bio
-
 KAIST develops technology for selective RNA modification in living cells and animals
· A team led by Professor Won Do Heo from the Department of Biological Sciences, KAIST, has developed a pioneering technology that selectively acetylates specific RNA molecules in living cells and tissues.
· The platform uses RNA-targeting CRISPR tools in combination with RNA-modifying enzymes to chemically modify only the intended RNA.
· The method opens new possibilities for gene therapy by enabling precise control of disease-related RNA without affecting the rest of the transcriptome.
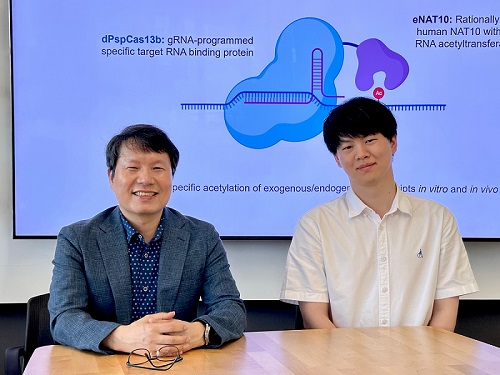
< Photo 1. (From left) Professor Won Do Heo and Jihwan Yu, a Ph.D. Candidate of the Department of Biological Sciences >
CRISPR-Cas13, a powerful RNA-targeting technology is gaining increasing attention as a next-generation gene therapy platform due to its precision and reduced side effects. Utilizing this system, researchers at KAIST have now developed the world’s first technology capable of selectively acetylating (chemically modifying) specific RNA molecules among countless transcripts within living cells. This breakthrough enables precise, programmable control of RNA function and is expected to open new avenues in RNA-based therapeutic development.
KAIST (President Kwang Hyung Lee) announced that a research team led by Professor Won Do Heo in the Department of Biological Sciences has recently developed a groundbreaking technology capable of selectively acetylating specific RNA molecules within the human body using the CRISPR-Cas13 system—an RNA-targeting platform gaining increasing attention in the fields of gene regulation and RNA-based therapeutics.
RNA molecules can undergo chemical modifications—the addition of specific chemical groups—which alter their function and behavior without changing the underlying nucleotide sequence. However, some of these modifications, a critical layer of post-transcriptional gene regulation, remain poorly understood. Among them, N4-acetylcytidine (ac4C) has been particularly enigmatic, with ongoing debate about its existence and function in human messenger RNA (mRNA), the RNA that encodes proteins.
To address this gap, the KAIST research team developed a targeted RNA acetylation system, named dCas13-eNAT10. This platform combines a catalytically inactive Cas13 enzyme (dCas13) that guides the system to specific RNA targets, with a hyperactive variant of the NAT10 enzyme (eNAT10), which performs RNA acetylation. This approach enables precise acetylation of only the desired RNA molecules among the vast pool of transcripts within the cell.
< Figure 1. Development of hyperactive variant eNAT10 through NAT10 protein engineering. By engineering the NAT10 protein, which performs RNA acetylation in human cells, based on its domain and structure, eNAT10 was developed, showing approximately a 3-fold increase in RNA acetylation activity compared to the wild-type enzyme. >
Using this system, the researchers demonstrated that guide RNAs could direct the dCas13-eNAT10 complex to acetylate specific RNA targets, and acetylation significantly increased protein expression from the modified mRNA. Moreover, the study revealed, for the first time, that RNA acetylation plays a role in intracellular RNA localization, facilitating the export of RNA from the nucleus to the cytoplasm—a critical step in gene expression regulation.
To validate its therapeutic potential, the team successfully delivered the targeted RNA acetylation system into the livers of live mice using adeno-associated virus (AAV), a commonly used gene therapy vector. This marks the first demonstration of in vivo RNA modification, extending the applicability of RNA chemical modification tools from cell culture models to living organisms.
< Figure 2. Acetylation of various RNA in cells using dCas13-eNAT10 fusion protein. Utilizing the CRISPR-Cas13 system, which can precisely target specific RNA through guide RNA, a dCas13-eNAT10 fusion protein was created, demonstrating its ability to specifically acetylate various endogenous RNA at different locations within cells. >
Professor Won Do Heo, who previously developed COVID-19 treatment technology using RNA gene scissors and technology to activate RNA gene scissors with light, stated, "Existing RNA chemical modification research faced difficulties in controlling specificity, temporality, and spatiality. However, this new technology allows selective acetylation of desired RNA, opening the door for accurate and detailed research into the functions of RNA acetylation." He added, "The RNA chemical modification technology developed in this study can be widely used as an RNA-based therapeutic agent and a tool for regulating RNA functions in living organisms in the future."
< Figure 3. In vivo delivery of targeted RNA acetylation system. The targeted RNA acetylation system was encoded in an AAV vector, commonly used in gene therapy, and delivered intravenously to adult mice, showing that target RNA in liver tissue was specifically acetylated according to the guide RNA. >
This research, with Ph.D. candidate Jihwan Yu from the Department of Biological Sciences at KAIST as the first author, was published in the journal Nature Chemical Biology on June 2, 2025. (Title: Programmable RNA acetylation with CRISPR-Cas13, Impact factor: 12.9, DOI: https://doi.org/10.1038/s41589-025-01922-3)
This research was supported by the Samsung Future Technology Foundation and the Bio & Medical Technology Development Program of the National Research Foundation of Korea.
2025.06.10 View 970
KAIST develops technology for selective RNA modification in living cells and animals
· A team led by Professor Won Do Heo from the Department of Biological Sciences, KAIST, has developed a pioneering technology that selectively acetylates specific RNA molecules in living cells and tissues.
· The platform uses RNA-targeting CRISPR tools in combination with RNA-modifying enzymes to chemically modify only the intended RNA.
· The method opens new possibilities for gene therapy by enabling precise control of disease-related RNA without affecting the rest of the transcriptome.
< Photo 1. (From left) Professor Won Do Heo and Jihwan Yu, a Ph.D. Candidate of the Department of Biological Sciences >
CRISPR-Cas13, a powerful RNA-targeting technology is gaining increasing attention as a next-generation gene therapy platform due to its precision and reduced side effects. Utilizing this system, researchers at KAIST have now developed the world’s first technology capable of selectively acetylating (chemically modifying) specific RNA molecules among countless transcripts within living cells. This breakthrough enables precise, programmable control of RNA function and is expected to open new avenues in RNA-based therapeutic development.
KAIST (President Kwang Hyung Lee) announced that a research team led by Professor Won Do Heo in the Department of Biological Sciences has recently developed a groundbreaking technology capable of selectively acetylating specific RNA molecules within the human body using the CRISPR-Cas13 system—an RNA-targeting platform gaining increasing attention in the fields of gene regulation and RNA-based therapeutics.
RNA molecules can undergo chemical modifications—the addition of specific chemical groups—which alter their function and behavior without changing the underlying nucleotide sequence. However, some of these modifications, a critical layer of post-transcriptional gene regulation, remain poorly understood. Among them, N4-acetylcytidine (ac4C) has been particularly enigmatic, with ongoing debate about its existence and function in human messenger RNA (mRNA), the RNA that encodes proteins.
To address this gap, the KAIST research team developed a targeted RNA acetylation system, named dCas13-eNAT10. This platform combines a catalytically inactive Cas13 enzyme (dCas13) that guides the system to specific RNA targets, with a hyperactive variant of the NAT10 enzyme (eNAT10), which performs RNA acetylation. This approach enables precise acetylation of only the desired RNA molecules among the vast pool of transcripts within the cell.
< Figure 1. Development of hyperactive variant eNAT10 through NAT10 protein engineering. By engineering the NAT10 protein, which performs RNA acetylation in human cells, based on its domain and structure, eNAT10 was developed, showing approximately a 3-fold increase in RNA acetylation activity compared to the wild-type enzyme. >
Using this system, the researchers demonstrated that guide RNAs could direct the dCas13-eNAT10 complex to acetylate specific RNA targets, and acetylation significantly increased protein expression from the modified mRNA. Moreover, the study revealed, for the first time, that RNA acetylation plays a role in intracellular RNA localization, facilitating the export of RNA from the nucleus to the cytoplasm—a critical step in gene expression regulation.
To validate its therapeutic potential, the team successfully delivered the targeted RNA acetylation system into the livers of live mice using adeno-associated virus (AAV), a commonly used gene therapy vector. This marks the first demonstration of in vivo RNA modification, extending the applicability of RNA chemical modification tools from cell culture models to living organisms.
< Figure 2. Acetylation of various RNA in cells using dCas13-eNAT10 fusion protein. Utilizing the CRISPR-Cas13 system, which can precisely target specific RNA through guide RNA, a dCas13-eNAT10 fusion protein was created, demonstrating its ability to specifically acetylate various endogenous RNA at different locations within cells. >
Professor Won Do Heo, who previously developed COVID-19 treatment technology using RNA gene scissors and technology to activate RNA gene scissors with light, stated, "Existing RNA chemical modification research faced difficulties in controlling specificity, temporality, and spatiality. However, this new technology allows selective acetylation of desired RNA, opening the door for accurate and detailed research into the functions of RNA acetylation." He added, "The RNA chemical modification technology developed in this study can be widely used as an RNA-based therapeutic agent and a tool for regulating RNA functions in living organisms in the future."
< Figure 3. In vivo delivery of targeted RNA acetylation system. The targeted RNA acetylation system was encoded in an AAV vector, commonly used in gene therapy, and delivered intravenously to adult mice, showing that target RNA in liver tissue was specifically acetylated according to the guide RNA. >
This research, with Ph.D. candidate Jihwan Yu from the Department of Biological Sciences at KAIST as the first author, was published in the journal Nature Chemical Biology on June 2, 2025. (Title: Programmable RNA acetylation with CRISPR-Cas13, Impact factor: 12.9, DOI: https://doi.org/10.1038/s41589-025-01922-3)
This research was supported by the Samsung Future Technology Foundation and the Bio & Medical Technology Development Program of the National Research Foundation of Korea.
2025.06.10 View 970 -
 A 10-Month Journey of Tiny Flaps Completed: A Special Family Returns to KAIST Duck Pond
On the morning of June 9, 2025, gentle activity stirred early around the KAIST campus duck pond. It was the day a special family of ducks—and two goslings—were to be released back into the pond after spending a month in a temporary shelter. One by one, the ducklings cautiously emerged from their box, waddling toward the water's edge and scanning their surroundings, followed closely by their mother.
< The landscape manager from the KAIST Facilities Team releases the ducks and goslings. >
The mother duck, once a rescued loner who couldn’t integrate with the flock, returned triumphantly as the head of a new family—caring for both ducklings and goslings. Students and faculty looked on quietly, welcoming them back and reflecting on their remarkable 10-month journey.
The story began in July 2024, as a student filed a report of spotting two ducklings wandering near the pond without a mother. Based on their soft down, flat beaks, and lack of fear around humans, it was presumed they had been abandoned. Professor Won Do Heo of the Department of Biological Sciences—affectionately known as the “Goose Dad”—and the KAIST Facilities Team quickly stepped in to rescue them. After about a month of care, the ducklings were released back into the pond.
< On June 9, the day of the release, KAIST President Kwang-Hyung Lee (left), the former “Goose Dad,” and Professor Won Do Heo (right), the current “Goose Dad,” watched the flock as they freely wobbled about. >
At first, the ducklings seemed to adapt, but they started distancing themselves from the established goose flock. One eventually disappeared, and the remaining duckling was found injured by the pond during winter. Although KAIST typically avoids making human interference in the natural ecosystem, an exception was made to save the young duck’s life. It was put under the care of Professor Heo and the Facilities Team to regain its health within a month.
In the spring, the healed duck began laying eggs. Professor Heo supported the process by adjusting its diet, avoiding further intervention. On Children’s Day, May 5, the duck’s eggs hatched. The once-isolated duck had become a mother. Ten days later, on May 15, four goslings also hatched from the resident goose flock. With new life flourishing, the pond was more vibrant than ever.
< Rescued baby goslings near the pond, alongside the duck family that took them in. The mother duck—once a vulnerable duckling herself—had grown strong enough to care for others in need. >
But just days later, the mother goose disappeared, and two goslings—still unable to swim—were found shivering by the pond. Dahyeon Byeon, a student from Seoul National University who came for a visit on that day, reported this upon sighting, prompting another rescue. The vulnerable goslings were brought to the shelter to stay with the duck family.
Initially, the interspecies cohabitation was uneasy. But the mother duck did not reject the goslings. Slowly, they began to eat and sleep together, forming a new kind of family. After a month, they were released together into the pond—and to everyone’s surprise, the existing goose flock accepted both the goslings and the duck family.
< A peaceful moment for the duck family. The baby goslings naturally followed the mother duck. >
It took ten months for this family to return. From abandonment and injury to healing, birth, and unexpected bonds, this was more than a story of survival. It was a journey of transformation. The duck family’s ten-month saga is a quiet miracle—written in small moments of crisis, care, and connection—and a lasting memory on the KAIST campus.
< The resident goose flock at KAIST’s pond naturally accepted the returning duck and goslings as part of their group. >
2025.06.10 View 1070
A 10-Month Journey of Tiny Flaps Completed: A Special Family Returns to KAIST Duck Pond
On the morning of June 9, 2025, gentle activity stirred early around the KAIST campus duck pond. It was the day a special family of ducks—and two goslings—were to be released back into the pond after spending a month in a temporary shelter. One by one, the ducklings cautiously emerged from their box, waddling toward the water's edge and scanning their surroundings, followed closely by their mother.
< The landscape manager from the KAIST Facilities Team releases the ducks and goslings. >
The mother duck, once a rescued loner who couldn’t integrate with the flock, returned triumphantly as the head of a new family—caring for both ducklings and goslings. Students and faculty looked on quietly, welcoming them back and reflecting on their remarkable 10-month journey.
The story began in July 2024, as a student filed a report of spotting two ducklings wandering near the pond without a mother. Based on their soft down, flat beaks, and lack of fear around humans, it was presumed they had been abandoned. Professor Won Do Heo of the Department of Biological Sciences—affectionately known as the “Goose Dad”—and the KAIST Facilities Team quickly stepped in to rescue them. After about a month of care, the ducklings were released back into the pond.
< On June 9, the day of the release, KAIST President Kwang-Hyung Lee (left), the former “Goose Dad,” and Professor Won Do Heo (right), the current “Goose Dad,” watched the flock as they freely wobbled about. >
At first, the ducklings seemed to adapt, but they started distancing themselves from the established goose flock. One eventually disappeared, and the remaining duckling was found injured by the pond during winter. Although KAIST typically avoids making human interference in the natural ecosystem, an exception was made to save the young duck’s life. It was put under the care of Professor Heo and the Facilities Team to regain its health within a month.
In the spring, the healed duck began laying eggs. Professor Heo supported the process by adjusting its diet, avoiding further intervention. On Children’s Day, May 5, the duck’s eggs hatched. The once-isolated duck had become a mother. Ten days later, on May 15, four goslings also hatched from the resident goose flock. With new life flourishing, the pond was more vibrant than ever.
< Rescued baby goslings near the pond, alongside the duck family that took them in. The mother duck—once a vulnerable duckling herself—had grown strong enough to care for others in need. >
But just days later, the mother goose disappeared, and two goslings—still unable to swim—were found shivering by the pond. Dahyeon Byeon, a student from Seoul National University who came for a visit on that day, reported this upon sighting, prompting another rescue. The vulnerable goslings were brought to the shelter to stay with the duck family.
Initially, the interspecies cohabitation was uneasy. But the mother duck did not reject the goslings. Slowly, they began to eat and sleep together, forming a new kind of family. After a month, they were released together into the pond—and to everyone’s surprise, the existing goose flock accepted both the goslings and the duck family.
< A peaceful moment for the duck family. The baby goslings naturally followed the mother duck. >
It took ten months for this family to return. From abandonment and injury to healing, birth, and unexpected bonds, this was more than a story of survival. It was a journey of transformation. The duck family’s ten-month saga is a quiet miracle—written in small moments of crisis, care, and connection—and a lasting memory on the KAIST campus.
< The resident goose flock at KAIST’s pond naturally accepted the returning duck and goslings as part of their group. >
2025.06.10 View 1070 -
 KAIST Research Team Develops Electronic Ink for Room-Temperature Printing of High-Resolution, Variable-Stiffness Electronics
A team of researchers from KAIST and Seoul National University has developed a groundbreaking electronic ink that enables room-temperature printing of variable-stiffness circuits capable of switching between rigid and soft modes. This advancement marks a significant leap toward next-generation wearable, implantable, and robotic devices.
< Photo 1. (From left) Professor Jae-Woong Jeong and PhD candidate Simok Lee of the School of Electrical Engineering, (in separate bubbles, from left) Professor Gun-Hee Lee of Pusan National University, Professor Seongjun Park of Seoul National University, Professor Steve Park of the Department of Materials Science and Engineering>
Variable-stiffness electronics are at the forefront of adaptive technology, offering the ability for a single device to transition between rigid and soft modes depending on its use case. Gallium, a metal known for its high rigidity contrast between solid and liquid states, is a promising candidate for such applications. However, its use has been hindered by challenges including high surface tension, low viscosity, and undesirable phase transitions during manufacturing.
On June 4th, a research team led by Professor Jae-Woong Jeong from the School of Electrical Engineering at KAIST, Professor Seongjun Park from the Digital Healthcare Major at Seoul National University, and Professor Steve Park from the Department of Materials Science and Engineering at KAIST introduced a novel liquid metal electronic ink. This ink allows for micro-scale circuit printing – thinner than a human hair – at room temperature, with the ability to reversibly switch between rigid and soft modes depending on temperature.
The new ink combines printable viscosity with excellent electrical conductivity, enabling the creation of complex, high-resolution multilayer circuits comparable to commercial printed circuit boards (PCBs). These circuits can dynamically change stiffness in response to temperature, presenting new opportunities for multifunctional electronics, medical technologies, and robotics.
Conventional electronics typically have fixed form factors – either rigid for durability or soft for wearability. Rigid devices like smartphones and laptops offer robust performance but are uncomfortable when worn, while soft electronics are more comfortable but lack precise handling. As demand grows for devices that can adapt their stiffness to context, variable-stiffness electronics are becoming increasingly important.
< Figure 1. Fabrication process of stable, high-viscosity electronic ink by dispersing micro-sized gallium particles in a polymer matrix (left). High-resolution large-area circuit printing process through pH-controlled chemical sintering (right). >
To address this challenge, the researchers focused on gallium, which melts just below body temperature. Solid gallium is quite stiff, while its liquid form is fluid and soft. Despite its potential, gallium’s use in electronic printing has been limited by its high surface tension and instability when melted.
To overcome these issues, the team developed a pH-controlled liquid metal ink printing process. By dispersing micro-sized gallium particles into a hydrophilic polyurethane matrix using a neutral solvent (dimethyl sulfoxide, or DMSO), they created a stable, high-viscosity ink suitable for precision printing. During post-print heating, the DMSO decomposes to form an acidic environment, which removes the oxide layer on the gallium particles. This triggers the particles to coalesce into electrically conductive networks with tunable mechanical properties.
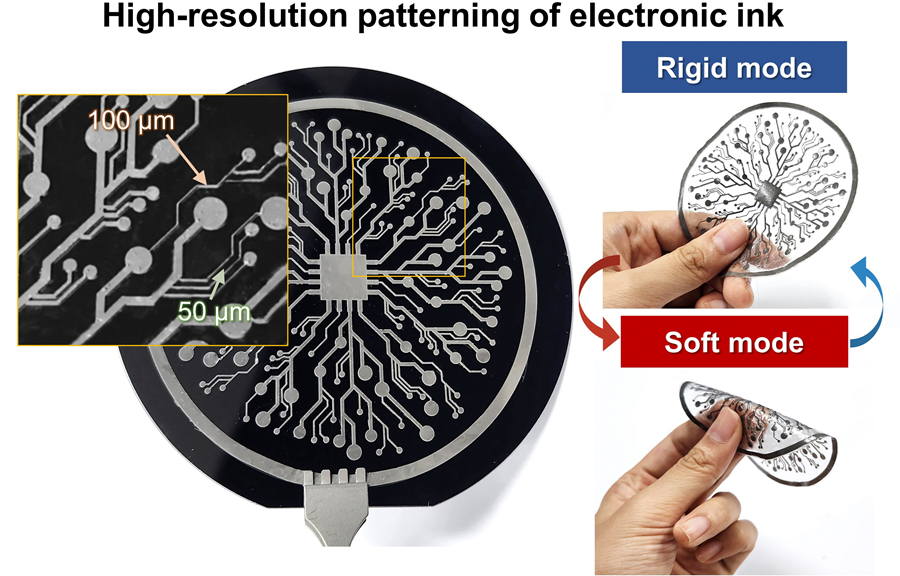
The resulting printed circuits exhibit fine feature sizes (~50 μm), high conductivity (2.27 × 10⁶ S/m), and a stiffness modulation ratio of up to 1,465 – allowing the material to shift from plastic-like rigidity to rubber-like softness. Furthermore, the ink is compatible with conventional printing techniques such as screen printing and dip coating, supporting large-area and 3D device fabrication.
< Figure 2. Key features of the electronic ink. (i) High-resolution printing and multilayer integration capability. (ii) Batch fabrication capability through large-area screen printing. (iii) Complex three-dimensional structure printing capability through dip coating. (iv) Excellent electrical conductivity and stiffness control capability.>
The team demonstrated this technology by developing a multi-functional device that operates as a rigid portable electronic under normal conditions but transforms into a soft wearable healthcare device when attached to the body. They also created a neural probe that remains stiff during surgical insertion for accurate positioning but softens once inside brain tissue to reduce inflammation – highlighting its potential for biomedical implants.
< Figure 3. Variable stiffness wearable electronics with high-resolution circuits and multilayer structure comparable to commercial printed circuit boards (PCBs). Functions as a rigid portable electronic device at room temperature, then transforms into a wearable healthcare device by softening at body temperature upon skin contact.>
“The core achievement of this research lies in overcoming the longstanding challenges of liquid metal printing through our innovative technology,” said Professor Jeong. “By controlling the ink’s acidity, we were able to electrically and mechanically connect printed gallium particles, enabling the room-temperature fabrication of high-resolution, large-area circuits with tunable stiffness. This opens up new possibilities for future personal electronics, medical devices, and robotics.”
< Figure 4. Body-temperature softening neural probe implemented by coating electronic ink on an optical waveguide structure. (Left) Remains rigid during surgery for precise manipulation and brain insertion, then softens after implantation to minimize mechanical stress on the brain and greatly enhance biocompatibility. (Right) >
This research was published in Science Advances under the title, “Phase-Change Metal Ink with pH-Controlled Chemical Sintering for Versatile and Scalable Fabrication of Variable Stiffness Electronics.” The work was supported by the National Research Foundation of Korea, the Boston-Korea Project, and the BK21 FOUR Program.
2025.06.04 View 1765
KAIST Research Team Develops Electronic Ink for Room-Temperature Printing of High-Resolution, Variable-Stiffness Electronics
A team of researchers from KAIST and Seoul National University has developed a groundbreaking electronic ink that enables room-temperature printing of variable-stiffness circuits capable of switching between rigid and soft modes. This advancement marks a significant leap toward next-generation wearable, implantable, and robotic devices.
< Photo 1. (From left) Professor Jae-Woong Jeong and PhD candidate Simok Lee of the School of Electrical Engineering, (in separate bubbles, from left) Professor Gun-Hee Lee of Pusan National University, Professor Seongjun Park of Seoul National University, Professor Steve Park of the Department of Materials Science and Engineering>
Variable-stiffness electronics are at the forefront of adaptive technology, offering the ability for a single device to transition between rigid and soft modes depending on its use case. Gallium, a metal known for its high rigidity contrast between solid and liquid states, is a promising candidate for such applications. However, its use has been hindered by challenges including high surface tension, low viscosity, and undesirable phase transitions during manufacturing.
On June 4th, a research team led by Professor Jae-Woong Jeong from the School of Electrical Engineering at KAIST, Professor Seongjun Park from the Digital Healthcare Major at Seoul National University, and Professor Steve Park from the Department of Materials Science and Engineering at KAIST introduced a novel liquid metal electronic ink. This ink allows for micro-scale circuit printing – thinner than a human hair – at room temperature, with the ability to reversibly switch between rigid and soft modes depending on temperature.
The new ink combines printable viscosity with excellent electrical conductivity, enabling the creation of complex, high-resolution multilayer circuits comparable to commercial printed circuit boards (PCBs). These circuits can dynamically change stiffness in response to temperature, presenting new opportunities for multifunctional electronics, medical technologies, and robotics.
Conventional electronics typically have fixed form factors – either rigid for durability or soft for wearability. Rigid devices like smartphones and laptops offer robust performance but are uncomfortable when worn, while soft electronics are more comfortable but lack precise handling. As demand grows for devices that can adapt their stiffness to context, variable-stiffness electronics are becoming increasingly important.
< Figure 1. Fabrication process of stable, high-viscosity electronic ink by dispersing micro-sized gallium particles in a polymer matrix (left). High-resolution large-area circuit printing process through pH-controlled chemical sintering (right). >
To address this challenge, the researchers focused on gallium, which melts just below body temperature. Solid gallium is quite stiff, while its liquid form is fluid and soft. Despite its potential, gallium’s use in electronic printing has been limited by its high surface tension and instability when melted.
To overcome these issues, the team developed a pH-controlled liquid metal ink printing process. By dispersing micro-sized gallium particles into a hydrophilic polyurethane matrix using a neutral solvent (dimethyl sulfoxide, or DMSO), they created a stable, high-viscosity ink suitable for precision printing. During post-print heating, the DMSO decomposes to form an acidic environment, which removes the oxide layer on the gallium particles. This triggers the particles to coalesce into electrically conductive networks with tunable mechanical properties.
The resulting printed circuits exhibit fine feature sizes (~50 μm), high conductivity (2.27 × 10⁶ S/m), and a stiffness modulation ratio of up to 1,465 – allowing the material to shift from plastic-like rigidity to rubber-like softness. Furthermore, the ink is compatible with conventional printing techniques such as screen printing and dip coating, supporting large-area and 3D device fabrication.
< Figure 2. Key features of the electronic ink. (i) High-resolution printing and multilayer integration capability. (ii) Batch fabrication capability through large-area screen printing. (iii) Complex three-dimensional structure printing capability through dip coating. (iv) Excellent electrical conductivity and stiffness control capability.>
The team demonstrated this technology by developing a multi-functional device that operates as a rigid portable electronic under normal conditions but transforms into a soft wearable healthcare device when attached to the body. They also created a neural probe that remains stiff during surgical insertion for accurate positioning but softens once inside brain tissue to reduce inflammation – highlighting its potential for biomedical implants.
< Figure 3. Variable stiffness wearable electronics with high-resolution circuits and multilayer structure comparable to commercial printed circuit boards (PCBs). Functions as a rigid portable electronic device at room temperature, then transforms into a wearable healthcare device by softening at body temperature upon skin contact.>
“The core achievement of this research lies in overcoming the longstanding challenges of liquid metal printing through our innovative technology,” said Professor Jeong. “By controlling the ink’s acidity, we were able to electrically and mechanically connect printed gallium particles, enabling the room-temperature fabrication of high-resolution, large-area circuits with tunable stiffness. This opens up new possibilities for future personal electronics, medical devices, and robotics.”
< Figure 4. Body-temperature softening neural probe implemented by coating electronic ink on an optical waveguide structure. (Left) Remains rigid during surgery for precise manipulation and brain insertion, then softens after implantation to minimize mechanical stress on the brain and greatly enhance biocompatibility. (Right) >
This research was published in Science Advances under the title, “Phase-Change Metal Ink with pH-Controlled Chemical Sintering for Versatile and Scalable Fabrication of Variable Stiffness Electronics.” The work was supported by the National Research Foundation of Korea, the Boston-Korea Project, and the BK21 FOUR Program.
2025.06.04 View 1765 -
 KAIST-UIUC researchers develop a treatment platform to disable the ‘biofilm’ shield of superbugs
< (From left) Ph.D. Candidate Joo Hun Lee (co-author), Professor Hyunjoon Kong (co-corresponding author) and Postdoctoral Researcher Yujin Ahn (co-first author) from the Department of Chemical and Biomolecular Engineering of the University of Illinois at Urbana-Champaign and Ju Yeon Chung (co-first author) from the Integrated Master's and Doctoral Program, and Professor Hyun Jung Chung (co-corresponding author) from the Department of Biological Sciences of KAIST >
A major cause of hospital-acquired infections, the super bacteria Methicillin-resistant Staphylococcus aureus (MRSA), not only exhibits strong resistance to existing antibiotics but also forms a dense biofilm that blocks the effects of external treatments. To meet this challenge, KAIST researchers, in collaboration with an international team, successfully developed a platform that utilizes microbubbles to deliver gene-targeted nanoparticles capable of break ing down the biofilms, offering an innovative solution for treating infections resistant to conventional antibiotics.
KAIST (represented by President Kwang Hyung Lee) announced on May 29 that a research team led by Professor Hyun Jung Chung from the Department of Biological Sciences, in collaboration with Professor Hyunjoon Kong's team at the University of Illinois, has developed a microbubble-based nano-gene delivery platform (BTN MB) that precisely delivers gene suppressors into bacteria to effectively remove biofilms formed by MRSA.
The research team first designed short DNA oligonucleotides that simultaneously suppress three major MRSA genes, related to—biofilm formation (icaA), cell division (ftsZ), and antibiotic resistance (mecA)—and engineered nanoparticles (BTN) to effectively deliver them into the bacteria.
< Figure 1. Effective biofilm treatment using biofilm-targeting nanoparticles controlled by microbubbler system. Schematic illustration of BTN delivery with microbubbles (MB), enabling effective permeation of ASOs targeting bacterial genes within biofilms infecting skin wounds. Gene silencing of targets involved in biofilm formation, bacterial proliferation, and antibiotic resistance leads to effective biofilm removal and antibacterial efficacy in vivo. >
In addition, microbubbles (MB) were used to increase the permeability of the microbial membrane, specifically the biofilm formed by MRSA. By combining these two technologies, the team implemented a dual-strike strategy that fundamentally blocks bacterial growth and prevents resistance acquisition.
This treatment system operates in two stages. First, the MBs induce pressure changes within the bacterial biofilm, allowing the BTNs to penetrate. Then, the BTNs slip through the gaps in the biofilm and enter the bacteria, delivering the gene suppressors precisely. This leads to gene regulation within MRSA, simultaneously blocking biofilm regeneration, cell proliferation, and antibiotic resistance expression.
In experiments conducted in a porcine skin model and a mouse wound model infected with MRSA biofilm, the BTN MB treatment group showed a significant reduction in biofilm thickness, as well as remarkable decreases in bacterial count and inflammatory responses.
< Figure 2. (a) Schematic illustration on the evaluation of treatment efficacy of BTN-MB gene therapy. (b) Reduction in MRSA biofilm mass via simultaneous inhibition of multiple genes. (c, d) Antibacterial efficacy of BTN-MB over time in a porcine skin infection biofilm model. (e) Schematic of the experimental setup to verify antibacterial efficacy in a mouse skin wound infection model. (f) Wound healing effects in mice. (g) Antibacterial effects at the wound site. (h) Histological analysis results. >
These results are difficult to achieve with conventional antibiotic monotherapy and demonstrate the potential for treating a wide range of resistant bacterial infections.
Professor Hyun Jung Chung of KAIST, who led the research, stated, “This study presents a new therapeutic solution that combines nanotechnology, gene suppression, and physical delivery strategies to address superbug infections that existing antibiotics cannot resolve. We will continue our research with the aim of expanding its application to systemic infections and various other infectious diseases.”
< (From left) Ju Yeon Chung from the Integrated Master's and Doctoral Program, and Professor Hyun Jung Chung from the Department of Biological Sciences >
The study was co-first authored by Ju Yeon Chung, a graduate student in the Department of Biological Sciences at KAIST, and Dr. Yujin Ahn from the University of Illinois. The study was published online on May 19 in the journal, Advanced Functional Materials.
※ Paper Title: Microbubble-Controlled Delivery of Biofilm-Targeting Nanoparticles to Treat MRSA Infection ※ DOI: https://doi.org/10.1002/adfm.202508291
This study was supported by the National Research Foundation and the Ministry of Health and Welfare, Republic of Korea; and the National Science Foundation and National Institutes of Health, USA.
2025.05.29 View 1490
KAIST-UIUC researchers develop a treatment platform to disable the ‘biofilm’ shield of superbugs
< (From left) Ph.D. Candidate Joo Hun Lee (co-author), Professor Hyunjoon Kong (co-corresponding author) and Postdoctoral Researcher Yujin Ahn (co-first author) from the Department of Chemical and Biomolecular Engineering of the University of Illinois at Urbana-Champaign and Ju Yeon Chung (co-first author) from the Integrated Master's and Doctoral Program, and Professor Hyun Jung Chung (co-corresponding author) from the Department of Biological Sciences of KAIST >
A major cause of hospital-acquired infections, the super bacteria Methicillin-resistant Staphylococcus aureus (MRSA), not only exhibits strong resistance to existing antibiotics but also forms a dense biofilm that blocks the effects of external treatments. To meet this challenge, KAIST researchers, in collaboration with an international team, successfully developed a platform that utilizes microbubbles to deliver gene-targeted nanoparticles capable of break ing down the biofilms, offering an innovative solution for treating infections resistant to conventional antibiotics.
KAIST (represented by President Kwang Hyung Lee) announced on May 29 that a research team led by Professor Hyun Jung Chung from the Department of Biological Sciences, in collaboration with Professor Hyunjoon Kong's team at the University of Illinois, has developed a microbubble-based nano-gene delivery platform (BTN MB) that precisely delivers gene suppressors into bacteria to effectively remove biofilms formed by MRSA.
The research team first designed short DNA oligonucleotides that simultaneously suppress three major MRSA genes, related to—biofilm formation (icaA), cell division (ftsZ), and antibiotic resistance (mecA)—and engineered nanoparticles (BTN) to effectively deliver them into the bacteria.
< Figure 1. Effective biofilm treatment using biofilm-targeting nanoparticles controlled by microbubbler system. Schematic illustration of BTN delivery with microbubbles (MB), enabling effective permeation of ASOs targeting bacterial genes within biofilms infecting skin wounds. Gene silencing of targets involved in biofilm formation, bacterial proliferation, and antibiotic resistance leads to effective biofilm removal and antibacterial efficacy in vivo. >
In addition, microbubbles (MB) were used to increase the permeability of the microbial membrane, specifically the biofilm formed by MRSA. By combining these two technologies, the team implemented a dual-strike strategy that fundamentally blocks bacterial growth and prevents resistance acquisition.
This treatment system operates in two stages. First, the MBs induce pressure changes within the bacterial biofilm, allowing the BTNs to penetrate. Then, the BTNs slip through the gaps in the biofilm and enter the bacteria, delivering the gene suppressors precisely. This leads to gene regulation within MRSA, simultaneously blocking biofilm regeneration, cell proliferation, and antibiotic resistance expression.
In experiments conducted in a porcine skin model and a mouse wound model infected with MRSA biofilm, the BTN MB treatment group showed a significant reduction in biofilm thickness, as well as remarkable decreases in bacterial count and inflammatory responses.
< Figure 2. (a) Schematic illustration on the evaluation of treatment efficacy of BTN-MB gene therapy. (b) Reduction in MRSA biofilm mass via simultaneous inhibition of multiple genes. (c, d) Antibacterial efficacy of BTN-MB over time in a porcine skin infection biofilm model. (e) Schematic of the experimental setup to verify antibacterial efficacy in a mouse skin wound infection model. (f) Wound healing effects in mice. (g) Antibacterial effects at the wound site. (h) Histological analysis results. >
These results are difficult to achieve with conventional antibiotic monotherapy and demonstrate the potential for treating a wide range of resistant bacterial infections.
Professor Hyun Jung Chung of KAIST, who led the research, stated, “This study presents a new therapeutic solution that combines nanotechnology, gene suppression, and physical delivery strategies to address superbug infections that existing antibiotics cannot resolve. We will continue our research with the aim of expanding its application to systemic infections and various other infectious diseases.”
< (From left) Ju Yeon Chung from the Integrated Master's and Doctoral Program, and Professor Hyun Jung Chung from the Department of Biological Sciences >
The study was co-first authored by Ju Yeon Chung, a graduate student in the Department of Biological Sciences at KAIST, and Dr. Yujin Ahn from the University of Illinois. The study was published online on May 19 in the journal, Advanced Functional Materials.
※ Paper Title: Microbubble-Controlled Delivery of Biofilm-Targeting Nanoparticles to Treat MRSA Infection ※ DOI: https://doi.org/10.1002/adfm.202508291
This study was supported by the National Research Foundation and the Ministry of Health and Welfare, Republic of Korea; and the National Science Foundation and National Institutes of Health, USA.
2025.05.29 View 1490 -
 KAIST Develops Virtual Staining Technology for 3D Histopathology
Moving beyond traditional methods of observing thinly sliced and stained cancer tissues, a collaborative international research team led by KAIST has successfully developed a groundbreaking technology. This innovation uses advanced optical techniques combined with an artificial intelligence-based deep learning algorithm to create realistic, virtually stained 3D images of cancer tissue without the need for serial sectioning nor staining. This breakthrough is anticipated to pave the way for next-generation non-invasive pathological diagnosis.
< Photo 1. (From left) Juyeon Park (Ph.D. Candidate, Department of Physics), Professor YongKeun Park (Department of Physics) (Top left) Professor Su-Jin Shin (Gangnam Severance Hospital), Professor Tae Hyun Hwang (Vanderbilt University School of Medicine) >
KAIST (President Kwang Hyung Lee) announced on the 26th that a research team led by Professor YongKeun Park of the Department of Physics, in collaboration with Professor Su-Jin Shin's team at Yonsei University Gangnam Severance Hospital, Professor Tae Hyun Hwang's team at Mayo Clinic, and Tomocube's AI research team, has developed an innovative technology capable of vividly displaying the 3D structure of cancer tissues without separate staining.
For over 200 years, conventional pathology has relied on observing cancer tissues under a microscope, a method that only shows specific cross-sections of the 3D cancer tissue. This has limited the ability to understand the three-dimensional connections and spatial arrangements between cells.
To overcome this, the research team utilized holotomography (HT), an advanced optical technology, to measure the 3D refractive index information of tissues. They then integrated an AI-based deep learning algorithm to successfully generate virtual H&E* images.* H&E (Hematoxylin & Eosin): The most widely used staining method for observing pathological tissues. Hematoxylin stains cell nuclei blue, and eosin stains cytoplasm pink.
The research team quantitatively demonstrated that the images generated by this technology are highly similar to actual stained tissue images. Furthermore, the technology exhibited consistent performance across various organs and tissues, proving its versatility and reliability as a next-generation pathological analysis tool.
< Figure 1. Comparison of conventional 3D tissue pathology procedure and the 3D virtual H&E staining technology proposed in this study. The traditional method requires preparing and staining dozens of tissue slides, while the proposed technology can reduce the number of slides by up to 10 times and quickly generate H&E images without the staining process. >
Moreover, by validating the feasibility of this technology through joint research with hospitals and research institutions in Korea and the United States, utilizing Tomocube's holotomography equipment, the team demonstrated its potential for full-scale adoption in real-world pathological research settings.
Professor YongKeun Park stated, "This research marks a major advancement by transitioning pathological analysis from conventional 2D methods to comprehensive 3D imaging. It will greatly enhance biomedical research and clinical diagnostics, particularly in understanding cancer tumor boundaries and the intricate spatial arrangements of cells within tumor microenvironments."
< Figure 2. Results of AI-based 3D virtual H&E staining and quantitative analysis of pathological tissue. The virtually stained images enabled 3D reconstruction of key pathological features such as cell nuclei and glandular lumens. Based on this, various quantitative indicators, including cell nuclear distribution, volume, and surface area, could be extracted. >
This research, with Juyeon Park, a student of the Integrated Master’s and Ph.D. Program at KAIST, as the first author, was published online in the prestigious journal Nature Communications on May 22.
(Paper title: Revealing 3D microanatomical structures of unlabeled thick cancer tissues using holotomography and virtual H&E staining.
[https://doi.org/10.1038/s41467-025-59820-0]
This study was supported by the Leader Researcher Program of the National Research Foundation of Korea, the Global Industry Technology Cooperation Center Project of the Korea Institute for Advancement of Technology, and the Korea Health Industry Development Institute.
2025.05.26 View 2424
KAIST Develops Virtual Staining Technology for 3D Histopathology
Moving beyond traditional methods of observing thinly sliced and stained cancer tissues, a collaborative international research team led by KAIST has successfully developed a groundbreaking technology. This innovation uses advanced optical techniques combined with an artificial intelligence-based deep learning algorithm to create realistic, virtually stained 3D images of cancer tissue without the need for serial sectioning nor staining. This breakthrough is anticipated to pave the way for next-generation non-invasive pathological diagnosis.
< Photo 1. (From left) Juyeon Park (Ph.D. Candidate, Department of Physics), Professor YongKeun Park (Department of Physics) (Top left) Professor Su-Jin Shin (Gangnam Severance Hospital), Professor Tae Hyun Hwang (Vanderbilt University School of Medicine) >
KAIST (President Kwang Hyung Lee) announced on the 26th that a research team led by Professor YongKeun Park of the Department of Physics, in collaboration with Professor Su-Jin Shin's team at Yonsei University Gangnam Severance Hospital, Professor Tae Hyun Hwang's team at Mayo Clinic, and Tomocube's AI research team, has developed an innovative technology capable of vividly displaying the 3D structure of cancer tissues without separate staining.
For over 200 years, conventional pathology has relied on observing cancer tissues under a microscope, a method that only shows specific cross-sections of the 3D cancer tissue. This has limited the ability to understand the three-dimensional connections and spatial arrangements between cells.
To overcome this, the research team utilized holotomography (HT), an advanced optical technology, to measure the 3D refractive index information of tissues. They then integrated an AI-based deep learning algorithm to successfully generate virtual H&E* images.* H&E (Hematoxylin & Eosin): The most widely used staining method for observing pathological tissues. Hematoxylin stains cell nuclei blue, and eosin stains cytoplasm pink.
The research team quantitatively demonstrated that the images generated by this technology are highly similar to actual stained tissue images. Furthermore, the technology exhibited consistent performance across various organs and tissues, proving its versatility and reliability as a next-generation pathological analysis tool.
< Figure 1. Comparison of conventional 3D tissue pathology procedure and the 3D virtual H&E staining technology proposed in this study. The traditional method requires preparing and staining dozens of tissue slides, while the proposed technology can reduce the number of slides by up to 10 times and quickly generate H&E images without the staining process. >
Moreover, by validating the feasibility of this technology through joint research with hospitals and research institutions in Korea and the United States, utilizing Tomocube's holotomography equipment, the team demonstrated its potential for full-scale adoption in real-world pathological research settings.
Professor YongKeun Park stated, "This research marks a major advancement by transitioning pathological analysis from conventional 2D methods to comprehensive 3D imaging. It will greatly enhance biomedical research and clinical diagnostics, particularly in understanding cancer tumor boundaries and the intricate spatial arrangements of cells within tumor microenvironments."
< Figure 2. Results of AI-based 3D virtual H&E staining and quantitative analysis of pathological tissue. The virtually stained images enabled 3D reconstruction of key pathological features such as cell nuclei and glandular lumens. Based on this, various quantitative indicators, including cell nuclear distribution, volume, and surface area, could be extracted. >
This research, with Juyeon Park, a student of the Integrated Master’s and Ph.D. Program at KAIST, as the first author, was published online in the prestigious journal Nature Communications on May 22.
(Paper title: Revealing 3D microanatomical structures of unlabeled thick cancer tissues using holotomography and virtual H&E staining.
[https://doi.org/10.1038/s41467-025-59820-0]
This study was supported by the Leader Researcher Program of the National Research Foundation of Korea, the Global Industry Technology Cooperation Center Project of the Korea Institute for Advancement of Technology, and the Korea Health Industry Development Institute.
2025.05.26 View 2424 -
 KAIST to Develop a Korean-style ChatGPT Platform Specifically Geared Toward Medical Diagnosis and Drug Discovery
On May 23rd, KAIST (President Kwang-Hyung Lee) announced that its Digital Bio-Health AI Research Center (Director: Professor JongChul Ye of KAIST Kim Jaechul Graduate School of AI) has been selected for the Ministry of Science and ICT's 'AI Top-Tier Young Researcher Support Program (AI Star Fellowship Project).' With a total investment of ₩11.5 billion from May 2025 to December 2030, the center will embark on the full-scale development of AI technology and a platform capable of independently inferring and determining the kinds of diseases, and discovering new drugs.
< Photo. On May 20th, a kick-off meeting for the AI Star Fellowship Project was held at KAIST Kim Jaechul Graduate School of AI’s Yangjae Research Center with the KAIST research team and participating organizations of Samsung Medical Center, NAVER Cloud, and HITS. [From left to right in the front row] Professor Jaegul Joo (KAIST), Professor Yoonjae Choi (KAIST), Professor Woo Youn Kim (KAIST/HITS), Professor JongChul Ye (KAIST), Professor Sungsoo Ahn (KAIST), Dr. Haanju Yoo (NAVER Cloud), Yoonho Lee (KAIST), HyeYoon Moon (Samsung Medical Center), Dr. Su Min Kim (Samsung Medical Center) >
This project aims to foster an innovative AI research ecosystem centered on young researchers and develop an inferential AI agent that can utilize and automatically expand specialized knowledge systems in the bio and medical fields.
Professor JongChul Ye of the Kim Jaechul Graduate School of AI will serve as the lead researcher, with young researchers from KAIST including Professors Yoonjae Choi, Kimin Lee, Sungsoo Ahn, and Chanyoung Park, along with mid-career researchers like Professors Jaegul Joo and Woo Youn Kim, jointly undertaking the project. They will collaborate with various laboratories within KAIST to conduct comprehensive research covering the entire cycle from the theoretical foundations of AI inference to its practical application.
Specifically, the main goals include: - Building high-performance inference models that integrate diverse medical knowledge systems to enhance the precision and reliability of diagnosis and treatment. - Developing a convergence inference platform that efficiently combines symbol-based inference with neural network models. - Securing AI technology for new drug development and biomarker discovery based on 'cell ontology.'
Furthermore, through close collaboration with industry and medical institutions such as Samsung Medical Center, NAVER Cloud, and HITS Co., Ltd., the project aims to achieve: - Clinical diagnostic AI utilizing medical knowledge systems. - AI-based molecular target exploration for new drug development. - Commercialization of an extendible AI inference platform.
Professor JongChul Ye, Director of KAIST's Digital Bio-Health AI Research Center, stated, "At a time when competition in AI inference model development is intensifying, it is a great honor for KAIST to lead the development of AI technology specialized in the bio and medical fields with world-class young researchers." He added, "We will do our best to ensure that the participating young researchers reach a world-leading level in terms of research achievements after the completion of this seven-year project starting in 2025."
The AI Star Fellowship is a newly established program where post-doctoral researchers and faculty members within seven years of appointment participate as project leaders (PLs) to independently lead research. Multiple laboratories within a university and demand-side companies form a consortium to operate the program.
Through this initiative, KAIST plans to nurture bio-medical convergence AI talent and simultaneously promote the commercialization of core technologies in collaboration with Samsung Medical Center, NAVER Cloud, and HITS.
2025.05.26 View 2892
KAIST to Develop a Korean-style ChatGPT Platform Specifically Geared Toward Medical Diagnosis and Drug Discovery
On May 23rd, KAIST (President Kwang-Hyung Lee) announced that its Digital Bio-Health AI Research Center (Director: Professor JongChul Ye of KAIST Kim Jaechul Graduate School of AI) has been selected for the Ministry of Science and ICT's 'AI Top-Tier Young Researcher Support Program (AI Star Fellowship Project).' With a total investment of ₩11.5 billion from May 2025 to December 2030, the center will embark on the full-scale development of AI technology and a platform capable of independently inferring and determining the kinds of diseases, and discovering new drugs.
< Photo. On May 20th, a kick-off meeting for the AI Star Fellowship Project was held at KAIST Kim Jaechul Graduate School of AI’s Yangjae Research Center with the KAIST research team and participating organizations of Samsung Medical Center, NAVER Cloud, and HITS. [From left to right in the front row] Professor Jaegul Joo (KAIST), Professor Yoonjae Choi (KAIST), Professor Woo Youn Kim (KAIST/HITS), Professor JongChul Ye (KAIST), Professor Sungsoo Ahn (KAIST), Dr. Haanju Yoo (NAVER Cloud), Yoonho Lee (KAIST), HyeYoon Moon (Samsung Medical Center), Dr. Su Min Kim (Samsung Medical Center) >
This project aims to foster an innovative AI research ecosystem centered on young researchers and develop an inferential AI agent that can utilize and automatically expand specialized knowledge systems in the bio and medical fields.
Professor JongChul Ye of the Kim Jaechul Graduate School of AI will serve as the lead researcher, with young researchers from KAIST including Professors Yoonjae Choi, Kimin Lee, Sungsoo Ahn, and Chanyoung Park, along with mid-career researchers like Professors Jaegul Joo and Woo Youn Kim, jointly undertaking the project. They will collaborate with various laboratories within KAIST to conduct comprehensive research covering the entire cycle from the theoretical foundations of AI inference to its practical application.
Specifically, the main goals include: - Building high-performance inference models that integrate diverse medical knowledge systems to enhance the precision and reliability of diagnosis and treatment. - Developing a convergence inference platform that efficiently combines symbol-based inference with neural network models. - Securing AI technology for new drug development and biomarker discovery based on 'cell ontology.'
Furthermore, through close collaboration with industry and medical institutions such as Samsung Medical Center, NAVER Cloud, and HITS Co., Ltd., the project aims to achieve: - Clinical diagnostic AI utilizing medical knowledge systems. - AI-based molecular target exploration for new drug development. - Commercialization of an extendible AI inference platform.
Professor JongChul Ye, Director of KAIST's Digital Bio-Health AI Research Center, stated, "At a time when competition in AI inference model development is intensifying, it is a great honor for KAIST to lead the development of AI technology specialized in the bio and medical fields with world-class young researchers." He added, "We will do our best to ensure that the participating young researchers reach a world-leading level in terms of research achievements after the completion of this seven-year project starting in 2025."
The AI Star Fellowship is a newly established program where post-doctoral researchers and faculty members within seven years of appointment participate as project leaders (PLs) to independently lead research. Multiple laboratories within a university and demand-side companies form a consortium to operate the program.
Through this initiative, KAIST plans to nurture bio-medical convergence AI talent and simultaneously promote the commercialization of core technologies in collaboration with Samsung Medical Center, NAVER Cloud, and HITS.
2025.05.26 View 2892 -
 Life Springs at KAIST: A Tale of Two Special Campus Families
A Gift of Life on Teachers' Day: Baby Geese Born at KAIST Pond
On Teachers' Day, a meaningful miracle of life arrived at the KAIST campus. A pair of geese gave birth to two goslings by the duck pond.
< On Teachers' Day, a pair of geese and their goslings leisurely swim in the pond. >
The baby goslings, covered in yellow down, began exploring the pond's edge, scurrying about, while their aunt geese steadfastly stood by. Their curious glances, watchful gazes, playful hops on waterside rocks, and the procession of babies swimming behind their parents in the water melted the hearts of onlookers.
< As night falls on the duck pond, the goose family gathers among the reeds. >
This special new life, born on Teachers' Day, seems to symbolize the day's meaning of "care" and "growth." This wondrous scene of life brought warm comfort and joy to KAIST members, adding the inspiration of nature to a campus that is a space for research and learning.
< Under the protection of the adult geese, the goslings take their first steps, exploring the pond's grassy areas and rocks. >
This adorable family is already roaming the area leisurely, like the pond's owners. With the joy of life added to the spring-filled pond, warm smiles are spreading across the KAIST campus.
< The geese look around, surveying their surroundings, while caring for their goslings. >
The pond has now become a small but special haven for students and staff. This goose family, arriving on Teachers' Day, quietly reminds us of the meaning of care and learning conveyed by nature.
< The goose family shows care and growth, and warm moments together are anticipated. >
---
On Children's Day 2025, a Duck Becomes a Mother
In July 2024, a special guest arrived at the KAIST campus. With soft yellow down, waddling gait, and a flat beak, it was undeniably a baby duck. However, for some reason, its mother was nowhere to be seen. Given that it wasn't afraid of people and followed them well, it was clear that someone had abandoned the duck.
Fortunately, the baby duck was safely rescued thanks to prompt reporting by students.
< Two ducks found on a corner of campus, immediately after their rescue in summer 2024. >
The ducks, newly integrated into KAIST, seemed to adapt relatively peacefully to campus life. As new additions, they couldn't blend in with the existing goose flock that had settled on campus, but the geese didn't ostracize them either. Perhaps because they were awkward neighbors, there was hope that the ducks would soon join the existing goose flock.
< Following their rescue based on a student's report in summer 2024, the ducks adapted to campus life under the protection of the campus facility team and Professor Won Do Heo. >
Professor Won Do Heo of the Department of Biological Sciences, widely known as "Goose Dad," stepped forward to protect them along with the KAIST facility team. Professor Heo is well-known for consistently observing and protecting the campus geese and ducks, which are practically symbols of KAIST. Thanks to the care of the staff and Professor Heo, the two ducks were safely released back onto campus approximately one month after their rescue.
< A moment on campus: Before winter, the ducks lived separately from the goose flock, maintaining a certain distance. While there were no conflicts, they rarely socialized. >
However, as winter passed, sad news arrived. One duck went missing, and the remaining one was found injured by the pond. While the policy of the facility team and Professor Heo was to minimize intervention to allow campus animals to maintain their natural state, saving the injured duck was the top priority. After being isolated again for a month of recovery, the duck fully recovered and was able to greet spring under the sun.
< The mother duck left alone in winter: One went missing, and the remaining one was found injured. After indoor isolation and recovery, she was released back onto campus in the spring. >
As spring, the ducks' breeding season, began, Professor Heo decided to offer a little more help. When signs of egg-laying appeared, he consistently provided "special meals for pregnant mothers" throughout March. On the morning of May 5th, Children's Day, 28 days after the mother duck began incubating her eggs with the care and attention of KAIST members, new life finally hatched. It was a precious outcome achieved solely by the duck that had survived abandonment and injury, with no special protection other than food.
The duck, having overcome hardship and injury to stand alone, has now formed a new family. Although there is still some distance from the existing goose flock, it is expected that they will naturally find their place in the campus ecosystem, as KAIST's geese are not aggressive or exclusive. The KAIST goose flock already has experience protecting and raising five ducklings.
< A new beginning by the pond on Children's Day: On the morning of May 5th, the 28th day of incubation, four ducklings hatched by the pond. This was a natural hatching, achieved without protective equipment. >
A single duck brought a special spring to the KAIST campus on Children's Day. The outcome achieved by that small life, leading to the birth of a new family, also symbolizes the harmonious coexistence of people and animals on the KAIST campus. The careful intervention of KAIST members, providing only the necessary assistance from rescue to hatching, makes us reconsider what "desirable coexistence between animals and people" truly means.
2025.05.21 View 2078
Life Springs at KAIST: A Tale of Two Special Campus Families
A Gift of Life on Teachers' Day: Baby Geese Born at KAIST Pond
On Teachers' Day, a meaningful miracle of life arrived at the KAIST campus. A pair of geese gave birth to two goslings by the duck pond.
< On Teachers' Day, a pair of geese and their goslings leisurely swim in the pond. >
The baby goslings, covered in yellow down, began exploring the pond's edge, scurrying about, while their aunt geese steadfastly stood by. Their curious glances, watchful gazes, playful hops on waterside rocks, and the procession of babies swimming behind their parents in the water melted the hearts of onlookers.
< As night falls on the duck pond, the goose family gathers among the reeds. >
This special new life, born on Teachers' Day, seems to symbolize the day's meaning of "care" and "growth." This wondrous scene of life brought warm comfort and joy to KAIST members, adding the inspiration of nature to a campus that is a space for research and learning.
< Under the protection of the adult geese, the goslings take their first steps, exploring the pond's grassy areas and rocks. >
This adorable family is already roaming the area leisurely, like the pond's owners. With the joy of life added to the spring-filled pond, warm smiles are spreading across the KAIST campus.
< The geese look around, surveying their surroundings, while caring for their goslings. >
The pond has now become a small but special haven for students and staff. This goose family, arriving on Teachers' Day, quietly reminds us of the meaning of care and learning conveyed by nature.
< The goose family shows care and growth, and warm moments together are anticipated. >
---
On Children's Day 2025, a Duck Becomes a Mother
In July 2024, a special guest arrived at the KAIST campus. With soft yellow down, waddling gait, and a flat beak, it was undeniably a baby duck. However, for some reason, its mother was nowhere to be seen. Given that it wasn't afraid of people and followed them well, it was clear that someone had abandoned the duck.
Fortunately, the baby duck was safely rescued thanks to prompt reporting by students.
< Two ducks found on a corner of campus, immediately after their rescue in summer 2024. >
The ducks, newly integrated into KAIST, seemed to adapt relatively peacefully to campus life. As new additions, they couldn't blend in with the existing goose flock that had settled on campus, but the geese didn't ostracize them either. Perhaps because they were awkward neighbors, there was hope that the ducks would soon join the existing goose flock.
< Following their rescue based on a student's report in summer 2024, the ducks adapted to campus life under the protection of the campus facility team and Professor Won Do Heo. >
Professor Won Do Heo of the Department of Biological Sciences, widely known as "Goose Dad," stepped forward to protect them along with the KAIST facility team. Professor Heo is well-known for consistently observing and protecting the campus geese and ducks, which are practically symbols of KAIST. Thanks to the care of the staff and Professor Heo, the two ducks were safely released back onto campus approximately one month after their rescue.
< A moment on campus: Before winter, the ducks lived separately from the goose flock, maintaining a certain distance. While there were no conflicts, they rarely socialized. >
However, as winter passed, sad news arrived. One duck went missing, and the remaining one was found injured by the pond. While the policy of the facility team and Professor Heo was to minimize intervention to allow campus animals to maintain their natural state, saving the injured duck was the top priority. After being isolated again for a month of recovery, the duck fully recovered and was able to greet spring under the sun.
< The mother duck left alone in winter: One went missing, and the remaining one was found injured. After indoor isolation and recovery, she was released back onto campus in the spring. >
As spring, the ducks' breeding season, began, Professor Heo decided to offer a little more help. When signs of egg-laying appeared, he consistently provided "special meals for pregnant mothers" throughout March. On the morning of May 5th, Children's Day, 28 days after the mother duck began incubating her eggs with the care and attention of KAIST members, new life finally hatched. It was a precious outcome achieved solely by the duck that had survived abandonment and injury, with no special protection other than food.
The duck, having overcome hardship and injury to stand alone, has now formed a new family. Although there is still some distance from the existing goose flock, it is expected that they will naturally find their place in the campus ecosystem, as KAIST's geese are not aggressive or exclusive. The KAIST goose flock already has experience protecting and raising five ducklings.
< A new beginning by the pond on Children's Day: On the morning of May 5th, the 28th day of incubation, four ducklings hatched by the pond. This was a natural hatching, achieved without protective equipment. >
A single duck brought a special spring to the KAIST campus on Children's Day. The outcome achieved by that small life, leading to the birth of a new family, also symbolizes the harmonious coexistence of people and animals on the KAIST campus. The careful intervention of KAIST members, providing only the necessary assistance from rescue to hatching, makes us reconsider what "desirable coexistence between animals and people" truly means.
2025.05.21 View 2078 -
 2025 National Strategic Technology Innovation Forum Held - Seeking ROK-U.S. Cooperation
The Future Institute for National Strategic Technology and Policy (FINST&P) at KAIST will host the 'National Strategic Technology* Innovation Forum for 1st half of 2025' on Thursday, May 22, at the Chung Kunmo Conference Hall in the Academic and Culture Building (E9) at the KAIST Main Campus in Daejeon.
* National Strategic Technologies: Technologies recognized for their strategic importance in terms of diplomacy and security, with significant impact on the national economy and related industries, and serving as the foundation for future innovation, including the creation of new technologies and industries. Currently, 12 major technologies such as AI, advanced bio, quantum, and semiconductors, and 50 detailed key technologies are being selected and supported (「Special Act on Fostering National Strategic Technologies」).
This forum will examine the policy direction for fostering national strategic technologies in South Korea amidst rapidly changing international dynamics, such as escalating conflict between the United States and China and increasing global security uncertainties. Furthermore, it will discuss ways to strengthen technology innovation between South Korea and the United States to secure scientific and technological sovereignty and future growth engines.
The forum will feature: △An opening address by KAIST President Kwang-Hyung Lee △Congratulatory remarks by Minister Sang-im Yoo of the Ministry of Science and ICT △A keynote speech by Robert D. Atkinson, President of the Information Technology and Innovation Foundation (ITIF) of the U.S. Subsequently, △Part 1, ‘ROK-U.S. Science and Technology Cooperation,’ will share the latest global trends in national strategic technologies and discuss ROK-U.S. science and technology cooperation under the U.S.-China technology hegemony structure.
Following this, △Part 2, ‘ROK-U.S. Cooperation in Key Detailed Technology Fields,’ will analyze R&D trends and current issues focusing on major national strategic technologies, and derive action-oriented policy tasks that South Korea can pursue based on ROK-U.S. cooperation.
< National Strategic Technology Innovation Forum Poster >
Each session of Part 1 and Part 2 will consist of presentations by domestic and international experts, followed by a comprehensive discussion and Q&A with the audience, promising more in-depth discussions.
Robert D. Atkinson, President of the U.S. Information Technology and Innovation Foundation (ITIF), in his keynote speech ‘The Trump 2.0 Era: South Korea's New Growth Strategy,’ suggests that South Korea should shift from its existing export-oriented growth to a new growth strategy based on broad technological innovation, and promote technological innovation by improving "shadow regulations" imposed by social practices.
The first presenter in Part 1, Stephen Ezell, Vice President for Global Innovation Policy at ITIF, emphasizes in ‘U.S.-China Conflict: South Korea's Response and Global Implications’ that South Korea must overcome the crisis by improving overall national productivity and fostering a competitive service industry.
Following this, Kyungjin Song, Country Representative of The Asia Foundation Korea Office, suggests in ‘Strengthening ROK-U.S. Strategic Technology Partnership Cooperation’ that as global technological hegemony competition changes the diplomatic and security landscape, ROK-U.S. cooperation should advance towards an institutional and sustainable cooperation foundation through a multi-layered partnership structure involving both countries' parliaments, industries, academia, and civil society.
Jaemin Jung, Dean of the College of Humanities and Social Sciences at KAIST, in ‘The Value of Humanities, Social Sciences, and Arts in the Age of Artificial Intelligence,’ explains the role and importance of the KAIST College of Humanities and Social Sciences in connecting technological innovation with human-centered values, as responsible technological development of artificial intelligence (AI) is difficult without insights into humans, society, and culture, presenting examples through AI joint research projects conducted with MIT.
As the first presenter in Part 2, Yong-hee Kim, Director of the Future Institute for National Strategic Technology and Policy (FINST&P) at KAIST, in ‘ROK-U.S. Cooperation for Truly Sustainable Next-Generation Nuclear Power,’ states that many countries or companies are pursuing nuclear power for carbon neutrality and energy security. He suggests that to achieve sustainable nuclear power, three major issues—safety, spent fuel, and uranium resources—need to be resolved, and the molten salt fast reactor (MSFR), an advanced reactor, can be an effective solution.*Molten Salt Fast Reactor (MSFR): A type of Generation IV nuclear reactor that uses molten salt as nuclear fuel and coolant in a fast neutron reactor.
Byung Hee Hong, Professor at Seoul National University's Department of Chemistry, predicts in ‘Innovation in Strategic Industries Led by Graphene Mass Production Technology’ that graphene is a ‘dream new material’ that will overcome the limitations of existing technologies. If South Korea succeeds in mass-producing graphene, it will bring tremendous innovation across key industries such as AI semiconductors and sensors, quantum computing, and biomedical.
Finally, Hoi-Jun Yoo, Distinguished Professor at the KAIST Graduate School of Artificial Intelligence Semiconductor, in ‘The Present and Future of AI Semiconductors,’ explains that with the full-scale utilization of large-scale AI like ChatGPT, semiconductor design is tending to reorganize from a computation-centric to a memory-centric approach. He then presents the direction and feasibility of mid-to-long-term strategies for the competitive development of Korean AI semiconductors.
KAIST President Kwang-Hyung Lee stated the purpose of the event, saying, "As national strategic technology is a core agenda directly linked to our nation's future growth, KAIST will continue to provide a platform for science and technology and policy to communicate, together with domestic and international industry-academia-research institutions."
This event is co-hosted with the U.S. think tank Information Technology and Innovation Foundation (ITIF), which has played a leading role in science and technology innovation policy, with the sponsorship of the Ministry of Science and ICT.
2025.05.16 View 1180
2025 National Strategic Technology Innovation Forum Held - Seeking ROK-U.S. Cooperation
The Future Institute for National Strategic Technology and Policy (FINST&P) at KAIST will host the 'National Strategic Technology* Innovation Forum for 1st half of 2025' on Thursday, May 22, at the Chung Kunmo Conference Hall in the Academic and Culture Building (E9) at the KAIST Main Campus in Daejeon.
* National Strategic Technologies: Technologies recognized for their strategic importance in terms of diplomacy and security, with significant impact on the national economy and related industries, and serving as the foundation for future innovation, including the creation of new technologies and industries. Currently, 12 major technologies such as AI, advanced bio, quantum, and semiconductors, and 50 detailed key technologies are being selected and supported (「Special Act on Fostering National Strategic Technologies」).
This forum will examine the policy direction for fostering national strategic technologies in South Korea amidst rapidly changing international dynamics, such as escalating conflict between the United States and China and increasing global security uncertainties. Furthermore, it will discuss ways to strengthen technology innovation between South Korea and the United States to secure scientific and technological sovereignty and future growth engines.
The forum will feature: △An opening address by KAIST President Kwang-Hyung Lee △Congratulatory remarks by Minister Sang-im Yoo of the Ministry of Science and ICT △A keynote speech by Robert D. Atkinson, President of the Information Technology and Innovation Foundation (ITIF) of the U.S. Subsequently, △Part 1, ‘ROK-U.S. Science and Technology Cooperation,’ will share the latest global trends in national strategic technologies and discuss ROK-U.S. science and technology cooperation under the U.S.-China technology hegemony structure.
Following this, △Part 2, ‘ROK-U.S. Cooperation in Key Detailed Technology Fields,’ will analyze R&D trends and current issues focusing on major national strategic technologies, and derive action-oriented policy tasks that South Korea can pursue based on ROK-U.S. cooperation.
< National Strategic Technology Innovation Forum Poster >
Each session of Part 1 and Part 2 will consist of presentations by domestic and international experts, followed by a comprehensive discussion and Q&A with the audience, promising more in-depth discussions.
Robert D. Atkinson, President of the U.S. Information Technology and Innovation Foundation (ITIF), in his keynote speech ‘The Trump 2.0 Era: South Korea's New Growth Strategy,’ suggests that South Korea should shift from its existing export-oriented growth to a new growth strategy based on broad technological innovation, and promote technological innovation by improving "shadow regulations" imposed by social practices.
The first presenter in Part 1, Stephen Ezell, Vice President for Global Innovation Policy at ITIF, emphasizes in ‘U.S.-China Conflict: South Korea's Response and Global Implications’ that South Korea must overcome the crisis by improving overall national productivity and fostering a competitive service industry.
Following this, Kyungjin Song, Country Representative of The Asia Foundation Korea Office, suggests in ‘Strengthening ROK-U.S. Strategic Technology Partnership Cooperation’ that as global technological hegemony competition changes the diplomatic and security landscape, ROK-U.S. cooperation should advance towards an institutional and sustainable cooperation foundation through a multi-layered partnership structure involving both countries' parliaments, industries, academia, and civil society.
Jaemin Jung, Dean of the College of Humanities and Social Sciences at KAIST, in ‘The Value of Humanities, Social Sciences, and Arts in the Age of Artificial Intelligence,’ explains the role and importance of the KAIST College of Humanities and Social Sciences in connecting technological innovation with human-centered values, as responsible technological development of artificial intelligence (AI) is difficult without insights into humans, society, and culture, presenting examples through AI joint research projects conducted with MIT.
As the first presenter in Part 2, Yong-hee Kim, Director of the Future Institute for National Strategic Technology and Policy (FINST&P) at KAIST, in ‘ROK-U.S. Cooperation for Truly Sustainable Next-Generation Nuclear Power,’ states that many countries or companies are pursuing nuclear power for carbon neutrality and energy security. He suggests that to achieve sustainable nuclear power, three major issues—safety, spent fuel, and uranium resources—need to be resolved, and the molten salt fast reactor (MSFR), an advanced reactor, can be an effective solution.*Molten Salt Fast Reactor (MSFR): A type of Generation IV nuclear reactor that uses molten salt as nuclear fuel and coolant in a fast neutron reactor.
Byung Hee Hong, Professor at Seoul National University's Department of Chemistry, predicts in ‘Innovation in Strategic Industries Led by Graphene Mass Production Technology’ that graphene is a ‘dream new material’ that will overcome the limitations of existing technologies. If South Korea succeeds in mass-producing graphene, it will bring tremendous innovation across key industries such as AI semiconductors and sensors, quantum computing, and biomedical.
Finally, Hoi-Jun Yoo, Distinguished Professor at the KAIST Graduate School of Artificial Intelligence Semiconductor, in ‘The Present and Future of AI Semiconductors,’ explains that with the full-scale utilization of large-scale AI like ChatGPT, semiconductor design is tending to reorganize from a computation-centric to a memory-centric approach. He then presents the direction and feasibility of mid-to-long-term strategies for the competitive development of Korean AI semiconductors.
KAIST President Kwang-Hyung Lee stated the purpose of the event, saying, "As national strategic technology is a core agenda directly linked to our nation's future growth, KAIST will continue to provide a platform for science and technology and policy to communicate, together with domestic and international industry-academia-research institutions."
This event is co-hosted with the U.S. think tank Information Technology and Innovation Foundation (ITIF), which has played a leading role in science and technology innovation policy, with the sponsorship of the Ministry of Science and ICT.
2025.05.16 View 1180 -
 Decoding Fear: KAIST Identifies An Affective Brain Circuit Crucial for Fear Memory Formation by Non-nociceptive Threat Stimulus
Fear memories can form in the brain following exposure to threatening situations such as natural disasters, accidents, or violence. When these memories become excessive or distorted, they can lead to severe mental health disorders, including post-traumatic stress disorder (PTSD), anxiety disorders, and depression. However, the mechanisms underlying fear memory formation triggered by affective pain rather than direct physical pain have remained largely unexplored – until now.
A KAIST research team has identified, for the first time, a brain circuit specifically responsible for forming fear memories in the absence of physical pain, marking a significant advance in understanding how psychological distress is processed and drives fear memory formation in the brain. This discovery opens the door to the development of targeted treatments for trauma-related conditions by addressing the underlying neural pathways.
< Photo 1. (from left) Professor Jin-Hee Han, Dr. Junho Han and Ph.D. Candidate Boin Suh of the Department of Biological Sciences >
KAIST (President Kwang-Hyung Lee) announced on May 15th that the research team led by Professor Jin-Hee Han in the Department of Biological Sciences has identified the pIC-PBN circuit*, a key neural pathway involved in forming fear memories triggered by psychological threats in the absence of sensory pain. This groundbreaking work was conducted through experiments with mice.*pIC–PBN circuit: A newly identified descending neural pathway from the posterior insular cortex (pIC) to the parabrachial nucleus (PBN), specialized for transmitting psychological threat information.
Traditionally, the lateral parabrachial nucleus (PBN) has been recognized as a critical part of the ascending pain pathway, receiving pain signals from the spinal cord. However, this study reveals a previously unknown role for the PBN in processing fear induced by non-painful psychological stimuli, fundamentally changing our understanding of its function in the brain.
This work is considered the first experimental evidence that 'emotional distress' and 'physical pain' are processed through different neural circuits to form fear memories, making it a significant contribution to the field of neuroscience. It clearly demonstrates the existence of a dedicated pathway (pIC-PBN) for transmitting emotional distress.
The study's first author, Dr. Junho Han, shared the personal motivation behind this research: “Our dog, Lego, is afraid of motorcycles. He never actually crashed into one, but ever since having a traumatizing event of having a motorbike almost run into him, just hearing the sound now triggers a fearful response. Humans react similarly – even if you didn’t have a personal experience of being involved in an accident, a near-miss or exposure to alarming media can create lasting fear memories, which may eventually lead to PTSD.”
He continued, “Until now, fear memory research has mainly relied on experimental models involving physical pain. However, much of real-world human fears arise from psychological threats, rather than from direct physical harm. Despite this, little was known about the brain circuits responsible for processing these psychological threats that can drive fear memory formation.”
To investigate this, the research team developed a novel fear conditioning model that utilizes visual threat stimuli instead of electrical shocks. In this model, mice were exposed to a rapidly expanding visual disk on a ceiling screen, simulating the threat of an approaching predator. This approach allowed the team to demonstrate that fear memories can form in response to a non-nociceptive, psychological threat alone, without the need for physical pain.
< Figure 1. Artificial activation of the posterior insular cortex (pIC) to lateral parabrachial nucleus (PBN) neural circuit induces anxiety-like behaviors and fear memory formation in mice. >
Using advanced chemogenetic and optogenetic techniques, the team precisely controlled neuronal activity, revealing that the lateral parabrachial nucleus (PBN) is essential to form fear memories in response to visual threats. They further traced the origin of these signals to the posterior insular cortex (pIC), a region known to process negative emotions and pain, confirming a direct connection between the two areas.
The study also showed that inhibiting the pIC–PBN circuit significantly reduced fear memory formation in response to visual threats, without affecting innate fear responses or physical pain-based learning. Conversely, artificially activating this circuit alone was sufficient to drive fear memory formation, confirming its role as a key pathway for processing psychological threat information.
< Figure 2. Schematic diagram of brain neural circuits transmitting emotional & physical pain threat signals. Visual threat stimuli do not involve physical pain but can create an anxious state and form fear memory through the affective pain signaling pathway. >
Professor Jin-Hee Han commented, “This study lays an important foundation for understanding how emotional distress-based mental disorders, such as PTSD, panic disorder, and anxiety disorder, develop, and opens new possibilities for targeted treatment approaches.”
The findings, authored by Dr. Junho Han (first author), Ph.D. candidate Boin Suh (second author), and Dr. Jin-Hee Han (corresponding author) of the Department of Biological Sciences, were published online in the international journal Science Advances on May 9, 2025.※ Paper Title: A top-down insular cortex circuit crucial for non-nociceptive fear learning. Science Advances (https://doi.org/10.1101/2024.10.14.618356)※ Author Information: Junho Han (first author), Boin Suh (second author), and Jin-Hee Han (corresponding author)
This research was supported by grants from the National Research Foundation of Korea (NRF-2022M3E5E8081183 and NRF-2017M3C7A1031322).
2025.05.15 View 2600
Decoding Fear: KAIST Identifies An Affective Brain Circuit Crucial for Fear Memory Formation by Non-nociceptive Threat Stimulus
Fear memories can form in the brain following exposure to threatening situations such as natural disasters, accidents, or violence. When these memories become excessive or distorted, they can lead to severe mental health disorders, including post-traumatic stress disorder (PTSD), anxiety disorders, and depression. However, the mechanisms underlying fear memory formation triggered by affective pain rather than direct physical pain have remained largely unexplored – until now.
A KAIST research team has identified, for the first time, a brain circuit specifically responsible for forming fear memories in the absence of physical pain, marking a significant advance in understanding how psychological distress is processed and drives fear memory formation in the brain. This discovery opens the door to the development of targeted treatments for trauma-related conditions by addressing the underlying neural pathways.
< Photo 1. (from left) Professor Jin-Hee Han, Dr. Junho Han and Ph.D. Candidate Boin Suh of the Department of Biological Sciences >
KAIST (President Kwang-Hyung Lee) announced on May 15th that the research team led by Professor Jin-Hee Han in the Department of Biological Sciences has identified the pIC-PBN circuit*, a key neural pathway involved in forming fear memories triggered by psychological threats in the absence of sensory pain. This groundbreaking work was conducted through experiments with mice.*pIC–PBN circuit: A newly identified descending neural pathway from the posterior insular cortex (pIC) to the parabrachial nucleus (PBN), specialized for transmitting psychological threat information.
Traditionally, the lateral parabrachial nucleus (PBN) has been recognized as a critical part of the ascending pain pathway, receiving pain signals from the spinal cord. However, this study reveals a previously unknown role for the PBN in processing fear induced by non-painful psychological stimuli, fundamentally changing our understanding of its function in the brain.
This work is considered the first experimental evidence that 'emotional distress' and 'physical pain' are processed through different neural circuits to form fear memories, making it a significant contribution to the field of neuroscience. It clearly demonstrates the existence of a dedicated pathway (pIC-PBN) for transmitting emotional distress.
The study's first author, Dr. Junho Han, shared the personal motivation behind this research: “Our dog, Lego, is afraid of motorcycles. He never actually crashed into one, but ever since having a traumatizing event of having a motorbike almost run into him, just hearing the sound now triggers a fearful response. Humans react similarly – even if you didn’t have a personal experience of being involved in an accident, a near-miss or exposure to alarming media can create lasting fear memories, which may eventually lead to PTSD.”
He continued, “Until now, fear memory research has mainly relied on experimental models involving physical pain. However, much of real-world human fears arise from psychological threats, rather than from direct physical harm. Despite this, little was known about the brain circuits responsible for processing these psychological threats that can drive fear memory formation.”
To investigate this, the research team developed a novel fear conditioning model that utilizes visual threat stimuli instead of electrical shocks. In this model, mice were exposed to a rapidly expanding visual disk on a ceiling screen, simulating the threat of an approaching predator. This approach allowed the team to demonstrate that fear memories can form in response to a non-nociceptive, psychological threat alone, without the need for physical pain.
< Figure 1. Artificial activation of the posterior insular cortex (pIC) to lateral parabrachial nucleus (PBN) neural circuit induces anxiety-like behaviors and fear memory formation in mice. >
Using advanced chemogenetic and optogenetic techniques, the team precisely controlled neuronal activity, revealing that the lateral parabrachial nucleus (PBN) is essential to form fear memories in response to visual threats. They further traced the origin of these signals to the posterior insular cortex (pIC), a region known to process negative emotions and pain, confirming a direct connection between the two areas.
The study also showed that inhibiting the pIC–PBN circuit significantly reduced fear memory formation in response to visual threats, without affecting innate fear responses or physical pain-based learning. Conversely, artificially activating this circuit alone was sufficient to drive fear memory formation, confirming its role as a key pathway for processing psychological threat information.
< Figure 2. Schematic diagram of brain neural circuits transmitting emotional & physical pain threat signals. Visual threat stimuli do not involve physical pain but can create an anxious state and form fear memory through the affective pain signaling pathway. >
Professor Jin-Hee Han commented, “This study lays an important foundation for understanding how emotional distress-based mental disorders, such as PTSD, panic disorder, and anxiety disorder, develop, and opens new possibilities for targeted treatment approaches.”
The findings, authored by Dr. Junho Han (first author), Ph.D. candidate Boin Suh (second author), and Dr. Jin-Hee Han (corresponding author) of the Department of Biological Sciences, were published online in the international journal Science Advances on May 9, 2025.※ Paper Title: A top-down insular cortex circuit crucial for non-nociceptive fear learning. Science Advances (https://doi.org/10.1101/2024.10.14.618356)※ Author Information: Junho Han (first author), Boin Suh (second author), and Jin-Hee Han (corresponding author)
This research was supported by grants from the National Research Foundation of Korea (NRF-2022M3E5E8081183 and NRF-2017M3C7A1031322).
2025.05.15 View 2600 -
 KAIST Discovers Protein Switch that Turns Anti-Viral Immune Response On and Off
Even after the COVID-19 pandemic, various new infectious diseases continue to emerge, posing ongoing viral threats that demand robust and sustained immune defenses. However, excessive immune reactions can also harm body tissues, causing significant health issues. KAIST and an international research team have discovered a critical protein that acts as a 'switch' regulating immune responses to viruses. This breakthrough is expected to lay the groundwork for future infectious disease responses and autoimmune disease treatment strategies.
KAIST (President Kwang-Hyung Lee) announced on May 14 that a joint research team led by Professor Yoosik Kim from the Department of Chemical and Biomolecular Engineering at KAIST and Professor Seunghee Cha from University of Florida has discovered the mechanism by which double-stranded RNA derived from mitochondria amplifies immune responses. They identified the protein SLIRP as an 'immune switch' that regulates this process, playing a crucial role in both viral infections and autoimmune diseases.
< (From left) Master's candidate Yewon Yang, Professor Yoosik Kim and Ph.D. candidate Doyeong Ku of the Department of Chemical and Biomolecular Engineering >
Autoimmune diseases arise when the immune system fails to differentiate between external pathogens and the body's own molecules, leading to self-directed attacks. Despite extensive research, the precise causes of excessive inflammatory conditions like Sjögren’s syndrome and systemic lupus erythematosus remain unclear, and effective treatments are still limited.
To uncover the molecular mechanisms driving immune hyperactivation and to identify potential regulatory factors, the research team led by Professor Yoosik Kim focused on mitochondrial double-stranded RNA (mt-dsRNA), a genetic immunogenic material produced within cellular organelles. Since mt-dsRNA structurally resembles viral RNA, it can mistakenly trigger immune responses even in the absence of an actual viral infection.
The team discovered that SLIRP, a key regulator of mt-dsRNA, amplifies immune responses by stabilizing the RNA. They confirmed that SLIRP expression increases in experimental models simulating the tissues of autoimmune disease patients and viral infections. Conversely, suppressing SLIRP significantly reduced the immune response, underscoring its role as a critical factor in immune amplification.
This study also demonstrated the dual function of SLIRP in different contexts. In cells infected with human beta coronavirus OC43 and encephalomyocarditis virus (EMCV), SLIRP suppression led to reduced antiviral responses and increased viral replication. Meanwhile, in the blood and salivary gland cells of Sjögren’s syndrome patients, where both SLIRP and mt-dsRNA levels were elevated, suppressing SLIRP alleviated the abnormal immune response.
These findings highlight SLIRP as a key molecular switch that regulates immune responses in both infections and autoimmune diseases.
< Figure 1. Schematic diagram of antiviral signal amplification by SLIRP: SLIRP-based mt-dsRNA induction, cytoplasmic accumulation, and strong interferon response induction by positive feedback of immune response activation. Confirmation of the immune regulatory function of SLIRP in defense against autoimmune diseases Sjögren's syndrome, coronavirus, and encephalomyocarditis virus infection. >
Professor Yoosik Kim remarked, "Through this study, we have identified SLIRP as a crucial protein that drives immune amplification via mt-dsRNAs. Given its dual role in autoimmune diseases and viral infections, SLIRP presents a promising target for immune regulation therapies across various inflammatory disease contexts."
The study, with Ph.D. student Do-Young Ku (first author) and M.S. student Ye-Won Yang (second author) from the Department of Chemical and Biomolecular Engineering at KAIST as primary contributors, was published online in the journal Cell Reports on April 19, 2025.
※ Paper title: SLIRP amplifies antiviral signaling via positive feedback regulation and contributes to autoimmune diseases※ Main authors: Do-Young Ku (KAIST, first author), Ye-Won Yang (KAIST, second author), Seunghee Cha (University of Florida, corresponding author), Yoosik Kim (KAIST, corresponding author)
This study was supported by the Ministry of Health and Welfare's Public Health Technology Research Program and the National Institutes of Health (NIH) through Research Project (R01) funding.
2025.05.14 View 2311
KAIST Discovers Protein Switch that Turns Anti-Viral Immune Response On and Off
Even after the COVID-19 pandemic, various new infectious diseases continue to emerge, posing ongoing viral threats that demand robust and sustained immune defenses. However, excessive immune reactions can also harm body tissues, causing significant health issues. KAIST and an international research team have discovered a critical protein that acts as a 'switch' regulating immune responses to viruses. This breakthrough is expected to lay the groundwork for future infectious disease responses and autoimmune disease treatment strategies.
KAIST (President Kwang-Hyung Lee) announced on May 14 that a joint research team led by Professor Yoosik Kim from the Department of Chemical and Biomolecular Engineering at KAIST and Professor Seunghee Cha from University of Florida has discovered the mechanism by which double-stranded RNA derived from mitochondria amplifies immune responses. They identified the protein SLIRP as an 'immune switch' that regulates this process, playing a crucial role in both viral infections and autoimmune diseases.
< (From left) Master's candidate Yewon Yang, Professor Yoosik Kim and Ph.D. candidate Doyeong Ku of the Department of Chemical and Biomolecular Engineering >
Autoimmune diseases arise when the immune system fails to differentiate between external pathogens and the body's own molecules, leading to self-directed attacks. Despite extensive research, the precise causes of excessive inflammatory conditions like Sjögren’s syndrome and systemic lupus erythematosus remain unclear, and effective treatments are still limited.
To uncover the molecular mechanisms driving immune hyperactivation and to identify potential regulatory factors, the research team led by Professor Yoosik Kim focused on mitochondrial double-stranded RNA (mt-dsRNA), a genetic immunogenic material produced within cellular organelles. Since mt-dsRNA structurally resembles viral RNA, it can mistakenly trigger immune responses even in the absence of an actual viral infection.
The team discovered that SLIRP, a key regulator of mt-dsRNA, amplifies immune responses by stabilizing the RNA. They confirmed that SLIRP expression increases in experimental models simulating the tissues of autoimmune disease patients and viral infections. Conversely, suppressing SLIRP significantly reduced the immune response, underscoring its role as a critical factor in immune amplification.
This study also demonstrated the dual function of SLIRP in different contexts. In cells infected with human beta coronavirus OC43 and encephalomyocarditis virus (EMCV), SLIRP suppression led to reduced antiviral responses and increased viral replication. Meanwhile, in the blood and salivary gland cells of Sjögren’s syndrome patients, where both SLIRP and mt-dsRNA levels were elevated, suppressing SLIRP alleviated the abnormal immune response.
These findings highlight SLIRP as a key molecular switch that regulates immune responses in both infections and autoimmune diseases.
< Figure 1. Schematic diagram of antiviral signal amplification by SLIRP: SLIRP-based mt-dsRNA induction, cytoplasmic accumulation, and strong interferon response induction by positive feedback of immune response activation. Confirmation of the immune regulatory function of SLIRP in defense against autoimmune diseases Sjögren's syndrome, coronavirus, and encephalomyocarditis virus infection. >
Professor Yoosik Kim remarked, "Through this study, we have identified SLIRP as a crucial protein that drives immune amplification via mt-dsRNAs. Given its dual role in autoimmune diseases and viral infections, SLIRP presents a promising target for immune regulation therapies across various inflammatory disease contexts."
The study, with Ph.D. student Do-Young Ku (first author) and M.S. student Ye-Won Yang (second author) from the Department of Chemical and Biomolecular Engineering at KAIST as primary contributors, was published online in the journal Cell Reports on April 19, 2025.
※ Paper title: SLIRP amplifies antiviral signaling via positive feedback regulation and contributes to autoimmune diseases※ Main authors: Do-Young Ku (KAIST, first author), Ye-Won Yang (KAIST, second author), Seunghee Cha (University of Florida, corresponding author), Yoosik Kim (KAIST, corresponding author)
This study was supported by the Ministry of Health and Welfare's Public Health Technology Research Program and the National Institutes of Health (NIH) through Research Project (R01) funding.
2025.05.14 View 2311 -
 KAIST Develops Novel Catalyst With 100-Fold Platinum Efficiency
Propylene, a key building block used in producing plastics, textiles, automotive components, and electronics, is essential to the petrochemical industry. A KAIST research team has developed a novel catalyst that dramatically enhances the efficiency of propylene production while significantly reducing costs.
< Photo. Professor Minkee Choi (left), and Ph.D. Candidate Susung Lee (right) of the Department of Chemical and Biomolecular Engineering >
KAIST (represented by President Kwang-Hyung Lee) announced on the 12th of May that a research group led by Professor Minkee Choi from the Department of Chemical and Biomolecular Engineering has successfully developed a new catalyst using inexpensive metals—gallium (Ga) and alumina (Al₂O₃)—with only a trace amount of platinum (100 ppm, or 0.01%). Remarkably, this new catalyst outperforms conventional industrial catalysts that use high concentrations of platinum (10,000 ppm).
Propylene is commonly produced through the propane dehydrogenation (PDH) process, which removes hydrogen from propane. Platinum has long been used as a catalyst in PDH due to its high efficiency in breaking carbon-hydrogen bonds and facilitating hydrogen removal. However, platinum is costly and suffers from performance degradation over repeated use.
To address this, the KAIST team engineered a catalyst that incorporates only a minimal amount of platinum, relying on gallium and alumina as the primary components.
< Figure 1. Schematic diagram showing the catalytic cooperation between gallium (Ga) and platinum (Pt) >
The core mechanism of the catalyst involves a cooperative function between the metals: gallium activates the C–H bond in propane to produce propylene, while platinum bonds the residual hydrogen atoms on the surface to form hydrogen gas (H₂), which is then released. This division of roles allows for high catalytic efficiency despite the drastic reduction in platinum content.
The researchers identified an optimal platinum-to-gallium ratio that delivered peak performance and provided a scientific rationale and quantitative metrics to predict this ideal composition.
Additionally, the team addressed a major limitation of traditional platinum catalysts: sintering—the agglomeration of platinum particles during repeated use, which causes performance loss. By adding a small amount of cerium (Ce), the researchers successfully suppressed this aggregation. As a result, the new catalyst maintained stable performance even after more than 20 reaction-regeneration cycles.
< Figure 2. Performance comparison of KAIST's newly developed catalyst (100 ppm platinum) and existing commercial platinum catalyst (10,000 ppm platinum) >
Professor Choi stated, “This research demonstrates the possibility of reducing platinum usage to 1/100th of current levels without compromising, and even enhancing, performance. It presents significant economic and environmental advantages, including lower catalyst costs, extended replacement intervals, and reduced catalyst waste.”
He added, “We are planning to evaluate this technology for large-scale process demonstration and commercialization. If adopted in industry, it could greatly improve the economic viability and efficiency of propylene production.”
The study was led by Professor Minkee Choi as corresponding author, with Ph.D. candidate Susung Lee as the first author. The findings were published in the Journal of the American Chemical Society (JACS), a leading journal in chemistry and chemical engineering, on February 13.※ Paper title: Ideal Bifunctional Catalysis for Propane Dehydrogenation over Pt-Promoted Gallia-Alumina and Minimized Use of Precious Elements※ DOI: https://pubs.acs.org/doi/10.1021/jacs.4c13787
The research was supported by the National Research Foundation of Korea and Hanwha Solutions Corporation.
2025.05.12 View 1409
KAIST Develops Novel Catalyst With 100-Fold Platinum Efficiency
Propylene, a key building block used in producing plastics, textiles, automotive components, and electronics, is essential to the petrochemical industry. A KAIST research team has developed a novel catalyst that dramatically enhances the efficiency of propylene production while significantly reducing costs.
< Photo. Professor Minkee Choi (left), and Ph.D. Candidate Susung Lee (right) of the Department of Chemical and Biomolecular Engineering >
KAIST (represented by President Kwang-Hyung Lee) announced on the 12th of May that a research group led by Professor Minkee Choi from the Department of Chemical and Biomolecular Engineering has successfully developed a new catalyst using inexpensive metals—gallium (Ga) and alumina (Al₂O₃)—with only a trace amount of platinum (100 ppm, or 0.01%). Remarkably, this new catalyst outperforms conventional industrial catalysts that use high concentrations of platinum (10,000 ppm).
Propylene is commonly produced through the propane dehydrogenation (PDH) process, which removes hydrogen from propane. Platinum has long been used as a catalyst in PDH due to its high efficiency in breaking carbon-hydrogen bonds and facilitating hydrogen removal. However, platinum is costly and suffers from performance degradation over repeated use.
To address this, the KAIST team engineered a catalyst that incorporates only a minimal amount of platinum, relying on gallium and alumina as the primary components.
< Figure 1. Schematic diagram showing the catalytic cooperation between gallium (Ga) and platinum (Pt) >
The core mechanism of the catalyst involves a cooperative function between the metals: gallium activates the C–H bond in propane to produce propylene, while platinum bonds the residual hydrogen atoms on the surface to form hydrogen gas (H₂), which is then released. This division of roles allows for high catalytic efficiency despite the drastic reduction in platinum content.
The researchers identified an optimal platinum-to-gallium ratio that delivered peak performance and provided a scientific rationale and quantitative metrics to predict this ideal composition.
Additionally, the team addressed a major limitation of traditional platinum catalysts: sintering—the agglomeration of platinum particles during repeated use, which causes performance loss. By adding a small amount of cerium (Ce), the researchers successfully suppressed this aggregation. As a result, the new catalyst maintained stable performance even after more than 20 reaction-regeneration cycles.
< Figure 2. Performance comparison of KAIST's newly developed catalyst (100 ppm platinum) and existing commercial platinum catalyst (10,000 ppm platinum) >
Professor Choi stated, “This research demonstrates the possibility of reducing platinum usage to 1/100th of current levels without compromising, and even enhancing, performance. It presents significant economic and environmental advantages, including lower catalyst costs, extended replacement intervals, and reduced catalyst waste.”
He added, “We are planning to evaluate this technology for large-scale process demonstration and commercialization. If adopted in industry, it could greatly improve the economic viability and efficiency of propylene production.”
The study was led by Professor Minkee Choi as corresponding author, with Ph.D. candidate Susung Lee as the first author. The findings were published in the Journal of the American Chemical Society (JACS), a leading journal in chemistry and chemical engineering, on February 13.※ Paper title: Ideal Bifunctional Catalysis for Propane Dehydrogenation over Pt-Promoted Gallia-Alumina and Minimized Use of Precious Elements※ DOI: https://pubs.acs.org/doi/10.1021/jacs.4c13787
The research was supported by the National Research Foundation of Korea and Hanwha Solutions Corporation.
2025.05.12 View 1409 -
 Editing Parkinson's Disease – KAIST Makes World's First Discovery of an Inflammatory RNA Editing Enzyme through Co-work with UCL Researchers
< Professor Minee Choi of the Department of Brain and Cognitive Sciences (top left). Professor Sonia Gandhi (top right) and Professor Klenerman of the University College London (bottom right) >
Parkinson's disease (PD) is a neurodegenerative disorder in which the α-synuclein protein abnormally aggregates within brain cells, causing neuronal damage. Through international collaboration, researchers at KAIST have revealed that RNA editing plays a crucial role in regulating neuroinflammation, a key pathology of Parkinson's disease.
KAIST (represented by President Kwang-Hyung Lee) announced on the 27th of April that a research team led by Professor Minee L. Choi from the Department of Brain and Cognitive Sciences, in collaboration with University College London (UCL) and the Francis Crick Institute, discovered that the RNA editing enzyme ADAR1 plays an important role in controlling immune responses in astrocytes, glial cells that trigger protective reactions in the brain, and demonstrated that this mechanism is critically involved in the progression of Parkinson’s disease.
Professor Choi's research team created a co-culture model composed of astrocytes and neurons derived from stem cells originating from Parkinson's disease patients, in order to study the inflammatory responses of brain immune cells. They then treated the model with α-synuclein aggregates, which are known to cause Parkinson’s disease, and analyzed how the immune cells' inflammatory responses changed.
< Figure 1. Schematic diagram of the inflammatory RNA editing model in Parkinson's disease >
As a result, it was found that early pathological forms of α-synuclein, known as oligomers, activated the Toll-like receptor pathway, which acts as a danger sensor in astrocytes, as well as the interferon response pathway, an immune signaling network that combats viruses and pathogens. During this process, the RNA editing enzyme ADAR1 was expressed and transformed into an isoform with an altered protein structure and function.
Notably, the RNA editing activity of ADAR1, which normally functions to regulate immune responses during viral infections by converting adenosine (A) to inosine (I) through a process known as A-to-I RNA editing, was found to be abnormally focused on genes that cause inflammation rather than operating under normal conditions. This phenomenon was observed not only in the patient-derived neuron models but also in postmortem brain tissues from actual Parkinson’s disease patients.
< Figure 2. Experimental design and inflammatory response induction in astrocytes following treatment with α-synuclein oligomers (abnormally folded protein fragments) >
This directly proves that the dysregulation of RNA editing induces chronic inflammatory responses in astrocytes, ultimately leading to neuronal toxicity and pathological progression.
This study is significant in that it newly identified the regulation of RNA editing within astrocytes as a key mechanism behind neuroinflammatory responses. In particular, it suggests that ADAR1 could serve as a novel genetic target for the treatment of Parkinson’s disease.
It is also noteworthy that the study reflected actual pathological characteristics of patients by utilizing patient-specific induced pluripotent stem cell-based precision models for brain diseases.
Professor Minee L. Choi stated, “This study demonstrates that the regulator of inflammation caused by protein aggregation operates at the new layer of RNA editing, offering a completely different therapeutic strategy from existing approaches to Parkinson's disease treatment." She further emphasized, “RNA editing technology could become an important turning point in the development of therapeutics for neuroinflammation.”
< Figure 3. When treated with α-synuclein oligomers, the causative agent of Parkinson's disease, A-to-I RNA editing is induced to change genetic information by ADAR in patient-derived stem cell-differentiated glial cells, confirming that α-synuclein is likely to be associated with the progression of Parkinson's disease through RNA editing >
This study was published in Science Advances on April 11, with Professor Choi listed as a co-first author.
Paper Title: Astrocytic RNA editing regulates the host immune response to alpha-synuclein, Science Advances Vol.11, Issue 15. (DOI:10.1126/sciadv.adp8504)
Lead Authors: Karishma D’Sa (UCL, Co-First Author), Minee L. Choi (KAIST, Co-First Author), Mina Ryten (UCL, Corresponding Author), Sonia Gandhi (Francis Crick Institute, University of Cambridge, Corresponding Author)
This research was supported by the Brain Research Program and the Excellent Young Researcher Program of the National Research Foundation of Korea, as well as KAIST’s Daekyo Cognitive Enhancement Program.
2025.05.02 View 3571
Editing Parkinson's Disease – KAIST Makes World's First Discovery of an Inflammatory RNA Editing Enzyme through Co-work with UCL Researchers
< Professor Minee Choi of the Department of Brain and Cognitive Sciences (top left). Professor Sonia Gandhi (top right) and Professor Klenerman of the University College London (bottom right) >
Parkinson's disease (PD) is a neurodegenerative disorder in which the α-synuclein protein abnormally aggregates within brain cells, causing neuronal damage. Through international collaboration, researchers at KAIST have revealed that RNA editing plays a crucial role in regulating neuroinflammation, a key pathology of Parkinson's disease.
KAIST (represented by President Kwang-Hyung Lee) announced on the 27th of April that a research team led by Professor Minee L. Choi from the Department of Brain and Cognitive Sciences, in collaboration with University College London (UCL) and the Francis Crick Institute, discovered that the RNA editing enzyme ADAR1 plays an important role in controlling immune responses in astrocytes, glial cells that trigger protective reactions in the brain, and demonstrated that this mechanism is critically involved in the progression of Parkinson’s disease.
Professor Choi's research team created a co-culture model composed of astrocytes and neurons derived from stem cells originating from Parkinson's disease patients, in order to study the inflammatory responses of brain immune cells. They then treated the model with α-synuclein aggregates, which are known to cause Parkinson’s disease, and analyzed how the immune cells' inflammatory responses changed.
< Figure 1. Schematic diagram of the inflammatory RNA editing model in Parkinson's disease >
As a result, it was found that early pathological forms of α-synuclein, known as oligomers, activated the Toll-like receptor pathway, which acts as a danger sensor in astrocytes, as well as the interferon response pathway, an immune signaling network that combats viruses and pathogens. During this process, the RNA editing enzyme ADAR1 was expressed and transformed into an isoform with an altered protein structure and function.
Notably, the RNA editing activity of ADAR1, which normally functions to regulate immune responses during viral infections by converting adenosine (A) to inosine (I) through a process known as A-to-I RNA editing, was found to be abnormally focused on genes that cause inflammation rather than operating under normal conditions. This phenomenon was observed not only in the patient-derived neuron models but also in postmortem brain tissues from actual Parkinson’s disease patients.
< Figure 2. Experimental design and inflammatory response induction in astrocytes following treatment with α-synuclein oligomers (abnormally folded protein fragments) >
This directly proves that the dysregulation of RNA editing induces chronic inflammatory responses in astrocytes, ultimately leading to neuronal toxicity and pathological progression.
This study is significant in that it newly identified the regulation of RNA editing within astrocytes as a key mechanism behind neuroinflammatory responses. In particular, it suggests that ADAR1 could serve as a novel genetic target for the treatment of Parkinson’s disease.
It is also noteworthy that the study reflected actual pathological characteristics of patients by utilizing patient-specific induced pluripotent stem cell-based precision models for brain diseases.
Professor Minee L. Choi stated, “This study demonstrates that the regulator of inflammation caused by protein aggregation operates at the new layer of RNA editing, offering a completely different therapeutic strategy from existing approaches to Parkinson's disease treatment." She further emphasized, “RNA editing technology could become an important turning point in the development of therapeutics for neuroinflammation.”
< Figure 3. When treated with α-synuclein oligomers, the causative agent of Parkinson's disease, A-to-I RNA editing is induced to change genetic information by ADAR in patient-derived stem cell-differentiated glial cells, confirming that α-synuclein is likely to be associated with the progression of Parkinson's disease through RNA editing >
This study was published in Science Advances on April 11, with Professor Choi listed as a co-first author.
Paper Title: Astrocytic RNA editing regulates the host immune response to alpha-synuclein, Science Advances Vol.11, Issue 15. (DOI:10.1126/sciadv.adp8504)
Lead Authors: Karishma D’Sa (UCL, Co-First Author), Minee L. Choi (KAIST, Co-First Author), Mina Ryten (UCL, Corresponding Author), Sonia Gandhi (Francis Crick Institute, University of Cambridge, Corresponding Author)
This research was supported by the Brain Research Program and the Excellent Young Researcher Program of the National Research Foundation of Korea, as well as KAIST’s Daekyo Cognitive Enhancement Program.
2025.05.02 View 3571