Jeung+Ku+Kang
-
 KAIST Develops a Fire-risk Free Self-Powered Hydrogen Production System
KAIST researchers have developed a new hydrogen production system that overcomes the current limitations of green hydrogen production. By using a water-splitting system with an aqueous electrolyte, this system is expected to block fire risks and enable stable hydrogen production.
KAIST (represented by President Kwang Hyung Lee) announced on the 22nd of October that a research team led by Professor Jeung Ku Kang from the Department of Materials Science and Engineering developed a self-powered hydrogen production system based on a high-performance zinc-air battery*.
*Zinc-air battery: A primary battery that absorbs oxygen from the air and uses it as an oxidant. Its advantage is long life, but its low electromotive force is a disadvantage.
Hydrogen (H₂) is a key raw material for synthesizing high-value-added substances, and it is gaining attention as a clean fuel with an energy density (142 MJ/kg) more than three times higher than traditional fossil fuels (gasoline, diesel, etc.). However, most current hydrogen production methods impose environmental burden as they emit carbon dioxide (CO₂).
While green hydrogen can be produced by splitting water using renewable energy sources such as solar cells and wind power, these sources are subject to irregular power generation due to weather and temperature fluctuations, leading to low water-splitting efficiency.
To overcome this, air batteries that can emit sufficient voltage (greater than 1.23V) for water splitting have been gaining attention. However, achieving sufficient capacity requires expensive precious metal catalysts and the performance of the catalyst materials becomes significantly degraded during prolonged charge and discharge cycles. Thus, it is essential to develop catalysts that are effective for the water-splitting reactions (oxygen and hydrogen evolution) and materials that can stabilize the repeated charge and discharge reactions (oxygen reduction and evolution) in zinc-air battery electrodes.
In response, Professor Kang's research team proposed a method to synthesize a non-precious metal catalyst material (G-SHELL) that is effective for three different catalytic reactions (oxygen evolution, hydrogen evolution, and oxygen reduction) by growing nano-sized, metal-organic frameworks on graphene oxide.
The team incorporated the developed catalyst material into the air cathode of a zinc-air battery, confirming that it achieved approximately five times higher energy density (797Wh/kg), high power characteristics (275.8mW/cm²), and long-term stability even under repeated charge and discharge conditions compared to conventional batteries.
Additionally, the zinc-air battery, which operates using an aqueous electrolyte, is safe from fire risks. It is expected that this system can be applied as a next-generation energy storage device when linked with water electrolysis systems, offering an environmentally friendly method for hydrogen production.
< Figure 1. Illustrations of a trifunctional graphene-sandwiched heterojunction-embedded layered lattice (G-SHELL) structure. Schematic representation of a) synthesis procedures of G-SHELL from a zeolitic imidazole framework, b) hollow core-layered shell structure with trifunctional sites for oxygen reduction evolution (ORR), oxygen evolution reaction (OER), and hydrogen evolution reaction (HER), and c) heterojunctions, eterojunction-induced internal electric fields, and the corresponding band structure. >
Professor Kang explained, "By developing a catalyst material with high activity and durability for three different electrochemical catalytic reactions at low temperatures using simple methods, the self-powered hydrogen production system we implemented based on zinc-air batteries presents a new breakthrough to overcome the current limitations of green hydrogen production."
<Figure 2. Electrochemical performance of a ZAB-driven water-splitting cell with G-SHELL. Diagram of a self-driven water-splitting cell integrated by combining a ZAB with an alkaline water electrolyzer.>
PhD candidate Dong Won Kim and Jihoon Kim, a master's student in the Department of Materials Science and Engineering at KAIST, were co-first authors of this research, which was published in the international journal Advanced Science on September 17th in the multidisciplinary field of materials science. (Paper Title: “Trifunctional Graphene-Sandwiched Heterojunction-Embedded Layered Lattice Electrocatalyst for High Performance in Zn-Air Battery-Driven Water Splitting”)
This research was supported by the Nano and Material Technology Development Program of the Ministry of Science and ICT and the National Research Foundation of Korea’s Future Technology Research Laboratory.
2024.10.22 View 7372
KAIST Develops a Fire-risk Free Self-Powered Hydrogen Production System
KAIST researchers have developed a new hydrogen production system that overcomes the current limitations of green hydrogen production. By using a water-splitting system with an aqueous electrolyte, this system is expected to block fire risks and enable stable hydrogen production.
KAIST (represented by President Kwang Hyung Lee) announced on the 22nd of October that a research team led by Professor Jeung Ku Kang from the Department of Materials Science and Engineering developed a self-powered hydrogen production system based on a high-performance zinc-air battery*.
*Zinc-air battery: A primary battery that absorbs oxygen from the air and uses it as an oxidant. Its advantage is long life, but its low electromotive force is a disadvantage.
Hydrogen (H₂) is a key raw material for synthesizing high-value-added substances, and it is gaining attention as a clean fuel with an energy density (142 MJ/kg) more than three times higher than traditional fossil fuels (gasoline, diesel, etc.). However, most current hydrogen production methods impose environmental burden as they emit carbon dioxide (CO₂).
While green hydrogen can be produced by splitting water using renewable energy sources such as solar cells and wind power, these sources are subject to irregular power generation due to weather and temperature fluctuations, leading to low water-splitting efficiency.
To overcome this, air batteries that can emit sufficient voltage (greater than 1.23V) for water splitting have been gaining attention. However, achieving sufficient capacity requires expensive precious metal catalysts and the performance of the catalyst materials becomes significantly degraded during prolonged charge and discharge cycles. Thus, it is essential to develop catalysts that are effective for the water-splitting reactions (oxygen and hydrogen evolution) and materials that can stabilize the repeated charge and discharge reactions (oxygen reduction and evolution) in zinc-air battery electrodes.
In response, Professor Kang's research team proposed a method to synthesize a non-precious metal catalyst material (G-SHELL) that is effective for three different catalytic reactions (oxygen evolution, hydrogen evolution, and oxygen reduction) by growing nano-sized, metal-organic frameworks on graphene oxide.
The team incorporated the developed catalyst material into the air cathode of a zinc-air battery, confirming that it achieved approximately five times higher energy density (797Wh/kg), high power characteristics (275.8mW/cm²), and long-term stability even under repeated charge and discharge conditions compared to conventional batteries.
Additionally, the zinc-air battery, which operates using an aqueous electrolyte, is safe from fire risks. It is expected that this system can be applied as a next-generation energy storage device when linked with water electrolysis systems, offering an environmentally friendly method for hydrogen production.
< Figure 1. Illustrations of a trifunctional graphene-sandwiched heterojunction-embedded layered lattice (G-SHELL) structure. Schematic representation of a) synthesis procedures of G-SHELL from a zeolitic imidazole framework, b) hollow core-layered shell structure with trifunctional sites for oxygen reduction evolution (ORR), oxygen evolution reaction (OER), and hydrogen evolution reaction (HER), and c) heterojunctions, eterojunction-induced internal electric fields, and the corresponding band structure. >
Professor Kang explained, "By developing a catalyst material with high activity and durability for three different electrochemical catalytic reactions at low temperatures using simple methods, the self-powered hydrogen production system we implemented based on zinc-air batteries presents a new breakthrough to overcome the current limitations of green hydrogen production."
<Figure 2. Electrochemical performance of a ZAB-driven water-splitting cell with G-SHELL. Diagram of a self-driven water-splitting cell integrated by combining a ZAB with an alkaline water electrolyzer.>
PhD candidate Dong Won Kim and Jihoon Kim, a master's student in the Department of Materials Science and Engineering at KAIST, were co-first authors of this research, which was published in the international journal Advanced Science on September 17th in the multidisciplinary field of materials science. (Paper Title: “Trifunctional Graphene-Sandwiched Heterojunction-Embedded Layered Lattice Electrocatalyst for High Performance in Zn-Air Battery-Driven Water Splitting”)
This research was supported by the Nano and Material Technology Development Program of the Ministry of Science and ICT and the National Research Foundation of Korea’s Future Technology Research Laboratory.
2024.10.22 View 7372 -
 KAIST Develops Sodium Battery Capable of Rapid Charging in Just a Few Seconds
Sodium (Na), which is over 500 times more abundant than lithium (Li), has recently garnered significant attention for its potential in sodium-ion battery technologies. However, existing sodium-ion batteries face fundamental limitations, including lower power output, constrained storage properties, and longer charging times, necessitating the development of next-generation energy storage materials.
On the 11th of April, KAIST (represented by President Kwang Hyung Lee) announced that a research team led by Professor Jeung Ku Kang from the Department of Materials Science and Engineering had developed a high-energy, high-power hybrid sodium-ion battery capable of rapid charging.
The innovative hybrid energy storage system integrates anode materials typically used in batteries with cathodes suitable for supercapacitors. This combination allows the device to achieve both high storage capacities and rapid charge-discharge rates, positioning it as a viable next-generation alternative to lithium-ion batteries.
However, the development of a hybrid battery with high energy and high power density requires an improvement to the slow energy storage rate of battery-type anodes as well as the enhancement of the relatively low capacity of supercapacitor-type cathode materials.
< Figure 1. Schematic synthetic procedures of high-capacity/high-rate anode and cathode materials for a sodium-ion hybrid energy storages (SIHES) and their proposed energy storage mechanisms. Synthetic procedures for (a) ultrafine iron sulfide-embedded S-doped carbon/graphene (FS/C/G) anode and (b) zeolitic imidazolate framework-derived porous carbon (ZDPC) cathode materials. (c) Proposed energy storage mechanisms of Na+ ions in FS/C/G anode and ClO-4 ions in ZDPC cathode for an SIHES. >
To account for this, Professor Kang's team utilized two distinct metal-organic frameworks for the optimized synthesis of hybrid batteries. This approach led to the development of an anode material with improved kinetics through the inclusion of fine active materials in porous carbon derived from metal-organic frameworks. Additionally, a high-capacity cathode material was synthesized, and the combination of the cathode and anode materials allowed for the development of a sodium-ion storage system optimizing the balance and minimizing the disparities in energy storage rates between the electrodes.
The assembled full cell, comprising the newly developed anode and cathode, forms a high-performance hybrid sodium-ion energy storage device. This device surpasses the energy density of commercial lithium-ion batteries and exhibits the characteristics of supercapacitors' power density. It is expected to be suitable for rapid charging applications ranging from electric vehicles to smart electronic devices and aerospace technologies.
< Figure 2. Electrochemical characterizations of FS/C/G-20//ZDPC SIHES full cells (left). Ragone plots for FS/C/G-20//ZDPC (this work) and other previously reported sodium-ion electrochemical energy storage devices (right). >
Professor Kang noted that the hybrid sodium-ion energy storage device, capable of rapid charging and achieving an energy density of 247 Wh/kg and a power density of 34,748 W/kg, represents a breakthrough in overcoming the current limitations of energy storage systems. He anticipates broader applications across various electronic devices, including electric vehicles.
This research, co-authored by KAIST doctoral candidates Jong Hui Choi and Dong Won Kim, was published in the international journal Energy Storage Materials on March 29 with the title "Low-crystallinity conductive multivalence iron sulfide-embedded S-doped anode and high-surface-area O-doped cathode of 3D porous N-rich graphitic carbon frameworks for high-performance sodium-ion hybrid energy storages."
The study was conducted with support from the Ministry of Science and ICT and the National Research Foundation of Korea through the Nanomaterial Technology Development Project.
2024.04.18 View 20114
KAIST Develops Sodium Battery Capable of Rapid Charging in Just a Few Seconds
Sodium (Na), which is over 500 times more abundant than lithium (Li), has recently garnered significant attention for its potential in sodium-ion battery technologies. However, existing sodium-ion batteries face fundamental limitations, including lower power output, constrained storage properties, and longer charging times, necessitating the development of next-generation energy storage materials.
On the 11th of April, KAIST (represented by President Kwang Hyung Lee) announced that a research team led by Professor Jeung Ku Kang from the Department of Materials Science and Engineering had developed a high-energy, high-power hybrid sodium-ion battery capable of rapid charging.
The innovative hybrid energy storage system integrates anode materials typically used in batteries with cathodes suitable for supercapacitors. This combination allows the device to achieve both high storage capacities and rapid charge-discharge rates, positioning it as a viable next-generation alternative to lithium-ion batteries.
However, the development of a hybrid battery with high energy and high power density requires an improvement to the slow energy storage rate of battery-type anodes as well as the enhancement of the relatively low capacity of supercapacitor-type cathode materials.
< Figure 1. Schematic synthetic procedures of high-capacity/high-rate anode and cathode materials for a sodium-ion hybrid energy storages (SIHES) and their proposed energy storage mechanisms. Synthetic procedures for (a) ultrafine iron sulfide-embedded S-doped carbon/graphene (FS/C/G) anode and (b) zeolitic imidazolate framework-derived porous carbon (ZDPC) cathode materials. (c) Proposed energy storage mechanisms of Na+ ions in FS/C/G anode and ClO-4 ions in ZDPC cathode for an SIHES. >
To account for this, Professor Kang's team utilized two distinct metal-organic frameworks for the optimized synthesis of hybrid batteries. This approach led to the development of an anode material with improved kinetics through the inclusion of fine active materials in porous carbon derived from metal-organic frameworks. Additionally, a high-capacity cathode material was synthesized, and the combination of the cathode and anode materials allowed for the development of a sodium-ion storage system optimizing the balance and minimizing the disparities in energy storage rates between the electrodes.
The assembled full cell, comprising the newly developed anode and cathode, forms a high-performance hybrid sodium-ion energy storage device. This device surpasses the energy density of commercial lithium-ion batteries and exhibits the characteristics of supercapacitors' power density. It is expected to be suitable for rapid charging applications ranging from electric vehicles to smart electronic devices and aerospace technologies.
< Figure 2. Electrochemical characterizations of FS/C/G-20//ZDPC SIHES full cells (left). Ragone plots for FS/C/G-20//ZDPC (this work) and other previously reported sodium-ion electrochemical energy storage devices (right). >
Professor Kang noted that the hybrid sodium-ion energy storage device, capable of rapid charging and achieving an energy density of 247 Wh/kg and a power density of 34,748 W/kg, represents a breakthrough in overcoming the current limitations of energy storage systems. He anticipates broader applications across various electronic devices, including electric vehicles.
This research, co-authored by KAIST doctoral candidates Jong Hui Choi and Dong Won Kim, was published in the international journal Energy Storage Materials on March 29 with the title "Low-crystallinity conductive multivalence iron sulfide-embedded S-doped anode and high-surface-area O-doped cathode of 3D porous N-rich graphitic carbon frameworks for high-performance sodium-ion hybrid energy storages."
The study was conducted with support from the Ministry of Science and ICT and the National Research Foundation of Korea through the Nanomaterial Technology Development Project.
2024.04.18 View 20114 -
 Energy Storage Using Oxygen to Boost Battery Performance
Researchers have presented a novel electrode material for advanced energy storage device that is directly charged with oxygen from the air. Professor Jeung Ku Kang’s team synthesized and preserved the sub-nanometric particles of atomic cluster sizes at high mass loadings within metal-organic frameworks (MOF) by controlling the behavior of reactants at the molecular level. This new strategy ensures high performance for lithium-oxygen batteries, acclaimed as a next-generation energy storage technology and widely used in electric vehicles.
Lithium-oxygen batteries in principle can generate ten times higher energy densities than conventional lithium-ion batteries, but they suffer from very poor cyclability. One of the methods to improve cycle stability is to reduce the overpotential of electrocatalysts in cathode electrodes. When the size of an electrocatalyst material is reduced to the atomic level, the increased surface energy leads to increased activity while significantly accelerating the material’s agglomeration.
As a solution to this challenge, Professor Kang from the Department of Materials Science and Engineering aimed to maintain the improved activity by stabilizing atomic-scale sized electrocatalysts into the sub-nanometric spaces. This is a novel strategy for simultaneously producing and stabilizing atomic-level electrocatalysts within metal-organic frameworks (MOFs).
Metal-organic frameworks continuously assemble metal ions and organic linkers.
The team controlled hydrogen affinities between water molecules to separate them and transfer the isolated water molecules one by one through the sub-nanometric pores of MOFs. The transferred water molecules reacted with cobalt ions to form di-nuclear cobalt hydroxide under precisely controlled synthetic conditions, then the atomic-level cobalt hydroxide is stabilized inside the sub-nanometric pores.
The di-nuclear cobalt hydroxide that is stabilized in the sub-nanometric pores of metal-organic frameworks (MOFs) reduced the overpotential by 63.9% and showed ten-fold improvements in the life cycle.
Professor Kang said, “Simultaneously generating and stabilizing atomic-level electrocatalysts within MOFs can diversify materials according to numerous combinations of metal and organic linkers. It can expand not only the development of electrocatalysts, but also various research fields such as photocatalysts, medicine, the environment, and petrochemicals.”
This study was reported in Advanced Science (Title: Autogenous Production and Stabilization of Highly Loaded Sub-Nanometric Particles within Multishell Hollow Metal-Organic Frameworks and Their Utilization for High Performance in Li-O2 Batteries).
This research was mainly supported by the Global Frontier R&D Program of the Ministry of Science, ICT & Planning (Grant No. 2013M3A6B1078884) funded by the Ministry of Science, ICT & Future Planning, and the National Research Foundation of Korea (Grant No. 2019M3E6A1104196).
Profile:Professor Jeung Ku Kang
jeungku@kaist.ac.kr
http://nanosf.kaist.ac.kr/
Nano Materials Simulation and Fabrication Laboratory
Department of Materials Science and Engineering
KAIST
2020.06.15 View 15164
Energy Storage Using Oxygen to Boost Battery Performance
Researchers have presented a novel electrode material for advanced energy storage device that is directly charged with oxygen from the air. Professor Jeung Ku Kang’s team synthesized and preserved the sub-nanometric particles of atomic cluster sizes at high mass loadings within metal-organic frameworks (MOF) by controlling the behavior of reactants at the molecular level. This new strategy ensures high performance for lithium-oxygen batteries, acclaimed as a next-generation energy storage technology and widely used in electric vehicles.
Lithium-oxygen batteries in principle can generate ten times higher energy densities than conventional lithium-ion batteries, but they suffer from very poor cyclability. One of the methods to improve cycle stability is to reduce the overpotential of electrocatalysts in cathode electrodes. When the size of an electrocatalyst material is reduced to the atomic level, the increased surface energy leads to increased activity while significantly accelerating the material’s agglomeration.
As a solution to this challenge, Professor Kang from the Department of Materials Science and Engineering aimed to maintain the improved activity by stabilizing atomic-scale sized electrocatalysts into the sub-nanometric spaces. This is a novel strategy for simultaneously producing and stabilizing atomic-level electrocatalysts within metal-organic frameworks (MOFs).
Metal-organic frameworks continuously assemble metal ions and organic linkers.
The team controlled hydrogen affinities between water molecules to separate them and transfer the isolated water molecules one by one through the sub-nanometric pores of MOFs. The transferred water molecules reacted with cobalt ions to form di-nuclear cobalt hydroxide under precisely controlled synthetic conditions, then the atomic-level cobalt hydroxide is stabilized inside the sub-nanometric pores.
The di-nuclear cobalt hydroxide that is stabilized in the sub-nanometric pores of metal-organic frameworks (MOFs) reduced the overpotential by 63.9% and showed ten-fold improvements in the life cycle.
Professor Kang said, “Simultaneously generating and stabilizing atomic-level electrocatalysts within MOFs can diversify materials according to numerous combinations of metal and organic linkers. It can expand not only the development of electrocatalysts, but also various research fields such as photocatalysts, medicine, the environment, and petrochemicals.”
This study was reported in Advanced Science (Title: Autogenous Production and Stabilization of Highly Loaded Sub-Nanometric Particles within Multishell Hollow Metal-Organic Frameworks and Their Utilization for High Performance in Li-O2 Batteries).
This research was mainly supported by the Global Frontier R&D Program of the Ministry of Science, ICT & Planning (Grant No. 2013M3A6B1078884) funded by the Ministry of Science, ICT & Future Planning, and the National Research Foundation of Korea (Grant No. 2019M3E6A1104196).
Profile:Professor Jeung Ku Kang
jeungku@kaist.ac.kr
http://nanosf.kaist.ac.kr/
Nano Materials Simulation and Fabrication Laboratory
Department of Materials Science and Engineering
KAIST
2020.06.15 View 15164 -
 'The 2016 Top 100 Research Projects in Korea'
The Ministry of Science, ICT and Future Planning (MSIP) of Korea recently released a list of the 2016 Top 100 Research Projects in Korea. The list included the work of KAIST Professors Dong-Ho Cho of the School of Electrical Engineering, Jeung Ku Kang of the Graduate School of Energy, Environment, Water and Sustainability (EEWS), and Sang Yup Lee of the Chemical and Biomolecular Engineering Department.
Experts from academia, universities, and industries selected the 100 research projects, among 620 projects recommended by various government offices, in consideration of their contribution to the growth of science and technology in the nation.
Professor Cho conducts research on the development of 5G mobile communication systems based on the pattern polarization beam-division multiple access method.
Professor Kang works on the production of highly efficient energy materials and equipment by controlling them at the electron and atomic level.
Professor Lee focuses on the creation of strategies to produce important chemicals through a biological approach, i.e., microorganisms, which will help develop the means to mitigate climate change.
The MISP will publish a book that describes in detail each research project and will distribute copies of it to the National Assembly of Korea, libraries, and other public organizations.
For more information on the list, please go to www.ntis.go.kr.
Pictured from left to right are Professors Dong-Ho Cho, Jeung Ku Kang, and Sang Yup Lee.
2016.07.21 View 5928
'The 2016 Top 100 Research Projects in Korea'
The Ministry of Science, ICT and Future Planning (MSIP) of Korea recently released a list of the 2016 Top 100 Research Projects in Korea. The list included the work of KAIST Professors Dong-Ho Cho of the School of Electrical Engineering, Jeung Ku Kang of the Graduate School of Energy, Environment, Water and Sustainability (EEWS), and Sang Yup Lee of the Chemical and Biomolecular Engineering Department.
Experts from academia, universities, and industries selected the 100 research projects, among 620 projects recommended by various government offices, in consideration of their contribution to the growth of science and technology in the nation.
Professor Cho conducts research on the development of 5G mobile communication systems based on the pattern polarization beam-division multiple access method.
Professor Kang works on the production of highly efficient energy materials and equipment by controlling them at the electron and atomic level.
Professor Lee focuses on the creation of strategies to produce important chemicals through a biological approach, i.e., microorganisms, which will help develop the means to mitigate climate change.
The MISP will publish a book that describes in detail each research project and will distribute copies of it to the National Assembly of Korea, libraries, and other public organizations.
For more information on the list, please go to www.ntis.go.kr.
Pictured from left to right are Professors Dong-Ho Cho, Jeung Ku Kang, and Sang Yup Lee.
2016.07.21 View 5928 -
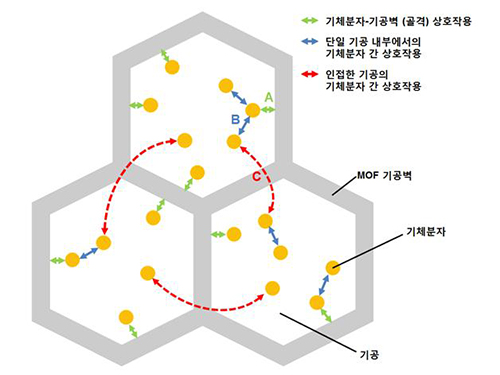 A New Way to Look at MOFs
An international research team composed of researchers from KAIST (led by Professors Osamu Terasaki and Jeung Ku Kang at the Graduate School of Energy, Environment, Water and Sustainability) and other universities, including UC Berkeley, has recently published research results on the adsorption process of metal-organic frameworks (MOFs) in Nature (November 9, 2015).
MOFs are porous three-dimensional crystals with a high internal surface area, which have a wide range of applications involving adsorption such as hydrogen, methane, or carbon dioxide storage. In the paper entitled “Extra Adsorption and Adsorbate Superlattice Formation in Metal-organic Frameworks,” the research team described their observation of a very specific interpore interaction process in MOFs.
For additional information, please see:
A New Way to Look at MOFs
International study challenges prevailing view on how metal organic frameworks store gases
EurekAlert, November 9, 2015
http://www.eurekalert.org/pub_releases/2015-11/dbnl-anw110915.php
(Courtesy of the US Department of Energy and Lawrence Berkeley National Laboratory news release)
2015.11.13 View 9333
A New Way to Look at MOFs
An international research team composed of researchers from KAIST (led by Professors Osamu Terasaki and Jeung Ku Kang at the Graduate School of Energy, Environment, Water and Sustainability) and other universities, including UC Berkeley, has recently published research results on the adsorption process of metal-organic frameworks (MOFs) in Nature (November 9, 2015).
MOFs are porous three-dimensional crystals with a high internal surface area, which have a wide range of applications involving adsorption such as hydrogen, methane, or carbon dioxide storage. In the paper entitled “Extra Adsorption and Adsorbate Superlattice Formation in Metal-organic Frameworks,” the research team described their observation of a very specific interpore interaction process in MOFs.
For additional information, please see:
A New Way to Look at MOFs
International study challenges prevailing view on how metal organic frameworks store gases
EurekAlert, November 9, 2015
http://www.eurekalert.org/pub_releases/2015-11/dbnl-anw110915.php
(Courtesy of the US Department of Energy and Lawrence Berkeley National Laboratory news release)
2015.11.13 View 9333