research
-
 KAIST Nanosatellite LINK Launched to the ISS
Courtesy: United Launch Alliance
The KAIST nanosatellite LINK (Little Intelligent Nanosatellite of KAIST) was successfully launched on an Atlas V booster aboard the NASA CRS-7 Mission on April 18 at Space Launch Complex 41, Cape Canaveral Air Force Station in Florida. The KAIST nanosatellite was developed by the research team led by Professor Hyochoong Bang of the Department of Aerospace Engineering.
Aboard the flight to the ISS (International Space Station) were 28 satellites including LINK. They are part of the QB50 Project, an international educational initiative which aims to deploy an array of CubeSat-mounted sensors into Earth’s thermosphere. The project is funded by the European Commission and managed by the von Karman Institute for Fluid Dynamics in Belgium.
The small satellites are hitching a lift into orbit aboard the unmanned resupply spacecraft Cygnus, with a total mass of 83 kilograms. Built to CubeSat specifications, Cygnus will deploy four of the spacecraft following its departure from the space station. LINK will conduct its scientific mission for three months at the station.
The majority of QB50 satellites carry one of three standard instrument packages, consisting of a primary instrument and an array of thermistors, thermocouples, and resistant temperature detectors. LINK is a two-unit CubeSat and weighs two kilograms. It carries an ion-neutral mass spectrometer (INMS), which measures the mass of ions and neutral atoms, as the primary payload of the QB50 project. The secondary payload is two Langmuir probes, which are in-house sensors (m-NLP) developed by Professor Kyong Wook Min’s team of the Department of Physics at KAIST. These are all geared toward collecting long-term continuous in-situ measurements of conditions in Earth’s lower thermosphere.
Professor Bang said, “The QB50 Project is being used for educational purposes. However, the LINK launch will bring a new breakthrough toward collecting information on Earth’s lower thermosphere. Building on these experiences of designing and launching the CubeSat will serve as an opportunity to verify the research results made in our lab firsthand in space.”
(Caption: LINK (Little Intelligent Nanosatellite of KAIST) was launched on an Atlast V booster aboard the NASA CRS-7 Mission on April 18.)
2017.04.25 View 9586
KAIST Nanosatellite LINK Launched to the ISS
Courtesy: United Launch Alliance
The KAIST nanosatellite LINK (Little Intelligent Nanosatellite of KAIST) was successfully launched on an Atlas V booster aboard the NASA CRS-7 Mission on April 18 at Space Launch Complex 41, Cape Canaveral Air Force Station in Florida. The KAIST nanosatellite was developed by the research team led by Professor Hyochoong Bang of the Department of Aerospace Engineering.
Aboard the flight to the ISS (International Space Station) were 28 satellites including LINK. They are part of the QB50 Project, an international educational initiative which aims to deploy an array of CubeSat-mounted sensors into Earth’s thermosphere. The project is funded by the European Commission and managed by the von Karman Institute for Fluid Dynamics in Belgium.
The small satellites are hitching a lift into orbit aboard the unmanned resupply spacecraft Cygnus, with a total mass of 83 kilograms. Built to CubeSat specifications, Cygnus will deploy four of the spacecraft following its departure from the space station. LINK will conduct its scientific mission for three months at the station.
The majority of QB50 satellites carry one of three standard instrument packages, consisting of a primary instrument and an array of thermistors, thermocouples, and resistant temperature detectors. LINK is a two-unit CubeSat and weighs two kilograms. It carries an ion-neutral mass spectrometer (INMS), which measures the mass of ions and neutral atoms, as the primary payload of the QB50 project. The secondary payload is two Langmuir probes, which are in-house sensors (m-NLP) developed by Professor Kyong Wook Min’s team of the Department of Physics at KAIST. These are all geared toward collecting long-term continuous in-situ measurements of conditions in Earth’s lower thermosphere.
Professor Bang said, “The QB50 Project is being used for educational purposes. However, the LINK launch will bring a new breakthrough toward collecting information on Earth’s lower thermosphere. Building on these experiences of designing and launching the CubeSat will serve as an opportunity to verify the research results made in our lab firsthand in space.”
(Caption: LINK (Little Intelligent Nanosatellite of KAIST) was launched on an Atlast V booster aboard the NASA CRS-7 Mission on April 18.)
2017.04.25 View 9586 -
 Processable High Internal Phase Pickering Emulsion Using Depletion Attraction
Professor Siyoung Choi’s research team from the KAIST Department of Chemical & Biomolecular Engineering used physical force to successfully produce a stable emulsion.
Emulsions, commonly known as cosmetic products, refer to stably dispersed structures of oil droplets in water (or water droplets in oil). Pickering emulsions refer to emulsions stabilized using solid particles, instead of detergent. Traditionally, it is said that water and oil do not mix. Until recently, detergent was added to mix oil and water for dispersion. Emulsions have traditionally been produced using this technique and are currently used for products such as mayonnaise, sun block, and lotion.
On the other hand, Pickering emulsions have been used after stabilization of chemical treatments on solid particle surfaces to enhance adsorption power. However, there were limitations in its application, since the treatment process is complex and its applicable range remains limited. Instead of chemical treatment on Pickering emulsion surfaces, the research team mixed small macromolecules a few nanometer in size with larger solid particles (tens of nanometers to a few micrometers). This induced depletion force was used to successfully stabilize the emulsion.
Depletion force refers to the force a large number of small particles induces to aggregate the bigger particles, in order to secure free space for themselves. In short, the force induces an attraction between larger particles. Until now, depletion force could only be applied to solids and solid particles. However, the research team used macromolecules and large particles such as solid particles and oil droplets to show the applicability of depletion force between solids and liquids. By introducing macromolecules that act as smaller particles, hydrophilic solid particles enhanced the adsorption of solid particles to the oil droplet surface, while preventing dissociation from the particle surface, resulting in the maintenance of a stable state.
The research team confirmed the possibility of the simple production of various porous macromolecular materials using stable Pickering emulsions. Such porous macromolecules are expected to be applicable in separation film, systems engineering, drug delivery, and sensors, given their large surface area.
Professor KyuHan Kim, the first author said, “Until now, depletion force has only been used between solid colloid particles. This research has scientific significance since it is the first example of using depletion force between solid particles and liquid droplets.”
Professor Choi said, “Beyond its academic significance, this technology could contribute to industries and national competitiveness.” He continued, “Since this technology uses physical force, not chemical, to produce stable emulsion, it can be used regardless of the type of solid particle and macromolecule. Further, it could be used in customized porous material production for special purposes.”
The research was published in Nature Communications online on February 1. In particular, this research is significant since an undergraduate student, Subeen Kim, participated in the project as a second author through the KAIST Undergraduate Research Program (URP). This research was funded by the National Research Foundation of Korea.
(Figure 1: Images of the inner structure of porous macromolecules produced using the new technology)
(Figure 2: Images showing the measurement of rheological properties of Pickering emulsions and system processability)
(Figure 3: Images showing a stable Pickering emulsion system)
2017.04.19 View 9785
Processable High Internal Phase Pickering Emulsion Using Depletion Attraction
Professor Siyoung Choi’s research team from the KAIST Department of Chemical & Biomolecular Engineering used physical force to successfully produce a stable emulsion.
Emulsions, commonly known as cosmetic products, refer to stably dispersed structures of oil droplets in water (or water droplets in oil). Pickering emulsions refer to emulsions stabilized using solid particles, instead of detergent. Traditionally, it is said that water and oil do not mix. Until recently, detergent was added to mix oil and water for dispersion. Emulsions have traditionally been produced using this technique and are currently used for products such as mayonnaise, sun block, and lotion.
On the other hand, Pickering emulsions have been used after stabilization of chemical treatments on solid particle surfaces to enhance adsorption power. However, there were limitations in its application, since the treatment process is complex and its applicable range remains limited. Instead of chemical treatment on Pickering emulsion surfaces, the research team mixed small macromolecules a few nanometer in size with larger solid particles (tens of nanometers to a few micrometers). This induced depletion force was used to successfully stabilize the emulsion.
Depletion force refers to the force a large number of small particles induces to aggregate the bigger particles, in order to secure free space for themselves. In short, the force induces an attraction between larger particles. Until now, depletion force could only be applied to solids and solid particles. However, the research team used macromolecules and large particles such as solid particles and oil droplets to show the applicability of depletion force between solids and liquids. By introducing macromolecules that act as smaller particles, hydrophilic solid particles enhanced the adsorption of solid particles to the oil droplet surface, while preventing dissociation from the particle surface, resulting in the maintenance of a stable state.
The research team confirmed the possibility of the simple production of various porous macromolecular materials using stable Pickering emulsions. Such porous macromolecules are expected to be applicable in separation film, systems engineering, drug delivery, and sensors, given their large surface area.
Professor KyuHan Kim, the first author said, “Until now, depletion force has only been used between solid colloid particles. This research has scientific significance since it is the first example of using depletion force between solid particles and liquid droplets.”
Professor Choi said, “Beyond its academic significance, this technology could contribute to industries and national competitiveness.” He continued, “Since this technology uses physical force, not chemical, to produce stable emulsion, it can be used regardless of the type of solid particle and macromolecule. Further, it could be used in customized porous material production for special purposes.”
The research was published in Nature Communications online on February 1. In particular, this research is significant since an undergraduate student, Subeen Kim, participated in the project as a second author through the KAIST Undergraduate Research Program (URP). This research was funded by the National Research Foundation of Korea.
(Figure 1: Images of the inner structure of porous macromolecules produced using the new technology)
(Figure 2: Images showing the measurement of rheological properties of Pickering emulsions and system processability)
(Figure 3: Images showing a stable Pickering emulsion system)
2017.04.19 View 9785 -
 Tactile Sensor for Robot Skin Advanced by KAIST Team
The joint research team of Professors Jung Kim and Inkyu Park from the Department of Mechanical Engineering developed a tactile sensor that can act as skin for robots using silicon and carbon materials. This technology produced a sensor that can absorb shock and distinguish various forms of touch, and it is hoped to be used as robot skin in the future.
Skin serves an important role as the largest organ of the human body. As well as protecting major organs from external shock, skin also measures and distinguishes delicate tactile information and transfer it to the nervous system.
Current robotic sensory technology allows robots to have visual and auditory systems at nearly similar levels to human capacity, but there are limitations in tactile sensors that can detect changes in the environment throughout the body. To apply skin with similar functions as humans to robots, it is essential to develop skin sensor technology with high flexibility and high shock absorption. Another limitation for developing robot skin was connecting numerous sensors all over the body using electric wiring.
To overcome this problem, the research team combined silicon and carbon nanotubes (CNT) to produce a composite, which was then used in combination with a medical imaging technique called electrical impedance tomography (EIT). This led to technology that can distinguish various forms of force over a large area without electrical wiring.
The sensing material can distinguish the location and the size of various forms by touch, and thus can be applied to robot skin that can absorb shock as well as serves as a 3D computer interface and tactile sensor. It can withstand strong force such as a hammer strike, and can be re-used even after partial damage to the sensor by filling and hardening the damaged region with composite. Further, the sensor can be made by filling a 3D shape frame with silicon-nanotube composite. Using this technology, new forms of computer interaces can be developed with both curbed and flat surfaces.
This research was conducted through a collaboration between Professor Park, an expert in nanostructures and sensors, and Professor Kim, an expert in bio-robotics. Hence, the technology is likely to be applied in real products.
Professor Kim said, “Flexible tactile sensors can not only be directly adhered to the body, but they also provides information on modified states in multiple dimensions”. He continued, “This technology will contribute to the soft robot industry in the areas of robot skin and the field of wearable medical appliances.”
Professor Park said, “This technology implemented a next-generation user interface through the integration of functional nano-composite material and computer tomography.”
This research was published in Scientific Reports, a sister journal of Nature, online on January 25. This research was conducted as joint research by first author Hyo-Sang Lee, as well as Donguk Kwon and Ji-seung Cho, and was funded by the Ministry of Science, ICT and Future Planning.
(Fiigrue 1: Robotic hand responding to resistance via a connection with the developed tactile sensor)
(Figure 2: Manufacturing process for pressure-resistant composite using silicon rubber and carbon nanotubes)
(Figure 3: Computer interface using pressure-resistant composite)
2017.04.17 View 13313
Tactile Sensor for Robot Skin Advanced by KAIST Team
The joint research team of Professors Jung Kim and Inkyu Park from the Department of Mechanical Engineering developed a tactile sensor that can act as skin for robots using silicon and carbon materials. This technology produced a sensor that can absorb shock and distinguish various forms of touch, and it is hoped to be used as robot skin in the future.
Skin serves an important role as the largest organ of the human body. As well as protecting major organs from external shock, skin also measures and distinguishes delicate tactile information and transfer it to the nervous system.
Current robotic sensory technology allows robots to have visual and auditory systems at nearly similar levels to human capacity, but there are limitations in tactile sensors that can detect changes in the environment throughout the body. To apply skin with similar functions as humans to robots, it is essential to develop skin sensor technology with high flexibility and high shock absorption. Another limitation for developing robot skin was connecting numerous sensors all over the body using electric wiring.
To overcome this problem, the research team combined silicon and carbon nanotubes (CNT) to produce a composite, which was then used in combination with a medical imaging technique called electrical impedance tomography (EIT). This led to technology that can distinguish various forms of force over a large area without electrical wiring.
The sensing material can distinguish the location and the size of various forms by touch, and thus can be applied to robot skin that can absorb shock as well as serves as a 3D computer interface and tactile sensor. It can withstand strong force such as a hammer strike, and can be re-used even after partial damage to the sensor by filling and hardening the damaged region with composite. Further, the sensor can be made by filling a 3D shape frame with silicon-nanotube composite. Using this technology, new forms of computer interaces can be developed with both curbed and flat surfaces.
This research was conducted through a collaboration between Professor Park, an expert in nanostructures and sensors, and Professor Kim, an expert in bio-robotics. Hence, the technology is likely to be applied in real products.
Professor Kim said, “Flexible tactile sensors can not only be directly adhered to the body, but they also provides information on modified states in multiple dimensions”. He continued, “This technology will contribute to the soft robot industry in the areas of robot skin and the field of wearable medical appliances.”
Professor Park said, “This technology implemented a next-generation user interface through the integration of functional nano-composite material and computer tomography.”
This research was published in Scientific Reports, a sister journal of Nature, online on January 25. This research was conducted as joint research by first author Hyo-Sang Lee, as well as Donguk Kwon and Ji-seung Cho, and was funded by the Ministry of Science, ICT and Future Planning.
(Fiigrue 1: Robotic hand responding to resistance via a connection with the developed tactile sensor)
(Figure 2: Manufacturing process for pressure-resistant composite using silicon rubber and carbon nanotubes)
(Figure 3: Computer interface using pressure-resistant composite)
2017.04.17 View 13313 -
 Nuclease-Resistant Hybrid Nanoflowers
An eco-friendly method to synthesize DNA-copper nanoflowers with high load efficiencies, low cytotoxicity, and strong resistance against nucleases has been developed by Professor Hyun Gyu Park in the Department of Chemical and Biomolecular Engineering and his collaborators.
The research team successfully formed a flower-shaped nanostructure in an eco-friendly condition by using interactions between copper ions and DNA containing amide and amine groups. The resulting nanoflowers exhibit high DNA loading capacities in addition to low cytotoxicity.
Flower-shaped nanocrystals called nanoflowers have gained attention for their distinct features of high surface roughness and high surface area to volume ratios. The nanoflowers have been used in many areas including catalysis, electronics, and analytical chemistry.
Of late, research breakthroughs were made in the generation of hybrid inorganic-organic nanoflowers containing various enzymes as organic components. The hybridization with inorganic materials greatly enhanced enzymatic activity, stability, and durability compared to the corresponding free enzymes.
Generally, the formation of protein nanocrystals requires high heat treatment so it has limitations for achieving the high loading capacities of intact DNA.
The research team addressed the issue, focusing on the fact that nucleic acids with well-defined structures and selective recognition properties also contain amide and amine groups in their nucleobases. They proved that flower-like structures could be formed by using nucleic acids as a synthetic template, which paved the way to synthesize the hybrid nanoflowers containing DNA as an organic component in an eco-friendly condition.
The team also confirmed that this synthetic method can be universally applied to any DNA sequences containing amide and amine groups. They said their approach is quite unique considering that the majority of previous works focused on the utilization of DNA as a linker to assemble the nanomaterials. They said the method has several advantageous features. First, the ‘green’ synthetic procedure doesn’t involve any toxic chemicals, and shows low cytotoxicity and strong resistance against nucleases. Second, the obtained nanoflowers exhibit exceptionally high DNA loading capacities.
Above all, such superior features of hybrid nanoflowers enabled the sensitive detection of various molecules including phenol, hydrogen peroxide, and glucose. DNA-copper nanoflowers showed even higher peroxidase activity than those of protein-copper nanoflowers, which may be due to the larger surface area of the flower- shaped structures, creating a greater chance for applying them in the field of sensing of detection of hydrogen peroxide.
The research team expects that their research will create diverse applications in many areas including biosensors and will be further applied into therapeutic applications.
Professor Park said, “The inorganic component in the hybrid nanoflowers not only exhibits low cytotoxicity, but also protects the encapsulated DNA from being cleaved by endonuclease enzymes. Using this feature, the nanostructure will be applied into developing gene therapeutic carriers.”
This research was co-led by Professor Moon Il Kim at Gachon University and KAIST graduate Ki Soo Park, currently a professor at Konkuk University, is the first author. The research was featured as the front cover article of the Journal of Materials Chemistry B on March 28, Issue 12, published by the Royal Society of Chemistry.
The research was funded by the Mid-Career Researcher Support Program of the National Research Foundation of Korea and the Global Frontier Project of the Ministry of Science, ICT & Future Planning.
(Figure: (A) Schematic illustration of the formation of nuclease-resistant DNA–inorganic nanoflowers. (B) SEM images showing time-dependent growth of DNA-nanoflowers. The concentration of A-rich ssDNA (Table S1, ESI†) was 0.25 mM.)
2017.04.14 View 9953
Nuclease-Resistant Hybrid Nanoflowers
An eco-friendly method to synthesize DNA-copper nanoflowers with high load efficiencies, low cytotoxicity, and strong resistance against nucleases has been developed by Professor Hyun Gyu Park in the Department of Chemical and Biomolecular Engineering and his collaborators.
The research team successfully formed a flower-shaped nanostructure in an eco-friendly condition by using interactions between copper ions and DNA containing amide and amine groups. The resulting nanoflowers exhibit high DNA loading capacities in addition to low cytotoxicity.
Flower-shaped nanocrystals called nanoflowers have gained attention for their distinct features of high surface roughness and high surface area to volume ratios. The nanoflowers have been used in many areas including catalysis, electronics, and analytical chemistry.
Of late, research breakthroughs were made in the generation of hybrid inorganic-organic nanoflowers containing various enzymes as organic components. The hybridization with inorganic materials greatly enhanced enzymatic activity, stability, and durability compared to the corresponding free enzymes.
Generally, the formation of protein nanocrystals requires high heat treatment so it has limitations for achieving the high loading capacities of intact DNA.
The research team addressed the issue, focusing on the fact that nucleic acids with well-defined structures and selective recognition properties also contain amide and amine groups in their nucleobases. They proved that flower-like structures could be formed by using nucleic acids as a synthetic template, which paved the way to synthesize the hybrid nanoflowers containing DNA as an organic component in an eco-friendly condition.
The team also confirmed that this synthetic method can be universally applied to any DNA sequences containing amide and amine groups. They said their approach is quite unique considering that the majority of previous works focused on the utilization of DNA as a linker to assemble the nanomaterials. They said the method has several advantageous features. First, the ‘green’ synthetic procedure doesn’t involve any toxic chemicals, and shows low cytotoxicity and strong resistance against nucleases. Second, the obtained nanoflowers exhibit exceptionally high DNA loading capacities.
Above all, such superior features of hybrid nanoflowers enabled the sensitive detection of various molecules including phenol, hydrogen peroxide, and glucose. DNA-copper nanoflowers showed even higher peroxidase activity than those of protein-copper nanoflowers, which may be due to the larger surface area of the flower- shaped structures, creating a greater chance for applying them in the field of sensing of detection of hydrogen peroxide.
The research team expects that their research will create diverse applications in many areas including biosensors and will be further applied into therapeutic applications.
Professor Park said, “The inorganic component in the hybrid nanoflowers not only exhibits low cytotoxicity, but also protects the encapsulated DNA from being cleaved by endonuclease enzymes. Using this feature, the nanostructure will be applied into developing gene therapeutic carriers.”
This research was co-led by Professor Moon Il Kim at Gachon University and KAIST graduate Ki Soo Park, currently a professor at Konkuk University, is the first author. The research was featured as the front cover article of the Journal of Materials Chemistry B on March 28, Issue 12, published by the Royal Society of Chemistry.
The research was funded by the Mid-Career Researcher Support Program of the National Research Foundation of Korea and the Global Frontier Project of the Ministry of Science, ICT & Future Planning.
(Figure: (A) Schematic illustration of the formation of nuclease-resistant DNA–inorganic nanoflowers. (B) SEM images showing time-dependent growth of DNA-nanoflowers. The concentration of A-rich ssDNA (Table S1, ESI†) was 0.25 mM.)
2017.04.14 View 9953 -
 Improving Traffic Safety with a Crowdsourced Traffic Violation Reporting App
KAIST researchers revealed that crowdsourced traffic violation reporting with smartphone-based continuous video capturing can dramatically change the current practice of policing activities on the road and will significantly improve traffic safety.
Professor Uichin Lee of the Department of Industrial and Systems Engineering and the Graduate School of Knowledge Service Engineering at KAIST and his research team designed and evaluated Mobile Roadwatch, a mobile app that helps citizen record traffic violation with their smartphones and report the recorded videos to the police.
This app supports continuous video recording just like onboard vehicle dashboard cameras. Mobile Roadwatch allows drivers to safely capture traffic violations by simply touching a smartphone screen while driving. The captured videos are automatically tagged with contextual information such as location and time. This information will be used as important evidence for the police to ticket the violators. All of the captured videos can be conveniently reviewed, allowing users to decide which events to report to the police.
The team conducted a two-week field study to understand how drivers use Mobile Roadwatch. They found that the drivers tended to capture all traffic risks regardless of the level of their involvement and the seriousness of the traffic risks. However, when it came to actual reporting, they tended to report only serious traffic violations, which could have led to car accidents, such as traffic signal violations and illegal U-turns. After receiving feedback about their reports from the police, drivers typically felt very good about their contributions to traffic safety.
At the same time, some drivers felt pleased to know that the offenders received tickets since they thought these offenders deserved to be ticketed. While participating in the Mobile Roadwatch campaign, drivers reported that they tried to drive as safely as possible and abide by traffic laws. This was because they wanted to be as fair as possible so that they could capture others’ violations without feeling guilty. They were also afraid that other drivers might capture their violations.
Professor Lee said, “Our study participants answered that Mobile Roadwatch served as a very useful tool for reporting traffic violations, and they were highly satisfied with its features. Beyond simple reporting, our tool can be extended to support online communities, which help people actively discuss various local safety issues and work with the police and local authorities to solve these safety issues.”
Korea and India were the early adaptors supporting video-based reporting of traffic violations to the police. In recent years, the number of reports has dramatically increased. For example, Korea’s ‘Looking for a Witness’ (released in April 2015) received more than half million reported violations as of November 2016. In the US, authorities started tapping into smartphone recordings by releasing video-based reporting apps such as ICE Blackbox and Mobile Justice. Professor Lee said that the existing services cannot be used while driving, because none of the existing services support continuous video recording and safe event capturing behind the wheel.
Professor Lee’s team has been incorporating advanced computer vision techniques into Mobile Roadwatch for automatically capturing traffic violations and safety risks, including potholes and obstacles. The researchers will present their results in May at the ACM CHI Conference on Human Factors in Computing Systems (CHI 2017) in Denver, CO, USA. Their research was supported by the KAIST-KUSTAR fund.
(Caption: A driver is trying to capture an event by touching a screen. The Mobile Radwatch supports continuous video recording and safe event captureing behind the wheel.)
2017.04.10 View 11601
Improving Traffic Safety with a Crowdsourced Traffic Violation Reporting App
KAIST researchers revealed that crowdsourced traffic violation reporting with smartphone-based continuous video capturing can dramatically change the current practice of policing activities on the road and will significantly improve traffic safety.
Professor Uichin Lee of the Department of Industrial and Systems Engineering and the Graduate School of Knowledge Service Engineering at KAIST and his research team designed and evaluated Mobile Roadwatch, a mobile app that helps citizen record traffic violation with their smartphones and report the recorded videos to the police.
This app supports continuous video recording just like onboard vehicle dashboard cameras. Mobile Roadwatch allows drivers to safely capture traffic violations by simply touching a smartphone screen while driving. The captured videos are automatically tagged with contextual information such as location and time. This information will be used as important evidence for the police to ticket the violators. All of the captured videos can be conveniently reviewed, allowing users to decide which events to report to the police.
The team conducted a two-week field study to understand how drivers use Mobile Roadwatch. They found that the drivers tended to capture all traffic risks regardless of the level of their involvement and the seriousness of the traffic risks. However, when it came to actual reporting, they tended to report only serious traffic violations, which could have led to car accidents, such as traffic signal violations and illegal U-turns. After receiving feedback about their reports from the police, drivers typically felt very good about their contributions to traffic safety.
At the same time, some drivers felt pleased to know that the offenders received tickets since they thought these offenders deserved to be ticketed. While participating in the Mobile Roadwatch campaign, drivers reported that they tried to drive as safely as possible and abide by traffic laws. This was because they wanted to be as fair as possible so that they could capture others’ violations without feeling guilty. They were also afraid that other drivers might capture their violations.
Professor Lee said, “Our study participants answered that Mobile Roadwatch served as a very useful tool for reporting traffic violations, and they were highly satisfied with its features. Beyond simple reporting, our tool can be extended to support online communities, which help people actively discuss various local safety issues and work with the police and local authorities to solve these safety issues.”
Korea and India were the early adaptors supporting video-based reporting of traffic violations to the police. In recent years, the number of reports has dramatically increased. For example, Korea’s ‘Looking for a Witness’ (released in April 2015) received more than half million reported violations as of November 2016. In the US, authorities started tapping into smartphone recordings by releasing video-based reporting apps such as ICE Blackbox and Mobile Justice. Professor Lee said that the existing services cannot be used while driving, because none of the existing services support continuous video recording and safe event capturing behind the wheel.
Professor Lee’s team has been incorporating advanced computer vision techniques into Mobile Roadwatch for automatically capturing traffic violations and safety risks, including potholes and obstacles. The researchers will present their results in May at the ACM CHI Conference on Human Factors in Computing Systems (CHI 2017) in Denver, CO, USA. Their research was supported by the KAIST-KUSTAR fund.
(Caption: A driver is trying to capture an event by touching a screen. The Mobile Radwatch supports continuous video recording and safe event captureing behind the wheel.)
2017.04.10 View 11601 -
 Crowdsourcing-Based Global Indoor Positioning System
Research team of Professor Dong-Soo Han of the School of Computing Intelligent Service Lab at KAIST developed a system for providing global indoor localization using Wi-Fi signals. The technology uses numerous smartphones to collect fingerprints of location data and label them automatically, significantly reducing the cost of constructing an indoor localization system while maintaining high accuracy.
The method can be used in any building in the world, provided the floor plan is available and there are Wi-Fi fingerprints to collect. To accurately collect and label the location information of the Wi-Fi fingerprints, the research team analyzed indoor space utilization. This led to technology that classified indoor spaces into places used for stationary tasks (resting spaces) and spaces used to reach said places (transient spaces), and utilized separate algorithms to optimally and automatically collect location labelling data.
Years ago, the team implemented a way to automatically label resting space locations from signals collected in various contexts such as homes, shops, and offices via the users’ home or office address information. The latest method allows for the automatic labelling of transient space locations such as hallways, lobbies, and stairs using unsupervised learning, without any additional location information. Testing in KAIST’s N5 building and the 7th floor of N1 building manifested the technology is capable of accuracy up to three or four meters given enough training data. The accuracy level is comparable to technology using manually-labeled location information.
Google, Microsoft, and other multinational corporations collected tens of thousands of floor plans for their indoor localization projects. Indoor radio map construction was also attempted by the firms but proved more difficult. As a result, existing indoor localization services were often plagued by inaccuracies. In Korea, COEX, Lotte World Tower, and other landmarks provide comparatively accurate indoor localization, but most buildings suffer from the lack of radio maps, preventing indoor localization services.
Professor Han said, “This technology allows the easy deployment of highly accurate indoor localization systems in any building in the world. In the near future, most indoor spaces will be able to provide localization services, just like outdoor spaces.” He further added that smartphone-collected Wi-Fi fingerprints have been unutilized and often discarded, but now they should be treated as invaluable resources, which create a new big data field of Wi-Fi fingerprints. This new indoor navigation technology is likely to be valuable to Google, Apple, or other global firms providing indoor positioning services globally. The technology will also be valuable for helping domestic firms provide positioning services.
Professor Han added that “the new global indoor localization system deployment technology will be added to KAILOS, KAIST’s indoor localization system.” KAILOS was released in 2014 as KAIST’s open platform for indoor localization service, allowing anyone in the world to add floor plans to KAILOS, and collect the building’s Wi-Fi fingerprints for a universal indoor localization service. As localization accuracy improves in indoor environments, despite the absence of GPS signals, applications such as location-based SNS, location-based IoT, and location-based O2O are expected to take off, leading to various improvements in convenience and safety. Integrated indoor-outdoor navigation services are also visible on the horizon, fusing vehicular navigation technology with indoor navigation.
Professor Han’s research was published in IEEE Transactions on Mobile Computing (TMC) in November in 2016.
For more, please visit http://ieeexplore.ieee.org/stamp/stamp.jsp?arnumber=7349230http://ieeexplore.ieee.org/document/7805133/
2017.04.06 View 10542
Crowdsourcing-Based Global Indoor Positioning System
Research team of Professor Dong-Soo Han of the School of Computing Intelligent Service Lab at KAIST developed a system for providing global indoor localization using Wi-Fi signals. The technology uses numerous smartphones to collect fingerprints of location data and label them automatically, significantly reducing the cost of constructing an indoor localization system while maintaining high accuracy.
The method can be used in any building in the world, provided the floor plan is available and there are Wi-Fi fingerprints to collect. To accurately collect and label the location information of the Wi-Fi fingerprints, the research team analyzed indoor space utilization. This led to technology that classified indoor spaces into places used for stationary tasks (resting spaces) and spaces used to reach said places (transient spaces), and utilized separate algorithms to optimally and automatically collect location labelling data.
Years ago, the team implemented a way to automatically label resting space locations from signals collected in various contexts such as homes, shops, and offices via the users’ home or office address information. The latest method allows for the automatic labelling of transient space locations such as hallways, lobbies, and stairs using unsupervised learning, without any additional location information. Testing in KAIST’s N5 building and the 7th floor of N1 building manifested the technology is capable of accuracy up to three or four meters given enough training data. The accuracy level is comparable to technology using manually-labeled location information.
Google, Microsoft, and other multinational corporations collected tens of thousands of floor plans for their indoor localization projects. Indoor radio map construction was also attempted by the firms but proved more difficult. As a result, existing indoor localization services were often plagued by inaccuracies. In Korea, COEX, Lotte World Tower, and other landmarks provide comparatively accurate indoor localization, but most buildings suffer from the lack of radio maps, preventing indoor localization services.
Professor Han said, “This technology allows the easy deployment of highly accurate indoor localization systems in any building in the world. In the near future, most indoor spaces will be able to provide localization services, just like outdoor spaces.” He further added that smartphone-collected Wi-Fi fingerprints have been unutilized and often discarded, but now they should be treated as invaluable resources, which create a new big data field of Wi-Fi fingerprints. This new indoor navigation technology is likely to be valuable to Google, Apple, or other global firms providing indoor positioning services globally. The technology will also be valuable for helping domestic firms provide positioning services.
Professor Han added that “the new global indoor localization system deployment technology will be added to KAILOS, KAIST’s indoor localization system.” KAILOS was released in 2014 as KAIST’s open platform for indoor localization service, allowing anyone in the world to add floor plans to KAILOS, and collect the building’s Wi-Fi fingerprints for a universal indoor localization service. As localization accuracy improves in indoor environments, despite the absence of GPS signals, applications such as location-based SNS, location-based IoT, and location-based O2O are expected to take off, leading to various improvements in convenience and safety. Integrated indoor-outdoor navigation services are also visible on the horizon, fusing vehicular navigation technology with indoor navigation.
Professor Han’s research was published in IEEE Transactions on Mobile Computing (TMC) in November in 2016.
For more, please visit http://ieeexplore.ieee.org/stamp/stamp.jsp?arnumber=7349230http://ieeexplore.ieee.org/document/7805133/
2017.04.06 View 10542 -
 Improving Silver Nanowires for FTCEs with Flash Light Interactions
Flexible transparent conducting electrodes (FTCEs) are an essential element of flexible optoelectronics for next-generation wearable displays, augmented reality (AR), and the Internet of Things (IoTs). Silver nanowires (Ag NWs) have received a great deal of attention as future FTCEs due to their great flexibility, material stability, and large-scale productivity. Despite these advantages, Ag NWs have drawbacks such as high wire-to-wire contact resistance and poor adhesion to substrates, resulting in severe power consumption and the delamination of FTCEs.
A research team led by Professor Keon Jae Lee of the Materials Science and Engineering Department at KAIST and Dr. Hong-Jin Park from BSP Inc., has developed high-performance Ag NWs (sheet resistance ~ 5 Ω/sq, transmittance 90 % at λ = 550 nm) with strong adhesion on plastic (interfacial energy of 30.7 J∙m-2) using flash light-material interactions.
The broad ultraviolet (UV) spectrum of a flash light enables the localized heating at the junctions of nanowires (NWs), which results in the fast and complete welding of Ag NWs. Consequently, the Ag NWs demonstrate six times higher conductivity than that of the pristine NWs. In addition, the near-infrared (NIR) of the flash lamp melted the interface between the Ag NWs and a polyethylene terephthalate (PET) substrate, dramatically enhancing the adhesion force of the Ag NWs to the PET by 310 %.
Professor Lee said, “Light interaction with nanomaterials is an important field for future flexible electronics since it can overcome thermal limit of plastics, and we are currently expanding our research into light-inorganic interactions.”
Meanwhile, BSP Inc., a laser manufacturing company and a collaborator of this work, has launched new flash lamp equipment for flexible applications based on the Professor Lee’s research.
The results of this work entitled “Flash-Induced Self-Limited Plasmonic Welding of Ag NW Network for Transparent Flexible Energy Harvester (DOI: 10.1002/adma.201603473)” were published in the February 2, 2017 issue of Advanced Materials as the cover article.
Professor Lee also contributed an invited review in the same journal of the April 3, 2017 online issue, “Laser-Material Interactions for Flexible Applications (DOI:10.1002/adma.201606586),” overviewing the recent advances in light interactions with flexible nanomaterials.
References
[1] Advanced Materials, February 2, 2017, Flash-Induced Self-Limited Plasmonic Welding of Ag NW network for Transparent Flexible Energy Harvester http://onlinelibrary.wiley.com/doi/10.1002/adma.201603473/epdf
[2] Advanced Materials, April 3, 2017, Laser-Material Interactions for Flexible Applications
http://onlinelibrary.wiley.com/doi/10.1002/adma.201606586/abstract
For further inquiries on research:
keonlee@kaist.ac.kr (Keon Jae Lee), hjpark@bsptech.co.kr (Hong-Jin Park)
Picture 1: Artistic Rendtition of Light Interaction with Nanomaterials
(This image shows flash-induced plasmonic interactions with nanowires to improve silver nanowires (Ag NWs).)
Picture 2: Ag NW/PET Film
(This picture shows the Ag NWs on a polyethylene terephthalate (PET) film after the flash-induced plasmonic thermal process.)
2017.04.05 View 11548
Improving Silver Nanowires for FTCEs with Flash Light Interactions
Flexible transparent conducting electrodes (FTCEs) are an essential element of flexible optoelectronics for next-generation wearable displays, augmented reality (AR), and the Internet of Things (IoTs). Silver nanowires (Ag NWs) have received a great deal of attention as future FTCEs due to their great flexibility, material stability, and large-scale productivity. Despite these advantages, Ag NWs have drawbacks such as high wire-to-wire contact resistance and poor adhesion to substrates, resulting in severe power consumption and the delamination of FTCEs.
A research team led by Professor Keon Jae Lee of the Materials Science and Engineering Department at KAIST and Dr. Hong-Jin Park from BSP Inc., has developed high-performance Ag NWs (sheet resistance ~ 5 Ω/sq, transmittance 90 % at λ = 550 nm) with strong adhesion on plastic (interfacial energy of 30.7 J∙m-2) using flash light-material interactions.
The broad ultraviolet (UV) spectrum of a flash light enables the localized heating at the junctions of nanowires (NWs), which results in the fast and complete welding of Ag NWs. Consequently, the Ag NWs demonstrate six times higher conductivity than that of the pristine NWs. In addition, the near-infrared (NIR) of the flash lamp melted the interface between the Ag NWs and a polyethylene terephthalate (PET) substrate, dramatically enhancing the adhesion force of the Ag NWs to the PET by 310 %.
Professor Lee said, “Light interaction with nanomaterials is an important field for future flexible electronics since it can overcome thermal limit of plastics, and we are currently expanding our research into light-inorganic interactions.”
Meanwhile, BSP Inc., a laser manufacturing company and a collaborator of this work, has launched new flash lamp equipment for flexible applications based on the Professor Lee’s research.
The results of this work entitled “Flash-Induced Self-Limited Plasmonic Welding of Ag NW Network for Transparent Flexible Energy Harvester (DOI: 10.1002/adma.201603473)” were published in the February 2, 2017 issue of Advanced Materials as the cover article.
Professor Lee also contributed an invited review in the same journal of the April 3, 2017 online issue, “Laser-Material Interactions for Flexible Applications (DOI:10.1002/adma.201606586),” overviewing the recent advances in light interactions with flexible nanomaterials.
References
[1] Advanced Materials, February 2, 2017, Flash-Induced Self-Limited Plasmonic Welding of Ag NW network for Transparent Flexible Energy Harvester http://onlinelibrary.wiley.com/doi/10.1002/adma.201603473/epdf
[2] Advanced Materials, April 3, 2017, Laser-Material Interactions for Flexible Applications
http://onlinelibrary.wiley.com/doi/10.1002/adma.201606586/abstract
For further inquiries on research:
keonlee@kaist.ac.kr (Keon Jae Lee), hjpark@bsptech.co.kr (Hong-Jin Park)
Picture 1: Artistic Rendtition of Light Interaction with Nanomaterials
(This image shows flash-induced plasmonic interactions with nanowires to improve silver nanowires (Ag NWs).)
Picture 2: Ag NW/PET Film
(This picture shows the Ag NWs on a polyethylene terephthalate (PET) film after the flash-induced plasmonic thermal process.)
2017.04.05 View 11548 -
 Expanding the Genetic Code of Mus Musculus
Professor Hee-Sung Park of the Department of Chemistry, who garnered attention for his novel strategy of installing authentic post-translational modifications into recombinant proteins, expanded his research portfolio to another level. Professor Park’s team was the first to report the generation of a mouse strain with an expanded genetic code, allowing site-specific incorporation of unnatural amino acids.
Professor Park published the research on the new chemical biology method for achieving selective chemical modifications in proteins in Science last September. The research team, this time in collaboration with Professor Chan Bae Park of the Department of Physiology at the Ajou University School of Medicine, demonstrated temporal and spatial control of protein acetylation in various organs of the transgenic mouse using a recombinant green fluorescent protein as a model protein. This research was published in the online edition of Nature Communications on February 21.
This approach enables the rapid onset of position-specific acetylation of a target protein at any developmental stage, facilitating temporal and spatial control of protein acetylation in various organs of the transgenic mouse. Such temporal and spatial control of protein acetylation will be of prime importance for investigating many essential biological processes and human diseases at the tissue and organism level.
Almost all human proteins, the products of about 25,000 genes, are known to undergo various post-translational modifications during and after synthesis. Post-translation modifications regulate the function of cellular proteins, playing a key role in many essential processes such as delivering signals and body growth. However, the unusual protein modifications, aroused from genetic and/or environmental factors, trigger severe diseases including cancer, dementia, and diabetes.
The team inserted transgenes into the mouse genome to allocate the site-specific addition of unnatural amino acids. The researchers inserted a modified version of lysine into the house mice, which allowed for the control of the acetylation. They used recombinant green fluorescent proteins from transgenic house mice as models for control of the acetylation.
The team was also able to regulate the acetylation of specific temporal and spatial frames in the mice, restraining the abnormality in proteins to certain organs such as the liver and kidneys. The research team said the strategy will provide a powerful tool for systematic in vivo study of cellular proteins in the most commonly used mammalian model organisms for human physiology and disease. Professor Park said, “This method can be easily extended to generate a wide range of custom-made transgenic mouse strains for further investigating diverse proteins of interest.” He added, “This method can be further extended to generate a wide range of custom-made transgenic mouse strains, opening a new paradigm for investigating anti-cancer and cerebral disease treatments.
This work was supported by grants from KAIST Systems Healthcare and the Medicinal Bioconvergence Research Center and the Intelligent Synthetic Biology Center of the Global Frontier Project funded by the Ministry of Science, ICT & Future Planning and the Ministry of Food and Drug Safety.
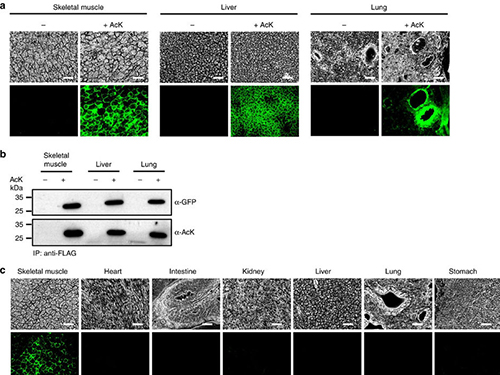
(Figure:Temporal and spatial control of in vivo protein acetylation)
(a) Temporal expression of acetylated GFPuv in the AcK-GFPamber mouse. The expression of GFPuv in skeletal muscle, liver, and lung tissues was detected only in the AcK-injected mouse. Scale bar, 200 µm. (b) Western blotting of anti-FLAG-immunoprecipitated proteins from tissues of the AcK-GFPamber mouse. Acetylated GFPuv was produced after AcK injection. (c) Spatial expression of acetylated GFPuv in the AcK-GFPamber mouse. Acetylated GFPuv was observed only in skeletal muscle when AcK was directly delivered to the tissues. Sacle bar, 200 µm.
2017.03.27 View 10524
Expanding the Genetic Code of Mus Musculus
Professor Hee-Sung Park of the Department of Chemistry, who garnered attention for his novel strategy of installing authentic post-translational modifications into recombinant proteins, expanded his research portfolio to another level. Professor Park’s team was the first to report the generation of a mouse strain with an expanded genetic code, allowing site-specific incorporation of unnatural amino acids.
Professor Park published the research on the new chemical biology method for achieving selective chemical modifications in proteins in Science last September. The research team, this time in collaboration with Professor Chan Bae Park of the Department of Physiology at the Ajou University School of Medicine, demonstrated temporal and spatial control of protein acetylation in various organs of the transgenic mouse using a recombinant green fluorescent protein as a model protein. This research was published in the online edition of Nature Communications on February 21.
This approach enables the rapid onset of position-specific acetylation of a target protein at any developmental stage, facilitating temporal and spatial control of protein acetylation in various organs of the transgenic mouse. Such temporal and spatial control of protein acetylation will be of prime importance for investigating many essential biological processes and human diseases at the tissue and organism level.
Almost all human proteins, the products of about 25,000 genes, are known to undergo various post-translational modifications during and after synthesis. Post-translation modifications regulate the function of cellular proteins, playing a key role in many essential processes such as delivering signals and body growth. However, the unusual protein modifications, aroused from genetic and/or environmental factors, trigger severe diseases including cancer, dementia, and diabetes.
The team inserted transgenes into the mouse genome to allocate the site-specific addition of unnatural amino acids. The researchers inserted a modified version of lysine into the house mice, which allowed for the control of the acetylation. They used recombinant green fluorescent proteins from transgenic house mice as models for control of the acetylation.
The team was also able to regulate the acetylation of specific temporal and spatial frames in the mice, restraining the abnormality in proteins to certain organs such as the liver and kidneys. The research team said the strategy will provide a powerful tool for systematic in vivo study of cellular proteins in the most commonly used mammalian model organisms for human physiology and disease. Professor Park said, “This method can be easily extended to generate a wide range of custom-made transgenic mouse strains for further investigating diverse proteins of interest.” He added, “This method can be further extended to generate a wide range of custom-made transgenic mouse strains, opening a new paradigm for investigating anti-cancer and cerebral disease treatments.
This work was supported by grants from KAIST Systems Healthcare and the Medicinal Bioconvergence Research Center and the Intelligent Synthetic Biology Center of the Global Frontier Project funded by the Ministry of Science, ICT & Future Planning and the Ministry of Food and Drug Safety.
(Figure:Temporal and spatial control of in vivo protein acetylation)
(a) Temporal expression of acetylated GFPuv in the AcK-GFPamber mouse. The expression of GFPuv in skeletal muscle, liver, and lung tissues was detected only in the AcK-injected mouse. Scale bar, 200 µm. (b) Western blotting of anti-FLAG-immunoprecipitated proteins from tissues of the AcK-GFPamber mouse. Acetylated GFPuv was produced after AcK injection. (c) Spatial expression of acetylated GFPuv in the AcK-GFPamber mouse. Acetylated GFPuv was observed only in skeletal muscle when AcK was directly delivered to the tissues. Sacle bar, 200 µm.
2017.03.27 View 10524 -
 First Mutations in Human Life Discovered
The earliest mutations of human life have been observed by research team led by the Wellcome Trust Sanger Institute and their collaborators. Analyzing genomes from adult cells, the scientists could look back in time to reveal how each embryo developed.
Research team of the Sanger Institute including Professor Young Seok Ju of the Graduate School of Medical Science and Engineering at KAIST published an article of “Somatic Mutations Reveal Asymmetric Cellular Dynamics in the Early Human Embryo” in Nature on March 22.
The study shows that from the two-cell stage of the human embryo, one of these cells becomes more dominant than the other and leads to a higher proportion of the adult body.
A longstanding question for researchers has been what happens in the very early human development as this has proved impossible to study directly. Now, researchers have analyzed the whole genome sequences of blood samples (collected from 279 individuals with breast cancer) and discovered 163 mutations that occurred very early in the embryonic development of those people.
Once identified, the researchers used mutations from the first, second and third divisions of the fertilized egg to calculate which proportion of adult cells resulted from each of the first two cells in the embryo. They found that these first two cells contribute differently to the whole body. One cell gives rise to about 70 percent of the adult body tissues, whereas the other cell has a more minor contribution, leading to about 30percent of the tissues. This skewed contribution continues for some cells in the second and third generation too.
Originally pinpointed in normal blood cells from cancer patients, the researchers then looked for these mutations in cancer samples that had been surgically removed from the patients during treatment. Unlike normal tissues composed of multiple somatic cell clones, a cancer develops from one mutant cell. Therefore, each proposed embryonic mutation should either be present in all of the cancer cells in a tumor, or none of them. This proved to be the case, and by using these cancer samples, the researchers were able to validate that the mutations had originated during early development.
Dr. Young Seok Ju, first author from the Wellcome Trust Sanger Institute and KAIST, said: "This is the first time that anyone has seen where mutations arise in the very early human development. It is like finding a needle in a haystack. There are just a handful of these mutations, compared with millions of inherited genetic variations, and finding them allowed us to track what happened during embryogenesis."
Dr. Inigo Martincorena, from the Sanger Institute, said: "Having identified the mutations, we were able to use statistical analysis to better understand cell dynamics during embryo development. We determined the relative contribution of the first embryonic cells to the adult blood cell pool and found one dominant cell - that led to 70 percent of the blood cells - and one minor cell. We also sequenced normal lymph and breast cells, and the results suggested that the dominant cell also contributes to these other tissues at a similar level. This opens an unprecedented window into the earliest stages of human development."
During this study, the researchers were also able to measure the rate of mutation in early human development for the first time, up to three generations of cell division. Previous researchers had estimated one mutation per cell division, but this study measured three mutations for each cell doubling, in every daughter cell.
Mutations during the development of the embryo occur by two processes - known as mutational signatures 1 and 5. These mutations are fairly randomly distributed through the genome, and the vast majority of them will not affect the developing embryo. However, a mutation that occurs in an important gene can lead to disease such as developmental disorders.
Professor Sir Mike Stratton, lead author on the paper and Director of the Sanger Institute, said: "This is a significant step forward in widening the range of biological insights that can be extracted using genome sequences and mutations. Essentially, the mutations are archaeological traces of embryonic development left in our adult tissues, so if we can find and interpret them, we can understand human embryology better. This is just one early insight into human development, with hopefully many more to come in the future."
(Figure 1. Detection of somatic mutations acquired in early human embryogenesis )
(Figure 2. Unequal contributions of early embryonic cells to adult somatic tissues )
2017.03.23 View 8337
First Mutations in Human Life Discovered
The earliest mutations of human life have been observed by research team led by the Wellcome Trust Sanger Institute and their collaborators. Analyzing genomes from adult cells, the scientists could look back in time to reveal how each embryo developed.
Research team of the Sanger Institute including Professor Young Seok Ju of the Graduate School of Medical Science and Engineering at KAIST published an article of “Somatic Mutations Reveal Asymmetric Cellular Dynamics in the Early Human Embryo” in Nature on March 22.
The study shows that from the two-cell stage of the human embryo, one of these cells becomes more dominant than the other and leads to a higher proportion of the adult body.
A longstanding question for researchers has been what happens in the very early human development as this has proved impossible to study directly. Now, researchers have analyzed the whole genome sequences of blood samples (collected from 279 individuals with breast cancer) and discovered 163 mutations that occurred very early in the embryonic development of those people.
Once identified, the researchers used mutations from the first, second and third divisions of the fertilized egg to calculate which proportion of adult cells resulted from each of the first two cells in the embryo. They found that these first two cells contribute differently to the whole body. One cell gives rise to about 70 percent of the adult body tissues, whereas the other cell has a more minor contribution, leading to about 30percent of the tissues. This skewed contribution continues for some cells in the second and third generation too.
Originally pinpointed in normal blood cells from cancer patients, the researchers then looked for these mutations in cancer samples that had been surgically removed from the patients during treatment. Unlike normal tissues composed of multiple somatic cell clones, a cancer develops from one mutant cell. Therefore, each proposed embryonic mutation should either be present in all of the cancer cells in a tumor, or none of them. This proved to be the case, and by using these cancer samples, the researchers were able to validate that the mutations had originated during early development.
Dr. Young Seok Ju, first author from the Wellcome Trust Sanger Institute and KAIST, said: "This is the first time that anyone has seen where mutations arise in the very early human development. It is like finding a needle in a haystack. There are just a handful of these mutations, compared with millions of inherited genetic variations, and finding them allowed us to track what happened during embryogenesis."
Dr. Inigo Martincorena, from the Sanger Institute, said: "Having identified the mutations, we were able to use statistical analysis to better understand cell dynamics during embryo development. We determined the relative contribution of the first embryonic cells to the adult blood cell pool and found one dominant cell - that led to 70 percent of the blood cells - and one minor cell. We also sequenced normal lymph and breast cells, and the results suggested that the dominant cell also contributes to these other tissues at a similar level. This opens an unprecedented window into the earliest stages of human development."
During this study, the researchers were also able to measure the rate of mutation in early human development for the first time, up to three generations of cell division. Previous researchers had estimated one mutation per cell division, but this study measured three mutations for each cell doubling, in every daughter cell.
Mutations during the development of the embryo occur by two processes - known as mutational signatures 1 and 5. These mutations are fairly randomly distributed through the genome, and the vast majority of them will not affect the developing embryo. However, a mutation that occurs in an important gene can lead to disease such as developmental disorders.
Professor Sir Mike Stratton, lead author on the paper and Director of the Sanger Institute, said: "This is a significant step forward in widening the range of biological insights that can be extracted using genome sequences and mutations. Essentially, the mutations are archaeological traces of embryonic development left in our adult tissues, so if we can find and interpret them, we can understand human embryology better. This is just one early insight into human development, with hopefully many more to come in the future."
(Figure 1. Detection of somatic mutations acquired in early human embryogenesis )
(Figure 2. Unequal contributions of early embryonic cells to adult somatic tissues )
2017.03.23 View 8337 -
 Furniture That Learns to Move by Itself
A novel strategy for displacing large objects by attaching relatively small vibration sources. After learning how several random bursts of vibration affect an object's pose, an optimization algorithm discovers the optimal sequence of vibration patterns required to (slowly but surely) move the object to a specified position. Displacements of large objects induced by vibration are a common occurrence, but generally result in unpredictable motion. Think, for instance, of an unbalanced front-loading washing machine. For controlled movement, wheels or legs are usually preferred.
Professor Daniel Saakes of the Department of Industrial Design and his team explored a strategy for moving everyday objects by harvesting external vibration rather than using a mechanical system with wheels. This principle may be useful for displacing large objects in situations where attaching wheels or complete lifting is impossible – assuming the speed of the process is not a concern.
His team designed vibration modules that can be easily attached to furniture and objects, and this could be a welcomed creation for people with limited mobility, including the elderly. Embedding these vibration modules as part of mass-produced objects may provide a low-cost way to make almost any object mobile.
Vibration as a principle for directed locomotion has been previously applied in micro-robots. For instance, the three-legged Kilobots move thanks to centrifugal forces alternatively generated by a pair of vibrations on two of its legs. The unbalanced weight transforms the robot into a ratchet and the resulting motion is deterministic with respect to the input vibration. To the best of our knowledge, we are the first to add vibratory actuators to deterministically steer large objects regardless of their structural properties.
The perturbation resulting from a particular pattern of vibration depends on a myriad of parameters, including but not limited to the microscopic properties of the contact surfaces. The key challenge is to empirically discover and select the sequence of vibration patterns to bring the object to the target pose.
Their approach is as follows. In the first step we systematically explore the object’s response by manipulating the amplitudes of the motors. This generates a pool of available moves (translations and rotations). We then calculate from this pool the most efficient way (either in terms of length or number of moves) to go from pose A to pose B using optimization strategies, such as genetic algorithms. The learning process may be repeated from time to time to account for changes in the mechanical response, at least for the patterns of vibration that contribute more to the change.
Prototype modules are made with eccentric rotating motors (type 345-002 Precision Microdrive) with a nominal force of 115g, which proved sufficient to shake (and eventually locomote) four-legged IKEA chairs and small furniture such as tables and stools. The motors are powered by NiMH batteries and communicate wirelessly with a low-cost ESP8266 WiFi module. The team designed modules that are externally attached using straps as well as motors embedded in furniture.
To study the general method, the team employed an overhead camera to track the chair and generate the pool of available moves. The team demonstrated that the system discovered pivot-like gaits and others. However, as one can imagine, using a pre-computed sequence to move to a target pose does not end up providing perfect matches. This is because the contact properties vary with location. Although this can be considered a secondary disturbance, it may in certain cases be mandatory to recompute the matrix of moves every now and then. The chair could, for instance, move into a wet area, over plastic carpet, etc.
The principle and application in furniture is called “ratchair” as a portmanteau combining “Ratchet” and “Chair”. Ratchair was demonstrated at the 2016 ACM Siggraph Emerging Technologies and won the DC-EXPO award jointly organized by the Japanese Ministry of Economy, Trade and Industry (METI) and the Digital Content Association of Japan (DCAJ). At the DCEXPO Exhibition, Fall 2016, the work was one of 20 Innovative Technologies and the only non-Japanese contribution.
*This article is from the KAIST Breakthroughs, research newsletter from the College of Engineering.
For more stories of the KAIST Breakthroughs, please visit http://breakthroughs.kaist.ac.kr
http://mid.kaist.ac.kr/projects/ratchair/
http://s2016.siggraph.org/content/emerging-technologies
https://www.dcexpo.jp/ko/15184
Figure 1. The vibration modules embedded and attached to furniture.
Figure 2. A close-up of the vibration module.
Figure 3. A close-up of the embedded modules.
Figure 4. A close-up of the vibration motor.
2017.03.23 View 9965
Furniture That Learns to Move by Itself
A novel strategy for displacing large objects by attaching relatively small vibration sources. After learning how several random bursts of vibration affect an object's pose, an optimization algorithm discovers the optimal sequence of vibration patterns required to (slowly but surely) move the object to a specified position. Displacements of large objects induced by vibration are a common occurrence, but generally result in unpredictable motion. Think, for instance, of an unbalanced front-loading washing machine. For controlled movement, wheels or legs are usually preferred.
Professor Daniel Saakes of the Department of Industrial Design and his team explored a strategy for moving everyday objects by harvesting external vibration rather than using a mechanical system with wheels. This principle may be useful for displacing large objects in situations where attaching wheels or complete lifting is impossible – assuming the speed of the process is not a concern.
His team designed vibration modules that can be easily attached to furniture and objects, and this could be a welcomed creation for people with limited mobility, including the elderly. Embedding these vibration modules as part of mass-produced objects may provide a low-cost way to make almost any object mobile.
Vibration as a principle for directed locomotion has been previously applied in micro-robots. For instance, the three-legged Kilobots move thanks to centrifugal forces alternatively generated by a pair of vibrations on two of its legs. The unbalanced weight transforms the robot into a ratchet and the resulting motion is deterministic with respect to the input vibration. To the best of our knowledge, we are the first to add vibratory actuators to deterministically steer large objects regardless of their structural properties.
The perturbation resulting from a particular pattern of vibration depends on a myriad of parameters, including but not limited to the microscopic properties of the contact surfaces. The key challenge is to empirically discover and select the sequence of vibration patterns to bring the object to the target pose.
Their approach is as follows. In the first step we systematically explore the object’s response by manipulating the amplitudes of the motors. This generates a pool of available moves (translations and rotations). We then calculate from this pool the most efficient way (either in terms of length or number of moves) to go from pose A to pose B using optimization strategies, such as genetic algorithms. The learning process may be repeated from time to time to account for changes in the mechanical response, at least for the patterns of vibration that contribute more to the change.
Prototype modules are made with eccentric rotating motors (type 345-002 Precision Microdrive) with a nominal force of 115g, which proved sufficient to shake (and eventually locomote) four-legged IKEA chairs and small furniture such as tables and stools. The motors are powered by NiMH batteries and communicate wirelessly with a low-cost ESP8266 WiFi module. The team designed modules that are externally attached using straps as well as motors embedded in furniture.
To study the general method, the team employed an overhead camera to track the chair and generate the pool of available moves. The team demonstrated that the system discovered pivot-like gaits and others. However, as one can imagine, using a pre-computed sequence to move to a target pose does not end up providing perfect matches. This is because the contact properties vary with location. Although this can be considered a secondary disturbance, it may in certain cases be mandatory to recompute the matrix of moves every now and then. The chair could, for instance, move into a wet area, over plastic carpet, etc.
The principle and application in furniture is called “ratchair” as a portmanteau combining “Ratchet” and “Chair”. Ratchair was demonstrated at the 2016 ACM Siggraph Emerging Technologies and won the DC-EXPO award jointly organized by the Japanese Ministry of Economy, Trade and Industry (METI) and the Digital Content Association of Japan (DCAJ). At the DCEXPO Exhibition, Fall 2016, the work was one of 20 Innovative Technologies and the only non-Japanese contribution.
*This article is from the KAIST Breakthroughs, research newsletter from the College of Engineering.
For more stories of the KAIST Breakthroughs, please visit http://breakthroughs.kaist.ac.kr
http://mid.kaist.ac.kr/projects/ratchair/
http://s2016.siggraph.org/content/emerging-technologies
https://www.dcexpo.jp/ko/15184
Figure 1. The vibration modules embedded and attached to furniture.
Figure 2. A close-up of the vibration module.
Figure 3. A close-up of the embedded modules.
Figure 4. A close-up of the vibration motor.
2017.03.23 View 9965 -
 A Transport Technology for Nanowires Thermally Treated at 700 Celsius Degrees
Professor Jun-Bo Yoon and his research team of the Department of Electrical Engineering at KAIST developed a technology for transporting thermally treated nanowires to a flexible substrate and created a high performance device for collecting flexible energy by using the new technology.
Mr. Min-Ho Seo, a Ph.D. candidate, participated in this study as the first author. The results were published online on January 30th in ACS Nano, an international journal in the field of nanoscience and engineering. (“Versatile Transfer of an Ultralong and Seamless Nanowire Array Crystallized at High Temperature for Use in High-performance Flexible Devices,” DOI: 10.1021/acsnano.6b06842)
Nanowires are one of the most representative nanomaterials. They have wire structures with dimensions in nanometers. The nanowires are widely used in the scientific and engineering fields due to their prominent physical and chemical properties that depend on a one-dimensional structure, and their high applicability.
Nanowires have much higher performance if their structure has unique features such as an excellent arrangement and a longer-than-average length. Many researchers are thus actively participating in the research for making nanowires without much difficulty, analyzing them, and developing them for high performance application devices.
Scientists have recently favored a research topic on making nanowires chemically and physically on a flexible substrate and applies the nanowires to a flexible electric device such as a high performance wearable sensor.
The existing technology, however, mixed nanowires from a chemical synthesis with a solution and spread the mixture on a flexible substrate. The resultant distribution was random, and it was difficult to produce a high performance device based on the structural advantages of nanowires. In addition, the technology used a cutting edge nano-process and flexible materials, but this was not economically beneficial. The production of stable materials at a temperature of 700 Celsius degrees or higher is unattainable, a great challenge for the application.
To solve this problem, the research team developed a new nano-transfer technology that combines a silicon nano-grating board with a large surface area and a nano-sacrificial layer process. A nano-sacrificial layer exists between nanowires and a nano-grating board, which acts as the mold for the nano-transfer. The new technology allows the device undergo thermal treatment. After this, the layer disappears when the nanowires are transported to a flexible substrate.
This technology also permits the stable production of nanowires with secured properties at an extremely high temperature. In this case, the nanowires are neatly organized on a flexible substrate. The research team used the technology to manufacture barium carbonate nanowires on top of the flexible substrate. The wires secured their properties at a temperature of 700℃ or above. The team employed the collection of wearable energy to obtain much higher electrical energy than that of an energy collecting device designed based on regular barium titanate nanowires.
The researchers said that their technology is built upon a semiconductor process, known as Physical Vapor Deposition that allows various materials such as ceramics and semiconductors to be used for flexible substrates of nanowires. They expected that high performance flexible electric devices such as flexible transistors and thermoelectric elements can be produced with this method.
Mr. Seo said, “In this study, we transported nanowire materials with developed properties on a flexible substrate and showed an increase in device performance. Our technology will be fundamental to the production of various nanowires on a flexible substrate as well as the feasibility of making high performance wearable electric devices.”
This research was supported by the Leap Research Support Program of the National Research Foundation of Korea.
Fig. 1. Transcription process of new, developed nanowires (a) and a fundamental mimetic diagram of a nano-sacrificial layer (b)
Fig. 2. Transcription results from using gold (AU) nanowires. The categories of the results were (a) optical images, (b) physical signals, (c) cross-sectional images from a scanning electron microscope (SEM), and (d-f) an electric verification of whether the perfectly arranged nanowires were made on a large surface.
Fig. 3. Transcription from using barium titanate (BaTiO3) nanowires. The results were (a) optical images, (b-e) top images taken from an SEM in various locations, and (f, g) property analysis.
Fig. 4. Mimetic diagram of the energy collecting device from using a BaTiO3 nanowire substrate and an optical image of the experiment for the miniature energy collecting device attached to an index finger.
2017.03.22 View 10187
A Transport Technology for Nanowires Thermally Treated at 700 Celsius Degrees
Professor Jun-Bo Yoon and his research team of the Department of Electrical Engineering at KAIST developed a technology for transporting thermally treated nanowires to a flexible substrate and created a high performance device for collecting flexible energy by using the new technology.
Mr. Min-Ho Seo, a Ph.D. candidate, participated in this study as the first author. The results were published online on January 30th in ACS Nano, an international journal in the field of nanoscience and engineering. (“Versatile Transfer of an Ultralong and Seamless Nanowire Array Crystallized at High Temperature for Use in High-performance Flexible Devices,” DOI: 10.1021/acsnano.6b06842)
Nanowires are one of the most representative nanomaterials. They have wire structures with dimensions in nanometers. The nanowires are widely used in the scientific and engineering fields due to their prominent physical and chemical properties that depend on a one-dimensional structure, and their high applicability.
Nanowires have much higher performance if their structure has unique features such as an excellent arrangement and a longer-than-average length. Many researchers are thus actively participating in the research for making nanowires without much difficulty, analyzing them, and developing them for high performance application devices.
Scientists have recently favored a research topic on making nanowires chemically and physically on a flexible substrate and applies the nanowires to a flexible electric device such as a high performance wearable sensor.
The existing technology, however, mixed nanowires from a chemical synthesis with a solution and spread the mixture on a flexible substrate. The resultant distribution was random, and it was difficult to produce a high performance device based on the structural advantages of nanowires. In addition, the technology used a cutting edge nano-process and flexible materials, but this was not economically beneficial. The production of stable materials at a temperature of 700 Celsius degrees or higher is unattainable, a great challenge for the application.
To solve this problem, the research team developed a new nano-transfer technology that combines a silicon nano-grating board with a large surface area and a nano-sacrificial layer process. A nano-sacrificial layer exists between nanowires and a nano-grating board, which acts as the mold for the nano-transfer. The new technology allows the device undergo thermal treatment. After this, the layer disappears when the nanowires are transported to a flexible substrate.
This technology also permits the stable production of nanowires with secured properties at an extremely high temperature. In this case, the nanowires are neatly organized on a flexible substrate. The research team used the technology to manufacture barium carbonate nanowires on top of the flexible substrate. The wires secured their properties at a temperature of 700℃ or above. The team employed the collection of wearable energy to obtain much higher electrical energy than that of an energy collecting device designed based on regular barium titanate nanowires.
The researchers said that their technology is built upon a semiconductor process, known as Physical Vapor Deposition that allows various materials such as ceramics and semiconductors to be used for flexible substrates of nanowires. They expected that high performance flexible electric devices such as flexible transistors and thermoelectric elements can be produced with this method.
Mr. Seo said, “In this study, we transported nanowire materials with developed properties on a flexible substrate and showed an increase in device performance. Our technology will be fundamental to the production of various nanowires on a flexible substrate as well as the feasibility of making high performance wearable electric devices.”
This research was supported by the Leap Research Support Program of the National Research Foundation of Korea.
Fig. 1. Transcription process of new, developed nanowires (a) and a fundamental mimetic diagram of a nano-sacrificial layer (b)
Fig. 2. Transcription results from using gold (AU) nanowires. The categories of the results were (a) optical images, (b) physical signals, (c) cross-sectional images from a scanning electron microscope (SEM), and (d-f) an electric verification of whether the perfectly arranged nanowires were made on a large surface.
Fig. 3. Transcription from using barium titanate (BaTiO3) nanowires. The results were (a) optical images, (b-e) top images taken from an SEM in various locations, and (f, g) property analysis.
Fig. 4. Mimetic diagram of the energy collecting device from using a BaTiO3 nanowire substrate and an optical image of the experiment for the miniature energy collecting device attached to an index finger.
2017.03.22 View 10187 -
 Highly-Efficient Photoelectrochemical CO2 Reduction
Direct CO2 conversion has continuously attracted a great deal of attention as a technology to produce fuels and chemical building blocks from renewable energy resources. Specifically, substances such as carbon feedstocks and fuels can be produced by utilizing sunlight, water, and CO2 as semiconductors and a water interface through photoelectrochemical CO2 reduction.
A KAIST research team demonstrated a novel photoelectrode structure for highly-selective and efficient photoelectrochemical CO2 reduction reactions. The research team led by Professor Jihun Oh of the Graduate School of EEWS (Energy, Environment, Water and Sustainability) presented a Si photoelectrode with a nanoporous Au thin film that is capable of reducing CO2 to CO with 90 percent selectivity in aqueous solution. The research team’s technology will provide a basic framework for designing the semiconductor photoelectrode structure necessary for photoelectrochemical conversion.
In order to achieve steady conversion of CO2, it is necessary to use a high-performance catalyst to lower overpotential. Among the metal catalysts, Au is known to be an electrocatalyst that converts CO2 to CO.
Conventionally, bare Au, as a catalyst, produces a lot of hydrogen gas due to its low CO selectivity. In addition, the high cost of Au remains a challenge in using the catalyst. Professor Oh’s research team addressed the issue by creating a nanoporous Au thin film formed by the electrochemical reduction of an anodized Au thin film.
As a result, the team could demonstrate an efficient, selective photoelectrochemical reduction reaction of CO2 to CO using electrochemically-treated Au thin films on a Si photoelectrode. The electrochemical reduction on anodized Au thin films forms a nanoporous thin layer exhibiting many grain boundaries of nanoparticles on the Au surface. This dramatically improves the selectivity of the reduction reaction with a maximum CO faradaic efficiency of over 90% at low overpotential and durability.
The research team also used an Au thin film of about 200 nanometers, 50,000 times thinner than previously reported nanostructured Au catalysts, resulting in a cost-effective catalyst. When depositing the catalyst on the semiconductor surface in the type of nanoparticles, the substrate of the thin film will be affected in the course of electrochemical reduction.
Thus, the research team designed a new Si photoelectrode with mesh-type co-catalysts that are independently wired at the front and back of the photoelectrode without influencing the photoelectrode, and made it possible for electrochemical reduction.
Due to the superior CO2 reduction reaction activity of the nanoporous Au mesh and high photovoltage from Si, the Si photoelectrode with the nanoporous Au thin film mesh shows conversion of CO2 to CO with 91% Faradaic efficiency at positive potential than CO equilibrium potential.
Professor Oh explained, “This technology will serve as a platform for diverse semiconductors and catalysts. Researchers can further improve the solar-to-CO2 conversion efficiency using this technology.
Dr. Jun Tae Song, the first author continued, “This new approach made it possible to develop a simple but very important type of electrode structure. It is the first time to achieve CO2 conversion at the potential lower than equilibrium potential. We believe that our research will contribute to efficient CO2 conversion.”
This research was published in the inside front cover of Advanced Energy Materials on February 8, 2017. The research was funded and supported by the Korea Carbon Capture & Sequestration R&D Center. Professor Sung-Yoon Chung of the EEWS also participated in this research.
(Figure: Schematic diagram of a Si photoelectrode that patterns with mesh-type nanoporous Au)
2017.03.08 View 10596
Highly-Efficient Photoelectrochemical CO2 Reduction
Direct CO2 conversion has continuously attracted a great deal of attention as a technology to produce fuels and chemical building blocks from renewable energy resources. Specifically, substances such as carbon feedstocks and fuels can be produced by utilizing sunlight, water, and CO2 as semiconductors and a water interface through photoelectrochemical CO2 reduction.
A KAIST research team demonstrated a novel photoelectrode structure for highly-selective and efficient photoelectrochemical CO2 reduction reactions. The research team led by Professor Jihun Oh of the Graduate School of EEWS (Energy, Environment, Water and Sustainability) presented a Si photoelectrode with a nanoporous Au thin film that is capable of reducing CO2 to CO with 90 percent selectivity in aqueous solution. The research team’s technology will provide a basic framework for designing the semiconductor photoelectrode structure necessary for photoelectrochemical conversion.
In order to achieve steady conversion of CO2, it is necessary to use a high-performance catalyst to lower overpotential. Among the metal catalysts, Au is known to be an electrocatalyst that converts CO2 to CO.
Conventionally, bare Au, as a catalyst, produces a lot of hydrogen gas due to its low CO selectivity. In addition, the high cost of Au remains a challenge in using the catalyst. Professor Oh’s research team addressed the issue by creating a nanoporous Au thin film formed by the electrochemical reduction of an anodized Au thin film.
As a result, the team could demonstrate an efficient, selective photoelectrochemical reduction reaction of CO2 to CO using electrochemically-treated Au thin films on a Si photoelectrode. The electrochemical reduction on anodized Au thin films forms a nanoporous thin layer exhibiting many grain boundaries of nanoparticles on the Au surface. This dramatically improves the selectivity of the reduction reaction with a maximum CO faradaic efficiency of over 90% at low overpotential and durability.
The research team also used an Au thin film of about 200 nanometers, 50,000 times thinner than previously reported nanostructured Au catalysts, resulting in a cost-effective catalyst. When depositing the catalyst on the semiconductor surface in the type of nanoparticles, the substrate of the thin film will be affected in the course of electrochemical reduction.
Thus, the research team designed a new Si photoelectrode with mesh-type co-catalysts that are independently wired at the front and back of the photoelectrode without influencing the photoelectrode, and made it possible for electrochemical reduction.
Due to the superior CO2 reduction reaction activity of the nanoporous Au mesh and high photovoltage from Si, the Si photoelectrode with the nanoporous Au thin film mesh shows conversion of CO2 to CO with 91% Faradaic efficiency at positive potential than CO equilibrium potential.
Professor Oh explained, “This technology will serve as a platform for diverse semiconductors and catalysts. Researchers can further improve the solar-to-CO2 conversion efficiency using this technology.
Dr. Jun Tae Song, the first author continued, “This new approach made it possible to develop a simple but very important type of electrode structure. It is the first time to achieve CO2 conversion at the potential lower than equilibrium potential. We believe that our research will contribute to efficient CO2 conversion.”
This research was published in the inside front cover of Advanced Energy Materials on February 8, 2017. The research was funded and supported by the Korea Carbon Capture & Sequestration R&D Center. Professor Sung-Yoon Chung of the EEWS also participated in this research.
(Figure: Schematic diagram of a Si photoelectrode that patterns with mesh-type nanoporous Au)
2017.03.08 View 10596