Department+of+Chemistry
-
 Hyosung R&DB Labs to Teach Special Class on High Molecule Chemistry for the Fall Semester
The Department of Chemistry in collaboration with the Hyosung Group’s R&DB Labs will open a ‘special class on high molecule chemistry’ for Masters and Ph.D. candidates. The class, led by researchers at Hyosung’s R&D think tank, will provide the latest market and technology trends in the molecule chemical industry during the fall semester.
Hyosung joined this special industry program in an effort to enhance students’ hands-on understanding of new technologies that will emerge in the global market. During the semester, Hyosung plans to present the technology portfolios on their brand new materials of TAC film, membrane, and carbon fiber as well as the existing products leading the world in market share such as spandex, tire cords.
Hyosung plans to recruit students who previously took courses led by Hyosung researchers. President Tu-Won Chang of Hyosung R&DB said, “This program is designed to foster highly qualified R&D personnel especially catering to our company’s needs and market demands. We will continue to share our company’s market analysis and R&D know-how with outstanding universities.
2017.09.07 View 5470
Hyosung R&DB Labs to Teach Special Class on High Molecule Chemistry for the Fall Semester
The Department of Chemistry in collaboration with the Hyosung Group’s R&DB Labs will open a ‘special class on high molecule chemistry’ for Masters and Ph.D. candidates. The class, led by researchers at Hyosung’s R&D think tank, will provide the latest market and technology trends in the molecule chemical industry during the fall semester.
Hyosung joined this special industry program in an effort to enhance students’ hands-on understanding of new technologies that will emerge in the global market. During the semester, Hyosung plans to present the technology portfolios on their brand new materials of TAC film, membrane, and carbon fiber as well as the existing products leading the world in market share such as spandex, tire cords.
Hyosung plans to recruit students who previously took courses led by Hyosung researchers. President Tu-Won Chang of Hyosung R&DB said, “This program is designed to foster highly qualified R&D personnel especially catering to our company’s needs and market demands. We will continue to share our company’s market analysis and R&D know-how with outstanding universities.
2017.09.07 View 5470 -
 Material-Independent Nanocoating Antimicrobial Spray Significantly Extends the Shelf Life of Produce
The edible coating on produce has drawn a great deal of attention in the food and agricultural industry. It could not only prolong postharvest shelf life of produce against external changes in the environment but also provide additional nutrients to be useful for human health. However, most versions of the coating have had intrinsic limitations in their practical application. First, highly specific interactions between coating materials and target surfaces are required for a stable and durable coating. Even further, the coating of bulk substrates, such as fruits, is time consuming or is not achievable in the conventional solution-based coating. In this respect, material-independent and rapid coating strategies are highly demanded.
The research team led by Professor Insung Choi of the Department of Chemistry developed a sprayable nanocoating technique using plant-derived polyphenol that can be applied to any surface. This new nanocoating process can be completed in seconds to form nanometer-thick films, allowing for the coating of commodity goods, such as shoe insoles and fruits, in a controlled fashion. For example, spray-coated mandarin oranges and strawberries show significantly-prolonged postharvest shelf life, suggesting the practical potential in edible coatings of perishable produce.
The technology has been patented and is currently being commercialized for widespread use as a means of preserving produce. The research results have recently been published in Scientific Reports on Aug 1.
Polyphenols, a metabolite of photosynthesis, possess several hydroxyl groups and are found in a large number of plants showing excellent antioxidant properties. They have been widely used as a nontoxic food additive and are known to exhibit antibacterial, as well as potential anti-carcinogenic capabilities. Polyphenols can also be used with iron ions, which are naturally found in the body, to form an adhesive complex, which has been used in leather tanning, ink, etc.
The research team combined these chemical properties of polyphenol-iron complexes with spray techniques to develop their nanocoating technology. Compared to conventional immersion coating methods, which dip substrates in specialized coating solutions, this spray technique can coat the select areas more quickly. The spray also prevents cross contamination, which is a big concern for immersion methods. The research team has showcased the spray’s ability to coat a variety of different materials, including metals, plastics, glass, as well as textile fabrics. The polyphenol complex has been used to form antifogging films on corrective lenses, as well as antifungal treatments for shoe soles, demonstrating the versatility of their technique.
Furthermore, the spray has been used to coat produce with a naturally antibacterial, edible film. The coatings significantly improved the shelf life of tangerines and strawberries, preserving freshness beyond 28 days and 58 hours, respectively. (Uncoated fruit decomposed and became moldy under the same conditions). See the image below.
a –I, II: Uncoated and coated tangerines incubated for 14 and 28 days in daily-life settings
b –I: Uncoated and coated strawberries incubated for 58 hours in daily-life settings
b –II: Statistical investigation of the resulting edibility.
Professor Choi said, “Nanocoating technologies are still in their infancy, but they have untapped potential for exciting applications. As we have shown, nanocoatings can be easily adapted for several different uses, and the creative combination of existing nanomaterials and coating methods can synergize to unlock this potential.”
2017.08.10 View 9674
Material-Independent Nanocoating Antimicrobial Spray Significantly Extends the Shelf Life of Produce
The edible coating on produce has drawn a great deal of attention in the food and agricultural industry. It could not only prolong postharvest shelf life of produce against external changes in the environment but also provide additional nutrients to be useful for human health. However, most versions of the coating have had intrinsic limitations in their practical application. First, highly specific interactions between coating materials and target surfaces are required for a stable and durable coating. Even further, the coating of bulk substrates, such as fruits, is time consuming or is not achievable in the conventional solution-based coating. In this respect, material-independent and rapid coating strategies are highly demanded.
The research team led by Professor Insung Choi of the Department of Chemistry developed a sprayable nanocoating technique using plant-derived polyphenol that can be applied to any surface. This new nanocoating process can be completed in seconds to form nanometer-thick films, allowing for the coating of commodity goods, such as shoe insoles and fruits, in a controlled fashion. For example, spray-coated mandarin oranges and strawberries show significantly-prolonged postharvest shelf life, suggesting the practical potential in edible coatings of perishable produce.
The technology has been patented and is currently being commercialized for widespread use as a means of preserving produce. The research results have recently been published in Scientific Reports on Aug 1.
Polyphenols, a metabolite of photosynthesis, possess several hydroxyl groups and are found in a large number of plants showing excellent antioxidant properties. They have been widely used as a nontoxic food additive and are known to exhibit antibacterial, as well as potential anti-carcinogenic capabilities. Polyphenols can also be used with iron ions, which are naturally found in the body, to form an adhesive complex, which has been used in leather tanning, ink, etc.
The research team combined these chemical properties of polyphenol-iron complexes with spray techniques to develop their nanocoating technology. Compared to conventional immersion coating methods, which dip substrates in specialized coating solutions, this spray technique can coat the select areas more quickly. The spray also prevents cross contamination, which is a big concern for immersion methods. The research team has showcased the spray’s ability to coat a variety of different materials, including metals, plastics, glass, as well as textile fabrics. The polyphenol complex has been used to form antifogging films on corrective lenses, as well as antifungal treatments for shoe soles, demonstrating the versatility of their technique.
Furthermore, the spray has been used to coat produce with a naturally antibacterial, edible film. The coatings significantly improved the shelf life of tangerines and strawberries, preserving freshness beyond 28 days and 58 hours, respectively. (Uncoated fruit decomposed and became moldy under the same conditions). See the image below.
a –I, II: Uncoated and coated tangerines incubated for 14 and 28 days in daily-life settings
b –I: Uncoated and coated strawberries incubated for 58 hours in daily-life settings
b –II: Statistical investigation of the resulting edibility.
Professor Choi said, “Nanocoating technologies are still in their infancy, but they have untapped potential for exciting applications. As we have shown, nanocoatings can be easily adapted for several different uses, and the creative combination of existing nanomaterials and coating methods can synergize to unlock this potential.”
2017.08.10 View 9674 -
 Total Synthesis of Flueggenine C via an Accelerated Intermolecular Rauhut-Currier Reaction
The first total synthesis of dimeric securinega alkaloid (-)-flueggenine C was completed via an accelerated intermolecular Rauhut–Currier (RC) reaction. The research team led by Professor Sunkyu Han in the Department of Chemistry succeeded in synthesizing the natural product by reinventing the conventional RC reaction.
The total synthesis of natural products refers to the process of synthesizing secondary metabolites isolated from living organisms in the laboratory through a series of chemical reactions. Each stage of chemical reaction needs to be successful to produce the final target molecule, and thus the process requires high levels of patience and creativity. For that reason, the researchers working on natural products total synthesis are often called “molecular artists”.
Despite numerous reports on the total synthesis of monomeric securinegas, the synthesis of dimeric securinegas, whose monomeric units are connected by a putative enzymatic RC reaction, has not been reported to date.
The team used a Rauhut-Currier (RC) reaction, a carboncarbon bond forming a reaction between two Michael acceptors first reported by Rauhut and Currier in 1963, to successfully synthesize a dimeric natural product, flueggenine C. This new work featured the first application of an intermolecular RC reaction in total synthesis.
The conventional intermolecular RC reaction was driven non-selectively by a toxic nucleophilic catalyst at a high temperature of over 150°C and a highly concentrated reaction mixture, and thus has never been applied to natural products total synthesis. To overcome this long-standing problem, the research team placed a nucleophilic moiety at the γ-position of the enone derivative. As a result, the RC reaction could be induced by the simple addition of a base at ambient temperature and dilute solution, without the need of a nucleophilic catalyst. Using this newly discovered reactivity, the team successfully synthesized the natural product (-)-flueggenine C from commercially available amino acid derivative in 12 steps.
Professor Han said, “Our key finding regarding the remarkably improved reactivity and selectivity of the intermolecular RC reaction will serve as a significant stepping stone in allowing this reaction to be considered a practical and reliable chemical tool with broad applicability in natural products, pharmaceuticals, and materials syntheses. ”
This research was led by Ph.D. candidate Sangbin Jeon and was published in The Journal of the American Chemical Society (JACS) on May 10. This research was funded by KAIST start-up funds, HRHR (High-Risk High-Return), RED&B (Research, Education, Development & Business) projects, the National Research Foundation of Korea, and the Institute for Basic Science.
(Figure 1: Representative dimeric/oligomeric securinega alkaloids)
(Figure 2: Our reinvented Rauhut-Currier reaction)
(Figure 3: Total Synthesis of (-)-flueggenine C)
2017.05.23 View 9917
Total Synthesis of Flueggenine C via an Accelerated Intermolecular Rauhut-Currier Reaction
The first total synthesis of dimeric securinega alkaloid (-)-flueggenine C was completed via an accelerated intermolecular Rauhut–Currier (RC) reaction. The research team led by Professor Sunkyu Han in the Department of Chemistry succeeded in synthesizing the natural product by reinventing the conventional RC reaction.
The total synthesis of natural products refers to the process of synthesizing secondary metabolites isolated from living organisms in the laboratory through a series of chemical reactions. Each stage of chemical reaction needs to be successful to produce the final target molecule, and thus the process requires high levels of patience and creativity. For that reason, the researchers working on natural products total synthesis are often called “molecular artists”.
Despite numerous reports on the total synthesis of monomeric securinegas, the synthesis of dimeric securinegas, whose monomeric units are connected by a putative enzymatic RC reaction, has not been reported to date.
The team used a Rauhut-Currier (RC) reaction, a carboncarbon bond forming a reaction between two Michael acceptors first reported by Rauhut and Currier in 1963, to successfully synthesize a dimeric natural product, flueggenine C. This new work featured the first application of an intermolecular RC reaction in total synthesis.
The conventional intermolecular RC reaction was driven non-selectively by a toxic nucleophilic catalyst at a high temperature of over 150°C and a highly concentrated reaction mixture, and thus has never been applied to natural products total synthesis. To overcome this long-standing problem, the research team placed a nucleophilic moiety at the γ-position of the enone derivative. As a result, the RC reaction could be induced by the simple addition of a base at ambient temperature and dilute solution, without the need of a nucleophilic catalyst. Using this newly discovered reactivity, the team successfully synthesized the natural product (-)-flueggenine C from commercially available amino acid derivative in 12 steps.
Professor Han said, “Our key finding regarding the remarkably improved reactivity and selectivity of the intermolecular RC reaction will serve as a significant stepping stone in allowing this reaction to be considered a practical and reliable chemical tool with broad applicability in natural products, pharmaceuticals, and materials syntheses. ”
This research was led by Ph.D. candidate Sangbin Jeon and was published in The Journal of the American Chemical Society (JACS) on May 10. This research was funded by KAIST start-up funds, HRHR (High-Risk High-Return), RED&B (Research, Education, Development & Business) projects, the National Research Foundation of Korea, and the Institute for Basic Science.
(Figure 1: Representative dimeric/oligomeric securinega alkaloids)
(Figure 2: Our reinvented Rauhut-Currier reaction)
(Figure 3: Total Synthesis of (-)-flueggenine C)
2017.05.23 View 9917 -
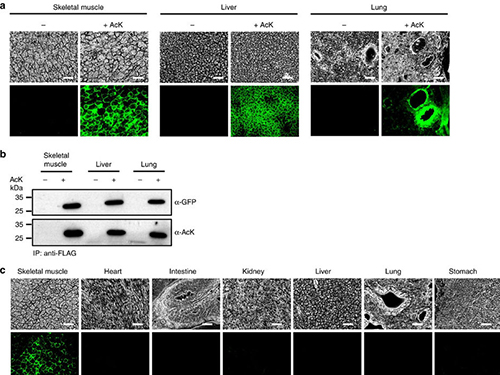 Expanding the Genetic Code of Mus Musculus
Professor Hee-Sung Park of the Department of Chemistry, who garnered attention for his novel strategy of installing authentic post-translational modifications into recombinant proteins, expanded his research portfolio to another level. Professor Park’s team was the first to report the generation of a mouse strain with an expanded genetic code, allowing site-specific incorporation of unnatural amino acids.
Professor Park published the research on the new chemical biology method for achieving selective chemical modifications in proteins in Science last September. The research team, this time in collaboration with Professor Chan Bae Park of the Department of Physiology at the Ajou University School of Medicine, demonstrated temporal and spatial control of protein acetylation in various organs of the transgenic mouse using a recombinant green fluorescent protein as a model protein. This research was published in the online edition of Nature Communications on February 21.
This approach enables the rapid onset of position-specific acetylation of a target protein at any developmental stage, facilitating temporal and spatial control of protein acetylation in various organs of the transgenic mouse. Such temporal and spatial control of protein acetylation will be of prime importance for investigating many essential biological processes and human diseases at the tissue and organism level.
Almost all human proteins, the products of about 25,000 genes, are known to undergo various post-translational modifications during and after synthesis. Post-translation modifications regulate the function of cellular proteins, playing a key role in many essential processes such as delivering signals and body growth. However, the unusual protein modifications, aroused from genetic and/or environmental factors, trigger severe diseases including cancer, dementia, and diabetes.
The team inserted transgenes into the mouse genome to allocate the site-specific addition of unnatural amino acids. The researchers inserted a modified version of lysine into the house mice, which allowed for the control of the acetylation. They used recombinant green fluorescent proteins from transgenic house mice as models for control of the acetylation.
The team was also able to regulate the acetylation of specific temporal and spatial frames in the mice, restraining the abnormality in proteins to certain organs such as the liver and kidneys. The research team said the strategy will provide a powerful tool for systematic in vivo study of cellular proteins in the most commonly used mammalian model organisms for human physiology and disease. Professor Park said, “This method can be easily extended to generate a wide range of custom-made transgenic mouse strains for further investigating diverse proteins of interest.” He added, “This method can be further extended to generate a wide range of custom-made transgenic mouse strains, opening a new paradigm for investigating anti-cancer and cerebral disease treatments.
This work was supported by grants from KAIST Systems Healthcare and the Medicinal Bioconvergence Research Center and the Intelligent Synthetic Biology Center of the Global Frontier Project funded by the Ministry of Science, ICT & Future Planning and the Ministry of Food and Drug Safety.
(Figure:Temporal and spatial control of in vivo protein acetylation)
(a) Temporal expression of acetylated GFPuv in the AcK-GFPamber mouse. The expression of GFPuv in skeletal muscle, liver, and lung tissues was detected only in the AcK-injected mouse. Scale bar, 200 µm. (b) Western blotting of anti-FLAG-immunoprecipitated proteins from tissues of the AcK-GFPamber mouse. Acetylated GFPuv was produced after AcK injection. (c) Spatial expression of acetylated GFPuv in the AcK-GFPamber mouse. Acetylated GFPuv was observed only in skeletal muscle when AcK was directly delivered to the tissues. Sacle bar, 200 µm.
2017.03.27 View 10080
Expanding the Genetic Code of Mus Musculus
Professor Hee-Sung Park of the Department of Chemistry, who garnered attention for his novel strategy of installing authentic post-translational modifications into recombinant proteins, expanded his research portfolio to another level. Professor Park’s team was the first to report the generation of a mouse strain with an expanded genetic code, allowing site-specific incorporation of unnatural amino acids.
Professor Park published the research on the new chemical biology method for achieving selective chemical modifications in proteins in Science last September. The research team, this time in collaboration with Professor Chan Bae Park of the Department of Physiology at the Ajou University School of Medicine, demonstrated temporal and spatial control of protein acetylation in various organs of the transgenic mouse using a recombinant green fluorescent protein as a model protein. This research was published in the online edition of Nature Communications on February 21.
This approach enables the rapid onset of position-specific acetylation of a target protein at any developmental stage, facilitating temporal and spatial control of protein acetylation in various organs of the transgenic mouse. Such temporal and spatial control of protein acetylation will be of prime importance for investigating many essential biological processes and human diseases at the tissue and organism level.
Almost all human proteins, the products of about 25,000 genes, are known to undergo various post-translational modifications during and after synthesis. Post-translation modifications regulate the function of cellular proteins, playing a key role in many essential processes such as delivering signals and body growth. However, the unusual protein modifications, aroused from genetic and/or environmental factors, trigger severe diseases including cancer, dementia, and diabetes.
The team inserted transgenes into the mouse genome to allocate the site-specific addition of unnatural amino acids. The researchers inserted a modified version of lysine into the house mice, which allowed for the control of the acetylation. They used recombinant green fluorescent proteins from transgenic house mice as models for control of the acetylation.
The team was also able to regulate the acetylation of specific temporal and spatial frames in the mice, restraining the abnormality in proteins to certain organs such as the liver and kidneys. The research team said the strategy will provide a powerful tool for systematic in vivo study of cellular proteins in the most commonly used mammalian model organisms for human physiology and disease. Professor Park said, “This method can be easily extended to generate a wide range of custom-made transgenic mouse strains for further investigating diverse proteins of interest.” He added, “This method can be further extended to generate a wide range of custom-made transgenic mouse strains, opening a new paradigm for investigating anti-cancer and cerebral disease treatments.
This work was supported by grants from KAIST Systems Healthcare and the Medicinal Bioconvergence Research Center and the Intelligent Synthetic Biology Center of the Global Frontier Project funded by the Ministry of Science, ICT & Future Planning and the Ministry of Food and Drug Safety.
(Figure:Temporal and spatial control of in vivo protein acetylation)
(a) Temporal expression of acetylated GFPuv in the AcK-GFPamber mouse. The expression of GFPuv in skeletal muscle, liver, and lung tissues was detected only in the AcK-injected mouse. Scale bar, 200 µm. (b) Western blotting of anti-FLAG-immunoprecipitated proteins from tissues of the AcK-GFPamber mouse. Acetylated GFPuv was produced after AcK injection. (c) Spatial expression of acetylated GFPuv in the AcK-GFPamber mouse. Acetylated GFPuv was observed only in skeletal muscle when AcK was directly delivered to the tissues. Sacle bar, 200 µm.
2017.03.27 View 10080 -
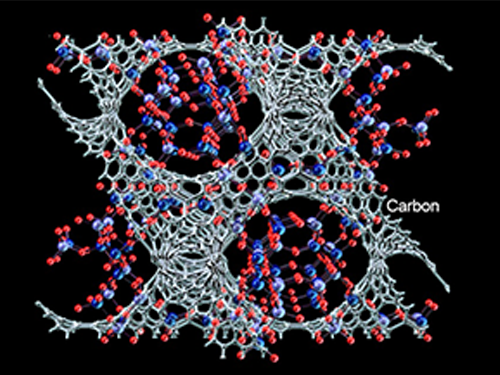 Synthesized Microporous 3D Graphene-like Carbons
Distinguished Professor Ryong Ryoo of the Chemistry Department at KAIST, who is also the Director of the Center for Nanomaterials and Carbon Materials at the Institute for Basic Science (IBS), and his research team have recently published their research results entitled "Lanthanum-catalysed Synthesis of Microporous 3D Graphene-like Carbons in a Zeolite Template" on June 29, 2016 in Nature on a new method to synthesize carbons having graphene structures with 3D periodic micropores, a trait resulted from using a zeolite as a template for the synthesis. The research team expects this technology to find a range of useful applications such as in batteries and catalysts.
Graphene, an allotrope of carbon, which was discovered more than a decade ago, has led to myriad research that seeks to unlock its vast potential. Zeolites, commonly used microporous solid catalysts in the petrochemical industry, have recently attracted attention in the field of material science as a template for carbon synthesis. Zeolites’ individual crystal is distinguished by its unique 1 nanometer (nm)-size pore structures. These structures facilitate the accommodation of carbon nanotubes inside zeolites. In their paper, the research team showed that these nanoporous systems are an ideal template for the carbon synthesis of three-dimensional (3D) graphene architecture, but zeolite pores are too small to accommodate bulky molecular compounds like polyaromatic and furfuryl alcohol that are often used in carbon synthesis. Small molecules like ethylene and acetylene can be used as a carbon source to achieve successful carbonization within zeolite pores, but it comes at a great cost. The high temperatures required for the synthesis cause reactions of carbons being deposited randomly on the external surfaces of zeolites as well as their internal pore walls, resulting in coke deposition and consequently, causing serious diffusion limitations in the zeolite pores.
The team from the IBS Center for Nanomaterials and Carbon Materials solved this conundrum with a novel approach. First author Dr. KIM Kyoungsoo explains: “The zeolite-template carbon synthesis has existed for a long time, but the problem with temperatures has foiled many scientists from extracting their full potential. Here, our team sought to find the answer by embedding lanthanum ions (La3+), a silvery-white metal element, in zeolite pores. This lowers the temperature required for the carbonization of ethylene or acetylene. Graphene-like sp2 carbon structures can be selectively formed inside the zeolite template, without carbon deposition at the external surfaces. After the zeolite template is removed, the carbon framework exhibits the electrical conductivity two orders of magnitude higher than amorphous mesoporous carbon, which is a pretty astonishing result. This highly efficient synthesis strategy based on the lanthanum ions renders the carbon framework to be formed in pores with a less than 1 nm diameter, just like as easily reproducible as in mesoporous templates. This provides a general method to synthesize carbon nanostructures with various topologies corresponding to the template zeolite pore topologies, such as FAU, EMT, beta, LTL, MFI, and LTA. Also, all the synthesis can be readily scaled up, which is important for practical applications in areas of batteries, fuel storage, and other zeolite-like catalyst supports.”
The research team began their experiment by utilizing La3+ ions. Dr. KIM elucidates why this silvery-white element proved so beneficial to the team, “La3+ ions are unreducible under carbonization process condition, so they can stay inside the zeolite pores instead of moving to the outer zeolite surface in the form of reduced metal particles. Within the pores, they can stabilize ethylene and the pyrocondensation intermediately to form a carbon framework in zeolites.”
In order to test this hypothesis, the team compared the amount of carbon deposited in La3+-containing form of Y zeolite (LaY) sample against a host of other samples such as NaY and HY. The experimental results indicate that all the LaY, NaY, and HY zeolite samples show rapid carbon deposition at 800°C. However, as the temperature decreases, there appears to be a dramatic difference between the different ionic forms of zeolites. At 600°C, the LaY zeolite is still active as a carbon deposition template. In contrast, both NaY and HY lose their carbon deposition functions almost completely.
The results, according to their paper published in Nature, highlight a catalytic effect of lanthanum for carbonization. By making graphene with 3D periodic nanoporous architectures, it promises a wide range of useful applications such as in batteries and catalysts but due to the lack of efficient synthetic strategies, such applications have not yet been successful. By taking advantage of the pore-selective carbon filling at decreased temperatures, the synthesis can readily be scaled up for studies requiring bulk quantities of carbon, in particular high electrical conductivity, which is a highly sought aspect for the production of batteries.
YouTube Link: https://youtu.be/lkNiHiB8lBk
Image 1: (Top to Bottom) Zeolite Template: Microporous Aluminosilicate; Zeolite ion exchanged with La3+ ions in aqueous solution; and Zeolite Template with La3+ ions
Image 2: (Top to Bottom) Catalytic carbonization progressed at La3+ ions-exchanged sites using ethylene as a carbon precursor. Carbon is highlighted in grey; Zeolite template removed in an acid solution (HF/ HCl); Microporous 3D graphene-like carbon
2016.07.01 View 9765
Synthesized Microporous 3D Graphene-like Carbons
Distinguished Professor Ryong Ryoo of the Chemistry Department at KAIST, who is also the Director of the Center for Nanomaterials and Carbon Materials at the Institute for Basic Science (IBS), and his research team have recently published their research results entitled "Lanthanum-catalysed Synthesis of Microporous 3D Graphene-like Carbons in a Zeolite Template" on June 29, 2016 in Nature on a new method to synthesize carbons having graphene structures with 3D periodic micropores, a trait resulted from using a zeolite as a template for the synthesis. The research team expects this technology to find a range of useful applications such as in batteries and catalysts.
Graphene, an allotrope of carbon, which was discovered more than a decade ago, has led to myriad research that seeks to unlock its vast potential. Zeolites, commonly used microporous solid catalysts in the petrochemical industry, have recently attracted attention in the field of material science as a template for carbon synthesis. Zeolites’ individual crystal is distinguished by its unique 1 nanometer (nm)-size pore structures. These structures facilitate the accommodation of carbon nanotubes inside zeolites. In their paper, the research team showed that these nanoporous systems are an ideal template for the carbon synthesis of three-dimensional (3D) graphene architecture, but zeolite pores are too small to accommodate bulky molecular compounds like polyaromatic and furfuryl alcohol that are often used in carbon synthesis. Small molecules like ethylene and acetylene can be used as a carbon source to achieve successful carbonization within zeolite pores, but it comes at a great cost. The high temperatures required for the synthesis cause reactions of carbons being deposited randomly on the external surfaces of zeolites as well as their internal pore walls, resulting in coke deposition and consequently, causing serious diffusion limitations in the zeolite pores.
The team from the IBS Center for Nanomaterials and Carbon Materials solved this conundrum with a novel approach. First author Dr. KIM Kyoungsoo explains: “The zeolite-template carbon synthesis has existed for a long time, but the problem with temperatures has foiled many scientists from extracting their full potential. Here, our team sought to find the answer by embedding lanthanum ions (La3+), a silvery-white metal element, in zeolite pores. This lowers the temperature required for the carbonization of ethylene or acetylene. Graphene-like sp2 carbon structures can be selectively formed inside the zeolite template, without carbon deposition at the external surfaces. After the zeolite template is removed, the carbon framework exhibits the electrical conductivity two orders of magnitude higher than amorphous mesoporous carbon, which is a pretty astonishing result. This highly efficient synthesis strategy based on the lanthanum ions renders the carbon framework to be formed in pores with a less than 1 nm diameter, just like as easily reproducible as in mesoporous templates. This provides a general method to synthesize carbon nanostructures with various topologies corresponding to the template zeolite pore topologies, such as FAU, EMT, beta, LTL, MFI, and LTA. Also, all the synthesis can be readily scaled up, which is important for practical applications in areas of batteries, fuel storage, and other zeolite-like catalyst supports.”
The research team began their experiment by utilizing La3+ ions. Dr. KIM elucidates why this silvery-white element proved so beneficial to the team, “La3+ ions are unreducible under carbonization process condition, so they can stay inside the zeolite pores instead of moving to the outer zeolite surface in the form of reduced metal particles. Within the pores, they can stabilize ethylene and the pyrocondensation intermediately to form a carbon framework in zeolites.”
In order to test this hypothesis, the team compared the amount of carbon deposited in La3+-containing form of Y zeolite (LaY) sample against a host of other samples such as NaY and HY. The experimental results indicate that all the LaY, NaY, and HY zeolite samples show rapid carbon deposition at 800°C. However, as the temperature decreases, there appears to be a dramatic difference between the different ionic forms of zeolites. At 600°C, the LaY zeolite is still active as a carbon deposition template. In contrast, both NaY and HY lose their carbon deposition functions almost completely.
The results, according to their paper published in Nature, highlight a catalytic effect of lanthanum for carbonization. By making graphene with 3D periodic nanoporous architectures, it promises a wide range of useful applications such as in batteries and catalysts but due to the lack of efficient synthetic strategies, such applications have not yet been successful. By taking advantage of the pore-selective carbon filling at decreased temperatures, the synthesis can readily be scaled up for studies requiring bulk quantities of carbon, in particular high electrical conductivity, which is a highly sought aspect for the production of batteries.
YouTube Link: https://youtu.be/lkNiHiB8lBk
Image 1: (Top to Bottom) Zeolite Template: Microporous Aluminosilicate; Zeolite ion exchanged with La3+ ions in aqueous solution; and Zeolite Template with La3+ ions
Image 2: (Top to Bottom) Catalytic carbonization progressed at La3+ ions-exchanged sites using ethylene as a carbon precursor. Carbon is highlighted in grey; Zeolite template removed in an acid solution (HF/ HCl); Microporous 3D graphene-like carbon
2016.07.01 View 9765 -
 Efficient Methane C-H Bond Activated by KAIST and UPenn Teams
Professor Mu-Hyun Baik of the Chemistry Department at KAIST and his team collaborated with an international team to discover a novel chemical reaction, carbon-hydrogen borylation using methane, and their research results were published in the March 25th issue of Science.
For details, please refer to the following press release from the Institute for Basic Sciences (IBS) in Korea and the University of Pennsylvania in the United States.
Efficient Methane C-H Bond Activation Achieved for the First Time
The Institute for Basic Science, March 24, 2016
Penn Chemists Lay Groundwork for Countless New, Cleaner Uses of Methane
University of Pennsylvania, March 24, 2016
2016.03.25 View 10109
Efficient Methane C-H Bond Activated by KAIST and UPenn Teams
Professor Mu-Hyun Baik of the Chemistry Department at KAIST and his team collaborated with an international team to discover a novel chemical reaction, carbon-hydrogen borylation using methane, and their research results were published in the March 25th issue of Science.
For details, please refer to the following press release from the Institute for Basic Sciences (IBS) in Korea and the University of Pennsylvania in the United States.
Efficient Methane C-H Bond Activation Achieved for the First Time
The Institute for Basic Science, March 24, 2016
Penn Chemists Lay Groundwork for Countless New, Cleaner Uses of Methane
University of Pennsylvania, March 24, 2016
2016.03.25 View 10109 -
 KAIST Develops New Technique for Chiral Activity in Molecules
Professor Hyunwoo Kim of the Chemistry Department and his research team have developed a technique that can easily analyze the optical activity of charged compounds by using nuclear magnetic resonance (NMR) spectroscopy. The research finding entitled “H NMR Chiral Analysis of Charged Molecules via Ion Pairing with Aluminum Complexes” was published online in the October 19th issue of The Journal of the American Chemical Society.
The technique relies on observation of the behavior of optical isomers. Molecules with the same composition that are mirror images of each other are optical isomers. For example, the building blocks of all living organisms, amino acids, are a single optical isomer. In our bodies, optical isomers bring different physiological changes due to their distinct optical activities. Therefore, controlling and analyzing the optical activities are critical when developing a new drug.
High-performance liquid chromatography (HPLC) is the de facto standard of analyzing the optical activity of a compound. However, HPLC is very expensive that many laboratories can’t afford to have. In addition, with the machine, one analysis may take 30 minutes to one hour to complete. It lacks in signal sensitivity and chemical decomposition, and the application is limited to nonpolar compounds.
Usually adopted in analyzing the structure of a chemical compound, NMR spectroscopy requires only one to five minutes per single analysis. Since it is essential for analyzing the molecular structure, many chemistry labs have NMR equipment. However, until this technique was invented, no other research team had reported an effective way of using the NMR spectroscopy to decompose the signal of chiral activity of a compound.
The research team uses negatively-charged metal compounds in NMR spectroscopy. The technique employs negatively-charged metal compounds which bond ionically to positively- and negatively-charged optical compounds. As a result, the NMR spectroscopy can distinguish the signal from chiral activity. Not only can it analyze various chemicals without structural constraints, but it can also be used for both nonpolar and polar solvents.
As many compounds for new drugs have functional groups, which can be charged, this analysis method can be directly employed in the development process of drugs. Professor Kim said, “A revolutionary analysis method has been developed using simple chemical principles. I hope that our method will be applied to the development of new medicine.”
This research was sponsored by the Center for Nanomaterials and Chemical Reactions at the Institute for Basic Science and the Supercomputing Research Center of KAIST.
Picture 1: Separations of NMR Signals of Chemicals due to Interaction with Metal Compounds
Picture 2: Separations of NMR Signals in Different Chemicals
2015.11.20 View 12193
KAIST Develops New Technique for Chiral Activity in Molecules
Professor Hyunwoo Kim of the Chemistry Department and his research team have developed a technique that can easily analyze the optical activity of charged compounds by using nuclear magnetic resonance (NMR) spectroscopy. The research finding entitled “H NMR Chiral Analysis of Charged Molecules via Ion Pairing with Aluminum Complexes” was published online in the October 19th issue of The Journal of the American Chemical Society.
The technique relies on observation of the behavior of optical isomers. Molecules with the same composition that are mirror images of each other are optical isomers. For example, the building blocks of all living organisms, amino acids, are a single optical isomer. In our bodies, optical isomers bring different physiological changes due to their distinct optical activities. Therefore, controlling and analyzing the optical activities are critical when developing a new drug.
High-performance liquid chromatography (HPLC) is the de facto standard of analyzing the optical activity of a compound. However, HPLC is very expensive that many laboratories can’t afford to have. In addition, with the machine, one analysis may take 30 minutes to one hour to complete. It lacks in signal sensitivity and chemical decomposition, and the application is limited to nonpolar compounds.
Usually adopted in analyzing the structure of a chemical compound, NMR spectroscopy requires only one to five minutes per single analysis. Since it is essential for analyzing the molecular structure, many chemistry labs have NMR equipment. However, until this technique was invented, no other research team had reported an effective way of using the NMR spectroscopy to decompose the signal of chiral activity of a compound.
The research team uses negatively-charged metal compounds in NMR spectroscopy. The technique employs negatively-charged metal compounds which bond ionically to positively- and negatively-charged optical compounds. As a result, the NMR spectroscopy can distinguish the signal from chiral activity. Not only can it analyze various chemicals without structural constraints, but it can also be used for both nonpolar and polar solvents.
As many compounds for new drugs have functional groups, which can be charged, this analysis method can be directly employed in the development process of drugs. Professor Kim said, “A revolutionary analysis method has been developed using simple chemical principles. I hope that our method will be applied to the development of new medicine.”
This research was sponsored by the Center for Nanomaterials and Chemical Reactions at the Institute for Basic Science and the Supercomputing Research Center of KAIST.
Picture 1: Separations of NMR Signals of Chemicals due to Interaction with Metal Compounds
Picture 2: Separations of NMR Signals in Different Chemicals
2015.11.20 View 12193 -
 The Real Time Observation of the Birth of a Molecule
From right to left: Dr. Kyung-Hwan Kim, Professor Hyotcherl Lhee, and Jong-Gu Kim, a Ph.D. candidate
Professor Hyotcherl Lhee of the Department of Chemistry at KAIST and Japanese research teams jointly published their research results showing that they have succeeded in the direct observation of how atoms form a molecule in the online issue of Nature on February 19, 2015.
The researchers used water in which gold atoms ([Au(CN) 2- ]) are dissolved and fired X-ray pulses over the specimen in femtosecond timescales to study chemical reactions taking place among the gold atoms. They were able to examine in real time the instant process of how gold atoms bond together to become a molecule, to a trimer or tetramer state.
This direct viewing of the formation of a gold trimer complex ([Au(CN) 2- ] 3 ) will provide an opportunity to understand complex chemical and biological systems.
For details, please see the following press release that was distributed by the High Energy Accelerator Research Organization, KEK, in Japan:
Direct Observation of Bond Formations
February 18, 2015
A collaboration between researchers from KEK, the Institute for Basic Science (IBS), the Korea Advanced Institute of Science and Technology (KAIST), RIKEN, and the Japan Synchrotron Radiation Research Institute (JASRI) used the SACLA X-ray free electron laser (XFEL) facility for a real time visualization of the birth of a molecular that occurs via photoinduced formation of a chemical bonds. This achievement was published in the online version of the scientific journal “Nature” (published on 19 February 2015).
Direct “observation” of the bond making, through a chemical reaction, has been longstanding dream for chemists. However, the distance between atoms is very small, at about 100 picometer, and the bonding is completed very quickly, taking less than one picosecond (ps). Hence, previously, one could only imagine the bond formation between atoms while looking at the chemical reaction progressing in the test-tube.
In this study, the research group focused on the process of photoinduced bond formation between gold (Au) ions dissolved in water. In the ground state (S 0 state in Fig. 1) Au ions that are weakly bound to each other by an electron affinity and aligned in a bent geometry. Upon a photoexcitation, the S 0 state rapidly converts into an excited (S 1 state in Fig. 1) state where Au-Au covalent bonds are formed among Au ions aligned in a linear geometry. Subsequently, the S 1 state transforms to a triplet state (T 1 state in Fig. 1) in 1.6 ps while accompanying further contraction of Au-Au bonds by 0.1 Å. Later, the T 1 state of the trimer converts to a tetramer (tetramer state in Fig. 1) on nanosecond time scale. Finally, the Au ions returned to their original loosely interacting bent structure.
In this research, the direct observation of a very fast chemical reaction, induced by the photo-excitation, was succeeded (Fig. 2, 3). Therefore, this method is expected to be a fundamental technology for understanding the light energy conversion reaction. The research group is actively working to apply this method to the development of viable renewable energy resources, such as a photocatalysts for artificial photosynthesis using sunlight.
This research was supported by the X-ray Free Electron Laser Priority Strategy Program of the MEXT, PRESTO of the JST, and the the Innovative Areas "Artificial Photosynthesis (AnApple)" grant from the Japan Society for the Promotion of Science (JSPS).
Publication: Nature , 518 (19 February 2015)
Title: Direct observation of bond formation in solution with femtosecond X-ray scattering
Authors: K. H. Kim 1 , J. G. Kim 1 , S. Nozawa 1 , T. Sato 1 , K. Y. Oang, T. W. Kim, H. Ki, J. Jo, S. Park, C. Song, T. Sato, K. Ogawa, T. Togashi, K. Tono, M. Yabashi, T. Ishikawa, J. Kim, R. Ryoo, J. Kim, H. Ihee, S. Adachi. ※ 1: These authors contributed equally to the work.
DOI: 10.1038/nature14163
Figure 1. Structure of a gold cyano trimer complex (Au(CN) 2 - ) 3 .
Figure 2. Observed changes in the molecular structure of the gold complex
Figure 3. Schematic view of the research of photo-chemical reactions by the molecular movie
2015.02.27 View 13147
The Real Time Observation of the Birth of a Molecule
From right to left: Dr. Kyung-Hwan Kim, Professor Hyotcherl Lhee, and Jong-Gu Kim, a Ph.D. candidate
Professor Hyotcherl Lhee of the Department of Chemistry at KAIST and Japanese research teams jointly published their research results showing that they have succeeded in the direct observation of how atoms form a molecule in the online issue of Nature on February 19, 2015.
The researchers used water in which gold atoms ([Au(CN) 2- ]) are dissolved and fired X-ray pulses over the specimen in femtosecond timescales to study chemical reactions taking place among the gold atoms. They were able to examine in real time the instant process of how gold atoms bond together to become a molecule, to a trimer or tetramer state.
This direct viewing of the formation of a gold trimer complex ([Au(CN) 2- ] 3 ) will provide an opportunity to understand complex chemical and biological systems.
For details, please see the following press release that was distributed by the High Energy Accelerator Research Organization, KEK, in Japan:
Direct Observation of Bond Formations
February 18, 2015
A collaboration between researchers from KEK, the Institute for Basic Science (IBS), the Korea Advanced Institute of Science and Technology (KAIST), RIKEN, and the Japan Synchrotron Radiation Research Institute (JASRI) used the SACLA X-ray free electron laser (XFEL) facility for a real time visualization of the birth of a molecular that occurs via photoinduced formation of a chemical bonds. This achievement was published in the online version of the scientific journal “Nature” (published on 19 February 2015).
Direct “observation” of the bond making, through a chemical reaction, has been longstanding dream for chemists. However, the distance between atoms is very small, at about 100 picometer, and the bonding is completed very quickly, taking less than one picosecond (ps). Hence, previously, one could only imagine the bond formation between atoms while looking at the chemical reaction progressing in the test-tube.
In this study, the research group focused on the process of photoinduced bond formation between gold (Au) ions dissolved in water. In the ground state (S 0 state in Fig. 1) Au ions that are weakly bound to each other by an electron affinity and aligned in a bent geometry. Upon a photoexcitation, the S 0 state rapidly converts into an excited (S 1 state in Fig. 1) state where Au-Au covalent bonds are formed among Au ions aligned in a linear geometry. Subsequently, the S 1 state transforms to a triplet state (T 1 state in Fig. 1) in 1.6 ps while accompanying further contraction of Au-Au bonds by 0.1 Å. Later, the T 1 state of the trimer converts to a tetramer (tetramer state in Fig. 1) on nanosecond time scale. Finally, the Au ions returned to their original loosely interacting bent structure.
In this research, the direct observation of a very fast chemical reaction, induced by the photo-excitation, was succeeded (Fig. 2, 3). Therefore, this method is expected to be a fundamental technology for understanding the light energy conversion reaction. The research group is actively working to apply this method to the development of viable renewable energy resources, such as a photocatalysts for artificial photosynthesis using sunlight.
This research was supported by the X-ray Free Electron Laser Priority Strategy Program of the MEXT, PRESTO of the JST, and the the Innovative Areas "Artificial Photosynthesis (AnApple)" grant from the Japan Society for the Promotion of Science (JSPS).
Publication: Nature , 518 (19 February 2015)
Title: Direct observation of bond formation in solution with femtosecond X-ray scattering
Authors: K. H. Kim 1 , J. G. Kim 1 , S. Nozawa 1 , T. Sato 1 , K. Y. Oang, T. W. Kim, H. Ki, J. Jo, S. Park, C. Song, T. Sato, K. Ogawa, T. Togashi, K. Tono, M. Yabashi, T. Ishikawa, J. Kim, R. Ryoo, J. Kim, H. Ihee, S. Adachi. ※ 1: These authors contributed equally to the work.
DOI: 10.1038/nature14163
Figure 1. Structure of a gold cyano trimer complex (Au(CN) 2 - ) 3 .
Figure 2. Observed changes in the molecular structure of the gold complex
Figure 3. Schematic view of the research of photo-chemical reactions by the molecular movie
2015.02.27 View 13147 -
 Eggshell-like Cell Encapsulation and Degradation Technology Developed
Some bacteria form endospores on cell walls to protect their DNA in case of nutrient deficiency. When an endospore meets a suitable environment for survival, the cell can revert to the original state from which it can reproduce.
The technique that can artificially control such phenomenon was developed by an international team of researchers. At first, a cell is wrapped and preserved like an egg. When the cell is needed, the technique allows the endospore to decompose while it is alive. Future applications for this technique include cell-based biosensor, cell therapy, and biocatalyst processes.
Professors Insung Choi and Younghoon Lee from the Department of Chemistry at KAIST as well as and Professor Frank Caruso from the University of Melbourne developed this technique which permits a cell to stay alive by coating it with film on a nanometer scale and then to be decomposed while it is alive.
The research finding was published in the November 10th issue of Angewandte Chemie International Edition as the lead article.
Cell encapsulation allows researchers to capture a cell in a tight capsule while it is alive. It is highly recognized in cell-based applications where the control of cell stability and cell-division is the biggest issue.
Traditional cell encapsulation methods utilized organic film or inorganic capsules that are made of organic film moldings. Although these films tightly closed around the cell, because they were not easily decomposable, it was difficult to apply the method.
The research team succeeded in encapsulating each cell in a metal-polyphenol film by mixing tannic acid and iron ion solution with yeast cells.
Usually extracted from oak barks or grape peels, tannic acid is a natural substance. It forms a metal-polyphenol film within ten seconds when it meets iron ions due to its high affinity with cells. Cells encapsulated with this film presented high survival rates. Since the film forms quickly in a simple manner, it was possible to obtain large amount of encapsulated cells.
The research team also found that the metal-polyphenol film was stable in neutral pH, but is easily degradable under a weak acidic condition. Using this property, they were able to control cell division by restoring the cell to its pre-encapsulated state at a desired moment.
Protecting the cell from the external environment like an egg shell, the metal-polyphenol film protected the cell against foreign conditions such as lytic enzymes, extended exposure to UV radiation, and silver nanoparticles. The research indicated that the encapsulated cells had a high survival rate even under extreme environments.
Professor Lee said that “not only the cells remain alive during the encapsulation stage, but also they can be protected under extreme environment.” He added, “This is an advanced cell encapsulation technology that allows controlling cell-division of those cells through responsive shell degradation on-demand.”
Professor Choi commented, “Although the cell encapsulation technology is still in its infancy, as the technology matures the application of cell-manipulation technology will be actualized.” He highlighted that “it will serve as a breakthrough to problems faced by cell-based applications.”
Sponsored by the Ministry of Science, ICT and Future Planning and the National Research Foundation of Korea, the research was led by two Master’s candidates, Ji Hun Park and Kyung Hwan Kim, under the joint guidance of research professors from KAIST and the University of Melbourne.
Figure 1: Lead article of Angewandte Chemie
Background: Shows a live native yeast (in green) encapsulated in a metal-polyphenol film (in red) illustrating the vitality of the yeast
Front: A native yeast at each encapsulation stage
Pictured on the bottom left is a cell prior to encapsulation. Following the red arrow, the native yeast is in purple to show metal-polyphenol film formed around the cell. The cell after the green arrow is a visualization of the degradation of the film in weak acidic condition.
Figure 2: A mimetic diagram of cell encapsulation with a metal-polyphenol film
Top: A native yeast before encapsulation
Middle: A native yeast encapsulated with Tannic Acid-Fe (III) Nanoshell – cell-division of the encapsulated cell is controlled by pH and the shell is protected against silver nanoparticle, lytic enzyme, and UV-C
Bottom: Shell degradation on-demand depending on pH
2014.11.18 View 10916
Eggshell-like Cell Encapsulation and Degradation Technology Developed
Some bacteria form endospores on cell walls to protect their DNA in case of nutrient deficiency. When an endospore meets a suitable environment for survival, the cell can revert to the original state from which it can reproduce.
The technique that can artificially control such phenomenon was developed by an international team of researchers. At first, a cell is wrapped and preserved like an egg. When the cell is needed, the technique allows the endospore to decompose while it is alive. Future applications for this technique include cell-based biosensor, cell therapy, and biocatalyst processes.
Professors Insung Choi and Younghoon Lee from the Department of Chemistry at KAIST as well as and Professor Frank Caruso from the University of Melbourne developed this technique which permits a cell to stay alive by coating it with film on a nanometer scale and then to be decomposed while it is alive.
The research finding was published in the November 10th issue of Angewandte Chemie International Edition as the lead article.
Cell encapsulation allows researchers to capture a cell in a tight capsule while it is alive. It is highly recognized in cell-based applications where the control of cell stability and cell-division is the biggest issue.
Traditional cell encapsulation methods utilized organic film or inorganic capsules that are made of organic film moldings. Although these films tightly closed around the cell, because they were not easily decomposable, it was difficult to apply the method.
The research team succeeded in encapsulating each cell in a metal-polyphenol film by mixing tannic acid and iron ion solution with yeast cells.
Usually extracted from oak barks or grape peels, tannic acid is a natural substance. It forms a metal-polyphenol film within ten seconds when it meets iron ions due to its high affinity with cells. Cells encapsulated with this film presented high survival rates. Since the film forms quickly in a simple manner, it was possible to obtain large amount of encapsulated cells.
The research team also found that the metal-polyphenol film was stable in neutral pH, but is easily degradable under a weak acidic condition. Using this property, they were able to control cell division by restoring the cell to its pre-encapsulated state at a desired moment.
Protecting the cell from the external environment like an egg shell, the metal-polyphenol film protected the cell against foreign conditions such as lytic enzymes, extended exposure to UV radiation, and silver nanoparticles. The research indicated that the encapsulated cells had a high survival rate even under extreme environments.
Professor Lee said that “not only the cells remain alive during the encapsulation stage, but also they can be protected under extreme environment.” He added, “This is an advanced cell encapsulation technology that allows controlling cell-division of those cells through responsive shell degradation on-demand.”
Professor Choi commented, “Although the cell encapsulation technology is still in its infancy, as the technology matures the application of cell-manipulation technology will be actualized.” He highlighted that “it will serve as a breakthrough to problems faced by cell-based applications.”
Sponsored by the Ministry of Science, ICT and Future Planning and the National Research Foundation of Korea, the research was led by two Master’s candidates, Ji Hun Park and Kyung Hwan Kim, under the joint guidance of research professors from KAIST and the University of Melbourne.
Figure 1: Lead article of Angewandte Chemie
Background: Shows a live native yeast (in green) encapsulated in a metal-polyphenol film (in red) illustrating the vitality of the yeast
Front: A native yeast at each encapsulation stage
Pictured on the bottom left is a cell prior to encapsulation. Following the red arrow, the native yeast is in purple to show metal-polyphenol film formed around the cell. The cell after the green arrow is a visualization of the degradation of the film in weak acidic condition.
Figure 2: A mimetic diagram of cell encapsulation with a metal-polyphenol film
Top: A native yeast before encapsulation
Middle: A native yeast encapsulated with Tannic Acid-Fe (III) Nanoshell – cell-division of the encapsulated cell is controlled by pH and the shell is protected against silver nanoparticle, lytic enzyme, and UV-C
Bottom: Shell degradation on-demand depending on pH
2014.11.18 View 10916 -
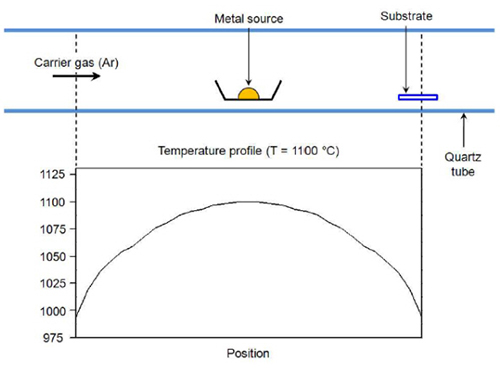 Development of Gold Nanowire Probe Needle with an Increased Sensitivity
Professor Bongsoo Kim
The newly developed nano-probe needle’s thickness estimates only at 0.1 micrometers with an increased 1,000-fold sensitivity and spatial resolution of 1mm.
Professor Bongsoo Kim and his research team from the Department of Chemistry, KASIT, including the first author, Dr. Mijeong Kang, succeeded in measuring nerve signals of a mouse using the world’s thinnest nano-probe needle made of single-crystal gold nanowires.
The newly developed nano-probe needle possesses the thickness of 100 nanometers (nm), which shows 1,000 times more sensitivity than the conventional nerve probe needles, as well as accurately measures nerve signals with an extremely fine resolution of less than 1mm. Unlike the existing probe needles that cause neural tissues to be damaged during insertion, the new nano-probe needle minimizes the damage and thus can detect large nerve signals.
The brain neural probe, which collects and analyzes electrical nerve signals generated in the brain, is the most essential element in brain research. A neural probe should minimize tissue damages, but needs to possess a good electrical sensitivity.
The researchers first applied heat on the gold, which is the necessary material for a probe, until it turned to a vapor phase. Then, the gold evaporation slug was transported to a colder board and left to form single-crystal gold nano structures by condensation. Because the new gold nanowire, produced by using this principle, is a flawless single crystal structure, it shows strong and flexible properties.
Professor Kim and his team applied the nano-probe needle into the brain of a mouse that has been administered a drug to induce epilepsy. They were able to find the exact area in the brain that triggers epilepsy. Furthermore, the researchers also detected neural signal changes in the brain of the mouse when it encountered the intrusion of a stranger mouse.
Professor Bongsoo Kim commented the meaning of his research:
“The new nano-probe needle is able to detect signals from a single nerve cell with high sensitivity while preserving the nerve cells intact. The probe needle will be useful for creating a precise three-dimensional brain map, as well as providing electrical treatment for brain diseases such as dementia and Parkinson's disease.”
This research results were published online in the August 12, 2014 edition of ACS Nano.
2014.09.06 View 6999
Development of Gold Nanowire Probe Needle with an Increased Sensitivity
Professor Bongsoo Kim
The newly developed nano-probe needle’s thickness estimates only at 0.1 micrometers with an increased 1,000-fold sensitivity and spatial resolution of 1mm.
Professor Bongsoo Kim and his research team from the Department of Chemistry, KASIT, including the first author, Dr. Mijeong Kang, succeeded in measuring nerve signals of a mouse using the world’s thinnest nano-probe needle made of single-crystal gold nanowires.
The newly developed nano-probe needle possesses the thickness of 100 nanometers (nm), which shows 1,000 times more sensitivity than the conventional nerve probe needles, as well as accurately measures nerve signals with an extremely fine resolution of less than 1mm. Unlike the existing probe needles that cause neural tissues to be damaged during insertion, the new nano-probe needle minimizes the damage and thus can detect large nerve signals.
The brain neural probe, which collects and analyzes electrical nerve signals generated in the brain, is the most essential element in brain research. A neural probe should minimize tissue damages, but needs to possess a good electrical sensitivity.
The researchers first applied heat on the gold, which is the necessary material for a probe, until it turned to a vapor phase. Then, the gold evaporation slug was transported to a colder board and left to form single-crystal gold nano structures by condensation. Because the new gold nanowire, produced by using this principle, is a flawless single crystal structure, it shows strong and flexible properties.
Professor Kim and his team applied the nano-probe needle into the brain of a mouse that has been administered a drug to induce epilepsy. They were able to find the exact area in the brain that triggers epilepsy. Furthermore, the researchers also detected neural signal changes in the brain of the mouse when it encountered the intrusion of a stranger mouse.
Professor Bongsoo Kim commented the meaning of his research:
“The new nano-probe needle is able to detect signals from a single nerve cell with high sensitivity while preserving the nerve cells intact. The probe needle will be useful for creating a precise three-dimensional brain map, as well as providing electrical treatment for brain diseases such as dementia and Parkinson's disease.”
This research results were published online in the August 12, 2014 edition of ACS Nano.
2014.09.06 View 6999 -
 Review of organophosphonate nerve agent remediation and sensing chemistry
Professor David Churchill, Dept. of Chemistry, KAIST
Scientists in Daejeon, South Korea and Lexington, Kentucky (USA) have recently published a review on the subject of nerve agent remediation and probing chemistry (Chemical Reviews, DOI:10.1021/cr100193y). This article endeavored to pursue organophosphonate nerve agent chemistry deeply and comprehensively and to reflect that decontamination / sensing and nerve agents / pesticides are quite inextricable: when one tries to degrade nerve agents one also needs to detect what components are still present “downstream,” etc. Nerve agents and many pesticides also share a common generalized organophosphate / -phosphonate structure.
Also, the use of simulant molecules (mimics) and a consideration of the closely related organophosphonate pesticides were also treated comprehensively in the Review. The authors reached back into the literature when developing some sections to make important connections to the contemporary topics of interest. The review also includes industrial insights.
Kibong Kim, Olga G. Tsay and David G. Churchill of the Department of Chemistry at KAIST and David A. Atwood of the Department of Chemistry of the University of Kentucky endeavored to "make a variety of connections in research strategies and (sub-) fields to present what is still possible, fruitful, practical, and necessary and to facilitate a current comprehensive molecular level understanding of organophosphonate degradation and sensing," Churchill says.
The authors feel that for the time being, researchers in varying research areas “can use this manuscript effectively when considering future research directions.”
2011.09.19 View 9548
Review of organophosphonate nerve agent remediation and sensing chemistry
Professor David Churchill, Dept. of Chemistry, KAIST
Scientists in Daejeon, South Korea and Lexington, Kentucky (USA) have recently published a review on the subject of nerve agent remediation and probing chemistry (Chemical Reviews, DOI:10.1021/cr100193y). This article endeavored to pursue organophosphonate nerve agent chemistry deeply and comprehensively and to reflect that decontamination / sensing and nerve agents / pesticides are quite inextricable: when one tries to degrade nerve agents one also needs to detect what components are still present “downstream,” etc. Nerve agents and many pesticides also share a common generalized organophosphate / -phosphonate structure.
Also, the use of simulant molecules (mimics) and a consideration of the closely related organophosphonate pesticides were also treated comprehensively in the Review. The authors reached back into the literature when developing some sections to make important connections to the contemporary topics of interest. The review also includes industrial insights.
Kibong Kim, Olga G. Tsay and David G. Churchill of the Department of Chemistry at KAIST and David A. Atwood of the Department of Chemistry of the University of Kentucky endeavored to "make a variety of connections in research strategies and (sub-) fields to present what is still possible, fruitful, practical, and necessary and to facilitate a current comprehensive molecular level understanding of organophosphonate degradation and sensing," Churchill says.
The authors feel that for the time being, researchers in varying research areas “can use this manuscript effectively when considering future research directions.”
2011.09.19 View 9548 -
 Spintronics: A high wire act by Nanowerk News
An article by Nanowerk News on the integration of ferromagnetic nanowire arrays on grapheme substrates was published. Professor Bong-Soo Kim from the Department of Chemistry, KAIST, led the research in conjunction with Hanyang University and Samsung in Korea.
http://www.nanowerk.com/news/newsid=22204.php
Posted: Jul 25th, 2011
Spintronics: A high wire act
(Nanowerk News) Graphene is a promising material for a wide range of applications due to its remarkable mechanical and electronic properties. An application of particular interest is spin-based electronics, or spintronics, in which the spin orientation of an electron is used to perform circuit functions in addition to its charge. Bongsoo Kim and colleagues from KAIST, Hanyang University and Samsung in Korea now report the integration of ferromagnetic nanowire arrays on graphene substrates, opening up a route for the construction of graphene-based spintronic devices using nanowires as spin-injecting contacts ("Epitaxially Integrating Ferromagnetic Fe1.3Ge Nanowire Arrays on Few-Layer Graphene").
The spin of an electron is a property that, like charge, can be used to encode, process and transport information. However, spin information is easily lost in most media, which has made spintronics difficult to realize in practice. In graphene, on the other hand, spin can be preserved for longer due to its peculiar electron transport properties. "Low intrinsic spin–orbit coupling, long spin diffusion lengths and vanishing hyperfine interaction are features of graphene that make it a promising medium for spin transport," explains Kim.
Scanning electron microscopy image of vertical iron germanide nanowires grown on graphene. (© ACS 2011)
A prerequisite for the realization of spintronic devices based on graphene is its integration with ferromagnetic contacts to allow spin injection. Kim and his co-workers found that nanowires of iron germanide (Fe1.3Ge) serve as efficient contacts for this purpose. "Iron germanide nanowires show low resistivity and room-temperature ferromagnetism, and they are compatible with existing complementary metal–oxide–semiconductor technologies," says Kim.
To produce the atomically well-defined interfacial contact between the nanowires and the graphene surface needed for optimum device performance, the researchers deposited the contacts by an epitaxial method based on chemical vapor transport. Through careful adjustment of deposition parameters such as carrier gas flow rate and reaction temperature, the researchers produced vertically aligned nanowires that are closely lattice-matched to the graphene sheets (see image).
Initially preparing the graphene sheets on a substrate of silicon oxide allowed the researchers to isolate the final nanowire–graphene structure by etching and then transfer it to another substrate, greatly expanding the versatility of the approach. It is a delicate process, however. "It is necessary to transfer the graphene films onto the substrate very carefully in order to avoid folding and wrinkling of the graphene," says Kim.
Source: Tokyo Institute of Technology
2011.07.26 View 11464
Spintronics: A high wire act by Nanowerk News
An article by Nanowerk News on the integration of ferromagnetic nanowire arrays on grapheme substrates was published. Professor Bong-Soo Kim from the Department of Chemistry, KAIST, led the research in conjunction with Hanyang University and Samsung in Korea.
http://www.nanowerk.com/news/newsid=22204.php
Posted: Jul 25th, 2011
Spintronics: A high wire act
(Nanowerk News) Graphene is a promising material for a wide range of applications due to its remarkable mechanical and electronic properties. An application of particular interest is spin-based electronics, or spintronics, in which the spin orientation of an electron is used to perform circuit functions in addition to its charge. Bongsoo Kim and colleagues from KAIST, Hanyang University and Samsung in Korea now report the integration of ferromagnetic nanowire arrays on graphene substrates, opening up a route for the construction of graphene-based spintronic devices using nanowires as spin-injecting contacts ("Epitaxially Integrating Ferromagnetic Fe1.3Ge Nanowire Arrays on Few-Layer Graphene").
The spin of an electron is a property that, like charge, can be used to encode, process and transport information. However, spin information is easily lost in most media, which has made spintronics difficult to realize in practice. In graphene, on the other hand, spin can be preserved for longer due to its peculiar electron transport properties. "Low intrinsic spin–orbit coupling, long spin diffusion lengths and vanishing hyperfine interaction are features of graphene that make it a promising medium for spin transport," explains Kim.
Scanning electron microscopy image of vertical iron germanide nanowires grown on graphene. (© ACS 2011)
A prerequisite for the realization of spintronic devices based on graphene is its integration with ferromagnetic contacts to allow spin injection. Kim and his co-workers found that nanowires of iron germanide (Fe1.3Ge) serve as efficient contacts for this purpose. "Iron germanide nanowires show low resistivity and room-temperature ferromagnetism, and they are compatible with existing complementary metal–oxide–semiconductor technologies," says Kim.
To produce the atomically well-defined interfacial contact between the nanowires and the graphene surface needed for optimum device performance, the researchers deposited the contacts by an epitaxial method based on chemical vapor transport. Through careful adjustment of deposition parameters such as carrier gas flow rate and reaction temperature, the researchers produced vertically aligned nanowires that are closely lattice-matched to the graphene sheets (see image).
Initially preparing the graphene sheets on a substrate of silicon oxide allowed the researchers to isolate the final nanowire–graphene structure by etching and then transfer it to another substrate, greatly expanding the versatility of the approach. It is a delicate process, however. "It is necessary to transfer the graphene films onto the substrate very carefully in order to avoid folding and wrinkling of the graphene," says Kim.
Source: Tokyo Institute of Technology
2011.07.26 View 11464