bio
-
 Metabolically Engineered Bacterium Produces Lutein
A research group at KAIST has engineered a bacterial strain capable of producing lutein. The research team applied systems metabolic engineering strategies, including substrate channeling and electron channeling, to enhance the production of lutein in an engineered Escherichia coli strain. The strategies will be also useful for the efficient production of other industrially important natural products used in the food, pharmaceutical, and cosmetic industries.
Figure: Systems metabolic engineering was employed to construct and optimize the metabolic pathways for lutein production, and substrate channeling and electron channeling strategies were additionally employed to increase the production of the lutein with high productivity.
Lutein is classified as a xanthophyll chemical that is abundant in egg yolk, fruits, and vegetables. It protects the eye from oxidative damage from radiation and reduces the risk of eye diseases including macular degeneration and cataracts. Commercialized products featuring lutein are derived from the extracts of the marigold flower, which is known to harbor abundant amounts of lutein. However, the drawback of lutein production from nature is that it takes a long time to grow and harvest marigold flowers. Furthermore, it requires additional physical and chemical-based extractions with a low yield, which makes it economically unfeasible in terms of productivity. The high cost and low yield of these bioprocesses has made it difficult to readily meet the demand for lutein.
These challenges inspired the metabolic engineers at KAIST, including researchers Dr. Seon Young Park, Ph.D. Candidate Hyunmin Eun, and Distinguished Professor Sang Yup Lee from the Department of Chemical and Biomolecular Engineering. The team’s study entitled “Metabolic engineering of Escherichia coli with electron channeling for the production of natural products” was published in Nature Catalysis on August 5, 2022.
This research details the ability to produce lutein from E. coli with a high yield using a cheap carbon source, glycerol, via systems metabolic engineering. The research group focused on solving the bottlenecks of the biosynthetic pathway for lutein production constructed within an individual cell. First, using systems metabolic engineering, which is an integrated technology to engineer the metabolism of a microorganism, lutein was produced when the lutein biosynthesis pathway was introduced, albeit in very small amounts.
To improve the productivity of lutein production, the bottleneck enzymes within the metabolic pathway were first identified. It turned out that metabolic reactions that involve a promiscuous enzyme, an enzyme that is involved in two or more metabolic reactions, and electron-requiring cytochrome P450 enzymes are the main bottleneck steps of the pathway inhibiting lutein biosynthesis.
To overcome these challenges, substrate channeling, a strategy to artificially recruit enzymes in physical proximity within the cell in order to increase the local concentrations of substrates that can be converted into products, was employed to channel more metabolic flux towards the target chemical while reducing the formation of unwanted byproducts.
Furthermore, electron channeling, a strategy similar to substrate channeling but differing in terms of increasing the local concentrations of electrons required for oxidoreduction reactions mediated by P450 and its reductase partners, was applied to further streamline the metabolic flux towards lutein biosynthesis, which led to the highest titer of lutein production achieved in a bacterial host ever reported. The same electron channeling strategy was successfully applied for the production of other natural products including nootkatone and apigenin in E. coli, showcasing the general applicability of the strategy in the research field.
“It is expected that this microbial cell factory-based production of lutein will be able to replace the current plant extraction-based process,” said Dr. Seon Young Park, the first author of the paper. She explained that another important point of the research is that integrated metabolic engineering strategies developed from this study can be generally applicable for the efficient production of other natural products useful as pharmaceuticals or nutraceuticals.
“As maintaining good health in an aging society is becoming increasingly important, we expect that the technology and strategies developed here will play pivotal roles in producing other valuable natural products of medical or nutritional importance,” explained Distinguished Professor Sang Yup Lee.
This work was supported by the Cooperative Research Program for Agriculture Science & Technology Development funded by the Rural Development Administration of Korea, with further support from the Development of Next-generation Biorefinery Platform Technologies for Leading Bio-based Chemicals Industry Project and by the Development of Platform Technologies of Microbial Cell Factories for the Next-generation Biorefineries Project of the National Research Foundation funded by the Ministry of Science and ICT of Korea.
2022.08.05 View 11070
Metabolically Engineered Bacterium Produces Lutein
A research group at KAIST has engineered a bacterial strain capable of producing lutein. The research team applied systems metabolic engineering strategies, including substrate channeling and electron channeling, to enhance the production of lutein in an engineered Escherichia coli strain. The strategies will be also useful for the efficient production of other industrially important natural products used in the food, pharmaceutical, and cosmetic industries.
Figure: Systems metabolic engineering was employed to construct and optimize the metabolic pathways for lutein production, and substrate channeling and electron channeling strategies were additionally employed to increase the production of the lutein with high productivity.
Lutein is classified as a xanthophyll chemical that is abundant in egg yolk, fruits, and vegetables. It protects the eye from oxidative damage from radiation and reduces the risk of eye diseases including macular degeneration and cataracts. Commercialized products featuring lutein are derived from the extracts of the marigold flower, which is known to harbor abundant amounts of lutein. However, the drawback of lutein production from nature is that it takes a long time to grow and harvest marigold flowers. Furthermore, it requires additional physical and chemical-based extractions with a low yield, which makes it economically unfeasible in terms of productivity. The high cost and low yield of these bioprocesses has made it difficult to readily meet the demand for lutein.
These challenges inspired the metabolic engineers at KAIST, including researchers Dr. Seon Young Park, Ph.D. Candidate Hyunmin Eun, and Distinguished Professor Sang Yup Lee from the Department of Chemical and Biomolecular Engineering. The team’s study entitled “Metabolic engineering of Escherichia coli with electron channeling for the production of natural products” was published in Nature Catalysis on August 5, 2022.
This research details the ability to produce lutein from E. coli with a high yield using a cheap carbon source, glycerol, via systems metabolic engineering. The research group focused on solving the bottlenecks of the biosynthetic pathway for lutein production constructed within an individual cell. First, using systems metabolic engineering, which is an integrated technology to engineer the metabolism of a microorganism, lutein was produced when the lutein biosynthesis pathway was introduced, albeit in very small amounts.
To improve the productivity of lutein production, the bottleneck enzymes within the metabolic pathway were first identified. It turned out that metabolic reactions that involve a promiscuous enzyme, an enzyme that is involved in two or more metabolic reactions, and electron-requiring cytochrome P450 enzymes are the main bottleneck steps of the pathway inhibiting lutein biosynthesis.
To overcome these challenges, substrate channeling, a strategy to artificially recruit enzymes in physical proximity within the cell in order to increase the local concentrations of substrates that can be converted into products, was employed to channel more metabolic flux towards the target chemical while reducing the formation of unwanted byproducts.
Furthermore, electron channeling, a strategy similar to substrate channeling but differing in terms of increasing the local concentrations of electrons required for oxidoreduction reactions mediated by P450 and its reductase partners, was applied to further streamline the metabolic flux towards lutein biosynthesis, which led to the highest titer of lutein production achieved in a bacterial host ever reported. The same electron channeling strategy was successfully applied for the production of other natural products including nootkatone and apigenin in E. coli, showcasing the general applicability of the strategy in the research field.
“It is expected that this microbial cell factory-based production of lutein will be able to replace the current plant extraction-based process,” said Dr. Seon Young Park, the first author of the paper. She explained that another important point of the research is that integrated metabolic engineering strategies developed from this study can be generally applicable for the efficient production of other natural products useful as pharmaceuticals or nutraceuticals.
“As maintaining good health in an aging society is becoming increasingly important, we expect that the technology and strategies developed here will play pivotal roles in producing other valuable natural products of medical or nutritional importance,” explained Distinguished Professor Sang Yup Lee.
This work was supported by the Cooperative Research Program for Agriculture Science & Technology Development funded by the Rural Development Administration of Korea, with further support from the Development of Next-generation Biorefinery Platform Technologies for Leading Bio-based Chemicals Industry Project and by the Development of Platform Technologies of Microbial Cell Factories for the Next-generation Biorefineries Project of the National Research Foundation funded by the Ministry of Science and ICT of Korea.
2022.08.05 View 11070 -
 KAIST Research Team Proves How a Neurotransmitter may be the Key in Controlling Alzheimer’s Toxicity
With nearly 50 million dementia patients worldwide, and Alzheimers’s disease is the most common neurodegenerative disease. Its main symptom is the impairment of general cognitive abilities, including the ability to speak or to remember. The importance of finding a cure is widely understood with increasingly aging population and the life expectancy being ever-extended. However, even the cause of the grim disease is yet to be given a clear definition.
A KAIST research team in the Department of Chemistry led by professor Mi Hee Lim took on a lead to discovered a new role for somatostatin, a protein-based neurotransmitter, in reducing the toxicity caused in the pathogenic mechanism taken towards development of Alzheimer’s disease. The study was published in the July issue of Nature Chemistry under the title, “Conformational and functional changes of the native neuropeptide somatostatin occur in the presence of copper and amyloid-β”.
According to the amyloid hypothesis, the abnormal deposition of Aβ proteins causes death of neuronal cells. While Aβ agglomerations make up most of the aged plaques through fibrosis, in recent studies, high concentrations of transitional metal were found in the plaques from Alzheimer’s patients.
This suggests a close interaction between metallic ions and Aβ, which accelerates the fibrosis of proteins. Copper in particular is a redox-activating transition metal that can produce large amounts of oxygen and cause serious oxidative stress on cell organelles. Aβ proteins and transition metals can closely interact with neurotransmitters at synapses, but the direct effects of such abnormalities on the structure and function of neurotransmitters are yet to be understood.
Figure 1. Functional shift of somatostatin (SST) by factors in the pathogenesis of Alzheimer's disease.
Figure 2. Somatostatin’s loss-of-function as neurotransmitter. a. Schematic diagram of SST auto-aggregation due to Alzheimer's pathological factors. b. SST’s aggregation by copper ions. c. Coordination-prediction structure and N-terminal folding of copper-SST. d. Inhibition of SST receptor binding specificity by metals.
In their research, Professor Lim’s team discovered that when somatostatin, the protein-based neurotransmitter, is met with copper, Aβ, and metal-Aβ complexes, self-aggregates and ceases to perform its innate function of transmitting neural signals, but begins to attenuate the toxicity and agglomeration of metal-Aβ complexes.
Figure 3. Gain-of-function of somatostatin (SST) in the dementia setting. a. Prediction of docking of SST and amyloid beta. b. SST making metal-amyloid beta aggregates into an amorphous form. c. Cytotoxic mitigation effect of SST. d. SST mitigating the interaction between amyloid beta protein with the cell membrane.
This research, by Dr. Jiyeon Han et al. from the KAIST Department of Chemistry, revealed the coordination structure between copper and somatostatin at a molecular level through which it suggested the agglomeration mechanism, and discovered the effects of somatostatin on Aβ agglomeration path depending on the presence or absence of metals. The team has further confirmed somatostatin’s receptor binding, interactions with cell membranes, and effects on cell toxicity for the first time to receive international attention.
Professor Mi Hee Lim said, “This research has great significance in having discovered a new role of neurotransmitters in the pathogenesis of Alzheimer’s disease.” “We expect this research to contribute to defining the pathogenic network of neurodegenerative diseases caused by aging, and to the development of future biomarkers and medicine,” she added.
This research was conducted jointly by Professor Seung-Hee Lee’s team of KAIST Department of Biological Sciences, Professor Kiyoung Park’s Team of KAIST Department of Chemistry, and Professor Yulong Li’s team of Peking University.
The research was funded by Basic Science Research Program of the National Research Foundation of Korea and KAIST.
For more information about the research team, visit the website: https://sites.google.com/site/miheelimlab/1-professor-mi-hee-lim.
2022.07.29 View 15770
KAIST Research Team Proves How a Neurotransmitter may be the Key in Controlling Alzheimer’s Toxicity
With nearly 50 million dementia patients worldwide, and Alzheimers’s disease is the most common neurodegenerative disease. Its main symptom is the impairment of general cognitive abilities, including the ability to speak or to remember. The importance of finding a cure is widely understood with increasingly aging population and the life expectancy being ever-extended. However, even the cause of the grim disease is yet to be given a clear definition.
A KAIST research team in the Department of Chemistry led by professor Mi Hee Lim took on a lead to discovered a new role for somatostatin, a protein-based neurotransmitter, in reducing the toxicity caused in the pathogenic mechanism taken towards development of Alzheimer’s disease. The study was published in the July issue of Nature Chemistry under the title, “Conformational and functional changes of the native neuropeptide somatostatin occur in the presence of copper and amyloid-β”.
According to the amyloid hypothesis, the abnormal deposition of Aβ proteins causes death of neuronal cells. While Aβ agglomerations make up most of the aged plaques through fibrosis, in recent studies, high concentrations of transitional metal were found in the plaques from Alzheimer’s patients.
This suggests a close interaction between metallic ions and Aβ, which accelerates the fibrosis of proteins. Copper in particular is a redox-activating transition metal that can produce large amounts of oxygen and cause serious oxidative stress on cell organelles. Aβ proteins and transition metals can closely interact with neurotransmitters at synapses, but the direct effects of such abnormalities on the structure and function of neurotransmitters are yet to be understood.
Figure 1. Functional shift of somatostatin (SST) by factors in the pathogenesis of Alzheimer's disease.
Figure 2. Somatostatin’s loss-of-function as neurotransmitter. a. Schematic diagram of SST auto-aggregation due to Alzheimer's pathological factors. b. SST’s aggregation by copper ions. c. Coordination-prediction structure and N-terminal folding of copper-SST. d. Inhibition of SST receptor binding specificity by metals.
In their research, Professor Lim’s team discovered that when somatostatin, the protein-based neurotransmitter, is met with copper, Aβ, and metal-Aβ complexes, self-aggregates and ceases to perform its innate function of transmitting neural signals, but begins to attenuate the toxicity and agglomeration of metal-Aβ complexes.
Figure 3. Gain-of-function of somatostatin (SST) in the dementia setting. a. Prediction of docking of SST and amyloid beta. b. SST making metal-amyloid beta aggregates into an amorphous form. c. Cytotoxic mitigation effect of SST. d. SST mitigating the interaction between amyloid beta protein with the cell membrane.
This research, by Dr. Jiyeon Han et al. from the KAIST Department of Chemistry, revealed the coordination structure between copper and somatostatin at a molecular level through which it suggested the agglomeration mechanism, and discovered the effects of somatostatin on Aβ agglomeration path depending on the presence or absence of metals. The team has further confirmed somatostatin’s receptor binding, interactions with cell membranes, and effects on cell toxicity for the first time to receive international attention.
Professor Mi Hee Lim said, “This research has great significance in having discovered a new role of neurotransmitters in the pathogenesis of Alzheimer’s disease.” “We expect this research to contribute to defining the pathogenic network of neurodegenerative diseases caused by aging, and to the development of future biomarkers and medicine,” she added.
This research was conducted jointly by Professor Seung-Hee Lee’s team of KAIST Department of Biological Sciences, Professor Kiyoung Park’s Team of KAIST Department of Chemistry, and Professor Yulong Li’s team of Peking University.
The research was funded by Basic Science Research Program of the National Research Foundation of Korea and KAIST.
For more information about the research team, visit the website: https://sites.google.com/site/miheelimlab/1-professor-mi-hee-lim.
2022.07.29 View 15770 -
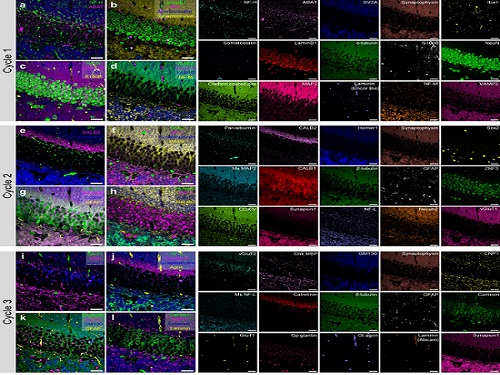 PICASSO Technique Drives Biological Molecules into Technicolor
The new imaging approach brings current imaging colors from four to more than 15 for mapping overlapping proteins
Pablo Picasso’s surreal cubist artistic style shifted common features into unrecognizable scenes, but a new imaging approach bearing his namesake may elucidate the most complicated subject: the brain. Employing artificial intelligence to clarify spectral color blending of tiny molecules used to stain specific proteins and other items of research interest, the PICASSO technique, allows researchers to use more than 15 colors to image and parse our overlapping proteins.
The PICASSO developers, based in Korea, published their approach on May 5 in Nature Communications.
Fluorophores — the staining molecules — emit specific colors when excited by a light, but if more than four fluorophores are used, their emitted colors overlap and blend. Researchers previously developed techniques to correct this spectral overlap by precisely defining the matrix of mixed and unmixed images. This measurement depends on reference spectra, found by identifying clear images of only one fluorophore-stained specimen or of multiple, identically prepared specimens that only contain a single fluorophore each.
“Such reference spectra measurement could be complicated to perform in highly heterogeneous specimens, such as the brain, due to the highly varied emission spectra of fluorophores depending on the subregions from which the spectra were measured,” said co-corresponding author Young-Gyu Yoon, professor in the School of Electrical Engineering at KAIST. He explained that the subregions would each need their own spectra reference measurements, making for an inefficient, time-consuming process. “To address this problem, we developed an approach that does not require reference spectra measurements.”
The approach is the “Process of ultra-multiplexed Imaging of biomolecules viA the unmixing of the Signals of Spectrally Overlapping fluorophores,” also known as PICASSO. Ultra-multiplexed imaging refers to visualizing the numerous individual components of a unit. Like a cinema multiplex in which each theater plays a different movie, each protein in a cell has a different role. By staining with fluorophores, researchers can begin to understand those roles.
“We devised a strategy based on information theory; unmixing is performed by iteratively minimizing the mutual information between mixed images,” said co-corresponding author Jae-Byum Chang, professor in the Department of Materials Science and Engineering, KAIST. “This allows us to get away with the assumption that the spatial distribution of different proteins is mutually exclusive and enables accurate information unmixing.”
To demonstrate PICASSO’s capabilities, the researchers applied the technique to imaging a mouse brain. With a single round of staining, they performed 15-color multiplexed imaging of a mouse brain. Although small, mouse brains are still complex, multifaceted organs that can take significant resources to map. According to the researchers, PICASSO can improve the capabilities of other imaging techniques and allow for the use of even more fluorophore colors.
Using one such imaging technique in combination with PICASSO, the team achieved 45-color multiplexed imaging of the mouse brain in only three staining and imaging cycles, according to Yoon.
“PICASSO is a versatile tool for the multiplexed biomolecule imaging of cultured cells, tissue slices and clinical specimens,” Chang said. “We anticipate that PICASSO will be useful for a broad range of applications for which biomolecules’ spatial information is important. One such application the tool would be useful for is revealing the cellular heterogeneities of tumor microenvironments, especially the heterogeneous populations of immune cells, which are closely related to cancer prognoses and the efficacy of cancer therapies.”
The Samsung Research Funding & Incubation Center for Future Technology supported this work. Spectral imaging was performed at the Korea Basic Science Institute Western Seoul Center.
-PublicationJunyoung Seo, Yeonbo Sim, Jeewon Kim, Hyunwoo Kim, In Cho, Hoyeon Nam, Yong-Gyu Yoon, Jae-Byum Chang, “PICASSO allows ultra-multiplexed fluorescence imaging of spatiallyoverlapping proteins without reference spectra measurements,” May 5, Nature Communications (doi.org/10.1038/s41467-022-30168-z)
-ProfileProfessor Jae-Byum ChangDepartment of Materials Science and EngineeringCollege of EngineeringKAIST
Professor Young-Gyu YoonSchool of Electrical EngineeringCollege of EngineeringKAIST
2022.06.22 View 11286
PICASSO Technique Drives Biological Molecules into Technicolor
The new imaging approach brings current imaging colors from four to more than 15 for mapping overlapping proteins
Pablo Picasso’s surreal cubist artistic style shifted common features into unrecognizable scenes, but a new imaging approach bearing his namesake may elucidate the most complicated subject: the brain. Employing artificial intelligence to clarify spectral color blending of tiny molecules used to stain specific proteins and other items of research interest, the PICASSO technique, allows researchers to use more than 15 colors to image and parse our overlapping proteins.
The PICASSO developers, based in Korea, published their approach on May 5 in Nature Communications.
Fluorophores — the staining molecules — emit specific colors when excited by a light, but if more than four fluorophores are used, their emitted colors overlap and blend. Researchers previously developed techniques to correct this spectral overlap by precisely defining the matrix of mixed and unmixed images. This measurement depends on reference spectra, found by identifying clear images of only one fluorophore-stained specimen or of multiple, identically prepared specimens that only contain a single fluorophore each.
“Such reference spectra measurement could be complicated to perform in highly heterogeneous specimens, such as the brain, due to the highly varied emission spectra of fluorophores depending on the subregions from which the spectra were measured,” said co-corresponding author Young-Gyu Yoon, professor in the School of Electrical Engineering at KAIST. He explained that the subregions would each need their own spectra reference measurements, making for an inefficient, time-consuming process. “To address this problem, we developed an approach that does not require reference spectra measurements.”
The approach is the “Process of ultra-multiplexed Imaging of biomolecules viA the unmixing of the Signals of Spectrally Overlapping fluorophores,” also known as PICASSO. Ultra-multiplexed imaging refers to visualizing the numerous individual components of a unit. Like a cinema multiplex in which each theater plays a different movie, each protein in a cell has a different role. By staining with fluorophores, researchers can begin to understand those roles.
“We devised a strategy based on information theory; unmixing is performed by iteratively minimizing the mutual information between mixed images,” said co-corresponding author Jae-Byum Chang, professor in the Department of Materials Science and Engineering, KAIST. “This allows us to get away with the assumption that the spatial distribution of different proteins is mutually exclusive and enables accurate information unmixing.”
To demonstrate PICASSO’s capabilities, the researchers applied the technique to imaging a mouse brain. With a single round of staining, they performed 15-color multiplexed imaging of a mouse brain. Although small, mouse brains are still complex, multifaceted organs that can take significant resources to map. According to the researchers, PICASSO can improve the capabilities of other imaging techniques and allow for the use of even more fluorophore colors.
Using one such imaging technique in combination with PICASSO, the team achieved 45-color multiplexed imaging of the mouse brain in only three staining and imaging cycles, according to Yoon.
“PICASSO is a versatile tool for the multiplexed biomolecule imaging of cultured cells, tissue slices and clinical specimens,” Chang said. “We anticipate that PICASSO will be useful for a broad range of applications for which biomolecules’ spatial information is important. One such application the tool would be useful for is revealing the cellular heterogeneities of tumor microenvironments, especially the heterogeneous populations of immune cells, which are closely related to cancer prognoses and the efficacy of cancer therapies.”
The Samsung Research Funding & Incubation Center for Future Technology supported this work. Spectral imaging was performed at the Korea Basic Science Institute Western Seoul Center.
-PublicationJunyoung Seo, Yeonbo Sim, Jeewon Kim, Hyunwoo Kim, In Cho, Hoyeon Nam, Yong-Gyu Yoon, Jae-Byum Chang, “PICASSO allows ultra-multiplexed fluorescence imaging of spatiallyoverlapping proteins without reference spectra measurements,” May 5, Nature Communications (doi.org/10.1038/s41467-022-30168-z)
-ProfileProfessor Jae-Byum ChangDepartment of Materials Science and EngineeringCollege of EngineeringKAIST
Professor Young-Gyu YoonSchool of Electrical EngineeringCollege of EngineeringKAIST
2022.06.22 View 11286 -
 New Polymer Mesophase Structure Discovered
Bilayer-folded lamellar mesophase induced by random polymer sequence
Polymers, large molecules made up of repeating smaller molecules called monomers, are found in nearly everything we use in our day-to-day lives. Polymers can be natural or created synthetically. Natural polymers, also called biopolymers, include DNA, proteins, and materials like silk, gelatin, and collagen. Synthetic polymers make up many different kinds of materials, including plastic, that are used in constructing everything from toys to industrial fiber cables to brake pads.
As polymers are formed through a process called polymerization, the monomers are connected through a chain. As the chain develops, the structure of the polymer determines its unique physical and chemical properties. Researchers are continually studying polymers, how they form, how they are structured, and how they develop these unique properties. By understanding this information, scientists can develop new uses for polymers and create new materials that can be used in a wide variety of industries.
In a paper published in Nature Communications on May 4, researchers describe a new structure found in an aqueous solution of an amphiphilic copolymer, called a bilayer-folded lamellar mesophase, that has been discovered through a random copolymer sequence.
“A new mesophase is an important discovery as it shows a new way for molecules to self-organize,” said Professor Myungeun Seo at the Department of Chemistry at KAIST. “We were particularly thrilled to identify this bilayer-folded lamellar phase because pure bilayer membranes are difficult to fold thermodynamically.”
Researchers think that this mesophase structure comes from the sequence of the monomers within the copolymer. The way the different monomers arrange themselves in the chain that makes up a copolymer is important and can have implications for what the copolymer can do. Many copolymers are random, which means that their structure relies on how the monomers interact with each other. In this case, the interaction between the hydrophobic monomers associates the copolymer chains to conceal the hydrophobic domain from water. As the structure gets more complex, researchers have found that a visible order develops so that monomers can be matched up with the right pair.
“While we tend to think random means disorder, here we showed that a periodic order can spontaneously arise from the random copolymer sequence based on their collective behavior,” said Professor Seo. “We believe this comes from the sequence matching problem: finding a perfectly complementary pair for a long sequence is nearly impossible.”
This is what creates the unique structure of this newly discovered mesophase. The copolymer spontaneously folds and creates a multilamellar structure that is separated by water. A multilamellar structure refers to plate-like folds and the folded layers stack on top of each other. The resulting mesophase is birefringent, meaning light refracts through it, it is similar to liquid crystalline, and viscoelastic, which means that it is both viscous and elastic at the same time.
Looking ahead, researchers hope to learn more about this new mesophase and figure out how to control the outcome. Once more is understood about the mesophase and how it is formed, it’s possible that new mesophases could be discovered as more sequences are researched. “One of the obvious questions for us is how to control the folding frequency and adjust the folded height, which we are currently working to address. Ultimately, we want to understand how different multinary sequences can associate with another to create order and apply the knowledge to develop new materials,” said Professor Seo. The National Research Foundation, the Ministry of Education, and the Ministry of Science and ICT of Korea funded this research.
-PublicationMinjoong Shin, Hayeon Kim, Geonhyeong Park, Jongmin Park, Hyungju Ahn, Dong Ki Yoon, Eunji Lee, Myungeun Seo, “Bilayer-folded lamellar mesophase induced by random polymersequence,” May 4, 2022, Nature Communications (https://doi.org/10.1038/s41467-022-30122-z)
-ProfileProfessor Myungeun SeoMacromolecular Materials Chemistry Lab (https://nanopsg.kaist.ac.kr/)Department of ChemistryCollege of Natural SciencesKAIST
2022.06.17 View 9775
New Polymer Mesophase Structure Discovered
Bilayer-folded lamellar mesophase induced by random polymer sequence
Polymers, large molecules made up of repeating smaller molecules called monomers, are found in nearly everything we use in our day-to-day lives. Polymers can be natural or created synthetically. Natural polymers, also called biopolymers, include DNA, proteins, and materials like silk, gelatin, and collagen. Synthetic polymers make up many different kinds of materials, including plastic, that are used in constructing everything from toys to industrial fiber cables to brake pads.
As polymers are formed through a process called polymerization, the monomers are connected through a chain. As the chain develops, the structure of the polymer determines its unique physical and chemical properties. Researchers are continually studying polymers, how they form, how they are structured, and how they develop these unique properties. By understanding this information, scientists can develop new uses for polymers and create new materials that can be used in a wide variety of industries.
In a paper published in Nature Communications on May 4, researchers describe a new structure found in an aqueous solution of an amphiphilic copolymer, called a bilayer-folded lamellar mesophase, that has been discovered through a random copolymer sequence.
“A new mesophase is an important discovery as it shows a new way for molecules to self-organize,” said Professor Myungeun Seo at the Department of Chemistry at KAIST. “We were particularly thrilled to identify this bilayer-folded lamellar phase because pure bilayer membranes are difficult to fold thermodynamically.”
Researchers think that this mesophase structure comes from the sequence of the monomers within the copolymer. The way the different monomers arrange themselves in the chain that makes up a copolymer is important and can have implications for what the copolymer can do. Many copolymers are random, which means that their structure relies on how the monomers interact with each other. In this case, the interaction between the hydrophobic monomers associates the copolymer chains to conceal the hydrophobic domain from water. As the structure gets more complex, researchers have found that a visible order develops so that monomers can be matched up with the right pair.
“While we tend to think random means disorder, here we showed that a periodic order can spontaneously arise from the random copolymer sequence based on their collective behavior,” said Professor Seo. “We believe this comes from the sequence matching problem: finding a perfectly complementary pair for a long sequence is nearly impossible.”
This is what creates the unique structure of this newly discovered mesophase. The copolymer spontaneously folds and creates a multilamellar structure that is separated by water. A multilamellar structure refers to plate-like folds and the folded layers stack on top of each other. The resulting mesophase is birefringent, meaning light refracts through it, it is similar to liquid crystalline, and viscoelastic, which means that it is both viscous and elastic at the same time.
Looking ahead, researchers hope to learn more about this new mesophase and figure out how to control the outcome. Once more is understood about the mesophase and how it is formed, it’s possible that new mesophases could be discovered as more sequences are researched. “One of the obvious questions for us is how to control the folding frequency and adjust the folded height, which we are currently working to address. Ultimately, we want to understand how different multinary sequences can associate with another to create order and apply the knowledge to develop new materials,” said Professor Seo. The National Research Foundation, the Ministry of Education, and the Ministry of Science and ICT of Korea funded this research.
-PublicationMinjoong Shin, Hayeon Kim, Geonhyeong Park, Jongmin Park, Hyungju Ahn, Dong Ki Yoon, Eunji Lee, Myungeun Seo, “Bilayer-folded lamellar mesophase induced by random polymersequence,” May 4, 2022, Nature Communications (https://doi.org/10.1038/s41467-022-30122-z)
-ProfileProfessor Myungeun SeoMacromolecular Materials Chemistry Lab (https://nanopsg.kaist.ac.kr/)Department of ChemistryCollege of Natural SciencesKAIST
2022.06.17 View 9775 -
 Professor Jae-Woong Jeong Receives Hyonwoo KAIST Academic Award
Professor Jae-Woong Jeong from the School of Electrical Engineering was selected for the Hyonwoo KAIST Academic Award, funded by the HyonWoo Cultural Foundation (Chairman Soo-il Kwak, honorary professor at Seoul National University Business School).
The Hyonwoo KAIST Academic Award, presented for the first time in 2021, is an award newly founded by the donations of Chairman Soo-il Kwak of the HyonWoo Cultural Foundation, who aims to reward excellent KAIST scholars who have made outstanding academic achievements.
Every year, through the strict evaluations of the selection committee of the HyonWoo Cultural Foundation and the faculty reward recommendation board, KAIST will choose one faculty member that may represent the school with their excellent academic achievement, and reward them with a plaque and 100 million won.
Professor Jae-Woong Jeong, the winner of this year’s award, developed the first IoT-based wireless remote brain neural network control system to overcome brain diseases, and has been leading the field. The research was published in 2021 in Nature Biomedical Engineering, one of world’s best scientific journals, and has been recognized as a novel technology that suggested a new vision for the automation of brain research and disease treatment. This study, led by Professor Jeong’s research team, was part of the KAIST College of Engineering Global Initiative Interdisciplinary Research Project, and was jointly studied by Washington University School of Medicine through an international research collaboration. The technology was introduced more than 60 times through both domestic and international media, including Medical Xpress, MBC News, and Maeil Business News.
Professor Jeong has also developed a wirelessly chargeable soft machine for brain transplants, and the results were published in Nature Communications. He thereby opened a new paradigm for implantable semi-permanent devices for transplants, and is making unprecedented research achievements.
2022.06.13 View 9269
Professor Jae-Woong Jeong Receives Hyonwoo KAIST Academic Award
Professor Jae-Woong Jeong from the School of Electrical Engineering was selected for the Hyonwoo KAIST Academic Award, funded by the HyonWoo Cultural Foundation (Chairman Soo-il Kwak, honorary professor at Seoul National University Business School).
The Hyonwoo KAIST Academic Award, presented for the first time in 2021, is an award newly founded by the donations of Chairman Soo-il Kwak of the HyonWoo Cultural Foundation, who aims to reward excellent KAIST scholars who have made outstanding academic achievements.
Every year, through the strict evaluations of the selection committee of the HyonWoo Cultural Foundation and the faculty reward recommendation board, KAIST will choose one faculty member that may represent the school with their excellent academic achievement, and reward them with a plaque and 100 million won.
Professor Jae-Woong Jeong, the winner of this year’s award, developed the first IoT-based wireless remote brain neural network control system to overcome brain diseases, and has been leading the field. The research was published in 2021 in Nature Biomedical Engineering, one of world’s best scientific journals, and has been recognized as a novel technology that suggested a new vision for the automation of brain research and disease treatment. This study, led by Professor Jeong’s research team, was part of the KAIST College of Engineering Global Initiative Interdisciplinary Research Project, and was jointly studied by Washington University School of Medicine through an international research collaboration. The technology was introduced more than 60 times through both domestic and international media, including Medical Xpress, MBC News, and Maeil Business News.
Professor Jeong has also developed a wirelessly chargeable soft machine for brain transplants, and the results were published in Nature Communications. He thereby opened a new paradigm for implantable semi-permanent devices for transplants, and is making unprecedented research achievements.
2022.06.13 View 9269 -
 2022 KAIST Research Day Recognizes 10 Outstanding Researches
On May 31, the 2022 KAIST Research Day was held at the Jeong Geun-mo Conference Hall at KAIST’s main campus.
Since 2016, Research Day has been a yearly festival for researchers at KAIST. By introducing major research achievements and providing opportunities for information exchanges in R&D, it aims to create an atmosphere for mutual cooperation and communication amongst researchers, thereby vitalizing interdisciplinary research.
At this year’s event, 10 faculty members and their representative research achievements were rewarded. As the winner of the Grand Prize for Research, Professor Il-Doo Kim (Department of Materials Science and Engineering) gave a lecture on his topic, “Ultrasensitive flexible chemical sensor”.
With rising attention being given to environmental safety and healthcare, the importance of mobile sensors for trace amounts of molecules that can quickly raise hazard signals and allow early diagnosis from breath analysis have been brought to light. The lecture will break down ultrasensitive chemical sensor development cases, and introduced how gas sensor technologies developed at KAIST in particular are being applied at semiconductor and display fabrication plants for environmental and safety analyses and hazard prevention.
Professor Il-Doo Kim is a recognized researcher for his inventive achievements in the fields of respiratory gas sensor technology for early disease monitoring, and ordered nanofiber membranes for antiviral and fine dust filters.
Professor Kim has so far published 343 international research papers, received 56 journal covers, been awarded 230 domestic and international patents, and completed 12 technology transfers. He has also received a presidential award on the 51st invention day in 2016, Scientist of the Year Award selected by reporters in 2019, and has been named a fellow in the engineering division of the Korean Academy of Science and Technology in 2022.
Professor Kwang-Hyun Cho at the Department of Bio and Brain Engineering and Professor Doh Chang Lee at the Department of Chemical and Biomolecular Engineering were each awarded the Research Award, and Professor Dongsoo Han at the School of Computing received the Innovation Award.
Professors Buhm Soon Park at the Graduate School of Science and Technology Policy, Changick Kim at the School of Electrical Engineering and Hyun Jung Cho at the School of Digital Humanities and Computational Social Sciences received the Interdisciplinary Research Award as a team.
The passion and experiences of the awardees are to be introduced to undergraduate and graduate students as well as fellow researchers through a pre-recorded video lecture, while the lecture of the winner of the grand prize will be delivered on site.
Meanwhile, the top ten R&D achievements of KAIST selected excellent research outcomes from the natural and biological sciences including “Polariton-based PT symmetry laser that turns loss into gain” (Professor Yong-Hoon Cho at the Department of Physics), “Solution to the Riemann Problem including weak shock waves in 1-dimensional space” (Professor Moon-Jin Kang at the Department of Mathematical Sciences), and “Characterization of immune reaction in COVID-19 patients” (Professor Eui-Cheol Shin at the Graduate School of Medical Science and Engineering.)
Awardees from the engineering field included “Fluid surface stabilization technology using plasma jet” (Professor Wonho Choe at the Department of Nuclear and Quantum Engineering, “Visual recognition technology using event-based cameras” (Professor Kuk-Jin Yoon at theDepartment of Mechanical Engineering, “Artificial sensory system development through neural signal mimicry” (Professor Seongjun Park at the Department of Bio and Brain Engineering, “Mott transition material-based ultrahigh speed, low-power, and deformation-resistant true random number generator” (Professor Kyung Min Kim at the Department of Materials Science and Engineering, “Investment service design based on Aline: ESG” (Professor Sangsu Lee at the Department of Industrial Design), “Structural color printing technology without chemical colorings” (Professor Shin-Hyun Kim at the Department of Chemical and Biomolecular Engineering), and “Differentiable transient optical transfer simulation” (Professor Minhyuk Kim at the School of Computing)
To encourage the participation of members of KAIST, all parts of the ceremony will be broadcast live through YouTube in both English and Korean.” He added, “Offline audiences will congratulate the awardees at Fusion Hall in the KI Building and gain research ideas.”
2022.06.10 View 9551
2022 KAIST Research Day Recognizes 10 Outstanding Researches
On May 31, the 2022 KAIST Research Day was held at the Jeong Geun-mo Conference Hall at KAIST’s main campus.
Since 2016, Research Day has been a yearly festival for researchers at KAIST. By introducing major research achievements and providing opportunities for information exchanges in R&D, it aims to create an atmosphere for mutual cooperation and communication amongst researchers, thereby vitalizing interdisciplinary research.
At this year’s event, 10 faculty members and their representative research achievements were rewarded. As the winner of the Grand Prize for Research, Professor Il-Doo Kim (Department of Materials Science and Engineering) gave a lecture on his topic, “Ultrasensitive flexible chemical sensor”.
With rising attention being given to environmental safety and healthcare, the importance of mobile sensors for trace amounts of molecules that can quickly raise hazard signals and allow early diagnosis from breath analysis have been brought to light. The lecture will break down ultrasensitive chemical sensor development cases, and introduced how gas sensor technologies developed at KAIST in particular are being applied at semiconductor and display fabrication plants for environmental and safety analyses and hazard prevention.
Professor Il-Doo Kim is a recognized researcher for his inventive achievements in the fields of respiratory gas sensor technology for early disease monitoring, and ordered nanofiber membranes for antiviral and fine dust filters.
Professor Kim has so far published 343 international research papers, received 56 journal covers, been awarded 230 domestic and international patents, and completed 12 technology transfers. He has also received a presidential award on the 51st invention day in 2016, Scientist of the Year Award selected by reporters in 2019, and has been named a fellow in the engineering division of the Korean Academy of Science and Technology in 2022.
Professor Kwang-Hyun Cho at the Department of Bio and Brain Engineering and Professor Doh Chang Lee at the Department of Chemical and Biomolecular Engineering were each awarded the Research Award, and Professor Dongsoo Han at the School of Computing received the Innovation Award.
Professors Buhm Soon Park at the Graduate School of Science and Technology Policy, Changick Kim at the School of Electrical Engineering and Hyun Jung Cho at the School of Digital Humanities and Computational Social Sciences received the Interdisciplinary Research Award as a team.
The passion and experiences of the awardees are to be introduced to undergraduate and graduate students as well as fellow researchers through a pre-recorded video lecture, while the lecture of the winner of the grand prize will be delivered on site.
Meanwhile, the top ten R&D achievements of KAIST selected excellent research outcomes from the natural and biological sciences including “Polariton-based PT symmetry laser that turns loss into gain” (Professor Yong-Hoon Cho at the Department of Physics), “Solution to the Riemann Problem including weak shock waves in 1-dimensional space” (Professor Moon-Jin Kang at the Department of Mathematical Sciences), and “Characterization of immune reaction in COVID-19 patients” (Professor Eui-Cheol Shin at the Graduate School of Medical Science and Engineering.)
Awardees from the engineering field included “Fluid surface stabilization technology using plasma jet” (Professor Wonho Choe at the Department of Nuclear and Quantum Engineering, “Visual recognition technology using event-based cameras” (Professor Kuk-Jin Yoon at theDepartment of Mechanical Engineering, “Artificial sensory system development through neural signal mimicry” (Professor Seongjun Park at the Department of Bio and Brain Engineering, “Mott transition material-based ultrahigh speed, low-power, and deformation-resistant true random number generator” (Professor Kyung Min Kim at the Department of Materials Science and Engineering, “Investment service design based on Aline: ESG” (Professor Sangsu Lee at the Department of Industrial Design), “Structural color printing technology without chemical colorings” (Professor Shin-Hyun Kim at the Department of Chemical and Biomolecular Engineering), and “Differentiable transient optical transfer simulation” (Professor Minhyuk Kim at the School of Computing)
To encourage the participation of members of KAIST, all parts of the ceremony will be broadcast live through YouTube in both English and Korean.” He added, “Offline audiences will congratulate the awardees at Fusion Hall in the KI Building and gain research ideas.”
2022.06.10 View 9551 -
 Now You Can See Floral Scents!
Optical interferometry visualizes how often lilies emit volatile organic compounds
Have you ever thought about when flowers emit their scents?
KAIST mechanical engineers and biological scientists directly visualized how often a lily releases a floral scent using a laser interferometry method. These measurement results can provide new insights for understanding and further exploring the biosynthesis and emission mechanisms of floral volatiles.
Why is it important to know this? It is well known that the fragrance of flowers affects their interactions with pollinators, microorganisms, and florivores. For instance, many flowering plants can tune their scent emission rates when pollinators are active for pollination. Petunias and the wild tobacco Nicotiana attenuata emit floral scents at night to attract night-active pollinators. Thus, visualizing scent emissions can help us understand the ecological evolution of plant-pollinator interactions.
Many groups have been trying to develop methods for scent analysis. Mass spectrometry has been one widely used method for investigating the fragrance of flowers. Although mass spectrometry reveals the quality and quantity of floral scents, it is impossible to directly measure the releasing frequency. A laser-based gas detection system and a smartphone-based detection system using chemo-responsive dyes have also been used to measure volatile organic compounds (VOCs) in real-time, but it is still hard to measure the time-dependent emission rate of floral scents.
However, the KAIST research team co-led by Professor Hyoungsoo Kim from the Department of Mechanical Engineering and Professor Sang-Gyu Kim from the Department of Biological Sciences measured a refractive index difference between the vapor of the VOCs of lilies and the air to measure the emission frequency. The floral scent vapor was detected and the refractive index of air was 1.0 while that of the major floral scent of a linalool lily was 1.46.
Professor Hyoungsoo Kim said, “We expect this technology to be further applicable to various industrial sectors such as developing it to detect hazardous substances in a space.” The research team also plans to identify the DNA mechanism that controls floral scent secretion.
The current work entitled “Real-time visualization of scent accumulation reveals the frequency of floral scent emissions” was published in ‘Frontiers in Plant Science’ on April 18, 2022. (https://doi.org/10.3389/fpls.2022.835305).
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF-2021R1A2C2007835), the Rural Development Administration (PJ016403), and the KAIST-funded Global Singularity Research PREP-Program.
-Publication:H. Kim, G. Lee, J. Song, and S.-G. Kim, "Real-time visualization of scent accumulation reveals the frequency of floral scent emissions," Frontiers in Plant Science 18, 835305 (2022) (https://doi.org/10.3389/fpls.2022.835305)
-Profile:Professor Hyoungsoo Kimhttp://fil.kaist.ac.kr
@MadeInH on TwitterDepartment of Mechanical EngineeringKAIST
Professor Sang-Gyu Kimhttps://sites.google.com/view/kimlab/home Department of Biological SciencesKAIST
2022.05.25 View 10651
Now You Can See Floral Scents!
Optical interferometry visualizes how often lilies emit volatile organic compounds
Have you ever thought about when flowers emit their scents?
KAIST mechanical engineers and biological scientists directly visualized how often a lily releases a floral scent using a laser interferometry method. These measurement results can provide new insights for understanding and further exploring the biosynthesis and emission mechanisms of floral volatiles.
Why is it important to know this? It is well known that the fragrance of flowers affects their interactions with pollinators, microorganisms, and florivores. For instance, many flowering plants can tune their scent emission rates when pollinators are active for pollination. Petunias and the wild tobacco Nicotiana attenuata emit floral scents at night to attract night-active pollinators. Thus, visualizing scent emissions can help us understand the ecological evolution of plant-pollinator interactions.
Many groups have been trying to develop methods for scent analysis. Mass spectrometry has been one widely used method for investigating the fragrance of flowers. Although mass spectrometry reveals the quality and quantity of floral scents, it is impossible to directly measure the releasing frequency. A laser-based gas detection system and a smartphone-based detection system using chemo-responsive dyes have also been used to measure volatile organic compounds (VOCs) in real-time, but it is still hard to measure the time-dependent emission rate of floral scents.
However, the KAIST research team co-led by Professor Hyoungsoo Kim from the Department of Mechanical Engineering and Professor Sang-Gyu Kim from the Department of Biological Sciences measured a refractive index difference between the vapor of the VOCs of lilies and the air to measure the emission frequency. The floral scent vapor was detected and the refractive index of air was 1.0 while that of the major floral scent of a linalool lily was 1.46.
Professor Hyoungsoo Kim said, “We expect this technology to be further applicable to various industrial sectors such as developing it to detect hazardous substances in a space.” The research team also plans to identify the DNA mechanism that controls floral scent secretion.
The current work entitled “Real-time visualization of scent accumulation reveals the frequency of floral scent emissions” was published in ‘Frontiers in Plant Science’ on April 18, 2022. (https://doi.org/10.3389/fpls.2022.835305).
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF-2021R1A2C2007835), the Rural Development Administration (PJ016403), and the KAIST-funded Global Singularity Research PREP-Program.
-Publication:H. Kim, G. Lee, J. Song, and S.-G. Kim, "Real-time visualization of scent accumulation reveals the frequency of floral scent emissions," Frontiers in Plant Science 18, 835305 (2022) (https://doi.org/10.3389/fpls.2022.835305)
-Profile:Professor Hyoungsoo Kimhttp://fil.kaist.ac.kr
@MadeInH on TwitterDepartment of Mechanical EngineeringKAIST
Professor Sang-Gyu Kimhttps://sites.google.com/view/kimlab/home Department of Biological SciencesKAIST
2022.05.25 View 10651 -
 Neuromorphic Memory Device Simulates Neurons and Synapses
Simultaneous emulation of neuronal and synaptic properties promotes the development of brain-like artificial intelligence
Researchers have reported a nano-sized neuromorphic memory device that emulates neurons and synapses simultaneously in a unit cell, another step toward completing the goal of neuromorphic computing designed to rigorously mimic the human brain with semiconductor devices.
Neuromorphic computing aims to realize artificial intelligence (AI) by mimicking the mechanisms of neurons and synapses that make up the human brain. Inspired by the cognitive functions of the human brain that current computers cannot provide, neuromorphic devices have been widely investigated. However, current Complementary Metal-Oxide Semiconductor (CMOS)-based neuromorphic circuits simply connect artificial neurons and synapses without synergistic interactions, and the concomitant implementation of neurons and synapses still remains a challenge. To address these issues, a research team led by Professor Keon Jae Lee from the Department of Materials Science and Engineering implemented the biological working mechanisms of humans by introducing the neuron-synapse interactions in a single memory cell, rather than the conventional approach of electrically connecting artificial neuronal and synaptic devices.
Similar to commercial graphics cards, the artificial synaptic devices previously studied often used to accelerate parallel computations, which shows clear differences from the operational mechanisms of the human brain. The research team implemented the synergistic interactions between neurons and synapses in the neuromorphic memory device, emulating the mechanisms of the biological neural network. In addition, the developed neuromorphic device can replace complex CMOS neuron circuits with a single device, providing high scalability and cost efficiency.
The human brain consists of a complex network of 100 billion neurons and 100 trillion synapses. The functions and structures of neurons and synapses can flexibly change according to the external stimuli, adapting to the surrounding environment. The research team developed a neuromorphic device in which short-term and long-term memories coexist using volatile and non-volatile memory devices that mimic the characteristics of neurons and synapses, respectively. A threshold switch device is used as volatile memory and phase-change memory is used as a non-volatile device. Two thin-film devices are integrated without intermediate electrodes, implementing the functional adaptability of neurons and synapses in the neuromorphic memory.
Professor Keon Jae Lee explained, "Neurons and synapses interact with each other to establish cognitive functions such as memory and learning, so simulating both is an essential element for brain-inspired artificial intelligence. The developed neuromorphic memory device also mimics the retraining effect that allows quick learning of the forgotten information by implementing a positive feedback effect between neurons and synapses.”
This result entitled “Simultaneous emulation of synaptic and intrinsic plasticity using a memristive synapse” was published in the May 19, 2022 issue of Nature Communications.
-Publication:Sang Hyun Sung, Tae Jin Kim, Hyera Shin, Tae Hong Im, and Keon Jae Lee (2022) “Simultaneous emulation of synaptic and intrinsic plasticity using a memristive synapse,” Nature Communications May 19, 2022 (DOI: 10.1038/s41467-022-30432-2)
-Profile:Professor Keon Jae Leehttp://fand.kaist.ac.kr
Department of Materials Science and EngineeringKAIST
2022.05.20 View 15103
Neuromorphic Memory Device Simulates Neurons and Synapses
Simultaneous emulation of neuronal and synaptic properties promotes the development of brain-like artificial intelligence
Researchers have reported a nano-sized neuromorphic memory device that emulates neurons and synapses simultaneously in a unit cell, another step toward completing the goal of neuromorphic computing designed to rigorously mimic the human brain with semiconductor devices.
Neuromorphic computing aims to realize artificial intelligence (AI) by mimicking the mechanisms of neurons and synapses that make up the human brain. Inspired by the cognitive functions of the human brain that current computers cannot provide, neuromorphic devices have been widely investigated. However, current Complementary Metal-Oxide Semiconductor (CMOS)-based neuromorphic circuits simply connect artificial neurons and synapses without synergistic interactions, and the concomitant implementation of neurons and synapses still remains a challenge. To address these issues, a research team led by Professor Keon Jae Lee from the Department of Materials Science and Engineering implemented the biological working mechanisms of humans by introducing the neuron-synapse interactions in a single memory cell, rather than the conventional approach of electrically connecting artificial neuronal and synaptic devices.
Similar to commercial graphics cards, the artificial synaptic devices previously studied often used to accelerate parallel computations, which shows clear differences from the operational mechanisms of the human brain. The research team implemented the synergistic interactions between neurons and synapses in the neuromorphic memory device, emulating the mechanisms of the biological neural network. In addition, the developed neuromorphic device can replace complex CMOS neuron circuits with a single device, providing high scalability and cost efficiency.
The human brain consists of a complex network of 100 billion neurons and 100 trillion synapses. The functions and structures of neurons and synapses can flexibly change according to the external stimuli, adapting to the surrounding environment. The research team developed a neuromorphic device in which short-term and long-term memories coexist using volatile and non-volatile memory devices that mimic the characteristics of neurons and synapses, respectively. A threshold switch device is used as volatile memory and phase-change memory is used as a non-volatile device. Two thin-film devices are integrated without intermediate electrodes, implementing the functional adaptability of neurons and synapses in the neuromorphic memory.
Professor Keon Jae Lee explained, "Neurons and synapses interact with each other to establish cognitive functions such as memory and learning, so simulating both is an essential element for brain-inspired artificial intelligence. The developed neuromorphic memory device also mimics the retraining effect that allows quick learning of the forgotten information by implementing a positive feedback effect between neurons and synapses.”
This result entitled “Simultaneous emulation of synaptic and intrinsic plasticity using a memristive synapse” was published in the May 19, 2022 issue of Nature Communications.
-Publication:Sang Hyun Sung, Tae Jin Kim, Hyera Shin, Tae Hong Im, and Keon Jae Lee (2022) “Simultaneous emulation of synaptic and intrinsic plasticity using a memristive synapse,” Nature Communications May 19, 2022 (DOI: 10.1038/s41467-022-30432-2)
-Profile:Professor Keon Jae Leehttp://fand.kaist.ac.kr
Department of Materials Science and EngineeringKAIST
2022.05.20 View 15103 -
 Anonymous Donor Makes a Gift of Property Valued at 30 Billion KRW
The KAIST Development Foundation announced on May 9 that an anonymous donor in his 50s made a gift of real estate valued at 30 billion KRW. This is the first donation from an anonymous benefactor on such a grand scale. The benefactor expressed his wishes to fund scholarships for students in need and R&D for medical and bio sciences.
According to the Development Foundation official, the benefactor is reported to have said that he felt burdened that he earned much more than he needed and was looking for the right way to share his assets. The benefactor refused to hold an official donation ceremony and meeting with high-level university administrators.
The donor believes that KAIST is filled with young and dynamic energy, saying, “I would like to help KAIST move forward and create breakthroughs that will benefit the nation as well as all humanity.”
Before making up his mind to give his asset to KAIST, he had planned to establish his own social foundation but he changed his mind. “I decided that an investment in education would be the best investment,” he said. He explained that he was inspired by his KAIST graduate friend who is running a company. He was deeply motivated to help KAIST after witnessing the KAIST graduate’s passion for conducting his business.
After receiving the gift, KAIST President Kwang Hyung Lee was thankful for the full support and trust of the benefactor. “We will spare no effort to foster next-generation talents and advance science and technology in the field of biomedicine.”
2022.05.11 View 6133
Anonymous Donor Makes a Gift of Property Valued at 30 Billion KRW
The KAIST Development Foundation announced on May 9 that an anonymous donor in his 50s made a gift of real estate valued at 30 billion KRW. This is the first donation from an anonymous benefactor on such a grand scale. The benefactor expressed his wishes to fund scholarships for students in need and R&D for medical and bio sciences.
According to the Development Foundation official, the benefactor is reported to have said that he felt burdened that he earned much more than he needed and was looking for the right way to share his assets. The benefactor refused to hold an official donation ceremony and meeting with high-level university administrators.
The donor believes that KAIST is filled with young and dynamic energy, saying, “I would like to help KAIST move forward and create breakthroughs that will benefit the nation as well as all humanity.”
Before making up his mind to give his asset to KAIST, he had planned to establish his own social foundation but he changed his mind. “I decided that an investment in education would be the best investment,” he said. He explained that he was inspired by his KAIST graduate friend who is running a company. He was deeply motivated to help KAIST after witnessing the KAIST graduate’s passion for conducting his business.
After receiving the gift, KAIST President Kwang Hyung Lee was thankful for the full support and trust of the benefactor. “We will spare no effort to foster next-generation talents and advance science and technology in the field of biomedicine.”
2022.05.11 View 6133 -
 Machine Learning-Based Algorithm to Speed up DNA Sequencing
The algorithm presents the first full-fledged, short-read alignment software that leverages learned indices for solving the exact match search problem for efficient seeding
The human genome consists of a complete set of DNA, which is about 6.4 billion letters long. Because of its size, reading the whole genome sequence at once is challenging. So scientists use DNA sequencers to produce hundreds of millions of DNA sequence fragments, or short reads, up to 300 letters long. Then the DNA sequencer assembles all the short reads like a giant jigsaw puzzle to reconstruct the entire genome sequence. Even with very fast computers, this job can take hours to complete.
A research team at KAIST has achieved up to 3.45x faster speeds by developing the first short-read alignment software that uses a recent advance in machine-learning called a learned index.
The research team reported their findings on March 7, 2022 in the journal Bioinformatics. The software has been released as open source and can be found on github (https://github.com/kaist-ina/BWA-MEME).
Next-generation sequencing (NGS) is a state-of-the-art DNA sequencing method. Projects are underway with the goal of producing genome sequencing at population scale. Modern NGS hardware is capable of generating billions of short reads in a single run. Then the short reads have to be aligned with the reference DNA sequence. With large-scale DNA sequencing operations running hundreds of next-generation sequences, the need for an efficient short read alignment tool has become even more critical. Accelerating the DNA sequence alignment would be a step toward achieving the goal of population-scale sequencing. However, existing algorithms are limited in their performance because of their frequent memory accesses.
BWA-MEM2 is a popular short-read alignment software package currently used to sequence the DNA. However, it has its limitations. The state-of-the-art alignment has two phases – seeding and extending. During the seeding phase, searches find exact matches of short reads in the reference DNA sequence. During the extending phase, the short reads from the seeding phase are extended. In the current process, bottlenecks occur in the seeding phase. Finding the exact matches slows the process.
The researchers set out to solve the problem of accelerating the DNA sequence alignment. To speed the process, they applied machine learning techniques to create an algorithmic improvement. Their algorithm, BWA-MEME (BWA-MEM emulated) leverages learned indices to solve the exact match search problem. The original software compared one character at a time for an exact match search. The team’s new algorithm achieves up to 3.45x faster speeds in seeding throughput over BWA-MEM2 by reducing the number of instructions by 4.60x and memory accesses by 8.77x. “Through this study, it has been shown that full genome big data analysis can be performed faster and less costly than conventional methods by applying machine learning technology,” said Professor Dongsu Han from the School of Electrical Engineering at KAIST.
The researchers’ ultimate goal was to develop efficient software that scientists from academia and industry could use on a daily basis for analyzing big data in genomics. “With the recent advances in artificial intelligence and machine learning, we see so many opportunities for designing better software for genomic data analysis. The potential is there for accelerating existing analysis as well as enabling new types of analysis, and our goal is to develop such software,” added Han.
Whole genome sequencing has traditionally been used for discovering genomic mutations and identifying the root causes of diseases, which leads to the discovery and development of new drugs and cures. There could be many potential applications. Whole genome sequencing is used not only for research, but also for clinical purposes. “The science and technology for analyzing genomic data is making rapid progress to make it more accessible for scientists and patients. This will enhance our understanding about diseases and develop a better cure for patients of various diseases.”
The research was funded by the National Research Foundation of the Korean government’s Ministry of Science and ICT.
-PublicationYoungmok Jung, Dongsu Han, “BWA-MEME:BWA-MEM emulated with a machine learning approach,” Bioinformatics, Volume 38, Issue 9, May 2022
(https://doi.org/10.1093/bioinformatics/btac137)
-ProfileProfessor Dongsu HanSchool of Electrical EngineeringKAIST
2022.05.10 View 10320
Machine Learning-Based Algorithm to Speed up DNA Sequencing
The algorithm presents the first full-fledged, short-read alignment software that leverages learned indices for solving the exact match search problem for efficient seeding
The human genome consists of a complete set of DNA, which is about 6.4 billion letters long. Because of its size, reading the whole genome sequence at once is challenging. So scientists use DNA sequencers to produce hundreds of millions of DNA sequence fragments, or short reads, up to 300 letters long. Then the DNA sequencer assembles all the short reads like a giant jigsaw puzzle to reconstruct the entire genome sequence. Even with very fast computers, this job can take hours to complete.
A research team at KAIST has achieved up to 3.45x faster speeds by developing the first short-read alignment software that uses a recent advance in machine-learning called a learned index.
The research team reported their findings on March 7, 2022 in the journal Bioinformatics. The software has been released as open source and can be found on github (https://github.com/kaist-ina/BWA-MEME).
Next-generation sequencing (NGS) is a state-of-the-art DNA sequencing method. Projects are underway with the goal of producing genome sequencing at population scale. Modern NGS hardware is capable of generating billions of short reads in a single run. Then the short reads have to be aligned with the reference DNA sequence. With large-scale DNA sequencing operations running hundreds of next-generation sequences, the need for an efficient short read alignment tool has become even more critical. Accelerating the DNA sequence alignment would be a step toward achieving the goal of population-scale sequencing. However, existing algorithms are limited in their performance because of their frequent memory accesses.
BWA-MEM2 is a popular short-read alignment software package currently used to sequence the DNA. However, it has its limitations. The state-of-the-art alignment has two phases – seeding and extending. During the seeding phase, searches find exact matches of short reads in the reference DNA sequence. During the extending phase, the short reads from the seeding phase are extended. In the current process, bottlenecks occur in the seeding phase. Finding the exact matches slows the process.
The researchers set out to solve the problem of accelerating the DNA sequence alignment. To speed the process, they applied machine learning techniques to create an algorithmic improvement. Their algorithm, BWA-MEME (BWA-MEM emulated) leverages learned indices to solve the exact match search problem. The original software compared one character at a time for an exact match search. The team’s new algorithm achieves up to 3.45x faster speeds in seeding throughput over BWA-MEM2 by reducing the number of instructions by 4.60x and memory accesses by 8.77x. “Through this study, it has been shown that full genome big data analysis can be performed faster and less costly than conventional methods by applying machine learning technology,” said Professor Dongsu Han from the School of Electrical Engineering at KAIST.
The researchers’ ultimate goal was to develop efficient software that scientists from academia and industry could use on a daily basis for analyzing big data in genomics. “With the recent advances in artificial intelligence and machine learning, we see so many opportunities for designing better software for genomic data analysis. The potential is there for accelerating existing analysis as well as enabling new types of analysis, and our goal is to develop such software,” added Han.
Whole genome sequencing has traditionally been used for discovering genomic mutations and identifying the root causes of diseases, which leads to the discovery and development of new drugs and cures. There could be many potential applications. Whole genome sequencing is used not only for research, but also for clinical purposes. “The science and technology for analyzing genomic data is making rapid progress to make it more accessible for scientists and patients. This will enhance our understanding about diseases and develop a better cure for patients of various diseases.”
The research was funded by the National Research Foundation of the Korean government’s Ministry of Science and ICT.
-PublicationYoungmok Jung, Dongsu Han, “BWA-MEME:BWA-MEM emulated with a machine learning approach,” Bioinformatics, Volume 38, Issue 9, May 2022
(https://doi.org/10.1093/bioinformatics/btac137)
-ProfileProfessor Dongsu HanSchool of Electrical EngineeringKAIST
2022.05.10 View 10320 -
 VP Sang Yup Lee Receives Honorary Doctorate from DTU
Vice President for Research, Distinguished Professor Sang Yup Lee at the Department of Chemical & Biomolecular Engineering, was awarded an honorary doctorate from the Technical University of Denmark (DTU) during the DTU Commemoration Day 2022 on April 29. The event drew distinguished guests, students, and faculty including HRH The Crown Prince Frederik Andre Henrik Christian and DTU President Anders Bjarklev.
Professor Lee was recognized for his exceptional scholarship in the field of systems metabolic engineering, which led to the development of microcell factories capable of producing a wide range of fuels, chemicals, materials, and natural compounds, many for the first time.
Professor Lee said in his acceptance speech that KAIST’s continued partnership with DTU in the field of biotechnology will lead to significant contributions in the global efforts to respond to climate change and promote green growth.
DTU CPO and CSO Dina Petronovic Nielson, who heads DTU Biosustain, also lauded Professor Lee saying, “It is not only a great honor for Professor Lee to be induced at DTU but also great honor for DTU to have him.”
Professor Lee also gave commemorative lectures at DTU Biosustain in Lingby and the Bio Innovation Research Institute at the Novo Nordisk Foundation in Copenhagen while in Denmark.
DTU, one of the leading science and technology universities in Europe, has been awarding honorary doctorates since 1921, including to Nobel laureate in chemistry Professor Frances Arnold at Caltech. Professor Lee is the first Korean to receive an honorary doctorate from DTU.
2022.05.03 View 11685
VP Sang Yup Lee Receives Honorary Doctorate from DTU
Vice President for Research, Distinguished Professor Sang Yup Lee at the Department of Chemical & Biomolecular Engineering, was awarded an honorary doctorate from the Technical University of Denmark (DTU) during the DTU Commemoration Day 2022 on April 29. The event drew distinguished guests, students, and faculty including HRH The Crown Prince Frederik Andre Henrik Christian and DTU President Anders Bjarklev.
Professor Lee was recognized for his exceptional scholarship in the field of systems metabolic engineering, which led to the development of microcell factories capable of producing a wide range of fuels, chemicals, materials, and natural compounds, many for the first time.
Professor Lee said in his acceptance speech that KAIST’s continued partnership with DTU in the field of biotechnology will lead to significant contributions in the global efforts to respond to climate change and promote green growth.
DTU CPO and CSO Dina Petronovic Nielson, who heads DTU Biosustain, also lauded Professor Lee saying, “It is not only a great honor for Professor Lee to be induced at DTU but also great honor for DTU to have him.”
Professor Lee also gave commemorative lectures at DTU Biosustain in Lingby and the Bio Innovation Research Institute at the Novo Nordisk Foundation in Copenhagen while in Denmark.
DTU, one of the leading science and technology universities in Europe, has been awarding honorary doctorates since 1921, including to Nobel laureate in chemistry Professor Frances Arnold at Caltech. Professor Lee is the first Korean to receive an honorary doctorate from DTU.
2022.05.03 View 11685 -
 President-Elect Suk-Yeol Yoon Meets and Talks with KAIST Students
President-Elect Yoon stresses science and technology-powered economic growth during his visit to KAIST
Korean President-elect Suk-Yeol Yoon stressed that semiconductors are the key strategical industry that will take the lead during the fourth industrial revolution powered by AI and data during a meeting with KAIST graduate students on April 29. President-elect Yoon promised systemic policy support for making science and technology breakthroughs possible and better rewarding young researchers who are devoted to advances in R&D during his meeting at KAIST.
Before he met with the students, he toured the National Nanofab Center, which is affiliated with KAIST, and was briefed on the center’s role and responsibilities.
President-elect Yoon, who will take office on May 10, said that the best way to ensure prompt growth in Korea’s aging society hinges on advances in science and technology. “All-out investments in science and technology will help us move forward to improve people’s quality of life and lessen the social divide,” he explained.
Eight Master’s and PhD candidates majoring in nuclear engineering, AI robotics, semiconductors, electrical engineering, aerospace, and bioengineering attended the meeting with President-elect Yoon. The students asked for help dealing with the challenges they are experiencing while researching and called for deregulation in the process of forming startups.
PhD candidate Jae Wan Cho from the Department Nuclear and Quantum Engineering stressed the importance of energy security. He asked for the prompt development of new types of nuclear reactors such as small modular reactors, adding, “Korea has very excellent technologies in nuclear plant construction and parts manufacturing, but lags behind in the new types of nuclear reactors. This sector will develop new energy markets and create synergy along with the shipbuilding industry, which will emerge as new pillars of our export.”
Student entrepreneurs such as PhD candidate Kwang Min Kim from the Department of Bio and Brain Engineering and PhD candidate Dong Yoon Shin from the Department of Mechanical and Aerospace Engineering asked for more deregulation in the process of creating startups. PhD candidate Dong Hon Lee from the School of Electrical Engineering pointed out the insecure future caused by the ‘special research fellow system,’ where the number of fellows who have been designated alternative military service has drastically decreased.
2022.05.02 View 6696
President-Elect Suk-Yeol Yoon Meets and Talks with KAIST Students
President-Elect Yoon stresses science and technology-powered economic growth during his visit to KAIST
Korean President-elect Suk-Yeol Yoon stressed that semiconductors are the key strategical industry that will take the lead during the fourth industrial revolution powered by AI and data during a meeting with KAIST graduate students on April 29. President-elect Yoon promised systemic policy support for making science and technology breakthroughs possible and better rewarding young researchers who are devoted to advances in R&D during his meeting at KAIST.
Before he met with the students, he toured the National Nanofab Center, which is affiliated with KAIST, and was briefed on the center’s role and responsibilities.
President-elect Yoon, who will take office on May 10, said that the best way to ensure prompt growth in Korea’s aging society hinges on advances in science and technology. “All-out investments in science and technology will help us move forward to improve people’s quality of life and lessen the social divide,” he explained.
Eight Master’s and PhD candidates majoring in nuclear engineering, AI robotics, semiconductors, electrical engineering, aerospace, and bioengineering attended the meeting with President-elect Yoon. The students asked for help dealing with the challenges they are experiencing while researching and called for deregulation in the process of forming startups.
PhD candidate Jae Wan Cho from the Department Nuclear and Quantum Engineering stressed the importance of energy security. He asked for the prompt development of new types of nuclear reactors such as small modular reactors, adding, “Korea has very excellent technologies in nuclear plant construction and parts manufacturing, but lags behind in the new types of nuclear reactors. This sector will develop new energy markets and create synergy along with the shipbuilding industry, which will emerge as new pillars of our export.”
Student entrepreneurs such as PhD candidate Kwang Min Kim from the Department of Bio and Brain Engineering and PhD candidate Dong Yoon Shin from the Department of Mechanical and Aerospace Engineering asked for more deregulation in the process of creating startups. PhD candidate Dong Hon Lee from the School of Electrical Engineering pointed out the insecure future caused by the ‘special research fellow system,’ where the number of fellows who have been designated alternative military service has drastically decreased.
2022.05.02 View 6696