research
-
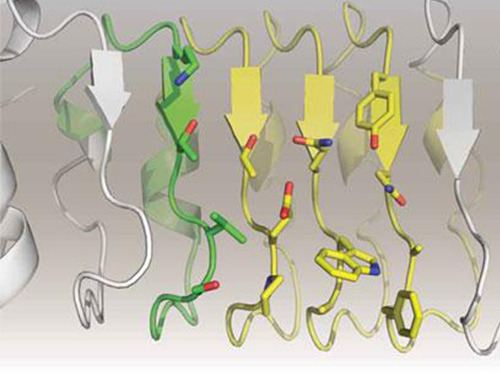 Artificial Antibody-based Therapeutic Candidate for Lung Cancer Developed
Professor Hak-Sung Kim of Biological Sciences at KAIST publishes a cover article on artificial antibody in "Molecular Therapy".
Repebody-based lung cancer therapeutic drug candidate developed Repebody-based protein demonstrates the possibility of the development of a new drug
KAIST Biological Sciences Department’s Professor Hak-Sung Kim, in collaboration with Professor Eun-Kyung Cho from the College of Medicine at Chungnam National University, has successfully developed an artificial antibody-based, or repebody, cancer therapeutic candidate. These research results were published as a cover paper of the July edition of Molecular Therapy.
The repebody developed by Professor Kim and his team strongly binds to interleukin-6, a cancer-causing factor. It has also been confirmed that the repebody can significantly inhibit the proliferation of cancer cells in non-small-cell lung cancer animal model.
Numerous multinational pharmaceutical and biotechnology companies have invested astronomical amounts of money in research for the development of protein therapeutics with low side effects and high efficacy. More than 20 kinds of such therapeutics are currently under clinical trials, and over 100 drugs are under clinical demonstration. Among these, the majority is antibody-based therapeutics, and most of the investments are heavily concentrated in this field. However, antibody production cost is very high because it has large molecular weights and complex structural properties, and this makes it difficult to engineer. Consequently, the development costs a great deal of time and money.
In order to overcome the existing limitations of antibody-based therapeutics, Professor Kim and his team have developed a new artificial antibody, or repebody, which was published in Proceedings of the National Academy of Sciences (PNAS) in 2012. Based on this research, they have succeeded in developing a therapeutic candidate for treating non-small-cell lung cancer with a specifically strong cohesion to the cancer-causing factor, interleukin-6.
Interleukin-6 is a crucial substance within the body that is involved in immune and inflammatory-related signals. When abnormally expressed, it activates various carcinogenic pathways and promotes tumor growth and metastasis. Because of its importance, multinational pharmaceutical companies are heavily investing in developing therapeutics that can inhibit the signaling of interleukin-6.
In this study, Professor Kim and his team observed that a repebody consists of repeated modules, and they conceived a module-based affinity amplification technology that can effectively increase the binding affinity with the disease target. The developed therapeutic candidate has been confirmed in cell and animal experiments to show low immunogenicity, as well as to strongly inhibit the proliferation of non-small-cell lung cancer.
Furthermore, by investigating the complex structure of the repebody with interleukin-6, Professor Kim has identified its mechanism, which demonstrated the potential for therapeutic development. The researchers are currently carrying out pre-clinical trials for acquiring permission to perform clinical trials on animals with non-small-cell lung cancer. The repebody can be developed into a new protein drug after demonstrating its safety and efficacy.
Professor Hak-Sung Kim and his team have confirmed that the repebody can be utilized as a new protein drug, and this will be a significant contribution to Korea’s protein drugs and biotechnology industry development.
The research was supported by the Future Pioneer Industry project and sponsored by the Ministry of Science, ICT and Future Planning.
Figure 1. Professor Kim’s article published as the cover article of July edition of Molecular Therapy
Figure 2. Clinical proof of the repebody’s inhibition of cancer growth using animal models
2014.07.14 View 14368
Artificial Antibody-based Therapeutic Candidate for Lung Cancer Developed
Professor Hak-Sung Kim of Biological Sciences at KAIST publishes a cover article on artificial antibody in "Molecular Therapy".
Repebody-based lung cancer therapeutic drug candidate developed Repebody-based protein demonstrates the possibility of the development of a new drug
KAIST Biological Sciences Department’s Professor Hak-Sung Kim, in collaboration with Professor Eun-Kyung Cho from the College of Medicine at Chungnam National University, has successfully developed an artificial antibody-based, or repebody, cancer therapeutic candidate. These research results were published as a cover paper of the July edition of Molecular Therapy.
The repebody developed by Professor Kim and his team strongly binds to interleukin-6, a cancer-causing factor. It has also been confirmed that the repebody can significantly inhibit the proliferation of cancer cells in non-small-cell lung cancer animal model.
Numerous multinational pharmaceutical and biotechnology companies have invested astronomical amounts of money in research for the development of protein therapeutics with low side effects and high efficacy. More than 20 kinds of such therapeutics are currently under clinical trials, and over 100 drugs are under clinical demonstration. Among these, the majority is antibody-based therapeutics, and most of the investments are heavily concentrated in this field. However, antibody production cost is very high because it has large molecular weights and complex structural properties, and this makes it difficult to engineer. Consequently, the development costs a great deal of time and money.
In order to overcome the existing limitations of antibody-based therapeutics, Professor Kim and his team have developed a new artificial antibody, or repebody, which was published in Proceedings of the National Academy of Sciences (PNAS) in 2012. Based on this research, they have succeeded in developing a therapeutic candidate for treating non-small-cell lung cancer with a specifically strong cohesion to the cancer-causing factor, interleukin-6.
Interleukin-6 is a crucial substance within the body that is involved in immune and inflammatory-related signals. When abnormally expressed, it activates various carcinogenic pathways and promotes tumor growth and metastasis. Because of its importance, multinational pharmaceutical companies are heavily investing in developing therapeutics that can inhibit the signaling of interleukin-6.
In this study, Professor Kim and his team observed that a repebody consists of repeated modules, and they conceived a module-based affinity amplification technology that can effectively increase the binding affinity with the disease target. The developed therapeutic candidate has been confirmed in cell and animal experiments to show low immunogenicity, as well as to strongly inhibit the proliferation of non-small-cell lung cancer.
Furthermore, by investigating the complex structure of the repebody with interleukin-6, Professor Kim has identified its mechanism, which demonstrated the potential for therapeutic development. The researchers are currently carrying out pre-clinical trials for acquiring permission to perform clinical trials on animals with non-small-cell lung cancer. The repebody can be developed into a new protein drug after demonstrating its safety and efficacy.
Professor Hak-Sung Kim and his team have confirmed that the repebody can be utilized as a new protein drug, and this will be a significant contribution to Korea’s protein drugs and biotechnology industry development.
The research was supported by the Future Pioneer Industry project and sponsored by the Ministry of Science, ICT and Future Planning.
Figure 1. Professor Kim’s article published as the cover article of July edition of Molecular Therapy
Figure 2. Clinical proof of the repebody’s inhibition of cancer growth using animal models
2014.07.14 View 14368 -
 KAIST Researchers Develops Sensor That Reads Emotional States of Users
A piloerection monitoring sensor attached on the skin
The American Institute of Physics distributed a press release dated June 24, 2014 on a research paper written by a KAIST research team, which was published in its journal entitled Applied Physics Letters (APL). APL features concise, up-to-date reports in significant new findings in applied physics.
According to the release, “KAIST researchers have developed a flexible, wearable 20 mm x 20 mm polymer sensor that can directly measure the degree and occurrence on the skin of goose bumps, which is caused by sudden changes in body temperature or emotional states.”
The lead researcher was Professor Young-Ho Cho from the Department of Bio and Brain Engineering at KAIST.
If you would like to read the press release, please go to the link below:
American Institute of Physics, June 24, 2014
“New technology: The goose bump sensor”
http://www.eurekalert.org/pub_releases/2014-06/aiop-ntt062314.php
2014.06.26 View 9286
KAIST Researchers Develops Sensor That Reads Emotional States of Users
A piloerection monitoring sensor attached on the skin
The American Institute of Physics distributed a press release dated June 24, 2014 on a research paper written by a KAIST research team, which was published in its journal entitled Applied Physics Letters (APL). APL features concise, up-to-date reports in significant new findings in applied physics.
According to the release, “KAIST researchers have developed a flexible, wearable 20 mm x 20 mm polymer sensor that can directly measure the degree and occurrence on the skin of goose bumps, which is caused by sudden changes in body temperature or emotional states.”
The lead researcher was Professor Young-Ho Cho from the Department of Bio and Brain Engineering at KAIST.
If you would like to read the press release, please go to the link below:
American Institute of Physics, June 24, 2014
“New technology: The goose bump sensor”
http://www.eurekalert.org/pub_releases/2014-06/aiop-ntt062314.php
2014.06.26 View 9286 -
 The First Demonstration of a Self-powered Cardiac Pacemaker
As the number of pacemakers implanted each year reaches into the millions worldwide, improving the lifespan of pacemaker batteries has been of great concern for developers and manufacturers. Currently, pacemaker batteries last seven years on average, requiring frequent replacements, which may pose patients to a potential risk involved in medical procedures.
A research team from the Korea Advanced Institute of Science and Technology (KAIST), headed by Professor Keon Jae Lee of the Department of Materials Science and Engineering at KAIST and Professor Boyoung Joung, M.D. of the Division of Cardiology at Severance Hospital of Yonsei University, has developed a self-powered artificial cardiac pacemaker that is operated semi-permanently by a flexible piezoelectric nanogenerator.
The artificial cardiac pacemaker is widely acknowledged as medical equipment that is integrated into the human body to regulate the heartbeats through electrical stimulation to contract the cardiac muscles of people who suffer from arrhythmia. However, repeated surgeries to replace pacemaker batteries have exposed elderly patients to health risks such as infections or severe bleeding during operations.
The team’s newly designed flexible piezoelectric nanogenerator directly stimulated a living rat’s heart using electrical energy converted from the small body movements of the rat. This technology could facilitate the use of self-powered flexible energy harvesters, not only prolonging the lifetime of cardiac pacemakers but also realizing real-time heart monitoring.
The research team fabricated high-performance flexible nanogenerators utilizing a bulk single-crystal PMN-PT thin film (iBULe Photonics). The harvested energy reached up to 8.2 V and 0.22 mA by bending and pushing motions, which were high enough values to directly stimulate the rat’s heart.
Professor Keon Jae Lee said:
“For clinical purposes, the current achievement will benefit the development of self-powered cardiac pacemakers as well as prevent heart attacks via the real-time diagnosis of heart arrhythmia. In addition, the flexible piezoelectric nanogenerator could also be utilized as an electrical source for various implantable medical devices.”
This research result was described in the April online issue of Advanced Materials (“Self-Powered Cardiac Pacemaker Enabled by Flexible Single Crystalline PMN-PT Piezoelectric Energy Harvester”: http://onlinelibrary.wiley.com/doi/10.1002/adma.201400562/abstract).
Youtube link: http://www.youtube.com/watch?v=ZWYT2cU_Mog&feature=youtu.be
Picture Caption: A self-powered cardiac pacemaker is enabled by a flexible piezoelectric energy harvester.
2014.06.25 View 18064
The First Demonstration of a Self-powered Cardiac Pacemaker
As the number of pacemakers implanted each year reaches into the millions worldwide, improving the lifespan of pacemaker batteries has been of great concern for developers and manufacturers. Currently, pacemaker batteries last seven years on average, requiring frequent replacements, which may pose patients to a potential risk involved in medical procedures.
A research team from the Korea Advanced Institute of Science and Technology (KAIST), headed by Professor Keon Jae Lee of the Department of Materials Science and Engineering at KAIST and Professor Boyoung Joung, M.D. of the Division of Cardiology at Severance Hospital of Yonsei University, has developed a self-powered artificial cardiac pacemaker that is operated semi-permanently by a flexible piezoelectric nanogenerator.
The artificial cardiac pacemaker is widely acknowledged as medical equipment that is integrated into the human body to regulate the heartbeats through electrical stimulation to contract the cardiac muscles of people who suffer from arrhythmia. However, repeated surgeries to replace pacemaker batteries have exposed elderly patients to health risks such as infections or severe bleeding during operations.
The team’s newly designed flexible piezoelectric nanogenerator directly stimulated a living rat’s heart using electrical energy converted from the small body movements of the rat. This technology could facilitate the use of self-powered flexible energy harvesters, not only prolonging the lifetime of cardiac pacemakers but also realizing real-time heart monitoring.
The research team fabricated high-performance flexible nanogenerators utilizing a bulk single-crystal PMN-PT thin film (iBULe Photonics). The harvested energy reached up to 8.2 V and 0.22 mA by bending and pushing motions, which were high enough values to directly stimulate the rat’s heart.
Professor Keon Jae Lee said:
“For clinical purposes, the current achievement will benefit the development of self-powered cardiac pacemakers as well as prevent heart attacks via the real-time diagnosis of heart arrhythmia. In addition, the flexible piezoelectric nanogenerator could also be utilized as an electrical source for various implantable medical devices.”
This research result was described in the April online issue of Advanced Materials (“Self-Powered Cardiac Pacemaker Enabled by Flexible Single Crystalline PMN-PT Piezoelectric Energy Harvester”: http://onlinelibrary.wiley.com/doi/10.1002/adma.201400562/abstract).
Youtube link: http://www.youtube.com/watch?v=ZWYT2cU_Mog&feature=youtu.be
Picture Caption: A self-powered cardiac pacemaker is enabled by a flexible piezoelectric energy harvester.
2014.06.25 View 18064 -
 Professor Won Do Heo on LED Light Technology for Controlling Proteins in Living Cells
With the newly developed LED technology, Professor Won Do Heo at the College of Life Science and Bioengineering, KAIST, was able to suppress cell migration and division when cells are exposed to LED light. This suggests a breakthrough to apply in future cancer cell research.
Professor Heo talked about the impact of his research in the following excerpt from a news article:
“We are already conducting research on the spread of cancer, as well as brain science in animal models with the Light-Activated Reversible Inhibition by Assembled Trap. I believe this technology will be a breakthrough in investigating cancer treatments and the function of neurons in a complex neural network, which existing technologies have not been able to do.”
From EE Times Europe, June 19, 2014
“LED Light Technology Controls Proteins in Living Cells”
http://www.ledlighting-eetimes.com/en/led-light-technology-controls-proteins-in-living-cells.html?cmp_id=7&news_id=222909336
2014.06.22 View 8533
Professor Won Do Heo on LED Light Technology for Controlling Proteins in Living Cells
With the newly developed LED technology, Professor Won Do Heo at the College of Life Science and Bioengineering, KAIST, was able to suppress cell migration and division when cells are exposed to LED light. This suggests a breakthrough to apply in future cancer cell research.
Professor Heo talked about the impact of his research in the following excerpt from a news article:
“We are already conducting research on the spread of cancer, as well as brain science in animal models with the Light-Activated Reversible Inhibition by Assembled Trap. I believe this technology will be a breakthrough in investigating cancer treatments and the function of neurons in a complex neural network, which existing technologies have not been able to do.”
From EE Times Europe, June 19, 2014
“LED Light Technology Controls Proteins in Living Cells”
http://www.ledlighting-eetimes.com/en/led-light-technology-controls-proteins-in-living-cells.html?cmp_id=7&news_id=222909336
2014.06.22 View 8533 -
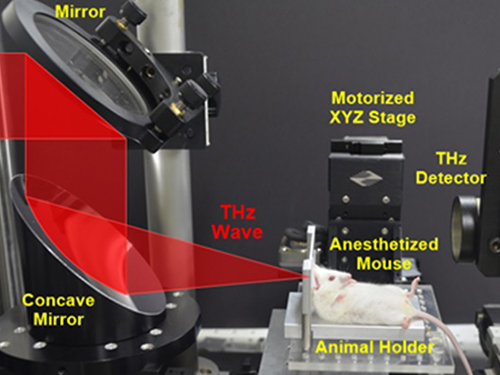 First Instance of Negative Effects from Terahertz-Range Electromagnetic Waves
Professor Philhan Kim
Electromagnetic waves (EM-wave) in the terahertz range were widely regarded as the “dream wavelength” due to its perceived neutrality. Its application was also wider than X-rays. However, KAIST scientists have discovered negative effects from terahertz EM-waves.
Professor Philhan Kim of KAIST’s Graduate School of Nanoscience and Technology and Dr. Young-wook Jeong of the Korea Atomic Energy Research Institute (KAERI) observed inflammation of animal skin tissue when exposed to terahertz EM-waves.
The results were published in the online edition of Optics Express (May 19, 20104).
Terahertz waves range from 0.1 to 10 terahertz and have a longer wavelength than visible or infrared light. Commonly used to see through objects like the X-ray, it was believed that the low energy of terahertz waves did not inflict any harm on the human body.
Despite being applied for security checks, next-generation wireless communications, and medical imaging technology, little research has been conducted in proving its safety and impact. Conventional research failed to predict the exact impact of terahertz waves on organic tissues as only artificially cultured cells were used.
The research team at KAERI developed a high power terahertz EM-wave generator that can be used on live organisms. A high power generator was necessary in applications such as biosensors and required up to 10 times greater power than currently used telecommunications EM-wave. Simultaneously, a KAIST research team developed a high speed, high resolution video-laser microscope that can distinguish cells within the organism.
The experiment exposed 30 minutes of terahertz EM-wave on genetically modified mice and found six times the normal number of inflammation cells in the skin tissue after six hours. It was the first instance where negative side effects of terahertz EM-wave were observed.
Professor Kim commented that “the research has set a standard for how we can use the terahertz EM-wave safely” and that “we will use this research to analyze and understand the effects of other EM-waves on organisms.”
2014.06.20 View 10787
First Instance of Negative Effects from Terahertz-Range Electromagnetic Waves
Professor Philhan Kim
Electromagnetic waves (EM-wave) in the terahertz range were widely regarded as the “dream wavelength” due to its perceived neutrality. Its application was also wider than X-rays. However, KAIST scientists have discovered negative effects from terahertz EM-waves.
Professor Philhan Kim of KAIST’s Graduate School of Nanoscience and Technology and Dr. Young-wook Jeong of the Korea Atomic Energy Research Institute (KAERI) observed inflammation of animal skin tissue when exposed to terahertz EM-waves.
The results were published in the online edition of Optics Express (May 19, 20104).
Terahertz waves range from 0.1 to 10 terahertz and have a longer wavelength than visible or infrared light. Commonly used to see through objects like the X-ray, it was believed that the low energy of terahertz waves did not inflict any harm on the human body.
Despite being applied for security checks, next-generation wireless communications, and medical imaging technology, little research has been conducted in proving its safety and impact. Conventional research failed to predict the exact impact of terahertz waves on organic tissues as only artificially cultured cells were used.
The research team at KAERI developed a high power terahertz EM-wave generator that can be used on live organisms. A high power generator was necessary in applications such as biosensors and required up to 10 times greater power than currently used telecommunications EM-wave. Simultaneously, a KAIST research team developed a high speed, high resolution video-laser microscope that can distinguish cells within the organism.
The experiment exposed 30 minutes of terahertz EM-wave on genetically modified mice and found six times the normal number of inflammation cells in the skin tissue after six hours. It was the first instance where negative side effects of terahertz EM-wave were observed.
Professor Kim commented that “the research has set a standard for how we can use the terahertz EM-wave safely” and that “we will use this research to analyze and understand the effects of other EM-waves on organisms.”
2014.06.20 View 10787 -
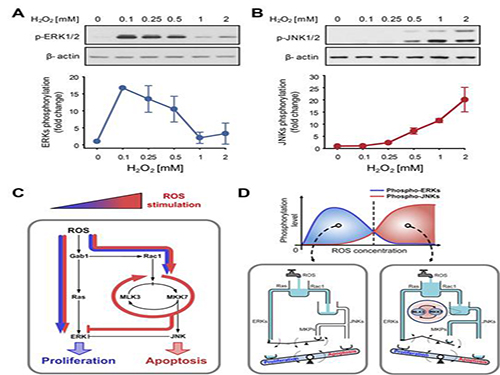 A mechanism for how reactive oxygen species cause cell responses studied
A research team led by Professor Kwang-Hyun Cho of the Department of Biology and Brain Engineering, KAIST, and Dr. Gi-Sun Kwon of the Korea Research Institute of Bioscience and Biotechnology succeeded in proving the mechanism behind the determination of cell life in relation to reactive oxygen species.
The results of the venture were published in the June 3rd edition of Science Signaling. The title of the research paper is “MLK3 is part of a feedback mechanism that regulates different cellular responses to reactive oxygen species.”
The research team discovered that the molecular switch that determines the division of apoptosis of a cell was based on MLK3 feedback mechanism. MLK stands for mixed-lineage kinase.
Under sufficient stress, the mechanism instructs the cell to undergo the division but in an overly stressful environment, the mechanism stops the cell division and instead, induces apoptosis. This discovery is expected to be a breakthrough in illnesses related to the concentration of the reactive oxygen species (ROS).
At low concentration of ROS, the protein associated with cell division, ERK (extracellular-signal-regulated kinase), is activated while as the ROS concentration increases, JNK (c-Jun N-terminal protein kinases), responsible for apoptosis, becomes activated. Furthermore, through computer simulation analysis and mathematical modeling, in tandem with molecular cell biology experiments, the MLK3 based feedback mechanism was the fundamental molecular switch that determines the balance between ERK and JNK, and ultimately the cell’s responses.
Professor Cho commented that “the contradicting cell responses to ROS had remained a mystery, but with the system biology, an approach in which information technology and biotechnology converge, such riddles can be resolved. We expect that the proven mechanism will be used to overcome aging or cancer growth as a result of ROS in the near future.”
Picture shows the process of identifying cell responses caused by reactive oxygen species.
2014.06.13 View 10389
A mechanism for how reactive oxygen species cause cell responses studied
A research team led by Professor Kwang-Hyun Cho of the Department of Biology and Brain Engineering, KAIST, and Dr. Gi-Sun Kwon of the Korea Research Institute of Bioscience and Biotechnology succeeded in proving the mechanism behind the determination of cell life in relation to reactive oxygen species.
The results of the venture were published in the June 3rd edition of Science Signaling. The title of the research paper is “MLK3 is part of a feedback mechanism that regulates different cellular responses to reactive oxygen species.”
The research team discovered that the molecular switch that determines the division of apoptosis of a cell was based on MLK3 feedback mechanism. MLK stands for mixed-lineage kinase.
Under sufficient stress, the mechanism instructs the cell to undergo the division but in an overly stressful environment, the mechanism stops the cell division and instead, induces apoptosis. This discovery is expected to be a breakthrough in illnesses related to the concentration of the reactive oxygen species (ROS).
At low concentration of ROS, the protein associated with cell division, ERK (extracellular-signal-regulated kinase), is activated while as the ROS concentration increases, JNK (c-Jun N-terminal protein kinases), responsible for apoptosis, becomes activated. Furthermore, through computer simulation analysis and mathematical modeling, in tandem with molecular cell biology experiments, the MLK3 based feedback mechanism was the fundamental molecular switch that determines the balance between ERK and JNK, and ultimately the cell’s responses.
Professor Cho commented that “the contradicting cell responses to ROS had remained a mystery, but with the system biology, an approach in which information technology and biotechnology converge, such riddles can be resolved. We expect that the proven mechanism will be used to overcome aging or cancer growth as a result of ROS in the near future.”
Picture shows the process of identifying cell responses caused by reactive oxygen species.
2014.06.13 View 10389 -
 An Exploratory Study on Smartphone Abuse among College Students
Professor Uichin Lee
Professor Uichin Lee of the Department of Knowledge Service Engineering, KAIST, and his research team developed a system that automatically diagnoses the levels of smartphone addiction based on an analysis of smartphone use records.
Professor Lee investigated the usage patterns of 95 smartphone users (college students) by conducting surveys and interviews and collecting logged data. The research team divided participants into “risk” and “non-risk” groups based on a self-reported rating scale to evaluate their abuse of smartphones. As a result, 36 students were categorized as “high risk” and 59 were categorized as “low risk.”
The researchers collected over 50,000 hours of smartphone use encompassing power levels, screen, battery status, application use, internet use, calling, and texting.
The results showed that the “high risk” group used only 1~2 applications, focusing on mobile messengers (Kakotalk, etc.) and SNS (Facebook, etc.).
In addition, a relationship was found between alarm function and addiction levels. Users who set alarms for Kakaotalk messages and SNS comments used smartphones for an additional 38 minutes per day on average.
Results also showed that “high risk” students were on their smartphones for 4 hours and 13 minutes per day, 46 minutes longer than “low risk” students who used smartphones for 3 hours and 27 minutes. The difference was prevalent during 6 am and noon, and 6pm and midnight. In addition, “high risk” students accessed their smartphones 11.4 times more than “low risk” students.
Based on the collected data, Professor Lee developed an automatic system that distinguished users into “high risk” or “low risk” categories with 80% accuracy. The new system is expected to give an early diagnosis of addiction to smartphone users, thereby allowing for early treatment and intervention before the user becomes addicted.
Professor Lee commented that, "the conventional addiction analysis based on self-analysis surveys did not provide real-time data and were largely inaccurate. The new system overcomes these limitations through data science and personal big data analysis" and that he is "developing an application that monitors smartphone abuse."
Figure 1. Usage amount: overall and application-specific results
Figure 2. Usage frequency: overall and application-specific results
Figure 3. Overall diurnal usage time and frequency
2014.06.05 View 8417
An Exploratory Study on Smartphone Abuse among College Students
Professor Uichin Lee
Professor Uichin Lee of the Department of Knowledge Service Engineering, KAIST, and his research team developed a system that automatically diagnoses the levels of smartphone addiction based on an analysis of smartphone use records.
Professor Lee investigated the usage patterns of 95 smartphone users (college students) by conducting surveys and interviews and collecting logged data. The research team divided participants into “risk” and “non-risk” groups based on a self-reported rating scale to evaluate their abuse of smartphones. As a result, 36 students were categorized as “high risk” and 59 were categorized as “low risk.”
The researchers collected over 50,000 hours of smartphone use encompassing power levels, screen, battery status, application use, internet use, calling, and texting.
The results showed that the “high risk” group used only 1~2 applications, focusing on mobile messengers (Kakotalk, etc.) and SNS (Facebook, etc.).
In addition, a relationship was found between alarm function and addiction levels. Users who set alarms for Kakaotalk messages and SNS comments used smartphones for an additional 38 minutes per day on average.
Results also showed that “high risk” students were on their smartphones for 4 hours and 13 minutes per day, 46 minutes longer than “low risk” students who used smartphones for 3 hours and 27 minutes. The difference was prevalent during 6 am and noon, and 6pm and midnight. In addition, “high risk” students accessed their smartphones 11.4 times more than “low risk” students.
Based on the collected data, Professor Lee developed an automatic system that distinguished users into “high risk” or “low risk” categories with 80% accuracy. The new system is expected to give an early diagnosis of addiction to smartphone users, thereby allowing for early treatment and intervention before the user becomes addicted.
Professor Lee commented that, "the conventional addiction analysis based on self-analysis surveys did not provide real-time data and were largely inaccurate. The new system overcomes these limitations through data science and personal big data analysis" and that he is "developing an application that monitors smartphone abuse."
Figure 1. Usage amount: overall and application-specific results
Figure 2. Usage frequency: overall and application-specific results
Figure 3. Overall diurnal usage time and frequency
2014.06.05 View 8417 -
 KAIST Made Great Improvements of Nanogenerator Power Efficiency
The energy efficiency of a piezoelectric nanogenerator developed by KAIST has increased by almost 40 times, one step closer toward the commercialization of flexible energy harvesters that can supply power infinitely to wearable, implantable electronic devices.
NANOGENERATORS are innovative self-powered energy harvesters that convert kinetic energy created from vibrational and mechanical sources into electrical power, removing the need of external circuits or batteries for electronic devices. This innovation is vital in realizing sustainable energy generation in isolated, inaccessible, or indoor environments and even in the human body.
Nanogenerators, a flexible and lightweight energy harvester on a plastic substrate, can scavenge energy from the extremely tiny movements of natural resources and human body such as wind, water flow, heartbeats, and diaphragm and respiration activities to generate electrical signals. The generators are not only self-powered, flexible devices but also can provide permanent power sources to implantable biomedical devices, including cardiac pacemakers and deep brain stimulators.
However, poor energy efficiency and a complex fabrication process have posed challenges to the commercialization of nanogenerators. Keon Jae Lee, Associate Professor of Materials Science and Engineering at KAIST, and his colleagues have recently proposed a solution by developing a robust technique to transfer a high-quality piezoelectric thin film from bulk sapphire substrates to plastic substrates using laser lift-off (LLO).
Applying the inorganic-based laser lift-off (LLO) process, the research team produced a large-area PZT thin film nanogenerators on flexible substrates (2cm x 2cm).
“We were able to convert a high-output performance of ~250 V from the slight mechanical deformation of a single thin plastic substrate. Such output power is just enough to turn on 100 LED lights,” Keon Jae Lee explained.
The self-powered nanogenerators can also work with finger and foot motions. For example, under the irregular and slight bending motions of a human finger, the measured current signals had a high electric power of ~8.7 μA. In addition, the piezoelectric nanogenerator has world-record power conversion efficiency, almost 40 times higher than previously reported similar research results, solving the drawbacks related to the fabrication complexity and low energy efficiency.
Lee further commented,
“Building on this concept, it is highly expected that tiny mechanical motions, including human body movements of muscle contraction and relaxation, can be readily converted into electrical energy and, furthermore, acted as eternal power sources.”
The research team is currently studying a method to build three-dimensional stacking of flexible piezoelectric thin films to enhance output power, as well as conducting a clinical experiment with a flexible nanogenerator.
This research result, entitled “Highly-efficient, Flexible Piezoelectric PZT Thin Film Nanogenerator on Plastic Substrates,” was published as the cover article of the April issue of Advanced Materials. (http://onlinelibrary.wiley.com/doi/10.1002/adma.201305659/abstract)
YouTube Link: http://www.youtube.com/watch?v=G_Fny7Xb9ig
Over 100 LEDs operated by self-powered flexible piezoelectric thin film nanogenerator
Flexible PZT thin film nanogenerator using inorganic-based laser lift-off process
Photograph of large-area PZT thin film nanogenerator (3.5cm × 3.5cm) on a curved glass tube and 105 commercial LEDs operated by self-powered flexible piezoelectric energy harvester
2014.05.19 View 15796
KAIST Made Great Improvements of Nanogenerator Power Efficiency
The energy efficiency of a piezoelectric nanogenerator developed by KAIST has increased by almost 40 times, one step closer toward the commercialization of flexible energy harvesters that can supply power infinitely to wearable, implantable electronic devices.
NANOGENERATORS are innovative self-powered energy harvesters that convert kinetic energy created from vibrational and mechanical sources into electrical power, removing the need of external circuits or batteries for electronic devices. This innovation is vital in realizing sustainable energy generation in isolated, inaccessible, or indoor environments and even in the human body.
Nanogenerators, a flexible and lightweight energy harvester on a plastic substrate, can scavenge energy from the extremely tiny movements of natural resources and human body such as wind, water flow, heartbeats, and diaphragm and respiration activities to generate electrical signals. The generators are not only self-powered, flexible devices but also can provide permanent power sources to implantable biomedical devices, including cardiac pacemakers and deep brain stimulators.
However, poor energy efficiency and a complex fabrication process have posed challenges to the commercialization of nanogenerators. Keon Jae Lee, Associate Professor of Materials Science and Engineering at KAIST, and his colleagues have recently proposed a solution by developing a robust technique to transfer a high-quality piezoelectric thin film from bulk sapphire substrates to plastic substrates using laser lift-off (LLO).
Applying the inorganic-based laser lift-off (LLO) process, the research team produced a large-area PZT thin film nanogenerators on flexible substrates (2cm x 2cm).
“We were able to convert a high-output performance of ~250 V from the slight mechanical deformation of a single thin plastic substrate. Such output power is just enough to turn on 100 LED lights,” Keon Jae Lee explained.
The self-powered nanogenerators can also work with finger and foot motions. For example, under the irregular and slight bending motions of a human finger, the measured current signals had a high electric power of ~8.7 μA. In addition, the piezoelectric nanogenerator has world-record power conversion efficiency, almost 40 times higher than previously reported similar research results, solving the drawbacks related to the fabrication complexity and low energy efficiency.
Lee further commented,
“Building on this concept, it is highly expected that tiny mechanical motions, including human body movements of muscle contraction and relaxation, can be readily converted into electrical energy and, furthermore, acted as eternal power sources.”
The research team is currently studying a method to build three-dimensional stacking of flexible piezoelectric thin films to enhance output power, as well as conducting a clinical experiment with a flexible nanogenerator.
This research result, entitled “Highly-efficient, Flexible Piezoelectric PZT Thin Film Nanogenerator on Plastic Substrates,” was published as the cover article of the April issue of Advanced Materials. (http://onlinelibrary.wiley.com/doi/10.1002/adma.201305659/abstract)
YouTube Link: http://www.youtube.com/watch?v=G_Fny7Xb9ig
Over 100 LEDs operated by self-powered flexible piezoelectric thin film nanogenerator
Flexible PZT thin film nanogenerator using inorganic-based laser lift-off process
Photograph of large-area PZT thin film nanogenerator (3.5cm × 3.5cm) on a curved glass tube and 105 commercial LEDs operated by self-powered flexible piezoelectric energy harvester
2014.05.19 View 15796 -
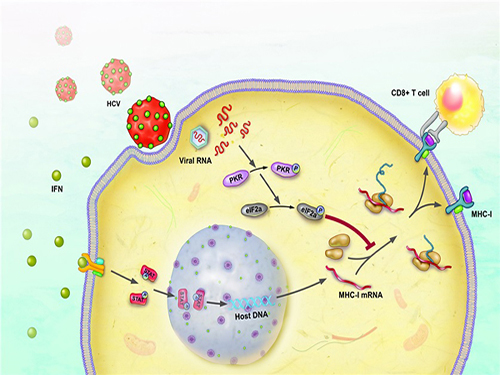 Immune Evasion Mechanism of Hepatitis C Virus Revealed
Professor Ui-Cheol Shin
Inhibiting major histocompatibility complex [MHC] class I protein expression, T cell immune response is evaded.
The research will be a great help to the development of C hepatitis vaccine.
Roughly 1-2% of the population in Korea is known to be infected with Hepatitis C. Most Hepatitis C Virus (HCV) infections progress to a chronic disease and can cause liver cirrhosis or liver cancer, which may lead to death.
Unlike Hepatitis type A or B, there is no vaccine for Hepatitis C Virus and therefore avoiding exposure to the virus is the best known method of prevention. However, a team of researchers at KAIST has produced research results, which may contribute significantly to the vaccine development.
KAIST Graduate School of Medical Sciences & Engineering’ Professor Ui-Cheol Shin and his team have successfully identified why Hepatitis C Virus does not cause an immune response within the human body. The research results were published in the May edition of The Journal of Gastroenterology, a world-renowned journal in the field of gastroenterology.
The immune response occurs to eliminate the virus that has invaded our body. During this process, a major histocompatibility complex [MHC] class I plays a key role in inducing T cell response, which is needed for the elimination of virus-infected cells.
When a cell is infected by a virus, a substance called interferon causes the increased expression of major histocompatibility complex class I. T cell recognizes the increased MHC class I and therefore finds the virus-infected cells.
However, the effect that Hepatitis C Virus has on major histocompatibility complex class I has not been clearly identified until now.
The research team has revealed, using a cell culture for infection systems, that the Hepatitis C Virus suppresses the expression of major histocompatibility complex class I. Also, the mechanism to prove that HCV activates a protein called PKR within the cell to inhibit MHC class I protein expression was identified at a molecular level.
In this study, researchers established the hypothesis that regulating PKR protein in the cell can enhance the T cell immune response, which was then proved through experiments.
Professor Ui-Cheol Shin said, “There are a lot of new drugs to treat Hepatitis C Virus, while its vaccine has not been developed yet. Revealing the HCV immune evasion mechanism will help stimulate momentum for the HCV vaccine development.”
The first author of the journal, Dr. Won-Seok Kang is a graduate from Yonsei College of Medicine. After earning his medical degree, he has continued his training as a ‘doctor-scientist’ at KAIST Graduate School of Medical Sciences & Engineering to study Hepatitis C Virus immune evasion mechanism in this research.
Hepatitis C Virus activates PKR-eIF2a pathway, which inhibits the major histocompatibility complex class I, and therefore weakens the T cell activation to the viral activity.
2014.05.19 View 11874
Immune Evasion Mechanism of Hepatitis C Virus Revealed
Professor Ui-Cheol Shin
Inhibiting major histocompatibility complex [MHC] class I protein expression, T cell immune response is evaded.
The research will be a great help to the development of C hepatitis vaccine.
Roughly 1-2% of the population in Korea is known to be infected with Hepatitis C. Most Hepatitis C Virus (HCV) infections progress to a chronic disease and can cause liver cirrhosis or liver cancer, which may lead to death.
Unlike Hepatitis type A or B, there is no vaccine for Hepatitis C Virus and therefore avoiding exposure to the virus is the best known method of prevention. However, a team of researchers at KAIST has produced research results, which may contribute significantly to the vaccine development.
KAIST Graduate School of Medical Sciences & Engineering’ Professor Ui-Cheol Shin and his team have successfully identified why Hepatitis C Virus does not cause an immune response within the human body. The research results were published in the May edition of The Journal of Gastroenterology, a world-renowned journal in the field of gastroenterology.
The immune response occurs to eliminate the virus that has invaded our body. During this process, a major histocompatibility complex [MHC] class I plays a key role in inducing T cell response, which is needed for the elimination of virus-infected cells.
When a cell is infected by a virus, a substance called interferon causes the increased expression of major histocompatibility complex class I. T cell recognizes the increased MHC class I and therefore finds the virus-infected cells.
However, the effect that Hepatitis C Virus has on major histocompatibility complex class I has not been clearly identified until now.
The research team has revealed, using a cell culture for infection systems, that the Hepatitis C Virus suppresses the expression of major histocompatibility complex class I. Also, the mechanism to prove that HCV activates a protein called PKR within the cell to inhibit MHC class I protein expression was identified at a molecular level.
In this study, researchers established the hypothesis that regulating PKR protein in the cell can enhance the T cell immune response, which was then proved through experiments.
Professor Ui-Cheol Shin said, “There are a lot of new drugs to treat Hepatitis C Virus, while its vaccine has not been developed yet. Revealing the HCV immune evasion mechanism will help stimulate momentum for the HCV vaccine development.”
The first author of the journal, Dr. Won-Seok Kang is a graduate from Yonsei College of Medicine. After earning his medical degree, he has continued his training as a ‘doctor-scientist’ at KAIST Graduate School of Medical Sciences & Engineering to study Hepatitis C Virus immune evasion mechanism in this research.
Hepatitis C Virus activates PKR-eIF2a pathway, which inhibits the major histocompatibility complex class I, and therefore weakens the T cell activation to the viral activity.
2014.05.19 View 11874 -
 Clear Display Technology Under Sunlight Developed
The late Professor Seung-Man Yang
The last paper of the late Professor Seung-Man Yang, who was a past master of colloids and fluid mechanics
Practical patterning technology of the next generation optical materials, photonic crystals
The mineral opal does not possess any pigments, but it appears colorful to our eyes. This is because only a particular wavelength is reflected due to the regular nano-structure of its surface. The material that causes selective reflection of the light is called photonic crystals.
The deceased Professor Seung-Man Yang and his research team from KAIST’s Chemical and Biomolecular Engineering Department ha ve developed micro-pattern technology using photolithographic process. This can accelerate the commercialization of photonic crystals, which is hailed as the next generation optics material.
The research results were published in the April 16th edition of Advanced Materials, known as the most prestigious world-renowned journal in the field of materials science.
The newly developed photonic crystal micro-pattern could be used as a core material for the next generation reflective display that is clearly visible even under sunlight. Since it does not require a separate light source, a single charge is enough to last for several days.
Until now, many scientists have endeavored to make photonic crystals artificially, however, most were produced in a lump and therefore lacked efficiency. Also, the low mechanical stability of the formed structure prevented from commercialization.
In order to solve these problems, the research team has copied the nano-structure of opals.
Glass beads were arranged in the same nano-structure as the opal on top of the photoresist material undergoing photocuring by ultraviolet light. The glass beads were installed in the photoresist materials, and UV light was selectively exposed on micro regions. The remaining region was developed by photolithographic process to successfully produce photonic crystals in micro-patterns.
The co-author of the research, KAIST Chemical and Biomolecular Engineering Department’s Professor Sin-Hyeon Kim, said, “Combining the semiconductor process technology with photonic crystal pattern technology can secure the practical applications for photonic crystals.”He also predicted “This technology can be used as the key optical material that configures the next generation reflective color display device with very low power consumption.”
The late Professor Seung-Man Yang was a world-renowned expert in the field of colloids and fluid mechanics. Professor Yang published over 193 papers in international journals and continued his research until his passing in last September.
He received Du Pont Science and Technology Award in 2007, KAIST Person of the Year 2008, Gyeong-Am Academy Award in 2009, as well as the President’s Award of the Republic of Korea in March 2014. The researchers devoted the achievement of this year’s research to Professor Yang in his honor.
Research was conducted by KAIST Photonic-fluidic Integrated Devices Research Team, as a part of the Creative Research Program funded by the Ministry of Science, ICT and Future Planning, Republic of Korea.
Figure 1. Opal [left] and the nano glass bead arrangement structure within the opal [right]
Figure 2. Process chart of the photonic crystal micro-pattern formation based on photolithography
Figure 3. Opal structure [left] and inverted structure of the opal [right]
Figure 4. Photonic crystal micro-pattern in solid colors
Figure 5. Photonic crystal micro-pattern that reflects two different crystals (Red, Green) [left] and pixelated pattern of photonic crystal in three primary colors (Red, Green, Blue) [right] that is applicable to reflective displays
2014.05.14 View 14023
Clear Display Technology Under Sunlight Developed
The late Professor Seung-Man Yang
The last paper of the late Professor Seung-Man Yang, who was a past master of colloids and fluid mechanics
Practical patterning technology of the next generation optical materials, photonic crystals
The mineral opal does not possess any pigments, but it appears colorful to our eyes. This is because only a particular wavelength is reflected due to the regular nano-structure of its surface. The material that causes selective reflection of the light is called photonic crystals.
The deceased Professor Seung-Man Yang and his research team from KAIST’s Chemical and Biomolecular Engineering Department ha ve developed micro-pattern technology using photolithographic process. This can accelerate the commercialization of photonic crystals, which is hailed as the next generation optics material.
The research results were published in the April 16th edition of Advanced Materials, known as the most prestigious world-renowned journal in the field of materials science.
The newly developed photonic crystal micro-pattern could be used as a core material for the next generation reflective display that is clearly visible even under sunlight. Since it does not require a separate light source, a single charge is enough to last for several days.
Until now, many scientists have endeavored to make photonic crystals artificially, however, most were produced in a lump and therefore lacked efficiency. Also, the low mechanical stability of the formed structure prevented from commercialization.
In order to solve these problems, the research team has copied the nano-structure of opals.
Glass beads were arranged in the same nano-structure as the opal on top of the photoresist material undergoing photocuring by ultraviolet light. The glass beads were installed in the photoresist materials, and UV light was selectively exposed on micro regions. The remaining region was developed by photolithographic process to successfully produce photonic crystals in micro-patterns.
The co-author of the research, KAIST Chemical and Biomolecular Engineering Department’s Professor Sin-Hyeon Kim, said, “Combining the semiconductor process technology with photonic crystal pattern technology can secure the practical applications for photonic crystals.”He also predicted “This technology can be used as the key optical material that configures the next generation reflective color display device with very low power consumption.”
The late Professor Seung-Man Yang was a world-renowned expert in the field of colloids and fluid mechanics. Professor Yang published over 193 papers in international journals and continued his research until his passing in last September.
He received Du Pont Science and Technology Award in 2007, KAIST Person of the Year 2008, Gyeong-Am Academy Award in 2009, as well as the President’s Award of the Republic of Korea in March 2014. The researchers devoted the achievement of this year’s research to Professor Yang in his honor.
Research was conducted by KAIST Photonic-fluidic Integrated Devices Research Team, as a part of the Creative Research Program funded by the Ministry of Science, ICT and Future Planning, Republic of Korea.
Figure 1. Opal [left] and the nano glass bead arrangement structure within the opal [right]
Figure 2. Process chart of the photonic crystal micro-pattern formation based on photolithography
Figure 3. Opal structure [left] and inverted structure of the opal [right]
Figure 4. Photonic crystal micro-pattern in solid colors
Figure 5. Photonic crystal micro-pattern that reflects two different crystals (Red, Green) [left] and pixelated pattern of photonic crystal in three primary colors (Red, Green, Blue) [right] that is applicable to reflective displays
2014.05.14 View 14023 -
 Binding Regulatory Mechanism of Protein Biomolecules Revealed
Professor Hak-Sung Kim
A research team led by Professor Hak-Sung Kim of Biological Sciences, KAIST, and Dr. Mun-Hyeong Seo, KAIST, has revealed a regulatory mechanism that controls the binding affinity of protein’s biomolecules, which is crucial for the protein to recognize molecules and carry out functions within the body.
The research results were published in the April 24th online edition of Nature Communications.
The protein, represented by enzyme, antibody, or hormones, specifically recognizes a variety of biomolecules in all organisms and implements signaling or immune response to precisely adjust and maintain important biological processes. The protein binding affinity of biomolecules plays a crucial role in determining the duration of the bond between two molecules, and hence to determine and control the in-vivo function of proteins.
The researchers have noted that, during the process of proteins’ recognizing biomolecules, the protein binding affinity of biomolecules is closely linked not only to the size of non-covalent interaction between two molecules, but also to the unique kinetic properties of proteins.
To identify the basic mechanism that determines the protein binding affinity of biomolecules, Professor Kim and his research team have made mutation in the allosteric site of protein to create a variety of mutant proteins with the same chemical binding surface, but with the binding affinity vastly differing from 10 to 100 times. The allosteric site of the protein refers to a region which does not directly bind with biomolecules, but crucially influences the biomolecule recognition site.
Using real-time analysis at the single-molecule level of unique kinetic properties of the produced mutant proteins, the researchers were able to identify that the protein binding affinity of biomolecules is directly associated with the protein’s specific kinetic characteristics, its structure opening rate.
Also, by proving that unique characteristics of the protein can be changed at the allosteric site, instead of protein’s direct binding site with biomolecules, the researchers have demonstrated a new methodology of regulating the in-vivo function of proteins.
The researchers expect that these results will contribute greatly to a deeper understanding of protein’s nature that governs various life phenomena and help evaluate the proof of interpreting protein binding affinity of biomolecules from the perspective of protein kinetics.
Professor Kim said, “Until now, the protein binding affinity of biomolecules was determined by a direct interaction between two molecules. Our research has identified an important fact that the structure opening rate of proteins also plays a crucial role in determining their binding affinity.”
[Picture]
A correlation graph of opening rate (kopening) and binding affinity (kd) between protein’s stable, open state and its unstable, partially closed state.
2014.05.02 View 10919
Binding Regulatory Mechanism of Protein Biomolecules Revealed
Professor Hak-Sung Kim
A research team led by Professor Hak-Sung Kim of Biological Sciences, KAIST, and Dr. Mun-Hyeong Seo, KAIST, has revealed a regulatory mechanism that controls the binding affinity of protein’s biomolecules, which is crucial for the protein to recognize molecules and carry out functions within the body.
The research results were published in the April 24th online edition of Nature Communications.
The protein, represented by enzyme, antibody, or hormones, specifically recognizes a variety of biomolecules in all organisms and implements signaling or immune response to precisely adjust and maintain important biological processes. The protein binding affinity of biomolecules plays a crucial role in determining the duration of the bond between two molecules, and hence to determine and control the in-vivo function of proteins.
The researchers have noted that, during the process of proteins’ recognizing biomolecules, the protein binding affinity of biomolecules is closely linked not only to the size of non-covalent interaction between two molecules, but also to the unique kinetic properties of proteins.
To identify the basic mechanism that determines the protein binding affinity of biomolecules, Professor Kim and his research team have made mutation in the allosteric site of protein to create a variety of mutant proteins with the same chemical binding surface, but with the binding affinity vastly differing from 10 to 100 times. The allosteric site of the protein refers to a region which does not directly bind with biomolecules, but crucially influences the biomolecule recognition site.
Using real-time analysis at the single-molecule level of unique kinetic properties of the produced mutant proteins, the researchers were able to identify that the protein binding affinity of biomolecules is directly associated with the protein’s specific kinetic characteristics, its structure opening rate.
Also, by proving that unique characteristics of the protein can be changed at the allosteric site, instead of protein’s direct binding site with biomolecules, the researchers have demonstrated a new methodology of regulating the in-vivo function of proteins.
The researchers expect that these results will contribute greatly to a deeper understanding of protein’s nature that governs various life phenomena and help evaluate the proof of interpreting protein binding affinity of biomolecules from the perspective of protein kinetics.
Professor Kim said, “Until now, the protein binding affinity of biomolecules was determined by a direct interaction between two molecules. Our research has identified an important fact that the structure opening rate of proteins also plays a crucial role in determining their binding affinity.”
[Picture]
A correlation graph of opening rate (kopening) and binding affinity (kd) between protein’s stable, open state and its unstable, partially closed state.
2014.05.02 View 10919 -
 Thermoelectric generator on glass fabric for wearable electronic devices
Wearable computers or devices have been hailed as the next generation of mobile electronic gadgets, from smart watches to smart glasses to smart pacemakers. For electronics to be worn by a user, they must be light, flexible, and equipped with a power source, which could be a portable, long-lasting battery or no battery at all but a generator. How to supply power in a stable and reliable manner is one of the most critical issues to commercialize wearable devices.
A team of KAIST researchers headed by Byung Jin Cho, a professor of electrical engineering, proposed a solution to this problem by developing a glass fabric-based thermoelectric (TE) generator that is extremely light and flexible and produces electricity from the heat of the human body. In fact, it is so flexible that the allowable bending radius of the generator is as low as 20 mm. There are no changes in performance even if the generator bends upward and downward for up to 120 cycles.
To date, two types of TE generators have been developed based either on organic or inorganic materials. The organic-based TE generators use polymers that are highly flexible and compatible with human skin, ideal for wearable electronics. The polymers, however, have a low power output. Inorganic-based TE generators produce a high electrical energy, but they are heavy, rigid, and bulky.
Professor Cho came up with a new concept and design technique to build a flexible TE generator that minimizes thermal energy loss but maximizes power output. His team synthesized liquid-like pastes of n-type (Bi2Te3) and p-type (Sb2Te3) TE materials and printed them onto a glass fabric by applying a screen printing technique. The pastes permeated through the meshes of the fabric and formed films of TE materials in a range of thickness of several hundreds of microns. As a result, hundreds of TE material dots (in combination of n and p types) were printed and well arranged on a specific area of the glass fabric.
Professor Cho explained that his TE generator has a self-sustaining structure, eliminating thick external substrates (usually made of ceramic or alumina) that hold inorganic TE materials. These substrates have taken away a great portion of thermal energy, a serious setback which causes low output power.
He also commented,
"For our case, the glass fabric itself serves as the upper and lower substrates of a TE generator, keeping the inorganic TE materials in between. This is quite a revolutionary approach to design a generator. In so doing, we were able to significantly reduce the weight of our generator (~0.13g/cm2), which is an essential element for wearable electronics."
When using KAIST's TE generator (with a size of 10 cm x 10 cm) for a wearable wristband device, it will produce around 40 mW electric power based on the temperature difference of 31 °F between human skin and the surrounding air.
Professor Cho further described about the merits of the new generator:
"Our technology presents an easy and simple way of fabricating an extremely flexible, light, and high-performance TE generator. We expect that this technology will find further applications in scale-up systems such as automobiles, factories, aircrafts, and vessels where we see abundant thermal energy being wasted."
This research result was published online in the March 14th issue of Energy & Environmental Science and was entitled "Wearable Thermoelectric Generator Fabricated on Glass Fabric."
Youtube Link: http://www.youtube.com/watch?v=BlN9lvEzCuw&feature=youtu.be
[Picture Captions]
Caption 1: The picture shows a high-performance wearable thermoelectric generator that is extremely flexible and light.
Caption 2: A thermoelectric generator developed as a wristband. The generator can be easily curved along with the shape of human body.
Caption 3: KAIST’s thermoelectric generator can be bent as many as 120 times, but it still shows the same high performance.
2014.04.21 View 21532
Thermoelectric generator on glass fabric for wearable electronic devices
Wearable computers or devices have been hailed as the next generation of mobile electronic gadgets, from smart watches to smart glasses to smart pacemakers. For electronics to be worn by a user, they must be light, flexible, and equipped with a power source, which could be a portable, long-lasting battery or no battery at all but a generator. How to supply power in a stable and reliable manner is one of the most critical issues to commercialize wearable devices.
A team of KAIST researchers headed by Byung Jin Cho, a professor of electrical engineering, proposed a solution to this problem by developing a glass fabric-based thermoelectric (TE) generator that is extremely light and flexible and produces electricity from the heat of the human body. In fact, it is so flexible that the allowable bending radius of the generator is as low as 20 mm. There are no changes in performance even if the generator bends upward and downward for up to 120 cycles.
To date, two types of TE generators have been developed based either on organic or inorganic materials. The organic-based TE generators use polymers that are highly flexible and compatible with human skin, ideal for wearable electronics. The polymers, however, have a low power output. Inorganic-based TE generators produce a high electrical energy, but they are heavy, rigid, and bulky.
Professor Cho came up with a new concept and design technique to build a flexible TE generator that minimizes thermal energy loss but maximizes power output. His team synthesized liquid-like pastes of n-type (Bi2Te3) and p-type (Sb2Te3) TE materials and printed them onto a glass fabric by applying a screen printing technique. The pastes permeated through the meshes of the fabric and formed films of TE materials in a range of thickness of several hundreds of microns. As a result, hundreds of TE material dots (in combination of n and p types) were printed and well arranged on a specific area of the glass fabric.
Professor Cho explained that his TE generator has a self-sustaining structure, eliminating thick external substrates (usually made of ceramic or alumina) that hold inorganic TE materials. These substrates have taken away a great portion of thermal energy, a serious setback which causes low output power.
He also commented,
"For our case, the glass fabric itself serves as the upper and lower substrates of a TE generator, keeping the inorganic TE materials in between. This is quite a revolutionary approach to design a generator. In so doing, we were able to significantly reduce the weight of our generator (~0.13g/cm2), which is an essential element for wearable electronics."
When using KAIST's TE generator (with a size of 10 cm x 10 cm) for a wearable wristband device, it will produce around 40 mW electric power based on the temperature difference of 31 °F between human skin and the surrounding air.
Professor Cho further described about the merits of the new generator:
"Our technology presents an easy and simple way of fabricating an extremely flexible, light, and high-performance TE generator. We expect that this technology will find further applications in scale-up systems such as automobiles, factories, aircrafts, and vessels where we see abundant thermal energy being wasted."
This research result was published online in the March 14th issue of Energy & Environmental Science and was entitled "Wearable Thermoelectric Generator Fabricated on Glass Fabric."
Youtube Link: http://www.youtube.com/watch?v=BlN9lvEzCuw&feature=youtu.be
[Picture Captions]
Caption 1: The picture shows a high-performance wearable thermoelectric generator that is extremely flexible and light.
Caption 2: A thermoelectric generator developed as a wristband. The generator can be easily curved along with the shape of human body.
Caption 3: KAIST’s thermoelectric generator can be bent as many as 120 times, but it still shows the same high performance.
2014.04.21 View 21532