Daejeon
-
 Professor Kyu-Young Whang Donates Toward the 50th Anniversary Memorial Building
Distinguished Professor Kyu-Young Whang from the School of Computing made a gift of 100 million KRW toward the construction of the 50th Anniversary Memorial Building during a ceremony on November 3 at the Daejeon campus. "As a member of the first class of KAIST, I feel very delighted to play a part in the fundraising campaign for the 50th anniversary celebration. This is also a token of appreciation to my alma mater and I look forward to alumni and the KAIST community joining this campaign," said Professor Emeritus Whang. KAIST will name the Kyu-Young Whang and Jonghae Song Christian Seminar Room at the 50th Anniversary Memorial Building. The ground will be broken in 2022 for construction of the building.
2020.11.04 View 8052
Professor Kyu-Young Whang Donates Toward the 50th Anniversary Memorial Building
Distinguished Professor Kyu-Young Whang from the School of Computing made a gift of 100 million KRW toward the construction of the 50th Anniversary Memorial Building during a ceremony on November 3 at the Daejeon campus. "As a member of the first class of KAIST, I feel very delighted to play a part in the fundraising campaign for the 50th anniversary celebration. This is also a token of appreciation to my alma mater and I look forward to alumni and the KAIST community joining this campaign," said Professor Emeritus Whang. KAIST will name the Kyu-Young Whang and Jonghae Song Christian Seminar Room at the 50th Anniversary Memorial Building. The ground will be broken in 2022 for construction of the building.
2020.11.04 View 8052 -
 Slippery When Wet: Fish and Seaweed Inspire Ships to Reduce Fluid Friction
Faster ships could be on the horizon after KAIST scientists develop a slippery surface inspired by fish and seaweed to reduce the hull's drag through the water.
Long-distance cargo ships lose a significant amount of energy due to fluid friction. Looking to the drag reduction mechanisms employed by aquatic life can provide inspiration on how to improve efficiency.
Fish and seaweed secrete a layer of mucus to create a slippery surface, reducing their friction as they travel through water. A potential way to mimic this is by creating lubricant-infused surfaces covered with cavities. As the cavities are continuously filled with the lubricant, a layer is formed over the surface.
Though this method has previously been shown to work, reducing drag by up to 18%, the underlying physics is not fully understood. KAIST researchers in collaboration with a team of researchers from POSTECH conducted simulations of this process to help explain the effects, and their findings were published in the journal Physics of Fluids on September 15.
The group looked at the average speed of a cargo ship with realistic material properties and simulated how it behaves under various lubrication setups. Specifically, they monitored the effects of the open area of the lubricant-filled cavities, as well as the thickness of the cavity lids.
They found that for larger open areas, the lubricant spreads more than it does with smaller open areas, leading to a slipperier surface. On the other hand, the lid thickness does not have much of an effect on the slip, though a thicker lid does create a thicker lubricant buildup layer.
Professor Emeritus Hyung Jin Sung from the KAIST Department of Mechanical Engineering who led this study said, “Our investigation of the hydrodynamics of a lubricant layer and how it results in drag reduction with a slippery surface in a basic configuration has provided significant insight into the benefits of a lubricant-infused surface.”
Now that they have worked on optimizing the lubricant secretion design, the authors hope it can be implemented in real-life marine vehicles.
“If the present design parameters are adopted, the drag reduction rate will increase significantly,” Professor Sung added.
This work was supported by the National Research Foundation (NRF) of Korea.
Source:
Materials provided by American Institute of Physics.
Publication:
Kim, Seung Joong, et al. (2020). A lubricant-infused slip surface for drag reduction. Physics of Fluids. Available online at https://doi.org/10.1063/5.0018460
Profile:
Hyung Jin Sung
Professor Emeritus
hyungjin@kaist.ac.kr
http://flow.kaist.ac.kr/index.php
Flow Control Lab. (FCL)
Department of Mechanical Engineering
http://kaist.ac.kr
Korea Advanced Institute of Science and Technology (KAIST)
Daejeon, Republic of Korea
(END)
2020.10.12 View 9209
Slippery When Wet: Fish and Seaweed Inspire Ships to Reduce Fluid Friction
Faster ships could be on the horizon after KAIST scientists develop a slippery surface inspired by fish and seaweed to reduce the hull's drag through the water.
Long-distance cargo ships lose a significant amount of energy due to fluid friction. Looking to the drag reduction mechanisms employed by aquatic life can provide inspiration on how to improve efficiency.
Fish and seaweed secrete a layer of mucus to create a slippery surface, reducing their friction as they travel through water. A potential way to mimic this is by creating lubricant-infused surfaces covered with cavities. As the cavities are continuously filled with the lubricant, a layer is formed over the surface.
Though this method has previously been shown to work, reducing drag by up to 18%, the underlying physics is not fully understood. KAIST researchers in collaboration with a team of researchers from POSTECH conducted simulations of this process to help explain the effects, and their findings were published in the journal Physics of Fluids on September 15.
The group looked at the average speed of a cargo ship with realistic material properties and simulated how it behaves under various lubrication setups. Specifically, they monitored the effects of the open area of the lubricant-filled cavities, as well as the thickness of the cavity lids.
They found that for larger open areas, the lubricant spreads more than it does with smaller open areas, leading to a slipperier surface. On the other hand, the lid thickness does not have much of an effect on the slip, though a thicker lid does create a thicker lubricant buildup layer.
Professor Emeritus Hyung Jin Sung from the KAIST Department of Mechanical Engineering who led this study said, “Our investigation of the hydrodynamics of a lubricant layer and how it results in drag reduction with a slippery surface in a basic configuration has provided significant insight into the benefits of a lubricant-infused surface.”
Now that they have worked on optimizing the lubricant secretion design, the authors hope it can be implemented in real-life marine vehicles.
“If the present design parameters are adopted, the drag reduction rate will increase significantly,” Professor Sung added.
This work was supported by the National Research Foundation (NRF) of Korea.
Source:
Materials provided by American Institute of Physics.
Publication:
Kim, Seung Joong, et al. (2020). A lubricant-infused slip surface for drag reduction. Physics of Fluids. Available online at https://doi.org/10.1063/5.0018460
Profile:
Hyung Jin Sung
Professor Emeritus
hyungjin@kaist.ac.kr
http://flow.kaist.ac.kr/index.php
Flow Control Lab. (FCL)
Department of Mechanical Engineering
http://kaist.ac.kr
Korea Advanced Institute of Science and Technology (KAIST)
Daejeon, Republic of Korea
(END)
2020.10.12 View 9209 -
 Fundraising for the 50th Anniversary Memorial Building Kicks Off
KAIST started the fundraising campaign to construct the 50th Anniversary Memorial Building. This is one of the projects and events the 50th Anniversary Commemorative Committee established to celebrate the anniversary.
The ground will be broken in 2022 after raising approximately 50 billion KRW through 2021. The five-story building will be the latest addition to the KAIST campus. To highlight the campus’s history, the new building will connect the N5 (Basic Experiment & Research) and N2 (Administration Branch) buildings, the first buildings on the main Daejeon campus after its main campus moved from Seoul in 1987. Currently, the College of Business remains at the Seoul campus.
The 50th Anniversary Memorial Building will connect the two buildings in the shape of C, and represent KAIST’s C3 core value of Challenging, Creating, and Caring. The concept of this building was designed by Professor Sang-Min Bae from the Department of Industrial Design.
The 50th Anniversary Commemorative Committee said the Memorial Building will reflect the spirit of its core values. The first floor will accommodate the auditorium and exhibition hall, showcasing the latest achievements in KAIST innovation and convergence research as well as alumni startups and companies. The second floor will be an education space for entrepreneurship and video studios. An area for delivering creative education platforms such as Education 4.0 will be prepared on the third floor. The fourth floor will be used for global leadership education. The fifth floor will house the KAIST Club, a lounge for alumni and the Global Strategy Institute.
Co-Chair of the Fundraising & PR Sub-Committee of the KAIST 50th Anniversary Commemorative Committee and Former Vice President for Planning and Budget Seung-Bin Park and current Vice President for Planning and Budget Suchan Chae reiterated the importance of extending the infrastructure of the campus, saying that investments in the infrastructure will expand the university’s future growth potential. In a letter to kick off the fundraising efforts last month, they called for support from the entire KAIST community to help construct the new memorial building that will produce global talents and help young scientists make their dreams come true.
To donate, click here
2020.10.07 View 9886
Fundraising for the 50th Anniversary Memorial Building Kicks Off
KAIST started the fundraising campaign to construct the 50th Anniversary Memorial Building. This is one of the projects and events the 50th Anniversary Commemorative Committee established to celebrate the anniversary.
The ground will be broken in 2022 after raising approximately 50 billion KRW through 2021. The five-story building will be the latest addition to the KAIST campus. To highlight the campus’s history, the new building will connect the N5 (Basic Experiment & Research) and N2 (Administration Branch) buildings, the first buildings on the main Daejeon campus after its main campus moved from Seoul in 1987. Currently, the College of Business remains at the Seoul campus.
The 50th Anniversary Memorial Building will connect the two buildings in the shape of C, and represent KAIST’s C3 core value of Challenging, Creating, and Caring. The concept of this building was designed by Professor Sang-Min Bae from the Department of Industrial Design.
The 50th Anniversary Commemorative Committee said the Memorial Building will reflect the spirit of its core values. The first floor will accommodate the auditorium and exhibition hall, showcasing the latest achievements in KAIST innovation and convergence research as well as alumni startups and companies. The second floor will be an education space for entrepreneurship and video studios. An area for delivering creative education platforms such as Education 4.0 will be prepared on the third floor. The fourth floor will be used for global leadership education. The fifth floor will house the KAIST Club, a lounge for alumni and the Global Strategy Institute.
Co-Chair of the Fundraising & PR Sub-Committee of the KAIST 50th Anniversary Commemorative Committee and Former Vice President for Planning and Budget Seung-Bin Park and current Vice President for Planning and Budget Suchan Chae reiterated the importance of extending the infrastructure of the campus, saying that investments in the infrastructure will expand the university’s future growth potential. In a letter to kick off the fundraising efforts last month, they called for support from the entire KAIST community to help construct the new memorial building that will produce global talents and help young scientists make their dreams come true.
To donate, click here
2020.10.07 View 9886 -
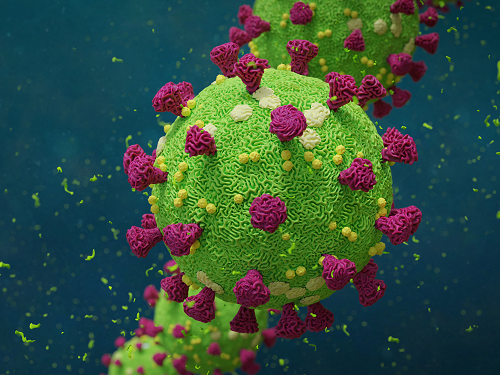 Biomarker Predicts Who Will Have Severe COVID-19
- Airway cell analyses showing an activated immune axis could pinpoint the COVID-19 patients who will most benefit from targeted therapies.-
KAIST researchers have identified key markers that could help pinpoint patients who are bound to get a severe reaction to COVID-19 infection. This would help doctors provide the right treatments at the right time, potentially saving lives. The findings were published in the journal Frontiers in Immunology on August 28.
People’s immune systems react differently to infection with SARS-CoV-2, the virus that causes COVID-19, ranging from mild to severe, life-threatening responses.
To understand the differences in responses, Professor Heung Kyu Lee and PhD candidate Jang Hyun Park from the Graduate School of Medical Science and Engineering at KAIST analysed ribonucleic acid (RNA) sequencing data extracted from individual airway cells of healthy controls and of mildly and severely ill patients with COVID-19. The data was available in a public database previously published by a group of Chinese researchers.
“Our analyses identified an association between immune cells called neutrophils and special cell receptors that bind to the steroid hormone glucocorticoid,” Professor Lee explained. “This finding could be used as a biomarker for predicting disease severity in patients and thus selecting a targeted therapy that can help treat them at an appropriate time,” he added.
Severe illness in COVID-19 is associated with an exaggerated immune response that leads to excessive airway-damaging inflammation. This condition, known as acute respiratory distress syndrome (ARDS), accounts for 70% of deaths in fatal COVID-19 infections.
Scientists already know that this excessive inflammation involves heightened neutrophil recruitment to the airways, but the detailed mechanisms of this reaction are still unclear.
Lee and Park’s analyses found that a group of immune cells called myeloid cells produced excess amounts of neutrophil-recruiting chemicals in severely ill patients, including a cytokine called tumour necrosis factor (TNF) and a chemokine called CXCL8.
Further RNA analyses of neutrophils in severely ill patients showed they were less able to recruit very important T cells needed for attacking the virus. At the same time, the neutrophils produced too many extracellular molecules that normally trap pathogens, but damage airway cells when produced in excess.
The researchers additionally found that the airway cells in severely ill patients were not expressing enough glucocorticoid receptors. This was correlated with increased CXCL8 expression and neutrophil recruitment.
Glucocorticoids, like the well-known drug dexamethasone, are anti-inflammatory agents that could play a role in treating COVID-19. However, using them in early or mild forms of the infection could suppress the necessary immune reactions to combat the virus. But if airway damage has already happened in more severe cases, glucocorticoid treatment would be ineffective.
Knowing who to give this treatment to and when is really important. COVID-19 patients showing reduced glucocorticoid receptor expression, increased CXCL8 expression, and excess neutrophil recruitment to the airways could benefit from treatment with glucocorticoids to prevent airway damage. Further research is needed, however, to confirm the relationship between glucocorticoids and neutrophil inflammation at the protein level.
“Our study could serve as a springboard towards more accurate and reliable COVID-19 treatments,” Professor Lee said.
This work was supported by the National Research Foundation of Korea, and Mobile Clinic Module Project funded by KAIST.
Figure. Low glucocorticoid receptor (GR) expression led to excessive inflammation and lung damage by neutrophils through enhancing the expression of CXCL8 and other cytokines.
Image credit: Professor Heung Kyu Lee, KAIST. Created with Biorender.com.
Image usage restrictions: News organizations may use or redistribute these figures and image, with proper attribution, as part of news coverage of this paper only.
-Publication:
Jang Hyun Park, and Heung Kyu Lee. (2020). Re-analysis of Single Cell Transcriptome Reveals That the NR3C1-CXCL8-Neutrophil Axis Determines the Severity of COVID-19. Frontiers in Immunology, Available online at https://doi.org/10.3389/fimmu.2020.02145
-Profile: Heung Kyu Lee
Associate Professor
heungkyu.lee@kaist.ac.kr
https://www.heungkyulee.kaist.ac.kr/
Laboratory of Host Defenses
Graduate School of Medical Science and Engineering (GSMSE)
The Center for Epidemic Preparedness at KAIST Institute
http://kaist.ac.kr
Korea Advanced Institute of Science and Technology (KAIST)
Daejeon, Republic of Korea
Profile: Jang Hyun Park
PhD Candidate
janghyun.park@kaist.ac.kr
GSMSE, KAIST
2020.09.17 View 19543
Biomarker Predicts Who Will Have Severe COVID-19
- Airway cell analyses showing an activated immune axis could pinpoint the COVID-19 patients who will most benefit from targeted therapies.-
KAIST researchers have identified key markers that could help pinpoint patients who are bound to get a severe reaction to COVID-19 infection. This would help doctors provide the right treatments at the right time, potentially saving lives. The findings were published in the journal Frontiers in Immunology on August 28.
People’s immune systems react differently to infection with SARS-CoV-2, the virus that causes COVID-19, ranging from mild to severe, life-threatening responses.
To understand the differences in responses, Professor Heung Kyu Lee and PhD candidate Jang Hyun Park from the Graduate School of Medical Science and Engineering at KAIST analysed ribonucleic acid (RNA) sequencing data extracted from individual airway cells of healthy controls and of mildly and severely ill patients with COVID-19. The data was available in a public database previously published by a group of Chinese researchers.
“Our analyses identified an association between immune cells called neutrophils and special cell receptors that bind to the steroid hormone glucocorticoid,” Professor Lee explained. “This finding could be used as a biomarker for predicting disease severity in patients and thus selecting a targeted therapy that can help treat them at an appropriate time,” he added.
Severe illness in COVID-19 is associated with an exaggerated immune response that leads to excessive airway-damaging inflammation. This condition, known as acute respiratory distress syndrome (ARDS), accounts for 70% of deaths in fatal COVID-19 infections.
Scientists already know that this excessive inflammation involves heightened neutrophil recruitment to the airways, but the detailed mechanisms of this reaction are still unclear.
Lee and Park’s analyses found that a group of immune cells called myeloid cells produced excess amounts of neutrophil-recruiting chemicals in severely ill patients, including a cytokine called tumour necrosis factor (TNF) and a chemokine called CXCL8.
Further RNA analyses of neutrophils in severely ill patients showed they were less able to recruit very important T cells needed for attacking the virus. At the same time, the neutrophils produced too many extracellular molecules that normally trap pathogens, but damage airway cells when produced in excess.
The researchers additionally found that the airway cells in severely ill patients were not expressing enough glucocorticoid receptors. This was correlated with increased CXCL8 expression and neutrophil recruitment.
Glucocorticoids, like the well-known drug dexamethasone, are anti-inflammatory agents that could play a role in treating COVID-19. However, using them in early or mild forms of the infection could suppress the necessary immune reactions to combat the virus. But if airway damage has already happened in more severe cases, glucocorticoid treatment would be ineffective.
Knowing who to give this treatment to and when is really important. COVID-19 patients showing reduced glucocorticoid receptor expression, increased CXCL8 expression, and excess neutrophil recruitment to the airways could benefit from treatment with glucocorticoids to prevent airway damage. Further research is needed, however, to confirm the relationship between glucocorticoids and neutrophil inflammation at the protein level.
“Our study could serve as a springboard towards more accurate and reliable COVID-19 treatments,” Professor Lee said.
This work was supported by the National Research Foundation of Korea, and Mobile Clinic Module Project funded by KAIST.
Figure. Low glucocorticoid receptor (GR) expression led to excessive inflammation and lung damage by neutrophils through enhancing the expression of CXCL8 and other cytokines.
Image credit: Professor Heung Kyu Lee, KAIST. Created with Biorender.com.
Image usage restrictions: News organizations may use or redistribute these figures and image, with proper attribution, as part of news coverage of this paper only.
-Publication:
Jang Hyun Park, and Heung Kyu Lee. (2020). Re-analysis of Single Cell Transcriptome Reveals That the NR3C1-CXCL8-Neutrophil Axis Determines the Severity of COVID-19. Frontiers in Immunology, Available online at https://doi.org/10.3389/fimmu.2020.02145
-Profile: Heung Kyu Lee
Associate Professor
heungkyu.lee@kaist.ac.kr
https://www.heungkyulee.kaist.ac.kr/
Laboratory of Host Defenses
Graduate School of Medical Science and Engineering (GSMSE)
The Center for Epidemic Preparedness at KAIST Institute
http://kaist.ac.kr
Korea Advanced Institute of Science and Technology (KAIST)
Daejeon, Republic of Korea
Profile: Jang Hyun Park
PhD Candidate
janghyun.park@kaist.ac.kr
GSMSE, KAIST
2020.09.17 View 19543 -
 Advanced NVMe Controller Technology for Next Generation Memory Devices
KAIST researchers advanced non-volatile memory express (NVMe) controller technology for next generation information storage devices, and made this new technology named ‘OpenExpress’ freely available to all universities and research institutes around the world to help reduce the research cost in related fields.
NVMe is a communication protocol made for high-performance storage devices based on a peripheral component interconnect-express (PCI-E) interface. NVMe has been developed to take the place of the Serial AT Attachment (SATA) protocol, which was developed to process data on hard disk drives (HDDs) and did not perform well in solid state drives (SSDs).
Unlike HDDs that use magnetic spinning disks, SSDs use semiconductor memory, allowing the rapid reading and writing of data. SSDs also generate less heat and noise, and are much more compact and lightweight.
Since data processing in SSDs using NVMe is up to six times faster than when SATA is used, NVMe has become the standard protocol for ultra-high speed and volume data processing, and is currently used in many flash-based information storage devices.
Studies on NVMe continue at both the academic and industrial levels, however, its poor accessibility is a drawback. Major information and communications technology (ICT) companies around the world expend astronomical costs to procure intellectual property (IP) related to hardware NVMe controllers, necessary for the use of NVMe. However, such IP is not publicly disclosed, making it difficult to be used by universities and research institutes for research purposes.
Although a small number of U.S. Silicon Valley startups provide parts of their independently developed IP for research, the cost of usage is around 34,000 USD per month. The costs skyrocket even further because each copy of single-use source code purchased for IP modification costs approximately 84,000 USD.
In order to address these issues, a group of researchers led by Professor Myoungsoo Jung from the School of Electrical Engineering at KAIST developed a next generation NVMe controller technology that achieved parallel data input/output processing for SSDs in a fully hardware automated form.
The researchers presented their work at the 2020 USENIX Annual Technical Conference (USENIX ATC ’20) in July, and released it as an open research framework named ‘OpenExpress.’
This NVMe controller technology developed by Professor Jung’s team comprises a wide range of basic hardware IP and key NVMe IP cores. To examine its actual performance, the team made an NVMe hardware controller prototype using OpenExpress, and designed all logics provided by OpenExpress to operate at high frequency.
The field-programmable gate array (FPGA) memory card prototype developed using OpenExpress demonstrated increased input/output data processing capacity per second, supporting up to 7 gigabit per second (GB/s) bandwidth. This makes it suitable for ultra-high speed and volume next generation memory device research.
In a test comparing various storage server loads on devices, the team’s FPGA also showed 76% higher bandwidth and 68% lower input/output delay compared to Intel’s new high performance SSD (Optane SSD), which is sufficient for many researchers studying systems employing future memory devices. Depending on user needs, silicon devices can be synthesized as well, which is expected to further enhance performance.
The NVMe controller technology of Professor Jung’s team can be freely used and modified under the OpenExpress open-source end-user agreement for non-commercial use by all universities and research institutes. This makes it extremely useful for research on next-generation memory compatible NVMe controllers and software stacks.
“With the product of this study being disclosed to the world, universities and research institutes can now use controllers that used to be exclusive for only the world’s biggest companies, at no cost,ˮ said Professor Jung. He went on to stress, “This is a meaningful first step in research of information storage device systems such as high-speed and volume next generation memory.”
This work was supported by a grant from MemRay, a company specializing in next generation memory development and distribution.
More details about the study can be found at http://camelab.org.
Image credit: Professor Myoungsoo Jung, KAIST
Image usage restrictions: News organizations may use or redistribute these figures and image, with proper attribution, as part of news coverage of this paper only.
-Publication:
Myoungsoo Jung. (2020). OpenExpress: Fully Hardware Automated Open Research Framework for Future Fast NVMe Devices. Presented in the Proceedings of the 2020 USENIX Annual Technical Conference (USENIX ATC ’20), Available online at https://www.usenix.org/system/files/atc20-jung.pdf
-Profile: Myoungsoo Jung
Associate Professor
m.jung@kaist.ac.kr
http://camelab.org
Computer Architecture and Memory Systems Laboratory
School of Electrical Engineering
http://kaist.ac.kr
Korea Advanced Institute of Science and Technology (KAIST)
Daejeon, Republic of Korea
(END)
2020.09.04 View 13148
Advanced NVMe Controller Technology for Next Generation Memory Devices
KAIST researchers advanced non-volatile memory express (NVMe) controller technology for next generation information storage devices, and made this new technology named ‘OpenExpress’ freely available to all universities and research institutes around the world to help reduce the research cost in related fields.
NVMe is a communication protocol made for high-performance storage devices based on a peripheral component interconnect-express (PCI-E) interface. NVMe has been developed to take the place of the Serial AT Attachment (SATA) protocol, which was developed to process data on hard disk drives (HDDs) and did not perform well in solid state drives (SSDs).
Unlike HDDs that use magnetic spinning disks, SSDs use semiconductor memory, allowing the rapid reading and writing of data. SSDs also generate less heat and noise, and are much more compact and lightweight.
Since data processing in SSDs using NVMe is up to six times faster than when SATA is used, NVMe has become the standard protocol for ultra-high speed and volume data processing, and is currently used in many flash-based information storage devices.
Studies on NVMe continue at both the academic and industrial levels, however, its poor accessibility is a drawback. Major information and communications technology (ICT) companies around the world expend astronomical costs to procure intellectual property (IP) related to hardware NVMe controllers, necessary for the use of NVMe. However, such IP is not publicly disclosed, making it difficult to be used by universities and research institutes for research purposes.
Although a small number of U.S. Silicon Valley startups provide parts of their independently developed IP for research, the cost of usage is around 34,000 USD per month. The costs skyrocket even further because each copy of single-use source code purchased for IP modification costs approximately 84,000 USD.
In order to address these issues, a group of researchers led by Professor Myoungsoo Jung from the School of Electrical Engineering at KAIST developed a next generation NVMe controller technology that achieved parallel data input/output processing for SSDs in a fully hardware automated form.
The researchers presented their work at the 2020 USENIX Annual Technical Conference (USENIX ATC ’20) in July, and released it as an open research framework named ‘OpenExpress.’
This NVMe controller technology developed by Professor Jung’s team comprises a wide range of basic hardware IP and key NVMe IP cores. To examine its actual performance, the team made an NVMe hardware controller prototype using OpenExpress, and designed all logics provided by OpenExpress to operate at high frequency.
The field-programmable gate array (FPGA) memory card prototype developed using OpenExpress demonstrated increased input/output data processing capacity per second, supporting up to 7 gigabit per second (GB/s) bandwidth. This makes it suitable for ultra-high speed and volume next generation memory device research.
In a test comparing various storage server loads on devices, the team’s FPGA also showed 76% higher bandwidth and 68% lower input/output delay compared to Intel’s new high performance SSD (Optane SSD), which is sufficient for many researchers studying systems employing future memory devices. Depending on user needs, silicon devices can be synthesized as well, which is expected to further enhance performance.
The NVMe controller technology of Professor Jung’s team can be freely used and modified under the OpenExpress open-source end-user agreement for non-commercial use by all universities and research institutes. This makes it extremely useful for research on next-generation memory compatible NVMe controllers and software stacks.
“With the product of this study being disclosed to the world, universities and research institutes can now use controllers that used to be exclusive for only the world’s biggest companies, at no cost,ˮ said Professor Jung. He went on to stress, “This is a meaningful first step in research of information storage device systems such as high-speed and volume next generation memory.”
This work was supported by a grant from MemRay, a company specializing in next generation memory development and distribution.
More details about the study can be found at http://camelab.org.
Image credit: Professor Myoungsoo Jung, KAIST
Image usage restrictions: News organizations may use or redistribute these figures and image, with proper attribution, as part of news coverage of this paper only.
-Publication:
Myoungsoo Jung. (2020). OpenExpress: Fully Hardware Automated Open Research Framework for Future Fast NVMe Devices. Presented in the Proceedings of the 2020 USENIX Annual Technical Conference (USENIX ATC ’20), Available online at https://www.usenix.org/system/files/atc20-jung.pdf
-Profile: Myoungsoo Jung
Associate Professor
m.jung@kaist.ac.kr
http://camelab.org
Computer Architecture and Memory Systems Laboratory
School of Electrical Engineering
http://kaist.ac.kr
Korea Advanced Institute of Science and Technology (KAIST)
Daejeon, Republic of Korea
(END)
2020.09.04 View 13148 -
 Before Eyes Open, They Get Ready to See
- Spontaneous retinal waves can generate long-range horizontal connectivity in visual cortex. -
A KAIST research team’s computational simulations demonstrated that the waves of spontaneous neural activity in the retinas of still-closed eyes in mammals develop long-range horizontal connections in the visual cortex during early developmental stages.
This new finding featured in the August 19 edition of Journal of Neuroscience as a cover article has resolved a long-standing puzzle for understanding visual neuroscience regarding the early organization of functional architectures in the mammalian visual cortex before eye-opening, especially the long-range horizontal connectivity known as “feature-specific” circuitry.
To prepare the animal to see when its eyes open, neural circuits in the brain’s visual system must begin developing earlier. However, the proper development of many brain regions involved in vision generally requires sensory input through the eyes.
In the primary visual cortex of the higher mammalian taxa, cortical neurons of similar functional tuning to a visual feature are linked together by long-range horizontal circuits that play a crucial role in visual information processing.
Surprisingly, these long-range horizontal connections in the primary visual cortex of higher mammals emerge before the onset of sensory experience, and the mechanism underlying this phenomenon has remained elusive.
To investigate this mechanism, a group of researchers led by Professor Se-Bum Paik from the Department of Bio and Brain Engineering at KAIST implemented computational simulations of early visual pathways using data obtained from the retinal circuits in young animals before eye-opening, including cats, monkeys, and mice.
From these simulations, the researchers found that spontaneous waves propagating in ON and OFF retinal mosaics can initialize the wiring of long-range horizontal connections by selectively co-activating cortical neurons of similar functional tuning, whereas equivalent random activities cannot induce such organizations.
The simulations also showed that emerged long-range horizontal connections can induce the patterned cortical activities, matching the topography of underlying functional maps even in salt-and-pepper type organizations observed in rodents. This result implies that the model developed by Professor Paik and his group can provide a universal principle for the developmental mechanism of long-range horizontal connections in both higher mammals as well as rodents.
Professor Paik said, “Our model provides a deeper understanding of how the functional architectures in the visual cortex can originate from the spatial organization of the periphery, without sensory experience during early developmental periods.”
He continued, “We believe that our findings will be of great interest to scientists working in a wide range of fields such as neuroscience, vision science, and developmental biology.”
This work was supported by the National Research Foundation of Korea (NRF). Undergraduate student Jinwoo Kim participated in this research project and presented the findings as the lead author as part of the Undergraduate Research Participation (URP) Program at KAIST.
Figures and image credit: Professor Se-Bum Paik, KAIST
Image usage restrictions: News organizations may use or redistribute these figures and image, with proper attribution, as part of news coverage of this paper only.
Publication:
Jinwoo Kim, Min Song, and Se-Bum Paik. (2020). Spontaneous retinal waves generate long-range horizontal connectivity in visual cortex. Journal of Neuroscience, Available online athttps://www.jneurosci.org/content/early/2020/07/17/JNEUROSCI.0649-20.2020
Profile: Se-Bum Paik
Assistant Professor
sbpaik@kaist.ac.kr
http://vs.kaist.ac.kr/
VSNN Laboratory
Department of Bio and Brain Engineering
Program of Brain and Cognitive Engineering
http://kaist.ac.kr
Korea Advanced Institute of Science and Technology (KAIST) Daejeon, Republic of Korea
Profile: Jinwoo Kim
Undergraduate Student
bugkjw@kaist.ac.kr
Department of Bio and Brain Engineering, KAIST
Profile: Min Song
Ph.D. Candidate
night@kaist.ac.kr
Program of Brain and Cognitive Engineering, KAIST
(END)
2020.08.25 View 16001
Before Eyes Open, They Get Ready to See
- Spontaneous retinal waves can generate long-range horizontal connectivity in visual cortex. -
A KAIST research team’s computational simulations demonstrated that the waves of spontaneous neural activity in the retinas of still-closed eyes in mammals develop long-range horizontal connections in the visual cortex during early developmental stages.
This new finding featured in the August 19 edition of Journal of Neuroscience as a cover article has resolved a long-standing puzzle for understanding visual neuroscience regarding the early organization of functional architectures in the mammalian visual cortex before eye-opening, especially the long-range horizontal connectivity known as “feature-specific” circuitry.
To prepare the animal to see when its eyes open, neural circuits in the brain’s visual system must begin developing earlier. However, the proper development of many brain regions involved in vision generally requires sensory input through the eyes.
In the primary visual cortex of the higher mammalian taxa, cortical neurons of similar functional tuning to a visual feature are linked together by long-range horizontal circuits that play a crucial role in visual information processing.
Surprisingly, these long-range horizontal connections in the primary visual cortex of higher mammals emerge before the onset of sensory experience, and the mechanism underlying this phenomenon has remained elusive.
To investigate this mechanism, a group of researchers led by Professor Se-Bum Paik from the Department of Bio and Brain Engineering at KAIST implemented computational simulations of early visual pathways using data obtained from the retinal circuits in young animals before eye-opening, including cats, monkeys, and mice.
From these simulations, the researchers found that spontaneous waves propagating in ON and OFF retinal mosaics can initialize the wiring of long-range horizontal connections by selectively co-activating cortical neurons of similar functional tuning, whereas equivalent random activities cannot induce such organizations.
The simulations also showed that emerged long-range horizontal connections can induce the patterned cortical activities, matching the topography of underlying functional maps even in salt-and-pepper type organizations observed in rodents. This result implies that the model developed by Professor Paik and his group can provide a universal principle for the developmental mechanism of long-range horizontal connections in both higher mammals as well as rodents.
Professor Paik said, “Our model provides a deeper understanding of how the functional architectures in the visual cortex can originate from the spatial organization of the periphery, without sensory experience during early developmental periods.”
He continued, “We believe that our findings will be of great interest to scientists working in a wide range of fields such as neuroscience, vision science, and developmental biology.”
This work was supported by the National Research Foundation of Korea (NRF). Undergraduate student Jinwoo Kim participated in this research project and presented the findings as the lead author as part of the Undergraduate Research Participation (URP) Program at KAIST.
Figures and image credit: Professor Se-Bum Paik, KAIST
Image usage restrictions: News organizations may use or redistribute these figures and image, with proper attribution, as part of news coverage of this paper only.
Publication:
Jinwoo Kim, Min Song, and Se-Bum Paik. (2020). Spontaneous retinal waves generate long-range horizontal connectivity in visual cortex. Journal of Neuroscience, Available online athttps://www.jneurosci.org/content/early/2020/07/17/JNEUROSCI.0649-20.2020
Profile: Se-Bum Paik
Assistant Professor
sbpaik@kaist.ac.kr
http://vs.kaist.ac.kr/
VSNN Laboratory
Department of Bio and Brain Engineering
Program of Brain and Cognitive Engineering
http://kaist.ac.kr
Korea Advanced Institute of Science and Technology (KAIST) Daejeon, Republic of Korea
Profile: Jinwoo Kim
Undergraduate Student
bugkjw@kaist.ac.kr
Department of Bio and Brain Engineering, KAIST
Profile: Min Song
Ph.D. Candidate
night@kaist.ac.kr
Program of Brain and Cognitive Engineering, KAIST
(END)
2020.08.25 View 16001 -
 Deep Learning-Based Cough Recognition Model Helps Detect the Location of Coughing Sounds in Real Time
The Center for Noise and Vibration Control at KAIST announced that their coughing detection camera recognizes where coughing happens, visualizing the locations. The resulting cough recognition camera can track and record information about the person who coughed, their location, and the number of coughs on a real-time basis.
Professor Yong-Hwa Park from the Department of Mechanical Engineering developed a deep learning-based cough recognition model to classify a coughing sound in real time. The coughing event classification model is combined with a sound camera that visualizes their locations in public places. The research team said they achieved a best test accuracy of 87.4 %.
Professor Park said that it will be useful medical equipment during epidemics in public places such as schools, offices, and restaurants, and to constantly monitor patients’ conditions in a hospital room.
Fever and coughing are the most relevant respiratory disease symptoms, among which fever can be recognized remotely using thermal cameras. This new technology is expected to be very helpful for detecting epidemic transmissions in a non-contact way. The cough event classification model is combined with a sound camera that visualizes the cough event and indicates the location in the video image.
To develop a cough recognition model, a supervised learning was conducted with a convolutional neural network (CNN). The model performs binary classification with an input of a one-second sound profile feature, generating output to be either a cough event or something else.
In the training and evaluation, various datasets were collected from Audioset, DEMAND, ETSI, and TIMIT. Coughing and others sounds were extracted from Audioset, and the rest of the datasets were used as background noises for data augmentation so that this model could be generalized for various background noises in public places.
The dataset was augmented by mixing coughing sounds and other sounds from Audioset and background noises with the ratio of 0.15 to 0.75, then the overall volume was adjusted to 0.25 to 1.0 times to generalize the model for various distances.
The training and evaluation datasets were constructed by dividing the augmented dataset by 9:1, and the test dataset was recorded separately in a real office environment.
In the optimization procedure of the network model, training was conducted with various combinations of five acoustic features including spectrogram, Mel-scaled spectrogram and Mel-frequency cepstrum coefficients with seven optimizers. The performance of each combination was compared with the test dataset. The best test accuracy of 87.4% was achieved with Mel-scaled Spectrogram as the acoustic feature and ASGD as the optimizer.
The trained cough recognition model was combined with a sound camera. The sound camera is composed of a microphone array and a camera module. A beamforming process is applied to a collected set of acoustic data to find out the direction of incoming sound source. The integrated cough recognition model determines whether the sound is cough or not. If it is, the location of cough is visualized as a contour image with a ‘cough’ label at the location of the coughing sound source in a video image.
A pilot test of the cough recognition camera in an office environment shows that it successfully distinguishes cough events and other events even in a noisy environment. In addition, it can track the location of the person who coughed and count the number of coughs in real time. The performance will be improved further with additional training data obtained from other real environments such as hospitals and classrooms.
Professor Park said, “In a pandemic situation like we are experiencing with COVID-19, a cough detection camera can contribute to the prevention and early detection of epidemics in public places. Especially when applied to a hospital room, the patient's condition can be tracked 24 hours a day and support more accurate diagnoses while reducing the effort of the medical staff."
This study was conducted in collaboration with SM Instruments Inc.
Profile: Yong-Hwa Park, Ph.D.
Associate Professor
yhpark@kaist.ac.kr
http://human.kaist.ac.kr/
Human-Machine Interaction Laboratory (HuMaN Lab.)
Department of Mechanical Engineering (ME)
Korea Advanced Institute of Science and Technology (KAIST)
https://www.kaist.ac.kr/en/
Daejeon 34141, Korea
Profile: Gyeong Tae Lee
PhD Candidate
hansaram@kaist.ac.kr
HuMaN Lab., ME, KAIST
Profile: Seong Hu Kim
PhD Candidate
tjdgnkim@kaist.ac.kr
HuMaN Lab., ME, KAIST
Profile: Hyeonuk Nam
PhD Candidate
frednam@kaist.ac.kr
HuMaN Lab., ME, KAIST
Profile: Young-Key Kim
CEO
sales@smins.co.kr
http://en.smins.co.kr/
SM Instruments Inc.
Daejeon 34109, Korea
(END)
2020.08.13 View 19663
Deep Learning-Based Cough Recognition Model Helps Detect the Location of Coughing Sounds in Real Time
The Center for Noise and Vibration Control at KAIST announced that their coughing detection camera recognizes where coughing happens, visualizing the locations. The resulting cough recognition camera can track and record information about the person who coughed, their location, and the number of coughs on a real-time basis.
Professor Yong-Hwa Park from the Department of Mechanical Engineering developed a deep learning-based cough recognition model to classify a coughing sound in real time. The coughing event classification model is combined with a sound camera that visualizes their locations in public places. The research team said they achieved a best test accuracy of 87.4 %.
Professor Park said that it will be useful medical equipment during epidemics in public places such as schools, offices, and restaurants, and to constantly monitor patients’ conditions in a hospital room.
Fever and coughing are the most relevant respiratory disease symptoms, among which fever can be recognized remotely using thermal cameras. This new technology is expected to be very helpful for detecting epidemic transmissions in a non-contact way. The cough event classification model is combined with a sound camera that visualizes the cough event and indicates the location in the video image.
To develop a cough recognition model, a supervised learning was conducted with a convolutional neural network (CNN). The model performs binary classification with an input of a one-second sound profile feature, generating output to be either a cough event or something else.
In the training and evaluation, various datasets were collected from Audioset, DEMAND, ETSI, and TIMIT. Coughing and others sounds were extracted from Audioset, and the rest of the datasets were used as background noises for data augmentation so that this model could be generalized for various background noises in public places.
The dataset was augmented by mixing coughing sounds and other sounds from Audioset and background noises with the ratio of 0.15 to 0.75, then the overall volume was adjusted to 0.25 to 1.0 times to generalize the model for various distances.
The training and evaluation datasets were constructed by dividing the augmented dataset by 9:1, and the test dataset was recorded separately in a real office environment.
In the optimization procedure of the network model, training was conducted with various combinations of five acoustic features including spectrogram, Mel-scaled spectrogram and Mel-frequency cepstrum coefficients with seven optimizers. The performance of each combination was compared with the test dataset. The best test accuracy of 87.4% was achieved with Mel-scaled Spectrogram as the acoustic feature and ASGD as the optimizer.
The trained cough recognition model was combined with a sound camera. The sound camera is composed of a microphone array and a camera module. A beamforming process is applied to a collected set of acoustic data to find out the direction of incoming sound source. The integrated cough recognition model determines whether the sound is cough or not. If it is, the location of cough is visualized as a contour image with a ‘cough’ label at the location of the coughing sound source in a video image.
A pilot test of the cough recognition camera in an office environment shows that it successfully distinguishes cough events and other events even in a noisy environment. In addition, it can track the location of the person who coughed and count the number of coughs in real time. The performance will be improved further with additional training data obtained from other real environments such as hospitals and classrooms.
Professor Park said, “In a pandemic situation like we are experiencing with COVID-19, a cough detection camera can contribute to the prevention and early detection of epidemics in public places. Especially when applied to a hospital room, the patient's condition can be tracked 24 hours a day and support more accurate diagnoses while reducing the effort of the medical staff."
This study was conducted in collaboration with SM Instruments Inc.
Profile: Yong-Hwa Park, Ph.D.
Associate Professor
yhpark@kaist.ac.kr
http://human.kaist.ac.kr/
Human-Machine Interaction Laboratory (HuMaN Lab.)
Department of Mechanical Engineering (ME)
Korea Advanced Institute of Science and Technology (KAIST)
https://www.kaist.ac.kr/en/
Daejeon 34141, Korea
Profile: Gyeong Tae Lee
PhD Candidate
hansaram@kaist.ac.kr
HuMaN Lab., ME, KAIST
Profile: Seong Hu Kim
PhD Candidate
tjdgnkim@kaist.ac.kr
HuMaN Lab., ME, KAIST
Profile: Hyeonuk Nam
PhD Candidate
frednam@kaist.ac.kr
HuMaN Lab., ME, KAIST
Profile: Young-Key Kim
CEO
sales@smins.co.kr
http://en.smins.co.kr/
SM Instruments Inc.
Daejeon 34109, Korea
(END)
2020.08.13 View 19663 -
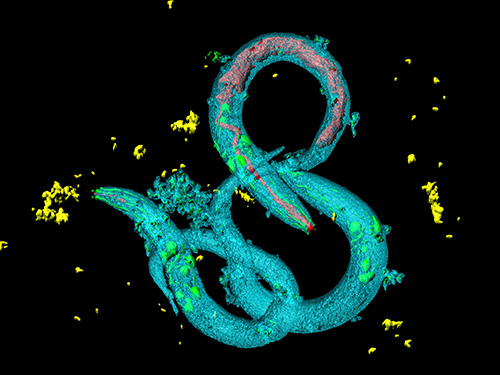 Tinkering with Roundworm Proteins Offers Hope for Anti-aging Drugs
- The somatic nuclear protein kinase VRK-1 increases the worm’s lifespan through AMPK activation, and this mechanism can be applied to promoting human longevity, the study reveals. -
KAIST researchers have been able to dial up and down creatures’ lifespans by altering the activity of proteins found in roundworm cells that tell them to convert sugar into energy when their cellular energy is running low. Humans also have these proteins, offering up the intriguing possibilities for developing longevity-promoting drugs. These new findings were published on July 1 in Science Advances.
The roundworm Caenorhabditis elegans (C. elegans), a millimeter-long nematode commonly used in lab testing, enjoyed a boost in its lifespan when researchers tinkered with a couple of proteins involved in monitoring the energy use by its cells.
The proteins VRK-1 and AMPK work in tandem in roundworm cells, with the former telling the latter to get to work by sticking a phosphate molecule, composed of one phosphorus and four oxygen atoms, on it. In turn, AMPK’s role is to monitor energy levels in cells, when cellular energy is running low. In essence, VRK-1 regulates AMPK, and AMPK regulates the cellular energy status.
Using a range of different biological research tools, including introducing foreign genes into the worm, a group of researchers led by Professor Seung-Jae V. Lee from the Department of Biological Sciences at KAIST were able to dial up and down the activity of the gene that tells cells to produce the VRK-1 protein. This gene has remained pretty much unchanged throughout evolution. Most complex organisms have this same gene, including humans.
Lead author of the study Sangsoon Park and his colleagues confirmed that the overexpression, or increased production, of the VRK-1 protein boosted the lifespan of the C. elegans, which normally lives just two to three weeks, and the inhibition of VRK-1 production reduced its lifespan.
The research team found that the activity of the VRK-1-to-AMPK cellular-energy monitoring process is increased in low cellular energy status by reduced mitochondrial respiration, the set of metabolic chemical reactions that make use of the oxygen the worm breathes to convert macronutrients from food into the energy “currency” that cells spend to do everything they need to do.
It is already known that mitochondria, the energy-producing engine rooms in cells, play a crucial role in aging, and declines in the functioning of mitochondria are associated with age-related diseases. At the same time, the mild inhibition of mitochondrial respiration has been shown to promote longevity in a range of species, including flies and mammals.
When the research team performed similar tinkering with cultured human cells, they found they could also replicate this ramping up and down of the VRK-1-to-AMPK process that occurs in roundworms.
“This raises the intriguing possibility that VRK-1 also functions as a factor in governing human longevity, and so perhaps we can start developing longevity-promoting drugs that alter the activity of VRK-1,” explained Professor Lee.
At the very least, the research points us in an interesting direction for investigating new therapeutic strategies to combat metabolic disorders by targeting the modulation of VRK-1. Metabolic disorders involve the disruption of chemical reactions in the body, including diseases of the mitochondria.
But before metabolic disorder therapeutics or longevity drugs can be contemplated by scientists, further research still needs to be carried out to better understand how VRK-1 works to activate AMPK, as well as figure out the precise mechanics of how AMPK controls cellular energy.
This work was supported by the National Research Foundation (NRF), and the Ministry of Science and ICT (MSIT) of Korea.
Image credit: Seung-Jae V. LEE, KAIST.
Image usage restrictions: News organizations may use or redistribute this image, with proper attribution, as part of news coverage of this paper only.
Publication:
Park, S., et al. (2020) ‘VRK-1 extends life span by activation of AMPK via phosphorylation’. Science Advances, Volume 6. No. 27, eaaw7824. Available online at https://doi.org/10.1126/sciadv.aaw7824
Profile: Seung-Jae V. Lee, Ph.D.
Professor
seungjaevlee@kaist.ac.kr
https://sites.google.com/view/mgakaist
Molecular Genetics of Aging Laboratory
Department of Biological Sciences
Korea Advanced Institute of Science and Technology (KAIST)
https://www.kaist.ac.krDaejeon 34141, Korea
(END)
2020.07.31 View 14178
Tinkering with Roundworm Proteins Offers Hope for Anti-aging Drugs
- The somatic nuclear protein kinase VRK-1 increases the worm’s lifespan through AMPK activation, and this mechanism can be applied to promoting human longevity, the study reveals. -
KAIST researchers have been able to dial up and down creatures’ lifespans by altering the activity of proteins found in roundworm cells that tell them to convert sugar into energy when their cellular energy is running low. Humans also have these proteins, offering up the intriguing possibilities for developing longevity-promoting drugs. These new findings were published on July 1 in Science Advances.
The roundworm Caenorhabditis elegans (C. elegans), a millimeter-long nematode commonly used in lab testing, enjoyed a boost in its lifespan when researchers tinkered with a couple of proteins involved in monitoring the energy use by its cells.
The proteins VRK-1 and AMPK work in tandem in roundworm cells, with the former telling the latter to get to work by sticking a phosphate molecule, composed of one phosphorus and four oxygen atoms, on it. In turn, AMPK’s role is to monitor energy levels in cells, when cellular energy is running low. In essence, VRK-1 regulates AMPK, and AMPK regulates the cellular energy status.
Using a range of different biological research tools, including introducing foreign genes into the worm, a group of researchers led by Professor Seung-Jae V. Lee from the Department of Biological Sciences at KAIST were able to dial up and down the activity of the gene that tells cells to produce the VRK-1 protein. This gene has remained pretty much unchanged throughout evolution. Most complex organisms have this same gene, including humans.
Lead author of the study Sangsoon Park and his colleagues confirmed that the overexpression, or increased production, of the VRK-1 protein boosted the lifespan of the C. elegans, which normally lives just two to three weeks, and the inhibition of VRK-1 production reduced its lifespan.
The research team found that the activity of the VRK-1-to-AMPK cellular-energy monitoring process is increased in low cellular energy status by reduced mitochondrial respiration, the set of metabolic chemical reactions that make use of the oxygen the worm breathes to convert macronutrients from food into the energy “currency” that cells spend to do everything they need to do.
It is already known that mitochondria, the energy-producing engine rooms in cells, play a crucial role in aging, and declines in the functioning of mitochondria are associated with age-related diseases. At the same time, the mild inhibition of mitochondrial respiration has been shown to promote longevity in a range of species, including flies and mammals.
When the research team performed similar tinkering with cultured human cells, they found they could also replicate this ramping up and down of the VRK-1-to-AMPK process that occurs in roundworms.
“This raises the intriguing possibility that VRK-1 also functions as a factor in governing human longevity, and so perhaps we can start developing longevity-promoting drugs that alter the activity of VRK-1,” explained Professor Lee.
At the very least, the research points us in an interesting direction for investigating new therapeutic strategies to combat metabolic disorders by targeting the modulation of VRK-1. Metabolic disorders involve the disruption of chemical reactions in the body, including diseases of the mitochondria.
But before metabolic disorder therapeutics or longevity drugs can be contemplated by scientists, further research still needs to be carried out to better understand how VRK-1 works to activate AMPK, as well as figure out the precise mechanics of how AMPK controls cellular energy.
This work was supported by the National Research Foundation (NRF), and the Ministry of Science and ICT (MSIT) of Korea.
Image credit: Seung-Jae V. LEE, KAIST.
Image usage restrictions: News organizations may use or redistribute this image, with proper attribution, as part of news coverage of this paper only.
Publication:
Park, S., et al. (2020) ‘VRK-1 extends life span by activation of AMPK via phosphorylation’. Science Advances, Volume 6. No. 27, eaaw7824. Available online at https://doi.org/10.1126/sciadv.aaw7824
Profile: Seung-Jae V. Lee, Ph.D.
Professor
seungjaevlee@kaist.ac.kr
https://sites.google.com/view/mgakaist
Molecular Genetics of Aging Laboratory
Department of Biological Sciences
Korea Advanced Institute of Science and Technology (KAIST)
https://www.kaist.ac.krDaejeon 34141, Korea
(END)
2020.07.31 View 14178 -
 KAIST Receives $57 Million Donation to Enhance Research
The largest amount since the opening of KAIST will fund ‘Singularity Professors’
KAIST Development Foundation Chairman Soo-Young Lee made a gift of real estate estimated at approximately $57 million on July 23. This is the largest donation KAIST has received since it was founded in 1971.
The fund will establish the “Soo-Young Lee Science Education Foundation” and the proceeds of the foundation will go to the “Singularity Professors” as necessary resources to help make discoveries and design new approaches to accelerate breakthroughs.
“KAIST should be the institute that will produce first Korean Nobel laureate in the field of science. I hope this fund will be utilized to enable Korea to stand out in this challenging time by accomplishing breakthroughs nobody has never imagined,” said Chairman Lee during the donation ceremony at KAIST’s campus in Daejeon.
This is Chairman Lee’s third donation following the $6.7 million donation in 2012 and the $830,000 donation in 2016. Chairman Lee began her career as a journalist in 1963. In 1981, she started her own business by launching Kwangwon Ranch and became a successful businesswoman. In 1988, Chairman Lee established the real estate company Kwangwon Industries. After receiving an honorary doctorate from KAIST in 2012, she has served as the chairman of the KAIST Development Foundation from 2013. Chairman Lee expressed her intention to make another donation to KAIST in the near future during the news conference.
“People matter most for advancing the world. KAIST has a very distinct mission to foster the brightest minds and will drive the nation to move forward. I have worked with KAIST for quite long time so that I have a strong belief that KAIST is the one that will not only make contributions to Korea but also to all humanity,” she explained.
“For example, about one-fourth of the R&D manpower at Samsung Electronics is from KAIST. In 2019, Samsung Electronics recorded a revenue of approximately $206 billion which accounted for about 16% of national GDP. KAIST is the one that fosters global talents who are working at global company such as Samsung and many others.”
KAIST President Sung-Chul Shin also expressed his deep respect for Chairman Lee’s decision, saying that the entire KAIST community will make every effort to keep up Chairman Lee’s noble idea encouraging KAIST to push forward and help realize KAIST’s role and mission.
(END)
2020.07.23 View 10588
KAIST Receives $57 Million Donation to Enhance Research
The largest amount since the opening of KAIST will fund ‘Singularity Professors’
KAIST Development Foundation Chairman Soo-Young Lee made a gift of real estate estimated at approximately $57 million on July 23. This is the largest donation KAIST has received since it was founded in 1971.
The fund will establish the “Soo-Young Lee Science Education Foundation” and the proceeds of the foundation will go to the “Singularity Professors” as necessary resources to help make discoveries and design new approaches to accelerate breakthroughs.
“KAIST should be the institute that will produce first Korean Nobel laureate in the field of science. I hope this fund will be utilized to enable Korea to stand out in this challenging time by accomplishing breakthroughs nobody has never imagined,” said Chairman Lee during the donation ceremony at KAIST’s campus in Daejeon.
This is Chairman Lee’s third donation following the $6.7 million donation in 2012 and the $830,000 donation in 2016. Chairman Lee began her career as a journalist in 1963. In 1981, she started her own business by launching Kwangwon Ranch and became a successful businesswoman. In 1988, Chairman Lee established the real estate company Kwangwon Industries. After receiving an honorary doctorate from KAIST in 2012, she has served as the chairman of the KAIST Development Foundation from 2013. Chairman Lee expressed her intention to make another donation to KAIST in the near future during the news conference.
“People matter most for advancing the world. KAIST has a very distinct mission to foster the brightest minds and will drive the nation to move forward. I have worked with KAIST for quite long time so that I have a strong belief that KAIST is the one that will not only make contributions to Korea but also to all humanity,” she explained.
“For example, about one-fourth of the R&D manpower at Samsung Electronics is from KAIST. In 2019, Samsung Electronics recorded a revenue of approximately $206 billion which accounted for about 16% of national GDP. KAIST is the one that fosters global talents who are working at global company such as Samsung and many others.”
KAIST President Sung-Chul Shin also expressed his deep respect for Chairman Lee’s decision, saying that the entire KAIST community will make every effort to keep up Chairman Lee’s noble idea encouraging KAIST to push forward and help realize KAIST’s role and mission.
(END)
2020.07.23 View 10588 -
 Atomic Force Microscopy Reveals Nanoscale Dental Erosion from Beverages
KAIST researchers used atomic force microscopy to quantitatively evaluate how acidic and sugary drinks affect human tooth enamel at the nanoscale level. This novel approach is useful for measuring mechanical and morphological changes that occur over time during enamel erosion induced by beverages.
Enamel is the hard-white substance that forms the outer part of a tooth. It is the hardest substance in the human body, even stronger than bone. Its resilient surface is 96 percent mineral, the highest percentage of any body tissue, making it durable and damage-resistant. The enamel acts as a barrier to protect the soft inner layers of the tooth, but can become susceptible to degradation by acids and sugars.
Enamel erosion occurs when the tooth enamel is overexposed to excessive consumption of acidic and sugary food and drinks. The loss of enamel, if left untreated, can lead to various tooth conditions including stains, fractures, sensitivity, and translucence. Once tooth enamel is damaged, it cannot be brought back. Therefore, thorough studies on how enamel erosion starts and develops, especially at the initial stages, are of high scientific and clinical relevance for dental health maintenance.
A research team led by Professor Seungbum Hong from the Department of Materials Science and Engineering at KAIST reported a new method of applying atomic force microscopy (AFM) techniques to study the nanoscale characterization of this early stage of enamel erosion. This study was introduced in the Journal of the Mechanical Behavior of Biomedical Materials (JMBBM) on June 29.
AFM is a very-high-resolution type of scanning probe microscopy (SPM), with demonstrated resolution on the order of fractions of a nanometer (nm) that is equal to one billionth of a meter. AFM generates images by scanning a small cantilever over the surface of a sample, and this can precisely measure the structure and mechanical properties of the sample, such as surface roughness and elastic modulus.
The co-lead authors of the study, Dr. Panpan Li and Dr. Chungik Oh, chose three commercially available popular beverages, Coca-Cola®, Sprite®, and Minute Maid® orange juice, and immersed tooth enamel in these drinks over time to analyze their impacts on human teeth and monitor the etching process on tooth enamel.
Five healthy human molars were obtained from volunteers between age 20 and 35 who visited the KAIST Clinic. After extraction, the teeth were preserved in distilled water before the experiment. The drinks were purchased and opened right before the immersion experiment, and the team utilized AFM to measure the surface topography and elastic modulus map.
The researchers observed that the surface roughness of the tooth enamel increased significantly as the immersion time increased, while the elastic modulus of the enamel surface decreased drastically. It was demonstrated that the enamel surface roughened five times more when it was immersed in beverages for 10 minutes, and that the elastic modulus of tooth enamel was five times lower after five minutes in the drinks.
Additionally, the research team found preferential etching in scratched tooth enamel. Brushing your teeth too hard and toothpastes with polishing particles that are advertised to remove dental biofilms can cause scratches on the enamel surface, which can be preferential sites for etching, the study revealed.
Professor Hong said, “Our study shows that AFM is a suitable technique to characterize variations in the morphology and mechanical properties of dental erosion quantitatively at the nanoscale level.”
This work was supported by the National Research Foundation (NRF), the Ministry of Science and ICT (MSIT), and the KUSTAR-KAIST Institute of Korea.
A dentist at the KAIST Clinic, Dr. Suebean Cho, Dr. Sangmin Shin from the Smile Well Dental, and Professor Kack-Kyun Kim at the Seoul National University School of Dentistry also collaborated in this project.
Publication:
Li, P., et al. (2020) ‘Nanoscale effects of beverages on enamel surface of human teeth: An atomic force microscopy study’. Journal of the Mechanical Behavior of Biomedical Materials (JMBBM), Volume 110. Article No. 103930. Available online at https://doi.org/10.1016/j.jmbbm.2020.103930
Profile: Seungbum Hong, Ph.D.
Associate Professor
seungbum@kaist.ac.kr
http://mii.kaist.ac.kr/
Materials Imaging and Integration (MII) Lab.
Department of Materials Science and Engineering (MSE)
Korea Advanced Institute of Science and Technology (KAIST)
https://www.kaist.ac.kr
Daejeon 34141, Korea
(END)
2020.07.21 View 15196
Atomic Force Microscopy Reveals Nanoscale Dental Erosion from Beverages
KAIST researchers used atomic force microscopy to quantitatively evaluate how acidic and sugary drinks affect human tooth enamel at the nanoscale level. This novel approach is useful for measuring mechanical and morphological changes that occur over time during enamel erosion induced by beverages.
Enamel is the hard-white substance that forms the outer part of a tooth. It is the hardest substance in the human body, even stronger than bone. Its resilient surface is 96 percent mineral, the highest percentage of any body tissue, making it durable and damage-resistant. The enamel acts as a barrier to protect the soft inner layers of the tooth, but can become susceptible to degradation by acids and sugars.
Enamel erosion occurs when the tooth enamel is overexposed to excessive consumption of acidic and sugary food and drinks. The loss of enamel, if left untreated, can lead to various tooth conditions including stains, fractures, sensitivity, and translucence. Once tooth enamel is damaged, it cannot be brought back. Therefore, thorough studies on how enamel erosion starts and develops, especially at the initial stages, are of high scientific and clinical relevance for dental health maintenance.
A research team led by Professor Seungbum Hong from the Department of Materials Science and Engineering at KAIST reported a new method of applying atomic force microscopy (AFM) techniques to study the nanoscale characterization of this early stage of enamel erosion. This study was introduced in the Journal of the Mechanical Behavior of Biomedical Materials (JMBBM) on June 29.
AFM is a very-high-resolution type of scanning probe microscopy (SPM), with demonstrated resolution on the order of fractions of a nanometer (nm) that is equal to one billionth of a meter. AFM generates images by scanning a small cantilever over the surface of a sample, and this can precisely measure the structure and mechanical properties of the sample, such as surface roughness and elastic modulus.
The co-lead authors of the study, Dr. Panpan Li and Dr. Chungik Oh, chose three commercially available popular beverages, Coca-Cola®, Sprite®, and Minute Maid® orange juice, and immersed tooth enamel in these drinks over time to analyze their impacts on human teeth and monitor the etching process on tooth enamel.
Five healthy human molars were obtained from volunteers between age 20 and 35 who visited the KAIST Clinic. After extraction, the teeth were preserved in distilled water before the experiment. The drinks were purchased and opened right before the immersion experiment, and the team utilized AFM to measure the surface topography and elastic modulus map.
The researchers observed that the surface roughness of the tooth enamel increased significantly as the immersion time increased, while the elastic modulus of the enamel surface decreased drastically. It was demonstrated that the enamel surface roughened five times more when it was immersed in beverages for 10 minutes, and that the elastic modulus of tooth enamel was five times lower after five minutes in the drinks.
Additionally, the research team found preferential etching in scratched tooth enamel. Brushing your teeth too hard and toothpastes with polishing particles that are advertised to remove dental biofilms can cause scratches on the enamel surface, which can be preferential sites for etching, the study revealed.
Professor Hong said, “Our study shows that AFM is a suitable technique to characterize variations in the morphology and mechanical properties of dental erosion quantitatively at the nanoscale level.”
This work was supported by the National Research Foundation (NRF), the Ministry of Science and ICT (MSIT), and the KUSTAR-KAIST Institute of Korea.
A dentist at the KAIST Clinic, Dr. Suebean Cho, Dr. Sangmin Shin from the Smile Well Dental, and Professor Kack-Kyun Kim at the Seoul National University School of Dentistry also collaborated in this project.
Publication:
Li, P., et al. (2020) ‘Nanoscale effects of beverages on enamel surface of human teeth: An atomic force microscopy study’. Journal of the Mechanical Behavior of Biomedical Materials (JMBBM), Volume 110. Article No. 103930. Available online at https://doi.org/10.1016/j.jmbbm.2020.103930
Profile: Seungbum Hong, Ph.D.
Associate Professor
seungbum@kaist.ac.kr
http://mii.kaist.ac.kr/
Materials Imaging and Integration (MII) Lab.
Department of Materials Science and Engineering (MSE)
Korea Advanced Institute of Science and Technology (KAIST)
https://www.kaist.ac.kr
Daejeon 34141, Korea
(END)
2020.07.21 View 15196 -
 COVID-19 Update: Fall Semester to Continue Offering Classes Online
KAIST announced that the university would continue online classes through the fall semester. However, the university will conduct additional in-person classes for upper-level undergraduate lab classes and some graduate courses where on-site interaction was deemed to be highly necessary. Some 600-level graduate courses at the Daejeon campus and graduate courses at the Seoul campus will carry out both in-person and online classes. The fall semester will start from August 31.
Provost and Executive Vice President Kwang Hyung Lee announced the fall semester plan in his letter to the entire student body on July 9. He said that the university decided to continue with online classes in consideration of the safety of KAIST community members and the current status of the COVID-19 spread. However, he said the new plan will help students choose class options between in-person and online classes.
“Although the number of classes with two versions is limited, we believe this will help many students continue learning without the sustained face-to-face contact that is inherent in residential education,” Provost Lee said.
In-person classes conducted in the fall semester will also be provided online for students who are not available for in-person classes. Students may choose the type of the classes they prefer according to their situation, among only the courses that will offer two versions. Professors will decide if they will conduct two versions of their classes. The Office of Academic Affairs is collecting the professors’ applications for conducting both versions until July 24.
KAIST offered real-time online classes and pre-recorded KLMS (KAIST Learning Management System) classes during the spring semester with a very limited number of in-person lab classes for graduate courses and these two versions of online class will continue for fall semester.
Provost Lee asked the students who will take the in-person classes to strictly observe all precaution measures as the university will do its best to abide by the government guidelines against the Covid-19 in preparation for the fall semester.
“We will continue to make appropriate and safe accommodations for them,” said Provost Lee.
Those who need to reside in on-campus dormitories are required to be approved for moving. The applications will open after all the in-person class schedules are fixed next month. However, students who were approved for staying in the dormitories last semester can move in without additional approval procedures for the fall semester.
(END)
2020.07.10 View 10479
COVID-19 Update: Fall Semester to Continue Offering Classes Online
KAIST announced that the university would continue online classes through the fall semester. However, the university will conduct additional in-person classes for upper-level undergraduate lab classes and some graduate courses where on-site interaction was deemed to be highly necessary. Some 600-level graduate courses at the Daejeon campus and graduate courses at the Seoul campus will carry out both in-person and online classes. The fall semester will start from August 31.
Provost and Executive Vice President Kwang Hyung Lee announced the fall semester plan in his letter to the entire student body on July 9. He said that the university decided to continue with online classes in consideration of the safety of KAIST community members and the current status of the COVID-19 spread. However, he said the new plan will help students choose class options between in-person and online classes.
“Although the number of classes with two versions is limited, we believe this will help many students continue learning without the sustained face-to-face contact that is inherent in residential education,” Provost Lee said.
In-person classes conducted in the fall semester will also be provided online for students who are not available for in-person classes. Students may choose the type of the classes they prefer according to their situation, among only the courses that will offer two versions. Professors will decide if they will conduct two versions of their classes. The Office of Academic Affairs is collecting the professors’ applications for conducting both versions until July 24.
KAIST offered real-time online classes and pre-recorded KLMS (KAIST Learning Management System) classes during the spring semester with a very limited number of in-person lab classes for graduate courses and these two versions of online class will continue for fall semester.
Provost Lee asked the students who will take the in-person classes to strictly observe all precaution measures as the university will do its best to abide by the government guidelines against the Covid-19 in preparation for the fall semester.
“We will continue to make appropriate and safe accommodations for them,” said Provost Lee.
Those who need to reside in on-campus dormitories are required to be approved for moving. The applications will open after all the in-person class schedules are fixed next month. However, students who were approved for staying in the dormitories last semester can move in without additional approval procedures for the fall semester.
(END)
2020.07.10 View 10479 -
 X-ray Scattering Shines Light on Protein Folding
- Multiple forms of a non-functional, unfolded protein follow different pathways and timelines to reach its folded, functional state, a study reveals. -
KAIST researchers have used an X-ray method to track how proteins fold, which could improve computer simulations of this process, with implications for understanding diseases and improving drug discovery. Their findings were reported in the Proceedings of the National Academy of Sciences of the United States of America (PNAS) on June 30.
When proteins are translated from their DNA codes, they quickly transform from a non-functional, unfolded state into their folded, functional state. Problems in folding can lead to diseases like Alzheimer’s and Parkinson’s.
“Protein folding is one of the most important biological processes, as it forms the functioning 3D protein structure,” explained the physical chemist Hyotcherl Ihee of the Department of Chemistry at KAIST. Dr. Tae Wu Kim, the lead author of this research from Ihee’s group, added, “Understanding the mechanisms of protein folding is important, and could pave the way for disease study and drug development.”
Ihee’s team developed an approach using an X-ray scattering technique to uncover how the protein cytochrome c folds from its initial unfolded state. This protein is composed of a chain of 104 amino acids with an iron-containing heme molecule. It is often used for protein folding studies.
The researchers placed the protein in a solution and shined ultraviolet light on it. This process provides electrons to cytochrome c, reducing the iron within it from the ferric to the ferrous form, which initiates folding. As this was happening, the researchers beamed X-rays at very short intervals onto the sample. The X-rays scattered off all the atomic pairs in the sample and a detector continuously recorded the X-ray scattering patterns. The X-ray scattering patterns provided direct information regarding the 3D protein structure and the changes made in these patterns over time showed real-time motion of the protein during the folding process.
The team found cytochrome c proteins initially exist in a wide variety of unfolded states. Once the folding process is triggered, they stop by a group of intermediates within 31.6 microseconds, and then those intermediates follow different pathways with different folding times to reach an energetically stable folded state.
“We don’t know if this diversity in folding paths can be generalized to other proteins,” Ihee confessed. He continued, “However, we believe that our approach can be used to study other protein folding systems.”
Ihee hopes this approach can improve the accuracy of models that simulate protein interactions by including information on their unstructured states. These simulations are important as they can help identify barriers to proper folding and predict a protein’s folded state given its amino acid sequence. Ultimately, the models could help clarify how some diseases develop and how drugs interact with various protein structures.
Ihee’s group collaborated with Professor Young Min Rhee at the KAIST Department of Chemistry, and this work was supported by the National Research Foundation of Korea (NRF) and the Institute for Basic Science (IBS).
Figure. The scientists found that non-functional unfolded forms of the protein cytochrome c follow different pathways and timelines to reach a stable functional folded state.
Publications:
Kim, T. W., et al. (2020) ‘Protein folding from heterogeneous unfolded state revealed by time-resolved X-ray solution scattering’. PNAS. Volume 117. Issue 26. Page 14996-15005. Available online at https://doi.org/10.1073/pnas.1913442117
Profile: Hyotcherl Ihee, Ph.D.
Professor
hyotcherl.ihee@kaist.ac.kr
http://time.kaist.ac.kr/
Ihee Laboratory
Department of Chemistry
KAIST
https://www.kaist.ac.kr
Daejeon 34141, Korea
Profile: Young Min Rhee, Ph.D.
Professor
ymrhee@kaist.ac.kr
http://singlet.kaist.ac.kr
Rhee Research Group
Department of Chemistry
KAIST
https://www.kaist.ac.kr
Daejeon 34141, Korea
(END)
2020.07.09 View 18223
X-ray Scattering Shines Light on Protein Folding
- Multiple forms of a non-functional, unfolded protein follow different pathways and timelines to reach its folded, functional state, a study reveals. -
KAIST researchers have used an X-ray method to track how proteins fold, which could improve computer simulations of this process, with implications for understanding diseases and improving drug discovery. Their findings were reported in the Proceedings of the National Academy of Sciences of the United States of America (PNAS) on June 30.
When proteins are translated from their DNA codes, they quickly transform from a non-functional, unfolded state into their folded, functional state. Problems in folding can lead to diseases like Alzheimer’s and Parkinson’s.
“Protein folding is one of the most important biological processes, as it forms the functioning 3D protein structure,” explained the physical chemist Hyotcherl Ihee of the Department of Chemistry at KAIST. Dr. Tae Wu Kim, the lead author of this research from Ihee’s group, added, “Understanding the mechanisms of protein folding is important, and could pave the way for disease study and drug development.”
Ihee’s team developed an approach using an X-ray scattering technique to uncover how the protein cytochrome c folds from its initial unfolded state. This protein is composed of a chain of 104 amino acids with an iron-containing heme molecule. It is often used for protein folding studies.
The researchers placed the protein in a solution and shined ultraviolet light on it. This process provides electrons to cytochrome c, reducing the iron within it from the ferric to the ferrous form, which initiates folding. As this was happening, the researchers beamed X-rays at very short intervals onto the sample. The X-rays scattered off all the atomic pairs in the sample and a detector continuously recorded the X-ray scattering patterns. The X-ray scattering patterns provided direct information regarding the 3D protein structure and the changes made in these patterns over time showed real-time motion of the protein during the folding process.
The team found cytochrome c proteins initially exist in a wide variety of unfolded states. Once the folding process is triggered, they stop by a group of intermediates within 31.6 microseconds, and then those intermediates follow different pathways with different folding times to reach an energetically stable folded state.
“We don’t know if this diversity in folding paths can be generalized to other proteins,” Ihee confessed. He continued, “However, we believe that our approach can be used to study other protein folding systems.”
Ihee hopes this approach can improve the accuracy of models that simulate protein interactions by including information on their unstructured states. These simulations are important as they can help identify barriers to proper folding and predict a protein’s folded state given its amino acid sequence. Ultimately, the models could help clarify how some diseases develop and how drugs interact with various protein structures.
Ihee’s group collaborated with Professor Young Min Rhee at the KAIST Department of Chemistry, and this work was supported by the National Research Foundation of Korea (NRF) and the Institute for Basic Science (IBS).
Figure. The scientists found that non-functional unfolded forms of the protein cytochrome c follow different pathways and timelines to reach a stable functional folded state.
Publications:
Kim, T. W., et al. (2020) ‘Protein folding from heterogeneous unfolded state revealed by time-resolved X-ray solution scattering’. PNAS. Volume 117. Issue 26. Page 14996-15005. Available online at https://doi.org/10.1073/pnas.1913442117
Profile: Hyotcherl Ihee, Ph.D.
Professor
hyotcherl.ihee@kaist.ac.kr
http://time.kaist.ac.kr/
Ihee Laboratory
Department of Chemistry
KAIST
https://www.kaist.ac.kr
Daejeon 34141, Korea
Profile: Young Min Rhee, Ph.D.
Professor
ymrhee@kaist.ac.kr
http://singlet.kaist.ac.kr
Rhee Research Group
Department of Chemistry
KAIST
https://www.kaist.ac.kr
Daejeon 34141, Korea
(END)
2020.07.09 View 18223