research
-
 Enhanced Video Quality despite Poor Network Conditions
(from left: Jaehong Kim, Youngmok Jung, Hyunho Yeo, Professor Dongsu Han and Professor Jinwoo Shin)
Professor Jinwoo Shin and Professor Dongsu Han from the School of Electrical Engineering developed neural adaptive content-aware internet video delivery. This technology is a novel method that combines adaptive streaming over HTTP, the video transmission system adopted by YouTube and Netflix, with a deep learning model.
This technology is expected to create an internet environment where users can enjoy watching 4K and AV/VR videos with high-quality and high-definition (HD) videos even with weak internet connections.
Thanks to video streaming services, internet video has experienced remarkable growth; nevertheless, users often suffer from low video quality due to unfavorable network conditions. Currently, existing adaptive streaming systems adjust the quality of the video in real time, accommodating the continuously changing internet bandwidth. Various algorithms are being researched for adaptive streaming systems, but there is an inherent limitation; that is, high-quality videos cannot be streamed in poor network environments regardless of which algorithm is used.
By incorporating super-resolution in adaptive streaming, the team overcame the limit of existing content distribution networks, of which their quality relies too much on the bandwidth. In the conventional method, the server that provides the video splits a video into certain lengths of time in advance. But the novel system introduced by the team allows the downloading of neural network segments. To facilitate this method, the video server needs to provide deep neural networks for each video segment as well as sizes of Deep Neural Networks (DNN) according to the specifications of the user’s computing capacity.
The largest neural network size is two megabytes, which is considerably smaller than video. When downloading the neural network from the user’s video player, it is split into several segments. Even its partial download is sufficient for a slightly comprised super-resolution.
While playing the video, the system converts the low quality video to a high-quality version by employing super-resolution based on deep convolution neural networks (CNN). The entire process is done in real time, and users can enjoy the high-definition video.
Even with a 17% smaller bandwidth, the system can provide the Quality of Experience equivalent to the latest adaptive streaming service. At a given internet bandwidth, it can provide 43% higher average QoE than the latest service.
Using a deep learning method allows this system to achieve a higher level of compression than the existing video compression methods. Their technology was recognized as a next-generation internet video system that applies super-resolution based on a deep convolution neural network to online videos.
Professor Han said, “So far, it has only been implemented on desktops, but we will further develop applications that work in mobile devices as well. This technology has been applied to the same video transmission systems used by streaming channels such as YouTube and Netflix, and thus shows good signs for practicability.”
This research, led by Hyunho Yeo, Youngmok Jung and Jaehong Kim, was presented at the 13th UNSENIX OSDI conference on October 10 2018 and completed for filing international patent application.
For further information, please click here.
Figure 1. Image quality before (left) and after (right) the technology application
Figure 2. The technology Concept
Figure 3. A transition from low-quality to high quality video after video transmission from the video server
2019.01.22 View 7453
Enhanced Video Quality despite Poor Network Conditions
(from left: Jaehong Kim, Youngmok Jung, Hyunho Yeo, Professor Dongsu Han and Professor Jinwoo Shin)
Professor Jinwoo Shin and Professor Dongsu Han from the School of Electrical Engineering developed neural adaptive content-aware internet video delivery. This technology is a novel method that combines adaptive streaming over HTTP, the video transmission system adopted by YouTube and Netflix, with a deep learning model.
This technology is expected to create an internet environment where users can enjoy watching 4K and AV/VR videos with high-quality and high-definition (HD) videos even with weak internet connections.
Thanks to video streaming services, internet video has experienced remarkable growth; nevertheless, users often suffer from low video quality due to unfavorable network conditions. Currently, existing adaptive streaming systems adjust the quality of the video in real time, accommodating the continuously changing internet bandwidth. Various algorithms are being researched for adaptive streaming systems, but there is an inherent limitation; that is, high-quality videos cannot be streamed in poor network environments regardless of which algorithm is used.
By incorporating super-resolution in adaptive streaming, the team overcame the limit of existing content distribution networks, of which their quality relies too much on the bandwidth. In the conventional method, the server that provides the video splits a video into certain lengths of time in advance. But the novel system introduced by the team allows the downloading of neural network segments. To facilitate this method, the video server needs to provide deep neural networks for each video segment as well as sizes of Deep Neural Networks (DNN) according to the specifications of the user’s computing capacity.
The largest neural network size is two megabytes, which is considerably smaller than video. When downloading the neural network from the user’s video player, it is split into several segments. Even its partial download is sufficient for a slightly comprised super-resolution.
While playing the video, the system converts the low quality video to a high-quality version by employing super-resolution based on deep convolution neural networks (CNN). The entire process is done in real time, and users can enjoy the high-definition video.
Even with a 17% smaller bandwidth, the system can provide the Quality of Experience equivalent to the latest adaptive streaming service. At a given internet bandwidth, it can provide 43% higher average QoE than the latest service.
Using a deep learning method allows this system to achieve a higher level of compression than the existing video compression methods. Their technology was recognized as a next-generation internet video system that applies super-resolution based on a deep convolution neural network to online videos.
Professor Han said, “So far, it has only been implemented on desktops, but we will further develop applications that work in mobile devices as well. This technology has been applied to the same video transmission systems used by streaming channels such as YouTube and Netflix, and thus shows good signs for practicability.”
This research, led by Hyunho Yeo, Youngmok Jung and Jaehong Kim, was presented at the 13th UNSENIX OSDI conference on October 10 2018 and completed for filing international patent application.
For further information, please click here.
Figure 1. Image quality before (left) and after (right) the technology application
Figure 2. The technology Concept
Figure 3. A transition from low-quality to high quality video after video transmission from the video server
2019.01.22 View 7453 -
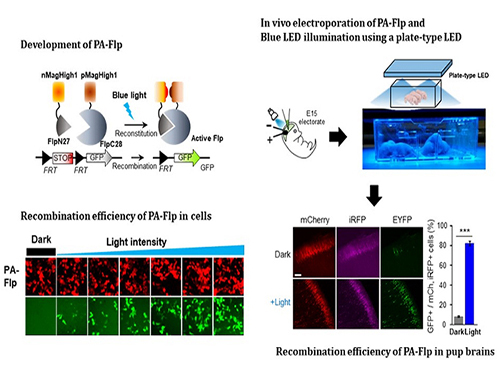 Noninvasive Light-Sensitive Recombinase for Deep Brain Genetic Manipulation
A KAIST team presented a noninvasive light-sensitive photoactivatable recombinase suitable for genetic manipulation in vivo. The highly light-sensitive property of photoactivatable Flp recombinase will be ideal for controlling genetic manipulation in deep mouse brain regions by illumination with a noninvasive light-emitting diode. This easy-to-use optogenetic module made by Professor Won Do Heo and his team will provide a side-effect free and expandable genetic manipulation tool for neuroscience research.
Spatiotemporal control of gene expression has been acclaimed as a valuable strategy for identifying functions of genes with complex neural circuits. Studies of complex brain functions require highly sophisticated and robust technologies that enable specific labeling and rapid genetic modification in live animals. A number of approaches for controlling the activity of proteins or expression of genes in a spatiotemporal manner using light, small molecules, hormones, and peptides have been developed for manipulating intact circuits or functions.
Among them, recombination-employing, chemically inducible systems are the most commonly used in vivo gene-modification systems. Other approaches include selective or conditional Cre-activation systems within subsets of green fluorescent protein-expressing cells or dual-promoter-driven intersectional populations of cells.
However, these methods are limited by the considerable time and effort required to establish knock-in mouse lines and by constraints on spatiotemporal control, which relies on a limited set of available genetic promoters and transgenic mouse resources.
Beyond these constraints, optogenetic approaches allow the activity of genetically defined neurons in the mouse brain to be controlled with high spatiotemporal resolution. However, an optogenetic module for gene-manipulation capable of revealing the spatiotemporal functions of specific target genes in the mouse brain has remained a challenge.
In the study published at Nature Communication on Jan. 18, the team featured photoactivatable Flp recombinase by searching out split sites of Flp recombinase that were not previously identified, being capable of reconstitution to be active. The team validated the highly light-sensitive, efficient performance of photoactivatable Flp recombinase through precise light targeting by showing transgene expression within anatomically confined mouse brain regions.
The concept of local genetic labeling presented here suggests a new approach for genetically identifying subpopulations of cells defined by the spatial and temporal characteristics of light delivery. To date, an optogenetic module for gene-manipulation capable of revealing spatiotemporal functions of specific target genes in the mouse brain has remained out of reach and no such light-inducible Flp system has been developed. Accordingly, the team sought to develop a photoactivatable Flp recombinase that takes full advantage of the high spatiotemporal control offered by light stimulation.
This activation through noninvasive light illumination deep inside the brain is advantageous in that it avoids chemical or optic fiber implantation-mediated side effects, such as off-target cytotoxicity or physical lesions that might influence animal physiology or behaviors. The technique provides expandable utilities for transgene expression systems upon Flp recombinase activity in vivo, by designing a viral vector for minimal leaky expression influenced by viral nascent promoters.
The team demonstrated the utility of PA-Flp as a noninvasive in vivo optogenetic manipulation tool for use in the mouse brain, even applicable for deep brain structures as it can reach the hippocampus or medial septum using external LED light illumination.
The study is the result of five years of research by Professor Heo, who has led the bio-imaging and optogenetics fields by developing his own bio-imaging and optogenetics technologies. “It will be a great advantage to control specific gene expression desired by LEDs with little physical and chemical stimulation that can affect the physiological phenomenon in living animals,” he explained.
2019.01.22 View 7986
Noninvasive Light-Sensitive Recombinase for Deep Brain Genetic Manipulation
A KAIST team presented a noninvasive light-sensitive photoactivatable recombinase suitable for genetic manipulation in vivo. The highly light-sensitive property of photoactivatable Flp recombinase will be ideal for controlling genetic manipulation in deep mouse brain regions by illumination with a noninvasive light-emitting diode. This easy-to-use optogenetic module made by Professor Won Do Heo and his team will provide a side-effect free and expandable genetic manipulation tool for neuroscience research.
Spatiotemporal control of gene expression has been acclaimed as a valuable strategy for identifying functions of genes with complex neural circuits. Studies of complex brain functions require highly sophisticated and robust technologies that enable specific labeling and rapid genetic modification in live animals. A number of approaches for controlling the activity of proteins or expression of genes in a spatiotemporal manner using light, small molecules, hormones, and peptides have been developed for manipulating intact circuits or functions.
Among them, recombination-employing, chemically inducible systems are the most commonly used in vivo gene-modification systems. Other approaches include selective or conditional Cre-activation systems within subsets of green fluorescent protein-expressing cells or dual-promoter-driven intersectional populations of cells.
However, these methods are limited by the considerable time and effort required to establish knock-in mouse lines and by constraints on spatiotemporal control, which relies on a limited set of available genetic promoters and transgenic mouse resources.
Beyond these constraints, optogenetic approaches allow the activity of genetically defined neurons in the mouse brain to be controlled with high spatiotemporal resolution. However, an optogenetic module for gene-manipulation capable of revealing the spatiotemporal functions of specific target genes in the mouse brain has remained a challenge.
In the study published at Nature Communication on Jan. 18, the team featured photoactivatable Flp recombinase by searching out split sites of Flp recombinase that were not previously identified, being capable of reconstitution to be active. The team validated the highly light-sensitive, efficient performance of photoactivatable Flp recombinase through precise light targeting by showing transgene expression within anatomically confined mouse brain regions.
The concept of local genetic labeling presented here suggests a new approach for genetically identifying subpopulations of cells defined by the spatial and temporal characteristics of light delivery. To date, an optogenetic module for gene-manipulation capable of revealing spatiotemporal functions of specific target genes in the mouse brain has remained out of reach and no such light-inducible Flp system has been developed. Accordingly, the team sought to develop a photoactivatable Flp recombinase that takes full advantage of the high spatiotemporal control offered by light stimulation.
This activation through noninvasive light illumination deep inside the brain is advantageous in that it avoids chemical or optic fiber implantation-mediated side effects, such as off-target cytotoxicity or physical lesions that might influence animal physiology or behaviors. The technique provides expandable utilities for transgene expression systems upon Flp recombinase activity in vivo, by designing a viral vector for minimal leaky expression influenced by viral nascent promoters.
The team demonstrated the utility of PA-Flp as a noninvasive in vivo optogenetic manipulation tool for use in the mouse brain, even applicable for deep brain structures as it can reach the hippocampus or medial septum using external LED light illumination.
The study is the result of five years of research by Professor Heo, who has led the bio-imaging and optogenetics fields by developing his own bio-imaging and optogenetics technologies. “It will be a great advantage to control specific gene expression desired by LEDs with little physical and chemical stimulation that can affect the physiological phenomenon in living animals,” he explained.
2019.01.22 View 7986 -
 A Comprehensive Metabolic Map for Bio-Based Chemicals Production
A KAIST research team completed a metabolic map that charts all available strategies and pathways of chemical reactions that lead to the production of various industrial bio-based chemicals.
The team was led by Distinguished Professor Sang Yup Lee, who has produced high-quality metabolic engineering and systems engineering research for decades, and made the hallmark chemicals map after seven years of studies.
The team presented a very detailed analysis on metabolic engineering for the production of a wide range of industrial chemicals, fuels, and materials. Surveying the current trends in the bio-based production of chemicals in industrial biotechnology, the team thoroughly examined the current status of industrial chemicals produced using biological and/or chemical reactions.
This comprehensive map is expected to serve as a blueprint for the visual and intuitive inspection of biological and/or chemical reactions for the production of interest from renewable resources. The team also compiled an accompanying poster to visually present the synthetic pathways of chemicals in the context of their microbial metabolism.
As metabolic engineering has become increasing powerful in addressing limited fossil resources, climate change, and other environmental issues, the number of microbially produced chemicals using biomass as a carbon source has increased substantially. The sustainable production of industrial chemicals and materials has been explored with micro-organisms as cell factories and renewable nonfood biomass as raw materials for alternative petroleum. The engineering of these micro-organism has increasingly become more efficient and effective with the help of metabolic engineering – a practice of engineering using the metabolism of living organisms to produce a desired metabolite.
With the establishment of systems metabolic engineering – the integration of metabolic engineering with tools and strategies from systems biology, synthetic biology and evolutionary engineering – the speed at which micro-organisms are being engineered has reached an unparalleled pace.
In order to evaluate the current state at which metabolically engineered micro-organisms can produce a large portfolio of industrial chemicals, the team conducted an extensive review of the literature and mapped them out on a poster. This resulting poster, termed the bio-based chemicals map, presents synthetic pathways for industrial chemicals, which consist of biological and/or chemical reactions.
Industrial chemicals and their production routes are presented in the context of central carbon metabolic pathways as these key metabolites serve as precursors for the chemicals to be produced. The resulting biochemical map allows the detection and analysis of optimal synthetic pathways for a given industrial chemical. In addition to the poster, the authors have compiled a list of chemicals that have successfully been produced using micro-organisms and a list of the corresponding companies producing them commercially. This thorough review of the literature and the accompanying analytical summary will be an important resource for researchers interested in the production of chemicals from renewable biomass sources.
Metabolically engineered micro-organisms have already made a huge contribution toward the sustainable production of chemicals using renewable resources. Professor Lee said he wanted a detailed survey of the current state and capacity of bio-based chemicals production.
“We are so excited that this review and poster will expand further discussion on the production of important chemicals through engineered micro-organisms and also combined biological and chemical means in a more sustainable manner,” he explained.
This work was supported by the Technology Development Program to Solve Climate Changes on Systems Metabolic Engineering for Biofineries from the Ministry of Science and ICT through the National Research Foundation of Korea.
For further information, Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering at KAIST ( leesy@kaist.ac.kr , Tel: +82-42-350-3930)
Figure: Bio-based chemicals production through biological and chemical routes. This metabolic map describes representative chemicals that can be produced either by biological and/or chemical means. Red arrows represent chemical routes and blue arrows represent biological routes. Intermediate metabolites in the metabolism of a living organism can serve as a platform toward the production of industrially relevant chemicals. A more comprehensive map presented by the team can be found as a poster in the review.
2019.01.15 View 7946
A Comprehensive Metabolic Map for Bio-Based Chemicals Production
A KAIST research team completed a metabolic map that charts all available strategies and pathways of chemical reactions that lead to the production of various industrial bio-based chemicals.
The team was led by Distinguished Professor Sang Yup Lee, who has produced high-quality metabolic engineering and systems engineering research for decades, and made the hallmark chemicals map after seven years of studies.
The team presented a very detailed analysis on metabolic engineering for the production of a wide range of industrial chemicals, fuels, and materials. Surveying the current trends in the bio-based production of chemicals in industrial biotechnology, the team thoroughly examined the current status of industrial chemicals produced using biological and/or chemical reactions.
This comprehensive map is expected to serve as a blueprint for the visual and intuitive inspection of biological and/or chemical reactions for the production of interest from renewable resources. The team also compiled an accompanying poster to visually present the synthetic pathways of chemicals in the context of their microbial metabolism.
As metabolic engineering has become increasing powerful in addressing limited fossil resources, climate change, and other environmental issues, the number of microbially produced chemicals using biomass as a carbon source has increased substantially. The sustainable production of industrial chemicals and materials has been explored with micro-organisms as cell factories and renewable nonfood biomass as raw materials for alternative petroleum. The engineering of these micro-organism has increasingly become more efficient and effective with the help of metabolic engineering – a practice of engineering using the metabolism of living organisms to produce a desired metabolite.
With the establishment of systems metabolic engineering – the integration of metabolic engineering with tools and strategies from systems biology, synthetic biology and evolutionary engineering – the speed at which micro-organisms are being engineered has reached an unparalleled pace.
In order to evaluate the current state at which metabolically engineered micro-organisms can produce a large portfolio of industrial chemicals, the team conducted an extensive review of the literature and mapped them out on a poster. This resulting poster, termed the bio-based chemicals map, presents synthetic pathways for industrial chemicals, which consist of biological and/or chemical reactions.
Industrial chemicals and their production routes are presented in the context of central carbon metabolic pathways as these key metabolites serve as precursors for the chemicals to be produced. The resulting biochemical map allows the detection and analysis of optimal synthetic pathways for a given industrial chemical. In addition to the poster, the authors have compiled a list of chemicals that have successfully been produced using micro-organisms and a list of the corresponding companies producing them commercially. This thorough review of the literature and the accompanying analytical summary will be an important resource for researchers interested in the production of chemicals from renewable biomass sources.
Metabolically engineered micro-organisms have already made a huge contribution toward the sustainable production of chemicals using renewable resources. Professor Lee said he wanted a detailed survey of the current state and capacity of bio-based chemicals production.
“We are so excited that this review and poster will expand further discussion on the production of important chemicals through engineered micro-organisms and also combined biological and chemical means in a more sustainable manner,” he explained.
This work was supported by the Technology Development Program to Solve Climate Changes on Systems Metabolic Engineering for Biofineries from the Ministry of Science and ICT through the National Research Foundation of Korea.
For further information, Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering at KAIST ( leesy@kaist.ac.kr , Tel: +82-42-350-3930)
Figure: Bio-based chemicals production through biological and chemical routes. This metabolic map describes representative chemicals that can be produced either by biological and/or chemical means. Red arrows represent chemical routes and blue arrows represent biological routes. Intermediate metabolites in the metabolism of a living organism can serve as a platform toward the production of industrially relevant chemicals. A more comprehensive map presented by the team can be found as a poster in the review.
2019.01.15 View 7946 -
 Technology to Control Near-Field Thermal Radiation
(from left clockwise: Professor Seung Seob Lee, Professor Bong Jae Lee, PhD Mikyung Lim and PhD candidate Jaeman Song)
A KAIST research team succeeded in measuring and controlling the near-field thermal radiation between metallo-dielectric (MD) multilayer structures.
Their thermal radiation control technology can be applied to next-generation semiconductor packaging, thermophotovoltaic cells and thermal management systems. It also has the potential to be applied to a sustainable energy source for IoT sensors.
In the nanoscale gaps, thermal radiation between objects increases greatly with closer distances. The amount of heat transfer in this scale was found to be from 1,000 to 10,000 times greater than the blackbody radiation heat transfer, which was once considered the theoretical maximum for the rate of thermal radiation. This phenomenon is called near-field thermal radiation. With recent developments in nanotechnology, research into near-field thermal radiation between various materials has been actively carried out.
Surface polariton coupling generated from nanostructures has been of particular interest because it enhances the amount of near-field thermal radiation between two objects, and allows the spectral control of near-field thermal radiation. This advantage has motivated much of the recent theoretical research on the application of near-field thermal radiation using nanostructures, such as thin films, multilayer nanostructures, and nanowires. Nevertheless, thus far, most of the studies have focused on measuring near-field thermal radiation between isotropic materials.
A joint team led by Professor Bong Jae Lee and Professor Seung Seob Lee from the Department of Mechanical Engineering succeeded in measuring near-field thermal radiation according to the vacuum distance between MD multilayer nanostructures by using a custom MEMS (Micro-Electro-Mechanical Systems)-device-integrated platform with three-axis nanopositioner.
MD multilayer nanostructures refer to structures in which metal and dielectric layers with regular thickness alternate. The MD single-layer pair is referred to as a unit cell, and the ratio of the thickness occupied by the metal layer in the unit cell is called the fill factor.
By measuring the near-field thermal radiation with a varying number of unit cells and the fill factor of the multilayer nanostructures, the team demonstrated that the surface plasmon polariton coupling enhances near-field thermal radiation greatly, and allows spectral control over the heat transfer.
Professor B. J. Lee said, “The isotropic materials that have so far been studied experimentally had limited spectral control over the near-field thermal radiation. Our near-field thermal radiation control technology using multilayer nanostructures is expected to become the first step toward developing various near-field thermal radiation applications.”
This research, led by PhD Mikyung Lim and PhD candidate Jaeman Song, was published in Nature Communications on October 16.
Figure 1. Experimental setup for measuring near-field thermal radiation between MD multilayers
Figure 2. Investigation of manipulated near-field heat flux by modifying the surface conditions with MD multilayers
2019.01.04 View 6968
Technology to Control Near-Field Thermal Radiation
(from left clockwise: Professor Seung Seob Lee, Professor Bong Jae Lee, PhD Mikyung Lim and PhD candidate Jaeman Song)
A KAIST research team succeeded in measuring and controlling the near-field thermal radiation between metallo-dielectric (MD) multilayer structures.
Their thermal radiation control technology can be applied to next-generation semiconductor packaging, thermophotovoltaic cells and thermal management systems. It also has the potential to be applied to a sustainable energy source for IoT sensors.
In the nanoscale gaps, thermal radiation between objects increases greatly with closer distances. The amount of heat transfer in this scale was found to be from 1,000 to 10,000 times greater than the blackbody radiation heat transfer, which was once considered the theoretical maximum for the rate of thermal radiation. This phenomenon is called near-field thermal radiation. With recent developments in nanotechnology, research into near-field thermal radiation between various materials has been actively carried out.
Surface polariton coupling generated from nanostructures has been of particular interest because it enhances the amount of near-field thermal radiation between two objects, and allows the spectral control of near-field thermal radiation. This advantage has motivated much of the recent theoretical research on the application of near-field thermal radiation using nanostructures, such as thin films, multilayer nanostructures, and nanowires. Nevertheless, thus far, most of the studies have focused on measuring near-field thermal radiation between isotropic materials.
A joint team led by Professor Bong Jae Lee and Professor Seung Seob Lee from the Department of Mechanical Engineering succeeded in measuring near-field thermal radiation according to the vacuum distance between MD multilayer nanostructures by using a custom MEMS (Micro-Electro-Mechanical Systems)-device-integrated platform with three-axis nanopositioner.
MD multilayer nanostructures refer to structures in which metal and dielectric layers with regular thickness alternate. The MD single-layer pair is referred to as a unit cell, and the ratio of the thickness occupied by the metal layer in the unit cell is called the fill factor.
By measuring the near-field thermal radiation with a varying number of unit cells and the fill factor of the multilayer nanostructures, the team demonstrated that the surface plasmon polariton coupling enhances near-field thermal radiation greatly, and allows spectral control over the heat transfer.
Professor B. J. Lee said, “The isotropic materials that have so far been studied experimentally had limited spectral control over the near-field thermal radiation. Our near-field thermal radiation control technology using multilayer nanostructures is expected to become the first step toward developing various near-field thermal radiation applications.”
This research, led by PhD Mikyung Lim and PhD candidate Jaeman Song, was published in Nature Communications on October 16.
Figure 1. Experimental setup for measuring near-field thermal radiation between MD multilayers
Figure 2. Investigation of manipulated near-field heat flux by modifying the surface conditions with MD multilayers
2019.01.04 View 6968 -
 Ultrathin Digital Camera Inspired by Xenos Peckii Eyes
(Professor Ki-Hun Jeong from the Department of Bio and Brain Engineering)
The visual system of Xenos peckii, an endoparasite of paper wasps, demonstrates distinct benefits for high sensitivity and high resolution, differing from the compound eyes of most insects. Taking their unique features, a KAIST team developed an ultrathin digital camera that emulates the unique eyes of Xenos peckii.
The ultrathin digital camera offers a wide field of view and high resolution in a slimmer body compared to existing imaging systems. It is expected to support various applications, such as monitoring equipment, medical imaging devices, and mobile imaging systems.
Professor Ki-Hun Jeong from the Department of Bio and Brain Engineering and his team are known for mimicking biological visual organs. The team’s past research includes an LED lens based on the abdominal segments of fireflies and biologically inspired anti-reflective structures.
Recently, the demand for ultrathin digital cameras has increased, due to the miniaturization of electronic and optical devices. However, most camera modules use multiple lenses along the optical axis to compensate for optical aberrations, resulting in a larger volume as well as a thicker total track length of digital cameras. Resolution and sensitivity would be compromised if these modules were to be simply reduced in size and thickness.
To address this issue, the team have developed micro-optical components, inspired from the visual system of Xenos peckii, and combined them with a CMOS (complementary metal oxide semiconductor) image sensor to achieve an ultrathin digital camera.
This new camera, measuring less than 2mm in thickness, emulates the eyes of Xenos peckii by using dozens of microprism arrays and microlens arrays. A microprism and microlens pair form a channel and the light-absorbing medium between the channels reduces optical crosstalk. Each channel captures the partial image at slightly different orientation, and the retrieved partial images are combined into a single image, thereby ensuring a wide field of view and high resolution.
Professor Jeong said, “We have proposed a novel method of fabricating an ultrathin camera. As the first insect-inspired, ultrathin camera that integrates a microcamera on a conventional CMOS image sensor array, our study will have a significant impact in optics and related fields.”
This research, led by PhD candidates Dongmin Keum and Kyung-Won Jang, was published in Light: Science & Applications on October 24, 2018.
Figure 1. Natural Xenos peckii eye and the biological inspiration for the ultrathin digital camera (Light: Science & Applications 2018)
Figure 2. Optical images captured by the bioinspired ultrathin digital camera (Light: Science & Applications 2018)
2018.12.31 View 9496
Ultrathin Digital Camera Inspired by Xenos Peckii Eyes
(Professor Ki-Hun Jeong from the Department of Bio and Brain Engineering)
The visual system of Xenos peckii, an endoparasite of paper wasps, demonstrates distinct benefits for high sensitivity and high resolution, differing from the compound eyes of most insects. Taking their unique features, a KAIST team developed an ultrathin digital camera that emulates the unique eyes of Xenos peckii.
The ultrathin digital camera offers a wide field of view and high resolution in a slimmer body compared to existing imaging systems. It is expected to support various applications, such as monitoring equipment, medical imaging devices, and mobile imaging systems.
Professor Ki-Hun Jeong from the Department of Bio and Brain Engineering and his team are known for mimicking biological visual organs. The team’s past research includes an LED lens based on the abdominal segments of fireflies and biologically inspired anti-reflective structures.
Recently, the demand for ultrathin digital cameras has increased, due to the miniaturization of electronic and optical devices. However, most camera modules use multiple lenses along the optical axis to compensate for optical aberrations, resulting in a larger volume as well as a thicker total track length of digital cameras. Resolution and sensitivity would be compromised if these modules were to be simply reduced in size and thickness.
To address this issue, the team have developed micro-optical components, inspired from the visual system of Xenos peckii, and combined them with a CMOS (complementary metal oxide semiconductor) image sensor to achieve an ultrathin digital camera.
This new camera, measuring less than 2mm in thickness, emulates the eyes of Xenos peckii by using dozens of microprism arrays and microlens arrays. A microprism and microlens pair form a channel and the light-absorbing medium between the channels reduces optical crosstalk. Each channel captures the partial image at slightly different orientation, and the retrieved partial images are combined into a single image, thereby ensuring a wide field of view and high resolution.
Professor Jeong said, “We have proposed a novel method of fabricating an ultrathin camera. As the first insect-inspired, ultrathin camera that integrates a microcamera on a conventional CMOS image sensor array, our study will have a significant impact in optics and related fields.”
This research, led by PhD candidates Dongmin Keum and Kyung-Won Jang, was published in Light: Science & Applications on October 24, 2018.
Figure 1. Natural Xenos peckii eye and the biological inspiration for the ultrathin digital camera (Light: Science & Applications 2018)
Figure 2. Optical images captured by the bioinspired ultrathin digital camera (Light: Science & Applications 2018)
2018.12.31 View 9496 -
 Sound-based Touch Input Technology for Smart Tables and Mirrors
(from left: MS candidate Anish Byanjankar, Research Assistant Professor Hyosu Kim and Professor Insik Shin)
Time passes so quickly, especially in the morning. Your hands are so busy brushing your teeth and checking the weather on your smartphone. You might wish that your mirror could turn into a touch screen and free up your hands. That wish can be achieved very soon. A KAIST team has developed a smartphone-based touch sound localization technology to facilitate ubiquitous interactions, turning objects like furniture and mirrors into touch input tools.
This technology analyzes touch sounds generated from a user’s touch on a surface and identifies the location of the touch input. For instance, users can turn surrounding tables or walls into virtual keyboards and write lengthy e-mails much more conveniently by using only the built-in microphone on their smartphones or tablets. Moreover, family members can enjoy a virtual chessboard or enjoy board games on their dining tables.
Additionally, traditional smart devices such as smart TVs or mirrors, which only provide simple screen display functions, can play a smarter role by adding touch input function support (see the image below).
Figure 1.Examples of using touch input technology: By using only smartphone, you can use surrounding objects as a touch screen anytime and anywhere.
The most important aspect of enabling the sound-based touch input method is to identify the location of touch inputs in a precise manner (within about 1cm error). However, it is challenging to meet these requirements, mainly because this technology can be used in diverse and dynamically changing environments. Users may use objects like desks, walls, or mirrors as touch input tools and the surrounding environments (e.g. location of nearby objects or ambient noise level) can be varied. These environmental changes can affect the characteristics of touch sounds.
To address this challenge, Professor Insik Shin from the School of Computing and his team focused on analyzing the fundamental properties of touch sounds, especially how they are transmitted through solid surfaces.
On solid surfaces, sound experiences a dispersion phenomenon that makes different frequency components travel at different speeds. Based on this phenomenon, the team observed that the arrival time difference (TDoA) between frequency components increases in proportion to the sound transmission distance, and this linear relationship is not affected by the variations of surround environments.
Based on these observations, Research Assistant Professor Hyosu Kim proposed a novel sound-based touch input technology that records touch sounds transmitted through solid surfaces, then conducts a simple calibration process to identify the relationship between TDoA and the sound transmission distance, finally achieving accurate touch input localization.
The accuracy of the proposed system was then measured. The average localization error was lower than about 0.4 cm on a 17-inch touch screen. Particularly, it provided a measurement error of less than 1cm, even with a variety of objects such as wooden desks, glass mirrors, and acrylic boards and when the position of nearby objects and noise levels changed dynamically. Experiments with practical users have also shown positive responses to all measurement factors, including user experience and accuracy.
Professor Shin said, “This is novel touch interface technology that allows a touch input system just by installing three to four microphones, so it can easily turn nearby objects into touch screens.”
The proposed system was presented at ACM SenSys, a top-tier conference in the field of mobile computing and sensing, and was selected as a best paper runner-up in November 2018.
(The demonstration video of the sound-based touch input technology)
2018.12.26 View 9884
Sound-based Touch Input Technology for Smart Tables and Mirrors
(from left: MS candidate Anish Byanjankar, Research Assistant Professor Hyosu Kim and Professor Insik Shin)
Time passes so quickly, especially in the morning. Your hands are so busy brushing your teeth and checking the weather on your smartphone. You might wish that your mirror could turn into a touch screen and free up your hands. That wish can be achieved very soon. A KAIST team has developed a smartphone-based touch sound localization technology to facilitate ubiquitous interactions, turning objects like furniture and mirrors into touch input tools.
This technology analyzes touch sounds generated from a user’s touch on a surface and identifies the location of the touch input. For instance, users can turn surrounding tables or walls into virtual keyboards and write lengthy e-mails much more conveniently by using only the built-in microphone on their smartphones or tablets. Moreover, family members can enjoy a virtual chessboard or enjoy board games on their dining tables.
Additionally, traditional smart devices such as smart TVs or mirrors, which only provide simple screen display functions, can play a smarter role by adding touch input function support (see the image below).
Figure 1.Examples of using touch input technology: By using only smartphone, you can use surrounding objects as a touch screen anytime and anywhere.
The most important aspect of enabling the sound-based touch input method is to identify the location of touch inputs in a precise manner (within about 1cm error). However, it is challenging to meet these requirements, mainly because this technology can be used in diverse and dynamically changing environments. Users may use objects like desks, walls, or mirrors as touch input tools and the surrounding environments (e.g. location of nearby objects or ambient noise level) can be varied. These environmental changes can affect the characteristics of touch sounds.
To address this challenge, Professor Insik Shin from the School of Computing and his team focused on analyzing the fundamental properties of touch sounds, especially how they are transmitted through solid surfaces.
On solid surfaces, sound experiences a dispersion phenomenon that makes different frequency components travel at different speeds. Based on this phenomenon, the team observed that the arrival time difference (TDoA) between frequency components increases in proportion to the sound transmission distance, and this linear relationship is not affected by the variations of surround environments.
Based on these observations, Research Assistant Professor Hyosu Kim proposed a novel sound-based touch input technology that records touch sounds transmitted through solid surfaces, then conducts a simple calibration process to identify the relationship between TDoA and the sound transmission distance, finally achieving accurate touch input localization.
The accuracy of the proposed system was then measured. The average localization error was lower than about 0.4 cm on a 17-inch touch screen. Particularly, it provided a measurement error of less than 1cm, even with a variety of objects such as wooden desks, glass mirrors, and acrylic boards and when the position of nearby objects and noise levels changed dynamically. Experiments with practical users have also shown positive responses to all measurement factors, including user experience and accuracy.
Professor Shin said, “This is novel touch interface technology that allows a touch input system just by installing three to four microphones, so it can easily turn nearby objects into touch screens.”
The proposed system was presented at ACM SenSys, a top-tier conference in the field of mobile computing and sensing, and was selected as a best paper runner-up in November 2018.
(The demonstration video of the sound-based touch input technology)
2018.12.26 View 9884 -
 Fabrication of Shape-conformable Batteries with 3D-Printing
(from left: Dr. Bok Yeop Ahn, Dr. Chanhoon Kim, Professor Il-Doo Kim and Professor Jennifer A. Lewis)
Flexible, wireless electronic devices are rapidly emerging and have reached the level of commercialization; nevertheless, most of battery shapes are limited to either spherical and/or rectangular structures, which results in inefficient space use. Professor Il-Doo Kim’s team from the Department of Materials Science at KAIST has successfully developed technology to significantly enhance the variability of battery design through collaboration research with Professor Jennifer A. Lewis and her team from the School of Engineering and Applied Sciences at Harvard University.
Most of the battery shapes today are optimized for coin cell and/or pouch cells. Since the battery as an energy storage device occupies most of the space in microelectronic devices with different designs, new technology to freely change the shape of the battery is required.
The KAIST-Harvard research collaboration team has successfully manufactured various kinds of battery shapes, such as ring-type, H, and U shape, using 3D printing technology. And through the research collaboration with Dr. Youngmin Choi at the Korea Research Institute of Chemical Technology (KRICT), 3D-printed batteries were applied to small-scale wearable electronic devices (wearable light sensor rings).
The research group has adopted environmentally friendly aqueous Zn-ion batteries to make customized battery packs. This system, which uses Zn2+ instead of Li+ as charge carriers, is much safer compared with the conventional lithium rechargeable batteries that use highly inflammable organic electrolytes. Moreover, the processing conditions of lithium-ion batteries are very complicated because organic solvents can ignite upon exposure to moisture and oxygen.
As the aqueous Zn-ion batteries adopted by the research team are stable upon contact with atmospheric moisture and oxygen, they can be fabricated in the ambient air condition, and have advantages in packaging since packaged plastic does not dissolve in water even when plastic packaging is applied using a 3D printer.
To fabricate a stable cathode that can be modulated in various forms and allows high charge-discharge, the research team fabricated a carbon fiber current collector using electrospinning process and uniformly coated electrochemically active polyaniline conductive polymer on the surface of carbon fiber for a current collector-active layer integrated cathode. The cathode, based on conductive polyaniline consisting of a 3D structure, exhibits very fast charging speeds (50% of the charge in two minutes) and can be fabricated without the detachment of active cathode materials, so various battery forms with high mechanical stability can be manufactured.
Prof. Kim said, “Zn-ion batteries employing aqueous electrolytes have the advantage of fabrication under ambient conditions, so it is easy to fabricate the customized battery packs using 3D printing.”
“3D-printed batteries can be easily applied for niche applications such as wearable, personalized, miniaturized micro-robots, and implantable medical devices or microelectronic storage devices with unique designs,” added Professor Lewis.
With Dr. Chanhoon Kim in the Department of Materials Science and Engineering at KAIST and Dr. Bok Yeop Ahn School of Engineering and Applied Sciences at Harvard University participating as equally contributing first authors, this work was published in the December issue of ACS Nano.
This work was financially supported by the Global Research Laboratory (NRF-2015K1A1A2029679) and Wearable Platform Materials Technology Center (2016R1A5A1009926).
Figure 1.Fabrication of shape-conformable batteries based on 3D-printing technology and the application of polyaniline carbon nanofiber cathodes and wearable electronic devices
Figure 2.Fabricated shape-conformable batteries based on a 3D-printing method
Meanwhile, Professor Il-Doo Kim was recently appointed as an Associate Editor of ACS Nano, a highly renowned journal in the field of nanoscience.
Professor Kim said, “It is my great honor to be an Associate Editor of the highly renowned journal ACS Nano, which has an impact factor reaching 13.709 with 134,596 citations as of 2017. Through the editorial activities in the fields of energy, I will dedicate myself to improving the prominence of KAIST and expanding the scope of Korea’s science and technology. I will also contribute to carrying out more international collaborations with world-leading research groups.”
(Associate Editor of ACS Nano Professor Il-Doo Kim)
2018.12.20 View 11629
Fabrication of Shape-conformable Batteries with 3D-Printing
(from left: Dr. Bok Yeop Ahn, Dr. Chanhoon Kim, Professor Il-Doo Kim and Professor Jennifer A. Lewis)
Flexible, wireless electronic devices are rapidly emerging and have reached the level of commercialization; nevertheless, most of battery shapes are limited to either spherical and/or rectangular structures, which results in inefficient space use. Professor Il-Doo Kim’s team from the Department of Materials Science at KAIST has successfully developed technology to significantly enhance the variability of battery design through collaboration research with Professor Jennifer A. Lewis and her team from the School of Engineering and Applied Sciences at Harvard University.
Most of the battery shapes today are optimized for coin cell and/or pouch cells. Since the battery as an energy storage device occupies most of the space in microelectronic devices with different designs, new technology to freely change the shape of the battery is required.
The KAIST-Harvard research collaboration team has successfully manufactured various kinds of battery shapes, such as ring-type, H, and U shape, using 3D printing technology. And through the research collaboration with Dr. Youngmin Choi at the Korea Research Institute of Chemical Technology (KRICT), 3D-printed batteries were applied to small-scale wearable electronic devices (wearable light sensor rings).
The research group has adopted environmentally friendly aqueous Zn-ion batteries to make customized battery packs. This system, which uses Zn2+ instead of Li+ as charge carriers, is much safer compared with the conventional lithium rechargeable batteries that use highly inflammable organic electrolytes. Moreover, the processing conditions of lithium-ion batteries are very complicated because organic solvents can ignite upon exposure to moisture and oxygen.
As the aqueous Zn-ion batteries adopted by the research team are stable upon contact with atmospheric moisture and oxygen, they can be fabricated in the ambient air condition, and have advantages in packaging since packaged plastic does not dissolve in water even when plastic packaging is applied using a 3D printer.
To fabricate a stable cathode that can be modulated in various forms and allows high charge-discharge, the research team fabricated a carbon fiber current collector using electrospinning process and uniformly coated electrochemically active polyaniline conductive polymer on the surface of carbon fiber for a current collector-active layer integrated cathode. The cathode, based on conductive polyaniline consisting of a 3D structure, exhibits very fast charging speeds (50% of the charge in two minutes) and can be fabricated without the detachment of active cathode materials, so various battery forms with high mechanical stability can be manufactured.
Prof. Kim said, “Zn-ion batteries employing aqueous electrolytes have the advantage of fabrication under ambient conditions, so it is easy to fabricate the customized battery packs using 3D printing.”
“3D-printed batteries can be easily applied for niche applications such as wearable, personalized, miniaturized micro-robots, and implantable medical devices or microelectronic storage devices with unique designs,” added Professor Lewis.
With Dr. Chanhoon Kim in the Department of Materials Science and Engineering at KAIST and Dr. Bok Yeop Ahn School of Engineering and Applied Sciences at Harvard University participating as equally contributing first authors, this work was published in the December issue of ACS Nano.
This work was financially supported by the Global Research Laboratory (NRF-2015K1A1A2029679) and Wearable Platform Materials Technology Center (2016R1A5A1009926).
Figure 1.Fabrication of shape-conformable batteries based on 3D-printing technology and the application of polyaniline carbon nanofiber cathodes and wearable electronic devices
Figure 2.Fabricated shape-conformable batteries based on a 3D-printing method
Meanwhile, Professor Il-Doo Kim was recently appointed as an Associate Editor of ACS Nano, a highly renowned journal in the field of nanoscience.
Professor Kim said, “It is my great honor to be an Associate Editor of the highly renowned journal ACS Nano, which has an impact factor reaching 13.709 with 134,596 citations as of 2017. Through the editorial activities in the fields of energy, I will dedicate myself to improving the prominence of KAIST and expanding the scope of Korea’s science and technology. I will also contribute to carrying out more international collaborations with world-leading research groups.”
(Associate Editor of ACS Nano Professor Il-Doo Kim)
2018.12.20 View 11629 -
 Highly Scalable Process to Obtain Stable 2D Nanosheet Dispersion
(Professor Do Hyun Kim and his team)
A KAIST team developed technology that allows the mass production of two-dimensional (2D) nanomaterial dispersion by utilizing the characteristic shearing force of hydraulic power.
The 2D nanosheet dispersion can be directly applied to solution-based processes to manufacture devices for electronics as well as energy storage and conversion. It is expected to be used in these devices with improved performance.
There have been numerous researches on the mass production of various 2D nanomaterial because they show outstanding physical and chemical characteristics when they are truly 2D.
With strong mechanical force or chemical reaction only, each existing exfoliation method has its limitation to make 2D material when the scale of manufacturing increases. They also face the issues of high cost and long process time.
Moreover, 2D nanosheets by the exfoliation have the tendency of agglomeration due to the surface energy. Usually, organic solvent or surfactant is required to obtain high yield and concentration of 2D material by minimizing agglomeration.
After several years of research, Professor Do Hyun Kim in the Department of Chemical and Biomolecular Engineering and his team verified that optimized shearing in their reactor provided the highest efficiency for the exfoliation of nanomaterial. For the increased reactor capacity, they selected a flow and a dispersive agent to develop a high-speed, mass-production process to get 2D nanosheets by physical exfoliation with an aqueous solution.
The team proposed a flow reactor based on Taylor-Couette flow, which has the advantage of high shear rate and mixing efficiency even under large reactor capacity.
In this research, Professor Young-Kyu Han at Dongguk University-Seoul carried out the Ab initio calculation to select the dispersive agent. According to his calculation, an ionic liquid can stabilize and disperse 2D nanomaterial even in a small concentration. This calculation could maximize the exfoliating efficiency.
Professor Bong Gill Choi at Kangwon National University carried out the evaluation of device made of resulting dispersion. The team used a membrane filtration process to make a flexible and highly conductive film of 2D material. The film was then applied to produce an electrode for the supercapacitor device with very high capacity per volume. They also confirmed its stability in their supercapacitor device.
Additionally, they applied dispersive nanomaterials including graphene, molybdenum disulfide (MoS₂), and boron nitride (BN) to inkjet printer ink and realized micrometer-thick nanomaterial patterns on A4 paper. The graphene ink showed no loss of electrical property after printing without additional heat treatment.
Professor Kim said, “This new technology for the high-speed mass production of nanomaterials can easily be applied to various 2D nanomaterials. It will accelerate the production of highly efficient devices for optoelectronics, biosensors, and energy storage/conversion units with low cost.”
This research, led by Dr. Jae-Min Jeong, was published in Advanced Functional Materials on August 12.
Figure 1. The cover page of Advanced Functional Materials
2018.12.19 View 6356
Highly Scalable Process to Obtain Stable 2D Nanosheet Dispersion
(Professor Do Hyun Kim and his team)
A KAIST team developed technology that allows the mass production of two-dimensional (2D) nanomaterial dispersion by utilizing the characteristic shearing force of hydraulic power.
The 2D nanosheet dispersion can be directly applied to solution-based processes to manufacture devices for electronics as well as energy storage and conversion. It is expected to be used in these devices with improved performance.
There have been numerous researches on the mass production of various 2D nanomaterial because they show outstanding physical and chemical characteristics when they are truly 2D.
With strong mechanical force or chemical reaction only, each existing exfoliation method has its limitation to make 2D material when the scale of manufacturing increases. They also face the issues of high cost and long process time.
Moreover, 2D nanosheets by the exfoliation have the tendency of agglomeration due to the surface energy. Usually, organic solvent or surfactant is required to obtain high yield and concentration of 2D material by minimizing agglomeration.
After several years of research, Professor Do Hyun Kim in the Department of Chemical and Biomolecular Engineering and his team verified that optimized shearing in their reactor provided the highest efficiency for the exfoliation of nanomaterial. For the increased reactor capacity, they selected a flow and a dispersive agent to develop a high-speed, mass-production process to get 2D nanosheets by physical exfoliation with an aqueous solution.
The team proposed a flow reactor based on Taylor-Couette flow, which has the advantage of high shear rate and mixing efficiency even under large reactor capacity.
In this research, Professor Young-Kyu Han at Dongguk University-Seoul carried out the Ab initio calculation to select the dispersive agent. According to his calculation, an ionic liquid can stabilize and disperse 2D nanomaterial even in a small concentration. This calculation could maximize the exfoliating efficiency.
Professor Bong Gill Choi at Kangwon National University carried out the evaluation of device made of resulting dispersion. The team used a membrane filtration process to make a flexible and highly conductive film of 2D material. The film was then applied to produce an electrode for the supercapacitor device with very high capacity per volume. They also confirmed its stability in their supercapacitor device.
Additionally, they applied dispersive nanomaterials including graphene, molybdenum disulfide (MoS₂), and boron nitride (BN) to inkjet printer ink and realized micrometer-thick nanomaterial patterns on A4 paper. The graphene ink showed no loss of electrical property after printing without additional heat treatment.
Professor Kim said, “This new technology for the high-speed mass production of nanomaterials can easily be applied to various 2D nanomaterials. It will accelerate the production of highly efficient devices for optoelectronics, biosensors, and energy storage/conversion units with low cost.”
This research, led by Dr. Jae-Min Jeong, was published in Advanced Functional Materials on August 12.
Figure 1. The cover page of Advanced Functional Materials
2018.12.19 View 6356 -
 Optimal Immuno-Therapeutic Strategies for Liver Cancer
KAIST medical scientists have presented a heterogeneity of immune cell exhaustion in the cancer environment, providing evidence and rationale for designing optimal strategies for immune checkpoint inhibitors in liver cancer patients.
They succeeded in distinguishing the hepatocellular carcinoma group from the exhausted tumor infiltrating immune cell composition of liver cancer patients. The study, conducted in collaboration with Asan Medical Center, confirmed the applicability for liver cancer patients, providing a new path for personalized precision medicine as well as a new model for translational research.
Our immune system is able to destroy cancerous cells in our body, however sometimes cancer cells can adapt and mutate, effectively hiding from our immune system. One of the mechanisms that has evolved to prevent eradication by the immune system is to functionally silence effector T cells, termed T-cell exhaustion, that is mainly mediated by immune checkpoint molecules such as PD-1, TIM-3, and LAG-3.
Recent breakthroughs and encouraging clinical results with various immune checkpoint inhibitors (ICIs), such as anti-PD-1 monoclonal antibodies (mAbs) and anti-CTLA-4 mAbs, have demonstrated tremendous potential to cure cancers through the immune activation of exhausted T cells. Immune checkpoint inhibitors showed significant clinical benefits for several types of cancers, leading to their wide application in clinical practice.
Anti-PD1 blocking antibodies are one of the most representative agents in this class of drug. However, it has been challenging to precisely understand the biological and clinical significance of T-cell exhaustion in cancer. A KAIST research team led by Professor Su-Hyung Park reported the heterogeneity of T-cell exhaustion in hepatocellular carcinoma (HCC) and its potential clinical implications in Gastroenterology on December 4.
The team revealed that heterogeneous T-cell exhaustion status is determined by the differential PD-1 expression levels in CD8+ T cells in liver cancer patients. The authors found that tumor-infiltrating CD8+ T cells with high PD-1 expression from liver cancer patients are functionally impaired and co-express other immune checkpoint receptors such as TIM-3 and/or LAG3, compared to those with low PD-1 expression.
Moreover, based on these results, the authors suggested that liver cancer patients can be classified into two distinct subgroups. Patients having high PD-1 expression levels in the tumor microenvironment showed more aggressive tumor features and biomarkers predicting a favorable response to anti-PD1 therapy. The research team also demonstrated that only liver cancer patients having high PD-1 expression are susceptible to combined immune checkpoint blockade-based therapies.
Prof. Park said, “The new classification of liver cancer patients identified by this study can be utilized as a biomarker to predict the response of current cancer immunotherapy targeting the PD-1 pathway.” He also said they will continue to conduct research on T-cell exhaustion and activation in various types of cancer, which could lead to a better understanding of T-cell response against cancer, thereby providing evidence for future cancer immunotherapy to achieve the ultimate goal to prolong the survival of cancer patients.
2018.12.18 View 5675
Optimal Immuno-Therapeutic Strategies for Liver Cancer
KAIST medical scientists have presented a heterogeneity of immune cell exhaustion in the cancer environment, providing evidence and rationale for designing optimal strategies for immune checkpoint inhibitors in liver cancer patients.
They succeeded in distinguishing the hepatocellular carcinoma group from the exhausted tumor infiltrating immune cell composition of liver cancer patients. The study, conducted in collaboration with Asan Medical Center, confirmed the applicability for liver cancer patients, providing a new path for personalized precision medicine as well as a new model for translational research.
Our immune system is able to destroy cancerous cells in our body, however sometimes cancer cells can adapt and mutate, effectively hiding from our immune system. One of the mechanisms that has evolved to prevent eradication by the immune system is to functionally silence effector T cells, termed T-cell exhaustion, that is mainly mediated by immune checkpoint molecules such as PD-1, TIM-3, and LAG-3.
Recent breakthroughs and encouraging clinical results with various immune checkpoint inhibitors (ICIs), such as anti-PD-1 monoclonal antibodies (mAbs) and anti-CTLA-4 mAbs, have demonstrated tremendous potential to cure cancers through the immune activation of exhausted T cells. Immune checkpoint inhibitors showed significant clinical benefits for several types of cancers, leading to their wide application in clinical practice.
Anti-PD1 blocking antibodies are one of the most representative agents in this class of drug. However, it has been challenging to precisely understand the biological and clinical significance of T-cell exhaustion in cancer. A KAIST research team led by Professor Su-Hyung Park reported the heterogeneity of T-cell exhaustion in hepatocellular carcinoma (HCC) and its potential clinical implications in Gastroenterology on December 4.
The team revealed that heterogeneous T-cell exhaustion status is determined by the differential PD-1 expression levels in CD8+ T cells in liver cancer patients. The authors found that tumor-infiltrating CD8+ T cells with high PD-1 expression from liver cancer patients are functionally impaired and co-express other immune checkpoint receptors such as TIM-3 and/or LAG3, compared to those with low PD-1 expression.
Moreover, based on these results, the authors suggested that liver cancer patients can be classified into two distinct subgroups. Patients having high PD-1 expression levels in the tumor microenvironment showed more aggressive tumor features and biomarkers predicting a favorable response to anti-PD1 therapy. The research team also demonstrated that only liver cancer patients having high PD-1 expression are susceptible to combined immune checkpoint blockade-based therapies.
Prof. Park said, “The new classification of liver cancer patients identified by this study can be utilized as a biomarker to predict the response of current cancer immunotherapy targeting the PD-1 pathway.” He also said they will continue to conduct research on T-cell exhaustion and activation in various types of cancer, which could lead to a better understanding of T-cell response against cancer, thereby providing evidence for future cancer immunotherapy to achieve the ultimate goal to prolong the survival of cancer patients.
2018.12.18 View 5675 -
 Characteristics of Submesoscale Geophysical Turbulence Reported
A KAIST research team has reported some of unique characteristics and driving forces behind submesoscale geophysical turbulence. Using big data analysis on ocean surface currents and chlorophyll concentrations observed using coastal radars and satellites has brought better understanding of oceanic processes in space and time scales of O(1) kilometer and O(1) hour. The outcomes of this work will lead to improved tracking of water-borne materials and performance in global and regional climate prediction models.
In 2012, United States National Aeronautics and Space Administration (NASA) released a movie clip called “Perpetual Oceans”, which visualized ocean circulation obtained from satellite altimeter-derived sea surface height observations over two and a half years. When the movie was released to the public, it received a great deal of attention because the circulation patterns were strikingly similar to “The Starry Night” by Vincent van Gogh.
“Perpetual Oceans” is full of vortical flow patterns describing the oceanic turbulent motions at mesoscale (a scale of 100 km or larger). Meanwhile, Professor Sung Yong Kim from the Department of Mechanical Engineering and his team focused on the study of the oceanic turbulence at sub-mesoscale (space and time scales of 1 to 100 km and hours).
Sub-mesoscale processes are important because they contribute to the vertical transport of oceanic tracers, mass, buoyancy, and nutrients and rectify both the mixed layer structure and upper ocean stratification. These process studies have been primarily based on numerical simulations because traditional in situ ocean measurements can be limited in their capability to resolve the detailed horizontal and vertical structures of these processes.
The team conducted big data analysis on hourly observations of one-year ocean surface current maps and five-year chlorophyll concentration maps, obtained from remote sensing instruments such as coastal high-frequency radars (HFRs) and geostationary ocean color imagery (GOCI) to examine the unique characteristics of oceanic submesoscale processes.
The team analyzed the slope change of the wavenumber energy spectra of the observations in terms of season and sampling directions. Through the analysis, the team proved that energy cascade (a phenomenon in which large-scale energy transfers to small-scale energy or vice-versa during the turbulent energy transit) occurs in the spatial scale of 10 km in the forward and inverse directions. This is driven by baroclinic instability as opposed to the mesoscale eddy-driven frontogenesis at the O(100) km scale based on the observed regional submesoscale circulations.
This work will contribute to the parameterization of physical phenomenon of sub-mesoscale in the field of global high-resolution modeling within ocean physics and atmospheric as well as climate change. Based on the understanding of the principle of sub-mesoscale surface circulation, practical applications can be further derived for radioactivity, oil spill recovery, and marine pollutant tracking.
Moreover, the data used in this research was based on long-term observations on sub-mesoscale surface currents and concentrations of chlorophyll, which may reflect the submesoscale processes actively generated in the subpolar front off the east coast of Korea. Hence, this study can potentially be beneficial for integrated big data analyses using high-resolution coastal radar-derived surface currents and satellite-derived products and motivate interdisciplinary research between ocean physics and biology.
This research was published as two companion papers in the Journal of Geophysical Research: Oceans on August 6, 2018. (doi:10.1002/2016JC012517; doi:10.1002/2017JC013732)
Figure 1.'The Starry Night' of Van Gogh and the 'Perpetual Ocean' created by NASA's Goddard Space Flight Center.
Figure 2. A schematic diagram of the energy cascades in forward and backward directions and the spatial scale where the energy is injected.
Figure 3. A snapshot of the chlorophyll concentration map derived from geostationary ocean color imagery (GOCI) off the east coast of Korea presenting several examples of sub-mesoscale turbulent flows.
Figure 4. Energy spectra of the HFR-derived surface currents and GOCI-derived chlorophyll concentrations and the temporal variability of spectral decay slopes in the cross-shore and along-shore directions.
2018.12.13 View 6022
Characteristics of Submesoscale Geophysical Turbulence Reported
A KAIST research team has reported some of unique characteristics and driving forces behind submesoscale geophysical turbulence. Using big data analysis on ocean surface currents and chlorophyll concentrations observed using coastal radars and satellites has brought better understanding of oceanic processes in space and time scales of O(1) kilometer and O(1) hour. The outcomes of this work will lead to improved tracking of water-borne materials and performance in global and regional climate prediction models.
In 2012, United States National Aeronautics and Space Administration (NASA) released a movie clip called “Perpetual Oceans”, which visualized ocean circulation obtained from satellite altimeter-derived sea surface height observations over two and a half years. When the movie was released to the public, it received a great deal of attention because the circulation patterns were strikingly similar to “The Starry Night” by Vincent van Gogh.
“Perpetual Oceans” is full of vortical flow patterns describing the oceanic turbulent motions at mesoscale (a scale of 100 km or larger). Meanwhile, Professor Sung Yong Kim from the Department of Mechanical Engineering and his team focused on the study of the oceanic turbulence at sub-mesoscale (space and time scales of 1 to 100 km and hours).
Sub-mesoscale processes are important because they contribute to the vertical transport of oceanic tracers, mass, buoyancy, and nutrients and rectify both the mixed layer structure and upper ocean stratification. These process studies have been primarily based on numerical simulations because traditional in situ ocean measurements can be limited in their capability to resolve the detailed horizontal and vertical structures of these processes.
The team conducted big data analysis on hourly observations of one-year ocean surface current maps and five-year chlorophyll concentration maps, obtained from remote sensing instruments such as coastal high-frequency radars (HFRs) and geostationary ocean color imagery (GOCI) to examine the unique characteristics of oceanic submesoscale processes.
The team analyzed the slope change of the wavenumber energy spectra of the observations in terms of season and sampling directions. Through the analysis, the team proved that energy cascade (a phenomenon in which large-scale energy transfers to small-scale energy or vice-versa during the turbulent energy transit) occurs in the spatial scale of 10 km in the forward and inverse directions. This is driven by baroclinic instability as opposed to the mesoscale eddy-driven frontogenesis at the O(100) km scale based on the observed regional submesoscale circulations.
This work will contribute to the parameterization of physical phenomenon of sub-mesoscale in the field of global high-resolution modeling within ocean physics and atmospheric as well as climate change. Based on the understanding of the principle of sub-mesoscale surface circulation, practical applications can be further derived for radioactivity, oil spill recovery, and marine pollutant tracking.
Moreover, the data used in this research was based on long-term observations on sub-mesoscale surface currents and concentrations of chlorophyll, which may reflect the submesoscale processes actively generated in the subpolar front off the east coast of Korea. Hence, this study can potentially be beneficial for integrated big data analyses using high-resolution coastal radar-derived surface currents and satellite-derived products and motivate interdisciplinary research between ocean physics and biology.
This research was published as two companion papers in the Journal of Geophysical Research: Oceans on August 6, 2018. (doi:10.1002/2016JC012517; doi:10.1002/2017JC013732)
Figure 1.'The Starry Night' of Van Gogh and the 'Perpetual Ocean' created by NASA's Goddard Space Flight Center.
Figure 2. A schematic diagram of the energy cascades in forward and backward directions and the spatial scale where the energy is injected.
Figure 3. A snapshot of the chlorophyll concentration map derived from geostationary ocean color imagery (GOCI) off the east coast of Korea presenting several examples of sub-mesoscale turbulent flows.
Figure 4. Energy spectra of the HFR-derived surface currents and GOCI-derived chlorophyll concentrations and the temporal variability of spectral decay slopes in the cross-shore and along-shore directions.
2018.12.13 View 6022 -
 AI-based Digital Watermarking to Beat Fake News
(from left: PhD candidates Ji-Hyeon Kang, Seungmin Mun, Sangkeun Ji and Professor Heung-Kyu Lee)
The illegal use of images has been a prevalent issue along with the rise of distributing fake news, which all create social and economic problems. Here, a KAIST team succeeded in embedding and detecting digital watermarks based on deep neural learning artificial intelligence, which adaptively responds to a variety of attack types, such as removing watermarks and hacking. Their research shows that this technology reached a level of reliability for technology commercialization.
Conventional watermarking technologies show limitations in terms of practicality, technology scalability, and usefulness because they require a predetermined set of conditions, such as the attack type and intensity. They are designed and implemented in a way to satisfy specific conditions.
In addition to those limitations, the technology itself is vulnerable to security issues because upgraded hacking technologies are constantly emerging, such as watermark removal, copying, and substitution.
Professor Heung-Kyu Lee from the School of Computing and his team provided a web service that responds to new attacks through deep neural learning artificial intelligence. It also serves as a two-dimensional image watermarking technique based on neural networks with high security derived from the nonlinear characteristics of artificial neural networks. To protect images from varying viewpoints, the service offers a depth-image-based rendering (DIBR) three-dimensional image watermarking technique.
Lastly, they provided a stereoscopic three-dimensional (S3D) image watermarking technique that minimizes visual fatigue due to the embedded watermarks. Their two-dimensional image watermarking technology is the first of its kind to be based upon artificial neural works. It acquires robustness through educating the artificial neural networking on various attack scenarios.
At the same time, the team has greatly improved on existing security vulnerabilities by acquiring high security against watermark hacking through the deep structure of artificial neural networks. They have also developed a watermarking technique embedded whenever needed to provide proof during possible disagreements.
Users can upload their images to the web service and insert the watermarks. When necessary, they can detect the watermarks for proof in any dispute.
Moreover, this technology provides services, including simulation tools, watermark adjustment, and image quality comparisons before and after the watermark is embedded.
This study maximized the usefulness of watermarking technology by facilitating additional editing and demonstrating robustness against hacking.
Hence, this technology can be applied in a variety of contents for certification, authentication, distinction tracking, and copyrights. It can contribute to spurring the content industry and promoting a digital society by reducing the socio-economic losses caused by the use of various illegal image materials in the future.
Professor Lee said, “Disputes related to images are now beyond the conventional realm of copyrights. Recently, their interest has rapidly expanded due to the issues of authentication, certification, integrity inspection, and distribution tracking because of the fake video problem. We will lead digital watermarking research that can overcome the technical limitations of conventional watermarking techniques.”
This technology has only been conducted in labs thus far, but it is now open to the public after years of study. His team has been conducting a test run on the webpage (click).Moving forward from testing the technology under specific lab conditions, it will be applied to a real environment setting where constant changes pervade.
1. Figure. 2D image using the watermarking technique: a) original image b) watermark-embedded image c) signal from the embedded watermark
Figure 2. Result of watermark detection according to the password
Figure 3. Example of a center image using the DIBR 3D image watermarking technique: a) original image b) depth image c) watermark-embedded image d) signal from the embedded watermark
Figure 4. Example of using the S3D image watermarking technique: a) original left image b) original right image c) watermark-embedded left image d) watermark-embedded right image e) signal from the embedded watermark (left) f) signal from the embedded watermark (right)
2018.12.05 View 5610
AI-based Digital Watermarking to Beat Fake News
(from left: PhD candidates Ji-Hyeon Kang, Seungmin Mun, Sangkeun Ji and Professor Heung-Kyu Lee)
The illegal use of images has been a prevalent issue along with the rise of distributing fake news, which all create social and economic problems. Here, a KAIST team succeeded in embedding and detecting digital watermarks based on deep neural learning artificial intelligence, which adaptively responds to a variety of attack types, such as removing watermarks and hacking. Their research shows that this technology reached a level of reliability for technology commercialization.
Conventional watermarking technologies show limitations in terms of practicality, technology scalability, and usefulness because they require a predetermined set of conditions, such as the attack type and intensity. They are designed and implemented in a way to satisfy specific conditions.
In addition to those limitations, the technology itself is vulnerable to security issues because upgraded hacking technologies are constantly emerging, such as watermark removal, copying, and substitution.
Professor Heung-Kyu Lee from the School of Computing and his team provided a web service that responds to new attacks through deep neural learning artificial intelligence. It also serves as a two-dimensional image watermarking technique based on neural networks with high security derived from the nonlinear characteristics of artificial neural networks. To protect images from varying viewpoints, the service offers a depth-image-based rendering (DIBR) three-dimensional image watermarking technique.
Lastly, they provided a stereoscopic three-dimensional (S3D) image watermarking technique that minimizes visual fatigue due to the embedded watermarks. Their two-dimensional image watermarking technology is the first of its kind to be based upon artificial neural works. It acquires robustness through educating the artificial neural networking on various attack scenarios.
At the same time, the team has greatly improved on existing security vulnerabilities by acquiring high security against watermark hacking through the deep structure of artificial neural networks. They have also developed a watermarking technique embedded whenever needed to provide proof during possible disagreements.
Users can upload their images to the web service and insert the watermarks. When necessary, they can detect the watermarks for proof in any dispute.
Moreover, this technology provides services, including simulation tools, watermark adjustment, and image quality comparisons before and after the watermark is embedded.
This study maximized the usefulness of watermarking technology by facilitating additional editing and demonstrating robustness against hacking.
Hence, this technology can be applied in a variety of contents for certification, authentication, distinction tracking, and copyrights. It can contribute to spurring the content industry and promoting a digital society by reducing the socio-economic losses caused by the use of various illegal image materials in the future.
Professor Lee said, “Disputes related to images are now beyond the conventional realm of copyrights. Recently, their interest has rapidly expanded due to the issues of authentication, certification, integrity inspection, and distribution tracking because of the fake video problem. We will lead digital watermarking research that can overcome the technical limitations of conventional watermarking techniques.”
This technology has only been conducted in labs thus far, but it is now open to the public after years of study. His team has been conducting a test run on the webpage (click).Moving forward from testing the technology under specific lab conditions, it will be applied to a real environment setting where constant changes pervade.
1. Figure. 2D image using the watermarking technique: a) original image b) watermark-embedded image c) signal from the embedded watermark
Figure 2. Result of watermark detection according to the password
Figure 3. Example of a center image using the DIBR 3D image watermarking technique: a) original image b) depth image c) watermark-embedded image d) signal from the embedded watermark
Figure 4. Example of using the S3D image watermarking technique: a) original left image b) original right image c) watermark-embedded left image d) watermark-embedded right image e) signal from the embedded watermark (left) f) signal from the embedded watermark (right)
2018.12.05 View 5610 -
 Reducing the Drag Force of a Moving Body Underwater
(from left: Professor Yeunwoo Cho and PhD Jaeho Chung)
Professor Yeunwoo Cho and his team from the Department of Mechanical Engineering developed new technology that reduces the drag force of a moving body in a still fluid by using the supercavitation phenomenon.
When a body moves in air, the frictional drag is lower than that of the same body moving in water. Therefore, the body that moves in water can reduce the drag significantly when it is completely enveloped in a gaseous cavity.
The team used compressed air to create so-called supercavitation, which is a phenomenon created by completely enveloping a body in a single large gaseous cavity. The drag force exerted on the body is then measured.
As a result, the team confirmed that the drag force for a moving body enveloped in air is about 25% of the drag force for a moving body without envelopment.
These results can be applied for developing high-speed underwater vehicles and the development of air-lubricated, high-speed vessels.
The team expects that the results can be applied for developing high-speed underwater vehicles and the development of air lubrication for a ship’s hull.
This research, led by PhD Jaeho Chung, was published in the Journal of Fluid Mechanics as a cover article on November 10, 2018.
Figure 1. The cover article of the Journal of Fluid Mechanics Vol. 854
2018.12.04 View 4769
Reducing the Drag Force of a Moving Body Underwater
(from left: Professor Yeunwoo Cho and PhD Jaeho Chung)
Professor Yeunwoo Cho and his team from the Department of Mechanical Engineering developed new technology that reduces the drag force of a moving body in a still fluid by using the supercavitation phenomenon.
When a body moves in air, the frictional drag is lower than that of the same body moving in water. Therefore, the body that moves in water can reduce the drag significantly when it is completely enveloped in a gaseous cavity.
The team used compressed air to create so-called supercavitation, which is a phenomenon created by completely enveloping a body in a single large gaseous cavity. The drag force exerted on the body is then measured.
As a result, the team confirmed that the drag force for a moving body enveloped in air is about 25% of the drag force for a moving body without envelopment.
These results can be applied for developing high-speed underwater vehicles and the development of air-lubricated, high-speed vessels.
The team expects that the results can be applied for developing high-speed underwater vehicles and the development of air lubrication for a ship’s hull.
This research, led by PhD Jaeho Chung, was published in the Journal of Fluid Mechanics as a cover article on November 10, 2018.
Figure 1. The cover article of the Journal of Fluid Mechanics Vol. 854
2018.12.04 View 4769