BIO
-
 KAIST and the University of Minnesota-Twin Cities Partner for Research and Education Collaboration
President Steve Kang of KAIST and President Eric W. Kaler of the University of Minnesota-Twin Cities (United States) signed a memorandum of understanding to create exchange programs for students and faculty and to conduct joint research in the field of health and food. The following is an excerpt from President Kaler’s blog (https://storify.com/UMNstory/globalumn-hksk#edaadf) on his visit of KAIST on November 18, 2015:
A visit to the Korea Advanced Institute of Science and Technology
About 90 miles from Seoul—and more than that two-and-a-half-hours of a bus ride through the rugged early-morning traffic of South Korea’s capital city—sits Daejeon, Korea’s sixth largest city and home to KAIST, the Korea Advanced Institute of Science and Technology. Today, President Kaler and the small University of Minnesota delegation accompanying him visited what’s considered Korea’s MIT, a place focused on research and known to push the limits toward the future.
Fingernail heart monitors? Wireless anesthetic-monitoring devices?
KAIST is working on them.
The overlap of interests—from biomedical engineering to nanotechnology to robotics—between KAIST (pronounced “Kyst”) and the U are remarkable. Smartphone apps to monitor human health and GPS-driven robots to serve military interests or deliver packages were among the developing inventions that KAIST scientists showed to Kaler.
And even the personal relationships seem to illustrate the cliché of a small world and the natural affinity of Minnesota and KAIST.
KAIST’s President Sang Mo Kang was once the head of the University of Illinois’ department of electrical and computer engineering, and he and Kaler—a renowned chemical engineer before becoming the U’s president—hit it off … despite disagreeing about the potential outcome of Saturday’s Illinois-Gophers football game.
Accompanying Kaler on the day’s journey, meetings, and signing of a Memorandum of Understanding between the two schools to advance collaborations was U Associate Professor Sang Hyun Oh. Oh happens to be a physics graduate of this very KAIST and is now a rising star in Minnesota’s Department of Electrical and Computer Engineering.
The two sides agreed to focus on matching scholars on their respective campuses to discuss the sorts of research the two institutions can partner on. The idea of “Grand Challenges,” at the core of the U’s Twin Cities campus Strategic Plan, has fascinated Korean higher education leaders during Kaler’s weeklong visit, and KAIST’s leadership was interested in the health and food research, two U strengths.
###
2015.12.04 View 9579
KAIST and the University of Minnesota-Twin Cities Partner for Research and Education Collaboration
President Steve Kang of KAIST and President Eric W. Kaler of the University of Minnesota-Twin Cities (United States) signed a memorandum of understanding to create exchange programs for students and faculty and to conduct joint research in the field of health and food. The following is an excerpt from President Kaler’s blog (https://storify.com/UMNstory/globalumn-hksk#edaadf) on his visit of KAIST on November 18, 2015:
A visit to the Korea Advanced Institute of Science and Technology
About 90 miles from Seoul—and more than that two-and-a-half-hours of a bus ride through the rugged early-morning traffic of South Korea’s capital city—sits Daejeon, Korea’s sixth largest city and home to KAIST, the Korea Advanced Institute of Science and Technology. Today, President Kaler and the small University of Minnesota delegation accompanying him visited what’s considered Korea’s MIT, a place focused on research and known to push the limits toward the future.
Fingernail heart monitors? Wireless anesthetic-monitoring devices?
KAIST is working on them.
The overlap of interests—from biomedical engineering to nanotechnology to robotics—between KAIST (pronounced “Kyst”) and the U are remarkable. Smartphone apps to monitor human health and GPS-driven robots to serve military interests or deliver packages were among the developing inventions that KAIST scientists showed to Kaler.
And even the personal relationships seem to illustrate the cliché of a small world and the natural affinity of Minnesota and KAIST.
KAIST’s President Sang Mo Kang was once the head of the University of Illinois’ department of electrical and computer engineering, and he and Kaler—a renowned chemical engineer before becoming the U’s president—hit it off … despite disagreeing about the potential outcome of Saturday’s Illinois-Gophers football game.
Accompanying Kaler on the day’s journey, meetings, and signing of a Memorandum of Understanding between the two schools to advance collaborations was U Associate Professor Sang Hyun Oh. Oh happens to be a physics graduate of this very KAIST and is now a rising star in Minnesota’s Department of Electrical and Computer Engineering.
The two sides agreed to focus on matching scholars on their respective campuses to discuss the sorts of research the two institutions can partner on. The idea of “Grand Challenges,” at the core of the U’s Twin Cities campus Strategic Plan, has fascinated Korean higher education leaders during Kaler’s weeklong visit, and KAIST’s leadership was interested in the health and food research, two U strengths.
###
2015.12.04 View 9579 -
 Dr. Ryu of KAIST Receives the S-Oil Outstanding Paper Award
Dr. Je-Kyung Ryu of KAIST’s Department of Physics has been awarded the S-Oil Outstanding Paper Award for his doctoral dissertation’s originality and applicability.
Professor Tae-Young Yoon of Physics is his doctoral advisor.
The award ceremony took place on November 25, 2015 at the Press Center in Seoul.
This S-Oil Outstanding Paper Award, jointly sponsored by the Korean Academy of Science and Technology (KAST) and the Scholastic University Presidential Association, was established to foster young talented scientists in basic science and to advance the field.
The award is given every other year for each of the fields of physics, chemistry, mathematics, biology, and earth sciences.
With the award, Dr. Ryu received a research grant of USD 8,600.
He discovered, for the first time in the world, how NSF (N-ethylmaleimide-sensitive factor), a protein involved in a vesicular transport in cellular activities, disassembles a SNARE (soluble NSF attachment protein receptor) complex, using a unimolecular biophysics method.
Unlike the existing studies, he proposed a model in which NSF disassembles SNARE complexes at one step, and as a result, provided evidence of how the SNARE complex influenced the fusion of biological membranes.
His research was published in the scientific journal Science issued on March 27, 2015. The title of the paper is “Spring-loaded Unraveling of a Single SNARE Complex by NSF in One Round of ATP Turnover.”
2015.11.27 View 10938
Dr. Ryu of KAIST Receives the S-Oil Outstanding Paper Award
Dr. Je-Kyung Ryu of KAIST’s Department of Physics has been awarded the S-Oil Outstanding Paper Award for his doctoral dissertation’s originality and applicability.
Professor Tae-Young Yoon of Physics is his doctoral advisor.
The award ceremony took place on November 25, 2015 at the Press Center in Seoul.
This S-Oil Outstanding Paper Award, jointly sponsored by the Korean Academy of Science and Technology (KAST) and the Scholastic University Presidential Association, was established to foster young talented scientists in basic science and to advance the field.
The award is given every other year for each of the fields of physics, chemistry, mathematics, biology, and earth sciences.
With the award, Dr. Ryu received a research grant of USD 8,600.
He discovered, for the first time in the world, how NSF (N-ethylmaleimide-sensitive factor), a protein involved in a vesicular transport in cellular activities, disassembles a SNARE (soluble NSF attachment protein receptor) complex, using a unimolecular biophysics method.
Unlike the existing studies, he proposed a model in which NSF disassembles SNARE complexes at one step, and as a result, provided evidence of how the SNARE complex influenced the fusion of biological membranes.
His research was published in the scientific journal Science issued on March 27, 2015. The title of the paper is “Spring-loaded Unraveling of a Single SNARE Complex by NSF in One Round of ATP Turnover.”
2015.11.27 View 10938 -
 More Donations Arrive to Establish the New Medicine Research and Development Center on Campus
A raft of businesses continues to make donations to establish a new medicine research and development center on campus. The Department of Biological Sciences at KAIST is leading the fundraising campaign.
On November 9, 2015, Nikon Instruments Korea Co., Ltd. contributed USD 8,500 to the fundraising, followed by Carl Zeiss AG and Three-Shine Inc., which donated USD 12,800 and 8,500, respectively.
Bruno Lin, an Executive Director at Carl Zeiss AG in Korea, said, “I’m very glad to participate in this fundraising initiative for the Biological Sciences Department at KAIST, one rapidly reaching out to the world.”
From the left in the picture are Vice President Tae-Hoon Kim, Director Gyu-Hyeok Lee, and Executive Director Bruno Lin of Carl Zeiss AG, Byung-Ha Oh, Dean of the Biological Sciences Department, and Professor Eunjoon Kim.
From the left in the picture are Byung-Ha Oh, Dean of the Biological Sciences Department, President Chun-Gui Park of Three-Shine Inc., and Professor Daesoo Kim.
President Chun of Three-Shine Inc., said, “We hope that the Department of Biological Sciences at KAIST, aided by the construction of new research center, will produce practical research achievements and stand on the frontier of new medicine development research in Korea.”
The New Medicine Research and Development Center will be equipped with state-of-the-art, purpose-built research facilities to support convergent, interdisciplinary research in biomedicine.
2015.11.27 View 8648
More Donations Arrive to Establish the New Medicine Research and Development Center on Campus
A raft of businesses continues to make donations to establish a new medicine research and development center on campus. The Department of Biological Sciences at KAIST is leading the fundraising campaign.
On November 9, 2015, Nikon Instruments Korea Co., Ltd. contributed USD 8,500 to the fundraising, followed by Carl Zeiss AG and Three-Shine Inc., which donated USD 12,800 and 8,500, respectively.
Bruno Lin, an Executive Director at Carl Zeiss AG in Korea, said, “I’m very glad to participate in this fundraising initiative for the Biological Sciences Department at KAIST, one rapidly reaching out to the world.”
From the left in the picture are Vice President Tae-Hoon Kim, Director Gyu-Hyeok Lee, and Executive Director Bruno Lin of Carl Zeiss AG, Byung-Ha Oh, Dean of the Biological Sciences Department, and Professor Eunjoon Kim.
From the left in the picture are Byung-Ha Oh, Dean of the Biological Sciences Department, President Chun-Gui Park of Three-Shine Inc., and Professor Daesoo Kim.
President Chun of Three-Shine Inc., said, “We hope that the Department of Biological Sciences at KAIST, aided by the construction of new research center, will produce practical research achievements and stand on the frontier of new medicine development research in Korea.”
The New Medicine Research and Development Center will be equipped with state-of-the-art, purpose-built research facilities to support convergent, interdisciplinary research in biomedicine.
2015.11.27 View 8648 -
 Establishment of System Metabolic Engineering Strategies
Although conventional petrochemical processes have generated chemicals and materials which have been useful to mankind, they have also triggered a variety of environmental problems including climate change and relied too much on nonrenewable natural resources. To ameliorate this, researchers have actively pursued the development of industrial microbial strains around the globe in order to overproduce industrially useful chemicals and materials from microbes using renewable biomass. This discipline is called metabolic engineering.
Thanks to advances in genetic engineering and our knowledge of cellular metabolism, conventional metabolic engineering efforts have succeeded to a certain extent in developing microbial strains that overproduce bioproducts at an industrial level. However, many metabolic engineering projects launched in academic labs do not reach commercial markets due to a failure to fully integrate industrial bioprocesses.
In response to this, Distinguished Professor Sang Yup Lee and Dr. Hyun Uk Kim, both from the Department of Chemical and Biomolecular Engineering at KAIST, have recently suggested ten general strategies of systems metabolic engineering to successfully develop industrial microbial strains. Systems metabolic engineering differs from conventional metabolic engineering by incorporating traditional metabolic engineering approaches along with tools of other fields, such as systems biology, synthetic biology, and molecular evolution.
The ten strategies of systems metabolic engineering have been featured in Nature Biotechnology released online in October 2015, which is entitled "Systems strategies for developing industrial microbial strains."
The strategies cover economic, state-of-the-art biological techniques and traditional bioprocess aspects. Specifically, they consist of: 1) project design including economic evaluation of a target bioproduct; 2) selection of host strains to be used for overproduction of a bioproduct; 3) metabolic pathway reconstruction for bioproducts that are not naturally produced in the selected host strains; 4) increasing tolerance of a host strain against the bioproduct; 5) removing negative regulatory circuits in the microbial host limiting overproduction of a bioproduct; 6) rerouting intracellular fluxes to optimize cofactor and precursor availability necessary for the bioproduct formation; 7) diagnosing and optimizing metabolic fluxes towards product formation; 8) diagnosis and optimization of microbial culture conditions including carbon sources; 9) system-wide gene manipulation to further increase the host strain's production performance using high-throughput genome-scale engineering and computational tools; and 10) scale-up fermentation of the developed strain and diagnosis for the reproducibility of the strain's production performance.
These ten strategies were articulated with successful examples of the production of L-arginine using Corynebacterium glutamicum, 1,4-butanediol using Escherichia coli, and L-lysine and bio-nylon using C. glutamicum.
Professor Sang Yup Lee said, "At the moment, the chance of commercializing microbial strains developed in academic labs is very low. The strategies of systems metabolic engineering outlined in this analysis can serve as guidelines when developing industrial microbial strains. We hope that these strategies contribute to improving opportunities to commercialize microbial strains developed in academic labs with drastically reduced costs and efforts, and that a large fraction of petroleum-based processes will be replaced with sustainable bioprocesses."
Lee S. Y. & Kim, H. U. Systems Strategies for Developing Industrial Microbial Strains. Nature Biotechnology (2015).
This work was supported by the Technology Development Program to Solve Climate Change on Systems Metabolic Engineering for Biorefineries (NRF-2012M1A2A2026556) and by the Intelligent Synthetic Biology Center through the Global Frontier Project (2011-0031963) from the Ministry of Science, ICT and Future Planning (MSIP), Korea, and through the National Research Foundation (NRF) of Korea. This work was also supported by the Novo Nordisk Foundation.
Picture: Concept of the Systems Metabolic Engineering Framework
(a) Three major bioprocess stages (b) Considerations in systems metabolic engineering to optimize the whole bioprocess. List of considerations for the strain development and fermentation contribute to improving microbial strain's production performance (red), whereas those for the separation and purification help in reducing overall operation costs by facilitating the downstream process (blue). Some of the considerations can be repeated in the course of systems metabolic engineering.
2015.10.19 View 11893
Establishment of System Metabolic Engineering Strategies
Although conventional petrochemical processes have generated chemicals and materials which have been useful to mankind, they have also triggered a variety of environmental problems including climate change and relied too much on nonrenewable natural resources. To ameliorate this, researchers have actively pursued the development of industrial microbial strains around the globe in order to overproduce industrially useful chemicals and materials from microbes using renewable biomass. This discipline is called metabolic engineering.
Thanks to advances in genetic engineering and our knowledge of cellular metabolism, conventional metabolic engineering efforts have succeeded to a certain extent in developing microbial strains that overproduce bioproducts at an industrial level. However, many metabolic engineering projects launched in academic labs do not reach commercial markets due to a failure to fully integrate industrial bioprocesses.
In response to this, Distinguished Professor Sang Yup Lee and Dr. Hyun Uk Kim, both from the Department of Chemical and Biomolecular Engineering at KAIST, have recently suggested ten general strategies of systems metabolic engineering to successfully develop industrial microbial strains. Systems metabolic engineering differs from conventional metabolic engineering by incorporating traditional metabolic engineering approaches along with tools of other fields, such as systems biology, synthetic biology, and molecular evolution.
The ten strategies of systems metabolic engineering have been featured in Nature Biotechnology released online in October 2015, which is entitled "Systems strategies for developing industrial microbial strains."
The strategies cover economic, state-of-the-art biological techniques and traditional bioprocess aspects. Specifically, they consist of: 1) project design including economic evaluation of a target bioproduct; 2) selection of host strains to be used for overproduction of a bioproduct; 3) metabolic pathway reconstruction for bioproducts that are not naturally produced in the selected host strains; 4) increasing tolerance of a host strain against the bioproduct; 5) removing negative regulatory circuits in the microbial host limiting overproduction of a bioproduct; 6) rerouting intracellular fluxes to optimize cofactor and precursor availability necessary for the bioproduct formation; 7) diagnosing and optimizing metabolic fluxes towards product formation; 8) diagnosis and optimization of microbial culture conditions including carbon sources; 9) system-wide gene manipulation to further increase the host strain's production performance using high-throughput genome-scale engineering and computational tools; and 10) scale-up fermentation of the developed strain and diagnosis for the reproducibility of the strain's production performance.
These ten strategies were articulated with successful examples of the production of L-arginine using Corynebacterium glutamicum, 1,4-butanediol using Escherichia coli, and L-lysine and bio-nylon using C. glutamicum.
Professor Sang Yup Lee said, "At the moment, the chance of commercializing microbial strains developed in academic labs is very low. The strategies of systems metabolic engineering outlined in this analysis can serve as guidelines when developing industrial microbial strains. We hope that these strategies contribute to improving opportunities to commercialize microbial strains developed in academic labs with drastically reduced costs and efforts, and that a large fraction of petroleum-based processes will be replaced with sustainable bioprocesses."
Lee S. Y. & Kim, H. U. Systems Strategies for Developing Industrial Microbial Strains. Nature Biotechnology (2015).
This work was supported by the Technology Development Program to Solve Climate Change on Systems Metabolic Engineering for Biorefineries (NRF-2012M1A2A2026556) and by the Intelligent Synthetic Biology Center through the Global Frontier Project (2011-0031963) from the Ministry of Science, ICT and Future Planning (MSIP), Korea, and through the National Research Foundation (NRF) of Korea. This work was also supported by the Novo Nordisk Foundation.
Picture: Concept of the Systems Metabolic Engineering Framework
(a) Three major bioprocess stages (b) Considerations in systems metabolic engineering to optimize the whole bioprocess. List of considerations for the strain development and fermentation contribute to improving microbial strain's production performance (red), whereas those for the separation and purification help in reducing overall operation costs by facilitating the downstream process (blue). Some of the considerations can be repeated in the course of systems metabolic engineering.
2015.10.19 View 11893 -
 Professor Ki-Jun Jeong Wins the 2015 Dam Yeun Academic Award
The 11th Dam Yeun Academic Award presented by the Korean Society for Biotechnology and Bioengineering (KSBB) to a biologist under 45 years old went to Professor Ki-Jun Jeong of the Chemical and Biomolecular Engineering Department at KAIST.
The award ceremony took place on October 13, 2015, at the annual conference of KSBB held at Songdo Convensia in Incheon City.
Each year KSBB announces the recipient of the award based on the publications by researchers in the last five years at peer-reviewed international journals or KSBB Journal as well as the record of patent registration and technology transfers.
Professor Jeong is recognized for his pioneering research in protein, antibody, cellular engineering, and protein displays and chips.
2015.10.19 View 10648
Professor Ki-Jun Jeong Wins the 2015 Dam Yeun Academic Award
The 11th Dam Yeun Academic Award presented by the Korean Society for Biotechnology and Bioengineering (KSBB) to a biologist under 45 years old went to Professor Ki-Jun Jeong of the Chemical and Biomolecular Engineering Department at KAIST.
The award ceremony took place on October 13, 2015, at the annual conference of KSBB held at Songdo Convensia in Incheon City.
Each year KSBB announces the recipient of the award based on the publications by researchers in the last five years at peer-reviewed international journals or KSBB Journal as well as the record of patent registration and technology transfers.
Professor Jeong is recognized for his pioneering research in protein, antibody, cellular engineering, and protein displays and chips.
2015.10.19 View 10648 -
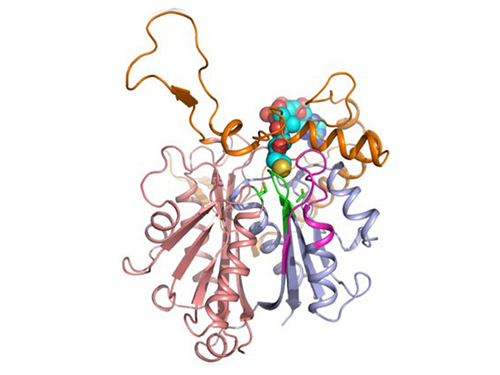 Discovery of Redox-Switch of KEenzyme Involved in N-Butanol Biosynthesis
Research teams at KAIST and Kyungpook National University (KNU) have succeeded in uncovering the redox-switch of thiolase, a key enzyme for n-butanol production in Clostridium acetobutylicum, one of the best known butanol-producing bacteria.
Biological n-butanol production was first reported by Louis Pasteur in 1861, and the bioprocess was industrialized usingClostridium acetobutylicum. The fermentation process by Clostridium strains has been known to be the most efficient one for n-butanol production. Due to growing world-wide issues such as energy security and climate change, the biological production of n-butanol has been receiving much renewed interest. This is because n-butanol possesses much better fuel characteristics compared to ethanol, such as higher energy content (29.2 MJ/L vs 19.6 MJ/L), less corrosiveness, less hygroscopy, and the ease with which it can be blended with gasoline and diesel.
In the paper published in Nature Communications, a broad-scope, online-only, and open access journal issued by the Nature Publishing Group (NPG), on September 22, 2015, Professor Kyung-Jin Kim at the School of Life Sciences, KNU, and Distinguished Professor Sang Yup Lee at the Department of Chemical and Biomolecular Engineering, KAIST, have proved that the redox-switch of thiolase plays a role in a regulation of metabolic flux in C. acetobutylicum by using in silico modeling and simulation tools.
The research team has redesigned thiolase with enhanced activity on the basis of the 3D structure of the wild-type enzyme. To reinforce a metabolic flux toward butanol production, the metabolic network of C. acetobutylicum strain was engineered with the redesigned enzyme. The combination of the discovery of 3D enzyme structure and systems metabolic engineering approaches resulted in increased n-butanol production in C. acetobutylicum, which allows the production of this important industrial chemical to be cost competitive.
Professors Kim and Lee said, "We have reported the 3D structure of C. acetobutylicum thiolase-a key enzyme involved in n-butanol biosynthesis, for the first time. Further study will be done to produce butanol more economically on the basis of the 3D structure of C. acetobutylicum thiolase."
This work was published online in Nature Communications on September 22, 2015.
Reference: Kim et al. "Redox-switch regulatory mechanism of thiolase from Clostridium acetobutylicum," Nature Communications
This research was supported by the Technology Development Program to Solve Climate Changes from the Ministry of Education, Science and Technology (MEST), Korea, the National Research Foundation of Korea, and the Advanced Biomass Center through the Global Frontier Research Program of the MEST, Korea.
For further information, contact Dr. Sang Yup Lee, Distinguished Professor, KAIST, Daejeon, Korea (leesy@kaist.ac.kr, +82-42-350-3930); and Dr. Kyung-Jin Kim, Professor, KNU, Daegu, Korea (kkim@knu.ac.kr, +82-53-950-6088).
Figure 1: A redox-switch of thiolase involves in butanol biosynthesis in Clostridium acetobutylicum. Thiolase condenses two acetyl-CoA molecules for initiating four carbon flux towards butanol.
Figure 2: Thiolase catalyzes the condensation reaction of acetyl-CoA to acetoacetyl-CoA. Two catalytic cysteine residues at 88th and 378th are oxidized and formed an intermolecular disulfide bond in an oxidized status, which results in inactivation of the enzyme for n-butanol biosynthesis. The intermolecular disulfide bond is broken enabling the n-butanol biosynthesis, when the environment status is reduced.
2015.09.23 View 11712
Discovery of Redox-Switch of KEenzyme Involved in N-Butanol Biosynthesis
Research teams at KAIST and Kyungpook National University (KNU) have succeeded in uncovering the redox-switch of thiolase, a key enzyme for n-butanol production in Clostridium acetobutylicum, one of the best known butanol-producing bacteria.
Biological n-butanol production was first reported by Louis Pasteur in 1861, and the bioprocess was industrialized usingClostridium acetobutylicum. The fermentation process by Clostridium strains has been known to be the most efficient one for n-butanol production. Due to growing world-wide issues such as energy security and climate change, the biological production of n-butanol has been receiving much renewed interest. This is because n-butanol possesses much better fuel characteristics compared to ethanol, such as higher energy content (29.2 MJ/L vs 19.6 MJ/L), less corrosiveness, less hygroscopy, and the ease with which it can be blended with gasoline and diesel.
In the paper published in Nature Communications, a broad-scope, online-only, and open access journal issued by the Nature Publishing Group (NPG), on September 22, 2015, Professor Kyung-Jin Kim at the School of Life Sciences, KNU, and Distinguished Professor Sang Yup Lee at the Department of Chemical and Biomolecular Engineering, KAIST, have proved that the redox-switch of thiolase plays a role in a regulation of metabolic flux in C. acetobutylicum by using in silico modeling and simulation tools.
The research team has redesigned thiolase with enhanced activity on the basis of the 3D structure of the wild-type enzyme. To reinforce a metabolic flux toward butanol production, the metabolic network of C. acetobutylicum strain was engineered with the redesigned enzyme. The combination of the discovery of 3D enzyme structure and systems metabolic engineering approaches resulted in increased n-butanol production in C. acetobutylicum, which allows the production of this important industrial chemical to be cost competitive.
Professors Kim and Lee said, "We have reported the 3D structure of C. acetobutylicum thiolase-a key enzyme involved in n-butanol biosynthesis, for the first time. Further study will be done to produce butanol more economically on the basis of the 3D structure of C. acetobutylicum thiolase."
This work was published online in Nature Communications on September 22, 2015.
Reference: Kim et al. "Redox-switch regulatory mechanism of thiolase from Clostridium acetobutylicum," Nature Communications
This research was supported by the Technology Development Program to Solve Climate Changes from the Ministry of Education, Science and Technology (MEST), Korea, the National Research Foundation of Korea, and the Advanced Biomass Center through the Global Frontier Research Program of the MEST, Korea.
For further information, contact Dr. Sang Yup Lee, Distinguished Professor, KAIST, Daejeon, Korea (leesy@kaist.ac.kr, +82-42-350-3930); and Dr. Kyung-Jin Kim, Professor, KNU, Daegu, Korea (kkim@knu.ac.kr, +82-53-950-6088).
Figure 1: A redox-switch of thiolase involves in butanol biosynthesis in Clostridium acetobutylicum. Thiolase condenses two acetyl-CoA molecules for initiating four carbon flux towards butanol.
Figure 2: Thiolase catalyzes the condensation reaction of acetyl-CoA to acetoacetyl-CoA. Two catalytic cysteine residues at 88th and 378th are oxidized and formed an intermolecular disulfide bond in an oxidized status, which results in inactivation of the enzyme for n-butanol biosynthesis. The intermolecular disulfide bond is broken enabling the n-butanol biosynthesis, when the environment status is reduced.
2015.09.23 View 11712 -
 KAIST's Mathematician Reveals the Mechanism for Sustaining Biological Rhythms
Our bodies have a variety of biological clocks that follow rhythms or oscillations with periods ranging from seconds to days. For example, our hearts beat every second, and cells divide periodically. The circadian clock located in the hypothalamus generates twenty-four hour rhythms, timing our sleep and hormone release. How do these biological clocks or circuits generate and sustain the stable rhythms that are essential to life?
Jae Kyoung Kim, who is an assistant professor in the Department of Mathematical Sciences at KAIST, has predicted how these biological circuits generate rhythms and control their robustness, utilizing mathematical modeling based on differential equations and stochastic parameter sampling. Based on his prediction, using synthetic biology, a research team headed by Matthew Bennett of Rice University constructed a novel biological circuit that spans two genetically engineered strains of bacteria, one serves as an activator and the other as a repressor to regulate gene expression across multiple cell types, and found that the circuit generates surprisingly robust rhythms under various conditions.
The results of the research conducted in collaboration with KAIST (Korea Institute of Science and Technology), Rice University, and the University of Houston were published in Science (August 28, 2015 issue). The title of the paper is "Emergent Genetic Oscillations in a Synthetic Microbial Consortium" .
The top-down research approach, which focuses on identifying the components of biological circuits, limits our understanding of the mechanisms in which the circuits generate rhythms. Synthetic biology, a rapidly growing field at the interface of biosciences and engineering, however, uses a bottom-up approach.
Synthetic biologists can create complex circuits out of simpler components, and some of these new genetic circuits are capable of fluctuation to regulate gene production. In the same way that electrical engineers understand how an electrical circuit works as they construct batteries, resistors, and wires, synthetic biologists can understand better about biological circuits if they put them together using genes and proteins. However, due to the complexity of biological systems, both experiments and mathematical modeling need to be applied hand in hand to design these biological circuits and understand their function.
In this research, an interdisciplinary approach proved that a synthetic intercellular singling circuit generates robust rhythms to create a cooperative microbial system. Specifically, Kim's mathematical analysis suggested, and experiments confirmed, that the presence of negative feedback loops in addition to a core transcriptional negative feedback loop can explain the robustness of rhythms in this system. This result provides important clues about the fundamental mechanism of robust rhythm generation in biological systems.
Furthermore, rather than constructing the entire circuit inside a single bacterial strain, the circuit was split among two strains of Escherichia coli bacterium. When the strains were grown together, the bacteria exchanged information, completing the circuit. Thus, this research also shows how, by regulating individual cells within the system, complex biological systems can be controlled, which in turn influences each other (e.g., the gut microbiome in humans).
###
Ye Chen, a graduate student in Bennett's laboratory at Rice University, and Jae Kyoung Kim, an assistant professor at KAIST and a former postdoctoral fellow at Ohio State University, are the lead authors of the paper. The co-authors are Rice graduate student Andrew Hirning and Krešimir Josic?, a professor of mathematics at the University of Houston. Bennett is the Assistant Professor of the Biochemistry and Cell Biology Department at Rice University.
About the researcher: While Jae Kyoung Kim is a mathematician, he has also solved various biological puzzles in collaboration with various experimental laboratories of Matthew Bennett at Rice University, David Virshup at Duke and the National University of Singapore, Carla Finkielstein at Virginia Polytechnic Institute and State University, Choo-Gon Lee at the Florida State University, Seung-Hee Yoo at the Medical School of the University of Texas, Toru Takumi at RIKEN Brain Science Institute, and Travis Wager at Pfizer Inc. He has used non-linear dynamics and stochastic analysis to understand the function of biochemical networks in biological systems. In particular, he is interested in mechanisms generating and regulating biological rhythms.
Picture 1: This schematic image is the design of a biological circuit between two strains of bacteria and the part of differential equations used to understand the function of the biological circuit.
Picture 2: The core transcriptional negative feedback loop and additional negative feedback loop in the biological circuit (picture 1) generate robust rhythms. The snapshots correspond the red dots in the time series graph.
2015.08.31 View 9314
KAIST's Mathematician Reveals the Mechanism for Sustaining Biological Rhythms
Our bodies have a variety of biological clocks that follow rhythms or oscillations with periods ranging from seconds to days. For example, our hearts beat every second, and cells divide periodically. The circadian clock located in the hypothalamus generates twenty-four hour rhythms, timing our sleep and hormone release. How do these biological clocks or circuits generate and sustain the stable rhythms that are essential to life?
Jae Kyoung Kim, who is an assistant professor in the Department of Mathematical Sciences at KAIST, has predicted how these biological circuits generate rhythms and control their robustness, utilizing mathematical modeling based on differential equations and stochastic parameter sampling. Based on his prediction, using synthetic biology, a research team headed by Matthew Bennett of Rice University constructed a novel biological circuit that spans two genetically engineered strains of bacteria, one serves as an activator and the other as a repressor to regulate gene expression across multiple cell types, and found that the circuit generates surprisingly robust rhythms under various conditions.
The results of the research conducted in collaboration with KAIST (Korea Institute of Science and Technology), Rice University, and the University of Houston were published in Science (August 28, 2015 issue). The title of the paper is "Emergent Genetic Oscillations in a Synthetic Microbial Consortium" .
The top-down research approach, which focuses on identifying the components of biological circuits, limits our understanding of the mechanisms in which the circuits generate rhythms. Synthetic biology, a rapidly growing field at the interface of biosciences and engineering, however, uses a bottom-up approach.
Synthetic biologists can create complex circuits out of simpler components, and some of these new genetic circuits are capable of fluctuation to regulate gene production. In the same way that electrical engineers understand how an electrical circuit works as they construct batteries, resistors, and wires, synthetic biologists can understand better about biological circuits if they put them together using genes and proteins. However, due to the complexity of biological systems, both experiments and mathematical modeling need to be applied hand in hand to design these biological circuits and understand their function.
In this research, an interdisciplinary approach proved that a synthetic intercellular singling circuit generates robust rhythms to create a cooperative microbial system. Specifically, Kim's mathematical analysis suggested, and experiments confirmed, that the presence of negative feedback loops in addition to a core transcriptional negative feedback loop can explain the robustness of rhythms in this system. This result provides important clues about the fundamental mechanism of robust rhythm generation in biological systems.
Furthermore, rather than constructing the entire circuit inside a single bacterial strain, the circuit was split among two strains of Escherichia coli bacterium. When the strains were grown together, the bacteria exchanged information, completing the circuit. Thus, this research also shows how, by regulating individual cells within the system, complex biological systems can be controlled, which in turn influences each other (e.g., the gut microbiome in humans).
###
Ye Chen, a graduate student in Bennett's laboratory at Rice University, and Jae Kyoung Kim, an assistant professor at KAIST and a former postdoctoral fellow at Ohio State University, are the lead authors of the paper. The co-authors are Rice graduate student Andrew Hirning and Krešimir Josic?, a professor of mathematics at the University of Houston. Bennett is the Assistant Professor of the Biochemistry and Cell Biology Department at Rice University.
About the researcher: While Jae Kyoung Kim is a mathematician, he has also solved various biological puzzles in collaboration with various experimental laboratories of Matthew Bennett at Rice University, David Virshup at Duke and the National University of Singapore, Carla Finkielstein at Virginia Polytechnic Institute and State University, Choo-Gon Lee at the Florida State University, Seung-Hee Yoo at the Medical School of the University of Texas, Toru Takumi at RIKEN Brain Science Institute, and Travis Wager at Pfizer Inc. He has used non-linear dynamics and stochastic analysis to understand the function of biochemical networks in biological systems. In particular, he is interested in mechanisms generating and regulating biological rhythms.
Picture 1: This schematic image is the design of a biological circuit between two strains of bacteria and the part of differential equations used to understand the function of the biological circuit.
Picture 2: The core transcriptional negative feedback loop and additional negative feedback loop in the biological circuit (picture 1) generate robust rhythms. The snapshots correspond the red dots in the time series graph.
2015.08.31 View 9314 -
 Nature Biotechnology Nominates Sang Yup Lee of KAIST for Top 20 Translational Researchers of 2014
Nature Biotechnology, recognized as the most prestigious journal in the field of biotechnology, has released today its list of the Top 20 Translational Researchers of 2014. Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering at KAIST (Korea Advanced Institute of Science and Technology) ranked seventh in the list. He is the only Asian researcher listed.
The journal, in partnership with IP Checkups, a patent analytics firm, presents an annual ranking of researchers based on their paper and patent output. The list includes, among others, each researcher’s most-cited patent in the past five years and their H index, a measurement to evaluate the impact of a researcher’s published work utilizing citation analysis. (More details can be found at http://www.nature.com/bioent/2015/150801/full/bioe.2015.9.html.)
American institutions made up the majority of the list, with 18 universities and research institutes, and the remainder was filled by KAIST in Korea and the Commonwealth Scientific and Industrial Research Organization (CSIRO) in Australia.
Globally known as a leading researcher in systems metabolic engineering, Professor Lee has published more than 500 journal papers and 580 patents. He has received many awards, including the Citation Classic Award, Elmer Gaden Award, Merck Metabolic Engineering Award, ACS Marvin Johnson Award, SIMB Charles Thom Award, POSCO TJ Park Prize, Amgen Biochemical Engineering Award, and the Ho Am Prize in Engineering.
2015.08.27 View 12131
Nature Biotechnology Nominates Sang Yup Lee of KAIST for Top 20 Translational Researchers of 2014
Nature Biotechnology, recognized as the most prestigious journal in the field of biotechnology, has released today its list of the Top 20 Translational Researchers of 2014. Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering at KAIST (Korea Advanced Institute of Science and Technology) ranked seventh in the list. He is the only Asian researcher listed.
The journal, in partnership with IP Checkups, a patent analytics firm, presents an annual ranking of researchers based on their paper and patent output. The list includes, among others, each researcher’s most-cited patent in the past five years and their H index, a measurement to evaluate the impact of a researcher’s published work utilizing citation analysis. (More details can be found at http://www.nature.com/bioent/2015/150801/full/bioe.2015.9.html.)
American institutions made up the majority of the list, with 18 universities and research institutes, and the remainder was filled by KAIST in Korea and the Commonwealth Scientific and Industrial Research Organization (CSIRO) in Australia.
Globally known as a leading researcher in systems metabolic engineering, Professor Lee has published more than 500 journal papers and 580 patents. He has received many awards, including the Citation Classic Award, Elmer Gaden Award, Merck Metabolic Engineering Award, ACS Marvin Johnson Award, SIMB Charles Thom Award, POSCO TJ Park Prize, Amgen Biochemical Engineering Award, and the Ho Am Prize in Engineering.
2015.08.27 View 12131 -
 Dr. Hyundoo Hwang Receives a Tenured Position at Monterrey Institute of Technology and Higher Education
Hyundoo Hwang, a former graduate student in the Department of Bio & Brain Engineering at KAIST, has been granted a tenured position at the Monterrey Institute of Technology and Higher Education (ITSEM), Mexico.
Dr. Hwang received his bachelor’s, master’s, and doctoral degree at KAIST and started his professorship at Ulsan National Institute of Science & Technology (UNIST) in Korea. He continued his research in the United States as a professor at Georgia Institute of Technology. He has been acknowledged for the development of an advanced nanotechnology for the diagnosis of rare diseases and research in cell signals. He is one of the leading researchers in an international research project in microelectromechanical systems (MEMS) with participation by researchers from over ten countries. He has been active in commercializing biosensor technology in the U.S. and Mexico.
Since its establishment in 1943, ITSEM has grown to 33 campuses in 25 cities in Mexico. It is the largest university in Latin America with over 90,000 students (47% of its graduate students has oversea research experience). It recruits over 5,000 international students and professors every year.
Dr. Hwang will begin teaching at ITSEM as a professor in the Department of Biomedical Engineering (Ingeniería Biomédica) this fall. He will also conduct research in nano- and micro-technology as a member of Sensors and Devices research group.
Professor Gwang Hyun Cho, head of KAIST's Department of Bio and Brain Engineering said that Dr. Hwang’s tenure professorship at ITSEM demonstrated that the academic program at KAIST—from undergraduate to doctoral—was on par with the international standard. He hoped that more talents from the department would seek academic careers in internationally renowned universities around the world.
2015.08.13 View 7813
Dr. Hyundoo Hwang Receives a Tenured Position at Monterrey Institute of Technology and Higher Education
Hyundoo Hwang, a former graduate student in the Department of Bio & Brain Engineering at KAIST, has been granted a tenured position at the Monterrey Institute of Technology and Higher Education (ITSEM), Mexico.
Dr. Hwang received his bachelor’s, master’s, and doctoral degree at KAIST and started his professorship at Ulsan National Institute of Science & Technology (UNIST) in Korea. He continued his research in the United States as a professor at Georgia Institute of Technology. He has been acknowledged for the development of an advanced nanotechnology for the diagnosis of rare diseases and research in cell signals. He is one of the leading researchers in an international research project in microelectromechanical systems (MEMS) with participation by researchers from over ten countries. He has been active in commercializing biosensor technology in the U.S. and Mexico.
Since its establishment in 1943, ITSEM has grown to 33 campuses in 25 cities in Mexico. It is the largest university in Latin America with over 90,000 students (47% of its graduate students has oversea research experience). It recruits over 5,000 international students and professors every year.
Dr. Hwang will begin teaching at ITSEM as a professor in the Department of Biomedical Engineering (Ingeniería Biomédica) this fall. He will also conduct research in nano- and micro-technology as a member of Sensors and Devices research group.
Professor Gwang Hyun Cho, head of KAIST's Department of Bio and Brain Engineering said that Dr. Hwang’s tenure professorship at ITSEM demonstrated that the academic program at KAIST—from undergraduate to doctoral—was on par with the international standard. He hoped that more talents from the department would seek academic careers in internationally renowned universities around the world.
2015.08.13 View 7813 -
 A Technology Holding Company Establishes Two Companies Based on Technologies Developed at KAIST
Mirae Holdings is a technology holding company created by four science and technology universities, KAIST, DIGIST (Daegu Gyeongbuk Institute of Science and Technology), GIST (Gwangju Institute of Science and Technology), and UNIST (Ulsan National Institute of Science and Technology) in 2014 to commercialize the universities’ research achievements. The company identifies promising technologies for commercialization, makes business plans, establishes venture capitals, and invests in startup companies.
Over the past year, Mirae Holdings has established two venture companies based on the technologies developed at KAIST. In September 2014, it founded Cresem Inc., a company used the anisotropic conductive film (ACF) bonding technology, which was developed by Professor Kyung-Wook Paik of the Material Science and Engineering Department at KAIST. Cresem provides a technology to bond electronic parts ultrasonically. The company is expected to have 860,000 USD worth of sales within the first year of its launching.
Last June, Mirae Holdings created another company, Doctor Kitchen, with the technology developed by Professor Gwan-Su Yi of the Bio and Brain Engineering Department at KAIST. Doctor Kitchen supplies precooked food, which helps diabetic patients regulate their diet. The company offers a personalized diet plan to customers so that they can effectively manage their disease and monitor their blood sugar level efficiently.
The Chief Executive Officer of Mirae Holdings, Young-Ho Kim, said, “We can assist KAIST researchers who aspire to create a company based on their research outcomes through various stages of startup services such as making business plans, securing venture capitals, and networking with existing businesses.”
Young-Ho Kim (left in the picture), the Chief Executive Officer of Mirae Holdings, holds a certificate of company registration with Sang-Min Oh (right in the picture), the Chief Executive Officer of Cresem.
Young-Ho Kim (left in the picture), the Chief Executive Officer of Mirae Holdings, holds a certificate of company registration with Jae-Yeun Park (right in the picture), the Chief Executive Officer of Dr. Kitchen.
2015.07.29 View 14502
A Technology Holding Company Establishes Two Companies Based on Technologies Developed at KAIST
Mirae Holdings is a technology holding company created by four science and technology universities, KAIST, DIGIST (Daegu Gyeongbuk Institute of Science and Technology), GIST (Gwangju Institute of Science and Technology), and UNIST (Ulsan National Institute of Science and Technology) in 2014 to commercialize the universities’ research achievements. The company identifies promising technologies for commercialization, makes business plans, establishes venture capitals, and invests in startup companies.
Over the past year, Mirae Holdings has established two venture companies based on the technologies developed at KAIST. In September 2014, it founded Cresem Inc., a company used the anisotropic conductive film (ACF) bonding technology, which was developed by Professor Kyung-Wook Paik of the Material Science and Engineering Department at KAIST. Cresem provides a technology to bond electronic parts ultrasonically. The company is expected to have 860,000 USD worth of sales within the first year of its launching.
Last June, Mirae Holdings created another company, Doctor Kitchen, with the technology developed by Professor Gwan-Su Yi of the Bio and Brain Engineering Department at KAIST. Doctor Kitchen supplies precooked food, which helps diabetic patients regulate their diet. The company offers a personalized diet plan to customers so that they can effectively manage their disease and monitor their blood sugar level efficiently.
The Chief Executive Officer of Mirae Holdings, Young-Ho Kim, said, “We can assist KAIST researchers who aspire to create a company based on their research outcomes through various stages of startup services such as making business plans, securing venture capitals, and networking with existing businesses.”
Young-Ho Kim (left in the picture), the Chief Executive Officer of Mirae Holdings, holds a certificate of company registration with Sang-Min Oh (right in the picture), the Chief Executive Officer of Cresem.
Young-Ho Kim (left in the picture), the Chief Executive Officer of Mirae Holdings, holds a certificate of company registration with Jae-Yeun Park (right in the picture), the Chief Executive Officer of Dr. Kitchen.
2015.07.29 View 14502 -
 KAIST to support the Genetic Donguibogam Research Project for global market entry of a new natural drug produced by Green Cross Corporation HS
In the wake of the spread of the Middle East Respiratory Syndrome (MERS), sales of immune-enhancing products in Korea such as red and white ginseng have risen dramatically. Ginseng is one of Korea’s major health supplement it exports, but due to the lack of precise scientific knowledge of its mechanism, sales of ginseng account for less than 2% of the global market share.
The Genetic Donguibogam Research Project represents a group of research initiatives to study genes and environmental factors that contribute to diseases and to discover alternative treatments through Eastern medicine. The project is being led by KAIST’s Department of Bio & Brain Engineering Professor Do-Heon Lee.
Professor Lee and Chief Executive Officer Young-Hyo Yoo of Green Cross Corporation HS, a Korean pharmaceutical company, signed a memorandum of understanding (MOU), as well as a non-disclosure agreement (NDA) to develop a naturally derived drug with an enhanced ginsenoside, pharmacological compounds of ginseng, for the global market entry of BST204 on June 10, 2015.
Donguibogam is the traditional Korean source for the principles and practice of Eastern medicine, which was compiled by the royal physician Heo Jun and first published in 1613 during the Joseon Dynasty of Korea.
Cooperating with Green Cross Co., HS, KAIST researchers will use a multi-component, multi-target (MCMT)-based development platform to produce the new natural drug, BST204. This cooperation is expected to assist the entry of the drug into the European market.
Green Cross Co., HS has applied a bio-conversion technique to ginseng to develop BST204, which is a drug with enhanced active constituent of aginsenosides. The drug is the first produced by any Korean pharmaceutical company to complete the first phase of clinical trials in Germany and is about to start the second phase of trials.
Professor Do-Heon Lee, the Director of the project said, “Genetic Donguibogam Research Project seeks to create new innovative healthcare material for the future using integrated fundamental technologies such as virtual human body computer modelling and multi-omics to explain the mechanism in which natural ingredients affect the human body.” He continued, “Especially, by employing the virtual human body computer modelling, we can develop an innovative new technology that will greatly assist Korean pharmaceutical industry and make it the platform technology in entering global markets.”
Young-Hyo Yoo, the CEO of Green Cross Co., HS, said, “For a new naturally derived drug to be acknowledged in the global market, such as Europe and the US, its mechanism, as well as its effectiveness and safety, should be proven. However, it is difficult and costly to explain the mechanism in which the complex composition of a natural substance influences the body. Innovative technology is needed to solve this problem.”
Professor Do-Heon Lee (left in the picture), the Director of Genetic Donguibogam Research Project, stands abreast Young-Hyo Yoo (right in the picture), the CEO of Green Cross Co., HS.
2015.06.10 View 10382
KAIST to support the Genetic Donguibogam Research Project for global market entry of a new natural drug produced by Green Cross Corporation HS
In the wake of the spread of the Middle East Respiratory Syndrome (MERS), sales of immune-enhancing products in Korea such as red and white ginseng have risen dramatically. Ginseng is one of Korea’s major health supplement it exports, but due to the lack of precise scientific knowledge of its mechanism, sales of ginseng account for less than 2% of the global market share.
The Genetic Donguibogam Research Project represents a group of research initiatives to study genes and environmental factors that contribute to diseases and to discover alternative treatments through Eastern medicine. The project is being led by KAIST’s Department of Bio & Brain Engineering Professor Do-Heon Lee.
Professor Lee and Chief Executive Officer Young-Hyo Yoo of Green Cross Corporation HS, a Korean pharmaceutical company, signed a memorandum of understanding (MOU), as well as a non-disclosure agreement (NDA) to develop a naturally derived drug with an enhanced ginsenoside, pharmacological compounds of ginseng, for the global market entry of BST204 on June 10, 2015.
Donguibogam is the traditional Korean source for the principles and practice of Eastern medicine, which was compiled by the royal physician Heo Jun and first published in 1613 during the Joseon Dynasty of Korea.
Cooperating with Green Cross Co., HS, KAIST researchers will use a multi-component, multi-target (MCMT)-based development platform to produce the new natural drug, BST204. This cooperation is expected to assist the entry of the drug into the European market.
Green Cross Co., HS has applied a bio-conversion technique to ginseng to develop BST204, which is a drug with enhanced active constituent of aginsenosides. The drug is the first produced by any Korean pharmaceutical company to complete the first phase of clinical trials in Germany and is about to start the second phase of trials.
Professor Do-Heon Lee, the Director of the project said, “Genetic Donguibogam Research Project seeks to create new innovative healthcare material for the future using integrated fundamental technologies such as virtual human body computer modelling and multi-omics to explain the mechanism in which natural ingredients affect the human body.” He continued, “Especially, by employing the virtual human body computer modelling, we can develop an innovative new technology that will greatly assist Korean pharmaceutical industry and make it the platform technology in entering global markets.”
Young-Hyo Yoo, the CEO of Green Cross Co., HS, said, “For a new naturally derived drug to be acknowledged in the global market, such as Europe and the US, its mechanism, as well as its effectiveness and safety, should be proven. However, it is difficult and costly to explain the mechanism in which the complex composition of a natural substance influences the body. Innovative technology is needed to solve this problem.”
Professor Do-Heon Lee (left in the picture), the Director of Genetic Donguibogam Research Project, stands abreast Young-Hyo Yoo (right in the picture), the CEO of Green Cross Co., HS.
2015.06.10 View 10382 -
 Professor Sang-Yup Lee Receives the Order of Service Merit Red Stripes from the Korean Government
The government of the Republic of Korea named Professor Sang-Yup Lee of the Department of Chemical and Bio-molecular Engineering at KAIST as the fiftieth recipient of the Order of Service Merit Red Stripes on May 19, 2015.
This medal is awarded to government employees, officials, and teachers in recognition of their contributions to public services including education.
Professor Lee is regarded as a leading scientist in the field of metabolic engineering, genomics, proteomics, metabolomics, and bioinformatics on microorganism producing various primary and secondary metabolites. He contributed significantly to the advancement of bio-based engineering research in Korea.
In addition, his research in microorganism metabolic engineering propelled him to the front of his field, making him the world’s founder of systems metabolic engineering, inventing numerous technologies in strain development.
Professor Lee has received many patent rights in bioprocess engineering. While at KAIST, he applied for 585 patents and registered 227 patents. In particular, he has applied for 135 patents and registered 99 patents in the past five years, successfully turning research results into commercial applications.
Professor Lee said, “I’m glad to contribute to the development of Korean science and technology as a researcher and teacher. I would like to share this honor with my students, master’s and doctoral students in particular, because without their support, it wouldn’t have been possible to pull off the highest level of research results recognized by this medal.”
2015.05.21 View 9668
Professor Sang-Yup Lee Receives the Order of Service Merit Red Stripes from the Korean Government
The government of the Republic of Korea named Professor Sang-Yup Lee of the Department of Chemical and Bio-molecular Engineering at KAIST as the fiftieth recipient of the Order of Service Merit Red Stripes on May 19, 2015.
This medal is awarded to government employees, officials, and teachers in recognition of their contributions to public services including education.
Professor Lee is regarded as a leading scientist in the field of metabolic engineering, genomics, proteomics, metabolomics, and bioinformatics on microorganism producing various primary and secondary metabolites. He contributed significantly to the advancement of bio-based engineering research in Korea.
In addition, his research in microorganism metabolic engineering propelled him to the front of his field, making him the world’s founder of systems metabolic engineering, inventing numerous technologies in strain development.
Professor Lee has received many patent rights in bioprocess engineering. While at KAIST, he applied for 585 patents and registered 227 patents. In particular, he has applied for 135 patents and registered 99 patents in the past five years, successfully turning research results into commercial applications.
Professor Lee said, “I’m glad to contribute to the development of Korean science and technology as a researcher and teacher. I would like to share this honor with my students, master’s and doctoral students in particular, because without their support, it wouldn’t have been possible to pull off the highest level of research results recognized by this medal.”
2015.05.21 View 9668