AR
-
 Team KAIST placed among top two at MBZIRC Maritime Grand Challenge
Representing Korean Robotics at Sea: KAIST’s 26-month strife rewarded
Team KAIST placed among top two at MBZIRC Maritime Grand Challenge
- Team KAIST, composed of students from the labs of Professor Jinwhan Kim of the Department of Mechanical Engineering and Professor Hyunchul Shim of the School of Electrical and Engineering, came through the challenge as the first runner-up winning the prize money totaling up to $650,000 (KRW 860 million).
- Successfully led the autonomous collaboration of unmanned aerial and maritime vehicles using cutting-edge robotics and AI technology through to the final round of the competition held in Abu Dhabi from January 10 to February 6, 2024.
KAIST (President Kwang-Hyung Lee), reported on the 8th that Team KAIST, led by students from the labs of Professor Jinwhan Kim of the Department of Mechanical Engineering and Professor Hyunchul Shim of the School of Electrical Engineering, with Pablo Aviation as a partner, won a total prize money of $650,000 (KRW 860 million) at the Maritime Grand Challenge by the Mohamed Bin Zayed International Robotics Challenge (MBZIRC), finishing first runner-up.
This competition, which is the largest ever robotics competition held over water, is sponsored by the government of the United Arab Emirates and organized by ASPIRE, an organization under the Abu Dhabi Ministry of Science, with a total prize money of $3 million.
In the competition, which started at the end of 2021, 52 teams from around the world participated and five teams were selected to go on to the finals in February 2023 after going through the first and second stages of screening. The final round was held from January 10 to February 6, 2024, using actual unmanned ships and drones in a secluded sea area of 10 km2 off the coast of Abu Dhabi, the capital of the United Arab Emirates. A total of 18 KAIST students and Professor Jinwhan Kim and Professor Hyunchul Shim took part in this competition at the location at Abu Dhabi.
Team KAIST will receive $500,000 in prize money for taking second place in the final, and the team’s prize money totals up to $650,000 including $150,000 that was as special midterm award for finalists.
The final mission scenario is to find the target vessel on the run carrying illegal cargoes among many ships moving within the GPS-disabled marine surface, and inspect the deck for two different types of stolen cargo to recover them using the aerial vehicle to bring the small cargo and the robot manipulator topped on an unmanned ship to retrieve the larger one. The true aim of the mission is to complete it through autonomous collaboration of the unmanned ship and the aerial vehicle without human intervention throughout the entire mission process. In particular, since GPS cannot be used in this competition due to regulations, Professor Jinwhan Kim's research team developed autonomous operation techniques for unmanned ships, including searching and navigating methods using maritime radar, and Professor Hyunchul Shim's research team developed video-based navigation and a technology to combine a small autonomous robot with a drone.
The final mission is to retrieve cargo on board a ship fleeing at sea through autonomous collaboration between unmanned ships and unmanned aerial vehicles without human intervention. The overall mission consists the first stage of conducting the inspection to find the target ship among several ships moving at sea and the second stage of conducting the intervention mission to retrieve the cargoes on the deck of the ship. Each team was given a total of three opportunities, and the team that completed the highest-level mission in the shortest time during the three attempts received the highest score.
In the first attempt, KAIST was the only team to succeed in the first stage search mission, but the competition began in earnest as the Croatian team also completed the first stage mission in the second attempt. As the competition schedule was delayed due to strong winds and high waves that continued for several days, the organizers decided to hold the finals with the three teams, including the Team KAIST and the team from Croatia’s the University of Zagreb, which completed the first stage of the mission, and Team Fly-Eagle, a team of researcher from China and UAE that partially completed the first stage. The three teams were given the chance to proceed to the finals and try for the third attempt, and in the final competition, the Croatian team won, KAIST took the second place, and the combined team of UAE-China combined team took the third place. The final prize to be given for the winning team is set at $2 million with $500,000 for the runner-up team, and $250,000 for the third-place.
Professor Jinwhan Kim of the Department of Mechanical Engineering, who served as the advisor for Team KAIST, said, “I would like to express my gratitude and congratulations to the students who put in a huge academic and physical efforts in preparing for the competition over the past two years. I feel rewarded because, regardless of the results, every bit of efforts put into this up to this point will become the base of their confidence and a valuable asset in their growth into a great researcher.” Sol Han, a doctoral student in mechanical engineering who served as the team leader, said, “I am disappointed of how narrowly we missed out on winning at the end, but I am satisfied with the significance of the output we’ve got and I am grateful to the team members who worked hard together for that.”
HD Hyundai, Rainbow Robotics, Avikus, and FIMS also participated as sponsors for Team KAIST's campaign.
2024.02.09 View 15601
Team KAIST placed among top two at MBZIRC Maritime Grand Challenge
Representing Korean Robotics at Sea: KAIST’s 26-month strife rewarded
Team KAIST placed among top two at MBZIRC Maritime Grand Challenge
- Team KAIST, composed of students from the labs of Professor Jinwhan Kim of the Department of Mechanical Engineering and Professor Hyunchul Shim of the School of Electrical and Engineering, came through the challenge as the first runner-up winning the prize money totaling up to $650,000 (KRW 860 million).
- Successfully led the autonomous collaboration of unmanned aerial and maritime vehicles using cutting-edge robotics and AI technology through to the final round of the competition held in Abu Dhabi from January 10 to February 6, 2024.
KAIST (President Kwang-Hyung Lee), reported on the 8th that Team KAIST, led by students from the labs of Professor Jinwhan Kim of the Department of Mechanical Engineering and Professor Hyunchul Shim of the School of Electrical Engineering, with Pablo Aviation as a partner, won a total prize money of $650,000 (KRW 860 million) at the Maritime Grand Challenge by the Mohamed Bin Zayed International Robotics Challenge (MBZIRC), finishing first runner-up.
This competition, which is the largest ever robotics competition held over water, is sponsored by the government of the United Arab Emirates and organized by ASPIRE, an organization under the Abu Dhabi Ministry of Science, with a total prize money of $3 million.
In the competition, which started at the end of 2021, 52 teams from around the world participated and five teams were selected to go on to the finals in February 2023 after going through the first and second stages of screening. The final round was held from January 10 to February 6, 2024, using actual unmanned ships and drones in a secluded sea area of 10 km2 off the coast of Abu Dhabi, the capital of the United Arab Emirates. A total of 18 KAIST students and Professor Jinwhan Kim and Professor Hyunchul Shim took part in this competition at the location at Abu Dhabi.
Team KAIST will receive $500,000 in prize money for taking second place in the final, and the team’s prize money totals up to $650,000 including $150,000 that was as special midterm award for finalists.
The final mission scenario is to find the target vessel on the run carrying illegal cargoes among many ships moving within the GPS-disabled marine surface, and inspect the deck for two different types of stolen cargo to recover them using the aerial vehicle to bring the small cargo and the robot manipulator topped on an unmanned ship to retrieve the larger one. The true aim of the mission is to complete it through autonomous collaboration of the unmanned ship and the aerial vehicle without human intervention throughout the entire mission process. In particular, since GPS cannot be used in this competition due to regulations, Professor Jinwhan Kim's research team developed autonomous operation techniques for unmanned ships, including searching and navigating methods using maritime radar, and Professor Hyunchul Shim's research team developed video-based navigation and a technology to combine a small autonomous robot with a drone.
The final mission is to retrieve cargo on board a ship fleeing at sea through autonomous collaboration between unmanned ships and unmanned aerial vehicles without human intervention. The overall mission consists the first stage of conducting the inspection to find the target ship among several ships moving at sea and the second stage of conducting the intervention mission to retrieve the cargoes on the deck of the ship. Each team was given a total of three opportunities, and the team that completed the highest-level mission in the shortest time during the three attempts received the highest score.
In the first attempt, KAIST was the only team to succeed in the first stage search mission, but the competition began in earnest as the Croatian team also completed the first stage mission in the second attempt. As the competition schedule was delayed due to strong winds and high waves that continued for several days, the organizers decided to hold the finals with the three teams, including the Team KAIST and the team from Croatia’s the University of Zagreb, which completed the first stage of the mission, and Team Fly-Eagle, a team of researcher from China and UAE that partially completed the first stage. The three teams were given the chance to proceed to the finals and try for the third attempt, and in the final competition, the Croatian team won, KAIST took the second place, and the combined team of UAE-China combined team took the third place. The final prize to be given for the winning team is set at $2 million with $500,000 for the runner-up team, and $250,000 for the third-place.
Professor Jinwhan Kim of the Department of Mechanical Engineering, who served as the advisor for Team KAIST, said, “I would like to express my gratitude and congratulations to the students who put in a huge academic and physical efforts in preparing for the competition over the past two years. I feel rewarded because, regardless of the results, every bit of efforts put into this up to this point will become the base of their confidence and a valuable asset in their growth into a great researcher.” Sol Han, a doctoral student in mechanical engineering who served as the team leader, said, “I am disappointed of how narrowly we missed out on winning at the end, but I am satisfied with the significance of the output we’ve got and I am grateful to the team members who worked hard together for that.”
HD Hyundai, Rainbow Robotics, Avikus, and FIMS also participated as sponsors for Team KAIST's campaign.
2024.02.09 View 15601 -
 A KAIST Research Team Develops a Novel “Bone Bandage” Material for Cracked Bones
Bone regeneration is a complex process, and existing methods to aid regeneration including transplants and growth factor transmissions face limitations such as the high cost. But recently, a piezoelectric material that can promote the growth of bone tissue has been developed.
A KAIST research team led by Professor Seungbum Hong from the Department of Materials Science and Engineering (DMSE) announced on January 25 the development of a biomimetic scaffold that generates electrical signals upon the application of pressure by utilizing the unique osteogenic ability of hydroxyapatite (HAp). This research was conducted in collaboration with a team led by Professor Jangho Kim from the Department of Convergence Biosystems Engineering at Chonnam National University.
HAp is a basic calcium phosphate material found in bones and teeth. This biocompatible mineral substance is also known to prevent tooth decay and is often used in toothpaste.
Previous studies on piezoelectric scaffolds confirmed the effects of piezoelectricity on promoting bone regeneration and improving bone fusion in various polymer-based materials, but were limited in simulating the complex cellular environment required for optimal bone tissue regeneration. However, this research suggests a new method for utilizing the unique osteogenic abilities of HAp to develop a material that mimics the environment for bone tissue in a living body.
< Figure 1. Design and characterization of piezoelectrically and topographically originated biomimetic scaffolds. (a) Schematic representation of the enhanced bone regeneration mechanism through electrical and topographical cues provided by HAp-incorporated P(VDF-TrFE) scaffolds. (b) Schematic diagram of the fabrication process. >
The research team developed a manufacturing process that fuses HAp with a polymer film. The flexible and free-standing scaffold developed through this process demonstrated its remarkable potential for promoting bone regeneration through in-vitro and in-vivo experiments in rats.
The team also identified the principles of bone regeneration that their scaffold is based on. Using atomic force microscopy (AFM), they analysed the electrical properties of the scaffold and evaluated the detailed surface properties related to cell shape and cell skeletal protein formation. They also investigated the effects of piezoelectricity and surface properties on the expression of growth factors.
Professor Hong from KAIST’s DMSE said, “We have developed a HAp-based piezoelectric composite material that can act like a ‘bone bandage’ through its ability to accelerate bone regeneration.” He added, “This research not only suggests a new direction for designing biomaterials, but is also significant in having explored the effects of piezoelectricity and surface properties on bone regeneration.”
This research, conducted by co-first authors Soyun Joo and Soyeon Kim from Professor Hong’s group, was published on ACS Applied Materials & Interfaces on January 4 under the title “Piezoelectrically and Topographically Engineered Scaffolds for Accelerating Bone Regeneration”. From Professor Kim’s group, Ph.D. candidate Yonghyun Gwon also participated as co-first author, and Professor Kim himself as a corresponding author.
< Figure 2. Analysis of piezoelectric and surface properties of the biomimetic scaffolds using atomic force microscopy. (a) PFM amplitude and phase images of box-poled composite scaffolds. The white bar represents 2 μm. (b) 3D representations of composite scaffolds paired with typical 2D line sections. (c) In vivo bone regeneration micro-CT analysis, (d) schematic representation of filler-derived electrical origins in bone regeneration. >
This research was supported by the KAIST Research and Development Team, the KUSTAR-KAIST Joint Research Center, the KAIST Global Singularity Project, and the government-funded Basic Research Project by the National Research Foundation of Korea.
2024.02.01 View 9773
A KAIST Research Team Develops a Novel “Bone Bandage” Material for Cracked Bones
Bone regeneration is a complex process, and existing methods to aid regeneration including transplants and growth factor transmissions face limitations such as the high cost. But recently, a piezoelectric material that can promote the growth of bone tissue has been developed.
A KAIST research team led by Professor Seungbum Hong from the Department of Materials Science and Engineering (DMSE) announced on January 25 the development of a biomimetic scaffold that generates electrical signals upon the application of pressure by utilizing the unique osteogenic ability of hydroxyapatite (HAp). This research was conducted in collaboration with a team led by Professor Jangho Kim from the Department of Convergence Biosystems Engineering at Chonnam National University.
HAp is a basic calcium phosphate material found in bones and teeth. This biocompatible mineral substance is also known to prevent tooth decay and is often used in toothpaste.
Previous studies on piezoelectric scaffolds confirmed the effects of piezoelectricity on promoting bone regeneration and improving bone fusion in various polymer-based materials, but were limited in simulating the complex cellular environment required for optimal bone tissue regeneration. However, this research suggests a new method for utilizing the unique osteogenic abilities of HAp to develop a material that mimics the environment for bone tissue in a living body.
< Figure 1. Design and characterization of piezoelectrically and topographically originated biomimetic scaffolds. (a) Schematic representation of the enhanced bone regeneration mechanism through electrical and topographical cues provided by HAp-incorporated P(VDF-TrFE) scaffolds. (b) Schematic diagram of the fabrication process. >
The research team developed a manufacturing process that fuses HAp with a polymer film. The flexible and free-standing scaffold developed through this process demonstrated its remarkable potential for promoting bone regeneration through in-vitro and in-vivo experiments in rats.
The team also identified the principles of bone regeneration that their scaffold is based on. Using atomic force microscopy (AFM), they analysed the electrical properties of the scaffold and evaluated the detailed surface properties related to cell shape and cell skeletal protein formation. They also investigated the effects of piezoelectricity and surface properties on the expression of growth factors.
Professor Hong from KAIST’s DMSE said, “We have developed a HAp-based piezoelectric composite material that can act like a ‘bone bandage’ through its ability to accelerate bone regeneration.” He added, “This research not only suggests a new direction for designing biomaterials, but is also significant in having explored the effects of piezoelectricity and surface properties on bone regeneration.”
This research, conducted by co-first authors Soyun Joo and Soyeon Kim from Professor Hong’s group, was published on ACS Applied Materials & Interfaces on January 4 under the title “Piezoelectrically and Topographically Engineered Scaffolds for Accelerating Bone Regeneration”. From Professor Kim’s group, Ph.D. candidate Yonghyun Gwon also participated as co-first author, and Professor Kim himself as a corresponding author.
< Figure 2. Analysis of piezoelectric and surface properties of the biomimetic scaffolds using atomic force microscopy. (a) PFM amplitude and phase images of box-poled composite scaffolds. The white bar represents 2 μm. (b) 3D representations of composite scaffolds paired with typical 2D line sections. (c) In vivo bone regeneration micro-CT analysis, (d) schematic representation of filler-derived electrical origins in bone regeneration. >
This research was supported by the KAIST Research and Development Team, the KUSTAR-KAIST Joint Research Center, the KAIST Global Singularity Project, and the government-funded Basic Research Project by the National Research Foundation of Korea.
2024.02.01 View 9773 -
 KAIST Professor Jiyun Lee becomes the first Korean to receive the Thurlow Award from the American Institute of Navigation
< Distinguished Professor Jiyun Lee from the KAIST Department of Aerospace Engineering >
KAIST (President Kwang-Hyung Lee) announced on January 27th that Distinguished Professor Jiyun Lee from the KAIST Department of Aerospace Engineering had won the Colonel Thomas L. Thurlow Award from the American Institute of Navigation (ION) for her achievements in the field of satellite navigation.
The American Institute of Navigation (ION) announced Distinguished Professor Lee as the winner of the Thurlow Award at its annual awards ceremony held in conjunction with its international conference in Long Beach, California on January 25th. This is the first time a person of Korean descent has received the award.
The Thurlow Award was established in 1945 to honor Colonel Thomas L. Thurlow, who made significant contributions to the development of navigation equipment and the training of navigators. This award aims to recognize an individual who has made an outstanding contribution to the development of navigation and it is awarded to one person each year. Past recipients include MIT professor Charles Stark Draper, who is well-known as the father of inertial navigation and who developed the guidance computer for the Apollo moon landing project.
Distinguished Professor Jiyun Lee was recognized for her significant contributions to technological advancements that ensure the safety of satellite-based navigation systems for aviation. In particular, she was recognized as a world authority in the field of navigation integrity architecture design, which is essential for ensuring the stability of intelligent transportation systems and autonomous unmanned systems. Distinguished Professor Lee made a groundbreaking contribution to help ensure the safety of satellite-based navigation systems from ionospheric disturbances, including those affected by sudden changes in external factors such as the solar and space environment.
She has achieved numerous scientific discoveries in the field of ionospheric research, while developing new ionospheric threat modeling methods, ionospheric anomaly monitoring and mitigation techniques, and integrity and availability assessment techniques for next-generation augmented navigation systems. She also contributed to the international standardization of technology through the International Civil Aviation Organization (ICAO).
Distinguished Professor Lee and her research group have pioneered innovative navigation technologies for the safe and autonomous operation of unmanned aerial vehicles (UAVs) and urban air mobility (UAM). She was the first to propose and develop a low-cost navigation satellite system (GNSS) augmented architecture for UAVs with a near-field network operation concept that ensures high integrity, and a networked ground station-based augmented navigation system for UAM. She also contributed to integrity design techniques, including failure monitoring and integrity risk assessment for multi-sensor integrated navigation systems.
< Professor Jiyoon Lee upon receiving the Thurlow Award >
Bradford Parkinson, professor emeritus at Stanford University and winner of the 1986 Thurlow Award, who is known as the father of GPS, congratulated Distinguished Professor Lee upon hearing that she was receiving the Thurlow Award and commented that her innovative research has addressed many important topics in the field of navigation and her solutions are highly innovative and highly regarded.
Distinguished Professor Lee said, “I am very honored and delighted to receive this award with its deep history and tradition in the field of navigation.” She added, “I will strive to help develop the future mobility industry by securing safe and sustainable navigation technology.”
2024.01.26 View 9109
KAIST Professor Jiyun Lee becomes the first Korean to receive the Thurlow Award from the American Institute of Navigation
< Distinguished Professor Jiyun Lee from the KAIST Department of Aerospace Engineering >
KAIST (President Kwang-Hyung Lee) announced on January 27th that Distinguished Professor Jiyun Lee from the KAIST Department of Aerospace Engineering had won the Colonel Thomas L. Thurlow Award from the American Institute of Navigation (ION) for her achievements in the field of satellite navigation.
The American Institute of Navigation (ION) announced Distinguished Professor Lee as the winner of the Thurlow Award at its annual awards ceremony held in conjunction with its international conference in Long Beach, California on January 25th. This is the first time a person of Korean descent has received the award.
The Thurlow Award was established in 1945 to honor Colonel Thomas L. Thurlow, who made significant contributions to the development of navigation equipment and the training of navigators. This award aims to recognize an individual who has made an outstanding contribution to the development of navigation and it is awarded to one person each year. Past recipients include MIT professor Charles Stark Draper, who is well-known as the father of inertial navigation and who developed the guidance computer for the Apollo moon landing project.
Distinguished Professor Jiyun Lee was recognized for her significant contributions to technological advancements that ensure the safety of satellite-based navigation systems for aviation. In particular, she was recognized as a world authority in the field of navigation integrity architecture design, which is essential for ensuring the stability of intelligent transportation systems and autonomous unmanned systems. Distinguished Professor Lee made a groundbreaking contribution to help ensure the safety of satellite-based navigation systems from ionospheric disturbances, including those affected by sudden changes in external factors such as the solar and space environment.
She has achieved numerous scientific discoveries in the field of ionospheric research, while developing new ionospheric threat modeling methods, ionospheric anomaly monitoring and mitigation techniques, and integrity and availability assessment techniques for next-generation augmented navigation systems. She also contributed to the international standardization of technology through the International Civil Aviation Organization (ICAO).
Distinguished Professor Lee and her research group have pioneered innovative navigation technologies for the safe and autonomous operation of unmanned aerial vehicles (UAVs) and urban air mobility (UAM). She was the first to propose and develop a low-cost navigation satellite system (GNSS) augmented architecture for UAVs with a near-field network operation concept that ensures high integrity, and a networked ground station-based augmented navigation system for UAM. She also contributed to integrity design techniques, including failure monitoring and integrity risk assessment for multi-sensor integrated navigation systems.
< Professor Jiyoon Lee upon receiving the Thurlow Award >
Bradford Parkinson, professor emeritus at Stanford University and winner of the 1986 Thurlow Award, who is known as the father of GPS, congratulated Distinguished Professor Lee upon hearing that she was receiving the Thurlow Award and commented that her innovative research has addressed many important topics in the field of navigation and her solutions are highly innovative and highly regarded.
Distinguished Professor Lee said, “I am very honored and delighted to receive this award with its deep history and tradition in the field of navigation.” She added, “I will strive to help develop the future mobility industry by securing safe and sustainable navigation technology.”
2024.01.26 View 9109 -
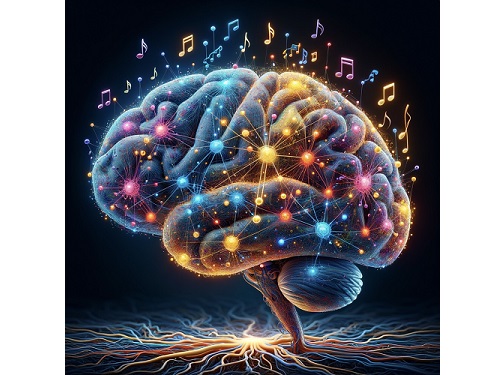 KAIST Research Team Breaks Down Musical Instincts with AI
Music, often referred to as the universal language, is known to be a common component in all cultures. Then, could ‘musical instinct’ be something that is shared to some degree despite the extensive environmental differences amongst cultures?
On January 16, a KAIST research team led by Professor Hawoong Jung from the Department of Physics announced to have identified the principle by which musical instincts emerge from the human brain without special learning using an artificial neural network model.
Previously, many researchers have attempted to identify the similarities and differences between the music that exist in various different cultures, and tried to understand the origin of the universality. A paper published in Science in 2019 had revealed that music is produced in all ethnographically distinct cultures, and that similar forms of beats and tunes are used. Neuroscientist have also previously found out that a specific part of the human brain, namely the auditory cortex, is responsible for processing musical information.
Professor Jung’s team used an artificial neural network model to show that cognitive functions for music forms spontaneously as a result of processing auditory information received from nature, without being taught music. The research team utilized AudioSet, a large-scale collection of sound data provided by Google, and taught the artificial neural network to learn the various sounds. Interestingly, the research team discovered that certain neurons within the network model would respond selectively to music. In other words, they observed the spontaneous generation of neurons that reacted minimally to various other sounds like those of animals, nature, or machines, but showed high levels of response to various forms of music including both instrumental and vocal.
The neurons in the artificial neural network model showed similar reactive behaviours to those in the auditory cortex of a real brain. For example, artificial neurons responded less to the sound of music that was cropped into short intervals and were rearranged. This indicates that the spontaneously-generated music-selective neurons encode the temporal structure of music. This property was not limited to a specific genre of music, but emerged across 25 different genres including classic, pop, rock, jazz, and electronic.
< Figure 1. Illustration of the musicality of the brain and artificial neural network (created with DALL·E3 AI based on the paper content) >
Furthermore, suppressing the activity of the music-selective neurons was found to greatly impede the cognitive accuracy for other natural sounds. That is to say, the neural function that processes musical information helps process other sounds, and that ‘musical ability’ may be an instinct formed as a result of an evolutionary adaptation acquired to better process sounds from nature.
Professor Hawoong Jung, who advised the research, said, “The results of our study imply that evolutionary pressure has contributed to forming the universal basis for processing musical information in various cultures.” As for the significance of the research, he explained, “We look forward for this artificially built model with human-like musicality to become an original model for various applications including AI music generation, musical therapy, and for research in musical cognition.” He also commented on its limitations, adding, “This research however does not take into consideration the developmental process that follows the learning of music, and it must be noted that this is a study on the foundation of processing musical information in early development.”
< Figure 2. The artificial neural network that learned to recognize non-musical natural sounds in the cyber space distinguishes between music and non-music. >
This research, conducted by first author Dr. Gwangsu Kim of the KAIST Department of Physics (current affiliation: MIT Department of Brain and Cognitive Sciences) and Dr. Dong-Kyum Kim (current affiliation: IBS) was published in Nature Communications under the title, “Spontaneous emergence of rudimentary music detectors in deep neural networks”.
This research was supported by the National Research Foundation of Korea.
2024.01.23 View 9184
KAIST Research Team Breaks Down Musical Instincts with AI
Music, often referred to as the universal language, is known to be a common component in all cultures. Then, could ‘musical instinct’ be something that is shared to some degree despite the extensive environmental differences amongst cultures?
On January 16, a KAIST research team led by Professor Hawoong Jung from the Department of Physics announced to have identified the principle by which musical instincts emerge from the human brain without special learning using an artificial neural network model.
Previously, many researchers have attempted to identify the similarities and differences between the music that exist in various different cultures, and tried to understand the origin of the universality. A paper published in Science in 2019 had revealed that music is produced in all ethnographically distinct cultures, and that similar forms of beats and tunes are used. Neuroscientist have also previously found out that a specific part of the human brain, namely the auditory cortex, is responsible for processing musical information.
Professor Jung’s team used an artificial neural network model to show that cognitive functions for music forms spontaneously as a result of processing auditory information received from nature, without being taught music. The research team utilized AudioSet, a large-scale collection of sound data provided by Google, and taught the artificial neural network to learn the various sounds. Interestingly, the research team discovered that certain neurons within the network model would respond selectively to music. In other words, they observed the spontaneous generation of neurons that reacted minimally to various other sounds like those of animals, nature, or machines, but showed high levels of response to various forms of music including both instrumental and vocal.
The neurons in the artificial neural network model showed similar reactive behaviours to those in the auditory cortex of a real brain. For example, artificial neurons responded less to the sound of music that was cropped into short intervals and were rearranged. This indicates that the spontaneously-generated music-selective neurons encode the temporal structure of music. This property was not limited to a specific genre of music, but emerged across 25 different genres including classic, pop, rock, jazz, and electronic.
< Figure 1. Illustration of the musicality of the brain and artificial neural network (created with DALL·E3 AI based on the paper content) >
Furthermore, suppressing the activity of the music-selective neurons was found to greatly impede the cognitive accuracy for other natural sounds. That is to say, the neural function that processes musical information helps process other sounds, and that ‘musical ability’ may be an instinct formed as a result of an evolutionary adaptation acquired to better process sounds from nature.
Professor Hawoong Jung, who advised the research, said, “The results of our study imply that evolutionary pressure has contributed to forming the universal basis for processing musical information in various cultures.” As for the significance of the research, he explained, “We look forward for this artificially built model with human-like musicality to become an original model for various applications including AI music generation, musical therapy, and for research in musical cognition.” He also commented on its limitations, adding, “This research however does not take into consideration the developmental process that follows the learning of music, and it must be noted that this is a study on the foundation of processing musical information in early development.”
< Figure 2. The artificial neural network that learned to recognize non-musical natural sounds in the cyber space distinguishes between music and non-music. >
This research, conducted by first author Dr. Gwangsu Kim of the KAIST Department of Physics (current affiliation: MIT Department of Brain and Cognitive Sciences) and Dr. Dong-Kyum Kim (current affiliation: IBS) was published in Nature Communications under the title, “Spontaneous emergence of rudimentary music detectors in deep neural networks”.
This research was supported by the National Research Foundation of Korea.
2024.01.23 View 9184 -
 A KAIST Research Team Observes the Processes of Memory and Cognition in Real Time
The human brain contains approximately 86 billion neurons and 600 trillion synapses that exchange signals between the neurons to help us control the various functions of the brain including cognition, emotion, and memory. Interestingly, the number of synapses decrease with age or as a result of diseases like Alzheimer’s, and research on synapses thus attracts a lot of attention. However, limitations have existed in observing the dynamics of synapse structures in real time.
On January 9, a joint research team led by Professor Won Do Heo from the KAIST Department of Biological Sciences, Professor Hyung-Bae Kwon from Johns Hopkins School of Medicine, and Professor Sangkyu Lee from the Institute for Basic Science (IBS) revealed that they have developed the world’s first technique to allow a real-time observation of synapse formation, extinction, and alterations.
Professor Heo’s team conjugated dimerization-dependent fluorescent proteins (ddFP) to synapses in order to observe the process in which synapses create connections between neurons in real time. The team named this technique SynapShot, by combining the words ‘synapse’ and snapshot’, and successfully tracked and observed the live formation and extinction processes of synapses as well as their dynamic changes.
< Figure 1. To observe dynamically changing synapses, dimerization-dependent fluorescent protein (ddFP) was expressed to observe flourescent signals upon synapse formation as ddFP enables fluorescence detection through reversible binding to pre- and postsynaptic terminals. >
Through a joint research project, the teams led by Professor Heo and Professor Sangkyu Lee at IBS together designed a SynapShot with green and red fluorescence, and were able to easily distinguish the synapse connecting two different neurons. Additionally, by combining an optogenetic technique that can control the function of a molecule using light, the team was able to observe the changes in the synapses while simultaneously inducing certain functions of the neurons using light.
Through more joint research with the team led by Professor Hyung-Bae Kwon at the Johns Hopkins School of Medicine, Professor Heo’s team induced several situations on live mice, including visual discrimination training, exercise, and anaesthesia, and used SynapShot to observe the changes in the synapses during each situation in real time. The observations revealed that each synapse could change fairly quickly and dynamically. This was the first-ever case in which the changes in synapses were observed in a live mammal.
< Figure 2. Microscopic photos observed through changes of the flourescence of the synapse sensor (SynapShot) by cultivating the neurons of an experimental rat and expressing the SynapShot. The changes in the synapse that is created when the pre- and post-synaptic terminals come into contact and the synapse that disappears after a certain period of time are measured by the fluorescence of the SynapShot. >
Professor Heo said, “Our group developed SynapShot through a collaboration with domestic and international research teams, and have opened up the possibility for first-hand live observations of the quick and dynamic changes of synapses, which was previously difficult to do. We expect this technique to revolutionize research methodology in the neurological field, and play an important role in brightening the future of brain science.”
This research, conducted by co-first authors Seungkyu Son (Ph.D. candidate), Jinsu Lee (Ph.D. candidate) and Dr. Kanghoon Jung from Johns Hopkins, was published in the online edition of Nature Methods on January 8 under the title “Real-time visualization of structural dynamics of synapses in live cells in vivo”, and will be printed in the February volume.
< Figure 3. Simultaneous use of green-SynapShot and red-SynapShot to distinguish and observe synapses with one post-terminal and different pre-terminals. >
< Figure 4. Dimer-dependent fluorescent protein (ddFP) exists as a green fluorescent protein as well as a red fluorescent protein, and can be applied together with blue light-activated optogenetic technology. After activating Tropomyosin receptor kinase B (TrkB) by blue light using optogenetic technology, the strengthening of synaptic connections through signals of brain-derived neurotrophic factor is observed using red-SynapShot. >
< Figure 5. Micrographs showing real-time changing synapses in the visual cortex of mice trained through visual training using in vivo imaging techniques such as two-photon microscopy as well as at the cellular level. >
This research was supported by Mid-Sized Research Funds and the Singularity Project from KAIST, and by IBS.
2024.01.18 View 8620
A KAIST Research Team Observes the Processes of Memory and Cognition in Real Time
The human brain contains approximately 86 billion neurons and 600 trillion synapses that exchange signals between the neurons to help us control the various functions of the brain including cognition, emotion, and memory. Interestingly, the number of synapses decrease with age or as a result of diseases like Alzheimer’s, and research on synapses thus attracts a lot of attention. However, limitations have existed in observing the dynamics of synapse structures in real time.
On January 9, a joint research team led by Professor Won Do Heo from the KAIST Department of Biological Sciences, Professor Hyung-Bae Kwon from Johns Hopkins School of Medicine, and Professor Sangkyu Lee from the Institute for Basic Science (IBS) revealed that they have developed the world’s first technique to allow a real-time observation of synapse formation, extinction, and alterations.
Professor Heo’s team conjugated dimerization-dependent fluorescent proteins (ddFP) to synapses in order to observe the process in which synapses create connections between neurons in real time. The team named this technique SynapShot, by combining the words ‘synapse’ and snapshot’, and successfully tracked and observed the live formation and extinction processes of synapses as well as their dynamic changes.
< Figure 1. To observe dynamically changing synapses, dimerization-dependent fluorescent protein (ddFP) was expressed to observe flourescent signals upon synapse formation as ddFP enables fluorescence detection through reversible binding to pre- and postsynaptic terminals. >
Through a joint research project, the teams led by Professor Heo and Professor Sangkyu Lee at IBS together designed a SynapShot with green and red fluorescence, and were able to easily distinguish the synapse connecting two different neurons. Additionally, by combining an optogenetic technique that can control the function of a molecule using light, the team was able to observe the changes in the synapses while simultaneously inducing certain functions of the neurons using light.
Through more joint research with the team led by Professor Hyung-Bae Kwon at the Johns Hopkins School of Medicine, Professor Heo’s team induced several situations on live mice, including visual discrimination training, exercise, and anaesthesia, and used SynapShot to observe the changes in the synapses during each situation in real time. The observations revealed that each synapse could change fairly quickly and dynamically. This was the first-ever case in which the changes in synapses were observed in a live mammal.
< Figure 2. Microscopic photos observed through changes of the flourescence of the synapse sensor (SynapShot) by cultivating the neurons of an experimental rat and expressing the SynapShot. The changes in the synapse that is created when the pre- and post-synaptic terminals come into contact and the synapse that disappears after a certain period of time are measured by the fluorescence of the SynapShot. >
Professor Heo said, “Our group developed SynapShot through a collaboration with domestic and international research teams, and have opened up the possibility for first-hand live observations of the quick and dynamic changes of synapses, which was previously difficult to do. We expect this technique to revolutionize research methodology in the neurological field, and play an important role in brightening the future of brain science.”
This research, conducted by co-first authors Seungkyu Son (Ph.D. candidate), Jinsu Lee (Ph.D. candidate) and Dr. Kanghoon Jung from Johns Hopkins, was published in the online edition of Nature Methods on January 8 under the title “Real-time visualization of structural dynamics of synapses in live cells in vivo”, and will be printed in the February volume.
< Figure 3. Simultaneous use of green-SynapShot and red-SynapShot to distinguish and observe synapses with one post-terminal and different pre-terminals. >
< Figure 4. Dimer-dependent fluorescent protein (ddFP) exists as a green fluorescent protein as well as a red fluorescent protein, and can be applied together with blue light-activated optogenetic technology. After activating Tropomyosin receptor kinase B (TrkB) by blue light using optogenetic technology, the strengthening of synaptic connections through signals of brain-derived neurotrophic factor is observed using red-SynapShot. >
< Figure 5. Micrographs showing real-time changing synapses in the visual cortex of mice trained through visual training using in vivo imaging techniques such as two-photon microscopy as well as at the cellular level. >
This research was supported by Mid-Sized Research Funds and the Singularity Project from KAIST, and by IBS.
2024.01.18 View 8620 -
 KAIST Research team develops anti-icing film that only requires sunlight
A KAIST research team has developed an anti-icing and de-icing film coating technology that can apply the photothermal effect of gold nanoparticles to industrial sites without the need for heating wires, periodic spray or oil coating of anti-freeze substances, and substrate design alterations.
The group led by Professor Hyoungsoo Kim from the Department of Mechanical Engineering (Fluid & Interface Laboratory) and Professor Dong Ki Yoon from the Department of Chemistry (Soft Material Assembly Group) revealed on January 3 to have together developed an original technique that can uniformly pattern gold nanorod (GNR) particles in quadrants through simple evaporation, and have used this to develop an anti-icing and de-icing surface.
Many scientists in recent years have tried to control substrate surfaces through various coating techniques, and those involving the patterning of functional nanomaterials have gained special attention. In particular, GNR is considered a promising candidate nanomaterial for its biocompatibility, chemical stability, relatively simple synthesis, and its stable and unique property of surface plasmon resonance. To maximize the performance of GNR, it is important to achieve a high uniformity during film deposition, and a high level of rod alignment. However, achieving both criteria has thus far been a difficult challenge.
< Figure 1. Conceptual image to display Hydrodynamic mechanisms for the formation of a homogeneous quadrant cellulose nanocrystal(CNC) matrix. >
To solve this, the joint research team utilized cellulose nanocrystal (CNC), a next-generation functional nanomaterial that can easily be extracted from nature. By co-assembling GNR on CNC quadrant templates, the team could uniformly dry the film and successfully obtain a GNR film with a uniform alignment in a ring-shape. Compared to existing coffee-ring films, the highly uniform and aligned GNR film developed through this research showed enhanced plasmonic photothermal properties, and the team showed that it could carry out anti-icing and de-icing functions by simply irradiating light in the visible wavelength range.
< Figure 2. Optical and thermal performance evaluation results of gold nanorod film and demonstration of plasmonic heater for anti-icing and de-icing. >
Professor Hyoungsoo Kim said, “This technique can be applied to plastic, as well as flexible surfaces. By using it on exterior materials and films, it can generate its own heat energy, which would greatly save energy through voluntary thermal energy harvesting across various applications including cars, aircrafts, and windows in residential or commercial spaces, where frosting becomes a serious issue in the winter.” Professor Dong Ki Yoon added, “This research is significant in that we can now freely pattern the CNC-GNR composite, which was previously difficult to create into films, over a large area. We can utilize this as an anti-icing material, and if we were to take advantage of the plasmonic properties of gold, we can also use it like stained-glass to decorate glass surfaces.”
This research was conducted by Ph.D. candidate Jeongsu Pyeon from the Department of Mechanical Engineering, and his co-first author Dr. Soon Mo Park (a KAIST graduate, currently a post-doctoral associate at Cornell University), and was pushed in the online volume of Nature Communication on December 8, 2023 under the title “Plasmonic Metasurfaces of Cellulose Nanocrystal Matrices with Quadrants of Aligned Gold Nanorods for Photothermal Anti-Icing." Recognized for its achievement, the research was also selected as an editor’s highlight for the journals Materials Science and Chemistry, and Inorganic and Physical Chemistry.
This research was supported by the Individual Basic Mid-Sized Research Fund from the National Research Foundation of Korea and the Center for Multiscale Chiral Architectures.
2024.01.16 View 12878
KAIST Research team develops anti-icing film that only requires sunlight
A KAIST research team has developed an anti-icing and de-icing film coating technology that can apply the photothermal effect of gold nanoparticles to industrial sites without the need for heating wires, periodic spray or oil coating of anti-freeze substances, and substrate design alterations.
The group led by Professor Hyoungsoo Kim from the Department of Mechanical Engineering (Fluid & Interface Laboratory) and Professor Dong Ki Yoon from the Department of Chemistry (Soft Material Assembly Group) revealed on January 3 to have together developed an original technique that can uniformly pattern gold nanorod (GNR) particles in quadrants through simple evaporation, and have used this to develop an anti-icing and de-icing surface.
Many scientists in recent years have tried to control substrate surfaces through various coating techniques, and those involving the patterning of functional nanomaterials have gained special attention. In particular, GNR is considered a promising candidate nanomaterial for its biocompatibility, chemical stability, relatively simple synthesis, and its stable and unique property of surface plasmon resonance. To maximize the performance of GNR, it is important to achieve a high uniformity during film deposition, and a high level of rod alignment. However, achieving both criteria has thus far been a difficult challenge.
< Figure 1. Conceptual image to display Hydrodynamic mechanisms for the formation of a homogeneous quadrant cellulose nanocrystal(CNC) matrix. >
To solve this, the joint research team utilized cellulose nanocrystal (CNC), a next-generation functional nanomaterial that can easily be extracted from nature. By co-assembling GNR on CNC quadrant templates, the team could uniformly dry the film and successfully obtain a GNR film with a uniform alignment in a ring-shape. Compared to existing coffee-ring films, the highly uniform and aligned GNR film developed through this research showed enhanced plasmonic photothermal properties, and the team showed that it could carry out anti-icing and de-icing functions by simply irradiating light in the visible wavelength range.
< Figure 2. Optical and thermal performance evaluation results of gold nanorod film and demonstration of plasmonic heater for anti-icing and de-icing. >
Professor Hyoungsoo Kim said, “This technique can be applied to plastic, as well as flexible surfaces. By using it on exterior materials and films, it can generate its own heat energy, which would greatly save energy through voluntary thermal energy harvesting across various applications including cars, aircrafts, and windows in residential or commercial spaces, where frosting becomes a serious issue in the winter.” Professor Dong Ki Yoon added, “This research is significant in that we can now freely pattern the CNC-GNR composite, which was previously difficult to create into films, over a large area. We can utilize this as an anti-icing material, and if we were to take advantage of the plasmonic properties of gold, we can also use it like stained-glass to decorate glass surfaces.”
This research was conducted by Ph.D. candidate Jeongsu Pyeon from the Department of Mechanical Engineering, and his co-first author Dr. Soon Mo Park (a KAIST graduate, currently a post-doctoral associate at Cornell University), and was pushed in the online volume of Nature Communication on December 8, 2023 under the title “Plasmonic Metasurfaces of Cellulose Nanocrystal Matrices with Quadrants of Aligned Gold Nanorods for Photothermal Anti-Icing." Recognized for its achievement, the research was also selected as an editor’s highlight for the journals Materials Science and Chemistry, and Inorganic and Physical Chemistry.
This research was supported by the Individual Basic Mid-Sized Research Fund from the National Research Foundation of Korea and the Center for Multiscale Chiral Architectures.
2024.01.16 View 12878 -
 KAIST develops an artificial muscle device that produces force 34 times its weight
- Professor IlKwon Oh’s research team in KAIST’s Department of Mechanical Engineering developed a soft fluidic switch using an ionic polymer artificial muscle that runs with ultra-low power to lift objects 34 times greater than its weight.
- Its light weight and small size make it applicable to various industrial fields such as soft electronics, smart textiles, and biomedical devices by controlling fluid flow with high precision, even in narrow spaces.
Soft robots, medical devices, and wearable devices have permeated our daily lives. KAIST researchers have developed a fluid switch using ionic polymer artificial muscles that operates at ultra-low power and produces a force 34 times greater than its weight. Fluid switches control fluid flow, causing the fluid to flow in a specific direction to invoke various movements.
KAIST (President Kwang-Hyung Lee) announced on the 4th of January that a research team under Professor IlKwon Oh from the Department of Mechanical Engineering has developed a soft fluidic switch that operates at ultra-low voltage and can be used in narrow spaces.
Artificial muscles imitate human muscles and provide flexible and natural movements compared to traditional motors, making them one of the basic elements used in soft robots, medical devices, and wearable devices. These artificial muscles create movements in response to external stimuli such as electricity, air pressure, and temperature changes, and in order to utilize artificial muscles, it is important to control these movements precisely.
Switches based on existing motors were difficult to use within limited spaces due to their rigidity and large size. In order to address these issues, the research team developed an electro-ionic soft actuator that can control fluid flow while producing large amounts of force, even in a narrow pipe, and used it as a soft fluidic switch.
< Figure 1. The separation of fluid droplets using a soft fluid switch at ultra-low voltage. >
The ionic polymer artificial muscle developed by the research team is composed of metal electrodes and ionic polymers, and it generates force and movement in response to electricity. A polysulfonated covalent organic framework (pS-COF) made by combining organic molecules on the surface of the artificial muscle electrode was used to generate an impressive amount of force relative to its weight with ultra-low power (~0.01V).
As a result, the artificial muscle, which was manufactured to be as thin as a hair with a thickness of 180 µm, produced a force more than 34 times greater than its light weight of 10 mg to initiate smooth movement. Through this, the research team was able to precisely control the direction of fluid flow with low power.
< Figure 2. The synthesis and use of pS-COF as a common electrode-electrolyte host for electroactive soft fluid switches. A) The synthesis schematic of pS-COF. B) The schematic diagram of the operating principle of the electrochemical soft switch. C) The schematic diagram of using a pS-COF-based electrochemical soft switch to control fluid flow in dynamic operation. >
Professor IlKwon Oh, who led this research, said, “The electrochemical soft fluidic switch that operate at ultra-low power can open up many possibilities in the fields of soft robots, soft electronics, and microfluidics based on fluid control.” He added, “From smart fibers to biomedical devices, this technology has the potential to be immediately put to use in a variety of industrial settings as it can be easily applied to ultra-small electronic systems in our daily lives.”
The results of this study, in which Dr. Manmatha Mahato, a research professor in the Department of Mechanical Engineering at KAIST, participated as the first author, were published in the international academic journal Science Advances on December 13, 2023. (Paper title: Polysulfonated Covalent Organic Framework as Active Electrode Host for Mobile Cation Guests in Electrochemical Soft Actuator)
This research was conducted with support from the National Research Foundation of Korea's Leader Scientist Support Project (Creative Research Group) and Future Convergence Pioneer Project.
* Paper DOI: https://www.science.org/doi/abs/10.1126/sciadv.adk9752
2024.01.11 View 12547
KAIST develops an artificial muscle device that produces force 34 times its weight
- Professor IlKwon Oh’s research team in KAIST’s Department of Mechanical Engineering developed a soft fluidic switch using an ionic polymer artificial muscle that runs with ultra-low power to lift objects 34 times greater than its weight.
- Its light weight and small size make it applicable to various industrial fields such as soft electronics, smart textiles, and biomedical devices by controlling fluid flow with high precision, even in narrow spaces.
Soft robots, medical devices, and wearable devices have permeated our daily lives. KAIST researchers have developed a fluid switch using ionic polymer artificial muscles that operates at ultra-low power and produces a force 34 times greater than its weight. Fluid switches control fluid flow, causing the fluid to flow in a specific direction to invoke various movements.
KAIST (President Kwang-Hyung Lee) announced on the 4th of January that a research team under Professor IlKwon Oh from the Department of Mechanical Engineering has developed a soft fluidic switch that operates at ultra-low voltage and can be used in narrow spaces.
Artificial muscles imitate human muscles and provide flexible and natural movements compared to traditional motors, making them one of the basic elements used in soft robots, medical devices, and wearable devices. These artificial muscles create movements in response to external stimuli such as electricity, air pressure, and temperature changes, and in order to utilize artificial muscles, it is important to control these movements precisely.
Switches based on existing motors were difficult to use within limited spaces due to their rigidity and large size. In order to address these issues, the research team developed an electro-ionic soft actuator that can control fluid flow while producing large amounts of force, even in a narrow pipe, and used it as a soft fluidic switch.
< Figure 1. The separation of fluid droplets using a soft fluid switch at ultra-low voltage. >
The ionic polymer artificial muscle developed by the research team is composed of metal electrodes and ionic polymers, and it generates force and movement in response to electricity. A polysulfonated covalent organic framework (pS-COF) made by combining organic molecules on the surface of the artificial muscle electrode was used to generate an impressive amount of force relative to its weight with ultra-low power (~0.01V).
As a result, the artificial muscle, which was manufactured to be as thin as a hair with a thickness of 180 µm, produced a force more than 34 times greater than its light weight of 10 mg to initiate smooth movement. Through this, the research team was able to precisely control the direction of fluid flow with low power.
< Figure 2. The synthesis and use of pS-COF as a common electrode-electrolyte host for electroactive soft fluid switches. A) The synthesis schematic of pS-COF. B) The schematic diagram of the operating principle of the electrochemical soft switch. C) The schematic diagram of using a pS-COF-based electrochemical soft switch to control fluid flow in dynamic operation. >
Professor IlKwon Oh, who led this research, said, “The electrochemical soft fluidic switch that operate at ultra-low power can open up many possibilities in the fields of soft robots, soft electronics, and microfluidics based on fluid control.” He added, “From smart fibers to biomedical devices, this technology has the potential to be immediately put to use in a variety of industrial settings as it can be easily applied to ultra-small electronic systems in our daily lives.”
The results of this study, in which Dr. Manmatha Mahato, a research professor in the Department of Mechanical Engineering at KAIST, participated as the first author, were published in the international academic journal Science Advances on December 13, 2023. (Paper title: Polysulfonated Covalent Organic Framework as Active Electrode Host for Mobile Cation Guests in Electrochemical Soft Actuator)
This research was conducted with support from the National Research Foundation of Korea's Leader Scientist Support Project (Creative Research Group) and Future Convergence Pioneer Project.
* Paper DOI: https://www.science.org/doi/abs/10.1126/sciadv.adk9752
2024.01.11 View 12547 -
 A KAIST Research Team Develops High-Performance Stretchable Solar Cells
With the market for wearable electric devices growing rapidly, stretchable solar cells that can function under strain have received considerable attention as an energy source. To build such solar cells, it is necessary that their photoactive layer, which converts light into electricity, shows high electrical performance while possessing mechanical elasticity. However, satisfying both of these two requirements is challenging, making stretchable solar cells difficult to develop.
On December 26, a KAIST research team from the Department of Chemical and Biomolecular Engineering (CBE) led by Professor Bumjoon Kim announced the development of a new conductive polymer material that achieved both high electrical performance and elasticity while introducing the world’s highest-performing stretchable organic solar cell.
Organic solar cells are devices whose photoactive layer, which is responsible for the conversion of light into electricity, is composed of organic materials. Compared to existing non-organic material-based solar cells, they are lighter and flexible, making them highly applicable for wearable electrical devices. Solar cells as an energy source are particularly important for building electrical devices, but high-efficiency solar cells often lack flexibility, and their application in wearable devices have therefore been limited to this point.
The team led by Professor Kim conjugated a highly stretchable polymer to an electrically conductive polymer with excellent electrical properties through chemical bonding, and developed a new conductive polymer with both electrical conductivity and mechanical stretchability. This polymer meets the highest reported level of photovoltaic conversion efficiency (19%) using organic solar cells, while also showing 10 times the stretchability of existing devices. The team thereby built the world’s highest performing stretchable solar cell that can be stretched up to 40% during operation, and demonstrated its applicability for wearable devices.
< Figure 1. Chemical structure of the newly developed conductive polymer and performance of stretchable organic solar cells using the material. >
Professor Kim said, “Through this research, we not only developed the world’s best performing stretchable organic solar cell, but it is also significant that we developed a new polymer that can be applicable as a base material for various electronic devices that needs to be malleable and/or elastic.”
< Figure 2. Photovoltaic efficiency and mechanical stretchability of newly developed polymers compared to existing polymers. >
This research, conducted by KAIST researchers Jin-Woo Lee and Heung-Goo Lee as first co-authors in cooperation with teams led by Professor Taek-Soo Kim from the Department of Mechanical Engineering and Professor Sheng Li from the Department of CBE, was published in Joule on December 1 (Paper Title: Rigid and Soft Block-Copolymerized Conjugated Polymers Enable High-Performance Intrinsically-Stretchable Organic Solar Cells).
This research was supported by the National Research Foundation of Korea.
2024.01.04 View 10596
A KAIST Research Team Develops High-Performance Stretchable Solar Cells
With the market for wearable electric devices growing rapidly, stretchable solar cells that can function under strain have received considerable attention as an energy source. To build such solar cells, it is necessary that their photoactive layer, which converts light into electricity, shows high electrical performance while possessing mechanical elasticity. However, satisfying both of these two requirements is challenging, making stretchable solar cells difficult to develop.
On December 26, a KAIST research team from the Department of Chemical and Biomolecular Engineering (CBE) led by Professor Bumjoon Kim announced the development of a new conductive polymer material that achieved both high electrical performance and elasticity while introducing the world’s highest-performing stretchable organic solar cell.
Organic solar cells are devices whose photoactive layer, which is responsible for the conversion of light into electricity, is composed of organic materials. Compared to existing non-organic material-based solar cells, they are lighter and flexible, making them highly applicable for wearable electrical devices. Solar cells as an energy source are particularly important for building electrical devices, but high-efficiency solar cells often lack flexibility, and their application in wearable devices have therefore been limited to this point.
The team led by Professor Kim conjugated a highly stretchable polymer to an electrically conductive polymer with excellent electrical properties through chemical bonding, and developed a new conductive polymer with both electrical conductivity and mechanical stretchability. This polymer meets the highest reported level of photovoltaic conversion efficiency (19%) using organic solar cells, while also showing 10 times the stretchability of existing devices. The team thereby built the world’s highest performing stretchable solar cell that can be stretched up to 40% during operation, and demonstrated its applicability for wearable devices.
< Figure 1. Chemical structure of the newly developed conductive polymer and performance of stretchable organic solar cells using the material. >
Professor Kim said, “Through this research, we not only developed the world’s best performing stretchable organic solar cell, but it is also significant that we developed a new polymer that can be applicable as a base material for various electronic devices that needs to be malleable and/or elastic.”
< Figure 2. Photovoltaic efficiency and mechanical stretchability of newly developed polymers compared to existing polymers. >
This research, conducted by KAIST researchers Jin-Woo Lee and Heung-Goo Lee as first co-authors in cooperation with teams led by Professor Taek-Soo Kim from the Department of Mechanical Engineering and Professor Sheng Li from the Department of CBE, was published in Joule on December 1 (Paper Title: Rigid and Soft Block-Copolymerized Conjugated Polymers Enable High-Performance Intrinsically-Stretchable Organic Solar Cells).
This research was supported by the National Research Foundation of Korea.
2024.01.04 View 10596 -
 KAIST presents strategies for environmentally friendly and sustainable polyamides production
- Provides current research trends in bio-based polyamide production
- Research on bio-based polyamides production gains importance for achieving a carbon-neutral society
Global industries focused on carbon neutrality, under the slogan "Net-Zero," are gaining increasing attention. In particular, research on microbial production of polymers, replacing traditional chemical methods with biological approaches, is actively progressing.
Polyamides, represented by nylon, are linear polymers widely used in various industries such as automotive, electronics, textiles, and medical fields. They possess beneficial properties such as high tensile strength, electrical insulation, heat resistance, wear resistance, and biocompatibility. Since the commercialization of nylon in 1938, approximately 7 million tons of polyamides are produced worldwide annually. Considering their broad applications and significance, producing polyamides through bio-based methods holds considerable environmental and industrial importance.
KAIST (President Kwang-Hyung Lee) announced that a research team led by Distinguished Professor Sang Yup Lee, including Dr. Jong An Lee and doctoral candidate Ji Yeon Kim from the Department of Chemical and Biomolecular Engineering, published a paper titled "Current Advancements in Bio-Based Production of Polyamides”. The paper was featured on the cover of the monthly issue of "Trends in Chemistry” by Cell Press.
As part of climate change response technologies, bio-refineries involve using biotechnological and chemical methods to produce industrially important chemicals and biofuels from renewable biomass without relying on fossil resources. Notably, systems metabolic engineering, pioneered by KAIST's Distinguished Professor Sang Yup Lee, is a research field that effectively manipulates microbial metabolic pathways to produce valuable chemicals, forming the core technology for bio-refineries. The research team has successfully developed high-performance strains producing a variety of compounds, including succinic acid, biodegradable plastics, biofuels, and natural products, using systems metabolic engineering tools and strategies.
The research team predicted that if bio-based polyamide production technology, which is widely used in the production of clothing and textiles, becomes widespread, it will attract attention as a future technology that can respond to the climate crisis due to its environment-friendly production technology. In this study, the research team comprehensively reviewed the bio-based polyamide production strategies. They provided insights into the advancements in polyamide monomer production using metabolically engineered microorganisms and highlighted the recent trends in bio-based polyamide advancements utilizing these monomers. Additionally, they reviewed the strategies for synthesizing bio-based polyamides through chemical conversion of natural oils and discussed the biodegradability and recycling of the polyamides. Furthermore, the paper presented the future direction in which metabolic engineering can be applied for the bio-based polyamide production, contributing to environmentally friendly and sustainable society.
Ji Yeon Kim, the co-first author of this paper from KAIST, stated "The importance of utilizing systems metabolic engineering tools and strategies for bio-based polyamides production is becoming increasingly prominent in achieving carbon neutrality."
Professor Sang Yup Lee emphasized, "Amid growing concerns about climate change, the significance of environmentally friendly and sustainable industrial development is greater than ever. Systems metabolic engineering is expected to have a significant impact not only on the chemical industry but also in various fields."
< [Figure 1] A schematic overview of the overall process for polyamides production >
This paper by Dr. Jong An Lee, PhD student Ji Yeon Kim, Dr. Jung Ho Ahn, and Master Yeah-Ji Ahn from the Department of Chemical and Biomolecular Engineering at KAIST was published in the December issue of 'Trends in Chemistry', an authoritative review journal in the field of chemistry published by Cell. It was published on December 7 as the cover paper and featured review.
※ Paper title: Current advancements in the bio-based production of polyamides
※ Author information: Jong An Lee, Ji Yeon Kim, Jung Ho Ahn, Yeah-Ji Ahn, and Sang Yup Lee
This research was conducted with the support from the development of platform technologies of microbial cell factories for the next-generation biorefineries project and C1 gas refinery program by Korean Ministry of Science and ICT.
< [Figure 2] Cover paper of the December issue of Trends in Chemistry >
2023.12.21 View 8640
KAIST presents strategies for environmentally friendly and sustainable polyamides production
- Provides current research trends in bio-based polyamide production
- Research on bio-based polyamides production gains importance for achieving a carbon-neutral society
Global industries focused on carbon neutrality, under the slogan "Net-Zero," are gaining increasing attention. In particular, research on microbial production of polymers, replacing traditional chemical methods with biological approaches, is actively progressing.
Polyamides, represented by nylon, are linear polymers widely used in various industries such as automotive, electronics, textiles, and medical fields. They possess beneficial properties such as high tensile strength, electrical insulation, heat resistance, wear resistance, and biocompatibility. Since the commercialization of nylon in 1938, approximately 7 million tons of polyamides are produced worldwide annually. Considering their broad applications and significance, producing polyamides through bio-based methods holds considerable environmental and industrial importance.
KAIST (President Kwang-Hyung Lee) announced that a research team led by Distinguished Professor Sang Yup Lee, including Dr. Jong An Lee and doctoral candidate Ji Yeon Kim from the Department of Chemical and Biomolecular Engineering, published a paper titled "Current Advancements in Bio-Based Production of Polyamides”. The paper was featured on the cover of the monthly issue of "Trends in Chemistry” by Cell Press.
As part of climate change response technologies, bio-refineries involve using biotechnological and chemical methods to produce industrially important chemicals and biofuels from renewable biomass without relying on fossil resources. Notably, systems metabolic engineering, pioneered by KAIST's Distinguished Professor Sang Yup Lee, is a research field that effectively manipulates microbial metabolic pathways to produce valuable chemicals, forming the core technology for bio-refineries. The research team has successfully developed high-performance strains producing a variety of compounds, including succinic acid, biodegradable plastics, biofuels, and natural products, using systems metabolic engineering tools and strategies.
The research team predicted that if bio-based polyamide production technology, which is widely used in the production of clothing and textiles, becomes widespread, it will attract attention as a future technology that can respond to the climate crisis due to its environment-friendly production technology. In this study, the research team comprehensively reviewed the bio-based polyamide production strategies. They provided insights into the advancements in polyamide monomer production using metabolically engineered microorganisms and highlighted the recent trends in bio-based polyamide advancements utilizing these monomers. Additionally, they reviewed the strategies for synthesizing bio-based polyamides through chemical conversion of natural oils and discussed the biodegradability and recycling of the polyamides. Furthermore, the paper presented the future direction in which metabolic engineering can be applied for the bio-based polyamide production, contributing to environmentally friendly and sustainable society.
Ji Yeon Kim, the co-first author of this paper from KAIST, stated "The importance of utilizing systems metabolic engineering tools and strategies for bio-based polyamides production is becoming increasingly prominent in achieving carbon neutrality."
Professor Sang Yup Lee emphasized, "Amid growing concerns about climate change, the significance of environmentally friendly and sustainable industrial development is greater than ever. Systems metabolic engineering is expected to have a significant impact not only on the chemical industry but also in various fields."
< [Figure 1] A schematic overview of the overall process for polyamides production >
This paper by Dr. Jong An Lee, PhD student Ji Yeon Kim, Dr. Jung Ho Ahn, and Master Yeah-Ji Ahn from the Department of Chemical and Biomolecular Engineering at KAIST was published in the December issue of 'Trends in Chemistry', an authoritative review journal in the field of chemistry published by Cell. It was published on December 7 as the cover paper and featured review.
※ Paper title: Current advancements in the bio-based production of polyamides
※ Author information: Jong An Lee, Ji Yeon Kim, Jung Ho Ahn, Yeah-Ji Ahn, and Sang Yup Lee
This research was conducted with the support from the development of platform technologies of microbial cell factories for the next-generation biorefineries project and C1 gas refinery program by Korean Ministry of Science and ICT.
< [Figure 2] Cover paper of the December issue of Trends in Chemistry >
2023.12.21 View 8640 -
 A KAIST Team Develops Selective Transfer Printing Technology for MicroLEDs
- A KAIST research team led by Professor Keon Jae Lee demonstrates the transfer printing of a large number of micro-sized inorganic semiconductor chips via the selective modulation of micro-vacuum force.
MicroLEDs are a light source for next-generation displays that utilize inorganic LED chips with a size of less than 100 μm. MicroLEDs have attracted a great deal of attention due to their superior electrical/optical properties, reliability, and stability compared to conventional displays such as LCD, OLED, and QD. To commercialize microLEDs, transfer printing technology is essential for rearranging microLED dies from a growth substrate onto the final substrate with a desired layout and precise alignment. However, previous transfer methods still have many challenges such as the need for additional adhesives, misalignment, low transfer yield, and chip damage.
Professor Lee’s research team has developed a micro-vacuum assisted selective transfer printing (µVAST) technology to transfer a large number of microLED chips by adjusting the micro-vacuum suction force.
The key technology relies on a laser-induced etching (LIE) method for forming 20 μm-sized micro-hole arrays with a high aspect ratio on glass substrates at fabrication speed of up to 7,000 holes per second. The LIE-drilled glass is connected to the vacuum channels, controlling the micro-vacuum force at desired hole arrays to selectively pick up and release the microLEDs. The micro-vacuum assisted transfer printing accomplishes a higher adhesion switchability compared to previous transfer methods, enabling the assembly of micro-sized semiconductors with various heterogeneous materials, sizes, shapes, and thicknesses onto arbitrary substrates with high transfer yields.
< Figure 01. Concept of micro-vacuum assisted selective transfer printing (μVAST). >
Professor Keon Jae Lee said, “The micro-vacuum assisted transfer provides an interesting tool for large-scale, selective integration of microscale high-performance inorganic semiconductors. Currently, we are investigating the transfer printing of commercial microLED chips with an ejector system for commercializing next-generation displays (Large screen TVs, flexible/stretchable devices) and wearable phototherapy patches.”
This result titled “Universal selective transfer printing via micro-vacuum force” was published in Nature Communications on November 26th, 2023. (DOI: 10.1038/S41467-023-43342-8)
< Figure 02. Universal transfer printing of thin-film semiconductors via μVAST. >
< Figure 03. Flexible devices fabricated by μVAST. >
Title: Entire process including LIE and µVAST
Vimeo link: https://vimeo.com/894430416?share=copy
2023.12.19 View 5348
A KAIST Team Develops Selective Transfer Printing Technology for MicroLEDs
- A KAIST research team led by Professor Keon Jae Lee demonstrates the transfer printing of a large number of micro-sized inorganic semiconductor chips via the selective modulation of micro-vacuum force.
MicroLEDs are a light source for next-generation displays that utilize inorganic LED chips with a size of less than 100 μm. MicroLEDs have attracted a great deal of attention due to their superior electrical/optical properties, reliability, and stability compared to conventional displays such as LCD, OLED, and QD. To commercialize microLEDs, transfer printing technology is essential for rearranging microLED dies from a growth substrate onto the final substrate with a desired layout and precise alignment. However, previous transfer methods still have many challenges such as the need for additional adhesives, misalignment, low transfer yield, and chip damage.
Professor Lee’s research team has developed a micro-vacuum assisted selective transfer printing (µVAST) technology to transfer a large number of microLED chips by adjusting the micro-vacuum suction force.
The key technology relies on a laser-induced etching (LIE) method for forming 20 μm-sized micro-hole arrays with a high aspect ratio on glass substrates at fabrication speed of up to 7,000 holes per second. The LIE-drilled glass is connected to the vacuum channels, controlling the micro-vacuum force at desired hole arrays to selectively pick up and release the microLEDs. The micro-vacuum assisted transfer printing accomplishes a higher adhesion switchability compared to previous transfer methods, enabling the assembly of micro-sized semiconductors with various heterogeneous materials, sizes, shapes, and thicknesses onto arbitrary substrates with high transfer yields.
< Figure 01. Concept of micro-vacuum assisted selective transfer printing (μVAST). >
Professor Keon Jae Lee said, “The micro-vacuum assisted transfer provides an interesting tool for large-scale, selective integration of microscale high-performance inorganic semiconductors. Currently, we are investigating the transfer printing of commercial microLED chips with an ejector system for commercializing next-generation displays (Large screen TVs, flexible/stretchable devices) and wearable phototherapy patches.”
This result titled “Universal selective transfer printing via micro-vacuum force” was published in Nature Communications on November 26th, 2023. (DOI: 10.1038/S41467-023-43342-8)
< Figure 02. Universal transfer printing of thin-film semiconductors via μVAST. >
< Figure 03. Flexible devices fabricated by μVAST. >
Title: Entire process including LIE and µVAST
Vimeo link: https://vimeo.com/894430416?share=copy
2023.12.19 View 5348 -
 KAIST introduces eco-friendly technologies for plastic production and biodegradation
- A research team under Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering published a paper in Nature Microbiology on the overview and trends of plastic production and degradation technology using microorganisms.
- Eco-friendly and sustainable plastic production and degradation technology using microorganisms as a core technology to achieve a plastic circular economy was presented.
Plastic is one of the important materials in modern society, with approximately 460 million tons produced annually and with expected production reaching approximately 1.23 billion tons in 2060. However, since 1950, plastic waste totaling more than 6.3 billion tons has been generated, and it is believed that more than 140 million tons of plastic waste has accumulated in the aquatic environment. Recently, the seriousness of microplastic pollution has emerged, not only posing a risk to the marine ecosystem and human health, but also worsening global warming by inhibiting the activity of marine plankton, which play an important role in lowering the Earth's carbon dioxide concentration.
KAIST President Kwang-Hyung Lee announced on December 11 that a research team under Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering had published a paper titled 'Sustainable production and degradation of plastics using microbes', which covers the latest technologies for producing plastics and processing waste plastics in an eco-friendly manner using microorganisms.
As the international community moves to solve this plastic problem, various efforts are being made, including 175 countries participating to conclude a legally binding agreement with the goal of ending plastic pollution by 2024. Various technologies are being developed for sustainable plastic production and processing, and among them, biotechnology using microorganisms is attracting attention.
Microorganisms have the ability to naturally produce or decompose certain compounds, and this ability is maximized through biotechnologies such as metabolic engineering and enzyme engineering to produce plastics from renewable biomass resources instead of fossil raw materials and to decompose waste plastics.
Accordingly, the research team comprehensively analyzed the latest microorganism-based technologies for the sustainable production and decomposition of plastics and presented how they actually contribute to solving the plastic problem. Based on this, they presented limitations, prospects, and research directions of the technologies for achieving a circular economy for plastics.
Microorganism-based technologies for various plastics range from widely used synthetic plastics such as polyethylene (PE) to promising bioplastics such as natural polymers derived from microorganisms (polyhydroxyalkanoate (PHA)) that are completely biodegradable in the natural environment and do not pose a risk of microplastic generation. Commercialization statuses and latest technologies were also discussed. In addition, the technology to decompose these plastics using microorganisms and their enzymes and the upcycling technology to convert them into other useful compounds after decomposition were introduced, highlighting the competitiveness and potential of technology using microorganisms.
First author So Young Choi, a research assistant professor in the Department of Chemical and Biomolecular Engineering at KAIST, said, “In the future, we will be able to easily find eco-friendly plastics made using microorganisms all around us,” and corresponding author Distinguished Professor Sang Yup Lee said, “Plastic can be made more sustainable. It is important to use plastics responsibly to protect the environment and simultaneously achieve economic and social development through the new plastics industry, and we look forward to the improved performance of microbial metabolic engineering technology.”
This paper was published on November 30th in the online edition of Nature Microbiology.
※ Paper Title : Sustainable production and degradation of plastics using microbes
Authors: So Young Choi, Youngjoon Lee, Hye Eun Yu, In Jin Cho, Minju Kang & Sang Yup Lee
2023.12.11 View 8416
KAIST introduces eco-friendly technologies for plastic production and biodegradation
- A research team under Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering published a paper in Nature Microbiology on the overview and trends of plastic production and degradation technology using microorganisms.
- Eco-friendly and sustainable plastic production and degradation technology using microorganisms as a core technology to achieve a plastic circular economy was presented.
Plastic is one of the important materials in modern society, with approximately 460 million tons produced annually and with expected production reaching approximately 1.23 billion tons in 2060. However, since 1950, plastic waste totaling more than 6.3 billion tons has been generated, and it is believed that more than 140 million tons of plastic waste has accumulated in the aquatic environment. Recently, the seriousness of microplastic pollution has emerged, not only posing a risk to the marine ecosystem and human health, but also worsening global warming by inhibiting the activity of marine plankton, which play an important role in lowering the Earth's carbon dioxide concentration.
KAIST President Kwang-Hyung Lee announced on December 11 that a research team under Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering had published a paper titled 'Sustainable production and degradation of plastics using microbes', which covers the latest technologies for producing plastics and processing waste plastics in an eco-friendly manner using microorganisms.
As the international community moves to solve this plastic problem, various efforts are being made, including 175 countries participating to conclude a legally binding agreement with the goal of ending plastic pollution by 2024. Various technologies are being developed for sustainable plastic production and processing, and among them, biotechnology using microorganisms is attracting attention.
Microorganisms have the ability to naturally produce or decompose certain compounds, and this ability is maximized through biotechnologies such as metabolic engineering and enzyme engineering to produce plastics from renewable biomass resources instead of fossil raw materials and to decompose waste plastics.
Accordingly, the research team comprehensively analyzed the latest microorganism-based technologies for the sustainable production and decomposition of plastics and presented how they actually contribute to solving the plastic problem. Based on this, they presented limitations, prospects, and research directions of the technologies for achieving a circular economy for plastics.
Microorganism-based technologies for various plastics range from widely used synthetic plastics such as polyethylene (PE) to promising bioplastics such as natural polymers derived from microorganisms (polyhydroxyalkanoate (PHA)) that are completely biodegradable in the natural environment and do not pose a risk of microplastic generation. Commercialization statuses and latest technologies were also discussed. In addition, the technology to decompose these plastics using microorganisms and their enzymes and the upcycling technology to convert them into other useful compounds after decomposition were introduced, highlighting the competitiveness and potential of technology using microorganisms.
First author So Young Choi, a research assistant professor in the Department of Chemical and Biomolecular Engineering at KAIST, said, “In the future, we will be able to easily find eco-friendly plastics made using microorganisms all around us,” and corresponding author Distinguished Professor Sang Yup Lee said, “Plastic can be made more sustainable. It is important to use plastics responsibly to protect the environment and simultaneously achieve economic and social development through the new plastics industry, and we look forward to the improved performance of microbial metabolic engineering technology.”
This paper was published on November 30th in the online edition of Nature Microbiology.
※ Paper Title : Sustainable production and degradation of plastics using microbes
Authors: So Young Choi, Youngjoon Lee, Hye Eun Yu, In Jin Cho, Minju Kang & Sang Yup Lee
2023.12.11 View 8416 -
 KAIST-UCSD researchers build an enzyme discovering AI
- A joint research team led by Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering and Bernhard Palsson of UCSD developed ‘DeepECtransformer’, an artificial intelligence that can predict Enzyme Commission (EC) number of proteins.
- The AI is tasked to discover new enzymes that have not been discovered yet, which would allow prediction for a total of 5,360 types of Enzyme Commission (EC) numbers
- It is expected to be used in the development of microbial cell factories that produce environmentally friendly chemicals as a core technology for analyzing the metabolic network of a genome.
While E. coli is one of the most studied organisms, the function of 30% of proteins that make up E. coli has not yet been clearly revealed. For this, an artificial intelligence was used to discover 464 types of enzymes from the proteins that were unknown, and the researchers went on to verify the predictions of 3 types of proteins were successfully identified through in vitro enzyme assay.
KAIST (President Kwang-Hyung Lee) announced on the 24th that a joint research team comprised of Gi Bae Kim, Ji Yeon Kim, Dr. Jong An Lee and Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering at KAIST, and Dr. Charles J. Norsigian and Professor Bernhard O. Palsson of the Department of Bioengineering at UCSD has developed DeepECtransformer, an artificial intelligence that can predict the enzyme functions from the protein sequence, and has established a prediction system by utilizing the AI to quickly and accurately identify the enzyme function.
Enzymes are proteins that catalyze biological reactions, and identifying the function of each enzyme is essential to understanding the various chemical reactions that exist in living organisms and the metabolic characteristics of those organisms. Enzyme Commission (EC) number is an enzyme function classification system designed by the International Union of Biochemistry and Molecular Biology, and in order to understand the metabolic characteristics of various organisms, it is necessary to develop a technology that can quickly analyze enzymes and EC numbers of the enzymes present in the genome.
Various methodologies based on deep learning have been developed to analyze the features of biological sequences, including protein function prediction, but most of them have a problem of a black box, where the inference process of AI cannot be interpreted. Various prediction systems that utilize AI for enzyme function prediction have also been reported, but they do not solve this black box problem, or cannot interpret the reasoning process in fine-grained level (e.g., the level of amino acid residues in the enzyme sequence).
The joint team developed DeepECtransformer, an AI that utilizes deep learning and a protein homology analysis module to predict the enzyme function of a given protein sequence. To better understand the features of protein sequences, the transformer architecture, which is commonly used in natural language processing, was additionally used to extract important features about enzyme functions in the context of the entire protein sequence, which enabled the team to accurately predict the EC number of the enzyme. The developed DeepECtransformer can predict a total of 5360 EC numbers.
The joint team further analyzed the transformer architecture to understand the inference process of DeepECtransformer, and found that in the inference process, the AI utilizes information on catalytic active sites and/or the cofactor binding sites which are important for enzyme function. By analyzing the black box of DeepECtransformer, it was confirmed that the AI was able to identify the features that are important for enzyme function on its own during the learning process.
"By utilizing the prediction system we developed, we were able to predict the functions of enzymes that had not yet been identified and verify them experimentally," said Gi Bae Kim, the first author of the paper. "By using DeepECtransformer to identify previously unknown enzymes in living organisms, we will be able to more accurately analyze various facets involved in the metabolic processes of organisms, such as the enzymes needed to biosynthesize various useful compounds or the enzymes needed to biodegrade plastics." he added.
"DeepECtransformer, which quickly and accurately predicts enzyme functions, is a key technology in functional genomics, enabling us to analyze the function of entire enzymes at the systems level," said Professor Sang Yup Lee. He added, “We will be able to use it to develop eco-friendly microbial factories based on comprehensive genome-scale metabolic models, potentially minimizing missing information of metabolism.”
The joint team’s work on DeepECtransformer is described in the paper titled "Functional annotation of enzyme-encoding genes using deep learning with transformer layers" written by Gi Bae Kim, Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering of KAIST and their colleagues. The paper was published via peer-review on the 14th of November on “Nature Communications”.
This research was conducted with the support by “the Development of next-generation biorefinery platform technologies for leading bio-based chemicals industry project (2022M3J5A1056072)” and by “Development of platform technologies of microbial cell factories for the next-generation biorefineries project (2022M3J5A1056117)” from National Research Foundation supported by the Korean Ministry of Science and ICT (Project Leader: Distinguished Professor Sang Yup Lee, KAIST).
< Figure 1. The structure of DeepECtransformer's artificial neural network >
2023.11.24 View 7638
KAIST-UCSD researchers build an enzyme discovering AI
- A joint research team led by Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering and Bernhard Palsson of UCSD developed ‘DeepECtransformer’, an artificial intelligence that can predict Enzyme Commission (EC) number of proteins.
- The AI is tasked to discover new enzymes that have not been discovered yet, which would allow prediction for a total of 5,360 types of Enzyme Commission (EC) numbers
- It is expected to be used in the development of microbial cell factories that produce environmentally friendly chemicals as a core technology for analyzing the metabolic network of a genome.
While E. coli is one of the most studied organisms, the function of 30% of proteins that make up E. coli has not yet been clearly revealed. For this, an artificial intelligence was used to discover 464 types of enzymes from the proteins that were unknown, and the researchers went on to verify the predictions of 3 types of proteins were successfully identified through in vitro enzyme assay.
KAIST (President Kwang-Hyung Lee) announced on the 24th that a joint research team comprised of Gi Bae Kim, Ji Yeon Kim, Dr. Jong An Lee and Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering at KAIST, and Dr. Charles J. Norsigian and Professor Bernhard O. Palsson of the Department of Bioengineering at UCSD has developed DeepECtransformer, an artificial intelligence that can predict the enzyme functions from the protein sequence, and has established a prediction system by utilizing the AI to quickly and accurately identify the enzyme function.
Enzymes are proteins that catalyze biological reactions, and identifying the function of each enzyme is essential to understanding the various chemical reactions that exist in living organisms and the metabolic characteristics of those organisms. Enzyme Commission (EC) number is an enzyme function classification system designed by the International Union of Biochemistry and Molecular Biology, and in order to understand the metabolic characteristics of various organisms, it is necessary to develop a technology that can quickly analyze enzymes and EC numbers of the enzymes present in the genome.
Various methodologies based on deep learning have been developed to analyze the features of biological sequences, including protein function prediction, but most of them have a problem of a black box, where the inference process of AI cannot be interpreted. Various prediction systems that utilize AI for enzyme function prediction have also been reported, but they do not solve this black box problem, or cannot interpret the reasoning process in fine-grained level (e.g., the level of amino acid residues in the enzyme sequence).
The joint team developed DeepECtransformer, an AI that utilizes deep learning and a protein homology analysis module to predict the enzyme function of a given protein sequence. To better understand the features of protein sequences, the transformer architecture, which is commonly used in natural language processing, was additionally used to extract important features about enzyme functions in the context of the entire protein sequence, which enabled the team to accurately predict the EC number of the enzyme. The developed DeepECtransformer can predict a total of 5360 EC numbers.
The joint team further analyzed the transformer architecture to understand the inference process of DeepECtransformer, and found that in the inference process, the AI utilizes information on catalytic active sites and/or the cofactor binding sites which are important for enzyme function. By analyzing the black box of DeepECtransformer, it was confirmed that the AI was able to identify the features that are important for enzyme function on its own during the learning process.
"By utilizing the prediction system we developed, we were able to predict the functions of enzymes that had not yet been identified and verify them experimentally," said Gi Bae Kim, the first author of the paper. "By using DeepECtransformer to identify previously unknown enzymes in living organisms, we will be able to more accurately analyze various facets involved in the metabolic processes of organisms, such as the enzymes needed to biosynthesize various useful compounds or the enzymes needed to biodegrade plastics." he added.
"DeepECtransformer, which quickly and accurately predicts enzyme functions, is a key technology in functional genomics, enabling us to analyze the function of entire enzymes at the systems level," said Professor Sang Yup Lee. He added, “We will be able to use it to develop eco-friendly microbial factories based on comprehensive genome-scale metabolic models, potentially minimizing missing information of metabolism.”
The joint team’s work on DeepECtransformer is described in the paper titled "Functional annotation of enzyme-encoding genes using deep learning with transformer layers" written by Gi Bae Kim, Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering of KAIST and their colleagues. The paper was published via peer-review on the 14th of November on “Nature Communications”.
This research was conducted with the support by “the Development of next-generation biorefinery platform technologies for leading bio-based chemicals industry project (2022M3J5A1056072)” and by “Development of platform technologies of microbial cell factories for the next-generation biorefineries project (2022M3J5A1056117)” from National Research Foundation supported by the Korean Ministry of Science and ICT (Project Leader: Distinguished Professor Sang Yup Lee, KAIST).
< Figure 1. The structure of DeepECtransformer's artificial neural network >
2023.11.24 View 7638