LLO
-
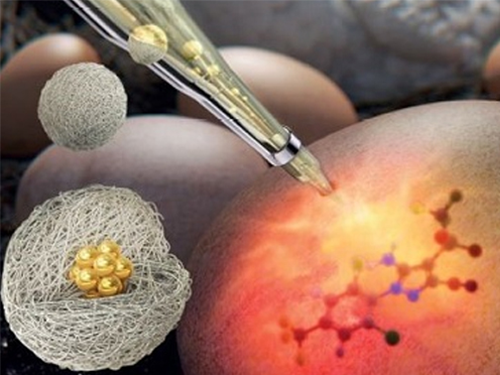 A Molecular Sensor for In-Situ Analysis of Complex Biological Fluids
A KAIST research group presented a molecular sensor with a microbead format for the rapid in-situ detection of harmful molecules in biological fluids or foods in a collaboration with a Korea Institute of Materials Science (KIMS) research group. As the sensor is designed to selectively concentrate charged small molecules and amplify the Raman signal, no time-consuming pretreatment of samples is required.
Raman spectra are commonly known as molecular fingerprints. However, their low intensity has restricted their use in molecular detection, especially for low concentrations. Raman signals can be dramatically amplified by locating the molecules on the surface of metal nanostructures where the electromagnetic field is strongly localized. However, it is still challenging to use Raman signals for the detection of small molecules dissolved in complex biological fluids. Adhesive proteins irreversibly adsorb on the metal surface, which prevents the access of small target molecules onto the metal surface. Therefore, it was a prerequisite to purify the samples before analysis. However, it takes a long time and is expensive.
A joint team from Professor Shin-Hyun Kim’s group in KAIST and Dr. Dong-Ho Kim’s group in KIMS has addressed the issue by encapsulating agglomerates of gold nanoparticles using a hydrogel. The hydrogel has three-dimensional network structures so that molecules smaller than the mesh are selectively permeable. Therefore, the hydrogel can exclude relatively large proteins, while allowing the infusion of small molecules. Therefore, the surface of gold nanoparticles remains intact against proteins, which accommodates small molecules. In particular, the charged hydrogel enables the concentration of oppositely-charged small molecules. That is, the purification is autonomously done by the materials, removing the need for time-consuming pretreatment. As a result, the Raman signal of small molecules can be selectively amplified in the absence of adhesive proteins.
Using the molecular sensors, the research team demonstrated the direct detection of fipronil sulfone dissolved in an egg without sample pretreatment. Recently, insecticide-contaminated eggs have spread in Europe, South Korea, and other countries, threatening health and causing social chaos. Fipronil is one of the most commonly used insecticides for veterinary medicine to combat fleas. The fipronil is absorbed through the chicken skin, from which a metabolite, fipronil sulfone, accumulates in the eggs. As the fipronil sulfone carries partial negative charges, it can be concentrated using positively-charged microgels while excluding adhesive proteins in eggs, such as ovalbumin, ovoglobulin, and ovomucoid. Therefore, the Raman spectrum of fipronil sulfone can be directly measured. The limit of direct detection of fipronil sulfone dissolved in an egg was measured at 0.05 ppm.
Professor Kim said, “The molecular sensors can be used not only for the direct detection of harmful molecules in foods but also for residual drugs or biomarkers in blood or urine.” Dr. Dong-Ho Kim said, “It will be possible to save time and cost as no sample treatment is required.”
This research was led by graduate student Dong Jae Kim and an article entitled “SERS-Active Charged Microgels for Size- and Charge-Selective Molecular Analysis of Complex Biological Samples” was published on October 4, 2018 in Small and featured on the inside cover of the journal.
Figure 1. Schematic illustrating the concentration of charged small molecules and the exclusion of large adhesive proteins using a charged hydrogel microbead containing an agglomerate of gold nanoparticles. The Raman signal of the small molecules is selectively amplified by the agglomerate.
Figure 2. Confocal laser scanning microscope images showing the concentration of oppositely charged molecules, where negatively-charged microgels are denoted by red circles and positively-charged microgels are denoted by blue circles. Green fluorescence originates from negatively-charged dye molecules and red fluorescence originates from positively-charged dye molecules.
Figure 3. Raman spectra measured from fipronil sulfone-spiked eggs, where the concentrations of fipronil sulfone are denoted; 0 ppm indicates no fipronil sulfone in the egg. The characteristic peaks of fipronil sulfone are denoted by the dotted lines.
2018.10.23 View 6849
A Molecular Sensor for In-Situ Analysis of Complex Biological Fluids
A KAIST research group presented a molecular sensor with a microbead format for the rapid in-situ detection of harmful molecules in biological fluids or foods in a collaboration with a Korea Institute of Materials Science (KIMS) research group. As the sensor is designed to selectively concentrate charged small molecules and amplify the Raman signal, no time-consuming pretreatment of samples is required.
Raman spectra are commonly known as molecular fingerprints. However, their low intensity has restricted their use in molecular detection, especially for low concentrations. Raman signals can be dramatically amplified by locating the molecules on the surface of metal nanostructures where the electromagnetic field is strongly localized. However, it is still challenging to use Raman signals for the detection of small molecules dissolved in complex biological fluids. Adhesive proteins irreversibly adsorb on the metal surface, which prevents the access of small target molecules onto the metal surface. Therefore, it was a prerequisite to purify the samples before analysis. However, it takes a long time and is expensive.
A joint team from Professor Shin-Hyun Kim’s group in KAIST and Dr. Dong-Ho Kim’s group in KIMS has addressed the issue by encapsulating agglomerates of gold nanoparticles using a hydrogel. The hydrogel has three-dimensional network structures so that molecules smaller than the mesh are selectively permeable. Therefore, the hydrogel can exclude relatively large proteins, while allowing the infusion of small molecules. Therefore, the surface of gold nanoparticles remains intact against proteins, which accommodates small molecules. In particular, the charged hydrogel enables the concentration of oppositely-charged small molecules. That is, the purification is autonomously done by the materials, removing the need for time-consuming pretreatment. As a result, the Raman signal of small molecules can be selectively amplified in the absence of adhesive proteins.
Using the molecular sensors, the research team demonstrated the direct detection of fipronil sulfone dissolved in an egg without sample pretreatment. Recently, insecticide-contaminated eggs have spread in Europe, South Korea, and other countries, threatening health and causing social chaos. Fipronil is one of the most commonly used insecticides for veterinary medicine to combat fleas. The fipronil is absorbed through the chicken skin, from which a metabolite, fipronil sulfone, accumulates in the eggs. As the fipronil sulfone carries partial negative charges, it can be concentrated using positively-charged microgels while excluding adhesive proteins in eggs, such as ovalbumin, ovoglobulin, and ovomucoid. Therefore, the Raman spectrum of fipronil sulfone can be directly measured. The limit of direct detection of fipronil sulfone dissolved in an egg was measured at 0.05 ppm.
Professor Kim said, “The molecular sensors can be used not only for the direct detection of harmful molecules in foods but also for residual drugs or biomarkers in blood or urine.” Dr. Dong-Ho Kim said, “It will be possible to save time and cost as no sample treatment is required.”
This research was led by graduate student Dong Jae Kim and an article entitled “SERS-Active Charged Microgels for Size- and Charge-Selective Molecular Analysis of Complex Biological Samples” was published on October 4, 2018 in Small and featured on the inside cover of the journal.
Figure 1. Schematic illustrating the concentration of charged small molecules and the exclusion of large adhesive proteins using a charged hydrogel microbead containing an agglomerate of gold nanoparticles. The Raman signal of the small molecules is selectively amplified by the agglomerate.
Figure 2. Confocal laser scanning microscope images showing the concentration of oppositely charged molecules, where negatively-charged microgels are denoted by red circles and positively-charged microgels are denoted by blue circles. Green fluorescence originates from negatively-charged dye molecules and red fluorescence originates from positively-charged dye molecules.
Figure 3. Raman spectra measured from fipronil sulfone-spiked eggs, where the concentrations of fipronil sulfone are denoted; 0 ppm indicates no fipronil sulfone in the egg. The characteristic peaks of fipronil sulfone are denoted by the dotted lines.
2018.10.23 View 6849 -
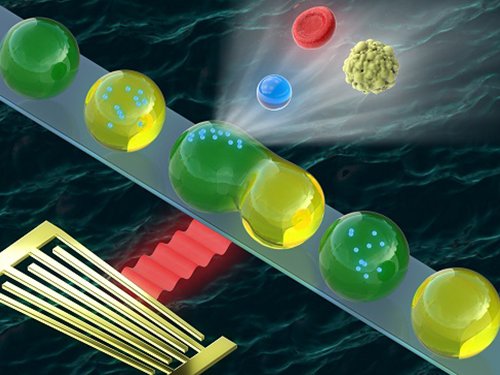 Washing and Enrichment of Micro-Particles Encapsulated in Droplets
Researchers developed microfluidic technology for the washing and enrichment of in-droplet micro-particles. They presented the technology using a microfluidic chip based on surface acoustic wave (SAW)-driven acoustic radiation force (ARF).
The team demonstrated the first instance of acoustic in-droplet micro-particle washing with a particle recovery rate of approximately 90 percent. They further extended the applicability of the proposed method to in-droplet particle enrichment with the unprecedented abilities to increase the in-droplet particle quantity and exchange the droplet dispersed phase.
This proposed method enabled on-chip, label-free, continuous, and selective in-droplet micro-particle manipulation. The team demonstrated the first instance of in-droplet micro-particle washing between two types of alternating droplets in a simple microchannel, proving that the method can increase the particle quantity, which has not been achieved by previously reported methods.
The study aimed to develop an in-droplet micro-particle washing and enrichment method based on SAW-driven ARF. When a droplet containing particles is exposed to an acoustic field, both the droplet and suspended particles experience ARF arising from inhomogeneous wave scattering at the liquid-liquid and liquid-solid interfaces. Unlike previous in-droplet particle manipulation methods, this method allows simultaneous and precise control over the droplets and suspended particles. Moreover, the proposed acoustic method does not require labelled particles, such as magnetic particles, and employs a simple microchannel geometry.
Microfluidic sample washing has emerged as an alternative to centrifugation because the limitations of centrifugation-based washing methods can be addressed using continuous washing processes. It also has considerable potential and importance in a variety of applications such as single-cell/particle assays, high-throughput screening of rare samples, and cell culture medium exchange.
Compared to continuous flow-based microfluidic methods, droplet-based microfluidic sample washing has been rarely explored due to technological difficulties. On-chip, in-droplet sample washing requires sample transfer across the droplet interface composed of two immiscible fluids. This process involves simultaneous and precise control over the encapsulated sample and droplet interface during the medium exchange of the in-droplet sample.
Sample encapsulation within individual microscale droplets offers isolated microenvironments for the samples. Experimental uncertainties due to cross-contamination and Taylor dispersion between multiple reagents can be reduced in droplet-based microfluidics.
This is the first research achievement made by the Acousto-Microfluidics Research Center for Next-Generation Healthcare, the cross-generation collaborative lab KAIST opened in May. This novel approach pairs senior and junior faculty members for sustaining the research legacy even after the senior researcher retires. The research center, which paired Chair Professor Hyung Jin Sung and Professors Hyoungsoo Kim and Yeunwoo Cho, made a breakthrough in microfluidics along with PhD candidate Jinsoo Park. The study was featured as the cover of Lab on a Chip published by Royal Society of Chemistry.
Jinsoo Park, first author of the study, believes this technology will may serve as an in-droplet sample preparation platform with in-line integration of other droplet microfluidic components. Chair Professor Sung said, “The proposed acoustic method will offer new perspectives on sample washing and enrichment by performing the operation in microscale droplets.”
Figure 1. (a) A microfluidic device for in-droplet micro-particle washing and enrichment; (b) alternatingly produced droplets of two kinds at a double T-junction; (c) a droplet and encapsulated micro-particles exposed to surface acoustic wave-driven acoustic radiation force; (d-h) sequential processes of in-droplet micro-particle washing and enrichment operation.
2018.10.19 View 8339
Washing and Enrichment of Micro-Particles Encapsulated in Droplets
Researchers developed microfluidic technology for the washing and enrichment of in-droplet micro-particles. They presented the technology using a microfluidic chip based on surface acoustic wave (SAW)-driven acoustic radiation force (ARF).
The team demonstrated the first instance of acoustic in-droplet micro-particle washing with a particle recovery rate of approximately 90 percent. They further extended the applicability of the proposed method to in-droplet particle enrichment with the unprecedented abilities to increase the in-droplet particle quantity and exchange the droplet dispersed phase.
This proposed method enabled on-chip, label-free, continuous, and selective in-droplet micro-particle manipulation. The team demonstrated the first instance of in-droplet micro-particle washing between two types of alternating droplets in a simple microchannel, proving that the method can increase the particle quantity, which has not been achieved by previously reported methods.
The study aimed to develop an in-droplet micro-particle washing and enrichment method based on SAW-driven ARF. When a droplet containing particles is exposed to an acoustic field, both the droplet and suspended particles experience ARF arising from inhomogeneous wave scattering at the liquid-liquid and liquid-solid interfaces. Unlike previous in-droplet particle manipulation methods, this method allows simultaneous and precise control over the droplets and suspended particles. Moreover, the proposed acoustic method does not require labelled particles, such as magnetic particles, and employs a simple microchannel geometry.
Microfluidic sample washing has emerged as an alternative to centrifugation because the limitations of centrifugation-based washing methods can be addressed using continuous washing processes. It also has considerable potential and importance in a variety of applications such as single-cell/particle assays, high-throughput screening of rare samples, and cell culture medium exchange.
Compared to continuous flow-based microfluidic methods, droplet-based microfluidic sample washing has been rarely explored due to technological difficulties. On-chip, in-droplet sample washing requires sample transfer across the droplet interface composed of two immiscible fluids. This process involves simultaneous and precise control over the encapsulated sample and droplet interface during the medium exchange of the in-droplet sample.
Sample encapsulation within individual microscale droplets offers isolated microenvironments for the samples. Experimental uncertainties due to cross-contamination and Taylor dispersion between multiple reagents can be reduced in droplet-based microfluidics.
This is the first research achievement made by the Acousto-Microfluidics Research Center for Next-Generation Healthcare, the cross-generation collaborative lab KAIST opened in May. This novel approach pairs senior and junior faculty members for sustaining the research legacy even after the senior researcher retires. The research center, which paired Chair Professor Hyung Jin Sung and Professors Hyoungsoo Kim and Yeunwoo Cho, made a breakthrough in microfluidics along with PhD candidate Jinsoo Park. The study was featured as the cover of Lab on a Chip published by Royal Society of Chemistry.
Jinsoo Park, first author of the study, believes this technology will may serve as an in-droplet sample preparation platform with in-line integration of other droplet microfluidic components. Chair Professor Sung said, “The proposed acoustic method will offer new perspectives on sample washing and enrichment by performing the operation in microscale droplets.”
Figure 1. (a) A microfluidic device for in-droplet micro-particle washing and enrichment; (b) alternatingly produced droplets of two kinds at a double T-junction; (c) a droplet and encapsulated micro-particles exposed to surface acoustic wave-driven acoustic radiation force; (d-h) sequential processes of in-droplet micro-particle washing and enrichment operation.
2018.10.19 View 8339 -
 Skin Hardness to Estimate Better Human Thermal Status
(Professor Young-Ho Cho and Researcher Sunghyun Yoon)
Under the same temperature and humidity, human thermal status may vary due to individual body constitution and climatic environment. A KAIST research team previously developed a wearable sweat rate sensor for human thermal comfort monitoring. Furthering the development, this time they proposed skin hardness as an additional, independent physiological sign to estimate human thermal status more accurately. This novel approach can be applied to developing systems incorporating human-machine interaction, which requires accurate information about human thermal status.
Professor Young-Ho Cho and his team from the Department of Bio and Brain Engineering had previously studied skin temperature and sweat rate to determine human thermal comfort, and developed a watch-type sweat rate sensor that accurately and steadily detects thermal comfort last February (title: Wearable Sweat Rate Sensors for Human Thermal Comfort Monitoring ).
However, skin temperature and sweat rate are still not enough to estimate exact human thermal comfort. Hence, an additional indicator is required for enhancing the accuracy and reliability of the estimation and the team selected skin hardness. When people feel hot or cold, arrector pili muscles connected to hair follicles contract and expand, and skin hardness comes from this contraction and relaxation of the muscles. Based on the phenomenon of changing skin hardness, the team proposed skin hardness as a new indicator for measuring human thermal sensation.
With this new estimation model using three physiological signs for estimating human thermal status, the team conducted human experiments and verified that skin hardness is effective and independent from the two conventional physiological signs. Adding skin hardness to the conventional model can reduce errors by 23.5%, which makes its estimation more reliable.
The team will develop a sensor that detects skin hardness and applies it to cognitive air-conditioning and heating systems that better interact with humans than existing systems.
Professor Cho said, “Introducing this new indicator, skin hardness, elevates the reliability of measuring human thermal comfort regardless of individual body constitution and climatic environment. Based on this method, we can develop a personalized air conditioning and heating system that will allow affective interaction between humans and machines by sharing both physical and mental health conditions and emotions.”
This research, led by researchers Sunghyun Yoon and Jai Kyoung Sim, was published in Scientific Reports, Vol.8, Article No.12027 on August 13, 2018. (pp.1-6)
Figure 1. Measuring human thermal status through skin hardness
Figure 2. The instrument used for measuring human thermal status through skin hardness
2018.10.17 View 6880
Skin Hardness to Estimate Better Human Thermal Status
(Professor Young-Ho Cho and Researcher Sunghyun Yoon)
Under the same temperature and humidity, human thermal status may vary due to individual body constitution and climatic environment. A KAIST research team previously developed a wearable sweat rate sensor for human thermal comfort monitoring. Furthering the development, this time they proposed skin hardness as an additional, independent physiological sign to estimate human thermal status more accurately. This novel approach can be applied to developing systems incorporating human-machine interaction, which requires accurate information about human thermal status.
Professor Young-Ho Cho and his team from the Department of Bio and Brain Engineering had previously studied skin temperature and sweat rate to determine human thermal comfort, and developed a watch-type sweat rate sensor that accurately and steadily detects thermal comfort last February (title: Wearable Sweat Rate Sensors for Human Thermal Comfort Monitoring ).
However, skin temperature and sweat rate are still not enough to estimate exact human thermal comfort. Hence, an additional indicator is required for enhancing the accuracy and reliability of the estimation and the team selected skin hardness. When people feel hot or cold, arrector pili muscles connected to hair follicles contract and expand, and skin hardness comes from this contraction and relaxation of the muscles. Based on the phenomenon of changing skin hardness, the team proposed skin hardness as a new indicator for measuring human thermal sensation.
With this new estimation model using three physiological signs for estimating human thermal status, the team conducted human experiments and verified that skin hardness is effective and independent from the two conventional physiological signs. Adding skin hardness to the conventional model can reduce errors by 23.5%, which makes its estimation more reliable.
The team will develop a sensor that detects skin hardness and applies it to cognitive air-conditioning and heating systems that better interact with humans than existing systems.
Professor Cho said, “Introducing this new indicator, skin hardness, elevates the reliability of measuring human thermal comfort regardless of individual body constitution and climatic environment. Based on this method, we can develop a personalized air conditioning and heating system that will allow affective interaction between humans and machines by sharing both physical and mental health conditions and emotions.”
This research, led by researchers Sunghyun Yoon and Jai Kyoung Sim, was published in Scientific Reports, Vol.8, Article No.12027 on August 13, 2018. (pp.1-6)
Figure 1. Measuring human thermal status through skin hardness
Figure 2. The instrument used for measuring human thermal status through skin hardness
2018.10.17 View 6880 -
 Permanent, Wireless Self-charging System Using NIR Band
(Professor Jung-Yong Lee from the Graduate School of Energy, Environment, Water and Sustainability)
As wearable devices are emerging, there are numerous studies on wireless charging systems. Here, a KAIST research team has developed a permanent, wireless self-charging platform for low-power wearable electronics by converting near-infrared (NIR) band irradiation to electrical energy. This novel technology can be applied to flexible, wearable charging systems without needing any attachments.
Colloidal-quantum-dots (CQDs) are promising materials for manufacturing semiconductors; in particular, PbS-based CQDs have facile optical tunability from the visible to infrared wavelength region. Hence, they can be applied to various devices, such as lighting, photovoltaics (PVs), and photodetectors.
Continuous research on CQD-based optoelectronic devices has increased their power conversion efficiency (PCE) to 12%; however, applicable fields have not yet been found for them. Meanwhile, wearable electronic devices commonly face the problem of inconvenient charging systems because users have to constantly charge batteries attached to an energy source.
A joint team led by Professor Jung-Yong Lee from the Graduate School of Energy, Environment, Water and Sustainability and Jang Wok Choi from Seoul National University decided to apply CQD PVs, which have high quantum efficiency in NIR band to self-charging systems on wearable devices.
They employed a stable and efficient NIR energy conversion strategy. The system was comprised of a PbS CQD-based PV module, a flexible interdigitated lithium-ion battery, and various types of NIR-transparent films.
The team removed the existing battery from the already commercialized wearable healthcare bracelet and replaced it with the proposed self-charging system. They confirmed that the system can be applied to a low power wearable device via the NIR band.
There have been numerous platforms using solar irradiation, but the newly developed platform has more advantages because it allows conventional devices to be much more comfortable to wear and charged easily in everyday life using various irradiation sources for constant charging.
With this aspect, the proposed platform facilitates more flexible designs, which are the important component for actual commercialization. It also secures higher photostability and efficient than existing structures.
Professor Lee said, “By using the NIR band, we proposed a new approach to solve charging system issues of wearable devices. I believe that this platform will be a novel platform for energy conversion and that its application can be further extended to various fields, including mobiles, IoTs, and drones.”
This research, led by PhD Se-Woong Baek and M.S. candidate Jungmin Cho, was published in Advanced Materials on May 11.
Figure 1. a) Conceptual NIR-driven self-charging system including a flexible CQD PVs module and an interdigitatedly structured LIB. b) Photographic images of a conventional wearable healthcare bracelet and a self-charging system-integrated wearable device.
Figure 2. Illustration of the CQD PVs structure and performance of the wireless self-charging platform.
2018.10.08 View 8910
Permanent, Wireless Self-charging System Using NIR Band
(Professor Jung-Yong Lee from the Graduate School of Energy, Environment, Water and Sustainability)
As wearable devices are emerging, there are numerous studies on wireless charging systems. Here, a KAIST research team has developed a permanent, wireless self-charging platform for low-power wearable electronics by converting near-infrared (NIR) band irradiation to electrical energy. This novel technology can be applied to flexible, wearable charging systems without needing any attachments.
Colloidal-quantum-dots (CQDs) are promising materials for manufacturing semiconductors; in particular, PbS-based CQDs have facile optical tunability from the visible to infrared wavelength region. Hence, they can be applied to various devices, such as lighting, photovoltaics (PVs), and photodetectors.
Continuous research on CQD-based optoelectronic devices has increased their power conversion efficiency (PCE) to 12%; however, applicable fields have not yet been found for them. Meanwhile, wearable electronic devices commonly face the problem of inconvenient charging systems because users have to constantly charge batteries attached to an energy source.
A joint team led by Professor Jung-Yong Lee from the Graduate School of Energy, Environment, Water and Sustainability and Jang Wok Choi from Seoul National University decided to apply CQD PVs, which have high quantum efficiency in NIR band to self-charging systems on wearable devices.
They employed a stable and efficient NIR energy conversion strategy. The system was comprised of a PbS CQD-based PV module, a flexible interdigitated lithium-ion battery, and various types of NIR-transparent films.
The team removed the existing battery from the already commercialized wearable healthcare bracelet and replaced it with the proposed self-charging system. They confirmed that the system can be applied to a low power wearable device via the NIR band.
There have been numerous platforms using solar irradiation, but the newly developed platform has more advantages because it allows conventional devices to be much more comfortable to wear and charged easily in everyday life using various irradiation sources for constant charging.
With this aspect, the proposed platform facilitates more flexible designs, which are the important component for actual commercialization. It also secures higher photostability and efficient than existing structures.
Professor Lee said, “By using the NIR band, we proposed a new approach to solve charging system issues of wearable devices. I believe that this platform will be a novel platform for energy conversion and that its application can be further extended to various fields, including mobiles, IoTs, and drones.”
This research, led by PhD Se-Woong Baek and M.S. candidate Jungmin Cho, was published in Advanced Materials on May 11.
Figure 1. a) Conceptual NIR-driven self-charging system including a flexible CQD PVs module and an interdigitatedly structured LIB. b) Photographic images of a conventional wearable healthcare bracelet and a self-charging system-integrated wearable device.
Figure 2. Illustration of the CQD PVs structure and performance of the wireless self-charging platform.
2018.10.08 View 8910 -
 Scientist of October, Professor Haeshin Lee
(Professor Haeshin Lee from the Department of Chemistry)
Professor Haeshin Lee from the Department of Chemistry received the ‘Science and Technology Award of October’ from the Ministry of Science and ICT and the National Research Foundation of Korea for his contribution to developing an antibleeding injection needle. This novel outcome will fundamentally prevent the problem of secondary infections of AIDS, Ebola and Hepatitis viruses transmitting from patients to medical teams.
This needle’s surface is coated with hemostatic materials. Its concept is simple and the key to this technology is to make materials that are firmly coated on the needle so that they can endure frictional force when being injected into skin and blood vessels. Moreover, the materials should be adhesive to skin and the interior of blood vessels, but harmless to humans.
Professor Lee found a solution from natural polymer ingredients. Catecholamine can be found in mussels. Professor Lee conjugated catechol groups on the chitosan backbone. He applied this mussel-inspired adhesive polymer Chitosan-catechol, which immediately forms an adhesive layer with blood, as a bioadhesion for the antibleeding injection needle.
Professor Lee said, “Chitosan-catechol, which copies the adhesive mechanism of mussels, shows high solubility in physiological saline as well as great mucoadhesion. Hence, it is perfectly suitable for coating the injection needle. Combining it with proteins allows for efficient drug delivery to the heart, which is a challenging injection location, so it will be also useful for treating incurable heart disease.”
2018.10.05 View 11458
Scientist of October, Professor Haeshin Lee
(Professor Haeshin Lee from the Department of Chemistry)
Professor Haeshin Lee from the Department of Chemistry received the ‘Science and Technology Award of October’ from the Ministry of Science and ICT and the National Research Foundation of Korea for his contribution to developing an antibleeding injection needle. This novel outcome will fundamentally prevent the problem of secondary infections of AIDS, Ebola and Hepatitis viruses transmitting from patients to medical teams.
This needle’s surface is coated with hemostatic materials. Its concept is simple and the key to this technology is to make materials that are firmly coated on the needle so that they can endure frictional force when being injected into skin and blood vessels. Moreover, the materials should be adhesive to skin and the interior of blood vessels, but harmless to humans.
Professor Lee found a solution from natural polymer ingredients. Catecholamine can be found in mussels. Professor Lee conjugated catechol groups on the chitosan backbone. He applied this mussel-inspired adhesive polymer Chitosan-catechol, which immediately forms an adhesive layer with blood, as a bioadhesion for the antibleeding injection needle.
Professor Lee said, “Chitosan-catechol, which copies the adhesive mechanism of mussels, shows high solubility in physiological saline as well as great mucoadhesion. Hence, it is perfectly suitable for coating the injection needle. Combining it with proteins allows for efficient drug delivery to the heart, which is a challenging injection location, so it will be also useful for treating incurable heart disease.”
2018.10.05 View 11458 -
 Flexible Piezoelectric Acoustic Sensors for Speaker Recognition
A KAIST research team led by Professor Keon Jae Lee from the Department of Material Science and Engineering has developed a machine learning-based acoustic sensor for speaker recognition.
Acoustic sensors were spotlighted as one of the most intuitive bilateral communication devices between humans and machines. However, conventional acoustic sensors use a condenser-type device for measuring capacitance between two conducting layers, resulting in low sensitivity, short recognition distance, and low speaker recognition rates.
The team fabricated a flexible piezoelectric membrane by mimicking the basilar membrane in the human cochlear. Resonant frequencies vibrate corresponding regions of the trapezoidal piezoelectric membrane, which converts voice to electrical signal with a highly sensitive self-powered acoustic sensor.
This multi-channel piezoelectric acoustic sensor exhibits sensitivity more than two times higher and allows for more abundant voice information compared to conventional acoustic sensors, which can detect minute sounds from farther distances. In addition, the acoustic sensor can achieve a 97.5% speaker recognition rate using a machine learning algorithm, reducing by 75% error rate than the reference microphone.
AI speaker recognition is the next big thing for future individual customized services. However, conventional technology attempts to improve recognition rates by using software upgrades, resulting in limited speaker recognition rates. The team enhanced the speaker recognition system by replacing the existing hardware with an innovative flexible piezoelectric acoustic sensor. Further software improvement of the piezoelectric acoustic sensor will significantly increase the speaker and voice recognition rate in diverse environments.
Professor Lee said, “Highly sensitive self-powered acoustic sensors for speaker recognition can be used for personalized voice services such as smart home appliances, AI secretaries, always-on IoT, biometric authentication, and FinTech.”
These research “Basilar Membrane-Inspired Self-Powered Acoustic Sensor” and “Machine Learning-based Acoustic Sensor for Speaker Recognition” were published in the September 2018 issue of Nano Energy.
Firgure 1: A flexible piezoelectric acoustic sensor mimicking the human cochlear.
Figure 2: Speaker recognition with a machine learning algorithm.
2018.10.04 View 7840
Flexible Piezoelectric Acoustic Sensors for Speaker Recognition
A KAIST research team led by Professor Keon Jae Lee from the Department of Material Science and Engineering has developed a machine learning-based acoustic sensor for speaker recognition.
Acoustic sensors were spotlighted as one of the most intuitive bilateral communication devices between humans and machines. However, conventional acoustic sensors use a condenser-type device for measuring capacitance between two conducting layers, resulting in low sensitivity, short recognition distance, and low speaker recognition rates.
The team fabricated a flexible piezoelectric membrane by mimicking the basilar membrane in the human cochlear. Resonant frequencies vibrate corresponding regions of the trapezoidal piezoelectric membrane, which converts voice to electrical signal with a highly sensitive self-powered acoustic sensor.
This multi-channel piezoelectric acoustic sensor exhibits sensitivity more than two times higher and allows for more abundant voice information compared to conventional acoustic sensors, which can detect minute sounds from farther distances. In addition, the acoustic sensor can achieve a 97.5% speaker recognition rate using a machine learning algorithm, reducing by 75% error rate than the reference microphone.
AI speaker recognition is the next big thing for future individual customized services. However, conventional technology attempts to improve recognition rates by using software upgrades, resulting in limited speaker recognition rates. The team enhanced the speaker recognition system by replacing the existing hardware with an innovative flexible piezoelectric acoustic sensor. Further software improvement of the piezoelectric acoustic sensor will significantly increase the speaker and voice recognition rate in diverse environments.
Professor Lee said, “Highly sensitive self-powered acoustic sensors for speaker recognition can be used for personalized voice services such as smart home appliances, AI secretaries, always-on IoT, biometric authentication, and FinTech.”
These research “Basilar Membrane-Inspired Self-Powered Acoustic Sensor” and “Machine Learning-based Acoustic Sensor for Speaker Recognition” were published in the September 2018 issue of Nano Energy.
Firgure 1: A flexible piezoelectric acoustic sensor mimicking the human cochlear.
Figure 2: Speaker recognition with a machine learning algorithm.
2018.10.04 View 7840 -
 Spray Coated Tactile Sensor on a 3-D Surface for Robotic Skin
Robots will be able to conduct a wide variety of tasks as well as humans if they can be given tactile sensing capabilities.
A KAIST research team has reported a stretchable pressure insensitive strain sensor by using an all solution-based process. The solution-based process is easily scalable to accommodate for large areas and can be coated as a thin-film on 3-dimensional irregularly shaped objects via spray coating. These conditions make their processing technique unique and highly suitable for robotic electronic skin or wearable electronic applications.
The making of electronic skin to mimic the tactile sensing properties of human skin is an active area of research for various applications such as wearable electronics, robotics, and prosthetics. One of the major challenges in electronic skin research is differentiating various external stimuli, particularly between strain and pressure. Another issue is uniformly depositing electrical skin on 3-dimensional irregularly shaped objects.
To overcome these issues, the research team led by Professor Steve Park from the Department of Materials Science and Engineering and Professor Jung Kim from the Department of Mechanical Engineering developed electronic skin that can be uniformly coated on 3-dimensional surfaces and distinguish mechanical stimuli. The new electronic skin can also distinguish mechanical stimuli analogous to human skin. The structure of the electronic skin was designed to respond differently under applied pressure and strain. Under applied strain, conducting pathways undergo significant conformational changes, considerably changing the resistance. On the other hand, under applied pressure, negligible conformational change in the conducting pathway occurs; e-skin is therefore non-responsive to pressure. The research team is currently working on strain insensitive pressure sensors to use with the developed strain sensors.
The research team also spatially mapped the local strain without the use of patterned electrode arrays utilizing electrical impedance tomography (EIT). By using EIT, it is possible to minimize the number of electrodes, increase durability, and enable facile fabrication onto 3-dimensional surfaces.
Professor Park said, “Our electronic skin can be mass produced at a low cost and can easily be coated onto complex 3-dimensional surfaces. It is a key technology that can bring us closer to the commercialization of electronic skin for various applications in the near future.”
The result of this work entitled “Pressure Insensitive Strain Sensor with Facile Solution-based Process for Tactile Sensing Applications” was published in the August issue of ACS Nano as a cover article.
(Figure: Detecting mechanical stimuli using electrical impedance tomography.)
2018.09.21 View 8926
Spray Coated Tactile Sensor on a 3-D Surface for Robotic Skin
Robots will be able to conduct a wide variety of tasks as well as humans if they can be given tactile sensing capabilities.
A KAIST research team has reported a stretchable pressure insensitive strain sensor by using an all solution-based process. The solution-based process is easily scalable to accommodate for large areas and can be coated as a thin-film on 3-dimensional irregularly shaped objects via spray coating. These conditions make their processing technique unique and highly suitable for robotic electronic skin or wearable electronic applications.
The making of electronic skin to mimic the tactile sensing properties of human skin is an active area of research for various applications such as wearable electronics, robotics, and prosthetics. One of the major challenges in electronic skin research is differentiating various external stimuli, particularly between strain and pressure. Another issue is uniformly depositing electrical skin on 3-dimensional irregularly shaped objects.
To overcome these issues, the research team led by Professor Steve Park from the Department of Materials Science and Engineering and Professor Jung Kim from the Department of Mechanical Engineering developed electronic skin that can be uniformly coated on 3-dimensional surfaces and distinguish mechanical stimuli. The new electronic skin can also distinguish mechanical stimuli analogous to human skin. The structure of the electronic skin was designed to respond differently under applied pressure and strain. Under applied strain, conducting pathways undergo significant conformational changes, considerably changing the resistance. On the other hand, under applied pressure, negligible conformational change in the conducting pathway occurs; e-skin is therefore non-responsive to pressure. The research team is currently working on strain insensitive pressure sensors to use with the developed strain sensors.
The research team also spatially mapped the local strain without the use of patterned electrode arrays utilizing electrical impedance tomography (EIT). By using EIT, it is possible to minimize the number of electrodes, increase durability, and enable facile fabrication onto 3-dimensional surfaces.
Professor Park said, “Our electronic skin can be mass produced at a low cost and can easily be coated onto complex 3-dimensional surfaces. It is a key technology that can bring us closer to the commercialization of electronic skin for various applications in the near future.”
The result of this work entitled “Pressure Insensitive Strain Sensor with Facile Solution-based Process for Tactile Sensing Applications” was published in the August issue of ACS Nano as a cover article.
(Figure: Detecting mechanical stimuli using electrical impedance tomography.)
2018.09.21 View 8926 -
 President Shin Presents Opportunities & Challenges of the 4IR at the Summer Davos Forum
(President Shin makes a keynote speech at the 2018 Summer Davos Forum in China on Sept.20.)
KAIST co-hosted the Asia Session with the World Economic Forum during the 2018 Summer Davos Forum in Tianjin, China from September 18 through 20. The session highlighted regional collaboration in Asia to promote inclusive growth in the Fourth Industrial Revolution.
KAIST is working closely with the WEF to take the lead in the Fourth Industrial Revolution. Last July, KAIST established the Fourth Industrial Revolution Information Center (FIRIC) at the KAIST Institute and signed an MOU with the Center for the Fourth Industrial Revolution (C4IR) at the WEF in October. The session is a follow-up event KAIST and the C4IR agreed to last year during the Roundtable Session held in Seoul.
Many experts in new emerging industries as well as many project directors, including Director Murat Sonmez of the C4IR, attended the session KAIST hosted. Director Chizuru Suga at the C4IR in Japan, Director Danil Kerimi in China, and Director Shailesh Sharda in India also attended the session and discussed ways to expand collaboration and networks among the countries.
In his keynote speech at the session on September 20, President Sung-Chul Shin presented how the Korean government is trying to drive the economy by strategically investing in focused industries in the new global industrial environment. President Shin introduced the government’s strategic roadmap to build the competitiveness of emerging technologies such as AI, blockchain, and precision medicine.
He also stressed that the three components of innovation, collaboration, and speed should be prioritized in all sectors for the successful realization of the Fourth Industrial Revolution. For instance, innovation in education, research, and technology commercialization, expansive domestic and international collaboration beyond the private and public sectors, speedy deregulation, and efficient governance will all be critical.
He also said that KAIST will launch new pilot collaboration projects along with the WEF soon. “We paved the way for leading the network with major countries including Japan and India for advancing the Fourth Industrial Revolution through this session,” President Shin said.
2018.09.21 View 9054
President Shin Presents Opportunities & Challenges of the 4IR at the Summer Davos Forum
(President Shin makes a keynote speech at the 2018 Summer Davos Forum in China on Sept.20.)
KAIST co-hosted the Asia Session with the World Economic Forum during the 2018 Summer Davos Forum in Tianjin, China from September 18 through 20. The session highlighted regional collaboration in Asia to promote inclusive growth in the Fourth Industrial Revolution.
KAIST is working closely with the WEF to take the lead in the Fourth Industrial Revolution. Last July, KAIST established the Fourth Industrial Revolution Information Center (FIRIC) at the KAIST Institute and signed an MOU with the Center for the Fourth Industrial Revolution (C4IR) at the WEF in October. The session is a follow-up event KAIST and the C4IR agreed to last year during the Roundtable Session held in Seoul.
Many experts in new emerging industries as well as many project directors, including Director Murat Sonmez of the C4IR, attended the session KAIST hosted. Director Chizuru Suga at the C4IR in Japan, Director Danil Kerimi in China, and Director Shailesh Sharda in India also attended the session and discussed ways to expand collaboration and networks among the countries.
In his keynote speech at the session on September 20, President Sung-Chul Shin presented how the Korean government is trying to drive the economy by strategically investing in focused industries in the new global industrial environment. President Shin introduced the government’s strategic roadmap to build the competitiveness of emerging technologies such as AI, blockchain, and precision medicine.
He also stressed that the three components of innovation, collaboration, and speed should be prioritized in all sectors for the successful realization of the Fourth Industrial Revolution. For instance, innovation in education, research, and technology commercialization, expansive domestic and international collaboration beyond the private and public sectors, speedy deregulation, and efficient governance will all be critical.
He also said that KAIST will launch new pilot collaboration projects along with the WEF soon. “We paved the way for leading the network with major countries including Japan and India for advancing the Fourth Industrial Revolution through this session,” President Shin said.
2018.09.21 View 9054 -
 KAIST Develops VRFB with Longer Durability
(from left: PhD candidate Soohyun Kim, Professor Hee-Tak Kim and PhD candidate Junghoon Choi)
There has been growing demand for large-scale storage for energy produced from renewable energy sources in an efficient and stable way. To meet this demand, a KAIST research team developed a new vanadium redox-flow battery (VRFB) with 15 times greater capacity retention and five times longer durability. This VRFB battery can be an excellent candidate for a large-scale rechargeable battery with no risk of explosion.
The VRFB has received much attention for its high efficiency and reliability with the absence of cross-contamination. However, it has the limitation of having insufficient charge and discharge efficiency and a low capacity retention rate because its perfluorinated membrane is very permeable to any active materials. To minimize energy loss, it needs a membrane that has low vanadium ion permeability and high ion conductivity. Hence, there was an attempt to incorporate a hydrocarbon membrane that has low cost and high ion selectivity but it turned out that the VO₂+ caused chemical degradation, which led to shortening the battery life drastically.
To develop a membrane with pore sizes smaller than the hydrated size of vanadium ions yet larger than that of the protons, a research team co-led by Professor Hee-Tae Jung and Professor Hee-Tak Kim from the Department of Chemical and Biomolecular Engineering implemented a graphene-oxide framework (GOF) membrane by cross-linking graphene oxide nanosheets. They believed that GOF, having strong ion selectivity, would be a good candidate for the membrane component for the VRFB. The interlayer spacing between the GO sheets limited moisture expansion and provided selective ion permeation.
The GOF membrane increased the capacity retention of the VRFB, which showed a 15 times higher rate than that of perfluorinated membranes. Its cycling stability was also enhanced up to five times, compared to conventional hydrocarbon membranes.
These pore-sized-tuned graphene oxide frameworks will allow pore-sized tuning of membranes and will be applicable to electrochemical systems that utilize ions of various sizes, such as rechargeable batteries and sensors.
Professor Kim said, “Developing a membrane that prevents the mixing of positive and negative active materials has been a chronic issue in the field of redox-flow batteries. Through this research, we showed that nanotechnology can prevent this crossover issue and membrane degradation. I believe that this technology can be applied to various rechargeable batteries requiring large-scale storage.”
This research was published in Nano Letters on May 3.
Figure 1. Electrochemical performances of the VRFBs with Nafion 115, SPAES (sulfonated poly), and GOF/SPAES: discharge capacity
Figure 2. Schematic of the selective ion transfer of hydrated vanadium ions and protons in the GOF membrane and the molecular structure of the GOF membrane, showing that the GO nanosheets are cross-linked with EDA (ethylenediamine)
2018.09.20 View 7859
KAIST Develops VRFB with Longer Durability
(from left: PhD candidate Soohyun Kim, Professor Hee-Tak Kim and PhD candidate Junghoon Choi)
There has been growing demand for large-scale storage for energy produced from renewable energy sources in an efficient and stable way. To meet this demand, a KAIST research team developed a new vanadium redox-flow battery (VRFB) with 15 times greater capacity retention and five times longer durability. This VRFB battery can be an excellent candidate for a large-scale rechargeable battery with no risk of explosion.
The VRFB has received much attention for its high efficiency and reliability with the absence of cross-contamination. However, it has the limitation of having insufficient charge and discharge efficiency and a low capacity retention rate because its perfluorinated membrane is very permeable to any active materials. To minimize energy loss, it needs a membrane that has low vanadium ion permeability and high ion conductivity. Hence, there was an attempt to incorporate a hydrocarbon membrane that has low cost and high ion selectivity but it turned out that the VO₂+ caused chemical degradation, which led to shortening the battery life drastically.
To develop a membrane with pore sizes smaller than the hydrated size of vanadium ions yet larger than that of the protons, a research team co-led by Professor Hee-Tae Jung and Professor Hee-Tak Kim from the Department of Chemical and Biomolecular Engineering implemented a graphene-oxide framework (GOF) membrane by cross-linking graphene oxide nanosheets. They believed that GOF, having strong ion selectivity, would be a good candidate for the membrane component for the VRFB. The interlayer spacing between the GO sheets limited moisture expansion and provided selective ion permeation.
The GOF membrane increased the capacity retention of the VRFB, which showed a 15 times higher rate than that of perfluorinated membranes. Its cycling stability was also enhanced up to five times, compared to conventional hydrocarbon membranes.
These pore-sized-tuned graphene oxide frameworks will allow pore-sized tuning of membranes and will be applicable to electrochemical systems that utilize ions of various sizes, such as rechargeable batteries and sensors.
Professor Kim said, “Developing a membrane that prevents the mixing of positive and negative active materials has been a chronic issue in the field of redox-flow batteries. Through this research, we showed that nanotechnology can prevent this crossover issue and membrane degradation. I believe that this technology can be applied to various rechargeable batteries requiring large-scale storage.”
This research was published in Nano Letters on May 3.
Figure 1. Electrochemical performances of the VRFBs with Nafion 115, SPAES (sulfonated poly), and GOF/SPAES: discharge capacity
Figure 2. Schematic of the selective ion transfer of hydrated vanadium ions and protons in the GOF membrane and the molecular structure of the GOF membrane, showing that the GO nanosheets are cross-linked with EDA (ethylenediamine)
2018.09.20 View 7859 -
 Using Donut-shaped Lithium Sulfide for Higher Performing Batteries
(from left: Research Professor Fangmin Ye and Professor Hee-Tak Kim)
A KAIST research team developed a lithium-sulfur battery with a doughnut-shaped active material structure showing a record lifecycle of over 600 cycles. Having higher energy density and lower production cost than a lithium-ion battery (LIB), it can be used in electric vehicles that require a longer battery life.
There has been an intense research conducted for developing lithium-sulfur batteries with high energy density because LIBs only allow for a very short travel distance per charge. However, Li-S batteries are still unable to provide a longer lifecycle due to the poor reversibility of the lithium metal cathode.
To tackle this issue, Professor Hee-Tak Kim from the Department of Chemical and Biomolecular Engineering and his team used lithium sulfide (Li₂S) cathodes and combine them with graphite anodes to enhance energy density and lifecycles for the batteries.
Yet, lithium sulfide is costly and, so far, there has not been an electrode architecture and electrolyte design that enables a longer lifecycle between the graphite anodes and lithium sulfide cathodes.
Hence, the team produced a doughnut-shaped lithium sulfide cathode active material from low-cost lithium sulfide developed from raw materials. They have also developed a lithium sulfide ion battery with a graphite anode and lithium sulfide cathode using a high concentration salt electrolyte.
This doughnut-shaped lithium sulfide showed outstanding charge and discharge reversibility through improving the transfer of lithium ions. Its highly concentrated salt electrolyte formed a stable film on the surface of the graphite electrode, which showed strong durability.
Through this technology, the team achieved 30% higher energy density than that of conventional LIBs and secured a lifecycle of more than 600 cycles. This doughnut-shaped lithium sulfide-based electrode can be manufactured using low-cost raw materials and a single heat treatment process. The electrode can also be applied to existing LIBs.
Professor Kim said, “We have demonstrated that applying low-cost sulfur compounds to LIBs can improve both energy density and the lifecycle simultaneously.”
This research, led by Research Professor Fangmin Ye, was published in Advanced Science on May 7.
Figure 1. Structural characterization of Li₂SO₄/CNT and Li₂S/CNT electrodes and suggested mechanism for the formation of the holey-Li₂S nanoarchitecture
2018.09.19 View 6055
Using Donut-shaped Lithium Sulfide for Higher Performing Batteries
(from left: Research Professor Fangmin Ye and Professor Hee-Tak Kim)
A KAIST research team developed a lithium-sulfur battery with a doughnut-shaped active material structure showing a record lifecycle of over 600 cycles. Having higher energy density and lower production cost than a lithium-ion battery (LIB), it can be used in electric vehicles that require a longer battery life.
There has been an intense research conducted for developing lithium-sulfur batteries with high energy density because LIBs only allow for a very short travel distance per charge. However, Li-S batteries are still unable to provide a longer lifecycle due to the poor reversibility of the lithium metal cathode.
To tackle this issue, Professor Hee-Tak Kim from the Department of Chemical and Biomolecular Engineering and his team used lithium sulfide (Li₂S) cathodes and combine them with graphite anodes to enhance energy density and lifecycles for the batteries.
Yet, lithium sulfide is costly and, so far, there has not been an electrode architecture and electrolyte design that enables a longer lifecycle between the graphite anodes and lithium sulfide cathodes.
Hence, the team produced a doughnut-shaped lithium sulfide cathode active material from low-cost lithium sulfide developed from raw materials. They have also developed a lithium sulfide ion battery with a graphite anode and lithium sulfide cathode using a high concentration salt electrolyte.
This doughnut-shaped lithium sulfide showed outstanding charge and discharge reversibility through improving the transfer of lithium ions. Its highly concentrated salt electrolyte formed a stable film on the surface of the graphite electrode, which showed strong durability.
Through this technology, the team achieved 30% higher energy density than that of conventional LIBs and secured a lifecycle of more than 600 cycles. This doughnut-shaped lithium sulfide-based electrode can be manufactured using low-cost raw materials and a single heat treatment process. The electrode can also be applied to existing LIBs.
Professor Kim said, “We have demonstrated that applying low-cost sulfur compounds to LIBs can improve both energy density and the lifecycle simultaneously.”
This research, led by Research Professor Fangmin Ye, was published in Advanced Science on May 7.
Figure 1. Structural characterization of Li₂SO₄/CNT and Li₂S/CNT electrodes and suggested mechanism for the formation of the holey-Li₂S nanoarchitecture
2018.09.19 View 6055 -
 Effective Drug Delivery to Heart with Tannic Acid
(Professor Haeshin Lee from the Department of Chemistry) Typical methods of drug delivery to the heart require surgical procedures involving incisions in the chest wall and bones. To efficiently treat cardiovascular and related vascular diseases without surgery, a KAIST research team developed a heart-targeting drug delivery technology using tannin acid via intravenous systemic injection. This method can be applied to the development of a variety of new protein-based drugs.
Cardiovascular-circulatory disease is currently the second leading cause of death in Korea. A typical example of this disease is myocardial infarction caused by poor oxygen and nutrient supply due to narrowed coronary arteries and poor blood flow to the heart.
Although there have been numerous research projects to develop chemotherapeutic drugs and therapeutic proteins, clinics still rely on surgical procedures. Drug delivery can be an alternative, but it is quite challenging because ceaseless dynamic cycles of the heart and massive exchanges of blood mean administered therapeutics do not stay inside the heart very long.
Professor Haeshin Lee from the Department of Chemistry and his team employed tannic acid (TA), which is known for giving bitter taste to wines. It is one of the most abundant polyphenols and can be easily found in plants, such as fruits, vegetables, cacao, and others. TA has also been used as a multifunctional coating molecule.
Using these properties of TA, the team complexed protein and peptide therapeutics with tannic acid and succeeded in targeting protein and peptide therapeutics to the heart. TA, coated on the surface of a granulated protein complex, helps maintain cardiac function because it adheres to extracellular matrices, elastin, and collagens in heart tissues allowing the protein to stay attached to the heart tissue for a longer period.
The team confirmed that these Tannic-acid-modified proteins stay in blood vessels five days longer than with protein-only injections. Additionally they found that TA-protein complexes do not show any cardiac toxicity and do not cause noticeable pathology.
The team has been continuously developing biomaterials for medical applications by testing various polyphenolic materials that feature adhesive and coating properties, including tannic acid. They have injected a mixture of TA and fibroblast growth factors (FGF) into animal models with myocardial infarctions. After four weeks, they confirmed that the infarction was reduced and the left ventricular pressure and cardiac output were almost normalized.
Professor Lee said, “Although there have been numerous drugs related to heart disease, so far there has not been efficient drug delivery to the heart so this technology will be able to reformulate existing drugs into new and more efficient drugs.”
This research, jointly led by Dr. Ki-Suk Kim from the Predictive Model Research Center, was published in Nature Biomedical Engineering on April 30 ( http://www.nature.com/articles/s41551-018-0227-9 ).
Figure 1. Schematic for the heart-targeting mechanism of TANNylated protein nanocomplexes: (1) size-dependent permeation, (2) phenolic (that is, TA), and (3) internalization by internalization by myoblasts
Figure 2. Effect of TA based protein complexes on cardiac cell transport efficiency and viral gene expression efficiency and therapeutic function in animal models with myocardial infarction
2018.09.18 View 5756
Effective Drug Delivery to Heart with Tannic Acid
(Professor Haeshin Lee from the Department of Chemistry) Typical methods of drug delivery to the heart require surgical procedures involving incisions in the chest wall and bones. To efficiently treat cardiovascular and related vascular diseases without surgery, a KAIST research team developed a heart-targeting drug delivery technology using tannin acid via intravenous systemic injection. This method can be applied to the development of a variety of new protein-based drugs.
Cardiovascular-circulatory disease is currently the second leading cause of death in Korea. A typical example of this disease is myocardial infarction caused by poor oxygen and nutrient supply due to narrowed coronary arteries and poor blood flow to the heart.
Although there have been numerous research projects to develop chemotherapeutic drugs and therapeutic proteins, clinics still rely on surgical procedures. Drug delivery can be an alternative, but it is quite challenging because ceaseless dynamic cycles of the heart and massive exchanges of blood mean administered therapeutics do not stay inside the heart very long.
Professor Haeshin Lee from the Department of Chemistry and his team employed tannic acid (TA), which is known for giving bitter taste to wines. It is one of the most abundant polyphenols and can be easily found in plants, such as fruits, vegetables, cacao, and others. TA has also been used as a multifunctional coating molecule.
Using these properties of TA, the team complexed protein and peptide therapeutics with tannic acid and succeeded in targeting protein and peptide therapeutics to the heart. TA, coated on the surface of a granulated protein complex, helps maintain cardiac function because it adheres to extracellular matrices, elastin, and collagens in heart tissues allowing the protein to stay attached to the heart tissue for a longer period.
The team confirmed that these Tannic-acid-modified proteins stay in blood vessels five days longer than with protein-only injections. Additionally they found that TA-protein complexes do not show any cardiac toxicity and do not cause noticeable pathology.
The team has been continuously developing biomaterials for medical applications by testing various polyphenolic materials that feature adhesive and coating properties, including tannic acid. They have injected a mixture of TA and fibroblast growth factors (FGF) into animal models with myocardial infarctions. After four weeks, they confirmed that the infarction was reduced and the left ventricular pressure and cardiac output were almost normalized.
Professor Lee said, “Although there have been numerous drugs related to heart disease, so far there has not been efficient drug delivery to the heart so this technology will be able to reformulate existing drugs into new and more efficient drugs.”
This research, jointly led by Dr. Ki-Suk Kim from the Predictive Model Research Center, was published in Nature Biomedical Engineering on April 30 ( http://www.nature.com/articles/s41551-018-0227-9 ).
Figure 1. Schematic for the heart-targeting mechanism of TANNylated protein nanocomplexes: (1) size-dependent permeation, (2) phenolic (that is, TA), and (3) internalization by internalization by myoblasts
Figure 2. Effect of TA based protein complexes on cardiac cell transport efficiency and viral gene expression efficiency and therapeutic function in animal models with myocardial infarction
2018.09.18 View 5756 -
 Distinguished Professor Sang Yup Lee Announced as the Eni Award Recipient
(Distinguished Professor Sang Yup Lee)
Distinguished Professor Sang Yup Lee from the Department of Chemical and Biomolecular Engineering will be awarded the 2018 Eni Advanced Environmental Solutions Prize in recognition of his innovations in the fields of energy and environment. The award ceremony will take place at the Quirinal Palace, the official residence of Italian President Sergio Mattarella, who will also be attending on October 22.
Eni, an Italian multinational energy corporation established the Eni Award in 2008 to promote technological and research innovation of efficient and sustainable energy resources. The Advanced Environmental Solutions Prize is one of the three categories of the Eni Award. The other two categories are Energy Transition and Energy Frontiers. The Award for Advanced Environmental Solutions recognizes a researcher or group of scientists that has achieved internationally significant R&D results in the field of environmental protection and recovery. The Eni Award is referred to as the Nobel Award in the fields of energy and environment.
Professor Lee, a pioneering leader in systems metabolic engineering was honored with the award for his developing engineered bacteria to produce chemical products, fuels, and non-food biomass materials sustainably and with a low environmental impact. He has leveraged the technology to develop microbial bioprocesses for the sustainable and environmentally friendly production of chemicals, fuels, and materials from non-food renewable biomass.
The award committee said that they considered the following elements in assessing Professor Lee’s achievement: the scientific relevance and the research innovation level; the impact on the energy system in terms of sustainability as well as fairer and broader access to energy; and the adequacy between technological and economic aspects.
Professor Lee, who already won two other distinguished prizes such as the George Washington Carver Award and the PV Danckwerts Memorial Lecture Award this year, said, “I am so glad that the international academic community as well as global industry leaders came to recognize our work that our students and research team has made for decades.”
Dr. Lee’s lab has been producing a lot of chemicals in environmentally friendly ways. Among them, many were biologically produced for the first time and some of these processes have been already commercialized. “We will continue to strive for research outcomes with two objectives: First, to develop bio-based processes suitable for sustainable chemical industry. The other is to contribute to the human healthcare system through development of platform technologies integrating medicine and nutrition,” he added.
2018.09.12 View 8558
Distinguished Professor Sang Yup Lee Announced as the Eni Award Recipient
(Distinguished Professor Sang Yup Lee)
Distinguished Professor Sang Yup Lee from the Department of Chemical and Biomolecular Engineering will be awarded the 2018 Eni Advanced Environmental Solutions Prize in recognition of his innovations in the fields of energy and environment. The award ceremony will take place at the Quirinal Palace, the official residence of Italian President Sergio Mattarella, who will also be attending on October 22.
Eni, an Italian multinational energy corporation established the Eni Award in 2008 to promote technological and research innovation of efficient and sustainable energy resources. The Advanced Environmental Solutions Prize is one of the three categories of the Eni Award. The other two categories are Energy Transition and Energy Frontiers. The Award for Advanced Environmental Solutions recognizes a researcher or group of scientists that has achieved internationally significant R&D results in the field of environmental protection and recovery. The Eni Award is referred to as the Nobel Award in the fields of energy and environment.
Professor Lee, a pioneering leader in systems metabolic engineering was honored with the award for his developing engineered bacteria to produce chemical products, fuels, and non-food biomass materials sustainably and with a low environmental impact. He has leveraged the technology to develop microbial bioprocesses for the sustainable and environmentally friendly production of chemicals, fuels, and materials from non-food renewable biomass.
The award committee said that they considered the following elements in assessing Professor Lee’s achievement: the scientific relevance and the research innovation level; the impact on the energy system in terms of sustainability as well as fairer and broader access to energy; and the adequacy between technological and economic aspects.
Professor Lee, who already won two other distinguished prizes such as the George Washington Carver Award and the PV Danckwerts Memorial Lecture Award this year, said, “I am so glad that the international academic community as well as global industry leaders came to recognize our work that our students and research team has made for decades.”
Dr. Lee’s lab has been producing a lot of chemicals in environmentally friendly ways. Among them, many were biologically produced for the first time and some of these processes have been already commercialized. “We will continue to strive for research outcomes with two objectives: First, to develop bio-based processes suitable for sustainable chemical industry. The other is to contribute to the human healthcare system through development of platform technologies integrating medicine and nutrition,” he added.
2018.09.12 View 8558