research
-
 Green Technology for Data Centers: Ultra-low Power 100 Gbps Ethernet Integrated Circuit Developed
A new integrated circuit (IC), consuming only 0.75W of electricity, will reduce the power usage of data chips installed at data centers by one-third.
Each day, billions of people surf the Internet for information, entertainment, and educational content. The Internet contains an immeasurable amount of information and knowledge generated every minute all around the world that is readily available to everyone with a click of a computer mouse. The real magic of the Internet, however, lies in data centers, where hundreds of billions of data are stored and distributed to designated users around the clock.
Today, almost every business or organization either has its own data centers or outsources data center services to a third party. These centers house highly specialized equipment responsible for the support of computers, networks, data storage, and business security. Accordingly, the operational cost of data centers is tremendous because they consume a large amount of electricity.
Data centers can consume up to 100 times more energy than a standard office building. Data center energy consumption doubled from 2000 to 2006, reaching more than 60 billion kilowatt hours per year. If the current usage and technology trends continue, the energy consumption of data centers in the US will reach 8% of the country’s total electric power consumption by 2020.
A research team at the Korea Advanced Institute of Science and Technology (KAIST) and Terasquare, Inc. (
http://www.terasquare.co.kr
), a spin-off company of the university,
developed an extremely low-powered integrated circuit for Ethernet that consumes less than 0.75W of electricity but is able to send and receive data at the high speed of 100 gigabits per second (Gbps). The research team, headed by Hyeon-Min Bae, assistant professor of electrical engineering at KAIST, claims that the new microchip uses only one-third of the electricity consumed by the currently installed chips at data centers, thereby helping the centers to save energy.
Integrated circuits are embedded on communication modules that are inserted into a line card. Data centers have numerous line cards to build a network including routers and switches. Currently, 8W ICs are the most common in the market, and they consume a lot of energy and require the largest modules (112 cm
2
of CFP), decreasing the port density of line cards and, thus, limiting the amount of data transmission.
The ultra-low-power-circuit, 100-gigabit, full-transceiver CDR, is the world’s first solution that can be loaded to the smallest communication modules (20 cm
2
of CFP4 or 16 cm
2
of
QSFP28), the next-generation chips for data centers. Compared with other chip producers, the 100 Gbps CDR is a greener version of the technology that improves the energy efficiency of data centers while maintaining the high speed of data transmission.
Professor Hyeon-Min Bae said, “When we demonstrate our chip in September of this year at one of the leading companies that manufacture optical communication components and systems, they said that our product is two years ahead of those of our competitors. We plan to produce the chip from 2014 and expect that it will lead the 100 Gbps Ethernet IC market, which is expected to grow to USD 1 billion by 2017.”
The commercial model of the IC was first introduced at the 39
th
European Conference and Exhibition on Optical Communication (ECOC), the largest optical communication forum for new results and developments in Europe, held from September 22-26 at ExCeL London, an international exhibition and convention center.
Professor Bae added, “We received positive responses to our ultra-low-power 100-Gbps Ethernet IC at the ECOC. The chip will be used not only for a particular industry but also for many of next-generation, super-high-speed information communications technologies, such as high-speed USB, high-definition multimedia interface (HDMI), and TV interface.”
Before joining KAIST, Hyeon-Min Bae worked for many years at Finisar as a researcher who designed and developed the world’s first super-high-speed circuit, the 100 Gbps Ethernet IC.
2013.11.25 View 10493
Green Technology for Data Centers: Ultra-low Power 100 Gbps Ethernet Integrated Circuit Developed
A new integrated circuit (IC), consuming only 0.75W of electricity, will reduce the power usage of data chips installed at data centers by one-third.
Each day, billions of people surf the Internet for information, entertainment, and educational content. The Internet contains an immeasurable amount of information and knowledge generated every minute all around the world that is readily available to everyone with a click of a computer mouse. The real magic of the Internet, however, lies in data centers, where hundreds of billions of data are stored and distributed to designated users around the clock.
Today, almost every business or organization either has its own data centers or outsources data center services to a third party. These centers house highly specialized equipment responsible for the support of computers, networks, data storage, and business security. Accordingly, the operational cost of data centers is tremendous because they consume a large amount of electricity.
Data centers can consume up to 100 times more energy than a standard office building. Data center energy consumption doubled from 2000 to 2006, reaching more than 60 billion kilowatt hours per year. If the current usage and technology trends continue, the energy consumption of data centers in the US will reach 8% of the country’s total electric power consumption by 2020.
A research team at the Korea Advanced Institute of Science and Technology (KAIST) and Terasquare, Inc. (
http://www.terasquare.co.kr
), a spin-off company of the university,
developed an extremely low-powered integrated circuit for Ethernet that consumes less than 0.75W of electricity but is able to send and receive data at the high speed of 100 gigabits per second (Gbps). The research team, headed by Hyeon-Min Bae, assistant professor of electrical engineering at KAIST, claims that the new microchip uses only one-third of the electricity consumed by the currently installed chips at data centers, thereby helping the centers to save energy.
Integrated circuits are embedded on communication modules that are inserted into a line card. Data centers have numerous line cards to build a network including routers and switches. Currently, 8W ICs are the most common in the market, and they consume a lot of energy and require the largest modules (112 cm
2
of CFP), decreasing the port density of line cards and, thus, limiting the amount of data transmission.
The ultra-low-power-circuit, 100-gigabit, full-transceiver CDR, is the world’s first solution that can be loaded to the smallest communication modules (20 cm
2
of CFP4 or 16 cm
2
of
QSFP28), the next-generation chips for data centers. Compared with other chip producers, the 100 Gbps CDR is a greener version of the technology that improves the energy efficiency of data centers while maintaining the high speed of data transmission.
Professor Hyeon-Min Bae said, “When we demonstrate our chip in September of this year at one of the leading companies that manufacture optical communication components and systems, they said that our product is two years ahead of those of our competitors. We plan to produce the chip from 2014 and expect that it will lead the 100 Gbps Ethernet IC market, which is expected to grow to USD 1 billion by 2017.”
The commercial model of the IC was first introduced at the 39
th
European Conference and Exhibition on Optical Communication (ECOC), the largest optical communication forum for new results and developments in Europe, held from September 22-26 at ExCeL London, an international exhibition and convention center.
Professor Bae added, “We received positive responses to our ultra-low-power 100-Gbps Ethernet IC at the ECOC. The chip will be used not only for a particular industry but also for many of next-generation, super-high-speed information communications technologies, such as high-speed USB, high-definition multimedia interface (HDMI), and TV interface.”
Before joining KAIST, Hyeon-Min Bae worked for many years at Finisar as a researcher who designed and developed the world’s first super-high-speed circuit, the 100 Gbps Ethernet IC.
2013.11.25 View 10493 -
 Observation of a water strider led to a new method of measuring properties of Nano films
Even the mechanical properties of Nano films of a few nanometers thick can be measured
Posted online Nature Communications on the 3rd of October
The joint research team of KAIST’s Department of Mechanical Engineering’s Professor Taek-Soo Kim and Doctor Seung-Min Hyun of the Nano mechanics laboratory of Korea Institute of Machinery and Materials has developed a new method to evaluate mechanical properties of Nano films using the characteristics of water surfaces.
The research findings have been posted on the online edition of Nature Communications on the 3rd of October. The technology can obtain accurate results by directly measuring the mechanical properties such as the strength and elasticity of Nano films. Academia and the industry expect the simplicity of the technology to present a new paradigm in the evaluation of mechanical properties of Nano films.
Evaluation of the mechanical properties of Nano films is essential not only in predicting the reliability of semiconductors and displays, but also in finding new phenomena in the Nano world. However, mechanical strength was difficult to test since the test demands the falling of objects to the ground to measure their strength, and nano films can easily break in the process.
The research team observed insects such as water striders freely floating on the surface of the water. The team used the properties of water, large surface tension and low viscosity, to float a 55 nanometers (nm) gold Nano film to successfully measure its mechanical properties without damaging it. The technology could be used to measure the mechanical properties of not only various types of Nano films but also films only a few nm thick.
Professor Taek-Soo Kim said, “We effectively performed an evaluation of the mechanical characteristics of Nano films, which was difficult in the past, by developing a new strength test using the properties of water.” He continued to say, “The team plans to discover the mechanical properties of 2D Nano films such as graphene that could not have been measured with the existing strength test methods.”
The research by KAIST’s Department of Mechanical Engineering’s graduate student Jae-Han Kim (lead author) under the supervision of Professor Taek-Soo Kim and Doctor Seung-Min Hyun of Korea Institute of Machinery and Materials was sponsored by the National Research Foundation of Korea.
Evaluation process of mechanical properties of Nano films by using the characteristics of water surfaces
Dr Seung-Min Hyun, Jae-Han Kim, and Professor Taek-Soo Kim from left to right
2013.11.11 View 9831
Observation of a water strider led to a new method of measuring properties of Nano films
Even the mechanical properties of Nano films of a few nanometers thick can be measured
Posted online Nature Communications on the 3rd of October
The joint research team of KAIST’s Department of Mechanical Engineering’s Professor Taek-Soo Kim and Doctor Seung-Min Hyun of the Nano mechanics laboratory of Korea Institute of Machinery and Materials has developed a new method to evaluate mechanical properties of Nano films using the characteristics of water surfaces.
The research findings have been posted on the online edition of Nature Communications on the 3rd of October. The technology can obtain accurate results by directly measuring the mechanical properties such as the strength and elasticity of Nano films. Academia and the industry expect the simplicity of the technology to present a new paradigm in the evaluation of mechanical properties of Nano films.
Evaluation of the mechanical properties of Nano films is essential not only in predicting the reliability of semiconductors and displays, but also in finding new phenomena in the Nano world. However, mechanical strength was difficult to test since the test demands the falling of objects to the ground to measure their strength, and nano films can easily break in the process.
The research team observed insects such as water striders freely floating on the surface of the water. The team used the properties of water, large surface tension and low viscosity, to float a 55 nanometers (nm) gold Nano film to successfully measure its mechanical properties without damaging it. The technology could be used to measure the mechanical properties of not only various types of Nano films but also films only a few nm thick.
Professor Taek-Soo Kim said, “We effectively performed an evaluation of the mechanical characteristics of Nano films, which was difficult in the past, by developing a new strength test using the properties of water.” He continued to say, “The team plans to discover the mechanical properties of 2D Nano films such as graphene that could not have been measured with the existing strength test methods.”
The research by KAIST’s Department of Mechanical Engineering’s graduate student Jae-Han Kim (lead author) under the supervision of Professor Taek-Soo Kim and Doctor Seung-Min Hyun of Korea Institute of Machinery and Materials was sponsored by the National Research Foundation of Korea.
Evaluation process of mechanical properties of Nano films by using the characteristics of water surfaces
Dr Seung-Min Hyun, Jae-Han Kim, and Professor Taek-Soo Kim from left to right
2013.11.11 View 9831 -
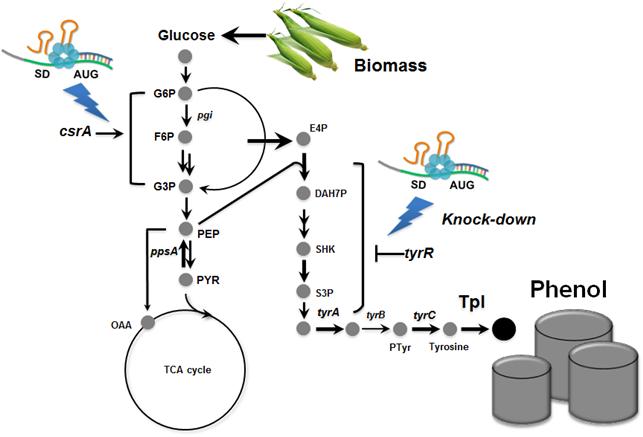 Metabolically engineered E. coli producing phenol
Many chemicals we use in everyday life are derived from fossil resources. Due to the increasing concerns on the use of fossil resources, there has been much interest in producing chemicals from renewable resources through biotechnology.
Phenol is an important commodity chemical, and is a starting material for the production of numerous industrial chemicals and polymers, including bisphenol A and phenolic resins, and others. At present, the production of phenol entirely depends on the chemical synthesis from benzene, and its annual production exceeds 8 million tons worldwide. Microbial production of phenol seems to be a non-viable process considering the high toxicity of phenol to the cell.
In the paper published online in Biotechnology Journal, a Korean research team led by Distinguished Professor Sang Yup Lee at the Department of Chemical and Biomolecular Engineering from the Korea Advanced Institute of Science and Technology (KAIST) reported the successful development of an engineered Escherichia coli (E. coli) strain which can produce phenol from glucose. E. coli has been a workhorse for biological production of various value-added compounds such as succinic acid and 1,4-butanediol in industrial scale. However, due to its low tolerance to phenol, E. coli was not considered a viable host strain for the biological production of phenol.
Professor Lee"s team, a leading research group in metabolic engineering, noted the genetic and physiological differences of various E. coli strains and investigated 18 different E. coli strains with respect to phenol tolerance and engineered all of the 18 strains simultaneously. If the traditional genetic engineering methods were used, this work would have taken years to do. To overcome this challenge, the research team used synthetic small RNA (sRNA) technology they recently developed (Nature Biotechnology, vol 31, pp 170-174, 2013). The sRNA technology allowed the team to screen 18 E. coli strains with respect to the phenol tolerance, and the activities of the metabolic pathway and enzyme involved in the production of phenol. The research team also metabolically engineered the E. coli strains to increase carbon flux toward phenol and finally generated an engineered E. coli strain which can produce phenol from glucose.
Furthermore, the team developed a biphasic extractive fermentation process to minimize the toxicity of phenol to E. coli cells. Glycerol tributyrate was found to have low toxicity to E. coli and allowed efficient extraction of phenol from the culture broth. Through the biphasic fed-batch fermentation using glycerol tributyrate as an in situ extractant, the final engineered E. coli strain produced phenol to the highest titer and productivity reported (3.8 g/L and 0.18 g/L/h, respectively). The strategy used for the strain development and the fermentation process will serve as a framework for metabolic engineering of microorganisms for the production of toxic chemicals from renewable resources.
This work was supported by the Intelligent Synthetic Biology Center through the Global Frontier Project (2011-0031963) of the Ministry of Science, ICT & Future Planning through the National Research Foundation of Korea.
Process of Phenol Production
2013.11.05 View 11463
Metabolically engineered E. coli producing phenol
Many chemicals we use in everyday life are derived from fossil resources. Due to the increasing concerns on the use of fossil resources, there has been much interest in producing chemicals from renewable resources through biotechnology.
Phenol is an important commodity chemical, and is a starting material for the production of numerous industrial chemicals and polymers, including bisphenol A and phenolic resins, and others. At present, the production of phenol entirely depends on the chemical synthesis from benzene, and its annual production exceeds 8 million tons worldwide. Microbial production of phenol seems to be a non-viable process considering the high toxicity of phenol to the cell.
In the paper published online in Biotechnology Journal, a Korean research team led by Distinguished Professor Sang Yup Lee at the Department of Chemical and Biomolecular Engineering from the Korea Advanced Institute of Science and Technology (KAIST) reported the successful development of an engineered Escherichia coli (E. coli) strain which can produce phenol from glucose. E. coli has been a workhorse for biological production of various value-added compounds such as succinic acid and 1,4-butanediol in industrial scale. However, due to its low tolerance to phenol, E. coli was not considered a viable host strain for the biological production of phenol.
Professor Lee"s team, a leading research group in metabolic engineering, noted the genetic and physiological differences of various E. coli strains and investigated 18 different E. coli strains with respect to phenol tolerance and engineered all of the 18 strains simultaneously. If the traditional genetic engineering methods were used, this work would have taken years to do. To overcome this challenge, the research team used synthetic small RNA (sRNA) technology they recently developed (Nature Biotechnology, vol 31, pp 170-174, 2013). The sRNA technology allowed the team to screen 18 E. coli strains with respect to the phenol tolerance, and the activities of the metabolic pathway and enzyme involved in the production of phenol. The research team also metabolically engineered the E. coli strains to increase carbon flux toward phenol and finally generated an engineered E. coli strain which can produce phenol from glucose.
Furthermore, the team developed a biphasic extractive fermentation process to minimize the toxicity of phenol to E. coli cells. Glycerol tributyrate was found to have low toxicity to E. coli and allowed efficient extraction of phenol from the culture broth. Through the biphasic fed-batch fermentation using glycerol tributyrate as an in situ extractant, the final engineered E. coli strain produced phenol to the highest titer and productivity reported (3.8 g/L and 0.18 g/L/h, respectively). The strategy used for the strain development and the fermentation process will serve as a framework for metabolic engineering of microorganisms for the production of toxic chemicals from renewable resources.
This work was supported by the Intelligent Synthetic Biology Center through the Global Frontier Project (2011-0031963) of the Ministry of Science, ICT & Future Planning through the National Research Foundation of Korea.
Process of Phenol Production
2013.11.05 View 11463 -
 KAIST announced a novel technology to produce gasoline by a metabolically engineered microorganism
A major scientific breakthrough in the development of renewable energy sources and other important chemicals; The research team succeeded in producing 580 mg of gasoline per liter of cultured broth by converting in vivo generated fatty acids
For many decades, we have been relying on fossil resources to produce liquid fuels such as gasoline, diesel, and many industrial and consumer chemicals for daily use. However, increasing strains on natural resources as well as environmental issues including global warming have triggered a strong interest in developing sustainable ways to obtain fuels and chemicals.
Gasoline, the petroleum-derived product that is most widely used as a fuel for transportation, is a mixture of hydrocarbons, additives, and blending agents. The hydrocarbons, called alkanes, consist only of carbon and hydrogen atoms. Gasoline has a combination of straight-chain and branched-chain alkanes (hydrocarbons) consisted of 4-12 carbon atoms linked by direct carbon-carbon bonds.
Previously, through metabolic engineering of Escherichia coli (E. coli), there have been a few research results on the production of long-chain alkanes, which consist of 13-17 carbon atoms, suitable for replacing diesel. However, there has been no report on the microbial production of short-chain alkanes, a possible substitute for gasoline.
In the paper (entitled "Microbial Production of Short-chain Alkanes") published online in Nature on September 29, a Korean research team led by Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering at the Korea Advanced Institute of Science and Technology (KAIST) reported, for the first time, the development of a novel strategy for microbial gasoline production through metabolic engineering of E. coli.
The research team engineered the fatty acid metabolism to provide the fatty acid derivatives that are shorter than normal intracellular fatty acid metabolites, and introduced a novel synthetic pathway for the biosynthesis of short-chain alkanes. This allowed the development of platform E. coli strain capable of producing gasoline for the first time. Furthermore, this platform strain, if desired, can be modified to produce other products such as short-chain fatty esters and short-chain fatty alcohols.
In this paper, the Korean researchers described detailed strategies for 1) screening of enzymes associated with the production of fatty acids, 2) engineering of enzymes and fatty acid biosynthetic pathways to concentrate carbon flux towards the short-chain fatty acid production, and 3) converting short-chain fatty acids to their corresponding alkanes (gasoline) by introducing a novel synthetic pathway and optimization of culture conditions. Furthermore, the research team showed the possibility of producing fatty esters and alcohols by introducing responsible enzymes into the same platform strain.
Professor Sang Yup Lee said, "It is only the beginning of the work towards sustainable production of gasoline. The titer is rather low due to the low metabolic flux towards the formation of short-chain fatty acids and their derivatives. We are currently working on increasing the titer, yield and productivity of bio-gasoline. Nonetheless, we are pleased to report, for the first time, the production of gasoline through the metabolic engineering of E. coli, which we hope will serve as a basis for the metabolic engineering of microorganisms to produce fuels and chemicals from renewable resources."
This research was supported by the Advanced Biomass Research and Development Center of Korea (ABC-2010-0029799) through the Global Frontier Research Program of the Ministry of Science, ICT and Future Planning (MSIP) through the National Research Foundation (NRF), Republic of Korea. Systems metabolic engineering work was supported by the Technology Development Program to Solve Climate Changes on Systems Metabolic Engineering for Biorefineries (NRF-2012-C1AAA001-2012M1A2A2026556) by MSIP through NRF.
Short-Chain Alkanes Generated from Renewable Biomass
This diagram shows the metabolic engineering of Escherichia coli for the production of short-chain alkanes (gasoline) from renewable biomass.
Nature Cover Page (September 29th, 2013)
2013.11.04 View 13487
KAIST announced a novel technology to produce gasoline by a metabolically engineered microorganism
A major scientific breakthrough in the development of renewable energy sources and other important chemicals; The research team succeeded in producing 580 mg of gasoline per liter of cultured broth by converting in vivo generated fatty acids
For many decades, we have been relying on fossil resources to produce liquid fuels such as gasoline, diesel, and many industrial and consumer chemicals for daily use. However, increasing strains on natural resources as well as environmental issues including global warming have triggered a strong interest in developing sustainable ways to obtain fuels and chemicals.
Gasoline, the petroleum-derived product that is most widely used as a fuel for transportation, is a mixture of hydrocarbons, additives, and blending agents. The hydrocarbons, called alkanes, consist only of carbon and hydrogen atoms. Gasoline has a combination of straight-chain and branched-chain alkanes (hydrocarbons) consisted of 4-12 carbon atoms linked by direct carbon-carbon bonds.
Previously, through metabolic engineering of Escherichia coli (E. coli), there have been a few research results on the production of long-chain alkanes, which consist of 13-17 carbon atoms, suitable for replacing diesel. However, there has been no report on the microbial production of short-chain alkanes, a possible substitute for gasoline.
In the paper (entitled "Microbial Production of Short-chain Alkanes") published online in Nature on September 29, a Korean research team led by Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering at the Korea Advanced Institute of Science and Technology (KAIST) reported, for the first time, the development of a novel strategy for microbial gasoline production through metabolic engineering of E. coli.
The research team engineered the fatty acid metabolism to provide the fatty acid derivatives that are shorter than normal intracellular fatty acid metabolites, and introduced a novel synthetic pathway for the biosynthesis of short-chain alkanes. This allowed the development of platform E. coli strain capable of producing gasoline for the first time. Furthermore, this platform strain, if desired, can be modified to produce other products such as short-chain fatty esters and short-chain fatty alcohols.
In this paper, the Korean researchers described detailed strategies for 1) screening of enzymes associated with the production of fatty acids, 2) engineering of enzymes and fatty acid biosynthetic pathways to concentrate carbon flux towards the short-chain fatty acid production, and 3) converting short-chain fatty acids to their corresponding alkanes (gasoline) by introducing a novel synthetic pathway and optimization of culture conditions. Furthermore, the research team showed the possibility of producing fatty esters and alcohols by introducing responsible enzymes into the same platform strain.
Professor Sang Yup Lee said, "It is only the beginning of the work towards sustainable production of gasoline. The titer is rather low due to the low metabolic flux towards the formation of short-chain fatty acids and their derivatives. We are currently working on increasing the titer, yield and productivity of bio-gasoline. Nonetheless, we are pleased to report, for the first time, the production of gasoline through the metabolic engineering of E. coli, which we hope will serve as a basis for the metabolic engineering of microorganisms to produce fuels and chemicals from renewable resources."
This research was supported by the Advanced Biomass Research and Development Center of Korea (ABC-2010-0029799) through the Global Frontier Research Program of the Ministry of Science, ICT and Future Planning (MSIP) through the National Research Foundation (NRF), Republic of Korea. Systems metabolic engineering work was supported by the Technology Development Program to Solve Climate Changes on Systems Metabolic Engineering for Biorefineries (NRF-2012-C1AAA001-2012M1A2A2026556) by MSIP through NRF.
Short-Chain Alkanes Generated from Renewable Biomass
This diagram shows the metabolic engineering of Escherichia coli for the production of short-chain alkanes (gasoline) from renewable biomass.
Nature Cover Page (September 29th, 2013)
2013.11.04 View 13487 -
 A powerful strategy for developing microbial cell factories by employing synthetic small RNAs
The current systems for the production of chemicals, fuels and materials heavily rely on the use of fossil resources. Due to the increasing concerns on climate change and other environmental problems, however, there has been much interest in developing biorefineries for the production of such chemicals, fuels and materials from renewable resources. For the biorefineries to be competitive with the traditional fossil resource-based refineries, development of high performance microorganisms is the most important as it will affect the overall economics of the process most significantly. Metabolic engineering, which can be defined as purposeful modification of cellular metabolic and regulatory networks with an aim to improve the production of a desired product, has been successfully employed to improve the performance of the cell. However, it is not trivial to engineer the cellular metabolism and regulatory circuits in the cell due to their high complexity.
In metabolic engineering, it is important to find the genes that need to be amplified and attenuated in order to increase the product formation rate while minimizing the production of undesirable byproducts. Gene knock-out experiments are often performed to delete those metabolic fluxes that will consequently result in the increase of the desired product formation. However, gene knock-out experiments require much effort and time to perform, and are difficult to do for a large number of genes. Furthermore, the gene knock-out experiments performed in one strain cannot be transferred to another organism and thus the whole experimental process has to be repeated. This is a big problem in developing a high performance microbial cell factory because it is required to find the best platform strain among many different strains. Therefore, researchers have been eager to develop a strategy that allows rapid identification of multiple genes to be attenuated in multiple strains at the same time.
A Korean research team led by Distinguished Professor Sang Yup Lee at the Department of Chemical and Biomolecular Engineering from the Korea Advanced Institute of Science and Technology (KAIST) reported the development of a strategy for efficiently developing microbial cell factories by employing synthetic small RNAs (sRNAs). They first reported the development of such system in Nature Biotechnology last February. This strategy of employing synthetic sRNAs in metabolic engineering has been receiving great interest worldwide as it allows easy, rapid, high-throughput, tunable, and un-doable knock-down of multiple genes in multiple strains at the same time. The research team published a paper online on August 8 as a cover page (September issue) in Nature Protocols, describing the detailed strategy and protocol to employ synthetic sRNAs for metabolic engineering.
In this paper, researchers described the detailed step-by-step protocol for synthetic sRNA-based gene expression control, including the sRNA design principles. Tailor-made synthetic sRNAs can be easily manipulated by using conventional gene cloning method. The use of synthetic sRNAs for gene expression regulation provides several advantages such as portability, conditionality, and tunability in high-throughput experiments. Plasmid-based synthetic sRNA expression system does not leave any scar on the chromosome, and can be easily transferred to many other host strains to be examined. Thus, the construction of libraries and examination of different host strains are much easier than the conventional hard-coded gene manipulation systems. Also, the expression of genes can be conditionally repressed by controlling the production of synthetic sRNAs. Synthetic sRNAs possessing different repression efficiencies make it possible to finely tune the gene expression levels as well. Furthermore, synthetic sRNAs allow knock-down of the expression of essential genes, which was not possible by conventional gene knock-out experiments.
Synthetic sRNAs can be utilized for diverse experiments where gene expression regulation is needed. One of promising applications is high-throughput screening of the target genes to be manipulated and multiple strains simultaneously to enhance the production of chemicals and materials of interest. Such simultaneous optimization of gene targets and strains has been one of the big challenges in metabolic engineering. Another application is to fine tune the expression of the screened genes for flux optimization, which would enhance chemical production further by balancing the flux between biomass formation and target chemical production. Synthetic sRNAs can also be applied to finely regulating genetic interactions in a circuit or network, which is essential in synthetic biology. Once a sRNA scaffold-harboring plasmid is constructed, tailor-made, synthetic sRNAs can be made within 3-4 days, followed by the desired application experiments.
Dr. Eytan Zlotorynski, an editor at Nature Protocols, said "This paper describes the detailed protocol for the design and applications of synthetic sRNA. The method, which has many advantages, is likely to become common practice, and prove useful for metabolic engineering and synthetic biology studies."
This paper published in Nature Protocols will be useful for all researchers in academia and industry who are interested in the use of synthetic sRNAs for fundamental and applied biological and biotechnological studies.
This work was supported by the Technology Development Program to Solve Climate Changes on Systems Metabolic Engineering for Biorefineries (NRF-2012-C1AAA001-2012M1A2A2026556) and the Intelligent Synthetic Biology Center through the Global Frontier Project (2011-0031963) of the Ministry of Science, ICT and Future Planning through the National Research Foundation of Korea.
2013.10.31 View 10979
A powerful strategy for developing microbial cell factories by employing synthetic small RNAs
The current systems for the production of chemicals, fuels and materials heavily rely on the use of fossil resources. Due to the increasing concerns on climate change and other environmental problems, however, there has been much interest in developing biorefineries for the production of such chemicals, fuels and materials from renewable resources. For the biorefineries to be competitive with the traditional fossil resource-based refineries, development of high performance microorganisms is the most important as it will affect the overall economics of the process most significantly. Metabolic engineering, which can be defined as purposeful modification of cellular metabolic and regulatory networks with an aim to improve the production of a desired product, has been successfully employed to improve the performance of the cell. However, it is not trivial to engineer the cellular metabolism and regulatory circuits in the cell due to their high complexity.
In metabolic engineering, it is important to find the genes that need to be amplified and attenuated in order to increase the product formation rate while minimizing the production of undesirable byproducts. Gene knock-out experiments are often performed to delete those metabolic fluxes that will consequently result in the increase of the desired product formation. However, gene knock-out experiments require much effort and time to perform, and are difficult to do for a large number of genes. Furthermore, the gene knock-out experiments performed in one strain cannot be transferred to another organism and thus the whole experimental process has to be repeated. This is a big problem in developing a high performance microbial cell factory because it is required to find the best platform strain among many different strains. Therefore, researchers have been eager to develop a strategy that allows rapid identification of multiple genes to be attenuated in multiple strains at the same time.
A Korean research team led by Distinguished Professor Sang Yup Lee at the Department of Chemical and Biomolecular Engineering from the Korea Advanced Institute of Science and Technology (KAIST) reported the development of a strategy for efficiently developing microbial cell factories by employing synthetic small RNAs (sRNAs). They first reported the development of such system in Nature Biotechnology last February. This strategy of employing synthetic sRNAs in metabolic engineering has been receiving great interest worldwide as it allows easy, rapid, high-throughput, tunable, and un-doable knock-down of multiple genes in multiple strains at the same time. The research team published a paper online on August 8 as a cover page (September issue) in Nature Protocols, describing the detailed strategy and protocol to employ synthetic sRNAs for metabolic engineering.
In this paper, researchers described the detailed step-by-step protocol for synthetic sRNA-based gene expression control, including the sRNA design principles. Tailor-made synthetic sRNAs can be easily manipulated by using conventional gene cloning method. The use of synthetic sRNAs for gene expression regulation provides several advantages such as portability, conditionality, and tunability in high-throughput experiments. Plasmid-based synthetic sRNA expression system does not leave any scar on the chromosome, and can be easily transferred to many other host strains to be examined. Thus, the construction of libraries and examination of different host strains are much easier than the conventional hard-coded gene manipulation systems. Also, the expression of genes can be conditionally repressed by controlling the production of synthetic sRNAs. Synthetic sRNAs possessing different repression efficiencies make it possible to finely tune the gene expression levels as well. Furthermore, synthetic sRNAs allow knock-down of the expression of essential genes, which was not possible by conventional gene knock-out experiments.
Synthetic sRNAs can be utilized for diverse experiments where gene expression regulation is needed. One of promising applications is high-throughput screening of the target genes to be manipulated and multiple strains simultaneously to enhance the production of chemicals and materials of interest. Such simultaneous optimization of gene targets and strains has been one of the big challenges in metabolic engineering. Another application is to fine tune the expression of the screened genes for flux optimization, which would enhance chemical production further by balancing the flux between biomass formation and target chemical production. Synthetic sRNAs can also be applied to finely regulating genetic interactions in a circuit or network, which is essential in synthetic biology. Once a sRNA scaffold-harboring plasmid is constructed, tailor-made, synthetic sRNAs can be made within 3-4 days, followed by the desired application experiments.
Dr. Eytan Zlotorynski, an editor at Nature Protocols, said "This paper describes the detailed protocol for the design and applications of synthetic sRNA. The method, which has many advantages, is likely to become common practice, and prove useful for metabolic engineering and synthetic biology studies."
This paper published in Nature Protocols will be useful for all researchers in academia and industry who are interested in the use of synthetic sRNAs for fundamental and applied biological and biotechnological studies.
This work was supported by the Technology Development Program to Solve Climate Changes on Systems Metabolic Engineering for Biorefineries (NRF-2012-C1AAA001-2012M1A2A2026556) and the Intelligent Synthetic Biology Center through the Global Frontier Project (2011-0031963) of the Ministry of Science, ICT and Future Planning through the National Research Foundation of Korea.
2013.10.31 View 10979 -
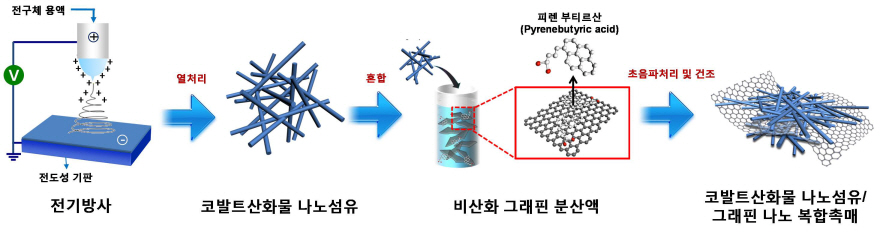 Core Technology for Lithium Air Secondary Battery Developed
KAIST-Kyonggi University joint research team developed composite catalyst out of nano fiber and graphene
Five times improvement in capacity compared to lithium-ion secondary battery, driving 800 km at maximum
The core technology for lithium air secondary battery, the next generation high capacity battery, has been developed.
A research team formed by KAIST Department of Materials Science’s Professors Il-Doo Kim and Seokwoo Jeon, and Kyonggi University Department of Materials Science’s Professor Yong-Joon Park has created a lithium air secondary battery, with five times greater storage than the lithium-ion secondary battery, by developing a nano fiber-graphene composite catalyst. The research results are published in the August 8th online edition of Nano Letters.
A cathode of a lithium-ion battery consists of graphite and an anode of the battery consists of a lithium transition metal oxide. Lithium-ion batteries are widely used in mobile phones and laptops. However, lithium-ion batteries cannot support electric vehicles, providing energy for only 160 kilometers on one full charge. The lithium air secondary battery just developed by the research team uses lithium on the cathode and oxygen on the anode. It is earning a popular acknowledgement among the next generation secondary battery research community for having lightweight mass and high energy density.
However, lithium-ion batteries remain difficult to commercialize because of their short lifespan. Lithium and oxygen meet up to form lithium oxide (Li2O2) at discharge, and decompose again at charge. In a traditional lithium air battery, this cycle does not occur smoothly and results in high resistance, thereby reducing the lifespan of the battery. It is thus essential to develop high efficiency catalyst that facilitates the formation and decomposition of lithium oxides.
The research team used electric radiation to develop a nano composite catalyst by mixing cobalt oxide nano fiber and graphene. The performance of the battery has been maximized by settling nonoxidative graphene, which has high specific surface area and electrical conductivity, on catalyst active cobalt oxide nano fiber. Applying the nano composite catalyst on both poles of the lithium air battery resulted in an improved lifespan of over 80 recharge cycles with capacity greater than 100mAh/g, five times greater than a lithium ion battery. The newly discovered charge-discharge property is the highest among the reported performances of the lithium air battery so far.
The lithium air battery is cheap to make, as the main materials are metal oxide and graphene. “There are yet more issues to resolve such as stability, but we will collaborate with other organizations to open up the era of electronic vehicles,” said Professor Il-Doo Kim. “We hope to contribute to vitalizing the fields of next generation lithium air battery by leading nanocatalyst synthesis technology, one of the core materials in the fields of secondary battery,” Professor Kim spoke of his aspiration.
The graduate students participated in the research are Won-Hee Ryu, a postdoctorate at KAIST Department of Materials Science, Sungho Song, a PhD candidate at KAIST Department of Materials Science, and Taek-Han Yoon, a graduate student at Kyonggi University.
Picture I: Schematic Diagram of Lithium Air Battery Made of Nano Composite Catalysts
Picture II: Images of Cobalt Oxide Nano Fibers and Graphene Nano Composite Catalysts
Picture III: Images of Manufacturing Process of Cobalt Oxide Nano Fibers and Graphene Nano Composite Catalysts for Lithium Air Battery
2013.10.18 View 13243
Core Technology for Lithium Air Secondary Battery Developed
KAIST-Kyonggi University joint research team developed composite catalyst out of nano fiber and graphene
Five times improvement in capacity compared to lithium-ion secondary battery, driving 800 km at maximum
The core technology for lithium air secondary battery, the next generation high capacity battery, has been developed.
A research team formed by KAIST Department of Materials Science’s Professors Il-Doo Kim and Seokwoo Jeon, and Kyonggi University Department of Materials Science’s Professor Yong-Joon Park has created a lithium air secondary battery, with five times greater storage than the lithium-ion secondary battery, by developing a nano fiber-graphene composite catalyst. The research results are published in the August 8th online edition of Nano Letters.
A cathode of a lithium-ion battery consists of graphite and an anode of the battery consists of a lithium transition metal oxide. Lithium-ion batteries are widely used in mobile phones and laptops. However, lithium-ion batteries cannot support electric vehicles, providing energy for only 160 kilometers on one full charge. The lithium air secondary battery just developed by the research team uses lithium on the cathode and oxygen on the anode. It is earning a popular acknowledgement among the next generation secondary battery research community for having lightweight mass and high energy density.
However, lithium-ion batteries remain difficult to commercialize because of their short lifespan. Lithium and oxygen meet up to form lithium oxide (Li2O2) at discharge, and decompose again at charge. In a traditional lithium air battery, this cycle does not occur smoothly and results in high resistance, thereby reducing the lifespan of the battery. It is thus essential to develop high efficiency catalyst that facilitates the formation and decomposition of lithium oxides.
The research team used electric radiation to develop a nano composite catalyst by mixing cobalt oxide nano fiber and graphene. The performance of the battery has been maximized by settling nonoxidative graphene, which has high specific surface area and electrical conductivity, on catalyst active cobalt oxide nano fiber. Applying the nano composite catalyst on both poles of the lithium air battery resulted in an improved lifespan of over 80 recharge cycles with capacity greater than 100mAh/g, five times greater than a lithium ion battery. The newly discovered charge-discharge property is the highest among the reported performances of the lithium air battery so far.
The lithium air battery is cheap to make, as the main materials are metal oxide and graphene. “There are yet more issues to resolve such as stability, but we will collaborate with other organizations to open up the era of electronic vehicles,” said Professor Il-Doo Kim. “We hope to contribute to vitalizing the fields of next generation lithium air battery by leading nanocatalyst synthesis technology, one of the core materials in the fields of secondary battery,” Professor Kim spoke of his aspiration.
The graduate students participated in the research are Won-Hee Ryu, a postdoctorate at KAIST Department of Materials Science, Sungho Song, a PhD candidate at KAIST Department of Materials Science, and Taek-Han Yoon, a graduate student at Kyonggi University.
Picture I: Schematic Diagram of Lithium Air Battery Made of Nano Composite Catalysts
Picture II: Images of Cobalt Oxide Nano Fibers and Graphene Nano Composite Catalysts
Picture III: Images of Manufacturing Process of Cobalt Oxide Nano Fibers and Graphene Nano Composite Catalysts for Lithium Air Battery
2013.10.18 View 13243 -
 Nanowire Made of Diverse Materials May Become Marketable
- Technology to commercialize nanowire developed after 2 years of industrial-academic joint research -
- 2 million strands of 50nm-width, 20 cm-length nanowire mass producible in 2 hours –
A South Korean joint industrial-academic research team has developed the technology to put forward the commercialization of nanowire that is only a few nanometers wide. It is expected to be applied in various fields such as semiconductors, high performance sensors, and biodevices.
In cooperation with LG Innotek and the National Nanofab center, Professor Jun-Bo Yoon, from KAIST Department of Electrical Engineering, developed the technology to mass produce nanowire at any length with various materials. The research results are published on the online edition of Nano Letters on July 30th.
Nanowire has a long linear structure with its width at 100 nanometers at maximum. It is a multifunctional material that has yet undiscovered thermal, electric, and mechanical properties. Nanowire is highly acclaimed as a cutting-edge material with unique nano-level properties that can be applied in semiconductors, energy, biodevices, and optic devices.
Previously, nanowires had an extremely low synthesis rate that required three or four days to grow few millimeters. It was therefore difficult to produce the desired products using nanowires. Moreover, nanowires needed to be evenly arranged for practical application, but the traditional technology required complex post-treatment, not to mention the arrangement was not immaculate.
The research team applied semiconductor process instead of chemical synthesis to resolve these issues. The team first formed a pattern greater that of the target frequency by using a photo-engraving process on a silicon wafer board whose diameter was 20 centimeters, then repeatedly reduced the frequency to produce 100 nm ultrafine linear grid pattern. Based on this pattern, the research team applied the sputtering process to mass-produce nanowires in perfect shapes of 50 nm width and 20 cm maximum length.
The new technology requires neither a lengthy synthesis process nor post-cleaning to attain a perfectly aligned state. Thus, academic and industrial circles consider the technology has high possibilities for commercialization.
“The significance is in resolving the issues in traditional technology, such as low productivity, long manufacturing time, restrictions in material synthesis, and nanowire alignment,” commented Professor Yoon on this research. “Nanowires have not been widely applied in the industry, but this technology will bring forward the commercialization of high performance semiconductors, optic devices, and biodevices that make use of nanowires.”
2013.10.18 View 10452
Nanowire Made of Diverse Materials May Become Marketable
- Technology to commercialize nanowire developed after 2 years of industrial-academic joint research -
- 2 million strands of 50nm-width, 20 cm-length nanowire mass producible in 2 hours –
A South Korean joint industrial-academic research team has developed the technology to put forward the commercialization of nanowire that is only a few nanometers wide. It is expected to be applied in various fields such as semiconductors, high performance sensors, and biodevices.
In cooperation with LG Innotek and the National Nanofab center, Professor Jun-Bo Yoon, from KAIST Department of Electrical Engineering, developed the technology to mass produce nanowire at any length with various materials. The research results are published on the online edition of Nano Letters on July 30th.
Nanowire has a long linear structure with its width at 100 nanometers at maximum. It is a multifunctional material that has yet undiscovered thermal, electric, and mechanical properties. Nanowire is highly acclaimed as a cutting-edge material with unique nano-level properties that can be applied in semiconductors, energy, biodevices, and optic devices.
Previously, nanowires had an extremely low synthesis rate that required three or four days to grow few millimeters. It was therefore difficult to produce the desired products using nanowires. Moreover, nanowires needed to be evenly arranged for practical application, but the traditional technology required complex post-treatment, not to mention the arrangement was not immaculate.
The research team applied semiconductor process instead of chemical synthesis to resolve these issues. The team first formed a pattern greater that of the target frequency by using a photo-engraving process on a silicon wafer board whose diameter was 20 centimeters, then repeatedly reduced the frequency to produce 100 nm ultrafine linear grid pattern. Based on this pattern, the research team applied the sputtering process to mass-produce nanowires in perfect shapes of 50 nm width and 20 cm maximum length.
The new technology requires neither a lengthy synthesis process nor post-cleaning to attain a perfectly aligned state. Thus, academic and industrial circles consider the technology has high possibilities for commercialization.
“The significance is in resolving the issues in traditional technology, such as low productivity, long manufacturing time, restrictions in material synthesis, and nanowire alignment,” commented Professor Yoon on this research. “Nanowires have not been widely applied in the industry, but this technology will bring forward the commercialization of high performance semiconductors, optic devices, and biodevices that make use of nanowires.”
2013.10.18 View 10452 -
 Prof. Jiyun Lee receives the U.S. FAA Achievement Award
- Ensures the safe operation of aircraft by monitoring ionospheric changes caused by solar storms.
KAIST’s Aerospace Engineering Department’s Professor Jiyun Lee has received an award from the U.S. Federal Aviation Administration (FAA), in recognition of her work for developing a Global Positioning System (GPS) reinforcing system and improving Satellite Navigation technology.
A GPS reinforcing system provides real-time GPS location and integrity information within 1m ranges to enable the accurate and safe navigation of aircraft. However, when the sun reaches Solar Max, the amount of total electron increases rapidly in the ionosphere. This also increases the possibility of the position error of navigation using the GPS reinforcing system. In order to solve this problem, Professor Lee built an Ionosphere Danger model that monitors the changes in the ionosphere due to solar storm. The model has been implemented into original monitoring software that secures the safety of the GPS reinforcing system user. The research results were published in July 2012 in Radio Science, one of the most prestigious international journals in the field of geophysical studies.
The FAA Technical Center successfully verified Professor Lee’s software and the software is currently being distributed and used by major institutions around the globe, including Eurocontrol. It is expected that the software will be standardized after consultations with international organizations in the recent future.
Professor Lee said, “Satellite navigation is the core of future navigation technology. Since its utilization has been extended to aviation, marine, transportation, telecommunications, finance and other major national infrastructures, it is crucial to ensure the performance and reliability of the system … In the future, cooperation between nations will help to develop the worldwide service of the GPS reinforcing system.”
2013.10.12 View 10216
Prof. Jiyun Lee receives the U.S. FAA Achievement Award
- Ensures the safe operation of aircraft by monitoring ionospheric changes caused by solar storms.
KAIST’s Aerospace Engineering Department’s Professor Jiyun Lee has received an award from the U.S. Federal Aviation Administration (FAA), in recognition of her work for developing a Global Positioning System (GPS) reinforcing system and improving Satellite Navigation technology.
A GPS reinforcing system provides real-time GPS location and integrity information within 1m ranges to enable the accurate and safe navigation of aircraft. However, when the sun reaches Solar Max, the amount of total electron increases rapidly in the ionosphere. This also increases the possibility of the position error of navigation using the GPS reinforcing system. In order to solve this problem, Professor Lee built an Ionosphere Danger model that monitors the changes in the ionosphere due to solar storm. The model has been implemented into original monitoring software that secures the safety of the GPS reinforcing system user. The research results were published in July 2012 in Radio Science, one of the most prestigious international journals in the field of geophysical studies.
The FAA Technical Center successfully verified Professor Lee’s software and the software is currently being distributed and used by major institutions around the globe, including Eurocontrol. It is expected that the software will be standardized after consultations with international organizations in the recent future.
Professor Lee said, “Satellite navigation is the core of future navigation technology. Since its utilization has been extended to aviation, marine, transportation, telecommunications, finance and other major national infrastructures, it is crucial to ensure the performance and reliability of the system … In the future, cooperation between nations will help to develop the worldwide service of the GPS reinforcing system.”
2013.10.12 View 10216 -
 Therapy developed to induce Angiogenesis of Retina
- Junyeop Lee, Graduate School of Medical Sciences and Engineering
- Research results expected to be applied for treatment of diabetic retinopathy
A major clue to treatment of retinovascular disease, which causes blindness, has been found. The key to protection of the retinal nerve is the angiogenic protein that promotes healthy retinal vessel growth around the retina, which usually does not receive blood supply readily. This research offers a beginning to the possible improvement of therapy for diabetic retinopathy1 and retinopathy of prematurity2. Also important to the research is the fact that the ophthalmology specialist researcher, currently undergoing professional training, provided the results.
KAIST Graduate School of Medical Sciences and Engineering’s Junyeop Lee is the opthalmology specialist, who carried out the research under supervision by academic advisers Gyuyeong Go and Wookjun Yoo. The Ministry of Science, ICT and Future Planning as well as the National Research Foundation of Korea have funded his research. The research results have been published as a cover paper on ‘Science Translational Medicine’ on 18th August. This journal is a sister publication of Science, which is prestigious in the field of translational medicine that ties the basic science with clinical medicine. (Thesis title: Angiopoietin-1 Guides Directional Angiogenesis Through Integrin αvβ5 Signaling for Recovery of Ischemic Retinopathy)
The traditional treatment of diabetic retinopathy includes laser photocoagulation to destroy the retinal tissues or antibody therapeutics, which prevents vessel proliferation and blood leaking. The advantage of antibody therapeutics3 is that it retains the retinal nerves, however, it is not the fundamental solution but merely a temporary one, which requires repeated treatments.
The research team identified that Angiopoietin-14 protein, known as essential for growth and stabilization of vessels, also plays an important role in retinal vessel growth. The protein protects the retinal nerves, as well as provides improvement for retinal ischemia5 that is the root cause of vision loss due to retinal hemorrhages. It is expected to become a key to finding fundamental treatment method – by providing sufficient blood supply to the retina, thereby preserving the retinal nerve functions.
The results show that administration of Angiopoietin-1 to retinopathy mouse model promotes growth of healthy vessel growth, further preventing abnormal vessel growth, retinal hemorrhage and vision loss due to retinal ischemia.
Junyeop Lee said, “This research has identified that Angiopoietin-1 is an important factor in retinal vessel generation and stabilization. The paradigm will shift from traditional treatment method, which prevents vessel growth, to a new method that generates healthy vessels and strengthens vessel functions.”
1 Diabetic retinopathy: This retinovascular disease is a diabetic complication caused by insufficient blood supply. It is the major causes of blindness in adults.
2 Retinopathy of prematurity: The retinal vascular disease that occurs in premature infants with incomplete retinal vascular development. It is also the most common cause of blindness in children.
3 Antibody Therapeutics: Antibody developed to selectively inhibit abnormal blood vessel growth and leakage. Typical antibody therapeutics is Avastin and Lucentis, which hinder vascular endothelial growth factor (VEGF).
4 Angiopoietin-1: A critical growth factor that induces the production of healthy blood vessels and maintains the stability of the created vessel.
5 Retinal ischemia: State of ailment where retinal tissue blood supply is not sufficient.
Figure 1. Retinopathy mouse models show that, in comparison to the control group, the VEGF-Trap treatment and Angiopoietin-1 (Ang1) treatment groups significantly suppresses the pathological vascular proliferation. In addition, the Ang 1 group show vessel growth toward the central avascular area (region of retinal ischemia), which is not observed in VEGF-Trap treatment.
Figure 2. Reduced retinal ischemia, retinal bleeding and blood vessel normalization by Angiopoietin-1. Retinal ischemic region (arrow) and retinal bleeding significantly reduced in the Angiopoietin-1 (Ang1) treatment model in comparison to control group (left). The newly generated vessels in Ang 1 model are structurally supported by perivascular cells as normal retinal vessels do (right).
2013.10.12 View 12852
Therapy developed to induce Angiogenesis of Retina
- Junyeop Lee, Graduate School of Medical Sciences and Engineering
- Research results expected to be applied for treatment of diabetic retinopathy
A major clue to treatment of retinovascular disease, which causes blindness, has been found. The key to protection of the retinal nerve is the angiogenic protein that promotes healthy retinal vessel growth around the retina, which usually does not receive blood supply readily. This research offers a beginning to the possible improvement of therapy for diabetic retinopathy1 and retinopathy of prematurity2. Also important to the research is the fact that the ophthalmology specialist researcher, currently undergoing professional training, provided the results.
KAIST Graduate School of Medical Sciences and Engineering’s Junyeop Lee is the opthalmology specialist, who carried out the research under supervision by academic advisers Gyuyeong Go and Wookjun Yoo. The Ministry of Science, ICT and Future Planning as well as the National Research Foundation of Korea have funded his research. The research results have been published as a cover paper on ‘Science Translational Medicine’ on 18th August. This journal is a sister publication of Science, which is prestigious in the field of translational medicine that ties the basic science with clinical medicine. (Thesis title: Angiopoietin-1 Guides Directional Angiogenesis Through Integrin αvβ5 Signaling for Recovery of Ischemic Retinopathy)
The traditional treatment of diabetic retinopathy includes laser photocoagulation to destroy the retinal tissues or antibody therapeutics, which prevents vessel proliferation and blood leaking. The advantage of antibody therapeutics3 is that it retains the retinal nerves, however, it is not the fundamental solution but merely a temporary one, which requires repeated treatments.
The research team identified that Angiopoietin-14 protein, known as essential for growth and stabilization of vessels, also plays an important role in retinal vessel growth. The protein protects the retinal nerves, as well as provides improvement for retinal ischemia5 that is the root cause of vision loss due to retinal hemorrhages. It is expected to become a key to finding fundamental treatment method – by providing sufficient blood supply to the retina, thereby preserving the retinal nerve functions.
The results show that administration of Angiopoietin-1 to retinopathy mouse model promotes growth of healthy vessel growth, further preventing abnormal vessel growth, retinal hemorrhage and vision loss due to retinal ischemia.
Junyeop Lee said, “This research has identified that Angiopoietin-1 is an important factor in retinal vessel generation and stabilization. The paradigm will shift from traditional treatment method, which prevents vessel growth, to a new method that generates healthy vessels and strengthens vessel functions.”
1 Diabetic retinopathy: This retinovascular disease is a diabetic complication caused by insufficient blood supply. It is the major causes of blindness in adults.
2 Retinopathy of prematurity: The retinal vascular disease that occurs in premature infants with incomplete retinal vascular development. It is also the most common cause of blindness in children.
3 Antibody Therapeutics: Antibody developed to selectively inhibit abnormal blood vessel growth and leakage. Typical antibody therapeutics is Avastin and Lucentis, which hinder vascular endothelial growth factor (VEGF).
4 Angiopoietin-1: A critical growth factor that induces the production of healthy blood vessels and maintains the stability of the created vessel.
5 Retinal ischemia: State of ailment where retinal tissue blood supply is not sufficient.
Figure 1. Retinopathy mouse models show that, in comparison to the control group, the VEGF-Trap treatment and Angiopoietin-1 (Ang1) treatment groups significantly suppresses the pathological vascular proliferation. In addition, the Ang 1 group show vessel growth toward the central avascular area (region of retinal ischemia), which is not observed in VEGF-Trap treatment.
Figure 2. Reduced retinal ischemia, retinal bleeding and blood vessel normalization by Angiopoietin-1. Retinal ischemic region (arrow) and retinal bleeding significantly reduced in the Angiopoietin-1 (Ang1) treatment model in comparison to control group (left). The newly generated vessels in Ang 1 model are structurally supported by perivascular cells as normal retinal vessels do (right).
2013.10.12 View 12852 -
 KAIST to establish Ombudsperson system
KAIST has recently undergone a massive reorganization to achieve a streamlined system and highly efficient administration; and it will now implement the new “Ombudsperson” system to hear the opinions of the members of the university.
On September 9th, President Sungmo Kang held a ceremony to appoint Professors Sang-Young Shin and Hong-Gu Shim as the new “Ombudspersons”.
The previous Shinmungo system raised complaints and recommendations for improvements by members of the university, but this is the first time that KAIST has assigned a direct department for handling such matters.
The newly appointed Ombudspersons will review for the possibility of any unjust, irrational systems, violations of research ethics and such. It is their role to take a neutral stance and advise on the correction and improvement.
The merit of the Ombudsperson system is that diverse opinions can be reflected on the policy. The Ombudsperson guarantees the security of the contents of discussion so that anyone can share his or her opinion without fear of being recorded in documents.
It is expected that the Ombudsperson system will protect the interests of the individuals and thus contribute to making a “happy campus”.
President [Sungmo] Kang has said that the reason establishing the office of the Ombudsperson is “In order for KAIST to take a new leap toward the world, it is crucial to bring the minds of the members together…. Even the smallest voices must be heard to present solutions to make the university where everyone’s happy.”
In 1809, the Swedish Parliament appointed the first “Ombudsperson” to investigate and resolve civil complaints. Now, it is widely used in public institutions, corporations and universities to improve the communication and work efficiency of the members.
The new Ombudsmen: Prof. Sang-Young Shin (left) and Prof. Hong-Gu Shim (right)
2013.09.27 View 12782
KAIST to establish Ombudsperson system
KAIST has recently undergone a massive reorganization to achieve a streamlined system and highly efficient administration; and it will now implement the new “Ombudsperson” system to hear the opinions of the members of the university.
On September 9th, President Sungmo Kang held a ceremony to appoint Professors Sang-Young Shin and Hong-Gu Shim as the new “Ombudspersons”.
The previous Shinmungo system raised complaints and recommendations for improvements by members of the university, but this is the first time that KAIST has assigned a direct department for handling such matters.
The newly appointed Ombudspersons will review for the possibility of any unjust, irrational systems, violations of research ethics and such. It is their role to take a neutral stance and advise on the correction and improvement.
The merit of the Ombudsperson system is that diverse opinions can be reflected on the policy. The Ombudsperson guarantees the security of the contents of discussion so that anyone can share his or her opinion without fear of being recorded in documents.
It is expected that the Ombudsperson system will protect the interests of the individuals and thus contribute to making a “happy campus”.
President [Sungmo] Kang has said that the reason establishing the office of the Ombudsperson is “In order for KAIST to take a new leap toward the world, it is crucial to bring the minds of the members together…. Even the smallest voices must be heard to present solutions to make the university where everyone’s happy.”
In 1809, the Swedish Parliament appointed the first “Ombudsperson” to investigate and resolve civil complaints. Now, it is widely used in public institutions, corporations and universities to improve the communication and work efficiency of the members.
The new Ombudsmen: Prof. Sang-Young Shin (left) and Prof. Hong-Gu Shim (right)
2013.09.27 View 12782 -
 Jellyfish Exterminator Robot Developed
Formation Control demonstrated by JEROS
- Trial performance successfully completed with three assembly robots -
A team led by KAIST Civil and Environmental Engineering Department’s Professor Hyeon Myeong has just finished testing the cooperative assembly robot for jellyfish population control, named JEROS, in the field.
The rising number of accidents and financial losses by fishing industry, estimated at 300 billion won per year, caused by the recent swarm of jellyfish in coastal waters has been a major problem for many years. The research team led by Prof. Hyeon Myeong began developing an unmanned automated system capable of eradicating jellyfishin in 2009, and has since completed field-tests last year with success.
This year, JEROS’s performance and speed has been improved with the ability to work in formation as a cooperative group to efficiently exterminate jellyfish.
An unmanned aquatic robot JEROS with a mountable grinding part is buoyed by two cylindrical bodies that utilizes propulsion motors to move forward and reverse, as well as rotate 360 degrees. Furthermore, GIS (geographic information system)-based map data is used to specify the region for jellyfish extermination, which automatically calculates the path for the task. JEROS then navigates autonomously using a GPS (Global Positioning System) receiver and an INS(inertial navigation system).
The assembly robots maintain a set formation pattern, while calculating its course to perform jellyfish extermination. The advantage of this method is that there is no need for individual control of the robots. Only the leader robot requires the calculated path, and the other robots can simply follow in a formation by exchanging their location information via wireless communication (ZigBee method).
JEROS uses its propulsion speed to capture jellyfish into the grinding part on the bottom, which then suctions the jellyfish toward the propeller to be exterminated.
The field test results show that three assembly robots operating at 4 knots (7.2km/h) disposes jellyfish at the rate of about 900kg/h.
The research team has currently completed testing JEROS at Gyeongnam Masan Bay and is expected to further experiment and improve the performance at various environment and conditions.
JEROS may also be utilized for other purposes including marine patrols, prevention of oil spills and waste removal in the sea.
JEROS research has been funded by Ministry of Science, ICT and Future Planning and Ministry of Trade, Industry and Energy.
2013.09.27 View 18058
Jellyfish Exterminator Robot Developed
Formation Control demonstrated by JEROS
- Trial performance successfully completed with three assembly robots -
A team led by KAIST Civil and Environmental Engineering Department’s Professor Hyeon Myeong has just finished testing the cooperative assembly robot for jellyfish population control, named JEROS, in the field.
The rising number of accidents and financial losses by fishing industry, estimated at 300 billion won per year, caused by the recent swarm of jellyfish in coastal waters has been a major problem for many years. The research team led by Prof. Hyeon Myeong began developing an unmanned automated system capable of eradicating jellyfishin in 2009, and has since completed field-tests last year with success.
This year, JEROS’s performance and speed has been improved with the ability to work in formation as a cooperative group to efficiently exterminate jellyfish.
An unmanned aquatic robot JEROS with a mountable grinding part is buoyed by two cylindrical bodies that utilizes propulsion motors to move forward and reverse, as well as rotate 360 degrees. Furthermore, GIS (geographic information system)-based map data is used to specify the region for jellyfish extermination, which automatically calculates the path for the task. JEROS then navigates autonomously using a GPS (Global Positioning System) receiver and an INS(inertial navigation system).
The assembly robots maintain a set formation pattern, while calculating its course to perform jellyfish extermination. The advantage of this method is that there is no need for individual control of the robots. Only the leader robot requires the calculated path, and the other robots can simply follow in a formation by exchanging their location information via wireless communication (ZigBee method).
JEROS uses its propulsion speed to capture jellyfish into the grinding part on the bottom, which then suctions the jellyfish toward the propeller to be exterminated.
The field test results show that three assembly robots operating at 4 knots (7.2km/h) disposes jellyfish at the rate of about 900kg/h.
The research team has currently completed testing JEROS at Gyeongnam Masan Bay and is expected to further experiment and improve the performance at various environment and conditions.
JEROS may also be utilized for other purposes including marine patrols, prevention of oil spills and waste removal in the sea.
JEROS research has been funded by Ministry of Science, ICT and Future Planning and Ministry of Trade, Industry and Energy.
2013.09.27 View 18058 -
 Secondary, High Capacity Battery developed from Rice Husks
Rice husks, a waste product from rice polishing, has been successfully utilized as the silicon anode for use in high capacity lithium ion secondary batteries. The new silicon anode derived from rice husks exhibit superior output and lifespan.
Professor Choi Jang Wook (The Graduate School of Energy, Environment, Water and Sustainability (EEWS)) and Professor Park Seung Min (Department of Biochemistry) and their respective research teams separated naturally occurring, highly porous silica material within the rice husks and developed a 3-dimensional, highly porous silicon anode material.
The result of the research effort was published in the online edition of the Proceedings of the National Academy of Sciences (PNAS) journal, a world renowned journal in the field of natural sciences.
Silicon has attracted much attention as anode material for next generation lithium ion secondary batteries because it exhibits 3~5 times higher capacity than conventional graphene. The high capacity will pave the way to lithium secondary batteries with higher energy densities than conventional batteries. It is anticipated that the application of silicon batteries will yield electronic devices with a longer duration for use in addition to electronic vehicles boasting longer mileage.
The silicon anode is based on the 3-dimensional, highly porous structure of rice husks which remedies the problematic extreme volume expansion of conventional silicon anodes.
Utilization of inexpensive rice husks to create high value silicon anodes will cause a ripple effect on the industry and academia.
2013.08.23 View 12022
Secondary, High Capacity Battery developed from Rice Husks
Rice husks, a waste product from rice polishing, has been successfully utilized as the silicon anode for use in high capacity lithium ion secondary batteries. The new silicon anode derived from rice husks exhibit superior output and lifespan.
Professor Choi Jang Wook (The Graduate School of Energy, Environment, Water and Sustainability (EEWS)) and Professor Park Seung Min (Department of Biochemistry) and their respective research teams separated naturally occurring, highly porous silica material within the rice husks and developed a 3-dimensional, highly porous silicon anode material.
The result of the research effort was published in the online edition of the Proceedings of the National Academy of Sciences (PNAS) journal, a world renowned journal in the field of natural sciences.
Silicon has attracted much attention as anode material for next generation lithium ion secondary batteries because it exhibits 3~5 times higher capacity than conventional graphene. The high capacity will pave the way to lithium secondary batteries with higher energy densities than conventional batteries. It is anticipated that the application of silicon batteries will yield electronic devices with a longer duration for use in addition to electronic vehicles boasting longer mileage.
The silicon anode is based on the 3-dimensional, highly porous structure of rice husks which remedies the problematic extreme volume expansion of conventional silicon anodes.
Utilization of inexpensive rice husks to create high value silicon anodes will cause a ripple effect on the industry and academia.
2013.08.23 View 12022