bio
-
 'The 2016 Top 100 Research Projects in Korea'
The Ministry of Science, ICT and Future Planning (MSIP) of Korea recently released a list of the 2016 Top 100 Research Projects in Korea. The list included the work of KAIST Professors Dong-Ho Cho of the School of Electrical Engineering, Jeung Ku Kang of the Graduate School of Energy, Environment, Water and Sustainability (EEWS), and Sang Yup Lee of the Chemical and Biomolecular Engineering Department.
Experts from academia, universities, and industries selected the 100 research projects, among 620 projects recommended by various government offices, in consideration of their contribution to the growth of science and technology in the nation.
Professor Cho conducts research on the development of 5G mobile communication systems based on the pattern polarization beam-division multiple access method.
Professor Kang works on the production of highly efficient energy materials and equipment by controlling them at the electron and atomic level.
Professor Lee focuses on the creation of strategies to produce important chemicals through a biological approach, i.e., microorganisms, which will help develop the means to mitigate climate change.
The MISP will publish a book that describes in detail each research project and will distribute copies of it to the National Assembly of Korea, libraries, and other public organizations.
For more information on the list, please go to www.ntis.go.kr.
Pictured from left to right are Professors Dong-Ho Cho, Jeung Ku Kang, and Sang Yup Lee.
2016.07.21 View 5650
'The 2016 Top 100 Research Projects in Korea'
The Ministry of Science, ICT and Future Planning (MSIP) of Korea recently released a list of the 2016 Top 100 Research Projects in Korea. The list included the work of KAIST Professors Dong-Ho Cho of the School of Electrical Engineering, Jeung Ku Kang of the Graduate School of Energy, Environment, Water and Sustainability (EEWS), and Sang Yup Lee of the Chemical and Biomolecular Engineering Department.
Experts from academia, universities, and industries selected the 100 research projects, among 620 projects recommended by various government offices, in consideration of their contribution to the growth of science and technology in the nation.
Professor Cho conducts research on the development of 5G mobile communication systems based on the pattern polarization beam-division multiple access method.
Professor Kang works on the production of highly efficient energy materials and equipment by controlling them at the electron and atomic level.
Professor Lee focuses on the creation of strategies to produce important chemicals through a biological approach, i.e., microorganisms, which will help develop the means to mitigate climate change.
The MISP will publish a book that describes in detail each research project and will distribute copies of it to the National Assembly of Korea, libraries, and other public organizations.
For more information on the list, please go to www.ntis.go.kr.
Pictured from left to right are Professors Dong-Ho Cho, Jeung Ku Kang, and Sang Yup Lee.
2016.07.21 View 5650 -
 Doctoral Student Receives the Best Paper Award from the International Metabolic Engineering Conference 2016
So Young Choi, a Ph.D. candidate at the Department of Chemical and Biomolecular Engineering at KAIST, received the Student and Young Investigator Poster Award at the 11th International Metabolic Engineering Conference held in Awaji, Japan on June 26-30.
Choi received the award for her research on one-step fermentative production of Poly(lactate-co-glycolate) (PLGA) from carbohydrates in Escherichia coli, which was published in the April 2016 issue of Nature Biotechnology.
In her paper, she presented a novel technology to synthesize PLGA, a non-natural copolymer, through a biological production process. Because of its biodegradability, non-toxicity, and biocompatibility, PLGA is widely used in biomedical and therapeutic applications, including surgical sutures, prosthetic devices, drug delivery, and tissue engineering.
Employing a metabolic engineering approach, Choi manipulated the metabolic pathway of an Escherichia coli bacterium to convert glucose and xylose into the biosynthesis of PLGA within the cell. Previously, PLGA could be obtained only through chemical synthesis.
Choi said, “I’m thrilled to receive an award from a flagship conference of my research field. Mindful of this recognition, I will continue my research to produce meaningful results, thereby contributing to the development of science and technology in Korea.”
The International Metabolic Engineering Conference is a leading professional gathering where state-of-the-art developments and achievements made in the field of metabolic engineering are shared. With the participation of about 400 professionals from all around the world, the conference participants discussed this year’s theme of “Design, Synthesis and System Integration for Metabolic Engineering.”
2016.07.07 View 11987
Doctoral Student Receives the Best Paper Award from the International Metabolic Engineering Conference 2016
So Young Choi, a Ph.D. candidate at the Department of Chemical and Biomolecular Engineering at KAIST, received the Student and Young Investigator Poster Award at the 11th International Metabolic Engineering Conference held in Awaji, Japan on June 26-30.
Choi received the award for her research on one-step fermentative production of Poly(lactate-co-glycolate) (PLGA) from carbohydrates in Escherichia coli, which was published in the April 2016 issue of Nature Biotechnology.
In her paper, she presented a novel technology to synthesize PLGA, a non-natural copolymer, through a biological production process. Because of its biodegradability, non-toxicity, and biocompatibility, PLGA is widely used in biomedical and therapeutic applications, including surgical sutures, prosthetic devices, drug delivery, and tissue engineering.
Employing a metabolic engineering approach, Choi manipulated the metabolic pathway of an Escherichia coli bacterium to convert glucose and xylose into the biosynthesis of PLGA within the cell. Previously, PLGA could be obtained only through chemical synthesis.
Choi said, “I’m thrilled to receive an award from a flagship conference of my research field. Mindful of this recognition, I will continue my research to produce meaningful results, thereby contributing to the development of science and technology in Korea.”
The International Metabolic Engineering Conference is a leading professional gathering where state-of-the-art developments and achievements made in the field of metabolic engineering are shared. With the participation of about 400 professionals from all around the world, the conference participants discussed this year’s theme of “Design, Synthesis and System Integration for Metabolic Engineering.”
2016.07.07 View 11987 -
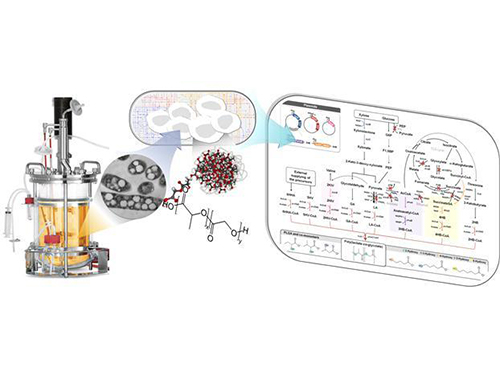 Top 10 Emerging Technologies by World Economic Forum
The World Economic Forum’s Meta-Council on Emerging Technologies announced its annual list of breakthrough technologies, the “Top 10 Emerging Technologies of 2016,” on June 23, 2016. The Meta-Council chose the top ten technologies based on the technologies’ potential to improve lives, transform industries, and safeguard the planet. The research field of systems metabolic engineering, founded by Distinguished Professor Sang Yup Lee of the Chemical and Biomolecular Engineering Department at KAIST, was also citied. Systems metabolic engineering, which combines elements of synthetic biology, systems biology, and evolutionary engineering, offers a sustainable process for the production of useful chemicals in an environmentally friendly way from plants such as inedible biomass, reducing the need of using fossil fuels. Details about the list follow below:
https://www.weforum.org/press/2016/06/battery-powered-villages-sociable-robots-rank-among-top-10-emerging-technologies-of-2016
The picture below shows the “systems metabolic engineering of E. coli for the production of PLGA." PLGA is poly(lactate-co-glycolate), which is widely used for biomedical applications, and has been made by chemical synthesis. Now it is possible to produce PLGA eco-friendly by one-step fermentation of a gut bacterium which is developed through systems metabolic engineering.
2016.06.27 View 12257
Top 10 Emerging Technologies by World Economic Forum
The World Economic Forum’s Meta-Council on Emerging Technologies announced its annual list of breakthrough technologies, the “Top 10 Emerging Technologies of 2016,” on June 23, 2016. The Meta-Council chose the top ten technologies based on the technologies’ potential to improve lives, transform industries, and safeguard the planet. The research field of systems metabolic engineering, founded by Distinguished Professor Sang Yup Lee of the Chemical and Biomolecular Engineering Department at KAIST, was also citied. Systems metabolic engineering, which combines elements of synthetic biology, systems biology, and evolutionary engineering, offers a sustainable process for the production of useful chemicals in an environmentally friendly way from plants such as inedible biomass, reducing the need of using fossil fuels. Details about the list follow below:
https://www.weforum.org/press/2016/06/battery-powered-villages-sociable-robots-rank-among-top-10-emerging-technologies-of-2016
The picture below shows the “systems metabolic engineering of E. coli for the production of PLGA." PLGA is poly(lactate-co-glycolate), which is widely used for biomedical applications, and has been made by chemical synthesis. Now it is possible to produce PLGA eco-friendly by one-step fermentation of a gut bacterium which is developed through systems metabolic engineering.
2016.06.27 View 12257 -
 KAIST to Participate in Summer Davos Forum 2016 in China
A group of KAIST researchers will share their insights on the future and challenges of the current technological innovations impacting all aspects of society, while showcasing their research excellence in artificial intelligence and robotics.
Scientific and technological breakthroughs are more important than ever as key agents to drive social, economic, and political changes and advancements in today’s world. The World Economic Forum (WEF), an international organization that provides one of the broadest engagement platforms to address issues of major concern to the global community, will discuss the effects of these breakthroughs at its 10th Annual Meeting of the New Champions, a.k.a., the Summer Davos Forum, in Tianjin, China, June 26-28, 2016.
Three professors from the Korea Advanced Institute of Science and Technology (KAIST) will join the Annual Meeting and offer their expertise in the fields of biotechnology, artificial intelligence, and robotics to explore the conference theme, “The Fourth Industrial Revolution and Its Transformational Impact.” The Fourth Industrial Revolution, a term coined by WEF founder, Klaus Schwab, is characterized by a range of new technologies that fuse the physical, digital, and biological worlds, such as the Internet of Things, cloud computing, and automation.
Distinguished Professor Sang Yup Lee of the Chemical and Biomolecular Engineering Department will speak at the Experts Reception to be held on June 25, 2016 on the topic of “The Summer Davos Forum and Science and Technology in Asia.” On June 27, 2016, he will participate in two separate discussion sessions.
In the first session entitled “What If Drugs Are Printed from the Internet?,” Professor Lee will discuss the impacts of advancements in biotechnology and 3D printing technology on the future of medicine with Nita A. Farahany, a Duke University professor. Clare Matterson, the Director of Strategy at Wellcome Trust in the United Kingdom, will serve as the moderator. The discussants will note recent developments made in the way patients receive their medicine, for example, downloading drugs directly from the internet and the production of yeast strains to make opioids for pain treatment through systems metabolic engineering. They will also suggest how these emerging technologies will transform the landscape of the pharmaceutical industry in the years to come.
In the second session, “Lessons for Life,” Professor Lee will talk about how to nurture life-long learning and creativity to support personal and professional growth necessary in an era of the new industrial revolution.
During the Annual Meeting, Professors Jong-Hwan Kim of the Electrical Engineering School and David Hyunchul Shim of the Aerospace Department will host, together with researchers from Carnegie Mellon University and AnthroTronix, an engineering research and development company, a technological exhibition on robotics. Professor Kim, the founder of the internally renowned Robot World Cup, will showcase his humanoid soccer-playing micro-robots and display their various cutting-edge technologies such as imaging processing, artificial intelligence, walking, and balancing. Professor Shim will present a human-like robotic piloting system, PIBOT, which autonomously operates a simulated flight program by employing control sticks and guiding an airplane from takeoff to landing.
In addition, the two professors will join Professor Lee, who is also a moderator, to host a KAIST-led session on June 26, 2016, entitled “Science in Depth: From Deep Learning to Autonomous Machines.” Professors Kim and Shim will explore new opportunities and challenges in their fields from machine learning to autonomous robotics, including unmanned vehicles and drones.
Since 2011, KAIST has participated in the World Economic Forum’s two flagship conferences, the January and June Davos Forums, to introduce outstanding talents, share their latest research achievements, and interact with global leaders.
KAIST President Steve Kang said, “It is important for KAIST to be involved in global forums that identify issues critical to humanity and seek answers to solve them, and where our skills and knowledge in science and technology can play a meaningful role. The Annual Meeting in China will become another venue to accomplish this.”
2016.06.27 View 13779
KAIST to Participate in Summer Davos Forum 2016 in China
A group of KAIST researchers will share their insights on the future and challenges of the current technological innovations impacting all aspects of society, while showcasing their research excellence in artificial intelligence and robotics.
Scientific and technological breakthroughs are more important than ever as key agents to drive social, economic, and political changes and advancements in today’s world. The World Economic Forum (WEF), an international organization that provides one of the broadest engagement platforms to address issues of major concern to the global community, will discuss the effects of these breakthroughs at its 10th Annual Meeting of the New Champions, a.k.a., the Summer Davos Forum, in Tianjin, China, June 26-28, 2016.
Three professors from the Korea Advanced Institute of Science and Technology (KAIST) will join the Annual Meeting and offer their expertise in the fields of biotechnology, artificial intelligence, and robotics to explore the conference theme, “The Fourth Industrial Revolution and Its Transformational Impact.” The Fourth Industrial Revolution, a term coined by WEF founder, Klaus Schwab, is characterized by a range of new technologies that fuse the physical, digital, and biological worlds, such as the Internet of Things, cloud computing, and automation.
Distinguished Professor Sang Yup Lee of the Chemical and Biomolecular Engineering Department will speak at the Experts Reception to be held on June 25, 2016 on the topic of “The Summer Davos Forum and Science and Technology in Asia.” On June 27, 2016, he will participate in two separate discussion sessions.
In the first session entitled “What If Drugs Are Printed from the Internet?,” Professor Lee will discuss the impacts of advancements in biotechnology and 3D printing technology on the future of medicine with Nita A. Farahany, a Duke University professor. Clare Matterson, the Director of Strategy at Wellcome Trust in the United Kingdom, will serve as the moderator. The discussants will note recent developments made in the way patients receive their medicine, for example, downloading drugs directly from the internet and the production of yeast strains to make opioids for pain treatment through systems metabolic engineering. They will also suggest how these emerging technologies will transform the landscape of the pharmaceutical industry in the years to come.
In the second session, “Lessons for Life,” Professor Lee will talk about how to nurture life-long learning and creativity to support personal and professional growth necessary in an era of the new industrial revolution.
During the Annual Meeting, Professors Jong-Hwan Kim of the Electrical Engineering School and David Hyunchul Shim of the Aerospace Department will host, together with researchers from Carnegie Mellon University and AnthroTronix, an engineering research and development company, a technological exhibition on robotics. Professor Kim, the founder of the internally renowned Robot World Cup, will showcase his humanoid soccer-playing micro-robots and display their various cutting-edge technologies such as imaging processing, artificial intelligence, walking, and balancing. Professor Shim will present a human-like robotic piloting system, PIBOT, which autonomously operates a simulated flight program by employing control sticks and guiding an airplane from takeoff to landing.
In addition, the two professors will join Professor Lee, who is also a moderator, to host a KAIST-led session on June 26, 2016, entitled “Science in Depth: From Deep Learning to Autonomous Machines.” Professors Kim and Shim will explore new opportunities and challenges in their fields from machine learning to autonomous robotics, including unmanned vehicles and drones.
Since 2011, KAIST has participated in the World Economic Forum’s two flagship conferences, the January and June Davos Forums, to introduce outstanding talents, share their latest research achievements, and interact with global leaders.
KAIST President Steve Kang said, “It is important for KAIST to be involved in global forums that identify issues critical to humanity and seek answers to solve them, and where our skills and knowledge in science and technology can play a meaningful role. The Annual Meeting in China will become another venue to accomplish this.”
2016.06.27 View 13779 -
 Unveiling the Distinctive Features of Industrial Microorganism
KAIST researchers have sequenced the whole genome of Clostridium tyrobutyricum, which has a higher tolerance to toxic chemicals, such as 1-butanol, compared to other clostridial bacterial strains.
Clostridium tyrobutyricum, a Gram-positive, anaerobic spore-forming bacterium, is considered a promising industrial host strain for the production of various chemicals including butyric acid which has many applications in different industries such as a precursor to biofuels. Despite such potential, C. tyrobutyricum has received little attention, mainly due to a limited understanding of its genotypic and metabolic characteristics at the genome level.
A Korean research team headed by Distinguished Professor Sang Yup Lee of the Chemical and Biomolecular Engineering Department at the Korea Advanced Institute of Science and Technology (KAIST) deciphered the genome sequence of C. tyrobutyricum and its proteome profiles during the course of batch fermentation. As a result, the research team learned that the bacterium is not only capable of producing a large amount of butyric acid but also can tolerate toxic compounds such as 1-butanol. The research results were published in mBio on June 14, 2016.
The team adopted a genoproteomic approach, combining genomics and proteomics, to investigate the metabolic features of C. tyrobutyricum. Unlike Clostridium acetobutylicum, the most widely used organism for 1-butanol production, C. tyrobutyricum has a novel butyrate-producing pathway and various mechanisms for energy conservation under anaerobic conditions. The expression of various metabolic genes, including those involved in butyrate formation, was analyzed using the “shotgun” proteome approach.
To date, the bio-based production of 1-butanol, a next-generation biofuel, has relied on several clostridial hosts including C. acetobutylicum and C. beijerinckii. However, these organisms have a low tolerance against 1-butanol even though they are naturally capable of producing it. C. tyrobutyricum cannot produce 1-butanol itself, but has a higher 1-butanol-tolerance and rapid uptake of monosaccharides, compared to those two species.
The team identified most of the genes involved in the central metabolism of C. tyrobutyricum from the whole-genome and shotgun proteome data, and this study will accelerate the bacterium’s engineering to produce useful chemicals including butyric acid and 1-butanol, replacing traditional bacterial hosts.
Professor Lee said,
“The unique metabolic features and energy conservation mechanisms of C. tyrobutyricum can be employed in the various microbial hosts we have previously developed to further improve their productivity and yield. Moreover, findings on C. tyrobutyricum revealed by this study will be the first step to directly engineer this bacterium.”
Director Jin-Woo Kim at the Platform Technology Division of the Ministry of Science, ICT and Future Planning of Korea, who oversees the Technology Development Program to Solve Climate Change, said,
“Over the years, Professor Lee’s team has researched the development of a bio-refinery system to produce natural and non-natural chemicals with the systems metabolic engineering of microorganisms. They were able to design strategies for the development of diverse industrial microbial strains to produce useful chemicals from inedible biomass-based carbon dioxide fixation. We believe the efficient production of butyric acid using a metabolic engineering approach will play an important role in the establishment of a bioprocess for chemical production.”
The title of the research paper is “Deciphering Clostridium tyrobutyricum Metabolism Based on the Who-Genome Sequence and Proteome Analyses.” (DOI: 10.1128/mBio.00743-16)
The lead authors are Joungmin Lee, a post-doctoral fellow in the BioProcess Research Center at KAIST, currently working in CJ CheilJedang Research Institute; Yu-Sin Jang, a research fellow in the BioProcess Research Center at KAIST, currently working at Gyeongsang National University as an assistant professor; and Mee-Jung Han, an assistant professor in the Environmental Engineering and Energy Department at Dongyang University. Jin Young Kim, a senior researcher at the Korea Basic Science Institute, also participated in the research.
This research was supported by the Technology Development Program to Solve Climate Change’s research project entitled “Systems Metabolic Engineering for Biorefineries” from the Ministry of Science, ICT and Future Planning through the National Research Foundation of Korea (NRF-2012M1A2A2026556).
Schematic Diagram of C. tyrobutyricum’s Genome Sequence and Its Proteome Profiles
The picture below shows the complete genome sequence, global protein expression profiles, and the genome-based metabolic characteristics during batch fermentation of C. tyrobutyricum.
2016.06.20 View 11593
Unveiling the Distinctive Features of Industrial Microorganism
KAIST researchers have sequenced the whole genome of Clostridium tyrobutyricum, which has a higher tolerance to toxic chemicals, such as 1-butanol, compared to other clostridial bacterial strains.
Clostridium tyrobutyricum, a Gram-positive, anaerobic spore-forming bacterium, is considered a promising industrial host strain for the production of various chemicals including butyric acid which has many applications in different industries such as a precursor to biofuels. Despite such potential, C. tyrobutyricum has received little attention, mainly due to a limited understanding of its genotypic and metabolic characteristics at the genome level.
A Korean research team headed by Distinguished Professor Sang Yup Lee of the Chemical and Biomolecular Engineering Department at the Korea Advanced Institute of Science and Technology (KAIST) deciphered the genome sequence of C. tyrobutyricum and its proteome profiles during the course of batch fermentation. As a result, the research team learned that the bacterium is not only capable of producing a large amount of butyric acid but also can tolerate toxic compounds such as 1-butanol. The research results were published in mBio on June 14, 2016.
The team adopted a genoproteomic approach, combining genomics and proteomics, to investigate the metabolic features of C. tyrobutyricum. Unlike Clostridium acetobutylicum, the most widely used organism for 1-butanol production, C. tyrobutyricum has a novel butyrate-producing pathway and various mechanisms for energy conservation under anaerobic conditions. The expression of various metabolic genes, including those involved in butyrate formation, was analyzed using the “shotgun” proteome approach.
To date, the bio-based production of 1-butanol, a next-generation biofuel, has relied on several clostridial hosts including C. acetobutylicum and C. beijerinckii. However, these organisms have a low tolerance against 1-butanol even though they are naturally capable of producing it. C. tyrobutyricum cannot produce 1-butanol itself, but has a higher 1-butanol-tolerance and rapid uptake of monosaccharides, compared to those two species.
The team identified most of the genes involved in the central metabolism of C. tyrobutyricum from the whole-genome and shotgun proteome data, and this study will accelerate the bacterium’s engineering to produce useful chemicals including butyric acid and 1-butanol, replacing traditional bacterial hosts.
Professor Lee said,
“The unique metabolic features and energy conservation mechanisms of C. tyrobutyricum can be employed in the various microbial hosts we have previously developed to further improve their productivity and yield. Moreover, findings on C. tyrobutyricum revealed by this study will be the first step to directly engineer this bacterium.”
Director Jin-Woo Kim at the Platform Technology Division of the Ministry of Science, ICT and Future Planning of Korea, who oversees the Technology Development Program to Solve Climate Change, said,
“Over the years, Professor Lee’s team has researched the development of a bio-refinery system to produce natural and non-natural chemicals with the systems metabolic engineering of microorganisms. They were able to design strategies for the development of diverse industrial microbial strains to produce useful chemicals from inedible biomass-based carbon dioxide fixation. We believe the efficient production of butyric acid using a metabolic engineering approach will play an important role in the establishment of a bioprocess for chemical production.”
The title of the research paper is “Deciphering Clostridium tyrobutyricum Metabolism Based on the Who-Genome Sequence and Proteome Analyses.” (DOI: 10.1128/mBio.00743-16)
The lead authors are Joungmin Lee, a post-doctoral fellow in the BioProcess Research Center at KAIST, currently working in CJ CheilJedang Research Institute; Yu-Sin Jang, a research fellow in the BioProcess Research Center at KAIST, currently working at Gyeongsang National University as an assistant professor; and Mee-Jung Han, an assistant professor in the Environmental Engineering and Energy Department at Dongyang University. Jin Young Kim, a senior researcher at the Korea Basic Science Institute, also participated in the research.
This research was supported by the Technology Development Program to Solve Climate Change’s research project entitled “Systems Metabolic Engineering for Biorefineries” from the Ministry of Science, ICT and Future Planning through the National Research Foundation of Korea (NRF-2012M1A2A2026556).
Schematic Diagram of C. tyrobutyricum’s Genome Sequence and Its Proteome Profiles
The picture below shows the complete genome sequence, global protein expression profiles, and the genome-based metabolic characteristics during batch fermentation of C. tyrobutyricum.
2016.06.20 View 11593 -
 Special Lecture by Professor Sung-Hou Kim of UC Berkeley
As part of its special lecture series, the Department of Biological Sciences at KAIST has invited Professor Sung-Hou Kim of the Department of Chemistry at the University of California, Berkeley, to lecture on his research in structural biology. He will speak twice on May 23 and 30, respectively, on the topics “Origin of Universe and Earth—A Narrative” and “Origin of Life and Human Species—A Narrative.”
Professor Kim's research addresses the structural basis of molecules to reveal how they communicate with each other to activate or inhibit particular processes in cell growth, cell differentiation, and cancer. Using the single-crystal X-ray diffraction technology, he discovered, for the first time in the world, the three-dimensional (3-D) structure of a transfer RNA (t-RNA) and received much praise for this work from the scientific community. Since then, he has been cited as a candidate for a Nobel Prize in Chemistry for many years.
He also examined the 3-D structures of a RAS protein in normal and cancer cells and identified the mutations of the RAS protein as a cause for cancer. His work has assisted in the development of target drugs for cancer treatment. In recent years, he has adopted a computational biology approach to study the structure and function of biological genomics, with which he has tried to predict disease-sensitive genes.
Professor Kim graduated from Seoul National University in 1962 and received his Ph.D. degree in chemistry from the University of Pittsburgh in the United States in 1966. He worked at the Massachusetts Institute of Technology (MIT) as a senior research scientist, and has taught at UC Berkeley since 1978.
2016.05.23 View 7735
Special Lecture by Professor Sung-Hou Kim of UC Berkeley
As part of its special lecture series, the Department of Biological Sciences at KAIST has invited Professor Sung-Hou Kim of the Department of Chemistry at the University of California, Berkeley, to lecture on his research in structural biology. He will speak twice on May 23 and 30, respectively, on the topics “Origin of Universe and Earth—A Narrative” and “Origin of Life and Human Species—A Narrative.”
Professor Kim's research addresses the structural basis of molecules to reveal how they communicate with each other to activate or inhibit particular processes in cell growth, cell differentiation, and cancer. Using the single-crystal X-ray diffraction technology, he discovered, for the first time in the world, the three-dimensional (3-D) structure of a transfer RNA (t-RNA) and received much praise for this work from the scientific community. Since then, he has been cited as a candidate for a Nobel Prize in Chemistry for many years.
He also examined the 3-D structures of a RAS protein in normal and cancer cells and identified the mutations of the RAS protein as a cause for cancer. His work has assisted in the development of target drugs for cancer treatment. In recent years, he has adopted a computational biology approach to study the structure and function of biological genomics, with which he has tried to predict disease-sensitive genes.
Professor Kim graduated from Seoul National University in 1962 and received his Ph.D. degree in chemistry from the University of Pittsburgh in the United States in 1966. He worked at the Massachusetts Institute of Technology (MIT) as a senior research scientist, and has taught at UC Berkeley since 1978.
2016.05.23 View 7735 -
 KAIST Hosts the 2016 IPFGRU
More than 120 senior representatives from 65 universities around the world will convene this month in Seoul to discuss the social responsibilities of higher education and strategic global partnerships among academia, research, and industry to advance socio-economic values.
Higher education as a driver of change to address the social and global challenges facing humanity in the 21st century has never been as important as it is today.
KAIST will raise the topic of higher education as a driver of social change, innovation, and entrepreneurship with the heads of global universities at its seventh international forum to be held on April 11-12, 2016 at the Grand Hyatt Hotel in Seoul, the Republic of Korea.
The 2016 International Presidential Forum on Global Research Universities (IPFGRU) will bring over 120 presidents and vice presidents of 65 research universities and institutes from 36 nations together to discuss the theme of “Social Responsibilities of Higher Education and Strategic Global Partnership.”
Presidents Sung-Mo Kang of KAIST, Jacques Biot of École Polytechnique in France, and Peretz Lavie of the Technion-Israel Institute of Technology will address the conference as plenary speakers.
President Kang will speak about KAIST’s initiatives to produce creative talents through student-centered education, entrepreneurship curricula, and the integration of humanities into cutting-edge research programs. His presentation, titled “The Fostering of Creative Talents and the Social Responsibility of Research Universities in the New Era,” introduces KAIST’s educational philosophy which can be represented as π. A broad range of understanding in basic disciplines (the horizontal line) is supported with one prong of in-depth knowledge in a chosen field and the other in entrepreneurial spirit.
KAIST graduates have demonstrated extraordinary leadership in research, academia, business, and public service. Nearly 25% of the research and development personnel at Samsung Electronics are KAIST Ph.D. holders. President Kang also describes KAIST’s latest endeavor to turn a university-led entrepreneurial activity into a stable business based on research outcomes and campus innovations. The K-School, a one-year master’s degree program on entrepreneurship and innovation, has just launched and is expecting to receive its first batch of students this fall. The K-School is envisioned to continue the university’s legacy as a major feeder for startups in Korea.
President Lavie will give a talk on “Fostering an Innovation and Entrepreneurship (I&E) Ecosystem in Israel,” in which he describes how the Technion-Israel Institute has become integral to the foundation of the nation’s I&E platform. Since its establishment in 1912, the university has become a key player in the growth of Israel’s industry, science, and technology while nurturing the majority of the nation’s top-notch researchers, innovators, and entrepreneurs. Technion graduates have created more than 2,000 companies in Israel alone, generating 100,000 jobs and USD 30 billion through mergers and acquisitions.
President Biot will offer his insights into how the future of global research universities will be widely impacted by the emergence of disruptions triggered by the Fourth Industrial Revolution. In his speech entitled “How to Prepare Our Universities for the New Era of Industry 4.0,” he emphasizes that universities should take multi-disciplinary approaches to tackle societal challenges given the complexity of today’s problems ranging from climate change to energy crises, pandemic diseases, and poverty. He argues that universities should identify the needs of students in “Generation Z” who, from birth, have been heavily exposed to the Internet and digital technologies and, thus, universities should develop new educational systems (i.e., University 4.0.) to better prepare these students to cope with Industry 4.0.
The IPFGRU consists of presentations and discussions addressing the following sub-topics:
- Seeking a New Model of Research Universities in a New Era: This session will explore the role of research universities as both innovation drivers and growth engines in an age of robotics, globalization, and digitally-driven markets. In addition, speakers will discuss how to prepare universities for the Industry 4.0 era, and how multidisciplinary approaches and open innovations will play a large part in facilitating translational research and technology transfer.
- Shared Challenges and Responsibilities from a Global Perspective: Universities will share their strategies, policies, and practices to respond to critical issues facing local and global communities such as youth unemployment, the environment, energy, inequality, and entrepreneurship.
- Strategic Global Partnerships for Sustainable Development: Panelists will discuss how to build productive and sustainable partnerships that can generate synergies between education and research.
- Insights into Higher Education: Trends and Development: Participants will examine how universities can stay relevant in an increasingly competitive higher education sector and can assist students to better adapt to opportunities and challenges posed by the new industry of digital transformation and exponentially-growing technologies.
Sung-Hyon Myaeng, the Associate Vice President of the International Office at KAIST and a Co-chair of the 2016 IPFGRU said,
“The IPFGRU was established in 2008 to promote excellence and innovation in higher education with presidents of leading research universities and key policy-makers in the private and public sectors from across the world. Since then, it has served as one of the largest university gatherings in Asia, allowing participants to cooperate and share their expertise, ideas, and best practices taking place in academia, industry, and government.”
“This year’s meeting has recorded the largest number of universities participating, including 28 European schools, 20 Asian institutions, and 8 schools from the Americas, which I believe reflects a sense of urgency that global universities share. One way or another, we must adapt to the rapidly transforming educational and research environment encompassing higher learning,” added Myaeng.
For more information, go to http://forum.kaist.ac.kr.
2016.04.08 View 10071
KAIST Hosts the 2016 IPFGRU
More than 120 senior representatives from 65 universities around the world will convene this month in Seoul to discuss the social responsibilities of higher education and strategic global partnerships among academia, research, and industry to advance socio-economic values.
Higher education as a driver of change to address the social and global challenges facing humanity in the 21st century has never been as important as it is today.
KAIST will raise the topic of higher education as a driver of social change, innovation, and entrepreneurship with the heads of global universities at its seventh international forum to be held on April 11-12, 2016 at the Grand Hyatt Hotel in Seoul, the Republic of Korea.
The 2016 International Presidential Forum on Global Research Universities (IPFGRU) will bring over 120 presidents and vice presidents of 65 research universities and institutes from 36 nations together to discuss the theme of “Social Responsibilities of Higher Education and Strategic Global Partnership.”
Presidents Sung-Mo Kang of KAIST, Jacques Biot of École Polytechnique in France, and Peretz Lavie of the Technion-Israel Institute of Technology will address the conference as plenary speakers.
President Kang will speak about KAIST’s initiatives to produce creative talents through student-centered education, entrepreneurship curricula, and the integration of humanities into cutting-edge research programs. His presentation, titled “The Fostering of Creative Talents and the Social Responsibility of Research Universities in the New Era,” introduces KAIST’s educational philosophy which can be represented as π. A broad range of understanding in basic disciplines (the horizontal line) is supported with one prong of in-depth knowledge in a chosen field and the other in entrepreneurial spirit.
KAIST graduates have demonstrated extraordinary leadership in research, academia, business, and public service. Nearly 25% of the research and development personnel at Samsung Electronics are KAIST Ph.D. holders. President Kang also describes KAIST’s latest endeavor to turn a university-led entrepreneurial activity into a stable business based on research outcomes and campus innovations. The K-School, a one-year master’s degree program on entrepreneurship and innovation, has just launched and is expecting to receive its first batch of students this fall. The K-School is envisioned to continue the university’s legacy as a major feeder for startups in Korea.
President Lavie will give a talk on “Fostering an Innovation and Entrepreneurship (I&E) Ecosystem in Israel,” in which he describes how the Technion-Israel Institute has become integral to the foundation of the nation’s I&E platform. Since its establishment in 1912, the university has become a key player in the growth of Israel’s industry, science, and technology while nurturing the majority of the nation’s top-notch researchers, innovators, and entrepreneurs. Technion graduates have created more than 2,000 companies in Israel alone, generating 100,000 jobs and USD 30 billion through mergers and acquisitions.
President Biot will offer his insights into how the future of global research universities will be widely impacted by the emergence of disruptions triggered by the Fourth Industrial Revolution. In his speech entitled “How to Prepare Our Universities for the New Era of Industry 4.0,” he emphasizes that universities should take multi-disciplinary approaches to tackle societal challenges given the complexity of today’s problems ranging from climate change to energy crises, pandemic diseases, and poverty. He argues that universities should identify the needs of students in “Generation Z” who, from birth, have been heavily exposed to the Internet and digital technologies and, thus, universities should develop new educational systems (i.e., University 4.0.) to better prepare these students to cope with Industry 4.0.
The IPFGRU consists of presentations and discussions addressing the following sub-topics:
- Seeking a New Model of Research Universities in a New Era: This session will explore the role of research universities as both innovation drivers and growth engines in an age of robotics, globalization, and digitally-driven markets. In addition, speakers will discuss how to prepare universities for the Industry 4.0 era, and how multidisciplinary approaches and open innovations will play a large part in facilitating translational research and technology transfer.
- Shared Challenges and Responsibilities from a Global Perspective: Universities will share their strategies, policies, and practices to respond to critical issues facing local and global communities such as youth unemployment, the environment, energy, inequality, and entrepreneurship.
- Strategic Global Partnerships for Sustainable Development: Panelists will discuss how to build productive and sustainable partnerships that can generate synergies between education and research.
- Insights into Higher Education: Trends and Development: Participants will examine how universities can stay relevant in an increasingly competitive higher education sector and can assist students to better adapt to opportunities and challenges posed by the new industry of digital transformation and exponentially-growing technologies.
Sung-Hyon Myaeng, the Associate Vice President of the International Office at KAIST and a Co-chair of the 2016 IPFGRU said,
“The IPFGRU was established in 2008 to promote excellence and innovation in higher education with presidents of leading research universities and key policy-makers in the private and public sectors from across the world. Since then, it has served as one of the largest university gatherings in Asia, allowing participants to cooperate and share their expertise, ideas, and best practices taking place in academia, industry, and government.”
“This year’s meeting has recorded the largest number of universities participating, including 28 European schools, 20 Asian institutions, and 8 schools from the Americas, which I believe reflects a sense of urgency that global universities share. One way or another, we must adapt to the rapidly transforming educational and research environment encompassing higher learning,” added Myaeng.
For more information, go to http://forum.kaist.ac.kr.
2016.04.08 View 10071 -
 Next-Generation Holographic Microscope for 3D Live Cell Imaging
KAIST researchers have developed a revolutionary bio-medical imaging tool, the HT-1, to view and analyze cells, which is commercially available.
Professor YongKeun Park of the Physics Department at KAIST and his research team have developed a powerful method for 3D imaging of live cells without staining. The researchers announced the launch of their new microscopic tool, the holotomography (HT)-1, to the global marketplace through a Korean start-up that Professor Park co-founded, TomoCube (www.tomocube.com).
Professor Park is a leading researcher in the field of biophotonics and has dedicated much of his research career to working on digital holographic microscopy technology. He collaborated with TomoCube’s R&D team to develop a state-of-the-art, 2D/3D/4D holographic microscope that would allow a real-time label-free visualization of biological cells and tissues.
The HT is an optical analogy of X-ray computed tomography (CT). Both X-ray CT and HT share the same physical principle—the inverse of wave scattering. The difference is that HT uses laser illumination whereas X-ray CT uses X-ray beams. From the measurement of multiple 2D holograms of a cell, coupled with various angles of laser illuminations, the 3D refractive index (RI) distribution of the cell can be reconstructed. The reconstructed 3D RI map provides structural and chemical information of the cell including mass, morphology, protein concentration, and dynamics of the cellular membrane.
The HT enables users to quantitatively and non-invasively investigate the intrinsic properties of biological cells, for example, dry mass and protein concentration. Some of the research team’s breakthroughs that have leveraged HT’s unique and special capabilities can be found in several recent publications, including a lead article on the simultaneous 3D visualization and position tracking of optically trapped particles which was published in Optica on April 20, 2015.
Current fluorescence confocal microscopy techniques require the use of exogenous labeling agents to render high-contrast molecular information. Therefore, drawbacks include possible photo-bleaching, photo-toxicity, and interference with normal molecular activities. Immune or stem cells that need to be reinjected into the body are considered particularly difficult to employ with fluorescence microscopy.
“As one of the two currently available, high-resolution tomographic microscopes in the world, I believe that the HT-1 is the best in class regarding specifications and functionality. Users can see 3D/4D live images of cells, without fixing, coating or staining cells. Sample preparation times are reduced from a few days or hours to just a few minutes,” said Professor Park.
Two Korean hospitals, Seoul National University Hospital in Bundang and Boramae Hospital in Seoul, are using this microscope currently. The research team has also introduced the HT-1 at the Photonics West Exhibition 2016 that took place on February 16-18 in San Francisco, USA.
Professor Park added, “Our technology has set a new paradigm for cell observation under a microscope. I expect that this tomographic microscopy will be more widely used in future in various areas of pharmaceuticals, neuroscience, immunology, hematology, and cell biology.”
Figure 1: HT-1 and Its Specifications
Figure 2: 3D Images of Representative Biological Cells Taken with the HT-1
2016.03.29 View 13898
Next-Generation Holographic Microscope for 3D Live Cell Imaging
KAIST researchers have developed a revolutionary bio-medical imaging tool, the HT-1, to view and analyze cells, which is commercially available.
Professor YongKeun Park of the Physics Department at KAIST and his research team have developed a powerful method for 3D imaging of live cells without staining. The researchers announced the launch of their new microscopic tool, the holotomography (HT)-1, to the global marketplace through a Korean start-up that Professor Park co-founded, TomoCube (www.tomocube.com).
Professor Park is a leading researcher in the field of biophotonics and has dedicated much of his research career to working on digital holographic microscopy technology. He collaborated with TomoCube’s R&D team to develop a state-of-the-art, 2D/3D/4D holographic microscope that would allow a real-time label-free visualization of biological cells and tissues.
The HT is an optical analogy of X-ray computed tomography (CT). Both X-ray CT and HT share the same physical principle—the inverse of wave scattering. The difference is that HT uses laser illumination whereas X-ray CT uses X-ray beams. From the measurement of multiple 2D holograms of a cell, coupled with various angles of laser illuminations, the 3D refractive index (RI) distribution of the cell can be reconstructed. The reconstructed 3D RI map provides structural and chemical information of the cell including mass, morphology, protein concentration, and dynamics of the cellular membrane.
The HT enables users to quantitatively and non-invasively investigate the intrinsic properties of biological cells, for example, dry mass and protein concentration. Some of the research team’s breakthroughs that have leveraged HT’s unique and special capabilities can be found in several recent publications, including a lead article on the simultaneous 3D visualization and position tracking of optically trapped particles which was published in Optica on April 20, 2015.
Current fluorescence confocal microscopy techniques require the use of exogenous labeling agents to render high-contrast molecular information. Therefore, drawbacks include possible photo-bleaching, photo-toxicity, and interference with normal molecular activities. Immune or stem cells that need to be reinjected into the body are considered particularly difficult to employ with fluorescence microscopy.
“As one of the two currently available, high-resolution tomographic microscopes in the world, I believe that the HT-1 is the best in class regarding specifications and functionality. Users can see 3D/4D live images of cells, without fixing, coating or staining cells. Sample preparation times are reduced from a few days or hours to just a few minutes,” said Professor Park.
Two Korean hospitals, Seoul National University Hospital in Bundang and Boramae Hospital in Seoul, are using this microscope currently. The research team has also introduced the HT-1 at the Photonics West Exhibition 2016 that took place on February 16-18 in San Francisco, USA.
Professor Park added, “Our technology has set a new paradigm for cell observation under a microscope. I expect that this tomographic microscopy will be more widely used in future in various areas of pharmaceuticals, neuroscience, immunology, hematology, and cell biology.”
Figure 1: HT-1 and Its Specifications
Figure 2: 3D Images of Representative Biological Cells Taken with the HT-1
2016.03.29 View 13898 -
 KAIST Offers Online Science Magnet High School Program
The Global Institute for Talented Education at KAIST has begun providing middle and high school students with in-depth online science education.
The institute receives applications until March 20, 2016. For details, please refer to the website: http://talented.kaist.ac.kr.
The program will run from March 21, 2016 to June 13, 2016.
Any middle and high school student can take courses on mathematics, science (physics, chemistry and biology), and information system (C language and Python computer language) based on their levels and needs. A total of 23 courses will be offered at the level of the first year of middle school to the second year of high school.
The online lecturers are drawn from science-magnet high schools nationwide. They will lead the classes to become more interactive with students, encouraging discussions and questions and answers.
KAIST students will also take part as tutors, helping the middle and high school students better understand the basic concept of the subjects they undertake and and to think creatively to solve problems.
About 500 top students will be chosen from the online course applicants to participate in a science camp hosted by KAIST during summer and winter vacations.
2016.03.14 View 5568
KAIST Offers Online Science Magnet High School Program
The Global Institute for Talented Education at KAIST has begun providing middle and high school students with in-depth online science education.
The institute receives applications until March 20, 2016. For details, please refer to the website: http://talented.kaist.ac.kr.
The program will run from March 21, 2016 to June 13, 2016.
Any middle and high school student can take courses on mathematics, science (physics, chemistry and biology), and information system (C language and Python computer language) based on their levels and needs. A total of 23 courses will be offered at the level of the first year of middle school to the second year of high school.
The online lecturers are drawn from science-magnet high schools nationwide. They will lead the classes to become more interactive with students, encouraging discussions and questions and answers.
KAIST students will also take part as tutors, helping the middle and high school students better understand the basic concept of the subjects they undertake and and to think creatively to solve problems.
About 500 top students will be chosen from the online course applicants to participate in a science camp hosted by KAIST during summer and winter vacations.
2016.03.14 View 5568 -
 Non-Natural Biomedical Polymers Produced from Microorganisms
KAIST researchers have developed metabolically engineered Escherichia coli strains to synthesize non-natural, biomedically important polymers including poly(lactate-co-glycolate) (PLGA), previously considered impossible to obtain from biobased materials.
Renewable non-food biomass could potentially replace petrochemical raw materials to produce energy sources, useful chemicals, or a vast array of petroleum-based end products such as plastics, lubricants, paints, fertilizers, and vitamin capsules. In recent years, biorefineries which transform non-edible biomass into fuel, heat, power, chemicals, and materials have received a great deal of attention as a sustainable alternative to decreasing the reliance on fossil fuels.
A research team headed by Distinguished Professor Sang Yup Lee of the Chemical and Biomolecular Engineering Department at KAIST has established a biorefinery system to create non-natural polymers from natural sources, allowing various plastics to be made in an environmentally-friendly and sustainable manner. The research results were published online in Nature Biotechnology on March 7, 2016. The print version will be issued in April 2016.
The research team adopted a systems metabolic engineering approach to develop a microorganism that can produce diverse non-natural, biomedically important polymers and succeeded in synthesizing poly(lactate-co-glycolate) (PLGA), a copolymer of two different polymer monomers, lactic and glycolic acid. PLGA is biodegradable, biocompatible, and non-toxic, and has been widely used in biomedical and therapeutic applications such as surgical sutures, prosthetic devices, drug delivery, and tissue engineering.
Inspired by the biosynthesis process for polyhydroxyalkanoates (PHAs), biologically-derived polyesters produced in nature by the bacterial fermentation of sugar or lipids, the research team designed a metabolic pathway for the biosynthesis of PLGA through microbial fermentation directly from carbohydrates in Escherichia coli (E. coli) strains.
The team had previously reported a recombinant E. coli producing PLGA by using the glyoxylate shunt pathway for the generation of glycolate from glucose, which was disclosed in their patents KR10-1575585-0000 (filing date of March 11, 2011), US08883463 and JP5820363. However, they discovered that the polymer content and glycolate fraction of PLGA could not be significantly enhanced via further engineering techniques. Thus, in this research, the team introduced a heterologous pathway to produce glycolate from xylose and succeeded in developing the recombinant E. coli producing PLGA and various novel copolymers much more efficiently.
In order to produce PLGA by microbial fermentation directly from carbohydrates, the team incorporated external and engineered enzymes as catalysts to co-polymerize PLGA while establishing a few additional metabolic pathways for the biosynthesis to produce a range of different non-natural polymers, some for the first time. This bio-based synthetic process for PLGA and other polymers could substitute for the existing complicated chemical production that involves the preparation and purification of precursors, chemical polymerization processes, and the elimination of metal catalysts.
Professor Lee and his team performed in silico genome-scale metabolic simulations of the E. coli cell to predict and analyze changes in the metabolic fluxes of cells which were caused by the introduction of external metabolic pathways. Based on these results, genes are manipulated to optimize metabolic fluxes by eliminating the genes responsible for byproducts formation and enhancing the expression levels of certain genes, thereby achieving the effective production of target polymers as well as stimulating cell growth.
The team utilized the structural basis of broad substrate specificity of the key synthesizing enzyme, PHA synthase, to incorporate various co-monomers with main and side chains of different lengths. These monomers were produced inside the cell by metabolic engineering, and then copolymerized to improve the material properties of PLGA. As a result, a variety of PLGA copolymers with different monomer compositions such as the US Food and Drug Administration (FDA)-approved monomers, 3-hydroxyburate, 4-hydroxyburate, and 6-hydroxyhexanoate, were produced. Newly applied bioplastics such as 5-hydroxyvalerate and 2-hydroxyisovalerate were also made.
The team employed a systems metabolic engineering application which, according to the researchers, is the first successful example of biological production of PGLA and several novel copolymers from renewable biomass by one-step direct fermentation of metabolically engineered E.coli.
Professor Lee said, “We presented important findings that non-natural polymers, such as PLGA which is commonly used for drug delivery or biomedical devices, were produced by a metabolically engineered gut bacterium. Our research is meaningful in that it proposes a platform strategy in metabolic engineering, which can be further utilized in the development of numerous non-natural, useful polymers.”
Director Ilsub Baek at the Platform Technology Division of the Ministry of Science, ICT and Future Planning of Korea, who oversees the Technology Development Program to Solve Climate Change, said, “Professor Lee has led one of our research projects, the Systems Metabolic Engineering for Biorefineries, which began as part of the Ministry’s Technology Development Program to Solve Climate Change. He and his team have continuously achieved promising results and been attracting greater interest from the global scientific community. As climate change technology grows more important, this research on the biological production of non-natural, high value polymers will have a great impact on science and industry.”
The title of the research paper is “One-step Fermentative Production of Poly(lactate-co-glycolate) from Carbohydrates in Escherichia coli (DOI: 10.1038/nbt.3485).” The lead authors are So Young Choi, a Ph.D. candidate in the Department of Chemical and Biomolecular Engineering at KAIST, and Si Jae Park, Assistant Professor of the Environmental Engineering and Energy Department at Myongji University. Won Jun Kim and Jung Eun Yang, both doctoral students in the Department of Chemical and Biomolecular Engineering at KAIST, also participated in the research.
This research was supported by the Technology Development Program to Solve Climate Change’s research project titled “Systems Metabolic Engineering for Biorefineries” from the Ministry of Science, ICT and Future Planning through the National Research Foundation of Korea (NRF-2012M1A2A2026556).
Figure: Production of PLGA and Other Non-Natural Copolymers
This schematic diagram shows the overall conceptualization of how metabolically engineered E. coli produced a variety of PLGAs with different monomer compositions, proposing the chemosynthetic process of non-natural polymers from biomass. The non-natural polymer PLGA and its other copolymers, which were produced by engineered bacteria developed by taking a systems metabolic engineering approach, accumulate in granule forms within a cell.
2016.03.08 View 12797
Non-Natural Biomedical Polymers Produced from Microorganisms
KAIST researchers have developed metabolically engineered Escherichia coli strains to synthesize non-natural, biomedically important polymers including poly(lactate-co-glycolate) (PLGA), previously considered impossible to obtain from biobased materials.
Renewable non-food biomass could potentially replace petrochemical raw materials to produce energy sources, useful chemicals, or a vast array of petroleum-based end products such as plastics, lubricants, paints, fertilizers, and vitamin capsules. In recent years, biorefineries which transform non-edible biomass into fuel, heat, power, chemicals, and materials have received a great deal of attention as a sustainable alternative to decreasing the reliance on fossil fuels.
A research team headed by Distinguished Professor Sang Yup Lee of the Chemical and Biomolecular Engineering Department at KAIST has established a biorefinery system to create non-natural polymers from natural sources, allowing various plastics to be made in an environmentally-friendly and sustainable manner. The research results were published online in Nature Biotechnology on March 7, 2016. The print version will be issued in April 2016.
The research team adopted a systems metabolic engineering approach to develop a microorganism that can produce diverse non-natural, biomedically important polymers and succeeded in synthesizing poly(lactate-co-glycolate) (PLGA), a copolymer of two different polymer monomers, lactic and glycolic acid. PLGA is biodegradable, biocompatible, and non-toxic, and has been widely used in biomedical and therapeutic applications such as surgical sutures, prosthetic devices, drug delivery, and tissue engineering.
Inspired by the biosynthesis process for polyhydroxyalkanoates (PHAs), biologically-derived polyesters produced in nature by the bacterial fermentation of sugar or lipids, the research team designed a metabolic pathway for the biosynthesis of PLGA through microbial fermentation directly from carbohydrates in Escherichia coli (E. coli) strains.
The team had previously reported a recombinant E. coli producing PLGA by using the glyoxylate shunt pathway for the generation of glycolate from glucose, which was disclosed in their patents KR10-1575585-0000 (filing date of March 11, 2011), US08883463 and JP5820363. However, they discovered that the polymer content and glycolate fraction of PLGA could not be significantly enhanced via further engineering techniques. Thus, in this research, the team introduced a heterologous pathway to produce glycolate from xylose and succeeded in developing the recombinant E. coli producing PLGA and various novel copolymers much more efficiently.
In order to produce PLGA by microbial fermentation directly from carbohydrates, the team incorporated external and engineered enzymes as catalysts to co-polymerize PLGA while establishing a few additional metabolic pathways for the biosynthesis to produce a range of different non-natural polymers, some for the first time. This bio-based synthetic process for PLGA and other polymers could substitute for the existing complicated chemical production that involves the preparation and purification of precursors, chemical polymerization processes, and the elimination of metal catalysts.
Professor Lee and his team performed in silico genome-scale metabolic simulations of the E. coli cell to predict and analyze changes in the metabolic fluxes of cells which were caused by the introduction of external metabolic pathways. Based on these results, genes are manipulated to optimize metabolic fluxes by eliminating the genes responsible for byproducts formation and enhancing the expression levels of certain genes, thereby achieving the effective production of target polymers as well as stimulating cell growth.
The team utilized the structural basis of broad substrate specificity of the key synthesizing enzyme, PHA synthase, to incorporate various co-monomers with main and side chains of different lengths. These monomers were produced inside the cell by metabolic engineering, and then copolymerized to improve the material properties of PLGA. As a result, a variety of PLGA copolymers with different monomer compositions such as the US Food and Drug Administration (FDA)-approved monomers, 3-hydroxyburate, 4-hydroxyburate, and 6-hydroxyhexanoate, were produced. Newly applied bioplastics such as 5-hydroxyvalerate and 2-hydroxyisovalerate were also made.
The team employed a systems metabolic engineering application which, according to the researchers, is the first successful example of biological production of PGLA and several novel copolymers from renewable biomass by one-step direct fermentation of metabolically engineered E.coli.
Professor Lee said, “We presented important findings that non-natural polymers, such as PLGA which is commonly used for drug delivery or biomedical devices, were produced by a metabolically engineered gut bacterium. Our research is meaningful in that it proposes a platform strategy in metabolic engineering, which can be further utilized in the development of numerous non-natural, useful polymers.”
Director Ilsub Baek at the Platform Technology Division of the Ministry of Science, ICT and Future Planning of Korea, who oversees the Technology Development Program to Solve Climate Change, said, “Professor Lee has led one of our research projects, the Systems Metabolic Engineering for Biorefineries, which began as part of the Ministry’s Technology Development Program to Solve Climate Change. He and his team have continuously achieved promising results and been attracting greater interest from the global scientific community. As climate change technology grows more important, this research on the biological production of non-natural, high value polymers will have a great impact on science and industry.”
The title of the research paper is “One-step Fermentative Production of Poly(lactate-co-glycolate) from Carbohydrates in Escherichia coli (DOI: 10.1038/nbt.3485).” The lead authors are So Young Choi, a Ph.D. candidate in the Department of Chemical and Biomolecular Engineering at KAIST, and Si Jae Park, Assistant Professor of the Environmental Engineering and Energy Department at Myongji University. Won Jun Kim and Jung Eun Yang, both doctoral students in the Department of Chemical and Biomolecular Engineering at KAIST, also participated in the research.
This research was supported by the Technology Development Program to Solve Climate Change’s research project titled “Systems Metabolic Engineering for Biorefineries” from the Ministry of Science, ICT and Future Planning through the National Research Foundation of Korea (NRF-2012M1A2A2026556).
Figure: Production of PLGA and Other Non-Natural Copolymers
This schematic diagram shows the overall conceptualization of how metabolically engineered E. coli produced a variety of PLGAs with different monomer compositions, proposing the chemosynthetic process of non-natural polymers from biomass. The non-natural polymer PLGA and its other copolymers, which were produced by engineered bacteria developed by taking a systems metabolic engineering approach, accumulate in granule forms within a cell.
2016.03.08 View 12797 -
 KAIST Commencement 2016
KAIST hosted its 2016 commencement ceremony on February 19, 2016 at the Sports Complex on campus.
KAIST celebrated the event with five thousand participants including graduating students, faculty, guests, Vice Minister Nam-Ki Hong of Science, ICT and Future Planning of Korea, Chairman Jang-Moo Lee of KAIST's Board of Trustees, and President Jeong-Sik Ko of the KAIST Alumni Association.
President Patrick Aebischer of the Swiss Federal Institute of Technology in Lausanne (EPFL), Switzerland, and the former Speaker of the National Assembly of Korea Chang-Hee Kang received honorary doctorates in science and technology for their contributions to the advancement of science and engineering in education and research.
KAIST granted 570 doctoral degrees, 1,329 master’s degrees, and 867 bachelor degrees on this day.
Yoon-Bum Lee of the Chemistry Department graduated with honors; Woo-Young Jin of the Mathematical Sciences Department received the Chairman’s Award of the KAIST Board and Eun-Hee Yoo of the Biological Sciences Department for the KAIST Presidential Award. Min-Hyun Cho and Yoon-Seok Chang were recipients of the President’s Award of the KAIST Alumni Association and the President’s Award of the University Supporting Association, respectively.
President Steve Kang addressed the ceremony and congratulated the graduates, saying,
“Now, your task is to make significant contributions to your communities: be leaders in your fields and remain active members of society. Given your academic knowledge and vision for the future, I encourage you to dream big.”
2016.02.23 View 43495
KAIST Commencement 2016
KAIST hosted its 2016 commencement ceremony on February 19, 2016 at the Sports Complex on campus.
KAIST celebrated the event with five thousand participants including graduating students, faculty, guests, Vice Minister Nam-Ki Hong of Science, ICT and Future Planning of Korea, Chairman Jang-Moo Lee of KAIST's Board of Trustees, and President Jeong-Sik Ko of the KAIST Alumni Association.
President Patrick Aebischer of the Swiss Federal Institute of Technology in Lausanne (EPFL), Switzerland, and the former Speaker of the National Assembly of Korea Chang-Hee Kang received honorary doctorates in science and technology for their contributions to the advancement of science and engineering in education and research.
KAIST granted 570 doctoral degrees, 1,329 master’s degrees, and 867 bachelor degrees on this day.
Yoon-Bum Lee of the Chemistry Department graduated with honors; Woo-Young Jin of the Mathematical Sciences Department received the Chairman’s Award of the KAIST Board and Eun-Hee Yoo of the Biological Sciences Department for the KAIST Presidential Award. Min-Hyun Cho and Yoon-Seok Chang were recipients of the President’s Award of the KAIST Alumni Association and the President’s Award of the University Supporting Association, respectively.
President Steve Kang addressed the ceremony and congratulated the graduates, saying,
“Now, your task is to make significant contributions to your communities: be leaders in your fields and remain active members of society. Given your academic knowledge and vision for the future, I encourage you to dream big.”
2016.02.23 View 43495 -
 Meditox Donates 600 Million KRW Scholarship
On February 17, a Korean biopharmaceutical company Meditox, headed by Chief Executive Officer (CEO) Hyun-Ho Jeong, signed a memorandum of understanding (MOU) with KAIST to establish the “Meditox Fellowship” and donated a total of 600 million Korean won (KRW) to the university to assist in promoting more scientists in the field of biology.
Meditox CEO Hyun-Ho Jeong, KAIST President Steve Kang, Dean of Life Science and Bioengineering College Jung-Hoe Kim, and Dean of the Department of Biological Sciences Byung-Ha Oh participated in the agreement ceremony.
According to the MOU, Meditox will donate 60,000,000 KRW over a ten year period, from which KAIST can draw on to grant scholarships for master’s and doctoral students.
The “Meditox Fellowship” will support promising and enthusiastic students whose finances limit their studies. The first scholarship students for 2016 were: Kwang-Uk Min, In-suk Yeo, Sung-ryung- Lee, Si-on Lee, and Jung-hyun Kim.
Meditox CEO Jeong, who graduated from KAIST’s Department of Biological Sciences, said, "I felt it was important to start the Meditox Fellowship at my alma mater to contribute to the cultivation of outstanding scientists in the field of biological sciences."
He also said that he would plan to launch projects that aim to support not only those who receive the scholarship but also the development of Korea’s biological sciences in general.
President Steve Kang (right) and Chief Executive Officer Hyun-Ho Jeong (left) of Meditox hold the signed memorandum of understanding together.
2016.02.18 View 11609
Meditox Donates 600 Million KRW Scholarship
On February 17, a Korean biopharmaceutical company Meditox, headed by Chief Executive Officer (CEO) Hyun-Ho Jeong, signed a memorandum of understanding (MOU) with KAIST to establish the “Meditox Fellowship” and donated a total of 600 million Korean won (KRW) to the university to assist in promoting more scientists in the field of biology.
Meditox CEO Hyun-Ho Jeong, KAIST President Steve Kang, Dean of Life Science and Bioengineering College Jung-Hoe Kim, and Dean of the Department of Biological Sciences Byung-Ha Oh participated in the agreement ceremony.
According to the MOU, Meditox will donate 60,000,000 KRW over a ten year period, from which KAIST can draw on to grant scholarships for master’s and doctoral students.
The “Meditox Fellowship” will support promising and enthusiastic students whose finances limit their studies. The first scholarship students for 2016 were: Kwang-Uk Min, In-suk Yeo, Sung-ryung- Lee, Si-on Lee, and Jung-hyun Kim.
Meditox CEO Jeong, who graduated from KAIST’s Department of Biological Sciences, said, "I felt it was important to start the Meditox Fellowship at my alma mater to contribute to the cultivation of outstanding scientists in the field of biological sciences."
He also said that he would plan to launch projects that aim to support not only those who receive the scholarship but also the development of Korea’s biological sciences in general.
President Steve Kang (right) and Chief Executive Officer Hyun-Ho Jeong (left) of Meditox hold the signed memorandum of understanding together.
2016.02.18 View 11609