bio
-
 KAIST to support the Genetic Donguibogam Research Project for global market entry of a new natural drug produced by Green Cross Corporation HS
In the wake of the spread of the Middle East Respiratory Syndrome (MERS), sales of immune-enhancing products in Korea such as red and white ginseng have risen dramatically. Ginseng is one of Korea’s major health supplement it exports, but due to the lack of precise scientific knowledge of its mechanism, sales of ginseng account for less than 2% of the global market share.
The Genetic Donguibogam Research Project represents a group of research initiatives to study genes and environmental factors that contribute to diseases and to discover alternative treatments through Eastern medicine. The project is being led by KAIST’s Department of Bio & Brain Engineering Professor Do-Heon Lee.
Professor Lee and Chief Executive Officer Young-Hyo Yoo of Green Cross Corporation HS, a Korean pharmaceutical company, signed a memorandum of understanding (MOU), as well as a non-disclosure agreement (NDA) to develop a naturally derived drug with an enhanced ginsenoside, pharmacological compounds of ginseng, for the global market entry of BST204 on June 10, 2015.
Donguibogam is the traditional Korean source for the principles and practice of Eastern medicine, which was compiled by the royal physician Heo Jun and first published in 1613 during the Joseon Dynasty of Korea.
Cooperating with Green Cross Co., HS, KAIST researchers will use a multi-component, multi-target (MCMT)-based development platform to produce the new natural drug, BST204. This cooperation is expected to assist the entry of the drug into the European market.
Green Cross Co., HS has applied a bio-conversion technique to ginseng to develop BST204, which is a drug with enhanced active constituent of aginsenosides. The drug is the first produced by any Korean pharmaceutical company to complete the first phase of clinical trials in Germany and is about to start the second phase of trials.
Professor Do-Heon Lee, the Director of the project said, “Genetic Donguibogam Research Project seeks to create new innovative healthcare material for the future using integrated fundamental technologies such as virtual human body computer modelling and multi-omics to explain the mechanism in which natural ingredients affect the human body.” He continued, “Especially, by employing the virtual human body computer modelling, we can develop an innovative new technology that will greatly assist Korean pharmaceutical industry and make it the platform technology in entering global markets.”
Young-Hyo Yoo, the CEO of Green Cross Co., HS, said, “For a new naturally derived drug to be acknowledged in the global market, such as Europe and the US, its mechanism, as well as its effectiveness and safety, should be proven. However, it is difficult and costly to explain the mechanism in which the complex composition of a natural substance influences the body. Innovative technology is needed to solve this problem.”
Professor Do-Heon Lee (left in the picture), the Director of Genetic Donguibogam Research Project, stands abreast Young-Hyo Yoo (right in the picture), the CEO of Green Cross Co., HS.
2015.06.10 View 10378
KAIST to support the Genetic Donguibogam Research Project for global market entry of a new natural drug produced by Green Cross Corporation HS
In the wake of the spread of the Middle East Respiratory Syndrome (MERS), sales of immune-enhancing products in Korea such as red and white ginseng have risen dramatically. Ginseng is one of Korea’s major health supplement it exports, but due to the lack of precise scientific knowledge of its mechanism, sales of ginseng account for less than 2% of the global market share.
The Genetic Donguibogam Research Project represents a group of research initiatives to study genes and environmental factors that contribute to diseases and to discover alternative treatments through Eastern medicine. The project is being led by KAIST’s Department of Bio & Brain Engineering Professor Do-Heon Lee.
Professor Lee and Chief Executive Officer Young-Hyo Yoo of Green Cross Corporation HS, a Korean pharmaceutical company, signed a memorandum of understanding (MOU), as well as a non-disclosure agreement (NDA) to develop a naturally derived drug with an enhanced ginsenoside, pharmacological compounds of ginseng, for the global market entry of BST204 on June 10, 2015.
Donguibogam is the traditional Korean source for the principles and practice of Eastern medicine, which was compiled by the royal physician Heo Jun and first published in 1613 during the Joseon Dynasty of Korea.
Cooperating with Green Cross Co., HS, KAIST researchers will use a multi-component, multi-target (MCMT)-based development platform to produce the new natural drug, BST204. This cooperation is expected to assist the entry of the drug into the European market.
Green Cross Co., HS has applied a bio-conversion technique to ginseng to develop BST204, which is a drug with enhanced active constituent of aginsenosides. The drug is the first produced by any Korean pharmaceutical company to complete the first phase of clinical trials in Germany and is about to start the second phase of trials.
Professor Do-Heon Lee, the Director of the project said, “Genetic Donguibogam Research Project seeks to create new innovative healthcare material for the future using integrated fundamental technologies such as virtual human body computer modelling and multi-omics to explain the mechanism in which natural ingredients affect the human body.” He continued, “Especially, by employing the virtual human body computer modelling, we can develop an innovative new technology that will greatly assist Korean pharmaceutical industry and make it the platform technology in entering global markets.”
Young-Hyo Yoo, the CEO of Green Cross Co., HS, said, “For a new naturally derived drug to be acknowledged in the global market, such as Europe and the US, its mechanism, as well as its effectiveness and safety, should be proven. However, it is difficult and costly to explain the mechanism in which the complex composition of a natural substance influences the body. Innovative technology is needed to solve this problem.”
Professor Do-Heon Lee (left in the picture), the Director of Genetic Donguibogam Research Project, stands abreast Young-Hyo Yoo (right in the picture), the CEO of Green Cross Co., HS.
2015.06.10 View 10378 -
 KAIST to Kick-Start the Exchange of Young Researchers with Northern European Universities
KAIST promotes research exchange and cooperation with three universities in Northern Europe.
KAIST has signed a letter of intent (LOI) for the mutual exchange of young researchers and cooperation to collaborate with KTH Royal Institute of Technology and Lund University, both based in Sweden on June 2, 2015, and with Aalto University in Finland on June 4, 2015.
This LOI was the result of the cooperative projects of Korea-Sweden and Korea-Finland Joint Committees on Science and Technology supervised by the Ministry of Science, ICT and Future Planning of Korea.
As agreed in the LOI, KAIST will conduct joint research projects with the three universities by providing students and researchers with opportunities to visit each other through internship programs and workshops and by sharing information on education and research.
Sung-Hyon Mayeng, the Associate Vice President of the International Relations Office at KAIST, said, “It’s an encouraging sign that universities and governments recognize the importance of increasing exchanges among academic and research communities. Expecting more vibrant relationships to be formed between KAIST and the three northern European universities in coming years, I hope that today’s agreement becomes a good basis to spur technological innovations that will not only benefit the regions but also the world.”
Established in 1827, the KTH Royal Institute of Technology is the largest and oldest technical university in Sweden, accounting for one-third of the nation’s technical research and engineering education capacity at university level. The university offers education and research programs from natural sciences to all branches of engineering including architecture, industrial management, and urban planning. According to the QS World University Rankings in 2014, KTH Royal Institute of Technology ranked 27th in engineering and 1st in Northern Europe.
Lund University, Sweden, is one of the oldest and most prestigious universities in northern Europe, consistently ranking among the world’s top 100 universities. In particular, its biological sciences and engineering have shown great strength, placing within the top 60 universities by the Times Higher Education (THE) World University Rankings. The university also receives the largest amount of research funding from the Swedish government.
Aalto University in Finland was created as a merger of three leading Finnish universities: the Helsinki University of Technology (established 1849), the Helsinki School of Economics (established 1904), and the University of Art and Design Helsinki (established 1871). The university nurtures the close collaborations across science, business, and arts to foster multi-disciplinary education and research.
2015.06.04 View 9712
KAIST to Kick-Start the Exchange of Young Researchers with Northern European Universities
KAIST promotes research exchange and cooperation with three universities in Northern Europe.
KAIST has signed a letter of intent (LOI) for the mutual exchange of young researchers and cooperation to collaborate with KTH Royal Institute of Technology and Lund University, both based in Sweden on June 2, 2015, and with Aalto University in Finland on June 4, 2015.
This LOI was the result of the cooperative projects of Korea-Sweden and Korea-Finland Joint Committees on Science and Technology supervised by the Ministry of Science, ICT and Future Planning of Korea.
As agreed in the LOI, KAIST will conduct joint research projects with the three universities by providing students and researchers with opportunities to visit each other through internship programs and workshops and by sharing information on education and research.
Sung-Hyon Mayeng, the Associate Vice President of the International Relations Office at KAIST, said, “It’s an encouraging sign that universities and governments recognize the importance of increasing exchanges among academic and research communities. Expecting more vibrant relationships to be formed between KAIST and the three northern European universities in coming years, I hope that today’s agreement becomes a good basis to spur technological innovations that will not only benefit the regions but also the world.”
Established in 1827, the KTH Royal Institute of Technology is the largest and oldest technical university in Sweden, accounting for one-third of the nation’s technical research and engineering education capacity at university level. The university offers education and research programs from natural sciences to all branches of engineering including architecture, industrial management, and urban planning. According to the QS World University Rankings in 2014, KTH Royal Institute of Technology ranked 27th in engineering and 1st in Northern Europe.
Lund University, Sweden, is one of the oldest and most prestigious universities in northern Europe, consistently ranking among the world’s top 100 universities. In particular, its biological sciences and engineering have shown great strength, placing within the top 60 universities by the Times Higher Education (THE) World University Rankings. The university also receives the largest amount of research funding from the Swedish government.
Aalto University in Finland was created as a merger of three leading Finnish universities: the Helsinki University of Technology (established 1849), the Helsinki School of Economics (established 1904), and the University of Art and Design Helsinki (established 1871). The university nurtures the close collaborations across science, business, and arts to foster multi-disciplinary education and research.
2015.06.04 View 9712 -
 KAIST International Food Festival
The KAIST International Students Association (KISA) hosted the 2015 International Food Festival in front of Creative Learning Building, KAIST, on May 22, 2015.
This was the 11th International Food Festival for KAIST where international students introduced food from their home countries to strengthen cultural exchanges with Korean students. This year’s festival was the biggest international festival in Daejeon in which around 500 students and staff from KAIST, Chungnam National University (CNU), the University of Science & Technology (UST), and the public participated.
KAIST’s President Steve Kang opened the festival with a welcoming speech, followed by congratulatory speeches by CNU President Sang-Chul Jung and UST President Un-Woo Lee.
The first section of the event was the food festival where around 40 kinds of food from ten countries including Kenya, Kazakhstan, India, and Turkey were presented. Students from each country offered cooking demonstrations in booths, and participants purchased the food.
Cheryl Wanderi, a Kenyan student who recently received a Master’s degree from KAIST’s Department of Bio and Brain Engineering last February said, “I am delighted to introduce Mandazi, a Kenyan donut, to not only Korean students but also other international students.”
The second half of the event consisted of cultural performances from different countries. There were eight teams performing including an Indonesian traditional Saman dance team, a Kazakh group that performed on traditional instruments, and an Azerbaijani K-POP dance team.
Sung-Hyon Myaeng, the Associate Vice President of KAIST’s International Office, said, “Despite their busy lives, students from three different universities planned this event to get to know each other. I hope international students and Korean students can come together and enjoy the festival.”
Edrick Kwek, the President of KISA, said, “This food festival is an event showing the cultural diversity of KAIST in the most splendid way.”
2015.05.27 View 10971
KAIST International Food Festival
The KAIST International Students Association (KISA) hosted the 2015 International Food Festival in front of Creative Learning Building, KAIST, on May 22, 2015.
This was the 11th International Food Festival for KAIST where international students introduced food from their home countries to strengthen cultural exchanges with Korean students. This year’s festival was the biggest international festival in Daejeon in which around 500 students and staff from KAIST, Chungnam National University (CNU), the University of Science & Technology (UST), and the public participated.
KAIST’s President Steve Kang opened the festival with a welcoming speech, followed by congratulatory speeches by CNU President Sang-Chul Jung and UST President Un-Woo Lee.
The first section of the event was the food festival where around 40 kinds of food from ten countries including Kenya, Kazakhstan, India, and Turkey were presented. Students from each country offered cooking demonstrations in booths, and participants purchased the food.
Cheryl Wanderi, a Kenyan student who recently received a Master’s degree from KAIST’s Department of Bio and Brain Engineering last February said, “I am delighted to introduce Mandazi, a Kenyan donut, to not only Korean students but also other international students.”
The second half of the event consisted of cultural performances from different countries. There were eight teams performing including an Indonesian traditional Saman dance team, a Kazakh group that performed on traditional instruments, and an Azerbaijani K-POP dance team.
Sung-Hyon Myaeng, the Associate Vice President of KAIST’s International Office, said, “Despite their busy lives, students from three different universities planned this event to get to know each other. I hope international students and Korean students can come together and enjoy the festival.”
Edrick Kwek, the President of KISA, said, “This food festival is an event showing the cultural diversity of KAIST in the most splendid way.”
2015.05.27 View 10971 -
 Professor Sang-Yup Lee Receives the Order of Service Merit Red Stripes from the Korean Government
The government of the Republic of Korea named Professor Sang-Yup Lee of the Department of Chemical and Bio-molecular Engineering at KAIST as the fiftieth recipient of the Order of Service Merit Red Stripes on May 19, 2015.
This medal is awarded to government employees, officials, and teachers in recognition of their contributions to public services including education.
Professor Lee is regarded as a leading scientist in the field of metabolic engineering, genomics, proteomics, metabolomics, and bioinformatics on microorganism producing various primary and secondary metabolites. He contributed significantly to the advancement of bio-based engineering research in Korea.
In addition, his research in microorganism metabolic engineering propelled him to the front of his field, making him the world’s founder of systems metabolic engineering, inventing numerous technologies in strain development.
Professor Lee has received many patent rights in bioprocess engineering. While at KAIST, he applied for 585 patents and registered 227 patents. In particular, he has applied for 135 patents and registered 99 patents in the past five years, successfully turning research results into commercial applications.
Professor Lee said, “I’m glad to contribute to the development of Korean science and technology as a researcher and teacher. I would like to share this honor with my students, master’s and doctoral students in particular, because without their support, it wouldn’t have been possible to pull off the highest level of research results recognized by this medal.”
2015.05.21 View 9662
Professor Sang-Yup Lee Receives the Order of Service Merit Red Stripes from the Korean Government
The government of the Republic of Korea named Professor Sang-Yup Lee of the Department of Chemical and Bio-molecular Engineering at KAIST as the fiftieth recipient of the Order of Service Merit Red Stripes on May 19, 2015.
This medal is awarded to government employees, officials, and teachers in recognition of their contributions to public services including education.
Professor Lee is regarded as a leading scientist in the field of metabolic engineering, genomics, proteomics, metabolomics, and bioinformatics on microorganism producing various primary and secondary metabolites. He contributed significantly to the advancement of bio-based engineering research in Korea.
In addition, his research in microorganism metabolic engineering propelled him to the front of his field, making him the world’s founder of systems metabolic engineering, inventing numerous technologies in strain development.
Professor Lee has received many patent rights in bioprocess engineering. While at KAIST, he applied for 585 patents and registered 227 patents. In particular, he has applied for 135 patents and registered 99 patents in the past five years, successfully turning research results into commercial applications.
Professor Lee said, “I’m glad to contribute to the development of Korean science and technology as a researcher and teacher. I would like to share this honor with my students, master’s and doctoral students in particular, because without their support, it wouldn’t have been possible to pull off the highest level of research results recognized by this medal.”
2015.05.21 View 9662 -
 Big Data Reveals the Secret of Classical Music Creation
Professor Juyong Park of the Graduate School of Culture Technology at KAIST and his research team have recently published the result of their study (“Topology and Evolution of the Network of Western Classical Music Composers”) on the dynamics of how classical music is created, stylized, and disseminated in EPJ Data Science online on April 22, 2015. For the press release issued by the journal, please go to the link below:
EPJ Data Science, May 6, 2015
“EPJ Data Science Highlight—Big Data Reveals Classical Music Creation Secrets”
http://www.epj.org/113-epj-ds/941-epjds-highlight-big-data-reveals-classical-music-creation-secrets
Researchers used big-data analysis and modelling technique to examine the complex, undercurrent network of classical music composers, which was constructed from the large volume of compact disc (CD) recordings data collected from an online retailer, ArkivMusic, and a music reference website, AllMusicGuide.
The study discovered that the basic characteristics of composers’ network are similar to many real-world networks, including the small-world property, the existence of a giant component, high clustering, and heavy-tailed degree distributions. The research team also found that composers collaborated and influenced each other and that composers’ networks grew over time.
The research showed that consumers of classical music CDs tend to listen together to the music of a certain group of different composers, offering a useful tool to understand how the music style and market develops. Based on this, the research team predicted the future of the classical music market would be centered on top composers, while maintaining diversity due to the growing number of new composers.
Professor Park said, “In recent years, technology greatly affects the way we consume culture and art. Accordingly, we see more and more artists and institutions try to incorporate technology into their creative process, and this will lead us to larger- and higher-quality data that can allow us to learn more about culture and art. The quantitative methodology we have demonstrated in our research will give us an opportunity to explore the nature of art and literature in novel ways.”
The European Physical Journal (EPJ) comprises a series of peer-reviewed journals, eleven in total, which cover physics and related subjects such as The Large Hadron Collider, condensed matter, particles, soft matter, and biological physics. The EPJ Data Science is the latest journal launched by EPJ.
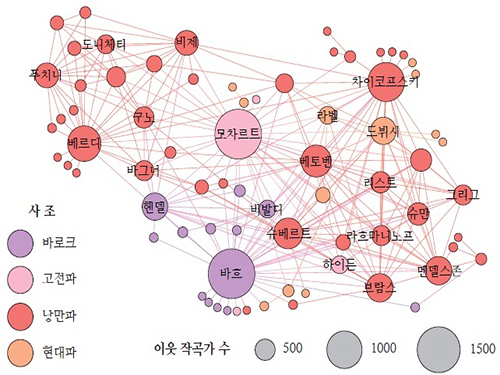
Figure: Backbone of the Composer Network
The composer-composer network backbone, projected from the CD-composer network, reveals the major component of the network. The node sizes represent the composers’ degrees, and the colors represent their active periods.
2015.05.07 View 11331
Big Data Reveals the Secret of Classical Music Creation
Professor Juyong Park of the Graduate School of Culture Technology at KAIST and his research team have recently published the result of their study (“Topology and Evolution of the Network of Western Classical Music Composers”) on the dynamics of how classical music is created, stylized, and disseminated in EPJ Data Science online on April 22, 2015. For the press release issued by the journal, please go to the link below:
EPJ Data Science, May 6, 2015
“EPJ Data Science Highlight—Big Data Reveals Classical Music Creation Secrets”
http://www.epj.org/113-epj-ds/941-epjds-highlight-big-data-reveals-classical-music-creation-secrets
Researchers used big-data analysis and modelling technique to examine the complex, undercurrent network of classical music composers, which was constructed from the large volume of compact disc (CD) recordings data collected from an online retailer, ArkivMusic, and a music reference website, AllMusicGuide.
The study discovered that the basic characteristics of composers’ network are similar to many real-world networks, including the small-world property, the existence of a giant component, high clustering, and heavy-tailed degree distributions. The research team also found that composers collaborated and influenced each other and that composers’ networks grew over time.
The research showed that consumers of classical music CDs tend to listen together to the music of a certain group of different composers, offering a useful tool to understand how the music style and market develops. Based on this, the research team predicted the future of the classical music market would be centered on top composers, while maintaining diversity due to the growing number of new composers.
Professor Park said, “In recent years, technology greatly affects the way we consume culture and art. Accordingly, we see more and more artists and institutions try to incorporate technology into their creative process, and this will lead us to larger- and higher-quality data that can allow us to learn more about culture and art. The quantitative methodology we have demonstrated in our research will give us an opportunity to explore the nature of art and literature in novel ways.”
The European Physical Journal (EPJ) comprises a series of peer-reviewed journals, eleven in total, which cover physics and related subjects such as The Large Hadron Collider, condensed matter, particles, soft matter, and biological physics. The EPJ Data Science is the latest journal launched by EPJ.
Figure: Backbone of the Composer Network
The composer-composer network backbone, projected from the CD-composer network, reveals the major component of the network. The node sizes represent the composers’ degrees, and the colors represent their active periods.
2015.05.07 View 11331 -
 2015 QS World University Rankings by Subject: KAIST's Chemical Engineering ranks 17th and 19th for Materials Science in the World
Chemical Engineering (1st in Korea)
1
MIT (US)
2
UC Berkeley (US)
3
Stanford University (US)
4
University of Cambridge (UK)
5
National University of Singapore (Singapore)
17
KAIST (Korea)
Materials Science and Engineering (1st in Korea)
1
MIT (US)
2
Stanford University (US)
3
UC Berkeley (US)
4
University of Cambridge (UK)
5
North Western University (US)
19
KAIST (Korea)
Electrical and Electronic Engineering (1st in Korea)
1
MIT (US)
2
Stanford University (US)
3
UC Berkeley (US)
4
Harvard University (US)
5
ETH Zurich – Swiss Federal Institute of Technology (Switzerland)
22
KAIST (Korea)
Civil and Structural Engineering (1st in Korea)
1
MIT (US)
2
Delft University of Technology (The Netherlands)
3
National University of Singapore (Singapore)
4
Imperial College London (UK)
5
University of Cambridge (UK)
22
KAIST (Korea)
Mechanical, Aeronautical and Manufacturing Engineering (1st in Korea)
1
MIT (US)
2
Stanford University (US)
3
University of Cambridge (UK)
4
UC Berkeley (US)
5
Michigan University (US)
26
KAIST (Korea)
Chemistry (2nd in Korea)
1
MIT (US)
2
UC Berkeley (US)
3
University of Cambridge (UK)
4
Harvard University (US)
5
University of Oxford (UK)
26
KAIST (Korea)
Computer Science and Information Systems (1st in Korea)
1
MIT (US)
2
Stanford University (US)
3
University of Oxford (UK)
4
Carnegie Mellon University (US)
Harvard University (US)
39
KAIST (Korea)
The QS World University Rankings released its 2015 rankings by subject on April 29, 2015.
According to the rankings, KAIST’s Chemical and Biomolecular Engineering and Materials Science Engineering were listed in the top 20 global universities, 17th and 19th, respectively.
KAIST took first place in six subjects among Korean universities, including electrical and electronic engineering; civil and structural engineering; mechanical, aeronautical and manufacturing engineering; and computer science and information systems.
The QS World University Rankings by Subject highlights the world’s top universities in a range of popular subject areas, covering 36 subjects as of this year. Published annually since 2011, the rankings are based on academic reputation, employer reputation, citation count, and research impact.
For a full list of the rankings: http://www.topuniversities.com/subject-rankings/2015
2015.04.29 View 6220
2015 QS World University Rankings by Subject: KAIST's Chemical Engineering ranks 17th and 19th for Materials Science in the World
Chemical Engineering (1st in Korea)
1
MIT (US)
2
UC Berkeley (US)
3
Stanford University (US)
4
University of Cambridge (UK)
5
National University of Singapore (Singapore)
17
KAIST (Korea)
Materials Science and Engineering (1st in Korea)
1
MIT (US)
2
Stanford University (US)
3
UC Berkeley (US)
4
University of Cambridge (UK)
5
North Western University (US)
19
KAIST (Korea)
Electrical and Electronic Engineering (1st in Korea)
1
MIT (US)
2
Stanford University (US)
3
UC Berkeley (US)
4
Harvard University (US)
5
ETH Zurich – Swiss Federal Institute of Technology (Switzerland)
22
KAIST (Korea)
Civil and Structural Engineering (1st in Korea)
1
MIT (US)
2
Delft University of Technology (The Netherlands)
3
National University of Singapore (Singapore)
4
Imperial College London (UK)
5
University of Cambridge (UK)
22
KAIST (Korea)
Mechanical, Aeronautical and Manufacturing Engineering (1st in Korea)
1
MIT (US)
2
Stanford University (US)
3
University of Cambridge (UK)
4
UC Berkeley (US)
5
Michigan University (US)
26
KAIST (Korea)
Chemistry (2nd in Korea)
1
MIT (US)
2
UC Berkeley (US)
3
University of Cambridge (UK)
4
Harvard University (US)
5
University of Oxford (UK)
26
KAIST (Korea)
Computer Science and Information Systems (1st in Korea)
1
MIT (US)
2
Stanford University (US)
3
University of Oxford (UK)
4
Carnegie Mellon University (US)
Harvard University (US)
39
KAIST (Korea)
The QS World University Rankings released its 2015 rankings by subject on April 29, 2015.
According to the rankings, KAIST’s Chemical and Biomolecular Engineering and Materials Science Engineering were listed in the top 20 global universities, 17th and 19th, respectively.
KAIST took first place in six subjects among Korean universities, including electrical and electronic engineering; civil and structural engineering; mechanical, aeronautical and manufacturing engineering; and computer science and information systems.
The QS World University Rankings by Subject highlights the world’s top universities in a range of popular subject areas, covering 36 subjects as of this year. Published annually since 2011, the rankings are based on academic reputation, employer reputation, citation count, and research impact.
For a full list of the rankings: http://www.topuniversities.com/subject-rankings/2015
2015.04.29 View 6220 -
 Fast, Accurate 3D Imaging to Track Optically-Trapped Particles
KAIST researchers published an article on the development of a novel technique to precisely track the 3-D positions of optically-trapped particles having complicated geometry in high speed in the April 2015 issue of Optica.
Optical tweezers have been used as an invaluable tool for exerting micro-scale force on microscopic particles and manipulating three-dimensional (3-D) positions of particles. Optical tweezers employ a tightly-focused laser whose beam diameter is smaller than one micrometer (1/100 of hair thickness), which generates attractive force on neighboring microscopic particles moving toward the beam focus. Controlling the positions of the beam focus enabled researchers to hold the particles and move them freely to other locations so they coined the name “optical tweezers.”
To locate the optically-trapped particles by a laser beam, optical microscopes have usually been employed. Optical microscopes measure light signals scattered by the optically-trapped microscopic particles and the positions of the particles in two dimensions. However, it was difficult to quantify the particles’ precise positions along the optic axis, the direction of the beam, from a single image, which is analogous to the difficulty of determining the front and rear positions of objects when closing an eye due to a lack of depth perception. Furthermore, it became more difficult to measure precisely 3-D positions of particles when scattered light signals were distorted by optically-trapped particles having complicated shapes or other particles occlude the target object along the optic axis.
Professor YongKeun Park and his research team in the Department of Physics at the Korea Advanced Institute of Science and Technology (KAIST) employed an optical diffraction tomography (ODT) technique to measure 3-D positions of optically-trapped particles in high speed. The principle of ODT is similar to X-ray CT imaging commonly used in hospitals for visualizing the internal organs of patients. Like X-ray CT imaging, which takes several images from various illumination angles, ODT measures 3-D images of optically-trapped particles by illuminating them with a laser beam in various incidence angles.
The KAIST team used optical tweezers to trap a glass bead with a diameter of 2 micrometers, and moved the bead toward a white blood cell having complicated internal structures. The team measured the 3-D dynamics of the white blood cell as it responded to an approaching glass bead via ODT in the high acquisition rate of 60 images per second. Since the white blood cell screens the glass bead along an optic axis, a conventionally-used optical microscope could not determine the 3-D positions of the glass bead. In contrast, the present method employing ODT localized the 3-D positions of the bead precisely as well as measured the composition of the internal materials of the bead and the white blood cell simultaneously.
Professor Park said, “Our technique has the advantage of measuring the 3-D positions and internal structures of optically-trapped particles in high speed without labelling exogenous fluorescent agents and can be applied in various fields including physics, optics, nanotechnology, and medical science.”
Kyoohyun Kim, the lead author of this paper (“Simultaneous 3D Visualization and Position Tracking of Optically Trapped Particles Using Optical Diffraction Tomography”), added, “This ODT technique can also apply to cellular-level surgeries where optical tweezers are used to manipulate intracellular organelles and to display in real time and in 3-D the images of the reaction of the cell membrane and nucleus during the operation or monitoring the recovery process of the cells from the surgery.”
The research results were published as the cover article in the April 2014 issue of Optica, the newest journal launched last year by the Optical Society of America (OSA) for rapid dissemination of high-impact results related to optics.
Figure 1: This picture shows the concept image of tweezing an optically-trapped glass bead on the cellular membrane of a white blood cell.
Figure 2: High-speed 3-D images produced from optical diffraction tomography technique
2015.04.24 View 12688
Fast, Accurate 3D Imaging to Track Optically-Trapped Particles
KAIST researchers published an article on the development of a novel technique to precisely track the 3-D positions of optically-trapped particles having complicated geometry in high speed in the April 2015 issue of Optica.
Optical tweezers have been used as an invaluable tool for exerting micro-scale force on microscopic particles and manipulating three-dimensional (3-D) positions of particles. Optical tweezers employ a tightly-focused laser whose beam diameter is smaller than one micrometer (1/100 of hair thickness), which generates attractive force on neighboring microscopic particles moving toward the beam focus. Controlling the positions of the beam focus enabled researchers to hold the particles and move them freely to other locations so they coined the name “optical tweezers.”
To locate the optically-trapped particles by a laser beam, optical microscopes have usually been employed. Optical microscopes measure light signals scattered by the optically-trapped microscopic particles and the positions of the particles in two dimensions. However, it was difficult to quantify the particles’ precise positions along the optic axis, the direction of the beam, from a single image, which is analogous to the difficulty of determining the front and rear positions of objects when closing an eye due to a lack of depth perception. Furthermore, it became more difficult to measure precisely 3-D positions of particles when scattered light signals were distorted by optically-trapped particles having complicated shapes or other particles occlude the target object along the optic axis.
Professor YongKeun Park and his research team in the Department of Physics at the Korea Advanced Institute of Science and Technology (KAIST) employed an optical diffraction tomography (ODT) technique to measure 3-D positions of optically-trapped particles in high speed. The principle of ODT is similar to X-ray CT imaging commonly used in hospitals for visualizing the internal organs of patients. Like X-ray CT imaging, which takes several images from various illumination angles, ODT measures 3-D images of optically-trapped particles by illuminating them with a laser beam in various incidence angles.
The KAIST team used optical tweezers to trap a glass bead with a diameter of 2 micrometers, and moved the bead toward a white blood cell having complicated internal structures. The team measured the 3-D dynamics of the white blood cell as it responded to an approaching glass bead via ODT in the high acquisition rate of 60 images per second. Since the white blood cell screens the glass bead along an optic axis, a conventionally-used optical microscope could not determine the 3-D positions of the glass bead. In contrast, the present method employing ODT localized the 3-D positions of the bead precisely as well as measured the composition of the internal materials of the bead and the white blood cell simultaneously.
Professor Park said, “Our technique has the advantage of measuring the 3-D positions and internal structures of optically-trapped particles in high speed without labelling exogenous fluorescent agents and can be applied in various fields including physics, optics, nanotechnology, and medical science.”
Kyoohyun Kim, the lead author of this paper (“Simultaneous 3D Visualization and Position Tracking of Optically Trapped Particles Using Optical Diffraction Tomography”), added, “This ODT technique can also apply to cellular-level surgeries where optical tweezers are used to manipulate intracellular organelles and to display in real time and in 3-D the images of the reaction of the cell membrane and nucleus during the operation or monitoring the recovery process of the cells from the surgery.”
The research results were published as the cover article in the April 2014 issue of Optica, the newest journal launched last year by the Optical Society of America (OSA) for rapid dissemination of high-impact results related to optics.
Figure 1: This picture shows the concept image of tweezing an optically-trapped glass bead on the cellular membrane of a white blood cell.
Figure 2: High-speed 3-D images produced from optical diffraction tomography technique
2015.04.24 View 12688 -
 KAIST Researchers Develops Hyper-Stretchable Elastic-Composite Energy Harvester
A research team led by Professor Keon Jae Lee (http://fand.kaist.ac.kr) of the Department of Materials Science and Engineering at KAIST has developed a hyper-stretchable elastic-composite energy harvesting device called a nanogenerator.
Flexible electronics have come into the market and are enabling new technologies like flexible displays in mobile phone, wearable electronics, and the Internet of Things (IoTs). However, is the degree of flexibility enough for most applications? For many flexible devices, elasticity is a very important issue. For example, wearable/biomedical devices and electronic skins (e-skins) should stretch to conform to arbitrarily curved surfaces and moving body parts such as joints, diaphragms, and tendons. They must be able to withstand the repeated and prolonged mechanical stresses of stretching. In particular, the development of elastic energy devices is regarded as critical to establish power supplies in stretchable applications. Although several researchers have explored diverse stretchable electronics, due to the absence of the appropriate device structures and correspondingly electrodes, researchers have not developed ultra-stretchable and fully-reversible energy conversion devices properly.
Recently, researchers from KAIST and Seoul National University (SNU) have collaborated and demonstrated a facile methodology to obtain a high-performance and hyper-stretchable elastic-composite generator (SEG) using very long silver nanowire-based stretchable electrodes. Their stretchable piezoelectric generator can harvest mechanical energy to produce high power output (~4 V) with large elasticity (~250%) and excellent durability (over 104 cycles). These noteworthy results were achieved by the non-destructive stress- relaxation ability of the unique electrodes as well as the good piezoelectricity of the device components. The new SEG can be applied to a wide-variety of wearable energy-harvesters to transduce biomechanical-stretching energy from the body (or machines) to electrical energy.
Professor Lee said, “This exciting approach introduces an ultra-stretchable piezoelectric generator. It can open avenues for power supplies in universal wearable and biomedical applications as well as self-powered ultra-stretchable electronics.”
This result was published online in the March issue of Advanced Materials, which is entitled “A Hyper-Stretchable Elastic-Composite Energy Harvester.”
YouTube Link: “A hyper-stretchable energy harvester”
https://www.youtube.com/watch?v=EBByFvPVRiU&feature=youtu.be
Figure: Top row: Schematics of hyper-stretchable elastic-composite generator enabled by very long silver nanowire-based stretchable electrodes.
Bottom row: The SEG energy harvester stretched by human hands over 200% strain.
2015.04.14 View 14739
KAIST Researchers Develops Hyper-Stretchable Elastic-Composite Energy Harvester
A research team led by Professor Keon Jae Lee (http://fand.kaist.ac.kr) of the Department of Materials Science and Engineering at KAIST has developed a hyper-stretchable elastic-composite energy harvesting device called a nanogenerator.
Flexible electronics have come into the market and are enabling new technologies like flexible displays in mobile phone, wearable electronics, and the Internet of Things (IoTs). However, is the degree of flexibility enough for most applications? For many flexible devices, elasticity is a very important issue. For example, wearable/biomedical devices and electronic skins (e-skins) should stretch to conform to arbitrarily curved surfaces and moving body parts such as joints, diaphragms, and tendons. They must be able to withstand the repeated and prolonged mechanical stresses of stretching. In particular, the development of elastic energy devices is regarded as critical to establish power supplies in stretchable applications. Although several researchers have explored diverse stretchable electronics, due to the absence of the appropriate device structures and correspondingly electrodes, researchers have not developed ultra-stretchable and fully-reversible energy conversion devices properly.
Recently, researchers from KAIST and Seoul National University (SNU) have collaborated and demonstrated a facile methodology to obtain a high-performance and hyper-stretchable elastic-composite generator (SEG) using very long silver nanowire-based stretchable electrodes. Their stretchable piezoelectric generator can harvest mechanical energy to produce high power output (~4 V) with large elasticity (~250%) and excellent durability (over 104 cycles). These noteworthy results were achieved by the non-destructive stress- relaxation ability of the unique electrodes as well as the good piezoelectricity of the device components. The new SEG can be applied to a wide-variety of wearable energy-harvesters to transduce biomechanical-stretching energy from the body (or machines) to electrical energy.
Professor Lee said, “This exciting approach introduces an ultra-stretchable piezoelectric generator. It can open avenues for power supplies in universal wearable and biomedical applications as well as self-powered ultra-stretchable electronics.”
This result was published online in the March issue of Advanced Materials, which is entitled “A Hyper-Stretchable Elastic-Composite Energy Harvester.”
YouTube Link: “A hyper-stretchable energy harvester”
https://www.youtube.com/watch?v=EBByFvPVRiU&feature=youtu.be
Figure: Top row: Schematics of hyper-stretchable elastic-composite generator enabled by very long silver nanowire-based stretchable electrodes.
Bottom row: The SEG energy harvester stretched by human hands over 200% strain.
2015.04.14 View 14739 -
 Anti-Cancer Therapy Delivering Drug to an Entire Tumor Developed
KAIST’s Department of Bio and Brain Engineering Professor Ji-Ho Park and his team successfully developed a new highly efficacious anti-cancer nanotechnology by delivering anti-cancer drugs uniformly to an entire tumor. Their research results were published in Nano Letters online on March 31, 2015.
To treat inoperable tumors, anti-cancer medicine is commonly used. However, efficient drug delivery to tumor cells is often difficult, treating an entire tumor with drugs even more so.
Using the existing drug delivery systems, including nanotechnology, a drug can be delivered only to tumor cells near blood vessels, leaving cells at the heart of a tumor intact. Since most drugs are injected into the bloodstream, tumor recurrence post medication is frequent.
Therefore, the team used liposomes that can fuse to the cell membrane and enter the cell. Once inside liposomes the drug can travel into the bloodstream, enter tumor cells near blood vessels, where they are loaded to exosomes, which are naturally occurring nanoparticles in the body. Since exosomes can travel between cells, the drug can be delivered efficiently into inner cells of the tumor.
Exosomes, which are secreted by cells that exist in the tumor microenvironment, is known to have an important role in tumor progression and metastasis since they transfer biological materials between cells. The research team started the investigation recognizing the possibility of delivering the anti-cancer drug to the entire tumor using exosomes.
The team injected the light-sensitive anti-cancer drug using their new delivery technique into experimental mice. The researchers applied light to the tumor site to activate the anti-cancer treatment and analyzed a tissue sample. They observed the effects of the anti-cancer drug in the entire tumor tissue.
The team’s results establish a ground-breaking foothold in drug delivery technology development that can be tailored to specific diseases by understanding its microenvironment. The work paves the way to more effective drug delivery systems for many chronic diseases, including cancer tumors that were difficult to treat due to the inability to penetrate deep into the tissue.
The team is currently conducting experiments with other anti-cancer drugs, which are being developed by pharmaceutical companies, using their tumor-penetrating drug delivery nanotechnology, to identify its effects on malignant tumors.
Professor Park said, “This research is the first to apply biological nanoparticles, exosomes that are continuously secreted and can transfer materials to neighboring cells, to deliver drugs directly to the heart of tumor.”
Picture: Incorporation of hydrophilic and hydrophobic compounds into membrane vesicles by engineering the parental cells via synthetic liposomes.
2015.04.07 View 12911
Anti-Cancer Therapy Delivering Drug to an Entire Tumor Developed
KAIST’s Department of Bio and Brain Engineering Professor Ji-Ho Park and his team successfully developed a new highly efficacious anti-cancer nanotechnology by delivering anti-cancer drugs uniformly to an entire tumor. Their research results were published in Nano Letters online on March 31, 2015.
To treat inoperable tumors, anti-cancer medicine is commonly used. However, efficient drug delivery to tumor cells is often difficult, treating an entire tumor with drugs even more so.
Using the existing drug delivery systems, including nanotechnology, a drug can be delivered only to tumor cells near blood vessels, leaving cells at the heart of a tumor intact. Since most drugs are injected into the bloodstream, tumor recurrence post medication is frequent.
Therefore, the team used liposomes that can fuse to the cell membrane and enter the cell. Once inside liposomes the drug can travel into the bloodstream, enter tumor cells near blood vessels, where they are loaded to exosomes, which are naturally occurring nanoparticles in the body. Since exosomes can travel between cells, the drug can be delivered efficiently into inner cells of the tumor.
Exosomes, which are secreted by cells that exist in the tumor microenvironment, is known to have an important role in tumor progression and metastasis since they transfer biological materials between cells. The research team started the investigation recognizing the possibility of delivering the anti-cancer drug to the entire tumor using exosomes.
The team injected the light-sensitive anti-cancer drug using their new delivery technique into experimental mice. The researchers applied light to the tumor site to activate the anti-cancer treatment and analyzed a tissue sample. They observed the effects of the anti-cancer drug in the entire tumor tissue.
The team’s results establish a ground-breaking foothold in drug delivery technology development that can be tailored to specific diseases by understanding its microenvironment. The work paves the way to more effective drug delivery systems for many chronic diseases, including cancer tumors that were difficult to treat due to the inability to penetrate deep into the tissue.
The team is currently conducting experiments with other anti-cancer drugs, which are being developed by pharmaceutical companies, using their tumor-penetrating drug delivery nanotechnology, to identify its effects on malignant tumors.
Professor Park said, “This research is the first to apply biological nanoparticles, exosomes that are continuously secreted and can transfer materials to neighboring cells, to deliver drugs directly to the heart of tumor.”
Picture: Incorporation of hydrophilic and hydrophobic compounds into membrane vesicles by engineering the parental cells via synthetic liposomes.
2015.04.07 View 12911 -
 Novel Photolithographic Technology Enabling 3D Control over Functional Shapes of Microstructures
Professor Shin-Hyun Kim and his research team in the Department of Chemical and Biomolecular Engineering at KAIST have developed a novel photolithographic technology enabling control over the functional shapes of micropatterns using oxygen diffusion.
The research was published online in the March 13th issue of Nature Communications and was selected as a featured image for the journal.
Photolithography is a standard optical process for transferring micropatterns on to a substrate by exposing specific regions of the photoresist layer to ultraviolet (UV) light. It is used widely throughout industries that require micropatterns, especially in the semiconductor manufacturing industry.
Conventional photolithography relied on photomasks which protected certain regions of the substrate from the input UV light. Areas covered by the photomasks remain intact with the base layer while the areas exposed to the UV light are washed away, thus creating a micropattern. This technology was limited to a two-dimensional, disc-shaped design as the boundaries between the exposed and roofed regions are always in a parallel arrangement with the direction of the light.
Professor Kim’s research team discovered that: 1) the areas exposed to UV light lowered the concentration of oxygen and thus resulted in oxygen diffusion; and 2) manipulation of the diffusion speed and direction allowed control of the growth, shape and size of the polymers. Based on these findings, the team developed a new photolithographic technology that enabled the production of micropatterns with three-dimensional structures in various shapes and sizes.
Oxygen was considered an inhibitor during photopolymerization. Photoresist under UV light creates radicals which initialize a chemical reaction. These radicals are eliminated with the presence of oxygen and thus prevents the reaction. This suggests that the photoresist must be exposed to UV light for an extended time to completely remove oxygen for a chemical reaction to begin.
The research team, however, exploited the presence of oxygen. While the region affected by the UV light lowered oxygen concentration, the concentration in the untouched region remained unchanged. This difference in the concentrations caused a diffusion of oxygen to the region under UV light.
When the speed of the oxygen flow is slow, the diffusion occurs in parallel with the direction of the UV light. When fast, the diffusion process develops horizontally, outward from the area affected by the UV light. Professor Kim and his team proved this phenomenon both empirically and theoretically. Furthermore, by injecting an external oxygen source, the team was able to manipulate diffusion strength and direction, and thus control the shape and size of the polymer. The use of the polymerization inhibitors enabled and facilitated the fabrication of complex, three-dimensional micropatterns.
Professor Kim said, “While 3D printing is considered an innovative manufacturing technology, it cannot be used for mass-production of microscopic products. The new photolithographic technology will have a broad impact on both the academia and industry especially because existing, conventional photolithographic equipment can be used for the development of more complex micropatterns.” His newest technology will enhance the manufacturing process of three-dimensional polymers which were considered difficult to be commercialized.
The research was also dedicated to the late Professor Seung-Man Yang of the Department of Chemical and Biomolecular Engineering at KAIST. He was considered one of the greatest scholars in Korea in the field of hydrodynamics and colloids.
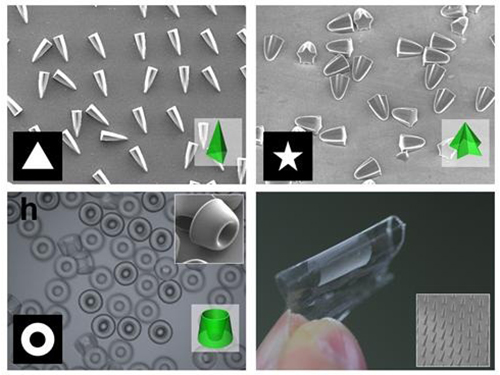
Picture 1: Featured Image of Nature Communications, March 2015
Picture 2: Polymers with various shapes and sizes produced with the new photolithographic technology developed by Professor Kim
2015.04.06 View 11561
Novel Photolithographic Technology Enabling 3D Control over Functional Shapes of Microstructures
Professor Shin-Hyun Kim and his research team in the Department of Chemical and Biomolecular Engineering at KAIST have developed a novel photolithographic technology enabling control over the functional shapes of micropatterns using oxygen diffusion.
The research was published online in the March 13th issue of Nature Communications and was selected as a featured image for the journal.
Photolithography is a standard optical process for transferring micropatterns on to a substrate by exposing specific regions of the photoresist layer to ultraviolet (UV) light. It is used widely throughout industries that require micropatterns, especially in the semiconductor manufacturing industry.
Conventional photolithography relied on photomasks which protected certain regions of the substrate from the input UV light. Areas covered by the photomasks remain intact with the base layer while the areas exposed to the UV light are washed away, thus creating a micropattern. This technology was limited to a two-dimensional, disc-shaped design as the boundaries between the exposed and roofed regions are always in a parallel arrangement with the direction of the light.
Professor Kim’s research team discovered that: 1) the areas exposed to UV light lowered the concentration of oxygen and thus resulted in oxygen diffusion; and 2) manipulation of the diffusion speed and direction allowed control of the growth, shape and size of the polymers. Based on these findings, the team developed a new photolithographic technology that enabled the production of micropatterns with three-dimensional structures in various shapes and sizes.
Oxygen was considered an inhibitor during photopolymerization. Photoresist under UV light creates radicals which initialize a chemical reaction. These radicals are eliminated with the presence of oxygen and thus prevents the reaction. This suggests that the photoresist must be exposed to UV light for an extended time to completely remove oxygen for a chemical reaction to begin.
The research team, however, exploited the presence of oxygen. While the region affected by the UV light lowered oxygen concentration, the concentration in the untouched region remained unchanged. This difference in the concentrations caused a diffusion of oxygen to the region under UV light.
When the speed of the oxygen flow is slow, the diffusion occurs in parallel with the direction of the UV light. When fast, the diffusion process develops horizontally, outward from the area affected by the UV light. Professor Kim and his team proved this phenomenon both empirically and theoretically. Furthermore, by injecting an external oxygen source, the team was able to manipulate diffusion strength and direction, and thus control the shape and size of the polymer. The use of the polymerization inhibitors enabled and facilitated the fabrication of complex, three-dimensional micropatterns.
Professor Kim said, “While 3D printing is considered an innovative manufacturing technology, it cannot be used for mass-production of microscopic products. The new photolithographic technology will have a broad impact on both the academia and industry especially because existing, conventional photolithographic equipment can be used for the development of more complex micropatterns.” His newest technology will enhance the manufacturing process of three-dimensional polymers which were considered difficult to be commercialized.
The research was also dedicated to the late Professor Seung-Man Yang of the Department of Chemical and Biomolecular Engineering at KAIST. He was considered one of the greatest scholars in Korea in the field of hydrodynamics and colloids.
Picture 1: Featured Image of Nature Communications, March 2015
Picture 2: Polymers with various shapes and sizes produced with the new photolithographic technology developed by Professor Kim
2015.04.06 View 11561 -
 Polymers with Highly Improved Light-transformation Efficiency
A joint Korean research team, led by Professor Bum-Joon Kim of the Department of Chemical and Biomolecular Engineering at KAIST and Professor Young-Woo Han of the Department of Nanofusion Engineering at Pusan National University, has developed a new type of electrically-conductive polymer for solar batteries with an improved light-transformation efficiency of up to 5%. The team considers it a viable replacement for existing plastic batteries for solar power which is viewed as the energy source of the future.
Polymer solar cells have greater structural stability and heat resistance compared to fullerene organic solar cells. However, they have lower light-transformation efficiency—below 4%—compared to 10% of the latter. The low efficiency is due to the failure of blending among the polymers that compose the active layer of the cell. This phenomenon deters the formation and movement of electrons and thus lowers light-transformation efficiency.
By manipulating the structure and concentration of conductive polymers, the team was able to effectively increase the polymer blending and increase light-transformation efficiency. The team was able to maximize the efficiency up to 6% which is the highest reported ratio.
Professor Kim said, “This research demonstrates that conductive polymer plastics can be used widely for solar cells and batteries for mobile devices.”
The research findings were published in the February 18th issue of the Journal of the American Chemical Society (JACS).
Picture: Flexible Solar Cell Polymer Developed by the Research Team
2015.04.05 View 12146
Polymers with Highly Improved Light-transformation Efficiency
A joint Korean research team, led by Professor Bum-Joon Kim of the Department of Chemical and Biomolecular Engineering at KAIST and Professor Young-Woo Han of the Department of Nanofusion Engineering at Pusan National University, has developed a new type of electrically-conductive polymer for solar batteries with an improved light-transformation efficiency of up to 5%. The team considers it a viable replacement for existing plastic batteries for solar power which is viewed as the energy source of the future.
Polymer solar cells have greater structural stability and heat resistance compared to fullerene organic solar cells. However, they have lower light-transformation efficiency—below 4%—compared to 10% of the latter. The low efficiency is due to the failure of blending among the polymers that compose the active layer of the cell. This phenomenon deters the formation and movement of electrons and thus lowers light-transformation efficiency.
By manipulating the structure and concentration of conductive polymers, the team was able to effectively increase the polymer blending and increase light-transformation efficiency. The team was able to maximize the efficiency up to 6% which is the highest reported ratio.
Professor Kim said, “This research demonstrates that conductive polymer plastics can be used widely for solar cells and batteries for mobile devices.”
The research findings were published in the February 18th issue of the Journal of the American Chemical Society (JACS).
Picture: Flexible Solar Cell Polymer Developed by the Research Team
2015.04.05 View 12146 -
 Mystery in Membrane Traffic How NSF Disassembles Single SNAR Complex Solved
KAIST researchers discovered that the protein N-ethylmaleimide-sensitive factor (NSF) unravels a single SNARE complex using one round ATP turnover by tearing the complex with a single burst, contradicting a previous theory that it unwinds in a processive manner.
In 2013, James E. Rothman, Randy W. Schekman, and Thomas C. Südhof won the Nobel Prize in Physiology or Medicine for their discoveries of molecular machineries for vesicle trafficking, a major transport system in cells for maintaining cellular processes. Vesicle traffic acts as a kind of “home-delivery service” in cells. Vesicles package and deliver materials such as proteins and hormones from one cell organelle to another. Then it releases its contents by fusing with the target organelle’s membrane. One example of vesicle traffic is in neuronal communications, where neurotransmitters are released from a neuron. Some of the key proteins for vesicle traffic discovered by the Nobel Prize winners were N-ethylmaleimide-sensitive factor (NSF), alpha-soluble NSF attachment protein (α-SNAP), and soluble SNAP receptors (SNAREs).
SNARE proteins are known as the minimal machinery for membrane fusion. To induce membrane fusion, the proteins combine to form a SNARE complex in a four helical bundle, and NSF and α-SNAP disassemble the SNARE complex for reuse. In particular, NSF can bind an energy source molecule, adenosine triphosphate (ATP), and the ATP-bound NSF develops internal tension via cleavage of ATP. This process is used to exert great force on SNARE complexes, eventually pulling them apart. However, although about 30 years have passed since the Nobel Prize winners’ discovery, how NSF/α-SNAP disassembled the SNARE complex remained a mystery to scientists due to a lack in methodology.
In a recent issue of Science, published on March 27, 2015, a research team, led by Tae-Young Yoon of the Department of Physics at the Korea Advanced Institute of Science and Technology (KAIST) and Reinhard Jahn of the Department of Neurobiology of the Max-Planck-Institute for Biophysical Chemistry, reports that NSF/α-SNAP disassemble a single SNARE complex using various single-molecule biophysical methods that allow them to monitor and manipulate individual protein complexes.
“We have learned that NSF releases energy in a burst within 20 milliseconds to “tear” the SNARE complex apart in a one-step global unfolding reaction, which is immediately followed by the release of SNARE proteins,” said Yoon.
Previously, it was believed that NSF disassembled a SNARE complex by unwinding it in a processive manner. Also, largely unexplained was how many cycles of ATP hydrolysis were required and how these cycles were connected to the disassembly of the SNARE complex.
Yoon added, “From our research, we found that NSF requires hydrolysis of ATPs that were already bound before it attached to the SNAREs—which means that only one round of an ATP turnover is sufficient for SNARE complex disassembly. Moreover, this is possible because NSF pulls a SNARE complex apart by building up the energy from individual ATPs and releasing it at once, yielding a “spring-loaded” mechanism.”
NSF is a member of the ATPases associated with various cellular activities family (AAA+ ATPase), which is essential for many cellular functions such as DNA replication and protein degradation, membrane fusion, microtubule severing, peroxisome biogenesis, signal transduction, and the regulation of gene expression. This research has added valuable new insights and hints for studying AAA+ ATPase proteins, which are crucial for various living beings.
The title of the research paper is “Spring-loaded unraveling of a single SNARE complex by NSF in one round of ATP turnover.” (DOI: 10.1126/science.aaa5267)
Youtube Link: https://www.youtube.com/watch?v=FqTSYHtyHWE&feature=youtu.be
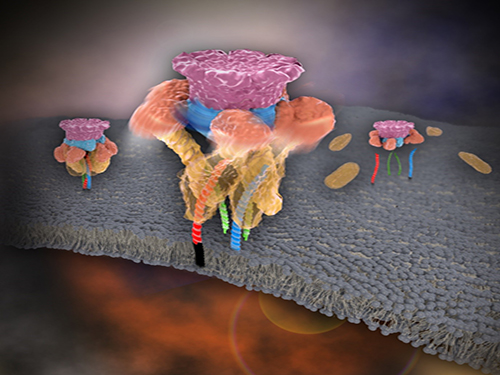
Picture 1. Working model of how NSF/α-SNAP disassemble a single SNARE complex
Picture 2. After neurotransmitter release, NSF disassembles a single SNARE complex using a single round of ATP turnover in a single burst reaction.
2015.03.28 View 11679
Mystery in Membrane Traffic How NSF Disassembles Single SNAR Complex Solved
KAIST researchers discovered that the protein N-ethylmaleimide-sensitive factor (NSF) unravels a single SNARE complex using one round ATP turnover by tearing the complex with a single burst, contradicting a previous theory that it unwinds in a processive manner.
In 2013, James E. Rothman, Randy W. Schekman, and Thomas C. Südhof won the Nobel Prize in Physiology or Medicine for their discoveries of molecular machineries for vesicle trafficking, a major transport system in cells for maintaining cellular processes. Vesicle traffic acts as a kind of “home-delivery service” in cells. Vesicles package and deliver materials such as proteins and hormones from one cell organelle to another. Then it releases its contents by fusing with the target organelle’s membrane. One example of vesicle traffic is in neuronal communications, where neurotransmitters are released from a neuron. Some of the key proteins for vesicle traffic discovered by the Nobel Prize winners were N-ethylmaleimide-sensitive factor (NSF), alpha-soluble NSF attachment protein (α-SNAP), and soluble SNAP receptors (SNAREs).
SNARE proteins are known as the minimal machinery for membrane fusion. To induce membrane fusion, the proteins combine to form a SNARE complex in a four helical bundle, and NSF and α-SNAP disassemble the SNARE complex for reuse. In particular, NSF can bind an energy source molecule, adenosine triphosphate (ATP), and the ATP-bound NSF develops internal tension via cleavage of ATP. This process is used to exert great force on SNARE complexes, eventually pulling them apart. However, although about 30 years have passed since the Nobel Prize winners’ discovery, how NSF/α-SNAP disassembled the SNARE complex remained a mystery to scientists due to a lack in methodology.
In a recent issue of Science, published on March 27, 2015, a research team, led by Tae-Young Yoon of the Department of Physics at the Korea Advanced Institute of Science and Technology (KAIST) and Reinhard Jahn of the Department of Neurobiology of the Max-Planck-Institute for Biophysical Chemistry, reports that NSF/α-SNAP disassemble a single SNARE complex using various single-molecule biophysical methods that allow them to monitor and manipulate individual protein complexes.
“We have learned that NSF releases energy in a burst within 20 milliseconds to “tear” the SNARE complex apart in a one-step global unfolding reaction, which is immediately followed by the release of SNARE proteins,” said Yoon.
Previously, it was believed that NSF disassembled a SNARE complex by unwinding it in a processive manner. Also, largely unexplained was how many cycles of ATP hydrolysis were required and how these cycles were connected to the disassembly of the SNARE complex.
Yoon added, “From our research, we found that NSF requires hydrolysis of ATPs that were already bound before it attached to the SNAREs—which means that only one round of an ATP turnover is sufficient for SNARE complex disassembly. Moreover, this is possible because NSF pulls a SNARE complex apart by building up the energy from individual ATPs and releasing it at once, yielding a “spring-loaded” mechanism.”
NSF is a member of the ATPases associated with various cellular activities family (AAA+ ATPase), which is essential for many cellular functions such as DNA replication and protein degradation, membrane fusion, microtubule severing, peroxisome biogenesis, signal transduction, and the regulation of gene expression. This research has added valuable new insights and hints for studying AAA+ ATPase proteins, which are crucial for various living beings.
The title of the research paper is “Spring-loaded unraveling of a single SNARE complex by NSF in one round of ATP turnover.” (DOI: 10.1126/science.aaa5267)
Youtube Link: https://www.youtube.com/watch?v=FqTSYHtyHWE&feature=youtu.be
Picture 1. Working model of how NSF/α-SNAP disassemble a single SNARE complex
Picture 2. After neurotransmitter release, NSF disassembles a single SNARE complex using a single round of ATP turnover in a single burst reaction.
2015.03.28 View 11679