AI
-
 KAIST presents a fundamental technology to remove metastatic traits from lung cancer cells
KAIST (President Kwang Hyung Lee) announced on January 30th that a research team led by Professor Kwang-Hyun Cho from the Department of Bio and Brain Engineering succeeded in using systems biology research to change the properties of carcinogenic cells in the lungs and eliminate both drug resistance and their ability to proliferate out to other areas of the body.
As the incidences of cancer increase within aging populations, cancer has become the most lethal disease threatening healthy life. Fatality rates are especially high when early detection does not happen in time and metastasis has occurred in various organs. In order to resolve this problem, a series of attempts were made to remove or lower the ability of cancer cells to spread, but they resulted in cancer cells in the intermediate state becoming more unstable and even more malignant, which created serious treatment challenges.
Professor Kwang-Hyun Cho's research team simulated various cancer cell states in the Epithelial-to-Mesenchymal Transition (EMT) of lung cancer cells, between epithelial cells without metastatic ability and mesenchymal cells with metastatic ability. A mathematical model of molecular network was established, and key regulators that could reverse the state of invasive and drug resistant mesenchymal cells back to the epithelial state were discovered through computer simulation analysis and molecular cell experiments. In particular, this process succeeded in properly reverting the mesenchymal lung cancer cells to a state where they were sensitive to chemotherapy treatment while avoiding the unstable EMT hybrid cell state in the middle process, which had remained a difficult problem.
The results of this research, in which KAIST Ph.D. student Namhee Kim, Dr. Chae Young Hwang, Researcher Taeyoung Kim, and Ph.D. student Hyunjin Kim participated, were published as an online paper in the international journal “Cancer Research” published by the American Association for Cancer Research (AACR) on January 30th. (Paper title: A cell fate reprogramming strategy reverses epithelial-to-mesenchymal transition of lung cancer cells while avoiding hybrid states)
Cells in an EMT hybrid state, which are caused by incomplete transitions during the EMT process in cancer cells, have the characteristics of both epithelial cells and mesenchymal cells, and are known to have high drug resistance and metastatic potential by acquiring high stem cell capacity. In particular, EMT is further enhanced through factors such as transforming growth factor-beta (TGF-β) secreted from the tumor microenvironment (TME) and, as a result, various cell states with high plasticity appear. Due to the complexity of EMT, it has been very difficult to completely reverse the transitional process of the mesenchymal cancer cells to an epithelial cell state in which metastatic ability and drug resistance are eliminated while avoiding the EMT hybrid cell state with high metastatic ability and drug resistance.
Professor Kwang-Hyun Cho's research team established a mathematical model of the gene regulation network that governs the complex process of EMT, and then applied large-scale computer simulation analysis and complex system network control technology to identify and verify 'p53', 'SMAD4', and 'ERK1' and 'ERK 2' (collectively ERKs) through molecular cell experiments as the three key molecular targets that can transform lung cancer cells in the mesenchymal cell state, reversed back to an epithelial cell state that no longer demonstrates the ability to metastasize, while avoiding the EMT hybrid cell state.
In particular, by analyzing the molecular regulatory mechanism of the complex EMT process at the system level, the key pathways were identified that were linked to the positive feedback that plays an important role in completely returning cancer cells to an epithelial cell state in which metastatic ability and drug resistance are removed.
This discovery is significant in that it proved that mesenchymal cells can be reverted to the state of epithelial cells under conditions where TGF-β stimulation are present, like they are in the actual environment where cancer tissue forms in the human body.
Abnormal EMT in cancer cells leads to various malignant traits such as the migration and invasion of cancer cells, changes in responsiveness to chemotherapy treatment, enhanced stem cell function, and the dissemination of cancer. In particular, the acquisition of the metastatic ability of cancer cells is a key determinant factor for the prognosis of cancer patients. The EMT reversal technology in lung cancer cells developed in this research is a new anti-cancer treatment strategy that reprograms cancer cells to eliminate their high plasticity and metastatic potential and increase their responsiveness to chemotherapy.
Professor Kwang-Hyun Cho said, "By succeeding in reversing the state of lung cancer cells that acquired high metastatic traits and resistance to drugs and reverting them to a treatable epithelial cell state with renewed sensitivity to chemotherapy, the research findings propose a new strategy for treatments that can improve the prognosis of cancer patients.”
Professor Kwang-Hyun Cho's research team was the first to present the principle of reversal treatment to revert cancer cells to normal cells, following through with the announcement of the results of their study that reverted colon cancer cells to normal colon cells in January of 2020, and also presenting successful re-programming research where the most malignant basal type breast cancer cells turned into less-malignant luminal type breast cancer cells that were treatable with hormonal therapies in January of 2022. This latest research result is the third in the development of reversal technology where lung cancer cells that had acquired metastatic traits returned to a state in which their metastatic ability was removed and drug sensitivity was enhanced.
This research was carried out with support from the Ministry of Science and ICT and the National Research Foundation of Korea's Basic Research in Science & Engineering Program for Mid-Career Researchers.
< Figure 1. Construction of the mathematical model of the regulatory network to represent the EMT phenotype based on the interaction between various molecules related to EMT.
(A) Professor Kwang-Hyun Cho's research team investigated numerous literatures and databases related to complex EMT, and based on comparative analysis of cell line data showing epithelial and mesenchymal cell conditions, they extracted key signaling pathways related to EMT and built a mathematical model of regulatory network (B) By comparing the results of computer simulation analysis and the molecular cell experiments, it was verified how well the constructed mathematical model simulated the actual cellular phenomena. >
< Figure 2. Understanding of various EMT phenotypes through large-scale computer simulation analysis and complex system network control technology.
(A) Through computer simulation analysis and experiments, Professor Kwang-Hyun Cho's research team found that complete control of EMT is impossible with single-molecule control alone. In particular, through comparison of the relative stability of attractors, it was revealed that the cell state exhibiting EMT hybrid characteristics has unstable properties. (B), (C) Based on these results, Prof. Cho’s team identified two feedbacks (positive feedback consisting of Snail-miR-34 and ZEB1-miR-200) that play an important role in avoiding the EMT hybrid state that appeared in the TGF-β-ON state. It was found through computer simulation analysis that the two feedbacks restore relatively high stability when the excavated p53 and SMAD4 are regulated. In addition, molecular cell experiments demonstrated that the expression levels of E-cad and ZEB1, which are representative phenotypic markers of EMT, changed similarly to the expression profile in the epithelial cell state, despite the TGF-β-ON state. >
< Figure 3. Complex molecular network analysis and discovery of reprogramming molecular targets for intact elimination of EMT hybrid features.
(A) Controlling the expression of p53 and SMAD4 in lung cancer cell lines was expected to overcome drug resistance, but contrary to expectations, chemotherapy responsiveness was not restored. (B) Professor Kwang-Hyun Cho's research team additionally analyzed computer simulations, genome data, and experimental results and found that high expression levels of TWIST1 and EPCAM were related to drug resistance. (C) Prof. Cho’s team identified three key molecular targets: p53, SMAD4 and ERK1 & ERK2. (D), (E) Furthermore, they identified a key pathway that plays an important role in completely reversing into epithelial cells while avoiding EMT hybrid characteristics, and confirmed through network analysis and attractor analysis that high stability of the key pathway was restored when the proposed molecular target was controlled. >
< Figure 4. Verification through experiments with lung cancer cell lines.
When p53 was activated and SMAD4 and ERK1/2 were inhibited in lung cancer cell lines, (A), (B) E-cad protein expression increased and ZEB1 protein expression decreased, and (C) mesenchymal cell status including TWIST1 and EPCAM and gene expression of markers related to stem cell potential characteristics were completely inhibited. In addition, (D) it was confirmed that resistance to chemotherapy treatment was also overcome as the cell state was reversed by the regulated target. >
< Figure 5. A schematic representation of the research results.
Prof. Cho’s research team identified key molecular regulatory pathways to avoid high plasticity formed by abnormal EMT of cancer cells and reverse it to an epithelial cell state through systems biology research. From this analysis, a reprogramming molecular target that can reverse the state of mesenchymal cells with acquired invasiveness and drug resistance to the state of epithelial cells with restored drug responsiveness was discovered.
For lung cancer cells, when a drug that enhances the expression of p53, one of the molecular targets discovered, and inhibits the expression of SMAD4 and ERK1 & ERK2 is administered, the molecular network of genes in the state of mesenchymal cells is modified, eventually eliminating metastatic ability and it is reprogrammed to turn into epithelial cells without the resistance to chemotherapy treatments. >
2023.01.30 View 20825
KAIST presents a fundamental technology to remove metastatic traits from lung cancer cells
KAIST (President Kwang Hyung Lee) announced on January 30th that a research team led by Professor Kwang-Hyun Cho from the Department of Bio and Brain Engineering succeeded in using systems biology research to change the properties of carcinogenic cells in the lungs and eliminate both drug resistance and their ability to proliferate out to other areas of the body.
As the incidences of cancer increase within aging populations, cancer has become the most lethal disease threatening healthy life. Fatality rates are especially high when early detection does not happen in time and metastasis has occurred in various organs. In order to resolve this problem, a series of attempts were made to remove or lower the ability of cancer cells to spread, but they resulted in cancer cells in the intermediate state becoming more unstable and even more malignant, which created serious treatment challenges.
Professor Kwang-Hyun Cho's research team simulated various cancer cell states in the Epithelial-to-Mesenchymal Transition (EMT) of lung cancer cells, between epithelial cells without metastatic ability and mesenchymal cells with metastatic ability. A mathematical model of molecular network was established, and key regulators that could reverse the state of invasive and drug resistant mesenchymal cells back to the epithelial state were discovered through computer simulation analysis and molecular cell experiments. In particular, this process succeeded in properly reverting the mesenchymal lung cancer cells to a state where they were sensitive to chemotherapy treatment while avoiding the unstable EMT hybrid cell state in the middle process, which had remained a difficult problem.
The results of this research, in which KAIST Ph.D. student Namhee Kim, Dr. Chae Young Hwang, Researcher Taeyoung Kim, and Ph.D. student Hyunjin Kim participated, were published as an online paper in the international journal “Cancer Research” published by the American Association for Cancer Research (AACR) on January 30th. (Paper title: A cell fate reprogramming strategy reverses epithelial-to-mesenchymal transition of lung cancer cells while avoiding hybrid states)
Cells in an EMT hybrid state, which are caused by incomplete transitions during the EMT process in cancer cells, have the characteristics of both epithelial cells and mesenchymal cells, and are known to have high drug resistance and metastatic potential by acquiring high stem cell capacity. In particular, EMT is further enhanced through factors such as transforming growth factor-beta (TGF-β) secreted from the tumor microenvironment (TME) and, as a result, various cell states with high plasticity appear. Due to the complexity of EMT, it has been very difficult to completely reverse the transitional process of the mesenchymal cancer cells to an epithelial cell state in which metastatic ability and drug resistance are eliminated while avoiding the EMT hybrid cell state with high metastatic ability and drug resistance.
Professor Kwang-Hyun Cho's research team established a mathematical model of the gene regulation network that governs the complex process of EMT, and then applied large-scale computer simulation analysis and complex system network control technology to identify and verify 'p53', 'SMAD4', and 'ERK1' and 'ERK 2' (collectively ERKs) through molecular cell experiments as the three key molecular targets that can transform lung cancer cells in the mesenchymal cell state, reversed back to an epithelial cell state that no longer demonstrates the ability to metastasize, while avoiding the EMT hybrid cell state.
In particular, by analyzing the molecular regulatory mechanism of the complex EMT process at the system level, the key pathways were identified that were linked to the positive feedback that plays an important role in completely returning cancer cells to an epithelial cell state in which metastatic ability and drug resistance are removed.
This discovery is significant in that it proved that mesenchymal cells can be reverted to the state of epithelial cells under conditions where TGF-β stimulation are present, like they are in the actual environment where cancer tissue forms in the human body.
Abnormal EMT in cancer cells leads to various malignant traits such as the migration and invasion of cancer cells, changes in responsiveness to chemotherapy treatment, enhanced stem cell function, and the dissemination of cancer. In particular, the acquisition of the metastatic ability of cancer cells is a key determinant factor for the prognosis of cancer patients. The EMT reversal technology in lung cancer cells developed in this research is a new anti-cancer treatment strategy that reprograms cancer cells to eliminate their high plasticity and metastatic potential and increase their responsiveness to chemotherapy.
Professor Kwang-Hyun Cho said, "By succeeding in reversing the state of lung cancer cells that acquired high metastatic traits and resistance to drugs and reverting them to a treatable epithelial cell state with renewed sensitivity to chemotherapy, the research findings propose a new strategy for treatments that can improve the prognosis of cancer patients.”
Professor Kwang-Hyun Cho's research team was the first to present the principle of reversal treatment to revert cancer cells to normal cells, following through with the announcement of the results of their study that reverted colon cancer cells to normal colon cells in January of 2020, and also presenting successful re-programming research where the most malignant basal type breast cancer cells turned into less-malignant luminal type breast cancer cells that were treatable with hormonal therapies in January of 2022. This latest research result is the third in the development of reversal technology where lung cancer cells that had acquired metastatic traits returned to a state in which their metastatic ability was removed and drug sensitivity was enhanced.
This research was carried out with support from the Ministry of Science and ICT and the National Research Foundation of Korea's Basic Research in Science & Engineering Program for Mid-Career Researchers.
< Figure 1. Construction of the mathematical model of the regulatory network to represent the EMT phenotype based on the interaction between various molecules related to EMT.
(A) Professor Kwang-Hyun Cho's research team investigated numerous literatures and databases related to complex EMT, and based on comparative analysis of cell line data showing epithelial and mesenchymal cell conditions, they extracted key signaling pathways related to EMT and built a mathematical model of regulatory network (B) By comparing the results of computer simulation analysis and the molecular cell experiments, it was verified how well the constructed mathematical model simulated the actual cellular phenomena. >
< Figure 2. Understanding of various EMT phenotypes through large-scale computer simulation analysis and complex system network control technology.
(A) Through computer simulation analysis and experiments, Professor Kwang-Hyun Cho's research team found that complete control of EMT is impossible with single-molecule control alone. In particular, through comparison of the relative stability of attractors, it was revealed that the cell state exhibiting EMT hybrid characteristics has unstable properties. (B), (C) Based on these results, Prof. Cho’s team identified two feedbacks (positive feedback consisting of Snail-miR-34 and ZEB1-miR-200) that play an important role in avoiding the EMT hybrid state that appeared in the TGF-β-ON state. It was found through computer simulation analysis that the two feedbacks restore relatively high stability when the excavated p53 and SMAD4 are regulated. In addition, molecular cell experiments demonstrated that the expression levels of E-cad and ZEB1, which are representative phenotypic markers of EMT, changed similarly to the expression profile in the epithelial cell state, despite the TGF-β-ON state. >
< Figure 3. Complex molecular network analysis and discovery of reprogramming molecular targets for intact elimination of EMT hybrid features.
(A) Controlling the expression of p53 and SMAD4 in lung cancer cell lines was expected to overcome drug resistance, but contrary to expectations, chemotherapy responsiveness was not restored. (B) Professor Kwang-Hyun Cho's research team additionally analyzed computer simulations, genome data, and experimental results and found that high expression levels of TWIST1 and EPCAM were related to drug resistance. (C) Prof. Cho’s team identified three key molecular targets: p53, SMAD4 and ERK1 & ERK2. (D), (E) Furthermore, they identified a key pathway that plays an important role in completely reversing into epithelial cells while avoiding EMT hybrid characteristics, and confirmed through network analysis and attractor analysis that high stability of the key pathway was restored when the proposed molecular target was controlled. >
< Figure 4. Verification through experiments with lung cancer cell lines.
When p53 was activated and SMAD4 and ERK1/2 were inhibited in lung cancer cell lines, (A), (B) E-cad protein expression increased and ZEB1 protein expression decreased, and (C) mesenchymal cell status including TWIST1 and EPCAM and gene expression of markers related to stem cell potential characteristics were completely inhibited. In addition, (D) it was confirmed that resistance to chemotherapy treatment was also overcome as the cell state was reversed by the regulated target. >
< Figure 5. A schematic representation of the research results.
Prof. Cho’s research team identified key molecular regulatory pathways to avoid high plasticity formed by abnormal EMT of cancer cells and reverse it to an epithelial cell state through systems biology research. From this analysis, a reprogramming molecular target that can reverse the state of mesenchymal cells with acquired invasiveness and drug resistance to the state of epithelial cells with restored drug responsiveness was discovered.
For lung cancer cells, when a drug that enhances the expression of p53, one of the molecular targets discovered, and inhibits the expression of SMAD4 and ERK1 & ERK2 is administered, the molecular network of genes in the state of mesenchymal cells is modified, eventually eliminating metastatic ability and it is reprogrammed to turn into epithelial cells without the resistance to chemotherapy treatments. >
2023.01.30 View 20825 -
 UAE Space Program Leaders named to be the 1st of the honorees of KAIST Alumni Association's special recognition for graduates of foreign nationality
The KAIST Alumni Association (Chairman, Chil-Hee Chung) announced on the 12th that the winners of the 2023 KAIST Distinguished Alumni Award and International Alumni Award has been selected.
The KAIST Distinguished Alumni Award, which produced the first recipient in 1992, is an award given to alumni who have contributed to the development of the nation and society, or who have glorified the honor of their alma mater with outstanding academic achievements and social and/or communal contributions.
On a special note, this year, there has been an addition to the honors, “the KAIST Distinguished International Alumni Award” to honor and encourage overseas alumni who are making their marks in the international community that will boost positive recognition of KAIST in the global setting and will later become a bridge that will expedite Korea's international efforts in the future.
As of 2022, the number of international students who succeeded in earning KAIST degrees has exceeded 1,700, and they are actively doing their part back in their home countries as leaders in various fields in which they belong, spanning from science and technology, to politics, industry and other corners of the society.
(From left) Omran Sharaf, the Assistant Minister of UAE Foreign Affairs and International Cooperation for Advanced Science and Technology, Amer Al Sayegh the Director General of Space Project at MBRSC, and Mohammed Al Harmi the Director General of Administration at MBRSC (Photos provided by the courtesy of MBRSC)
To celebrate and honor their outstanding achievements, the KAIST Alumni Association selected a team of three alumni of the United Arab Emirates (UAE) to receive the Distinguished International Alumni Award for the first time. The named honorees are Omran Sharaf, a master’s graduate from the Graduate School of Science and Technology Policy, and Amer Al Sayegh and Mohammed Al Harmi, master’s graduates of the Department of Aerospace Engineering - all three of the class of 2013 in leading positions in the UAE space program to lead the advancement of the science and technology of the country.
Currently, the three alums are in directorship of the Mohammed Bin Rashid Space Centre (MBRSC) with Mr. Omran Sharaf, who has recently been appointed as the Assistant Minister in charge of Advanced Science and Technology at the UAE Ministry of Foreign Affairs and International Cooperation, being the Project Director of the Emirates Mars Mission of MBRSC and Mr. Amer Al Sayegh in the Director General position in charge of Space Project and Mr. Mohammed Al Harmi, the Director General of Administration, at MBRSC.
They received technology transfer from “SatRec I”, Korea's first satellite system exporter and KAIST alumni company, for about 10 years from 2006, while carrying out their master’s studies at the same time.
Afterwards, they returned to UAE to lead the Emirates Mars Mission, which is already showing tangible progress including the successful launch of the Mars probe "Amal" (ال امل, meaning ‘Hope’ in Arabic), which was the first in the Arab world and the fifth in the world to successfully enter into orbit around Mars, and the UAE’s first independently developed Earth observation satellite "KhalifaSat".
An official from the KAIST Alumni Association said, "We selected the Distinguished International Alumni after evaluating their industrious leadership in promoting various space industry strategies, ranging from the development of Mars probes and Earth observation satellites, as well as lunar exploration, asteroid exploration, and Mars residence plans."
(From left) Joo-Sun Choi, President & CEO of Samsung Display Co. Ltd., Jung Goo Cho, the CEO of Green Power Co. Ltd., Jong Seung Park, the President of Agency for Defense Development (ADD), Kyunghyun Cho, Professor of New York University (NYU)
Also, four of the Korean graduates, Joo-Sun Choi, the CEO of Samsung Display, Jung Goo Cho, the CEO of Green Power Co. Ltd., Jong Seung Park, the President of Agency for Defense Development (ADD), and Kyunghyun Cho, a Professor of New York University (NYU), were selected as the winners of the “Distinguished Alumni Award”.
Mr. Joo-Sun Choi (Electrical and Electronic Engineering, M.S. in 1989, Ph.D. in 1995), the CEO of Samsung Display, led the successful development and mass-production of the world's first ultra-high-definition QD-OLED Displays, and preemptively transformed the structure of business of the industry and has been leading the way in technological innovation.
Mr. Jung Goo Cho (Electrical and Electronic Engineering, M.S. in 1988, Ph.D. in 1992), the CEO of Green Power Co. Ltd., developed wireless power technology for the first time in Korea in the early 2000s and applied it to semiconductor/display lines and led the wireless power charging technology in various fields, such as developing KAIST On-Line Electric Vehicles (OLEV) and commercializing the world's first wireless charger for 11kW electric vehicles.
Mr. Jong Seung Park (Mechanical Engineering, M.S. in 1988, Ph.D., in 1991), The President of ADD is an expert with abundant science and technology knowledge and organizational management capabilities. He is contributing greatly to national defense and security through science and technology.
Mr. Kyunghyun Cho (Computer Science, B.S., in 2009), the Professor of Computer Science and Data Science at NYU, is a world-renowned expert in Artificial Intelligence (AI), advancing the concept of 'Neural Machine Translation' in the field of natural language processing, to make great contributions to AI translation technology and related industries.
Chairman Chil-Hee Chung, the 26th Chair of KAIST Alumni Association “As each year goes by, I feel that the influence of KAIST alumni goes beyond science and technology to affect our society as a whole.” He went on to say, “This year, as it was more meaningful to extend the award to honor the international members of our Alums, we look forward to seeing more of our alumni continuing their social and academic endeavors to play an active role in the global stage in taking on the global challenges.”
The Ceremony for KAIST Distinguished Alumni and International Alumni Award Honorees will be conducted at the Annual New Year’s Event of KAIST Alumni Association for 2023 to be held on Friday, January 13th, at the Grand InterContinental Seoul Parnas.
2023.01.12 View 18732
UAE Space Program Leaders named to be the 1st of the honorees of KAIST Alumni Association's special recognition for graduates of foreign nationality
The KAIST Alumni Association (Chairman, Chil-Hee Chung) announced on the 12th that the winners of the 2023 KAIST Distinguished Alumni Award and International Alumni Award has been selected.
The KAIST Distinguished Alumni Award, which produced the first recipient in 1992, is an award given to alumni who have contributed to the development of the nation and society, or who have glorified the honor of their alma mater with outstanding academic achievements and social and/or communal contributions.
On a special note, this year, there has been an addition to the honors, “the KAIST Distinguished International Alumni Award” to honor and encourage overseas alumni who are making their marks in the international community that will boost positive recognition of KAIST in the global setting and will later become a bridge that will expedite Korea's international efforts in the future.
As of 2022, the number of international students who succeeded in earning KAIST degrees has exceeded 1,700, and they are actively doing their part back in their home countries as leaders in various fields in which they belong, spanning from science and technology, to politics, industry and other corners of the society.
(From left) Omran Sharaf, the Assistant Minister of UAE Foreign Affairs and International Cooperation for Advanced Science and Technology, Amer Al Sayegh the Director General of Space Project at MBRSC, and Mohammed Al Harmi the Director General of Administration at MBRSC (Photos provided by the courtesy of MBRSC)
To celebrate and honor their outstanding achievements, the KAIST Alumni Association selected a team of three alumni of the United Arab Emirates (UAE) to receive the Distinguished International Alumni Award for the first time. The named honorees are Omran Sharaf, a master’s graduate from the Graduate School of Science and Technology Policy, and Amer Al Sayegh and Mohammed Al Harmi, master’s graduates of the Department of Aerospace Engineering - all three of the class of 2013 in leading positions in the UAE space program to lead the advancement of the science and technology of the country.
Currently, the three alums are in directorship of the Mohammed Bin Rashid Space Centre (MBRSC) with Mr. Omran Sharaf, who has recently been appointed as the Assistant Minister in charge of Advanced Science and Technology at the UAE Ministry of Foreign Affairs and International Cooperation, being the Project Director of the Emirates Mars Mission of MBRSC and Mr. Amer Al Sayegh in the Director General position in charge of Space Project and Mr. Mohammed Al Harmi, the Director General of Administration, at MBRSC.
They received technology transfer from “SatRec I”, Korea's first satellite system exporter and KAIST alumni company, for about 10 years from 2006, while carrying out their master’s studies at the same time.
Afterwards, they returned to UAE to lead the Emirates Mars Mission, which is already showing tangible progress including the successful launch of the Mars probe "Amal" (ال امل, meaning ‘Hope’ in Arabic), which was the first in the Arab world and the fifth in the world to successfully enter into orbit around Mars, and the UAE’s first independently developed Earth observation satellite "KhalifaSat".
An official from the KAIST Alumni Association said, "We selected the Distinguished International Alumni after evaluating their industrious leadership in promoting various space industry strategies, ranging from the development of Mars probes and Earth observation satellites, as well as lunar exploration, asteroid exploration, and Mars residence plans."
(From left) Joo-Sun Choi, President & CEO of Samsung Display Co. Ltd., Jung Goo Cho, the CEO of Green Power Co. Ltd., Jong Seung Park, the President of Agency for Defense Development (ADD), Kyunghyun Cho, Professor of New York University (NYU)
Also, four of the Korean graduates, Joo-Sun Choi, the CEO of Samsung Display, Jung Goo Cho, the CEO of Green Power Co. Ltd., Jong Seung Park, the President of Agency for Defense Development (ADD), and Kyunghyun Cho, a Professor of New York University (NYU), were selected as the winners of the “Distinguished Alumni Award”.
Mr. Joo-Sun Choi (Electrical and Electronic Engineering, M.S. in 1989, Ph.D. in 1995), the CEO of Samsung Display, led the successful development and mass-production of the world's first ultra-high-definition QD-OLED Displays, and preemptively transformed the structure of business of the industry and has been leading the way in technological innovation.
Mr. Jung Goo Cho (Electrical and Electronic Engineering, M.S. in 1988, Ph.D. in 1992), the CEO of Green Power Co. Ltd., developed wireless power technology for the first time in Korea in the early 2000s and applied it to semiconductor/display lines and led the wireless power charging technology in various fields, such as developing KAIST On-Line Electric Vehicles (OLEV) and commercializing the world's first wireless charger for 11kW electric vehicles.
Mr. Jong Seung Park (Mechanical Engineering, M.S. in 1988, Ph.D., in 1991), The President of ADD is an expert with abundant science and technology knowledge and organizational management capabilities. He is contributing greatly to national defense and security through science and technology.
Mr. Kyunghyun Cho (Computer Science, B.S., in 2009), the Professor of Computer Science and Data Science at NYU, is a world-renowned expert in Artificial Intelligence (AI), advancing the concept of 'Neural Machine Translation' in the field of natural language processing, to make great contributions to AI translation technology and related industries.
Chairman Chil-Hee Chung, the 26th Chair of KAIST Alumni Association “As each year goes by, I feel that the influence of KAIST alumni goes beyond science and technology to affect our society as a whole.” He went on to say, “This year, as it was more meaningful to extend the award to honor the international members of our Alums, we look forward to seeing more of our alumni continuing their social and academic endeavors to play an active role in the global stage in taking on the global challenges.”
The Ceremony for KAIST Distinguished Alumni and International Alumni Award Honorees will be conducted at the Annual New Year’s Event of KAIST Alumni Association for 2023 to be held on Friday, January 13th, at the Grand InterContinental Seoul Parnas.
2023.01.12 View 18732 -
 KAIST to showcase a pack of KAIST Start-ups at CES 2023
- KAIST is to run an Exclusive Booth at the Venetian Expo (Hall G) in Eureka Park, at CES 2023, to be held in Las Vegas from Thursday, January 5th through Sunday, the 8th.
- Twelve businesses recently put together by KAIST faculty, alumni, and the start-ups given legal usage of KAIST technologies will be showcased.
- Out of the participating start-ups, the products by Fluiz and Hills Robotics were selected as the “CES Innovation Award 2023 Honoree”, scoring top in their respective categories.
On January 3, KAIST announced that there will be a KAIST booth at Consumer Electronics Show (CES) 2023, the most influential tech event in the world, to be held in Las Vegas from January 3 to 8.
At this exclusive corner, KAIST will introduce the technologies of KAIST start-ups over the exhibition period.
KAIST first started holding its exclusive booth in CES 2019 with five start-up businesses, following up at CES 2020 with 12 start-ups and at CES 2022 with 10 start-ups. At CES 2023, which would be KAIST’s fourth conference, KAIST will be accompanying 12 businesses including start-ups by the faculty members, alumni, and technology transfer companies that just began their businesses with technologies from their research findings that stands a head above others.
To maximize the publicity opportunity, KAIST will support each company’s marketing strategies through cooperation with the Korea International Trade Association (KITA), and provide an opportunity for the school and each startup to create global identity and exhibit the excellence of their technologies at the convention.
The following companies will be at the KAIST Booth in Eureka Park:
The twelve startups mentioned above aim to achieve global technology commecialization in their respective fields of expertise spanning from eXtended Reality (XR) and gaming, to AI and robotics, vehicle and transport, mobile platform, smart city, autonomous driving, healthcare, internet of thing (IoT), through joint research and development, technology transfer and investment attraction from world’s leading institutions and enterprises.
In particular, Fluiz and Hills Robotics won the CES Innovation Award as 2023 Honorees and is expected to attain greater achievements in the future.
A staff member from the KAIST Institute of Technology Value Creation said, “The KAIST Showcase for CES 2023 has prepared a new pitching space for each of the companies for their own IR efforts, and we hope that KAIST startups will actively and effectively market their products and technologies while they are at the convention. We hope it will help them utilize their time here to establish their name in presence here which will eventually serve as a good foothold for them and their predecessors to further global commercialization goals.”
2023.01.04 View 19674
KAIST to showcase a pack of KAIST Start-ups at CES 2023
- KAIST is to run an Exclusive Booth at the Venetian Expo (Hall G) in Eureka Park, at CES 2023, to be held in Las Vegas from Thursday, January 5th through Sunday, the 8th.
- Twelve businesses recently put together by KAIST faculty, alumni, and the start-ups given legal usage of KAIST technologies will be showcased.
- Out of the participating start-ups, the products by Fluiz and Hills Robotics were selected as the “CES Innovation Award 2023 Honoree”, scoring top in their respective categories.
On January 3, KAIST announced that there will be a KAIST booth at Consumer Electronics Show (CES) 2023, the most influential tech event in the world, to be held in Las Vegas from January 3 to 8.
At this exclusive corner, KAIST will introduce the technologies of KAIST start-ups over the exhibition period.
KAIST first started holding its exclusive booth in CES 2019 with five start-up businesses, following up at CES 2020 with 12 start-ups and at CES 2022 with 10 start-ups. At CES 2023, which would be KAIST’s fourth conference, KAIST will be accompanying 12 businesses including start-ups by the faculty members, alumni, and technology transfer companies that just began their businesses with technologies from their research findings that stands a head above others.
To maximize the publicity opportunity, KAIST will support each company’s marketing strategies through cooperation with the Korea International Trade Association (KITA), and provide an opportunity for the school and each startup to create global identity and exhibit the excellence of their technologies at the convention.
The following companies will be at the KAIST Booth in Eureka Park:
The twelve startups mentioned above aim to achieve global technology commecialization in their respective fields of expertise spanning from eXtended Reality (XR) and gaming, to AI and robotics, vehicle and transport, mobile platform, smart city, autonomous driving, healthcare, internet of thing (IoT), through joint research and development, technology transfer and investment attraction from world’s leading institutions and enterprises.
In particular, Fluiz and Hills Robotics won the CES Innovation Award as 2023 Honorees and is expected to attain greater achievements in the future.
A staff member from the KAIST Institute of Technology Value Creation said, “The KAIST Showcase for CES 2023 has prepared a new pitching space for each of the companies for their own IR efforts, and we hope that KAIST startups will actively and effectively market their products and technologies while they are at the convention. We hope it will help them utilize their time here to establish their name in presence here which will eventually serve as a good foothold for them and their predecessors to further global commercialization goals.”
2023.01.04 View 19674 -
 KAIST Offers Hope to Musicians with Dystonia
< Photo 1. Conductor and Pianist João Carlos Martins before the Recital at the Carnegie Hall preparing with his bionic gloves >
KAIST’s neuroscientist and professor, Dr. Daesoo Kim attended the “Conference for Musicians with Dystonia” supported by the World Health Organization (WHO) and the Carnegie Hall concert of legendary pianist João Carlos Martins, who is also a dystonia patient, to announce his team’s recent advancements toward finding a cure for dystonia.
On November 19, 2022, a “miracle concert” was held in Carnegie Hall. João Carlos Martins was a renowned world-class pianist in the 70s and 80s, but he had to put an end to his musical career due to focal dystonia in his fingers. But in 2020, he began using a bionic glove developed by industrial designer Ubiratã Bizarro Costa and after years of hard work he was back in Carnegie Hall as an 82-year-old man.
During the concert, he conducted the NOVUS NY orchestra in a performance of Bach, and later even played the piano himself. In particular, between his performances, he gave shout-outs to scientists studying dystonia including KAIST Professor Daesoo Kim, asking them to continue working towards curing rare diseases for musicians.
< Photo 2. Professor Daesoo Kim with Conductor and Pianist João Carlos Martins >
Musician’s dystonia affects 1-3% of musicians around the world and musicians make up approximately 5% of the total number of dystonia patients. Musicians who are no longer able to practice music due to the disease often experience stress and depression, which may even lead to suicide in extreme cases. Musicians are known to be particularly prone to such diseases due to excessive practice regimens, perfectionism, and even genetics. Currently, botulinum toxin (Botox) is used to suppress abnormal muscles, but muscle function suppression ultimately means that the musician is no longer able to play the instrument. João Carlos Martins himself underwent several Botox procedures and three brain surgeries, but saw no therapeutic results. This is why a new treatment was necessary.
Professor Daesoo Kim’s research team at KAIST took note of the fact that abnormal muscle tension is caused by excessive stress, and developed NT-1, a treatment that blocks the development of the symptoms of dystonia from the brain, allowing patients to use their muscles as they normally would. The research team published their findings in Science Advances in 2021, and João Carlos Martins invited Professor Daesoo Kim to the UN conference and his concert after reading this paper.
< Photo 3. Professor Daesoo Kim (3rd from the left) photographed with other guests at the recital including Dr. Dévora Kestel, the Director of the Mental Health and Substance Use at WHO, sharing the center with Conductor and Pianist João Carlos Martins >
During the UN conference held the day prior to the Carnegie Hall concert, Dr. Dévora Kestel, Director of the Mental Health and Substance Use at WHO, said, “Although dystonia is not as well-known, it is a common disease around the world, and needs our society’s attention and the devotion of many researchers.” Professor Daesoo Kim said, “NT-1 is a drug that blocks the cause of dystonia in the brain, and will allow musicians to continue practicing music. We aim to attain clinical approval in Korea by 2024.”
NT-1 is currently under development by NeuroTobe, a faculty-led start-up company at KAIST, headed by Professor Daesoo Kim as the CEO. The synthesis of the drug for clinical testing has been successfully completed, and it has shown excellent efficacy and safety through various rounds of animal testing. Unlike Botox, which takes a few days to show its therapeutic effects after receiving the procedure from a hospital, NT-1 shows its therapeutic effects within an hour after taking it. As a so-called “edible Botox”, it is expected to help treat various muscular diseases and ailments.
2022.12.27 View 13551
KAIST Offers Hope to Musicians with Dystonia
< Photo 1. Conductor and Pianist João Carlos Martins before the Recital at the Carnegie Hall preparing with his bionic gloves >
KAIST’s neuroscientist and professor, Dr. Daesoo Kim attended the “Conference for Musicians with Dystonia” supported by the World Health Organization (WHO) and the Carnegie Hall concert of legendary pianist João Carlos Martins, who is also a dystonia patient, to announce his team’s recent advancements toward finding a cure for dystonia.
On November 19, 2022, a “miracle concert” was held in Carnegie Hall. João Carlos Martins was a renowned world-class pianist in the 70s and 80s, but he had to put an end to his musical career due to focal dystonia in his fingers. But in 2020, he began using a bionic glove developed by industrial designer Ubiratã Bizarro Costa and after years of hard work he was back in Carnegie Hall as an 82-year-old man.
During the concert, he conducted the NOVUS NY orchestra in a performance of Bach, and later even played the piano himself. In particular, between his performances, he gave shout-outs to scientists studying dystonia including KAIST Professor Daesoo Kim, asking them to continue working towards curing rare diseases for musicians.
< Photo 2. Professor Daesoo Kim with Conductor and Pianist João Carlos Martins >
Musician’s dystonia affects 1-3% of musicians around the world and musicians make up approximately 5% of the total number of dystonia patients. Musicians who are no longer able to practice music due to the disease often experience stress and depression, which may even lead to suicide in extreme cases. Musicians are known to be particularly prone to such diseases due to excessive practice regimens, perfectionism, and even genetics. Currently, botulinum toxin (Botox) is used to suppress abnormal muscles, but muscle function suppression ultimately means that the musician is no longer able to play the instrument. João Carlos Martins himself underwent several Botox procedures and three brain surgeries, but saw no therapeutic results. This is why a new treatment was necessary.
Professor Daesoo Kim’s research team at KAIST took note of the fact that abnormal muscle tension is caused by excessive stress, and developed NT-1, a treatment that blocks the development of the symptoms of dystonia from the brain, allowing patients to use their muscles as they normally would. The research team published their findings in Science Advances in 2021, and João Carlos Martins invited Professor Daesoo Kim to the UN conference and his concert after reading this paper.
< Photo 3. Professor Daesoo Kim (3rd from the left) photographed with other guests at the recital including Dr. Dévora Kestel, the Director of the Mental Health and Substance Use at WHO, sharing the center with Conductor and Pianist João Carlos Martins >
During the UN conference held the day prior to the Carnegie Hall concert, Dr. Dévora Kestel, Director of the Mental Health and Substance Use at WHO, said, “Although dystonia is not as well-known, it is a common disease around the world, and needs our society’s attention and the devotion of many researchers.” Professor Daesoo Kim said, “NT-1 is a drug that blocks the cause of dystonia in the brain, and will allow musicians to continue practicing music. We aim to attain clinical approval in Korea by 2024.”
NT-1 is currently under development by NeuroTobe, a faculty-led start-up company at KAIST, headed by Professor Daesoo Kim as the CEO. The synthesis of the drug for clinical testing has been successfully completed, and it has shown excellent efficacy and safety through various rounds of animal testing. Unlike Botox, which takes a few days to show its therapeutic effects after receiving the procedure from a hospital, NT-1 shows its therapeutic effects within an hour after taking it. As a so-called “edible Botox”, it is expected to help treat various muscular diseases and ailments.
2022.12.27 View 13551 -
 Decoding Brain Signals to Control a Robotic Arm
Advanced brain-machine interface system successfully interprets arm movement directions from neural signals in the brain
Researchers have developed a mind-reading system for decoding neural signals from the brain during arm movement. The method, described in the journal Applied Soft Computing, can be used by a person to control a robotic arm through a brain-machine interface (BMI).
A BMI is a device that translates nerve signals into commands to control a machine, such as a computer or a robotic limb. There are two main techniques for monitoring neural signals in BMIs: electroencephalography (EEG) and electrocorticography (ECoG).
The EEG exhibits signals from electrodes on the surface of the scalp and is widely employed because it is non-invasive, relatively cheap, safe and easy to use. However, the EEG has low spatial resolution and detects irrelevant neural signals, which makes it difficult to interpret the intentions of individuals from the EEG.
On the other hand, the ECoG is an invasive method that involves placing electrodes directly on the surface of the cerebral cortex below the scalp. Compared with the EEG, the ECoG can monitor neural signals with much higher spatial resolution and less background noise. However, this technique has several drawbacks.
“The ECoG is primarily used to find potential sources of epileptic seizures, meaning the electrodes are placed in different locations for different patients and may not be in the optimal regions of the brain for detecting sensory and movement signals,” explained Professor Jaeseung Jeong, a brain scientist at KAIST. “This inconsistency makes it difficult to decode brain signals to predict movements.”
To overcome these problems, Professor Jeong’s team developed a new method for decoding ECoG neural signals during arm movement. The system is based on a machine-learning system for analysing and predicting neural signals called an ‘echo-state network’ and a mathematical probability model called the Gaussian distribution.
In the study, the researchers recorded ECoG signals from four individuals with epilepsy while they were performing a reach-and-grasp task. Because the ECoG electrodes were placed according to the potential sources of each patient’s epileptic seizures, only 22% to 44% of the electrodes were located in the regions of the brain responsible for controlling movement.
During the movement task, the participants were given visual cues, either by placing a real tennis ball in front of them, or via a virtual reality headset showing a clip of a human arm reaching forward in first-person view. They were asked to reach forward, grasp an object, then return their hand and release the object, while wearing motion sensors on their wrists and fingers. In a second task, they were instructed to imagine reaching forward without moving their arms.
The researchers monitored the signals from the ECoG electrodes during real and imaginary arm movements, and tested whether the new system could predict the direction of this movement from the neural signals. They found that the novel decoder successfully classified arm movements in 24 directions in three-dimensional space, both in the real and virtual tasks, and that the results were at least five times more accurate than chance. They also used a computer simulation to show that the novel ECoG decoder could control the movements of a robotic arm.
Overall, the results suggest that the new machine learning-based BCI system successfully used ECoG signals to interpret the direction of the intended movements. The next steps will be to improve the accuracy and efficiency of the decoder. In the future, it could be used in a real-time BMI device to help people with movement or sensory impairments.
This research was supported by the KAIST Global Singularity Research Program of 2021, Brain Research Program of the National Research Foundation of Korea funded by the Ministry of Science, ICT, and Future Planning, and the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education.
-PublicationHoon-Hee Kim, Jaeseung Jeong, “An electrocorticographic decoder for arm movement for brain-machine interface using an echo state network and Gaussian readout,” Applied SoftComputing online December 31, 2021 (doi.org/10.1016/j.asoc.2021.108393)
-ProfileProfessor Jaeseung JeongDepartment of Bio and Brain EngineeringCollege of EngineeringKAIST
2022.03.18 View 14883
Decoding Brain Signals to Control a Robotic Arm
Advanced brain-machine interface system successfully interprets arm movement directions from neural signals in the brain
Researchers have developed a mind-reading system for decoding neural signals from the brain during arm movement. The method, described in the journal Applied Soft Computing, can be used by a person to control a robotic arm through a brain-machine interface (BMI).
A BMI is a device that translates nerve signals into commands to control a machine, such as a computer or a robotic limb. There are two main techniques for monitoring neural signals in BMIs: electroencephalography (EEG) and electrocorticography (ECoG).
The EEG exhibits signals from electrodes on the surface of the scalp and is widely employed because it is non-invasive, relatively cheap, safe and easy to use. However, the EEG has low spatial resolution and detects irrelevant neural signals, which makes it difficult to interpret the intentions of individuals from the EEG.
On the other hand, the ECoG is an invasive method that involves placing electrodes directly on the surface of the cerebral cortex below the scalp. Compared with the EEG, the ECoG can monitor neural signals with much higher spatial resolution and less background noise. However, this technique has several drawbacks.
“The ECoG is primarily used to find potential sources of epileptic seizures, meaning the electrodes are placed in different locations for different patients and may not be in the optimal regions of the brain for detecting sensory and movement signals,” explained Professor Jaeseung Jeong, a brain scientist at KAIST. “This inconsistency makes it difficult to decode brain signals to predict movements.”
To overcome these problems, Professor Jeong’s team developed a new method for decoding ECoG neural signals during arm movement. The system is based on a machine-learning system for analysing and predicting neural signals called an ‘echo-state network’ and a mathematical probability model called the Gaussian distribution.
In the study, the researchers recorded ECoG signals from four individuals with epilepsy while they were performing a reach-and-grasp task. Because the ECoG electrodes were placed according to the potential sources of each patient’s epileptic seizures, only 22% to 44% of the electrodes were located in the regions of the brain responsible for controlling movement.
During the movement task, the participants were given visual cues, either by placing a real tennis ball in front of them, or via a virtual reality headset showing a clip of a human arm reaching forward in first-person view. They were asked to reach forward, grasp an object, then return their hand and release the object, while wearing motion sensors on their wrists and fingers. In a second task, they were instructed to imagine reaching forward without moving their arms.
The researchers monitored the signals from the ECoG electrodes during real and imaginary arm movements, and tested whether the new system could predict the direction of this movement from the neural signals. They found that the novel decoder successfully classified arm movements in 24 directions in three-dimensional space, both in the real and virtual tasks, and that the results were at least five times more accurate than chance. They also used a computer simulation to show that the novel ECoG decoder could control the movements of a robotic arm.
Overall, the results suggest that the new machine learning-based BCI system successfully used ECoG signals to interpret the direction of the intended movements. The next steps will be to improve the accuracy and efficiency of the decoder. In the future, it could be used in a real-time BMI device to help people with movement or sensory impairments.
This research was supported by the KAIST Global Singularity Research Program of 2021, Brain Research Program of the National Research Foundation of Korea funded by the Ministry of Science, ICT, and Future Planning, and the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education.
-PublicationHoon-Hee Kim, Jaeseung Jeong, “An electrocorticographic decoder for arm movement for brain-machine interface using an echo state network and Gaussian readout,” Applied SoftComputing online December 31, 2021 (doi.org/10.1016/j.asoc.2021.108393)
-ProfileProfessor Jaeseung JeongDepartment of Bio and Brain EngineeringCollege of EngineeringKAIST
2022.03.18 View 14883 -
 Five Projects Ranked in the Top 100 for National R&D Excellence
Five KAIST research projects were selected as the 2021 Top 100 for National R&D Excellence by the Ministry of Science and ICT and the Korea Institute of Science & Technology Evaluation and Planning.
The five projects are:-The development of E. coli that proliferates with only formic acid and carbon dioxide by Distinguished Professor Sang Yup Lee from the Department of Chemical and Biomolecular Engineering
-An original reverse aging technology that restores an old human skin cell into a younger one by Professor Kwang-Hyun Cho from the Department of Bio and Brain Engineering-The development of next-generation high-efficiency perovskite-silicon tandem solar cells by Professor Byungha Shin from the Department of Materials Science and Engineering-Research on the effects of ultrafine dust in the atmosphere has on energy consumption by Professor Jiyong Eom from the School of Business and Technology Management-Research on a molecular trigger that controls the phase transformation of bio materials by Professor Myungchul Kim from the Department of Bio and Brain Engineering
Started in 2006, an Evaluation Committee composed of experts in industries, universities, and research institutes has made the preliminary selections of the most outstanding research projects based on their significance as a scientific and technological development and their socioeconomic effects. The finalists went through an open public evaluation. The final 100 studies are from six fields: 18 from mechanics & materials, 26 from biology & marine sciences, 19 from ICT & electronics, 10 from interdisciplinary research, and nine from natural science and infrastructure.
The selected 100 studies will receive a certificate and an award plaque from the minister of MSIT as well as additional points for business and institutional evaluations according to appropriate regulations, and the selected researchers will be strongly recommended as candidates for national meritorious awards.
In particular, to help the 100 selected research projects become more accessible for the general public, their main contents will be provided in a free e-book ‘The Top 100 for National R&D Excellence of 2021’ that will be available from online booksellers.
2022.02.17 View 12953
Five Projects Ranked in the Top 100 for National R&D Excellence
Five KAIST research projects were selected as the 2021 Top 100 for National R&D Excellence by the Ministry of Science and ICT and the Korea Institute of Science & Technology Evaluation and Planning.
The five projects are:-The development of E. coli that proliferates with only formic acid and carbon dioxide by Distinguished Professor Sang Yup Lee from the Department of Chemical and Biomolecular Engineering
-An original reverse aging technology that restores an old human skin cell into a younger one by Professor Kwang-Hyun Cho from the Department of Bio and Brain Engineering-The development of next-generation high-efficiency perovskite-silicon tandem solar cells by Professor Byungha Shin from the Department of Materials Science and Engineering-Research on the effects of ultrafine dust in the atmosphere has on energy consumption by Professor Jiyong Eom from the School of Business and Technology Management-Research on a molecular trigger that controls the phase transformation of bio materials by Professor Myungchul Kim from the Department of Bio and Brain Engineering
Started in 2006, an Evaluation Committee composed of experts in industries, universities, and research institutes has made the preliminary selections of the most outstanding research projects based on their significance as a scientific and technological development and their socioeconomic effects. The finalists went through an open public evaluation. The final 100 studies are from six fields: 18 from mechanics & materials, 26 from biology & marine sciences, 19 from ICT & electronics, 10 from interdisciplinary research, and nine from natural science and infrastructure.
The selected 100 studies will receive a certificate and an award plaque from the minister of MSIT as well as additional points for business and institutional evaluations according to appropriate regulations, and the selected researchers will be strongly recommended as candidates for national meritorious awards.
In particular, to help the 100 selected research projects become more accessible for the general public, their main contents will be provided in a free e-book ‘The Top 100 for National R&D Excellence of 2021’ that will be available from online booksellers.
2022.02.17 View 12953 -
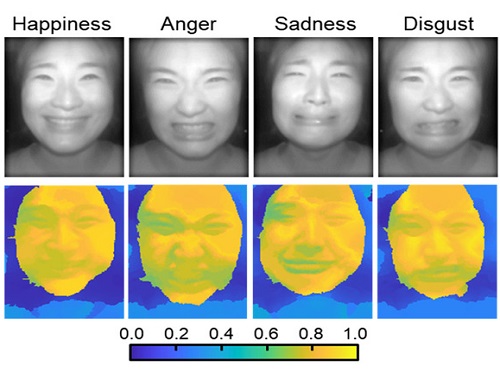 AI Light-Field Camera Reads 3D Facial Expressions
Machine-learned, light-field camera reads facial expressions from high-contrast illumination invariant 3D facial images
A joint research team led by Professors Ki-Hun Jeong and Doheon Lee from the KAIST Department of Bio and Brain Engineering reported the development of a technique for facial expression detection by merging near-infrared light-field camera techniques with artificial intelligence (AI) technology.
Unlike a conventional camera, the light-field camera contains micro-lens arrays in front of the image sensor, which makes the camera small enough to fit into a smart phone, while allowing it to acquire the spatial and directional information of the light with a single shot. The technique has received attention as it can reconstruct images in a variety of ways including multi-views, refocusing, and 3D image acquisition, giving rise to many potential applications.
However, the optical crosstalk between shadows caused by external light sources in the environment and the micro-lens has limited existing light-field cameras from being able to provide accurate image contrast and 3D reconstruction.
The joint research team applied a vertical-cavity surface-emitting laser (VCSEL) in the near-IR range to stabilize the accuracy of 3D image reconstruction that previously depended on environmental light. When an external light source is shone on a face at 0-, 30-, and 60-degree angles, the light field camera reduces 54% of image reconstruction errors. Additionally, by inserting a light-absorbing layer for visible and near-IR wavelengths between the micro-lens arrays, the team could minimize optical crosstalk while increasing the image contrast by 2.1 times.
Through this technique, the team could overcome the limitations of existing light-field cameras and was able to develop their NIR-based light-field camera (NIR-LFC), optimized for the 3D image reconstruction of facial expressions. Using the NIR-LFC, the team acquired high-quality 3D reconstruction images of facial expressions expressing various emotions regardless of the lighting conditions of the surrounding environment.
The facial expressions in the acquired 3D images were distinguished through machine learning with an average of 85% accuracy – a statistically significant figure compared to when 2D images were used. Furthermore, by calculating the interdependency of distance information that varies with facial expression in 3D images, the team could identify the information a light-field camera utilizes to distinguish human expressions.
Professor Ki-Hun Jeong said, “The sub-miniature light-field camera developed by the research team has the potential to become the new platform to quantitatively analyze the facial expressions and emotions of humans.” To highlight the significance of this research, he added, “It could be applied in various fields including mobile healthcare, field diagnosis, social cognition, and human-machine interactions.”
This research was published in Advanced Intelligent Systems online on December 16, under the title, “Machine-Learned Light-field Camera that Reads Facial Expression from High-Contrast and Illumination Invariant 3D Facial Images.” This research was funded by the Ministry of Science and ICT and the Ministry of Trade, Industry and Energy.
-Publication“Machine-learned light-field camera that reads fascial expression from high-contrast and illumination invariant 3D facial images,” Sang-In Bae, Sangyeon Lee, Jae-Myeong Kwon, Hyun-Kyung Kim. Kyung-Won Jang, Doheon Lee, Ki-Hun Jeong, Advanced Intelligent Systems, December 16, 2021 (doi.org/10.1002/aisy.202100182)
ProfileProfessor Ki-Hun JeongBiophotonic LaboratoryDepartment of Bio and Brain EngineeringKAIST
Professor Doheon LeeDepartment of Bio and Brain EngineeringKAIST
2022.01.21 View 14989
AI Light-Field Camera Reads 3D Facial Expressions
Machine-learned, light-field camera reads facial expressions from high-contrast illumination invariant 3D facial images
A joint research team led by Professors Ki-Hun Jeong and Doheon Lee from the KAIST Department of Bio and Brain Engineering reported the development of a technique for facial expression detection by merging near-infrared light-field camera techniques with artificial intelligence (AI) technology.
Unlike a conventional camera, the light-field camera contains micro-lens arrays in front of the image sensor, which makes the camera small enough to fit into a smart phone, while allowing it to acquire the spatial and directional information of the light with a single shot. The technique has received attention as it can reconstruct images in a variety of ways including multi-views, refocusing, and 3D image acquisition, giving rise to many potential applications.
However, the optical crosstalk between shadows caused by external light sources in the environment and the micro-lens has limited existing light-field cameras from being able to provide accurate image contrast and 3D reconstruction.
The joint research team applied a vertical-cavity surface-emitting laser (VCSEL) in the near-IR range to stabilize the accuracy of 3D image reconstruction that previously depended on environmental light. When an external light source is shone on a face at 0-, 30-, and 60-degree angles, the light field camera reduces 54% of image reconstruction errors. Additionally, by inserting a light-absorbing layer for visible and near-IR wavelengths between the micro-lens arrays, the team could minimize optical crosstalk while increasing the image contrast by 2.1 times.
Through this technique, the team could overcome the limitations of existing light-field cameras and was able to develop their NIR-based light-field camera (NIR-LFC), optimized for the 3D image reconstruction of facial expressions. Using the NIR-LFC, the team acquired high-quality 3D reconstruction images of facial expressions expressing various emotions regardless of the lighting conditions of the surrounding environment.
The facial expressions in the acquired 3D images were distinguished through machine learning with an average of 85% accuracy – a statistically significant figure compared to when 2D images were used. Furthermore, by calculating the interdependency of distance information that varies with facial expression in 3D images, the team could identify the information a light-field camera utilizes to distinguish human expressions.
Professor Ki-Hun Jeong said, “The sub-miniature light-field camera developed by the research team has the potential to become the new platform to quantitatively analyze the facial expressions and emotions of humans.” To highlight the significance of this research, he added, “It could be applied in various fields including mobile healthcare, field diagnosis, social cognition, and human-machine interactions.”
This research was published in Advanced Intelligent Systems online on December 16, under the title, “Machine-Learned Light-field Camera that Reads Facial Expression from High-Contrast and Illumination Invariant 3D Facial Images.” This research was funded by the Ministry of Science and ICT and the Ministry of Trade, Industry and Energy.
-Publication“Machine-learned light-field camera that reads fascial expression from high-contrast and illumination invariant 3D facial images,” Sang-In Bae, Sangyeon Lee, Jae-Myeong Kwon, Hyun-Kyung Kim. Kyung-Won Jang, Doheon Lee, Ki-Hun Jeong, Advanced Intelligent Systems, December 16, 2021 (doi.org/10.1002/aisy.202100182)
ProfileProfessor Ki-Hun JeongBiophotonic LaboratoryDepartment of Bio and Brain EngineeringKAIST
Professor Doheon LeeDepartment of Bio and Brain EngineeringKAIST
2022.01.21 View 14989 -
 AI Weather Forecasting Research Center Opens
The Kim Jaechul Graduate School of AI in collaboration with the National Institute of Meteorological Sciences (NIMS) under the National Meteorological Administration launched the AI Weather Forecasting Research Center last month.
The KAIST AI Weather Forecasting Research Center headed by Professor Seyoung Yoon was established with funding from from the AlphaWeather Development Research Project of the National Institute of Meteorological Sciences. KAIST was finally selected asas the project facilitator.
AlphaWeather is an AI system that utilizes and analyzes approximately approximately 150,000 ,000 pieces of weather information per hour to help weather forecasters produce accurate weather forecasts. The research center is composed of three research teams with the following goals: (a) developdevelop AI technology for precipitation nowcasting, (b) developdevelop AI technology for accelerating physical process-based numerical models, and (c) develop dAI technology for supporting weather forecasters. The teams consist of 15 staff member members from NIMS and 61 researchers from the Kim Jaechul Graduate School of AI at KAIST.
The research center is developing an AI algorithm for precipitation nowcasting (with up to six hours of lead time), which uses satellite images, radar reflectivity, and data collected from weather stations. It is also developing an AI algorithm for correcting biases in the prediction results from multiple numerical models. Finally, it is Finally, it is developing AI technology that supports weather forecasters by standardizing and automating repetitive manual processes. After verification, the the results obtained will be used by by the Korean National Weather Service as a next-generation forecasting/special-reporting system intelligence engine from 2026.
2022.01.10 View 8176
AI Weather Forecasting Research Center Opens
The Kim Jaechul Graduate School of AI in collaboration with the National Institute of Meteorological Sciences (NIMS) under the National Meteorological Administration launched the AI Weather Forecasting Research Center last month.
The KAIST AI Weather Forecasting Research Center headed by Professor Seyoung Yoon was established with funding from from the AlphaWeather Development Research Project of the National Institute of Meteorological Sciences. KAIST was finally selected asas the project facilitator.
AlphaWeather is an AI system that utilizes and analyzes approximately approximately 150,000 ,000 pieces of weather information per hour to help weather forecasters produce accurate weather forecasts. The research center is composed of three research teams with the following goals: (a) developdevelop AI technology for precipitation nowcasting, (b) developdevelop AI technology for accelerating physical process-based numerical models, and (c) develop dAI technology for supporting weather forecasters. The teams consist of 15 staff member members from NIMS and 61 researchers from the Kim Jaechul Graduate School of AI at KAIST.
The research center is developing an AI algorithm for precipitation nowcasting (with up to six hours of lead time), which uses satellite images, radar reflectivity, and data collected from weather stations. It is also developing an AI algorithm for correcting biases in the prediction results from multiple numerical models. Finally, it is Finally, it is developing AI technology that supports weather forecasters by standardizing and automating repetitive manual processes. After verification, the the results obtained will be used by by the Korean National Weather Service as a next-generation forecasting/special-reporting system intelligence engine from 2026.
2022.01.10 View 8176 -
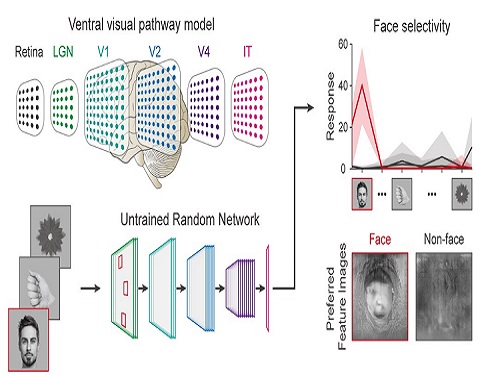 Face Detection in Untrained Deep Neural Networks
A KAIST team shows that primitive visual selectivity of faces can arise spontaneously in completely untrained deep neural networks
Researchers have found that higher visual cognitive functions can arise spontaneously in untrained neural networks. A KAIST research team led by Professor Se-Bum Paik from the Department of Bio and Brain Engineering has shown that visual selectivity of facial images can arise even in completely untrained deep neural networks.
This new finding has provided revelatory insights into mechanisms underlying the development of cognitive functions in both biological and artificial neural networks, also making a significant impact on our understanding of the origin of early brain functions before sensory experiences.
The study published in Nature Communications on December 16 demonstrates that neuronal activities selective to facial images are observed in randomly initialized deep neural networks in the complete absence of learning, and that they show the characteristics of those observed in biological brains.
The ability to identify and recognize faces is a crucial function for social behavior, and this ability is thought to originate from neuronal tuning at the single or multi-neuronal level. Neurons that selectively respond to faces are observed in young animals of various species, and this raises intense debate whether face-selective neurons can arise innately in the brain or if they require visual experience.
Using a model neural network that captures properties of the ventral stream of the visual cortex, the research team found that face-selectivity can emerge spontaneously from random feedforward wirings in untrained deep neural networks. The team showed that the character of this innate face-selectivity is comparable to that observed with face-selective neurons in the brain, and that this spontaneous neuronal tuning for faces enables the network to perform face detection tasks.
These results imply a possible scenario in which the random feedforward connections that develop in early, untrained networks may be sufficient for initializing primitive visual cognitive functions.
Professor Paik said, “Our findings suggest that innate cognitive functions can emerge spontaneously from the statistical complexity embedded in the hierarchical feedforward projection circuitry, even in the complete absence of learning”.
He continued, “Our results provide a broad conceptual advance as well as advanced insight into the mechanisms underlying the development of innate functions in both biological and artificial neural networks, which may unravel the mystery of the generation and evolution of intelligence.” This work was supported by the National Research Foundation of Korea (NRF) and by the KAIST singularity research project.
-PublicationSeungdae Baek, Min Song, Jaeson Jang, Gwangsu Kim, and Se-Bum Baik, “Face detection in untrained deep neural network,” Nature Communications 12, 7328 on Dec.16, 2021
(https://doi.org/10.1038/s41467-021-27606-9)
-ProfileProfessor Se-Bum PaikVisual System and Neural Network LaboratoryProgram of Brain and Cognitive EngineeringDepartment of Bio and Brain EngineeringCollege of EngineeringKAIST
2021.12.21 View 12302
Face Detection in Untrained Deep Neural Networks
A KAIST team shows that primitive visual selectivity of faces can arise spontaneously in completely untrained deep neural networks
Researchers have found that higher visual cognitive functions can arise spontaneously in untrained neural networks. A KAIST research team led by Professor Se-Bum Paik from the Department of Bio and Brain Engineering has shown that visual selectivity of facial images can arise even in completely untrained deep neural networks.
This new finding has provided revelatory insights into mechanisms underlying the development of cognitive functions in both biological and artificial neural networks, also making a significant impact on our understanding of the origin of early brain functions before sensory experiences.
The study published in Nature Communications on December 16 demonstrates that neuronal activities selective to facial images are observed in randomly initialized deep neural networks in the complete absence of learning, and that they show the characteristics of those observed in biological brains.
The ability to identify and recognize faces is a crucial function for social behavior, and this ability is thought to originate from neuronal tuning at the single or multi-neuronal level. Neurons that selectively respond to faces are observed in young animals of various species, and this raises intense debate whether face-selective neurons can arise innately in the brain or if they require visual experience.
Using a model neural network that captures properties of the ventral stream of the visual cortex, the research team found that face-selectivity can emerge spontaneously from random feedforward wirings in untrained deep neural networks. The team showed that the character of this innate face-selectivity is comparable to that observed with face-selective neurons in the brain, and that this spontaneous neuronal tuning for faces enables the network to perform face detection tasks.
These results imply a possible scenario in which the random feedforward connections that develop in early, untrained networks may be sufficient for initializing primitive visual cognitive functions.
Professor Paik said, “Our findings suggest that innate cognitive functions can emerge spontaneously from the statistical complexity embedded in the hierarchical feedforward projection circuitry, even in the complete absence of learning”.
He continued, “Our results provide a broad conceptual advance as well as advanced insight into the mechanisms underlying the development of innate functions in both biological and artificial neural networks, which may unravel the mystery of the generation and evolution of intelligence.” This work was supported by the National Research Foundation of Korea (NRF) and by the KAIST singularity research project.
-PublicationSeungdae Baek, Min Song, Jaeson Jang, Gwangsu Kim, and Se-Bum Baik, “Face detection in untrained deep neural network,” Nature Communications 12, 7328 on Dec.16, 2021
(https://doi.org/10.1038/s41467-021-27606-9)
-ProfileProfessor Se-Bum PaikVisual System and Neural Network LaboratoryProgram of Brain and Cognitive EngineeringDepartment of Bio and Brain EngineeringCollege of EngineeringKAIST
2021.12.21 View 12302 -
 Connecting the Dots to Find New Treatments for Breast Cancer
Systems biologists uncovered new ways of cancer cell reprogramming to treat drug-resistant cancers
Scientists at KAIST believe they may have found a way to reverse an aggressive, treatment-resistant type of breast cancer into a less dangerous kind that responds well to treatment. The study involved the use of mathematical models to untangle the complex genetic and molecular interactions that occur in the two types of breast cancer, but could be extended to find ways for treating many others. The study’s findings were published in the journal Cancer Research.
Basal-like tumours are the most aggressive type of breast cancer, with the worst prognosis. Chemotherapy is the only available treatment option, but patients experience high recurrence rates. On the other hand, luminal-A breast cancer responds well to drugs that specifically target a receptor on their cell surfaces, called estrogen receptor alpha (ERα).
KAIST systems biologist Kwang-Hyun Cho and colleagues analyzed the complex molecular and genetic interactions of basal-like and luminal-A breast cancers to find out if there might be a way to switch the former to the latter and give patients a better chance to respond to treatment.
To do this, they accessed large amounts of cancer and patient data to understand which genes and molecules are involved in the two types. They then input this data into a mathematical model that represents genes, proteins and molecules as dots and the interactions between them as lines. The model can be used to conduct simulations and see how interactions change when certain genes are turned on or off.
“There have been a tremendous number of studies trying to find therapeutic targets for treating basal-like breast cancer patients,” says Cho. “But clinical trials have failed due to the complex and dynamic nature of cancer. To overcome this issue, we looked at breast cancer cells as a complex network system and implemented a systems biological approach to unravel the underlying mechanisms that would allow us to reprogram basal-like into luminal-A breast cancer cells.”
Using this approach, followed by experimental validation on real breast cancer cells, the team found that turning off two key gene regulators, called BCL11A and HDAC1/2, switched a basal-like cancer signalling pathway into a different one used by luminal-A cancer cells. The switch reprograms the cancer cells and makes them more responsive to drugs that target ERα receptors. However, further tests will be needed to confirm that this also works in animal models and eventually humans.
“Our study demonstrates that the systems biological approach can be useful for identifying novel therapeutic targets,” says Cho.
The researchers are now expanding its breast cancer network model to include all breast cancer subtypes. Their ultimate aim is to identify more drug targets and to understand the mechanisms that could drive drug-resistant cells to turn into drug-sensitive ones.
This work was supported by the National Research Foundation of Korea, the Ministry of Science and ICT, Electronics and Telecommunications Research Institute, and the KAIST Grand Challenge 30 Project.
-Publication Sea R. Choi, Chae Young Hwang, Jonghoon Lee, and Kwang-Hyun Cho, “Network Analysis Identifies Regulators of Basal-like Breast Cancer Reprogramming and Endocrine TherapyVulnerability,” Cancer Research, November 30. (doi:10.1158/0008-5472.CAN-21-0621)
-ProfileProfessor Kwang-Hyun ChoLaboratory for Systems Biology and Bio-Inspired EngineeringDepartment of Bio and Brain EngineeringKAIST
2021.12.07 View 13210
Connecting the Dots to Find New Treatments for Breast Cancer
Systems biologists uncovered new ways of cancer cell reprogramming to treat drug-resistant cancers
Scientists at KAIST believe they may have found a way to reverse an aggressive, treatment-resistant type of breast cancer into a less dangerous kind that responds well to treatment. The study involved the use of mathematical models to untangle the complex genetic and molecular interactions that occur in the two types of breast cancer, but could be extended to find ways for treating many others. The study’s findings were published in the journal Cancer Research.
Basal-like tumours are the most aggressive type of breast cancer, with the worst prognosis. Chemotherapy is the only available treatment option, but patients experience high recurrence rates. On the other hand, luminal-A breast cancer responds well to drugs that specifically target a receptor on their cell surfaces, called estrogen receptor alpha (ERα).
KAIST systems biologist Kwang-Hyun Cho and colleagues analyzed the complex molecular and genetic interactions of basal-like and luminal-A breast cancers to find out if there might be a way to switch the former to the latter and give patients a better chance to respond to treatment.
To do this, they accessed large amounts of cancer and patient data to understand which genes and molecules are involved in the two types. They then input this data into a mathematical model that represents genes, proteins and molecules as dots and the interactions between them as lines. The model can be used to conduct simulations and see how interactions change when certain genes are turned on or off.
“There have been a tremendous number of studies trying to find therapeutic targets for treating basal-like breast cancer patients,” says Cho. “But clinical trials have failed due to the complex and dynamic nature of cancer. To overcome this issue, we looked at breast cancer cells as a complex network system and implemented a systems biological approach to unravel the underlying mechanisms that would allow us to reprogram basal-like into luminal-A breast cancer cells.”
Using this approach, followed by experimental validation on real breast cancer cells, the team found that turning off two key gene regulators, called BCL11A and HDAC1/2, switched a basal-like cancer signalling pathway into a different one used by luminal-A cancer cells. The switch reprograms the cancer cells and makes them more responsive to drugs that target ERα receptors. However, further tests will be needed to confirm that this also works in animal models and eventually humans.
“Our study demonstrates that the systems biological approach can be useful for identifying novel therapeutic targets,” says Cho.
The researchers are now expanding its breast cancer network model to include all breast cancer subtypes. Their ultimate aim is to identify more drug targets and to understand the mechanisms that could drive drug-resistant cells to turn into drug-sensitive ones.
This work was supported by the National Research Foundation of Korea, the Ministry of Science and ICT, Electronics and Telecommunications Research Institute, and the KAIST Grand Challenge 30 Project.
-Publication Sea R. Choi, Chae Young Hwang, Jonghoon Lee, and Kwang-Hyun Cho, “Network Analysis Identifies Regulators of Basal-like Breast Cancer Reprogramming and Endocrine TherapyVulnerability,” Cancer Research, November 30. (doi:10.1158/0008-5472.CAN-21-0621)
-ProfileProfessor Kwang-Hyun ChoLaboratory for Systems Biology and Bio-Inspired EngineeringDepartment of Bio and Brain EngineeringKAIST
2021.12.07 View 13210 -
 Industrial Liaison Program to Provide Comprehensive Consultation Services
The ILP’s one-stop solutions target all industrial sectors including conglomerates, small and medium-sized enterprises, venture companies, venture capital (VC) firms, and government-affiliated organizations.
The Industrial Liaison Center at KAIST launched the Industrial Liaison Program (ILP) on September 28, an industry-academic cooperation project to provide comprehensive solutions to industry partners. The Industrial Liaison Center will recruit member companies for this service every year, targeting all industrial sectors including conglomerates, small and medium-sized enterprises, venture companies, venture capital (VC) firms, and government-affiliated organizations.
The program plans to build a one-stop support system that can systematically share and use excellent resource information from KAIST’s research teams, R&D achievements, and infrastructure to provide member companies with much-needed services.
More than 40 KAIST professors with abundant academic-industrial collaboration experience will participate in the program. Experts from various fields with different points of view and experiences will jointly provide solutions to ILP member companies. To actively participate in academic-industrial liaisons and joint consultations, KAIST assigned 10 professors from related fields as program directors.
The program directors will come from four different fields including AI/robots (Professor Alice Oh, School from the School of Computing, Professor Young Jae Jang from the Department of Industrial & Systems Engineering, and Professor Yong-Hwa Park from Department of Mechanical Engineering), bio/medicine (Professor Daesoo Kim from Department of Biological Sciences and Professor YongKeun Park from Department of Physics), materials/electronics (Professor Sang Ouk Kim from the Department of Materials Science and Engineering and Professors Jun-Bo Yoon and Seonghwan Cho from the School of Electrical Engineering), and environment/energy (Professor Hee-Tak Kim from the Department of Biological Sciences and Professor Hoon Sohn from the Department of Civil and Environmental Engineering).
The transdisciplinary board of consulting professors that will lead technology innovation is composed of 30 professors including Professor Min-Soo Kim (School of Computing, AI), Professor Chan Hyuk Kim (Department of Biological Sciences, medicine), Professor Hae-Won Park (Department of Mechanical Engineering, robots), Professor Changho Suh (School of Electrical Engineering, electronics), Professor Haeshin Lee (Department of Chemistry, bio), Professor Il-Doo Kim (Department of Materials Science and Engineering, materials), Professor HyeJin Kim (School of Business Technology and Management), and Professor Byoung Pil Kim (School of Business Technology and Management, technology law)
The Head of the Industrial Liaison Center who is also in charge of the program, Professor Keon Jae Lee, said, “In a science and technology-oriented generation where technological supremacy determines national power, it is indispensable to build a new platform upon which innovative academic-industrial cooperation can be pushed forward in the fields of joint consultation, the development of academic-industrial projects, and the foundation of new industries.
He added, “KAIST professors carry out world-class research in many different fields and faculty members can come together through the ILP to communicate with representatives from industry to improve their corporations’ global competitiveness and further contribute to our nation’s interests by cultivating strong small enterprises
2021.09.30 View 8557
Industrial Liaison Program to Provide Comprehensive Consultation Services
The ILP’s one-stop solutions target all industrial sectors including conglomerates, small and medium-sized enterprises, venture companies, venture capital (VC) firms, and government-affiliated organizations.
The Industrial Liaison Center at KAIST launched the Industrial Liaison Program (ILP) on September 28, an industry-academic cooperation project to provide comprehensive solutions to industry partners. The Industrial Liaison Center will recruit member companies for this service every year, targeting all industrial sectors including conglomerates, small and medium-sized enterprises, venture companies, venture capital (VC) firms, and government-affiliated organizations.
The program plans to build a one-stop support system that can systematically share and use excellent resource information from KAIST’s research teams, R&D achievements, and infrastructure to provide member companies with much-needed services.
More than 40 KAIST professors with abundant academic-industrial collaboration experience will participate in the program. Experts from various fields with different points of view and experiences will jointly provide solutions to ILP member companies. To actively participate in academic-industrial liaisons and joint consultations, KAIST assigned 10 professors from related fields as program directors.
The program directors will come from four different fields including AI/robots (Professor Alice Oh, School from the School of Computing, Professor Young Jae Jang from the Department of Industrial & Systems Engineering, and Professor Yong-Hwa Park from Department of Mechanical Engineering), bio/medicine (Professor Daesoo Kim from Department of Biological Sciences and Professor YongKeun Park from Department of Physics), materials/electronics (Professor Sang Ouk Kim from the Department of Materials Science and Engineering and Professors Jun-Bo Yoon and Seonghwan Cho from the School of Electrical Engineering), and environment/energy (Professor Hee-Tak Kim from the Department of Biological Sciences and Professor Hoon Sohn from the Department of Civil and Environmental Engineering).
The transdisciplinary board of consulting professors that will lead technology innovation is composed of 30 professors including Professor Min-Soo Kim (School of Computing, AI), Professor Chan Hyuk Kim (Department of Biological Sciences, medicine), Professor Hae-Won Park (Department of Mechanical Engineering, robots), Professor Changho Suh (School of Electrical Engineering, electronics), Professor Haeshin Lee (Department of Chemistry, bio), Professor Il-Doo Kim (Department of Materials Science and Engineering, materials), Professor HyeJin Kim (School of Business Technology and Management), and Professor Byoung Pil Kim (School of Business Technology and Management, technology law)
The Head of the Industrial Liaison Center who is also in charge of the program, Professor Keon Jae Lee, said, “In a science and technology-oriented generation where technological supremacy determines national power, it is indispensable to build a new platform upon which innovative academic-industrial cooperation can be pushed forward in the fields of joint consultation, the development of academic-industrial projects, and the foundation of new industries.
He added, “KAIST professors carry out world-class research in many different fields and faculty members can come together through the ILP to communicate with representatives from industry to improve their corporations’ global competitiveness and further contribute to our nation’s interests by cultivating strong small enterprises
2021.09.30 View 8557 -
 Hydrogel-Based Flexible Brain-Machine Interface
The interface is easy to insert into the body when dry, but behaves ‘stealthily’ inside the brain when wet
Professor Seongjun Park’s research team and collaborators revealed a newly developed hydrogel-based flexible brain-machine interface. To study the structure of the brain or to identify and treat neurological diseases, it is crucial to develop an interface that can stimulate the brain and detect its signals in real time. However, existing neural interfaces are mechanically and chemically different from real brain tissue. This causes foreign body response and forms an insulating layer (glial scar) around the interface, which shortens its lifespan.
To solve this problem, the research team developed a ‘brain-mimicking interface’ by inserting a custom-made multifunctional fiber bundle into the hydrogel body. The device is composed not only of an optical fiber that controls specific nerve cells with light in order to perform optogenetic procedures, but it also has an electrode bundle to read brain signals and a microfluidic channel to deliver drugs to the brain.
The interface is easy to insert into the body when dry, as hydrogels become solid. But once in the body, the hydrogel will quickly absorb body fluids and resemble the properties of its surrounding tissues, thereby minimizing foreign body response.
The research team applied the device on animal models, and showed that it was possible to detect neural signals for up to six months, which is far beyond what had been previously recorded. It was also possible to conduct long-term optogenetic and behavioral experiments on freely moving mice with a significant reduction in foreign body responses such as glial and immunological activation compared to existing devices.
“This research is significant in that it was the first to utilize a hydrogel as part of a multifunctional neural interface probe, which increased its lifespan dramatically,” said Professor Park. “With our discovery, we look forward to advancements in research on neurological disorders like Alzheimer’s or Parkinson’s disease that require long-term observation.”
The research was published in Nature Communications on June 8, 2021. (Title: Adaptive and multifunctional hydrogel hybrid probes for long-term sensing and modulation of neural activity) The study was conducted jointly with an MIT research team composed of Professor Polina Anikeeva, Professor Xuanhe Zhao, and Dr. Hyunwoo Yook.
This research was supported by the National Research Foundation (NRF) grant for emerging research, Korea Medical Device Development Fund, KK-JRC Smart Project, KAIST Global Initiative Program, and Post-AI Project.
-PublicationPark, S., Yuk, H., Zhao, R. et al. Adaptive and multifunctional hydrogel hybrid probes for long-term sensing and modulation of neural activity. Nat Commun 12, 3435 (2021). https://doi.org/10.1038/s41467-021-23802-9
-ProfileProfessor Seongjun ParkBio and Neural Interfaces LaboratoryDepartment of Bio and Brain EngineeringKAIST
2021.07.13 View 14297
Hydrogel-Based Flexible Brain-Machine Interface
The interface is easy to insert into the body when dry, but behaves ‘stealthily’ inside the brain when wet
Professor Seongjun Park’s research team and collaborators revealed a newly developed hydrogel-based flexible brain-machine interface. To study the structure of the brain or to identify and treat neurological diseases, it is crucial to develop an interface that can stimulate the brain and detect its signals in real time. However, existing neural interfaces are mechanically and chemically different from real brain tissue. This causes foreign body response and forms an insulating layer (glial scar) around the interface, which shortens its lifespan.
To solve this problem, the research team developed a ‘brain-mimicking interface’ by inserting a custom-made multifunctional fiber bundle into the hydrogel body. The device is composed not only of an optical fiber that controls specific nerve cells with light in order to perform optogenetic procedures, but it also has an electrode bundle to read brain signals and a microfluidic channel to deliver drugs to the brain.
The interface is easy to insert into the body when dry, as hydrogels become solid. But once in the body, the hydrogel will quickly absorb body fluids and resemble the properties of its surrounding tissues, thereby minimizing foreign body response.
The research team applied the device on animal models, and showed that it was possible to detect neural signals for up to six months, which is far beyond what had been previously recorded. It was also possible to conduct long-term optogenetic and behavioral experiments on freely moving mice with a significant reduction in foreign body responses such as glial and immunological activation compared to existing devices.
“This research is significant in that it was the first to utilize a hydrogel as part of a multifunctional neural interface probe, which increased its lifespan dramatically,” said Professor Park. “With our discovery, we look forward to advancements in research on neurological disorders like Alzheimer’s or Parkinson’s disease that require long-term observation.”
The research was published in Nature Communications on June 8, 2021. (Title: Adaptive and multifunctional hydrogel hybrid probes for long-term sensing and modulation of neural activity) The study was conducted jointly with an MIT research team composed of Professor Polina Anikeeva, Professor Xuanhe Zhao, and Dr. Hyunwoo Yook.
This research was supported by the National Research Foundation (NRF) grant for emerging research, Korea Medical Device Development Fund, KK-JRC Smart Project, KAIST Global Initiative Program, and Post-AI Project.
-PublicationPark, S., Yuk, H., Zhao, R. et al. Adaptive and multifunctional hydrogel hybrid probes for long-term sensing and modulation of neural activity. Nat Commun 12, 3435 (2021). https://doi.org/10.1038/s41467-021-23802-9
-ProfileProfessor Seongjun ParkBio and Neural Interfaces LaboratoryDepartment of Bio and Brain EngineeringKAIST
2021.07.13 View 14297