NI
-
 Quantum Technology: the Next Game Changer?
The 6th KAIST Global Strategy Institute Forum explores how quantum technology has evolved into a new growth engine for the future
The participants of the 6th KAIST Global Strategy Institute (GSI) Forum on April 20 agreed that the emerging technology of quantum computing will be a game changer of the future. As KAIST President Kwang Hyung Lee said in his opening remarks, the future is quantum and that future is rapidly approaching. Keynote speakers and panelists presented their insights on the disruptive innovations we are already experiencing.
The three keynote speakers included Dr. Jerry M. Chow, IBM fellow and director of quantum infrastructure, Professor John Preskill from Caltech, and Professor Jungsang Kim from Duke University. They discussed the academic impact and industrial applications of quantum technology, and its prospects for the future.
Dr. Chow leads IBM Quantum’s hardware system development efforts, focusing on research and system deployment. Professor Preskill is one of the leading quantum information science and quantum computation scholars. He coined the term “quantum supremacy.” Professor Kim is the co-founder and CTO of IonQ Inc., which develops general-purpose trapped ion quantum computers and software to generate, optimize, and execute quantum circuits.
Two leading quantum scholars from KAIST, Professor June-Koo Kevin Rhee and Professor Youngik Sohn, and Professor Andreas Heinrich from the IBS Center for Quantum Nanoscience also participated in the forum as panelists. Professor Rhee is the founder of the nation’s first quantum computing software company and leads the AI Quantum Computing IT Research Center at KAIST.
During the panel session, Professor Rhee said that although it is undeniable the quantum computing will be a game changer, there are several challenges. For instance, the first actual quantum computer is NISQ (Noisy intermediate-scale quantum era), which is still incomplete. However, it is expected to outperform a supercomputer. Until then, it is important to advance the accuracy of quantum computation in order to offer super computation speeds.
Professor Sohn, who worked at PsiQuantum, detailed how quantum computers will affect our society. He said that PsiQuantum is developing silicon photonics that will control photons. We can’t begin to imagine how silicon photonics will transform our society. Things will change slowly but the scale would be massive.
The keynote speakers presented how quantum cryptography communications and its sensing technology will serve as disruptive innovations. Dr. Chow stressed that to realize the potential growth and innovation, additional R&D is needed. More research groups and scholars should be nurtured. Only then will the rich R&D resources be able to create breakthroughs in quantum-related industries. Lastly, the commercialization of quantum computing must be advanced, which will enable the provision of diverse services. In addition, more technological and industrial infrastructure must be built to better accommodate quantum computing.
Professor Preskill believes that quantum computing will benefit humanity. He cited two basic reasons for his optimistic prediction: quantum complexity and quantum error corrections. He explained why quantum computing is so powerful: quantum computer can easily solve the problems classically considered difficult, such as factorization. In addition, quantum computer has the potential to efficiently simulate all of the physical processes taking place in nature.
Despite such dramatic advantages, why does it seem so difficult? Professor Preskill believes this is because we want qubits (quantum bits or ‘qubits’ are the basic unit of quantum information) to interact very strongly with each other. Because computations can fail, we don’t want qubits to interact with the environment unless we can control and predict them.
As for quantum computing in the NISQ era, he said that NISQ will be an exciting tool for exploring physics. Professor Preskill does not believe that NISQ will change the world alone, rather it is a step forward toward more powerful quantum technologies in the future. He added that a potentially transformable, scalable quantum computer could still be decades away.
Professor Preskill said that a large number of qubits, higher accuracy, and better quality will require a significant investment. He said if we expand with better ideas, we can make a better system. In the longer term, quantum technology will bring significant benefits to the technological sectors and society in the fields of materials, physics, chemistry, and energy production.
Professor Kim from Duke University presented on the practical applications of quantum computing, especially in the startup environment. He said that although there is no right answer for the early applications of quantum computing, developing new approaches to solve difficult problems and raising the accessibility of the technology will be important for ensuring the growth of technology-based startups.
2022.04.21 View 14520
Quantum Technology: the Next Game Changer?
The 6th KAIST Global Strategy Institute Forum explores how quantum technology has evolved into a new growth engine for the future
The participants of the 6th KAIST Global Strategy Institute (GSI) Forum on April 20 agreed that the emerging technology of quantum computing will be a game changer of the future. As KAIST President Kwang Hyung Lee said in his opening remarks, the future is quantum and that future is rapidly approaching. Keynote speakers and panelists presented their insights on the disruptive innovations we are already experiencing.
The three keynote speakers included Dr. Jerry M. Chow, IBM fellow and director of quantum infrastructure, Professor John Preskill from Caltech, and Professor Jungsang Kim from Duke University. They discussed the academic impact and industrial applications of quantum technology, and its prospects for the future.
Dr. Chow leads IBM Quantum’s hardware system development efforts, focusing on research and system deployment. Professor Preskill is one of the leading quantum information science and quantum computation scholars. He coined the term “quantum supremacy.” Professor Kim is the co-founder and CTO of IonQ Inc., which develops general-purpose trapped ion quantum computers and software to generate, optimize, and execute quantum circuits.
Two leading quantum scholars from KAIST, Professor June-Koo Kevin Rhee and Professor Youngik Sohn, and Professor Andreas Heinrich from the IBS Center for Quantum Nanoscience also participated in the forum as panelists. Professor Rhee is the founder of the nation’s first quantum computing software company and leads the AI Quantum Computing IT Research Center at KAIST.
During the panel session, Professor Rhee said that although it is undeniable the quantum computing will be a game changer, there are several challenges. For instance, the first actual quantum computer is NISQ (Noisy intermediate-scale quantum era), which is still incomplete. However, it is expected to outperform a supercomputer. Until then, it is important to advance the accuracy of quantum computation in order to offer super computation speeds.
Professor Sohn, who worked at PsiQuantum, detailed how quantum computers will affect our society. He said that PsiQuantum is developing silicon photonics that will control photons. We can’t begin to imagine how silicon photonics will transform our society. Things will change slowly but the scale would be massive.
The keynote speakers presented how quantum cryptography communications and its sensing technology will serve as disruptive innovations. Dr. Chow stressed that to realize the potential growth and innovation, additional R&D is needed. More research groups and scholars should be nurtured. Only then will the rich R&D resources be able to create breakthroughs in quantum-related industries. Lastly, the commercialization of quantum computing must be advanced, which will enable the provision of diverse services. In addition, more technological and industrial infrastructure must be built to better accommodate quantum computing.
Professor Preskill believes that quantum computing will benefit humanity. He cited two basic reasons for his optimistic prediction: quantum complexity and quantum error corrections. He explained why quantum computing is so powerful: quantum computer can easily solve the problems classically considered difficult, such as factorization. In addition, quantum computer has the potential to efficiently simulate all of the physical processes taking place in nature.
Despite such dramatic advantages, why does it seem so difficult? Professor Preskill believes this is because we want qubits (quantum bits or ‘qubits’ are the basic unit of quantum information) to interact very strongly with each other. Because computations can fail, we don’t want qubits to interact with the environment unless we can control and predict them.
As for quantum computing in the NISQ era, he said that NISQ will be an exciting tool for exploring physics. Professor Preskill does not believe that NISQ will change the world alone, rather it is a step forward toward more powerful quantum technologies in the future. He added that a potentially transformable, scalable quantum computer could still be decades away.
Professor Preskill said that a large number of qubits, higher accuracy, and better quality will require a significant investment. He said if we expand with better ideas, we can make a better system. In the longer term, quantum technology will bring significant benefits to the technological sectors and society in the fields of materials, physics, chemistry, and energy production.
Professor Kim from Duke University presented on the practical applications of quantum computing, especially in the startup environment. He said that although there is no right answer for the early applications of quantum computing, developing new approaches to solve difficult problems and raising the accessibility of the technology will be important for ensuring the growth of technology-based startups.
2022.04.21 View 14520 -
 Professor Hyunjoo Jenny Lee to Co-Chair IEEE MEMS 2025
Professor Hyunjoo Jenny Lee from the School of Electrical Engineering has been appointed General Chair of the 38th IEEE MEMS 2025 (International Conference on Micro Electro Mechanical Systems). Professor Lee, who is 40, is the conference’s youngest General Chair to date and will work jointly with Professor Sheng-Shian Li of Taiwan’s National Tsing Hua University as co-chairs in 2025.
IEEE MEMS is a top-tier international conference on microelectromechanical systems and it serves as a core academic showcase for MEMS research and technology in areas such as microsensors and actuators.
With over 800 MEMS paper submissions each year, the conference only accepts and publishes about 250 of them after a rigorous review process recognized for its world-class prestige. Of all the submissions, fewer than 10% are chosen for oral presentations.
2022.04.18 View 9410
Professor Hyunjoo Jenny Lee to Co-Chair IEEE MEMS 2025
Professor Hyunjoo Jenny Lee from the School of Electrical Engineering has been appointed General Chair of the 38th IEEE MEMS 2025 (International Conference on Micro Electro Mechanical Systems). Professor Lee, who is 40, is the conference’s youngest General Chair to date and will work jointly with Professor Sheng-Shian Li of Taiwan’s National Tsing Hua University as co-chairs in 2025.
IEEE MEMS is a top-tier international conference on microelectromechanical systems and it serves as a core academic showcase for MEMS research and technology in areas such as microsensors and actuators.
With over 800 MEMS paper submissions each year, the conference only accepts and publishes about 250 of them after a rigorous review process recognized for its world-class prestige. Of all the submissions, fewer than 10% are chosen for oral presentations.
2022.04.18 View 9410 -
 KAIST Partners with Korea National Sport University
KAIST President Kwang Hyung Lee signed an MOU with Korea National Sport University (KNSU) President Yong-Kyu Ahn for collaboration in education and research in the fields of sports science and technology on April 5 at the KAIST main campus. The agreement also extends to student and credit exchanges between the two universities.
With this signing, KAIST plans to develop programs in which KAIST students can participate in the diverse sports classes and activities offered at KNSU.
Officials from KNSU said that this collaboration with KAIST will provide a new opportunity to recognize the importance of sports science more extensively. They added that KNSU will continue to foster more competitive sports talents who understand the convergence between sports science and technology.
The two universities also plan to conduct research on body mechanics optimizing athletes’ best performance, analyze how the muscles of different events’ athletes move, and will propose creative new solutions utilizing robot rehabilitation and AR technologies. It is expected that the research will extend to the physical performance betterment of the general public, especially for aged groups and the development of training solutions for musculoskeletal injury prevention as Korean society deals with its growing aging population.
President Lee said, “I look forward to the synergic impact when KAIST works together with the nation’s top sports university. We will make every effort to spearhead the wellbeing of the general public in our aging society as well as for growth of sports.”
President Ahn said, “The close collaboration between KAIST and KNSU will revitalize the sports community that has been staggering due to the Covid-19 pandemic and will contribute to the advancement of sports science in Korea.”
2022.04.07 View 6996
KAIST Partners with Korea National Sport University
KAIST President Kwang Hyung Lee signed an MOU with Korea National Sport University (KNSU) President Yong-Kyu Ahn for collaboration in education and research in the fields of sports science and technology on April 5 at the KAIST main campus. The agreement also extends to student and credit exchanges between the two universities.
With this signing, KAIST plans to develop programs in which KAIST students can participate in the diverse sports classes and activities offered at KNSU.
Officials from KNSU said that this collaboration with KAIST will provide a new opportunity to recognize the importance of sports science more extensively. They added that KNSU will continue to foster more competitive sports talents who understand the convergence between sports science and technology.
The two universities also plan to conduct research on body mechanics optimizing athletes’ best performance, analyze how the muscles of different events’ athletes move, and will propose creative new solutions utilizing robot rehabilitation and AR technologies. It is expected that the research will extend to the physical performance betterment of the general public, especially for aged groups and the development of training solutions for musculoskeletal injury prevention as Korean society deals with its growing aging population.
President Lee said, “I look forward to the synergic impact when KAIST works together with the nation’s top sports university. We will make every effort to spearhead the wellbeing of the general public in our aging society as well as for growth of sports.”
President Ahn said, “The close collaboration between KAIST and KNSU will revitalize the sports community that has been staggering due to the Covid-19 pandemic and will contribute to the advancement of sports science in Korea.”
2022.04.07 View 6996 -
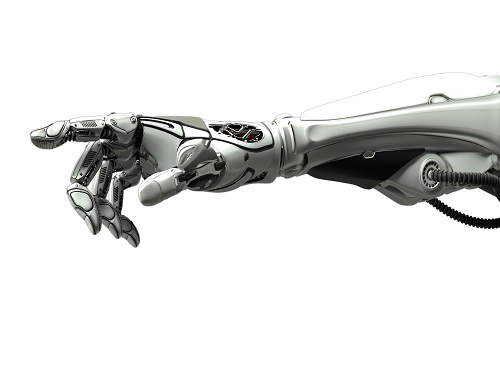 Decoding Brain Signals to Control a Robotic Arm
Advanced brain-machine interface system successfully interprets arm movement directions from neural signals in the brain
Researchers have developed a mind-reading system for decoding neural signals from the brain during arm movement. The method, described in the journal Applied Soft Computing, can be used by a person to control a robotic arm through a brain-machine interface (BMI).
A BMI is a device that translates nerve signals into commands to control a machine, such as a computer or a robotic limb. There are two main techniques for monitoring neural signals in BMIs: electroencephalography (EEG) and electrocorticography (ECoG).
The EEG exhibits signals from electrodes on the surface of the scalp and is widely employed because it is non-invasive, relatively cheap, safe and easy to use. However, the EEG has low spatial resolution and detects irrelevant neural signals, which makes it difficult to interpret the intentions of individuals from the EEG.
On the other hand, the ECoG is an invasive method that involves placing electrodes directly on the surface of the cerebral cortex below the scalp. Compared with the EEG, the ECoG can monitor neural signals with much higher spatial resolution and less background noise. However, this technique has several drawbacks.
“The ECoG is primarily used to find potential sources of epileptic seizures, meaning the electrodes are placed in different locations for different patients and may not be in the optimal regions of the brain for detecting sensory and movement signals,” explained Professor Jaeseung Jeong, a brain scientist at KAIST. “This inconsistency makes it difficult to decode brain signals to predict movements.”
To overcome these problems, Professor Jeong’s team developed a new method for decoding ECoG neural signals during arm movement. The system is based on a machine-learning system for analysing and predicting neural signals called an ‘echo-state network’ and a mathematical probability model called the Gaussian distribution.
In the study, the researchers recorded ECoG signals from four individuals with epilepsy while they were performing a reach-and-grasp task. Because the ECoG electrodes were placed according to the potential sources of each patient’s epileptic seizures, only 22% to 44% of the electrodes were located in the regions of the brain responsible for controlling movement.
During the movement task, the participants were given visual cues, either by placing a real tennis ball in front of them, or via a virtual reality headset showing a clip of a human arm reaching forward in first-person view. They were asked to reach forward, grasp an object, then return their hand and release the object, while wearing motion sensors on their wrists and fingers. In a second task, they were instructed to imagine reaching forward without moving their arms.
The researchers monitored the signals from the ECoG electrodes during real and imaginary arm movements, and tested whether the new system could predict the direction of this movement from the neural signals. They found that the novel decoder successfully classified arm movements in 24 directions in three-dimensional space, both in the real and virtual tasks, and that the results were at least five times more accurate than chance. They also used a computer simulation to show that the novel ECoG decoder could control the movements of a robotic arm.
Overall, the results suggest that the new machine learning-based BCI system successfully used ECoG signals to interpret the direction of the intended movements. The next steps will be to improve the accuracy and efficiency of the decoder. In the future, it could be used in a real-time BMI device to help people with movement or sensory impairments.
This research was supported by the KAIST Global Singularity Research Program of 2021, Brain Research Program of the National Research Foundation of Korea funded by the Ministry of Science, ICT, and Future Planning, and the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education.
-PublicationHoon-Hee Kim, Jaeseung Jeong, “An electrocorticographic decoder for arm movement for brain-machine interface using an echo state network and Gaussian readout,” Applied SoftComputing online December 31, 2021 (doi.org/10.1016/j.asoc.2021.108393)
-ProfileProfessor Jaeseung JeongDepartment of Bio and Brain EngineeringCollege of EngineeringKAIST
2022.03.18 View 14961
Decoding Brain Signals to Control a Robotic Arm
Advanced brain-machine interface system successfully interprets arm movement directions from neural signals in the brain
Researchers have developed a mind-reading system for decoding neural signals from the brain during arm movement. The method, described in the journal Applied Soft Computing, can be used by a person to control a robotic arm through a brain-machine interface (BMI).
A BMI is a device that translates nerve signals into commands to control a machine, such as a computer or a robotic limb. There are two main techniques for monitoring neural signals in BMIs: electroencephalography (EEG) and electrocorticography (ECoG).
The EEG exhibits signals from electrodes on the surface of the scalp and is widely employed because it is non-invasive, relatively cheap, safe and easy to use. However, the EEG has low spatial resolution and detects irrelevant neural signals, which makes it difficult to interpret the intentions of individuals from the EEG.
On the other hand, the ECoG is an invasive method that involves placing electrodes directly on the surface of the cerebral cortex below the scalp. Compared with the EEG, the ECoG can monitor neural signals with much higher spatial resolution and less background noise. However, this technique has several drawbacks.
“The ECoG is primarily used to find potential sources of epileptic seizures, meaning the electrodes are placed in different locations for different patients and may not be in the optimal regions of the brain for detecting sensory and movement signals,” explained Professor Jaeseung Jeong, a brain scientist at KAIST. “This inconsistency makes it difficult to decode brain signals to predict movements.”
To overcome these problems, Professor Jeong’s team developed a new method for decoding ECoG neural signals during arm movement. The system is based on a machine-learning system for analysing and predicting neural signals called an ‘echo-state network’ and a mathematical probability model called the Gaussian distribution.
In the study, the researchers recorded ECoG signals from four individuals with epilepsy while they were performing a reach-and-grasp task. Because the ECoG electrodes were placed according to the potential sources of each patient’s epileptic seizures, only 22% to 44% of the electrodes were located in the regions of the brain responsible for controlling movement.
During the movement task, the participants were given visual cues, either by placing a real tennis ball in front of them, or via a virtual reality headset showing a clip of a human arm reaching forward in first-person view. They were asked to reach forward, grasp an object, then return their hand and release the object, while wearing motion sensors on their wrists and fingers. In a second task, they were instructed to imagine reaching forward without moving their arms.
The researchers monitored the signals from the ECoG electrodes during real and imaginary arm movements, and tested whether the new system could predict the direction of this movement from the neural signals. They found that the novel decoder successfully classified arm movements in 24 directions in three-dimensional space, both in the real and virtual tasks, and that the results were at least five times more accurate than chance. They also used a computer simulation to show that the novel ECoG decoder could control the movements of a robotic arm.
Overall, the results suggest that the new machine learning-based BCI system successfully used ECoG signals to interpret the direction of the intended movements. The next steps will be to improve the accuracy and efficiency of the decoder. In the future, it could be used in a real-time BMI device to help people with movement or sensory impairments.
This research was supported by the KAIST Global Singularity Research Program of 2021, Brain Research Program of the National Research Foundation of Korea funded by the Ministry of Science, ICT, and Future Planning, and the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education.
-PublicationHoon-Hee Kim, Jaeseung Jeong, “An electrocorticographic decoder for arm movement for brain-machine interface using an echo state network and Gaussian readout,” Applied SoftComputing online December 31, 2021 (doi.org/10.1016/j.asoc.2021.108393)
-ProfileProfessor Jaeseung JeongDepartment of Bio and Brain EngineeringCollege of EngineeringKAIST
2022.03.18 View 14961 -
 CXL-Based Memory Disaggregation Technology Opens Up a New Direction for Big Data Solution Frameworks
A KAIST team’s compute express link (CXL) provides new insights on memory disaggregation and ensures direct access and high-performance capabilities
A team from the Computer Architecture and Memory Systems Laboratory (CAMEL) at KAIST presented a new compute express link (CXL) solution whose directly accessible, and high-performance memory disaggregation opens new directions for big data memory processing. Professor Myoungsoo Jung said the team’s technology significantly improves performance compared to existing remote direct memory access (RDMA)-based memory disaggregation.
CXL is a peripheral component interconnect-express (PCIe)-based new dynamic multi-protocol made for efficiently utilizing memory devices and accelerators. Many enterprise data centers and memory vendors are paying attention to it as the next-generation multi-protocol for the era of big data.
Emerging big data applications such as machine learning, graph analytics, and in-memory databases require large memory capacities. However, scaling out the memory capacity via a prior memory interface like double data rate (DDR) is limited by the number of the central processing units (CPUs) and memory controllers. Therefore, memory disaggregation, which allows connecting a host to another host’s memory or memory nodes, has appeared.
RDMA is a way that a host can directly access another host’s memory via InfiniBand, the commonly used network protocol in data centers. Nowadays, most existing memory disaggregation technologies employ RDMA to get a large memory capacity. As a result, a host can share another host’s memory by transferring the data between local and remote memory.
Although RDMA-based memory disaggregation provides a large memory capacity to a host, two critical problems exist. First, scaling out the memory still needs an extra CPU to be added. Since passive memory such as dynamic random-access memory (DRAM), cannot operate by itself, it should be controlled by the CPU. Second, redundant data copies and software fabric interventions for RDMA-based memory disaggregation cause longer access latency. For example, remote memory access latency in RDMA-based memory disaggregation is multiple orders of magnitude longer than local memory access.
To address these issues, Professor Jung’s team developed the CXL-based memory disaggregation framework, including CXL-enabled customized CPUs, CXL devices, CXL switches, and CXL-aware operating system modules. The team’s CXL device is a pure passive and directly accessible memory node that contains multiple DRAM dual inline memory modules (DIMMs) and a CXL memory controller. Since the CXL memory controller supports the memory in the CXL device, a host can utilize the memory node without processor or software intervention. The team’s CXL switch enables scaling out a host’s memory capacity by hierarchically connecting multiple CXL devices to the CXL switch allowing more than hundreds of devices. Atop the switches and devices, the team’s CXL-enabled operating system removes redundant data copy and protocol conversion exhibited by conventional RDMA, which can significantly decrease access latency to the memory nodes.
In a test comparing loading 64B (cacheline) data from memory pooling devices, CXL-based memory disaggregation showed 8.2 times higher data load performance than RDMA-based memory disaggregation and even similar performance to local DRAM memory. In the team’s evaluations for a big data benchmark such as a machine learning-based test, CXL-based memory disaggregation technology also showed a maximum of 3.7 times higher performance than prior RDMA-based memory disaggregation technologies.
“Escaping from the conventional RDMA-based memory disaggregation, our CXL-based memory disaggregation framework can provide high scalability and performance for diverse datacenters and cloud service infrastructures,” said Professor Jung. He went on to stress, “Our CXL-based memory disaggregation research will bring about a new paradigm for memory solutions that will lead the era of big data.”
-Profile: Professor Myoungsoo Jung Computer Architecture and Memory Systems Laboratory (CAMEL)http://camelab.org School of Electrical EngineeringKAIST
2022.03.16 View 25646
CXL-Based Memory Disaggregation Technology Opens Up a New Direction for Big Data Solution Frameworks
A KAIST team’s compute express link (CXL) provides new insights on memory disaggregation and ensures direct access and high-performance capabilities
A team from the Computer Architecture and Memory Systems Laboratory (CAMEL) at KAIST presented a new compute express link (CXL) solution whose directly accessible, and high-performance memory disaggregation opens new directions for big data memory processing. Professor Myoungsoo Jung said the team’s technology significantly improves performance compared to existing remote direct memory access (RDMA)-based memory disaggregation.
CXL is a peripheral component interconnect-express (PCIe)-based new dynamic multi-protocol made for efficiently utilizing memory devices and accelerators. Many enterprise data centers and memory vendors are paying attention to it as the next-generation multi-protocol for the era of big data.
Emerging big data applications such as machine learning, graph analytics, and in-memory databases require large memory capacities. However, scaling out the memory capacity via a prior memory interface like double data rate (DDR) is limited by the number of the central processing units (CPUs) and memory controllers. Therefore, memory disaggregation, which allows connecting a host to another host’s memory or memory nodes, has appeared.
RDMA is a way that a host can directly access another host’s memory via InfiniBand, the commonly used network protocol in data centers. Nowadays, most existing memory disaggregation technologies employ RDMA to get a large memory capacity. As a result, a host can share another host’s memory by transferring the data between local and remote memory.
Although RDMA-based memory disaggregation provides a large memory capacity to a host, two critical problems exist. First, scaling out the memory still needs an extra CPU to be added. Since passive memory such as dynamic random-access memory (DRAM), cannot operate by itself, it should be controlled by the CPU. Second, redundant data copies and software fabric interventions for RDMA-based memory disaggregation cause longer access latency. For example, remote memory access latency in RDMA-based memory disaggregation is multiple orders of magnitude longer than local memory access.
To address these issues, Professor Jung’s team developed the CXL-based memory disaggregation framework, including CXL-enabled customized CPUs, CXL devices, CXL switches, and CXL-aware operating system modules. The team’s CXL device is a pure passive and directly accessible memory node that contains multiple DRAM dual inline memory modules (DIMMs) and a CXL memory controller. Since the CXL memory controller supports the memory in the CXL device, a host can utilize the memory node without processor or software intervention. The team’s CXL switch enables scaling out a host’s memory capacity by hierarchically connecting multiple CXL devices to the CXL switch allowing more than hundreds of devices. Atop the switches and devices, the team’s CXL-enabled operating system removes redundant data copy and protocol conversion exhibited by conventional RDMA, which can significantly decrease access latency to the memory nodes.
In a test comparing loading 64B (cacheline) data from memory pooling devices, CXL-based memory disaggregation showed 8.2 times higher data load performance than RDMA-based memory disaggregation and even similar performance to local DRAM memory. In the team’s evaluations for a big data benchmark such as a machine learning-based test, CXL-based memory disaggregation technology also showed a maximum of 3.7 times higher performance than prior RDMA-based memory disaggregation technologies.
“Escaping from the conventional RDMA-based memory disaggregation, our CXL-based memory disaggregation framework can provide high scalability and performance for diverse datacenters and cloud service infrastructures,” said Professor Jung. He went on to stress, “Our CXL-based memory disaggregation research will bring about a new paradigm for memory solutions that will lead the era of big data.”
-Profile: Professor Myoungsoo Jung Computer Architecture and Memory Systems Laboratory (CAMEL)http://camelab.org School of Electrical EngineeringKAIST
2022.03.16 View 25646 -
 'Fingerprint' Machine Learning Technique Identifies Different Bacteria in Seconds
A synergistic combination of surface-enhanced Raman spectroscopy and deep learning serves as an effective platform for separation-free detection of bacteria in arbitrary media
Bacterial identification can take hours and often longer, precious time when diagnosing infections and selecting appropriate treatments. There may be a quicker, more accurate process according to researchers at KAIST. By teaching a deep learning algorithm to identify the “fingerprint” spectra of the molecular components of various bacteria, the researchers could classify various bacteria in different media with accuracies of up to 98%.
Their results were made available online on Jan. 18 in Biosensors and Bioelectronics, ahead of publication in the journal’s April issue.
Bacteria-induced illnesses, those caused by direct bacterial infection or by exposure to bacterial toxins, can induce painful symptoms and even lead to death, so the rapid detection of bacteria is crucial to prevent the intake of contaminated foods and to diagnose infections from clinical samples, such as urine. “By using surface-enhanced Raman spectroscopy (SERS) analysis boosted with a newly proposed deep learning model, we demonstrated a markedly simple, fast, and effective route to classify the signals of two common bacteria and their resident media without any separation procedures,” said Professor Sungho Jo from the School of Computing.
Raman spectroscopy sends light through a sample to see how it scatters. The results reveal structural information about the sample — the spectral fingerprint — allowing researchers to identify its molecules. The surface-enhanced version places sample cells on noble metal nanostructures that help amplify the sample’s signals.
However, it is challenging to obtain consistent and clear spectra of bacteria due to numerous overlapping peak sources, such as proteins in cell walls. “Moreover, strong signals of surrounding media are also enhanced to overwhelm target signals, requiring time-consuming and tedious bacterial separation steps,” said Professor Yeon Sik Jung from the Department of Materials Science and Engineering.
To parse through the noisy signals, the researchers implemented an artificial intelligence method called deep learning that can hierarchically extract certain features of the spectral information to classify data. They specifically designed their model, named the dual-branch wide-kernel network (DualWKNet), to efficiently learn the correlation between spectral features. Such an ability is critical for analyzing one-dimensional spectral data, according to Professor Jo.
“Despite having interfering signals or noise from the media, which make the general shapes of different bacterial spectra and their residing media signals look similar, high classification accuracies of bacterial types and their media were achieved,” Professor Jo said, explaining that DualWKNet allowed the team to identify key peaks in each class that were almost indiscernible in individual spectra, enhancing the classification accuracies. “Ultimately, with the use of DualWKNet replacing the bacteria and media separation steps, our method dramatically reduces analysis time.”
The researchers plan to use their platform to study more bacteria and media types, using the information to build a training data library of various bacterial types in additional media to reduce the collection and detection times for new samples.
“We developed a meaningful universal platform for rapid bacterial detection with the collaboration between SERS and deep learning,” Professor Jo said. “We hope to extend the use of our deep learning-based SERS analysis platform to detect numerous types of bacteria in additional media that are important for food or clinical analysis, such as blood.”
The National R&D Program, through a National Research Foundation of Korea grant funded by the Ministry of Science and ICT, supported this research.
-PublicationEojin Rho, Minjoon Kim, Seunghee H. Cho, Bongjae Choi, Hyungjoon Park, Hanhwi Jang, Yeon Sik Jung, Sungho Jo, “Separation-free bacterial identification in arbitrary media via deepneural network-based SERS analysis,” Biosensors and Bioelectronics online January 18, 2022 (doi.org/10.1016/j.bios.2022.113991)
-ProfileProfessor Yeon Sik JungDepartment of Materials Science and EngineeringKAIST
Professor Sungho JoSchool of ComputingKAIST
2022.03.04 View 24208
'Fingerprint' Machine Learning Technique Identifies Different Bacteria in Seconds
A synergistic combination of surface-enhanced Raman spectroscopy and deep learning serves as an effective platform for separation-free detection of bacteria in arbitrary media
Bacterial identification can take hours and often longer, precious time when diagnosing infections and selecting appropriate treatments. There may be a quicker, more accurate process according to researchers at KAIST. By teaching a deep learning algorithm to identify the “fingerprint” spectra of the molecular components of various bacteria, the researchers could classify various bacteria in different media with accuracies of up to 98%.
Their results were made available online on Jan. 18 in Biosensors and Bioelectronics, ahead of publication in the journal’s April issue.
Bacteria-induced illnesses, those caused by direct bacterial infection or by exposure to bacterial toxins, can induce painful symptoms and even lead to death, so the rapid detection of bacteria is crucial to prevent the intake of contaminated foods and to diagnose infections from clinical samples, such as urine. “By using surface-enhanced Raman spectroscopy (SERS) analysis boosted with a newly proposed deep learning model, we demonstrated a markedly simple, fast, and effective route to classify the signals of two common bacteria and their resident media without any separation procedures,” said Professor Sungho Jo from the School of Computing.
Raman spectroscopy sends light through a sample to see how it scatters. The results reveal structural information about the sample — the spectral fingerprint — allowing researchers to identify its molecules. The surface-enhanced version places sample cells on noble metal nanostructures that help amplify the sample’s signals.
However, it is challenging to obtain consistent and clear spectra of bacteria due to numerous overlapping peak sources, such as proteins in cell walls. “Moreover, strong signals of surrounding media are also enhanced to overwhelm target signals, requiring time-consuming and tedious bacterial separation steps,” said Professor Yeon Sik Jung from the Department of Materials Science and Engineering.
To parse through the noisy signals, the researchers implemented an artificial intelligence method called deep learning that can hierarchically extract certain features of the spectral information to classify data. They specifically designed their model, named the dual-branch wide-kernel network (DualWKNet), to efficiently learn the correlation between spectral features. Such an ability is critical for analyzing one-dimensional spectral data, according to Professor Jo.
“Despite having interfering signals or noise from the media, which make the general shapes of different bacterial spectra and their residing media signals look similar, high classification accuracies of bacterial types and their media were achieved,” Professor Jo said, explaining that DualWKNet allowed the team to identify key peaks in each class that were almost indiscernible in individual spectra, enhancing the classification accuracies. “Ultimately, with the use of DualWKNet replacing the bacteria and media separation steps, our method dramatically reduces analysis time.”
The researchers plan to use their platform to study more bacteria and media types, using the information to build a training data library of various bacterial types in additional media to reduce the collection and detection times for new samples.
“We developed a meaningful universal platform for rapid bacterial detection with the collaboration between SERS and deep learning,” Professor Jo said. “We hope to extend the use of our deep learning-based SERS analysis platform to detect numerous types of bacteria in additional media that are important for food or clinical analysis, such as blood.”
The National R&D Program, through a National Research Foundation of Korea grant funded by the Ministry of Science and ICT, supported this research.
-PublicationEojin Rho, Minjoon Kim, Seunghee H. Cho, Bongjae Choi, Hyungjoon Park, Hanhwi Jang, Yeon Sik Jung, Sungho Jo, “Separation-free bacterial identification in arbitrary media via deepneural network-based SERS analysis,” Biosensors and Bioelectronics online January 18, 2022 (doi.org/10.1016/j.bios.2022.113991)
-ProfileProfessor Yeon Sik JungDepartment of Materials Science and EngineeringKAIST
Professor Sungho JoSchool of ComputingKAIST
2022.03.04 View 24208 -
 KAA Recognizes 4 Distinguished Alumni of the Year
The KAIST Alumni Association (KAA) recognized four distinguished alumni of the year during a ceremony on February 25 in Seoul. The four Distinguished Alumni Awardees are Distinguished Professor Sukbok Chang from the KAIST Department of Chemistry, Hyunshil Ahn, head of the AI Economy Institute and an editorial writer at The Korea Economic Daily, CEO Hwan-ho Sung of PSTech, and President Hark Kyu Park of Samsung Electronics.
Distinguished Professor Sukbok Chang who received his MS from the Department of Chemistry in 1985 has been a pioneer in the novel field of ‘carbon-hydrogen bond activation reactions’. He has significantly contributed to raising Korea’s international reputation in natural sciences and received the Kyungam Academic Award in 2013, the 14th Korea Science Award in 2015, the 1st Science and Technology Prize of Korea Toray in 2018, and the Best Scientist/Engineer Award Korea in 2019. Furthermore, he was named as a Highly Cited Researcher who ranked in the top 1% of citations by field and publication year in the Web of Science citation index for seven consecutive years from 2015 to 2021, demonstrating his leadership as a global scholar.
Hyunshil Ahn, a graduate of the School of Business and Technology Management with an MS in 1985 and a PhD in 1987, was appointed as the first head of the AI Economy Institute when The Korea Economic Daily was the first Korean media outlet to establish an AI economy lab. He has contributed to creating new roles for the press and media in the 4th industrial revolution, and added to the popularization of AI technology through regulation reform and consulting on industrial policies.
PSTech CEO Hwan-ho Sung is a graduate of the School of Electrical Engineering where he received an MS in 1988 and a PhD in EMBA in 2008. He has run the electronics company PSTech for over 20 years and successfully localized the production of power equipment, which previously depended on foreign technology. His development of the world’s first power equipment that can be applied to new industries including semiconductors and displays was recognized through this award.
Samsung Electronics President Hark Kyu Park graduated from the School of Business and Technology Management with an MS in 1986. He not only enhanced Korea’s national competitiveness by expanding the semiconductor industry, but also established contract-based semiconductor departments at Korean universities including KAIST, Sungkyunkwan University, Yonsei University, and Postech, and semiconductor track courses at KAIST, Sogang University, Seoul National University, and Postech to nurture professional talents. He also led the national semiconductor coexistence system by leading private sector-government-academia collaborations to strengthen competence in semiconductors, and continues to make unconditional investments in strong small businesses.
KAA President Chilhee Chung said, “Thanks to our alumni contributing at the highest levels of our society, the name of our alma mater shines brighter. As role models for our younger alumni, I hope greater honours will follow our awardees in the future.”
2022.03.03 View 9699
KAA Recognizes 4 Distinguished Alumni of the Year
The KAIST Alumni Association (KAA) recognized four distinguished alumni of the year during a ceremony on February 25 in Seoul. The four Distinguished Alumni Awardees are Distinguished Professor Sukbok Chang from the KAIST Department of Chemistry, Hyunshil Ahn, head of the AI Economy Institute and an editorial writer at The Korea Economic Daily, CEO Hwan-ho Sung of PSTech, and President Hark Kyu Park of Samsung Electronics.
Distinguished Professor Sukbok Chang who received his MS from the Department of Chemistry in 1985 has been a pioneer in the novel field of ‘carbon-hydrogen bond activation reactions’. He has significantly contributed to raising Korea’s international reputation in natural sciences and received the Kyungam Academic Award in 2013, the 14th Korea Science Award in 2015, the 1st Science and Technology Prize of Korea Toray in 2018, and the Best Scientist/Engineer Award Korea in 2019. Furthermore, he was named as a Highly Cited Researcher who ranked in the top 1% of citations by field and publication year in the Web of Science citation index for seven consecutive years from 2015 to 2021, demonstrating his leadership as a global scholar.
Hyunshil Ahn, a graduate of the School of Business and Technology Management with an MS in 1985 and a PhD in 1987, was appointed as the first head of the AI Economy Institute when The Korea Economic Daily was the first Korean media outlet to establish an AI economy lab. He has contributed to creating new roles for the press and media in the 4th industrial revolution, and added to the popularization of AI technology through regulation reform and consulting on industrial policies.
PSTech CEO Hwan-ho Sung is a graduate of the School of Electrical Engineering where he received an MS in 1988 and a PhD in EMBA in 2008. He has run the electronics company PSTech for over 20 years and successfully localized the production of power equipment, which previously depended on foreign technology. His development of the world’s first power equipment that can be applied to new industries including semiconductors and displays was recognized through this award.
Samsung Electronics President Hark Kyu Park graduated from the School of Business and Technology Management with an MS in 1986. He not only enhanced Korea’s national competitiveness by expanding the semiconductor industry, but also established contract-based semiconductor departments at Korean universities including KAIST, Sungkyunkwan University, Yonsei University, and Postech, and semiconductor track courses at KAIST, Sogang University, Seoul National University, and Postech to nurture professional talents. He also led the national semiconductor coexistence system by leading private sector-government-academia collaborations to strengthen competence in semiconductors, and continues to make unconditional investments in strong small businesses.
KAA President Chilhee Chung said, “Thanks to our alumni contributing at the highest levels of our society, the name of our alma mater shines brighter. As role models for our younger alumni, I hope greater honours will follow our awardees in the future.”
2022.03.03 View 9699 -
 Commencement Ceremony Honors the Class of 2022
Third online commencement ceremony since the pandemic outbreak celebrates 2741 graduates
The 2022 commencement ceremony convened online on February 18 to celebrate and award degrees to the Class of 2022. The graduating class of 2022 included 663 PhDs, 1,383 Masters, and 695 Bachelors. The limited number of attendees included 86 graduate representatives and approximately 20 faculty members in senior leadership, as well as Korea’s Minister of Science and ICT Hyesook Lim. The ceremony was livestreamed on KAIST’s YouTube channel.
Valedictorian Ji-Young Lee from the Department of Physics received the Minister of Science and ICT’s Award. Yu-Jin Bang from the School of Business and Technology Management was the Awardee of the Chairman of the KAIST Board of Trustees and the KAIST Presidential Awardee was Jong-Hwan Lee from the Department of Mathematical Sciences.
KAIST conferred honorary doctorates to Honorary Chairman Jae-Chul Kim of Dongwon Group and Chairman Sung-Hwan Chang of Samsung Brush. Chairman Kim, whose donation funded the establishment of the Kim Jae-Chul Graduate School of AI, was awarded an honorary doctorate of science technology. Chairman Chang was awarded an honorary doctorate of business administration in recognition of his funding in the fields of medical science and engineering at KAIST.
This year’s undergraduate commencement speaker was Hye-Lin Park from the School of Computing. She has severe cerebral palsy and was the first student admitted to KAIST with a severe physical handicap.
“I loved mathematics and wanted to become a mathematician. When I learned programming in my second year, I was so mesmerized by it that I transferred to the School of Computing,” said Park, who plans to continue studying programming languages in graduate school at KAIST.
“I spent my entire life of 24 years in this beautiful wheelchair. Without the support and help of my parents, friends, and my special teachers who helped me move and study at the campus, I would not have made it this far,” said Park. For easier access to classrooms and facilities, KAIST started to remodel its facilities to make the entrance of buildings more wheelchair-friendly. Park made many suggestions to the Office of Student Life and the Facilities Management Office on how to ease mobility for handicapped people on campus. The physical education course that was required for graduation was also revised to stipulate exceptions.
Minister Lim stressed the role of young scientists and researchers in these times of digital transformation dominated by AI and the metaverse. She encouraged the graduates to carry out their dreams with warm hearts and challenging spirits.
KAIST President Kwang Hyung Lee also stressed the power of dreams, calling for graduates to dream big even in times of uncertainty.
“Humanity stands at an inflection point in history. The fourth industrial revolution and outbreak of Covid-19 have unfolded the grand global transformation. Although the future gives us new opportunities, it also comes with anxiety regarding the uncertainties ahead.”
“Dreams make your heart race and push us to live life to the fullest. Dreams will help you keep moving forward even in the face of adversity,” he said.
2022.02.18 View 12472
Commencement Ceremony Honors the Class of 2022
Third online commencement ceremony since the pandemic outbreak celebrates 2741 graduates
The 2022 commencement ceremony convened online on February 18 to celebrate and award degrees to the Class of 2022. The graduating class of 2022 included 663 PhDs, 1,383 Masters, and 695 Bachelors. The limited number of attendees included 86 graduate representatives and approximately 20 faculty members in senior leadership, as well as Korea’s Minister of Science and ICT Hyesook Lim. The ceremony was livestreamed on KAIST’s YouTube channel.
Valedictorian Ji-Young Lee from the Department of Physics received the Minister of Science and ICT’s Award. Yu-Jin Bang from the School of Business and Technology Management was the Awardee of the Chairman of the KAIST Board of Trustees and the KAIST Presidential Awardee was Jong-Hwan Lee from the Department of Mathematical Sciences.
KAIST conferred honorary doctorates to Honorary Chairman Jae-Chul Kim of Dongwon Group and Chairman Sung-Hwan Chang of Samsung Brush. Chairman Kim, whose donation funded the establishment of the Kim Jae-Chul Graduate School of AI, was awarded an honorary doctorate of science technology. Chairman Chang was awarded an honorary doctorate of business administration in recognition of his funding in the fields of medical science and engineering at KAIST.
This year’s undergraduate commencement speaker was Hye-Lin Park from the School of Computing. She has severe cerebral palsy and was the first student admitted to KAIST with a severe physical handicap.
“I loved mathematics and wanted to become a mathematician. When I learned programming in my second year, I was so mesmerized by it that I transferred to the School of Computing,” said Park, who plans to continue studying programming languages in graduate school at KAIST.
“I spent my entire life of 24 years in this beautiful wheelchair. Without the support and help of my parents, friends, and my special teachers who helped me move and study at the campus, I would not have made it this far,” said Park. For easier access to classrooms and facilities, KAIST started to remodel its facilities to make the entrance of buildings more wheelchair-friendly. Park made many suggestions to the Office of Student Life and the Facilities Management Office on how to ease mobility for handicapped people on campus. The physical education course that was required for graduation was also revised to stipulate exceptions.
Minister Lim stressed the role of young scientists and researchers in these times of digital transformation dominated by AI and the metaverse. She encouraged the graduates to carry out their dreams with warm hearts and challenging spirits.
KAIST President Kwang Hyung Lee also stressed the power of dreams, calling for graduates to dream big even in times of uncertainty.
“Humanity stands at an inflection point in history. The fourth industrial revolution and outbreak of Covid-19 have unfolded the grand global transformation. Although the future gives us new opportunities, it also comes with anxiety regarding the uncertainties ahead.”
“Dreams make your heart race and push us to live life to the fullest. Dreams will help you keep moving forward even in the face of adversity,” he said.
2022.02.18 View 12472 -
 President Lee Presents Plans to Nurture Next-Generation Talents
President Lee stressed that nurturing medical scientists, semiconductor R&D personnel, startup entrepreneurs, and global innovators are key missions he will continue to pursue during a news conference
KAIST President Kwang Hyung Lee said that nurturing medical scientists, semiconductor R&D personnel, startup entrepreneurs, and global innovators are key missions he will continue to pursue during an online news conference marking the 1st anniversary of him becoming the president on February 15.
He said that nurturing physician-scientists is the most critical mission for KAIST to help the nation create a new growth engine. He said KAIST will help the nation drive the bio-industry and provide medical science resources for the nation’s health sector. To this end, he said that KAIST will open its Medical Science and Technology School by 2026.
“We plan to expand the current Graduate School of Medical Science and Engineering into a new Medical Science and Technology School that will focus entirely on a condensed MD-PhD course converging the fields of AI, bio, and physics,” he said.
The school aims to foster medical scientists whose research results will eventually be commercialized. He said that the university is now discussing revisions to related laws and regulations with the government and other universities.
To supply human resources to the semiconductor industry, President Lee said the university will add a campus in Pyongtaek City that will serve as an advanced convergence research hub in the field of next generation semiconductors in collaboration with Samsung Electronics and the city of Pyongtaek. The three-stage opening plan projected the final opening of the campus by 2036. During the first stage, which will be completed by 2026, it will construct the campus infrastructure in Pyongtaek city where Samsung Semiconductors runs two massive semiconductor complexes. By 2031, it plans to launch the open research platform including a future cities research center and future vehicles research center. The campus will open the global industrial collaboration cluster hub by 2036.
In the global arena, President Lee said he is working to open the New York campus with stakeholders in the United States. He announced the plan last December that was endorsed by New York-based entrepreneur Hee-Nam Bae, the chairman of Big Continent Inc. President Lee and Chairman Lee signed an MOU for the funding to open the campus in New York.
“We are discussing how to facilitate the plan and best accommodate the interests and potential of our students. Many ideas and plans are on the table and we think it will take longer than expected to finalize the plan,” explained President Lee.
However, he added that the basic idea is to offer art tech and health technology programs as well as an AI-based finance MBA at the New York campus, in addition to it serving as the startup accelerator of KAIST.
President Lee stressed the importance of technology commercialization when successfully launching KAIST Holdings last month to help spinoffs of KAIST labs accelerate their end results. He said that KAIST Holdings will build a virtuous supporting system to commercialize the technology startups coming from KAIST.
“We plan to list at least 10 KAIST startups on the KOSDAQ and two on the NASDAQ by 2031. KAIST Holdings also aims to nurture companies valued at a total of one billion KRW and earn 100 billion KRW in technology fees by 2031.
2022.02.17 View 14047
President Lee Presents Plans to Nurture Next-Generation Talents
President Lee stressed that nurturing medical scientists, semiconductor R&D personnel, startup entrepreneurs, and global innovators are key missions he will continue to pursue during a news conference
KAIST President Kwang Hyung Lee said that nurturing medical scientists, semiconductor R&D personnel, startup entrepreneurs, and global innovators are key missions he will continue to pursue during an online news conference marking the 1st anniversary of him becoming the president on February 15.
He said that nurturing physician-scientists is the most critical mission for KAIST to help the nation create a new growth engine. He said KAIST will help the nation drive the bio-industry and provide medical science resources for the nation’s health sector. To this end, he said that KAIST will open its Medical Science and Technology School by 2026.
“We plan to expand the current Graduate School of Medical Science and Engineering into a new Medical Science and Technology School that will focus entirely on a condensed MD-PhD course converging the fields of AI, bio, and physics,” he said.
The school aims to foster medical scientists whose research results will eventually be commercialized. He said that the university is now discussing revisions to related laws and regulations with the government and other universities.
To supply human resources to the semiconductor industry, President Lee said the university will add a campus in Pyongtaek City that will serve as an advanced convergence research hub in the field of next generation semiconductors in collaboration with Samsung Electronics and the city of Pyongtaek. The three-stage opening plan projected the final opening of the campus by 2036. During the first stage, which will be completed by 2026, it will construct the campus infrastructure in Pyongtaek city where Samsung Semiconductors runs two massive semiconductor complexes. By 2031, it plans to launch the open research platform including a future cities research center and future vehicles research center. The campus will open the global industrial collaboration cluster hub by 2036.
In the global arena, President Lee said he is working to open the New York campus with stakeholders in the United States. He announced the plan last December that was endorsed by New York-based entrepreneur Hee-Nam Bae, the chairman of Big Continent Inc. President Lee and Chairman Lee signed an MOU for the funding to open the campus in New York.
“We are discussing how to facilitate the plan and best accommodate the interests and potential of our students. Many ideas and plans are on the table and we think it will take longer than expected to finalize the plan,” explained President Lee.
However, he added that the basic idea is to offer art tech and health technology programs as well as an AI-based finance MBA at the New York campus, in addition to it serving as the startup accelerator of KAIST.
President Lee stressed the importance of technology commercialization when successfully launching KAIST Holdings last month to help spinoffs of KAIST labs accelerate their end results. He said that KAIST Holdings will build a virtuous supporting system to commercialize the technology startups coming from KAIST.
“We plan to list at least 10 KAIST startups on the KOSDAQ and two on the NASDAQ by 2031. KAIST Holdings also aims to nurture companies valued at a total of one billion KRW and earn 100 billion KRW in technology fees by 2031.
2022.02.17 View 14047 -
 Research Finds Digital Music Streaming Consumption Dropped as a Result of Covid-19 and Lockdowns
Decline in human mobility has stunning consequences for content streaming
The Covid-19 pandemic and lockdowns significantly reduced the consumption of audio music streaming in many countries as people turned to video platforms. On average, audio music consumption decreased by 12.5% after the World Health Organization’s (WHO) pandemic declaration in March 2020.
Music streaming services were an unlikely area hit hard by the Covid-19 pandemic. New research in Marketing Science found that the drop in people’s mobility during the pandemic significantly reduced the consumption of audio music streaming. Instead, people turned more to video platforms.
“On average, audio music consumption decreased by more than 12% after the World Health Organization’s (WHO) pandemic declaration on March 11, 2020. As a result, during the pandemic, Spotify lost 838 million dollars of revenue in the first three quarters of 2020,” said Jaeung Sim, a PhD candidate in management engineering at KAIST and one of the authors of the research study on this phenomenon. “Our results showed that human mobility plays a much larger role in the audio consumption of music than previously thought.”
The study, “Frontiers: Virus Shook the Streaming Star: Estimating the Covid-19 Impact on Music Consumption,” conducted by Sim and Professor Daegon Cho of KAIST, Youngdeok Hwang of City University of New York, and Rahul Telang of Carnegie Mellon University, looked at online music streaming data for top songs for two years in 60 countries, as well as Covid-19 cases, lockdown statistics, and daily mobility data, to determine the nature of the changes.
The study showed how the pandemic adversely impacted music streaming services despite the common expectation that the pandemic would universally benefit online medias platforms. This implies that the substantially changing media consumption environment can place streaming music in fiercer competition with other media forms that offer more dynamic and vivid experiences to consumers.
The researchers found that music consumption through video platforms was positively associated with the severity of Covid-19, lockdown policies, and time spent at home.
-PublicationJaeung Sim, Daegon Cho, Youngdeok Hwang, and Rahul Telang,“Frontiers: Virus Shook the Streaming Star: Estimating the Covid-19 Impact on Music Consumption,” November 30 in Marketing Science online (doi.org/10.1287/mksc.2021.1321)
-Profile Professor Daegon ChoGraduate School of Information and Media ManagementCollege of BusinessKAIST
2022.02.15 View 12104
Research Finds Digital Music Streaming Consumption Dropped as a Result of Covid-19 and Lockdowns
Decline in human mobility has stunning consequences for content streaming
The Covid-19 pandemic and lockdowns significantly reduced the consumption of audio music streaming in many countries as people turned to video platforms. On average, audio music consumption decreased by 12.5% after the World Health Organization’s (WHO) pandemic declaration in March 2020.
Music streaming services were an unlikely area hit hard by the Covid-19 pandemic. New research in Marketing Science found that the drop in people’s mobility during the pandemic significantly reduced the consumption of audio music streaming. Instead, people turned more to video platforms.
“On average, audio music consumption decreased by more than 12% after the World Health Organization’s (WHO) pandemic declaration on March 11, 2020. As a result, during the pandemic, Spotify lost 838 million dollars of revenue in the first three quarters of 2020,” said Jaeung Sim, a PhD candidate in management engineering at KAIST and one of the authors of the research study on this phenomenon. “Our results showed that human mobility plays a much larger role in the audio consumption of music than previously thought.”
The study, “Frontiers: Virus Shook the Streaming Star: Estimating the Covid-19 Impact on Music Consumption,” conducted by Sim and Professor Daegon Cho of KAIST, Youngdeok Hwang of City University of New York, and Rahul Telang of Carnegie Mellon University, looked at online music streaming data for top songs for two years in 60 countries, as well as Covid-19 cases, lockdown statistics, and daily mobility data, to determine the nature of the changes.
The study showed how the pandemic adversely impacted music streaming services despite the common expectation that the pandemic would universally benefit online medias platforms. This implies that the substantially changing media consumption environment can place streaming music in fiercer competition with other media forms that offer more dynamic and vivid experiences to consumers.
The researchers found that music consumption through video platforms was positively associated with the severity of Covid-19, lockdown policies, and time spent at home.
-PublicationJaeung Sim, Daegon Cho, Youngdeok Hwang, and Rahul Telang,“Frontiers: Virus Shook the Streaming Star: Estimating the Covid-19 Impact on Music Consumption,” November 30 in Marketing Science online (doi.org/10.1287/mksc.2021.1321)
-Profile Professor Daegon ChoGraduate School of Information and Media ManagementCollege of BusinessKAIST
2022.02.15 View 12104 -
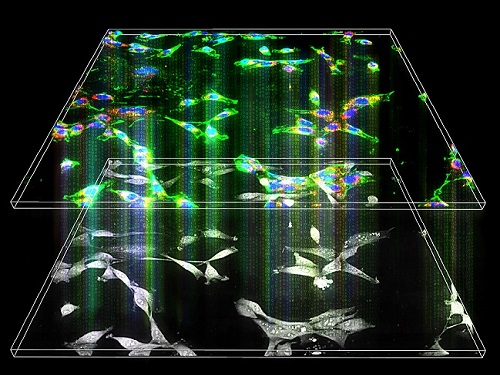 Label-Free Multiplexed Microtomography of Endogenous Subcellular Dynamics Using Deep Learning
AI-based holographic microscopy allows molecular imaging without introducing exogenous labeling agents
A research team upgraded the 3D microtomography observing dynamics of label-free live cells in multiplexed fluorescence imaging. The AI-powered 3D holotomographic microscopy extracts various molecular information from live unlabeled biological cells in real time without exogenous labeling or staining agents.
Professor YongKeum Park’s team and the startup Tomocube encoded 3D refractive index tomograms using the refractive index as a means of measurement. Then they decoded the information with a deep learning-based model that infers multiple 3D fluorescence tomograms from the refractive index measurements of the corresponding subcellular targets, thereby achieving multiplexed micro tomography. This study was reported in Nature Cell Biology online on December 7, 2021.
Fluorescence microscopy is the most widely used optical microscopy technique due to its high biochemical specificity. However, it needs to genetically manipulate or to stain cells with fluorescent labels in order to express fluorescent proteins. These labeling processes inevitably affect the intrinsic physiology of cells. It also has challenges in long-term measuring due to photobleaching and phototoxicity. The overlapped spectra of multiplexed fluorescence signals also hinder the viewing of various structures at the same time. More critically, it took several hours to observe the cells after preparing them.
3D holographic microscopy, also known as holotomography, is providing new ways to quantitatively image live cells without pretreatments such as staining. Holotomography can accurately and quickly measure the morphological and structural information of cells, but only provides limited biochemical and molecular information.
The 'AI microscope' created in this process takes advantage of the features of both holographic microscopy and fluorescence microscopy. That is, a specific image from a fluorescence microscope can be obtained without a fluorescent label. Therefore, the microscope can observe many types of cellular structures in their natural state in 3D and at the same time as fast as one millisecond, and long-term measurements over several days are also possible.
The Tomocube-KAIST team showed that fluorescence images can be directly and precisely predicted from holotomographic images in various cells and conditions. Using the quantitative relationship between the spatial distribution of the refractive index found by AI and the major structures in cells, it was possible to decipher the spatial distribution of the refractive index. And surprisingly, it confirmed that this relationship is constant regardless of cell type.
Professor Park said, “We were able to develop a new concept microscope that combines the advantages of several microscopes with the multidisciplinary research of AI, optics, and biology. It will be immediately applicable for new types of cells not included in the existing data and is expected to be widely applicable for various biological and medical research.”
When comparing the molecular image information extracted by AI with the molecular image information physically obtained by fluorescence staining in 3D space, it showed a 97% or more conformity, which is a level that is difficult to distinguish with the naked eye.
“Compared to the sub-60% accuracy of the fluorescence information extracted from the model developed by the Google AI team, it showed significantly higher performance,” Professor Park added.
This work was supported by the KAIST Up program, the BK21+ program, Tomocube, the National Research Foundation of Korea, and the Ministry of Science and ICT, and the Ministry of Health & Welfare.
-Publication
Hyun-seok Min, Won-Do Heo, YongKeun Park, et al. “Label-free multiplexed microtomography of endogenous subcellular dynamics using generalizable deep learning,” Nature Cell Biology (doi.org/10.1038/s41556-021-00802-x) published online December 07 2021.
-Profile
Professor YongKeun Park
Biomedical Optics Laboratory
Department of Physics
KAIST
2022.02.09 View 13901
Label-Free Multiplexed Microtomography of Endogenous Subcellular Dynamics Using Deep Learning
AI-based holographic microscopy allows molecular imaging without introducing exogenous labeling agents
A research team upgraded the 3D microtomography observing dynamics of label-free live cells in multiplexed fluorescence imaging. The AI-powered 3D holotomographic microscopy extracts various molecular information from live unlabeled biological cells in real time without exogenous labeling or staining agents.
Professor YongKeum Park’s team and the startup Tomocube encoded 3D refractive index tomograms using the refractive index as a means of measurement. Then they decoded the information with a deep learning-based model that infers multiple 3D fluorescence tomograms from the refractive index measurements of the corresponding subcellular targets, thereby achieving multiplexed micro tomography. This study was reported in Nature Cell Biology online on December 7, 2021.
Fluorescence microscopy is the most widely used optical microscopy technique due to its high biochemical specificity. However, it needs to genetically manipulate or to stain cells with fluorescent labels in order to express fluorescent proteins. These labeling processes inevitably affect the intrinsic physiology of cells. It also has challenges in long-term measuring due to photobleaching and phototoxicity. The overlapped spectra of multiplexed fluorescence signals also hinder the viewing of various structures at the same time. More critically, it took several hours to observe the cells after preparing them.
3D holographic microscopy, also known as holotomography, is providing new ways to quantitatively image live cells without pretreatments such as staining. Holotomography can accurately and quickly measure the morphological and structural information of cells, but only provides limited biochemical and molecular information.
The 'AI microscope' created in this process takes advantage of the features of both holographic microscopy and fluorescence microscopy. That is, a specific image from a fluorescence microscope can be obtained without a fluorescent label. Therefore, the microscope can observe many types of cellular structures in their natural state in 3D and at the same time as fast as one millisecond, and long-term measurements over several days are also possible.
The Tomocube-KAIST team showed that fluorescence images can be directly and precisely predicted from holotomographic images in various cells and conditions. Using the quantitative relationship between the spatial distribution of the refractive index found by AI and the major structures in cells, it was possible to decipher the spatial distribution of the refractive index. And surprisingly, it confirmed that this relationship is constant regardless of cell type.
Professor Park said, “We were able to develop a new concept microscope that combines the advantages of several microscopes with the multidisciplinary research of AI, optics, and biology. It will be immediately applicable for new types of cells not included in the existing data and is expected to be widely applicable for various biological and medical research.”
When comparing the molecular image information extracted by AI with the molecular image information physically obtained by fluorescence staining in 3D space, it showed a 97% or more conformity, which is a level that is difficult to distinguish with the naked eye.
“Compared to the sub-60% accuracy of the fluorescence information extracted from the model developed by the Google AI team, it showed significantly higher performance,” Professor Park added.
This work was supported by the KAIST Up program, the BK21+ program, Tomocube, the National Research Foundation of Korea, and the Ministry of Science and ICT, and the Ministry of Health & Welfare.
-Publication
Hyun-seok Min, Won-Do Heo, YongKeun Park, et al. “Label-free multiplexed microtomography of endogenous subcellular dynamics using generalizable deep learning,” Nature Cell Biology (doi.org/10.1038/s41556-021-00802-x) published online December 07 2021.
-Profile
Professor YongKeun Park
Biomedical Optics Laboratory
Department of Physics
KAIST
2022.02.09 View 13901 -
 Eco-Friendly Micro-Supercapacitors Using Fallen Leaves
Green micro-supercapacitors on a single leaf could easily be applied in wearable electronics, smart houses, and IoTs
A KAIST research team has developed graphene-inorganic-hybrid micro-supercapacitors made of fallen leaves using femtosecond laser direct writing. The rapid development of wearable electronics requires breakthrough innovations in flexible energy storage devices in which micro-supercapacitors have drawn a great deal of interest due to their high power density, long lifetimes, and short charging times. Recently, there has been an enormous increase in waste batteries owing to the growing demand and the shortened replacement cycle in consumer electronics. The safety and environmental issues involved in the collection, recycling, and processing of such waste batteries are creating a number of challenges.
Forests cover about 30 percent of the Earth’s surface and produce a huge amount of fallen leaves. This naturally occurring biomass comes in large quantities and is completely biodegradable, which makes it an attractive sustainable resource. Nevertheless, if the fallen leaves are left neglected instead of being used efficiently, they can contribute to fire hazards, air pollution, and global warming.
To solve both problems at once, a research team led by Professor Young-Jin Kim from the Department of Mechanical Engineering and Dr. Hana Yoon from the Korea Institute of Energy Research developed a novel technology that can create 3D porous graphene microelectrodes with high electrical conductivity by irradiating femtosecond laser pulses on the leaves in ambient air. This one-step fabrication does not require any additional materials or pre-treatment.
They showed that this technique could quickly and easily produce porous graphene electrodes at a low price, and demonstrated potential applications by fabricating graphene micro-supercapacitors to power an LED and an electronic watch. These results open up a new possibility for the mass production of flexible and green graphene-based electronic devices.
Professor Young-Jin Kim said, “Leaves create forest biomass that comes in unmanageable quantities, so using them for next-generation energy storage devices makes it possible for us to reuse waste resources, thereby establishing a virtuous cycle.”
This research was published in Advanced Functional Materials last month and was sponsored by the Ministry of Agriculture Food and Rural Affairs, the Korea Forest Service, and the Korea Institute of Energy Research.
-Publication
Truong-Son Dinh Le, Yeong A. Lee, Han Ku Nam, Kyu Yeon Jang, Dongwook Yang, Byunggi Kim, Kanghoon Yim, Seung Woo Kim, Hana Yoon, and Young-jin Kim, “Green Flexible Graphene-Inorganic-Hybrid Micro-Supercapacitors Made of Fallen Leaves Enabled by Ultrafast Laser Pulses," December 05, 2021, Advanced Functional Materials (doi.org/10.1002/adfm.202107768)
-ProfileProfessor Young-Jin KimUltra-Precision Metrology and Manufacturing (UPM2) LaboratoryDepartment of Mechanical EngineeringKAIST
2022.01.27 View 15084
Eco-Friendly Micro-Supercapacitors Using Fallen Leaves
Green micro-supercapacitors on a single leaf could easily be applied in wearable electronics, smart houses, and IoTs
A KAIST research team has developed graphene-inorganic-hybrid micro-supercapacitors made of fallen leaves using femtosecond laser direct writing. The rapid development of wearable electronics requires breakthrough innovations in flexible energy storage devices in which micro-supercapacitors have drawn a great deal of interest due to their high power density, long lifetimes, and short charging times. Recently, there has been an enormous increase in waste batteries owing to the growing demand and the shortened replacement cycle in consumer electronics. The safety and environmental issues involved in the collection, recycling, and processing of such waste batteries are creating a number of challenges.
Forests cover about 30 percent of the Earth’s surface and produce a huge amount of fallen leaves. This naturally occurring biomass comes in large quantities and is completely biodegradable, which makes it an attractive sustainable resource. Nevertheless, if the fallen leaves are left neglected instead of being used efficiently, they can contribute to fire hazards, air pollution, and global warming.
To solve both problems at once, a research team led by Professor Young-Jin Kim from the Department of Mechanical Engineering and Dr. Hana Yoon from the Korea Institute of Energy Research developed a novel technology that can create 3D porous graphene microelectrodes with high electrical conductivity by irradiating femtosecond laser pulses on the leaves in ambient air. This one-step fabrication does not require any additional materials or pre-treatment.
They showed that this technique could quickly and easily produce porous graphene electrodes at a low price, and demonstrated potential applications by fabricating graphene micro-supercapacitors to power an LED and an electronic watch. These results open up a new possibility for the mass production of flexible and green graphene-based electronic devices.
Professor Young-Jin Kim said, “Leaves create forest biomass that comes in unmanageable quantities, so using them for next-generation energy storage devices makes it possible for us to reuse waste resources, thereby establishing a virtuous cycle.”
This research was published in Advanced Functional Materials last month and was sponsored by the Ministry of Agriculture Food and Rural Affairs, the Korea Forest Service, and the Korea Institute of Energy Research.
-Publication
Truong-Son Dinh Le, Yeong A. Lee, Han Ku Nam, Kyu Yeon Jang, Dongwook Yang, Byunggi Kim, Kanghoon Yim, Seung Woo Kim, Hana Yoon, and Young-jin Kim, “Green Flexible Graphene-Inorganic-Hybrid Micro-Supercapacitors Made of Fallen Leaves Enabled by Ultrafast Laser Pulses," December 05, 2021, Advanced Functional Materials (doi.org/10.1002/adfm.202107768)
-ProfileProfessor Young-Jin KimUltra-Precision Metrology and Manufacturing (UPM2) LaboratoryDepartment of Mechanical EngineeringKAIST
2022.01.27 View 15084