NI
-
 Long Economic Depressions and Disparities Loom in the Wake of the COVID-19
"Global Cooperation for Managing Data Key to Mitigating the Impacts Around the World"
<Full recorded video of the GSI-IF2020>
The COVID-19 pandemic will lead to long economic depressions around the entire world. Experts predicted that the prevalent inequities among the countries, regions, and individuals will aggravate the economic crisis. However, crises always come with new opportunities and international cooperation and solidarity will help creating a new normal in the post-coronavirus era.
In a very basic but urgent step, global cooperation for managing data is the key to respond to COVID-19 since medicine and healthcare are intertwined with data science, said experts during an online international forum hosted by the Global Strategy Institute at KAIST on April 22.
KAIST launched its think-tank, the Global Strategy Institute (GSI), in February. The GSI aims to identify global issues proactively and help make breakthroughs well aligned with solid science-based policies.
The inaugural forum of the GSI focused on how the COVID-19 pandemic would impact socio-economic, scientific, and political landscapes, under the theme “Global Cooperation in the Coronavirus Era.”
In his opening remarks, KAIST President Sung-Chul Shin stressed that future global governance will be dominated by the power of science and technology. “If we can implement efficient policies together with troubleshooting technology for responding to future crises, we will emerge stronger than before,” he said.
President Shin said ‘the Korean model’, which is being recognized as a shining example for dealing with the pandemic, is the result of collaborations combining the creativity of the private sector, the public sector’s strong infrastructure, and the full support of the citizens. He added, “Without the technological prowess coming from the competent R&D power of Korea, we could not achieve these impressive results.”
“Creative collaboration among the private and public sectors, along with research universities from around the world, will help shore up global resilience against the epidemic. We should work together to build a world of growing prosperity,” President Shin said.
Prime Minister Sye-Kyun Chung, who is in charge of the Central Disaster and Safety Countermeasures Headquarters in Korea, stressed global solidarity in his welcoming remarks, saying that “We need to share information and rely on the strength of our connections, rather than retreating into nationalistic isolation.”
Peter Lee, Vice President of the Microsoft Healthcare, pointed out in his welcoming remarks three critical sectors for global cooperation: medicine and healthcare, public health and prevention, and life and the economy. He emphasized the rule of thumb for managing data, saying that data in these fields should be open, standardized, and shared among countries to combat this global pandemic.
During a keynote session, Director General of the International Vaccine Institute (IVI) Jerome Kim described the challenges that go along with developing a vaccine. Dr. Kim said that only 7% of vaccine candidates go through the clinical trial stages, and it will take five to 10 years to completely prove a new vaccine’s safety after completing three stages of clinical tests.
“It’s very challenging to develop the vaccine for COVID-19 within 12 to 15 months,” said Dr. Kim. He added that 78 out of 115 candidates are currently undergoing clinical trials around the world.
There are five groups, including Moderna, Inovio, Jenner Institute, CanSino, and the Beijing Institute of Biological Products, who are doing clinical trials in phases 1 and 2. “Given the fact that COVID-19 is a totally new type of virus, various stakeholders’ participation, such as the National Immunization Technical Advisory Groups, the WHO, and UNICEF, is needed to work together to benefit the entire world,” he pointed out.
Professor Edward Yoonjae Choi from the Graduate School of AI at KAIST shared how AI and data sciences are being utilized to interpret the major trends of the epidemic. His group mainly focuses on deep learning to model electronic health records (EHR) for disease predictions. Professor Choi said AI and machine learning would be crucial solutions and collaborative research projects will surely accelerate how quickly we can overcome the pandemic.
In addition, Dr. Kijung Shin’s group is interpreting the SIR (Susceptible, Infected, and Recovered) model in Korea to predict the number of infections and when people were infected. However, researchers noticed that they could not see the typical modeling in Korea for predicting the number of infections since the model disregarded the new variable of humans’ efforts to stop the spread the virus.
According to research by Professor Steven Whang’s group on social distancing and face mask distribution among vulnerable age groups, people in their 20s, 60s, and 70s followed the social distancing guidelines the most strictly. The research team analyzed the data provided by SK Telecom in the Gangnam district of Seoul. The data provided on people in their 70s, a group that accounted for half of all fatalities, showed that masks were generally well distributed nationwide.
Dr. Alexandros Papaspyrids, Tertiary Education Industry Director of the Asia region of Microsoft, said that despite all the disadvantages and problems related to remote education, we shouldn’t expect to return to the days before the COVID-19 any time soon. “We should accept the new normal and explore new opportunities in the new educational environment,” he said.
Hongtaek Yong, Deputy Minister at the Office of R&D Policy at the Ministry of Science and ICT presented the Korean government’s disease prevention and response policy and how they tried to mitigate the economic and social impact.
He stressed the government’s fast testing, tracing, and openness for successfully flattening the curve, adding that the government used an ICT-based approach in all aspects of their response. From early this year when the first patient was reported, the government aggressively encouraged the biotech industry to develop diagnostic kits and novel therapeutic medications.
As a result, five companies were able to produce genetic diagnostic reagents through the emergency approval. More notably, four of them are conducting massive R&D projects sponsored by the government and this is the result of the government’s continuous investment in R&D. Korea is the leader in R&D investment among the OECD countries.
According to Yong, the government’s big data project that was launched in 2017 continuously traces the trends of epidemics in Korea. The epidemiological studies based on the paths taken by suspected patients using credit card transaction made the difference in predicting the spread of the coronavirus and implementing countermeasures. The data has been provided to the Korea’s Center for Disease Control (CDC).
“In addition to the epidemics, we have so many other pending issues arising from digital and social equities, un-contact services, and job security. We are very open to collaborate and cooperate with other countries to deal with this global crisis,” Yong said.
During the subsequent panel discussions, David Dollar, a senior fellow at the Brookings Institution, said, “The global economy in the coronavirus era will not have a rapid V-shaped recovery, but rather will fall into a long depression for at least two years.” He pointed out that if countries practice protectionism like they did during the Great Depression, the recession will be even worse. Hence, he urged the international community, especially developed nations, to avoid protectionism, consider the economic difficulties of developing countries, and provide them with financial support.
Co-Director of the Center for Universal Education at the Brookings Institution Rebecca Winthrop raised concerns over the recent shift to online teaching and learning, claiming that insufficient infrastructures in low-income families in developing nations are already causing added educational disparities and provoking the inequity issue around the world. “The ways to provide quality education equally through faster and more effective means should be studied,” she said.
Professor Joungho Kim, the director of the KAIST GSI and the forum’s organizer, concluded the event by saying that this forum will be a valuable resource for everyone who is providing assistance to those in need, both during and after the COVID-19 pandemic.
(END)
2020.04.22 View 25966
Long Economic Depressions and Disparities Loom in the Wake of the COVID-19
"Global Cooperation for Managing Data Key to Mitigating the Impacts Around the World"
<Full recorded video of the GSI-IF2020>
The COVID-19 pandemic will lead to long economic depressions around the entire world. Experts predicted that the prevalent inequities among the countries, regions, and individuals will aggravate the economic crisis. However, crises always come with new opportunities and international cooperation and solidarity will help creating a new normal in the post-coronavirus era.
In a very basic but urgent step, global cooperation for managing data is the key to respond to COVID-19 since medicine and healthcare are intertwined with data science, said experts during an online international forum hosted by the Global Strategy Institute at KAIST on April 22.
KAIST launched its think-tank, the Global Strategy Institute (GSI), in February. The GSI aims to identify global issues proactively and help make breakthroughs well aligned with solid science-based policies.
The inaugural forum of the GSI focused on how the COVID-19 pandemic would impact socio-economic, scientific, and political landscapes, under the theme “Global Cooperation in the Coronavirus Era.”
In his opening remarks, KAIST President Sung-Chul Shin stressed that future global governance will be dominated by the power of science and technology. “If we can implement efficient policies together with troubleshooting technology for responding to future crises, we will emerge stronger than before,” he said.
President Shin said ‘the Korean model’, which is being recognized as a shining example for dealing with the pandemic, is the result of collaborations combining the creativity of the private sector, the public sector’s strong infrastructure, and the full support of the citizens. He added, “Without the technological prowess coming from the competent R&D power of Korea, we could not achieve these impressive results.”
“Creative collaboration among the private and public sectors, along with research universities from around the world, will help shore up global resilience against the epidemic. We should work together to build a world of growing prosperity,” President Shin said.
Prime Minister Sye-Kyun Chung, who is in charge of the Central Disaster and Safety Countermeasures Headquarters in Korea, stressed global solidarity in his welcoming remarks, saying that “We need to share information and rely on the strength of our connections, rather than retreating into nationalistic isolation.”
Peter Lee, Vice President of the Microsoft Healthcare, pointed out in his welcoming remarks three critical sectors for global cooperation: medicine and healthcare, public health and prevention, and life and the economy. He emphasized the rule of thumb for managing data, saying that data in these fields should be open, standardized, and shared among countries to combat this global pandemic.
During a keynote session, Director General of the International Vaccine Institute (IVI) Jerome Kim described the challenges that go along with developing a vaccine. Dr. Kim said that only 7% of vaccine candidates go through the clinical trial stages, and it will take five to 10 years to completely prove a new vaccine’s safety after completing three stages of clinical tests.
“It’s very challenging to develop the vaccine for COVID-19 within 12 to 15 months,” said Dr. Kim. He added that 78 out of 115 candidates are currently undergoing clinical trials around the world.
There are five groups, including Moderna, Inovio, Jenner Institute, CanSino, and the Beijing Institute of Biological Products, who are doing clinical trials in phases 1 and 2. “Given the fact that COVID-19 is a totally new type of virus, various stakeholders’ participation, such as the National Immunization Technical Advisory Groups, the WHO, and UNICEF, is needed to work together to benefit the entire world,” he pointed out.
Professor Edward Yoonjae Choi from the Graduate School of AI at KAIST shared how AI and data sciences are being utilized to interpret the major trends of the epidemic. His group mainly focuses on deep learning to model electronic health records (EHR) for disease predictions. Professor Choi said AI and machine learning would be crucial solutions and collaborative research projects will surely accelerate how quickly we can overcome the pandemic.
In addition, Dr. Kijung Shin’s group is interpreting the SIR (Susceptible, Infected, and Recovered) model in Korea to predict the number of infections and when people were infected. However, researchers noticed that they could not see the typical modeling in Korea for predicting the number of infections since the model disregarded the new variable of humans’ efforts to stop the spread the virus.
According to research by Professor Steven Whang’s group on social distancing and face mask distribution among vulnerable age groups, people in their 20s, 60s, and 70s followed the social distancing guidelines the most strictly. The research team analyzed the data provided by SK Telecom in the Gangnam district of Seoul. The data provided on people in their 70s, a group that accounted for half of all fatalities, showed that masks were generally well distributed nationwide.
Dr. Alexandros Papaspyrids, Tertiary Education Industry Director of the Asia region of Microsoft, said that despite all the disadvantages and problems related to remote education, we shouldn’t expect to return to the days before the COVID-19 any time soon. “We should accept the new normal and explore new opportunities in the new educational environment,” he said.
Hongtaek Yong, Deputy Minister at the Office of R&D Policy at the Ministry of Science and ICT presented the Korean government’s disease prevention and response policy and how they tried to mitigate the economic and social impact.
He stressed the government’s fast testing, tracing, and openness for successfully flattening the curve, adding that the government used an ICT-based approach in all aspects of their response. From early this year when the first patient was reported, the government aggressively encouraged the biotech industry to develop diagnostic kits and novel therapeutic medications.
As a result, five companies were able to produce genetic diagnostic reagents through the emergency approval. More notably, four of them are conducting massive R&D projects sponsored by the government and this is the result of the government’s continuous investment in R&D. Korea is the leader in R&D investment among the OECD countries.
According to Yong, the government’s big data project that was launched in 2017 continuously traces the trends of epidemics in Korea. The epidemiological studies based on the paths taken by suspected patients using credit card transaction made the difference in predicting the spread of the coronavirus and implementing countermeasures. The data has been provided to the Korea’s Center for Disease Control (CDC).
“In addition to the epidemics, we have so many other pending issues arising from digital and social equities, un-contact services, and job security. We are very open to collaborate and cooperate with other countries to deal with this global crisis,” Yong said.
During the subsequent panel discussions, David Dollar, a senior fellow at the Brookings Institution, said, “The global economy in the coronavirus era will not have a rapid V-shaped recovery, but rather will fall into a long depression for at least two years.” He pointed out that if countries practice protectionism like they did during the Great Depression, the recession will be even worse. Hence, he urged the international community, especially developed nations, to avoid protectionism, consider the economic difficulties of developing countries, and provide them with financial support.
Co-Director of the Center for Universal Education at the Brookings Institution Rebecca Winthrop raised concerns over the recent shift to online teaching and learning, claiming that insufficient infrastructures in low-income families in developing nations are already causing added educational disparities and provoking the inequity issue around the world. “The ways to provide quality education equally through faster and more effective means should be studied,” she said.
Professor Joungho Kim, the director of the KAIST GSI and the forum’s organizer, concluded the event by saying that this forum will be a valuable resource for everyone who is providing assistance to those in need, both during and after the COVID-19 pandemic.
(END)
2020.04.22 View 25966 -
 14-Day Drawing Challenge Helps Maintain a Sense of Connection Amid Prolonged Social Distancing
- “You need space, but you also need connections.” -
Schools and workplaces have closed and people are staying at home around the globe. Governments across the world have urged their people to keep a distance from others as a measure to slow the spread of the pandemic.
With the Korean government’s decision to extend the intensive social distancing campaign until at least April 19, people in Korea are advised to avoid nonessential trips, public facilities, and social gatherings for another two weeks or so.
This unprecedented prolonged social distancing drive leads people to feel fatigue and frustration. Such emotional stress is worse for those who live alone in a foreign country.
The International Scholar and Student Services (ISSS) Team at KAIST has been working around the clock to build a dedicated COVID-19 Mental Health Support Service to support the university’s international community on campus and abroad and help get them connected online.
As the COVID-19 situation lingers, there has been a growing demand for mental health support from many KAIST international members including 299 students who have been staying in Korea on their own and away from their families, as well as from those who could not return to campus from their overseas homes.
In response to this, the KAIST ISSS Team has been offering some special online events and programs that can help the KAIST international community stay feeling connected whereever they are, while still keeping a safe distance from each other.
For instance, the team is running an art-therapy program called ‘The 14-day Drawing Challenge’ March 30 through April 12. This program is online and individual-based, so it does not require any physical contact between participants.
Each participant is asked to draw a picture at home using the daily topics previously set by the ISSS Team over 14 days. The topics include (Day 1) self-portrait, (Day 2) spring flowers, (Day 3) if you could become anything…, (Day 4) funniest memory you have, (Day 5) animals at KAIST, (Day 6) something you love, (Day 7) country or city you want to visit, (Day 8) what’s for dinner? (Day 9) person you miss, (Day 10) your favorite place at KAIST, (Day 11) your feeling today, (Day 12) things in your favorite color, (Day 13) song lyrics, and (Day 14) your future self in 10 years.
Once all 14 pieces have been completed, submissions can be made online by sending an e-mail to the ISSS Team after scanning or taking a photo of each drawing. Selected submissions will be awarded small prizes for participation and shared through the university’s official website and SNS channels.
“All the participants need is paper, coloring tools, and their creativity and imagination. They don’t have to be a great artist to join this challenge. There is no right or wrong or good or bad. They just need to have fun drawing every day for two weeks, ease their coronavirus anxiety, and remain emotionally stable just like they did back in the normal days,” said Su-yeon Ahn, the manager of the KAIST ISSS Team. She added, “In times like these, you need space, but you also need connections. Our team wants our international students, professors, and researchers to build strong connections with each other, even online.”
Katherine Michelle Pena Santana, an M.S. candidate from the Department of Industrial and Systems Engineering who is taking part in ‘The 14-day Drawing Challenge,’ looked back and said, “Lately with the new coronavirus spreading around Korea and the entire world, I was feeling very anxious. I didn't get out of my room and lived by just looking at the same walls and creating some kind of a psychological burden on myself.”
Santana added that these kinds of activities could give many foreign members of KAIST an opportunity to not only relieve fear and stress, but also share each other’s experiences dealing with this pandemic. She explained that this is why she decided to participate in this challenge.
An undergraduate student from the Department of Physics, Ada Carpenter, appreciated the KAIST ISSS Team’s efforts to provide a variety of special online mental health support services to help the university’s international community socialize, while strictly following the government’s guidelines for social distancing. She expressed excitement about participating and said, “I’m so looking forward to the challenge of things that I wouldn’t normally draw.”
< Short Self-interview Video Clip Filmed by Ada Carpenter >
The COVID-19 Mental Health Support Service by the KAIST ISSS Team will be continually updated with new information and enhanced with other tools and support over the coming weeks and months. Some of the upcoming events and programs include ‘The Online Guitar Lessons’, ‘The Growing Houseplants Challenge’, and ‘The Any Song Challenge*’.
* The song titled “Any Song” by Korean rapper Zico has been gaining attention on social media thanks to many celebrities taking on the ‘Any Song Challenge’, performing a short dance to the chorus of the song and sharing it on social media.
(END)
2020.04.08 View 12571
14-Day Drawing Challenge Helps Maintain a Sense of Connection Amid Prolonged Social Distancing
- “You need space, but you also need connections.” -
Schools and workplaces have closed and people are staying at home around the globe. Governments across the world have urged their people to keep a distance from others as a measure to slow the spread of the pandemic.
With the Korean government’s decision to extend the intensive social distancing campaign until at least April 19, people in Korea are advised to avoid nonessential trips, public facilities, and social gatherings for another two weeks or so.
This unprecedented prolonged social distancing drive leads people to feel fatigue and frustration. Such emotional stress is worse for those who live alone in a foreign country.
The International Scholar and Student Services (ISSS) Team at KAIST has been working around the clock to build a dedicated COVID-19 Mental Health Support Service to support the university’s international community on campus and abroad and help get them connected online.
As the COVID-19 situation lingers, there has been a growing demand for mental health support from many KAIST international members including 299 students who have been staying in Korea on their own and away from their families, as well as from those who could not return to campus from their overseas homes.
In response to this, the KAIST ISSS Team has been offering some special online events and programs that can help the KAIST international community stay feeling connected whereever they are, while still keeping a safe distance from each other.
For instance, the team is running an art-therapy program called ‘The 14-day Drawing Challenge’ March 30 through April 12. This program is online and individual-based, so it does not require any physical contact between participants.
Each participant is asked to draw a picture at home using the daily topics previously set by the ISSS Team over 14 days. The topics include (Day 1) self-portrait, (Day 2) spring flowers, (Day 3) if you could become anything…, (Day 4) funniest memory you have, (Day 5) animals at KAIST, (Day 6) something you love, (Day 7) country or city you want to visit, (Day 8) what’s for dinner? (Day 9) person you miss, (Day 10) your favorite place at KAIST, (Day 11) your feeling today, (Day 12) things in your favorite color, (Day 13) song lyrics, and (Day 14) your future self in 10 years.
Once all 14 pieces have been completed, submissions can be made online by sending an e-mail to the ISSS Team after scanning or taking a photo of each drawing. Selected submissions will be awarded small prizes for participation and shared through the university’s official website and SNS channels.
“All the participants need is paper, coloring tools, and their creativity and imagination. They don’t have to be a great artist to join this challenge. There is no right or wrong or good or bad. They just need to have fun drawing every day for two weeks, ease their coronavirus anxiety, and remain emotionally stable just like they did back in the normal days,” said Su-yeon Ahn, the manager of the KAIST ISSS Team. She added, “In times like these, you need space, but you also need connections. Our team wants our international students, professors, and researchers to build strong connections with each other, even online.”
Katherine Michelle Pena Santana, an M.S. candidate from the Department of Industrial and Systems Engineering who is taking part in ‘The 14-day Drawing Challenge,’ looked back and said, “Lately with the new coronavirus spreading around Korea and the entire world, I was feeling very anxious. I didn't get out of my room and lived by just looking at the same walls and creating some kind of a psychological burden on myself.”
Santana added that these kinds of activities could give many foreign members of KAIST an opportunity to not only relieve fear and stress, but also share each other’s experiences dealing with this pandemic. She explained that this is why she decided to participate in this challenge.
An undergraduate student from the Department of Physics, Ada Carpenter, appreciated the KAIST ISSS Team’s efforts to provide a variety of special online mental health support services to help the university’s international community socialize, while strictly following the government’s guidelines for social distancing. She expressed excitement about participating and said, “I’m so looking forward to the challenge of things that I wouldn’t normally draw.”
< Short Self-interview Video Clip Filmed by Ada Carpenter >
The COVID-19 Mental Health Support Service by the KAIST ISSS Team will be continually updated with new information and enhanced with other tools and support over the coming weeks and months. Some of the upcoming events and programs include ‘The Online Guitar Lessons’, ‘The Growing Houseplants Challenge’, and ‘The Any Song Challenge*’.
* The song titled “Any Song” by Korean rapper Zico has been gaining attention on social media thanks to many celebrities taking on the ‘Any Song Challenge’, performing a short dance to the chorus of the song and sharing it on social media.
(END)
2020.04.08 View 12571 -
 Former Minister of Science and Technology Woo Sik Kim Elected as New Chairman of Board of Trustees
Dr. Woo Sik Kim, former Minister of Science and Technology and Deputy Prime Minister, was elected as the new chairman of the KAIST Board of Trustees on March 26. Dr. Kim will succeed Chairman Jang-Mu Lee, whose three-year term expired last month.
Dr. Kim is a chemical engineering professor who spent most of his academic career at Yonsei University from 1968. In 2000, he held the office of president of Yonsei University for four years before moving to the Presidential Office of President Roh Moo-Hyun as his chief of staff in 2004. After serving in the Blue House for two years, he served as the Minister of Science and Technology from 2006 to 2008.
An emeritus fellow of the National Academy of Engineering of Korea (NAEK), Chairman Kim also taught at KAIST as an invited distinguished professor from 2008 to 2010. He is currently the chairman of the Creativity Engineering Institute (CEI).
(END)
2020.04.06 View 15683
Former Minister of Science and Technology Woo Sik Kim Elected as New Chairman of Board of Trustees
Dr. Woo Sik Kim, former Minister of Science and Technology and Deputy Prime Minister, was elected as the new chairman of the KAIST Board of Trustees on March 26. Dr. Kim will succeed Chairman Jang-Mu Lee, whose three-year term expired last month.
Dr. Kim is a chemical engineering professor who spent most of his academic career at Yonsei University from 1968. In 2000, he held the office of president of Yonsei University for four years before moving to the Presidential Office of President Roh Moo-Hyun as his chief of staff in 2004. After serving in the Blue House for two years, he served as the Minister of Science and Technology from 2006 to 2008.
An emeritus fellow of the National Academy of Engineering of Korea (NAEK), Chairman Kim also taught at KAIST as an invited distinguished professor from 2008 to 2010. He is currently the chairman of the Creativity Engineering Institute (CEI).
(END)
2020.04.06 View 15683 -
 COVID-19 Update: Reaching Out to Help Local Schools’ Online Classes
After the Ministry of Education decided that all schools would conduct online classes from April 9, schools began to ramp up online education tools and systems. On March 30, the Ministry announced a three-phased opening for the schools, putting online classes for third-year middle and high school students first. The online classes will expand to first and second-year middle and high schoolers and the upper grades of elementary schools on April 16, followed by first to third graders of elementary schools on April 20.
Although some schools have already introduced online teaching and learning into the curriculum, most schools are still unprepared, raising concerns over possible educational disparities caused by insufficient infrastructures and a lack of training for teachers.
To counter these issues, KAIST rolled up its sleeves to help teachers from 38 local middle and high schools in the Yuseong District of Daejeon better prepare for their interactive online classes by providing a special training course from April 7 through 29. Following the course, approximately 40 undergraduate and graduate students will be assigned to schools to help them set up and better utilize the online educational program.
On April 3, Professor Youngsun Kwon, the Dean of KAIST Academy and the Director of the Center for Excellence in Learning and Teaching, gave a two-hour online interactive tutorial session on the utilization of the real-time video conferencing platform ‘Zoom’ for online classes. He shared best practices for checking attendance, running classes, and giving and marking quizzes and assignments. A total of 102 local middle and high school teachers attended this session.
“We feel very fortunate to reach out to teachers who are so passionate about learning online education methodology. We were pleasantly surprised to see this many educators show up for the tutorial session,” said Dean Kwon. He also appreciated KAIST students’ strong interests and support in the community outreach project in response to COVID-19 during these challenging times when social distancing is so critical.
He said more than 150 student volunteers signed up for this project 10 hours after his office posted the opening for volunteers on the KAIST intranet. “We will help front-line school teachers, strictly following the government’s guidelines for social distancing,” he added.
The students’ online class support group will provide additional help to schools that are inexperienced users of Zoom. The students will be those who are very familiar with online lectures using Zoom, and are fully acquainted with how to operate them. One or two of the students will be assigned to each school that requests support, and will directly help solve complications that stem from preparing and running the classes through online measures for safety reasons. The expenses for the support group’s activities will be fully covered by KAIST, and the period of support may be extended upon request.
KAIST has been offering approximately 1,200 courses remotely since the spring semester opened on March 16 and will do so until the COVID-19 situation stabilizes. Along with the provision of pre-recorded one-way lecture contents, real-time two-way lessons are being delivered through various video conferencing platforms including Zoom, YouTube Live, and Microsoft Teams. There were both minor and major technical issues at the beginning of the semester, caused by the instability of servers and system overloads as well as from users being inexperienced with the tools and systems. However, the class procedures have gradually stabilized and are now running better.
KAIST President Sung-Chul Shin said, “As the COVID-19 situation lingers, this is a more difficult time than ever, where all educational establishments and educators must quickly learn and apply new methods of education, often in insufficient preparation conditions.” He added, “KAIST will provide support for secondary schools in the region to quickly resolve the inconveniences caused by new users of online classes so that they may provide high-quality education.”
(END)
2020.04.06 View 9370
COVID-19 Update: Reaching Out to Help Local Schools’ Online Classes
After the Ministry of Education decided that all schools would conduct online classes from April 9, schools began to ramp up online education tools and systems. On March 30, the Ministry announced a three-phased opening for the schools, putting online classes for third-year middle and high school students first. The online classes will expand to first and second-year middle and high schoolers and the upper grades of elementary schools on April 16, followed by first to third graders of elementary schools on April 20.
Although some schools have already introduced online teaching and learning into the curriculum, most schools are still unprepared, raising concerns over possible educational disparities caused by insufficient infrastructures and a lack of training for teachers.
To counter these issues, KAIST rolled up its sleeves to help teachers from 38 local middle and high schools in the Yuseong District of Daejeon better prepare for their interactive online classes by providing a special training course from April 7 through 29. Following the course, approximately 40 undergraduate and graduate students will be assigned to schools to help them set up and better utilize the online educational program.
On April 3, Professor Youngsun Kwon, the Dean of KAIST Academy and the Director of the Center for Excellence in Learning and Teaching, gave a two-hour online interactive tutorial session on the utilization of the real-time video conferencing platform ‘Zoom’ for online classes. He shared best practices for checking attendance, running classes, and giving and marking quizzes and assignments. A total of 102 local middle and high school teachers attended this session.
“We feel very fortunate to reach out to teachers who are so passionate about learning online education methodology. We were pleasantly surprised to see this many educators show up for the tutorial session,” said Dean Kwon. He also appreciated KAIST students’ strong interests and support in the community outreach project in response to COVID-19 during these challenging times when social distancing is so critical.
He said more than 150 student volunteers signed up for this project 10 hours after his office posted the opening for volunteers on the KAIST intranet. “We will help front-line school teachers, strictly following the government’s guidelines for social distancing,” he added.
The students’ online class support group will provide additional help to schools that are inexperienced users of Zoom. The students will be those who are very familiar with online lectures using Zoom, and are fully acquainted with how to operate them. One or two of the students will be assigned to each school that requests support, and will directly help solve complications that stem from preparing and running the classes through online measures for safety reasons. The expenses for the support group’s activities will be fully covered by KAIST, and the period of support may be extended upon request.
KAIST has been offering approximately 1,200 courses remotely since the spring semester opened on March 16 and will do so until the COVID-19 situation stabilizes. Along with the provision of pre-recorded one-way lecture contents, real-time two-way lessons are being delivered through various video conferencing platforms including Zoom, YouTube Live, and Microsoft Teams. There were both minor and major technical issues at the beginning of the semester, caused by the instability of servers and system overloads as well as from users being inexperienced with the tools and systems. However, the class procedures have gradually stabilized and are now running better.
KAIST President Sung-Chul Shin said, “As the COVID-19 situation lingers, this is a more difficult time than ever, where all educational establishments and educators must quickly learn and apply new methods of education, often in insufficient preparation conditions.” He added, “KAIST will provide support for secondary schools in the region to quickly resolve the inconveniences caused by new users of online classes so that they may provide high-quality education.”
(END)
2020.04.06 View 9370 -
 Cyber MOU Signing with Zhejiang University
KAIST signed an MOU with Zhejiang University (ZJU) in China on March 25. This MOU signing ceremony took place via video conference due to the outbreak of COVID-19.
The collaboration with ZJU had already started with the signing of an MOU for cooperation in technology commercialization last December. Possible cooperation initiatives included facilitating joint start-up businesses, patent portfolios, and technology marketing.
With this general agreement signing, it is expected that the two institutes will expand mutual exchanges and collaborations at the institutional level for education and research.
President Sung-Chul Shin said, “We will work together to devise measures for the systematic advancement of cooperation in various directions, including education, research, and the commercialization of technologies.”
ZJU, a member of the C9 League known as China’s Ivy League, was established in 1897 and is located in the city of Hangzhou. Its population across 37 colleges and schools comprises 54,641 students and 3,741 faculty members. The university was ranked 6th in Asia and 54th in the world in the 2020 QS Rankings.
(END)
2020.03.30 View 16275
Cyber MOU Signing with Zhejiang University
KAIST signed an MOU with Zhejiang University (ZJU) in China on March 25. This MOU signing ceremony took place via video conference due to the outbreak of COVID-19.
The collaboration with ZJU had already started with the signing of an MOU for cooperation in technology commercialization last December. Possible cooperation initiatives included facilitating joint start-up businesses, patent portfolios, and technology marketing.
With this general agreement signing, it is expected that the two institutes will expand mutual exchanges and collaborations at the institutional level for education and research.
President Sung-Chul Shin said, “We will work together to devise measures for the systematic advancement of cooperation in various directions, including education, research, and the commercialization of technologies.”
ZJU, a member of the C9 League known as China’s Ivy League, was established in 1897 and is located in the city of Hangzhou. Its population across 37 colleges and schools comprises 54,641 students and 3,741 faculty members. The university was ranked 6th in Asia and 54th in the world in the 2020 QS Rankings.
(END)
2020.03.30 View 16275 -
 A Global Campaign of ‘Facts before Rumors’ on COVID-19 Launched
- A KAIST data scientist group responds to facts and rumors on COVID-19 for global awareness of the pandemic. -
Like the novel coronavirus, rumors have no borders. The world is fighting to contain the pandemic, but we also have to deal with the appalling spread of an infodemic that is as contagious as the virus. This infodemic, a pandemic of false information, is bringing chaos and extreme fear to the general public.
Professor Meeyoung Cha’s group at the School of Computing started a global campaign called ‘Facts before Rumors,’ to prevent the spread of false information from crossing borders. She explained, “We saw many rumors that had already been fact-checked long before in China and South Korea now begin to circulate in other countries, sometimes leading to detrimental results. We launched an official campaign, Facts before Rumors, to deliver COVID-19-related facts to countries where the number of cases is now increasing.” She released the first set of facts on March 26 via her Twitter account @nekozzang.
Professor Cha, a data scientist who has focused on detecting global fake news, is now part of the COVID-19 AI Task Force at the Global Strategy Institute at KAIST. She is also leading the Data Science Group at the Institute for Basic Science (IBS) as Chief Investigator.
Her research group worked in collaboration with the College of Nursing at Ewha Woman’s University to identify 15 claims about COVID-19 that circulated on social networks (SNS) and among the general public. The team fact-checked these claims based on information from the WHO and CDCs of Korea and the US. The research group is now working on translating the list of claims into Portuguese, Spanish, Persian, Chinese, Amharic, Hindi, and Vietnamese. Delivering facts before rumors, the team says, will help contain the disease and prevent any harm caused by misinformation.
The pandemic, which spread in China and South Korea before arriving in Europe and the US, is now moving into South America, Africa, and Southeast Asia. “We would like to play a part in preventing the further spread of the disease with the provision of only scientifically vetted, truthful facts,” said the team.
For this campaign, Professor Cha’s team investigated more than 200 rumored claims on COVID-19 in China during the early days of the pandemic. These claims spread in different levels: while some were only relevant locally or in larger regions of China, others propagated in Asia and are now spreading to countries that are currently most affected by the disease.
For example, the false claim which publicized that ‘Fireworks can help tame the virus in the air’ only spread in China. Other claims such as ‘Eating garlic helps people overcome the disease’ or ‘Gargling with salt water prevents the contraction of the disease,’ spread around the world even after being proved groundless.
The team noted, however, that the times at which these claims propagate are different from one country to another. “This opens up an opportunity to debunk rumors in some countries, even before they start to emerge,” said Professor Cha.
Kun-Woo Kim, a master’s candidate in the Department of Industrial Design who joined this campaign and designed the Facts before Rumors chart also expressed his hope that this campaign will help reduce the number of victims. He added, “I am very grateful to our scientists who quickly responded to the Fact Check in these challenging times.”
2020.03.27 View 15352
A Global Campaign of ‘Facts before Rumors’ on COVID-19 Launched
- A KAIST data scientist group responds to facts and rumors on COVID-19 for global awareness of the pandemic. -
Like the novel coronavirus, rumors have no borders. The world is fighting to contain the pandemic, but we also have to deal with the appalling spread of an infodemic that is as contagious as the virus. This infodemic, a pandemic of false information, is bringing chaos and extreme fear to the general public.
Professor Meeyoung Cha’s group at the School of Computing started a global campaign called ‘Facts before Rumors,’ to prevent the spread of false information from crossing borders. She explained, “We saw many rumors that had already been fact-checked long before in China and South Korea now begin to circulate in other countries, sometimes leading to detrimental results. We launched an official campaign, Facts before Rumors, to deliver COVID-19-related facts to countries where the number of cases is now increasing.” She released the first set of facts on March 26 via her Twitter account @nekozzang.
Professor Cha, a data scientist who has focused on detecting global fake news, is now part of the COVID-19 AI Task Force at the Global Strategy Institute at KAIST. She is also leading the Data Science Group at the Institute for Basic Science (IBS) as Chief Investigator.
Her research group worked in collaboration with the College of Nursing at Ewha Woman’s University to identify 15 claims about COVID-19 that circulated on social networks (SNS) and among the general public. The team fact-checked these claims based on information from the WHO and CDCs of Korea and the US. The research group is now working on translating the list of claims into Portuguese, Spanish, Persian, Chinese, Amharic, Hindi, and Vietnamese. Delivering facts before rumors, the team says, will help contain the disease and prevent any harm caused by misinformation.
The pandemic, which spread in China and South Korea before arriving in Europe and the US, is now moving into South America, Africa, and Southeast Asia. “We would like to play a part in preventing the further spread of the disease with the provision of only scientifically vetted, truthful facts,” said the team.
For this campaign, Professor Cha’s team investigated more than 200 rumored claims on COVID-19 in China during the early days of the pandemic. These claims spread in different levels: while some were only relevant locally or in larger regions of China, others propagated in Asia and are now spreading to countries that are currently most affected by the disease.
For example, the false claim which publicized that ‘Fireworks can help tame the virus in the air’ only spread in China. Other claims such as ‘Eating garlic helps people overcome the disease’ or ‘Gargling with salt water prevents the contraction of the disease,’ spread around the world even after being proved groundless.
The team noted, however, that the times at which these claims propagate are different from one country to another. “This opens up an opportunity to debunk rumors in some countries, even before they start to emerge,” said Professor Cha.
Kun-Woo Kim, a master’s candidate in the Department of Industrial Design who joined this campaign and designed the Facts before Rumors chart also expressed his hope that this campaign will help reduce the number of victims. He added, “I am very grateful to our scientists who quickly responded to the Fact Check in these challenging times.”
2020.03.27 View 15352 -
 Highly Efficient and Stable Double Layer Solar Cell Developed
Solar cells convert light into energy, but they can be inefficient and vulnerable to the environment, degrading with, ironically, too much light or other factors, including moisture and low temperature. An international research team has developed a new type of solar cell that can both withstand environmental hazards and is 26.7% efficient in power conversion.
They published their results on March 26 in Science.
The researchers, led by Byungha Shin, a professor from the Department of Materials Science and Engineering at KAIST, focused on developing a new class of light-absorbing material, called a wide bandgap perovskite. The material has a highly effective crystal structure that can process the power needs, but it can become problematic when exposed to environmental hazards, such as moisture. Researchers have made some progress increasing the efficiency of solar cells based on perovskite, but the material has greater potential than what was previously achieved.
To achieve better performance, Shin and his team built a double layer solar cell, called tandem, in which two or more light absorbers are stacked together to better utilize solar energy. To use perovskite in these tandem devices, the scientists modified the material’s optical property, which allows it to absorb a wider range of solar energy. Without the adjustment, the material is not as useful in achieving high performing tandem solar cells. The modification of the optical property of perovskite, however, comes with a penalty — the material becomes hugely vulnerable to the environment, in particular, to light.
To counteract the wide bandgap perovskite’s delicate nature, the researchers engineered combinations of molecules composing a two-dimensional layer in the perovskite, stabilizing the solar cells.
“We developed a high-quality wide bandgap perovskite material and, in combination with silicon solar cells, achieved world-class perovskite-silicon tandem cells,” Shin said.
The development was only possible due to the engineering method, in which the mixing ratio of the molecules building the two-dimensional layer are carefully controlled. In this case, the perovskite material not only improved efficiency of the resulting solar cell but also gained durability, retaining 80% of its initial power conversion capability even after 1,000 hours of continuous illumination. This is the first time such a high efficiency has been achieved with a wide bandgap perovskite single layer alone, according to Shin.
“Such high-efficiency wide bandgap perovskite is an essential technology for achieving ultra-high efficiency of perovskite-silicon tandem (double layer) solar cells,” Shin said. “The results also show the importance of bandgap matching of upper and lower cells in these tandem solar cells.”
The researchers, having stabilized the wide bandgap perovskite material, are now focused on developing even more efficient tandem solar cells that are expected to have more than 30% of power conversion efficiency, something that no one has achieved yet,
“Our ultimate goal is to develop ultra-high-efficiency tandem solar cells that contribute to the increase of shared solar energy among all energy sources,” Shin said. “We want to contribute to making the planet healthier.”
This work was supported by the National Research Foundation of Korea, the Korea Institute of Energy Technology Evaluation and Planning, the Ministry of Trade Industry and Energy of Korea, and the U.S. Department of Energy.
Other contributors include Daehan Kim, Jekyung Kim, Passarut Boonmongkolras, Seong Ryul Pae and Minkyu Kim, all of whom affiliated with the Department of Materials Science and Engineering at KAIST. Other authors include Byron W. Larson, Sean P. Dunfield, Chuanxiao Xiao, Jinhui Tong, Fei Zhang, Joseph J. Berry, Kai Zhu and Dong Hoe Kim, all of who are affiliated with the National Renewable Energy Laboratory in Colorado. Dunfield is also affiliated with the Materials Science and Engineering Program at the University of Colorado; Berry is also affiliated with the Department of Physics and the Renewable and Sustainable Energy Institute at the University of Colorado Boulder; and Kim is also affiliated with the Department of Nanotechnology and Advanced Materials Engineering at Sejong University. Hee Joon Jung and Vinayak Dravid of the Department of Materials Science and Engineering at Northwestern University; Ik Jae Park, Su Geun Ji and Jin Young Kim of the Department of Materials Science and Engineering at Seoul National University; and Seok Beom Kang of the Department of Nanotechnology and Advanced Materials Engineering of Sejong University also contributed.
Image credit: Professor Byungha Shin, KAIST
Image usage restrictions: News organizations may use or redistribute this image, with proper attribution, as part of news coverage of this paper only.
Publication: Kim et al. (2020) “Efficient, stable silicon tandem cells enabled by anion-engineered wide band gap perovskites”. Science. Available online at https://doi.org/10.1126/science.aba3433
Profile:
Byungha Shin
Professor
byungha@kaist.ac.kr
http://energymatlab.kaist.ac.kr/
Department of Materials Science and Engineering
KAIST
Profile:
Daehan Kim
Ph.D. Candidate
zxzx4592@kaist.ac.kr
http://energymatlab.kaist.ac.kr/
Department of Materials Science and Engineering
KAIST
(END)
2020.03.27 View 24760
Highly Efficient and Stable Double Layer Solar Cell Developed
Solar cells convert light into energy, but they can be inefficient and vulnerable to the environment, degrading with, ironically, too much light or other factors, including moisture and low temperature. An international research team has developed a new type of solar cell that can both withstand environmental hazards and is 26.7% efficient in power conversion.
They published their results on March 26 in Science.
The researchers, led by Byungha Shin, a professor from the Department of Materials Science and Engineering at KAIST, focused on developing a new class of light-absorbing material, called a wide bandgap perovskite. The material has a highly effective crystal structure that can process the power needs, but it can become problematic when exposed to environmental hazards, such as moisture. Researchers have made some progress increasing the efficiency of solar cells based on perovskite, but the material has greater potential than what was previously achieved.
To achieve better performance, Shin and his team built a double layer solar cell, called tandem, in which two or more light absorbers are stacked together to better utilize solar energy. To use perovskite in these tandem devices, the scientists modified the material’s optical property, which allows it to absorb a wider range of solar energy. Without the adjustment, the material is not as useful in achieving high performing tandem solar cells. The modification of the optical property of perovskite, however, comes with a penalty — the material becomes hugely vulnerable to the environment, in particular, to light.
To counteract the wide bandgap perovskite’s delicate nature, the researchers engineered combinations of molecules composing a two-dimensional layer in the perovskite, stabilizing the solar cells.
“We developed a high-quality wide bandgap perovskite material and, in combination with silicon solar cells, achieved world-class perovskite-silicon tandem cells,” Shin said.
The development was only possible due to the engineering method, in which the mixing ratio of the molecules building the two-dimensional layer are carefully controlled. In this case, the perovskite material not only improved efficiency of the resulting solar cell but also gained durability, retaining 80% of its initial power conversion capability even after 1,000 hours of continuous illumination. This is the first time such a high efficiency has been achieved with a wide bandgap perovskite single layer alone, according to Shin.
“Such high-efficiency wide bandgap perovskite is an essential technology for achieving ultra-high efficiency of perovskite-silicon tandem (double layer) solar cells,” Shin said. “The results also show the importance of bandgap matching of upper and lower cells in these tandem solar cells.”
The researchers, having stabilized the wide bandgap perovskite material, are now focused on developing even more efficient tandem solar cells that are expected to have more than 30% of power conversion efficiency, something that no one has achieved yet,
“Our ultimate goal is to develop ultra-high-efficiency tandem solar cells that contribute to the increase of shared solar energy among all energy sources,” Shin said. “We want to contribute to making the planet healthier.”
This work was supported by the National Research Foundation of Korea, the Korea Institute of Energy Technology Evaluation and Planning, the Ministry of Trade Industry and Energy of Korea, and the U.S. Department of Energy.
Other contributors include Daehan Kim, Jekyung Kim, Passarut Boonmongkolras, Seong Ryul Pae and Minkyu Kim, all of whom affiliated with the Department of Materials Science and Engineering at KAIST. Other authors include Byron W. Larson, Sean P. Dunfield, Chuanxiao Xiao, Jinhui Tong, Fei Zhang, Joseph J. Berry, Kai Zhu and Dong Hoe Kim, all of who are affiliated with the National Renewable Energy Laboratory in Colorado. Dunfield is also affiliated with the Materials Science and Engineering Program at the University of Colorado; Berry is also affiliated with the Department of Physics and the Renewable and Sustainable Energy Institute at the University of Colorado Boulder; and Kim is also affiliated with the Department of Nanotechnology and Advanced Materials Engineering at Sejong University. Hee Joon Jung and Vinayak Dravid of the Department of Materials Science and Engineering at Northwestern University; Ik Jae Park, Su Geun Ji and Jin Young Kim of the Department of Materials Science and Engineering at Seoul National University; and Seok Beom Kang of the Department of Nanotechnology and Advanced Materials Engineering of Sejong University also contributed.
Image credit: Professor Byungha Shin, KAIST
Image usage restrictions: News organizations may use or redistribute this image, with proper attribution, as part of news coverage of this paper only.
Publication: Kim et al. (2020) “Efficient, stable silicon tandem cells enabled by anion-engineered wide band gap perovskites”. Science. Available online at https://doi.org/10.1126/science.aba3433
Profile:
Byungha Shin
Professor
byungha@kaist.ac.kr
http://energymatlab.kaist.ac.kr/
Department of Materials Science and Engineering
KAIST
Profile:
Daehan Kim
Ph.D. Candidate
zxzx4592@kaist.ac.kr
http://energymatlab.kaist.ac.kr/
Department of Materials Science and Engineering
KAIST
(END)
2020.03.27 View 24760 -
 COVID-19 Update: Students and Professors Adjust to 1,200 Online Classes
- Approximately 1,200 online classes are being offered during the cyber semester. -
COVID-19 is transforming the way KAISTians live. Many restrictions imposed to contain the spread of the virus have us adjusting to a new environment swiftly. A cyber MOU signing ceremony with a foreign partner university took place on March 25, as did a cyber Board of Trustees Meeting on March 26. KAIST’s Main Campus is normally one of the most iconic picnic destinations for the citizens of Daejeon, but this is not the case this spring, as the campus has been temporarily closed to protect our own community as well as our neighboring communities.
KAIST has been offering approximately 1,200 courses remotely since this semester opened on March 16 and will do so until further notice. Students and faculty members are experiencing the newly emerging norms of remote education in this time of social distancing. This unexpected disruption might advance the new digital pedagogy at KAIST, which was already ahead of the curve with its online learning and teaching infrastructure.
Professor Youngsun Kwon, the Dean of KAIST Academy and the Director of the Center for Excellence in Learning and Teaching, said, “We had already initiated the KAIST Learning Management System (KLMS) in 2011 for introducing flipped learning, a student-centric creative-learning pedagogy. Since then, about nine percent of all our classes have been run using this methodology. Students pre-study the online streaming lecture materials that professors have uploaded in advance outside the classroom, and in-class activities are mainly group discussions and problem-solving activities.”
According to Dean Kwon, the university was planning to further introduce real-time online education from this spring semester and were in the process of setting up the system started from last year. “Our plan was to connect the real-time video conferencing service Zoom to our existing remote educational platform KLMS. However, things related to COVID-19 all happened so rapidly that we didn’t yet have a full-fledged connection,” said Dean Kwon.
Professors had to choose either to conduct their lectures remotely in the form of a pre-made one-way lesson or a real-time two-way lesson. They could also modify them using both platforms. Professor Youngchul Kim from the Department of Civil and Environmental Engineering said, “I had to also make some changes in my class activities and assignments. I removed a group design project and some tutorial workshops that were meant to provide students with hands-on experience using design tools such a 3D printer and a laser cutting system. Ironically, I found that students seem to focus on online lectures more intensely than I expected. I feel like students give me their thoughts and respond much quicker as well.”
Unfortunately, the online learning and teaching infrastructure and resources that had been put in place could not handle the overwhelming volume of classes being uploaded over very short period of time.
To handle the new demand, IT technicians are setting up the technical environment with stable servers to improve network traffic. For professors, teaching assistants, and students to teach and learn better in an online space, department offices have been lending spare equipment such as laptops, tablets, headsets, and webcams to those who do not have their own, based on availability. Academic support staff have also been pitching in by developing the best guidelines for online training.
“Even in these uncharted waters, all of the members of KAIST are doing their best to keep the ship steadily sailing in the right direction. I am very grateful for everyone’s efforts to make things work,” said Dean Kwon.
About 60% of the courses currently offered online are being uploaded using the non-real-time KLMS, and the remaining 40% are run in real time via Zoom. Each class runs for 50 minutes per academic credit, and comprises at least 25 minutes of lecture, a Q&A session, and a group discussion.
Students enrolled in the 481 courses that include experiments are asked to conduct their experiments individually after watching a 50-minute online lecture. Experimental, practical, and physical courses that are impossible to provide online have been cancelled or postponed until the next semester or summer/winter breaks.
“I find the online lessons quite convenient for the courses that I am taking this semester, especially the non-real-time ones, because I can watch the lecture videos over and over again even after the class has finished to understand the contents better,” said Jaymee Palma, an undergraduate student from the Department of Chemistry.
Ada Carpenter, an undergraduate student from the Department of Physics, added, “Students who normally feel uncomfortable speaking in class raise their questions on an online Q&A board more easily. Besides, I saw many other students asking questions and leading a discussion verbally as well. I think, when students join a synchronous Zoom classroom, they are more engaged than when just attending a regular lecture in a conventional classroom. It’s like everyone can sit in the front row of the class.”
Still, there are reportedly pedagogical, logistical, and technological challenges to these extraordinary educational measures. Some students express concerns about keeping up with professors and other students if they don’t have sufficient technological knowledge and skills. Some also cite the disadvantage of online classes having much less interaction and engagement among students and between professors and students than offline ones. “Fortunately, I think my professors are all excellent, so I can immerse myself well during all my cyber classes,” said Sang-Hyeon Lee, a graduate student from the School of Computing.
(END)
2020.03.26 View 11126
COVID-19 Update: Students and Professors Adjust to 1,200 Online Classes
- Approximately 1,200 online classes are being offered during the cyber semester. -
COVID-19 is transforming the way KAISTians live. Many restrictions imposed to contain the spread of the virus have us adjusting to a new environment swiftly. A cyber MOU signing ceremony with a foreign partner university took place on March 25, as did a cyber Board of Trustees Meeting on March 26. KAIST’s Main Campus is normally one of the most iconic picnic destinations for the citizens of Daejeon, but this is not the case this spring, as the campus has been temporarily closed to protect our own community as well as our neighboring communities.
KAIST has been offering approximately 1,200 courses remotely since this semester opened on March 16 and will do so until further notice. Students and faculty members are experiencing the newly emerging norms of remote education in this time of social distancing. This unexpected disruption might advance the new digital pedagogy at KAIST, which was already ahead of the curve with its online learning and teaching infrastructure.
Professor Youngsun Kwon, the Dean of KAIST Academy and the Director of the Center for Excellence in Learning and Teaching, said, “We had already initiated the KAIST Learning Management System (KLMS) in 2011 for introducing flipped learning, a student-centric creative-learning pedagogy. Since then, about nine percent of all our classes have been run using this methodology. Students pre-study the online streaming lecture materials that professors have uploaded in advance outside the classroom, and in-class activities are mainly group discussions and problem-solving activities.”
According to Dean Kwon, the university was planning to further introduce real-time online education from this spring semester and were in the process of setting up the system started from last year. “Our plan was to connect the real-time video conferencing service Zoom to our existing remote educational platform KLMS. However, things related to COVID-19 all happened so rapidly that we didn’t yet have a full-fledged connection,” said Dean Kwon.
Professors had to choose either to conduct their lectures remotely in the form of a pre-made one-way lesson or a real-time two-way lesson. They could also modify them using both platforms. Professor Youngchul Kim from the Department of Civil and Environmental Engineering said, “I had to also make some changes in my class activities and assignments. I removed a group design project and some tutorial workshops that were meant to provide students with hands-on experience using design tools such a 3D printer and a laser cutting system. Ironically, I found that students seem to focus on online lectures more intensely than I expected. I feel like students give me their thoughts and respond much quicker as well.”
Unfortunately, the online learning and teaching infrastructure and resources that had been put in place could not handle the overwhelming volume of classes being uploaded over very short period of time.
To handle the new demand, IT technicians are setting up the technical environment with stable servers to improve network traffic. For professors, teaching assistants, and students to teach and learn better in an online space, department offices have been lending spare equipment such as laptops, tablets, headsets, and webcams to those who do not have their own, based on availability. Academic support staff have also been pitching in by developing the best guidelines for online training.
“Even in these uncharted waters, all of the members of KAIST are doing their best to keep the ship steadily sailing in the right direction. I am very grateful for everyone’s efforts to make things work,” said Dean Kwon.
About 60% of the courses currently offered online are being uploaded using the non-real-time KLMS, and the remaining 40% are run in real time via Zoom. Each class runs for 50 minutes per academic credit, and comprises at least 25 minutes of lecture, a Q&A session, and a group discussion.
Students enrolled in the 481 courses that include experiments are asked to conduct their experiments individually after watching a 50-minute online lecture. Experimental, practical, and physical courses that are impossible to provide online have been cancelled or postponed until the next semester or summer/winter breaks.
“I find the online lessons quite convenient for the courses that I am taking this semester, especially the non-real-time ones, because I can watch the lecture videos over and over again even after the class has finished to understand the contents better,” said Jaymee Palma, an undergraduate student from the Department of Chemistry.
Ada Carpenter, an undergraduate student from the Department of Physics, added, “Students who normally feel uncomfortable speaking in class raise their questions on an online Q&A board more easily. Besides, I saw many other students asking questions and leading a discussion verbally as well. I think, when students join a synchronous Zoom classroom, they are more engaged than when just attending a regular lecture in a conventional classroom. It’s like everyone can sit in the front row of the class.”
Still, there are reportedly pedagogical, logistical, and technological challenges to these extraordinary educational measures. Some students express concerns about keeping up with professors and other students if they don’t have sufficient technological knowledge and skills. Some also cite the disadvantage of online classes having much less interaction and engagement among students and between professors and students than offline ones. “Fortunately, I think my professors are all excellent, so I can immerse myself well during all my cyber classes,” said Sang-Hyeon Lee, a graduate student from the School of Computing.
(END)
2020.03.26 View 11126 -
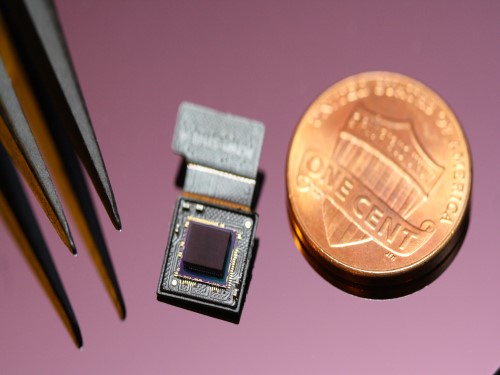 Ultrathin but Fully Packaged High-Resolution Camera
- Biologically inspired ultrathin arrayed camera captures super-resolution images. -
The unique structures of biological vision systems in nature inspired scientists to design ultracompact imaging systems. A research group led by Professor Ki-Hun Jeong have made an ultracompact camera that captures high-contrast and high-resolution images. Fully packaged with micro-optical elements such as inverted micro-lenses, multilayered pinhole arrays, and gap spacers on the image sensor, the camera boasts a total track length of 740 μm and a field of view of 73°.
Inspired by the eye structures of the paper wasp species Xenos peckii, the research team completely suppressed optical noise between micro-lenses while reducing camera thickness. The camera has successfully demonstrated high-contrast clear array images acquired from tiny micro lenses. To further enhance the image quality of the captured image, the team combined the arrayed images into one image through super-resolution imaging.
An insect’s compound eye has superior visual characteristics, such as a wide viewing angle, high motion sensitivity, and a large depth of field while maintaining a small volume of visual structure with a small focal length. Among them, the eyes of Xenos peckii and an endoparasite found on paper wasps have hundreds of photoreceptors in a single lens unlike conventional compound eyes. In particular, the eye structures of an adult Xenos peckii exhibit hundreds of photoreceptors on an individual eyelet and offer engineering inspiration for ultrathin cameras or imaging applications because they have higher visual acuity than other compound eyes.
For instance, Xenos peckii’s eye-inspired cameras provide a 50 times higher spatial resolution than those based on arthropod eyes. In addition, the effective image resolution of the Xenos peckii’s eye can be further improved using the image overlaps between neighboring eyelets. This unique structure offers higher visual resolution than other insect eyes.
The team achieved high-contrast and super-resolution imaging through a novel arrayed design of micro-optical elements comprising multilayered aperture arrays and inverted micro-lens arrays directly stacked over an image sensor. This optical component was integrated with a complementary metal oxide semiconductor image sensor.
This is first demonstration of super-resolution imaging which acquires a single integrated image with high contrast and high resolving power reconstructed from high-contrast array images. It is expected that this ultrathin arrayed camera can be applied for further developing mobile devices, advanced surveillance vehicles, and endoscopes.
Professor Jeong said, “This research has led to technological advances in imaging technology. We will continue to strive to make significant impacts on multidisciplinary research projects in the fields of microtechnology and nanotechnology, seeking inspiration from natural photonic structures.”
This work was featured in Light Science & Applications last month and was supported by the National Research Foundation (NRF) of and the Ministry of Health and Welfare (MOHW) of Korea.
Image credit: Professor Ki-Hun Jeong, KAIST
Image usage restrictions: News organizations may use or redistribute this image, with proper attribution, as part of news coverage of this paper only.
Publication:
Kisoo Kim, Kyung-Won Jang, Jae-Kwan Ryu, and Ki-Hun Jeong. (2020) “Biologically inspired ultrathin arrayed camera for high-contrast and high-resolution imaging”. Light Science & Applications. Volume 9. Article 28. Available online at https://doi.org/10.1038/s41377-020-0261-8
Profile:
Ki-Hun Jeong
Professor
kjeong@kaist.ac.kr
http://biophotonics.kaist.ac.kr/
Department of Bio and Brain Engineering
KAIST
Profile:
Kisoo Kim
Ph.D. Candidate
kisoo.kim1@kaist.ac.kr
http://biophotonics.kaist.ac.kr/
Department of Bio and Brain Engineering
KAIST
(END)
2020.03.23 View 22583
Ultrathin but Fully Packaged High-Resolution Camera
- Biologically inspired ultrathin arrayed camera captures super-resolution images. -
The unique structures of biological vision systems in nature inspired scientists to design ultracompact imaging systems. A research group led by Professor Ki-Hun Jeong have made an ultracompact camera that captures high-contrast and high-resolution images. Fully packaged with micro-optical elements such as inverted micro-lenses, multilayered pinhole arrays, and gap spacers on the image sensor, the camera boasts a total track length of 740 μm and a field of view of 73°.
Inspired by the eye structures of the paper wasp species Xenos peckii, the research team completely suppressed optical noise between micro-lenses while reducing camera thickness. The camera has successfully demonstrated high-contrast clear array images acquired from tiny micro lenses. To further enhance the image quality of the captured image, the team combined the arrayed images into one image through super-resolution imaging.
An insect’s compound eye has superior visual characteristics, such as a wide viewing angle, high motion sensitivity, and a large depth of field while maintaining a small volume of visual structure with a small focal length. Among them, the eyes of Xenos peckii and an endoparasite found on paper wasps have hundreds of photoreceptors in a single lens unlike conventional compound eyes. In particular, the eye structures of an adult Xenos peckii exhibit hundreds of photoreceptors on an individual eyelet and offer engineering inspiration for ultrathin cameras or imaging applications because they have higher visual acuity than other compound eyes.
For instance, Xenos peckii’s eye-inspired cameras provide a 50 times higher spatial resolution than those based on arthropod eyes. In addition, the effective image resolution of the Xenos peckii’s eye can be further improved using the image overlaps between neighboring eyelets. This unique structure offers higher visual resolution than other insect eyes.
The team achieved high-contrast and super-resolution imaging through a novel arrayed design of micro-optical elements comprising multilayered aperture arrays and inverted micro-lens arrays directly stacked over an image sensor. This optical component was integrated with a complementary metal oxide semiconductor image sensor.
This is first demonstration of super-resolution imaging which acquires a single integrated image with high contrast and high resolving power reconstructed from high-contrast array images. It is expected that this ultrathin arrayed camera can be applied for further developing mobile devices, advanced surveillance vehicles, and endoscopes.
Professor Jeong said, “This research has led to technological advances in imaging technology. We will continue to strive to make significant impacts on multidisciplinary research projects in the fields of microtechnology and nanotechnology, seeking inspiration from natural photonic structures.”
This work was featured in Light Science & Applications last month and was supported by the National Research Foundation (NRF) of and the Ministry of Health and Welfare (MOHW) of Korea.
Image credit: Professor Ki-Hun Jeong, KAIST
Image usage restrictions: News organizations may use or redistribute this image, with proper attribution, as part of news coverage of this paper only.
Publication:
Kisoo Kim, Kyung-Won Jang, Jae-Kwan Ryu, and Ki-Hun Jeong. (2020) “Biologically inspired ultrathin arrayed camera for high-contrast and high-resolution imaging”. Light Science & Applications. Volume 9. Article 28. Available online at https://doi.org/10.1038/s41377-020-0261-8
Profile:
Ki-Hun Jeong
Professor
kjeong@kaist.ac.kr
http://biophotonics.kaist.ac.kr/
Department of Bio and Brain Engineering
KAIST
Profile:
Kisoo Kim
Ph.D. Candidate
kisoo.kim1@kaist.ac.kr
http://biophotonics.kaist.ac.kr/
Department of Bio and Brain Engineering
KAIST
(END)
2020.03.23 View 22583 -
 Wearable Strain Sensor Using Light Transmittance Helps Measure Physical Signals Better
KAIST researchers have developed a novel wearable strain sensor based on the modulation of optical transmittance of a carbon nanotube (CNT)-embedded elastomer. The sensor is capable of sensitive, stable, and continuous measurement of physical signals. This technology, featured in the March 4th issue of ACS Applied Materials & Interfaces as a front cover article, shows great potential for the detection of subtle human motions and the real-time monitoring of body postures for healthcare applications.
A wearable strain sensor must have high sensitivity, flexibility, and stretchability, as well as low cost. Those used especially for health monitoring should also be tied to long-term solid performance, and be environmentally stable. Various stretchable strain sensors based on piezo-resistive and capacitive principles have been developed to meet all these requirements.
Conventional piezo-resistive strain sensors using functional nanomaterials, including CNTs as the most common example, have shown high sensitivity and great sensing performance. However, they suffer from poor long-term stability and linearity, as well as considerable signal hysteresis. As an alternative, piezo-capacitive strain sensors with better stability, lower hysteresis, and higher stretchability have been suggested. But due to the fact that piezo-capacitive strain sensors exhibit limited sensitivity and strong electromagnetic interference caused by the conductive objects in the surrounding environment, these conventional stretchable strain sensors are still facing limitations that are yet to be resolved.
A KAIST research team led by Professor Inkyu Park from the Department of Mechanical Engineering suggested that an optical-type stretchable strain sensor can be a good alternative to resolve the limitations of conventional piezo-resistive and piezo-capacitive strain sensors, because they have high stability and are less affected by environmental disturbances. The team then introduced an optical wearable strain sensor based on the light transmittance changes of a CNT-embedded elastomer, which further addresses the low sensitivity problem of conventional optical stretchable strain sensors.
In order to achieve a large dynamic range for the sensor, Professor Park and his researchers chose Ecoflex as an elastomeric substrate with good mechanical durability, flexibility, and attachability on human skin, and the new optical wearable strain sensor developed by the research group actually shows a wide dynamic range of 0 to 400%.
In addition, the researchers propagated the microcracks under tensile strain within the film of multi-walled CNTs embedded in the Ecoflex substrate, changing the optical transmittance of the film. By doing so, it was possible for them to develop a wearable strain sensor having a sensitivity 10 times higher than conventional optical stretchable strain sensors.
The proposed sensor has also passed the durability test with excellent results. The sensor’s response after 13,000 sets of cyclic loading was stable without any noticeable drift. This suggests that the sensor response can be used without degradation, even if the sensor is repeatedly used for a long time and in various environmental conditions.
Using the developed sensor, the research team could measure the finger bending motion and used it for robot control. They also developed a three-axes sensor array for body posture monitoring. The sensor was able to monitor human motions with small strains such as a pulse near the carotid artery and muscle movement around the mouth during pronunciation.
Professor Park said, “In this study, our group developed a new wearable strain sensor platform that overcomes many limitations of previously developed resistive, capacitive, and optical-type stretchable strain sensors. Our sensor could be widely used in a variety of fields including soft robotics, wearable electronics, electronic skin, healthcare, and even entertainment.”
This work was supported by the National Research Foundation (NRF) of Korea.
Publication:
Jimin Gu, Donguk Kwon, Junseong Ahn, and Inkyu Park. (2020) “Wearable Strain sensors Using Light Transmittance Change of Carbon Nanotube-Embedded Elastomers with Microcracks” ACS Applied Materials & Interfaces. Volume 12. Issue 9. Available online at https://doi.org/10.1021/acsami.9b18069
Profile:
Inkyu Park
Professor
inkyu@kaist.ac.kr
http://mintlab1.kaist.ac.kr
Micro/Nano Transducers Laboratory (MINT Lab)
Department of Mechanical Engineering (ME)Korea Advanced Institute of Science and Technology (KAIST)
Profile:
Jimin Gu
Ph.D. Candidate
mint9411@kaist.ac.kr
http://mintlab1.kaist.ac.kr
MINT Lab
KAIST ME
(END)
2020.03.20 View 23446
Wearable Strain Sensor Using Light Transmittance Helps Measure Physical Signals Better
KAIST researchers have developed a novel wearable strain sensor based on the modulation of optical transmittance of a carbon nanotube (CNT)-embedded elastomer. The sensor is capable of sensitive, stable, and continuous measurement of physical signals. This technology, featured in the March 4th issue of ACS Applied Materials & Interfaces as a front cover article, shows great potential for the detection of subtle human motions and the real-time monitoring of body postures for healthcare applications.
A wearable strain sensor must have high sensitivity, flexibility, and stretchability, as well as low cost. Those used especially for health monitoring should also be tied to long-term solid performance, and be environmentally stable. Various stretchable strain sensors based on piezo-resistive and capacitive principles have been developed to meet all these requirements.
Conventional piezo-resistive strain sensors using functional nanomaterials, including CNTs as the most common example, have shown high sensitivity and great sensing performance. However, they suffer from poor long-term stability and linearity, as well as considerable signal hysteresis. As an alternative, piezo-capacitive strain sensors with better stability, lower hysteresis, and higher stretchability have been suggested. But due to the fact that piezo-capacitive strain sensors exhibit limited sensitivity and strong electromagnetic interference caused by the conductive objects in the surrounding environment, these conventional stretchable strain sensors are still facing limitations that are yet to be resolved.
A KAIST research team led by Professor Inkyu Park from the Department of Mechanical Engineering suggested that an optical-type stretchable strain sensor can be a good alternative to resolve the limitations of conventional piezo-resistive and piezo-capacitive strain sensors, because they have high stability and are less affected by environmental disturbances. The team then introduced an optical wearable strain sensor based on the light transmittance changes of a CNT-embedded elastomer, which further addresses the low sensitivity problem of conventional optical stretchable strain sensors.
In order to achieve a large dynamic range for the sensor, Professor Park and his researchers chose Ecoflex as an elastomeric substrate with good mechanical durability, flexibility, and attachability on human skin, and the new optical wearable strain sensor developed by the research group actually shows a wide dynamic range of 0 to 400%.
In addition, the researchers propagated the microcracks under tensile strain within the film of multi-walled CNTs embedded in the Ecoflex substrate, changing the optical transmittance of the film. By doing so, it was possible for them to develop a wearable strain sensor having a sensitivity 10 times higher than conventional optical stretchable strain sensors.
The proposed sensor has also passed the durability test with excellent results. The sensor’s response after 13,000 sets of cyclic loading was stable without any noticeable drift. This suggests that the sensor response can be used without degradation, even if the sensor is repeatedly used for a long time and in various environmental conditions.
Using the developed sensor, the research team could measure the finger bending motion and used it for robot control. They also developed a three-axes sensor array for body posture monitoring. The sensor was able to monitor human motions with small strains such as a pulse near the carotid artery and muscle movement around the mouth during pronunciation.
Professor Park said, “In this study, our group developed a new wearable strain sensor platform that overcomes many limitations of previously developed resistive, capacitive, and optical-type stretchable strain sensors. Our sensor could be widely used in a variety of fields including soft robotics, wearable electronics, electronic skin, healthcare, and even entertainment.”
This work was supported by the National Research Foundation (NRF) of Korea.
Publication:
Jimin Gu, Donguk Kwon, Junseong Ahn, and Inkyu Park. (2020) “Wearable Strain sensors Using Light Transmittance Change of Carbon Nanotube-Embedded Elastomers with Microcracks” ACS Applied Materials & Interfaces. Volume 12. Issue 9. Available online at https://doi.org/10.1021/acsami.9b18069
Profile:
Inkyu Park
Professor
inkyu@kaist.ac.kr
http://mintlab1.kaist.ac.kr
Micro/Nano Transducers Laboratory (MINT Lab)
Department of Mechanical Engineering (ME)Korea Advanced Institute of Science and Technology (KAIST)
Profile:
Jimin Gu
Ph.D. Candidate
mint9411@kaist.ac.kr
http://mintlab1.kaist.ac.kr
MINT Lab
KAIST ME
(END)
2020.03.20 View 23446 -
 COVID-19 Update: All Undergrad Housing Closed
KAIST stepped up preventive measures against the outbreak of COVID-19 by closing all housing complexes for undergraduate students.
Provost Kwang-Hyung Lee, in an email to KAIST community members on March 12, advised all undergraduate students who had already moved in to the dormitories to move out by March 23. The university opened the spring semester on March 16, two weeks later than originally scheduled, due to the outbreak. All in-person classes have been shifted to online classes and this will continue until further notice.
“The dormitory would likely become the source of a COVID-19 cluster on the campus. Given the gravity of the current situation, we can’t help but make this unprecedented measure. It is fully for the best interests for our students’ health and safety. It saddens me to say that students are required to go back to their homes,” said Provost Lee. Dormitory fees will be refunded, and transportation and storage services will be provided for students who return back home. It has not yet been decided when they can return to the campus.
There are four exceptional cases for this special measure: 1. when a student does not have legal residency in Korea, 2. if a student’s legal residence is located in a severely affected region such as Daegu, Chongdo, and Kyongsan, 3. if students in their final semester before the graduation need to take a research class that is not available online, 4. if students have a very special reason that does not allow them to stay at home. Such students are required to meet the Associate Vice President of Student Life for approval of the exceptional stay.
Meanwhile, the first day of the online semester on March 16 saw an overwhelming amount of traffic on the remote educational platform, the KAIST learning management system (KLMS), and the real-time platform, Zoom. The two systems were both overloaded. The Dean of the KAIST Academy sent an email to the community, explaining the technical glitch causing the overload. He said his office had fixed the problem, allowing resumed access to the system from inside and outside the campus. Considered the nature of classes that are difficult or impossible to provide online, the university decided to cancel the some of physical training classes such as golf, dance sports, badminton, swimming, and tennis this semester.
Social distancing is another issue the university is enhancing throughout the campus. The university announced new lunch break shifts to disperse the dining hall crowds; the first shift is from 11:30 to 12:30 and the second shift is from 12:30 to 13:30, effective from March 17. The COVID-19 response bulletin also instructed KAIST community members to sit in a row, not face to face, when eating together with colleagues, and asked them to refrain from talking while eating. In addition, a total of 29 virus and fine duster filtering machines have been installed across the campus dining facilities.
The bulletin posted on March 13 restressed the importance of wearing a face mask in compact areas such as elevators and refrain the non-essential business or personal travel. Parents who need to take care of their children due to the closure of schools and day care centers are advised to work from home.
(END)
2020.03.16 View 10120
COVID-19 Update: All Undergrad Housing Closed
KAIST stepped up preventive measures against the outbreak of COVID-19 by closing all housing complexes for undergraduate students.
Provost Kwang-Hyung Lee, in an email to KAIST community members on March 12, advised all undergraduate students who had already moved in to the dormitories to move out by March 23. The university opened the spring semester on March 16, two weeks later than originally scheduled, due to the outbreak. All in-person classes have been shifted to online classes and this will continue until further notice.
“The dormitory would likely become the source of a COVID-19 cluster on the campus. Given the gravity of the current situation, we can’t help but make this unprecedented measure. It is fully for the best interests for our students’ health and safety. It saddens me to say that students are required to go back to their homes,” said Provost Lee. Dormitory fees will be refunded, and transportation and storage services will be provided for students who return back home. It has not yet been decided when they can return to the campus.
There are four exceptional cases for this special measure: 1. when a student does not have legal residency in Korea, 2. if a student’s legal residence is located in a severely affected region such as Daegu, Chongdo, and Kyongsan, 3. if students in their final semester before the graduation need to take a research class that is not available online, 4. if students have a very special reason that does not allow them to stay at home. Such students are required to meet the Associate Vice President of Student Life for approval of the exceptional stay.
Meanwhile, the first day of the online semester on March 16 saw an overwhelming amount of traffic on the remote educational platform, the KAIST learning management system (KLMS), and the real-time platform, Zoom. The two systems were both overloaded. The Dean of the KAIST Academy sent an email to the community, explaining the technical glitch causing the overload. He said his office had fixed the problem, allowing resumed access to the system from inside and outside the campus. Considered the nature of classes that are difficult or impossible to provide online, the university decided to cancel the some of physical training classes such as golf, dance sports, badminton, swimming, and tennis this semester.
Social distancing is another issue the university is enhancing throughout the campus. The university announced new lunch break shifts to disperse the dining hall crowds; the first shift is from 11:30 to 12:30 and the second shift is from 12:30 to 13:30, effective from March 17. The COVID-19 response bulletin also instructed KAIST community members to sit in a row, not face to face, when eating together with colleagues, and asked them to refrain from talking while eating. In addition, a total of 29 virus and fine duster filtering machines have been installed across the campus dining facilities.
The bulletin posted on March 13 restressed the importance of wearing a face mask in compact areas such as elevators and refrain the non-essential business or personal travel. Parents who need to take care of their children due to the closure of schools and day care centers are advised to work from home.
(END)
2020.03.16 View 10120 -
 3D Hierarchically Porous Nanostructured Catalyst Helps Efficiently Reduce CO2
- This new catalyst will bring CO2 one step closer to serving as a sustainable energy source. -
KAIST researchers developed a three-dimensional (3D) hierarchically porous nanostructured catalyst with carbon dioxide (CO2) to carbon monoxide (CO) conversion rate up to 3.96 times higher than that of conventional nanoporous gold catalysts. This new catalyst helps overcome the existing limitations of the mass transport that has been a major cause of decreases in the CO2 conversion rate, holding a strong promise for the large-scale and cost-effective electrochemical conversion of CO2 into useful chemicals.
As CO2 emissions increase and fossil fuels deplete globally, reducing and converting CO2 to clean energy electrochemically has attracted a great deal of attention as a promising technology. Especially due to the fact that the CO2 reduction reaction occurs competitively with hydrogen evolution reactions (HER) at similar redox potentials, the development of an efficient electrocatalyst for selective and robust CO2 reduction reactions has remained a key technological issue.
Gold (Au) is one of the most commonly used catalysts in CO2 reduction reactions, but the high cost and scarcity of Au pose obstacles for mass commercial applications. The development of nanostructures has been extensively studied as a potential approach to improving the selectivity for target products and maximizing the number of active stable sites, thus enhancing the energy efficiency.
However, the nanopores of the previously reported complex nanostructures were easily blocked by gaseous CO bubbles during aqueous reactions. The CO bubbles hindered mass transport of the reactants through the electrolyte, resulting in low CO2 conversion rates.
In the study published in the Proceedings of the National Academy of Sciences of the USA (PNAS) on March 4, a research group at KAIST led by Professor Seokwoo Jeon and Professor Jihun Oh from the Department of Materials Science and Engineering designed a 3D hierarchically porous Au nanostructure with two different sizes of macropores and nanopores. The team used proximity-field nanopatterning (PnP) and electroplating techniques that are effective for fabricating the 3D well-ordered nanostructures.
The proposed nanostructure, comprised of interconnected macroporous channels 200 to 300 nanometers (nm) wide and 10 nm nanopores, induces efficient mass transport through the interconnected macroporous channels as well as high selectivity by producing highly active stable sites from numerous nanopores.
As a result, its electrodes show a high CO selectivity of 85.8% at a low overpotential of 0.264 V and efficient mass activity that is up to 3.96 times higher than that of de-alloyed nanoporous Au electrodes.
“These results are expected to solve the problem of mass transfer in the field of similar electrochemical reactions and can be applied to a wide range of green energy applications for the efficient utilization of electrocatalysts,” said the researchers.
This work was supported by the National Research Foundation (NRF) of Korea.
Image credit: Professor Seokwoo Jeon and Professor Jihun Oh, KAIST
Image usage restrictions: News organizations may use or redistribute this image, with proper attribution, as part of news coverage of this paper only.
Publication:
Hyun et al. (2020) Hierarchically porous Au nanostructures with interconnected channels for efficient mass transport in electrocatalytic CO2 reduction. Proceedings of the National Academy of Sciences of the USA (PNAS). Available online at https://doi.org/10.1073/pnas.1918837117
Profile:
Seokwoo Jeon, PhD
Professor
jeon39@kaist.ac.kr
http://fdml.kaist.ac.kr
Department of Materials Science and Engineering (MSE)
https://www.kaist.ac.kr
Korea Advanced Institute of Science and Technology (KAIST)Daejeon, Republic of Korea
Profile:
Jihun Oh, PhD
Associate Professor
jihun.oh@kaist.ac.kr
http://les.kaist.ac.kr
Department of Materials Science and Engineering (MSE)
Department of Energy, Environment, Water and Sustainability (EEWS)
KAIST
Profile:
Gayea Hyun
PhD Candidate
cldywkd93@kaist.ac.kr
http://fdml.kaist.ac.kr
Flexible Devices and Metamaterials Laboratory (FDML)
Department of Materials Science and Engineering (MSE)
KAIST
Profile:
Jun Tae Song, PhD
Assistant Professor
song.juntae@cstf.kyushu-u.ac.jp
http://www.cstf.kyushu-u.ac.jp/~ishihara-lab/
Department of Applied Chemistry
https://www.kyushu-u.ac.jp
Kyushu UniversityFukuoka, Japan
(END)
2020.03.13 View 21001
3D Hierarchically Porous Nanostructured Catalyst Helps Efficiently Reduce CO2
- This new catalyst will bring CO2 one step closer to serving as a sustainable energy source. -
KAIST researchers developed a three-dimensional (3D) hierarchically porous nanostructured catalyst with carbon dioxide (CO2) to carbon monoxide (CO) conversion rate up to 3.96 times higher than that of conventional nanoporous gold catalysts. This new catalyst helps overcome the existing limitations of the mass transport that has been a major cause of decreases in the CO2 conversion rate, holding a strong promise for the large-scale and cost-effective electrochemical conversion of CO2 into useful chemicals.
As CO2 emissions increase and fossil fuels deplete globally, reducing and converting CO2 to clean energy electrochemically has attracted a great deal of attention as a promising technology. Especially due to the fact that the CO2 reduction reaction occurs competitively with hydrogen evolution reactions (HER) at similar redox potentials, the development of an efficient electrocatalyst for selective and robust CO2 reduction reactions has remained a key technological issue.
Gold (Au) is one of the most commonly used catalysts in CO2 reduction reactions, but the high cost and scarcity of Au pose obstacles for mass commercial applications. The development of nanostructures has been extensively studied as a potential approach to improving the selectivity for target products and maximizing the number of active stable sites, thus enhancing the energy efficiency.
However, the nanopores of the previously reported complex nanostructures were easily blocked by gaseous CO bubbles during aqueous reactions. The CO bubbles hindered mass transport of the reactants through the electrolyte, resulting in low CO2 conversion rates.
In the study published in the Proceedings of the National Academy of Sciences of the USA (PNAS) on March 4, a research group at KAIST led by Professor Seokwoo Jeon and Professor Jihun Oh from the Department of Materials Science and Engineering designed a 3D hierarchically porous Au nanostructure with two different sizes of macropores and nanopores. The team used proximity-field nanopatterning (PnP) and electroplating techniques that are effective for fabricating the 3D well-ordered nanostructures.
The proposed nanostructure, comprised of interconnected macroporous channels 200 to 300 nanometers (nm) wide and 10 nm nanopores, induces efficient mass transport through the interconnected macroporous channels as well as high selectivity by producing highly active stable sites from numerous nanopores.
As a result, its electrodes show a high CO selectivity of 85.8% at a low overpotential of 0.264 V and efficient mass activity that is up to 3.96 times higher than that of de-alloyed nanoporous Au electrodes.
“These results are expected to solve the problem of mass transfer in the field of similar electrochemical reactions and can be applied to a wide range of green energy applications for the efficient utilization of electrocatalysts,” said the researchers.
This work was supported by the National Research Foundation (NRF) of Korea.
Image credit: Professor Seokwoo Jeon and Professor Jihun Oh, KAIST
Image usage restrictions: News organizations may use or redistribute this image, with proper attribution, as part of news coverage of this paper only.
Publication:
Hyun et al. (2020) Hierarchically porous Au nanostructures with interconnected channels for efficient mass transport in electrocatalytic CO2 reduction. Proceedings of the National Academy of Sciences of the USA (PNAS). Available online at https://doi.org/10.1073/pnas.1918837117
Profile:
Seokwoo Jeon, PhD
Professor
jeon39@kaist.ac.kr
http://fdml.kaist.ac.kr
Department of Materials Science and Engineering (MSE)
https://www.kaist.ac.kr
Korea Advanced Institute of Science and Technology (KAIST)Daejeon, Republic of Korea
Profile:
Jihun Oh, PhD
Associate Professor
jihun.oh@kaist.ac.kr
http://les.kaist.ac.kr
Department of Materials Science and Engineering (MSE)
Department of Energy, Environment, Water and Sustainability (EEWS)
KAIST
Profile:
Gayea Hyun
PhD Candidate
cldywkd93@kaist.ac.kr
http://fdml.kaist.ac.kr
Flexible Devices and Metamaterials Laboratory (FDML)
Department of Materials Science and Engineering (MSE)
KAIST
Profile:
Jun Tae Song, PhD
Assistant Professor
song.juntae@cstf.kyushu-u.ac.jp
http://www.cstf.kyushu-u.ac.jp/~ishihara-lab/
Department of Applied Chemistry
https://www.kyushu-u.ac.jp
Kyushu UniversityFukuoka, Japan
(END)
2020.03.13 View 21001