Kenyan government asked to develop an industry-academy cooperation program in Konza Techno City. It reflects the growing industrial needs of Kenya KAIST, which will be located in the center of the Konza Technopolis. It is anticipated that the technopolis will create 16,675 jobs in the medium term and over 200,000 after completion, positioning Kenya as an ICT hub within the region.KAIST also shares a similar history of establishment with Kenya KAIST, as it will be built with a foreign loan. KAIST, created by the Korean government in 1971 to drive the economic engine through advancement of science and technology with a six-million USD loan from USAID, has now become a donor institution that hands down science and technology education systems including the construction of campuses to underdeveloped countries.The successful case of KAIST has been benchmarked by many countries for years. For instance, KAIST set up the curriculum of the nuclear engineering program at the Khalifa University of Science and Technology in UAE in 2010. In China, Chongqing University of Technology is running its electrical engineering and computer science programs based on the educational systems and curricula offered by KAIST from 2015. In October, KAIST also signed an MOU with the Prince Mohammad Bin Salman College of Cyber Security, AI, and Advanced Technologies in Saudi Arabia to provide the undergraduate program for robotics.Among all these programs benchmarking KAIST, Kenya KAIST clearly stands out, for it is carrying out a turnkey-based project that encompasses every aspect of institution building ranging from educational curriculum development to campus construction and supervision.President Sung-Chul Shin is extremely excited about finalizing the deal, remarking, “It is of great significance that KAIST’s successful development model has carved out a unique path to becoming a global leading university that will benefit other countries. In only a half century, we have transitioned from a receiver to a donor institution, as the country itself has done.”“KAIST will spare no effort for Kenya KAIST to become a successful science and technology university that will play a crucial role in Kenya’s national development. I believe Kenya KAIST will be an exemplary case of an ODA (Official Development Assistance) project based on the development of science and technology to benefit underdeveloped countries,” he added.
2018.12.03 View 11967 -
 From Concept to Reality: Changing Color of Light Using a Spatiotemporal Boundary
(from left: Professor Bumki Min, PhD candidate Jaehyeon Son and PhD Kanghee Lee)
A KAIST team developed an optical technique to change the color (frequency) of light using a spatiotemporal boundary. The research focuses on realizing a spatiotemporal boundary with a much higher degree of freedom than the results of previous studies by fabricating a thin metal structure on a semiconductor surface. Such a spatiotemporal boundary is expected to be applicable to an ultra-thin film type optical device capable of changing the color of light.
The optical frequency conversion device plays a key role in precision measurement and communication technology, and the device has been developed mainly based on optical nonlinearity.
If the intensity of light is very strong, the optical medium responds nonlinearly so the nonlinear optical phenomena, such as frequency doubling or frequency mixing, can be observed. Such optical nonlinear phenomena are realized usually by the interaction between a high-intensity laser and a nonlinear medium.
As an alternative method frequency conversion is observed by temporally modifying the optical properties of the medium through which light travels using an external stimulus. Since frequency conversion in this way can be observed even in weak light, such a technique could be particularly useful in communication technology.
However, rapid optical property modification of the medium by an external stimulus and subsequent light frequency conversion techniques have been researched only in the pertubative regime, and it has been difficult to realize these theoretical results in practical applications.
To realize such a conceptual idea, Professor Bumki Min from the Department of Mechanical Engineering and his team collaborated with Professor Wonju Jeon from the Department of Mechanical Engineering and Professor Fabian Rotermund from the Department of Physics. They developed an artificial optical material (metamaterial) by arranging a metal microstructure that mimics an atomic structure and succeeded in creating a spatiotemporal boundary by changing the optical property of the artificial material abruptly.
While previous studies only slightly modified the refractive index of the medium, this study provided a spatiotemporal boundary as a platform for freely designing and changing the spectral properties of the medium. Using this, the research team developed a device that can control the frequency of light to a large degree.
The research team said a spatiotemporal boundary, which was only conceptually considered in previous research and realized in the pertubative regime, was developed as a step that can be realized and applied.
Professor Min said, “The frequency conversion of light becomes designable and predictable, so our research could be applied in many optical applications. This research will present a new direction for time-variant media research projects in the field of optics.”
This research, led by PhD Kanghee Lee and PhD candidate Jaehyeon Son, was published online in Nature Photonics on October 8, 2018.
This work was supported by the National Research Foundation of Korea (NRF) through the government of Korea. The work was also supported by the Center for Advanced Meta-Materials (CAMM) funded by the Korea Government (MSIP) as the Global Frontier Project (NRF-2014M3A6B3063709).
Figure 1. The frequency conversion process of light using a spatiotemporal boundary.
Figure 2. The complex amplitude of light at the converted frequency with the variation of a spatiotemporal boundary.
2018.11.29 View 9410
From Concept to Reality: Changing Color of Light Using a Spatiotemporal Boundary
(from left: Professor Bumki Min, PhD candidate Jaehyeon Son and PhD Kanghee Lee)
A KAIST team developed an optical technique to change the color (frequency) of light using a spatiotemporal boundary. The research focuses on realizing a spatiotemporal boundary with a much higher degree of freedom than the results of previous studies by fabricating a thin metal structure on a semiconductor surface. Such a spatiotemporal boundary is expected to be applicable to an ultra-thin film type optical device capable of changing the color of light.
The optical frequency conversion device plays a key role in precision measurement and communication technology, and the device has been developed mainly based on optical nonlinearity.
If the intensity of light is very strong, the optical medium responds nonlinearly so the nonlinear optical phenomena, such as frequency doubling or frequency mixing, can be observed. Such optical nonlinear phenomena are realized usually by the interaction between a high-intensity laser and a nonlinear medium.
As an alternative method frequency conversion is observed by temporally modifying the optical properties of the medium through which light travels using an external stimulus. Since frequency conversion in this way can be observed even in weak light, such a technique could be particularly useful in communication technology.
However, rapid optical property modification of the medium by an external stimulus and subsequent light frequency conversion techniques have been researched only in the pertubative regime, and it has been difficult to realize these theoretical results in practical applications.
To realize such a conceptual idea, Professor Bumki Min from the Department of Mechanical Engineering and his team collaborated with Professor Wonju Jeon from the Department of Mechanical Engineering and Professor Fabian Rotermund from the Department of Physics. They developed an artificial optical material (metamaterial) by arranging a metal microstructure that mimics an atomic structure and succeeded in creating a spatiotemporal boundary by changing the optical property of the artificial material abruptly.
While previous studies only slightly modified the refractive index of the medium, this study provided a spatiotemporal boundary as a platform for freely designing and changing the spectral properties of the medium. Using this, the research team developed a device that can control the frequency of light to a large degree.
The research team said a spatiotemporal boundary, which was only conceptually considered in previous research and realized in the pertubative regime, was developed as a step that can be realized and applied.
Professor Min said, “The frequency conversion of light becomes designable and predictable, so our research could be applied in many optical applications. This research will present a new direction for time-variant media research projects in the field of optics.”
This research, led by PhD Kanghee Lee and PhD candidate Jaehyeon Son, was published online in Nature Photonics on October 8, 2018.
This work was supported by the National Research Foundation of Korea (NRF) through the government of Korea. The work was also supported by the Center for Advanced Meta-Materials (CAMM) funded by the Korea Government (MSIP) as the Global Frontier Project (NRF-2014M3A6B3063709).
Figure 1. The frequency conversion process of light using a spatiotemporal boundary.
Figure 2. The complex amplitude of light at the converted frequency with the variation of a spatiotemporal boundary.
2018.11.29 View 9410 -
 KAIST Shows Strong Performance in Crypto Contest Korea 2018
(Awardees at the ceremony for Crypto Contest Korea 2018)
A paper titled “Indifferentiability of Truncated Random Permutations” by PhD candidate Wonseok Choi and MS candidate Byeonghak Lee (under Professor Jooyoung Lee) from the KAIST Graduate School of Information Security (GSIS) won first place in Crypto Contest Korea 2018. Byeonghak Lee became a repeat winner since his paper titled “Tweakable Block Ciphers Secure Beyond the Birthday Bound in the Ideal Cipher Model” also received an award at Crypto Contest Korea 2017.
The contest, hosted by the Korea Cryptography Forum, the Korea Institute of Information Security & Cryptology, and the National Security Research Institute and sponsored by the National Intelligence Service, was held for promoting cryptography in Korea. The total prize money is fifty million won with ten million won going to the first place winners.
The contest was divided into three divisions: paper, problem solving, and idea. Among the three divisions, first place came from the paper division only.
Besides first place, KAIST students showed outstanding performance in the contest. PhD candidate Seongkwang Kim received participation prize while he also received special prizes with MS candidate Yeongmin Lee. The hacking club GoN (under Professor Sang Kil Cha), comprised of undergraduate students from the GSIS was awarded the grand prize in the division of problem solving.
The award ceremony was held during the Future Crypto Workshop 2018 on November 15. The awards ceremony for Crypto Expert Korea 2018 were also held there, and PhD candidate Ji-Eun Lee from the School of Computing and Byeonghak Lee received awards, the grand prize and runner-up prize respectively.
2018.11.27 View 10723
KAIST Shows Strong Performance in Crypto Contest Korea 2018
(Awardees at the ceremony for Crypto Contest Korea 2018)
A paper titled “Indifferentiability of Truncated Random Permutations” by PhD candidate Wonseok Choi and MS candidate Byeonghak Lee (under Professor Jooyoung Lee) from the KAIST Graduate School of Information Security (GSIS) won first place in Crypto Contest Korea 2018. Byeonghak Lee became a repeat winner since his paper titled “Tweakable Block Ciphers Secure Beyond the Birthday Bound in the Ideal Cipher Model” also received an award at Crypto Contest Korea 2017.
The contest, hosted by the Korea Cryptography Forum, the Korea Institute of Information Security & Cryptology, and the National Security Research Institute and sponsored by the National Intelligence Service, was held for promoting cryptography in Korea. The total prize money is fifty million won with ten million won going to the first place winners.
The contest was divided into three divisions: paper, problem solving, and idea. Among the three divisions, first place came from the paper division only.
Besides first place, KAIST students showed outstanding performance in the contest. PhD candidate Seongkwang Kim received participation prize while he also received special prizes with MS candidate Yeongmin Lee. The hacking club GoN (under Professor Sang Kil Cha), comprised of undergraduate students from the GSIS was awarded the grand prize in the division of problem solving.
The award ceremony was held during the Future Crypto Workshop 2018 on November 15. The awards ceremony for Crypto Expert Korea 2018 were also held there, and PhD candidate Ji-Eun Lee from the School of Computing and Byeonghak Lee received awards, the grand prize and runner-up prize respectively.
2018.11.27 View 10723 -
 Vietnamese Alumni of Korean S&T Universities Gather in Hanoi
(Vietnamese KAIST alumni gather in Hanoi on November 24.)
(Dr.Huong Minh Nguyen at the Institute of Biotechnology in the Vietnam Academy of Science and Technology (VAST). She is the representative of Vietnamese KAIST alumni.)
Some came from Ho Chi Minh and some even flew in from Singapore. For those KAIST alumni who gathered in Hanoi, the trip was well worth it. More than 100 Vietnamese alumni of KAIST, GIST, and UST attended the reunion in Hanoi on November 24. Presidents and vice president from three universities welcomed them and celebrated their successful careers after returning home or starting careers in other countries.
The reunion was co-hosted by KAIST, UST, and GIST in an effort to make a platform for continued networking for scientists who have studied at Korea’s science and technology universities. This joint reunion will be expected to include other science and technology universities and institutes in the future.
Among 1,873 international KAIST alumni from 106 countries, the number of Vietnamese graduates is the most dominant with 262 alumni, 14% of the total international alumni. Welcoming them, KAIST Vice President Soohyun Kim said that he was very impressed that all of the alumni are making a very impressive stride in their fields.
“You will be a big asset to make your country grow. You will also be a bridge for future collaborations with your institutions and KAIST and Korea. Vietnam holds great potential for future prosperity especially in science and technology and we look forward to seeing this network continue to benefit both countries.” Vice President Kim said all of the presidents shared the idea to make this gathering a regular event. “Other S&T universities will join to hold joint reunions in other countries in the future,” he added.
Dr. Huong Minh Nguyen at the Institute of Biotechnology in the Vietnam Academy of Science and Technology (VAST) is one of 22 KAIST alumni who joined the reunion and supported the idea. She is also the representative of Vietnamese KAIST alumni. Dr. Nguyen, who earned her MS and PhD in the Department of Biological Sciences in 2013, spent five and half years at KAIST. “All the members at the lab were taking care of each other. When I joined the lab back then, they treated me as a ‘baby sister’ in a family and our relationship grew like a sister and brotherhood. I still appreciate the bonding relationship we could make with our colleagues.”
She cites a supporting culture, a competent educational system that places a focus on practicality, and rich resources that help researchers try whatever they want as the distinct advantages of a KAIST education.
“I never heard my professor saying “No” to any of my suggestions in conducting research. Professors and lab members put all their efforts, resources, and facilities to getting my research started. They helped me obtain all the resources that I needed. It was a huge encouragement to me,” she added.
She started her Masters, along with four Vietnamese colleagues, right after graduating from the Hanoi University of Natural Sciences, a top science university in Vietnam. Their experience at KAIST during a 4-week exchange program during undergraduates made them decide their academic destination without any doubt. All of them finished their PhDs in 2013. Two of them moved back home and the two others are now in the US and Germany as postdoctoral fellows.
“At that time, I could go to other countries for my further studies. But I already experienced KAIST for a month when I was an undergraduate, so I was not hesitant to go to KAIST. All of the classes are in English so, for students who do not speak in Korean, language does not bring any problems in studying and conducting research.”
She said that KAIST alumni are enjoying very successful careers in Vietnam and many foreign countries. “We do not have any problems choosing our careers back home and other countries we wanted to work in.”
However, like many PhD candidates who feel pressure about their studies and an uncertain future, her days at KAIST also included challenging times while adjusting to Korean culture. She took Korean classes for three semesters voluntarily at the KAIST Language Center to better understand Korea and her lab members. Her efforts paid off well. She could easily communicate with her colleagues and felt she became a real part of the inner group. But the stress remained to prove herself in research and she still had to deal with some bias.
“I know some people think Vietnam is behind Korea. Many people think that we are not as good as Korean students because we are from Vietnam. I desperately wanted to prove that I am as good as my peers.” Studying together with Korean and other international PhD candidates, she realized that everyone has their own purposes and pressures. “Even though there are minor differences, every PhD candidate has the same issues with their uncertain futures. It was quite comforting when we shared that it’s not only my problem. To understand that it’s a problem we all share comforts us a lot and we came to support each other.
To better help students release such pressure and stress, she said the university needs to create more diverse institutional channels to communicate with them. “Looking back, I was younger and less competent to speak up about when we were stressed and needed to ask for help. I hope students can begin to release their pressure and stress through diverse channels and resolve the problems,” she said.
Asked about her future plans, she replied, “I don’t think I can do anything better than what I am doing now. I enjoy what I am doing now at my institute. But in the near very future, I want to visit Korea and KAIST again.”
2018.11.27 View 4616
Vietnamese Alumni of Korean S&T Universities Gather in Hanoi
(Vietnamese KAIST alumni gather in Hanoi on November 24.)
(Dr.Huong Minh Nguyen at the Institute of Biotechnology in the Vietnam Academy of Science and Technology (VAST). She is the representative of Vietnamese KAIST alumni.)
Some came from Ho Chi Minh and some even flew in from Singapore. For those KAIST alumni who gathered in Hanoi, the trip was well worth it. More than 100 Vietnamese alumni of KAIST, GIST, and UST attended the reunion in Hanoi on November 24. Presidents and vice president from three universities welcomed them and celebrated their successful careers after returning home or starting careers in other countries.
The reunion was co-hosted by KAIST, UST, and GIST in an effort to make a platform for continued networking for scientists who have studied at Korea’s science and technology universities. This joint reunion will be expected to include other science and technology universities and institutes in the future.
Among 1,873 international KAIST alumni from 106 countries, the number of Vietnamese graduates is the most dominant with 262 alumni, 14% of the total international alumni. Welcoming them, KAIST Vice President Soohyun Kim said that he was very impressed that all of the alumni are making a very impressive stride in their fields.
“You will be a big asset to make your country grow. You will also be a bridge for future collaborations with your institutions and KAIST and Korea. Vietnam holds great potential for future prosperity especially in science and technology and we look forward to seeing this network continue to benefit both countries.” Vice President Kim said all of the presidents shared the idea to make this gathering a regular event. “Other S&T universities will join to hold joint reunions in other countries in the future,” he added.
Dr. Huong Minh Nguyen at the Institute of Biotechnology in the Vietnam Academy of Science and Technology (VAST) is one of 22 KAIST alumni who joined the reunion and supported the idea. She is also the representative of Vietnamese KAIST alumni. Dr. Nguyen, who earned her MS and PhD in the Department of Biological Sciences in 2013, spent five and half years at KAIST. “All the members at the lab were taking care of each other. When I joined the lab back then, they treated me as a ‘baby sister’ in a family and our relationship grew like a sister and brotherhood. I still appreciate the bonding relationship we could make with our colleagues.”
She cites a supporting culture, a competent educational system that places a focus on practicality, and rich resources that help researchers try whatever they want as the distinct advantages of a KAIST education.
“I never heard my professor saying “No” to any of my suggestions in conducting research. Professors and lab members put all their efforts, resources, and facilities to getting my research started. They helped me obtain all the resources that I needed. It was a huge encouragement to me,” she added.
She started her Masters, along with four Vietnamese colleagues, right after graduating from the Hanoi University of Natural Sciences, a top science university in Vietnam. Their experience at KAIST during a 4-week exchange program during undergraduates made them decide their academic destination without any doubt. All of them finished their PhDs in 2013. Two of them moved back home and the two others are now in the US and Germany as postdoctoral fellows.
“At that time, I could go to other countries for my further studies. But I already experienced KAIST for a month when I was an undergraduate, so I was not hesitant to go to KAIST. All of the classes are in English so, for students who do not speak in Korean, language does not bring any problems in studying and conducting research.”
She said that KAIST alumni are enjoying very successful careers in Vietnam and many foreign countries. “We do not have any problems choosing our careers back home and other countries we wanted to work in.”
However, like many PhD candidates who feel pressure about their studies and an uncertain future, her days at KAIST also included challenging times while adjusting to Korean culture. She took Korean classes for three semesters voluntarily at the KAIST Language Center to better understand Korea and her lab members. Her efforts paid off well. She could easily communicate with her colleagues and felt she became a real part of the inner group. But the stress remained to prove herself in research and she still had to deal with some bias.
“I know some people think Vietnam is behind Korea. Many people think that we are not as good as Korean students because we are from Vietnam. I desperately wanted to prove that I am as good as my peers.” Studying together with Korean and other international PhD candidates, she realized that everyone has their own purposes and pressures. “Even though there are minor differences, every PhD candidate has the same issues with their uncertain futures. It was quite comforting when we shared that it’s not only my problem. To understand that it’s a problem we all share comforts us a lot and we came to support each other.
To better help students release such pressure and stress, she said the university needs to create more diverse institutional channels to communicate with them. “Looking back, I was younger and less competent to speak up about when we were stressed and needed to ask for help. I hope students can begin to release their pressure and stress through diverse channels and resolve the problems,” she said.
Asked about her future plans, she replied, “I don’t think I can do anything better than what I am doing now. I enjoy what I am doing now at my institute. But in the near very future, I want to visit Korea and KAIST again.”
2018.11.27 View 4616 -
 Silk Adhesive Paves the Way for Epidermal Electronics
(from left: Dr. Ji-Won Seo, Professor Hyunjoo Jenny Lee and PhD candidate, Hyojung Kim)
Producing effective epidermal electronics requires a strong, biocompatible interface between a biological surface and a sensor. Here, a KAIST team employed a calcium-modified silk fibroin as a biocompatible and strong adhesive. This technology led to the development of epidermal electronics with strong adhesion for patients who need drug injections and physiological monitoring over a long time.
Recently, biocompatible silk fibroins has been increasingly used for flexible substrates and water-soluble sacrificial layers because they allow structural modifications and are biodegradable. From previous studies, the team discovered the adhesive properties of silk fibroin via metal chelate bonding and the water-capturing of Ca ions.
Professor Hyunjoo Jenny Lee from the School of Electrical Engineering and her team explored ways to develop reusable, water-degradable, biocompatible and conductive epidermal electronics that can be attached to the human skin for long-term use. To overcome the limitations of conventional silk fibroin, the team introduced Ca ions to modify silk fibroin into a strong and biocompatible adhesive.
Calcium ions adopted in silk fibroins serve to capture water and enhance the cohesion force through metal chelation. Therefore, this endows viscoelasticity to previously a firm silk fibroin. This modified silk fibroin exhibits strong viscoelasticity and strong adhesiveness when physically attached to the human skin and various polymer substrates. Their developed silk adhesive is reusable, water-degradable, biocompatible, and conductive.
To test the effectiveness, the team employed the silk adhesive to fabricate an epidermal capacitive touch sensor that can be attached to the human skin. They verified the reusability of the sensor by performing attachment and detachment tests. They also confirmed that the physical adhesion of the Ca-modified silk facilitates its reusability and possesses high peel strength.
Furthermore, they tested the stretchability of the silk adhesive on bladder tissue. Although it is not an epidermal skin, bladder tissue is highly stretchable. Hence, it is a perfect target to measure the resistance-strain characteristic of the silk adhesive. When the bladder tissue was stretched, the resistive strain epidermal sensor corresponded to the tensile strain.
Showing high biocompatibility, the silk adhesive is suitable for interfacing with the human skin for a long period of time. Therefore, it can also be applied to a drug delivery epidermal system as well as an electrocardiogram (ECG) epidermal sensor.
Professor Lee said, “We are opening up a novel use for silk by developing reusable and biodegradable silk adhesive using biocompatible silk fibroin. This technology will contribute to the development of next-generation epidermal electronics as well as drug delivery systems.
This research, led by Dr. Ji-Won Seo and a PhD candidate, Hyojung Kim, was published in Advanced Functional Materials on September 5, 2018.
Figure 1. Schematic and photograph of a hydrogel patch adhered on the human skin through the silk adhesive
Figure 2. Cover page of Advanced Functional Materials
2018.11.21 View 7349
Silk Adhesive Paves the Way for Epidermal Electronics
(from left: Dr. Ji-Won Seo, Professor Hyunjoo Jenny Lee and PhD candidate, Hyojung Kim)
Producing effective epidermal electronics requires a strong, biocompatible interface between a biological surface and a sensor. Here, a KAIST team employed a calcium-modified silk fibroin as a biocompatible and strong adhesive. This technology led to the development of epidermal electronics with strong adhesion for patients who need drug injections and physiological monitoring over a long time.
Recently, biocompatible silk fibroins has been increasingly used for flexible substrates and water-soluble sacrificial layers because they allow structural modifications and are biodegradable. From previous studies, the team discovered the adhesive properties of silk fibroin via metal chelate bonding and the water-capturing of Ca ions.
Professor Hyunjoo Jenny Lee from the School of Electrical Engineering and her team explored ways to develop reusable, water-degradable, biocompatible and conductive epidermal electronics that can be attached to the human skin for long-term use. To overcome the limitations of conventional silk fibroin, the team introduced Ca ions to modify silk fibroin into a strong and biocompatible adhesive.
Calcium ions adopted in silk fibroins serve to capture water and enhance the cohesion force through metal chelation. Therefore, this endows viscoelasticity to previously a firm silk fibroin. This modified silk fibroin exhibits strong viscoelasticity and strong adhesiveness when physically attached to the human skin and various polymer substrates. Their developed silk adhesive is reusable, water-degradable, biocompatible, and conductive.
To test the effectiveness, the team employed the silk adhesive to fabricate an epidermal capacitive touch sensor that can be attached to the human skin. They verified the reusability of the sensor by performing attachment and detachment tests. They also confirmed that the physical adhesion of the Ca-modified silk facilitates its reusability and possesses high peel strength.
Furthermore, they tested the stretchability of the silk adhesive on bladder tissue. Although it is not an epidermal skin, bladder tissue is highly stretchable. Hence, it is a perfect target to measure the resistance-strain characteristic of the silk adhesive. When the bladder tissue was stretched, the resistive strain epidermal sensor corresponded to the tensile strain.
Showing high biocompatibility, the silk adhesive is suitable for interfacing with the human skin for a long period of time. Therefore, it can also be applied to a drug delivery epidermal system as well as an electrocardiogram (ECG) epidermal sensor.
Professor Lee said, “We are opening up a novel use for silk by developing reusable and biodegradable silk adhesive using biocompatible silk fibroin. This technology will contribute to the development of next-generation epidermal electronics as well as drug delivery systems.
This research, led by Dr. Ji-Won Seo and a PhD candidate, Hyojung Kim, was published in Advanced Functional Materials on September 5, 2018.
Figure 1. Schematic and photograph of a hydrogel patch adhered on the human skin through the silk adhesive
Figure 2. Cover page of Advanced Functional Materials
2018.11.21 View 7349 -
 Distinguished Professor Lee Re-Appointed As Co-Chair of the Global Future Council on Biotechnology
(Distinguished Professor Sang Yup Lee)
Distinguished Professor Sang Yup Lee from the Department of Chemical and Biomolecular Engineering was re-appointed as the co-chair of the Global Future Council on Biotechnology at the World Economic Forum during the annual GFC meeting in Dubai, UAE last week. Elizabeth O’Day, founder and CEO of Olaris Therapeutics, will co-chair the council with him for two years.
Professor Lee served as the inaugural co-chair of the council for two years from 2016 with Professor Feng Zhang from the Broad Institute of MIT and Harvard.
The World Economic Forum’s Global Future Councils on Biotechnology is comprised of 14 members and aims to identify policy opportunities to help accelerate new biotechnological discoveries and applications while guiding dialogue with consumers about their implications.
Professor Lee, who has made significant research breakthroughs in the field of systems metabolic engineering, received the George Washington Carver Award, the PV Danckwerts Memorial Lecture Award, and the Eni Award this year.
2018.11.19 View 4466
Distinguished Professor Lee Re-Appointed As Co-Chair of the Global Future Council on Biotechnology
(Distinguished Professor Sang Yup Lee)
Distinguished Professor Sang Yup Lee from the Department of Chemical and Biomolecular Engineering was re-appointed as the co-chair of the Global Future Council on Biotechnology at the World Economic Forum during the annual GFC meeting in Dubai, UAE last week. Elizabeth O’Day, founder and CEO of Olaris Therapeutics, will co-chair the council with him for two years.
Professor Lee served as the inaugural co-chair of the council for two years from 2016 with Professor Feng Zhang from the Broad Institute of MIT and Harvard.
The World Economic Forum’s Global Future Councils on Biotechnology is comprised of 14 members and aims to identify policy opportunities to help accelerate new biotechnological discoveries and applications while guiding dialogue with consumers about their implications.
Professor Lee, who has made significant research breakthroughs in the field of systems metabolic engineering, received the George Washington Carver Award, the PV Danckwerts Memorial Lecture Award, and the Eni Award this year.
2018.11.19 View 4466 -
 Novel Strategies to Transform a Commercially Available Iboga Alkaloid to Post-Iboga Alkaloids
(PhD candidate HyeonggeunLim, Professor Sunkyu Han, PhD candidate Sikwang Seong)
KAIST chemists have synthesized seven different iboga and post-iboga natural products from commercially available catharanthine by mirroring nature’s biosynthetic post-modification of the iboga skeleton. They devised a novel strategy to biosynthesize the natural products via a series of selective and efficient oxidation and rearrangement reactions. This will serve as a stepping stone for developing therapeutic medications against cancer and narcotics addiction.
The research team, led by Professor Sunkyu Han, conceptualized and coined the term “Post-Iboga” alkaloids to describe the natural products that are biosynthetically derived from iboga-type alkaloids, which are composed of rearranged indole and/or isoquinuclidine backbones.
Iboga alkaloids have attracted significant attention from the scientific community due to their intriguing polycyclic structures and potential therapeutic uses against drug addictions. Nature has evolved to add architectural repertoires to this family of secondary metabolites by diversifying the iboga frameworks.
Notable examples are the FDA-approved anticancer drugs vinblastine and vincristine, both derived by the oxidative dimerization of catharanthine and vindoline subunits. Admittedly, synthetic foci toward the biosynthetic iboga-derivatives have historically been on these aforementioned dimeric natural products.
Recent natural product isolation studies on Tabernaemontana corymbosa and Ervatamia officinalis species have resulted in discoveries of various secondary metabolites that are biosynthetically derived from iboga alkaloids. These recent outbursts of iboga-derived natural product isolation reports have kindled interests toward these family of natural products.
The research team utilized (+)-catharanthine, the starting material for the industrial production of the anticancer drug Navelbine®. Well-orchestrated oxidations at the C19 position and the indole moiety of the catharanthine derivative, followed by differential rearrangements under acidic conditions, provided synthetic samples of voatinggine and tabertinggine respectively.
On the other hand, opportune oxidations at the C19 position and the alpha position of the tertiary amine moiety of the catharantine derivative, followed by a transhemiaminalization, produced the first synthetic sample of chippiine/dippinine-type natural product, dippinine B.
It is important to note that the chippiine and dippinine-type alkaloids have been targeted among synthetic chemists for over 30 years but had not succumbed to synthesis prior to this report.
Professor Han believes that their study will serve as a blueprint for further explorations of the synthesis, biosynthesis, and pharmacology of this emerging family of natural products. This study was published in Chem on November 15, 2018 (DOI: 10.1016/j.chempr.2018.10.009).
2018.11.16 View 6584
Novel Strategies to Transform a Commercially Available Iboga Alkaloid to Post-Iboga Alkaloids
(PhD candidate HyeonggeunLim, Professor Sunkyu Han, PhD candidate Sikwang Seong)
KAIST chemists have synthesized seven different iboga and post-iboga natural products from commercially available catharanthine by mirroring nature’s biosynthetic post-modification of the iboga skeleton. They devised a novel strategy to biosynthesize the natural products via a series of selective and efficient oxidation and rearrangement reactions. This will serve as a stepping stone for developing therapeutic medications against cancer and narcotics addiction.
The research team, led by Professor Sunkyu Han, conceptualized and coined the term “Post-Iboga” alkaloids to describe the natural products that are biosynthetically derived from iboga-type alkaloids, which are composed of rearranged indole and/or isoquinuclidine backbones.
Iboga alkaloids have attracted significant attention from the scientific community due to their intriguing polycyclic structures and potential therapeutic uses against drug addictions. Nature has evolved to add architectural repertoires to this family of secondary metabolites by diversifying the iboga frameworks.
Notable examples are the FDA-approved anticancer drugs vinblastine and vincristine, both derived by the oxidative dimerization of catharanthine and vindoline subunits. Admittedly, synthetic foci toward the biosynthetic iboga-derivatives have historically been on these aforementioned dimeric natural products.
Recent natural product isolation studies on Tabernaemontana corymbosa and Ervatamia officinalis species have resulted in discoveries of various secondary metabolites that are biosynthetically derived from iboga alkaloids. These recent outbursts of iboga-derived natural product isolation reports have kindled interests toward these family of natural products.
The research team utilized (+)-catharanthine, the starting material for the industrial production of the anticancer drug Navelbine®. Well-orchestrated oxidations at the C19 position and the indole moiety of the catharanthine derivative, followed by differential rearrangements under acidic conditions, provided synthetic samples of voatinggine and tabertinggine respectively.
On the other hand, opportune oxidations at the C19 position and the alpha position of the tertiary amine moiety of the catharantine derivative, followed by a transhemiaminalization, produced the first synthetic sample of chippiine/dippinine-type natural product, dippinine B.
It is important to note that the chippiine and dippinine-type alkaloids have been targeted among synthetic chemists for over 30 years but had not succumbed to synthesis prior to this report.
Professor Han believes that their study will serve as a blueprint for further explorations of the synthesis, biosynthesis, and pharmacology of this emerging family of natural products. This study was published in Chem on November 15, 2018 (DOI: 10.1016/j.chempr.2018.10.009).
2018.11.16 View 6584 -
 New Anisotropic Conductive Film for Ultra-Fine Pitch Assembly Applications
(Professor Paik(right) and PhD Candidate Yoon)
Higher resolution display electronic devices increasingly needs ultra-fine pitch assemblies. On that account, display driver interconnection technology has become a major challenge for upscaling display electronics.
Researchers have moved to one step closer to realizing ultra-fine resolution for displays with a novel thermoplastic anchoring polymer layer structure. This new structure can significantly improve the ultra-fine pitch interconnection by effectively suppressing the movement of conductive particles. This film is expected to be applied to various mobile devices, large-sized OLED panels, and VR, among others.
A research team under Professor Kyung-Wook Paik in the Department of Materials developed an anchoring polymer layer structure that can effectively suppress the movement of conductive particles during the bonding process of the anisotropic conductive films (ACFs). The new structure will significantly improve the conductive particle capture rate, addressing electrical short problems in the ultra-fine pitch assembly process.
During the ultra-fine pitch bonding process, the conductive particles of conventional ACFs agglomerate between bumps and cause electrical short circuits. To overcome the electrical shortage problem caused by the free movement of conductive particles, higher tensile strength anchoring polymer layers incorporated with conductive particles were introduced into the ACFs to effectively prevent conductive particle movement.
The team used nylon to produce a single layer film with well-distributed and incorporated conductive particles. The higher tensile strength of nylon completely suppressed the movement of conductive particles, raising the capture rate of conductive particles from 33% of the conventional ACFs to 90%. The nylon films showed no short circuit problem during the Chip on Glass assembly. Even more, they obtained excellent electrical conductivity, high reliability, and low cost ACFs during the ultra-fine pitch applications.
Professor Paik believes this new type of ACFs can further be applied not only to VR, 4K and 8K UHD display products, but also to large-size OLED panels and mobile devices.
His team completed a prototype of the film supported by the ‘H&S High-Tech,’ a domestic company and the ‘Innopolis Foundation.’ The study, whose first author is PhD candidate Dal-Jin Yoon, is described in the October issue of IEEE TCPMT.
Figure 1: Schematic process of APL structure fabrication.
Figure 2: Proto-type production of APL ACFs.
2018.11.13 View 7120
New Anisotropic Conductive Film for Ultra-Fine Pitch Assembly Applications
(Professor Paik(right) and PhD Candidate Yoon)
Higher resolution display electronic devices increasingly needs ultra-fine pitch assemblies. On that account, display driver interconnection technology has become a major challenge for upscaling display electronics.
Researchers have moved to one step closer to realizing ultra-fine resolution for displays with a novel thermoplastic anchoring polymer layer structure. This new structure can significantly improve the ultra-fine pitch interconnection by effectively suppressing the movement of conductive particles. This film is expected to be applied to various mobile devices, large-sized OLED panels, and VR, among others.
A research team under Professor Kyung-Wook Paik in the Department of Materials developed an anchoring polymer layer structure that can effectively suppress the movement of conductive particles during the bonding process of the anisotropic conductive films (ACFs). The new structure will significantly improve the conductive particle capture rate, addressing electrical short problems in the ultra-fine pitch assembly process.
During the ultra-fine pitch bonding process, the conductive particles of conventional ACFs agglomerate between bumps and cause electrical short circuits. To overcome the electrical shortage problem caused by the free movement of conductive particles, higher tensile strength anchoring polymer layers incorporated with conductive particles were introduced into the ACFs to effectively prevent conductive particle movement.
The team used nylon to produce a single layer film with well-distributed and incorporated conductive particles. The higher tensile strength of nylon completely suppressed the movement of conductive particles, raising the capture rate of conductive particles from 33% of the conventional ACFs to 90%. The nylon films showed no short circuit problem during the Chip on Glass assembly. Even more, they obtained excellent electrical conductivity, high reliability, and low cost ACFs during the ultra-fine pitch applications.
Professor Paik believes this new type of ACFs can further be applied not only to VR, 4K and 8K UHD display products, but also to large-size OLED panels and mobile devices.
His team completed a prototype of the film supported by the ‘H&S High-Tech,’ a domestic company and the ‘Innopolis Foundation.’ The study, whose first author is PhD candidate Dal-Jin Yoon, is described in the October issue of IEEE TCPMT.
Figure 1: Schematic process of APL structure fabrication.
Figure 2: Proto-type production of APL ACFs.
2018.11.13 View 7120 -
 OUIC Presents the Six Most Promising Techs Transferrable to Local SMEs
KAIST will showcase the six most promising technologies for small and medium enterprises (SMEs) on November 14 in the Academic Cultural Complex. To strengthen the competitive edge of local SMEs in Daejeon, the Office of University-Industry made a survey of their technological needs and came up with the six most promising technologies. Developers will introduce their technologies during the session.Besides the introduction of the promising technologies, the session will also provide a program named University to Business (U2B) to match up technologies according to the SMEs’ needs. SMEs who wish to engage in technology transfers can receive counseling and other support programs during the session.First, Professor Seok-Hyung Bae from the Department of Industrial Design will present a technology for controlling cooperation robots. Professor Bae inserted flexible materials between the controllers to allow robots to use both hands stably and operate more accurately and swiftly. It can be applied to automatic robots, industrial robots, and service robots.Professor Hyun Myung from the Department of Civil & Environmental Engineering will demonstrate a robot navigation system in a dynamic indoor and outdoor environment, which can be applied to robotics in logistics, smart factories, and autonomous vehicles. Providing robust simultaneous localization and mapping systems, this technology shows high-performing navigation with low-cost sensors.Meanwhile, Professor Siyoung Choi from the Department of Chemical and Biomolecular Engineering will introduce a technology for forming stable adhesive emulsions. An emulsion is a stable mixture of water and oil. Conventionally, a small amount of surfactant is added to stabilize an emulsion. Here, Professor Choi developed a stable emulsion system without using any chemical substances. This technology can be applied to various fields, including the cosmetics, pharmaceutical, semiconductor, and painting industries. The session will also present smart IoTs platform technology developed by Professor Jinhong Yang from the KAIST Institute for IT Convergence. His technology minimizes errors occurring when multiple IoT devices are connected simultaneously. Professor Yong Keun Park from the Department of Physics will introduce a technology for measuring glycated hemoglobin by using the optical properties of red blood cells. This technology can be applied to make low-cost, small-sized measuring equipment. It can also be used for vitro diagnoses including diabetes, cardiovascular disorders, tumors, kidney disease, and infectious diseases. Professor Yong Man Ro from the School of Electrical Engineering will show technology for biometric access control. Conventional technologies for face recognition fall behind other biometrics. Professor Ro and his team developed a facial dynamics interpreting network which allows very accurate facial recognition by interpreting the relationships between facial local dynamics and estimating facial traits. This technology can be applied to security and communication in finance, computers, and information system.KAIST President Sung-Chul Shin said, “KAIST will continue to support SMEs to have stronger competitiveness in the market. Through technology transfer, we will drive innovation in technological commercialization where a university’s research and development creates economic value.”
2018.11.13 View 11016
OUIC Presents the Six Most Promising Techs Transferrable to Local SMEs
KAIST will showcase the six most promising technologies for small and medium enterprises (SMEs) on November 14 in the Academic Cultural Complex. To strengthen the competitive edge of local SMEs in Daejeon, the Office of University-Industry made a survey of their technological needs and came up with the six most promising technologies. Developers will introduce their technologies during the session.Besides the introduction of the promising technologies, the session will also provide a program named University to Business (U2B) to match up technologies according to the SMEs’ needs. SMEs who wish to engage in technology transfers can receive counseling and other support programs during the session.First, Professor Seok-Hyung Bae from the Department of Industrial Design will present a technology for controlling cooperation robots. Professor Bae inserted flexible materials between the controllers to allow robots to use both hands stably and operate more accurately and swiftly. It can be applied to automatic robots, industrial robots, and service robots.Professor Hyun Myung from the Department of Civil & Environmental Engineering will demonstrate a robot navigation system in a dynamic indoor and outdoor environment, which can be applied to robotics in logistics, smart factories, and autonomous vehicles. Providing robust simultaneous localization and mapping systems, this technology shows high-performing navigation with low-cost sensors.Meanwhile, Professor Siyoung Choi from the Department of Chemical and Biomolecular Engineering will introduce a technology for forming stable adhesive emulsions. An emulsion is a stable mixture of water and oil. Conventionally, a small amount of surfactant is added to stabilize an emulsion. Here, Professor Choi developed a stable emulsion system without using any chemical substances. This technology can be applied to various fields, including the cosmetics, pharmaceutical, semiconductor, and painting industries. The session will also present smart IoTs platform technology developed by Professor Jinhong Yang from the KAIST Institute for IT Convergence. His technology minimizes errors occurring when multiple IoT devices are connected simultaneously. Professor Yong Keun Park from the Department of Physics will introduce a technology for measuring glycated hemoglobin by using the optical properties of red blood cells. This technology can be applied to make low-cost, small-sized measuring equipment. It can also be used for vitro diagnoses including diabetes, cardiovascular disorders, tumors, kidney disease, and infectious diseases. Professor Yong Man Ro from the School of Electrical Engineering will show technology for biometric access control. Conventional technologies for face recognition fall behind other biometrics. Professor Ro and his team developed a facial dynamics interpreting network which allows very accurate facial recognition by interpreting the relationships between facial local dynamics and estimating facial traits. This technology can be applied to security and communication in finance, computers, and information system.KAIST President Sung-Chul Shin said, “KAIST will continue to support SMEs to have stronger competitiveness in the market. Through technology transfer, we will drive innovation in technological commercialization where a university’s research and development creates economic value.”
2018.11.13 View 11016 -
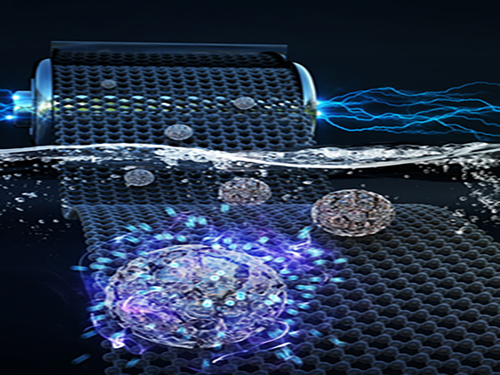 Faster and More Powerful Aqueous Hybrid Capacitor
(Professor Jeung Ku Kang from the Graduate School of EEWS)
A KAIST research team made it one step closer to realizing safe energy storage with high energy density, high power density, and a longer cycle life. This hybrid storage alternative shows power density 100 times faster than conventional batteries, allowing it to be charged within a few seconds. Hence, it is suitable for small portable electronic devices.
Conventional electrochemical energy storage systems, including lithium-ion batteries (LIBs), have a high voltage range and energy density, but are subject to safety issues raised by flammable organic electrolytes, which are used to ensure the beneficial properties. Additionally, they suffer from slow electrochemical reaction rates, which lead to a poor charging rate and low power density with a capacity that fades quickly, resulting in a short cycle life.
On the other hand, capacitors based on aqueous electrolytes are receiving a great deal of attention because they are considered to be safe and environmentally friendly alternatives. However, aqueous electrolytes lag behind energy storage systems based on organic electrolytes in terms of energy density due to their limited voltage range and low capacitance.
Hence, developing aqueous energy storage with high energy density and a long cycle life in addition to the high power density that enables fast charging is the most challenging task for advancing next-generation electrochemical energy storage devices.
Here, Professor Jeung Ku Kang from the Graduate School of Energy, Environment, Water and Sustainability and his team developed an aqueous hybrid capacitor (AHC) that boasts high energy density, high power, and excellent cycle stability by synthesizing two types of porous metal oxide nanoclusters on graphene to create positive and negative electrodes for AHCs.
The porous metal oxide nanoparticles are composed of nanoclusters as small as two to three nanometers and have mesopores that are smaller than five nanometers. In these porous structures, ions can be rapidly transferred to the material surfaces and a large number of ions can be stored inside the metal oxide particles very quickly due to their small particle size and large surface area.
The team applied porous manganese oxide on graphene for positive electrodes and porous iron oxide on graphene for negative electrodes to design an aqueous hybrid capacitor that can operate at an extended voltage range of 2V.
Professor Kang said, “This newly developed AHC with high capacity and power density driven from porous metal oxide electrodes will contribute to commercializing a new type of energy storage system. This technology allows ultra-fast charging within several seconds, making it suitable as a power source for mobile devices or electric vehicles where solar energy is directly stored as electricity.”
This research, co-led by Professor Hyung Mo Jeong from Kangwon National University, was published in Advanced Functional Materials on August 15, 2018.
Figure 1. Image that shows properties of porous metal oxide nanoparticles formed on graphene in the aqueous hybrid capacitor
2018.11.09 View 8669
Faster and More Powerful Aqueous Hybrid Capacitor
(Professor Jeung Ku Kang from the Graduate School of EEWS)
A KAIST research team made it one step closer to realizing safe energy storage with high energy density, high power density, and a longer cycle life. This hybrid storage alternative shows power density 100 times faster than conventional batteries, allowing it to be charged within a few seconds. Hence, it is suitable for small portable electronic devices.
Conventional electrochemical energy storage systems, including lithium-ion batteries (LIBs), have a high voltage range and energy density, but are subject to safety issues raised by flammable organic electrolytes, which are used to ensure the beneficial properties. Additionally, they suffer from slow electrochemical reaction rates, which lead to a poor charging rate and low power density with a capacity that fades quickly, resulting in a short cycle life.
On the other hand, capacitors based on aqueous electrolytes are receiving a great deal of attention because they are considered to be safe and environmentally friendly alternatives. However, aqueous electrolytes lag behind energy storage systems based on organic electrolytes in terms of energy density due to their limited voltage range and low capacitance.
Hence, developing aqueous energy storage with high energy density and a long cycle life in addition to the high power density that enables fast charging is the most challenging task for advancing next-generation electrochemical energy storage devices.
Here, Professor Jeung Ku Kang from the Graduate School of Energy, Environment, Water and Sustainability and his team developed an aqueous hybrid capacitor (AHC) that boasts high energy density, high power, and excellent cycle stability by synthesizing two types of porous metal oxide nanoclusters on graphene to create positive and negative electrodes for AHCs.
The porous metal oxide nanoparticles are composed of nanoclusters as small as two to three nanometers and have mesopores that are smaller than five nanometers. In these porous structures, ions can be rapidly transferred to the material surfaces and a large number of ions can be stored inside the metal oxide particles very quickly due to their small particle size and large surface area.
The team applied porous manganese oxide on graphene for positive electrodes and porous iron oxide on graphene for negative electrodes to design an aqueous hybrid capacitor that can operate at an extended voltage range of 2V.
Professor Kang said, “This newly developed AHC with high capacity and power density driven from porous metal oxide electrodes will contribute to commercializing a new type of energy storage system. This technology allows ultra-fast charging within several seconds, making it suitable as a power source for mobile devices or electric vehicles where solar energy is directly stored as electricity.”
This research, co-led by Professor Hyung Mo Jeong from Kangwon National University, was published in Advanced Functional Materials on August 15, 2018.
Figure 1. Image that shows properties of porous metal oxide nanoparticles formed on graphene in the aqueous hybrid capacitor
2018.11.09 View 8669 -
 Global Lounge Opens for Language and Cultural Exchange
KAIST celebrated the opening of its new Global Lounge on November 8. KAIST’s president, administrative executives, representatives of Korean and international student associations, and many other students attended the ceremony.
The Global Lounge, located on the fourth floor of the Academic Cultural Complex, was opened for students to share language and culture in a warm and pleasant environment. The Global Lounge will serve as a comfortable and open space. Students around the world can build networks by interacting with one another and freely sharing their ideas.
The lounge offers travel guidebooks and magazines for those who wish to learn more about countries around the world. It also boasts a room for seminars and a tearoom. The seminar room will be used for multipurpose space; while activities such as meditation, tea classes, and etiquette lessons will take place in the tearoom. One of the major highlights of the global lounge is that it exhibits cultural artifacts donated by embassies in Korea.
At the opening ceremony, President Sung-Chul Shin expressed the importance of communication and interaction among students by saying, “You can better embrace challenges and come up with creative ideas when you are interacting with others. I hope this Global Lounge serves as a multi-functional space for raising cultural awareness.”
2018.11.08 View 3010
Global Lounge Opens for Language and Cultural Exchange
KAIST celebrated the opening of its new Global Lounge on November 8. KAIST’s president, administrative executives, representatives of Korean and international student associations, and many other students attended the ceremony.
The Global Lounge, located on the fourth floor of the Academic Cultural Complex, was opened for students to share language and culture in a warm and pleasant environment. The Global Lounge will serve as a comfortable and open space. Students around the world can build networks by interacting with one another and freely sharing their ideas.
The lounge offers travel guidebooks and magazines for those who wish to learn more about countries around the world. It also boasts a room for seminars and a tearoom. The seminar room will be used for multipurpose space; while activities such as meditation, tea classes, and etiquette lessons will take place in the tearoom. One of the major highlights of the global lounge is that it exhibits cultural artifacts donated by embassies in Korea.
At the opening ceremony, President Sung-Chul Shin expressed the importance of communication and interaction among students by saying, “You can better embrace challenges and come up with creative ideas when you are interacting with others. I hope this Global Lounge serves as a multi-functional space for raising cultural awareness.”
2018.11.08 View 3010 -
 Team KAT Wins the Autonomous Car Challenge
(Team KAT receiving the Presidential Award)
A KAIST team won the 2018 International Autonomous Car Challenge for University Students held in Daegu on November 2.
Professor Seung-Hyun Kong from the ChoChunShik Graduate School of Green Transportation and his team participated in this contest with the team named KAT (KAIST Autonomous Technologies). The team received the Presidential Award with a fifty million won cash prize and an opportunity for a field trip abroad.
The competition was conducted on actual roads with Connected Autonomous Vehicles (CAV), which incorporate autonomous driving technologies and vehicle-to-everything (V2X) communication system.
In this contest, the autonomous vehicles were given a mission to pick up passengers or parcels. Through the V2X communication, the contest gave current location of the passengers or parcels, their destination, and service profitability according to distance and level of service difficulty.
The participating vehicles had to be equipped very accurate and robust navigation system since they had to drive on narrow roads as well as go through tunnels where GPS was not available. Moreover, they had to use camera-based recognition technology that was invulnerable to backlight as the contest was in the late afternoon.
The contest scored the mission in the following way: the vehicles get points if they pick up passengers and safely drop them off at their destination; on the other hand, points are deducted when they violate lanes or traffic lights. It will be a major black mark if a participant sitting in the driver’s seat needs to get involved in driving due to a technical issue.
Youngbo Shim of KAT said, “We believe that we got major points for technical superiority in autonomous driving and our algorithm for passenger selection.”
This contest, hosted by Ministry of Trade, Industry and Energy, was the first international competition for autonomous driving on actual roads. A total of nine teams participated in the final contest, four domestic teams and five teams allied with overseas universities such as Tsinghua University, Waseda University, and Nanyang Technological University.
Professor Kong said, “There is still a long way to go for fully autonomous vehicles that drive flexibly under congested traffic conditions. However, we will continue to our research in order to achieve high-quality autonomous driving technology.”
(Team KAT getting ready for the challenge)
2018.11.06 View 15051
Team KAT Wins the Autonomous Car Challenge
(Team KAT receiving the Presidential Award)
A KAIST team won the 2018 International Autonomous Car Challenge for University Students held in Daegu on November 2.
Professor Seung-Hyun Kong from the ChoChunShik Graduate School of Green Transportation and his team participated in this contest with the team named KAT (KAIST Autonomous Technologies). The team received the Presidential Award with a fifty million won cash prize and an opportunity for a field trip abroad.
The competition was conducted on actual roads with Connected Autonomous Vehicles (CAV), which incorporate autonomous driving technologies and vehicle-to-everything (V2X) communication system.
In this contest, the autonomous vehicles were given a mission to pick up passengers or parcels. Through the V2X communication, the contest gave current location of the passengers or parcels, their destination, and service profitability according to distance and level of service difficulty.
The participating vehicles had to be equipped very accurate and robust navigation system since they had to drive on narrow roads as well as go through tunnels where GPS was not available. Moreover, they had to use camera-based recognition technology that was invulnerable to backlight as the contest was in the late afternoon.
The contest scored the mission in the following way: the vehicles get points if they pick up passengers and safely drop them off at their destination; on the other hand, points are deducted when they violate lanes or traffic lights. It will be a major black mark if a participant sitting in the driver’s seat needs to get involved in driving due to a technical issue.
Youngbo Shim of KAT said, “We believe that we got major points for technical superiority in autonomous driving and our algorithm for passenger selection.”
This contest, hosted by Ministry of Trade, Industry and Energy, was the first international competition for autonomous driving on actual roads. A total of nine teams participated in the final contest, four domestic teams and five teams allied with overseas universities such as Tsinghua University, Waseda University, and Nanyang Technological University.
Professor Kong said, “There is still a long way to go for fully autonomous vehicles that drive flexibly under congested traffic conditions. However, we will continue to our research in order to achieve high-quality autonomous driving technology.”
(Team KAT getting ready for the challenge)
2018.11.06 View 15051